- 1Innovative Genomics Institute, University of California, Berkeley, Berkeley, CA, United States
- 2Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA, United States
Uterine fibroids (UFs) are the most common benign tumors of the female reproductive system, affecting 70%–80% of women by age 50. Early detection is challenging due to the absence of initial symptoms, and diagnosis primarily relies on ultrasound and magnetic resonance imaging (MRI). However, biomarker-driven approaches could enable earlier and more precise detection. This review explores emerging biomarkers and genetic factors in fibroid pathogenesis. Potential biomarkers, including PLP1, FOS, versican, LDH, and IGF-1, show promise for diagnosis and recurrence prediction. Genetic studies have identified key mutations in MED12, FH, HMGA2, and COL4A5-COL4A6, alongside genome-wide association studies (GWAS) that highlight fibroid risk loci. Interestingly, biomarkers may also be mutation-type specific, suggesting potential for more precise molecular classification. Gene therapy offers an innovative treatment approach but the genetic landscape of fibroids remains underexplored, limiting advancements in research and funding. Integrating biomarker-based diagnostics and genetic profiling could transform fibroid detection and management, reducing reliance on invasive procedures. This review highlights the urgent need for improved diagnostic tools, prognostic markers, and targeted therapies for uterine fibroids.
Uterine fibroids: a silent burden in women's health
Uterine fibroids (UFs), also known as leiomyomas or myomas, are benign tumors arising from the uncontrolled overgrowth of smooth muscle and connective tissue in the uterus. A hallmark of leiomyomas is the excessive deposition of a disorganized extracellular matrix (ECM), which normally provides structural support to tissues (1).
They are the most common benign tumors of the female reproductive system, affecting up to 70%–80% of women by the age of 50, with the highest prevalence occurring between 30 and 50 years of age. While some women remain asymptomatic, others experience a range of symptoms, including heavy menstrual bleeding, pelvic pain, frequent urination, and reproductive complications such as infertility or pregnancy loss. The symptoms often lead to limitations in daily activities, emotional distress and a reduced quality of life.
Despite their high prevalence, early diagnosis of fibroids is difficult due to the absence of detectable symptoms in the initial stages. Diagnosis is typically only possible after fibroids have grown large enough to be identified using imaging techniques such as ultrasound or MRI. Thyroid-stimulating hormone (TSH) (2) and prolactin levels (3) have been routinely used by gynecologists as part of the initial laboratory evaluation to exclude endocrine disorders that may mimic fibroid-related symptoms. While fibroids can occasionally coexist with thyroid nodules or, in rare cases, be associated with ectopic prolactin secretion, these hormones are not direct biomarkers of fibroids.
Fibroids are classified into four types based on their location within the uterus, each influencing symptoms differently (4):
• Intramural Fibroids: Most common type; they develop within the muscular wall of the uterus.
• Submucosal Fibroids: They are located just beneath the uterine lining and protruding into the uterine cavity; even small submucosal fibroids can lead to significant symptoms including heavy and prolonged menstrual bleeding.
• Subserosal Fibroids: They develop on the outer uterine surface and may exert pressure on surrounding pelvic organs.
• Pedunculated Fibroids: They are attached to the uterine wall by a stalk-like structure.
Treatment options for uterine fibroids vary based on factors such as size, location, severity of symptoms, and reproductive goals (4). Medications are used to manage symptoms by regulating hormones, reducing heavy menstrual bleeding, and shrinking fibroids. However, they do not eliminate fibroids completely. Minimally invasive procedures like uterine artery embolization (UAE) and MRI-guided focused ultrasound manage symptoms, while surgical options include myomectomy (fibroid removal while preserving the uterus) for fertility preservation and hysterectomy (complete removal of the uterus) as the only definitive cure. Although treatments through these procedures can be effective, recurrence rates remain a challenge. Studies suggest that 15%–33% of fibroids recur after myomectomy, with 10%–21% of women undergoing hysterectomy within five to ten years of myomectomy due to recurrence (5).
