- 1Department of Psychology, University of Kansas, Lawrence, KS, United States
- 2Department of Neurology, University of Kansas Alzheimer’s Disease Center, University of Kansas Medical Center, Fairway, KS, United States
Introduction: Two thirds of Alzheimer's disease (AD) patients are female. Genetic and chronic health risk factors for AD affect females more negatively compared to males.
Objective: This multimodal neuroimaging study aimed to examine sex differences in cognitively unimpaired older adults on: (1) amyloid-β via 18F-AV-45 Florbetapir PET imaging, (2) neurodegeneration via T1 weighted MRI volumetrics, (3) cerebral blood flow via ASL-MRI. We identified AD risk factors including genetic (APOE genotype status) and health markers (fasting glucose, mean arterial pressure, waist-to-hip ratio, and android and gynoid body fat) associated with neuroimaging outcomes for which we observed sex differences.
Methods: Participants were sedentary, amyloid-β positive older adults (N = 112, ages 65–87 years) without evidence of cognitive impairment (CDR = 0).
Results: Multivariate analysis of covariance models adjusted for intracranial volume, age, and years of education demonstrated lower volume [F (7, 102) = 2.67, p = 0.014] and higher blood flow F (6, 102) = 4.25, p ≤ 0.001) among females compared to males in regions of interest connected to AD pathology and the estrogen receptor network. We did not observe sex differences in amyloid-β levels. Higher than optimal waist to hip ratio was most strongly associated with lower volume among female participants.
Discussion: Findings suggest genetic and chronic health risk factors are associated with sex-specific AD neuroimaging biomarkers. Underlying sex-specific biological pathways may explain these findings. Our results highlight the importance of considering sex differences in neuroimaging studies and when developing effective interventions for AD prevention and risk reduction.
1 Introduction
The female sex has long been established as a major risk factor for late-onset Alzheimer's disease (AD) (1). Two thirds of patients with AD are women (2). Sex differences reported in the incidence and prevalence of AD have historically been attributed to women's longer life expectancy relative to men (3, 4). Further, research examining AD risk factors has historically adjusted for sex as a covariate in analyses but has given limited attention to describing or explaining sex and gender differences. Recent research suggests sex and gender differences in AD risk factors may better explain disparities in prevalence and has identified approximately 30 AD risk factors that affect men and women differently, with the female sex often more severely affected (5–7).
The prevalence rates of several AD risk factors (e.g., cerebrovascular events, depression, sleep disorders) are higher among women, especially after the age of 60 and during the postmenopausal period (8–13). One hypothesis proposed to explain this sex disparity suggests estrogen deprivation, as is observed in the menopause transition, may function as a physiological trigger of increased risk for AD given estrogen's (specifically 17b-estradiol) neuroprotective properties for the female brain (7, 14–17). The estrogen hypothesis emphasizes the numerous fundamental functions of estrogen highlighting an extensive network of estrogen receptors throughout the female brain, predominantly in the hypothalamus, hippocampus, and amygdala (18, 19), areas of the brain known to be associated with early AD pathophysiological changes. The most robust evidence for sex-differences in AD risk are found in genetic risk factors [i.e., Apolipoprotein E (APOE) genotype] and chronic health factors (e.g., cardiometabolic disease) (5).
The most studied and strongest genetic risk factor for late onset AD is APOE genotype status (20, 21). APOE gene expression has been shown to be more detrimental for female carriers of the APOE4 allele. Females that carry the APOE4 allele have 1.5 times higher risk for AD (1, 22, 23) and more amyloid-β plaques and neurofibrillary tangles compared to male APOE4 carriers (20). The literature has suggested an age-specific effect in females wherein female APOE4 carriers may have an early susceptibility to AD compared to males (24). Among cognitively intact older adult APOE4 carriers, females have greater decreases in hippocampal connectivity, and increased whole brain hypometabolism and atrophy compared to age matched male APOE4 carriers (25, 26). The mechanisms underlying the interaction between sex and APOE genotype remain unclear.
Cardiometabolic diseases (e.g., type 2 diabetes, metabolic syndrome) and their risk factors (e.g., hypertension, hyperlipidemia, non-optimal fasting glucose, obesity) associated with sedentary behavior are well-known risk factors for AD development and progression for both men and women (27–30). The development, symptoms, and treatment of cardiometabolic diseases differ by sex (6, 31–35). For example, microvascular disease contributes the most to cardiovascular disease in women compared to obstructive coronary artery disease in men (36). Additionally, men tend to develop cardiometabolic disease at an earlier age than women given that the menopause transition and loss of neuroprotective estrogen may accelerate the development of cardiometabolic diseases in postmenopausal women (37–39). Estrogen has demonstrated a protective effect on the brain through its vasodilatory effects, enhancing the production of sensitivity to vasodilatory factors (40, 41). Historically, the literature has suggested a greater impact of vascular disease for men's AD risk vs. women, yet given the growing body of literature on sex differences and cardiometabolic disease, it has been suggested that the diagnosis of hypertension, high cholesterol, and diabetes may put women at a higher risk for AD (6). However, more research is needed.
Emerging lines of evidence suggest one out of every three AD cases can be linked to modifiable risk factors (42). Thus, early detection and prevention efforts have dominated the field in addressing the AD epidemic. Given that many of these identified cardiometabolic disease risk factors are modifiable, it is important to fully understand sex differences, including how they contribute to AD-specific pathophysiological changes (amyloid-β accumulation, neurofibrillary tangles, and neuronal and synaptic loss) when developing effective AD risk reduction interventions. Through various imaging techniques, early detection of amyloid, tau, and neurodegeneration (A/T/N) biomarkers that begin in the brain before detectable clinical symptoms, can identify cognitively unimpaired individuals that may be at high risk for developing AD, and therefore benefit the most from interventions to prevent or delay the onset of AD.
When considering the evidence of amyloid-β accumulation beginning as early as 15–20 years before clinically observable cognitive decline and age being the strongest predictor for AD, this suggests amyloid-β accumulation may begin in or before midlife, which notably coincides with the menopause transition for women and the inherent loss of the neuroprotective 17b-estradiol (16, 43, 44). New evidence indicates the presence of sex differences among AD-related biomarkers start in midlife such that menopausal women exhibit more amyloid-β accumulation and decreased gray and white matter volumetrics among AD-specific regions of the brain compared to age-matched men (45–47). However, the research examining whether these early AD biomarker sex differences persist with aging among cognitively unimpaired older adults with consideration to postmenopausal women, remains mixed. Further, in vivo multi-modal imaging studies have shown 17b-estradiol densities among estrogen-regulated networks vary over the menopause transition, suggesting women's menopausal status is important to interpretation of neuroimaging results (48).
Studies that have examined sex differences in amyloid-β accumulation among cognitively unimpaired older adults have shown conflicting results including no sex differences (49, 50), older adult males exhibiting more amyloid-β accumulation (51), and older adult females exhibiting more amyloid-β accumulation (52). Sex differences among volumetric studies indicate cognitively unimpaired older adult males exhibit more age-related atrophy in the temporal regions of the brain compared to older adult women, including smaller hippocampus volume indicative of possible downstream AD neurodegeneration (49, 53, 54). However, review of longitudinal studies examining brain atrophy, amyloid-β accumulation, and cognitive decline have demonstrated sex differences in the progression of AD such that women may be protected relative to men during the prodromal AD phases, but exhibit faster progression rates of decline compared to men (5, 55).
In addition to the typical imaging techniques and biomarkers used to detect early pathophysiological processes of AD following the A/T/N classification system, decreased cerebral blood flow has been discussed as a novel AD biomarker given the hypothesis of vascular abnormality of AD in conjunction with the ability of ASL-MRI to detect tissues and cell damage that precede neurodegeneration (56–59). Further, cerebral blood flow is an important index of aging and it is well known that velocity decreases as a function of aging (60–62). Research has demonstrated sex differences in cerebral blood flow. An increased rate of cerebral blood flow in young adult women compared to age-matched men has been widely reported and observed using a variety of types of techniques [e.g., single photon emission computed tomography (SPECT), PET, Xenon-enhanced computed tomography, and ASL-MRI] (63–67). Few studies have examined sex differences in cerebral blood flow among older adults and those few report conflicting results (68, 69). One unique cross-sectional study looked at the rate of changes in cerebral blood flow across the lifespan in women and men (70). Results indicated sex-specific trajectories with aging such that women had a greater rate of decline of cerebral blood flow over the lifespan compared to men and that this disparity in the declining rate was most pronounced during ages 61–70 years old (70). For women, cerebral blood flow has been found to differ dependent on menopause stage, such that postmenopausal women demonstrate higher cerebral blood flow compared to other menopause stages (i.e., pre- and peri-menopausal) in certain brain regions (71).
Taken together, this research suggests contradictory results regarding sex differences in amyloid-β accumulation, AD related brain volumes, and cerebral blood flow in cognitively unimpaired older adults. These discrepancies suggest that there may be confounding variables that have yet to be fully explored, such as specific risk factors that may contribute to the findings and the timing of data collection relative to aging and menopause processes. For example, recent studies suggest that brain volume and connectivity change across the menstrual cycle (72, 73) and estrogen receptor density changes in estrogen regulated networks throughout the menopause transition (48), though these cycles are rarely considered in neuroimaging research. The underlying mechanistic explanations for these observed sex differences remain understudied. The role of sex hormones has been postulated as a potential contributing factor to these results (41) and examining the associations with sex specific risk factors may help with clarification.
For example, Rahman et al. (44) uniquely examined the relationship between sex-specific risks and AD biomarkers in a cohort of middle-aged adults. The study demonstrated higher amyloid-β deposition and lower MRI gray and white matter volumes in middle-aged women compared to age-matched men. Notably, hormonal risk factors, including menopause stage and hormone replacement therapy use, predicted these identified sex differences among the biomarkers when compared to other clinical, chronic health, and lifestyle AD risk factors (47).
The present study sought to investigate sex differences in the association of brain volume and cerebral blood flow with chronic health risk factors in a cohort of sedentary, amyloid-β positive, older adults. Our multimodal imaging study examined in vivo sex differences in a cohort of high-risk, cognitively unimpaired older adults on the following AD biomarkers: (1) amyloid-β on 18F-AV-45 Florbetapir PET imaging, (2) neurodegeneration via TI weighted MRI volumetrics, and (3) cerebral blood flow via ASL-MRI in AD-specific brain regions. Given that research suggests these brain biomarkers may indicate early detection of AD pathology, it is important to identify the most impactful risk factor associated with sex-driven AD biomarkers for each of the sexes to inform future hypotheses and research studies targeting optimization of AD prevention interventions. Therefore, we aimed to identify which of the literature supported sex-specific AD risk factors (genetic, cardiometabolic health factors) was most strongly associated with the identified sex-driven AD brain biomarker differences. Based on the estrogen hypothesis, we hypothesized that cognitively unimpaired older adult women would exhibit greater amyloid-β burden, reduced volume, and reduced cerebral blood flow in preclinical AD-specific brain regions (e.g., amygdala, hypothalamus, and parahippocampal gyrus) compared to men and that genetic (APOE genotype) and health risk factors (cardiometabolic disease) would predict these differences. Findings contribute to understanding the optimal timing of AD prevention trials while gaining insight regarding AD risk in the postmenopausal stage for women.
