- 1Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden
- 2Astrid Lindgren Children’s Hospital, Karolinska University Hospital, Stockholm, Sweden
- 3FUTURE, Center for Functional Tissue Reconstruction, University of Oslo, Oslo, Norway
- 4Fetal Medicine Unit, St George’s University Hospitals NHS Foundation Trust, University of London, London, United Kingdom
- 5Royal College of Obstetricians and Gynaecologists, London, United Kingdom
- 6Obstetrics and Gynaecology, Croydon University Hospitals NHS Trust, London, United Kingdom
- 7EGA Institute for Women's Health, Faculty of Population Health Sciences, University College London, London, United Kingdom
- 8Vascular Biology Research Centre, Molecular and Clinical Sciences Research Institute, St George’s University of London, London, United Kingdom
- 9Fetal Medicine Unit, Liverpool Women’s Hospital, Liverpool, United Kingdom
The climate crisis poses profound risks to women particularly during pregnancy. With rising global temperatures and increasing frequency of extreme weather events, there is an urgent need for health initiatives and guidelines tailored to the unique vulnerabilities of pregnant individuals. We conducted a review of English-language literature from 2000–2024 using PubMed, Scopus, and Web of Science, focusing on “climate change,” “pregnancy,” and “maternal health,” and included original studies, reviews. Relevant policy documents, including some published in 2025 were also included. We examine the multifaceted challenges posed by climate change, such as extreme weather events, water scarcity, malnutrition, and exposure to environmental pollutants like contaminated air and water, which directly and indirectly affect maternal and fetal health. The review explores the associations between these environmental stressors and adverse pregnancy outcomes, including preterm births, low birth weight, and developmental complications. These challenges are compounded in low-resource settings where healthcare infrastructure is limited, exacerbating inequities in maternal care. Furthermore, we focus on key areas for further investigation, including the long-term health effects of in-utero exposure to pollutants. The review addresses evidence-based strategies to reduce the environmental impact of healthcare through early interventions, innovation, and strengthened initiatives. It emphasises empowering healthcare professionals to educate others, raise awareness among policymakers, advocate for climate-conscious policies, and promote sustainable practices reducing the carbon footprint of the healthcare system, with a focus on the UK. In response to these pressing concerns, leading professional organizations, such as the Royal College of Obstetricians and Gynaecologists (RCOG) in the UK, and the American College of Obstetricians and Gynaecologists (ACOG) in the US, are prioritizing the intersection of climate change and women's health. Their initiatives, which aim to mitigate the climate-change impacts on pregnancies and fetal health by promoting research, raising awareness, and developing actionable strategies, are also highlighted. By amplifying awareness and global collaboration, the suggested strategies aim to protect maternal and fetal health in the face of an escalating climate crisis.
Introduction
Climate change and environmental pollution collectively pose a significant and escalating threat to human health. The World Health Organization (WHO) recognises these phenomena as among the most critical challenges to future global health, with pollution intensifying the effects of climate change and vice versa (1). The inter-governmental panel on climate change (IPCC) published the Sixth Assessment Report (AR6) in 2023, which identified that 3.3–3.6 billion people are vulnerable to the effects of climate change (2). According to the United Nations climate Promise, climate justice has many dimensions and women are disproportionately impacted by climate change, as they often have less access to resources that would help them adapt to and cope with sudden environmental changes (3). Notably, in a recent article based on discussions within the International Federation of Gynecology and Obstetrics (FIGO) Committee on Climate Change and Toxic Environmental Exposures, committee members emphasize that climate change should be addressed “in the context of women's reproductive health as a public health issue, a social justice issue, a human rights issue, an economic issue, a political issue, and a gender issue, one that demands our attention now for the health and well-being of this and future generations” (4).
Recent literature highlights the risks posed to women's health by various environmental factors, including both human-generated air pollution (5) and the adverse effects of climate change (6). Industrial pollution has degraded air quality and increased environmental contamination from plastics and microplastics (7–9, 12, 25, 145). In addition to the well-established direct impact of extreme climatic events and air pollution on maternal health and indirectly on water security and malnutrition, there is an emerging concern related to fetal exposure to pollutants such as black carbon particles and microplastics (10–13). Pregnant women already face increased physiological and psychosocial demands during pregnancy and are, therefore, more vulnerable to the increased stressors presented by climate change. This was emphasised by the recent WHO Call for Climate Action at the 2023 United Nations Climate Change Conference (COP28), which drew attention to the underestimated and underreported impact of climate events on vulnerable populations, including pregnant women, newborns and children.
This review aims to explore the relationship between climate change and environmental pollution and their impacts on maternal and fetal health, considering both direct and indirect stressors such as extreme weather events, resource insecurity, and exposure to pollutants. We will assess the latest literature, identify knowledge gaps, and suggest strategies to mitigate these effects through focused research and policy interventions. In doing so, this review highlights the urgent need for climate-conscious healthcare policies and stresses the vital role of maternal health within the broader context of climate justice initiatives.
This narrative review was developed as part of a planned meta-analysis on the effects of climate change on adverse pregnancy outcomes and maternal-fetal health. The search strategy included a comprehensive search of peer-reviewed literature and guidelines published between January 2000 and October 2024 using PubMed, Scopus, and Web of Science. Search terms comprised combinations of “climate change,” “pregnancy,” “maternal health,” and related concepts. Original research articles, systematic reviews and meta-analyses in English focusing on climate- and environmental- impacts on maternal-fetal health, were included, while policy documents, including some published in early 2025, were also considered.
The review also summarises the initiatives and position statements of medical association, such as the Royal and the American College of Obstetricians and Gynaecologists (RCOG, UK, and ACOG, US, respectively) to adapt to the effect of climate change and address its specific impacts on women's health, with a focus on healthcare systems in the UK.
Current evidence on the impact of climate change on adverse pregnancy outcomes
Adverse pregnancy outcomes include a wide range of conditions such as miscarriage, preterm birth (PTB), stillbirth, fetal growth restriction (FGR), or low birth weight (LBW), hypertensive disorders of pregnancy (HDP) including gestational hypertension (GH) and pre-eclampsia (PET), gestational diabetes (GDM), postpartum depression, and maternal death (14–17). Adverse pregnancy outcomes can result from additional factors, including maternal age, pre-existing health conditions, lifestyle factors such as smoking and substance abuse, inadequate prenatal care, deprived socioeconomic status, environmental exposures, and genetic factors. Recently, growing evidence suggests that adverse pregnancy outcomes are also directly and indirectly linked to the consequences of climate change and maternal environmental exposure to pollutants (5, 13, 18–25, 43, 55, 108–112, 116, 116, 119, 120, 127–129, 131–136, 138–140, 142–144, 148–153, 155–160, 162–165, 167, 169, 177, 178, 180–183) (Figure 1).
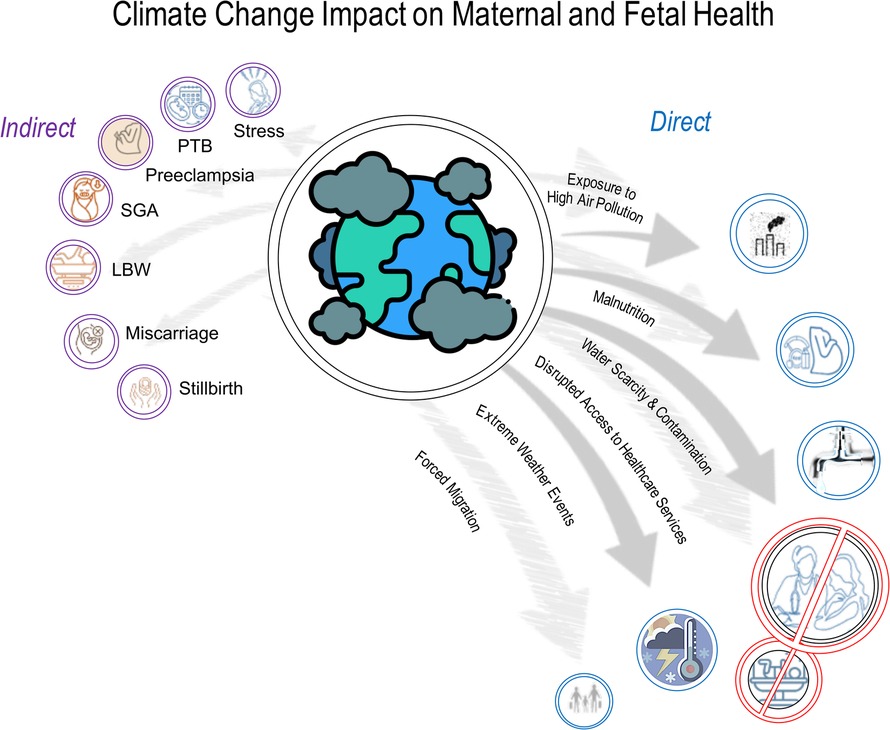
Figure 1. The frequency of climate-related emergencies, such as extreme weather events (heatwaves, floods), natural disasters, and disease outbreaks, is rising. Evidence increasingly links adverse pregnancy outcomes to climate change and maternal exposure to environmental pollutants. Disruptions in healthcare infrastructure after such events can delay or limit care for pregnant women, impacting fetal health. These adverse outcomes may include miscarriage, preterm birth (PTB), stillbirth, fetal growth restriction (FGR), low birth weight (LBW), hypertensive disorders of pregnancy (HDP) like gestational hypertension and pre-eclampsia, gestational diabetes, maternal stress, postpartum depression, and maternal death (5, 18–25, 43, 55, 108–112, 115–117, 119–120, 122–129, 131–136, 138–140, 142–144, 148–153, 155–166, 168, 169, 176–182).
