- Padma Inc., Wetzikon, Switzerland
Rigid pharmacopoeial regulations constrain traditional medicine, often excluding herbs due to strict compliance. Climate change and rising global demand threaten natural resources, while slow regulatory adaptation further restricts herbal ingredients. Narrow regulatory specificity and fragile resources create a rigidity trap, where regulations fail to accommodate the dynamic nature of traditional medicine. Traditional medical systems, such as Tibetan medicine, exhibit flexibility and resilience by utilizing diverse plant species for similar therapeutic effects—the functional spectrum of a formula. Rather than adhering to rigid pharmacopoeias, monographs should reflect the full functional spectrum of herbal materials historically and empirically used. Expanding pharmacopoeial monographs to reflect functional diversity preserves traditional formulas, safeguards medical knowledge, and enhances adaptability to modern challenges.
1 Introduction
Traditional medicine systems worldwide rely on plants as their primary natural resource for medicinal preparations, requiring a steady supply of herbal materials. However, as these products enter the global health market, they face increasing challenges due to stringent pharmaceutical regulations. Simultaneously, the availability of suitable herbal materials is declining, a challenge that, at first glance, may seem unrelated to regulatory constraints. This policy brief argues that these issues are closely interconnected: viewing medicinal biodiversity merely as a commercial and technical resource, disconnected from its ecological context, limits our ability to fully benefit from nature’s medicinal potential and to recognize how vulnerable this capacity is in the face of global environmental change (Theodoridi et al., 2023).
A key factor in this discussion is that many plants exert similar physiological effects, a principle long recognized in traditional medicine. This policy brief explores the concept of grouping medicinal plants based on their functional spectrum—an approach that could help navigate rigid pharmaceutical regulations. However, for this concept to be effectively applied, the classification and description of medicinal plants in traditional medicine textbooks must first be reconsidered.
Rigid pharmacopoeial structures limit traditional medicine’s adaptability, particularly as ecological challenges increase. This brief advocates for expanding herbal monographs to include all traditionally recognized species and emphasizing functional integrity over strict botanical classifications. Terminology should shift from “substitutes” to recognizing a broader functional spectrum. Integrating these updates into official regulations will enhance flexibility and preserve traditional medicine’s relevance.
2 Modern pharmacopoeias and the regulation of herbal materials in the context of ecological constraints
Herbal materials used in medicines and food supplements are subject to regulation by national health authorities. When these products enter the global health market, they encounter increasing challenges due to stringent pharmaceutical regulations. Similar regulatory frameworks exist in food law, given the comparable implications for safety and efficacy; however, for the purposes of this policy paper, the focus will remain on pharmaceutical regulations.
At the same time, the onset of the Anthropocene era introduces new challenges to the availability and sustainability of natural ingredients. Existing regulatory frameworks must adapt to these ecological constraints to ensure long-term sustainability. Currently, natural ingredients are caught in a trap between two opposing forces—on one hand, dwindling resources due to environmental pressures, and on the other, increasingly strict pharmaceutical regulations. This dynamic raises concerns about the long-term feasibility of herbal medicine production under the current regulatory paradigm.
2.1 Pharmacopoeias as national regulatory framework
Modern nation-states, along with select supranational organizations, establish official pharmacopoeias that serve as the primary regulatory framework for the pharmaceutical industry. For instance, the Council of Europe is responsible for publishing the European Pharmacopoeia, while countries such as the United States, China, and India have developed their own national pharmacopoeias. Additionally, some pharmacopoeias include monographs on herbal components and formulations used in traditional medical systems.
Monographs included in official national pharmacopoeias are legally binding and establish quality standards for active ingredients. These monographs provide regulatory authorities with the means to mandate and enforce compliance with precisely defined pharmaceutical standards. In the case of medicinal plants, regulations specify not only the plant species and the parts used but also the parameters that define their quality. This ensures consistency and safety in the use of herbal materials within medicinal products.
2.2 Increasingly rigid regulatory frameworks
Beyond national pharmacopoeias, a multitude of additional regulatory frameworks govern the production and distribution of pharmaceuticals. As an intergovernmental organization, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals (ICH) (www.ich.org) establishes internationally recognized “good practice” guidelines, including Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), and Good Distribution Practice (GDP). When it comes to the sourcing of herbal materials, Good Agricultural and Collection Practices (GACP) provide further regulatory oversight.
