- 1Department of Social Anthropology, University of Edinburgh, Edinburgh, United Kingdom
- 2Daknang Herbal Products, Riga, Latvia
Attempts to facilitate Sowa Rigpa practice in European countries are fairly recent and mostly happen under precarious circumstances, especially since the restrictive legal environment severely limits access to Sowa Rigpa medicinal preparations. Another issue is the incompatibility of common Sowa Rigpa pharmaceutical preparations in the form of powders and pills with modern lifestyles and tastes that, simple as it may seem, presents a major practical challenge for Tibetan medicine in the west. To remedy these limitations and incompatibilities, some more innovative ways of medicine production emerge that are supposed to increase accessibility. Looking at small-scale transnational collaborations between practitioners, laboratories, and companies as complex multi-sited entanglements, this paper follows the emergence of Daknang Herbal Products as a low-key industrial aspiration between Latvia and Nepal that attempts to realize calls for innovation and seeks to explore novel paths of engaging Sowa Rigpa medicinal preparations as food supplements in European contexts. By examining Daknang’s path toward the establishment of Sowa Rigpa pharmaceuticals as self-medicated reformulated extracts produced in Europe, this paper addresses the questions to what extent Sowa Rigpa medicines need to be transformed to enter western food supplement markets, and what kind of concerns and moral discourses about ‘negative aspirations’ surround the innovation of established forms of medicinal preparations. The aim is to show that looking at transnational business collaborations offers interesting perspectives for the Asian medicine industry that tend to be overlooked due to their small-scale nature, but which are nevertheless involved in the innovation of Asian medicines for global markets.
1 Introduction
Sowa Rigpa,1 or Tibetan medicine, draws heavily on its medicinal compounds—usually in pill or powder form—as one of its therapeutic options. It is common practice to offer a prescription of medicines to all patients and the medicinal treatments often take precedence in the overall therapeutic action.2 This therapeutic practice pattern, stemming from a strong textual and historic specialization in compound medicines, is rooted in the abundance of herbs and other potent medicinal substances from the Himalayan Mountain range.3 But it is also at the bottom of major frictions in its industrialization. On the one hand, the demand for herbs and other substances causes overharvesting and a general decline in the quality of the same (Craig, 2011; Kloos, 2015). On the other hand, the reliance on medicinal preparations that are produced by pharmacies in Asia, and consist of plant, mineral, or animal ingredients mainly native to Asian countries raises additional challenges for spreading the practice outside of Asia.
In many European countries, medicinal herbal products that have not been registered according to the EU Directive on Traditional Herbal Medicinal Products (DTHMP) 2004/24/EC are illegal and their prescription is prohibited. Whoever trespasses these laws through their import, prescription and sale risks substantial fines from customs or local authorities. Despite these precarious circumstances, Tibetan medicine including the prescription of medicinal compounds from Asian countries is practiced widely throughout Europe in legal grey zones or simply ‘under-the-radar’ both by resident and itinerant doctors.4 Apart from these extensive legal problems and limitations that Tibetan medicine encounters in European contexts, another challenge is the incompatibility of common Sowa Rigpa pharmaceutical preparations in the form of powders and pills with modern lifestyles and tastes. As patients in this context are not commonly used to the bitter tastes of medicines, they are likely to experience aversion to the mainly herbal compounds. This simple feature, while originating in the very ‘natural ingredients’ that are much appreciated in postmodern ideas of a gentle and benevolent nature that promote unprocessed foods (Coward, 1989; Siahpush, 1998) and thus contribute to the appeal of Tibetan medicine to lifestyle conscious western patients, also presents a major practical challenge for Tibetan medicine in the west.
Because of these insecurities and practical frictions that stand in the way of Sowa Rigpa’s spread on a global level some more innovative ways of medicine production emerge that are supposed to increase accessibility to the medical tradition not only through legitimizing preparations but also by producing them in a culturally adjusted form. Such efforts to adapt Sowa Rigpa to new circumstances and lifestyles are often transnational in nature. If undertaken with expertise and thoroughness, they can involve intimate collaborations between Sowa Rigpa doctors from countries with a long history of practice as well as companies, laboratories or production units in western countries. Whereas the task of the former is contributing knowledge, expertise, transmission, blessing, and other forms of efficacy as described in textual sources, the latter engages in adapting, producing, reinterpreting, standardizing, legalizing and marketing pharmaceutical products registered or declared as medicines, supplements, or foods produced in industrial settings in the west.
Such development efforts are often inspired by or go together with the Dalai Lama’s continuous calls for research (zhib ‘jug byas)5 and investigation (brtag dpyad byas) through means of modern science that could lead to modernized and new or extended medicinal knowledge (rig pa gsar pa) (Dalai Lama, 1992, 2006; see also Kloos, 2015, 125). These calls build a moral framework for many practitioners and also an international set of entrepreneurs who aspire to develop Tibetan medicine for a global market. However, the process of innovating and adapting products to international markets usually also involves substantial reinterpretation and modernization of products and production processes that can evoke fears of losing efficacy and eventually cause harm to Sowa Rigpa’s reputation. If such aspirations are established within commercial settings and seem ambitious in the sense that they go beyond the common practice and product framework, they are easily labeled as negative and practitioners accused of ‘immoral profiteering’ by fellow doctors or their social community. Yet, without denying its clear involvement in commercial activities of a capitalist and political nature (Janes, 1999; Craig and Adams, 2008; Gerke, 2019; Kloos et al., 2020), that “both trouble and reinforce” stereotypes of “beyond politics and profit” (Craig et al., 2019, 177), it is important to note that Sowa Rigpa’s strong moral foundations still shape the extent and direction of its commercialization and affect how practitioners navigate their involvement in business activities and questions of efficacy more broadly (Saxer, 2013; Kloos, 2020; Blaikie and Craig, 2022).
Looking at small-scale transnational collaborations between practitioners, laboratories, and companies as complex multi-sited entanglements, this paper follows the emergence of Daknang Herbal Products as a low-key industrial aspiration between Latvia and Nepal that attempts to realize calls for innovation and explores novel paths of engaging Sowa Rigpa medicinal preparations as food supplements in European contexts. By examining Daknang’s path toward the establishment of Sowa Rigpa pharmaceuticals as self-medicated reformulated extracts produced in Europe and its relation to transformations of Sowa Rigpa pharmaceuticals and production processes, this paper addresses the questions to what extent Sowa Rigpa medicines need to be transformed to enter EU food supplement markets, and what kind of concerns and moral discourses about ‘negative aspirations’ surround the innovation of established forms of medicinal preparations. The aim is to show that looking at transnational business collaborations offers interesting perspectives for the Asian medicine industry that tend to be overlooked due to their small-scale nature, but which are nevertheless involved in the innovation of Asian medicines for global markets.
To provide an overview of recent pharmaceutical developments in Sowa Rigpa that can be classified as reformulations, we will introduce the concept of reformulation as established by Pordié and Gaudillière (2014) to critically analyze Daknang’s attempts at reinterpreting Tibetan medicines as supplements for western tastes, lifestyles and ideals, and establishing itself as a brand6 catering to European customers. Thereby, Daknang extracts will be discussed as situated within a spectrum of innovation that follows certain ‘traditional’ notions of efficacy on the one hand, and other more modern concepts of quality and medicinal preparations on the other and we suggest that they can be thought of as bridging the two Sowa Rigpa medicinal categories of alcohol preparations (sman chang) and essence extracts (bcud len) while being reinterpreted rather than invented. Finally, we will address aspects of morality, efficacy and quality, and use the example of Daknang to take a more nuanced look at why innovations are generally viewed with skepticism, especially by Sowa Rigpa doctors themselves and to some extent by their local communities and patients.
This text is based on a collaboration and co-production of knowledge and text between Patricia Mundelius, an anthropologist who has been following the Sowa Rigpa practitioners involved in the development of Daknang within multiple field research periods in Nepal and Europe between 2014 and 2025, and Julija Sprisevska, a Tibetan medicine practitioner from Latvia, medical MD and co-establisher of Daknang Herbal Products. While this collaboration involves intimate commitments to the project of Daknang and its aims, we clearly intend to provide a complex and self-critical picture of the reformulations and intentions involved. The second author’s contribution is therefore focused on the technical aspects of describing (and categorizing) Daknang’s history, extracts and production process and its relation to classical sources. The first author contributed the ethnographic data and critical analysis surrounding the themes of innovation, reformulation, and morality. This text is thus largely based on ethnographic research by the first author on morality and commercialization in Kathmandu’s Sowa Rigpa as well as on transnational Sowa Rigpa practices between South Asia and Europe, including multiple interviews with around 40 practitioners from Nepal, India, and Europe, as well as other people involved in the practice, and hundreds of observations of Tibetan medicine consultations in Europe and Nepal.
2 Reformulating and innovating Asian medicines
As major assets for international markets and in line with the global trend of pharmaceuticalization (Nichter, 1996; Petryna and Kleinman, 2006; Busfield, 2010), Asian medicines have been subjected to extensive modifications over the past decades. These range from a standardization of ingredients and their proportions for mass production, to comparatively moderate external adaptations in the form of modified packaging and labeling (Gerke, 2019; Wang, 2020; Blaikie and Craig, 2022). The most extensive and overt transformations and specific product innovations happen when medicinal preparations—their ingredients, presentation and general ‘social life’ (Whyte et al., 2002)—are reformulated, reinvented and/or newly discovered. This process is often linked to the access of new markets or perceived changing health issues and usually involves fully commercialized production cycles with elaborate marketing strategies, consumer-oriented product designs and intellectual property protection. Pordié and Gaudillière (2014) first coined these extensively transformative production cycles within the ayurvedic industry as ‘reformulation regimes’ whose aims are “(a) to homogenize and control conventional preparations that are formulations containing as many as several dozen ingredients; (b) to simplify formulations in order to adapt them to mass production […] and to define therapeutic indications that combine, to some extent, ayurvedic and biomedical descriptions of the diseases; and (c) to change the formulations to adapt them to the global market” (Pordié and Gaudillière, 2014, 63). As reformulated and translated over-the-counter products (OTC), medical preparations become independent of the clinical practice where they originate from. A characteristic feature of these products is the combination of different medical paradigms, from biomedically defined conditions or symptoms to a ‘prospecting’ of relevant classical texts for corresponding conditions and suitable prescriptions. These are then listed and engineered into a new drug by recombining or simplifying ingredients and set up for testing and production. This process of drug discovery as ‘reverse engineering’ thus involves complex translations and interpretations and multiple shifts between epistemologies, nosologies and aetiologies that are not self-evident as is emphasized (Pordié and Gaudillière, 2014, 65–66). Instead, all of these processes demand critical study of differences and links between epistemologies and substantial time and other resources.