The changing biosphere and atmosphere threaten food security through droughts, floods, and heatwaves, reducing crop yields and making arable land inhospitable. This can disproportionately impact socioeconomically deprived populations who may lack the resources to adapt to changing conditions, worsening income inequalities and immediate health burden, and affect certain vulnerable groups, e.g., pregnant women (26, 27). Data from the 2019 Global Burden of Disease (GBD) study by the Institute for Health Metrics and Evaluation (IHME) highlight that maternal malnutrition remains a critical concern, especially in low Socio-Demographic Index (SDI) regions and among pregnant adolescents, underlining the need for targeted interventions (28, 121). SDI ranks regions from low to high based on income, education, and fertility rates; regions with low SDI scores often face limited healthcare access, higher disease rates, and lower life expectancy, highlighting areas needing interventions to reduce health disparities (29). Poverty intensifies malnutrition, with pregnancies being especially vulnerable, whereas the WHO stresses the importance of nutrition during the first 1,000 days from conception to age two for lifelong health (30). Women with low BMI face higher risks of FGR whereas maternal iron deficiency significantly increases maternal mortality accounting for at least 20% of deaths, often due to complications (27, 29, 31–36).
Emergency situations, including those caused by extreme weather events (such as heatwaves, floods, droughts, and wildfires), as well as natural disasters (e.g., earthquakes and tsunamis) and disease outbreaks, are increasing in frequency, intensity, and impact, partly due to climate change, and can directly affect maternal and fetal health (WHO, Climate Change and Health). Evidence is still limited but increasing (37), and recognising these challenges, future disaster preparedness efforts must prioritise swift and effective responses to safeguard maternal and fetal health amidst the growing impacts of climate change. Lessons from past disasters (38, 39), such as Hurricane Katrina left approximately 56,000 pregnant women and 75,000 infants directly affected by the devastation (40), and Nepal's earthquakes (41), stress the importance of timely government and humanitarian responses. Disruptions in healthcare infrastructure following natural disasters can lead to delayed or inadequate medical attention for pregnant women and fetal health (WHO, Climate Change and Health), with evidence suggesting increased risks of complications like preterm birth and LBW (42–44). Pregnant women and infants may also be susceptible to metabolic syndrome and impaired neurodevelopment in the aftermath of natural disasters (45–49).
Gestational age at heat exposure has been linked to adverse outcomes from both acute and chronic heat exposure (18, 50). Exposure to extreme heat during pregnancy has also been investigated to assess the risk of stillbirth (51). Heat-exposure is associated with the risk of miscarriage (52) and both low and high temperatures are associated with an increased risk of preterm birth (53). A specific threshold for temperature effects has not been identified, as most studies consider exposure to be temperatures above the 90th percentile for that population (50, 54–58). Proposed mechanisms include increased dehydration and impaired maternal thermoregulation. It remains unclear if heat exposure directly affects thermoregulation in pregnant women or acts as an external stressor triggering hormonal responses (59) as both pathways can impact fetal development and endocrine balance in neonates. Additionally, severe weather events related to climate change can elevate maternal stress levels, potentially leading to complications like preterm birth and LBW (60–63).
Climate change intensifies water security through altered weather patterns, leading to floods, droughts, and polar ice melt, which raises sea levels and salinates fresh water. Notably, access to safe drinking water remains a global issue, with 1 billion people lacking access (64), and a review of 14 studies linked water scarcity and poor sanitation to maternal mortality (65). Industrial pollution further contaminates fresh water, impacting hygiene and safety (66). A 2002 review found moderate evidence of water contaminants causing SGA, neural tube defects, miscarriage, and congenital abnormalities (11). In addition, two US-based studies showed that in New Jersey, contaminated water was linked to small-for-gestational-age (SGA) births and increased incidence of prematurity (67), whereas in Pennsylvania (USA), even low-level water contamination was linked to reduced fertility and adverse birth outcomes, such as low birth weight (68). Significant associations between water contaminants like BPA, phthalates, and PFAS with adverse obstetric outcomes and gynaecological pathology, including higher rates of infertility and endometriosis have also been reported (69).
Furthermore, and despite the rise in global concern around air pollution, global emissions continue to grow, and the problem persists. According to the WHO, household air pollution causes 3.2 million premature deaths annually, with over 99% of people living in areas exceeding WHO air quality standards (70). Ambient air pollution, primarily from motor vehicles, solid fuel burning, and industry, causes 4.2 million deaths per year. Air pollution is made up of a collection of solid and/or liquid materials of various sizes, ranging from a few nanometers in diameter, consisting of particulate matter (PM10 and PM2.5) and gaseous pollutants like sulphur dioxide, nitrogen oxides, ozone, and carbon monoxide (71). Associations between air pollution and pregnancy outcomes are complex due to various pollutants and confounding factors, but there is evidence associating certain types of air pollution and adverse birth outcomes, including, premature delivery, low birthweight, pre-eclampsia and miscarriage (5, 58, 72, 146). Notably, wildfire-specific PM2.5 exposure in the last four gestational weeks was shown to increase risks of preterm birth, stillbirth, and nonvertex presentation, whereas black carbon exposure was strongly linked to stillbirth, and the most vulnerable groups were female births, mothers with low socioeconomic status, and those with high biothermal exposure (18).
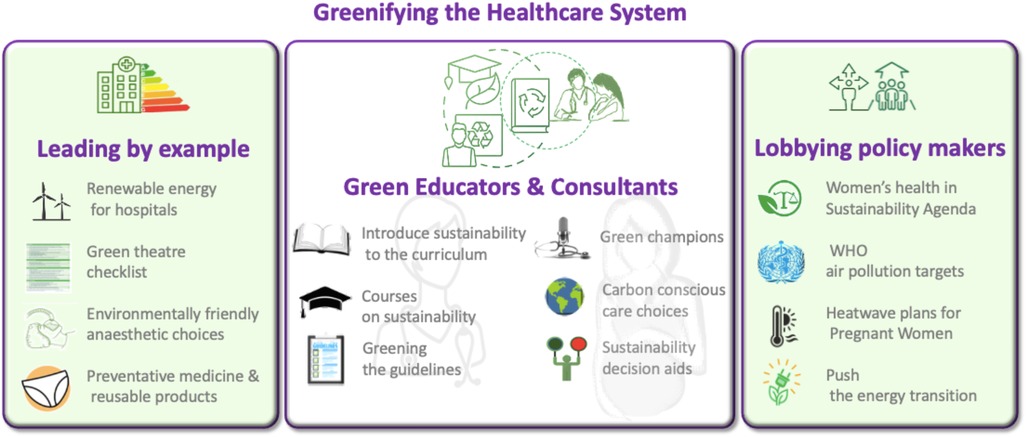
Raising awareness of climate and environmental change's impact on women's health, including transgenerational effects, is crucial [(73, 161–166, 168–182) and Figure 1]. Researchers and health organizations are examining all these climate change induced challenges, emphasizing the urgent need to protect pregnant women. Transdisciplinary research into the pregnancy exposome is essential to address health disparities, identify at-risk populations, and understand the molecular mechanisms behind climate-induced pregnancy complications (73). Moreover, efforts should focus on the urgency to protect women against the growing threats of a changing climate and environment, and strategies to understand these impacts while developing strategies to reduce, mitigate, or eliminate risks to maternal and fetal health (Figure 2) (4, 14, 40, 80, 113, 114, 118, 137, 141, 147, 154, 170). These efforts should prioritize minimizing the healthcare system's carbon footprint, implementing early interventions to reduce its environmental impact, empowering healthcare professionals to educate patients, and encouraging them to advocate for policy changes.
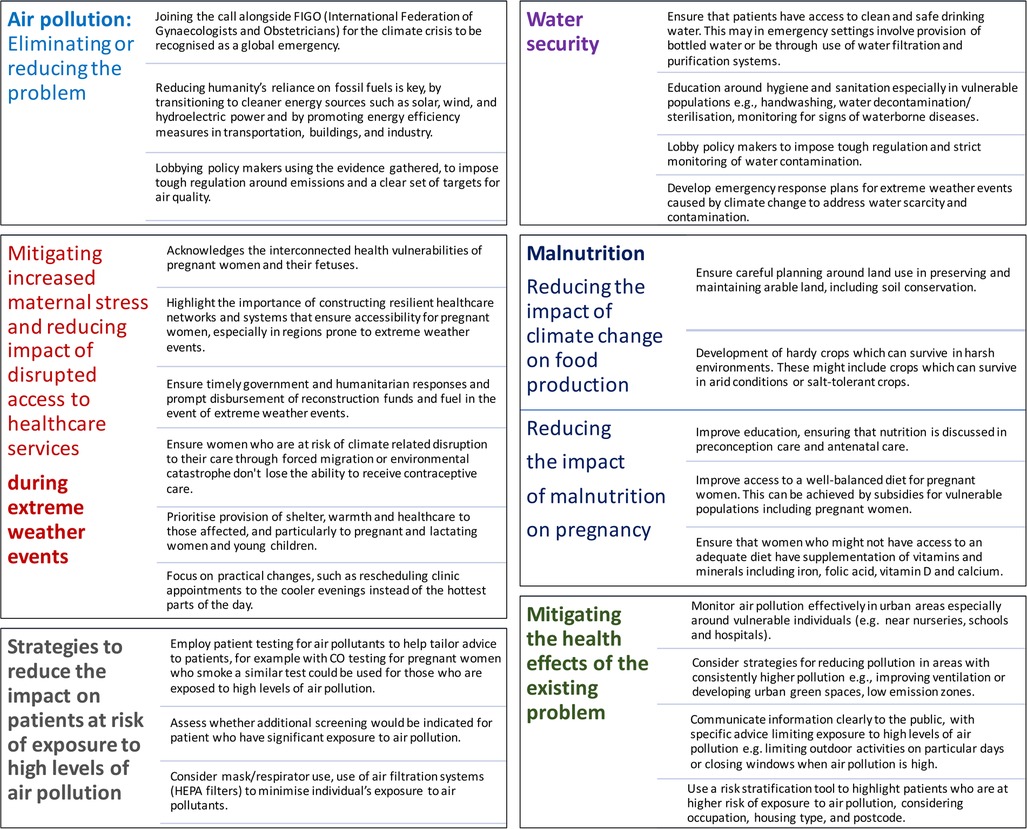
Figure 2. Strategies to mitigate climate change impacts on maternal health: Air pollution, water security, healthcare access, and nutrition.