Approximately 40%–50% of fibroids exhibit chromosomal abnormalities, indicating a significant genetic component. Family history also plays a role; women with a first-degree relative affected by fibroids have a higher risk of developing them. Non-genetic factors influencing fibroid development include hormonal imbalances, obesity, and lifestyle choices. The age of onset and severity of symptoms can vary based on these factors. Genetically predisposed individuals may develop fibroids at a younger age and experience more pronounced symptoms, while those with non-genetic risk factors might encounter a later onset with varying symptom severity. Understanding the interplay between genetic and non-genetic factors is crucial for personalized management and treatment of uterine fibroids (1, 6–11). This review summarizes advancements in blood-based biomarkers for the diagnosis of uterine fibroids and explores the genetic factors contributing to their development. To ensure a comprehensive and up-to-date synthesis of the literature, a structured search was conducted using PubMed and Google Scholar, mainly focusing on studies published in the last 10–15 years. Search terms included combinations of “uterine fibroids”, “leiomyoma”, “genetic predisposition”, “biomarkers”, “early diagnosis”, and “non-surgical treatment”. Reference lists of key articles were manually screened to capture additional relevant studies. Studies were selected based on their relevance to genetic and non-genetic risk factors, biomarker research, and emerging therapeutic approaches.
Advancements in biomarkers for the diagnosis of uterine fibroids
Efforts to identify reliable biomarkers for the diagnosis of uterine fibroids (UFs) are an ongoing process, aiming to improve early detection and develop molecular-based diagnostic tools that complement current imaging techniques. While ultrasound and MRI remain the clinical gold standards, molecular and genetic biomarkers will offer earlier diagnosis, enhanced prognostic accuracy, and personalized therapeutic strategies. Here, we describe potential biomarkers that could be used for the diagnosis of uterine fibroids.
PLP1, an X-linked gene encoding a major myelin protein in the central nervous system (CNS) is upregulated (12) and FOS, a non-X-linked gene encoding a transcription factor in the AP-1 complex is downregulated (13) at both mRNA and protein levels in uterine fibroid samples from Asian females undergoing myomectomy or hysterectomy. While the role of PLP1 in uterine fibroids remains unclear, the downregulation of FOS has been linked to extracellular matrix (ECM) remodeling and fibrotic changes, suggesting its potential involvement in UF pathogenesis (12). Serum versican levels were also significantly lower in women with uterine fibroids compared to healthy controls in an Eastern Indian cohort (14). Since excessive ECM deposition is a hallmark of uterine fibroids, testing versican, a key ECM proteoglycan, could provide valuable diagnostic insights. Note, however, that the above studies were conducted in patients of different ethnicities, which may contribute to biomarker variability.
The successful identification of biomarkers would also facilitate the study of uterine fibroid recurrence after initial treatment. LDH and IGF-1 are two such biomarkers whose protein levels decreased two days after uterus-preserving surgeries but increased again six months postoperatively. However, no clear correlation between fibroid recurrence and the upregulation of these markers was established, as testing did not extend beyond six months (15). Nonetheless, this approach holds promise for future studies on fibroid recurrence.
UFs are frequently associated with mutations in the mediator complex subunit 12 (MED12) gene, which plays a critical role in transcriptional regulation and cell proliferation. Given the involvement of MED12 mutations in fibroid pathogenesis, biomarker profiles may vary based on mutation status. Biomarkers like HPGDS (hematopoietic prostaglandin D synthase) and CBR3 (carbonyl reductase 3) may be specifically associated with MED12-mutated fibroids, suggesting that molecular classification could refine diagnostic and therapeutic strategies (16). MED12-mutant fibroids tend to be smaller but more numerous, with a rich extracellular matrix and poor vasculature, while wild-type fibroids exhibit higher vascularization and smooth muscle proliferation (17). These distinct phenotypic differences highlight the potential for personalized therapeutic strategies based on MED12 mutation status.
