Abstract
Objective:
This study aimed to develop a diagnostic model of multi-kinematic parameters for patients with amnestic mild cognitive impairment (aMCI).
Method:
In this cross-sectional study, 94 older adults were included (33 cognitively normal, CN; and 61 aMCI). We conducted neuropsychological battery tests, such as global cognition and cognitive domains, and collected gait parameters by an inertial-sensor gait analysis system. Multivariable regression models were used to identify the potential diagnostic variables for aMCI. Receiver operating characteristic (ROC) curves were applied to assess the diagnostic accuracy of kinematic parameters in discriminating aMCI from healthy subjects.
Results:
Multivariable regression showed that multi-kinematic parameters were the potential diagnostic variables for aMCI. The multi-kinematic parameter model, developed using Timed Up and Go (TUG) time, stride length, toe-off/heel stride angles, one-leg standing (OLS) time, and braking force, showed areas under ROC (AUC), 0.96 [95% confidence interval (CI), 0.905–0.857]; sensitivity, 0.90; and specificity, 0.91. In contrast, a single kinematic parameter’s sensitivity was 0.26–0.95 and specificity was 0.21–0.90. Notably, the separating capacity of multi-kinematic parameters was highly similar to Montreal Cognitive Assessment (MoCA; AUC: 0.96 vs. 0.95). Compared to cognitive domain tests, the separating ability was comparable to Auditory Verbal Learning Test (AVLT) and Boston Naming Test (BNT; AUC: 0.96 vs. 0.97; AUC: 0.96 vs. 0.94).
Conclusion:
We developed one diagnostic model of multi-kinematic parameters for patients with aMCI in Foshan.
Introduction
Alzheimer’s disease (AD), a neurodegenerative disease, is characterized by memory loss or multiple cognitive domain impairment. With rising longevity, AD is becoming a tremendous healthcare challenge worldwide. Although there have been many advances in pathogenesis and diagnosis of AD (Atri, 2019; Tiwari et al., 2019; Hameed et al., 2020). AD treatment is a challenge since there has been only symptomatic therapy available recently. Therefore, effective and accurate early screening of AD is crucial for intervention.
Alzheimer’s disease is a cognitive decline spectrum that includes preclinical and clinical stages. The disease course spans decades. Mild cognitive impairment (MCI) has been considered a prodromal stage of AD. It was divided into different subtypes depending on the impaired cognitive domains. Specifically, amnestic mild cognitive impairment (aMCI) was a transitional phase between normal aging and AD with a high AD conversion rate (Petersen, 2004). The familiar and widely used screening tools for aMCI are neuropsychological tests. The subjectivity and time consuming cannot be inadequate for current needs. Amyloid-42 of cerebrospinal fluid (CSF) and amyloid-positron emission tomography (PET) imaging has been increasingly accepted for the detection of aMCI (Hameed et al., 2020). However, given the invasiveness and high cost, CSF and PET imaging biomarkers were limited in clinical application (Guzman-Martinez et al., 2019). Therefore, more objective and convenient screening tools are necessary to identify aMCI accurately.
Recently, there has been a growing interest in motoric cognitive risk (MCR) syndrome, characterized as cognitive and motor dysfunction in older people (Verghese et al., 2013, 2014). Motor dysfunction is evaluated by multiple motion parameters usually, such as stride speed, stride length, stride time and variability, and so forth. Studies have found that motor and cognitive performance decline was paralleled in AD progression (Allali et al., 2016). In particular, stride speed was slow among individuals with early cognitive impairment (Knapstad et al., 2019), and stride length was shorter in patients with aMCI than in normal controls (Xie et al., 2019). Additionally, with the inertial-sensor gait analysis system integrated into applications, more spatial-temporal gait parameters were available (Prasanth et al., 2021).
There has been little research to investigate the diagnostic accuracy of gait parameters for identifying aMCI. Toosizadeh et al. (2019) applied objective tools for screening early AD and aMCI among oldest-old participants. The results showed that the cognitive status (both MCI and AD) was predicted with an area under the receiver operating characteristic (AUC) of 0.83 (sensitivity, 0.82; specificity, 0.72) (Toosizadeh et al., 2019). The task in the study was mainly based on elbow flexion with the dominant arm but not the gait parameters during walking. Thus, it was more suitable for the oldest-old or the elders with mobility impairments.
Additionally, several studies reported the diagnostic ability of motion parameters in MCI. Nielsen et al. (2018) suggested that the dual-task gait test, which was walking while simultaneously performing a cognitive challenge, had a high discriminative ability to separate patients with MCI from healthy controls (Nielsen et al., 2018). Another study evaluated MCI participants by a complex walking test. The results showed the sensitivity and specificity of motion parameters were 78 and 90% (Perrochon and Kemoun, 2014). The two studies showed that motion parameters had a high discriminative ability to separate MCI. However, MCI was a heterogeneous condition. It is necessary to focus on the aMCI subtype. In addition, motor functions were evaluated by either single parameter or complicated tasks in the two studies. The single parameter reflected a specific but not the overall motor function. In addition, the complex processes reduced the maneuverability in clinical practice. Hence, applying multi-kinematic parameters of relatively simple motor tasks might be preferable to distinguish aMCI from healthy elderly.
Therefore, this study investigated the diagnostic accuracy of multi-kinematic parameters collected by a sensor gait analysis system in simple gait tasks to distinguish aMCI.
Methods
Study design
This study is a cross-sectional study.
Participants
From 101 participants initially recruited, we excluded seven cases because of the diagnosis of AD, and the neuropsychological test data were unqualified (Figure 1). Ninety-four older adults were included for analysis. Sixty-one participants were diagnosed with aMCI at neurological clinics of First People’s Hospital of Foshan between January 2020 and December 2020. Thirty-three cognitive normal (CN) older adults with matching demographic information (age, sex, and education level) were enrolled in the communities. Demographic characteristics, medical history, and Hamilton Depression Scale (HAMD) scores were collected during face-to-face interviews.
FIGURE 1
Flow diagram of participants.