Climate action in high-income healthcare systems: lessons from the UK
High-income countries (HICs) bear particular responsibility for climate mitigation given their disproportionate carbon emissions and resource-intensive healthcare systems (74). The United Kingdom has emerged as a global leader in climate-conscious healthcare reform, with the National Health Service (NHS) implementing its landmark Net Zero Strategy in 2020, the world's first national health system commitment to carbon neutrality by 2040 (75). This initiative combines operational changes (e.g., low-carbon anaesthesia) with systemic interventions (e.g., supplier emissions requirements) demonstrating how industrialized nations can combine quality care with sustainability (76, 77). The UK experience also reveals both the promise and the challenges of healthcare decarbonization. While the NHS has reduced emissions by 30% since 2010 through innovations like virtual consultations and green surgical protocols, barriers persist in areas such as pharmaceutical supply chains and medical device lifecycle management (149). These lessons provide actionable insights for other HICs and LMICs facing comparable infrastructure challenges, from hospital energy use patterns to climate-vulnerable patient populations (76, 78). Therefore, the UK NHS model accentuates that healthcare sustainability requires both technological solutions and policy frameworks that can facilitate systemic change (79).
Reducing the carbon footprints of the healthcare system
The healthcare system should be leading by example in the implementation of carbon-neutral and emission-free provision of care and take responsibility for the supply chain (80). In total, the healthcare sector accounts for between 4%–5% of global carbon emissions (81, 82), thus, implementing sustainable practices in obstetrics can significantly reduce emissions and waste. Additionally, adopting energy-efficient measures, including transitioning hospitals to 100% renewable energy, as advocated and practiced by the RCOG, is crucial for sustainability.
Proper waste segregation and mindful practices, such as reduction of unused medical supplies disposal, can help lower the environmental impact of waste management. A recent systematic review assessed studies on the environmental impact of vaginal births, obstetric and gynaecological surgical procedures, menstrual products, vaginal specula and transportation to gynaecological oncologic consultations (83). The results showed that among the highest yielding mitigation strategies are displacing disposable with reusable materials and minimising content of surgical custom pack, whereas the lowest-yielding mitigation strategy was waste optimisation, including recycling. For caesarean sections, when excluding energy costs and analgesia, over half of the carbon impact comes from disposables, such as single-use drapes (84).
Professional bodies for obstetricians, gynaecologists, and anaesthetists should collaborate to develop standardized guidelines that prioritize women's health while effectively reducing the climate impact of maternity care. When inhalation anaesthesia is used, it can contribute up to 63% of the carbon footprint of perioperative departments in high-income countries, with anaesthetic gas emissions accounting for approximately 2%–5% of the total carbon footprint of the healthcare sector (75, 77, 85). When exploring alternative analgesia options, it is essential to prioritize patient safety and choice without compromising care (86). Additionally, careful selection of anaesthetics is vital, particularly by eliminating gases, such as desflurane, which lack significant clinical benefits but carry higher economic and environmental costs (87). Perhaps for maternity care where caesarean sections are more commonly performed under spinal anaesthesia, labour analgesia presents a more significant opportunity to reduce emissions. Nitrous oxide mixed with oxygen (Entonox) is widely used but has one of the worst carbon footprints. It was shown that for vaginal birth, using morphine resulted in a carbon footprint of 9.48 kg CO2e compared to 246.73 kg CO2e with Entonox (84). Strategies to reduce the impact of Entonox include reducing its use and replacing it with alternative analgesics, recapture or breakdown expired nitrous oxide and decommissioning leaky nitrous oxide manifolds. Notably, a recent study found that postnatal women had low awareness of nitrous oxide's environmental impact (88). However, after receiving information, 99% believed they had the right to be informed about its harmful effects when choosing analgesia.
Surgical interventions in obstetrics, such as caesarean sections, are highly carbon-intensive and generally have a larger carbon footprint than vaginal births when analgesia is excluded. This comparison is dependent on the type of analgesia used, with the use of nitrous oxide with oxygen during vaginal birth increasing its carbon footprint by 25 times (84), highlighting the complexity of assessing environmental impacts in maternity care. In the UK, the surgical Royal Colleges have developed evidence-based interventions to reduce the environmental impact of surgery (UKHACC). The approach accentuates preventative measures to reduce the need for surgery and promotes sustainability through the Intercollegiate Green Theatre Checklist (89). This strategy focuses on reusing equipment and minimising reliance on energy-intensive alternatives (90).
Reducing environmental impact of healthcare through early interventions and innovation
Preventative medicine is a crucial strategy to reduce the environmental impact of healthcare, as improved screening and preventative measures can help decrease the need for interventions. For example, pelvic floor exercises during and after pregnancy can reduce the rate of incontinence and long-term reliance on single-use continence pads (91). A recent study evaluated patient satisfaction on gynaecological examination with metal, plastic and biobased plastic vaginal specula, and investigated whether patients are willing to compromise on comfort for a more sustainable healthcare system (92). The results indicated a significant difference in favour of a biobased plastic speculum, with patients willing to compromise on comfort in favour of sustainability. While the willingness to use reusable specula is important, expecting women to endure increased discomfort for the sake of sustainability contradicts gender-transformative principles. Women should not be required to bear additional burdens in efforts to “greenify” healthcare systems. Instead of compromising comfort, alternatives such as self-sampling methods, miniature-imaging devices for cervical imaging and sampling, and redesigned specula should be explored. For example, innovative speculum designs that prioritize patient comfort while being compatible with high-level disinfection and autoclaving offer a sustainable, hygienic solution for gynaecological care (93–95).
Initiatives and calls to action
In the UK, the RCOG is working closely with key partners to develop and implement lower-carbon NHS care models (75, 96). The project actively involves individuals with lived experience of maternity services to ensure equitable and system-wide benefits. Supported by the findings of this project the RCOG seeks to integrate sustainability into care guidelines, including future Green-top Guidelines. As part of this effort, clear guidance should be given to patients on recommended safe, acceptable, and sustainable period products (97, 98).
Additionally, the RCOG, Royal College of Paediatricians and Child Health (RCPCH), and UK Health Alliance on Climate Change (UKHACC) have called on the UK government to create a heatwave plan that specifically addresses the needs of pregnant women and children. This call comes in response to evidence that severe weather events linked to climate change can increase maternal stress levels, potentially leading to complications (RCOG, RCPCH, and UKHACC call). In the US, ACOG recently released a committee statement on disaster preparedness, highlighting the critical needs of obstetric and gynaecologic patients during emergencies (99). Pregnant, postpartum, and breastfeeding women can receive guidance for emergency plans, including evacuation strategies, supply and birth kits, and access to important documents, from resources such as the CDC and the American Red Cross.
Healthcare professionals as patient educators
There is clear data linking climate change to negative maternal and fetal health and yet there has been limited success in implementing strategies to reduce its impact. When looking at implementing evidence-based practice, Morena et al. found clinical champions were a very successful way to reduce barriers to behavioural change (100). Clinical champions are individuals with great communication skills, committed to driving, advocating for, and supporting the implementation of initiatives while addressing and overcoming potential resistance at the organizational level (101). Green champions can lead initiatives such as waste reduction and energy efficiency, promoting eco-friendly practices in healthcare. They can stimulate a culture of climate-conscious care by educating colleagues both about the health impact of climate change, particularly on pregnant women, and the impact of sustainability on the reduction of carbon footprint. Additionally, they help pregnant women, and their families understand the importance of sustainable practices, encouraging healthier behaviours and environmental responsibility in maternal and fetal healthcare. Perhaps ensuring that maternity and women's health services are empowered with green champions who can spearhead organisational change would be a big step forward.
Healthcare professionals need training in sustainability and climate issues to effectively educate patients. In the UK, the RCOG is expanding sustainability education through training programs, an e-Learning course on climate change and healthcare, and workshops on integrating sustainability into patient discussions (RCOG, eLearning Catalogue). Additionally, the Centre for Sustainable Healthcare offers courses to deepen professionals' understanding of the direct and indirect impacts of climate change (102). As discussions around the mode of delivery and birth plans are highly personal and must be approached sensitively, decision aids can be valuable tools in guiding patients (103). Decision aids that incorporate clinical evidence alongside sustainability considerations can help inform patient choices, promoting environmentally conscious healthcare while maintaining high-quality, women-centered care.
Healthcare professionals raising awareness among policy makers
Despite growing efforts by health professionals to address climate-related health risks, evidence on the effectiveness of interventions is lacking and supporting literature remains scarce (104). Healthcare professionals have a duty to highlight health concerns, due to climate change and environmental pollution, in particular to policy makers (Figure 3). Medical colleges often act to coordinate and advocate for their patients when lobbying the government. In the UK, the RCOG, RCPCH, and UKHACC have jointly identified three urgent actions to protect child, fetal, and maternal health, as outlined in the Lancet Countdown policy brief (105). These include enforcing the ambitious air quality standards set by the WHO, developing heatwave response plans with a focus on vulnerable populations, and accelerating the energy transition by cutting fossil fuel subsidies and redirecting funds toward renewable energy. Similarly, the American College of Obstetricians and Gynaecologists (ACOG) has emphasized in a position statement the urgent need for clinical and community-based research on the health impacts of climate change, particularly on women and marginalized populations (106). AJOG also advocates for policies that reduce greenhouse gas emissions, promote environmentally responsible medical practices, and mitigate climate-related health risks, urging national and international leaders to take action and invest in solutions that protect women's health and well-being.
Further to these main policy goals, there are some more specific strategies to reduce the impact of climate change and environmental pollution on maternal and fetal health (Figure 2). These involve wider policies to reduce or prevent ongoing pollution, and solutions to mitigate the effects of air pollution, polluted water, poor diet, and extreme weather events on pregnant women and their pregnancies.
Discussion
The climate crisis presents significant risks to women, especially maternal and fetal health (Figure 1), with environmental exposure to pollutants disproportionately impacting women facing socio-economic hardships. Therefore, a woman-centered approach to healthcare is essential, focusing on inclusive, personalized care that addresses the unique physiological, emotional, cultural, and socio-economic needs of pregnant women. This approach is decisive as maternal health directly affects both women's health and the development of their pregnancies. Therefore, climate crisis-centered guidelines and policies should prioritize women's health across their lifespan, with a particular emphasis on maternal and fetal health during pregnancy and beyond.
