- 1Department of Public Health and Community Medicine, Tufts University School of Medicine, Boston, MA, United States
- 2School of Medicine, Tufts University School of Medicine, Boston, MA, United States
- 3Department of Public Health and Community Medicine, Universidad de Antioquia, Medellin, Columbia
- 4Cochrane US Mentoring Program, Boston, MA, United States
- 5US Mentoring Program, Boston, MA, United States
- 6WHO Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva, Switzerland
Background: Contraceptives play a crucial role in women's reproductive health, their hormonal components may be linked to cancer risks, specifically breast, and gynecological cancers. Given the high usage rates of hormonal contraceptives, it is vital to systematically evaluate their potential impact on cancer outcomes, especially among women with a family history of gynecological cancers.
Objectives: This study aims to evaluate the evidence on the association between modern contraceptive use and the risk of breast and reproductive cancers (ovarian, endometrial, and cervical cancer) among women of reproductive age, to inform healthcare providers, women, and program managers about cancer outcomes related to contraceptive use.
Methods: A systematic review was conducted according to PRISMA guidelines. Searches were performed in databases such as CINAHL, OVID Medline, EMBASE, and more from inception to February 2022. Eligible studies included randomized controlled trials, cohort studies, and case-control studies that compared cancer outcomes between contraceptive users and non-users. Data extraction, quality assessment, and meta-analyses were conducted following predefined protocols. Subgroup and sensitivity analyses examined variations in contraceptive methods, doses, and duration.
Results: A total of 51 studies were included, comprising 2 RCTs and 49 observational studies. The review identified a significant reduction in ovarian and endometrial cancer incidence among contraceptive users. Hormonal contraceptive users had a 36% lower risk of ovarian cancer (RR 0.64, 95% CI 0.60–0.68), with specific reductions seen in combined oral contraceptive users (RR 0.62, 95% CI 0.57–0.68) and hormonal IUD users (RR 0.68, 95% CI 0.48–0.96). The rate ratio of cervical cancer was higher among non- users compared to hormonal contraceptive users when we pooled the results (1.28, 95% CI 1.21, 1.35). No significant association was found between contraceptive use and breast cancer risk among healthy women (RR 1.00, 95% CI 0.94–1.06). However, BRCA1/2 mutation carriers using oral contraceptives showed a heightened risk of breast cancer (HR 1.39, 95% CI 1.15–1.67).
Conclusion: This systematic review highlights the protective effects of modern contraceptives against ovarian and endometrial cancers while identifying an increased risk of cervical. No significant breast cancer risk was found for healthy women, but BRCA1/2 mutation carriers faced increased risks. These findings underscore the need for personalized contraceptive counselling that considers cancer risk factors. Further research is needed to explore contraceptive impacts across different genetic profiles and dosing regimens.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, Prospero (CRD42022332647).
Background
Over the last two decades, the number of women using a modern contraceptive increased from 663 million to 851 million. By the year 2030, it is projected that an additional 70 million women will be using contraception (1). Modern contraceptives have therefore become an integral part of women's health globally.
Modern contraceptives come in three forms: short-acting, long-acting, and one-time barrier. Short-acting contraceptives include oral contraceptive pills (OCPs) (151 million users, 16%), injections (74 million users, 8%), and patches and vaginal rings (less than 15 million users, less than 2%). Long-acting contraceptives include intrauterine devices (IUDs) (159 million users, 17%), implanted devices (23 million users, 2%), and female sterilization (219 million users, 24%). One-time barrier contraceptives include sponges, diaphragms, cervical caps, spermicide, female condoms, and male condoms. Research shows that global use for all one-time barrier methods except for male condom use (189 million users, 21%) is low (1).
Hormonal methods of contraception contain either a combination of estrogen and progestin, or progestin only. Reproductive hormones of the ovaries, estrogen and progesterone, play a critical role in the genesis of breast, and gynecological cancers, as these two hormones are carcinogens (2). Worldwide, breast cancer are the top two most common cancer incidence in women following gynecological cancers: cervical 4th, endometrial/uterine 6th, and ovary 8th most common cancers (3).
Given the potential links between modern contraceptive use and cancer risk, it is critical for women, health care providers, and program managers to be well-informed about the benefits and risks of each method. The current medical eligibility criteria for safe contraceptive use does not have guidelines for women with a family history of gynecological cancers (see Supplementary Appendix 1 - A) (4). Despite the widespread use of contraceptives, guidelines for women with a family history of gynecological cancers are lacking. This study seeks to systematically evaluate evidence regarding the impact of contraceptives on cancer outcomes among women of reproductive age, emphasizing key factors influencing reproductive cancer risk. The review attempts to enhance awareness among women, healthcare providers, and program managers, facilitating informed decision-making regarding contraceptive choices in the context of cancer risks. This study aims to identify and evaluate evidence that focuses on the use of contraceptives and their impact on cancer outcomes among women of reproductive age. There are three factors that we considered in connection to reproductive cancer risk throughout this review: Contraceptive use, genetic background and lifestyle factors.
Methods
This systematic review is registered on Prospero (CRD42022332647). It is focused on quantitative evidence with reportable outcomes and measures of risk regarding the relationship between the use of contraceptives and cancer morbidity and mortality among women of reproductive age. This review also focused on evidence that is consistent within a specific population, intervention, comparison, outcome, and study design (PICO) or population, exposure, comparison, outcome and study design (PECO). The population of interest was women of reproductive age (14–49 years of age). When drawing data from interventional studies, all modern contraceptive methods that the WHO defines as effective and acceptable were included. Such methods include (1) short-acting hormonal contraception (e.g., OCPs, patches, and vaginal rings), (2) long-term contraception (e.g., hormonal or non-hormonal IUD, implants, and injections), (3) one-time barrier contraception (e.g., condoms, sponges, diaphragms, cervical caps, and spermicide), (4) permanent contraception (e.g., tubal ligation and vasectomy), and (5) emergency contraception (e.g., morning after pill or IUD). Contraceptive use of all types (as stated above) was considered the main exposure for observational studies. Any other study that investigated contraception in conjunction with other medications or modalities was excluded. We included studies whose comparison group consisted of non-users of contraception only. The outcome of interest was mortality and morbidity due to breast eproductive tract cancers (e.g., ovarian cancer, endometrial cancer, and cervical cancer). We included studies with any one of the following study designs: parallel or cluster randomized controlled trials (RCTs), controlled clinical trials, controlled before and after studies, interrupted time series studies, cohort or longitudinal analyses, regression discontinuity designs, and case- control studies.
To avoid publication bias, we searched for published or unpublished studies from inception to February 2022, with no language or geographical boundaries. Inception dates vary by database; we searched the CINAHL, OVID Medline, EMBASE, Psycho INFO, Maternity & Infant Care, LILACS, clinical trial.gov, web of science, SCOPUS, and CENTRAL Database. We also included WHO local databases as follows (see Supplementary Appendix 1 - B).
We checked the references of the reviews and references of the original RCTs to ensure no original study was missed. Our search strategy was designed by our Health Librarian at Tufts, and was approved by WHO counterpart (see Supplementary Appendix 1 - C). We used OpenGrey (https://opengrey.eu), Google, and Google Scholar to obtain relevant grey literature.
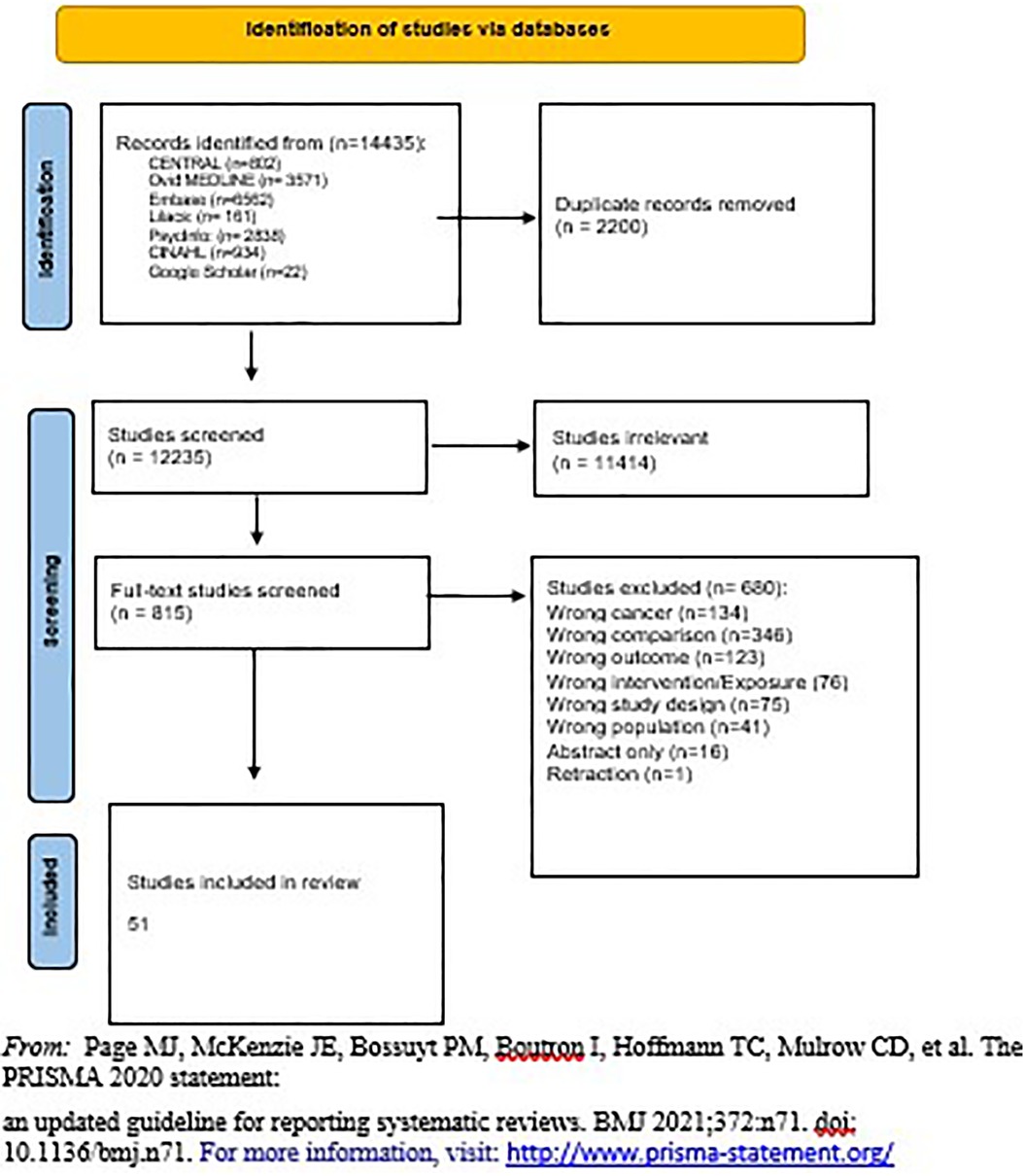
The retrieved review articles from the search strategy results were imported into Mendeley to remove duplicate copies and exported to Covidence software (Veritas Health Innovation, Melbourne, Australia). A PRISMA flow diagram was used to show the selection of the studies.