These new preparations are still marketed as Asian medicines and, as reformulated compounds or single-ingredient extracts, they mostly exhibit a clear distinction or ‘critical alterity’ to the main biomedical mode of harnessing isolated molecules as active ingredients. Yet, as the case of Chinese Propriety medicine demonstrates, “the potentially critical alterity of any formerly scholarly traditional medicine is more likely to be lost in those fields of health care that are both highly commercialized and polarized by the biomedical imperative” (Hsu, 2009, 112) and there is no clear boundary between ‘traditional’ Asian medicines and ‘modern’ biomedical drugs. Rather, preparations evolve alongside a spectrum, some more closely tied to what is known as ‘classical formulas,’7 others more apparently fitting into the reformulation regime and starkly contrasting their origin in design, representation and ingredients.
In Sowa Rigpa, pharmaceutical innovation also falls within a spectrum ranging from more cautious and conventional adaptation of existing formulas to the invention of new formulas or the manufacture of herbal products and dietary supplements. Most Sowa Rigpa medicines are compounds that consist of three to over a hundred plant, animal, or mineral ingredients and can be prepared as various medicinal preparations. Ingredients are classified according to several parameters of which the six tastes (ro)8 and eight potencies (nus pa)9 are most important as well as the basic differentiation of substances as either warming (drod) or cooling (bsil) in strength (stobs). These parameters provide the possibility for a systematic substitution as doctors or pharmacists can exchange ingredients with the same potency and taste if necessary. On the one hand thus, reformulations in the form of below-the-radar innovations (Madhavan, 2017) have been a more or less tacit and common part of the medical practice since its establishment. This is done by adding and substituting ingredients without losing the characteristics of the formula or changing measurements of ingredients according to the individual needs of a patient which combine factors such as personal constitution, age, or diet. For most efficacious treatment results, practitioners can add substances to existing formulas in a practice called kha tsar (kha tshar), but also just respond to the pragmatics of a medical practice that is highly dependent on the availability and affordability of medicinal ingredients and choose substitutes (tshab) for rare or unaffordable ones (Czaja, 2017). Tibetan medicine thus exhibits a kind of inherent versatility through its highly dynamic and adaptable practice of medicine compounding (sman sbyor) that exists in a continuous exchange with conditions and circumstances (Gerke, 2018, 180). As a consequence of this fundamental flexibility, Blaikie (2013, 2015) and Gerke (2018) also emphasize that there was never something like a uniform Sowa Rigpa medicinal tradition that involved a fixed set of identical preparations; but rather a wide variety of coexisting formulations for the same medicinal products that appear from different localized currents of tradition as assemblages of heterogeneous components manufactured according to different formulary texts and oral transmission lineages, and again adapted to socio-economic and ecological realities. However, such adaptation practices, even though they might fall into the category of ‘innovations,’ are still a very traditional form of practice that allowed Sowa Rigpa to spread to other countries with different climatic conditions and make use of the native resources. They have helped the medical system to be fluid, locally and individually adaptable while keeping its steady core of a common outline.
Beyond such ‘traditional innovations,’ reformulations commonly happen with an engagement of the techniques, concepts and mechanisms of modern science. And thus, Sowa Rigpa has undergone significant modernization and commercialization processes in most of its practice areas since the 1980s and is now considered one of the largest Asian medical industries (Kloos et al., 2020). Craig (2011) and Kloos (2015) argue that an engagement with modern science is also required when it comes to preserving Tibetan medicine by proving its efficacy and safety. Here, science is considered to be a tool that can strengthen the appeal of Tibetan medicine to a wider clientele who needs western scientific tests and explanations to trust and understand its value.
Likewise, the creation and innovation of Sowa Rigpa medicines and herbal products through modern technologies can be seen as a necessity that fulfils certain objectives connected to the preservation and spread of the medical system. These are to adapt the practice and its pharmaceuticals to different patient groups, sometimes within new social, ecological or legal conditions; to support Sowa Rigpa practice by generating profits that can be reinvested to subsidize prescription drugs or other necessary clinical or pharmaceutical purposes; as well as to promote Tibetan medicine internationally and spread awareness of the existence of this lesser known medical system and create some interest in the clinical practice and medical treatments for potential patients (Kloos, 2020, 173–174). These objectives are both capitalist and humanitarian in nature and are pursued, for instance, by the Dharamsala Men-Tsee-Khang as the largest institution for the study and research of Tibetan medicine in exile that has been modernizing its medicine production since the 1980s (Kloos, 2010, 2015). With its Sorig line of cosmetics, herbal teas, incense and a range of herbal supplements including some OTC medicines, the Men-Tsee-Khang demonstrates a leading role in developing and reformulating herbal products (Gerke, 2012b; Kloos, 2020). Marketed according to easily accessible symptom-oriented indications in Tibetan and English language, these products are geared toward national and international markets and are sold throughout the world via the internet or during international medical tours. Sorig products are surely not the only OTC Sowa Rigpa health products that have been created in recent decades; of noteworthy mention are Cheezheng’s pain relief plasters (Saxer, 2013, 185), the burgeoning commercial trade of precious pills (rin chen ril bu) which are now sold as colorfully packaged OTC tonics or ‘souvenirs from Tibet’ (Craig and Adams, 2008; Gerke, 2019), or the numerous new Sowa Rigpa health products developed and sold by individual small institutes and clinics. Yet, Sorig products have achieved an international outreach that is likely unmatched by other Sowa Rigpa products.
Within European markets, the Swiss company Padma AG has a leading role in creating Tibetan ‘alternative pharmaceuticals’ that are registered as food supplements or medicines and follow modern scientific ideas of pharmaceuticals (van der Valk, 2017). Products are recomposed according to Swiss and EU regulations and sold in capsule form like any other “ordinary Swiss pharmaceutical product” (Hofer, 2014, 62). Padma chose to blend into European supplement markets while successively attempting to establish their products as a choice for chronic ailments that tries to meet the level of biomedical medicines, fully tested and legally acknowledged (Schrempf, 2015, 304-5). However, establishing Asian medicines legally on European markets is not easy and the moderate success of companies selling pharmaceuticals and/or supplements has been documented to rely on multiple historical and cultural links established over long globalization periods (Kudlu and Nichter, 2019). In contrast to other Asian medicines, such as Chinese medicine or Ayurveda, Tibetan medicine arrived fairly recently in the west with an increase in globalizing activities since around the 1970s (Kloos, 2010, 98). Therefore, neither its epistemology nor its terminology is widely known. On the contrary, knowledge about the existence of the system in western countries tends to be confined to certain sub-groups mainly associated with Buddhism or Tibetan culture in general.10 And even if people are acquainted with the system, it is rarely the case that they understand the complex theories which do afford some deeper study. Likewise, the translation of Sowa Rigpa terminology and epistemology is by far not straightforward, and the translation of medical meaning affords a long process of interpretation and quite often results in vague approximations. Medical terms can be interpreted differently in different contexts and even seemingly obvious literal translations might encompass very different meanings on a physiological and functional level (Gerke, 2015, 21).
Another problem for integrating Tibetan medicines or supplements into western markets, is the correct translation and interpretation of indications (Schwabl and Vennos, 2015). Usually containing multiple ingredients, formulas possess a wide spectrum of indications, and it is difficult from a biomedical standpoint to connect these on a conceptual level. Moreover, formulas are commonly used in synergy with other formulas for various diseases but change their indications depending on the specific combinations.11 From a marketing perspective, it is certainly confusing for consumers to list all possible indications. Without prior knowledge of how Tibetan medicine views the body and mind as an interconnected and interdependent structure, it is difficult to see the connection between these potential indications.
In their endeavors, companies like Padma differ from online stores where one can order a wide range of mainly Tibetan medicine pills but often lacking an accurate legal basis. Whereas the latter mainly serve Tibetan medicine patients with medicinal supplies after a consultation,12 or simply sell herbal products that are deeply reformulated or invented,13 Padma aims at creating awareness and introduces Tibetan medicine to a wider public albeit in a highly translated and adapted version. While these attempts create accessibility and availability within a legal framework that otherwise severely restricts Tibetan medicine, they need to be critically evaluated and their impact on the representation and understanding of Tibetan medicine in the west should be considered. By discussing the example of Daknang Herbal Products as a small-scale transnational collaboration, this paper addresses similar transformative endeavors toward innovation and ‘new knowledge’ in terms of medicinal preparations that are clearly modified to suit new categories. The aim of the following chapters is to trace the history of Daknang as a small family business and to ask to what extent this company reformulated Tibetan medicines to adapt them to new settings and global markets while (dis)connecting to/from Tibetan medicine values and traditional forms of legitimization.
3 Establishing Daknang herbal products
Daknang Herbal Products, formally belonging to Pure Vision Sorig Ltd., was founded in 2016 near Riga, Latvia, as a small family business with the primary aspiration to promote Sowa Rigpa medicine in the west and to develop a suitable preparation to do so.14 The founders of Daknang had a long history of visiting and living in Nepal and a close relationship with Amchi Sherab Tenzin Barma,15 a Sowa Rigpa practitioner who has two clinics in and around Kathmandu, Nepal. Dr. Sherab has a liberal approach toward development and has been continuously searching for new pathways during the years of his practice.16
His initial impulse to develop extracts based on Sowa Rigpa classical formulas that would later on lead to the establishment of Daknang Herbal Products was the specific advice to develop tang, i.e., decoctions, he received from his teacher Trogawa Rinpoche in 1995/96 while he was studying and working at Chagpori Medical School in Darjeeling, India. This advice was further spurred by a speech that was given by the Dalai Lama during his graduation ceremony, as Dr. Sherab remembered:
“His Holiness the Dalai Lama was saying, ‘Oh, so you are all doctors now. You are graduates, right? Many teachers and students and more and more doctors are coming every year. And do you still have this ‘Agar 35′ or have you changed anything? Have you developed anything [new]? Everybody knows Agar 35; you can even get it online.’ Then he said, ‘Have you developed anything, or do you still have the same medicine?’ And then of course everybody is quiet, and no one answered. Then he said, ‘You should develop.’”