While individual efforts to protect pregnant women are important, climate change requires global strategies and solutions. Raising public awareness and advocating for policy reforms are vital to create meaningful change on a larger scale. Mental health support, management of chronic conditions, such as gestational diabetes and hypertension, and health equity must also be prioritized to ensure all women have access to quality care, regardless of their background. In addition, integrating sexual and reproductive health and human rights into climate policies is critical to enhance the well-being and health of women globally (2, 130).
Furthermore, advanced guidelines that incorporate AI-driven solutions, such as predictive algorithms for risk assessment and personalized healthcare recommendations, can significantly augment the precision of women's health and prenatal care. Thus, responsible AI deployment, aligned with climate justice principles, can help minimize environmental impact while improving healthcare delivery (107).
Moreover, healthcare systems can lead by adopting greener practices, engaging green champions, educators, and policymakers to prioritize sustainability. This includes positioning women's health at the forefront of the sustainability agenda, educating healthcare professionals about sustainable practices and greenifying healthcare operations (Figure 3). These initiatives will not only help mitigate climate crisis-related health risks but also ensure equitable healthcare access for these vulnerable groups, supporting the broader goal of climate justice.
Women's healthcare providers play a crucial role in educating patients about the adverse health impacts of climate change and advocating for sustainable practices. As some of the most trusted voices on climate issues, healthcare providers must lead by example, implementing climate change mitigation guidelines within clinical settings. By combining education with actionable interventions, they can empower patients and contribute to broader efforts to combat climate change's impact on public health.
While educating patients about climate change is important, true systemic change requires broader action beyond individual behaviour. OB-GYNs can amplify their roles through education, research, and advocacy (4), but we must also recognize the need for institutional and policy-level shifts. In line with this, the FIGO Opinion outlines four key recommendations for policymakers and stakeholders to reduce and prevent exposure to toxic chemicals globally, i.e., to reduce and prevent exposure to toxic chemicals: advocating for preventive policies, ensuring a healthy food system, integrating environmental health into healthcare, and promoting environmental justice (FIGO).
Balancing these systemic changes with patient education is essential for lasting impact. Recognising the climate crisis as a global emergency, researchers and healthcare providers should lead advocacy, research, capacity building, and education efforts to address its health consequences.
Author contributions
AS: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation. TH: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing, Validation. RT: Formal analysis, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. EJ: Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. AK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. September 28. Climate Change and Health (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (Accessed June 20, 2024).
2. Intergovernmental Panel on Climate Change, IPCC. Climate Change 2023: Synthesis Report. Summary for Policymakers (2023). Available at: https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_SPM.pdf (Accessed April 10, 2025).
3. United Nations Development Programme. Climate Change is a Matter of Justice. Here’s Why. Climate Promise (2023). Available at: https://climatepromise.undp.org/news-and-stories/climate-change-matter-justice-heres-why (Accessed December 7, 2024).
4. Giudice LC, Llamas-Clark EF, DeNicola N, Pandipati S, Zlatnik MG, Decena DCD, et al. Climate change, women’s health, and the role of obstetricians and gynecologists in leadership. Int J Gynaecol Obstet. (2021) 155(3):345–56. doi: 10.1002/ijgo.13958
5. Fussell JC, Jauniaux E, Smith RB, Burton GJ. Ambient air pollution and adverse birth outcomes: a review of underlying mechanisms. BJOG. (2024) 131(5):538–50. doi: 10.1111/1471-0528.17727
6. Romanello M, Napoli CD, Green C, Kennard H, Lampard P, Scamman D, et al. The 2023 report of the lancet countdown on health and climate change: the imperative for a health-centred response in a world facing irreversible harms. Lancet. (2023) 402(10419):2346–94. doi: 10.1016/S0140-6736(23)01859-7
7. Oliveri Conti G, Rapisarda P, Ferrante M. Relationship between climate change and environmental microplastics: a one health vision for the platysphere health. One Health Adv. (2024) 2:17. doi: 10.1186/s44280-024-00049-9
8. Parvez MS, Ullah H, Faruk O, Simon E, Czédli H. Role of microplastics in global warming and climate change: a review. Water Air Soil Pollut. (2024) 235:201. doi: 10.1007/s11270-024-07003-w
9. Karlsson O, Rocklöv J, Lehoux AP, Bergquist J, Rutgersson A, Blunt MJ, et al. The human exposome and health in the anthropocene. Int J Epidemiol. (2021) 50(2):378–89. doi: 10.1093/ije/dyaa231
10. Bongaerts E, Lecante LL, Bové H, Roeffaers MBJ, Ameloot M, Fowler PA, et al. Maternal exposure to ambient black carbon particles and their presence in maternal and fetal circulation and organs: an analysis of two independent population-based observational studies. Lancet Planet Health. (2022) 6(10):e804–11. doi: 10.1016/S2542-5196(22)00200-5
11. Bove F, Shim Y, Zeitz P. Drinking water contaminants and adverse pregnancy outcomes: a review. Environ Health Perspect. (2002) 110(Suppl 1):61–74. doi: 10.1289/ehp.02110s161
12. Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, et al. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ Pollut. (2019) 255(1):113122. doi: 10.1016/j.envpol.2019.113122
13. Jauniaux E, Wylie BJ, Verheijen E, Conry J, Papageorghiou A. Women’s health in the anthropocene. BJOG. (2024) 131(5):531–2. doi: 10.1111/1471-0528.17679
14. Royal College of Obstetricians and Gynaecologists (RCOG). Inequalities in Maternal and Perinatal Health (2021). Available at: https://www.rcog.org.uk (Accessed October 30, 2024).
15. American College of Obstetricians and Gynecologists (ACOG). Guidance on Maternal Health and Adverse Pregnancy Outcomes (2020). Available at: https://www.acog.org (Accessed October 30, 2024).
16. World Health Organization (WHO). WHO Recommendations on Maternal Health: Guidelines Approved by the WHO Guidelines Review Committee. Geneva: WHO (2017). Available at: https://www.who.int/publications/i/item/WHO-MCA-17.10 (Accessed October 30, 2024).
17. U.S. Environmental Protection Agency. America’s Children and the Environment: Third Edition—Health—Adverse Birth Outcomes (2014). Available at: https://www.epa.gov/sites/default/files/2015-06/documents/health-adverse-birth-outcomes.pdf (Accessed October 30, 2024).
18. Nyadanu SD, Foo D, Pereira G, Mickley LJ, Feng X, Bell ML. Short-term effects of wildfire-specific fine particulate matter and its carbonaceous components on perinatal outcomes: a multicentre cohort study in New South Wales, Australia. Environ Int. (2024) 191:109007. doi: 10.1016/j.envint.2024.109007
19. Zhang Y, Hu Y, Talarico R, Qiu X, Schwartz J, Fell DB, et al. Prenatal exposure to ambient air pollution and cerebral palsy. JAMA Netw Open. (2024) 7(7):e2420717. doi: 10.1001/jamanetworkopen.2024.20717
20. Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. Int J Epidemiol. (2012) 41(1):79–105. doi: 10.1093/ije/dyr154
21. Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. (2011) 31(3):363–73. doi: 10.1016/j.reprotox.2010.12.055
22. Palma-Gudiel H, Cirera F, Crispi F, Eixarch E, Fañanás L. The impact of prenatal insults on the human placental epigenome: a systematic review. Neurotoxicol Teratol. (2018) 66:80–93. doi: 10.1016/j.ntt.2018.01.001
23. Carré J, Gatimel N, Moreau J, Parinaud J, Léandri R. Does air pollution play a role in infertility?: a systematic review. Environ Health. (2017) 16:82. doi: 10.1186/s12940-017-0291-8
24. Segal TR, Giudice LC. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil Steril. (2019) 112(4):613–21. doi: 10.1016/j.fertnstert.2019.08.001
25. Zurub RE, Cariaco Y, Wade MG, Bainbridge SA. Microplastics exposure: implications for human fertility, pregnancy and child health. Front Endocrinol (Lausanne). (2024) 14:1330396. doi: 10.3389/fendo.2023.1330396
26. Butsch C, Beckers LM, Nilson E, Frassl M, Brennholt N, Kwiatkowski R, et al. Health impacts of extreme weather events—cascading risks in a changing climate. J Health Monit. (2023) 8(Suppl 4):33–56. doi: 10.25646/11652
27. Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. (2008) 371(9608):243–60. doi: 10.1016/S0140-6736(07)61690-0
28. Xu T, Dong C, Shao J, Huo C, Chen Z, Shi Z, et al. Global burden of maternal disorders attributable to malnutrition from 1990 to 2019 and predictions to 2035: worsening or improving? Front Nutr. (2024) 11:1343772. doi: 10.3389/fnut.2024.1343772
29. GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2162–203. doi: 10.1016/S0140-6736(24)00933-4 Erratum in: Lancet. 2024 July 20;404(10449):244. doi: 10.1016/S0140-6736(24)01458-2.38762324
30. World Health Organization. Air Quality, Energy and Health (2024). Available at: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts/types-of-pollutants (Accessed March 5, 2024).