Title and abstract screening was conducted using Covidence and our eligibility criteria. Our team of ten reviewers obtained a Kappa score of more than 7 in pairs. Upon inclusion, the studies were imported into the second stage of screening, a full-text screening for further assessment of their appropriateness. Studies that were approved after full-text review underwent quality appraisal and data extraction. One reviewer extracted the data while the other reviewed the data extraction for accuracy. The second data extractor was responsible for creating forest plots in Revman when feasible. Reasons for exclusion were documented in the PRISMA flow diagram. Covidence identified evaluation differences between reviewers at each stage of the selection process and, subsequently, disagreements were resolved by discussion.
Studies were separated into three major categories: RCTs, cohort, and case control. When we had two or more studies for a comparison and outcome, a forest plot was created. For studies not included in the forest plot, a short summary of the study was placed in Supplementary Appendix 1 - D (5–23).
Quality assessment
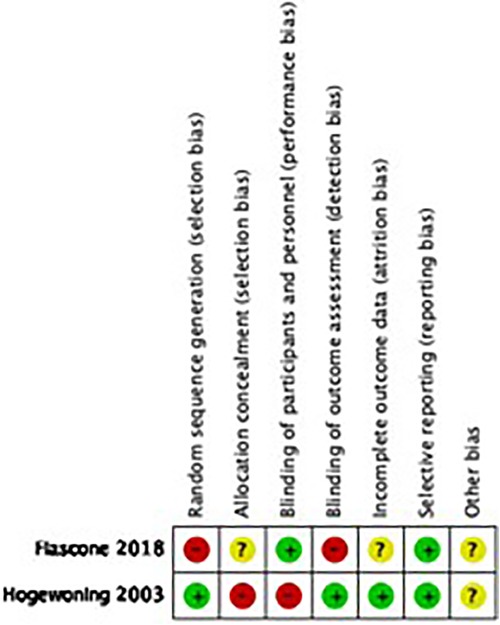
We assessed the risk of bias (high, moderate, or low) in each included study following EPOC criteria (for observational studies) and the Cochrane Handbook for Systematic Reviews of Interventions (24). For each RCT, we examined sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential biases. For quasi-randomized trials, we used the GRADE (Grades of Recommendation, Assessment, Development, and Evaluation) “risk of bias” framework, which is reported in the eligibility criteria, exposure measurement, outcome, confounding, and attrition rates (25).
For observational studies, we used the Dawn and Black quality assessment tool. This tool contains items such as clarity and external and internal validity (bias, exposure, and confounding) and culminates in a numerical score (26, 27).
Analysis
The unit of analysis was the individual participants in each study. The analysis of this review was limited to the analytical method used in the trial report (e.g., intent to treat, per protocol, or a modification of either type). Studies were combined for meta-analysis only when identical modern contraceptive devices, tools, or drugs; dosages; and regimens could be compared. We calculated the rate ratios (RR) and hazard ratios (HR) with a 95% confidence interval (95% CI) for each dichotomous outcome. We created forest plots for three-time points when possible: less than 5 years, more than 5 years, more than 10 years. If outcomes were reported for multiple reference periods, we reported the outcomes for the first and last reference periods only.
Subgroup analysis and sensitivity analysis
Subgroup analysis was conducted by comparing different types of contraception, dose, and route of administration when possible. We also conducted a sensitivity analysis to test the robustness of any results that appeared to be based on heterogeneous combinations by examining the effect of deleting each study. Finally, sensitivity analyses were conducted based on rates of loss to follow-up and any study with a rate of loss to follow-up over 20% was excluded.
Assessment of heterogeneity
We conducted a meta-analysis if two data points or more presented themselves for each comparison and outcome. We visually examined heterogeneity by comparing study designs, target populations, and primary outcome measures across included studies. We assessed the homogeneity of trials combined in a meta-analysis using both fixed-effects and random-effects models. The classical measure of heterogeneity is Cochran's Q, which was calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies. Q is distributed as a chi-square statistic, and the alpha level is set at 0.10. We then used the I2 score to identify the magnitude of heterogeneity. Any score of I2 above 50% was investigated for the clinical and methodological diversity of the studies.
GRADE profiler (GRADEpro 2020) was used to import data from Review Manager (Revman) 5.3 to create a “Summary of findings” table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach, which involves five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the evidence for each outcome.
Results
The Prisma chart in Figure 1 demonstrates the number of studies included in the search from different sources as well as the number of studies screened and included in the review. Separate publications discuss the connections between family planning methodologies and other non- reproductive health outcomes including irregular menstruation and changes in mental health.
The total number of included studies was 51, 2 of which were randomized clinical trials (RCTs) (7, 8) and 49 of which were observational studies (7 case-control studies (14, 15, 28–32) and 42 cohort studies (5, 6, 9–13, 16–23, 33–60). Supplementary Appendix 2 - Table S1 shows characteristics of the RCTs, including country of origin, year of publication, number of facilities, type of health facility, level of health facility, sample size, study design, population, type of contraception studied, the outcome of interest extracted, and quality of study based on design. Similar data (with exposure instead of intervention) was extracted for observational studies (Supplementary Appendix 2 - Table S2). Most of the studies were from 2000 onward, while eight were published before 2000.
Studies focused on either one form of contraception (OCPs, ring/patch, implant, injection, IUD, condoms, sterilization), a combination of contraceptives, or all HCs.
Comparisons were set based on available literature and the protocol on either all HCs vs. no contraceptive use or OC use vs. non-use. In cases where other types of contraceptives were studied, comparisons were made between use and non-use. Subgroup analysis can be seen is some of the forest plots where different contraceptives are used (e.g., OCPs vs. IUDs).
Outcomes of interest were morbidities and mortalities related to and reproductive cancers.
Quality assessment
Figure 2 reflects the quality assessment of included studies and is presented below.
Supplementary Appendix 2 -Table S3 shows the quality assessment of observational studies using Dawn and Black scoring system. We considered the overall quality of evidence to be moderate for our review (mean: 13.24 ± 2.25, median = 14 min = 5, max = 16).
Cohort studies
Ovarian cancer
We found 8 cohort studies that investigated OC use in relation to ovarian cancer rate (33–36, 39–41, 57), three investigated hormonal IUDs (36, 43, 46), two studies analyzed injectable contraception (36, 44), and three studies compared any hormonal contraceptive users to non- users (36, 39, 45).
