- 1Department of Entomology, China Agricultural University, Beijing, China
- 2Department of Entomology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, Pakistan
Spodoptera frugiperda (J.E. Smith), (Noctuidae, Lepidoptera), commonly known as fall armyworm (FAW), is a significant polyphagous pest that can cause considerable damage to various crops. Fundamental research on FAW is crucial and beneficial for creating an integrated management strategy. Lot of literatures are available on web to describe the fitness of FAW via conventional methods that deals the basic biology of FAW. However, there is currently a need to check the fitness for each stage of FAW using an advanced two-sex life table tool, which is crucial for creating efficient control strategies. The proposed study used an age-stage, two-sex life table to examine the lifetable parameters of FAW on four natural hosts: castor beans (Ricinus communis), potatoes (Solanum tuberosum), maize (Zea mays L.), and wheat (Triticum aestivium L.). The findings demonstrated that, despite notable variations in development and reproduction, the FAW completed its life cycle on each of the four studied hosts. The FAW that were fed maize performed at their best, showing shorter immature (egg-pupa) phases, longer lifespans, and better rates of adult reproduction. On maize, female FAW had the highest fecundity (2497.1 eggs/female), while on wheat, it was the lowest (675 eggs/female). With values of 532.8 (offspring individual-1), 0.21d-1, and 1.23 d-1, respectively, net reproductive rate, intrinsic rate of increase, and finite rate of increase peaked on maize, while the corresponding parameters were lowest on wheat (94.62 offspring individual-1, 0.11 d-1, and 1.12 d-1, respectively). This study indicates that all host plants can contribute to the development and outbreak of this pest in the absence of its primary host. Therefore, all potential host plants in the area should be thoroughly examined when developing an IPM program against said pest.
1 Introduction
Fall armyworm (FAW), Spodoptera frugiperda Smith, (Lepidoptera, Noctuidae), has high migratory ability and a wide host range. These attributes collectively play a significant role in causing economic losses to crops and pastures globally (1). The larvae of FAW consume the stems, foliage, and reproductive structures of the plants they inhabit (2). The FAW may prefer or be more effective on one plant species or a small number of host plants (3, 4). Due to its polyphagous nature, FAW can consume over 350 plants from 76 different families, including the Leguminosae, Compositae, and Gramineae (5).
The fall armyworm significantly threatens global food security, causing millions of dollars in losses to maize production areas worldwide (6–8). FAW targets crops from seedlings to maturity, causing physical damage that lowers maize yields. Additionally, it impacts other crops like potatoes, intensifying its economic consequences (9, 10). Farmers have experienced substantial economic losses due to FAW infestations. Prior to its outbreak, maize yields averaged 2–3 tons per acre; however, this dropped to less than 2 tons per acre following its spread (11–13). Native to America, particularly the United States and Argentina, and was initially discovered in central Africa in 2016 (14). It subsequently spread to India (15) and China by 2018; today, it is present across nearly all maize-growing regions of the country (1). In April 2019, for the first time in Sindh, Pakistan, the presence of FAW was confirmed on fodder corn, causing 100% damage to the maize crop (16). The FAW has two biotypes: the corn strain, which primarily invades maize, and the rice strain, which invades rice (17).
In tropical and subtropical areas around the world, FAW populations have developed insecticide resistance as a result of the careless application of pesticides (18), including Asia (7, 19–21). Over the past three decades, interest in behavioral manipulation as a pest management strategy has grown significantly, aiming to reduce dependence on broad-spectrum insecticides (22, 23). Cultivating standing crops is considered a safer and more sustainable alternative to insecticide application (24, 25).
Investigating how insect pests and host plants interact can reveal important details about how host plants affect herbivore biology, ecology, and population dynamics (26–28). Basic research on FAW is essential for creating a trustworthy Integrated Pest Management (IPM) strategy, which includes comprehending its behavior and the parameters of its age-stage, two-sex life table (9, 29, 30). A review article that includes all the components of an integrated pest management for FAW in maize crops was recently published by Babendreier et al. (2022) (31) Traditional life table studies often focus on female age-specific populations (32), but incorporating data on both males and females is essential for a comprehensive understanding of pest dynamics (33–37).
Even though research has been done on the biology of FAW on many hosts (38–42), its host preferences and two-sex life table characteristics are important and necessary to report for best management of FAW. Thus, the purpose of this study was to evaluate, in a laboratory setting, the host preference and age-stage, two-sex life table properties of FAW on the leaves of maize, wheat, castor beans, and potatoes. This study advances our knowledge of FAW and could aid in the creation of more potent control measures.
2 Material and method
2.1 Laboratory colony
In order to grow them, the FAW larvae were first taken from maize fields in Pakistan’s south Punjab region and taken to the Biological Control Laboratory at the Department of Entomology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University (BZU), Multan. For two or three generations prior to pupation, these larvae had been fed castor leaves. Larvae were raised at a photoperiod of 14:10 hours (L:D), a temperature of 26 ± 1°C, and a relative humidity (RH) of 65 ± 5%. After adult emergence, they were placed in transparent plastic jars (10.16×10.16×17.78cm). Cotton swap soaked in a honey solution (8% w/v) was provided as an artificial diet. Muslin cloth was tapped on two opposite inner sides of the jar as an ovipositional substrate. Each egg mass laid by females was collected and placed separately in a petri dish (2×6 cm) under laboratory-controlled conditions (9).
2.2 Plant source
Based on field observations in the various parts of south Punjab where these crops surrounding maize, four host plants were chosen: castor beans (Ricinus communis), potatoes (Solanum tuberosum), wheat (Triticum aestivium L.), and maize (Zea mays L.). The leaves of castor beans and potatoes were gathered from a farm close to Bosan Road in Multan. From the field of BZU Multan, Pakistan, wheat and maize leaves at the V5 stage (the growth point above ground and 1-1½” above the soil surface) were gathered. FAW were fed 30 cm of potato plants, 182.88 cm of caster beans, and the leaves of a V5 stage wheat plant.
2.3 Biological parameters
We looked at and contrasted the growth, survival, and reproduction of FAW fed on castor leaves, maize, potatoes, and wheat. Leaves were cut into disc shapes (2×6cm) except wheat leaves, which were cut into 7.62cm, and maize leaves were cut into 2×2cm and replaced with new leaves every 24 hours. Number of leaves varied with the larval instar. From the F3 generation, FAW eggs were collected, and neonates were housed individually in a 2x6 cm petri plate once they hatched. There were 50 replications for every host, and each host was regarded as a separate treatment. The presence of exuvium verified the existence of distinct FAW instars. After adult emergence, the male-female ratio was noted by their morphological characters in each treatment and pairs in different jars (10.16×10.16×17.78cm) to observe oviposition every 24 hours. Cotton swap soaked in honey solution (10% w/v) was provided as an artificial diet and it was changed daily. Up to the female’s death, the egg masses that each female laid were noted every day. After carefully transferring each egg mass to the plastic containers, the number of neonates that hatched was recorded. Fecundity, oviposition period, survival, and female lifespan were assessed.
2.4 Statistical analysis
Differences in biological parameters among treatments (different hosts) at each dose were analyzed separately using the Kruskal-Wallis test (P< 0.05). Post hoc pairwise comparisons were performed using Dunn’s test in SPSS Statistics 22.0 (SPSS Inc., Chicago, IL, USA). These non-parametric tests were employed after confirming through normality test that the data not followed the normal distribution. Using a TWO SEX-MS Chart, the life table parameters of FAW individuals were determined, age stage specific fxj: fecundity, lx: survival rate, sxj: specific survival rate, lxmx: maternity, mx: specific fecundity, exj: life expectancy, vxj: reproductive value, and population parameters, Net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T) (43) were calculated as (1 and 2):
by following the Euler–Lotka Equation 4, with age indexed from (44):
Life expectancy (exj) using the formula provided in Equation 7 (45).