Inclusion criteria of aMCI were as follows (Petersen, 2004): (1) subjective cognitive complaint, preferably confirmed by an informant; (2) a single domain or multi-domain of memory decline. Abnormal objective cognitive domain impairment was identified by a cutoff of 1.5 SD, below education and age matched-specific norms; (3) preserved activities of daily living were confirmed by a clinician’s interviews; and (4) Global Clinical Dementia Rating (CDR) = 0.5.
Exclusion criteria for all participants were as follows: (1) illiteracy; (2) neurologic disorder and other systematic diseases that would likely contribute to cognitive and motor deficits (history of stroke, Parkinson’s disease, epilepsy, brain trauma, etc.), active rheumatic and orthopedic diseases that affect lower limbs, and history of knee/hip replacement; and (3) use of neuroleptics or benzodiazepines and psychiatric comorbidity (e.g., significant depressive/anxiety).
Ethics approval was obtained from the Research Ethics Board of the First People’s Hospital of Foshan. All participants signed written consent forms at the time of registration.
Neuropsychological assessment
A neuropsychological test battery was carried out: global cognition was assessed using Montreal Cognitive Assessment (MoCA) (Julayanont et al., 2015) and CDR (Morris, 1993); memory with Auditory Verbal Learning Test (AVLT) that included immediate memory (AVLT_IM), short-delayed memory (AVLT_SDM, 5-min recalls), and long-delayed memory (AVLT_LDM, 20-min recalls) (Guo et al., 2001); language with Boston Naming Test (BNT); executive function with Stroop Color-Word Test (SCWT) that included part A, part B, and part C; attention with Symbol Digit Modalities Test (SDMT); and visual-spatial ability with Clock Drawing Test (CDT).
Gait measurements
The gait data were collected by the JiBuEn® gait analysis system (Tao et al., 2018; Gao et al., 2021). The system comprises wearable shoes and modules with the inertial Micro-Electro-Mechanical Systems (MEMS) sensors fixed under the shoe heel bottom, behind the upper and lower limbs, and wrist. The modules collected motion signals and transmitted them to a computer. The high-order low-pass filter and hexahedral calibration technique were employed in data preprocessing, which reduces high-frequency noise interference and installation errors produced by sensor devices. Moreover, the accumulative errors were also corrected based on the zero-correction algorithm. The final gait parameters were obtained by fusing acceleration data and posture, calculated using the quaternary complementary filtering technique.
Relative simple gait tasks, i.e., Free Walking, Timed Up and Go (TUG), and closed-eyed one-leg standing (OLS) tests, were implemented in this study. The gait parameters were collected during the gait tasks without cognitive challenge. Participants needed to walk on a walkway at their usual pace for the Free Walking test. The walkway was in a quiet, well-lit room. Participants wore specially made shoes walking for 30 s. The parameters of stride length, stride speed, cadence, stride time, toe-off/heel strike angles, and braking force came from the Free Walking test. TUG test measures in seconds, which was the time needed to rise from a chair, walk 3 m, turn around, and return to a seated position at a faster speed. Three times were tested for each subject. The mean time of three times was used for analysis. During the OLS test, participants were needed to stretch both arms to the sides, raise one leg about 5 cm from the floor, and stand up for as long as possible. The time of maintaining this position was measured. The subjects performed three attempts on each lower limb. The test ended when the subjects used the raised foot to touch the floor, moved the supporting leg on the ground, or observed a significant loss of balance. During the test, the examiner guarded the subject to prevent fell. The longest time of the three measurements was used for the analysis.
Statistical analysis
The comparison of characteristics used a t-test or chi-square test between the two groups. Multivariable regression models were used to identify the potential diagnostic variables for aMCI. Age, sex, education, body mass index (BMI), hypertension history, and diabetes history were adjusted. Receiver operating characteristic (ROC) curves, constructed using bootstrap resampling (times = 500), were used to assess the diagnostic accuracy of multi-kinematic parameters in discriminating aMCI from healthy subjects. We drew ROC curves to plot the true positive values (sensitivity) against the false positive values (1-specificity) for different possible cutoff values of the respective markers. The ROC curves illustrate the ability of various parameters to classify healthy controls and aMCI correctly. The AUC values were calculated to measure the parameter’s overall accuracy.
Analyses were conducted by the statistical software packages R1 (the R Foundation) and Empower Stats2 (X&Y Solutions, Inc., Boston, MA, United States). All the p-values <0.05 were considered statistically significant.
Results
Participants characteristics
In total, 94 participants were included in the study, i.e., 61 elderly diagnosed with aMCI. Clinical dates are presented in Table 1—no significant group differences in demographic characteristics, visual-spatial ability, cadence, stride speed, and stride time. Compared to CN, participants with aMCI had poorer cognitive performance (such as global cognition, memory, language, executive function, and attention), longer TUG time, shorter stride length, smaller toe-off/heel stride angle, worse braking force, and OLS performance.