2 Materials and methods
2.1 Participants
The present study is a secondary analysis utilizing the baseline data from the Alzheimer's Prevention through Exercise (APEX, NCT02000583) study, a 52-week exercise intervention conducted at the University of Kansas Alzheimer's Disease Research Center examining change in AD-related neuroimaging biomarkers. Baseline medical history, neuropsychological testing, and MRI and amyloid-β imaging with PET (18F-AV-45) were assessed in all participants during baseline evaluations. The APEX study recruited a convenience sample of 121 cognitively unimpaired, sedentary older adult participants. Inclusion criteria for APEX required age of 65 years and older, a Clinical Dementia Rating (CDR) Scale of 0, a Mini Mental State Examination (MMSE) score of 27 or greater, normal cognitive test performance on the Uniform Data Set neuropsychiatric battery (74, 75) for age and years of education (<1.5 SD below the mean), stable 30-day medication regimen, sedentary or underactive as defined by the Telephone Assessment of Physical Activity (76), and amyloid-β positive measured by PET (18F-AV-45). Persons with insulin-dependence, significant hearing or vision problems, clinically evident stroke, cancer in the previous 5 years, or recent history (<2 years) of major cardiorespiratory, musculoskeletal, or neuropsychiatric impairment, were excluded.
2.2 Genetic risk factors
APOE genotype status was determined from frozen whole blood samples (acid citrate dextrose anticoagulant) using an allelic discrimination assay to identify single nucleotide polymorphisms (ThermoFisher). We distinguished APOE4, APOE3, and APOE2 alleles using Taqman probes to APOE-defining polymorphisms: rs429358 (C_3084793_20) and rs7412 (C_904973_10). This was included in the model as the number of ε4 alleles (0, 1, 2).
2.3 Chronic health risk factors
The presence or absence of the following cardiovascular risk factors were included as predictive measures in the present analysis: (1) Optimal fasting glucose: A fasting glucose measurement was collected twice during the baseline visit and an average of these measurements was calculated. A fasting glucose level of above 100 mg/dl was considered non-optimal (77). (2) High mean arterial pressure: Mean arterial pressure was collected at the baseline visit and pressures that read above 100 was considered high (78). (3) Higher than optimal waist to hip ratio: Waist to hip ratio was calculated at the baseline visit and dichotomized as yes/no depending on whether it was above the sex-specific cutoff of greater than or equal to 0.85 in women and greater than or equal to 0.90 in men (79). A Dual-energy x-ray absorptiometry (DEXA) scan was completed during the baseline visit to measure total body, android (trunk and abdomen adipose tissue), and gynoid (hip and thigh adipose tissue) fat percentages. (4) Higher than optimal total body fat percentage: Total body fat percentage was calculated by dividing fat mass (g) by the sum of lean mass (g), bone mineral content (g), and fat mass (g). Total body fat percentage greater than 38% in women and greater than 25% in men was considered nonoptimal (80). (5) Total android fat percentage: Android fat percentages were calculated by dividing android fat (g) by total fat mass (g). (6) Total gynoid fat percentage: Gynoid fat percentages were calculated by dividing gynoid fat (g) by total fat mass (g).
2.4 AD brain biomarker imaging
For the proposed analysis, sex differences were examined among three neuroimaging techniques (18F-AV-45 PET imaging, T1 weighted MRI, and ASL-MRI, described in detail below).
2.4.1 Amyloid-β pet acquisition, processing, and analysis
All participants received Florbetapir PET scans obtained approximately 50 min after administration of intravenous florbetapir 18F-AV45 (370 MBq) acquired with the GE Discovery ST-16 PET/CT scanner. Two PET brain frames of five minutes in duration were acquired continuously, summed, and attenuation corrected. The mean PET signal across the brain was divided by the signal from whole cerebellum ROI to produce a Standardized Uptake Ratio (SUVR) image. Three experienced raters interpreted the images to determine amyloid status as “elevated” or “non-elevated” as previously described (81–84). Raters first reviewed raw PET images visually then examined the cerebellum normalized SUVRs in 6 cortical regions (anterior cingulate, posterior cingulate, precuneus, inferior medial frontal, lateral temporal, and superior parietal cortex) and projection maps comparing SUVRs to an atlas of amyloid negative scans (83). Inclusion criteria for the primary exercise trial was dependent on PET ratings and therefore completed prior to randomization. As such, PET ratings were blinded to groups/conditions by default. Participants for the primary exercise trial were eligible if they had an “elevated” scan or were in the “subthreshold” range which was defined as a mean cortical SUVR for the 6 ROIs >1.0, which represented the upper half of non-elevated scans [mean cortical SUVR for non-elevated scans (n = 166) 0.99 (0.06 SD)] (81). Therefore, participants included in the present secondary analysis were determined to have “sub-threshold” and “elevated” amyloid status as determined from the primary exercise trial. To remain consistent with universal quantitative standards, SUVR values were converted to Centiloid values (Centiloids = 196.9 × SUVR_wholecerebrefFBP−196.03) (85, 86). The Centiloid values for the sample met the ADNI standard amyloid-β positivity threshold (25.3 Centiloids/composite reference region). Therefore, participants included in the present secondary analysis were determined “amyloid-β positive.”
2.4.2 MRI acquisition, processing, and analysis
All participants received Siemens Skyra 3 T T1-weighted MRI of the brain (Tesla Skyra scanner; MP-RAGE 1 × 1 × 1.2 mm voxels, TR = 2,300 ms, TE = 2.98 ms, TI = 900 ms, FOV 256 × 256× mm, 9° flip angle; Pulsed ASL single-shot EPI 3.8 × 3.8 × 4.0 mm, TR = 3,400 ms, TE = 13 ms, TI = 700 ms, FOV 240 × 240× mm, 90° flip angle; ASL single-shot EPI 3.8 × 3.8 × 4.0 mm, TR = 3,400 ms, TE = 13 ms, TI = 700 ms, FOV 240 × 240 mm, 90° flip angle). To facilitate assessment across imaging modalities, participants’ sequences were co-registered to the anatomical native space using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12). The anatomical image was segmented using CAT12 and the Neuromorphometic atlas (https://neuro-jena.github.io) producing both regional volumes and region of interest (ROI) masks. These individualized ROI were used to extract mean values from the ASL-MRI and 18F-AV-45 PET SUVR images. ASL-MRI data were processed using the ASLTbx for SPM12 before mean blood flow values were extracted from each ROI. These MRI acquisition, processing, and analyses procedures were automated across participants and throughout the primary exercise trial to maintain quality control.
2.4.3 Brain regions of interest
Bilateral ROIs examined via 18F-AV-45 Florbetapir PET imaging included the anterior cingulate gyrus, lateral temporal lobe, precuneus, and superior parietal lobe. The anterior cingulate gyrus was primarily examined based on the results of the Rahman et al. (47) study that found amyloid-β deposition sex differences in this region among middle-aged adults. The additional neocortical areas (lateral temporal lobe, precuneus, and superior parietal lobe) in addition to a global measurement of amyloid-β deposition were included because of their identified association with preclinical AD according to the amyloid cascade hypothesis of AD (87, 88).
Bilateral ROIs examined via TI weighted MRI volumetrics included the hippocampus, amygdala, parahippocampal gyrus, entorhinal cortex, insula, and caudate. The hippocampus, amygdala, parahippocampal gyrus, ínsula, and caduate were primarily examined based on the findings of the Rahman et al. (47) study that demonstrated sex differences among these 5 regions in a cohort of middle-aged adults. The entorhinal cortex was also included as a ROI because it has been identified among the literature as one of the earliest brain regions found to be associated with Preclinical Alzheimer's disease via structural MRI, along with the hippocampus, amygdala, and parahippocampal gyrus (89). The hippocampus and the amygdala are also brain regions included in the estrogen receptor network and therefore considered ROI for the present study examining sex differences (90).
Bilateral ROIs examined via ASL-MRI included the hippocampus, amygdala, parahippocampal gyrus, temporal lobe, precuneus, anterior cingulate cortex, and superior parietal lobe. The precuneus, anterior cingulate cortex, and superior parietal lobe were primarily examined based on research that has found reduced cerebral blood flow in these three regions in patients with AD (91–94). The hippocampus, amygdala, parahippocampal gyrus, and temporal lobes were included regions due to their association with preclinical AD (89, 90).
2.5 Statistical analyses
2.5.1 Aim 1: AD brain biomarker differences by sex
To investigate regional differences between men and women in T1 weighted volumes (MRI), amyloid-β deposition (18F-AV-45 PET), and cerebral blood flow (ASL-MRI), we applied the following three Multivariate Analysis of Covariance (MANCOVA) models to examine whether regions differed by sex with age, education, and modality specific confounders (TIV = total intracranial volume, number of APOE4 alleles) included as covariates.
MANCOVA 1: Amyloid-β deposition (18F-AV-45 PET) of 5 ROIs∼Sex + Age + Education + APOE4 status
MANCOVA 2: T1 weighted volumes (MRI) of 6 ROIs∼Sex + Age + Education + TIV
MANCOVA 3: Cerebral blood flow (ASL-MRI) of 7 ROIs∼Sex + Age + Education
Univariate ANCOVAS corrected for multiple comparisons using Benjamini Hochberg's critical value for a false discovery rate of 5% were used to further examine significant group differences.
2.5.2 Aim 2: associations between sex-driven AD brain biomarker differences and AD risk factors (genetic, chronic health conditions)
To identify which of the risk factor variables were most strongly associated with sex differences in neuroimaging biomarkers identified in Aim 1, we used Least Absolute Shrinkage and Selection Operator (LASSO) regressions, a method used to obtain a subset of predictors that are, empirically, the strongest by excluding extraneous variables (95). The combination of different types of predictors (i.e., continuous vs. discrete) and some multicollinearity among the predictors (Table 1) further justified use of the LASSO regression vs. other statistical approaches (i.e., OLS regression, ridge regression) (96, 97). The LASSO regression approach was also used in an effort to remain consistent with the literature such that the Rahman et al. study used a LASSO regression approach to study a similar research question with a comparable ratio of sample size (150 participants) to number of predictors (15 predictors) to our study (47). Given the sample size and number of predictors, LASSO regressions within a k 2-fold cross validation using a leave one out predictive modeling framework were applied to the data to choose final models with the lowest minimum cross-validated error and identify the most essential predictors.