The Vxj was calculated by using the following equation (46).
3 Results
3.1 Life span of FAW
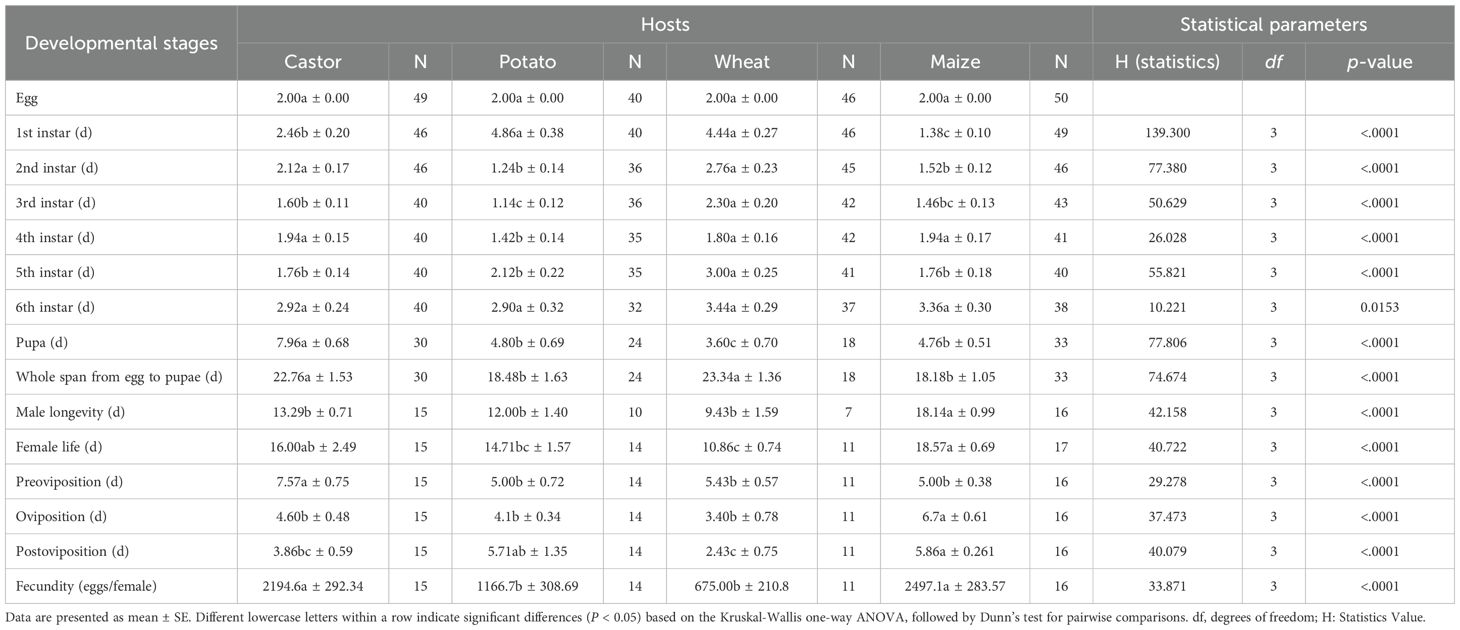
When fed on various host plants, the female reproductive capacity (FAW), adult lifespan, and development length for each immature stage differed considerably according to the Kruskal-Walli’s test and Dunn’s test at p < 0.05 (Table 1). Each egg stage lasted roughly two days since freshly hatched neonates were consistently recovered from egg masses. On maize and potatoes, every immature stage from the first instars to the pupa developed noticeably more quickly (all p < 0.05). When given different host plants as food, males (H = 42.158; df = 3, p < 0.0001) and females (H = 40.722; df = 3, p < 0.001) showed significantly varying adult longevity. When fed maize, the male and female lived the longest (18.14 and 18.57 days, respectively), whereas when fed wheat, they lived the shortest (9.43 and 10.86 days, respectively). Significant variations (H = 29.278; df = 3, and p < 0.0001) in the pre-oviposition period were noted when the FAW were fed on various plants (Table 1). Additionally, when FAW was fed on several host plants, the oviposition duration varied significantly (H = 37.473; df = 3, and p < 0.0001). On maize, the oviposition period peaked at 6.7 days, although other hosts displayed a comparable pattern. On several host plants, the fecundity of FAW varied considerably (H = 33.871; df =3, and p < 0.0001); Maize had the highest fecundity (2497.1 eggs/female), followed by castor (2194.6 eggs/female); the statistic ranks of potatoes (1166 eggs/female) and wheat (675 eggs/female) were comparable.
3.2 Population parameters
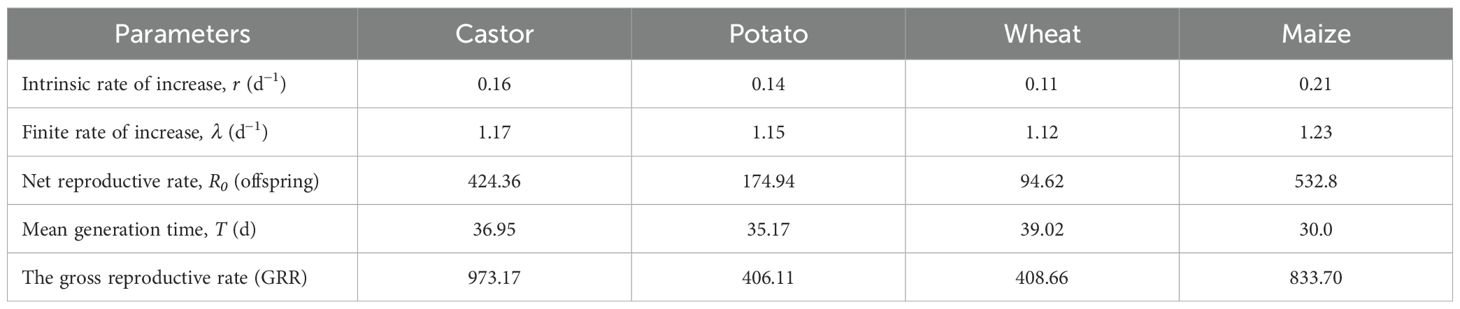
The intrinsic rate of increase (r), finite rate of increase (λ), the net reproductive rate (R0), and mean generation time (T) of FAW on different hosts were assessed using the bootstrap method, and are listed in Table 2. Statistical analysis showed that intrinsic rate of increase (r), finite rate of increase (λ), and net reproductive rate (R0) were higher on maize provided as food than with the other tested plants (Table 2). The mean generation time T was higher (39.02 d-1) on wheat, followed by castor and potato, while shorter on maize. When fed castor leaves, the FAW produced offspring with a Gross reproductive rate (GRR) of 973.17, followed by offspring fed maize, potatoes, and wheat with GRRs of 833.70, 408.66, and 406.11, respectively.
3.3 Survival rate
When fed maize, the greatest survival probability survival rate (sxj) of FAW pupa and eggs was 0.86 and 1, respectively. The survival probability of all larval stages was highest when they were nourished on Castor leaves. The maximum survival probability of males and females were 0.421 and 0.447, respectively on Maize leaves, while the least survival probability (0.189 and 0.290) was observed on wheat (Figure 1).