TABLE 1
| All (n = 94) |
NC (n = 33) |
aMCI (n = 61) |
P-value§ | |
| Demographic characteristics | ||||
| Age, y | 67.22 ± 4.36 | 68.03 ± 3.96 | 66.79 ± 4.53 | 0.188 |
| Sex (male), n% | 44 (46.81) | 14 (42.42) | 30 (49.18) | 0.531 |
| Education, n% | 0.503▲ | |||
| Primary school | 26 (27.66) | 8 (24.24) | 18 (29.51) | |
| Secondary school | 30 (31.91) | 9 (27.27) | 21 (34.43) | |
| Above secondary school | 38 (40.43) | 16 (48.48) | 22 (36.07) | |
| BMI, kg/m2 | 23.19 ± 2.70 | 22.94 ± 2.69 | 23.32 ± 2.71 | 0.526 |
| Hypertension history, yes, n% | 34 (36.17) | 15 (45.45) | 19 (31.15) | 0.168▲ |
| Diabetes history, yes, n% | 8 (8.51) | 5 (15.15) | 3 (4.92) | 0.090▲ |
| Neuropsychological assessment | ||||
| MoCA, scores | 24.37 ± 3.80 | 26.95 ± 2.08 | 22.80 ± 3.26 | < 0.001 |
| AVLT_immediate recall | 4.49 ± 1.90 | 5.76 ± 1.62 | 3.80 ± 1.68 | < 0.001 |
| AVLT-SD (5-min delayed recall) | 5.35 ± 2.61 | 7.33 ± 1.57 | 4.27 ± 2.43 | < 0.001 |
| AVLT-LD (20-min delayed recall) | 4.30 ± 2.90 | 6.58 ± 1.94 | 3.05 ± 2.56 | < 0.001 |
| CDT | 8.47 ± 2.07 | 9.00 ± 1.48 | 8.18 ± 2.30 | 0.069 |
| SCWT-A | 12.33 ± 9.06 | 12.09 ± 14.13 | 12.46 ± 4.30 | 0.853 |
| SCWT-B | 18.55 ± 6.97 | 16.03 ± 6.44 | 19.97 ± 6.90 | 0.009 |
| SCWT-C | 37.76 ± 18.25 | 32.76 ± 15.21 | 40.56 ± 19.31 | 0.049 |
| BNT | 20.91 ± 4.09 | 23.58 ± 3.00 | 19.45 ± 3.89 | < 0.001 |
| SDMT | 34.66 ± 10.71 | 39.82 ± 6.60 | 31.67 ± 11.52 | < 0.001 |
| Kinematics parameters | ||||
| TUG time, s | 15.26 ± 2.76 | 14.32 ± 2.36 | 15.76 ± 2.85 | 0.015 |
| Stride length, cm | ||||
| Left | 110.51 ± 15.80 | 115.85 ± 13.53 | 107.62 ± 16.28 | 0.014 |
| Right | 110.46 ± 14.96 | 111.70 ± 11.15 | 109.79 ± 16.71 | 0.557 |
| Stride speed, m/s | ||||
| Left | 0.88 ± 0.15 | 0.90 ± 0.14 | 0.87 ± 0.16 | 0.326 |
| Right | 0.88 ± 0.15 | 0.90 ± 0.14 | 0.87 ± 0.16 | 0.326 |
| Cadence, steps/min | ||||
| Left | 95.89 ± 11.02 | 95.32 ± 10.78 | 96.20 ± 11.23 | 0.713 |
| Right | 95.89 ± 11.02 | 95.32 ± 10.78 | 96.20 ± 11.23 | 0.713 |
| Stride time, s | ||||
| Left | 1.29 ± 0.17 | 1.31 ± 0.19 | 1.27 ± 0.16 | 0.351 |
| Right | 1.25 ± 0.16 | 1.24 ± 0.14 | 1.25 ± 0.16 | 0.715 |
| Toe stride angle, degrees | ||||
| Left | 41.36 ± 4.64 | 42.47 ± 4.05 | 40.76 ± 4.86 | 0.089 |
| Right | 41.17 ± 4.20 | 42.75 ± 3.63 | 40.31 ± 4.26 | 0.006 |
| Heel stride angle, degrees | ||||
| Left | 28.59 ± 9.47 | 31.46 ± 4.61 | 27.03 ± 10.99 | 0.030 |
| Right | 28.42 ± 9.29 | 30.89 ± 4.22 | 27.08 ± 10.92 | 0.057 |
| Braking force | 0.82 ± 0.10 | 0.86 ± 0.08 | 0.79 ± 0.10 | < 0.001 |
| One-leg standing test, s | ||||
| Right | 6.04 ± 8.06 | 7.45 ± 12.32 | 5.27 ± 4.15 | 0.212 |
| Left | 6.32 ± 9.22 | 9.18 ± 13.08 | 4.75 ± 5.71 | 0.026 |
Characteristics of the study participants.
BMI, body mass index; MoCA, Montreal Cognitive Assessment-Basic; AVLT, Auditory Verbal Learning Test; BNT, Boston Naming Test; SDMT, Symbol Digit Modalities Test; CDT, Clock Drawing Test; SCWT, Stroop Color-Word Test. Values of p < 0.05 are bolded. Bold font indicates they have statistical significance. §Comparison based on unpaired t-test or chi-square test (▲).
Multivariable regression of kinematic parameters and amnestic mild cognitive impairment
Table 2 shows that the potential diagnostic kinematic parameters for aMCI are TUG time, stride length, toe off/heel stride angle, OLS time, and braking force [TUG time, odds ratio (OR) = 1.31, 95% confidence interval (CI) = 1.04, 1.66; left stride length, OR = 0.95, 95% CI = 0.92, 0.99; right toe off stride angle, OR = 0.79, 95% CI = 0.66, 0.94; left heel stride angle, OR = 0.79, 95% CI = 0.68, 0.93; right heel stride angle, OR = 0.80, 95% CI = 0.69, 0.94; left leg standing time, OR = 0.93, 95% CI = 0.86, 1.00; and braking force, OR = 0.87, 95% CI = 0.80, 0.94].