The Dalai Lama was criticizing the mainstream approach among Sowa Rigpa practitioners he experienced as too conservative and closed up toward modern science. He urged practitioners to not only continue the well-trodden paths but invest resources for research and into furthering the development of different medicines in order to use the means of science and establish innovations or new knowledge as outlined before. Amchi Sherab perceives this instance as confirmation of Trogawa Rinpoche’s advice, who emphasized the necessity of broadening the different types of medicinal preparations. As outlined in the Gyüzhi (rgyud bzhi), Sowa Rigpa’s most important classical text, there are eight types of pacifying medicines (zhi byed kyi sman) with different characteristics and carrying agents (sman rta): decoctions (thang), powder (phye), pills (ril bu), pastes (lde gu), medicinal butters (sman mar), calcinated medicine (thal sman), concentrated decoction (khan da), and medicinal alcohols (sman chang). Each of these types of medicinal preparations is ascribed to different benefits according to distinct characteristics of disease, like acute or chronic, and the dominance of a specific nyépa. Within the context of Tibetan medicine in Nepal and India, only pills and sometimes powders are in more regular use for everyday medical practice. Yet, powders and pills, their taste, the impracticality of taking them with boiled warm water, or the simple fact that sending them to patients outside Nepal is often problematic, present certain obstacles that can discourage patients from consuming them, like Amchi Lektsok, a practitioner from Kathmandu illustrated while discussing Daknang extracts:
“These days people are more focused on easy things, everyone is busy, they do not have time. They have to go to work; they go out at 9 o’clock in the morning and they go home at 5 pm. They do not have the time to eat the medicine, and they do not have time to bring the medicine to somewhere outside. But they need like a quick relief or a quick heal, something like that. Thus, a different approach is very good.”
In contrast, the Gyüzhi describes many different kinds of thang, which can be applied more specifically to hot or cold disorders according to respective imbalances, but which require more detailed research on the composition of formulations.17 The practicalities of the same were, however, far from straightforward as Amchi Sherab had neither the means nor the capacity to realize this request for many years. Moreover, he explained that the form and taste of tang preparations themselves was not ideal yet as many of the classical formulas are very bitter, once prepared as a decoction cannot be stored for long and thus likewise impractical to be prepared fresh for every patient. Therefore, a different solution needed to be developed that presented itself in the form of a transnational collaboration between Nepal and Latvia.
Although this collaboration involved different family members from its beginning, Julija who had been training with Amchi Sherab for seven years to become a fully trained Tibetan medicine practitioner herself until 2015, independently picked up a similar impulse during a speech the Dalai Lama gave at a Tibetan medicine conference in 2012. Again, it was stressed that Sowa Rigpa needs to go hand in hand with western science and develop new pathways. Both Tibetan medicine practitioners involved in this collaboration thus felt a strong responsibility to follow the authority of teachers as well as the Dalai Lama. Finally, these different events and impulses triggered more serious thoughts about forms of Tibetan medicine preparations that could be more easily established in the west.
When the idea of creating herbal extracts based on Tibetan medicine formulas was born, this inspired other Latvians to join the efforts and provide some of the expertise necessary to build a company that had the capacity to develop, design, test, register and market extracts, most of them based on classical Sowa Rigpa formulas. A phase of trial and error followed in which suitable formulas, a carrying agent, as well as an extraction method were chosen with the investment of private financial resources as well as voluntary labor. Moreover, after assessing different extraction facilities in Nepal and Latvia, Daknang settled on a company near Riga as they provided better manufacturing standards, technology, and hygiene than similar facilities in Kathmandu. Whereas Daknang’s initial motivation for the production of extracts instead of traditional pills or capsules was to offer an alternative for a quick relief from common symptoms such as flatulence, insomnia and colds, it later began to redirect its aims to include the treatment of some common chronic symptoms as well. The preparations were tested within an informal testing protocol on volunteers out of the group of family, friends, and acquaintances in Latvia, as well as on some of Dr. Sherab’s patients in Nepal and Daknang finally began its commercial activities in 2019.18
4 Registering and selecting formulas
Up to now, Daknang has registered 19 extracts as food supplements as this was the only path available for a small business with limited financial means.19 In Latvia, the requirements for nutritional supplements are determined by the Regulations of the Cabinet of Ministers (Cabinet of Ministers, 2015). There are no harmonized rules regarding the registration of food supplements within the EU, and thus they are governed primarily by Member State legislations which vary in their extent of control and regulatory procedures (Anadón et al., 2021, 1242–1243). In Latvia food supplements can be placed on the market if they have been notified by the Food and Veterinary Authority and included in the dietary supplement register. Still, food supplements have to comply with the EU Directive 2002/46/EC on food supplements (EC, 2002), as well as with regulations on foodstuffs and food safety, such as the food labeling Directive 2003/89/EC (EC, 2003), and the Regulation on nutrition and health claims (EC, 2006). According to the latter, herbal products can be marketed within Europe if they are not carrying any health claims other than those already approved within the nutrition and health claims regulations and are included in the EU register on authorized health claims.20 Moreover, a general safety assessment of botanicals is laid out in the European Food Safety Authority report that contains a compendium of botanicals with possible concern for human health (EFSA, 2012).
One of the first crucial steps within the creation of Daknang supplements was the decision about location and set-up of production facilities as determined by financial resources as well as by the regulations on hygiene of foodstuffs (EC, 2004) that likewise need to be observed within supplement manufacturing. Opting for the extract preparations—instead of other forms of preparations like actual decoctions or dry herbal formulas produced in-house in capsules—did still align well with the request Dr. Sherab had received from his teacher and relieved the registration process. For the production and registration of dry herbal formulas or other foodstuffs it would have been essential to establish proper facilities that meet the criteria set by the European Commission and Latvian authorities. These concern basic equipment features and hygiene measures. However, such facilities would have required significant investments, and since Daknang’s resources are limited, they opted to collaborate with the L.E.V. factory.21 Through this partnership, Daknang could comply with regulatory requirements while utilizing the infrastructure and experience of a well-established production facility, freeing up resources for product development and navigating the supplement market. Additionally, since the L.E.V. manufacturing sites already meet all the regulation criteria, registering Tibetan medicine alcohol extracts produced within European legal boundaries became significantly easier than it would have been for extracts produced in and imported from Nepal.
The next step within the establishment of Daknang was the decision about what formulas should and could be included for the manufacturing and marketing of extracts. In general, Tibetan medicine offers hundreds of formulas, and the selection process for Daknang’s extracts involved extensive discussions among Dr. Sherab, the Daknang team, and L.E.V. specialists. Eventually, Daknang decided to include complex formulas, chülen (bcud len) formulas, i.e., revitalizing and rejuvenating essences, single ingredient extracts and Dr. Sherab’s own formulas, i.e., formulas received through transmissions from specific teachers as well as one formula that he composed himself, into the product range. Within this process, it became clear that several factors influenced which herbs and formulas could be chosen as OTC supplements for a European market and which ones not. Apart from the legal aspects as explained before, the first and most important criterion for selection was how ‘balanced’ (nyoms) a formula is in regard to its strength and how likely it is to cause further imbalance if taken incorrectly. Some formulas possess very strong cooling or warming qualities which translate into stronger impacts on the three nyépa and/or specific organs. These formulas might—if taken over a longer period or in higher dosages than specified—cause further disruptions to specific nyépa and lead to unwanted negative effects. Other criteria for selection were the question of how beneficial a formula would be for the specific socio-environmental conditions found in (Northern) European climates and the corresponding health needs, as well as which ingredients could be effectively extracted with alcohol.22
5 Reformulating Tibetan medicines as herbal extract supplements
Tibetan medical pharmacology is very complex and diverse. The Gyüzhi, describes a wide range of medicinal preparations tailored to meet the needs of individual patients, based on their unique constitution and the specific nature of their illnesses. As specified in the Root Tantra, decoctions and powders, for example, offer rapid absorption of herbal properties and are, therefore, especially beneficial for the treatment of tripa imbalances and more acute conditions such as infections and inflammations. Pills and powders are used for chronic conditions and béken disorders. Chülen essences are highly valued for their rejuvenating qualities. And alcohol-based remedies, or men chang, are especially effective for addressing lung and béken disorders. Alcohol is seen as a ‘warm’ and ‘sharp’ carrier that can help deliver the medicinal properties of herbs deep into the tissues and channels of the body, enhancing both absorption and efficacy. Using these various forms of medicines, Tibetan medicine practitioners can create more personalized treatment plans.
Alcohol extracts produced by modern technologies can be thought of as bridging Sowa Rigpa alcohol-based remedies and essence extracts, both conceptually and materially. In combining both the idea of essence extraction applied in the production of chülen as well as the carrier substance of medicinal alcohol as a signifier for men chang, Daknang produces alcohol-based herbal extracts that integrate—through modern technology—characteristics of two medicinal preparations described in the Gyüzhi. Therein, alcoholic preparations are made as tinctures and involve infusing herbs with different strengths of alcohol, such as chang (chang, similar to beer or wine) and arak (a rag, strong alcohol).23 The herbs used for preparing tinctures are ground and then added to the alcohol for a period of time.
Chülen preparations are essence extracts of various substances that are taken for prevention or rejuvenation and with the general aim of promoting longevity (Gerke, 2012a, 330). They can be prepared in all possible forms of medicinal preparations—pills, decoctions, medicinal butters etc.—as long as they contain the essence of substances. Thus, they are a medicinal category in themselves, based on the concept of taking something powerful, i.e., full of chü or vital essence, and using it to rejuvenate and revitalize the body and mind. If applied for prevention, taking chülen substances can also involve an intense cleansing of the body, fasting and meditative practices. They can, however, also be simply taken as a form of ‘supplement’ that is integrated into the normal diet and lifestyle and used for rejuvenation and increasing vitality (Gerke, 2012a, 356).