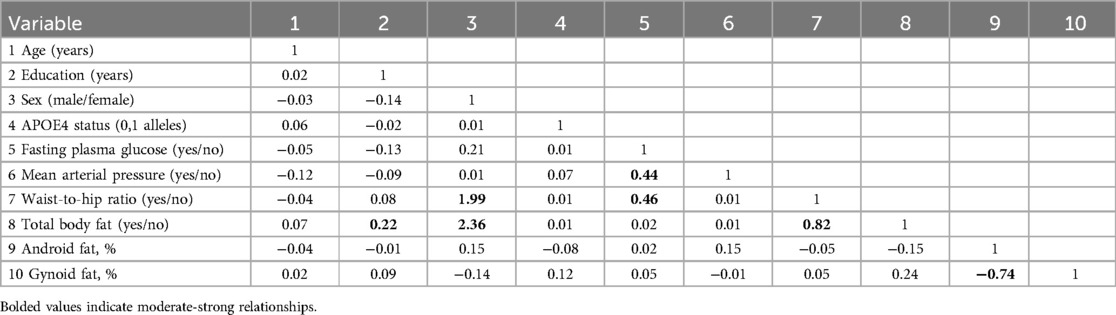
Table 1. Correlation matrix: Pearson correlation coefficients (for continuous variables)/point biserial coefficient (for one continuous variable and one dummy variable)/Phi coefficient (for two dummy variables).
Variables that were included as potential predictors include: APOE4 status (0, 1, 2 alleles), non-optimal fasting glucose (yes/no), elevated mean arterial pressure (yes/no), higher than optimal waist to hip ratio (yes/no), higher than optimal body fat percentage (yes/no), total android fat (percentage), total gynoid fat (percentage), age (years), and education (years). To identify the risk factors specific to sex, interaction terms between the sex variable and the risk factor variables were also included.
3 Results
3.1 Participants
Data from 121 participants were available for analysis. We excluded 9 participants (6 with incomplete genetic testing data and 3 with incomplete MRI data). The remaining 112 participants (ages 65–87 years) were examined, including 74 women and 38 men who were college educated on average and 95.5% of which identified as non-Hispanic, White. There were no quality control issues with the neuroimaging data from these 112 participants. Results from the Community Healthy Activities Model Program for Seniors (CHAMPS) self-report questionnaire suggest the sample did not engage in a substantial amount of physical activity and were primarily sedentary. Participants’ clinical, demographic, and anthropometric characteristics are given in Table 2. Notably, none of our participants in our sample carried 2 APOE4 alleles therefore APOE4 status in the subsequent models were reduced from three levels to two (0,1 alleles). Males and females did not differ by age, APOE4 carrier status, fasting plasma glucose, mean arterial pressure, or physical activity engagement. The female group was less educated (p = 0.049) and included a higher percentage of participants who identified as African American (p < 0.001), had a lower waist to hip ratio (p < 0.001), lower total body fat percentage (p < 0.001), higher android fat percentage (p < 0.001), and lower gynoid fat percentage (p < 0.001) than the male group.
3.2 Aim 1: AD brain biomarker differences by sex
Brain ROIs showing biomarker differences between sex groups were first identified among amyloid-β deposition (18F-AV-45 PET), T1 weighted volumetrics (MRI), and cerebral blood flow (ASL-MRI) among the whole sample.
3.2.1 Pet amyloid-β burden
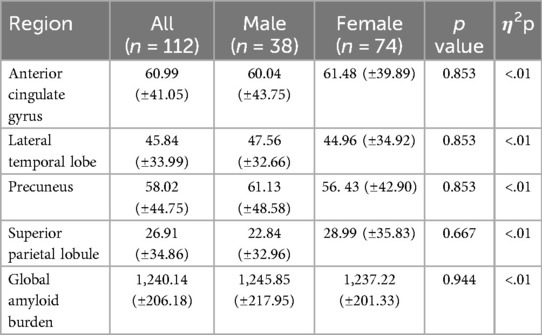
A Multivariate Analysis of Covariance (MANCOVA) of the relationship between sex and amyloid-β burden (Centiloid) in four hypothesized brain ROIs (anterior cingulate gyrus, lateral temporal lobe, precuneus, superior parietal lobule) and global amyloid-β burden adjusting for age, education, and APOE4 status revealed no group differences between men and women [F (5, 103) = 2.09, p = 0.071, η2p = .09], and post hoc individual ANCOVAs revealed no significant group differences for the these ROIs (Table 3).
Given the unequal sex sample sizes in this analysis (males = 38, females = 74), the Box's Test for Equivalence of Covariance Matrices (Box's M) was conducted and interpreted using p < .001 as a criterion (98, 99). The Box's M test was not significant (p = 0.120), suggesting the assumption of homogeneity of covariance matrices was met.
3.2.2 MRI volumes
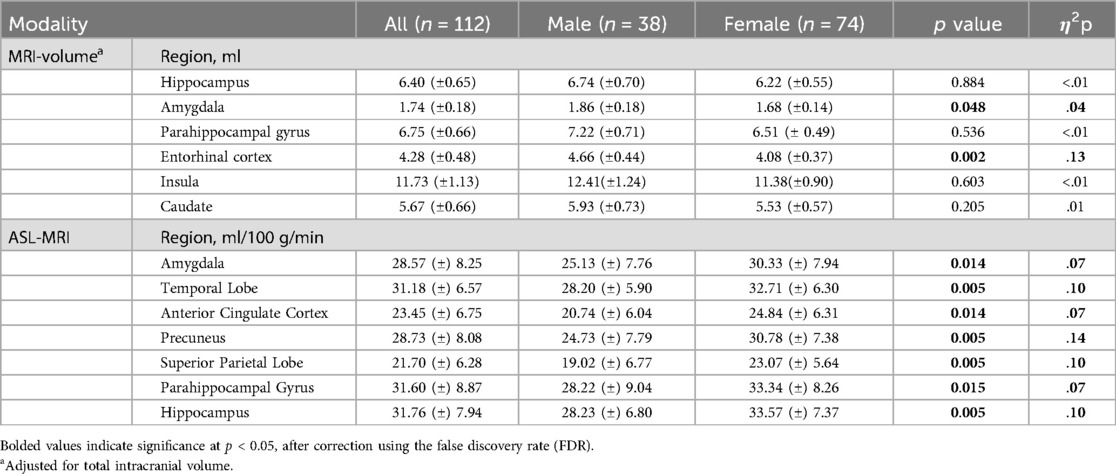
After adjusting for intracranial volume, age, and years of education, a MANCOVA yielded a significant group difference between male and female volumes in six hypothesized ROIs [F (6, 102) = 4.25, p < 0.001, η2p = .20] (Table 4). The Box's M test was not significant (p = 0.115), suggesting the assumption of homogeneity of covariance matrices was met. post hoc univariate ANCOVAs, corrected with the Benjamini-Hochberg critical value for a false discovery rate of 5%, among the six hypothesized ROIs revealed there was a statistically significant difference in volumes between males and females in the amygdala [F (1, 107) = 4.78, p < 0.048] and entorhinal cortex [F (1, 107) = 15.89, p ≤ 0.002]. Females exhibited lower volume in the amygdala (p < 0.048, 95% C.I. = 0.01, 0.13), and entorhinal cortex (p < 0.002, 95% C.I. = 0.17, 0.49) compared to males (Figure 1).
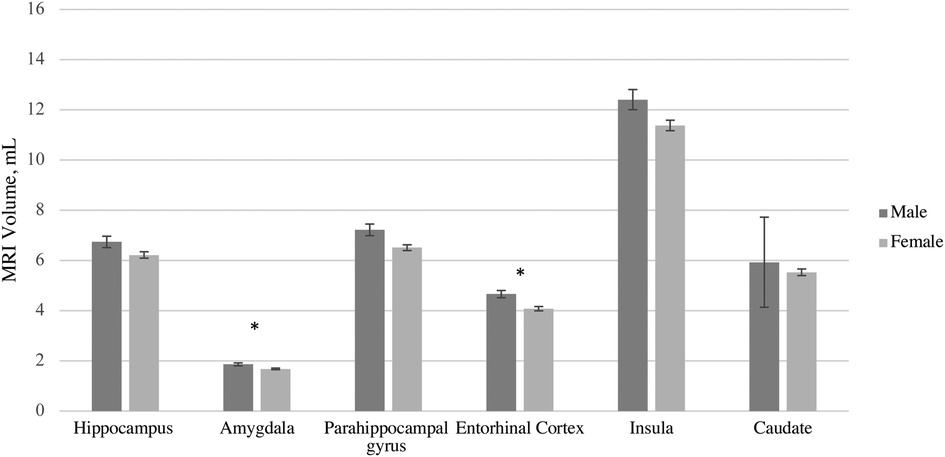
Figure 1. MRI volume Sex differences by region of interest accounting for differences in total volume, age, and education. Error bars represent 95% confidence intervals.
3.2.3 ASL-MRI cerebral blood flow
A MANCOVA of the relationship between sex and cerebral blood flow in hypothesized brain ROIs after adjusting for age and years of education was statistically significant, and the female group showed higher blood flow compared to the male group in several brain regions [F (7, 102) = 2.67, p = 0.014, η2p = .15] (Table 4). Given the unequal sex sample sizes in this analysis (males = 38, females = 74), the Box's Test for Equivalence of Covariance Matrices (Box's M) was conducted and interpreted as not significant (p = 0.002) using p < .001 as a criterion based on current literature, suggesting the assumption of homogeneity of covariance matrices was met (98, 99). post hoc univariate ANCOVAs, corrected with the Benjamini-Hochberg critical value for a false discovery rate of 5%, among the seven hypothesized ROIs revealed there was a statistically significant difference in cerebral blood flow between males and females in the amygdala [F (1, 108) = 8.70, p = 0.014], temporal lobe [F (1, 108) = 12.46, p = 0.005], anterior cingulate cortex [F (1, 108) = 8.65], p = 0.014), precuneus [F (1, 108) = 17.29, p = 0.005], superior parietal lobe [F (1, 108) = 11.93, p = 0.005], parahippocampal gyrus [F (1, 108) = 8.26, p = 0.015], and hippocampus [F (1, 108) = 11.66, p = .005]. Females exhibited higher blood flow in the amygdala (p = 0.014, 95% C.I. = −7.92, −1.55), temporal lobe (p < 0.005, 95% C.I. = −7.05, −1.97), anterior cingulate cortex (p = 0.014, 95% C.I. = −6.53, −1.27), precuneus (p = 0.005, 95% C.I. = −9.48, −3.36), superior parietal lobe (p = 0.005, 95% C.I. = −6.65, −1.80), parahippocampal gyrus (p = 0.004, 95% C.I. = −8.53, −1.56), and hippocampus (p = .005, 95% C.I. = −8.37 −2.22) compared to males (Figure 2).