TABLE 2
| Crude | Model I | Model II | |
|
|
|||
| OR (95%CI) P | |||
| TUG time | 1.23 (1.04, 1.47) 0.018 | 1.31 (1.04, 1.65) 0.020 | 1.31 (1.04, 1.66) 0.024 |
| Left stride length | 0.97 (0.94, 0.99) 0.018 | 0.96 (0.92, 0.99) 0.019 | 0.95 (0.92, 0.99) 0.019 |
| Right stride length | 0.99 (0.96, 1.02) 0.553 | 0.99 (0.95, 1.02) 0.447 | 0.98 (0.94, 1.02) 0.396 |
| Left stride speed | 0.24 (0.01, 4.05) 0.323 | 0.21 (0.01, 6.51) 0.375 | 0.19 (0.01, 6.49) 0.357 |
| Right stride speed | 0.24 (0.01, 4.05) 0.323 | 0.21 (0.01, 6.51) 0.375 | 0.19 (0.01, 6.49) 0.357 |
| Left cadence | 1.01 (0.97, 1.05) 0.709 | 1.02 (0.97, 1.07) 0.553 | 1.02 (0.96, 1.07) 0.569 |
| Right cadence | 1.01 (0.97, 1.05) 0.709 | 1.02 (0.97, 1.07) 0.553 | 1.02 (0.96, 1.07) 0.569 |
| Left stride time | 0.31 (0.03, 3.62) 0.348 | 0.20 (0.01, 3.98) 0.291 | 0.20 (0.01, 4.88) 0.324 |
| Right stride time | 1.68 (0.11, 26.82) 0.712 | 1.45 (0.03, 68.38) 0.849 | 1.13 (0.02, 57.81) 0.952 |
| Left toe off angle | 0.92 (0.83, 1.01) 0.092 | 0.90 (0.78, 1.03) 0.109 | 0.89 (0.77, 1.03) 0.113 |
| Right toe off angle | 0.86 (0.76, 0.96) 0.009 | 0.82 (0.69, 0.96) 0.015 | 0.79 (0.66, 0.94) 0.007 |
| Left heel stride angle | 0.88 (0.80, 0.98) 0.014 | 0.83 (0.73, 0.95) 0.007 | 0.79 (0.68, 0.93) 0.003 |
| Right heel stride angle | 0.90 (0.82, 1.00) 0.044 | 0.82 (0.71, 0.95) 0.007 | 0.80 (0.69, 0.94) 0.006 |
| Right leg standing time | 0.97 (0.91, 1.02) 0.242 | 0.97 (0.91, 1.04) 0.368 | 0.96 (0.90, 1.03) 0.254 |
| Left leg standing time | 0.95 (0.89, 1.00) 0.053 | 0.95 (0.88, 1.01) 0.093 | 0.93 (0.86, 1.00) 0.047 |
| Braking force | 0.92 (0.87, 0.97) 0.001 | 0.88 (0.82, 0.95) 0.000 | 0.87 (0.80, 0.94) 0.000 |
Regression analyses for kinematics parameters and amnestic mild cognitive impairment (aMCI).
Model I adjust age, sex, and education; Model II adjusts age, sex, education, BMI, Hypertension history, and diabetes history. Bold font indicates they have statistical significance.
Diagnostic accuracy of kinematic parameters for amnestic mild cognitive impairment
Receiver operating characteristic curves were plotted to compare how gait tests differentiated aMCI from healthy people. Multi-kinematic parameters showed an AUC of 0.83, specificity of 0.82, sensitivity of 0.75, and accuracy of 0.77. When age, sex, and education were combined, the multi-kinematic parameters showed an AUC of 0.96, specificity of 0.91, sensitivity of 0.90, and accuracy of 0.90. In contrast, one single kinematic parameter’s sensitivity and specificity were 0.26–0.95 and 0.21–0.90 (Figure 2 and Tables 3, 4).
FIGURE 2
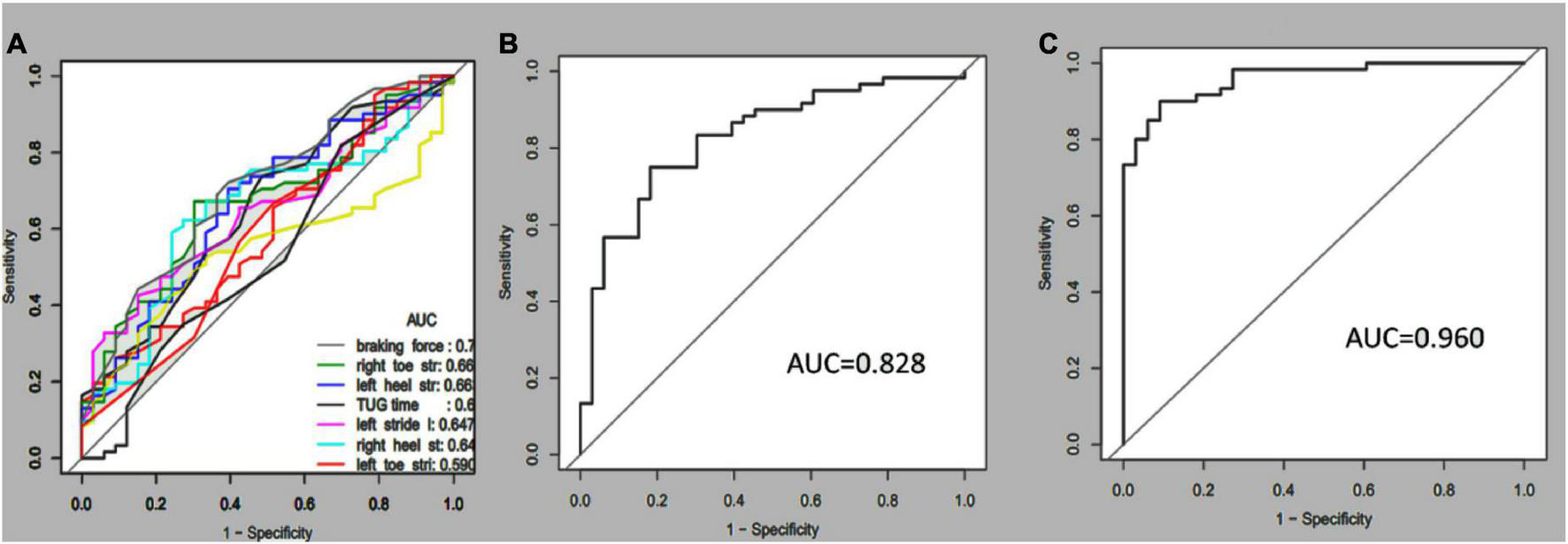
Receiver-operating characteristic (ROC) curves and corresponding area under the curve (AUC) of the single (A), multi-kinematic (B), parameters, and combined multi-kinematic parameters with age, sex, and education (C) to separate amnestic mild cognitive impairment (aMCI) from healthy controls.