At the L.E.V. factory, Daknang products are made using ultrasound-assisted extraction (UAE). This technique uses ultrasonic waves to enhance the extraction of bioactive compounds from the plant biomass (Gallo et al., 2018; Mehta et al., 2022; Liang et al., 2022).24 Various solvents can be used for extraction processes, such as water, ethanol, or acetone (Borges et al., 2020). After testing multiple formulas with extraction methods, including water, ethanol, glycerin, and CO2, Daknang chose to use organic grain alcohol (ethanol). These experiments were conducted with the InCell Ltd. laboratory, where biocomposition analyses and results were compared.25
Daknang products, produced through modern technology, are thus somewhat in-between textual descriptions of medicinal alcohols and essence extracts. They do not fully match either category but are instead diversely reformulated and partially invented through techno-scientific modernizations and western market adaptations. Within this process, the different product categories manufactured and sold undergo particular transformations: On the one hand, recipes of chülen and other complex formulas are still rooted in textual descriptions. Chülen preparations are transformed into extracts regardless of their different variants but as per instruction of “taking bcud len ‘in addition’ to a normal diet” (Gerke, 2012a, 357), they keep their potential supplementative function mentioned in the Gyüzhi as well as their composition. Complex formulas, on the other hand, are composed according to the chapter on medicinal alcohols in the Gyüzhi’s Subsequent Tantra. Yet as Daknang extracts, they are marketed as ‘emergency and preventative supplements’ to ease symptoms instead of treating diseases and thereby primarily receive a different function and identity.26 In general, Sowa Rigpa sources support the logic that whenever a compound is taken as fine powder, decoction, or tincture, its effects are faster and thus it can be applied more effectively in acute situations. Extracts as ‘Sowa Rigpa first-aid products’ are therefore an option that still connects to the principles of Sowa Rigpa epistemology that promotes a relief of symptoms in acute cases with preparations that can be more rapidly absorbed. Still, as OTC herbal extracts, they cannot be perceived as medicines that are used to cure imbalances of the three nyépa and specific diseases. Instead, they are sold independently and mainly according to biomedical symptoms and health claims as self-help preparations that can be randomly combined regardless of an underlying imbalance. They are thus reinterpreted and lose part of their efficacy within the medical system and treatment paradigm. Also, distributed online as natural and herbal food supplements, the production and marketing of these extracts is very similar to the criteria defined for the classical-polyherbal formulation regime (Schrempf, 2015, 296). Production according to the classical-polyherbal regime follows traditional standards to measure quality, like tasting,27 combined with the use of modern technologies, both of which apply to the Tibetan medicine extracts assembled in Nepal within Amchi Sherab’s pharmacy and manufactured in modern laboratories near Riga. They are also mainly based upon herbal ingredients,28 in contrast to many other compound formulas that do contain animal and mineral ingredients as the latter are often estimated to be of higher potency and thus efficacy than their herbal substitutes. Yet purely herbal products integrate better into the western supplement markets as they speak to modern preferences of unprocessed vegan diets and are usually considered a safe choice (Nichter and Thompson, 2006, 195).
Single ingredient extracts, on the other side, were never commonly used as medicines in the first instance, although the Gyüzhi describes the health benefits of using various single-ingredient preparations as foods in the Explanatory Tantra and as medicines in the Subsequent Tantra. As Daknang extracts, they are—partially or fully—inspired by a mixture of Tibetan cultural lores and food advice as well as perceived needs of western patients or market demands. For example, the first single-ingredient formula that Daknang registered in 2017 was Daknang 9 Himalayan Nettle Power.29 As it was initially difficult for Daknang to navigate the complex registration procedures and regulations, this simple extract helped to understand the challenges and provided the ground for further development. And nettle was chosen as the first extract because it is well known for its health benefits in the west, and brought a ‘Buddhist symbol’ to the company and its marketing approach as it is inspired by the famous story of the ‘Great Yogi Milarepa,’ who is said to have survived over many years by eating nettles (Quintman, 2013). Additionally, the single-ingredient extracts allowed Daknang to enter the food supplement market by using them as ‘anchor products.’ These products are well known by the general population and attract a larger clientele. Through these, it becomes easier to find Daknang’s online store and to learn about the complex Tibetan medicine formulas, which are currently the company’s focus.
With its single-ingredient products, Daknang most closely adapted to the modern supplement market as well as to biomedical conceptions of active ingredients that can be researched and tested for their efficacy. This stands in contrast to complex multi-compound formulas whose efficacy mechanisms are difficult to evaluate by modern testing regimes (Schwabl and Vennos, 2015). In a similar way, Daknang 10 Detox formula, also described as Life-strengthening Chülen (tshe stobs bcud len), has been created at the start of the company in order to attract a clientele that is familiar with the terms ‘detoxification’ and ‘purification’ and values alternative medicine ideas of ‘cleansing the body.’ Amchi Sherab, having traveled extensively within western countries and treated patients for many years, composed the formula in a way that corresponded to his ideas about the needs of western patients and without an equivalent in classical texts. Nevertheless, both kinds of preparations are placed in the same category as extracts produced according to classical recipes and become associated with the overall scope and efficacy of Tibetan medicine treatments. They are to a large extent ‘inventions as Tibetan medicines’ and reformulated in their make-up and identity since they were never in use as medicines or are completely novel creations. Thereby, they are revaluated as medicines and benefit to some degree from Sowa Rigpa’s reputation and strength as a medical system. Nowadays, however, the main focus of the company has shifted and most of the product range is not recomposed, but recipes are taken primarily from the formulas described in the Gyüzhi or from Amchi Sherab’s lineage transmission, although the other preparations are still being sold and continue to have a strategic market advantage. In their becoming, most of Daknang’s products are therefore partly ‘reverse engineered’—as defined before—with some of the classical formulas changing their function and their medicinal form and production regime, integrating both biomedical proceedings, technology and standards with Tibetan medical knowledge about ingredient composition and several steps to ensure quality and efficacy. They are thus reinterpreted into herbal extracts rather than invented.
6 Innovation as negative aspiration?
Despite calls for innovation from such highly respected Tibetan cultural figures as the Dalai Lama, its definite place in capitalist economies and its engagement with standardized industrial mass production, innovations have been a rather cautious agenda in the Sowa Rigpa industry when it comes to the development of medicines and if compared to other Asian medicine industries (Hsu, 2009; Madhavan, 2009; Pordié, 2015; Kudlu, 2022). As pointed out in the beginning, this is not to mean that there are no existing attempts of reformulations or reinterpretions for commercial means. Especially the Sowa Rigpa industry in China engages heavily in reinterpreting pharmaceuticals, mainly for national markets, the Dharamsala Men-Tsee-Khang steadily increases its Sorig product line that also contains some OTC medicines, and in Europe, Padma AG so far registered around thirteen reformulated supplements and medicines in various European countries. As previously mentioned, such reformulations of Tibetan medicines, and with them innovations, usually happen alongside a spectrum, some are adaptations, some are reinterpretations, and some are (re)inventions of the production process and/or the medicinal preparations—forms, ingredients, measurements, packaging, and marketing. Adaptations are a regular part of the medicine making and as such are considered ‘traditional,’ even though they are strictly speaking innovations. However, transformations that go beyond pragmatic adaptations to patients, surroundings or resources, that include (technological) modernizations which potentially interfere with a medicine’s potency or cross boundaries into the biomedical knowledge field are usually met with skepticism or outright disapproval (Janes, 1999, 2002; Craig and Adams, 2008; Blaikie, 2013; Kloos, 2015; Blaikie and Craig, 2022). They are often openly—or surreptitiously—accused of being led by ‘negative aspirations.’ This is a recurring response from practitioners or their communities from all geographical areas of Tibetan medicine and is based on the strong authority of and adherence to textual sources and the associated fears of losing certain standards. Most commonly, allegations and accusations of negative aspirations are associated with immoral profiteering or business-minded moralities on the one hand, and a threat to notions of efficacy and quality on the other. Both aspects have detailed and revered textual foundations that certainly are and have to be compromised to some extent in everyday lives and activities of practitioners but that still act as persistent guidelines and fluent boundaries for what is acceptable. In this last section, we will therefore discuss the questions why innovations are often met with skepticism and how they are framed as negative aspirations mainly among Sowa Rigpa practitioners as well as their local communities and look at how such conceptions are navigated by Daknang and Dr. Sherab himself.
6.1 Moral questions about global business
Morality is discussed in the 31st chapter of the Gyüzhi and advises practitioners on how to behave within social, clinical, and spiritual contexts as well as which personal habits and character traits are valued. Among practitioners, this textual guidance is often summarized in simple concepts, like possessing ‘a good heart’ (sems bzang) which translates into a pure motivation based on the Bodhicitta attitude (byang chub gyi sems), the wish to gain enlightenment for the benefit of all beings. It also emphasizes virtues such as altruism (gzhan phan) and compassion (snying rje) (Kloos, 2010, 2020) and is commonly set in contrast to a greedy and miserly ‘bad hearted’ (sems ngan) practitioner (Kloos, 2022, 51–53). Although gaining wealth (nor) is described in the Gyüzhi as one of the rewards for a good physician, above average profits do not seamlessly blend with the ideas of altruism and compassion and are a highly ambivalent topic which easily arouses judgments of being ‘money-minded’ or greedy (‘dod rngam). Even if one simply gives the impression of subduing oneself to a stigmatized business morality, this will quickly evoke criticism from the local community. General discourse therefore usually avoids the topic, but nevertheless it is very present in everyday actions and often highly debated in the context of local gossip not only in regard to an upscaling of medicine production in general, but also in relation to all kinds of activities that go beyond a common mode of practice and include aspirations to develop products as these could be sold for higher prices outside a treatment regime.
And indeed, particularly as goods for tourists and an (inter)national clientele who can afford spending money beyond common medical realms, luxury and wellness commodities are becoming increasingly popular, especially in such tourist places as Boudhanath30 where a mixture of exotic Tibetan culture and Buddhist spirituality supports the attraction toward Sowa Rigpa. Just behind Boudhanath Stupa31 in a recently opened shop called Nüpa, Daknang products are being sold together with goods such as dried Himalayan raspberries promoted as super foods and an array of cosmetics, essential oils and other wellness goods.32 They neatly fit into this carefully arranged setting for health-conscious consumers and also directly cater to Amchi Sherab’s large international patient clientele. But outside of Nüpa, Daknang extracts are hardly advertised, and even many Sowa Rigpa doctors in Kathmandu do not know exactly what these products are. Instead of actively promoting new medicinal preparations or discussing innovations with other practitioners, they are somewhat obscured, and customers happen to come across them accidentally.
Part of this reluctance in openly commercializing novelties is due to the sensibilities of appearing ostentatious and greedy. With his many unusual activities besides the development of extracts, Amchi Sherab’s activities have already been claimed money-minded by other practitioners or the local community. One of Kathmandu’s practitioners, for instance, recounted some accusations from a local journalist who complained about expensive prices at Amchi Sherab’s clinics, which reflects the doubts that are harbored by some practitioners:
“He (the journalist) claimed that in the name of Sowa Rigpa, they try to kind of loot the people and then he was saying that they were selling some medicine practices or pills very expensively. And how come they do that.”
However, other doctors also praise Amchi Sherab’s endeavors, especially when considering how much effort and time was invested in research and development, as most doctors in Kathmandu generally lack both time and resources to attempt in-depth development. Personally, Amchi Sherab wants to stay out of any business interactions that might describe him as greedy and is genuinely inspired by the development of Sowa Rigpa although, in practice, it is sometimes difficult to differentiate motivations, and his activities certainly do generate profits. In conversations with him, he often feels the need to emphasize his humbleness, which despite the seeming contradiction, fits his modest and easy-going character. And it is true that he is not earning any direct profits from the sale of Daknang extracts but instead receives some extracts in return. In this respect, this transnational collaboration serves him more for his inspirational goals and less on a monetary level.