Other technologies such as lipidomics provide additional diagnostic potential, with altered lipid profiles observed in UF patients (18). Urinary oxidative stress biomarkers have also been found to be upregulated in patients with fibroids, further supporting the role of metabolic changes in UF pathogenesis. Oxidative stress markers, such as lipid peroxidation (LOOH) products, advanced oxidation protein products (AOPPs), and carbonyl groups, are elevated in the sera of women with uterine fibroids, while antioxidant thiol levels are reduced, suggesting a role in fibroid development (17). Serum AOPP and carbonyl levels correlate with total fibroid weight and infertility duration, making them potential biomarkers for fibroid diagnosis and monitoring. Various other biomarkers have been investigated; however, no solid conclusions have been drawn (19).
These findings suggest that metabolic and molecular changes associated with fibroid development could be leveraged for novel diagnostic applications, particularly through blood plasma analysis. A systematic study involving diverse racial and age groups across different fibroid stages (no myoma, recurrent, and non-recurrent myoma), using both tissue and serum samples, is essential to identify a reliable and universally applicable biomarker for uterine fibroids.
Uterine fibroid genetics
Uterine fibroid genetics can be classified into two categories: somatic mutations, which are genetic changes acquired during an individual's lifetime, and germline mutations, which are inherited variations passed down from parents that may significantly influence fibroid risk. Since ethnicity plays a major role in the occurrence and symptoms of uterine fibroids, understanding germline mutations is crucial for further exploration and the development of targeted therapies. Studies indicate that women who develop uterine fibroids before the age of 30 are more likely to have a genetic predisposition, as genetically influenced traits tend to manifest earlier in life (20, 21). In this regard, patients with familial fibroids tend to present with more severe symptoms, multiple fibroids, and high VEGF-A expression (22).
Although many such mutations have been identified, no systematic study has determined the extent of their heritability. Two most frequently mutated genes are MED12 and Fumarate Hydratase (FH). With a frequency reported in the range of 50 to 85%, MED12 is the most frequently mutated gene in uterine leiomyomas. FH is known to alter a tumor suppressor mechanism for uterine leiomyomata (23). UF patients with FH mutations have an increased risk of renal cancer (24). Genetic predisposition to increased fat-free mass was found to be causally linked to a higher risk of uterine fibroids. Genes within the myocardin and cyclin-dependent kinase inhibitor 1A pathways involved in smooth muscle cell differentiation and proliferation appear to play a role in fibroid development (25). Other mutated genes include HMGA2 (encoding a high mobility group protein involved in transcriptional regulation) and COL4A5-COL4A6 (X-linked gene, encoding collagen proteins) genes (26).
Studies show that treatment response can vary based on the genetic subclass of uterine fibroids. For example, MED12 mutant fibroids are 4.4 times more likely to shrink than HMGA2 driven fibroids, highlighting the importance of genetic profiling in personalized fibroid treatment (27, 28).
Genome-wide association studies have aimed to identify genetic risk loci associated with uterine fibroids. One such study found shared genetic origins between uterine leiomyomata and endometriosis (29). However, for these findings to be truly valuable, risk loci must be identified across diverse ancestral backgrounds (30), the aberrant regulation of risk loci in uterine fibroids must be validated to explain the disease phenotype, and the identified genetic targets must be tested in vivo using cell line models or mouse models. In this regard, Buyukcelebi et al. advanced the field by integrating genetic risk loci with bulk and single-cell gene expression data, as well as serum protein levels, to identify potential molecular traits downstream of genetic risk loci. Their study found that in leiomyoma samples, smooth muscle cells, the cell type of origin for uterine fibroid tumors, contained the highest number of highly expressed GWAS target genes, with 40 percent of differentially expressed GWAS targets being selectively enriched in these cells.