TABLE 3
| AUC | 95%CI | Specificity | Sensitivity | Accuracy | |
| Single parameter | |||||
| TUG time | 0.651 | 0.534–0.766 | 0.515 | 0.737 | 0.659 |
| Left toe stride angle | 0.590 | 0.471–0.766 | 0.909 | 0.262 | 0.489 |
| Right toe stride angle | 0.668 | 0.556–0.779 | 0.697 | 0.672 | 0.680 |
| Left heel stride angle | 0.663 | 0.541–0.778 | 0.606 | 0.704 | 0.670 |
| Right heel stride angle | 0.646 | 0.528–0.762 | 0.727 | 0.623 | 0.659 |
| Left stride length | 0.646 | 0.535–0.758 | 0.848 | 0.426 | 0.574 |
| Right stride length | 0.544 | 0.427–0.660 | 0.667 | 0.525 | 0.574 |
| Braking force | 0.705 | 0.596–0.814 | 0.606 | 0.721 | 0.680 |
| Left OLS time | 0.584 | 0.460–0.706 | 0.212 | 0.950 | 0.688 |
| Right OLS time | 0.534 | 0.408–0.660 | 0.303 | 0.817 | 0.634 |
| Multiple parameters | 0.828 | 0.741–0.915 | 0.818 | 0.750 | 0.774 |
Diagnostic ability of single/multiple kinematic parameters in amnestic mild cognitive impairment (aMCI).
Bold values indicates they have statistical significance.
TABLE 4
| AUC | 95% CI | Specificity | Sensitivity | Accuracy | P § | |
| Model 1 | 0.758 | 0.659–0.857 | 0.878 | 0.606 | 0.702 | – |
| Model 1 + Multi-kinematic parameters | 0.960 | 0.905–0.981 | 0.909 | 0.900 | 0.903 | – |
| Model 1 + MoCA | 0.954 | 0.897–0.979 | 0.969 | 0.833 | 0.881 | 0.731 |
| Model 1 + AVLT_SD | 0.966 | 0.910–0.985 | 0.878 | 0.966 | 0.934 | 0.895 |
| Model 1 + AVLT_LD | 0.949 | 0.889–0.975 | 0.909 | 0.864 | 0.880 | 0.732 |
| Model 1 + BNT | 0.940 | 0.877–0.975 | 0.969 | 0.830 | 0.880 | 0.495 |
| Model 1 + SDMT | 0.842 | 0.739–0.917 | 0.697 | 0.892 | 0.820 | 0.006 |
| Model 1 + CDT | 0.772 | 0.663–0.858 | 0.878 | 0.593 | 0.695 | <0.001 |
| Model 1 + SCWT_B | 0.813 | 0.703–0.886 | 0.757 | 0.827 | 0.802 | 0.001 |
| Model 1 + SCWT_C | 0.800 | 0.689–0.874 | 0.818 | 0.724 | 0.758 | <0.001 |
Diagnostic accuracy of multi-kinematic parameters compared to neuropsychological tests in amnestic mild cognitive impairment (aMCI).
Model 1 included age, sex, and education. Bold font indicates they have statistical significance. §The p-value was the model compared to Model 1 + multi-kinematic parameters.
Furthermore, the separating capacity of combined multi-kinematic parameters was highly similar to MoCA (AUC: 0.96 vs. 0.95; specificity: 0.91 vs. 0.97; sensitivity: 0.90 vs. 0.83; p = 0.731). Compared to cognitive domains tests, the separating capacity was comparable to AVLT (AVLT_SDM, AUC: 0.96 vs. 0.97; specificity: 0.91 vs. 0.88; sensitivity: 0.90 vs. 0.97; p = 0.895; AVLT_LDM, AUC: 0.96 vs. 0.95; specificity: 0.91 vs. 0.91; sensitivity: 0.90 vs. 0.86; p = 0.732) and BNT (AUC: 0.96 vs. 0.94; specificity: 0.91 vs. 0.97; sensitivity: 0.90 vs. 0.83; p = 0.495), superior to SDMT, CDT, and SCWT (Figure 3 and Table 4).
FIGURE 3
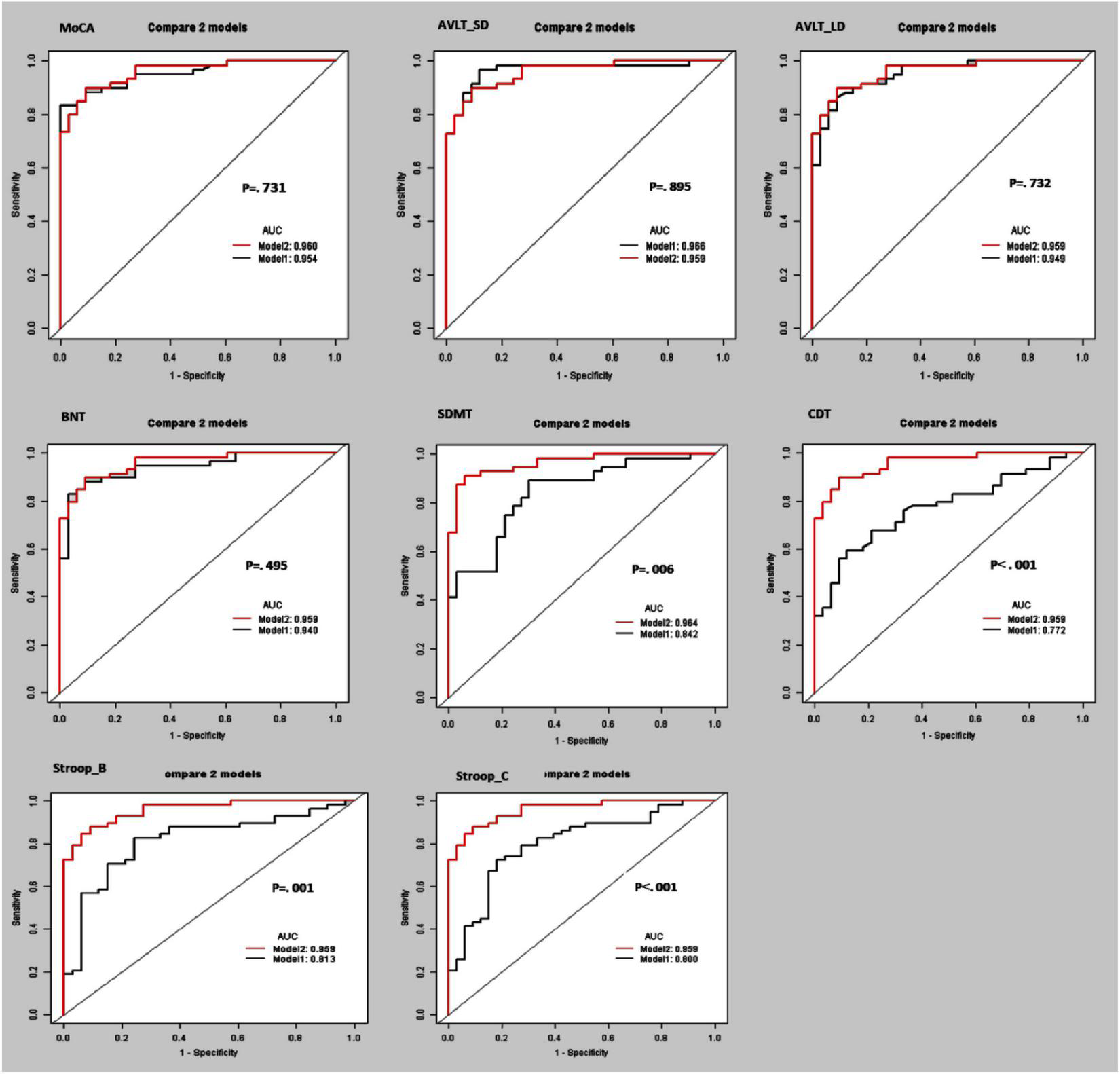
Receiver operating curve (ROC) curves and area under the ROC curve (AUC) of the multi-kinematic parameters (Model 2) when compared to neuropsychological tests (Model 1) to separate amnestic mild cognitive impairment (aMCI) from cognitive normal elderly.