Daknang itself has never been accused of any negative monetary aspirations, perhaps also due to the fact that its products are not openly advertised and are hardly known among practitioners in Nepal. As a business, it is clearly commercializing Tibetan medicine products, and the aim is to generate profits. Yet, its endeavors are also characterized by a lot of voluntary work and several purposes that stem out of an experienced need for innovation. And apart from that, as mentioned previously, Daknang is still struggling to establish itself on European markets and has hardly generated returns so far but incurred notable costs.33 Among European Sowa Rigpa practitioners and other people involved in facilitating the practice in Europe, Daknang is as yet also little known. However, the few encounters that could be observed by the first author during research within European Tibetan medicine networks indicated that scepticism toward Daknang products is less related to the commercialization of Tibetan medicine as such, but rather toward a suspected lack of efficacy due to the multiple reformulations of Daknang extracts.
6.2 Navigating ‘modern’ and ‘traditional’ efficacy
When the authors of this article met for the first time in 2014, it was in Amchi Sherab’s former clinic, just around the corner of Boudhanath Stupa and off one of the small busy roads often clogged by noisy traffic. While we were sitting next to each other on a low corner bench next to the consultation table, Julija was filling essential oils into small dark glass bottles directing the liquid with a plastic funnel. As a senior Sowa Rigpa student at that time, she was on duty in the clinic but as patients were more scarce in the afternoons, she had time to prepare some of the diverse range of herbal products, like teas, oils and cosmetics, which the clinic was selling in its anteroom and which were stocked on shelves opposite of the boards loaded with the common choice of pills. A medicine Buddha statue was sitting inside a cabinet draped with a kathak behind golden offering bowls. The consultation room smelled heavily of rose oil as Julija recounted a recent visit to a local retailer for essential oils. She lamented some of the practices that she found unhygienic: “Some of the oil spilled on the floor and one of the employees just took a sponge, mopped up the oil and squeezed it back into one of the containers. This is unacceptable, how can they put this oil back. There was so much dirt on the floor.” She looked up with a mixture of anger and resignation and carefully sealed some of the small glass bottles before putting them in a row on the table.
During the many conversations we have had since, the topic of hygiene and quality of medicines and pharmaceutical production processes came up regularly. Whereas Amchi Sherab sees the advantages of a different medicinal form mainly—though not exclusively—in providing an easily accessible preparation that is suitable for modern tastes and lifestyles, packaged with labels that contain instructions on how to take them and accessible outside of Nepal, Julija frequently pronounced the advantages of producing medicines in a clean and controlled environment. Being a student of Sowa Rigpa for these many years, she collected multiple instances in which her concepts of hygiene and quality did not meet local realities. Yet also other Sowa Rigpa doctors practicing in Nepal share these concerns to some extent. And producing Sowa Rigpa pharmaceuticals nowadays has become very much a struggle to gain control over quality and essentially to standardize medical efficacy (phan nus) (Kloos, 2015, 133–35). According to Sowa Rigpa epistemology, efficacy constitutes itself from material and immaterial aspects. It is on the one hand substantially determined by the observance of the ‘seven essential limbs’ (gces pa’i yan lag bdun) of the Gyüzhi’s Subsequent Tantra that include instructions on the correct harvesting period and location, time of day, specificities of what or which part of a plant to collect or even on the characteristics of the person collecting them. They also contain specific advice on how to store, dry, clean and detoxify certain plants which in turn can significantly impact the potency and safety of a medicinal preparation. Thus, material efficacy is a product of the observance of the steps creating, enhancing, or keeping potency and determining safety by following laid out instructions as closely as possible. Modernizations and the implementation of certain standards can, however, easily interfere with these traditional notions of efficacy (Craig, 2011, 2012; Saxer, 2013).
The immaterial aspects of efficacy, on the other hand, cover moral, psychosocial as well as karmic (las) dimensions, and are determined by a physician’s knowledge regarding diagnosis and treatment, but also by spiritual aspects mainly the ritual blessing of medicines and the doctor’s personal motivation, as well as the (karmic) connection between doctor and patient. As Craig (2012, 5) notes, efficacy is essentially an “inter-subjective phenomenon” which means that the outcomes of a therapeutic encounter are unsure before a patient experiences the effects (or not) of a medical treatment.
One common struggle that amchi nowadays encounter is a decline in the quality of raw materials as they are often unable to collect herbs themselves or even if they do have the time and resources, are confronted with the realities of environmental degradation that negatively affects the size, quantity and overall quality of plants that can be collected from the wild (Kloos, 2015, 133). Another aspect affecting the quality of Sowa Rigpa raw materials as well as of the medicinal products throughout their production process is the problem of contamination and pollution through pesticides, chemicals, heavy metals and—in the case of a large South Asian city—dust. In Kathmandu, most pharmacies or production facilities are located within city limits, and therefore, the environment plays a big role in the decline of medicines through exhaust from bypassing traffic or other small particles as it is common practice to dry herbs near busy roads or on the roofs of houses, exposing them to pollution. Plants are also often transported or stored in improper containers, allowing exposure to dirt, mold, or rodents. Additionally, ingredients are often improperly cleaned from impurities—such as sand, dirt, or outer bark—and handling procedures frequently lack cleanliness, with workers using unclean instruments or hands. Such practices raise concerns about the quality and safety of herbal ingredients and the quality of finished products.
Even though Amchi Sherab tries to control the quality in his pharmacy in Pharping—a village approximately one hour drive from Kathmandu that experiences less pollution—by, for instance, minimizing certain pollutants through installing dust shields for drying herbs or cultivating the overall amount of plants used for his production in his own plantations, settling Daknang’s production facilities in Latvia, still provides better manufacturing standards and hygiene than any facilities in Kathmandu would have done. More particularly, thus, Daknang was built as a small-scale collaboration that strategically combines quality control measures and connects manufacturing according to certain EU standards as well as Tibetan medicine’s sensorial evaluation of potency. It also tightly links geographic locations, that is Dr. Sherab’s Pure Vision Sorig pharmacy in Nepal and the Latvian L.E.V. extract factory, through the establishment of a continuous circulation of raw materials and processed extracts. Thus, nowadays, the raw materials, that is the herbs, are collected in Solukhumbu region in Nepal as well as in Arunashal Pradesh in India or are in certain cases cultivated on Dr. Sherab’s own lands, are then brought to Pharping where they are cleaned, dried, compounded into powdered formulas and blessed before being send to Latvia where the extracts are produced with UAE extraction technology.
While it is thus possible to follow textual Sowa Rigpa standards and notions of efficacy during the first half of the production process—through the control of raw materials, the blessing of substances within Buddhist rituals and prayers34 and a general evaluation of potency through the physician’s senses—the second half, the actual manufacturing of medicines, follows in out-of-reach laboratories that work according to their own standards of efficacy. And even if these standards lead to improved hygiene and product quality, as controlled production standards are intended to minimize contamination or impurity of ingredients and make products more homogeneous, the changes associated with compliance with such standards can be significant and can compromise traditional notions of what constitutes effective, high-quality medicines. In regard to the industrial Sowa Rigpa medicine production, points of criticism of machine-produced medicinal products include a reduction in quality due to heat in general, contact with metals or electricity from machines (Blaikie, 2013, 443). Even though it is unclear how the extraction process affects a medicine’s potency and thus efficacy, producing extracts in modern laboratories includes significant techno-scientific involvements through which some interference with traditional standards for efficacy cannot be excluded. Thus, despite its promises of increasing the quality and safety of medicinal preparations through controlling standards and extensive product testing, modern regulations convey a culture-and value-specific understanding of good medicines that often clashes with the Sowa Rigpa concept of phan nus (see Craig, 2011).
6.3 (Dis)connecting foundations
Another concern that relates to the question of ‘innovation as a negative aspiration’ is the reclassification of prescription medicines as supplements that become independent from their origins in clinical practice. As Blaikie (Blaikie, 2015, 13) demonstrates in the case of the precious pill Samnor (bsam nor), this can have unforeseen effects. Even though, the concept of supplements and self-help medicinal preparation is not new within Tibetan medicine and preparations like Norbu tea (nor bu mdun thang) have been in common use as an approximate to a self-applicable cold (prevention) remedy for some time, their interpretation, marketing and visual presentation certainly is. Not only are these medicines marketed according to biomedically defined symptoms and diseases, a translation process that sometimes neglects complex interrelations according to Sowa Rigpa epistemologies and can end up as substantially vague (Gerke, 2015; Czaja, 2017); but they are also reinvented as lifestyle products that feed into postmodern consumerist values and a materialist culture that can potentially support the very ills of a hectic modern lifestyle that Sowa Rigpa practitioners frequently frown upon. And more crucially, their reinterpretation as independent pharmaceuticals is a transfer in which they lose much of their efficacy, which is contained in the clinical diagnosis, their interaction with other treatment options and the general knowledge and advice of a practitioner. Instead of receiving a diagnosis based on individual constitution and specific imbalances of the three nyépa, as well as a treatment regime that would contain various synchronized medicinal preparations, dietary and behavioral advice and in some cases external therapies, patients or consumers now have to self-prescribe standardized products according to biomedically defined indications (Figure 1).

Figure 1. Daknang Instagram post (2024): Daknang 13 D-Equalize formula and its biomedically framed functions. Images created and Instragram page run by author JS.
Most common Sowa Rigpa treatment regimens given by a doctor combine a mixture of symptom management with the treatment of underlying imbalances as root causes for disease where this is necessary. Western supplement markets and biomedical mentalities, however, make a shift of focus to symptom management (only) a necessary precondition if an Asian medicine system wants to enter these markets. Such a shift in perspective contradicts much of the inherent efficacy of a system that does not only look at symptoms but at the manifold and complex causes that need to be addressed to establish healing and health and that otherwise focuses on long-term treatments of underlying imbalances or the prevention thereof. And a symptomatic self-diagnosed application of supplements surely cannot substitute a medical treatment with a doctor’s diagnosis. By shifting focus on those situations in which the relief of symptoms gains priority over the treatment of underlying root causes and in which acute but also chronic symptomatic afford specifically targeted measures, Sowa Rigpa supplements can most easily be established without losing their connection to their foundations as discussed in the context of reformulation. But they begin sharing similarities to a more symptom oriented biomedical treatment paradigm, a shift that significantly reduces the strength and perspective of the medical system they are associated with. Therefore, Daknang tries to navigate this reclassification with several strategies, for instance by offering health sets that are designed to mirror common Tibetan medical daily treatment courses, or by framing products as ‘first-aid’ and preventative options. They also rightly claim that they are not trying to establish the extracts as a substitute for longer term medical treatment, as this is clearly not the same and both doctors involved feel a responsibility to make this distinction. This is even more true since extracts on alcohol base cannot cover the whole realm of Sowa Rigpa treatment applications and the range of disorders that can be effectively addressed is severely limited.35 Daknang extracts are thus never quite comfortable when identified as Tibetan medicines, at least not in the full scope of its meaning. While this might be clear to an experienced patient of Tibetan medicine, to someone who has no previous experience with the medical practice and treatment, it might not be evident and Daknang products might be associated with the full extent and efficacy of Tibetan medicine.
