- 1Department of Virology, Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna, Brescia, Italy
- 2Department of Biological Sciences, Kean University, Union, NJ, United States
The larvae of five dipteran species were reared on artificial diets under controlled laboratory conditions. Usually, these species complete their life cycles in perishable, filthy, unhygienic, and foul-smelling natural diets, which hinder rearing work and affect the laboratory environment. More importantly, these unaltered foods do not allow for true conformity in rearing protocols. The addition of a standard artificial diet to rearing protocols would make it possible to conduct ecological, biological, and forensic investigations with greater accuracy and precision. To address this problem, we formulated a new artificial diet for larvae and tested its performance on five different fly species (Calliphora vicina, Lucilia sericata, Sarcophaga argyrostoma, Musca domestica, and Hermetia illucens). We compared the development of larvae reared on an artificial diet with that of larvae reared on beef liver and pig muscle. The results showed no differences in development time between the two groups. However, our results showed that the artificial diet facilitated the rearing of flies for forensic and medical purposes by standardizing the nutritional value of the diet, improving laboratory conditions, and providing a more hygienic and cost-effective food substrate.
1 Introduction
Rearing insects is an essential part of entomological research. The use of artificial diets has allowed researchers to conduct studies in the fields of biological control (1); sterile insect technologies, feed for other animals (2); bioreactors for the production of pharmaceuticals (3) and other recombinant proteins; food for people (4, 5), and in recent years, in forensic entomology (6–8). This last branch of entomology involves studying the life cycles of insects that are found on decaying human remains and can be used to determine the date and cause of death (9).
Families of insects belonging to the order Diptera, which are of forensic importance, are numerous and differ from each other in their ecological characteristics. In forensic entomology, it is important to estimate the postmortem interval (PMI) (10). A reliable estimate of PMI requires a proven assessment of insect development rates under controlled laboratory conditions. Because breeding conditions can considerably influence the growth and development of larvae (11), such influences should be considered appropriately to avoid miscalculation of the PMI.
Most studies of larval growth rates have focused on factors such as temperature (12–14), larval aggregation and crowding, and drug effects (15, 16). There has been considerable variation in food substrates in these studies, including mammalian muscle, ground beef, mouse/rat carcasses (17, 18), fish, or even artificial diets containing components such as whole milk powder, baking powder, and wheat germ. However, these food substrates have proven to be efficient for the breeding of only some dipterans of forensic interest and, therefore, are not functional for the possible standardization of breeding in the laboratory (19).
Currently, forensic laboratories rely on natural materials, such as liver or other meat products, to rear these insects (8). These types of food sources are impractical for crime laboratories as they are usually kept frozen before use and are not shelf-stable, presenting an additional challenge in the collection of entomological evidence (6). The use of these materials results in noxious odors and potential health hazards associated with their unclean and nonstandard nature. Furthermore, because of their different nutritional contents, the use of these substrates causes high variability in insect development, which affects the subsequent interpretation of the data (20).
There is great interest in necrophagous arthropods, both in relation to decomposition ecology and forensic investigations (21). However, the lack of standardization of laboratory diets, feeding, and rearing protocols makes it difficult to obtain accurate and univocal data from behavioral, medical, and forensic entomology studies (22). As an alternative, employing an artificial diet that can be stored long-term in a refrigerator with a specific nutritional value, and with performance that is similar to that of the diets currently in use, would provide a simple solution to this problem.
In this study, we proposed a new artificial diet to replace the natural diets commonly used for rearing forensically important flies. We tested this diet on two blow fly species (Diptera: Calliphoridae; Calliphora vicina, Lucilia sericata), one muscid species (Diptera: Muscidae; Musca domestica), one flesh fly species (Diptera: Sarcophagidae; Sarcophaga argyrostoma), and the black soldier fly (Diptera: Stratiomyidae; Hermetia illucens). These flies usually feed on fermenting or rotting organic materials of either animal or vegetable origin, including human corpses. Therefore, they are important indicators in forensic investigations (23).
2 Materials and methods
Adult Lucilia sericata, Calliphora vicina, Musca domestica, Sarcophaga argyrostoma, and Hermetia illucens individuals were initially collected from Reggio Emilia in northern Italy (Lat. 44°42′32.34″N, Long. 10°37′36.66″E) using aerial traps baited with pork meat and beef liver. Adults were identified based on their morphological characteristics, as previously described (24). Approximately 100–150 individuals were placed in insect dorms of 30 cm × 30 cm × 30 cm with water, granulated sucrose (10:1 ratio), and 100 g of fresh beef liver in a Petri dish for oviposition.
