- 1Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
- 2Medical Research Council (MRC) Versus Arthritis Centre for Musculoskeletal Ageing Research and the Research into Inflammatory Arthritis Centre Versus Arthritis, University of Birmingham, Birmingham, United Kingdom
- 3Rheumatology Department, Sandwell and West Birmingham National Health Service (NHS) Trust, Birmingham, United Kingdom
- 4National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre, University Hospitals Birmingham National Health Service (NHS) Foundation Trust and University of Birmingham, Birmingham, United Kingdom
There has been intense research focus on the biological mechanisms underlying the transition from health to disease for rheumatoid arthritis (RA) over recent years, and it is now well established that a state of autoimmunity precedes the development of symptoms for a large proportion of patients. This has led to an increased interest in the identification of at-risk groups and the potential for preventive intervention. The ability of several immunomodulatory agents to delay or prevent RA is under investigation and novel cellular therapies are in development. Preventive approaches are also being assessed in other chronic autoimmune diseases. For example, an anti-CD3 antibody has recently been shown to delay progression to type 1 diabetes in non-diabetic relatives of patients identified as being at high risk. The identification and treatment of individuals as being at risk of a disease where there is a degree of uncertainty around the potential for benefit is socially and ethically challenging. Recently reported difficulties in recruitment to RA prevention trials have underlined the importance of understanding the perspectives of at-risk individuals to identify barriers and facilitators that need to be addressed in order for preventive strategies to be acceptable. Understanding of their preferences for benefits and risks of preventive interventions can inform efficient intervention prioritization, prevention trial design and the development of informational resources for those at risk. In this review we summarize current knowledge of preferences for RA prevention and make recommendations for further research needed to ensure efficient development of preventive therapies and clinical implementation.
Introduction
Rheumatoid arthritis (RA) is an inflammatory disease that causes painful swelling of the joints, fatigue, depression, and extra-articular manifestations including accelerated cardiovascular disease. There is currently no cure, and long-term treatment is usually required to prevent joint erosion and loss of function (1). Although the introduction of biologic and targeted synthetic disease modifying anti-rheumatic drugs (b/ts DMARDs) has revolutionized management of RA, approximately 10-15% of patients do not respond to multiple sequential therapies (2). Risks of treatments for RA include infection and lung, liver and haematological toxicity. In addition to the disease burden experienced by patients, RA presents a significant socioeconomic burden (3, 4). There is thus a clear rationale for the development of a cure and/or preventive interventions for this condition.
It is established that early treatment of RA is associated with improved outcomes (5). This has led to increased focus on the earliest stages of disease development, including pre-clinical phases (6). Understanding of the biological mechanisms operating at articular and extra-articular sites in at-risk individuals has evolved rapidly (7), and algorithms to predict the development of clinical arthritis in at-risk populations have become increasingly sophisticated (8). Recognition of groups at risk of RA presents possibilities for preventive intervention. Such intervention could prevent or delay the onset of clinical arthritis, and also reduce the complex symptom burden often experienced before diagnosis (9). Intervention at this stage could also reduce RA severity if it were to subsequently develop.
The European Alliance of Associations for Rheumatology (EULAR) has provided recommendations for terminology to identify distinct at-risk phases (based on genetic and environmental risk factors, RA-related autoantibodies and symptoms) (10). Key target groups for preventive approaches may have one or more of the following: (a) genetic risk factors (e.g. risk is increased approximately fourfold in first-degree relatives [FDRs) (11)]; (b) environmental risk factors [e.g. smoking (12)]; (c) systemic autoimmunity associated with RA (typically indicated by rheumatoid factor and/or anti–citrullinated protein/peptide antibodies); (d) symptoms suggestive of underlying inflammation but without clinically apparent synovitis [clinically suspect arthralgias (CSAs) (13)]; or (e) early arthritis that does not fulfil RA classification criteria. Different approaches are likely to be appropriate at each phase. Primary prevention of seropositive RA would involve intervention to prevent development of systemic autoimmunity, while secondary prevention of seropositive RA would involve prevention of RA development in individuals with pre-existing systemic autoimmunity (6).
EULAR guidance for trials and observational studies in individuals at-risk of RA, based on expert consensus and evidence from systematic reviews (14, 15), is now available and the scene is set for progress towards a new paradigm of prevention, rather than treatment of RA (16). Evaluation of candidate preventive therapies for RA is a nascent research area, though early findings are promising. Whilst intramuscular glucocorticoid did not delay arthritis development in seropositive arthralgia patients (17), it prevented 10% of patients with early inflammatory polyarthritis from progressing to RA and delayed DMARD prescription (18). B-cell depletion with a single infusion of rituximab delayed, but did not prevent RA onset in individuals with seropositive arthralgia and either imaging synovitis or evidence of an acute phase response (19). The effects of time-limited courses of other immunomodulatory therapies, including abatacept (20) and hydroxychloroquine (21), on RA development are currently being assessed in other at-risk groups, including asymptomatic FDRs (21). Preventive treatments are also under investigation in other chronic autoimmune conditions. For example, an anti-CD3 antibody delayed progression to type 1 diabetes in non-diabetic relatives of patients identified as being at high risk based on the presence of diabetes-related autoantibodies and other risk factors (22).
Although trials of lifestyle interventions to prevent RA are currently lacking, Vitamin D supplementation for five years has been shown to reduce risk of autoimmune diseases (23). Omega 3 fatty acids have been inversely associated with the presence of RA-related autoantibodies (24, 25), though a prospective cohort study did not find an association between fish intake with RA development (26). There is a robust rationale for studies of smoking cessation to reduce risk of RA (12, 27, 28), and other interventions such as periodontal treatment and weight control have preventive potential (29, 30).