Other regulations also impact the use of herbal materials, particularly those aimed at protecting endangered species. For example, the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) (www.cites.org) sets restrictions on the harvesting and trade of certain plant species. Additionally, various positive and negative lists determine which plants may be legally used for medicinal or nutritional purposes. A notable example is the ASEAN negative list of substances (ASEAN, 2016), which explicitly prohibits the use of certain natural products.
As a result, an increasing number of traditional medicinal substances are being restricted or even completely banned for various regulatory, ecological, or safety-related reasons. Examples of such restricted substances include agarwood, musk, aconite, cannabis, bear bile, and betel nut, among others. These growing constraints pose significant challenges for traditional medicine, limiting access to ingredients that have been historically significant in various medical traditions.
2.3 New global challenges and static regulations
A major challenge facing traditional medicine today is the dwindling supply of essential medicinal plants due to climate change, overharvesting, and habitat loss. The combined effects of environmental shifts and rising global demand are placing unprecedented pressure on natural resources. Urgent action is required to ensure the sustainability of these vital plant species. Early indicators of this phenomenon are already evident: in some cases, plants that were once abundant and productive in specific regions now exhibit lower yields, have disappeared entirely, or have been replaced by other species. Climate change also affects the timing of harvests, the concentration of essential oils, and the size and characteristics of flowers and fruits. In certain cases, the profile of active compounds has shifted compared to previous years (Mishra and Sahu, 2022).
While nature adapts dynamically to these changing conditions, regulatory frameworks remain rigid, designed to enforce stable and consistent standards without accommodating environmental fluctuations or resource scarcity. As a result, the availability of medicinal plants for pharmaceutical use is increasingly constrained. Several examples from the European regulatory landscape illustrate this issue:
• Carthami flos (safflower): Currently, no safflower available on the market complies with the flavonoid spectrum prescribed by the European Pharmacopoeia. This is largely because growers prioritize safflower oil production, leading to variations in flower color and composition.
• Strychnos nux-vomica: The European commission recently lowered the permissible aflatoxin limit (European Commission, 2023) to a level that naturally dried Nux-vomica nuts cannot meet, effectively restricting its use.
• Foeniculum vulgare (fennel): The European Medicines Agency (EMA) has imposed restrictions on the medicinal consumption of fennel and other plants containing estragole due to concerns regarding the potential carcinogenicity of its essential oils (EMA, 2023).
Amending pharmacopoeial monographs is a slow and complex process, creating a fundamental conflict between two opposing forces: on one side, regulations that demand fixed standards—such as monographs and good practice guidelines—and on the other, the continuously adapting nature of medicinal plants. The rate of plant evolution far outpaces the speed at which regulatory frameworks can follow. As a result, those involved in the sourcing and use of herbal raw materials find themselves constrained by an increasingly restrictive system that struggles to accommodate the realities of a changing world, they are caught in the rigidity trap (Figure 1).