2.1 Formulation of the artificial diet
The ingredients of the artificial diet were powdered milk; casein; soy flour; yeast; and agar, which was used as the gelling agent. The diet was prepared by weighing and mixing all of the dry ingredients as follows: 50 g of soy flour, 10 g of casein, 15 g of powdered milk, and 15 g of Saccharomyces cerevisiae yeast powder We dissolved 10g of agar in 200ml of distilled water.
All ingredients were mixed thoroughly and added to the agar solution. The mixture was then transferred to a Petri dish (90 mm diameter). Propionic acid was nebulized into the mixture as a mound inhibitor. The diets were stored in a refrigerator until further use. Preliminary tests were conducted to determine the appropriate box size, number of eggs per box, and diet for optimal larval development and survival. We have tested the number of specimens per box to avoid overcrowding phenomena, and the pH of the artificial diet. The pH of the final composition is between 6.8 and 7.5, this range is optimal for larval growth as it positively influences palatability and the activation of enzymes involved in digestion (4)
2.2 Experimental design
The different types of food used in the study were beef liver, pig muscle, and the artificial diet. All experiments were performed in triplicate to avoid the potential effects of other factors, such as temperature and humidity. The experiments were conducted under a photoperiod of 16 h/8 h light/dark, 25 ± 1°C, and 70% ± 5 relative humidity.
Each experiment involved three containers (liver, meat, and artificial diet) containing 100 g of each food type. To complete the larval stage, the food substrate was added ad libitum.
For the deposition of eggs of Calliphoridae and larvae of Sarcophagidae, liver was used as a substrate, while for Muscidae and Stratiomyidae, the Gainesville diet (25) was used.
In each container, 200 eggs were added, and the containers were placed in a 2 L plastic box.
All three experiments were performed simultaneously. Once pupated, 10 pupae were randomly collected and weighed to determine the mean individual pupal weight. Developmental, larval, and pupal times, pupal weight, and the percentage of adult emergence were compared between diets. The emergence of adults, pupal weight, and completion of the larval and pupal stages were recorded daily.
2.3 Statistical analysis
For each species, the effects of time and diet on weight (expressed in µg) were analyzed using a general linear mixed effects model, with the “replicate” variable as a random effect and the Gaussian weight distribution as a link function, using the lmer function of the lme4 package (26). There is a growing consensus within the biomedical scientific community that a significant/non-significant dichotomy based on a predetermined p-value cutoff should not be used for the interpretation of results. As such, we focused on the extent of the estimated treatment effect and its uncertainty, and the confidence intervals of the model estimates were interpreted as compatibility intervals, as described by Artheinm (27), accordingly, no null-hypothesis significance testing was performed. If an interval includes zero, this suggests that a null effect is plausible.