A fundamental question remains regarding whether these genetic mutations are inherited or acquired during a person's lifetime. Chromosomal abnormalities are frequently observed in uterine fibroids (1, 24), further complicating the classification of genetic changes in fibroid development. Several other genes have also been linked to uterine leiomyoma development, and a multi-ethnic study identified fourteen genetic loci that may influence fibroid predisposition (7). These genes fall into three major categories: those involved in telomere length regulation, those involved in DNA damage response mechanisms, and those playing a role in genitourinary system development. Mutations or dysregulation in these genes may contribute to fibroid onset and progression.
A family history of fibroids does not necessarily guarantee that an individual will develop them, as hormonal factors also play a significant role. Both estrogen and progesterone directly stimulate fibroid growth, which becomes particularly evident during pregnancy when elevated hormone levels can trigger rapid fibroid expansion (31). Future research should focus on determining whether a common mechanistic link exists between various genetic alterations and hormonal pathways driving fibroid formation. Such findings could pave the way for targeted, personalized therapies that integrate genetic screening, biomarker-based diagnostics, and hormonal regulation strategies for effective fibroid management.
Gene therapy
Gene therapy presents an experimental but promising alternative with the potential for a lasting cure (32). To date, two main strategies have been explored in preclinical studies for uterine fibroids. The first involves suppressing the estrogen receptor in uterine fibroid cells, thereby reducing estrogen binding and receptor activity, which leads to decreased fibroid cell proliferation (11, 33, 34). The second approach utilizes targeted delivery of the herpes simplex virus thymidine kinase (HSV-TK) gene, followed by treatment with ganciclovir (GCV), which selectively induces cell death in fibroid cells (35).
One of the major challenges in developing gene therapy for uterine fibroids is identifying the appropriate target gene. This limitation may be addressed by conducting in vivo studies to identify and validate causal genes across patients of different racial backgrounds. While emerging research has begun identifying potential targets as mentioned above, these findings remain preliminary and require further validation.
On the other hand, a notable advantage of fibroid gene therapy is the potential for selective gene delivery to fibroid tissue. Once an appropriate target gene is identified, gene therapy could emerge as a viable therapeutic option. However, its clinical application remains distant, primarily due to the complex and less-explored genetic landscape of the disease, as well as the non-life-threatening nature of fibroids, which has limited research funding and focus.
Nevertheless, it is important to acknowledge the substantial impact fibroids can have on younger women's lifestyle and mental health. Living with fibroids often involves undergoing multiple medical procedures, extended recovery periods, and the emotional distress of recurrence, all of which can significantly affect quality of life (36, 37).
Discussion
Currently, uterine fibroids are diagnosed either during routine check-ups or when a patient presents with symptoms. By the time they are detected, fibroids have often already begun growing, initiating a treatment journey that primarily focuses on symptom management (38). In most cases, surgical treatment options eventually lead to either myomectomy (removal of fibroids while preserving the uterus) or hysterectomy (removal of the uterus) for long-term relief. For many women, this means facing difficult decisions, such as planning a family earlier than desired to avoid fibroid recurrence or, in severe cases, undergoing a hysterectomy at a young age. Early genetic screening, particularly for individuals with a family history of fibroids, may help assess the risk of fibroid development and enable earlier interventions. Additionally, the identification of serum and menstrual blood biomarkers has shown promise in research settings, but further validation is required before they can be adopted for routine early diagnosis and that could significantly advance the field of early fibroid diagnostics. While genetic predisposition plays a key role in the development of uterine fibroids, environmental and lifestyle factors such as vitamin D deficiency, dietary patterns and obesity may interact with genetic susceptibility to influence fibroid growth and severity. Understanding these factors allows for a more comprehensive view of fibroid pathogenesis and potential prevention strategies. Studies suggest that simple, non-invasive treatments, such as hormonal pills or addressing Vitamin D deficiency, may help slow fibroid progression if initiated early (39–42) but more clinical evidence is needed to support these preventative strategies. If such preventive measures are implemented from a young age, many women may be able to avoid surgery altogether. For those who do not respond to conventional treatments, gene therapy could offer a promising long-term solution. Analyzing fibroids removed during surgery may help identify mutational burden and could contribute to the development of preventive vaccines to reduce recurrence in future. However, this idea remains highly speculative and is not currently supported by clinical trials.