Whilst prevention of diseases such as RA has considerable potential to improve outcomes and reduce societal costs, the identification of individuals as being at risk, and the use of preventive treatment where there is a degree of uncertainty around disease development and progression, is ethically challenging (31, 32). Those at risk may face complex decisions around accepting predictive assessments and risks associated with immunomodulatory interventions in exchange for uncertain benefit. A recent trial of 40mg atorvastatin daily for three years to prevent arthritis development in seropositive arthralgia patients was terminated prematurely due to unwillingness to participate (33). A related qualitative study exploring barriers to trial participation highlighted perceptions that the need for treatment was low and outweighed by concerns about treatment risks and the burden of trial participation (34).
Understanding the perceptions and preferences of those at risk for preventive approaches is therefore essential to inform the development of balanced, tailored informational resources for those considering trial participation, and to support efficient clinical translation. There is increasing recognition of the value of information about patient preferences for decision-making by the pharmaceutical industry, regulatory agencies, and health technology assessors (35–37). Systematically collected data on patient preferences can support efficient, patient-focused medicine development, including target product profile development, endpoint selection, benefit-risk assessment, and regulatory approval (38, 39). The integration of patient preference information into drug development is more likely to result in treatments that are acceptable to patients. This is especially important in the context of disease prevention, where uptake and adherence to medications can be low (40, 41). Therefore, the objective of this article is to provide a narrative review of what is known about the perceptions and preferences of at-risk populations (EULAR at-risk stages a-d) and other key stakeholders for predictive and preventive strategies for RA, and identify opportunities for further investigation. The search strategy used to identify relevant literature is summarized in Supplementary Material.
Exploratory Qualitative Studies
A summary of published qualitative investigations exploring perceptions of predictive testing and/or preventive interventions for RA can be found in Table 1. Perceptions of predictive approaches have been studied in those with CSA (43, 48), asymptomatic individuals who have tested positive for RA-related autoantibodies (48), FDRs (44), the general public (49), and RA patients (who may be involved in providing access and/or information to FDRs) (45). Participants across these studies recognized the value of disease risk information in terms of increased self-awareness and also the potential for early or preventive treatment (15). However, several studies noted concerns around the uncertainty associated with disease development and potential for psychological distress (44, 45, 48). Mosor et al. (2020) reported that these concerns were particularly salient for participants with joint symptoms (48). However in another study, FDRs who received personalized risk education reported greater levels of reassurance than those who received standard RA risk information (50).
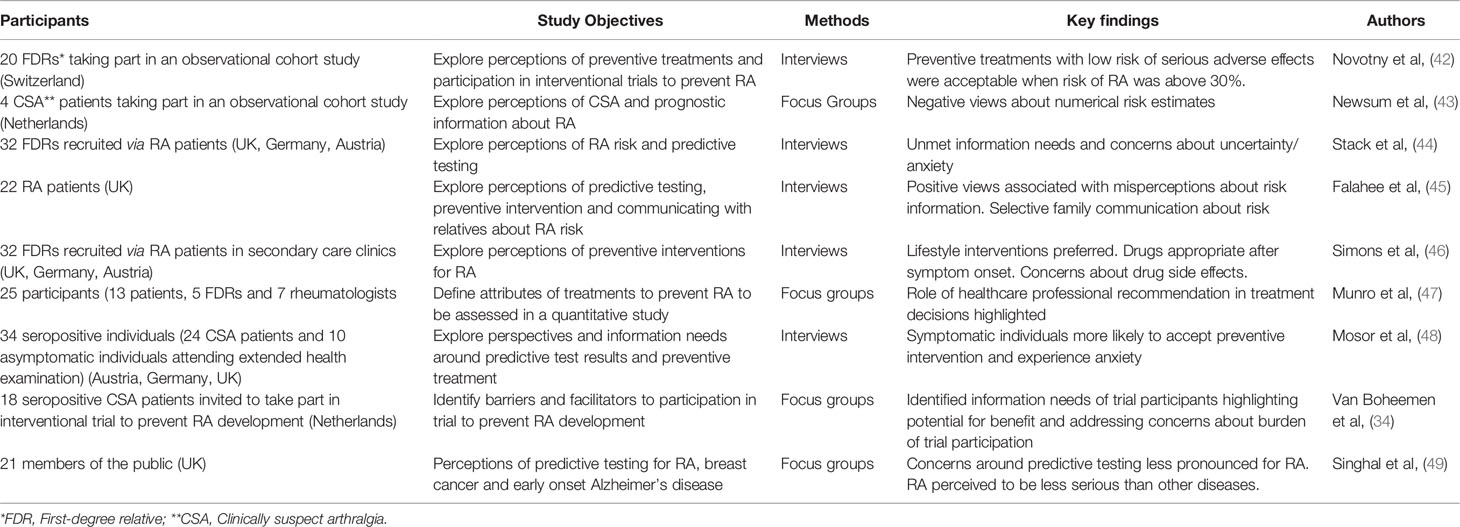
Table 1 Summary of published qualitative studies exploring perceptions/preferences of RA prediction/prevention.
In a focus group study of CSA patients, participants had negative views of the utility of numerical information about risk (43). Interview studies with FDRs (44) and patients (45) suggested that positive views of predictive testing for RA were associated with the misperception that such tests could rule in/out RA. Negative viewpoints were associated with an understanding of the probabilistic nature of risk information (45). In focus groups, members of the general public reflected misperceptions about the severity of RA that had been found in previous studies, and held beliefs that risk assessment was more appropriate for diseases that were perceived to be more serious (49). Lack of public awareness about the negative personal impact of RA was highlighted by RA patients as a potential barrier to predictive strategies (45). Several studies emphasized unmet needs for information about RA and risk factors for RA (44, 45, 48).