This holds even more true as Daknang initially decided to move away from Tibetan medicine descriptions of products and to adapt to the presentation and marketing of the food supplement market by prioritizing biomedical concepts and nutritional language. Within these markets, advertisements usually “emphasize the natural treatment of illness and suggest opportunities to maximize time and enhance performance, beauty, and overall fitness” (Nichter and Thompson, 2006, 197). The outcome of this initial strategy was a basic scientification of general effects, reducing Sowa Rigpa’s complexity for the cost of making it accessible to a western clientele. This approach seemed necessary in order to be found by customers and to actually sell products and survive as a company. However, instead of easing the path of establishing itself within western food supplement markets, the company realized that these descriptions did not appeal to their target audience—people looking for a profound, more traditional approach to health as Daknang frames it. Using food supplement terminology resulted in a disconnect from Sowa Rigpa epistemologies and a loss of Daknang’s uniqueness in the supplement market alike, and largely compromised Daknang’s aim of creating awareness for Tibetan medicine as a system. Thus, realizing that such an approach greatly jeopardizes both goals and authenticity, Daknang decided to change the strategy in 2023. Retaining some aspects of the previous approach, the new strategy combines an educational element consisting of the translation and explanation of basic Sowa Rigpa epistemology in a commentative form, combining (translated) Tibetan medical terminology as well as biomedically defined indications and biological mechanisms to describe the functioning of products (Figure 2).36 This form of hybridization is intended to make Tibetan medical knowledge accessible on the one hand and help Daknang to stay closer to its roots on the other. And even though the market adaptation through biomedical reinterpretation and general simplification is still evident, it can provide a general introduction to Tibetan medicine and create awareness for the medical system.
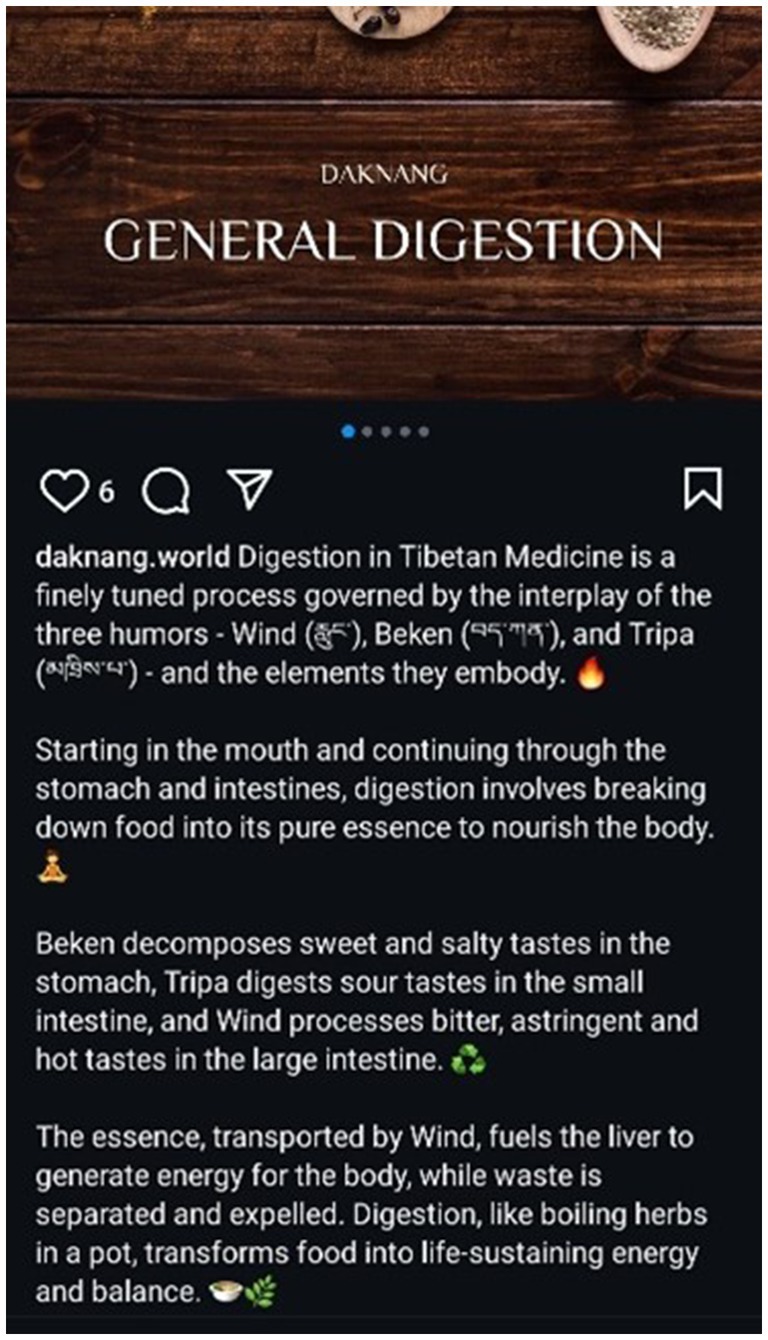
Figure 2. Daknang Instagram post (2024): Daknang educational post on ‘General Digestion’ according to Sowa Rigpa epistemology. Images created and Instragram page run by author JS.
7 Conclusion
The aim of this article was to highlight the many shifts, challenges and compromises that arise and must be overcome when attempting to transform an integral part of a centuries-old Asian medical system for new contexts. By looking at the example of Daknang Herbal Products, a company that has been established as a transnational collaboration between a Sowa Rigpa practitioner from Nepal and a Latvian laboratory involving a group of people inspired by the idea of making Tibetan medicines accessible to western health, lifestyles and tastes, this paper illustrated how Sowa Rigpa pharmaceuticals become reformulated into food supplements for global markets. Within this process, the common forms and make-ups of Tibetan medicines are diversely reformulated and innovated within a spectrum from adaptation to (re)invention. The latter can be rather associated with the disruptive steps of reverse engineering wherein researchers of large industrial firms are employed specifically to ‘mine’ Asian medical recipes from a largely biomedical point of view, finishing off a product by proposing an Asian medical identity (Pordié and Gaudillière, 2014, 70–71). Such cases where inventions mainly come from outside the medical system from which they supposedly originate and are used for purely commercial purposes provide legitimate cause for concern and some answers to the question why many Sowa Rigpa practitioners are cautious and skeptical about change. There is much to lose for the medical practice and system. At best, transformations provide support and relevance to a medical practice that is struggling to survive as its environment changes dramatically. At worst, such reformulations jeopardize essential moral values that form the identity of Tibetan medicine and, as a result, undermine the standards that make the medical system as a whole effective.
This paper provides a somewhat broader perspective on reformulation and the industrialization of Asian medicines by taking into account small-scale industrial aspirations. The example of Daknang’s endeavors is for sure not meant to serve as a blueprint for the reformulation of Tibetan medicines. Instead, it illustrates that transformations are learning processes of how to navigate possibilities and test boundaries, in practical, material, conceptual and moral terms. This process bridges not only several locations, different legal spaces, environments or specific health needs, but also and more importantly differing ideas and ideals of what makes efficacious medicines. As herbal extracts that are sold online, Daknang products are mainly adapted and reinterpreted to different legal surroundings and modern mentalities in the attempt to not only commercialize but likewise raise awareness for the possibilities of Tibetan medical healing. The motivation for establishing the company was thus very much one of venture and idealism. Nevertheless, Daknang’s mission has crossed boundaries that can provoke criticism, but also legitimate arguments about what is lost when such reinterpretations are realized. The process of developing innovative medicinal products that are sold as food supplements in new contexts poses risks that are not always easy to overcome. Especially the detachment of medicinal preparations from Sowa Rigpa medical experts, their specialized treatment and care as over-the-counter supplements, and sometimes even their dissociation from the knowledge system, is a critical step that is causing multiple concerns. Yet, this transformation stems out of an intimate transnational knowledge collaboration between Tibetan medicine doctors that is implemented with caution and care and with a foothold in the so called ‘tradition.’ It is thus an attempt to remain relevant by creating ‘new knowledge’ within changed conditions and for a new clientele.
Data availability statement
The datasets presented in this article are not readily available because data was created through interviews and observations. Requests to access the datasets should be directed to Patricia Mundelius, cC5tdW5kZWxpdXNAZWQuYWMudWs=.
Ethics statement
The studies involving humans were approved by the CAHSS Research ethics, College of Arts, Humanities and Social Sciences, University of Edinburgh, and by the Nepal Health Research Council. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PM: Writing – original draft, Writing – review & editing. JS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded in whole, or in part, by the Wellcome Trust [223322/Z/21/Z]. For the purpose of open access, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission.
Acknowledgments
The authors wish to thank all the Sowa Rigpa practitioners from Nepal, India and Europe, and especially Dr. Sherab Tenzin Barma, for their invaluable help and insights during the researching of this paper.
Conflict of interest
JS is the co-founder and manager of Pure Vision Sorig Ltd.
The remaining author, PM, declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^The term ‘Sowa Rigpa’ has gained more prominence over the last decades and there is an increasing tendency to use the term in scientific literature (Craig and Gerke, 2016). However, as we also want to reflect the realities of European contexts where the term ‘Tibetan medicine’ is predominant, we use both terms throughout this paper.
2. ^Changes in diet and lifestyle are of primary importance regarding therapeutic actions in Sowa Rigpa theory and, thus, can be applied as sole treatment measure for minor cases. Moreover, external therapies are sometimes used to treat more specific cases or as a complementary treatment method.
3. ^It should be noted that many of the major medicinal substances also stem from lowland India or other countries.
4. ^It is important to differentiate that providing dietary or lifestyle advice is not illegal, but only the import, prescription or selling of medicinal preparations that are not registered under the DTHMP.
5. ^All foreign language terms are transliterated Tibetan language terms, using the Wylie (1959) system of transliteration (in brackets) and the Tibetan and Himalayan Library Transliteration Converter (in text) https://www.thlib.org/reference/transliteration/wyconverter.php.