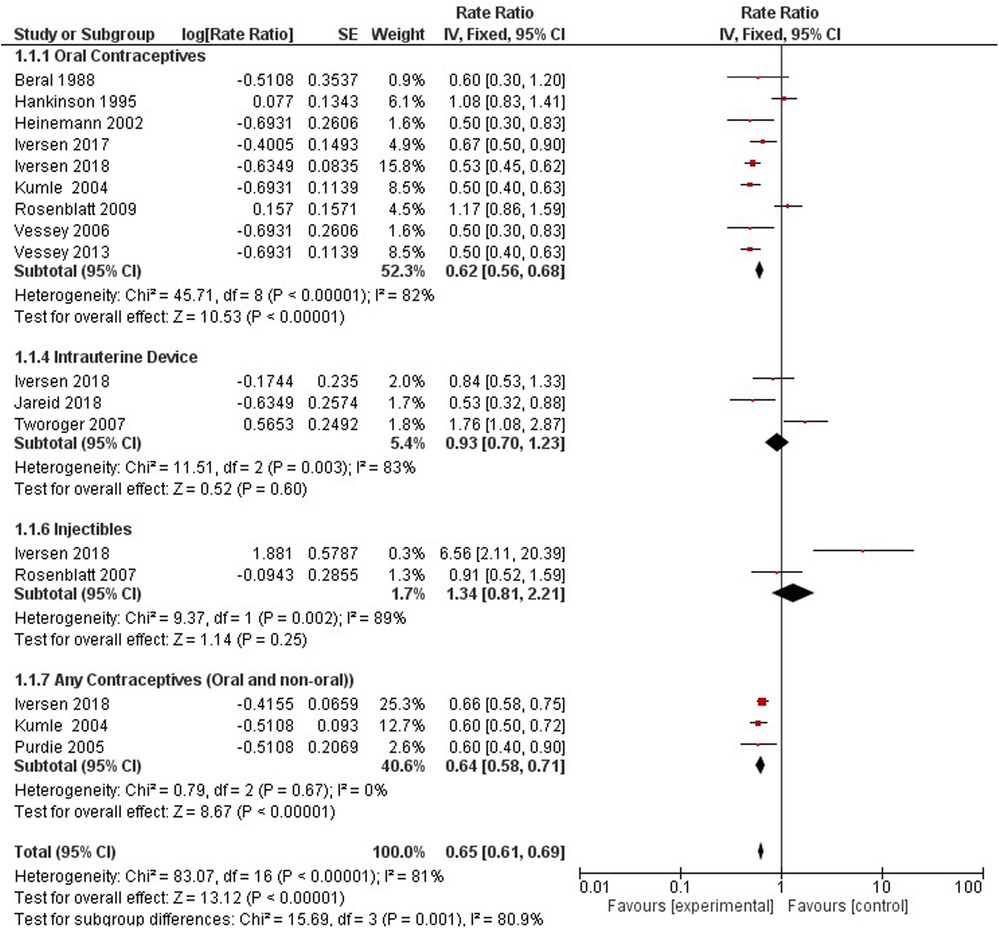
Compared with non-users, all hormonal contraceptive users had a 36% lower rate ratio of ovarian cancer (0.64, 95% CI 0.60, 0.68). We stratified the type of contraceptive use into OCP (0.62, 95% CI 0.57–0.68), hormonal IUS (0.68, 95% CI 0.48, 0.96), injectables (1.34, 95% CI 0.81, 2.21), and any contraceptives (oral or non-oral) (0.64, 95% CI 0.58, 0.71) based on what studies presented (Figure 3).
Five studies provided information on the hormonal contraception dosing regimens used among women and compared them to non-hormonal method users (35, 36, 39, 43, 44). Progestogen-only products include oral (Norethisterone, Levonorgestrel, Desogestrel) and non-oral (MPA depot, Implant, LNG-IUS) methods (36). Combined HC includes oral ethinylestradiol and non-oral patch and vaginal ring. (Supplementary Appendix 3-Figure S1) Norethisterone 50 mg ethinylestradiol, Levonorgestrel 50 ethinylestradiol, Norethisterone 30–35 mg ethinylestradiol, Drospirenone 20–35 mg ethinylestradiol, Noregestimate 35 mg ethinylestradiol, Norethisterone Progestin- Only Oral, Levonorgestrel Progestogen-Only Oral, Hydroxyprogesterone Caproate 5 mg Estradiol Valerate, and Progestin only methods (oral and non-oral) showed no significant effect on ovarian cancer risk. The following regimens were significantly associated with a reduced risk in ovarian cancer incidence compared to non-users: Levonorgestrel 30–35 mg ethinylestradiol (RR 0.33, 95% CI 0.18–0.61), Desogestrel 20–30 mg ethinylestradiol (RR 0.45, 95% CI 0.27–0.75), Gestodene 20–35 mg ethinylestradiol (RR 0.57, 95% CI 0.41–0.79), LNG-IUS Progestogen (RR 0.68, 95% CI 0.48–0.96), and any combined oral pills (RR 0.56, 95% CI 0.49–0.64). Over a 6-fold increase in ovarian cancer incidence was observed among women using MPA Depot Progestogen compared to non-users of hormonal contraception (RR 6.56, 95% CI 2.11–20.39).
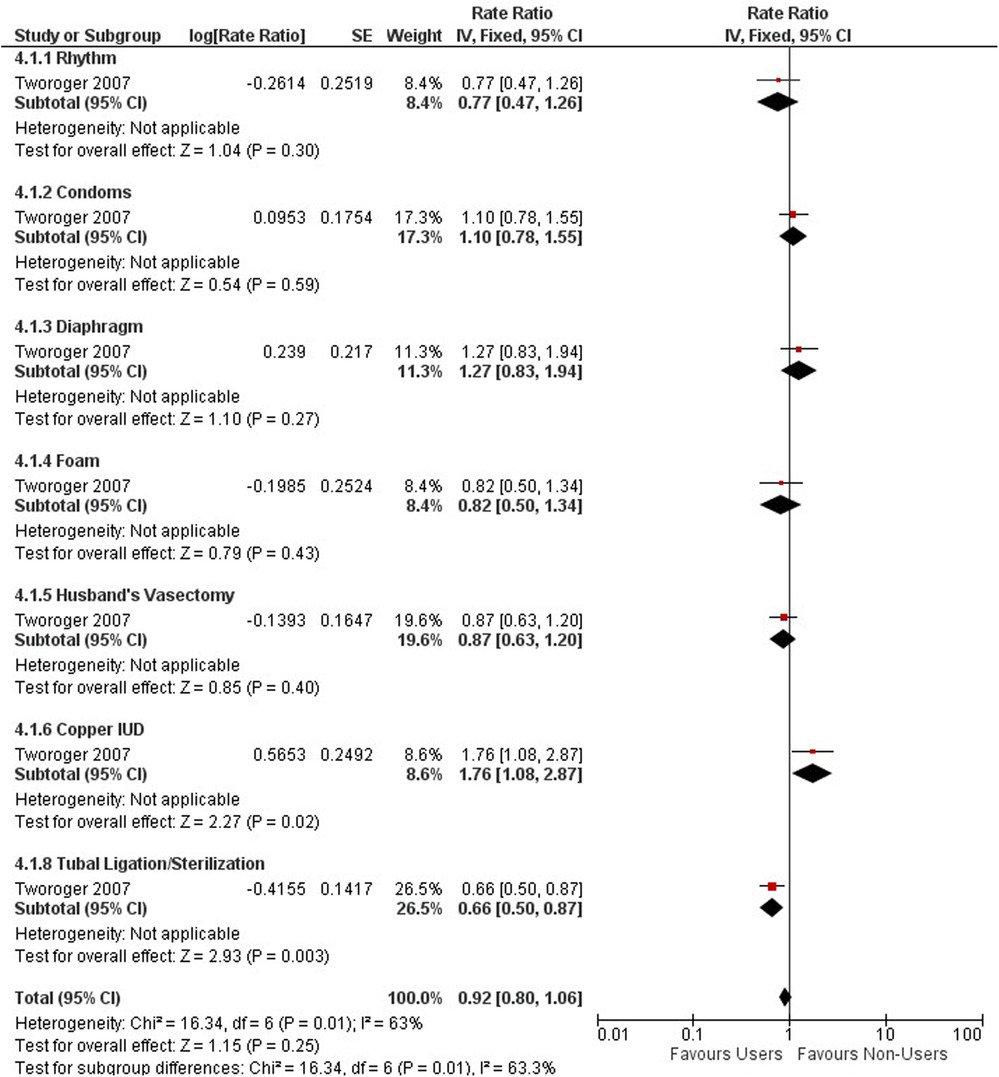
One study with 2,479,493 person-years from 107,900 women and 861 confirmed ovarian cancer cases (1,243 cases self-identified), investigated nonhormonal methods of contraception on the relative risk of ovarian cancer (46). Compared with non-users, there was no increased or decreased risk of ovarian cancer among non-hormonal method users (RR 0.92; 95% CI 0.80–1.06) (see Figure 4). We stratified the type of non-hormonal contraceptive methods and found significant protective effects for tubal ligation (RR 0.66; 95% CI 0.50–0.87). Conversely, we identified an increased risk by 76% in women using copper IUD's (RR 1.76; 95% CI 1.08–2.87).