3 Results
Data collected for six life parameters—larval and pupal time, pupal weight, percentage of adult emergence, death rate, and total developmental period—were compared for the three diets and five species. The “adequacy” of an insect rearing substrate is determined by several factors, including its physical and chemical composition and its ability to support growth and development. For this reason, the suitability of the three diets was determined using an index incorporating growth, development, fecundity (indicated by pupal weight), and survival as empirical factors, as described by Seth and Sharma (28) (Table 1).
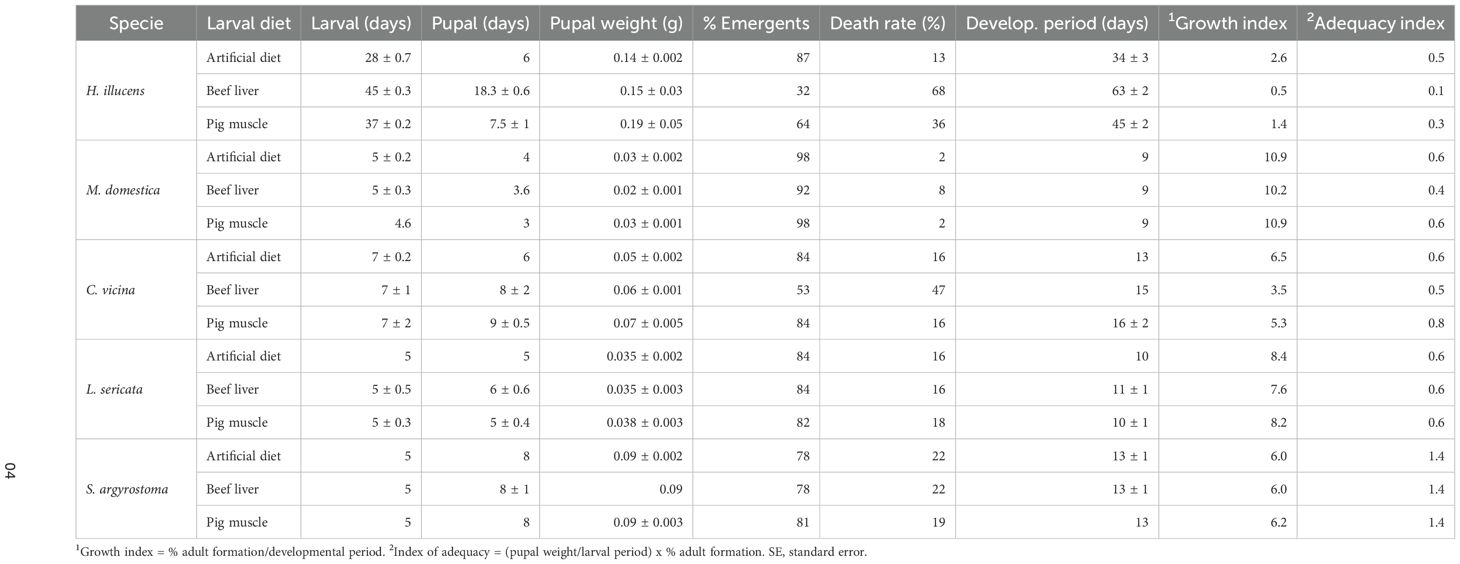
Table 1. Growth and development of Hermetia illucens (a), Musca domestica (b), Calliphora vicina (c), Lucilia sericata (d), and Sarcophaga argyrostoma (e) with three different diets.
In H. illucens and C. vicina, there were differences in the larval and pupal duration between larvae reared on the artificial diet and those reared on beef liver or pig muscle. Conversely, the development of S. argyrostoma, L. sericata, and M. domestica demonstrated similar performance on natural and artificial diets under laboratory conditions.
For most of the tested species, the growth index revealed that the performance of the artificial diet was better than that of the other natural substrates. Only M. domestica showed a high growth index in the pig muscle diet. The adequacy indices for all species were higher than or similar to those for insects reared on other natural substrates. C. vicina showed an adequacy index slightly higher than that of the artificial diet (0.8) when raised in pig muscle.
The results of the models for the effects of time and diet on pupal weight for different species are presented in a coefficient table, accompanied by a forest plot in Figure 1, along with a comprehensive description and guide to interpreting the results.
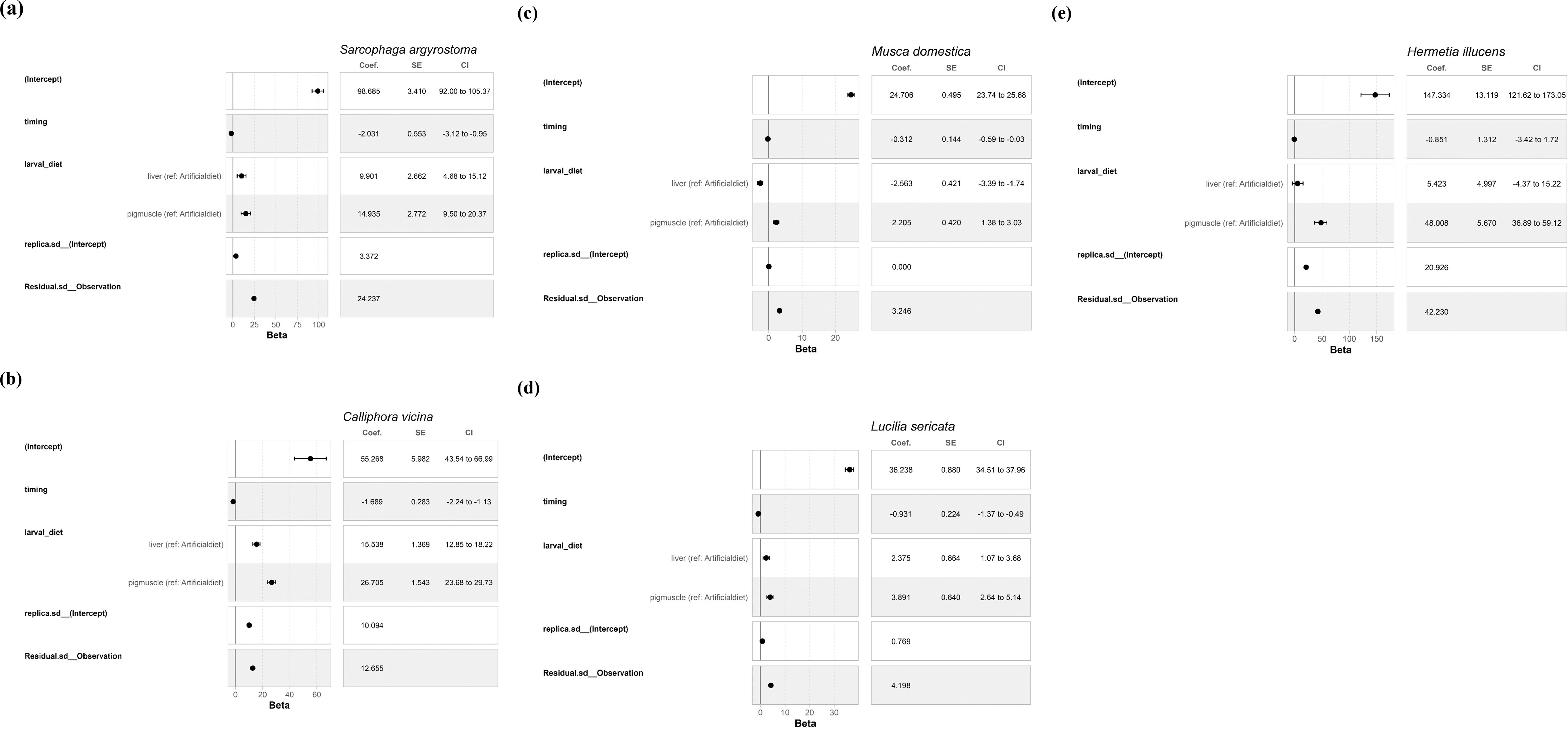
Figure 1. Results of the model for the effects of time and diet on pupal weight. Sarcophaga argyrostoma (a), Calliphora vicina (b), Musca domestica (c) Lucilia sericata (d), and Hermetia illucens (e). Intercept: This represents the estimated mean pupal weight (× 1,000) for the reference group (baseline larval diet, at time = 0).
For all bred species, an increase of one unit in time was associated with a decrease in pupal weight. The figures show that larvae reared on the liver and muscle diet produced heavier pupae than those reared on the artificial diet. The only exception was M. domestica, for which larvae raised on liver produced lighter pupae than those raised on the artificial diet. However, H. illucens larvae raised on the liver diet produced pupae that were 5.423 points heavier than those raised on the baseline diet. However, the plausible range varied from -4.39 to 15.18, indicating an effect that may be negative, close to zero, or positive. This suggested that there was uncertainty regarding whether a liver diet was beneficial. For S. argyrostoma, M. domestica, and L. sericata, the replicate effect was very small, and the compatibility interval was zero, suggesting that the replicates contributed little or no systematic variability in pupal weight. For the other species, the different experimental replicates showed clear differences in mean pupal weight, indicating that the replicates were a significant source of variability.