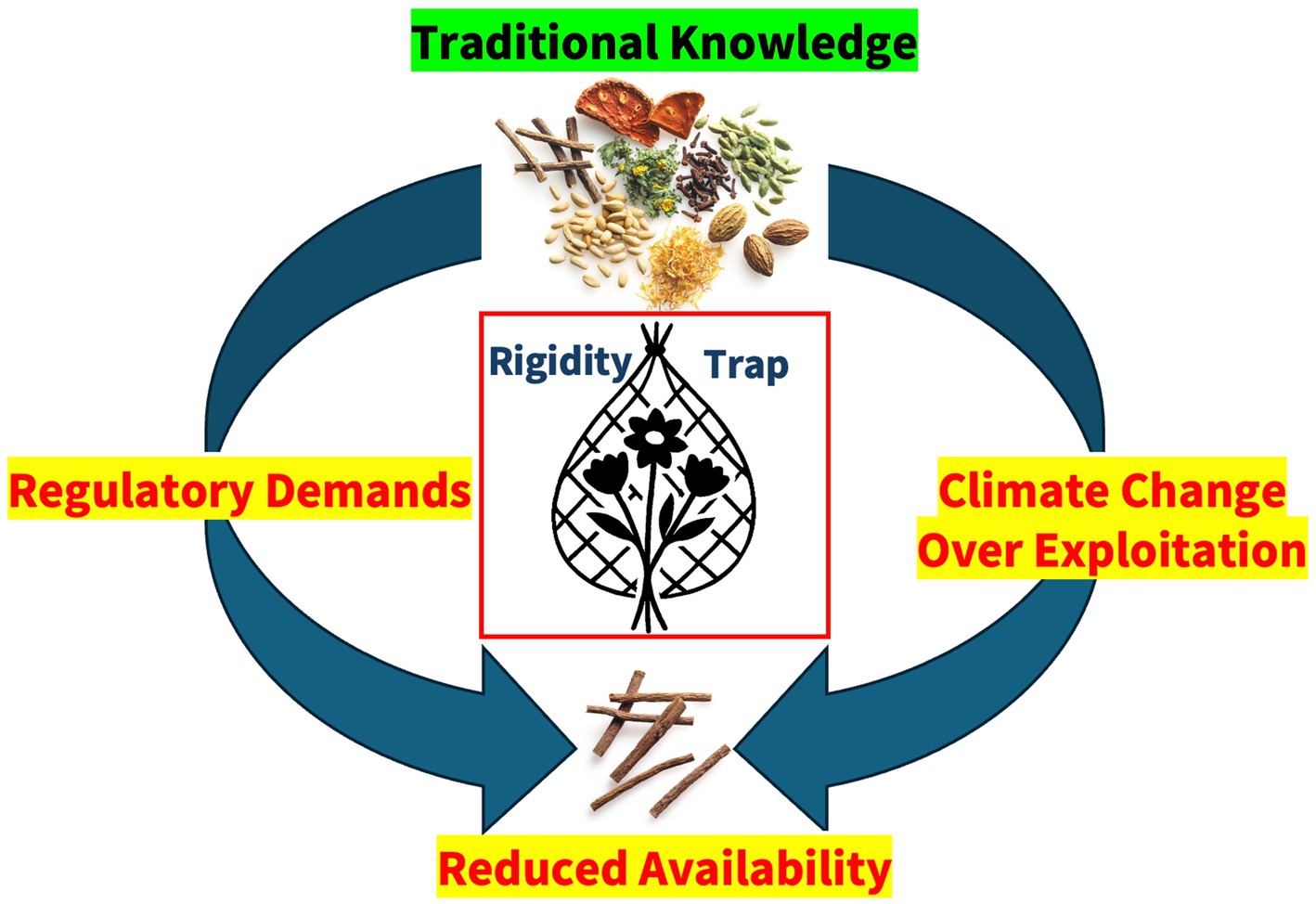
Figure 1. The rigidity trap: natural ingredients are caught in a trap between two opposing forces—on one hand, dwindling resources due to environmental pressures and exploitation, and on the other, increasingly strict pharmaceutical regulations.
3 The functional spectrum of plants
In an ideal pharmaceutical model, modern synthetic drugs are composed of a single, well-defined molecule with a specific mechanism of action. In contrast, herbal preparations inherently consist of a complex mixture of multiple chemical compounds, making them multicomponent drugs (Rostock and Saller, 2021). This complexity can be characterized in two key ways:
• Structural composition: Herbal mixtures consist of individual plants or component blends, often in low concentrations, categorized by chemical structure or botanical classification.
• Functional properties: These mixtures act through multiple pathways, exerting diverse effects with varying efficacies, engaging multiple drug-or organism-related interaction pathways. They exhibit pleiotropic properties, meaning they act through multiple independent mechanisms simultaneously. Their multi-target action profile allows them to exert therapeutic effects at different levels and through diverse biological pathways (Gertsch, 2011). Such herbal formulations create a functional signature (Gerke, 2018), making them valuable for disease management, prevention, and use as adaptogens or systemic medicines.
Despite differences in morphology and structural composition, many plant species display similar or even identical functional patterns, a phenomenon known as botanical plasticity (Schwabl and van der Valk, 2019). One possible explanation for this is the co-evolution of plants and animals, where diverse plant species have developed the ability to trigger analogous physiological responses in humans and other organisms. For instance, different plant species may share anti-inflammatory, antimicrobial, or digestive properties. Such a group of plants with overlapping action profiles can be referred to as a functional spectrum.
The concept of functional descriptions is not exclusive to modern biochemical and physiological mechanisms; it is also deeply embedded in the functional logic of traditional medical systems. A growing body of research investigates whether functional descriptions derived from traditional medical systems align with functional spectra identified through modern scientific methodologies (Dhondrup et al., 2020). Understanding these correlations could provide valuable insights for integrating traditional botanical knowledge into contemporary medical frameworks.
3.1 From specificity to flexibility
One constant across the diversity of traditional medicinal practices is the time-tested use and adaptation of natural resources. Throughout history, plants have been utilized in a multitude of ways across different cultures and historical periods. Traditional medical systems have endured for centuries, demonstrating both resilience and adaptability in the face of resource constraints and environmental fluctuations (Kloos and Blaikie, 2022). The materia medica of any given medical tradition exhibits significant regional variation, with different areas employing distinct plant species to achieve similar therapeutic goals. This diversity effectively expands the functional spectrum of medicinal plants.