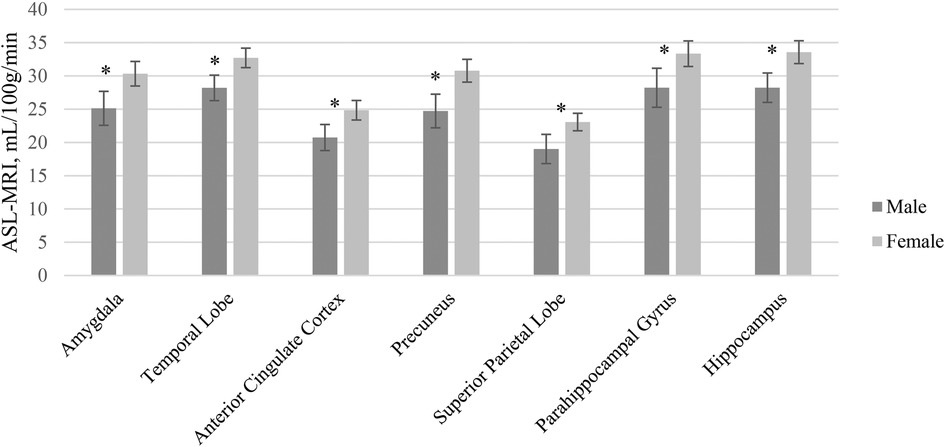
Figure 2. ASL-MRI Sex differences by region of interest accounting for differences in age and education. Error bars represent 95% confidence intervals.
3.3 Aim 2: associations between sex-driven brain biomarker differences and AD risk factors
LASSO regressions were used to identify which risk factor variables were most strongly associated with the Aim 1 identified brain ROIs showing neuroimaging biomarker differences between sex groups. LASSO regressions selected the most informative predictors for MRI volumetric measurements of the amygdala and entorhinal cortex as well as ASL-MRI cerebral blood flow measurements of the amygdala, temporal lobe, anterior cingular cortex, precuneus, superior parietal lobe, parahippocampal gyrus, and hippocampus regions. Ten AD risk factor variables were included as potential predictors including age, education, sex, APOE4 carrier status, non-optimal fasting plasma glucose, non-optimal mean arterial pressure, non-optimal waist-to-hip ratio, non-optimal total body fat percentage, android fat percentage, and gynoid fat percentage. To evaluate sex differences, interaction terms with each predictor multiplied by sex were included in the LASSO models (females = 1, males = 0).
3.3.1 MRI VOLUMES
Overall, all LASSO models for volumetric MRI ROIs performed well being able to explain a range of 63%–77% of the variation in the values of the training data coupled with appropriate RMSE values for each model. Table 5 lists the estimated coefficients and associated R2 and RMSE values for each model.
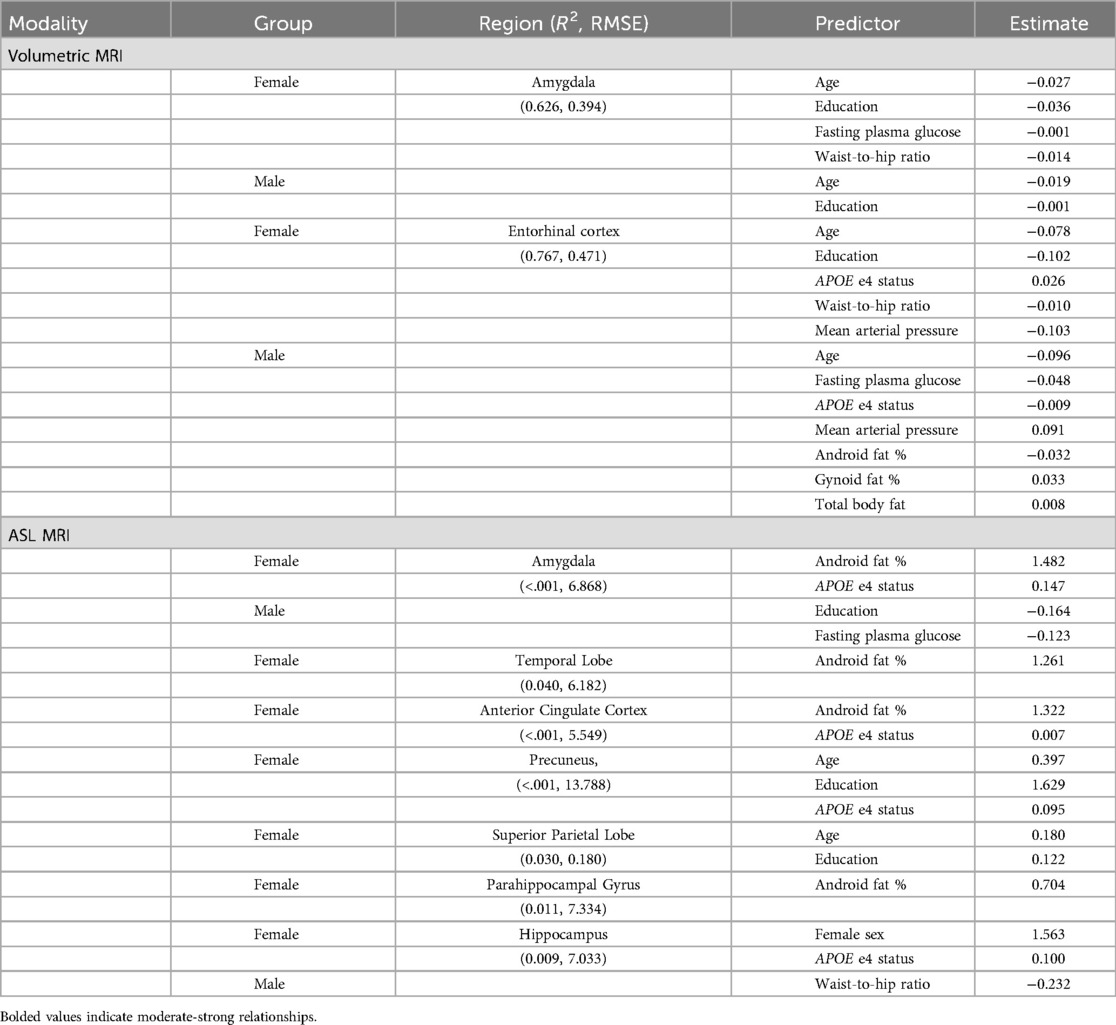
Table 5. LASSO regression estimation coefficient results by sex and imaging modality and region of interest: MRI measures.
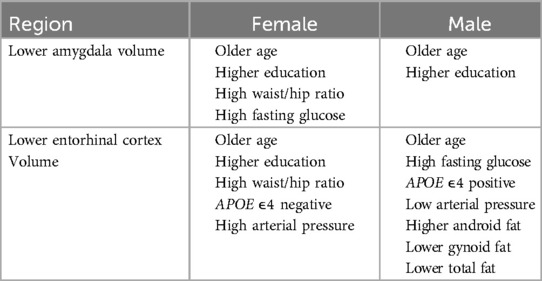
Older age predicted lower volumes in both amygdala and entorhinal cortex for both men and women. Higher education predicted lower amygdala volumes for both men and women, but only lower entorhinal volumes for women. Education was not significantly associated with entorhinal cortex volume for men. Carrying an APOE4 allele was associated with higher entorhinal cortex volume for females, but for males, carrying a APOE4 allele was associated with lower entorhinal cortex volume. The APOE4 allele was not significantly associated with amygdala volumes for either males or females.
Body composition variables were associated differently with brain volumes for males and females. For females, higher waist to hip ratio was associated with lower volumes in both amygdala and entorhinal cortex. For males, body composition was not significantly associated with amygdala volume. Meanwhile, for males, lower gynoid fat and total fat were associated with lower entorhinal cortex volume, but higher android fat was associated with lower entorhinal cortex volume.
We observed sex-specific patterns for glucose levels and arterial pressure. High fasting glucose levels were associated with lower amygdala volumes for females but were not associated with amygdala volumes for males. By contrast, high fasting glucose was associated with lower entorhinal cortex volumes for males, but not for females. High mean arterial pressure was associated with lower volume in the entorhinal cortex for women and higher volume for men. Arterial pressure was not associated with amygdala volume in males or females. The pattern of MRI volumetric association results by sex are summarized in Table 6.
3.3.2 ASL-MRI CEREBRAL BLOOD FLOW
Overall, the seven LASSO models for ASL-MRI cerebral blood flow of ROIs performed very poorly explaining a range of 0%–1% of the variation in the values of the training data. The models failed to accurately predict the most impactful risk factors associated with the ASL-MRI cerebral blood flow of these regions. Table 5 lists the estimated coefficients and associated R2 and RMSE values for each model.
4 Discussion
This multimodal neuroimaging study aimed to examine sex differences in preclinical AD biomarkers among amyloid positive, cognitively unimpaired, sedentary older adults. Several key findings emerged that support and extend existing literature. First, significant sex differences in MRI T1-weighted regional volumes and ASL-MRI measured blood flow were observed in regions linked to preclinical AD pathology and the estrogen receptor network. Second, AD MRI T1-weighted volumetric biomarker differences in the amygdala and entorhinal cortex were most consistently and strongly associated with impaired fasting glucose, higher than optimal waist to hip ratio, and APOE4 carrier status for females. Third, amyloid-β levels were comparable between females and males, likely due to the selection criteria of our study. Many of the implicated cardiometabolic risk factors are modifiable. Thus, these findings support the development of sex-specific strategies for modifiable risk factors during the AD preclinical stage.
4.1 Pet amyloid-β burden sex differences
We did not find any associations between sex and amyloid-β burden among the anterior cingulate gyrus, lateral temporal lobe, precuneus, superior parietal lobule or global amyloid-β burden after adjusting for age, education, and APOE4 status. It is important to note that we did not expect a large amount of variance among participants given the inclusion criteria of the primary APEX study required sub-threshold or elevated amyloid-β, which may have contributed to the lack of sex differences we observed. These results are consistent with literature using cross-sectional methodology among cognitively unimpaired older adults (100) and across the lifespan (49). Some research suggests sex differences in amyloid-β burden may exist but occur earlier in the aging process, such as during the menopause transition (47, 71). According to the estrogen hypothesis, estrogen may reduce aggregation of amyloid-β. This suggests that the decrease of estrogen during menopause may result in greater amyloid-β burden. Future studies may benefit from exploration of the potential effect of reproductive history, including years since menopause, and its relationship with amyloid-β burden among postmenopausal women. An alternative possibility for the lack of observed sex differences in amyloid-β may relate to a lack of control for AD family history in our study, for which prior studies for have demonstrated sex differences (101). Future studies should consider family history when examining the interaction between sex and asymptomatic cerebral β-amyloidosis.