4 Discussion
In recent years, several publications have focused on food quality as an additional biotic factor for plasticity in larval growth and development. Clark et al. (29) observed that larvae of the blow fly Lucilia sericata grew faster when reared on lung and heart in comparison to liver. El-Moaty and Kheirallah (30) presented a similar amount of variation for the same species when fed with brain, lung, liver, kidney, heart, meat, or intestine. Bernhardt et al. (31) investigated the larval growth and development of the blowfly C. vicina on human muscle tissue in comparison with common porcine tissues. Their results focused only on larval growth using natural substrates and did not consider the entire development cycle.
Several artificial diet formulations are available for different flies (32–36), especially in the field of forensic science.
The results of this study clearly showed that larval growth and development were supported by the diets tested, indicating that an artificial diet may effectively replace other protein-based substrates, such as liver or muscle, for rearing saprophagous flies.
The adequacy index of the artificial diet was similar to that of the other diets tested, indicating an optimum energetic and nutritional contribution. The percentage of adults fed the three diets that emerged was greater than 75%, demonstrating adaptation to the set of nutritional and physical–environmental conditions prevalent in the laboratory. However, it is worth emphasizing that greater emergence success was observed on artificial diets and in pig muscle than in beef liver. Indeed, for larvae reared on artificial diet, the adult emergence rate was between 78-98%, for larvae reared on pork muscle between 68-98% and for liver the range was between 32-92%. Some nutritional components of the artificial diet (proteins and fats provided by milk powder, casein, and yeast) may be more appropriate for nurturing and developing competent larvae and facilitating their survival. This pattern was also observed in another study (37). Pupal weight is a critical factor determining insect survivorship (38). Previous studies of L. sericata and Cochliomyia hominivorax (Diptera: Calliphoridae) have demonstrated the importance of pupal weight as a measure of colony fitness. For example, the primary screwworm pupates successfully only 3.9% of the time when the larval weight ranges between 21 and 25 mg but shows 100% successful pupation when the larvae weigh 56–60 mg (39). The pupal weight of L. sericata was helpful in differentiating the effect of diet on development, with the fresh beef liver diet producing the weighty pupae, the blood–yeast agar diet producing the smallest pupae, and the decomposed beef liver diet and powdered beef liver diet producing intermediate-sized pupae (40).