The ability to make reproductive choices without the looming pressure of fibroid-related complications would significantly alleviate the mental and emotional burden that many young women currently face. A crucial first step in this direction would be a comprehensive study of different ethnic populations to identify differentially expressed mRNAs and proteins in uterine fibroids. Such research could help uncover common fibroid pathogenesis pathways, potentially leading to targeted and personalized treatments. Breakthroughs in this area could mirror those seen in other complex disease fields (43).
Recent advancements in gene editing techniques have enabled the development of cellular and animal models for studying uterine fibroids (30, 44). Additionally, multiple ongoing mechanistic studies are shedding light on fibroid development and progression (27, 45–48). The majority of ongoing clinical trials focus on interventional treatments, yet a significant number have been terminated over the years. One of the main reasons for trial failure is poor patient accrual, indicating difficulty in recruiting enough suitable participants. This highlights the need for a more refined classification of uterine fibroid patients based on fibroid pathophysiology to ensure that treatments are tailored accordingly (Figure 1) and that clinical trials target specific patient subgroups for better outcomes.
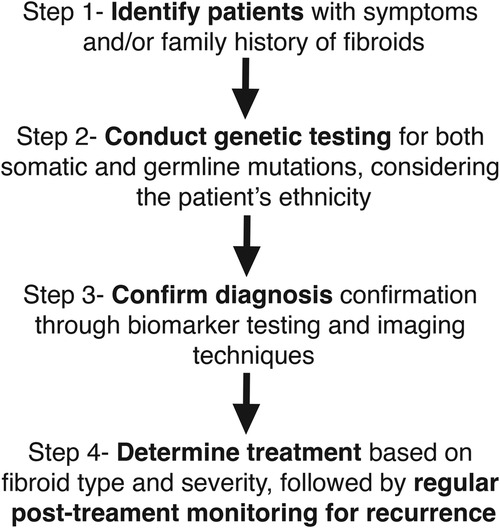
Figure 1. Integration of biomarkers and genetics in fibroid diagnosis: A proposed model. This figure illustrates a stepwise model for integrating biomarkers and genetic testing into the diagnosis and management of uterine fibroids. Step 1 involves identifying patients based on symptoms and/or family history of fibroids. Step 2 consists of genetic testing for both somatic and germline mutations, taking into account ethnic variations. Step 3 focuses on confirming the diagnosis through biomarker testing and imaging techniques. Step 4 determines treatment based on fibroid type and severity, with regular post-treatment monitoring for potential recurrence. This model highlights a personalized approach to fibroid diagnosis and management by incorporating genetic and biomarker-based strategies.
Author contributions
PM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Publication made possible in part by support from the Berkeley Research Impact Initiative (BRII) sponsored by the UC Berkeley Library.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Medikare V, Kandukuri LR, Ananthapur V, Deenadayal M, Nallari P. The genetic bases of uterine fibroids; a review. J Reprod Infertil. (2011) 12(3):181–91.23926501
2. Kim MH, Park YR, Lim DJ, Yoon KH, Kang MI, Cha BY, et al. The relationship between thyroid nodules and uterine fibroids. Endocr J. (2010) 57(7):615–21. doi: 10.1507/endocrj.k10e-024
3. Upreti R, Dray M, Elston MS. Uterine fibroid causing hyperprolactinemia and paradoxical prolactin rise with dopamine agonist: case report and systematic review. SN Compr Clin Med. (2020) 2(4):464–7. doi: 10.1007/s42399-020-00248-6
4. ucsfhealth.org. Fibroids (2025). Available at: https://www.ucsfhealth.org/conditions/fibroids (Accessed February 22, 2025).