The first qualitative study addressing perspectives on preventive treatments for RA found that most participants would accept a prophylactic treatment if their risk of developing RA was 30% or greater (42). However, the participants in that study were FDRs enrolled in a prospective observational cohort and their views may not be representative of other at-risk groups. Other studies of FDRs and RA patients (45–47) suggested that lifestyle interventions would be preferred over pharmaceutical therapies, highlighting concerns about medication side effects and beliefs that drug treatment is appropriate only after symptoms have developed. Such beliefs were echoed by Mosor et al. (2020) who reported that seropositive individuals without symptoms were less inclined to consider preventive treatments than those who were experiencing arthralgia (48). The focus group study by Munro et al. (2020) involving participants who were either RA patients, FDRs or rheumatologists also found that the precision of disease risk estimates and endorsement by a trusted healthcare professional would be important considerations when deciding whether to accept a preventive treatment for RA (47). No other qualitative studies published to date have addressed the perspectives of healthcare professionals.
Many of the themes described above were also found in interviews with autoantibody positive individuals with CSA who had been invited to take part in a trial of a treatment to reduce their risk of developing RA (34). Whilst potential for personal and societal benefit, along with detailed information and support from the individual’s physician, facilitated trial participation, barriers included beliefs about personal risk status and the need for treatment, and concerns about treatment-related harms and the perceived burden of trial participation.
Quantitative Investigations
Table 2 summarizes published quantitative investigations. A survey study found that over 50% of FDRs were definitely interested in taking a predictive test to quantify their risk of developing RA (52). Predictors of levels of interest included attitudes about risk knowledge, information-seeking preferences and beliefs that predictive testing could cause psychological harm. No other quantitative studies have addressed preferences for predictive testing for RA.
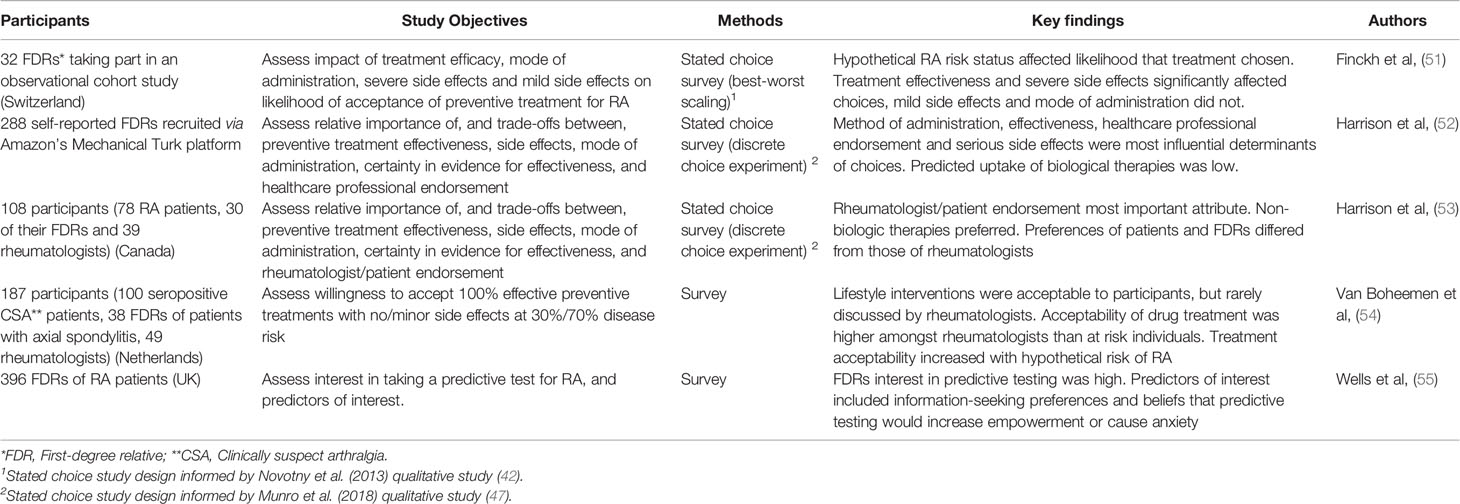
Table 2 Summary of published quantitative studies assessing perceptions/preferences of RA prediction/prevention.
Van Boheemen et al. (2020) surveyed willingness to use 100% effective preventive medications amongst seropositive arthralgia patients and rheumatologists (54). At 30% baseline risk of developing RA, 53% of patients and 74% of rheumatologists would be willing to use a preventive therapy with no side effects. At 70% baseline risk, this increased to 69% for patients and 92% for rheumatologists. A drug with minor side effects was acceptable to 26% of patients and 31% of rheumatologists when the baseline risk of RA was 30%; and to 40% of patients and 76% of rheumatologists when risk of RA was 70%. Patients’ willingness to make preventive lifestyle changes was high, though this was not often the focus of rheumatologists’ consultations (54).
Stated choice methods, where participants choose between hypothetical treatment options described by treatment attributes (e.g., risks, benefits, method of administration, etc.) with pre-specified levels that are varied systematically, provide quantitative information about the relative importance of treatment attributes, benefit/risk tradeoffs, preference heterogeneity, and predicted uptake. Such information can inform selection of outcomes and endpoints in clinical trials and also support stakeholder (e.g. regulator, HTA) decision-making (38, 39). Whilst stated preferences for RA treatments have been widely assessed (56) there are limited examples for RA prevention (57).
A best-worst scaling study of 32 FDRs enrolled in a prospective cohort in Switzerland reported that treatment effectiveness to reduce risk of RA and the likelihood of serious adverse effects were significant determinants of the likelihood that participants would choose a preventive treatment (51). Mild adverse events and the method of drug administration did not influence participants’ decisions. Preventive therapies were chosen 7%, 30% and 38% of the time when participants assumed a baseline risk status of 1%, 20%, and 40%, respectively (51).
A larger sample of self-reported FDRs took part in a Canadian discrete choice experiment (DCE) (52). Participants were asked to assume a 60% risk of developing RA. Method of administration, treatment effectiveness, healthcare professional preference and risk of serious side effects were the treatment attributes that most influenced participants’ choices. Latent class analysis identified three sub-groups of participants whose preferences were driven not only by treatment effectiveness, but also by safety aspects, healthcare professional endorsement and treatment convenience, respectively. Predicted uptake was high for non-biologic drugs such as hydroxychloroquine (84%), but low for atorvastatin and biologics (52).