4.2 MRI-volumetric sex differences
Our volumetric findings are consistent with the UK Biobank, the largest single-sample study of structural sex differences in the human brain ages 40–69 (102). The study found males generally had larger total brain volume and among similar brain regions of interest to the present study to include the hippocampus, nucleus accumbens, amygdala, caudate nucleus, dorsal pallidum, putamen, and thalamus, accounting for total intracranial volume. Interestingly, they also found women had thicker cortices compared to men globally and among these regions, except notably the entorhinal cortex which was found to be thicker among men. It is important to note that greater brain volume does not necessarily indicate better brain health and can be region specific (103). For example, greater amygdala volumes are associated with depression, while greater hippocampal volumes are associated with higher levels of education (104, 105). Future studies should independently examine sex differences among regional cortical thicknesses, which have been shown to be phenotypically independent from structural volume measurements (106). In a sample slightly younger than the current study, Rahman et al. and Mosconi et al. also demonstrated lower MRI gray and white matter volume among women in the amygdala compared to men. Contrary to our results, they also found women had significantly lower volumes in the hippocampus, parahippocampal gyrus, insula, and caudate in addition to the superior, middle, and orbital frontal gyrus, anterior cingulate (ACC), and putamen among 46–65 year old females compared to age-matched males (47, 71). Future studies with larger sample sizes and across age groups during post-menopause should explore the potential underlying mechanisms of sex hormones in volumetric sex differences accounting for reproductive system health history.
Other studies have found no sex differences among structural volumetric measurements of the hippocampus and amygdala among samples of healthy older adults (51, 107). Contrary to these studies, our sample included sedentary older adults with asymptomatic cerebral β-amyloidosis. Further, findings from the Buckley et al. review suggest a greater amyloid-β burden sensitivity in females relative to males such that females with asymptomatic cerebral β-amyloidosis had steeper cognitive decline and neurodegeneration compared to males (100). Taken in context of our volumetric findings, this may suggest faster neurodegeneration among women with preclinical AD risk factors compared to men. However, the cross-sectional design of the present study limits this interpretation, and longitudinal studies are needed to further investigate this potential sex difference in AD trajectory.
4.3 ASL-MRI cerebral blood flow sex differences
Our study found that older adult women with asymptomatic cerebral β-amyloidosis had higher blood flow in regions associated with AD pathology and estrogen receptor network ROIs compared to men. The mechanism explaining the interaction between sex and cerebral blood flow in our results remains unclear. It is important to note that greater blood flow does not necessarily indicate better brain health. Prominent theories in the aging neuroscience literature regarding compensation, maintenance, reserve, and age-related brain restructuring or reorganizing are examples of when higher blood flow in a certain region is not a marker of health (108, 109). Mosconi et al. demonstrated the role of estrogen on cerebral blood flow during the menopause stages. The study found higher cerebral blood flow in postmenopausal women compared to perimenopausal women in the supramarginal gyrus, middle and superior temporal gyrus, superior and inferior front gyrus of both hemispheres (71). In their discussion of these findings, they proposed a compensatory reaction to glucose hypometabolism, and increased ketone metabolism after menopause as an underlying reason for the increased cerebral blood flow in these women (71). These results could also be interpreted as suggesting that blood flow is reduced during the fluctuations of perimenopause. During post menopause, blood flow may be returning to normal or possibly higher functioning as part of the compensatory reaction. Given our postmenopausal sample was older than the Mosconi et al. study, in addition to our volumetric sex difference findings, there could be a similar compensatory process occurring for women later in the aging process.
4.4 Associations between sex-driven biomarker differences and AD risk factors
Only one other study to our knowledge has evaluated associations of demographic and cardiometabolic risk factors with MRI T1 weighted volumetric sex differences in cognitively unimpaired older adults (110) and none have examined predictors of ASL-MRI cerebral flood flow. In contrast to our methodological approach, Armstrong et al. followed its sample for 20 years and measured volumetric loss using cardiovascular predictors defined differently than in our study (110). They found that for males, hypertension and higher HDL cholesterol were protective against volume loss in the hippocampus and parahippocampal gyrus. In females, obesity (as measured by body mass index ≥30 kg/m2 vs. <30 kg/m2) was found to protect against volume loss in the temporal gray matter but hypertension was associated with steeper volumetric decline in gray matter compared to men (110). Although we did not replicate their hypertension findings in the hippocampus or parahippocampal gyrus, we also found sex differences to include high mean arterial pressure predicted higher volume in the entorhinal cortex for males and lower volume for females.
Contrary to their study, our study measured obesity using more precise methods including waist to hip ratio, DEXA measured total fat percentage, and type of fat distribution (android and gynoid fat percentages). Our study found that for men, more android fat but less gynoid fat and total fat was associated with smaller entorhinal cortex volumes. In contrast, we found for females, waist to hip ratios greater than or equal to 0.85 was associated with smaller amygdala and entorhinal cortex volumes and more android fat predicted higher cerebral blood flow. It is worth noting that measures of body composition have shown non-linear age-related associations with cognitive performance and dementia such that body fat or higher body weight may be protective at some life stages and deleterious at other stages (111). Thus, timing may be a critical element to understanding sex differences in the influence of cardiometabolic risk factors on the brain.
Weight distribution during aging differs between males and females and may further explain some of our findings. For males, adipose tissue slowly but consistently accrues in the trunk and abdomen during aging over time (android fat distribution). For females, prior to menopause, adipose tissues accrue in the hips and thighs (gynoid fat distribution). After menopause, gynoid fat mass stabilizes while android fat distribution steeply increases into older adulthood (112, 113). Android fat distribution is associated with visceral fat accumulation which is directly related to cardiovascular disease and metabolic disease compared to other types of fat, both of which have been recognized as risk factors for Alzheimer's disease. Females in our sample had significantly more android fat percentage on average compared to males, suggesting postmenopausal females in our sample have more visceral fat and susceptibility for increased cardiovascular risk factors.
Our sample was sedentary by design. Research has found sedentary behavior is associated with additional risk factors for Alzheimer's disease including impaired glucose and lipid metabolism (114). Sedentary behavior over time may lead to abdominal obesity and insulin resistance, the most prominent underlying risk factors for cardiovascular disease and metabolic syndrome (115–119). In addition, according to the estrogen hypothesis, postmenopausal women are also more susceptible to these cardiovascular and metabolic risk factors due to the loss of estrogen and its key protective effect for glucose transport regulation and aerobic glycolysis. This area of aging research may provide a potential understanding of the underlying cause for the association finding between waist to hip ratio with brain volume among AD pathology and estrogen receptor network region ROIs in our sample of sedentary older adult females. More research is warranted to further explain these findings.
We did not find any impactful risk factors associated with the ASL-MRI cerebral blood flow of these regions. The ASL-MRI technique is helpful with providing information on vascular physiology and neurodegeneration related to AD pathology (120). The present study was limited by the amount of risk factors included in the model directly related to vascular physiology. Future studies should consider including more risk factors, as acknowledged in our limitations section. Research has shown neurofibrillary tangle tau biomarkers and cerebral blood flow are correlated, independent of other well-known AD-related risk factors (i.e., APOE, amyloid, small vessel disease markers) (121–123). These studies suggest significant cerebral blood flow changes may be associated with more downstream AD pathology. Given our study sample consisted of cognitively unimpaired individuals that may be at high risk for developing AD, it may be too early to associate ASL-MRI measurements with any risk factors. More studies examining ASL-MRI in AD at-risk populations with consideration of tau are needed. Methodological limitations and inconsistencies of the ASL-MRI technique may have also contributed to our findings. The accuracy and reproducibility of results in studies using the ASL-MRI technique has been critiqued in the literature given its sensitivity to motion and reliance on the scanner's magnetic field strength (124). Studies also discussed concerns with lack of standardization in acquisition across the literature (125, 126).
4.5 Strengths and limitations
The current analysis was unique in that it included neuroimaging techniques that considered two of the three categories as part of the A/T/N biomarker classification scheme for AD pathology (43). Uniquely, it adds ASL-MRI imaging, a unique imaging technique for highlighting sex and age differences in cerebral blood flow. However, the current analysis is missing consideration of neurofibrillary tangle tau biomarkers, which is commonly measured by elevated CSF phosphorylated tau and elevated NFT-tau ligand uptake on PET imaging analyses. Future studies should consider examining sex differences among all three categories of the A/T/N biomarker AD pathology scheme in preclinical AD individuals.
While the LASSO regression statistical approach is designed for predication accuracy, literature also suggests concerns with variable selection consistency (127, 128). Certain techniques were used in the current study to accommodate for conducting a LASSO regression with a smaller sample size (i.e., k2 and leave-one-out cross validation) that we acknowledge may have oversimplified the model threatening the reliability of our Aim 2 findings (129). Results should be interpreted with caution and future replication of these findings are needed. Further, LASSO regressions are designed to estimate the relationships between variables and make predictions however do not estimate causal effects. Our study's purpose was to identify the most influential risk factor associated with sex-specific AD biomarkers to inform future hypotheses and studies. Future studies are needed to study the underlying mechanisms among these predicted associations.
The current study is cross-sectional in design, and therefore causal direction of associations cannot be determined. Longitudinal studies are needed to determine if the identified sex differences are predictive of dementia or suggest healthy brain aging differences over time, especially considering changes that result from the menopause transition. The use of regression analysis to examine a potential relationship between neuroimaging biomarkers and cardiovascular risk factors in a cross-sectional study is also problematic. Neurodegeneration can only be measured using longitudinal study designs. It also cannot be determined whether blood flow is increasing or decreasing over time for either men or women. Lastly, cardiovascular risk factors and health are cumulative over time therefore analyses examining health outcomes should be controlling for health and related behaviors over a lifetime to examine potential effects in later life.