In the last days of larval development, there is an accumulation of nutritional reserves in the larval body, which can be used during the subsequent development phases (41). These reserves accumulate in the adipose tissues. Therefore, the different levels of fat content in the diet can influence the size of both the larval and pupal body. In addition, effects on development times can also occur, leading the larvae to migrate and pupate early, leading to a misleading estimate of the PMI (42).
We found that the pupal weight was different between the three diets tested, except for S. argyrostoma, but this had no effect on the death rate. When reared on beef liver, the death rates were higher for H. illucens, M. domestica, and C. vicina. This may be explained by differences in the protein composition of the diets and the fact that H. illucens requires saccharides (43).
An evaluation of developmental time showed that the artificial diet did not cause differences in the egg-to-adult period, except for M. domestica and H. illucens. This confirmed the results of other studies in which the mass rearing of houseflies was possible on plant-based substrates consisting of 50% wheat bran, 30% alfalfa meal, and 20% corn meal. These substrates produced the higher survival-to-pupation ratios and greater pupal weights compared to those produced by animal waste (e.g. dairy, swine, or poultry manure) (44). The black soldier fly, H. illucens, although capable, like blow flies, of colonizing carrion, is considered a detritivore because its larvae feed on a variety of resources, including plant waste and restaurant food waste. When the larvae of this species are not reared on plant-based substances, they can extend their developmental period because of a lack of sufficient nutrients to survive through pupation and adult emergence.
Cook et al. (45) demonstrated that plant-based substrates are unsuitable for the mass rearing of C. vicina. C. vicina develops very slowly, even when soy flour is supplemented with other meat-based ingredients. Our study partially contradicts this result, showing that a diet supplemented with soy flour and the correct percentage of animal proteins represents a good alternative for the mass rearing of necrophagous insects.
Our data confirmed that all ingredients and their concentrations were likely indispensable for the growth of the five Dipteran species tested under the experimental conditions applied.
Fly rearing contributes to many aspects of our daily lives; for example, the production of human food and feed products, medicinal treatments for difficult-to-heal wounds, the transformation of large quantities of organic wastes that we generate daily into valuable products for the cosmetic or animal feed industries, the pollination of crops and their protection from pests (thereby avoiding the use of pesticides), and as evidence in criminal cases in the case of insects of forensic importance. Different diets affect the developmental time, size, and adult emergence of species of forensic importance (7, 8). This prompted us to prepare a standard diet on which they could be easily reared for research and other studies related to forensic entomology.
The artificial diet we developed was found to be most suitable for use in the laboratory. Its most relevant advantages are: 1) it has much less odor than liver or pig muscle diets. In fact, the gelling agent may have absorbed much of the metabolic waste, thereby reducing the amount of odor emitted during larval rearing; 2) it is simple to prepare and handle. All dry ingredients used to prepare the diet are commercially available from the indicated vendors; 3) it is less expensive to purchase in bulk and can be stored in a cool and dry place for an extended period, with no apparent loss of nutritional quality. However, our artificial diet has some disadvantages that need to be addressed. Although the artificial diet guarantees growth rates only like those of natural diets, thus offering advantages for forensic use, this also becomes a disadvantage for mass insect production. Our artificial diet likely does not guarantee the creation of populations dissimilar from natural ones, as it eliminates the differences that may exist in a natural population and influence development and evolution. Indeed, a short development period, high fertility, the laying of larger eggs highly enriched with nutrients, and the hatching rate are the proper parameters for determining a mass-rearing system, as well as key characteristics for evaluating artificial diets (46).