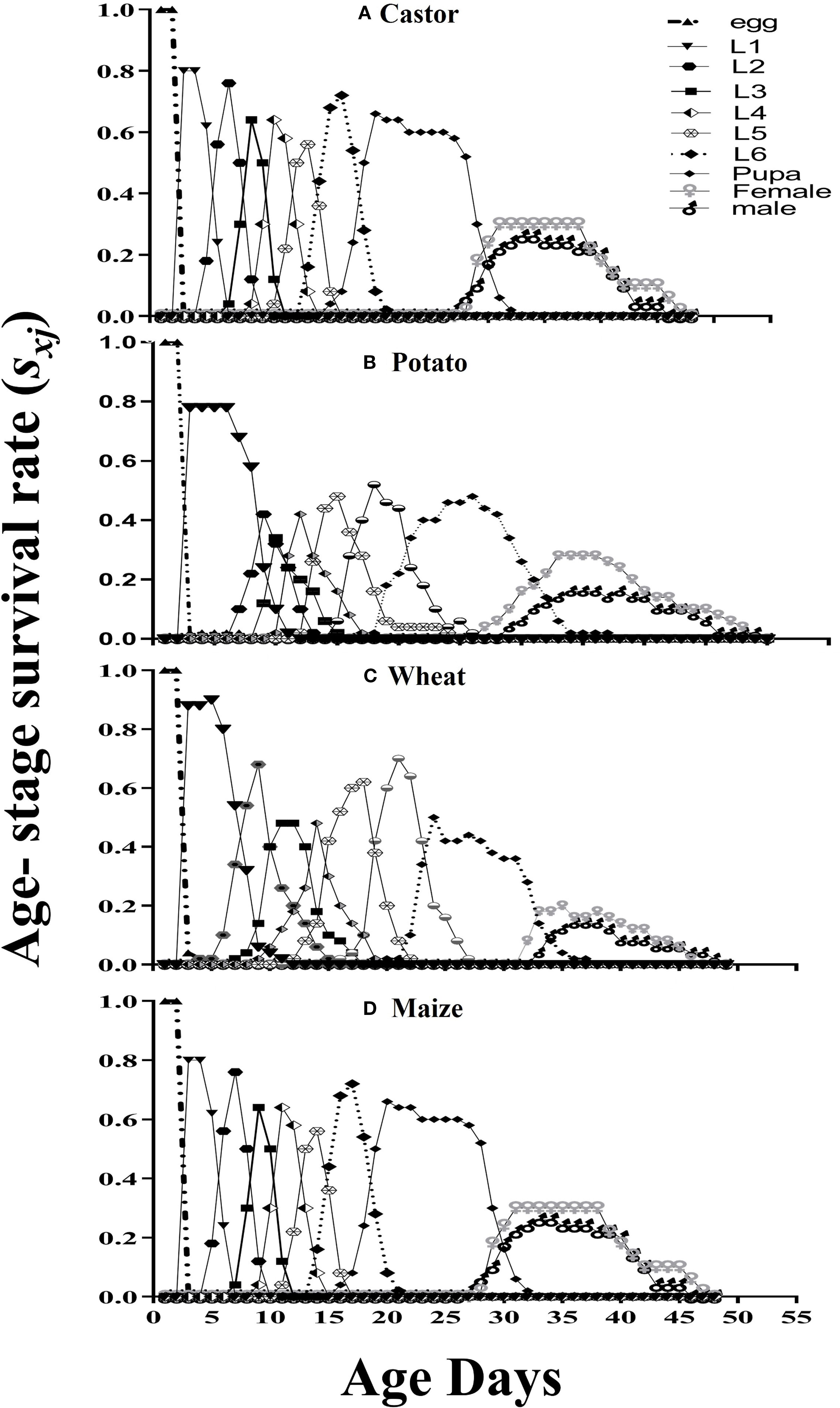
Figure 1. Age-stage survival rate (Sxj) for fall armyworm fed on four different host plants: (A) castor, (B) potato, (C) wheat, and (D) maize, under laboratory conditions.
3.4 Population survival rate and fecundity
The survival rate (lx), fecundity (fx), age-specific fecundity (mx), and age-specific maternity rate (lxmx) are plotted in Figure 2. The survival rate lx has shown maximum on both maize and castor than on wheat and potato. The fecundity (fx) showed that 317.22eggs on the 39th d, 167.64eggs on the 35thd, 197.25eggs on the 43thd, and 317.70eggs on the 30thday were laid on castor, potato wheat, and maize, respectively. The age-specific fecundity (mx) curve showed that reproduction began at 29d, 34d, 37d, and 38d in FAW fed on maize, potato, castor, and wheat, respectively. The age-specific maternity rate (lxmx) of FAW was maximum on maize followed by castor, potato, and wheat. The values of the reproductive value (vxj) of an adult female were recorded with the following trend: 813.61at the 28thd on maize, 739.89 at the 32d on castor, 408.44 on the 43rd day on potato, and 386.84 at the 55thd on wheat (Figure 3).
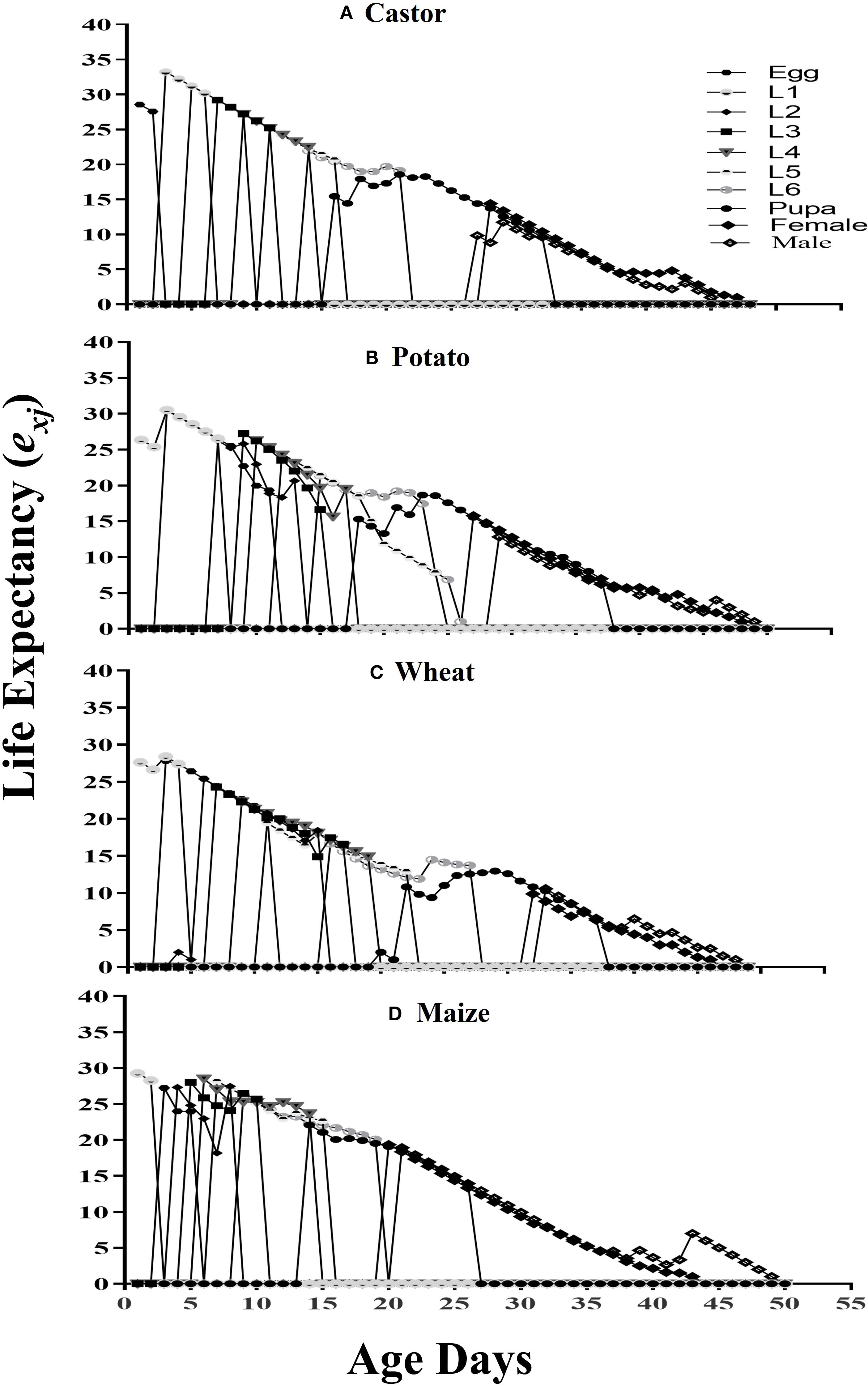
Figure 2. The survival rate (lx), the fecundity (fxj), the specific fecundity (mx) and the maternity (lxmx) of fall armyworm fed on four different host plants: (A) castor, (B) potato, (C) wheat, and (D) maize, under laboratory conditions.