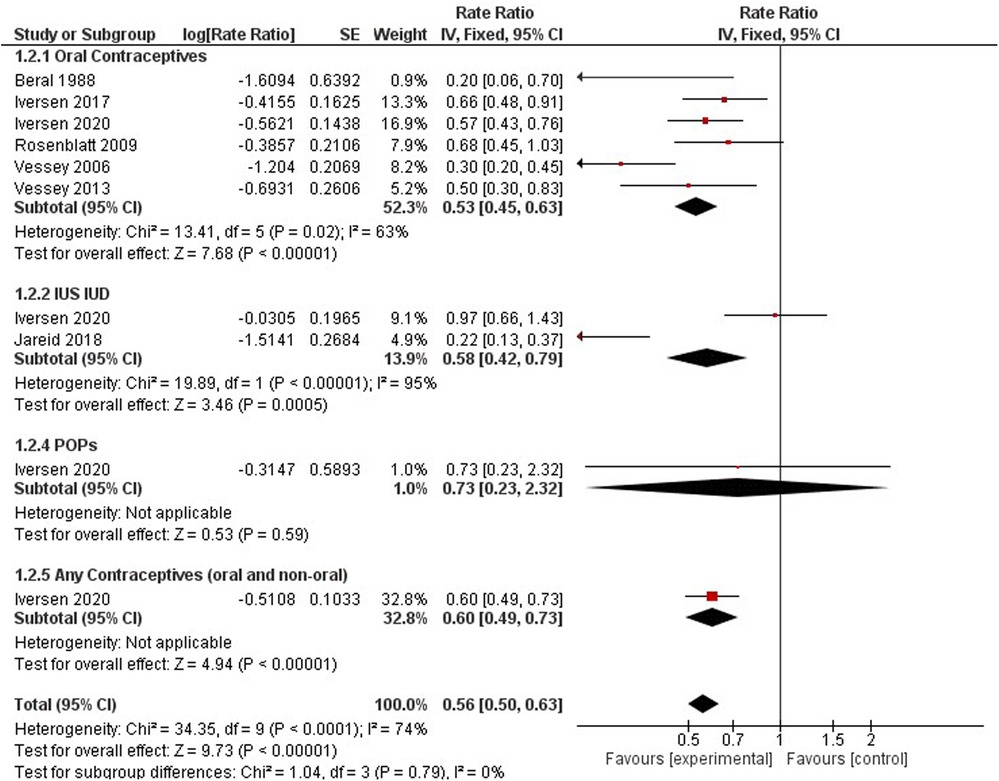
Endometrial cancer
Contraceptive users had a significantly lower risk of endometrial cancer incidence when compared to non-users (0.56, 95% CI 0.50, 0.63). We identified six studies on oral contraceptives (33, 35, 37, 40, 41, 57), two studies on hormonal IUS (36), one study on progestin-only products (37), and one study for any contraceptives (oral and non-oral) (37). The rate ratio for oral contraceptive users was 0.53 (95% CI 0.45, 0.63), for hormonal IUS 0.58 (95% CI 0.42, 0.79), for progestin-only products 0.73 (95% CI 0.23, 2.32) and for any contraception 0.61 (95% CI 0.49, 0.76). All hormonal contraception methods had a significant downward trend for endometrial incidence, except progestin-only products had no significant results (Figure 5).
Three studies investigated the associated risk incidence of endometrial cancer by comparing varying dosing regimens to non-users (37, 43, 44).
A significant protective effect against endometrial cancer incidence was observed among the following dosing regimens: Gestodene 20–35 mg ethinylestradiol (RR 0.27, 95% CI 0.12–0.61), LNG-IUS (Supplementary Appendix 3 - Figure S2).
Progestogen Only Non-oral (RR 0.22, 95% CI 0.13–0.37), Alpha-hydroxyprogesterone Caproate and 5 mg Estradiol Valerate (RR 0.27, 95% CI 0.12–0.61), and any combined oral pills (RR 0.66, 95% CI 0.48–0.91). A different cohort with 116, 429 females observed protective effects of estrogen or progestin formulations against endometrial cancer (HR 0.77; 95% CI 0.69–0.85) (60) (Figure 8).
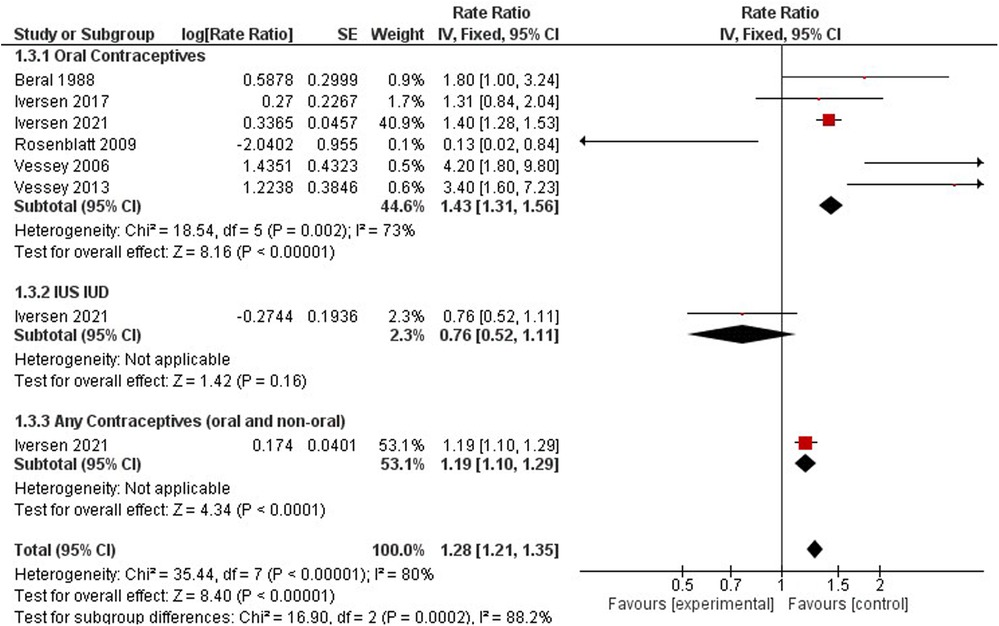
Cervical cancer
Cervical cancer was studied by six studies on oral contraceptives (33, 35, 38, 40, 41, 57), one study on hormonal IUS (38), and one study for any contraceptives (oral and non-oral) (38) (Figure 7). The rate ratio of cervical cancer was higher among non- users compared to hormonal contraceptive users when we pooled the results (1.28, 95% CI 1.21, 1.35). When stratifying the results, a significant increased risk was observed for OCP users (RR = 1.43, 95% CI 1.31, 1.56) and any users of oral and non-oral (RR = 1.19, 95% CI 1.10–1.29), while women using IUS showed no significant effect on cervical cancer risk incidence (RR = 0.76, 95% CI 0.52–1.11). In a comparison between non-users and hormonal IUD users, the risk ratio was 0.58(0.42, 0.79). Any contraceptive users reported one study only (37). These results had high heterogeneity (I2 = 80%) (Figure 6).
When comparing dosing regimens, a nonsignificant downward trend in cervical cancer incidence was observed by Rosenblatt et al., 2007 among Hydroxyprogesterone Caproate and 5 mg Estradiol Valerate users (RR 0.54, 95% CI 0.07–4.17), while an upward trend was observed by Iversen et al., 2017 for any combined oral contraceptive users (RR 1.31, 95% CI 0.82–1.94).
Gynecological cancer
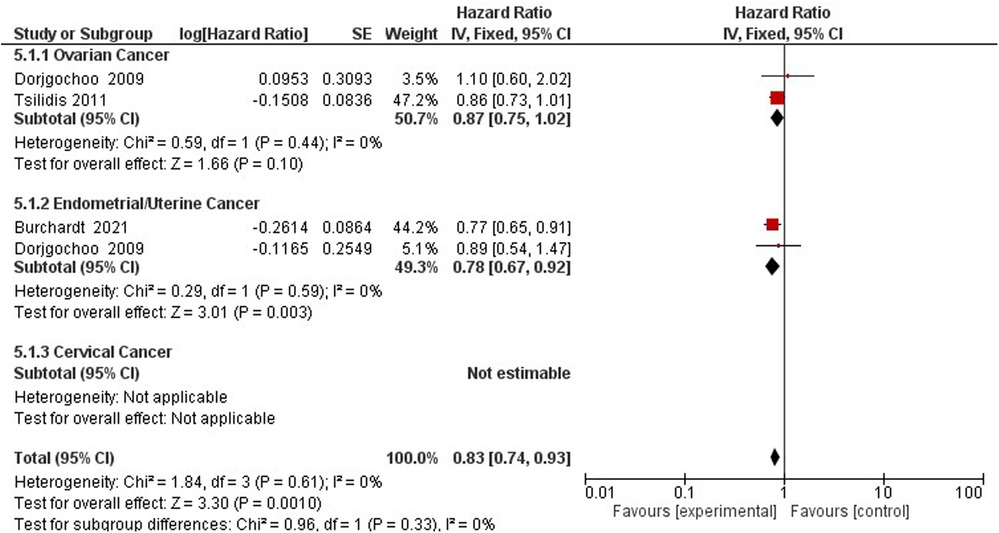
The association between gynecological cancer and the protective effect of tubal ligation was reported by four studies (47–50). Tubal ligation reduces the hazards of gynecological cancer risk by 20% (HR = 0.80, 95% CI 0.66–0.87) (Supplementary Appendix 3 - Figure S3).
Three studies assess the hazards of gynecological cancer among OCP users and non-users (22, 47, 60) (Figure 7). Compared to non-users, OCP use reduces the hazard of gynecological cancers of the ovaries and endometrium by 17% (HR 0.83; 95% CI 0.74–0.93) (Figure 8).
Four studies assessed the association between hormonal contraception usage and cancer mortality (42, 51–53). Compared to non-users, a reduced incidence of gynecological cancer mortality was observed among HC users (RR 0.60, 95% CI 0.51–0.71). When stratifying the results by gynecological cancer type, a 63% increase in cervical cancer mortality was observed among HC users (RR 1.63, 95% CI 1.07–2.49). Similarly, a cohort study containing 121,577 participants found a slight protective effect when comparing ever users of OCs to never users (HR 0.87; 95% CI 0.77–0.98) (59) (Supplementary Appendix 3 - Figure S4).