Discussion
The present study developed one diagnostic model of multi-kinematic parameters for patients with aMCI. The model used TUG time, stride length, toe-off/heel stride angles, OLS time, and braking force. These findings have been observed for the first time in the elderly with aMCI.
Previous evidence suggested that motor function was disturbances among the elderly with aMCI. Studies reported that aMCI had slow stride speed (Ali et al., 2022), short stride length, and reduced gait stability (Xie et al., 2019). In addition, OLS performance was significantly worse in aMCI than in the CN subjects (Fujisawa et al., 2017). Furthermore, a few studies attempted to explore if gait tests can be an essential clinical detection tool for MCI. Åhman et al. (2020) suggested that the TUG test might be a useful complementary tool for diagnosing MCI. In another study, gait tests could distinguish MCI from depressive elderly (Metzger et al., 2016). Interestingly, the dominant arm elbow flexion test screened early AD and aMCI among the oldest-old participants. Results showed that the parameters could discriminate aMCI with sensitivity and specificity of 0.82 and 0.72 (AUC = 0.83) (Toosizadeh et al., 2019).
Given these studies, it tends to use gait parameters to screen for preclinical AD. However, the problem of selecting gait tests and kinematic parameters remains unsolved. The kinematic parameters showed a remarkable distinguishability for cognitive impairment, whether tested by TUG, motor-cognitive tasks, or arm elbow flexion. However, the multi-task TUG test was based on traditional tests. It is complex and labor-intensive. The kinematic parameters in the studies indicated certain aspects but not the comprehensive motor function. In addition, the participants were MCI elderly in most of the studies. MCI was a heterogeneous cognitive disorder. The conversion rate was 10–15% per year (Eshkoor et al., 2015). Thus, it is crucial to give more attention to aMCI, a subtype of the highest possible converted to AD.
With advancements in sensor technology, it has been possible to conveniently and automatically collect multiple temporal-spatial kinetic parameters. The inertial-sensor gait analytic system collected real-time and high-precision gait information by integrating electronic components, such as pressure, curvature, accelerometer, and gyroscope. Combined with an integrated angle meter module worn on limbs, the dynamic angle information can be obtained by blending it with foot information. Therefore, the inertial-sensor gait analytic system automatically provides comprehensive knowledge of individual gait (Tao et al., 2018; Prasanth et al., 2021).
This study investigated the diagnostic ability of multi-kinematic parameters by an inertial-sensor gait analytic system in aMCI. We obtained a diagnostic model using multi-kinematic parameters, with a sensitivity of 0.90, specificity of 0.91, and accuracy of 0.90 (AUC = 0.96). The multi-kinematic parameters included TUG time, toe-off/heel stride angle, stride length, braking force, and OLS time. Previous studies well-documented the significance of these kinematic parameters. TUG test assesses essential mobility skills and strength, balance, and agility (Bennell et al., 2011). It is reliable, safe, and time-efficient to evaluate overall functional mobility. Short stride length variability and high stride length variability predicted adverse clinical events in older adults (Bytyçi and Henein, 2021). Based on the inertial-sensor gait analysis system, the toe-off and heel stride angles were indicators of falling risk (Kanzler et al., 2015). Braking force was a gait marker associated with gait stability. It was weaker in patients with AD when compared to healthy elderly (Cheng et al., 2020). OLS test is one of the balance tests (Michikawa et al., 2009). Short standing time was associated with repeatedly falling in MCI elderly. Hence, this study improved convenience and automation by an inertial-sensor gait analytic system while considering comprehensive and sensitive parameters to reflect motion functions.
Furthermore, the separating capacity of multi-kinematic parameters was highly similar to MoCA; when compared to cognitive domains tests, the separating ability was comparable to AVLT and BNT. It is well-known that substantial memory impairment is the characteristic of aMCI (Albert et al., 2011; Jester et al., 2021). Additionally, language decline was one characteristic of aMCI independent of memory decline (Sherman et al., 2021). The shared neural substrate was the neuropathology mechanism of motor-cognition function decline. Hippocampus (Rosso et al., 2017), gray matter volume of frontal (Doi et al., 2017), and cortical thickness of bilateral superior temporal gyri (Ali et al., 2022) were associated with cognition and motor simultaneously. Thus, the results also verified the diagnostic accuracy of multi-kinematic parameters in distinguishing aMCI.
Overall, our results suggested that (1) the multi-kinematic parameters, such as TUG time, stride length, toe-off/heel stride angles, OLS time, and braking force, were potential diagnostic kinematic parameters for aMCI. (2) The model of multi-kinematic parameters helps doctors to initially identify the high risk of aMCI. These findings have significant implications for early, objective, and convenient discerning aMCI. Moreover, the inertial-sensor gait analytic system assessed the multi-kinematic parameters that might be accurate and suitable for dynamic motor and cognitive function monitoring.