Our sample consisted of a preponderance of non-Hispanic White, educated, and high socioeconomic status older adults, diminishing the external validity of the results of the current study. However, our sample consisted of significantly more women who identified as African American (n = 5) compared to men (n = 0) (Table 2). African American populations are 2 times more likely than non-Hispanic white populations to be clinically diagnosed with Alzheimer's disease or dementia related disorders, with African American women shown to be at an even higher risk (130, 131). A recent review also suggested African American women may demonstrate more AD-related neurodegeneration and cerebral small vessel disease when compared to non-Hispanic white populations (132). Therefore, the sex differences identified for both volumetric MRI and ASL-MRI in our study may have been strengthened with the inclusion of significantly more African American women in our study sample. As for the Royse et al. meta-analysis findings suggested, more studies with larger, more diverse samples examining AD-related biomarkers and risk factors taking both sex and ethnoracial factors into consideration are needed. The imbalance between sample size of men and women and relatively small sample size of men may have limited the sex-associated difference analyses. However, the imbalanced sample size represents the ratio of Alzheimer's disease prevalence between men and women with two thirds of patients with AD being women. Our results are only generalizable to persons who identify as female or male and may not apply to trans-women or trans-men. Sex and gender exist on spectrums. Research on sex-differences must consider the health and aging experiences of trans-people or for persons who identify elsewhere on the sex/gender spectrum and examine factors unique to this population such as hormone therapy.
Another limitation of the current study was the binomial (yes/no) categorization of AD risk factors as predictive measures in the analysis including impaired fasting glucose, high mean arterial pressure, higher than optimal total body fat percentage, and higher than optimal waist to hip ratio. Although dichotomizing the risk factors aligned with typical approaches, we acknowledge it simplifies what is an underlying continuous process. Measurement imprecision inevitably results in some classification errors, particularly for values close to cutoff points. Future studies should consider the use of continuous biomarker variables to provide a more accurate representation of the severity of the risk factors.
The current study is limited by its lack of control for other well-established risk factors for AD with known sex and gender differences including other health conditions (e.g., dyslipidemia, hypercholesterolemia), health behaviors (e.g., diet, sleep, smoking), socioeconomic considerations (e.g., occupation, household income), and psychological factors (e.g., depression) (133–135). Another major limitation of the current study is its lack of reproductive history consideration, specifically menopause type (e.g., natural, medically or surgically induced) and treatment (e.g., hormone therapy) as potential contributors to AD risk. Natural menopause has been associated with AD-risk-specific neuropathology including amyloid-β deposition and decreased hippocampal volume (45, 46). Further, research has identified a greater risk for AD associated with early medically or surgically induced menopause that occurs prior to natural menopause (136, 137). A recent review and meta-analysis of research on hormone replacement therapy and its relationship to cognition and AD risk indicate a lack of benefits and potential harmful effects, except in women who undergo early surgically induced menopause. Current research suggests the formulation, route of administration, and timing of hormone replacement therapy initiation produce different effects and are critical to understanding the efficacy of hormone therapy for AD prevention (15, 138–149). Future studies should consider the combination of these risk factors, especially reproductive history, among one individual and the potential sex differences among these risk factors in relation to preclinical AD biomarkers.
5 Conclusion
In summary, we examined associations of AD risk factors with AD biomarkers by sex in our cohort of sedentary older adults. We found widespread sex differences in T1 MRI weighted volumetrics in addition to ASL-MRI blood flow. Sedentary older adult women with asymptomatic cerebral β-amyloidosis demonstrated smaller volumes in addition to higher blood flow in AD pathology and estrogen receptor network ROIs. Genetic and chronic health risk factors were most strongly associated with these sex-related differences for the female group only including higher than optimal waist to hip ratio was most strongly associated with lower volume. Future investigations with larger sample sizes should include reproductive history characteristics including hysterectomy status and hormone replacement in an effort to investigate a potential underlying sex-specific biological pathway to brain aging to explain these differences, as was found among midlife women in the Rahman et al. study (47). These findings highlight the importance of considering differences in patterns of neurodegeneration and brain blood flow among preclinical AD older adult males and females when developing effective AD interventions and prevention strategies.
Data availability statement
The datasets presented in this study can be found in online repositories. The data supporting the findings of this study are openly available in Harvard Dataverse at https://doi.org/10.7910/DVN/B9I1F8.
Ethics statement
The studies involving humans were approved by University of Kansas Medical Center Human Subjects Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GL: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. MK: Data curation, Formal analysis, Writing – review & editing. EV: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. JC: Project administration, Writing – review & editing. JM: Investigation, Project administration, Writing – review & editing. JB: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – review & editing. AW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Institutes of Health R01 AG043962 (JB); K99 AG050490 (JM) and gifts from Frank and Evangeline Thompson (JB), The Ann and Gary Dickinson Family Charitable Foundation, John and Marny Sherman, and Brad and Libby Bergman. Institutional infrastructure support for testing was provided in part by UL1 TR000001 (RB) and P30 AG035982 (RHS JB). Lilly Pharmaceuticals provided a grant to support F18-AV45 doses and partial scan costs (JB). AW is supported by an NIH grant from NIGMS and OD 1P20GM152280. MNK is supported by NIA grants T32 AG078114 and P30 AG072973. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. (1997) 278:1349–56. doi: 10.1001/jama.1997.03550160069041
2. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. (2017) 13:325–73. doi: 10.1016/j.jalz.2017.02.001
3. Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol. (2001) 153:132–6. doi: 10.1093/aje/153.2.132
4. Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the framingham study. Neurology. (1997) 49:1498–504. doi: 10.1212/wnl.49.6.1498
5. Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol. (2018) 14:457–69. doi: 10.1038/s41582-018-0032-9
6. Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. (2018) 14:1171–83. doi: 10.1016/j.jalz.2018.04.008
7. Rahman A, Jackson H, Hristov H, Isaacson RS, Saif N, Shetty T, et al. Sex and gender driven modifiers of Alzheimer’s: the role for estrogenic control across age, race, medical, and lifestyle risks. Front Aging Neurosci. (2019). 11:315. doi: 10.3389/fnagi.2019.00315
8. Cordonnier C, Sprigg N, Sandset EC, Pavlovic A, Sunnerhagen KS, Caso V, et al. Stroke in women—from evidence to inequalities. Nat Rev Neurol. (2017) 13:521–32. doi: 10.1038/nrneurol.2017.95
9. Gibson CL. Cerebral ischemic stroke: is gender important? J Cereb Blood Flow Metab. (2013) 33:1355–61. doi: 10.1038/jcbfm.2013.102
10. Longstreth WT, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. (2009) 23:291–4. doi: 10.1097/WAD.0b013e318199fc7a
11. Madsen TE, Khoury J, Alwell K, Moomaw CJ, Rademacher E, Flaherty ML, et al. Sex-specific stroke incidence over time in the greater cincinnati/northern Kentucky stroke study. Neurology. (2017) 89:990–6. doi: 10.1212/WNL.0000000000004325
12. Mallampalli MP, Carter CL. Exploring sex and gender differences in sleep health: a society for women’s health research report. J Womens Health (Larchmt). (2014) 23:553–62. doi: 10.1089/jwh.2014.4816
13. National Institute of Mental Health. Major depression. U.S. Department of Health and Human Services, National Institutes of Health (2021). Available at: https://www.nimh.nih.gov/health/statistics/major-depression (Accessed July 18, 2021).
14. Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. (2006) 26:10332–48. doi: 10.1523/JNEUROSCI.3369-06.2006
15. Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. (2008) 31:529–37. doi: 10.1016/j.tins.2008.07.003
16. Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. (2015) 11:393–405. doi: 10.1038/nrendo.2015.82
17. Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. (2014) 35:8–30. doi: 10.1016/j.yfrne.2013.08.001
18. Österlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. (2001) 64:251–67. doi: 10.1016/S0301-0082(00)00059-9
19. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. (2015) 9:37. doi: 10.3389/fnins.2015.00037
20. Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. (2004) 1019:24–8. doi: 10.1196/annals.1297.005
21. Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. (2009) 41:1088–93. doi: 10.1038/ng.440
22. Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s disease neuroimaging initiative investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. (2014) 75:563–73. doi: 10.1002/ana.24135
23. Kim S, Kim MJ, Kim S, Kang HS, Lim SW, Myung W, et al. Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: a CREDOS study. Compr Psychiatry. (2015) 62:114–22. doi: 10.1016/j.comppsych.2015.07.002
24. Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. (2017) 74:1178–89. doi: 10.1001/jamaneurol.2017.2188
25. Heise V, Filippini N, Trachtenberg AJ, Suri S, Ebmeier KP, Mackay CE. Apolipoprotein E genotype, gender and age modulate connectivity of the hippocampus in healthy adults. Neuroimage. (2014) 98:23–30. doi: 10.1016/j.neuroimage.2014.04.081
26. Sampedro F, Vilaplana E, de Leon MJ, Alcolea D, Pegueroles J, Montal V, et al. APOE -by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. (2015) 6:26663–74. doi: 10.18632/oncotarget.5185
27. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. (2011) 10:819–28. doi: 10.1016/S1474-4422(11)70072-2
28. Hamilton MT, Healy GN, Dunstan DW, Zderic TW, Owen N. Too little exercise and too much sitting: inactivity physiology and the need for new recommendations on sedentary behavior. Curr Cardio Risk Rep. (2008) 2:292–8. doi: 10.1007/s12170-008-0054-8
29. Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc. (2012) 44:225. doi: 10.1249/MSS.0b013e31822ac0c0
30. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. (2010) 38:105. doi: 10.1097/JES.0b013e3181e373a2
31. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes Mellitus. Endocr Rev. (2016) 37:278–316. doi: 10.1210/er.2015-1137
32. Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med. (2019) 25:1657–66. doi: 10.1038/s41591-019-0643-8