It has long been recognized that diets varying in nutritional composition influence insect development, reproduction, and longevity (47). Beyond providing sustenance and essential nutrients, the food ingested by insects supports the growth of numerous gut bacteria, resulting in a diverse and relatively stable microbial community (48). In this study, non-sterile diets were used to avoid alterations in the nutritional quality and physical properties of the diet components that can result from sterilization. This approach is aimed at preserving the microbial integrity of the artificial diet. Furthermore, as highlighted by Ponton et al. (49), diet quality and access to specific nutrients can affect both insect development and immune system performance. Inadequate nutrition during the larval stage may lead to a significantly weakened immune response. Future research will explore the potential effects of this artificial diet on the reproductive capacity of emerged adults. This strategy will contribute to the development of an artificial diet suitable not only for forensic entomology but also for broader applications, including the mass rearing of necrophagous and scavenging flies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
FD: Formal analysis, Writing – original draft, Validation, Conceptualization, Methodology, Investigation. MG: Writing – review & editing, Methodology, Conceptualization, Writing – original draft. AG: Methodology, Validation, Writing – review & editing. VT: Writing – review & editing, Formal analysis, Data curation. AL: Resources, Writing – review & editing. MD: Supervision, Resources, Writing – review & editing. AM: Supervision, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yazlovetsky IG. Development of artificial diets for entomophagous insects by understanding their nutrition and digestion. In: Anderson TE and Leppla NC, editors. Advances in Insect Rearing for Research & Pest Management. Westview Press, Boulder, CO (1992). p. 41–62.
2. Pérez-Grisales MS and Uribe Soto SI. Insects as sources of food and bioproducts: a review from Colombia. JoBAZ. (2022) 83:56. doi: 10.1186/s41936-022-00319-1
3. Cox MM. Recombinant protein vaccines produced in insect cells. Vaccine. (2012) 30:1759–66. doi: 10.1016/j.vaccine.2012.01.016
5. Anderson TE and Leppla NC eds. Advances in Insect Rearing for Research & Pest Management. Boulder, CO: Westview Press (1992) p. 33–40.
6. Weidner LM, Nigoghosian G, Hanau CG, and Jennings DE. Analysis of alternative food sources for rearing entomological evidence. J Med Entomology. (2020) 57:1407–10. doi: 10.1093/jme/tjaa060
7. Anderson GS. Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J Forensic Sci. (2000) 45:824–32. doi: 10.1520/JFS14778J
8. Byrd JH and Castner JL. Insects of forensic importance. In: Byrd JH and Castner JL, editors. Forensic Entomology: The Utility of Arthropods in Legal Investigations. CRC Press, Boca Raton, FL (2009). p. 43–5.
9. Sharma R, Garg RK, and Gaur J. Various methods for the estimation of the post mortem interval from Calliphoridae: A review. Egyptian J Forensic Sci. (2015) 5:1–12. doi: 10.1016/j.ejfs.2013.04.002
10. Joseph I, Mathew DG, Sathyan P, and Vargheese G. The use of insects in forensic investigations: An overview on the scope of forensic entomology. J Forensic Dent Sci. (2011) 3:89–91. doi: 10.4103/0975-1475.92154
11. Niederegger S, Astuschek J, and Mall G. Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci Int. (2010) 199:72–8. doi: 10.1016/j.forsciint.2010.03.015
12. Grassberger M and Reiter C. Effect of temperature on development of the forensically important holarctic blow fly Protophormia terraenovae (Robineau-Desvoidy) (Diptera: Calliphoridae). Forensic Sci Int. (2002) 128:177–82. doi: 10.1016/s0379-0738(02)00199-8
13. Ames C and Turner B. Low temperature episodes in development of blowflies: implications for postmortem interval estimation. Med veterinary entomology vol. (2003) 17:178–86. doi: 10.1046/j.1365-2915.2003.00421.x
14. Boatright SA and Tomberlin JK. Effects of temperature and tissue type on the development of Cochliomyia macellaria (Diptera: Calliphoridae). J Med entomology vol. (2010) 47:5. doi: 10.1603/me09206
15. Introna F, Campobasso CP, and Goff ML. Entomotoxicology. Forensic Sci Int. (2001) 120:42–7. doi: 10.1016/s0379-0738(01)00418-2