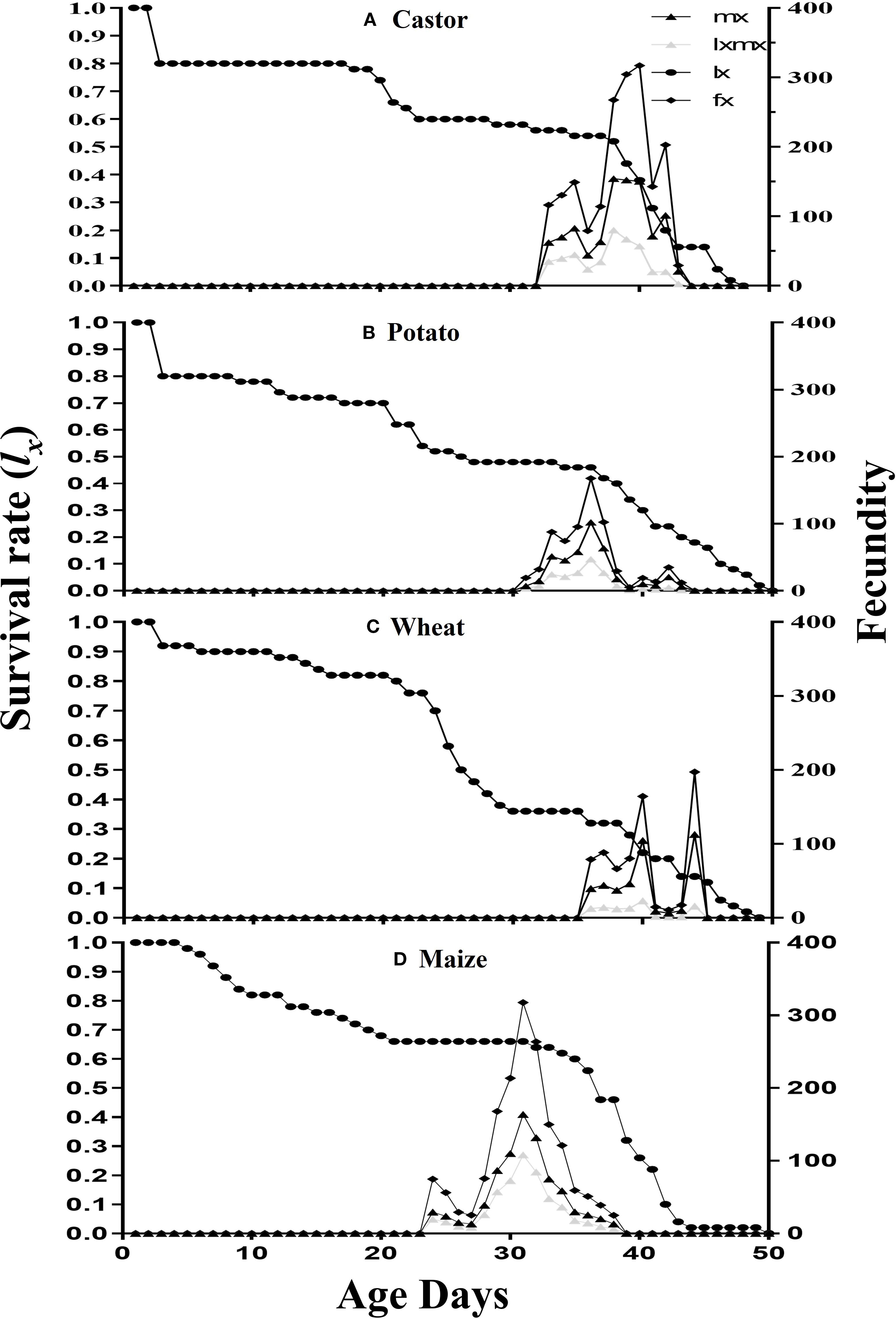
Figure 3. The reproductive value (Vxj) of fall armyworm fed on different host plants: (A) castor, (B) potato, (C) wheat, and (D) maize under laboratory conditions.
3.5 Life expectancy
The estimated duration of survival for an insect of age x and stage j is denoted by the life expectancy (exj) (Figure 4). Freshly deposited FAW eggs raised on maize, castor, potato, and wheat had respective life expectancies of 29.26, 33.2, 30.53, and 28.40.
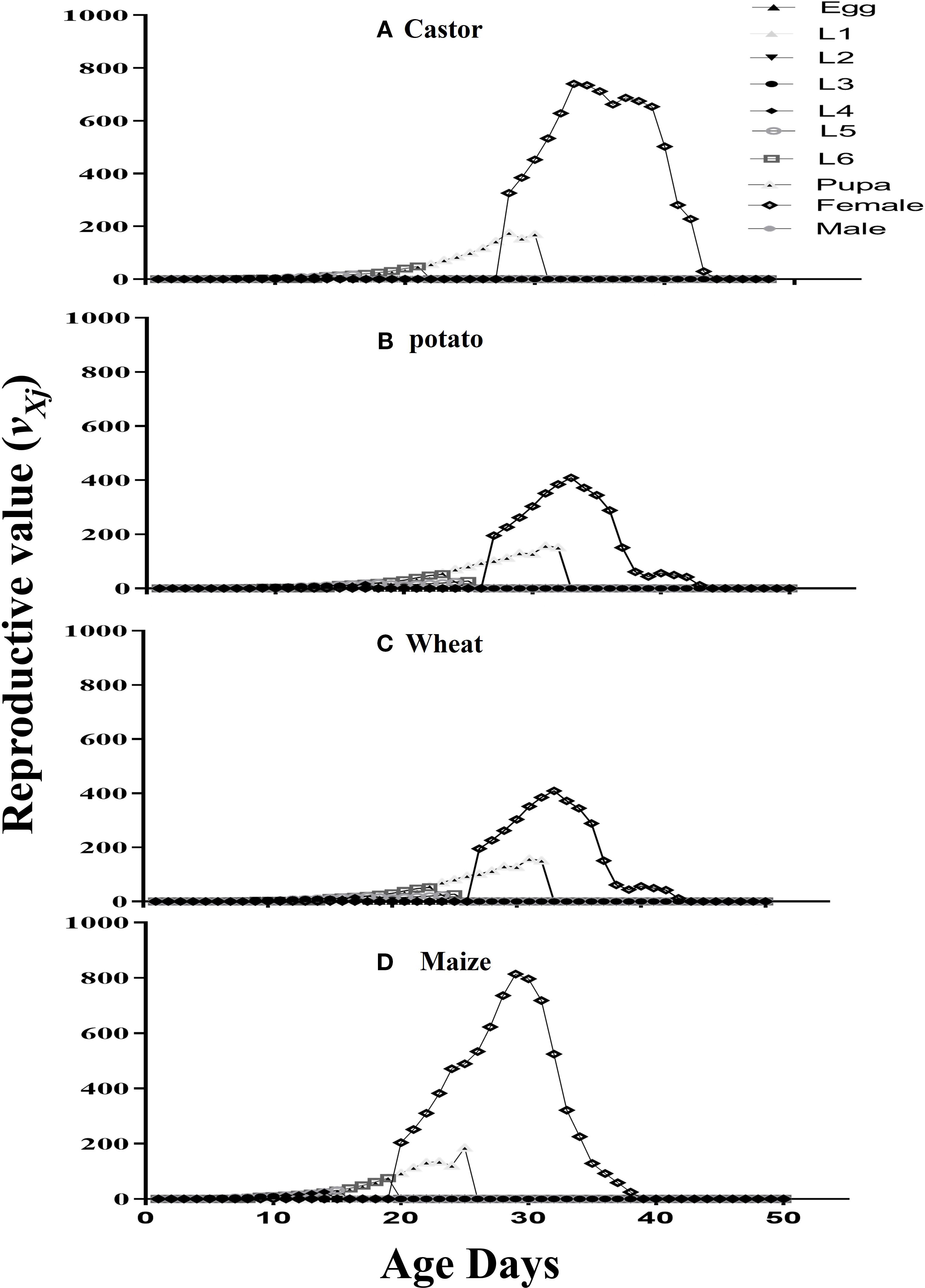
Figure 4. The life expectancy (exj) of fall armyworm fed on different host plants: (A) castor, (B) potato, (C) wheat, and (D) maize, under laboratory conditions.