31. Edelson PK, Cao D, James KE, Ngonzi J, Roberts DJ, Bebell LM, et al. Maternal anemia is associated with adverse maternal and neonatal outcomes in Mbarara, Uganda. J Matern Fetal Neonatal Med. (2023) 36(1):2190834. doi: 10.1080/14767058.2023.2190834
32. Montvignier Monnet A, Savoy D, Préaubert L, Hoffmann P, Bétry C. In underweight women, insufficient gestational weight gain is associated with adverse obstetric outcomes. Nutrients. (2022) 15(1):57. doi: 10.3390/nu15010057
33. Jardine J, Walker K, Gurol-Urganci I, Webster K, Muller P, Hawdon J, et al. National maternity and perinatal audit project team. Adverse pregnancy outcomes attributable to socioeconomic and ethnic inequalities in England: a national cohort study. Lancet. (2021) 398(10314):1905–12. doi: 10.1016/S0140-6736(21)01595-6 Erratum in: Lancet. 2021 November 20;398(10314):1874. doi: 10.1016/S0140-6736(21)02432-6.34735797
34. Daru J, Zamora J, Fernández-Félix BM, Vogel J, Oladapo OT, Morisaki N, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Health. (2018) 6(5):e548–54. doi: 10.1016/S2214-109X(18)30078-0
35. Sebire NJ, Jolly M, Harris J, Regan L, Robinson S. Is maternal underweight really a risk factor for adverse pregnancy outcome? A population-based study in London. BJOG. (2001) 108(1):61–6. doi: 10.1111/j.1471-0528.2001.00021.x
36. Fishman SM, Caulfield L, de Onis M, Hyder A, Mullany L, Black R, et al. Childhood and maternal underweight. In: Ezzati M, Lopez AD, Rodgers A, Murray CLJ, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected major Risk Factors. Geneva: World Health Organization (2004). p. 39–161. Available at: https://iris.who.int/bitstream/handle/10665/42792/9241580348_eng_Volume1.pdf?sequence=1&isAllowed=y (Accessed May 16, 2025).
37. Bansal A, Cherbuin N, Davis DL, Peek MJ, Wingett A, Christensen BK, et al. Heatwaves and wildfires suffocate our healthy start to life: time to assess impact and take action. Lancet Planet Health. (2023) 7(8):e718–25. doi: 10.1016/S2542-5196(23)00134-1
38. Olson DM, Brémault-Phillips S, King S, Metz GAS, Montesanti S, Olson JK, et al. Recent Canadian efforts to develop population-level pregnancy intervention studies to mitigate effects of natural disasters and other tragedies. J Dev Orig Health Dis. (2019) 10(1):108–14. doi: 10.1017/S2040174418001113
39. Reid CE. Invited perspective: what do we know about fetal-maternal health and health care needs after wildfires? Not nearly enough. Environ Health Perspect. (2022) 130(8):81304. doi: 10.1289/EHP11699
40. Callaghan WM, Rasmussen SA, Jamieson DJ, Ventura SJ, Farr SL, Sutton PD, et al. Health concerns of women and infants in times of natural disasters: lessons learned from Hurricane Katrina. Matern Child Health J. (2007) 11(4):307–11. doi: 10.1007/s10995-007-0177-4
41. Brunson J. Maternal, newborn, and child health after the 2015 Nepal earthquakes: an investigation of the long-term gendered impacts of disasters. Matern Child Health J. (2017) 21(12):2267–73. doi: 10.1007/s10995-017-2350-8
42. Basu R, Malig B, Ostro B. High ambient temperature and the risk of preterm delivery. Am J Epidemiol. (2010) 172(10):1108–17. doi: 10.1093/aje/kwq170
43. Bekkar B, Pacheco S, Basu R, DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. (2020) 3(6):e208243–e208243. doi: 10.1001/jamanetworkopen.2020.8243
44. Shankar K, Hwang K, Westcott JL, Saleem S, Ali SA, Jessani S, et al. Associations between ambient temperature and pregnancy outcomes from three south Asian sites of the global network maternal newborn health registry: a retrospective cohort study. BJOG. (2023) 130(Suppl 3):124–33. doi: 10.1111/1471-0528.17616
45. Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project ice storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J Am Acad Child Adolesc Psychiatry. (2008) 47(9):1063–72. doi: 10.1097/CHI.0b013e31817eec80
46. King S, Dancause K, Turcotte-Tremblay AM, Veru F, Laplante DP. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res C Embryo Today. (2012) 96(4):273–88. doi: 10.1002/bdrc.21026
47. Dancause KN, Veru F, Andersen RE, Laplante DP, King S. Prenatal stress due to a natural disaster predicts insulin secretion in adolescence. Early Hum Dev. (2013) 89(9):773–6. doi: 10.1016/j.earlhumdev.2013.06.006
48. Liu GT, Dancause KN, Elgbeili G, Laplante DP, King S. Disaster-related prenatal maternal stress explains increasing amounts of variance in body composition through childhood and adolescence: project ice storm. Environ Res. (2016) 150:1–7. doi: 10.1016/j.envres.2016.04.039
49. Veru F, Dancause K, Laplante DP, King S, Luheshi G. Prenatal maternal stress predicts reductions in CD4 + lymphocytes, increases in innate-derived cytokines, and a Th2 shift in adolescents: project ice storm. Physiol Behav. (2015) 144:137–45. doi: 10.1016/j.physbeh.2015.03.016
50. Bonell A, Part C, Okomo U, Cole R, Hajat S, Kovats S, et al. An expert review of environmental heat exposure and stillbirth in the face of climate change: clinical implications and priority issues. BJOG. (2024) 131(5):623–31. doi: 10.1111/1471-0528.17622
51. Sexton J, Andrews C, Carruthers S, Kumar S, Flenady V, Lieske S. Systematic review of ambient temperature exposure during pregnancy and stillbirth: methods and evidence. Environ Res. (2021) 197:111037. doi: 10.1016/j.envres.2021.111037
52. Rekha S, Nalini SJ, Bhuvana S, Kanmani S, Hirst JE, Venugopal V. Heat stress and adverse pregnancy outcome: prospective cohort study. BJOG. (2024) 131(5):612–22. doi: 10.1111/1471-0528.17680
53. Terada S, Nishimura H, Miyasaka N, Fujiwara T. Ambient temperature and preterm birth: a case-crossover study. BJOG. (2024) 131(5):632–40. doi: 10.1111/1471-0528.17720
54. Asamoah B, Kjellstrom T, Östergren PO. Is ambient heat exposure levels associated with miscarriage or stillbirths in hot regions? A cross-sectional study using survey data from the Ghana maternal health survey 2007. Int J Biometeorol. (2018) 62(3):319–30. doi: 10.1007/s00484-017-1402-5
55. Ranjbaran M, Mohammadi R, Yaseri M, Kamari M, Habibelahi A, Yazdani K. Effect of ambient air pollution and temperature on the risk of stillbirth: a distributed lag nonlinear time series analysis. J Environ Health Sci Eng. (2020) 18(2):1289–99. doi: 10.1007/s40201-020-00547-z
56. McElroy S, Ilango S, Dimitrova A, Gershunov A, Benmarhnia T. Extreme heat, preterm birth, and stillbirth: a global analysis across 14 lower-middle income countries. Environ Int. (2022) 158:106902. doi: 10.1016/j.envint.2021.106902
57. Khodadadi N, Dastoorpoor M, Khanjani N, Ghasemi A. Universal thermal climate index (UTCI) and adverse pregnancy outcomes in Ahvaz, Iran. Reprod Health. (2022) 19(1):33. doi: 10.1186/s12978-022-01344-7
58. Nyadanu SD, Tessema GA, Mullins B, Kumi-Boateng B, Ofosu AA, Pereira G. Prenatal exposure to long-term heat stress and stillbirth in Ghana: a within-space time-series analysis. Environ Res. (2023) 222:115385. doi: 10.1016/j.envres.2023.115385
59. Yüzen D, Graf I, Diemert A, Arck PC. Climate change and pregnancy complications: from hormones to the immune response. Front Endocrinol (Lausanne). (2023) 14:1149284. doi: 10.3389/fendo.2023.1149284
60. Verstraeten BSE, Elgbeili G, Hyde A, King S, Olson DM. Maternal mental health after a wildfire: effects of social support in the fort McMurray wood buffalo study. Can J Psychiatry. (2021) 66(8):710–8. doi: 10.1177/0706743720970859
61. Holstius DM, Reid CE, Jesdale BM, Morello-Frosch R. Birth weight following pregnancy during the 2003 southern California wildfires. Environ Health Perspect. (2012) 120(9):1340–5. doi: 10.1289/ehp.1104515,
62. Fernández ACG, Basilio E, Benmarhnia T, Roger J, Gaw SL, Robinson JF, et al. Retrospective analysis of wildfire smoke exposure and birth weight outcomes in the San Francisco bay area of California. Environ Res Health. (2023) 1(2):025009. doi: 10.1088/2752-5309/acd5f5
63. Jung EJ, Lim AY, Kim JH. Decreased birth weight after prenatal exposure to wildfires on the eastern coast of Korea in 2000. Epidemiol Health. (2023) 45:e2023003. doi: 10.4178/epih.e2023003
64. Akachi Y, Goodman D, Parker D. Global Climate Change and Child Health: A Review of Pathways, Impacts and Measures to Improve the Evidence Base. Innocenti Discussion Papers 2009. Florence: UNICEF Innocenti Research Centre (2009).
65. Benova L, Cumming O, Campbell OM. Systematic review and meta-analysis: association between water and sanitation environment and maternal mortality. Trop Med Int Health. (2014) 19(4):368–87. doi: 10.1111/tmi.12275
66. Damania R, Zaveri E. Hidden toxins: the effects of water quality on pregnancy and infant health. BJOG. (2024) 131(5):535–7. doi: 10.1111/1471-0528.17628
67. Currie J, Zivin JG, Meckel K, Neidell M, Schlenker W. Something in the water: contaminated drinking water and infant health. Can J Econ. (2013) 46(3):791–810. doi: 10.1111/caje.12039
68. DiSalvo R, Hill EA. Drinking water contaminant concentrations and birth outcomes. J Policy Anal Manage. (2023) 43(2):368–99. doi: 10.1002/pam.22558
69. Rashtian J, Chavkin DE, Merhi Z. Water and soil pollution as determinant of water and food quality/contamination and its impact on female fertility. Reprod Biol Endocrinol. (2019) 17(5):1–13. doi: 10.1186/s12958-018-0448-5
70. World Health Organization. Types of Pollutants (2021). Available at: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts/types-of-pollutants (Accessed June 20, 2024).