5. Kramer KJ, Ottum S, Gonullu D, Bell C, Ozbeki H, Berman JM, et al. Reoperation rates for recurrence of fibroids after abdominal myomectomy in women with large uterus. PLoS One. (2021) 16(12):e0261085. doi: 10.1371/journal.pone.0261085
6. Yang Q, Ciebiera M, Bariani MV, Ali M, Elkafas H, Boyer TG, et al. Comprehensive review of uterine fibroids: developmental origin, pathogenesis, and treatment. Endocr Rev. (2022) 43(4):678–719. doi: 10.1210/endrev/bnab039
7. Välimäki N, Kuisma H, Pasanen A, Heikinheimo O, Sjöberg J, Bützow R, et al. Genetic predisposition to uterine leiomyoma is determined by loci for genitourinary development and genome stability. Elife. (2018 7:e37110. doi: 10.7554/elife.37110
8. Dimmer O. News Center. (2024). New genes implicated in uterine fibroid development. Available at: https://news.feinberg.northwestern.edu/2024/03/13/new-genes-implicated-in-uterine-fibroid-development/ (Accessed February 22, 2025).
9. Peloso M. Brigham On a Mission. Brigham and Women’s Hospital. (2018). Unlocking the mysteries behind uterine fibroids. Available at: https://www.brighamhealthonamission.org/2018/11/28/unlocking-the-mysteries-behind-uterine-fibroids/ (Accessed February 22, 2025).
10. Senbadejo T, Abiola I, Paemka L. Probing genetics and environmental factors underlying uterine fibroid tumorigenesis in Ghana, West Africa. Res Ideas Outcomes. (2024) 10:e116907. doi: 10.3897/rio.10.e116907
11. Hassan MH, Salama SA, Zhang D, Arafa HMM, Hamada FMA, Fouad H, et al. Gene therapy targeting leiomyoma: adenovirus-mediated delivery of dominant-negative estrogen receptor gene shrinks uterine tumors in eker rat model. Fertil Steril. (2010) 93(1):239–50. doi: 10.1016/j.fertnstert.2008.09.086
12. Cai L, Liao Z, Li S, Wu R, Li J, Ren F, et al. PLP1 May serve as a potential diagnostic biomarker of uterine fibroids. Front Genet. (2022) 13:1045395. doi: 10.3389/fgene.2022.1045395
13. Cai L, Li J, Long R, Liao Z, Gong J, Zheng B, et al. An autophagy-related diagnostic biomarker for uterine fibroids: FOS. Front Med. (2023) 10:1153537. doi: 10.3389/fmed.2023.1153537
14. Jasti P, Kumari S, Singh S, Anudeep PP. Serum versican as a potential biomarker in patients with uterine fibroids: a study from eastern India. J Family Med Prim Care. (2023) 12(8):1704–9. doi: 10.4103/jfmpc.jfmpc_320_23
15. Jacob M, Richter R, Sehouli J, David M. Evaluation of biomarkers in Myoma patients: a prospective study investigating the role of LDH, CA 125, and IGF-1 after uterus-preserving surgical therapy. Gynecol Obstet Invest. (2021) 86(1–2):100–7. doi: 10.1159/000513045
16. Liu X, Liu Y, Zhao J, Liu Y. Screening of potential biomarkers in uterine leiomyomas disease via gene expression profiling analysis. Mol Med Rep. (2018) 17(5):6985–96. doi: 10.3892/mmr.2018.8756