4 Discussion
The biology of herbivorous insects, including FAW is significantly impacted by variations in the nutrient content of their host plants and also affects the shifting trends of their populations (9, 47–54). A plant is deemed more suitable when an insect feeds on it and shows signs of faster development and higher reproduction rates (55). Our findings showed that FAW completed its life cycle on all hosts, and the total duration of immature development (from egg to pupa) was shorter on maize than on other tested hosts. Additionally, adult longevity (both male and female) and fecundity were higher on maize than on other treatments. Xu et al.,(2019) observed that when FAW larvae were fed tobacco (a non-preferred host) instead of maize, their development time was extended, while survival rate and fecundity were decreased, which aligns with our findings (56). Likewise, Wu et al. (2020) reported that FAW exhibited faster development and heavier pupal weight and fecundity when reared on primary hosts such as wheat and maize (1). Similarly, another study revealed that FAW could complete its life cycle on both hosts (kidney beans and maize). The larval and pupal development duration was notably extended, while the adult lifespan was shortened on kidney beans compared to maize, with no difference in the oviposition rate (57). According to Acharya et al. (2022), maize had higher reproduction rates and a shorter mean generation time than rice and potatoes (58). Various factors can cause these differences among host plants, including extrinsic factors such as food source characteristics and intrinsic genetic characteristics (59–61). The reduced performance observed in other test plants may be linked to insufficient nutrition and the presence of certain insect-repelling compounds.
The life parameter statistics the intrinsic rate of increase (r), the finite rate of increase (λ), the net reproductive rate (Ro), and the mean generation time (T) offer valuable insights into the growth potential of a population in a specific environment (62). These parameters frequently change depending on the type of host plant and the environmental conditions of the area (42, 57, 63). Our findings showed that shorter developmental time, high oviposition period, and high fecundity rate of FAW reared on maize resulted in higher the intrinsic rate of increase (r), the finite rate of increase (λ), the net reproductive rate (R0), and lower the mean generation time (T) values. The findings are consistent with the previous study, which demonstrated that when FAW reared on maize, it exhibits a higher capacity for population growth (higher rates of r, l, R0, and a lower T) compared to tomato, cotton, or soybean (1). Similar findings on maize were reported by Wu et al. (62), with the exception that tomato had the highest net reproductive rate. Likewise, higher intrinsic rate of increase (r), the finite rate of increase (λ), the net reproductive rate (R0), and lower T values were recorded previously when FAW was raised on maize compared to potato and rice (58). A similar trend was observed when FAW was raised on maize instead of kidney beans (57). These trends may be attributed to the nutritional differences found in the host plants (64).
The survival probability of all larval instars was higher on wheat; however, the maximum survival rate of males and females was observed on maize in comparison with other hosts. The life expectance (exj) also varied among the four host plants. The life expectance (exj) of freshly laid eggs of FAW was greater on castor (33.2), followed by potato (30.53), maize (29.26), and wheat (28.40). Following our results, a previous study reported that the survival rates of newly hatched FAW neonates to adult age showed considerable variation across three host plants: maximum survival was observed on maize (98.31%), followed by potato (31.61%) and tobacco (8.13%) (9). The differences in results may be due to variations in laboratory conditions (temperature, humidity, and light), as well as sample preparation, handling, and measurement techniques. Moreover, genetic variations among maize cultivars or different strains of organisms could also influence the outcomes. Likewise, the survival rate and life expectancy of FAW were greater in maize compared to kidney beans (57). According to Altaf et al. (2022) reported that the Survival rate and life expectancy of FAW were higher in maize than in sorghum, wheat, and rice under laboratory conditions (65). These parameters are used to develop early warning models that predict the survival of insects at a certain age, timing, and amount of pest occurrence (28, 66).
To accomplish dependable Integrated Pest Management (IPM) (31), basic research of FAW are required, including behavior and age-stage, two-sex life table factors (9, 30). Traditional life table characteristics, which only offer data for female age-specific populations, are frequently used to study the development and survival of pests (32). Tobetter comprehend a pest, life table parameters for both males and females are required (33, 34, 36). Although the biology of FAW on several hosts has been researched (9, 38, 67), there is a dearth of information on your host and the two-sex life table characteristics of FAW. Furthermore, research on FAW’s preferred food has shown that growth, survival, and effectiveness are contingent upon the availability of food sources and favorable environmental conditions. To the best of our knowledge, however, no thorough data utilizing age-stage, two-sex life methods has previously been published on the fitness of the FAW on host (9, 49). Fall Armyworm (FAW) in maize is controlled via Integrated Pest Management (IPM), which takes a multifaceted strategy to reduce the pest’s negative effects on the environment (31, 68, 69). In order to interrupt the FAW life cycle, cultural methods like crop rotation, intercropping, and optimal planting schedules are essential, as are routine monitoring for early diagnosis and prompt interventions (70–73). Natural predators, parasitoids, entomopathogenic fungi and plant extracts are examples of biological control techniques that can naturally lower FAW populations and are more environmentally benign than chemical control (74–76). Since two-sex life table studies take into consideration both sexes when creating the appropriate population curve for future populations, they aid researchers in creating pest management plans to combat any pest (43, 45, 77, 78).This helps us comprehend the ecology and fitness of an insect pest and improve its management, as it was reported by Zafar et al. (2024) (79). The reproductive characteristics r, GRR λ, and R0values are important in determining how diet impacts the fitness of insect pests (34). If r is greater than 0, it might be the best population index to show how an insect has adapted to a food source (80, 81). Our research underscores the substantial impact of host plant variability on FAW’s life cycle and population dynamics. Understanding these dynamics can inform pest management strategies and contribute to more effective control measures by considering the host plant’s influence on insect fitness and population growth. Future research can involve the construction of life tables in field conditions to better understand the ecology of the FAW population in Asian maize cropping environments.