71. Aplin JD, Jones CJP, Jauniaux E. Environmental nanoparticles and placental research. BJOG. (2024) 131(5):551–4. doi: 10.1111/1471-0528.17695
72. Bachwenkizi J, Liu C, Meng X, Zhang L, Wang W, van Donkelaar A, et al. Maternal exposure to fine particulate matter and preterm birth and low birth weight in Africa. Environ Int. (2022) 160:107053. doi: 10.1016/j.envint.2021.107053
73. Girardi G, Bremer AA. Climate and environmental changes exacerbate health disparities in pregnant people and their offspring. How can we protect women and their babies? Birth Defects Res. (2024) 116(2):e2313. doi: 10.1002/bdr2.2313
74. Health Care Without Harm. Global Road Map for Health Care Decarbonization: A Navigational Tool for Achieving Zero Emissions with Climate Resilience and Health Equity (Executive Summary, Climate-Smart Health Care Series, Green Paper Number Two (2021). Available at: https://healthcareclimateaction.org/sites/default/files/2021-09/Road%20Map%20for%20Health%20Care%20Decarbonization%20Executive%20Summary.pdf (Accessed April 10, 2025).
75. NHS England. Delivering a Net Zero National Health Service (2020). Available at: https://www.england.nhs.uk/greenernhs/publication/delivering-a-net-zero-national-health-service/ (Accessed April 09, 2025).
76. MacNeill AJ, McGain F, Sherman JD. Planetary health care: a framework for sustainable health systems. Lancet Planet Health. (2021) 5(2):e66–8. doi: 10.1016/S2542-5196(21)00005-X. Erratum in: Lancet Planet Health. 2022 Jan;6(1):e7. doi: 10.1016/S2542-5196(21)00353-3.33581064
77. Tennison I, Roschnik S, Ashby B, Boyd R, Hamilton I, Oreszczyn T, et al. Health care’s response to climate change: a carbon footprint assessment of the NHS in England. Lancet Planet Health. (2021) 5(2):e84–92. doi: 10.1016/S2542-5196(20)30271-0
78. Rasheed FN, Baddley J, Prabhakaran P, De Barros EF, Reddy KS, Vianna NA, et al. Decarbonising healthcare in low and middle income countries: potential pathways to net zero emissions. Br Med J. (2021) 375:n1284. doi: 10.1136/bmj.n1284
79. Watts N, Amann M, Ayeb-Karlsson S, Belesova K, Bouley T, Boykoff M, et al. The lancet countdown on health and climate change. Lancet. (2023) 391(10120):581–630. doi: 10.1016/S0140-6736(23)01859-7
80. Royal College of Physicians. Royal College of Physicians Every Breath We Take: The Lifelong Impact of Air Pollution (2016). Available at: https://www.rcplondon.ac.uk/projects/outputs/every-breath-we-take-lifelong-impact-air-pollution (Accessed March 1, 2024).
81. Karliner J, Slotterback S, Boyd R, Ashby B, Steele K. Health care's climate footprint—how the health sector contributes to the global climate crisis and opportunities for action—Green paper number one in the climate-smart health care series. Health Care Without Harm (2019). https://noharm.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_092319.pdf (Accessed March 10, 2025).
82. Rodríguez-Jiménez L, Romero-Martín M, Spruell T, Steley Z, Gómez-Salgado J. The carbon footprint of healthcare settings: a systematic review. J Adv Nurs. (2023) 79(8):2830–44. doi: 10.1111/jan.15671
83. Cohen ES, Kouwenberg LHJA, Moody KS, Sperna Weiland NH, Kringos DS, Timmermans A, et al. Environmental sustainability in obstetrics and gynaecology: a systematic review. BJOG. (2024) 131(5):555–67. doi: 10.1111/1471-0528.17637
84. Spil NA, van Nieuwenhuizen KE, Rowe R, Thornton JG, Murphy E, Verheijen E, et al. The carbon footprint of different modes of birth in the UK and the Netherlands: an exploratory study using life cycle assessment. BJOG. (2024) 131(5):568–78. doi: 10.1111/1471-0528.17771
85. American Society of Anesthesiologists. Reduce Carbon Footprint from Inhaled Anesthesia with New Guidance Published (2022). Available at: https://www.asahq.org/about-asa/newsroom/news-releases/2022/06/reduce-carbon-footprint-from-inhaled-anesthesia-with-new-guidance-published (Accessed February 06, 2025).
86. Royal College of Obstetricians and Gynaecologists. Sustainability and Climate Change at the RCOG: Workshop. Royal College of Obstetricians and Gynaecologists (2024). https://www.rcog.org.uk/about-us/the-college-s-ambition-for-sustainability-and-climate-change/sustainability-and-climate-change-at-the-rcog-workshop/ (Accessed June 10, 2024).
87. Shelton CL, Sutton R, White SM. Desflurane in modern anaesthetic practice: walking on thin ice(caps)? Br J Anaesth. (2020) 125(6):852–6. doi: 10.1016/j.bja.2020.09.013
88. McGarrigle C, Hartigan S, Duffy O, Tan T. Perspectives on sustainable practices in the use of nitrous oxide for labour analgesia: a patient and staff survey. Eur J Anaesthesiol. (2024) 41(7):473–9. doi: 10.1097/EJA.0000000000002005
89. Royal College of Surgeons. The intercollegiate green theatre checklist. Bull R Coll Surg Engl. (2023) 105(1):25. doi: 10.1308/rcsbull.2023.25
90. Rizan C, Reed M, Mortimer F, Jones A, Stancliffe R, Bhutta M. Using surgical sustainability principles to improve planetary health and optimise surgical services following the COVID-19 pandemic. Bull R Coll Surg Engl. (2020) 102(5):177–81. doi: 10.1308/rcsbull.2020.148
91. Johannessen HH, Frøshaug BE, Lysåker PJG, Salvesen KÅ, Lukasse M, Mørkved S, et al. Regular antenatal exercise including pelvic floor muscle training reduces urinary incontinence 3 months postpartum-follow up of a randomized controlled trial. Acta Obstet Gynecol Scand. (2021) 100(2):294–301. doi: 10.1111/aogs.14010
92. Ten Buuren AAA, Poolman TB, Bongers MY, Bullens LM, Van Hanegem N, Klerkx WM, et al. Patient preferences for disposable and reusable vaginal specula and their willingness to compromise in the era of climate change: a cross-sectional study. BJOG. (2024) 131(5):684–9. doi: 10.1111/1471-0528.17733
93. Asiedu MN, Agudogo J, Krieger MS, Miros R, Proeschold-Bell RJ, Schmitt JW, et al. Design and preliminary analysis of a vaginal inserter for speculum-free cervical cancer screening. PLoS One. (2017) 12(5):e0177782. doi: 10.1371/journal.pone.0177782
94. U Delft. New Vaginal Speculum Design Might Motivate Women to go for Health Checkups (2025). Available at: https://www.tudelft.nl/en/ide/delft-design-stories/new-vaginal-speculum-design-might-motivate-women-to-go-for-health-checkups (Accessed February 6, 2025).
95. Yona Care. Yona Speculum: A More Comfortable Alternative (2025). Available at: https://yonacare.com/Speculum (Accessed February 6, 2025).
96. Royal College of Obstetricians and Gynaecologists. RCOG Announce New Collaborative Project: Taking Action to Deliver Low-Carbon, Equitable Maternity Care (2024). Available at: https://www.rcog.org.uk/news/rcog-announce-new-collaborative-project-taking-action-to-deliver-low-carbon-equitable-maternity-care/ (Accessed April 05, 2025).
97. Pokhrel D, Bhattarai S, Emgård M, Von Schickfus M, Forsberg BC, Biermann O. Acceptability and feasibility of using vaginal menstrual cups among schoolgirls in rural Nepal: a qualitative pilot study. Reprod Health. (2021) 18:20. doi: 10.1186/s12978-020-01036-0
98. Hennegan J, Dolan C, Steinfield L, Montgomery P. A qualitative understanding of the effects of reusable sanitary pads and puberty education: implications for future research and practice. Reprod Health. (2017) 14(1):78. doi: 10.1186/s12978-017-0339-9
99. American College of Obstetricians and Gynecologists. “Preparing for Disasters: Addressing Critical Obstetric and Gynecologic Needs of Patients.” Last Modified January 2025 (2025). Available at: https://www.acog.org/clinical/clinical-guidance/committee-statement/articles/2025/01/preparing-for-disasters-addressing-critical-obstetric-and-gynecologic-needs-of-patients (Accessed February 6, 2025).
100. Morena AL, Gaias LM, Larkin C. Understanding the role of clinical champions and their impact on clinician behavior change: the need for causal pathway mechanisms. Front Health Serv. (2022) 2:896885. doi: 10.3389/frhs.2022.896885
101. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci. (2015) 10:21. doi: 10.1186/s13012-015-0209-1
102. Sustainable Healthcare Coalition. Sustainable Healthcare (2024). Available at: https://sustainablehealthcare.org.uk/ (Accessed December 6, 2024).
103. National Institute for Health and Care Excellence. Decision Aid Process Guide (2018). Available at: https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/nice-guidance/shared-decision-making/decision-aid-process-guide.pdf (Accessed April 09, 2025).
104. Dupraz J, Burnand B. Role of health professionals regarding the impact of climate change on health-an exploratory review. Int J Environ Res Public Health. (2021) 18(6):3222. doi: 10.3390/ijerph18063222
105. RCOG, RCPCH and UKHACC. RCOG, RCPCH and UKHACC Call for Urgent Environmental Action to Protect the Health of Children, Pregnant Women and Their Babies. Released 21 October 2021. Available at: https://www.rcog.org.uk/news/rcog-rcpch-and-ukhacc-call-for-urgent-environmental-action-to-protect-the-health-of-children-pregnant-women-and-their-babies/ (Accessed May 20, 2024).
106. American College of Obstetricians and Gynecologists. Position Statement; Addressing Climate Change (2021). Available at: https://www.acog.org/clinical-information/policy-and-position-statements/position-statements/2021/addressing-climate-change (Accessed February 06, 2025).
107. World Economic Forum. Generative AI and Its Energy Emissions. World Economic Forum (2024). Available at: https://www.weforum.org/stories/2024/07/generative-ai-energy-emissions/ (Accessed December 7, 2024).