17. AlAshqar A, Lulseged B, Mason-Otey A, Liang J, Begum UAM, Afrin S, et al. Oxidative stress and antioxidants in uterine fibroids: pathophysiology and clinical implications. Antioxidants. (2023) 12(4):807. doi: 10.3390/antiox12040807
18. Tonoyan NM, Kozachenko IF, Frankevich VE, Chagovets VV, Stepanian AA, Adamyan LV. 3069 Analysis of the plasma lipid composition in patients with uterine Myoma and recurrent fibroids using mass spectrometry. J Minim Invasive Gynecol. (2019) 26(7):S181. doi: 10.1016/j.jmig.2019.09.343
19. Levy G, Hill MJ, Plowden TC, Catherino WH, Armstrong AY. Biomarkers in uterine leiomyoma. Fertil Steril. (2013) 99(4):1146–52. doi: 10.1016/j.fertnstert.2012.10.048
20. Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. (2013) 22(10):807–16. doi: 10.1089/jwh.2013.4334
21. Sato F, Mori M, Nishi M, Kudo R, Miyake H. Familial aggregation of uterine myomas in Japanese women. J Epidemiol. (2002) 12(3):249–53. doi: 10.2188/jea.12.249
22. Okolo SO, Gentry CC, Perrett CW, Maclean AB. Familial prevalence of uterine fibroids is associated with distinct clinical and molecular features. Hum Reprod. (2005) 20(8):2321–4. doi: 10.1093/humrep/dei049
23. Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. (2016) 34:3–12. doi: 10.1016/j.bpobgyn.2015.11.018
24. Kubínová K, Mára M, Horák P, Kuzel D. Genetic factors in etiology of uterine fibroids. Ceska Gynekol. (2012) 77(1):58–60.
25. Sliz E, Tyrmi JS, Rahmioglu N, Zondervan KT, Becker CM, FinnGen , et al. Evidence of a causal effect of genetic tendency to gain muscle mass on uterine leiomyomata. Nat Commun. (2023) 14(1):542. doi: 10.1038/s41467-023-35974-7
26. Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril. (2014) 102(3):621–9. doi: 10.1016/j.fertnstert.2014.06.050
27. Kajimura T, Serna VA, Amurgis E, Blumenfeld M, Kurita T. Patient-derived xenograft studies of fumarate hydratase (FH)-deficient uterine leiomyoma subtype. bioRxiv [Preprint]. (2022). doi: 10.1101/2022.07.27.501688
28. Goad J, Rudolph J, Wei JJ, Bulun SE, Chakravarti D, Rajkovic A. Single Cell atlas of uterine myometrium and leiomyomas reveals diverse and novel cell types of non-monoclonal origin. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.12.21.402313
29. Gallagher CS, Mäkinen N, Harris HR, Rahmioglu N, Uimari O, Cook JP, et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat Commun. (2019) 10(1):4857. doi: 10.1038/s41467-019-12536-4
30. Buyukcelebi K, Duval AJ, Abdula F, Elkafas H, Seker-Polat F, Adli M. Integrating leiomyoma genetics, epigenomics, and single-cell transcriptomics reveals causal genetic variants, genes, and cell types. Nat Commun. (2024) 15(1):1169. doi: 10.1038/s41467-024-45382-0
31. OASH | Office on Women’s Health. Uterine fibroids (2025). Available at: https://womenshealth.gov/a-z-topics/uterine-fibroids (Accessed February 22, 2025).