Nonbiologic drugs were also preferred in a similar survey in Canada of a sample including RA patients, FDRs and rheumatologists (53). 38% of patients/FDRs preferred no preventive treatment, compared with 12% of rheumatologists. The most important drivers of participants’ choice were shared decision-making (whether the treatment option was supported by the rheumatologist/patient), risks of serious side effects, and treatment effectiveness (53).
Finally, the protocol of a stated choice survey employing both a DCE and a probabilistic threshold technique to assess preferences for preventive treatments for RA has been published (58). That study recruits large samples of the general population via survey panels in the UK, Germany and Romania, and also recruits FDRs of confirmed RA patients. Initial findings from the DCE of the general population indicated that treatment effectiveness was the most important determinant of choice across countries, and the sample in Romania was more sensitive to treatment risks (59). Predicted uptake of profiles resembling RA prevention candidate therapies varied across countries, with a profile chosen to estimate abatacept being most likely treatment to be chosen in all three (59).
Discussion
The studies described in this narrative review highlight significant progress in our understanding of preferences for risk assessment and preventive interventions for RA (60). There are now a number of qualitative explorations across a range of stakeholder groups indicating perceived potential for benefit that is sometimes outweighed by concerns around the probability of RA development, treatment harms, uncertainty about effectiveness, and perceptions that preventive intervention with pharmaceutical products are not warranted for RA. The latter finding may reflect commonly held public misperceptions that RA is not a serious condition, and/or that it is a natural part of human ageing (61–63). Taken together these studies highlight an urgent need to provide at risk groups with accurate information about RA, RA risk and the risks and benefits associated with potential preventive strategies to support shared decision-making in the context of trial participation and effective clinical translation. Little is known about the perspectives of healthcare professionals in this context. As the implementation of preventive strategies for RA would require considerable reconfiguration of healthcare services, further studies are needed.
Whilst several studies have described a preference for lifestyle interventions over pharmaceutical therapies, and personalized risk education has been shown to increase risk-reducing health behaviours amongst FDRs of RA patients (64), interventional trials of potential preventive lifestyle interventions for RA (such as smoking cessation, periodontal treatment, weight loss and dietary change) are currently lacking.
There are fewer examples of quantitative studies. Choice-based methods have been applied to samples of FDRs and the public and provide initial evidence that preventive treatments for RA are acceptable to those assuming a hypothetical high-risk status. However, no quantitative studies have used stated choice methods to directly elicit the preferences of very high-risk populations (e.g., seropositive individuals with CSA) for either predictive tools or preventive treatments. Further research in this area is therefore needed to enable quantification of the relative importance of outcomes/intervention attributes, benefit/risk tradeoffs and predicted uptake of treatment profiles for this group. Such information would support patient-focused development of preventive therapies and enhance the likelihood of clinical impact. Importantly, no stated choice studies have quantified the degree of benefit required from preventive lifestyle interventions for RA in exchange for sustained behavioral change. This, is an important area for future research given that several studies have indicated that lifestyle interventions are preferred for prevention of RA. No studies to date have assessed preferences for combined lifestyle and pharmacological intervention.
All preference studies undertaken to date have focused on a single aspect of treatment effectiveness: reduction of the risk of RA development. None have investigated preferences for outcomes such as delay of the onset of RA, or reduction of subsequent RA severity. For symptomatic at-risk groups, important additional benefits may include reduction of symptoms such as arthralgia and fatigue. Further research is therefore needed to quantify the relative importance of these outcomes in high-risk populations. All existing studies were undertaken in Europe or North America. Further investigation is needed to assess preferences in different countries with different types of healthcare provision and also in low and middle income countries. Existing choice-based studies have not yet identified participant characteristics (e.g., gender; health literacy; and numeracy) associated with preference heterogeneity (52), though this is currently under investigation (58).
Comparisons across quantitative studies are limited by methodological heterogeneity. For example, where a treatment attribute describing healthcare professional endorsement or certainty of risk estimates is included in the experimental design it is likely to be an important determinant of participants choices (52, 53). Such considerations can be held constant in the treatment scenario to allow assessment of the relative importance of additional treatment characteristics.
The emergence of evidence-based recommendations to guide the use of preference studies for decision-making in the medical product lifecycle, such as those produced by the PREFER consortium (35), provides a framework for future studies in this area. PREFER has also contributed to an agenda for further refinement of stated preference study methodology. For example, the application of measures of psychological constructs to explain preference heterogeneity (65, 66), and the development of scenario-based interactive educational tools to deliver background information and training to preference study participants to support informed choices (67). These methodological considerations are particularly relevant in the context of RA prevention, where decision making by those at risk of developing RA about accepting treatment is likely to be highly preference sensitive, and influenced by underlying beliefs about RA, personal risk status and treatment risks and benefits. Therefore, the development of innovative educational tools to obtain informed preferences within preference elicitation studies of preventive interventions for RA could also be usefully applied to support shared decision-making in clinical settings.
Preventive strategies for other chronic conditions are routinely integrated into clinical practice, and many asymptomatic individuals accept preventive pharmaceutical treatments (e.g., statins and antihypertensive medications are widely prescribed to reduce risk of cardiovascular disease). A similar approach to RA could dramatically improve clinical outcomes with considerable cost savings. The development of treatments to achieve this that are acceptable to those at risk would represent an important paradigm shift. Such an achievement is more likely to be realized if it is informed by an understanding of stakeholder perspectives and underpinned by evidence that aligns with the treatment preferences of at-risk populations.
Author Contributions
Both authors contributed to the conception, writing and finalization of this article. MF wrote an initial draft. All authors contributed to the article and approved the submitted version.