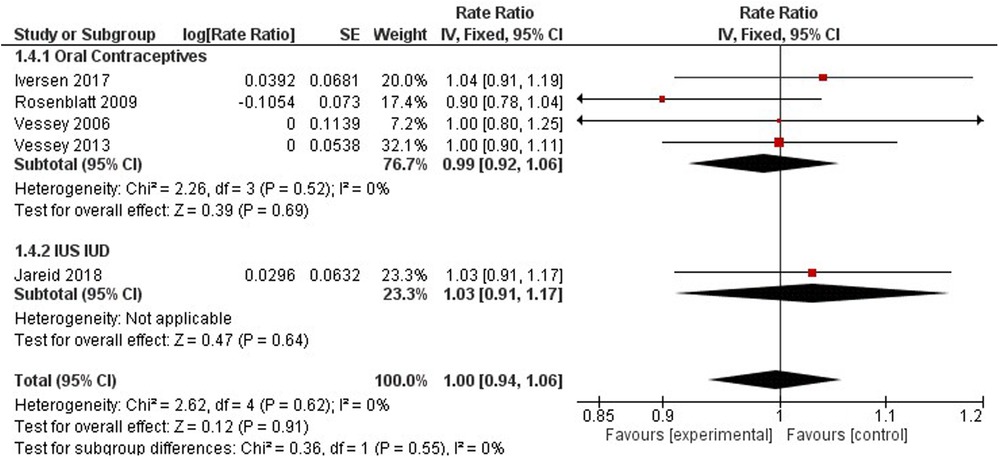
Breast cancer
Breast cancer was studied by four studies on oral contraceptives (35, 41, 57), and one study on hormonal IUS (43) (Figure 8). A pooled non-significant rate ratio was estimated (1.00, 95% CI 0.94, 1.06) for OCP and IUS users. Dosing regimens comparing HC users to non-users found no significant difference in breast cancer incidence among Hydroxyprogesterone Caproate and 5 mg Estradiol Valerate, LNG-IUS Progestogen-Only Non-Oral, and any Combined Oral Pill users with a pooled effect of 1.02 (95% CI 0.93–1.11) (35, 43, 44) (Figure 8).
Only one study observed the association between tubal ligation and breast cancer risk (47). No significant effect was observed among women who underwent tubal ligation and those who have not (RR 1.15, 95% CI 0.86–1.54). Three studies investigated regimen dosing to understand breast cancer risk among HC users (35, 43, 44). Compared to non-users, no significant difference in breast cancer incidence was observed among women using alpha- hydroxyprogesterone caproate and 5 mg estradiol valerate, LNG-IUS Progestogen-Only Non- Oral, and any combined oral pills (RR 1.02, 95% CI 0.93–1.11).
Additionally, breast cancer mortality was not significantly different between OCP users and non-users (1.00, 95% CI 0.89, 1.12) (Supplementary Appendix 3 - Figure S5).
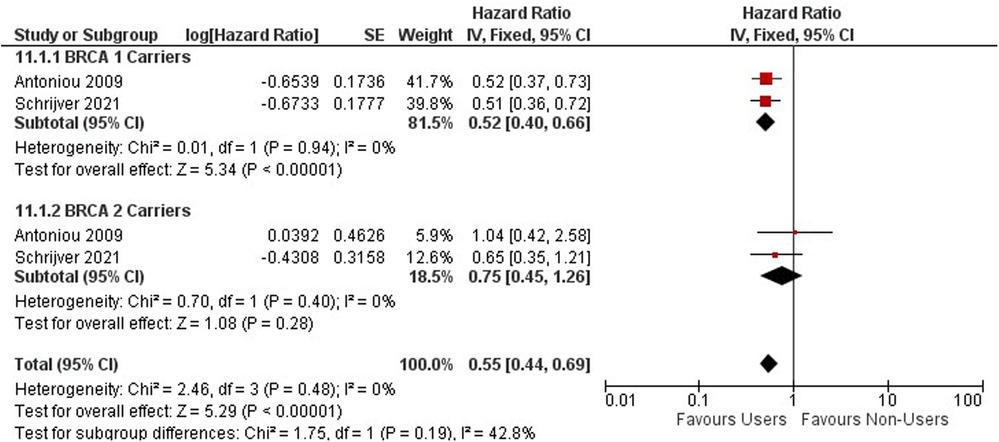
Contraceptives and hereditary cancers Ovarian cancer
Ovarian cancer risk among BRCA 1 and 2 mutation carriers using OCs was investigated within two cohort studies including 9,753 women (54, 58) (Figure 9). Among BRCA 1 and 2 mutation carriers, ever users of oral contraceptives are associated with a decreased risk of ovarian cancer (HR = 0.55, 95% CI 0.44, 0.69) (Figure 9).
Among BRCA 1 and 2 carriers using OCs for less than 5 years, no significant effect was observed (HR = 0.76; 95% CI 0.57, 1.02) Supplementary Appendix 3 - Figure S6.
A significant protective effect against ovarian cancer is observed among BRCA 1 and 2 Carriers who use oral contraceptives between 4 and 9 years (HR = 0.45; 95% CI 0.34, 0.61) (Supplementary Appendix 3 - Figure S7).
For BRCA 1 carriers, OCPs have a protective effect, while no difference in effect is detected among BRCA 2 carriers. BRCA 1 and 2 mutation carriers using oral contraceptives for greater than 10 years have a significant protective effect against the risk of ovarian cancer (HR = 0.37; 95% CI 0.26, 0.54). (Supplementary Appendix 3 - Figure S8).
Breast cancer
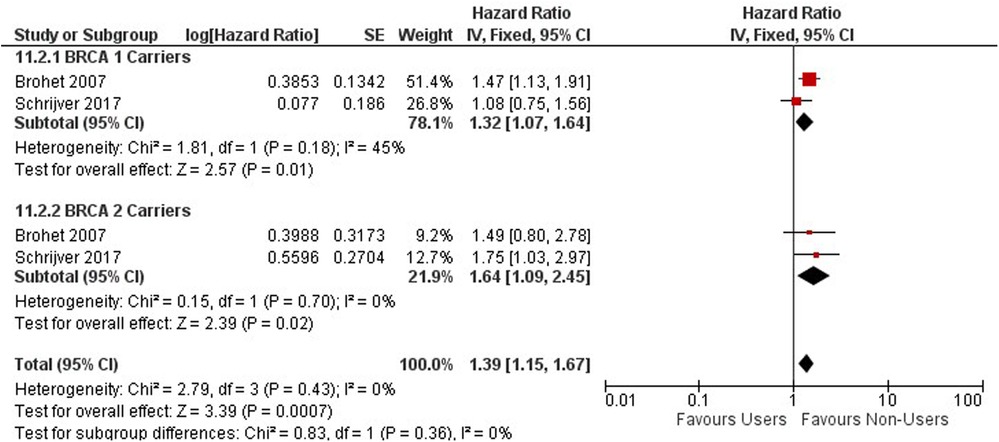
The risk of breast cancer association from OC use was observed among 11,432 BRCA 1 or 2 gene mutation carriers with two cohort studies conducted in the United Kingdom and Netherlands (55, 56) (Figure 10). Among BRCA 1 and 2 mutation carriers, ever users of oral contraceptives are associated with an increased risk of breast cancer compared to never users (HR = 1.39, 95% CI 1.15, 1.67).
Among BRCA 1 and 2 carriers using OCs for less than 5 years, an increased risk in breast cancer was observed compared to never users (HR = 1.33; 95% CI 1.07, 1.66) (Supplementary Appendix 3 - Figure S9).
A significant increase in risk of breast cancer is observed among BRCA 1 and 2 Carriers who use oral contraceptives between 4 and 9 years compared to never users (HR = 1.29; 95% CI 1.03, 1.61), however, the risk between carriers using OCs for less than 5 years and between 4 and 9 years are very similar as you compare them (Supplementary Appendix 3 - Figure S10).
BRCA 1 and 2 mutation carriers using oral contraceptives for greater than 10 years have an increased risk of breast cancer (HR = 1.47; 95% CI 1.18, 1.85). Long term OC use of greater than 10 years has a higher risk for mutation carriers as compared to those using them for less than 10 years (Supplementary Appendix 3 - Figure S11).
Duration gynecological cancers
Duration of hormonal contraception use among a sample of over 21 million women were accounted for in 6 cohort studies measuring the rate ratio of gynecological cancers among users and non-users to examine a dose-response relationship (10, 37, 39–41, 57) (Supplementary Appendix 3 - Figures S12, S13).
Among short-term hormonal contraception users who used HCs for less than 5 years, a significant protective effect was observed for ovarian (RR = 0.63; 95% CI 0.52, 0.75) and endometrial cancer (RR = 0.75; 95% CI 0.59, 0.94) outcomes, however, a harmful effect was observed for cervical cancer (RR = 2.55; 95% CI 1.17, 5.59) (Supplementary Appendix 3 - Figure S12).
Among long-term HC users who used HCs for at least 8 years or more, a similar pattern to short-term users was observed. A significant reduction in ovarian (RR = 0.36; 95% CI 0.27, 0.49) and endometrial cancers (RR = 0.26; 95% CI 0.19, 0.36) was observed. A harmful effect was observed for cervical cancer, and this risk increased from the initial risk that short-term users have (RR = 2.55 vs. 5.40; 95% CI 2.89, 10.89) (Supplementary Appendix 3 - Figure S13).