Tibetan medicine (Sowa Rigpa) serves as a compelling case study of a medical system that has continuously evolved while maintaining resilience in the face of adversity. Instead of relying on a single botanical species, Tibetan medicine has historically grouped ingredients by their functional properties, allowing for substitutions within the same therapeutic category. This reflects the core concept of the functional spectrum, which acknowledges the adaptability of traditional medical systems by recognizing that different plant species can serve comparable therapeutic functions.
At its foundation, this system employs a flexible approach to herbal materia medica, leveraging botanical plasticity to ensure the continued availability of key medicinal ingredients. A notable example illustrating this functional approach is the Tibetan medical ingredient “a gar ru,” which encompasses multiple, botanically unrelated species that transcend both species and regional boundaries (Schwabl and van der Valk, 2019). The functional spectrum of a gar ru includes:
• Aquilaria spp. (agarwood),
• Cinnamomum spp. (cinnamon wood),
• Syringa spp. (lilac wood),
• Caryopteris mongholica (Bunge) (Mongolian bluebeard),
• Carum carvi (L.) (caraway seeds).
This functional approach enables Tibetan medicine to maintain therapeutic efficacy despite environmental changes, trade restrictions, or resource scarcity, demonstrating a model of medical adaptability that could offer valuable insights for modern regulatory frameworks.
4 Recommendation: expand herbal monographs to acknowledge the functional spectrum
Modern formula textbooks and herbal pharmacopoeias on Asian medicine often adopt the standardized format of official pharmacopoeias, presenting monographs of ingredients and formulations in a style that mirrors conventional pharmaceutical documentation (Arya, 1998; Dawa, 1999; Dawa, 2009; Kletter and Kriechbaum, 2001). In this format, a traditionally recognized herbal substance is linked to a single, botanically defined species.
While this approach aims to bring clarity, it inadvertently suppresses knowledge by imposing a rigid framework that does not reflect the full functional spectrum of traditional medicine. Traditional herbal systems have historically employed multiple plant species for the same therapeutic purpose, varying across regions, time periods, and environmental conditions. However, by adhering strictly to single-species classifications, modern pharmacopoeias limit the understanding of traditional knowledge and restrict the adaptability of traditional medicine, particularly in the face of resource shortages due to climate change and biodiversity loss.
Rigid pharmacopoeial structures erode flexibility and limit the evolution of traditional medicine, further reinforcing the rigidity trap. To prevent this loss and preserve the richness of traditional materia medica, textbooks and regulatory frameworks should expand herbal monographs to incorporate a broader and more functional understanding of traditional medicinal ingredients. The following changes should be considered:
• Incorporating the full range of traditionally recognized ingredients: Instead of listing a single botanical species, pharmacopoeias should document all empirically used species that serve the same function, ensuring that traditional medical knowledge is preserved in its full scope. When associating traditional ingredients with botanical species, pharmacopoeias should include as many known variations as possible, recognizing the full functional spectrum of ingredients. All practically used species should be named to leverage their botanical plasticity.
• Emphasizing functional integrity: The functional properties of formulas should be prioritized, beginning with the traditional naming of the formula, describe the ingredients in traditional terms rather than rigid modern botanical classifications.
• Reframing terminology: Instead of categorizing alternative species as “substitutes” or “replacements” (Boesi, 2005; Czaja, 2018; Sabernig, 2011), monographs should recognize them as integral components of the functional spectrum. The focus should shift from rigid specificity to embracing the richness and diversity of the materia medica.
Nevertheless, the structure of the consensus-oriented pharmacopoeia should be preserved, particularly by clearly identifying the expert group responsible for drafting or revising each monograph. Assigning authorship and responsibility not only reinforces accountability but also serves as an important safeguard against the inclusion of anonymous, unverified, or artificially generated content. This transparency helps maintain the integrity of the pharmacopoeial standard in an era increasingly shaped by digital and AI-assisted knowledge production.
In a best case scenario these updates should also be incorporated into official pharmacopoeias and regulatory guidelines, allowing for greater flexibility in the classification and regulation of herbal ingredients. Regulatory frameworks for traditional herbal medicine should permit context-based ingredient selection, taking into account regional, ecological, and environmental factors rather than imposing one-size-fits-all standards.