5 Conclusions
The life table parameters of FAW were studied on different hosts i.e., maize, castor, potato, and wheat, via the age-stage two-sex method. Our findings revealed that the FAW was able to complete its life span on all four host plants; however, it exhibited a shorter pre-adult duration, a higher survival rate, and greater fecundity on maize when compared to castor, potato, and wheat. The interaction of all these parameters resulted in increased the net reproductive rate (R0), the intrinsic rate of increase (r), and the finite rate of increase (λ) in the FAW raised on maize, highlighting the pest’s strong adaptability to maize compared to other tested host plants. FAW performed much better in maize (primary host), the establishment of an integrated pest management program for FAW in maize is highly recommended to suppress its population and minimize economic losses. Furthermore, our results propose that castor, potato, and wheat can serve as alternative hosts for FAW, allowing it to complete its life cycle even when preferred crops are absent. Consequently, it is essential to monitor alternative host plants to observe their population changes and assess any potential crop damage.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
AT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. National Key Research and Development Program of China [2021YFD1400703].
Acknowledgments
The authors would like to thank Mr. Muhammad Shahzaib, Mr. Khalid Abbas, Mr. Adeel Mukhtar, Mr. Muhammad Usama Altaf and Ms. Aiman Ishfaq for their help during the work. Moreover, we are grateful to the Department of entomology, Faculty of Agricultural Sciences & Technology, Bahauddin Zakariya University Multan for providing support and facilities to perform the Research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang W, He P, Zhang Y, Liu T, Jing X, and Zhang S. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects. (2020) 11:639. doi: 10.3390/insects11090639
2. Cheng Y, Liu L, Li H, Yang X, and Shang S. Understanding the feeding behavior and identifying the plant parts preferences of fall armyworm on peanut seedlings. Agronomy. (2024) 14:2432. doi: 10.3390/agronomy14102432
3. Tiwari S. Host plant preference by the fall armyworm, Spodoptera frugiperda (JE Smith)(Noctuidae: Lepidoptera) on the range of potential host plant species. J Agric Forestry Univ. (2022) 5:25–33. doi: 10.3126/jafu.v5i1.48437
4. Buntin GD. A review of plant response to fall armyworm, Spodoptera frugiperda (JE Smith), injury in selected field and forage crops. Florida Entomologist. (1986) 69:549–59. doi: 10.2307/3495389
5. Montezano DG, Sosa-Gómez D, Specht A, Roque-Specht VF, Sousa-Silva JC, Paula-Moraes SD, et al. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr entomology. (2018) 26:286–300. doi: 10.4001/003.026.0286
6. CABI-led study provides comprehensive review of devastating fall armyworm pest . Available online at: https://www.cabi.org/news-article/cabi-led-study-provides-comprehensive-review-of-devastating-fall-armyworm-pest/ (Accessed 15 May 2023).
7. Prasanna B, Huesing JE, Peschke VM, Nagoshi RN, Jia X, Wu K, et al. Fall armyworm in Asia: invasion, impacts, and strategies for sustainable management. CIMMYT. (2021) 1–20.
8. Steensland A and Thompson TL. global agricultural productivity report: productivity in a time of pandemics. (2020).
9. Guo JF, Zhang MD, Gao ZP, Wang DJ, He KL, and Wang ZY. Comparison of larval performance and oviposition preference of Spodoptera frugiperda among three host plants: Potential risks to potato and tobacco crops. Insect Sci. (2021) 28:602–10. doi: 10.1111/1744-7917.12830
10. Ibrahim ES and Jimma E. Review on effect of American fall army worm and its management on maize as the world, SeniorSeminar paper, Jimma University Colledge of Agriculture and veterinary. (2018), Jimma, Ethiopia: Jimma University.
11. Abro Z, Kimathi E, De Groote H, Tefera T, Sevgan S, Niassy S, et al. Socioeconomic and health impacts of fall armyworm in Ethiopia. PloS One. (2021) 16:e0257736. doi: 10.1371/journal.pone.0257736
12. Kassie M, Wossen T, De Groote H, Tefera T, Sevgan S, and Balew S. Economic impacts of fall armyworm and its management strategies: evidence from southern Ethiopia. Eur Rev Agric Econ. (2020) 47:1473–501. doi: 10.1093/erae/jbz048
13. Tambo JA, Kansiime MK, Mugambi I, Agboyi LK, Beseh PK, and Day R. Economic impacts and management of fall armyworm (Spodoptera frugiperda) in smallholder agriculture: a panel data analysis for Ghana. CABI Agric Bioscience. (2023) 4:38. doi: 10.1186/s43170-023-00181-3
14. Goergen G, Kumar PL, Sankung SB, Togola A, and Tamò M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PloS One. (2016) 11:e0165632.
15. Shankar G and Adachi Y. First report of the occurrence of fall armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) on Ginger (Zingiber officinale) in Haveri district, Karnataka, India. J Entomology Zoology Stud. (2019) 7:78–80.
16. Gilal AA, Bashir L, Faheem M, Rajput A, Soomro JA, Kunbhar S, et al. First record of invasive fall armyworm (Spodoptera frugiperda (Smith)(Lepidoptera: Noctuidae)) in corn fields of Sindh, Pakistan. Pakistan J Agric Res. (2020) 33:247–52. doi: 10.17582/journal.pjar/2020/33.2.247.252
17. Pashley DP and Martin JA. Reproductive incompatibility between host strains of the fall armyworm (Lepidoptera: Noctuidae). Ann Entomological Soc America. (1987) 80:731–3. doi: 10.1093/aesa/80.6.731
18. Samanta S, Barman M, Thakur H, Chakraborty S, Upadhyaya G, Roy D, et al. Evidence of population expansion and insecticide resistance mechanism in invasive fall armyworm (Spodoptera frugiperda). BMC Biotechnol. (2023) 23:17. doi: 10.1186/s12896-023-00786-6
19. Hafeez M, Li X, Ullah F, Zhang Z, Zhang J, Huang J, et al. Characterization of indoxacarb resistance in the fall armyworm: selection, inheritance, cross-resistance, possible biochemical mechanisms, and fitness costs. Biology. (2022) 11:1718. doi: 10.3390/biology11121718
20. Chen HL, Hasnain A, Cheng QH, Xia LJ, Cai YH, Hu R, et al. Resistance monitoring and mechanism in the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) for chlorantraniliprole from Sichuan Province, China. Front Physiol. (2023) 14:1180655. doi: 10.3389/fphys.2023.1180655
21. Kumar NJ, Mandali R, Devaki K, and Reddy MG. Insecticide resistance monitoring of fall armyworm (Spodoptera frugiperda je smith) in chittoor district of andhra pradesh. Agric Sci. (2022) 8:239.