The current study has some limitations that need to be considered. Firstly, this study is a cross-sectional design with its inherent deficiency. A follow-up examination is necessary for revealing the association between multi-kinematic parameters and aMCI in depth. Secondly, we did not classify aMCI due to the sample size limitation. More subjects are needed to analyze the value of multi-kinematic parameters in future screening and diagnosis of simple aMCI.
Implications
This study has important clinical and practical implications for screening for early AD. The multi-kinematic parameters were automated and collected by an inertial-sensor-based gait analysis system. Specifically, the collections of the gait parameters were completed under simple gait tasks without cognitive challenge. If the findings are replicated in more extensive studies, they could represent a useful screening tool for early AD in clinical practice.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Board of the First People’s Hospital of Foshan. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SH, HX, SP, and XH contributed to the conception of the study and design, study supervision, interpretation of data, and manuscript preparation. CZ, YW, LC, and ST helped perform the analysis and critical revision of the manuscript. YL, PS, and JL contributed to acquiring kinetic parameters. WZ, QH, and BL contributed to purchasing neuropsychological evaluation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (Grant No. 2018YFC2001700), the Special Fund of the Foshan Summit plan (Grant No. 2019A011), and the Foshan Science and Technology Bureau Project (Grant No. 1920001001161).
Acknowledgments
We thank all participants in this study. We want to thank the team of the First People’s Hospital of Foshan who administered the cognitive evaluation and the gait monitoring.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Åhman H. B. Cedervall Y. Kilander L. Giedraitis V. Berglund L. McKee K. J. et al (2020). Dual-task tests discriminate between dementia, mild cognitive impairment, subjective cognitive impairment, and healthy controls - a cross-sectional cohort study.BMC Geriatr.20:258. 10.1186/s12877-020-01645-1
2
Albert M. S. DeKosky S. T. Dickson D. Dubois B. Feldman H. H. Fox N. C. et al (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease.Alzheimers Dement.7270–279. 10.1016/j.jalz.2011.03.008
3
Ali P. Labriffe M. Paisant P. Custaud M. A. Annweiler C. Dinomais M. (2022). Associations between gait speed and brain structure in amnestic mild cognitive impairment: a quantitative neuroimaging study.Brain Imag. Behav.16228–238. 10.1007/s11682-021-00496-7
4
Allali G. Annweiler C. Blumen H. M. Callisaya M. L. De Cock A. M. Kressig R. W. et al (2016). Gait phenotype from mild cognitive impairment to moderate dementia: results from the GOOD initiative.Eur. J. Neurol.23527–541. 10.1111/ene.12882
5
Atri A. (2019). The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management.Med. Clin. North Am.103263–293. 10.1016/j.mcna.2018.10.009
6
Bennell K. Dobson F. Hinman R. (2011). Measures of physical performance assessments: Self-Paced Walk Test (SPWT), Stair Climb Test (SCT), Six-Minute Walk Test (6MWT), Chair Stand Test (CST), Timed Up & Go (TUG), Sock Test, Lift and Carry Test (LCT), and Car Task.Arthritis Care Res.11S350–S370. 10.1002/acr.20538
7
Bytyçi I. Henein M. Y. (2021). Stride Length Predicts Adverse Clinical Events in Older Adults: A Systematic Review and Meta-Analysis.J. Clin. Med.10:2670. 10.3390/jcm10122670
8
Cheng Q. Wu M. Wu Y. Hu Y. Kwapong W. R. Shi X. et al (2020). Weaker Braking Force, A New Marker of Worse Gait Stability in Alzheimer Disease.Front. Aging Neurosci.12:554168. 10.3389/fnagi.2020.554168
9
Doi T. Blumen H. M. Verghese J. Shimada H. Makizako H. Tsutsumimoto K. et al (2017). Gray matter volume and dual-task gait performance in mild cognitive impairment.Brain Imag. Behav.11887–898. 10.1007/s11682-016-9562-1
10
Eshkoor S. A. Hamid T. A. Mun C. Y. Ng C. K. (2015). Mild cognitive impairment and its management in older people.Clin. Interv. Aging10687–693. 10.2147/CIA.S73922
11
Fujisawa C. Umegaki H. Okamoto K. Nakashima H. Kuzuya M. Toba K. et al (2017). Physical Function Differences Between the Stages From Normal Cognition to Moderate Alzheimer Disease.J. Am. Med. Dir. Assoc.18368.e9–368.e15. 10.1016/j.jamda.2016.12.079
12
Gao Q. Lv Z. Zhang X. Hou Y. Liu H. Mengyue W. G. et al (2021). Validation of the JiBuEn§system in measuring gait parameters.Adv. Intell. Syst. Comput.1378526–531.