16. Gunn JA, Shelley C, Lewis SW, Toop T, and Archer M. The Determination of Morphine in the Larvae of Calliphora stygia using Flow Injection Analysis and HPLC with Chemiluminescence Detection. J Analytical Toxicol. (2006) 30:519–23. doi: 10.1093/jat/30.8.519
17. Putman RJ. Dynamics of the blowfly, calliphora erythrocephala, within carrion. J Anim Ecol. (1977) 46:853–66. doi: 10.2307/3645
18. Tarone AM and Foran DR. Components of developmental plasticity in a Michigan population of Lucilia sericata (Diptera: Calliphoridae). J Med Entomology. (2006) 43:1023–33. doi: 10.1093/jmedent/43.5.1023
19. Niederegger S, Wartenberg N, Spiess R, and Mall G. Influence of food substrates on the development of the blowflies Calliphora vicina and Calliphora vomitoria (Diptera, Calliphoridae). Parasitol Res. (2013) 112:2847–53. doi: 10.1007/s00436-013-3456-6
20. Thyssen PJ, de Souza CM, Shimamoto PM, Salewski TB, and Moretti TC. Rates of development of immatures of three species of Chrysomya (Diptera: Calliphoridae) reared in different types of animal tissues: implications for estimating the postmortem interval. Parasitol Res. (2014) 113:3373–80. doi: 10.1007/s00436-014-4002-x
21. Dawson BM, Ueland M, Carter DO, Mclntyre D, and Barton PS. Bridging the gap between decomposition theory and forensic research on postmortem interval. Int J Legal Med. (2024) 138:509–18. doi: 10.1007/s00414-023-03060-8
22. Khatun R, Mostafa G, Haque E, Ahmad S, and Akte T. Formulation and evaluation of the efficacy of an artificial diet for two forensicallyimportant flies. Asian Australas. J Biosci Biotechnol. (2018) 3:86–92. doi: 10.3329/aajbb.v3i2.64786
23. Tachibana S-I and Numata H. An artificial diet for blow fly larvae, Lucilia sericata (Meigen) (Diptera: Calliphoridae). Appl Entomol Zool. (2001) 36:521–3. doi: 10.1303/aez.2001.521
24. Smith KGV. A Manual of Forensic Entomology Trustees of the British Museum. London, UK: Natural History and Cornell University Press (1986).
25. Hogsette JA. New diets for production of house flies and stable flies (Diptera: muscidae) in the laboratory. J Economic Entomology. (1992) 85:2291–4. doi: 10.1093/jee/85.6.2291
26. Larmarange J. _ggstats: Extension to ‘ggplot2’ for Plotting Stats_. R package version 0.6.0, https://CRAN.R-project.org/package=ggstats. In: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria (2024). Available online at: https://www.R-project.org. R language environment (R Core Team (2024) (Accessed January 27, 2025).
27. Amrhein V, Greenland S, McShane B, et al. Scientists rise up against statistical significance. Nature. (2019) 567:305–7. doi: 10.1038/d41586-019-00857-9
28. Seth RK and Sharma VP. Growth, development, reproductive competence and adult behaviour of Spodoptera litura (Lepidoptera: Noctuidae) reared on different diets. In: Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, vol. 126. Vienna, Austria: International Atomic Energy Agency (IAEA) (2002). p. 15–22.
29. Clark K, Evans L, and Wall R. Growth rates of the blowfly, Lucilia sericata, on different body tissues. Forensic Sci Int. (2006) 156:145–9. doi: 10.1016/j.forsciint.2004.12.025
30. El-Moaty ZA and Kheirallah AEM. Developmental variation of the blow fly Lucilia sericata (Meigen, 1826) (Diptera: Calliphoridae) by different substrate tissue types. J Asia-Pacific Entomology. (2013) 16:297–300. doi: 10.1016/j.aspen.2013.03.008
31. Bernhardt V, Schomerus C, Verhoff MA, and Amendt J. Of pigs and men—comparing the development of Calliphora vicina (Diptera: Calliphoridae) on human and porcine tissue. Int J Legal Med. (2017) 131:847–53. doi: 10.1007/s00414-016-1487-0
32. Pérez C, Segura NA, Patarroyo MA, and Bello FJ. Evaluating the biological cycle and reproductive and population parameters of calliphora vicina (Diptera: calliphoridae) reared on three different diets. J Med Entomology. (2016) 53:1268–75. doi: 10.1093/jme/tjw114
33. Idris AB, Abdullah M, and Lin TP. Effect of various diets on the development of scuttle fly, Megaselia scalaris (Loew) (Diptera: Phoridae), larvae and puparia and percent of adult emergence and longevity. Pak J Biol Sci. (2001) 4:321–5. doi: 10.3923/pjbs.2001.321.325