Breast cancer
There were three cohort studies with 275,988 participants examined for the association between duration of HC use and breast cancer incidence (Supplementary Appendix 3 - Figures S14, S15) (40, 41, 57). No significant relationship among short-term and long-term HC users was observed for breast cancer incidence.
Discussion
Summary of main results
There are significant reductions in the rate of ovarian and endometrial cancer incidence and mortality among contraceptive users. Short- and long-term methods showed a protective effect against ovarian and endometrial cancers. When comparing dosing regimens, HCs containing both progestins and estrogens have protective effects against ovarian and endometrial cancer. Conversely, HC users are at an increased risk of cervical cancer incidence and mortality, and this risk persists regardless of duration. Among healthy women with no genetic mutations, no significant difference in breast cancer incidence and mortality was reported among users and non-users of HC. Among mutation carriers (BRCA1/2) using OCs, an increased risk in breast cancer incidence was observed and this risk persists among short- and long-term users. For ovarian and cervical cancer, our case-control findings matched our cohort studies, however, for breast cancer, a significant risk was observed among OCP users.
Contraceptive use as a primary exposure for our review was self-reported in a majority of our studies. Recall bias and information bias are a major concern for studies that relied on self- reports of contraceptive use as this can potentially underestimate the true effect of contraceptive use on our outcomes of interest. Future studies should focus on techniques such as recording the on-time injection checklist or electronic pill count.
Highlighting the mechanism of action in each scenario outlined above is crucial. For example, the inhibition of ovulation is a key factor in providing protection against ovarian cancer, shedding light on why specific methods like DMPA may not confer the same level of protection as COCs. Nevertheless, it's important to note that this manuscript, while acknowledging the importance of theoretical mechanisms, refrains from delving extensively into the intricacies beyond the causality of the effects on different types of cancer.
Agreements and disagreements with other studies
Our results on contraceptives as a protective effect against ovarian cancer are confirmatory to the initial findings of Havrilesky 2013 (61). A previous study (Pragout et al.) reported an insignificant increased risk of cervical cancer, while our study found a significantly (28%) higher risk among users than non-users (62). In the systematic review conducted by Asthana, the overall odds ratio of invasive cancer on OC use was estimated as 1.51 (95% CI 1.35, 1.68) with 19 included studies, which is not very different from our study (63). In Pragout et al., a slight but significant increase in breast cancer risk among users was observed; however, in our study, no significant differences were observed between users and non-users, except for women who are BRCA1/2 carriers. A study (Conz et al.) systematically reviewed the relationship between use of the Levonorgestrel-releasing intrauterine system (LNG-IUS) and breast cancer risk with 26 studies. They found an increased risk in LNG-IUS users (pooled OR = 1.16, 95% CI 1.06–1.28) (64). However, we did not detect a significant risk in LNG-IUS users. Our study supports the previous findings from Pragout 2018 on the significant reduction of endometrial cancer. However, there is conflicting information about the roles IUD and tubal ligation play in the risk factor. For BRCA1/2 mutation carriers, Huber examined OC users’ risk of ovarian cancer and breast cancer compared to non-users in a systematic review (65). It reported a risk reduction in ovarian cancer, and a significant risk elevation in breast cancer, which is aligned with our findings.
Our study contributes nuanced insights into the complex relationship between contraceptive use and various types of cancer, offering both confirmatory evidence and novel findings that warrant further exploration and validation in future research endeavors.
Our disagreement with other studies can also be explained by the adopted methodology that we employed a comprehensive approach by searching across 19 databases to ensure inclusivity. Papers were included without geographical or language restrictions. The population of interest encompassed women of reproductive age (14–49 years). Interventional studies were included if they investigated modern contraceptive methods endorsed by the WHO, such as short-acting hormonal contraception, long-term contraception, one-time barrier contraception, permanent contraception, and emergency contraception. Observational studies considered contraceptive use of all types as the main exposure, excluding those investigating contraception alongside other medications or modalities. Comparison groups consisted solely of non-users of contraception. The primary outcome of interest was mortality and morbidity related to reproductive tract cancers. Eligible study designs included parallel, or cluster randomized controlled trials, controlled clinical trials, controlled before and after studies, interrupted time series studies, cohort or longitudinal analyses, regression discontinuity designs, and case-control studies.
Strengths
First, we searched the databases for all studies from their inception. Thus, we included studies with both outdated and up-to-date contraceptive methods, with broad coverage. Second, for each outcome category, our studies covered diseases and conditions commonly seen in women of reproductive age. For some of these outcomes, we have studies that yielded large pooled sample sizes and low loss to follow-up rates, allowing for higher statistical power, narrower confidence intervals, and more credible results. The prospective study design eliminated the possible influence of differential recall of contraception use.
Limitations
Cancer is a difficult outcome to measure as there is a long latency period between the time of exposure and the detection of an outcome. During these long latency periods, the methods and dosing regimens a woman uses can change over time. Our study did not account for the age at first use of contraceptives, time since last use, environmental exposures, or demographic characteristics. Included studies lacked information on specific HC regimens and dosing and HC methods (patch/ring, implants, and injectables) were often left out of analysis due to a lack of available data. There were limited studies examining the association between varying HC methods and dosing regimens for cervical and breast cancer outcomes. Most studies were on OC users, with limited studies looking at the association of long-term contraceptive types. As long-term contraceptive methods become more popular, it is essential to have more information on these methods. Heterogeneity was a significant issue in this study.
New knowledge this review brings to the field
This review provides new insights into the relationship between modern contraceptive use and cancer risk, focusing on the effects of birth control pills, and long-acting reversible contraceptives. We found a significant reduction in the risk of developing ovarian and endometrial cancers among contraceptive users, highlighting the potential benefits of these contraceptive methods. However, the review also reveals an increased risk of cervical cancer among contraceptive users, which persists regardless of the duration of use. These findings suggest the need for additional research into the link between hormonal contraceptive use and cancer incidence. The authors recommend that healthcare providers consider various factors such as age, physical and mental status, family history, and individual preferences when selecting contraceptive methods to maximize benefits and minimize risks.
Implications for practice
The findings of this research offer a comprehensive body of evidence on the utilization of contraception and its impact on various stakeholders, such as healthcare professionals, pharmaceutical companies, pharmacists, and others. The results indicate that aside from its primary role as a contraceptive, contraception may also have preventive or therapeutic properties for certain diseases or conditions. Nevertheless, the study highlights potential adverse effects that must be considered. Healthcare providers should evaluate several situational factors, such as age, physical and mental health status, and family history, to choose the most suitable contraceptive method for their female patients, ensuring maximum benefits and minimal risks. Recommendations include discussions about the woman's overall health and changes to medical history, continued monitoring and a complete physical examination at each follow-up visit checking for symptoms of blood pressure changes, weight changes, headaches, or abnormal vaginal bleeding (66).
Conclusion
This comprehensive review explores the intricate relationship between contraceptive use and cancer, revealing significant reductions in ovarian and endometrial cancer incidence and mortality among users. Short- and long-term methods exhibit protective effects against these cancers, especially hormonal contraceptives containing both progestins and estrogens. However, an increased risk of cervical cancer is observed among contraceptive users, persisting regardless of use duration. No significant differences in breast cancer risk were found for healthy women, but for BRCA1/2 carriers using oral contraceptives, an elevated risk was noted. The study's strengths lie in exhaustive database searches, broad coverage of diseases, and a prospective design, minimizing recall bias. Yet, limitations include the long latency of cancer, changing contraceptive methods, and limited information on specific regimens. Overall, this research offers nuanced insights and implications for healthcare practices, suggesting contraception's potential preventive or therapeutic properties for certain diseases. Future research should explore the benefits of different hormonal methods and doses, considering non- reproductive health and specific health conditions in study populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SJ: Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. JM: Data curation, Investigation, Methodology, Project administration, Software, Visualization, Writing – review & editing. AL: Data curation, Investigation, Methodology, Resources, Visualization, Writing – review & editing. CC: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – review & editing. JA: Data curation, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – review & editing. KM: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – review & editing. JB-M: Data curation, Investigation, Methodology, Software, Validation, Writing – review & editing. MS: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. OM: Conceptualization, Formal Analysis, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. JB: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. PA: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. MB: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. MA: Conceptualization, Funding acquisition, Methodology, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the USAID consolidated grant 7200GH21IO00005.
Acknowledgments
We gratefully acknowledge comments and suggestions from the WHO Technical Advisory Group (TAG) consisting of (listed in Alphabetical order): Dr. Ann Biddlecom; Dr. Harriet Birungi; Professor Herbert Peterson; Dr. Iqbal Shah; Dr. James Kiarie; Professor John Cleland; Dr. John Townsend; Dr. Manala Makua and Professor Sonalde Desai. We would like to specially acknowledge the support and guidance by Dr. James Kiarie (WHO) throughout the process to complete the project. We acknowledge the support of USAID who provided input on the research questions. USAID did not participate in the data abstraction, analysis or interpretation or the decision to submit it for publication. The analysis, interpretation, write up and decision to submit the paper was coordinated by the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research, WHO. All authors were consultants and one author is a staff member.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2024.1487820/full#supplementary-material
References
1. United Nations Department of Economic and Social Affairs PD. Contraceptive Use by Method 2019: Data Booklet (ST/ESA/SER.A/435). Published online 2019:28 (2019).