Funding
There was no specific funding for this work. KR is supported by the NIHR Birmingham Biomedical Research Centre.
Conflict of Interest
KR declares personal fees from Abbvie, Sanofi, and grant/research support from Bristol Myers Squibb.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.883287/full#supplementary-material
References
1. Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR Recommendations for the Management of Rheumatoid Arthritis With Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann Rheum Dis (2020) 79(6):685–99. doi: 10.1136/annrheumdis-2019-216655
2. Nagy G, Roodenrijs NMT, Welsing PM, Kedves M, Hamar A, van der Goes MC, et al. EULAR Definition of Difficult-to-Treat Rheumatoid Arthritis. Ann Rheum Dis (2021) 80(1):31–5. doi: 10.1136/annrheumdis-2020-217344
3. National Rheumatoid Arthritis Society and University of Chester. The Burden of Rheumatoid Arthritis Across Europe: A Socioeconomic Survey (BRASS) - Summary Report. Available at: https://www.nras.org.uk/data/files/Publications/Surveys%20Reports/UoC_HCD_BRASS%20Summary%20Report%20FINAL.pdf.
4. Hsieh P-H, Wu O, Geue C, McIntosh E, McInnes IB, Siebert S. Economic Burden of Rheumatoid Arthritis: A Systematic Review of Literature in Biologic Era. Ann Rheum Dis (2020) 79:771–7. doi: 10.1136/annrheumdis-2019-216243
5. van der Linden MP, le Cessie S, Raza K, van der Woude D, Knevel R, Huizinga TW, et al. Long-Term Impact of Delay in Assessment of Patients With Early Arthritis. Arthritis Rheumatol (2010) 62(12):3537–46. doi: 10.1002/art.27692
6. Raza K, Klareskog L, Holers VM. Predicting and Preventing the Development of Rheumatoid Arthritis. Rheumatology (2016) 55(1):1–3. doi: 10.1093/rheumatology/kev261
7. Tracy A, Buckley CD, Raza K. Pre-Symptomatic Autoimmunity in Rheumatoid Arthritis: When Does the Disease Start? Semin Immunopathol (2017) 39(4):423–35. doi: 10.1007/s00281-017-0620-6
8. van Boheemen L, van Schaardenburg D. Predicting Rheumatoid Arthritis in At-Risk Individuals. Clin Ther (2019) 41(7):1286–98. doi: 10.1016/j.clinthera.2019.04.017
9. Stack RJ, van Tuyl LHD, Sloots M, van de Stadt LA, Hoogland W, Maat B, et al. Symptom Complexes in Patients With Seropositive Arthralgia and in Patients Newly Diagnosed With Rheumatoid Arthritis: A Qualitative Exploration of Symptom Development. Rheumatology (2014) 53(9):1646–53. doi: 10.1093/rheumatology/keu159
10. Gerlag DM, Raza K, van Baarsen LGM, Brouwer E, Buckley CD, Burmester GR, et al. EULAR Recommendations for Terminology and Research in Individuals at Risk of Rheumatoid Arthritis: Report From the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis (2012) 71(5):638–41. doi: 10.1136/annrheumdis-2011-200990
11. Frisell T, Holmqvist M, Källberg H, Klareskog L, Alfredsson L, Askling J. Familial Risks and Heritability of Rheumatoid Arthritis: Role of Rheumatoid Factor/Anti–Citrullinated Protein Antibody Status, Number and Type of Affected Relatives, Sex, and Age. Arthritis Rheumatism (2013) 65(11):2773–82. doi: 10.1002/art.38097
12. Kallberg H, Ding B, Padyukov L, Bengtsson C, Ronnelid J, Klareskog L, et al. Smoking is a Major Preventable Risk Factor for Rheumatoid Arthritis: Estimations of Risks After Various Exposures to Cigarette Smoke. Ann Rheum Dis (2011) 70(3):508–11. doi: 10.1136/ard.2009.120899
13. van Steenbergen HW, Aletaha D, Beaart-van de Voorde LJJ, Brouwer E, Codreanu C, Combe B, et al. EULAR Definition of Arthralgia Suspicious for Progression to Rheumatoid Arthritis. Ann Rheum Dis (2017) 76:491–6. doi: 10.1136/annrheumdis-2016-209846
14. Mankia K, Siddle H, Di Matteo A, Alpízar-Rodríguez D, Kerry J, Kerschbaumer A, et al. A Core Set of Risk Factors in Individuals at Risk of Rheumatoid Arthritis: A Systematic Literature Review Informing the EULAR Points to Consider for Conducting Clinical Trials and Observational Studies in Individuals at Risk of Rheumatoid Arthritis. RMD Open (2021) 7(3). doi: 10.1136/rmdopen-2021-001768
15. Siddle HJ, Chapman LS, Mankia K, Zăbălan C, Kouloumas M, Raza K, et al. Perceptions and Experiences of Individuals at-Risk of Rheumatoid Arthritis (RA) Knowing About Their Risk of Developing RA and Being Offered Preventive Treatment: Systematic Review and Thematic Synthesis of Qualitative Studies. Ann Rheum Dis (2022) 81(2):159. doi: 10.1136/annrheumdis-2021-221160
16. Mankia K, Siddle HJ, Kerschbaumer A, Alpizar Rodriguez D, Catrina AI, Cañete JD, et al. EULAR Points to Consider for Conducting Clinical Trials and Observational Studies in Individuals at Risk of Rheumatoid Arthritis. Ann Rheum Dis (2021) 80(10):1286–98. doi: 10.1136/annrheumdis-2021-220884
17. Bos WH, Dijkmans BAC, Boers M, van de Stadt RJ, van Schaardenburg D. Effect of Dexamethasone on Autoantibody Levels and Arthritis Development in Patients With Arthralgia: A Randomised Trial. Ann Rheum Dis (2010) 69:571–4. doi: 10.1136/ard.2008.105767
18. Verstappen SMM, McCoy MJ, Roberts C, Dale NE, Hassell AB, Symmons DPM. Beneficial Effects of a 3-Week Course of Intramuscular Glucocorticoid Injections in Patients With Very Early Inflammatory Polyarthritis: Results of the STIVEA Trial. Ann Rheum Dis (2010) 69:503–9. doi: 10.1136/ard.2009.119149
19. Gerlag DM, Safy M, Maijer KI, Tang MW, Tas SW, Starmans-Kool MJF, et al. Effects of B-Cell Directed Therapy on the Preclinical Stage of Rheumatoid Arthritis: The PRAIRI Study. Ann Rheum Dis (2019) 78:179–85. doi: 10.1136/annrheumdis-2017-212763
20. Al-Laith M, Jasenecova M, Abraham S, Bosworth A, Bruce IN, Buckley CD, et al. Arthritis Prevention in the Pre-Clinical Phase of RA With Abatacept (the APIPPRA Study): A Multi-Centre, Randomised, Double-Blind, Parallel-Group, Placebo-Controlled Clinical Trial Protocol. Trials (2019) 20(1):429. doi: 10.1186/s13063-019-3403-7
21. Strategy to Prevent the Onset of Clinically-Apparent Rheumatoid Arthritis (StopRA) . Available at: https://clinicaltrials.gov/ct2/show/NCT02603146.
22. Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. New Engl J Med (2019) 381(7):603–13. doi: 10.1056/NEJMoa1902226
23. Hahn J, Cook NR, Alexander EK, Friedman S, Walter J, Bubes V, et al. Vitamin D and Marine Omega 3 Fatty Acid Supplementation and Incident Autoimmune Disease: VITAL Randomized Controlled Trial. BMJ (2022) 376. doi: 10.1136/bmj-2021-066452
24. Gan RW, Young KA, Zerbe GO, Demoruelle MK, Weisman MH, Buckner JH, et al. Lower Omega-3 Fatty Acids are Associated With the Presence of Anti-Cyclic Citrullinated Peptide Autoantibodies in a Population at Risk for Future Rheumatoid Arthritis: A Nested Case-Control Study. Rheumatol (Oxford England) (2016) 55(2):367–76. doi: 10.1093/rheumatology/kev266
25. Gan RW, Demoruelle MK, Deane KD, Weisman MH, Buckner JH, Gregersen PK, et al. Omega-3 Fatty Acids are Associated With a Lower Prevalence of Autoantibodies in Shared Epitope-Positive Subjects at Risk for Rheumatoid Arthritis. Ann Rheum Dis (2017) 76(1):147–52. doi: 10.1136/annrheumdis-2016-209154
26. Sparks JA, O’Reilly ÉVerifytat, Barbhaiya M, Tedeschi SK, Malspeis S, Lu B, et al. Association of Fish Intake and Smoking With Risk of Rheumatoid Arthritis and Age of Onset: A Prospective Cohort Study. BMC Musculoskel Dis (2019) 20(1):2. doi: 10.1186/s12891-018-2381-3
27. Kallberg H, Padyukov L, Plenge RM, Ronnelid J, Gregersen PK, van der Helm-van Mil AH, et al. Gene-Gene and Gene-Environment Interactions Involving HLA-DRB1, PTPN22, and Smoking in Two Subsets of Rheumatoid Arthritis. Am J Hum Genet (2007) 80(5):867–75. doi: 10.1086/516736
28. Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking Intensity, Duration, and Cessation, and the Risk of Rheumatoid Arthritis in Women. Am J Med (2006) 119(6):503.e1–9. doi: 10.1016/j.amjmed.2005.09.053
29. Unriza-Puin S, Bautista-Molano W, Lafaurie GI, Valle-Oñate R, Chalem P, Chila-Moreno L, et al. Are Obesity, ACPAs and Periodontitis Conditions That Influence the Risk of Developing Rheumatoid Arthritis in First-Degree Relatives? Clin Rheumatol (2017) 36(4):799–806. doi: 10.1007/s10067-016-3519-z
30. Zemedikun DT, Chandan JS, Raindi D, Rajgor AD, Gokhale KM, Thomas T, et al. Burden of Chronic Diseases Associated With Periodontal Diseases: A Retrospective Cohort Study Using UK Primary Care Data. BMJ Open (2021) 11. doi: 10.1136/bmjopen-2020-048296
31. Hansson M, Falahee M, Raza K. Genomic and Biological Risk Profiling: From Medicalization to Empowerment. In: Kihlbom U, Hansson M, Schicktanz S, editors. Ethical, Social and Psychological Impacts of Genomic Risk Communication. London: Routledge (2020).