33. Dong C, Zhou C, Fu C, Hao W, Ozaki A, Shrestha N, et al. Sex differences in the association between cardiovascular diseases and dementia subtypes: a prospective analysis of 464,616 UK biobank participants. Biol Sex Differ. (2022) 13:21. doi: 10.1186/s13293-022-00431-5
34. Volgman AS, Bairey Merz CN, Aggarwal NT, Bittner V, Bunch TJ, Gorelick PB, et al. Sex differences in cardiovascular disease and cognitive impairment: another health disparity for women? J Am Heart Assoc. (2019) 8:e013154. doi: 10.1161/JAHA.119.013154
35. Appelman Y, van Rijn BB, ten Haaf ME, Boersma E, Peters SAE. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. (2015) 241:211–8. doi: 10.1016/j.atherosclerosis.2015.01.027
36. Merz CN B, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. (2006) 47:S21–9. doi: 10.1016/j.jacc.2004.12.084
37. Kannel BW, Hjortland CM, Mcnamara MP, Gordon T. Menopause and risk of cardiovascular disease. Ann Intern Med. (2020) 85(4):447–52. doi: 10.7326/0003-4819-85-4-447
38. Heianza Y, Arase Y, Kodama S, Hsieh SD, Tsuji H, Saito K, et al. Effect of postmenopausal Status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: toranomon hospital health management center study 17 (TOPICS 17). Diabetes Care. (2013) 36:4007–14. doi: 10.2337/dc13-1048
39. Sutton-Tyrrell K, Lassila HC, Meilahn E, Bunker C, Matthews KA, Kuller LH. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. (1998) 29:1116–21. doi: 10.1161/01.STR.29.6.1116
40. Kaya E, Sahin FK, Köken G, Köse M, Cevrioglu AS. Acute effect of intranasal estrogen on cerebral and cerebellar perfusion in postmenopausal women. Maturitas. (2008) 59:72–82. doi: 10.1016/j.maturitas.2007.10.004
41. Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. J Appl Physiol (1985). (2006) 101:1252–61. doi: 10.1152/japplphysiol.01095.2005
42. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. (2014) 13:788–94. doi: 10.1016/S1474-4422(14)70136-X
43. Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. (2016) 87:539–47. doi: 10.1212/WNL.0000000000002923
44. Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. (2011) 7:280–92. doi: 10.1016/j.jalz.2011.03.003
45. Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, et al. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology. (2017) 89:1382–90. doi: 10.1212/WNL.0000000000004425
46. Mosconi L, Rahman A, Diaz I, Wu X, Scheyer O, Hristov HW, et al. Increased Alzheimer’s risk during the menopause transition: a 3-year longitudinal brain imaging study. PLoS One. (2018) 13:e0207885. doi: 10.1371/journal.pone.0207885
47. Rahman A, Schelbaum E, Hoffman K, Diaz I, Hristov H, Andrews R, et al. Sex-driven modifiers of Alzheimer risk: a multimodality brain imaging study. Neurology. (2020) 95:e166–78. doi: 10.1212/WNL.0000000000009781
48. Mosconi L, Nerattini M, Matthews DC, Jett S, Andy C, Williams S, et al. In vivo brain estrogen receptor density by neuroendocrine aging and relationships with cognition and symptomatology. Sci Rep. (2024) 14:12680. doi: 10.1038/s41598-024-62820-7
49. Jack CR, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. (2015) 72:511–9. doi: 10.1001/jamaneurol.2014.4821
50. Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. (2015) 313:1924–38. doi: 10.1001/jama.2015.4668
51. Cavedo E, Chiesa PA, Houot M, Ferretti MT, Grothe MJ, Teipel SJ, et al. Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer’s disease in cognitively normal older adults with subjective memory complaints. Alzheimers Dement. (2018) 14:1204–15. doi: 10.1016/j.jalz.2018.05.014
52. Gottesman RF, Schneider ALC, Zhou Y, Chen X, Green E, Gupta N, et al. The ARIC-PET amyloid imaging study. Neurology. (2016) 87:473–80. doi: 10.1212/WNL.0000000000002914
53. Vemuri P, Knopman DS, Lesnick TG, Przybelski SA, Mielke MM, Graff-Radford J, et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol. (2017) 74:718–26. doi: 10.1001/jamaneurol.2017.0244
54. Jack CR, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, et al. Age and sex specific prevalences of cerebral β-amyloidosis, tauopathy and neurodegeneration among clinically normal individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. (2017) 16:435–44. doi: 10.1016/S1474-4422(17)30077-7
55. Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E ε4 carriers. AJNR Am J Neuroradiol. (2013) 34:2287–93. doi: 10.3174/ajnr.A3601
56. Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J Alzheimers Dis. (2014) 42:S411–9. doi: 10.3233/JAD-141467
57. Hays CC, Zlatar ZZ, Wierenga CE. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell Mol Neurobiol. (2016) 36:167–79. doi: 10.1007/s10571-015-0261-z
58. Wang Z, Das SR, Xie SX, Arnold SE, Detre JA, Wolk DA. Arterial spin labeled MRI in prodromal Alzheimer’s disease: a multi-site study. NeuroImage: Clinical. (2013) 2:630–6. doi: 10.1016/j.nicl.2013.04.014
59. Khan TK. An algorithm for preclinical diagnosis of Alzheimer’s disease. Front Neurosci. (2018) 12:275. doi: 10.3389/fnins.2018.00275
60. Arnolds BJ, von Reutern GM. Transcranial doppler sonography. Examination technique and normal reference values. Ultrasound Med Biol. (1986) 12:115–23. doi: 10.1016/0301-5629(86)90016-5
61. Keage HAD, Churches OF, Kohler M, Pomeroy D, Luppino R, Bartolo ML, et al. Cerebrovascular function in aging and dementia: a systematic review of transcranial doppler studies. Dement Geriatr Cogn Disord Extra. (2012) 2:258–70. doi: 10.1159/000339234
62. Martin PJ, Evans DH, Naylor AR. Transcranial color-coded sonography of the basal cerebral circulation. Reference data from 115 volunteers. Stroke. (1994) 25:390–6. doi: 10.1161/01.str.25.2.390
63. Devous MD, Stokely EM, Chehabi HH, Bonte FJ. Normal distribution of regional cerebral blood flow measured by dynamic single-photon emission tomography. J Cereb Blood Flow Metab. (1986) 6:95–104. doi: 10.1038/jcbfm.1986.12
64. Esposito G, Van Horn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med. (1996) 37:559–64.8691239
65. Gur RE, Gur RC. Gender differences in regional cerebral blood flow. Schizophr Bull. (1990) 16:247–54. doi: 10.1093/schbul/16.2.247
66. Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. (2012) 68:912–22. doi: 10.1002/mrm.23286
67. Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. (2004) 51:736–43. doi: 10.1002/mrm.20023
68. Bakker SLM, de Leeuw F-E, den Heijer T, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral haemodynamics in the elderly: the rotterdam study. NED. (2004) 23:178–84. doi: 10.1159/000078503
69. Tegeler CH, Crutchfield K, Katsnelson M, Kim J, Tang R, Griffin LP, et al. Transcranial doppler velocities in a large, healthy population. J Neuroimaging. (2013) 23:466–72. doi: 10.1111/j.1552-6569.2012.00711.x
70. Alwatban MR, Aaron SE, Kaufman CS, Barnes JN, Brassard P, Ward JL, et al. Effects of age and sex on middle cerebral artery blood velocity and flow pulsatility index across the adult lifespan. J Appl Physiol. (2021) 130:1675–83. doi: 10.1152/japplphysiol.00926.2020
71. Mosconi L, Berti V, Dyke J, Schelbaum E, Jett S, Loughlin L, et al. Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Sci Rep. (2021) 11:10867. doi: 10.1038/s41598-021-90084-y
72. Barth C, Steele CJ, Mueller K, Rekkas VP, Arélin K, Pampel A, et al. In vivo dynamics of the human hippocampus across the menstrual cycle. Sci Rep. (2016) 6:1–9. doi: 10.1038/srep32833
73. Dubol M, Epperson CN, Sacher J, Pletzer B, Derntl B, Lanzenberger R, et al. Neuroimaging the menstrual cycle: a multimodal systematic review. Front Neuroendocrinol. (2021) 60:100878. doi: 10.1016/j.yfrne.2020.100878
74. Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, et al. Version 3 of the national Alzheimer’s coordinating center’s uniform data set. Alzheimer Dis Assoc Disord. (2018) 32:351–8. doi: 10.1097/WAD.0000000000000279
75. Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. (1993) 43:2412–4. doi: 10.1212/wnl.43.11.2412-a
76. Mayer CJ, Steinman L, Williams B, Topolski TD, LoGerfo J. Developing a telephone assessment of physical activity (TAPA) questionnaire for older adults. Prev Chronic Dis. (2008) 5:A24.18082013
77. Rao SS, Disraeli P, McGregor T. Impaired glucose tolerance and impaired fasting glucose. Am Fam Physician. (2004) 69(8):1961–8.15117017
78. Meng L, Yu W, Wang T, Zhang L, Heerdt PM, Gelb AW. Blood pressure targets in perioperative care. Hypertension. (2018) 72:806–17. doi: 10.1161/HYPERTENSIONAHA.118.11688
79. World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva: World Health Organization (2011). p. 8–11. Available at: https://iris.who.int/handle/10665/44583 (Accessed May 25, 2022).
80. Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. (2000) 72:694–701. doi: 10.1093/ajcn/72.3.694
81. Vidoni ED, Morris JK, Watts A, Perry M, Clutton J, Sciver AV, et al. Effect of aerobic exercise on amyloid accumulation in preclinical Alzheimer’s: a 1-year randomized controlled trial. PLoS One. (2021) 16:e0244893. doi: 10.1371/journal.pone.0244893
82. Harn NR, Hunt SL, Hill J, Vidoni E, Perry M, Burns JM. Augmenting amyloid PET interpretations with quantitative information improves consistency of early amyloid detection. Clin Nucl Med. (2017) 42:577–81. doi: 10.1097/RLU.0000000000001693
83. Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. (2012) 11:669–78. doi: 10.1016/S1474-4422(12)70142-4
84. Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer’s disease and cognitively normal subjects. J Nucl Med. (2012) 53:378–84. doi: 10.2967/jnumed.111.090340
85. Klunk WE, Koeppe RA, Price JC, Benzinger T, Devous MD, Jagust W, et al. The centiloid project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. (2015) 11:1–15.e4. doi: 10.1016/j.jalz.2014.07.003
86. Jagust WJ, Landau SM, Alzheimer’s Disease Neuroimaging Initiative. Temporal dynamics of β-amyloid accumulation in aging and Alzheimer disease. Neurology. (2021) 96:e1347–57. doi: 10.1212/WNL.0000000000011524
87. Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. (1991) 12:383–8. doi: 10.1016/0165-6147(91)90609-V
88. Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. (1992) 256:184–5. doi: 10.1126/science.1566067
89. Zhou Y, Tan C, Wen D, Sun H, Han W, Xu Y. The biomarkers for identifying preclinical Alzheimer’s disease via structural and functional magnetic resonance imaging. Front Aging Neurosci. (2016) 8:92. doi: 10.3389/fnagi.2016.00092
90. Maioli S, Leander K, Nilsson P, Nalvarte I. Estrogen receptors and the aging brain. Essays Biochem. (2021) 65:913–25. doi: 10.1042/EBC20200162
91. Alsop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer’s disease. J Alzheimers Dis. (2010) 20:871–80. doi: 10.3233/JAD-2010-091699
92. Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol. (2000) 47:93–100. doi: 10.1002/1531-8249(200001)47:1%3C93::AID-ANA15%3E3.0.CO;2-8
93. Johnson NA, Jahng G-H, Weiner MW, Miller BL, Chui HC, Jagust WJ, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. (2005) 234:851–9. doi: 10.1148/radiol.2343040197
94. Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild cognitive impairment and Alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. (2009) 250:856–66. doi: 10.1148/radiol.2503080751
95. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B Stat Methodol. (1996) 58:267–88. doi: 10.1111/j.2517-6161.1996.tb02080.x
96. Xi LJ, Guo ZY, Yang XK, Ping ZG. Application of LASSO and its extended method in variable selection of regression analysis. Zhonghua Yu Fang Yi Xue Za Zhi. (2023) 57:107–11. doi: 10.3760/cma.j.cn112150-20220117-00063
97. Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd eds. New York, NY: Springer Science & Business Media (2009).