2. Chen GG, Zeng Q, Tse GM. Estrogen and its receptors in cancer. Med Res Rev. (2008) 28(6):954–74. doi: 10.1002/med.20131
3. World Health Organization/International Agency for Research on Cancer. Estimated Number of New Cases in 2020, World, Females, All Ages. (2020). Available online at: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_grou p=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&i nclude_nmsc_other=1 (accessed March 21, 2023).
4. Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016 (US MEC). Published online 2020. Available online at: https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (accessed March 29, 2023).
5. Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol. (2018) 4(4):516–21. doi: 10.1001/jamaoncol.2017.4942
6. Hüsing A, Dossus L, Ferrari P, Tjønneland A, Hansen L, Fagherazzi G, et al. An epidemiological model for prediction of endometrial cancer risk in Europe. Eur J Epidemiol. (2016) 31(1):51–60. doi: 10.1007/s10654-015-0030-9
7. Hogewoning CJ, Bleeker MC, van den Brule AJ, Voorhorst FJ, Snijders PJ, Berkhof J, et al. Condom use promotes regression of cervical intraepithelial neoplasia and clearance of human papillomavirus: a randomized clinical trial. Int J Cancer. (2003) 107(5):811–6. doi: 10.1002/ijc.11474
8. Fiascone S, Danilack VA, Kao MJ, Cohen M, Singh K, Kalife E, et al. While women await surgery for type I endometrial cancer, depot medroxyprogesterone acetate reduces tumor glandular cellularity. Am J Obstet Gynecol. (2018) 219(4):381.e1–10. doi: 10.1016/j.ajog.2018.07.024
9. Gross TP, Schlesselman JJ. The estimated effect of oral contraceptive use on the cumulative risk of epithelial ovarian cancer. Obstet Gynecol. (1994) 83(3):419–24. doi: 10.1093/oxfordjournals.aje.a116419
10. Walker GR, Schlesselman JJ, Ness RB. Family history of cancer, oral contraceptive use, and ovarian cancer risk. Am J Obstet Gynecol. (2002) 186(1):8–14. doi: 10.1067/mob.2002.118657
11. Loopik DL, IntHout J, Melchers WJG, Massuger LFAG, Bekkers RLM, Siebers AG. Oral contraceptive and intrauterine device use and the risk of cervical intraepithelial neoplasia grade III or worse: a population-based study. Eur J Cancer Oxf Engl. (2020) 124:102–9. doi: 10.1016/j.ejca.2019.10.009
12. Miracle-McMahill HL, Calle EE, Kosinski AS, Rodriguez C, Wingo PA, Thun MJ, et al. Heath, tubal ligation and fatal ovarian cancer in a large prospective cohort study. Am J Epidemiol. (1997) 145(4):349–57. doi: 10.1093/oxfordjournals.aje.a009112
13. Sponholtz TR, Palmer JR, Rosenberg LA, Hatch EE, Adams-Campbell LL, Wise LA. Exogenous hormone use and endometrial cancer in U.S. Black women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. (2018) 27(5):558–65. doi: 10.1158/1055-9965.EPI-17-0722
14. Robbins CL, Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, et al. Influence of reproductive factors on mortality after epithelial ovarian cancer diagnosis. Cancer Epidemiol Biomarkers Prev. (2009) 18(7):2035–41. doi: 10.1158/1055-9965.EPI-09-0156
15. Rosenblatt KA, Thomas DB. Reduced risk of ovarian cancer in women with a tubal ligation or hysterectomy. The world health organization collaborative study of neoplasia and steroid contraceptives. Cancer Epidemiol Biomark Prev. (1996) 5(11):933–5.
16. Graff-Iversen S, Hammar N, Thelle DS, Tonstad S. Use of oral contraceptives and mortality during 14 years’ follow-up of Norwegian women. Scand J Public Health. (2006) 34(1):11–6. doi: 10.1080/14034940510032239
17. Iversen L, Hannaford PC, Elliott AM. Tubal sterilization, all-cause death, and cancer among women in the United Kingdom: evidence from the royal college of general practitioners’ oral contraception study. Am J Obstet Gynecol. (2007) 196(5):447.e1–e8. doi: 10.1016/j.ajog.2007.01.022
18. Merritt MA, Riboli E, Murphy N, Kadi M, Tjønneland A, Olsen A, et al. Reproductive factors and risk of mortality in the European prospective investigation into cancer and nutrition; a cohort study. BMC Med. (2015) 13:252. doi: 10.1186/s12916-015-0484-3
19. Shafrir AL, Schock H, Poole EM, Terry KL, Tamimi RM, Hankinson SE, et al. A prospective cohort study of oral contraceptive use and ovarian cancer among women in the United States born from 1947 to 1964. Cancer Causes Control. (2017) 28(5):371–83. doi: 10.1007/s10552-017-0876-0
20. Syrjänen K, Shabalova I, Petrovichev N, Kozachenko V, Zakharova T, Pajanidi J, et al. Oral contraceptives are not an independent risk factor for cervical intraepithelial neoplasia or high-risk human papillomavirus infections. Anticancer Res. (2006) 26(6C):4729–40.
21. Westreich D, Jamal N, Smith JS, Schulze D, Williams S, Michelow P, et al. Injectable and oral contraception and the incidence and progression of cervical disease in HIV-infected women in South Africa. Contraception. (2014) 89(4):286–91. doi: 10.1016/j.contraception.2013.12.011
22. Tsilidis KK, Allen NE, Key TJ, Dossus L, Lukanova A, Bakken K, et al. Oral contraceptive use and reproductive factors and risk of ovarian cancer in the European prospective investigation into cancer and nutrition. Br J Cancer. (2011) 105(9):1436–42. doi: 10.1038/bjc.2011.371
23. Grabrick DM, Hartmann LC, Cerhan JR, Vierkant RA, Therneau TM, Vachon CM, et al. Risk of breast cancer with oral contraceptive use in women with a family history of breast cancer. JAMA. (2000) 284(14):1791–8. doi: 10.1001/jama.284.14.1791
24. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. Cochrane (2022). (updated February 2022). Available online at: www.training.cochrane.org/handbook (accessed February 10, 2022).
25. Guyatt G, Oxman AD, Akl EA, Kunzd R, Vistc G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026
26. Walsh D, Downe S. Appraising the quality of qualitative research. Midwifery. (2006) 22(2):108. doi: 10.1016/j.midw.2005.05.004
27. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. (1998) 52(6):377–84. doi: 10.1136/jech.52.6.377
28. Karlsson T, Johansson T, Hoglund J, Ek WE, Johansson A. Time-Dependent effects of oral contraceptive use on breast, ovarian, and endometrial cancers. Cancer Res. (2021) 81(4):1153–62. doi: 10.1158/0008-5472.CAN-20-2476
29. Faber MT, Jensen A, Frederiksen K, Glud E, Høgdall E, Høgdall C, et al. Oral contraceptive use and impact of cumulative intake of estrogen and progestin on risk of ovarian cancer. Cancer Causes Control. (2013) 24(12):2197–206. doi: 10.1007/s10552-013-0296-8
30. Xia YY, Lubinski J, Rosen B, Moller P, Eisen A, Ainsworth P, et al. Contraceptive use and ovarian cancer risk in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Cancer Res. (2021) 81(13_Supplement):878–878. doi: 10.1158/1538-7445.AM2021-878
31. Vaisy A, Lotfinejad S, Zhian F. Risk of cancer with combined oral contraceptive use among Iranian women. Asian Pac J Cancer Prev. (2014) 15(14):5517–22. doi: 10.7314/APJCP.2014.15.14.5517
32. Zondervan KT, Carpenter LM, Painter R, Vessey MP. Oral contraceptives and cervical cancer-further findings from the Oxford family planning association contraceptive study. Br J Cancer. (1996) 73(10):1291–7. doi: 10.1038/bjc.1996.247
33. Beral V, Hannaford P, Kay C. Oral contraceptive use and malignancies of the genital tract. Results from the royal college of general practitioners’ oral contraception study. Lancet. (1988) 2(8624):1331–5. doi: 10.1016/S0140-6736(88)90869-0
34. Hankinson SE, Colditz GA, Hunter DJ, Willett WC, Stampfer MJ, Rosner B, et al. A prospective study of reproductive factors and risk of epithelial ovarian cancer. Cancer. (1995) 76(2):284–90. doi: 10.1002/1097-0142(19950715)76:2%3C284::aid-cncr2820760219%3E3.0.co;2-5
35. Iversen L, Sivasubramaniam S, Lee AJ, Fielding S, Hannaford PC. Lifetime cancer risk and combined oral contraceptives: the royal college of general Practitioners’ oral contraception study. Am J Obstet Gynecol. (2017) 216(6):580.e1–e9. doi: 10.1016/j.ajog.2017.02.002
36. Iversen L, Fielding S, Lidegaard O, Morch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. Br Med J. (2018) 362:k3609. doi: 10.1136/bmj.k3609
37. Iversen L, Fielding S, Lidegaard O, Hannaford PC. Contemporary hormonal contraception and risk of endometrial cancer in women younger than age 50: a retrospective cohort study of danish women. Contraception. (2020) 102(3):152–8. doi: 10.1016/j.contraception.2020.06.008