32. Falahee M, Simons G, Raza K, Stack RJ. Healthcare Professionals’ Perceptions of Risk in the Context of Genetic Testing for the Prediction of Chronic Disease: A Qualitative Metasynthesis. J Risk Res (2018) 21(2):129–66. doi: 10.1080/13669877.2016.1153503
33. van Boheemen L, Turk S, Beers-Tas MV, Bos W, Marsman D, Griep EN, et al. Atorvastatin is Unlikely to Prevent Rheumatoid Arthritis in High Risk Individuals: Results From the Prematurely Stopped STAtins to Prevent Rheumatoid Arthritis (STAPRA) Trial. RMD Open (2021) 7. doi: 10.1136/rmdopen-2021-001591
34. van Boheemen L, ter Wee MM, Seppen B, van Schaardenburg D. How to Enhance Recruitment of Individuals at Risk of Rheumatoid Arthritis Into Trials Aimed at Prevention: Understanding the Barriers and Facilitators. RMD Open (2021) 7. doi: 10.1136/rmdopen-2021-001592
35. de Bekker-Grob E, Berlin C, Levitan B, Raza K, Christoforidi K, Cleemput I, et al. Giving Patients’ Preferences a Voice in Medical Treatment Life Cycle: The PREFER Public–Private Project. Patient: Patient Centred Outcomes Res (2017) 10(3):263–6. doi: 10.1007/s40271-017-0222-3
36. Ho MP, Gonzalez JM, Lerner HP, Neuland CY, Whang JM, McMurry-Heath M, et al. Incorporating Patient-Preference Evidence Into Regulatory Decision Making. Surg Endosc (2015) 29(10):2984–93. doi: 10.1007/s00464-014-4044-2
37. Bouvy J, Cowie L, Lovett R, Morrison D, Livingstone H, Crabb N. Use of Patient Preference Studies in HTA Decision Making: A NICE Perspective. patient (2020) 13:145–9. doi: 10.1007/s40271-019-00408-4
38. Ho M, Saha A, McCleary KK, Levitan B, Christopher S, Zandlo K, et al. A Framework for Incorporating Patient Preferences Regarding Benefits and Risks Into Regulatory Assessment of Medical Technologies. Value Health J Int Soc Pharmacoeconomics Outcomes Res (2016) 19(6):746–50. doi: 10.1016/j.jval.2016.02.019
39. Marsh K, van Til JA, Molsen-David E, Juhnke C, Hawken N, Oehrlein EM, et al. Health Preference Research in Europe: A Review of Its Use in Marketing Authorization, Reimbursement, and Pricing Decisions-Report of the ISPOR Stated Preference Research Special Interest Group. Value Health J Int Soc Pharmacoeconomics Outcomes Res (2020) 23(7):831–41. doi: 10.1016/j.jval.2019.11.009
40. Smith SG, Sestak I, Forster A, Partridge A, Side L, Wolf MS, et al. Factors Affecting Uptake and Adherence to Breast Cancer Chemoprevention: A Systematic Review and Meta-Analysis. Ann Oncol (2016) 27(4):575–90. doi: 10.1093/annonc/mdv590
41. Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and Risk Indicators of Nonadherence to Statin Therapy: A Meta-Analysis. Can J Cardiol (2012) 28(5):574–80. doi: 10.1016/j.cjca.2012.05.007
42. Novotny F, Haeny S, Hudelson P, Escher M, Finckh A. Primary Prevention of Rheumatoid Arthritis: A Qualitative Study in a High-Risk Population. Joint Bone Spine (2013) 80(6):673–4. doi: 10.1016/j.jbspin.2013.05.005
43. Newsum EC, van der Helm-van Mil AH, Kaptein AA. Views on Clinically Suspect Arthralgia: A Focus Group Study. Clin Rheumatol (2016) 35(5):1347–52. doi: 10.1007/s10067-015-3038-3
44. Stack RJ, Stoffer M, Englbrecht M, Mosor E, Falahee M, Simons G, et al. Perceptions of Risk and Predictive Testing Held by the First-Degree Relatives of Patients With Rheumatoid Arthritis in England, Austria and Germany: A Qualitative Study. BMJ Open (2016) 6(6):e010555–e. doi: 10.1136/bmjopen-2015-010555
45. Falahee M, Simons G, Buckley CD, Hansson M, Stack RJ, Raza K. Patients' Perceptions of Their Relatives' Risk of Developing Rheumatoid Arthritis and of the Potential for Risk Communication, Prediction, and Modulation. Arthrit Care Res (2017) 69(10):1558–65. doi: 10.1002/acr.23179
46. Simons G, Stack RJ, Stoffer-Marx M, Englbrecht M, Mosor E, Buckley CD, et al. Perceptions of First-Degree Relatives of Patients With Rheumatoid Arthritis About Lifestyle Modifications and Pharmacological Interventions to Reduce the Risk of Rheumatoid Arthritis Development: A Qualitative Interview Study. BMC Rheumatol (2018) 2:31. doi: 10.1186/s41927-018-0038-3
47. Munro S, Spooner L, Milbers K, Hudson M, Koehn C, Harrison M. Perspectives of patients, first-degree relatives and rheumatologists on preventive treatments for rheumatoid arthritis: a qualitative analysis. BMC Rheumatol (2018) 2(1):18. doi: 10.1186/s41927-018-0026-7
48. Mosor E, Stoffer-Marx M, Steiner G, Raza K, Stack RJ, Simons G, et al. I Would Never Take Preventive Medication! Perspectives and Information Needs of People Who Underwent Predictive Tests for Rheumatoid Arthritis. Arthritis Care Res (Hoboken) (2020) 72(3):360–8. doi: 10.1002/acr.23841
49. Singhal J, Wells I, Simons G, Wöhlke S, Raza K, Falahee M. Public Perceptions of Predictive Testing for Rheumatoid Arthritis Compared to Breast Cancer and Early-Onset Alzheimer’s Disease: A Qualitative Study. BMC Rheumatol (2022) 6(1):14. doi: 10.1186/s41927-021-00244-w
50. Marshall AA, Zaccardelli A, Yu Z, Prado MG, Liu X, Miller Kroouze R, et al. Effect of Communicating Personalized Rheumatoid Arthritis Risk on Concern for Developing RA: A Randomized Controlled Trial. Patient Educ Couns (2019) 102(5):976–83. doi: 10.1016/j.pec.2018.12.011
51. Finckh A, Escher M, Liang MH, Bansback N. Preventive Treatments for Rheumatoid Arthritis: Issues Regarding Patient Preferences. Curr Rheumatol Rep (2016) 18(8):51. doi: 10.1007/s11926-016-0598-4