22. Foster And S and Harris M. Behavioral manipulation methods for insect pest-management. Annu Rev entomology. (1997) 42:123–46. doi: 10.1146/annurev.ento.42.1.123
23. Avosani S, Nieri R, Mazzoni V, Anfora G, Hamouche Z, Zippari C, et al. Intruding into a conversation: How behavioral manipulation could support management of Xylella fastidiosa and its insect vectors. J Pest Sci. (2024) 97:17–33. doi: 10.1007/s10340-023-01631-7
24. Bouri M, Arslan KS, and Şahin F. Climate-smart pest management in sustainable agriculture: Promises and challenges. Sustainability. (2023) 15:4592. doi: 10.3390/su15054592
25. Kalogiannidis S, Kalfas D, Chatzitheodoridis F, and Papaevangelou O. Role of crop-protection technologies in sustainable agricultural productivity and management. Land. (2022) 11:1680. doi: 10.3390/land11101680
26. Bruce TJ. Interplay between insects and plants: dynamic and complex interactions that have coevolved over millions of years but act in milliseconds. J Exp Bot. (2015) 66:455–65. doi: 10.1093/jxb/eru391
27. Stotz HU, Kroymann J, and Mitchell-Olds T. Plant-insect interactions. Curr Opin Plant Biol. (1999) 2:268–72. doi: 10.1016/S1369-5266(99)80048-X
28. Wang KY, Zhang Y, Wang HY, Xia XM, and Liu TX. Biology and life table studies of the oriental tobacco budworm, Helicoverpa assulta (Lepidoptera: Noctuidae), influenced by different larval diets. Insect Sci. (2008) 15:569–76. doi: 10.1111/j.1744-7917.2008.00247.x
29. Zhang QY, Zhang YL, Quandahor P, Gou YP, Li CC, Zhang KX, et al. Oviposition preference and age-stage, two-sex life table analysis of Spodoptera frugiperda (Lepidoptera: Noctuidae) on different maize varieties. Insects. (2023) 14:413. doi: 10.3390/insects14050413
30. Ali MP, Haque SS, Hossain MM, Bari MN, Kabir MMM, Roy TK, et al. Development and demographic parameters of Fall Armyworm (Spodoptera frugiperda JE Smith) when feeding on rice (Oryza sativa). CABI Agric Bioscience. (2023) 4:1–14.
31. Babendreier D, Toepfer S, Bateman M, and Kenis M. Potential management options for the invasive moth Spodoptera frugiperda in Europe. J Economic Entomology. (2022) 115:1772–82. doi: 10.1093/jee/toac089
33. Ali S, Li S, Jaleel W, Musa Khan M, Wang J, and Zhou X. Using a two-sex life table tool to calculate the fitness of Orius strigicollis as a predator of Pectinophora gossypiella. Insects. (2020) 11:275. doi: 10.3390/insects11050275
34. Chen Q, Li N, Wang X, Ma L, Huang J-B, and Huang G-H. Age-stage, two-sex life table of Parapoynx crisonalis (Lepidoptera: Pyralidae) at different temperatures. PloS One. (2017) 12:e0173380. doi: 10.1371/journal.pone.0173380
35. Jaleel W, Saeed S, Saeed Q, Naqqash MN, Sial MU, Aine QU, et al. Effects of three different cultivars of cruciferous plants on the age-stage, two-sex life table traits of Plutella xylostella (L. )(Lepidoptera: Plutellidae). Entomological Res. (2019) 49:151–7. doi: 10.1111/1748-5967.12270
36. Jaleel W, Tao X, Wang D, Lu L, and He Y. Using two-sex life table traits to assess the fruit preference and fitness of Bactrocera dorsalis (Diptera: Tephritidae). J Economic Entomology. (2018) 111:2936–45. doi: 10.1093/jee/toy243
37. Yin Jiao YJ, Cao YaZhong CY, Luo LiZhi LL, and Hu Yi HY. Effects of host plants on population increase of meadow moth, Loxostege sticticalis L. J Plant Prot Res. (2004) 31:173–8.
38. Gopalakrishnan R and Kalia VK. Biology and biometric characteristics of Spodoptera frugiperda (Lepidoptera: Noctuidae) reared on different host plants with regard to diet. Pest Manage Sci. (2022) 78:2043–51. doi: 10.1002/ps.6830
39. He LM, Wang TL, Chen YC, Ge SS, Wyckhuys KA, and Wu KM. Larval diet affects development and reproduction of East Asian strain of the fall armyworm, Spodoptera frugiperda. J Integr Agric. (2021) 20:736–44. doi: 10.1016/S2095-3119(19)62879-0
40. Jin T, Lin YY, Chi H, Xiang KP, Ma GC, Peng ZQ, et al. Comparative performance of the fall armyworm (Lepidoptera: Noctuidae) reared on various cereal-based artificial diets. J Economic Entomology. (2020) 113:2986–96. doi: 10.1093/jee/toaa198
41. Nandhini D, Deshmukh SS, Kalleshwaraswamy C, Satish K, and Sannathimmappa H. Effect of host plants on the biology and nutritional indices of fall armyworm, Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Anim Biol. (2023) 73:153–70. doi: 10.1163/15707563-bja10102
42. Silva D, Bueno A, Andrade K, Stecca C, Neves PMOJ, and Oliveira M. Biology and nutrition of Spodoptera frugiperda (Lepidoptera: Noctuidae) fed on different food sources. Scientia Agricola. (2017) 74:18–31. doi: 10.1590/1678-992x-2015-0160
43. Huang YB and Chi H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett)(Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. (2012) 19:263–73. doi: 10.1111/j.1744-7917.2011.01424.x
44. Goodman D. Optimal life histories, optimal notation, and the value of reproductive value. Am Nat. (1982) 119:803–23. doi: 10.1086/283956
45. Chi H and Su HY. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead)(Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer)(Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ entomology. (2006) 35:10–21. doi: 10.1603/0046-225X-35.1.10
46. Tuan SJ, Lee C-C, and Chi H. Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Manage Sci. (2014) 70:805–13. doi: 10.1002/ps.3618
47. Alami S, Naseri B, Golizadeh A, and Razmjou J. Age-stage, two-sex life table of the tomato looper, Chrysodeixis chalcites (Lepidoptera: Noctuidae), on different bean cultivars. Arthropod-Plant Interact. (2014) 8:475–84. doi: 10.1007/s11829-014-9330-3
48. Gharekhani G and Salek-Ebrahimi H. Life table parameters of Tuta absoluta (Lepidoptera: Gelechiidae) on different varieties of tomato. J Economic Entomology. (2014) 107:1765–70. doi: 10.1603/EC14059
49. Li DY, Zhi JR, Zhang T, Ye JQ, and Liang YJ. Effects of different host plants on the development and reproduction of. Spodoptera frugiperda. (2020) 42(2):311–7.
50. Nouri-Ganbalani G, Mardani-Talaee M, and Haji-Ramezani MR. Age-stage, two-sex life history of the golden twin spot moth, Chrysodeixis chalcites (Lepidoptera: Noctuidae), on six commercial tomato cultivars under laboratory conditions. Can Entomologist. (2016) 148:92–101. doi: 10.4039/tce.2015.16
51. Ribeiro L, Klock A, Nesi C, Luczkievicz F, Travi M, and Rech A. Adaptability and comparative biology of fall armyworm on maize and perennial forage species and relation with chemical-bromatological composition. Neotropical Entomology. (2020) 49:758–67. doi: 10.1007/s13744-020-00794-7
52. Sedighi L, Ranjbar Aghdam H, Imani S, and Shojai M. Age-stage two-sex life table analysis of Sesamia nonagrioides (Lep.: Noctuidae) reared on different host plants. Arch Phytopathol Plant Prot. (2017) 50:438–53. doi: 10.1080/03235408.2017.1323464
53. Umbanhowar J and Hastings A. The impact of resource limitation and the phenology of parasitoid attack on the duration of insect herbivore outbreaks. Theor Population Biol. (2002) 62:259–69. doi: 10.1006/tpbi.2002.1617
54. Yin WD, Qiu GS, Yan WT, Sun LN, Zhang HJ, Ma C-S, et al. Age-stage two-sex life tables of Panonychus ulmi (Acari: Tetranychidae), on different apple varieties. J Economic Entomology. (2013) 106:2118–25. doi: 10.1603/EC12491
55. Chen GM, Chi H, Wang RC, Wang YP, Xu YY, Li XD, et al. Demography and uncertainty of population growth of Conogethes punctiferalis (Lepidoptera: Crambidae) reared on five host plants with discussion on some life history statistics. J Economic Entomology. (2018) 111:2143–52. doi: 10.1093/jee/toy202
56. Xu P, Zhang D, Wang J, Wu K, Wang X, Wang X, et al. The host preference of Spodoptera frugiperda on maize and tobacco. Plant Prot. (2019) 45:61–4.