6. ^Daknang Herbal Products is the brand which is formally owned by the company Pure Vision Sorig Ltd. However, the latter only features in legal records and since there is no difference between the owners/founders and it is commonly thought of as Daknang since the name ‘Daknang’ is a reverse translation of ‘Pure Vision,’ we will keep using the former term throughout this paper when talking about the products, brand and company.
7. ^The term ‘classical formula’ is often applied to describe formulas that have their roots in classical texts and/or connect to oral lineage transmissions. In ayurvedic contexts, it is commonly used to distinguish the latter from new formulations as proprietary medicines. As Blaikie (2015, 8) points out the term employs connotations of tradition and stability over time as well as authenticity and purity as contrasting to commercialized and (re)invented modern preparations.
8. ^The six tastes are sweet, sour, salty, pungent, bitter, and astringent.
9. ^The eight potencies are heavy, oily, cooling, blunt, light, rough, pungent, and sharp.
10. ^Switzerland presents an exception here since the company Padma has been actively promoting Tibetan medicine there, as explained below.
11. ^For example, Agar 35 is one of the most popular formulas against lung (rlung, wind, pron. loong) imbalance. However, it is said in Tibetan medicinal texts that Agar 35 can be used for the trio of infection (gnyan), fever (tshad) and lung, among other benefits. It is also used for blood pressure and even for joint disorders (grum bu).Lung is one of the three nyépa or defective energies and often associated with the nervous system. The three nyépa, lung, tripa (mkhris pa, bile), béken (bad kan, phlegm) are psychophysiological processes in the body that may also work on a subtle level, and which are considered as the underlying factors that influence physical and mental health (Parfionovitch et al., 1992, 4).
12. ^An example is the online shop Menla https://menla.shop/page/legal-side.
13. ^Some preparations make use of the signifier ‘Tibetan’ but without any apparent connection to classical formulas, for instance https://bioherba.com/en/tibetan-herbs-of-life-100-capsules.html.
14. ^Daknang/Pure Vision Sorig Ltd. is formally owned by six shareholders. However, two of these shareholders, collectively holding 66% of the company’s shares, are responsible for its day-to-day management. These shareholders, Julija Sprisevska and Sergejs Sprisevskis, represent one family that founded the Daknang brand along with Alla Sprisevska and Aleksandr Sprisevskis, Julija’s parents. Julija Sprisevska serves as the primary brand ambassador and oversees product development and marketing. Sergejs Sprisevskis manages procurement, finances, and distribution. All other business functions are currently outsourced.
15. ^‘Amchi’ (em chi) refers to practitioners of Tibetan medicine. Throughout the text, the terms amchi, doctor, practitioner and physician are used interchangeably.
16. ^Besides his work as an amchi, he has joined international NGO projects or worked as a consultant. Thereby he might be described as one of the practitioners who go “well beyond archetypal descriptions” in their activities (Pordié, 2008, 9).
17. ^Medicinal texts state names and ingredients of formulas, yet often without details on exact preparation and measurements of ingredients which are either orally transmitted from a teacher to a student, or in this case experimentally researched.
18. ^Daknang products are sold worldwide through their online shop www.daknang.com.
19. ^Within the EU, an herbal medicine product has to be registered under the Directive on Traditional Herbal Medicinal Products (DTHMP) 2004/24/EC. This process is replete with challenges and very costly. Kudlu and Nichter (2019, 120) estimate that registration under the DTHMP would cost between $114,000 and $462,000 per product. During the registration process, Daknang encountered similar challenges to those met by other manufacturers navigating European legislation (Millard, 2008; Schwabl and Vennos, 2015). The main problems were the identification of the materia medica used in formulations within the western plant taxonomy and the question of how to deal with ingredients that are considered illegal under EU law.
20. ^https://ec.europa.eu/food/food-feed-portal/screen/health-claims/eu-register.
21. ^Ekstraktu rūpnīca https://lev-extracts.com/en/about-company/.
22. ^Dr. Sherab had many in-person meetings with L.E.V. specialists. Chongzhi 6 (cong zhi drug pa) was chosen as one of the initial formulas. However, after analyzing ingredients in this formula, L.E.V. specialists concluded that calcite, the key ingredient of the formulas, cannot be extracted and formulas containing calcite cannot be used.
23. ^Historically, the distillation techniques used for making arak frequently led to contaminants and dangerous byproducts like methanol. Nowadays, high-purity ethanol is used.
24. ^The principle of the UAE method is that ultrasonic waves (above the human hearing threshold, i.e., 20 kHz) generate cavitation bubbles in the liquid, which collapse and produce mechanical forces that disrupt plant cell walls, making it easier and more efficient to release the bioactive compounds (Gallo et al., 2018; Mehta et al., 2022). Moreover, the UAE method demonstrates a significantly higher extraction yield of bioactive compounds and enhanced therapeutic activity, making the product more potent (Frohlich et al., 2022).
25. ^With multiple studies demonstrating its superiority, ethanol seems to be the most effective solvent in producing the most bioactive components. Furthermore, alcohol is a natural preservative that greatly increases the extracts’ shelf life (Frohlich et al., 2022; Borges et al., 2020). After extensive consultations with the L.E.V. factory and biochemists from InCell, Daknang concluded that other solvents would require additional preservatives which they wanted to avoid.
26. ^While all medicines would usually be prescribed after a consultation with a doctor, there are some formulas that can and have been used for the self-applied treatment of acute (and chronic) symptoms. Some of the more common self-help preparations are Norbu 7 thang (nor bu mdun thang) applied for the prevention and relief of cold and cough or Semde (sems bde) for insomnia or anxiety. Many Tibetan and Himalayan people accustomed to Sowa Rigpa know about these formulas and sometimes use them as a self-help measure even though there is a definite awareness that Sowa Rigpa is not a symptom-oriented self-help method and underlying imbalances need to be diagnosed by a Sowa Rigpa physician for a long-term treatment success.
27. ^Taste is described in the Gyüshi as the most important sensory means to assess the potency of raw materials (ro nus) and to evaluate the quality and efficacy of materia medica (rdzas gyi nus pa). It is thus an integral part within the process of compounding medicine.
28. ^Except for honey (sbrang tsri) and shilajit (brag zhun mdog nag), a rock excretion that contains minerals.
29. ^Daknang does not follow the Tibetan formula tradition of using numbers indicating the number of ingredients used in the formula.
30. ^One of Kathmandu’s wards that has a large Tibetan and Himalayan population.
31. ^Boudhanath stupa is an important pilgrimage site within the Kathmandu valley.
32. ^Nüpa has been established by Amchi Sherab and his wife Pema Bhuti in 2023 to sell the diverse range of Pure Vision wellness goods along with healthy food choices that are supposed to create awareness for the perspective of ‘food as medicine.’
33. ^For annual reports with revenue statements see https://company.lursoft.lv/en/pure-vision-sorig/40103931204.
34. ^In this context, the medicines are usually blessed through personal Medicine Buddha rituals.
35. ^Whereas pills or powders can have hot or cold potencies, alcohol preparations and chülen are described invariably as having ‘warm’ and ‘sharp’ qualities. Since Daknang extracts combine both, they also carry these characteristics. This limits the formulas to addressing imbalances of lung and béken, excluding other imbalances such as tripa and blood (khrag).
36. ^The descriptions are combined based on classical Tibetan medicine texts, Dr. Sherab’s commentaries, and modern descriptions from Buryat doctors.
References
Anadón, A., Ares, I., Martínez-Larranaga, M.-R., and Martínez, M.-A. (2021). “Evaluation and regulation of food supplements: European perspective,” in Nutraceuticals: Efficacy, safety and toxicity. eds. R. C. Gupta, R. Lall, and A. Srivastava (London: Academic Press), 1241–1270.
Blaikie, C. (2013). Currents of tradition in Sowa Rigpa pharmacy. East Asian Sci. Technol. Soc. Int. J. 7, 425–451. doi: 10.1215/18752160-2332223
Blaikie, C. (2015). Wish-fulfilling Jewel pills: Tibetan medicines from exclusivity to ubiquity. Anthropol. Med. 22, 7–22. doi: 10.1080/13648470.2015.1004504
Blaikie, C., and Craig, S. R. (2022). “Making Tibetan medicine in Nepal: industrial aspirations, cooperative relations, and the limits of production,” in Asian medical industries: Contemporary perspectives on traditional pharmaceuticals. eds. S. Kloos and C. Blaikie (London: Routledge), 253–279.
Borges, A., José, H., Homem, V., and Simões, M. (2020). Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from acacia dealbata and Olea Europaea. Antibiotics 9:48. doi: 10.3390/antibiotics9020048
Busfield, J. (2010). A pill for every ill: explaining the expansion in medicine use. Soc. Sci. Med. 70, 934–941. doi: 10.1016/j.socscimed.2009.10.068
Cabinet of Ministers (2015). Republic of Latvia Cabinet Regulation No. 685: Requirements for Food Supplements. Available at: https://likumi.lv/ta/id/278387-prasibas-uztura-bagatinatajiem (Accessed November 02, 2024).
Craig, S. R. (2011). “Good” manufacturing by whose standards? Remaking concepts of quality, safety, and value in the production of Tibetan medicines. Anthropol. Q. 84, 331–378. doi: 10.1353/anq.2011.0027
Craig, S. R. (2012). Healing elements: Efficacy and the social ecologies of Tibetan medicine. Berkeley: University of California Press.
Craig, S. R., and Adams, V. (2008). Global pharma in the land of snows: Tibetan medicines, SARS, and identity politics across nations. Asian Med. 4, 1–28. doi: 10.1163/157342108X381205
Craig, S. R., and Gerke, B. (2016). Naming and forgetting: Sowa Rigpa and the territory of Asian medical systems. Med. Anthropol. Theory 3, 87–122. doi: 10.17157/mat.3.2.350
Craig, S. R., Gerke, B., and Sheldon, V. (2019). Sowa Rigpa Humanitarianism: Local logics of care within a global politics of compassion. Med. Anthropol. Q. 34, 174–191. doi: 10.1111/maq.12561
Czaja, O. (2017). The substitution of Materia Medica in Tibetan medicine: an inquiry into traditional Tibetan treatises. East Asian Sci. Technol. Med. 46, 119–212. doi: 10.1163/26669323-04601008
Dalai Lama. (1992). Nyin sman rtsis khang dbu brnyes pa’i dus dran gyi mdzad sgor chibs bsgyur bka’ drin bskyangs skabs sman rtsis las slob yongs la stsal pa’i bka’ slob. Available at: https://www.mentsee.org/pages/f56f40f60f0bf66fb3f7cf56f0d.php (Accessed September 09, 2024).