52. Harrison M, Spooner L, Bansback N, Milbers K, Koehn C, Shojania K, et al. Preventing Rheumatoid Arthritis: Preferences for and Predicted Uptake of Preventive Treatments Among High Risk Individuals. PloS One (2019) 14(4):e0216075. doi: 10.1371/journal.pone.0216075
53. Harrison M, Bansback N, Aguiar M, Koehn C, Shojania K, Finckh A, et al. Preferences for Treatments to Prevent Rheumatoid Arthritis in Canada and the Influence of Shared Decision-Making. Clin Rheumatol (2020) 39(10):2931–41. doi: 10.1007/s10067-020-05072-w
54. van Boheemen L, Bolt JW, ter Wee MM, de Jong HM, van de Sande MG, van Schaardenburg D. Patients’ and Rheumatologists’ Perceptions on Preventive Intervention in Rheumatoid Arthritis and Axial Spondyloarthritis. Arthritis Res Ther (2020) 22(1):217. doi: 10.1186/s13075-020-02314-9
55. Wells I, Zemedikun DT, Simons G, Stack RJ, Mallen CD, Raza K, et al. Predictors of Interest in Predictive Testing for Rheumatoid Arthritis Amongst First Degree Relatives of Rheumatoid Arthritis Patients. Rheumatol (Oxford) (2021). doi: 10.1093/rheumatology/keab890
56. Durand C, Eldoma M, Marshall DA, Bansback N, Hazlewood GS. Patient Preferences for Disease-Modifying Antirheumatic Drug Treatment in Rheumatoid Arthritis: A Systematic Review. J Rheumatol (2020) 47(2):176–87. doi: 10.3899/jrheum.181165
57. Simons G, Caplan J, DiSantostefano RL, Veldwijk J, Englbrecht M, Bywall KS, et al. Systematic Review of Quantitative Preference Studies of Treatments for Rheumatoid Arthritis Among Patients and at-Risk Populations. Arthritis Res Ther (2022) 24(1):55. doi: 10.1186/s13075-021-02707-4
58. Falahee M, Simons G, DiSantostefano RL, Valor Méndez L, Radawski C, Englbrecht M, et al. Treatment Preferences for Preventive Interventions for Rheumatoid Arthritis: Protocol of a Mixed Methods Case Study for the Innovative Medicines Initiative PREFER Project. BMJ Open (2021) 11. doi: 10.1136/bmjopen-2020-045851
59. Simons G, Veldwijk J DI, Santostefano R, Englbrecht M, Radawski C, Valor L, et al. OP0160-HPR Preferences For Treatments To Prevent Rheumatoid Arthritis: Discrete Choice Survey Of General Populations In United Kingdom, Germany, And Romania. Ann Rheum Dis (2021) 80:96–7. doi: 10.1136/annrheumdis-2021-eular.2168
60. Falahee M, Finckh A, Raza K, Harrison M. Preferences of Patients and At-Risk Individuals for Preventive Approaches to Rheumatoid Arthritis. Clin Ther (2019) 41(7):1346–54. doi: 10.1016/j.clinthera.2019.04.015
61. Simons G, Mallen CD, Kumar K, Stack RJ, Raza K. A Qualitative Investigation of the Barriers to Help-Seeking Among Members of the Public Presented With Symptoms of New-Onset Rheumatoid Arthritis. J Rheumatol (2015) 42:585–92. doi: 10.3899/jrheum.140913
62. Simons G, Mason A, Falahee M, Kumar K, Mallen CD, Raza K, et al. Qualitative Exploration of Illness Perceptions of Rheumatoid Arthritis in the General Public. Musculoskeletal Care (2017) 15(1):13–22. doi: 10.1002/msc.1135
63. Simons G, Belcher J, Morton C, Kumar K, Falahee M, Mallen CD, et al. Symptom Recognition and Perceived Urgency of Help-Seeking for Rheumatoid Arthritis and Other Diseases in the General Public: A Mixed Method Approach. Arthrit Care Res (2017) 69(5):633–41. doi: 10.1002/acr.22979
64. Sparks JA, Iversen MD, Yu Z, Triedman NA, Prado MG, Miller Kroouze R, et al. Disclosure of Personalized Rheumatoid Arthritis Risk Using Genetics, Biomarkers, and Lifestyle Factors to Motivate Health Behavior Improvements: A Randomized Controlled Trial. Arthrit Care Res (2018) 70(6):823–33. doi: 10.1002/acr.23411
65. Russo S, Monzani D, Pinto CA, Vergani L, Marton G, Falahee M, et al. Taking Into Account Patient Preferences: A Consensus Study on the Assessment of Psychological Dimensions Within Patient Preference Studies. Patient Prefer Adherence (2021) 15:1331–45. doi: 10.2147/PPA.S261615
66. Russo S, Jongerius C, Faccio F, Pizzoli SFM, Pinto CA, Veldwijk J, et al. Understanding Patients' Preferences: A Systematic Review of Psychological Instruments Used in Patients' Preference and Decision Studies. Value Health J Int Soc Pharmacoeconomics Outcomes Res (2019) 22(4):491–501. doi: 10.1016/j.jval.2018.12.007
67. Bywall KS, Veldwijk J, Hansson MG, Baecklund E, Raza K, Falahee M, et al. Does Being Exposed to an Educational Tool Influence Patient Preferences? The Influence of an Educational Tool on Patient Preferences Assessed by a Discrete Choice Experiment. Patient Educ Couns (2021) 104(10):2577–85. doi: 10.1016/j.pec.2021.03.013
Keywords: rheumatoid arthritis, prediction, prevention, at-risk groups, perceptions, preferences, choice - behaviour
Citation: Falahee M and Raza K (2022) Perspectives of at-Risk Individuals on Preventive Intervention for Rheumatoid Arthritis: A Mini Review. Front. Immunol. 13:883287. doi: 10.3389/fimmu.2022.883287
Received: 24 February 2022; Accepted: 07 April 2022;
Published: 29 April 2022.
Edited by:
Nancy J. Olsen, Penn State Milton S. Hershey Medical Center, United StatesReviewed by:
Guenter Steiner, Medical University of Vienna, AustriaCopyright © 2022 Falahee and Raza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie Falahee, m.falahee@bham.ac.uk
 Marie Falahee
Marie Falahee Karim Raza
Karim Raza