13
Guo Q. Lv C. Z. Hong Z. (2001). Reliability and validity of auditory verbal learning test on Chinese elderly patients.Chin. Ment. Health113–15. 10.3321/j.issn:1000-7091.1992.02.001
14
Guzman-Martinez L. Maccioni R. B. Farías G. A. Fuentes P. Navarrete L. P. (2019). Biomarkers for Alzheimer’s Disease.Curr. Alzheimer Res.16518–528. 10.2174/1567205016666190517121140
15
Hameed S. Fuh J. L. Senanarong V. Ebenezer E. G. M. Looi I. Dominguez J. C. et al (2020). Role of Fluid Biomarkers and PET Imaging in Early Diagnosis and its Clinical Implication in the Management of Alzheimer’s Disease.J. Alzheimers Dis. Rep.421–37. 10.3233/ADR-190143
16
Jester D. J. Andel R. Cechová K. Laczó J. Lerch O. Marková H. et al (2021). Cognitive Phenotypes of Older Adults with Subjective Cognitive Decline and Amnestic Mild Cognitive Impairment: The Czech Brain Aging Study.J. Int. Neuropsychol. Soc.27329–342. 10.1017/S1355617720001046
17
Julayanont P. Tangwongchai S. Hemrungrojn S. Tunvirachaisakul C. Phanthumchinda K. Hongsawat J. et al (2015). The Montreal Cognitive Assessment-Basic: A Screening Tool for Mild Cognitive Impairment in Illiterate and Low-Educated Elderly Adults.J. Am. Geriatr. Soc.632550–2554. 10.1111/jgs.13820
18
Kanzler C. M. Barth J. Rampp A. Schlarb H. Rott F. Klucken J. et al (2015). “Inertial sensor based and shoe size independent gait analysis including heel and toe clearance estimation,” in Conference: 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), (Italy: A.C. Milan), 5424–5427. 10.1109/EMBC.2015.7319618
19
Knapstad M. K. Steihaug O. M. Aaslund M. K. Nakling A. Naterstad I. F. Fladby T. et al (2019). Reduced Walking Speed in Subjective and Mild Cognitive Impairment: A Cross-Sectional Study.J. Geriatr. Phys. Ther.42E122–E128. 10.1519/JPT.0000000000000157
20
Metzger F. G. Hobert M. A. Ehlis A. C. Hasmann S. E. Hahn T. Eschweiler G. W. et al (2016). Dual Tasking for the Differentiation between Depression and Mild Cognitive Impairment.Front. Aging Neurosci.8:235. 10.3389/fnagi.2016.00235
21
Michikawa T. Nishiwaki Y. Takebayashi T. Toyama Y. (2009). One-leg standing test for elderly populations.J. Orthop. Sci.14675–685. 10.1007/s00776-009-1371-6
22
Morris J. C. (1993). The clinical dementia rating (CDR): current version and scoring rules.Neurology432412–2414. 10.1212/wnl.43.11.2412-a
23
Nielsen M. S. Simonsen A. H. Siersma V. Hasselbalch S. G. Hoegh P. (2018). The Diagnostic and Prognostic Value of a Dual-Tasking Paradigm in a Memory Clinic.J. Alzheimers Dis.611189–1199. 10.3233/JAD-161310
24
Perrochon A. Kemoun G. (2014). The Walking Trail-Making Test is an early detection tool for mild cognitive impairment.Clin. Interv. Aging9111–119. 10.2147/CIA.S53645
25
Petersen R. C. (2004). Mild cognitive impairment as a diagnostic entity.J. Intern. Med.256183–194. 10.1111/j.1365-2796.2004.01388.x
26
Prasanth H. Caban M. Keller U. Courtine G. Ijspeert A. Vallery H. et al (2021). Wearable Sensor-Based Real-Time Gait Detection: A Systematic Review.Sensors21:2727. 10.3390/s21082727
27
Rosso A. L. Verghese J. Metti A. L. Boudreau R. M. Aizenstein H. J. Kritchevsky S. et al (2017). Slowing gait and risk for cognitive impairment: The hippocampus as a shared neural substrate.Neurology89336–342. 10.1212/WNL.0000000000004153
28
Sherman J. C. Henderson C. R. Jr. Flynn S. Gair J. W. Lust B. (2021). Language Decline Characterizes Amnestic Mild Cognitive Impairment Independent of Cognitive Decline.J. Speech Lang. Hear. Res.644287–4307. 10.1044/2021_JSLHR-20-00503
29
Tao S. Zhang X. Cai H. Lv Z. Hu C. Xie H. (2018). Gait based biometric personal authentication by using MEMS inertial sensors.J. Amb. Intellig. Human. Comput.51705–1712. 10.1007/s12652-018-0880-6
30
Tiwari S. Atluri V. Kaushik A. Yndart A. Nair M. (2019). Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics.Int. J. Nanomed.145541–5554. 10.2147/IJN.S200490
31
Toosizadeh N. Ehsani H. Wendel C. Zamrini E. Connor K. O. Mohler J. (2019). Screening older adults for amnestic mild cognitive impairment and early-stage Alzheimer’s disease using upper-extremity dual-tasking.Sci. Rep.9:10911. 10.1038/s41598-019-46925-y
32
Verghese J. Annweiler C. Ayers E. Barzilai N. Beauchet O. Bennett D. A. et al (2014). Motoric cognitive risk syndrome: multicountry prevalence and dementia risk.Neurology83718–726. 10.1212/WNL.0000000000000717
33
Verghese J. Wang C. Richard B. Lipton R. H. (2013). Motoric cognitive risk syndrome and the risk of dementia.J. Gerontol. A. Biol. Sci. Med. Sci.68412–418. 10.1093/gerona/gls191
34
Xie H. Wang Y. Tao S. Huang S. Zhang C. Lv Z. (2019). Wearable Sensor-Based Daily Life Walking Assessment of Gait for Distinguishing Individuals With Amnestic Mild Cognitive Impairment.Front. Aging Neurosci.11:285.
Summary
Keywords
amnestic mild cognitive impairment, inertial-sensor gait analysis system, multi-kinematic parameters, diagnostic accuracy, receiver operating characteristic
Citation
Huang S, Hou X, Liu Y, Shang P, Luo J, Lv Z, Zhang W, Lin B, Huang Q, Tao S, Wang Y, Zhang C, Chen L, Pan S and Xie H (2022) Diagnostic accuracy of multi-component spatial-temporal gait parameters in older adults with amnestic mild cognitive impairment. Front. Hum. Neurosci. 16:911607. doi: 10.3389/fnhum.2022.911607
Received
02 April 2022
Accepted
15 August 2022
Published
15 September 2022
Volume
16 - 2022
Edited by
Kewei Chen, Banner Alzheimer’s Institute, United States
Reviewed by
Lijuan Gao, Southeast University, China; Weijie Huang, Beijing Normal University, China
Updates
Copyright
© 2022 Huang, Hou, Liu, Shang, Luo, Lv, Zhang, Lin, Huang, Tao, Wang, Zhang, Chen, Pan and Xie.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiqun Xie, haiqunx@foxmail.comSuyue Pan, pansuyue@smu.edu.cn
†These authors have contributed equally to this work and share first authorship
This article was submitted to Cognitive Neuroscience, a section of the journal Frontiers in Human Neuroscience
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.