98. Hahs-Vaughn DL. Applied Multivariate Statistical Concepts. New York: Routledge (2016). doi: 10.4324/9781315816685
100. Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, et al. Sex, amyloid, and APOE ε4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimer Dement. (2018) 14:1193–203. doi: 10.1016/j.jalz.2018.04.010
101. Villeneuve S, Vogel JW, Gonneaud J, Pichet Binette A, Rosa-Neto P, Gauthier S, et al. Proximity to parental symptom onset and amyloid-β burden in sporadic Alzheimer disease. JAMA Neurol. (2018) 75:608–19. doi: 10.1001/jamaneurol.2017.5135
102. Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, et al. Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex. (2018) 28:2959–75. doi: 10.1093/cercor/bhy109
103. Ritchie SJ, Dickie DA, Cox SR, del Valdes Hernandez MC, Corley J, Royle NA, et al. Brain volumetric changes and cognitive ageing during the eighth decade of life. Hum Brain Mapp. (2015) 36:4910–25. doi: 10.1002/hbm.22959
104. Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. (2008) 13:993–1000. doi: 10.1038/mp.2008.57
105. Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, et al. Hippocampal volume varies with educational attainment across the life-span. Front Hum Neurosci. (2012) 6:307. doi: 10.3389/fnhum.2012.00307
106. Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. (2010) 53:1135–46. doi: 10.1016/j.neuroimage.2009.12.028
107. Sambuco N. Sex differences in the aging brain? A voxel-based morphometry analysis of the hippocampus and the amygdala. Neuroreport. (2021) 32:1320–4. doi: 10.1097/WNR.0000000000001728
108. Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. (2018) 19:701–10. doi: 10.1038/s41583-018-0068-2
109. Morcom AM, Johnson W. Neural reorganization and compensation in aging. J Cogn Neurosci. (2015) 27:1275–85. doi: 10.1162/jocn_a_00783
110. Armstrong NM, An Y, Beason-Held L, Doshi J, Erus G, Ferrucci L, et al. Sex differences in brain aging and predictors of neurodegeneration in cognitively healthy older adults. Neurobiol Aging. (2019) 81:146–56. doi: 10.1016/j.neurobiolaging.2019.05.020
111. Chang S-H, Beason TS, Hunleth JM, Colditz GA. A systematic review of body fat distribution and mortality in older people. Maturitas. (2012) 72:175–91. doi: 10.1016/j.maturitas.2012.04.004
112. Mastaglia SR, Solis F, Bagur A, Mautalen C, Oliveri B. Increase in android fat mass with age in healthy women with normal body mass index. J Clin Densitom. (2012) 15:159–64. doi: 10.1016/j.jocd.2011.12.006
113. Trémollieres FA, Pouilles JM, Ribot CA. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am J Obstet Gynecol. (1996) 175:1594–600. doi: 10.1016/s0002-9378(96)70111-4
114. Yan S, Fu W, Wang C, Mao J, Liu B, Zou L, et al. Association between sedentary behavior and the risk of dementia: a systematic review and meta-analysis. Transl Psychiatry. (2020) 10:1–8. doi: 10.1038/s41398-020-0799-5
115. Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. (2000) 102:179–84. doi: 10.1161/01.CIR.102.2.179
116. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third national health and nutrition examination survey, 1988–1994. Arch Intern Med. (2003) 163:427–36. doi: 10.1001/archinte.163.4.427
117. Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the national cholesterol education program adult treatment panel III criteria for the metabolic syndrome. Diabetes. (2004) 53:2087–94. doi: 10.2337/diabetes.53.8.2087
118. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. (1988) 37:1595–607.3056758
119. Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. (1991) 34:416–22. doi: 10.1007/BF00403180
120. Thropp P, Phillips E, Jung Y, Thomas DL, Tosun D, Initiative for the ADN. Arterial spin labeling perfusion MRI in the Alzheimer’s disease neuroimaging initiative: past, present, and future. Alzheimers Dement. (2024) 20:8937–52. doi: 10.1002/alz.14310
121. Albrecht D, Isenberg AL, Stradford J, Monreal T, Sagare A, Pachicano M, et al. Associations between vascular function and tau PET are associated with global cognition and amyloid. J Neurosci. (2020) 40:8573–86. doi: 10.1523/JNEUROSCI.1230-20.2020
122. Rubinski A, Tosun D, Franzmeier N, Neitzel J, Frontzkowski L, Weiner M, et al. Lower cerebral perfusion is associated with tau-PET in the entorhinal cortex across the Alzheimer’s continuum. Neurobiol Aging. (2021) 102:111–8. doi: 10.1016/j.neurobiolaging.2021.02.003
123. Bryant AG, Manhard MK, Salat DH, Rosen BR, Hyman BT, Johnson KA, et al. Heterogeneity of tau deposition and microvascular involvement in MCI and AD. CAR. (2021) 18:711–20. doi: 10.1016/j.neuroimage.2008.06.006
124. Alsop DC, Casement M, de Bazelaire C, Fong T, Press DZ. Hippocampal hyperperfusion in Alzheimer’s disease. Neuroimage. (2008) 42:1267–74.18602481
125. Zhang L, Xie D, Li Y, Camargo A, Song D, Lu T, et al. Improving sensitivity of arterial spin labeling perfusion MRI in Alzheimer’s disease using transfer learning of deep learning-based ASL denoising. J Magn Reson Imaging. (2022) 55:1710–22. doi: 10.1002/jmri.27984
126. Le Heron CJ, Wright SL, Melzer TR, Myall DJ, MacAskill MR, Livingston L, et al. Comparing cerebral perfusion in Alzheimer’s disease and Parkinson’s disease dementia: an ASL-MRI study. J Cereb Blood Flow Metab. (2014) 34:964–70. doi: 10.1038/jcbfm.2014.40
127. Fan J, Li R. Variable selection via nonconcave penalized likelihood and its oracle properties. J Am Stat Assoc. (2001) 96:1348–60. doi: 10.1198/016214501753382273
128. van Erp S, Oberski DL, Mulder J. Shrinkage priors for Bayesian penalized regression. J Math Psychol. (2019) 89:31–50. doi: 10.1016/j.jmp.2018.12.004
130. Association A. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. (2019) 15:321–87. doi: 10.1016/j.jalz.2019.01.010
131. Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. (2019) 15:17–24. doi: 10.1016/j.jalz.2018.06.3063
132. Royse SK, Cohen AD, Snitz BE, Rosano C. Differences in Alzheimer’s disease and related dementias pathology among African American and Hispanic women: a qualitative literature review of biomarker studies. Front Syst Neurosci. (2021) 15:685957. doi: 10.3389/fnsys.2021.685957
133. Forno G D, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. (2005) 57:381–7. doi: 10.1002/ana.20405
134. Fuhrer R, Dufouil C, Dartigues JF, PAQUID Study. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID study. J Am Geriatr Soc. (2003) 51:1055–63. doi: 10.1046/j.1532-5415.2003.51352.x
135. Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN, et al. Risk profiles for mild cognitive impairment vary by age and sex: the Sydney memory and ageing study. Am J Geriatr Psychiatry. (2012) 20:854–65. doi: 10.1097/JGP.0b013e31825461b0
136. Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. (2007) 69:1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6
137. Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. (2014) 82:222–9. doi: 10.1212/WNL.0000000000000033
138. Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA, MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. (2005) 76:103–5. doi: 10.1136/jnnp.2003.024927
139. Shao H, Breitner JCS, Whitmer RA, Wang J, Hayden K, Wengreen H, et al. Hormone therapy and Alzheimer disease dementia: new findings from the cache county study. Neurology. (2012) 79:1846–52. doi: 10.1212/WNL.0b013e318271f823
140. Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. (2011) 69:163–9. doi: 10.1002/ana.22239
141. Marder K, Sano M. Estrogen to treat Alzheimer’s disease: too little, too late? So what’s a woman to do? Neurology. (2000) 54:2035–7. doi: 10.1212/wnl.54.11.2035
142. Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA. (2002) 288:2170–2. doi: 10.1001/jama.288.17.2170
143. Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, et al. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. (2013) 173:1429–36. doi: 10.1001/jamainternmed.2013.7727
144. Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS-cognitive and affective study. PLoS Med. (2015) 12:e1001833. discussion e1001833. doi: 10.1371/journal.pmed.1001833
145. Henderson VW, St John JA, Hodis HN, McCleary CA, Stanczyk FZ, Shoupe D, et al. Cognitive effects of estradiol after menopause: a randomized trial of the timing hypothesis. Neurology. (2016) 87:699–708. doi: 10.1212/WNL.0000000000002980
146. Boccardi M, Ghidoni R, Govoni S, Testa C, Benussi L, Bonetti M, et al. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a voxel-based morphometry study. Menopause. (2006) 13:584–91. doi: 10.1097/01.gme.0000196811.88505.10
147. Barha CK, Dalton GL, Galea LA. Low doses of 17alpha-estradiol and 17beta-estradiol facilitate, whereas higher doses of estrone and 17alpha- and 17beta-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. (2010) 35:547–59. doi: 10.1038/npp.2009.161
148. Barha CK, Galea LAM. The hormone therapy, premarin, impairs hippocampus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiol Aging. (2013) 34:986–1004. doi: 10.1016/j.neurobiolaging.2012.07.009
Keywords: Alzheimer's disease, sex differences, risk factors, biomarkers, neuroimaging
Citation: Losinski GM, Key MN, Vidoni ED, Clutton J, Morris JK, Burns JM and Watts A (2025) APOE4 and chronic health risk factors are associated with sex-specific preclinical Alzheimer’s disease neuroimaging biomarkers. Front. Glob. Women's Health 6:1531062. doi: 10.3389/fgwh.2025.1531062
Received: 19 November 2024; Accepted: 28 April 2025;
Published: 15 May 2025.
Edited by:
Jacob Raber, Oregon Health and Science University, United StatesCopyright: © 2025 Losinski, Key, Vidoni, Clutton, Morris, Burns and Watts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amber Watts, YW1iZXJ3YXR0c0BrdS5lZHU=
 Genna M. Losinski
Genna M. Losinski Mickeal N. Key
Mickeal N. Key Eric D. Vidoni
Eric D. Vidoni Jonathan Clutton2
Jonathan Clutton2 Jill K. Morris
Jill K. Morris Jeffrey M. Burns
Jeffrey M. Burns Amber Watts
Amber Watts