In the Anthropocene era, it is imperative to preserve and sustain the empirical, time-tested knowledge of traditional medicine (Glover, 2021). Emerging scientific disciplines, such as network pharmacology, offer promising new approaches that align with functional spectrum theory and can help bridge the gap between traditional and modern medical paradigms (Zhang et al., 2019). Looking ahead, the wealth of knowledge embedded in traditional medicine may prove crucial in addressing pressing global health challenges, such as the antibiotic resistance crisis, the rise of chronic and multi-morbid diseases, and the need for more adaptable therapeutic strategies. A functional approach to materia medica enables traditional medicine to adapt, preserving its knowledge while ensuring relevance in modern healthcare.
Author contributions
HS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author was advisor to the board of PADMA Inc.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
ASEAN. (2016). Guiding Principles for Inclusion into or Exclusion from the Negative List of Substances for Traditional Medicines (version 5). Available online at: https://asean.org/wp-content/uploads/2017/09/ASEAN-Guiding-Principles-for-Negative-List-TM-V5.0-with-disclaimer.pdf (Accessed October 15, 2024).
Czaja, O. (2018). The substitution of Materia Medica in Tibetan medicine: an inquiry into traditional Tibetan treatises. East Asian Sci. Technol. Med. 46, 119–212.
Dhondrup, W., Tidwell, T., Wang, X., Tso, D., Dhondrup, G., Luo, Q., et al. (2020). Tibetan medical informatics: an emerging field in Sowa Rigpa pharmacological & clinical research. J. Ethnopharmacol. 250:112481. doi: 10.1016/j.jep.2019.112481
EMA–European Medical Agency (2023). Public statement on the use of herbal medicinal products containing estragole. EMA/HMPC/137212/2005 Rev 1 Corr 1. Available online at: https://www.ema.europa.eu/en/documents/other/public-statement-use-herbal-medicinal-products-containing-estragole-revision-1_en.pdf (Accessed March 23, 2025).
European Commission (2023). Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32023R0915 (Accessed March 23, 2025).
Gerke, B. (2018). The signature of recipes: authorship, intertextuality and the epistemic genre of Tibetan formulas. Revue d’Etudes Tibétaines 45, 178–220.
Gertsch, J. (2011). Botanical drugs, synergy, and network pharmacology: forth and back to intelligent mixtures. Planta Med. 77, 1086–1098. doi: 10.1055/s-0030-1270904
Glover, D. M. (2021). Traditional medicines in a global economy: resource sustainability and resilience in the traditional Tibetan medical practice of ingredient substitution. Hum. Ecol. 49, 33–42. doi: 10.1007/s10745-020-00198-6
Kloos, S. T., and Blaikie, C. (2022). Asian medical industries: Contemporary perspectives on traditional pharmaceuticals. New York: Routledge.
Mishra, S., and Sahu, B. (2022). Pharmacognosy and climate change: impact on medicinal plants and conservation efforts. History Medicine 8, 396–412. doi: 10.17720/2409-5834.v8.2.2022.042
Rostock, M., and Saller, R. (2021). Phytotherapie und “herbal medicine”. Complement. Med. Res. 28, 281–283. doi: 10.1159/000518339
Sabernig, K. (2011). The substitution of rare ingredients in traditional Tibetan medicine on the basis of classical Tibetan texts, their use in modern formularies, and a case study from Amdo/Qinghai. Curare 34, 83–96.
Schwabl, H., and van der Valk, J. M. A. (2019). Challenging the biomedical notion of ‘active substance’: the botanical plasticity of Tibetan medical formulas. Himalaya 39, 208–218.
Theodoridi, S., Drakou, E. G., Hickler, T., Thines, M., and Nogues-Bravo, D. (2023). Evaluating natural medicinal resources and their exposure to global change. Lancet Planet Health 7, e155–e163. doi: 10.1016/S2542-5196(22)00317-5
Keywords: Sowa Rigpa, Tibetan herbal formulas, materia medica, Asian medicine, pharmacopoeia, substitution, functional spectrum
Citation: Schwabl H (2025) Escaping the rigidity trap: the functional spectrum of Tibetan formulas. Front. Hum. Dyn. 7:1537802. doi: 10.3389/fhumd.2025.1537802
Edited by:
Stephan Kloos, Austrian Academy of Sciences (OeAW), AustriaReviewed by:
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaEdoardo Andrea Cutolo, University of Verona, Italy
Copyright © 2025 Schwabl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Herbert Schwabl, aC5zY2h3YWJsQHBhZG1hLmNo
 Herbert Schwabl
Herbert Schwabl