38. Iversen L, Fielding S, Lidegaard Ø, Hannaford PC. Contemporary hormonal contraception and cervical cancer in women of reproductive age. Int J Cancer. (2021). doi: 10.1002/ijc.33585
39. Kumle M, Weiderpass E, Braaten T, Adami HO, Lund E, Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Risk for invasive and borderline epithelial ovarian neoplasias following use of hormonal contraceptives: the Norwegian-Swedish women’s lifestyle and health cohort study. Br J Cancer. (2004) 90(7):1386–91. doi: 10.1038/sj.bjc.6601715
40. Rosenblatt KA, Gao DL, Ray RM, Nelson ZC, Wernli KJ, Li W, et al. Oral contraceptives and the risk of all cancers combined and site-specific cancers in Shanghai. Cancer Causes Control. (2009) 20(1):27–34. doi: 10.1007/s10552-008-9213-y
41. Vessey M, Yeates D. Oral contraceptive use and cancer: final report from the Oxford-family planning association contraceptive study. Contraception. (2013) 88(6):678–83. doi: 10.1016/j.contraception.2013.08.008
42. Vessey M, Yeates D, Flynn S. Factors affecting mortality in a large cohort study with special reference to oral contraceptive use. Contraception. (2010) 82(3):221–9. doi: 10.1016/j.contraception.2010.04.006
43. Jareid M, Thalabard JC, Aarflot M, Bovelstad HM, Lund E, Braaten T. Levonorgestrel-releasing intrauterine system use is associated with a decreased risk of ovarian and endometrial cancer, without increased risk of breast cancer. Results from the NOWAC study. Gynecol Oncol. (2018) 149(1):127–32. doi: 10.1016/j.ygyno.2018.02.006
44. Rosenblatt KA, Gao DL, Ray RM, Nelson ZC, Wernli KJ, Li W, et al. Monthly injectable contraceptives and the risk of all cancers combined and site-specific cancers in Shanghai. Contraception. (2007) 76(1):40–4. doi: 10.1016/j.contraception.2007.03.004
45. Purdie. Risk of invasive epithelial ovarian neoplasia declined with each year of hormonal contraceptive use. Evid-Based Obstet Gynecol. (2005) 7(2):96–7. doi: 10.1016/j.ebobgyn.2005.03.008
46. Tworoger SS, Fairfield KM, Colditz GA, Rosner BA, Hankinson SE. Association of oral contraceptive use, other contraceptive methods, and infertility with ovarian cancer risk. Am J Epidemiol. (2007) 166(8):894–901. doi: 10.1093/aje/kwm157
47. Dorjgochoo T, Shu XO, Li HL, Qian HZ, Yang G, Cai H, et al. Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer. (2009) 124(10):2442–9. doi: 10.1002/ijc.24232
48. Falconer H, Yin L, Altman D. Association between tubal ligation and endometrial cancer risk: a Swedish population-based cohort study. Int J Cancer. (2018) 143(1):16–21. doi: 10.1002/ijc.31287
49. Winer I, Lehman A, Wactawski-Wende J, Robinson R, Simon M, Cote M. Tubal ligation and risk of endometrial cancer: findings from the women’s health initiative. Int J Gynecol Cancer. (2016) 26(3):464–71. doi: 10.1097/IGC.0000000000000651
50. Rice MS, Hankinson SE, Tworoger SS. Tubal ligation, hysterectomy, unilateral oophorectomy, and risk of ovarian cancer in the Nurses’ health studies. Fertil Steril. (2014) 102(1):192–198.e3. doi: 10.1016/j.fertnstert.2014.03.041
51. Beral V, Hermon C, Kay C, Hannaford P, Darby S, Reeves G. Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from royal college of general practitioners’ oral contraception study. Br Med J. (1999) 318(7176):96–100. doi: 10.1136/bmj.318.7176.96
52. Colditz GA. Oral contraceptive use and mortality during 12 years of follow-up: the Nurses’ health study. Ann Intern Med. (1994) 120(10):821–6. doi: 10.7326/0003-4819-120-10-199405150-00002
53. Hannaford PC, Iversen L, Macfarlane TV, Elliott AM, Angus V, Lee AJ. Mortality among contraceptive pill users: cohort evidence from royal college of general Practitioners’ oral contraception study. Br Med J. (2010) 340:c927. doi: 10.1136/bmj.c927
54. Schrijver LH, Antoniou AC, Olsson H, Mooij TM, Roos-Blom MJ, Azarang L, et al. Epidemiological study of familial breast cancer, gene etude prospective sein ovaire sein, hereditary breast and ovarian cancer research group Netherlands, and international BRCA1/2 carrier cohort study. Oral contraceptive use and ovarian cancer risk for BRCA1/2 mutation carriers: an international cohort study. Am J Obstet Gynecol. (2021) 225(1):51.e1–17. doi: 10.1016/j.ajog.2021.01.014
55. Brohet RM, Goldgar DE, Easton DF, Antoniou AC, Andrieu N, Chang-Claude J, et al. Oral contraceptives and breast cancer risk in the international BRCA1/2 carrier cohort study: a report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS collaborating group. J Clin Oncol. (2007) 25(25):3831–6. doi: 10.1200/JCO.2007.11.1179
56. Schrijver LH, Olsson H, Phillips KA, Terry MB, Goldgar DE, Kast K, et al. Oral contraceptive use and breast cancer risk: retrospective and prospective analyses from a BRCA1 and BRCA2 mutation carrier cohort study. JNCI Cancer Spectr. (2018) 2(2):pky023. doi: 10.1093/jncics/pky023 Erratum in: JNCI Cancer Spectr. 2018 2(3):pky041. doi: 10.1093/jncics/pky041.31360853
57. Vessey M, Painter R. Oral contraceptive use and cancer. Findings in a large cohort study, 1968–2004. Br J Cancer. (2006) 95(3):385–9. doi: 10.1038/sj.bjc.6603260
58. Antoniou AC, Rookus M, Andrieu N, Brohet R, Chang-Claude J, Peock S, et al. Reproductive and hormonal factors, and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: results from the international BRCA1/2 carrier cohort study. Cancer Epidemiol Biomarkers Prev. (2009) 18(2):601–10. doi: 10.1158/1055-9965.EPI-08-0546
59. Charlton BM, Rich-Edwards JW, Colditz GA, Missmer SA, Rosner BA, Hankinson SE, et al. Oral contraceptive use and mortality after 36 years of follow-up in the nurses’ health study: prospective cohort study. Br Med J. (2014) 349:g6356. doi: 10.1136/bmj.g6356
60. Burchardt NA, Shafrir AL, Kaaks R, Tworoger SS, Fortner RT. Oral contraceptive use by formulation and endometrial cancer risk among women born in 1947–1964: the nurses’ health study II, a prospective cohort study. Eur J Epidemiol. (2021) 36(8):827–39. doi: 10.1007/s10654-020-00705-5
61. Havrilesky LJ, Moorman PG, Lowery WJ, Gierisch JM, Coeytaux RR, Urrutia RP, et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet Gynecol. (2013) 122(1):139–47. doi: 10.1097/AOG.0b013e318291c235
62. Pragout D, Laurence V, Baffet H, Raccah-Tebeka B, Rousset-Jablonski C. [Contraception and cancer: cNGOF contraception guidelines]. Gynecol Obstet Fertil Senol. (2018) 46(12):834–44. doi: 10.1016/j.gofs.2018.10.010
63. Asthana S, Busa V, Labani S. Oral contraceptives use and risk of cervical cancer-a systematic review & meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2020) 247:163–75. doi: 10.1016/j.ejogrb.2020.02.014
64. Conz L, Mota BS, Bahamondes L, Teixeira Dória M, Françoise Mauricette Derchain S, Rieira R, et al. Levonorgestrel-releasing intrauterine system and breast cancer risk: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2020) 99(8):970–82. doi: 10.1111/aogs.13817
65. Huber D, Seitz S, Kast K, Emons G, Ortmann O. Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: a systematic review. Arch Gynecol Obstet. (2020) 301(4):875–84. doi: 10.1007/s00404-020-05458-w
66. International Federation of Gynecology and Obstetrics (FIGO), World Health Organization (WHO). MEC for Contraceptive Use. Available online at: https://www.figo.org/mec-for-contraceptive-use (accessed February 10, 2022).
Keywords: contraceptives, ovarian cancer, cervical cancer, endometrial cancer, breast cancer
Citation: Jahanfar S, Mortazavi J, Lapidow A, Cu C, Al Abosy J, Morris K, Becerra-Mateus JC, Steinfeldt M, Maurer O, Bohang J, Andrenacci P, Badawy M and Ali M (2024) Assessing the impact of contraceptive use on reproductive cancer risk among women of reproductive age—a systematic review. Front. Glob. Womens Health 5:1487820. doi: 10.3389/fgwh.2024.1487820
Received: 28 August 2024; Accepted: 24 October 2024;
Published: 13 November 2024.
Edited by:
Jen Sothornwit, Khon Kaen University, ThailandReviewed by:
Manuela Farris, Sapienza University of Rome, ItalyAyse Akin, Başkent University, Türkiye
Copyright: © 2024 Jahanfar, Mortazavi, Lapidow, Cu, Al Abosy, Morris, Becerra-Mateus, Steinfeldt, Maurer, Bohang, Andrenacci, Badawy and Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moazzam Ali, YWxpbW9hQHdoby5pbnQ=
 Shayesteh Jahanfar
Shayesteh Jahanfar Julie Mortazavi1
Julie Mortazavi1 Amy Lapidow
Amy Lapidow Paola Andrenacci
Paola Andrenacci Moazzam Ali
Moazzam Ali