108. Alvarado-Jiménez D, Donzelli G, Morales-Suarez-Varela M. A systematic review on the association between exposure to air particulate matter during pregnancy and the development of hypertensive disorders of pregnancy and gestational diabetes mellitus. Rev Environ Health. (2023) 39(4):619–41. doi: 10.1515/reveh-2022-0258
109. Bai W, Li Y, Niu Y, Ding Y, Yu X, Zhu B, et al. Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ Res. (2020) 185:109471. doi: 10.1016/j.envres.2020.109471
110. Bonzini M, Carugno M, Grillo P, Mensi C, Bertazzi PA, Pesatori AC. Impact of ambient air pollution on birth outcomes: systematic review of the current evidences. Med. Lav. (2010) 101(5):341–63. Available at: https://pubmed.ncbi.nlm.nih.gov/21105590/21105590
111. Bosetti C, Nieuwenhuijsen MJ, Gallus S, Cipriani S, La Vecchia C, Parazzini F. Ambient particulate matter and preterm birth or birth weight: a review of the literature. Arch Toxicol (2010) 84(6):447–60. doi: 10.1007/s00204-010-0514-z
112. Cao L, Wang L, Wu L, Wang T, Cui X, Yu L, et al. Particulate matter and hypertensive disorders in pregnancy: systematic review and meta-analysis. Public Health. (2021) 200:22–32. doi: 10.1016/j.puhe.2021.08.013
113. Carlsten C, Salvi S, Wong GWK, Chung KF. Personal strategies to minimise effects of air pollution on respiratory health: advice for providers, patients and the public. Eur Respir J. (2020) 55(6):1902056. doi: 10.1183/13993003.02056-2019
114. Dewey KG. Energy and protein requirements during lactation. Annu Rev Nutr. (1997) 17:19–36. doi: 10.1146/annurev.nutr.17.1.19
115. Edwards L, Wilkinson P, Rutter G, Milojevic A. Health effects in people relocating between environments of differing ambient air pollution concentrations: a literature review. Environ Pollut (2022) 292:118314. doi: 10.1016/j.envpol.2021.118314
116. Elshahidi MH. Outdoor air pollution and gestational diabetes mellitus: a systematic review and meta-analysis. Iran J Public Health. (2019) 48(1):9–19. doi: 10.18502/ijph.v48i1.778
117. Ergul A, Yuksel Ozgor B. Study of characteristics of pregnant women who are refugees and aged under 18 years. Cureus. (2023) 15(5):e39169. doi: 10.7759/cureus.39169
118. International Federation of Gynecology and Obstetrics (FIGO). Prenatal Exposure to Toxic Chemicals and Climate Change: FIGO Opinion (n.d). Available at: https://prhe.ucsf.edu/figo (Accessed March 8, 2025).
119. Ghosh R, Rankin J, Pless-Mulloli T, Glinianaia S. Does the effect of air pollution on pregnancy outcomes differ by gender? A systematic review. Environ Res. (2007) 105(3):400–8. doi: 10.1016/j.envres.2007.03.009
120. Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. (2004) 15(1):36–45. doi: 10.1097/01.ede.0000101023.41844
121. Global Burden of Disease Study 2021 (GBD 2021) Socio-Demographic Index (SDI) 1950–2021. Last Modified May 2024 (2021). Available at: https://ghdx.healthdata.org/record/global-burden-disease-study-2021-gbd-2021-socio-demographic-index-sdi-1950%E2%80%932021 (Accessed October 20, 2024).
122. Gogna P, Villeneuve PJ, Borghese MM, King WD. An exposure-response meta-analysis of ambient PM2.5 during pregnancy and preeclampsia. Environ Res. (2022) 210:112934. doi: 10.1016/j.envres.2022.112934
123. Gong C, Wang J, Bai Z, Rich DQ, Zhang Y. Maternal exposure to ambient PM2.5 and term birth weight: a systematic review and meta-analysis of effect estimates. Sci. Total Environ. (2022) 807:150744. doi: 10.1016/j.scitotenv.2021.150744
124. Grippo A, Zhang J, Chu L, Guo Y, Qiao L, Zhang J, et al. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev Environ Health. (2018) 33(3):247–64. doi: 10.1515/reveh-2017-0033
125. Harakow HI, Hvidman L, Wejse C, Eiset AH. Pregnancy complications among refugee women: a systematic review. Acta Obstet Gynecol Scand. (2021) 100(4):649–57. doi: 10.1111/aogs.14070
126. Heer K, Mahmoud L, Abdelmeguid H, Selvan K, Malvankar-Mehta MS. Prevalence, risk factors, and interventions of postpartum depression in refugees and asylum-seeking women: a systematic review and meta-analysis. Gynecol Obstet Invest. (2024) 89(1):11–21. doi: 10.1159/000535719
127. Heo S, Fong KC, Bell ML. Risk of particulate matter on birth outcomes in relation to maternal socio-economic factors: a systematic review. Environ Res Lett (2019) 14(12):123004. doi: 10.1088/1748-9326/ab4cd0
128. Hu CY, Gao X, Fang Y, Jiang W, Huang K, Hua XG, et al. Human epidemiological evidence about the association between air pollution exposure and gestational diabetes mellitus: systematic review and meta-analysis. Environ Res. (2020) 180:108843. doi: 10.1016/j.envres.2019.108843
129. Hu H, Ha S, Roth J, Kearney G, Talbott EO, Xu X. Ambient air pollution and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Atmos Environ. (2014) 97:336–45. doi: 10.1016/j.atmosenv.2014.08.027
130. International Planned Parenthood Federation. The Climate Crisis and Sexual and Reproductive Health and Rights. UN Women (2022). Available at: https://www.unwomen.org/sites/default/files/Headquarters/Attachments/Sections/CSW/66/EGM/Expert%20Papers/IPPF_CSW66%20Expert%20Paper.pdf (Accessed April 07, 2025).
131. Jacobs M, Zhang G, Chen S, Mullins B, Bell M, Jin L, et al. The association between ambient air pollution and selected adverse pregnancy outcomes in China: a systematic review. Sci Total Environ (2017) 579:1179–92. doi: 10.1016/j.scitotenv.2016.11.100
132. Ji Y, Song F, Xu B, Zhu Y, Lu C, Xia Y. Association between exposure to particulate matter during pregnancy and birthweight: a systematic review and a meta-analysis of birth cohort studies. J Biomed Res. (2017) 33:56. doi: 10.7555/JBR.31.20170038
133. Ju L, Li C, Yang M, Sun S, Zhang Q, Cao J, et al. Maternal air pollution exposure increases the risk of preterm birth: evidence from the meta-analysis of 24 cohort studies. Environ. Res. (2021) 202:111654. doi: 10.1016/j.envres.2021.111654
134. Koman PD, Hogan KA, Sampson N, Mandell R, Coombe CM, Tetteh MM, et al. Examining joint effects of air pollution exposure and social determinants of health in defining “at-risk” populations under the clean air act: susceptibility of pregnant women to hypertensive disorders of pregnancy. World Med Health Policy. (2018) 10(1):7–54. doi: 10.1002/wmh3.257
135. Knibbs LD. The health impacts of ambient air pollution in Australia: a systematic literature review. Intern. Med. J. (2021) 51(10):1567–79. doi: 10.1111/imj.15415
136. Lamichhane DK, Leem JH, Lee JY, Kim HC. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ. Health Toxicol. (2015) 30:e2015011. doi: 10.5620/eht.e2015011
137. Laumbach R, Meng Q, Kipen H. What can individuals do to reduce personal health risks from air pollution? J Thorac Dis. (2015) 7(1):96–107. doi: 10.3978/j.issn.2072-1439.2014.12.21
138. Liang W, Zhu H, Xu J, Zhao Z, Zhou L, Zhu Q, et al. Ambient air pollution and gestational diabetes mellitus: an updated systematic review and meta-analysis. Ecotoxicol Environ Saf. (2023) 255:114802. doi: 10.1016/j.ecoenv.2023.114802
139. Li C, Yang M, Zhu Z, Sun S, Zhang Q, Cao J, et al. Maternal exposure to air pollution and the risk of low birth weight: a meta-analysis of cohort studies. Environ. Res. (2020) 190:109970. doi: 10.1016/j.envres.2020.109970
140. Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ. Pollut. (2017) 227:596–605. doi: 10.1016/j.envpol.2017.03.055
141. Lin LY, Chuang HC, Liu IJ, Chen HW, Chuang KJ. Reducing indoor air pollution by air conditioning is associated with improvements in cardiovascular health among the general population. Sci Total Environ. (2013) 463:176–181. doi: 10.1016/j.scitotenv.2013.05.093
142. Liu C, Sun J, Liu Y, Liang H, Wang M, Wang C, et al. Different exposure levels of fine particulate matter and preterm birth: a meta-analysis based on cohort studies. Environ. Sci. Pollut. Res. Int. (2017) 24(22):17976–84. doi: 10.1007/s11356-017-9363-0
143. Luo D, Kuang T, Chen Y-X, Huang Y-H, Zhang H, Xia Y-Y. Air pollution and pregnancy outcomes based on exposure evaluation using a land use regression model: a systematic review. Taiwan. J. Obstet. Gynecol. (2021) 60(2):193–215. doi: 10.1016/j.tjog.2021.01.004
144. Malley CS, Kuylenstierna JC, Vallack HW, Henze DK, Blencowe H, Ashmore MR. Preterm birth associated with maternal fine particulate matter exposure: a global, regional and national assessment. Environ Int. (2017) 101:173–82. doi: 10.1016/j.envint.2017.01.023
145. Garcia MA, Liu R, Nihart A, Hayek EE, Castillo E, Barrozo ER, et al. Quantitation and identification of microplastics accumulation in human placental specimens using pyrolysis gas chromatography mass spectrometry. Toxicol Sci. (2024) 199:kfae021. doi: 10.1093/toxsci/kfae021
146. Mazumder H, Rimu FH, Shimul MH, Das J, Gain EP, Liaw W, et al. Maternal health outcomes associated with ambient air pollution: an umbrella review of systematic reviews and meta-analyses. Sci Total Environ. (2024) 914:169792. doi: 10.1016/j.scitotenv.2023.169792
147. MSI Reproductive Choices. The Impact of the Climate Crisis on Reproductive Choice (2023). Available at: https://www.msichoices.org/wp-content/uploads/2023/10/the-impact-of-the-climate-crisis-on-reproductive-choice-final.pdf (Accessed May 20 2024).