57. Xie W, Zhi J, Ye J, Zhou Y, Li C, Liang Y, et al. Age-stage, two-sex life table analysis of Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae) reared on maize and kidney bean. Chem Biol Technol Agric. (2021) 8:1–8. doi: 10.1186/s40538-021-00241-8
58. Acharya R, Malekera MJ, Dhungana SK, Sharma SR, and Lee KY. Impact of rice and potato host plants is higher on the reproduction than growth of corn strain fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects. (2022) 13:256. doi: 10.3390/insects13030256
59. Date P, Crowley-Gall A, Diefendorf AF, and Rollmann SM. Population differences in host plant preference and the importance of yeast and plant substrate to volatile composition. Ecol Evol. (2017) 7:3815–25. doi: 10.1002/ece3.2993
60. Mandal P, Mondal F, and Hossain M. Factors influences selection and adaptation of aphid to their host plant. J Plant Sci Crop Prot. (2020) 3:102.
61. Prager SM, Esquivel I, and Trumble JT. Factors influencing host plant choice and larval performance in Bactericera cockerelli. PloS One. (2014) 9:e94047. doi: 10.1371/journal.pone.0094047
62. Wu LH, Cao Z, Long GY, Yang XB, Wei ZY, Liao YJ, et al. Fitness of fall armyworm, Spodoptera frugiperda to three solanaceous vegetables. J Integr Agric. (2021) 20:755–63. doi: 10.1016/S2095-3119(20)63476-1
63. Ozgokçe MS, Chi H, Atlıhan R, and Kara H. Demography and population projection of Myzus persicae (Sulz.)(Hemiptera: Aphididae) on five pepper (Capsicum annuum L.) cultivars. Phytoparasitica. (2018) 46:153–67.
64. Kazemi M, TalebI CP, Shakiba MR, and Mashhadi JM. Biological responses of Russian wheat aphid, Diuraphis noxia (Mordvilko)(Homoptera: Aphididae) to different wheat varieties. J Agriculture Sci Technol. (2001) 3:249–55.
65. Altaf N, Idrees A, Ullah MI, Arshad M, Afzal A, Afzal M, et al. Biotic potential induced by different host plants in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects. (2022) 13:921. doi: 10.3390/insects13100921
66. Zhou Y, He H, and Xue F. The effects of photoperiod and temperature on diapause induction in the Tai’an population of cabbage beetle, Colaphellus bowringi Baly. Environ Entomol. (2018) 40:123–8.
67. Barros EM, Torres JB, Ruberson JR, and Oliveira MD. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomologia Experimentalis Applicata. (2010) 137:237–45. doi: 10.1111/j.1570-7458.2010.01058.x
68. Mooventhan P, Baskaran R, Kaushal J, and Kumar J. Integrated management of fall armyworm in maize Vol. 225. . Raipur, India: ICAR-National Institute of Biotic Stress Management (2019) p. 2–24.
69. Badhai S, Gupta AK, and Koiri B. Integrated management of fall armyworm (Spodoptera frugiperda) in maize crop. Rev Food Agric. (2020) 1:27–9. doi: 10.26480/rfna.01.2020.27.29
70. Koffi D, Agboka K, Adom M, Adjevi MKA, Tounou KA, and Meagher RL. Eco-friendly management of fall armyworm: can host-plant intercropping drive to a sustainable IPM? Int J Pest Manage. (2024), 1–10.
71. Mukanga M, Machuku O, Lwinya K, Lupapula M, Matimelo M, and Chilipa L. Effect of intercropping maize with legumes, oilseed crops and cucurbits, and perimeter cropping on fall armyworm (Spodoptera frugiperda) infestation in Zambia. J Agric Environ Sci. (2024) 13:16–28.
72. Taambaijim’d Mbaidiro J, Onzo A, Djenaissem A, and Mbaikoubou M. Influence of sowing dates on the population density of the fall armyworm Spodoptera frugiperda (JE. Smith) and its damage on maize plants in Chad. Int J Biol Chem Sci. (2023) 17:773–86.
73. Ullah MS, Sharmin D, Tumpa TA, Rashed MTNN, Mondal P, Akram MW, et al. Invasion, distribution, monitoring and farmers perception of fall armyworm (Spodoptera frugiperda) and farm-level management practices in Bangladesh. Insects. (2023) 14:343. doi: 10.3390/insects14040343
74. Abbas A, Ullah F, Hafeez M, Han X, Dara MZN, Gul H, et al. Biological control of fall armyworm, Spodoptera frugiperda. Agronomy. (2022) 12:2704. doi: 10.3390/agronomy12112704
75. Ballal CR, Kandan A, Varshney R, Gupta A, Shylesha A, Venkatesan T, et al. Biological control for fall armyworm management in Asia. CIMMYT. (2021) 34:131–5.
76. Babendreier D, Koku Agboyi L, Beseh P, Osae M, Nboyine J, Ofori SE, et al. The efficacy of alternative, environmentally friendly plant protection measures for control of fall armyworm, Spodoptera frugiperda, in maize. Insects. (2020) 11:240. doi: 10.3390/insects11040240
77. Chi H and Yang TC. Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer)(Homoptera: Aphididae). Environ entomology. (2003) 32:327–33. doi: 10.1603/0046-225X-32.2.327
78. Farooq M, Shakeel M, Iftikhar A, Shahid MR, and Zhu X. Age-stage, two-sex life tables of the lady beetle (Coleoptera: Coccinellidae) feeding on different aphid species. J Economic Entomology. (2018) 111:575–85. doi: 10.1093/jee/toy012
79. Zafar J, Shoukat RF, Zhu Z, Fu D, Xu X, and Jin F. Two-sex life table analysis for optimizing Beauveria bassiana application against Spodoptera exigua (Hübner)(Lepidoptera: Noctuidae). J Fungi. (2024) 10:469. doi: 10.3390/jof10070469
80. Frazier M, Huey RB, and Berrigan D. Thermodynamics constrains the evolution of insect population growth rates:”warmer is better. Am Nat. (2006) 168:512–20. doi: 10.1086/506977
Keywords: Age-stage two-sex life table, castor, fall armyworm, potato, reproduction, Spodoptera frugiperda, survival
Citation: Tajdar A, Cao C, Jaleel W, Zaka SM and Shi W (2025) Spodoptera frugiperda Smith fitness on four natural hosts using a two-sex life table in a controlled setting. Front. Insect Sci. 5:1548497. doi: 10.3389/finsc.2025.1548497
Received: 20 December 2024; Accepted: 12 September 2025;
Published: 01 October 2025.
Edited by:
Isabel Gomez, National Autonomous University of Mexico, MexicoReviewed by:
Luis F. Aristizabal, Consultant, Kailua-Kona, United StatesNishtha Nayyar, University of Kentucky, United States
Copyright © 2025 Tajdar, Cao, Jaleel, Zaka and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangpeng Shi, d3BzaGlAY2F1LmVkdS5jbg==; Syed Muhammad Zaka, emFrYV9lbnRvQGJ6dS5lZHUucGs=; Waqar Jaleel, d2FxYXI0bWVAeWFob28uY29t
 Alia Tajdar
Alia Tajdar Chuan Cao1
Chuan Cao1 Waqar Jaleel
Waqar Jaleel Syed Muhammad Zaka
Syed Muhammad Zaka Wangpeng Shi
Wangpeng Shi