32. Shtykalova SV, Egorova AA, Maretina MA, Freund SA, Baranov VS, Kiselev AV. Molecular genetic basis and prospects of gene therapy of uterine leiomyoma. Russ J Genet. (2021) 57(9):1002–16. doi: 10.1134/S1022795421090118
33. Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. (2004) 191(5):1621–31. doi: 10.1016/j.ajog.2004.04.022
34. Al-Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review. Hum Reprod Update. (2006) 12(4):385–400. doi: 10.1093/humupd/dml015
35. Shtykalova S, Egorova A, Maretina M, Baranov V, Kiselev A. Magnetic nanoparticles as a component of peptide-based DNA delivery system for suicide gene therapy of uterine leiomyoma. Bioengineering. (2022) 9(3):112. doi: 10.3390/bioengineering9030112
36. Soliman AM, Margolis MK, Castelli-Haley J, Fuldeore MJ, Owens CD, Coyne KS. Impact of uterine fibroid symptoms on health-related quality of life of US women: evidence from a cross-sectional survey. Curr Med Res Opin. (2017) 33(11):1971–8. doi: 10.1080/03007995.2017.1372107
37. Fernandez H, Ardaens K, Queval I, Solignac C. Impact of uterine fibroids on quality of life: a national cross-sectional survey. Eur J Obstet Gynecol Reprod Biol. (2018) 229:32–7. doi: 10.1016/j.ejogrb.2018.07.032
38. Krzyżanowski J, Paszkowski T, Szkodziak P, Woźniak S. Advancements and emerging therapies in the medical management of uterine fibroids: a comprehensive scoping review. Med Sci Monit. (2024) 30:e943614. doi: 10.12659/MSM.943614
39. Al-Hendy A, Badr M. Can vitamin D reduce the risk of uterine fibroids? Womens Health. (2014) 10(4):353–8. doi: 10.2217/WHE.14.24
40. Islam MS, Akhtar MM, Segars JH. Vitamin D deficiency and uterine fibroids: an opportunity for treatment or prevention? Fertil Steril. (2021) 115(5):1175–6. doi: 10.1016/j.fertnstert.2021.02.040
41. Hajhashemi M, Ansari M, Haghollahi F, Eslami B. The effect of vitamin D supplementation on the size of uterine leiomyoma in women with vitamin D deficiency. Caspian J Intern Med. (2019) 10(2):125–31. doi: 10.22088/cjim.10.2.125
42. Pludowski P, Grant WB, Konstantynowicz J, Holick MF. Editorial: classic and pleiotropic actions of vitamin D. Front Endocrinol (Lausanne). (2019) 10:341. doi: 10.3389/fendo.2019.00341
43. Elguindy A, Yacoub MH. The discovery of PCSK9 inhibitors: a tale of creativity and multifaceted translational research. Glob Cardiol Sci Pract. (2013) 2013(4):343–7. doi: 10.5339/gcsp.2013.39
44. Buyukcelebi K, Chen X, Abdula F, Duval A, Ozturk H, Seker-Polat F, et al. Engineered MED12 mutations drive uterine fibroid-like transcriptional and metabolic programs by altering the 3D genome compartmentalization. Res Sq. (2023) 14(1):4057. doi: 10.21203/rs.3.rs-2537075/v1
45. McWilliams MM, Koohestani F, Jefferson WN, Gunewardena S, Shivashankar K, Wertenberger RA, et al. Estrogen receptor alpha mediated repression of PRICKLE1 destabilizes REST and promotes uterine fibroid pathogenesis. bioRxiv [Preprint]. (2024). doi: 10.1101/2024.09.09.612036
46. Paul EN, Carpenter TJ, Pavliscak LA, Bennett AZ, Ochoa-Bernal MA, Fazleabas AT, et al. Unraveling the molecular landscape of uterine fibroids, insights into HMGA2 and stem cell involvement. bioRxiv [Preprint]. (2024). doi: 10.1101/2024.04.26.591351
47. George JW, Cancino RA, Griffin Miller JL, Qiu F, Lin Q, Rowley MJ , et al. Characterization of m6A modifiers and RNA modifications in uterine fibroids. Endocrinology. (2024) 165(8):bqae074. doi: 10.1210/endocr/bqae074
Keywords: uterine fibroid, biomarkers, precision medicine, leiomyoma, genetic predisposition, early diagnosis, women's health, quality of life
Citation: Mukherjee P (2025) Genetic and biomarker approaches to uterine fibroids: toward precision medicine. Front. Glob. Women's Health 6:1581823. doi: 10.3389/fgwh.2025.1581823
Received: 23 February 2025; Accepted: 2 April 2025;
Published: 22 April 2025.
Edited by:
Gaetano Riemma, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Yong Jin, The Second Affiliated Hospital of Soochow University, ChinaCopyright: © 2025 Mukherjee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pooja Mukherjee, bXVraGVyanBAYmVya2VsZXkuZWR1
 Pooja Mukherjee
Pooja Mukherjee