148. Nazarpour S, Ramezani Tehrani F, Valizadeh R, Amiri M. The relationship between air pollutants and gestational diabetes: an updated systematic review and meta-analysis. J Endocrinol Invest. (2023) 46(7):1317–32. doi: 10.1007/s40618-023-02037-z
149. NHS England. Health and Climate Change. Greener NHS. Available at: https://www.england.nhs.uk/greenernhs/national-ambition/national-commitments/ (Accessed April 10, 2025)
150. Pedersen M, Stayner L, Slama R, Sørensen M, Figueras F, Nieuwenhuijsen MJ, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. (2014) 64(3):494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545
151. Pourhoseini SA, Akbary A, Mahmoudi H, Akbari M, Heydari ST. Association between prenatal period exposure to ambient air pollutants and development of postpartum depression: a systematic review and meta-analysis. Int J Environ Health Res. (2024) 34(1):455–65. doi: 10.1080/09603123.2022.2153808
152. Rappazzo KM, Nichols JL, Rice RB, Luben TJ. Ozone exposure during early pregnancy and preterm birth: a systematic review and meta-analysis. Environ. Res. (2021) 198:111317. doi: 10.1016/j.envres.2021.111317
153. Ren Z, Yuan J, Luo Y, Wang J, Li Y. Association of air pollution and fine particulate matter (PM2.5) exposure with gestational diabetes: a systematic review and meta-analysis. Ann Transl Med. (2023) 11(1):23. doi: 10.21037/atm-22-6306
154. Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. (2019) 13(3):e0007213. doi: 10.1371/journal.pntd.0007213
155. Sapkota A, Chelikowsky AP, Nachman KE, Cohen AJ, Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and metaanalysis. Air Qual. Atmos. Health. (2010) 5(4):369–81. doi: 10.1007/s11869-010-0106-3
156. Shah PS, Balkhair T, Knowledge Synthesis Group on Determinants of Preterm, L. B. W. b. Air pollution and birth outcomes: a systematic review. Environ. Int. (2011) 37(2):498–516. doi: 10.1016/j.envint.2010.10.009
157. Siddika N, Balogun HA, Amegah AK, Jaakkola JJ. Prenatal ambient air pollution exposure and the risk of stillbirth: systematic review and meta-analysis of the empirical evidence. Occup. Environ. Med. (2016) 73(9):573–81. doi: 10.1136/oemed-2015-103086
158. Simoncic V, Enaux C, Deguen S, Kihal-Talantikite W. Adverse birth outcomes related to NO2 and PM exposure: european systematic review and meta-analysis. Int. J. Environ. Res. Publ. Health. (2020) 17(21):8116. doi: 10.3390/ijerph17218116
159. Stapleton SO, Nadin R, Watson C, Kellett J. Climate Change, Migration and Displacement: the Need for a Risk-Informed and Coherent Approach (2017). Overseas Development Institute; Available at: https://www.undp.org/sites/g/files/zskgke326/files/publications/MIgration_Report.pdf (Accessed April 10, 2025).
160. Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ. Res. (2012) 117:100–11. doi: 10.1016/j.envres.2012.05.007
161. Sun M, Yan W, Fang K, Chen D, Liu J, Chen Y, et al. The correlation between PM2.5 exposure and hypertensive disorders in pregnancy: a meta-analysis. Sci Total Environ. (2020) 703:134985. doi: 10.1016/j.scitotenv.2019.134985
162. Sun X, Luo X, Zhao C, Zhang B, Tao J, Yang Z, et al. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ. Pollut. (2016) 211:38–47. doi: 10.1016/j.envpol.2015.12.022
163. Sun X, Luo X, Zhao C, Chung Ng RW, Lim CE, Zhang B, et al. The association between fine particulate matter exposure during pregnancy and preterm birth: a meta-analysis. BMC Pregnancy Childbirth. (2015) 15:300. doi: 10.1186/s12884-015-0738-2
164. Thayamballi N, Habiba S, Laribi O, Ebisu K. Impact of maternal demographic and socioeconomic factors on the association between particulate matter and adverse birth outcomes: a systematic review and meta-analysis. J. Racial and Ethn. Health Disparities. (2020) 8:743–755. doi: 10.1007/s40615-020-00835-2
165. Trasande L, Malecha P, Attina TM. Particulate matter exposure and preterm birth: estimates of U.S. Attributable burden and economic costs. Environ Health Perspect. (2016) 124:12. doi: 10.1289/ehp.1510810
166. Tsoli S, Ploubidis GB, Kalantzi O-I. Particulate air pollution and birth weight: a systematic literature review. Atmos. Pollut. Res. (2019) 10(4):1084–122. doi: 10.1016/j.apr.2019.01.016
167. Uwak I, Olson N, Fuentes A, Moriarty M, Pulczinski J, Lam J, et al. Application of the navigation guide systematic review methodology to evaluate prenatal exposure to particulate matter air pollution and infant birth weight. Environ. Int. (2021) 148:106378. doi: 10.1016/j.envint.2021.106378
168. Walter CM, Schneider-Futschik EK, Lansbury NL, Sly PD, Head BW, Knibbs LD. The health impacts of ambient air pollution in Australia: a systematic literature review. Intern. Med. J. (2021) 51(10):1567–79. doi: 10.1111/imj.15415
169. Westergaard N, Gehring U, Slama R, Pedersen M. Ambient air pollution and low birth weight—are some women more vulnerable than others? Environ. Int. (2017) 104:146–54. doi: 10.1016/j.envint.2017.03.026
170. Wheeler S, Ateva E, Churchill R, Pleuss E, McCallon B, Storey A, et al. Short communication: the global health community needs to start planning for the impact of the climate crisis on maternal and newborn health. J. Clim. Change Health. (2022) 6:100131. doi: 10.1016/j.joclim.2022.100131
171. World Health Organization. Malnutrition, 1 March 2024 (2024). Available at: https://www.who.int/news-room/fact-sheets/detail/malnutrition (Accessed March 7, 2024).
172. World Health Organization. Environment, Climate Change and Health (2024). Available at: https://www.who.int/teams/environment-climate-change-and-health/emergencies (Accessed October 20, 2024).
173. World Health Organization. Climate Impacts of Air Pollution (2024). Available at: https://www.who.int/teams/environment-climate-change-and-health/air-quality-energy-and-health/health-impacts/climate-impacts-of-air-pollution (Accessed October 20, 2024).
174. World Health Organization. Call for Climate Action (2023). Available at: https://www.who.int/teams/environment-climate-change-and-health/call-for-climate-action (Accessed June 20, 2024).
175. Xie G, Sun L, Yang W, Wang R, Shang L, Yang L, et al. Maternal exposure to PM2.5 was linked to elevated risk of stillbirth. Chemosphere. (2021) 283:131169. doi: 10.1016/j.chemosphere.2021.131169
176. Yuan L, Zhang Y, Gao Y, Tian Y. Maternal fine particulate matter (PM2.5) exposure and adverse birth outcomes: an updated systematic review based on cohort studies. Environ. Sci. Pollut. Res. Int. (2019) 26(14):13963–83. doi: 10.1007/s11356-019-04644-x
177. Yu H, Yin Y, Zhang J, Zhou R. The impact of particulate matter 2.5 on the risk of preeclampsia: an updated systematic review and meta-analysis. Environ Sci Pollut Res Int. (2020) 27(30):37527–39. doi: 10.1007/s11356-020-10112-8
178. Yu S, Zhang M, Zhu J, Yang X, Bigambo FM, Snijders AM, et al. The effect of ambient ozone exposure on three types of diabetes: a meta-analysis. Environ Health. (2023) 22(1):32. doi: 10.1186/s12940-023-00981-0
179. Zhang H, Wang Q, He S, Wu K, Ren M, Dong H, et al. Ambient air pollution and gestational diabetes mellitus: a review of evidence from biological mechanisms to population epidemiology. Sci Total Environ. (2020) 719:137349. doi: 10.1016/j.scitotenv.2020.137349
180. Zhang H, Zhang X, Wang Q, Xu Y, Feng Y, Yu Z, et al. Ambient air pollution and stillbirth: an updated systematic review and meta-analysis of epidemiological studies. Environ. Pollut. (2021) 278:116752. doi: 10.1016/j.envpol.2021.116752
181. Zhang K, Lu Y, Zhao H, Guo J, Gehendra M, Qiu H, et al. Association between atmospheric particulate matter and adverse pregnancy outcomes in the population. Int J Clin Exp Med. (2016) 9:20594–604. Available at: https://www.researchgate.net/publication/311495830_Association_between_atmospheric_particulate_matter_and_adverse_pregnancy_outcomes_in_the_population
Keywords: climate change, pregnancy, maternal health, fetal development, environmental pollution, sustainable healthcare, UK healthcare system
Citation: Samara A, Hanton T, Thakar R, Jauniaux E and Khalil A (2025) Impact of climate change and environmental adversities on maternal and fetal health: the role of clinical practices and providers in mitigating effects and prioritising women's health in the UK. Front. Glob. Women's Health 6:1483938. doi: 10.3389/fgwh.2025.1483938
Received: 20 August 2024; Accepted: 28 April 2025;
Published: 13 June 2025.
Edited by:
Wendy R. Sheldon, Verge Research, United StatesReviewed by:
Tania Romo-Gonzalez, Universidad Veracruzana, MexicoDeborah L. Billings, University of South Carolina, United States
Rasha Dabash, Independent Researcher, Paris, France
Copyright: © 2025 Samara, Hanton, Thakar, Jauniaux and Khalil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athina Samara, YXRoaW5hc0B1aW8ubm8=; Asma Khalil, YWtoYWxpbEBzZ3VsLmFjLnVr
†These authors have contributed equally to this work
 Athina Samara
Athina Samara Thomas Hanton
Thomas Hanton Ranee Thakar5,6
Ranee Thakar5,6 Eric Jauniaux
Eric Jauniaux Asma Khalil
Asma Khalil