Dalai Lama. (2006). Nyin dge slob rnams la stsal ba’i bka’ slob. Available at: https://www.mentsee.org/pages/posts/f66fa4fb1f72f0bf63f7cf0b-f22f20f20f26-f5ffb3f0b-f21f21-f5af7af66-f22f29-f49f72f53f0bf51f42f7af0bf66fb3f7cf56f0bf62fa3f58f66f0bf63f0bf66fa9f63f0bf56f60f72f0bf56f40f60f0bf66fb3f7cf56f0d-643.php (Accessed September 09, 2024).
EC. (2002). Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002, on the approximation of the laws of the Member States relating to food supplements. Available at: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002L0046 (Accessed October 02, 2024).
EC. (2003). Directive 2003/89/EC of the European Parliament and of the Council of 10 November 2003: amending Directive 2000/13/EC as regards indication of the ingredients present in foodstuffs. Available at: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:308:0015:0018:EN:PDF (Accessed October 10, 2024).
EC. (2004). Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004, on the hygiene of foodstuffs. Available at: https://eur-lex.europa.eu/eli/reg/2004/852/oj/lav (Accessed November 15, 2024).
EC. (2006). Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Available at: https://eur-lex.europa.eu/eli/reg/2006/1925/oj (Accessed October 04, 2024).
EFSA (2012). Compendium of botanicals reported to contain naturally Occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 10:2663. doi: 10.2903/j.efsa.2012.2663
Frohlich, P. C., Santos, K. A., Hasan, S. D. M., and Da Silva, E. A. (2022). Evaluation of the Ethanolic ultrasound-assisted extraction from clove (Syzygium Aromaticum) leaves and chemical characterization of the extracts. Food Chem. 373:131351. doi: 10.1016/j.foodchem.2021.131351
Gallo, M., Ferrara, L., and Naviglio, D. (2018). Application of ultrasound in food science and technology: a perspective. Foods. 7:164. doi: 10.3390/foods7100164
Gerke, B. (2012a). ‘Treating the aged’ and ‘maintaining health’: locating bcud len practices in the four Tibetan medical Tantras. J. Int. Assoc. Buddh. Stud. 35, 329–362.
Gerke, B. (2012b). Treating essence with essence: re-inventing bcud len as vitalising dietary supplements in contemporary Tibetan medicine. Asian Med. 7, 196–224. doi: 10.1163/15734218-12341248
Gerke, B. (2015). “Introduction: challenges of translating Tibetan medical texts and medical histories,” in Das Letzte Tantra, aus “Die vier Tantra der Tibetischen Medizin”. ed. F. Ploberger (Schiedlberg: Bacopa), 17–29.
Gerke, B. (2018). The signature of recipes: authorship, intertextuality, and the epistemic genre of Tibetan formulas. Revue d’Etudes Tibétaines 45, 178–220.
Gerke, B. (2019). “Material presentations and cultural drug translations of contemporary Tibetan precious pills,” in Knowledge and context in Tibetan medicine. ed. W. A. McGrath (Leiden, Boston: Brill), 337–367.
Hofer, T. (2014). “Foundations of pharmacology and the compounding of Tibetan medicines,” in Bodies in balance: The art of Tibetan medicine. ed. T. Hofer (Seattle, London: University of Washington Press), 46–63.
Hsu, E. (2009). Chinese propriety medicines: an "alternative modernity?" the case of the anti-malarial substance artemisinin in East Africa. Med. Anthropol. 28, 111–140. doi: 10.1080/01459740902848303
Janes, C. R. (1999). The health transition, global modernity and the crisis of traditional medicine: the Tibetan case. Soc. Sci. Med. 48, 1803–1820. doi: 10.1016/S0277-9536(99)00082-9
Janes, C. R. (2002). Buddhism, science, and market: the globalisation of Tibetan medicine. Anthropol. Med. 9, 267–289. doi: 10.1080/13648470216337
Kloos, S. (2010). Tibetan medicine in exile: The ethics, politics and science of cultural survival. Doctoral Dissertation. Berkeley, San Francisco: University of California.
Kloos, S. (2015). “(Im-)potent knowledges. Preserving ‘traditional’ Tibetan medicine through modern science,” in Fugitive knowledge: The loss and preservation of knowledge in cultural contact zones. eds. A. Beer and G. Mackenthun (Münster, New York: Waxmann), 123–142.
Kloos, S. (2020). Humanitarianism from below: Sowa Rigpa, the traditional pharmaceutical industry, and Global Health. Med. Anthropol. 39, 167–181. doi: 10.1080/01459740.2019.1587423
Kloos, S. (2022). “Good medicines, bad hearts: the social role of the Amchi in a Buddhist Dard community,” in Healing at the periphery: Ethnographies of Tibetan medicine in India. eds. L. Pordié and S. Kloos (Durham, London: Duke University Press), 41–64.
Kloos, S., Madhavan, H., Tidwell, T., Blaikie, C., and Cuomo, M. (2020). The transnational Sowa Rigpa industry in Asia: new perspectives on an emerging economy. Soc. Sci. Med. 245:112617. doi: 10.1016/j.socscimed.2019.112617
Kudlu, C. (2022). “Globalising Ayurveda, Branding India: Implications for the Ayurvedic Pharmaceutical Industry,” in Asian Medical Industries: Contemporary Perspectives on Traditional Pharmaceuticals. eds. S. Kloos and C. Blaikie (London: Routledge), 139–168.
Kudlu, C., and Nichter, M. (2019). Indian imaginaries of Chinese success in the global herbal medicine market: a critical assessment. Asian Med. 14, 104–144. doi: 10.1163/15734218-12341437
Liang, Y., Yang, Y., Zheng, L., Zheng, X., Xiao, D., Wang, S., et al. (2022). Extraction of pectin from passion fruit Peel: composition, structural characterization and emulsion stability. Food. 11:3995. doi: 10.3390/foods11243995
Madhavan, H. (2009). “Commercialising traditional Medicine”: Ayurvedic manufacturing in Kerala. Econ. Polit. Wkly. 44, 44–51.
Madhavan, H. (2017). Below the radar innovations and emerging property right approaches in Tibetan medicine. J. World Intellectual Property 20, 239–257. doi: 10.1111/jwip.12084
Mehta, N., Jeyapriya, S., Kumar, P., Kumar, A. V., Umaraw, P., Kumar, S. K., et al. (2022). Ultrasound-assisted extraction and the encapsulation of bioactive components for food applications. Foods. 11:2973. doi: 10.3390/foods11192973
Millard, C. (2008). “The integration of Tibetan medicine in the United Kingdom: the clinics of the Tara Institute of Medicine,” in Tibetan Medicine in the Contemporary World: Global Politics of Medical Knowledge and Practice. ed. L. Pordié (London: Routledge), 189–214.
Nichter, M. (1996). “Pharmaceuticals, the commodification of health, and the health care-medicine use transition,” in Anthropology and international health: Asian care studies, theory and practice in medical anthropology and international health. eds. M. Nichter and M. Nichter (London and New York: Routledge), 265–326.
Nichter, M., and Thompson, J. J. (2006). For my wellness, not just my illness: North Americans' use of dietary supplements. Cult. Med. Psychiatry 30, 175–222. doi: 10.1007/s11013-006-9016-0
Parfionovitch, Y., Dorje, G., and Meyer, F. (Eds.) (1992). Tibetan Medical Paintings. Illustrations to the Blue Beryl Treatise of Sangye Gyamtso (1653-1705). London: Serindia Publications.
Petryna, A., and Kleinman, A. (2006). “The pharmaceutical Nexus,” in Global pharmaceuticals: Ethics, markets, practices. eds. A. Petryna, A. Lakoff, and A. Kleinman (Durham: Duke University Press), 1–32.
Pordié, L. (2008). “Tibetan medicine today: Neo-traditionalism as an analytical lens and a political tool,” in Tibetan medicine in the contemporary world: Global politics of medical knowledge and practice. ed. L. Pordié (London: Routledge), 3–32.
Pordié, L. (2015). Hangover free! The social and material trajectories of PartySmart. Anthropol. Med. 22, 34–48. doi: 10.1080/13648470.2015.1004773
Pordié, L., and Gaudillière, J.-P. (2014). The reformulation regime in drug discovery: revisiting Polyherbals and property rights in the Ayurvedic industry. East Asian Sci. Technol. Soc. Int. J. 8, 57–79. doi: 10.1215/18752160-2406053
Quintman, A. (2013). The Yogin and the Madman: Reading the Biographical Corpus of Tibet's Great Saint Milarepa. New York: Columbia University Press.
Saxer, M. (2013). Manufacturing Tibetan medicine: The creation of an industry and the moral economy of Tibetanness. New York, Oxford: Berghahn Books.
Schwabl, H., and Vennos, C. (2015). From medical tradition to traditional medicine: a Tibetan formula in the European framework. J. Ethnopharmacol. 167, 108–114. doi: 10.1016/j.jep.2014.10.033
Schrempf, M. (2015). Contested Issues of efficacy and safety between transnational formulation regimes of Tibetan medicines in china and europe. Asian Med. 10, 273–315. doi: 10.1163/15734218-12341360
Siahpush, M. (1998). Postmodern values, dissatisfaction with conventional medicine and popularity of alternative therapies. J. Sociol. 34, 58–70. doi: 10.1177/144078339803400106
van der Valk, Jan M. A. (2017). Alternative pharmaceuticals: The technoscientific becomings of Tibetan medicines in-between India and Switzerland. Doctoral Dissertation. Kent.
Wang, S. (2020). “Circumventing regulation and professional legitimization: the circulation of Chinese medicine between China and France,” in Circulation and governance of Asian medicine. eds. C. Coderey and L. Pordié (London, New York: Routledge), 139–156.
Whyte, S. R., van der Geest, S., and Hardon, A. (2002). The social lives of medicines. Cambridge: Cambridge University Press.
Keywords: Sowa Rigpa (Tibetan medicine), dietary supplements, innovation, herbal extracts, reformulation
Citation: Mundelius P and Sprisevska J (2025) Engaging transnational expertise: creating Sowa Rigpa supplements between South Asia and Europe. Front. Hum. Dyn. 7:1543183. doi: 10.3389/fhumd.2025.1543183
Edited by:
Calum Blaikie, Austrian Academy of Sciences (OeAW), AustriaReviewed by:
Barbara Gerke, University of Vienna, AustriaJan M. A. van der Valk, Independent researcher, Riemst, Belgium
Copyright © 2025 Mundelius and Sprisevska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patricia Mundelius, cC5tdW5kZWxpdXNAZWQuYWMudWs=
 Patricia Mundelius
Patricia Mundelius Julija Sprisevska2
Julija Sprisevska2