34. Zuha RM, Razak TA, Ahmad NW, and Omar B. Interaction effects of temperature and food on the development of forensically important fly, Megaselia scalaris (Loew) (Diptera: Phoridae). Parasitol Res. (2012) 111:2179–87. doi: 10.1007/s00436-012-3070-z
35. Rueda LC, Ortega LG, Segura NA, Acero VM, and Bello F. Lucilia sericata strain from Colombia: Experimental colonization, life tables and evaluation of two artificial diets of the blowfly Lucilia sericata (Meigen) (Diptera: Calliphoridae), Bogota, Colombia strain. Biol Res. (2010) 43:197–203. doi: 10.4067/S0716-97602010000200008
36. Loureiro MS, Oliveira VC, and Almeida JM. Desenvolvimento pós-embrionário de Pattonella intermutans (Thomson) (Diptera: Sarcophagidae) em diferentes dietas. Rev Bras Entomol. (2005) 49:127–129. doi: 10.1590/S0085-56262005000100014
37. Chaudhury MF, Chen H, Sagel A, and Skoda SR. Effects of new dietary ingredients used in artificial diet for screwworm larvae (Diptera: calliphoridae). J Economic Entomology. (2015) 108:1429–34. doi: 10.1093/jee/tov039
38. Davidowitz G, D’Amico LJ, and Nijhout HF. Critical weight in the development of insect body size. Evol Dev. (2003) 5:188–97. doi: 10.1046/j.1525-142X.2003.03026.x
39. Hightower B, Spates GE, and Garcia JJ. Growth and critical size at pupation for larvae of the screwworm developing in fresh wounds. J Econ Entomol. (1972) 65:1349–52. doi: 10.1093/jee/65.5.1349
40. Zheng L, Crippen TL, Dabney A, Gordy A, and Tomberlin JK. Evaluation of sterilized artificial diets for mass rearing the lucilia sericata (Diptera: calliphoridae). J Med Entomology. (2017) 54:1122–8. doi: 10.1093/jme/tjx091
41. Ujvari B, Wallman JF, Madsen T, Whelan M, and Hulbert AJ. Experimental studies of blowfly (Calliphora stygia) longevity: A little dietary fat is beneficial but too much is detrimental. Comp Biochem Physiol Part A: Mol Integr Physiol. (2009) 154. doi: 10.1016/j.cbpa.2009.07.012
42. Li X, Yang Y, Li G, Li H, Wang Q, and Wan L. The effect of dietary fat levels on the size and development of Chrysomya megacephala (Diptera: Calliphoridae). J Insect Sci. (2014) 14:174. doi: 10.1093/jisesa/ieu036
43. Kawasaki K, Ohkawa M, Zhao J, and Yano K. Effect of dietary meat content on weight gain, mortality, and pre-pupal rate in black soldier fly (Hermetia illucens) larvae. Insects. (2022) 13:229. doi: 10.3390/insects13030229
44. Miranda CD, Cammack JA, and Tomberlin JK. Large-scale production of house fly, Musca domestica (Diptera: Muscidae), larvae fed 3 manure types. J Econ Entomol. (2023) 116:1102–9. doi: 10.1093/jee/toad099
45. Cook DF, Tufail MS, and Voss SC. Can the necrophagous blow fly calliphora vicina (Diptera: calliphoridae) be reared on plant-based meal? Insects. (2024) 15:14. doi: 10.3390/insects15070551
46. Ali HMS, Mirza JH, Kamran M, and Alatawi FJ. Assessing different artificial diets to improve the life history parameters of the almond moth, ephestia cautella (Walker) (Lepidoptera: pyralidae). Agriculture. (2024) 14:2295. doi: 10.3390/agriculture14122295
47. Karley AJ, Douglas AE, and Parker WE. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J Exp Biol. (2002) 205:3009–18. doi: 10.1242/jeb.205.19.3009
48. Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol. (2015) 60:17–34. doi: 10.1146/annurev-ento-010814-020822
Keywords: Calliphoridae, artificial diet, insect rearing, black soldier fly, Muscidae
Citation: Defilippo F, Gemmellaro MD, Grisendi A, Tranquillo V, Lavazza A, Dottori M and Moreno A (2025) Formulation and evaluation of the efficacy of an artificial larval diet for rearing various species of flies under laboratory conditions. Front. Insect Sci. 5:1630472. doi: 10.3389/finsc.2025.1630472
Received: 17 May 2025; Accepted: 22 July 2025;
Published: 08 August 2025.
Edited by:
Ashfaq Sial, University of Georgia, United StatesReviewed by:
Jorge Contreras-Garduño, National Autonomous University of Mexico, MexicoAndrzej Grzywacz, Nicolaus Copernicus University, Poland
Maristella Mastore, University of Insubria, Italy
Copyright © 2025 Defilippo, Gemmellaro, Grisendi, Tranquillo, Lavazza, Dottori and Moreno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Defilippo, ZnJhbmNlc2NvLmRlZmlsaXBwb0BpenNsZXIuaXQ=
 Francesco Defilippo
Francesco Defilippo M. Denise Gemmellaro2
M. Denise Gemmellaro2 Vito Tranquillo
Vito Tranquillo Ana Moreno
Ana Moreno