- 1Liaoning Provincial Key Laboratory of Forest Protection, Liaoning Academy of Forestry Sciences, Shenyang, China
- 2Key Laboratory of Sustainable Forest Ecosystem Management-Ministry of Education, Northeast Forestry University, Harbin, China
Monochamus saltuarius is an important wood-boring pest of forests and a vector insect for the transmission of Bursaphelenchus xylophilus in China and other East Asian regions. To gain insight into the Mo. saltuarius olfactory system, we characterized the sizes and morphological characteristics of sensilla on antennae, maxillary palps, and labial palps of adults by scanning electron microscopy. Eight types of antennal sensilla were identified on the antennae: Böhm bristles (BBs), sensilla chaetica (SChs, with subtypes SChI and SChII), sensilla trichodea (STs, with subtypes STI, STII and STIII), sensilla auricillica (SAus), sensilla basiconica (SBs, with subtypes SBI and SBII), sensilla grooved peg (SGPs), dome shaped organs (DSOs), and cuticular pores (CPs); among these, BBs, STIs, STIIs, SChIs, and SChIIs may be mechanoreceptors, and STIIIs, SAus, SBIs, SBIIs, SGPs and CPs may be chemoreceptors. Seven sensillum types were identified on maxillary palps and labial palps: BBs, STs (with subtypes STII, and STIII), SChs, sensilla placodea (SPs), sensilla coeloconica (SCos), CPs, and sensilla twig basiconica (STBs, with subtypes STBI, STBII, STBIII, and STBIV), among which BBs, STIIs, and SChs may be mechanoreceptors, and STIIIs, SPs, CPs, STBIs, STBIIs, STBIIIs, and STIVs may be chemoreceptors. DSOs on the antennae and SCos on the palps may be hydroreceptors, and/or thermoreceptors. The types and densities of sensilla increased from the base to the tip of the antennae, and sensilla with chemical-sensing functions were concentrated mostly on the flagellum. Identification of these sensillum types provides a basis for analyzing the mechanisms of host recognition and environmental perception of Mo. saltuarius.
1 Introduction
Antennae, maxillary palps, and labial palps are the major organs with which insects perceive external stimuli, and their surfaces are covered with many different types of sensillum. These sensilla enable insects to perceive the external environment and carry out chemical communication, playing an important role in the acquisition of food, detection of host plants, avoidance of predators, identification of mates, and selection of oviposition sites (1–3). The sensillum is a specialized exoskeletal region that comprises formative cells, sensory neurons (which detect stimuli from the outer cuticular structure), and occasionally auxiliary cells (4). Specific sensilla respond to infrared radiation, CO2, chemical and mechanical stimuli, temperature, and humidity (5). They function as discrete sensory units and include a variety of types, each with distinct morphological characteristics. Sensilla often exhibit sexual dimorphism within a species, and antennae may be twice as long in males as in females, potentially as a result of male competition (6) or to enable the contact detection of female sex pheromones (7). The antennae, maxillary palps, and labial palps vary substantially in length and morphology; they function in the recognition of potential mates, host plants, and conspecifics and also help to mediate feeding and egg-laying behaviors (1, 3, 7, 8). Because evolutionary forces have shaped antennal architecture to optimize the perception of chemosensory signals (3), detailed structural characterization of antennae, maxillary palps, and labial palps is critical for understanding the chemical ecology of insects, including longhorned beetles.
The long-horned beetle Monochamus saltuarius Gebler (Coleoptera: Cerambycidae) is found in the Heilongjiang, Jilin, Liaoning, Inner Mongolia, Gansu, Xinjiang, Hebei, Shanxi, Shandong, and Zhejiang provinces of China (9–12). It is also present in Europe, Russia (Siberia and Sakhalin), Mongolia, South Korea, and Japan. In China, the predicted suitable habitats of Mo. saltuarius are mainly located north of 33° latitude (13). Its host species include Picea asperata, Pinus koraiensis, Pinus sylvestris var. mongholica, Larix spp., Abies fabri, and Pinus tabulaeformis (9, 10). The larvae of Mo. saltuarius penetrate into the host xylem and cut worm channels, thereby reducing wood value (14). The adults of Mo. saltuarius gnaw on the bark of branches, affecting the growth of standing trees. More importantly, Mo. saltuarius is a vector insect for the transmission of pine wood nematodes (Bursaphelenchus xylophilus) in Liaoning (11, 12), Japan (15, 16), South Korea (17, 18), and other East Asian regions. The maximum B. xylophilus carrier rate of Mo. saltuarius to pine wood was 95%, the maximum carrier number was 9528 (15), and the average carrier number was 7970 (12). Pine wood nematodes cause large numbers of pine deaths in these areas, leading to serious economic losses and ecological damage (12). Huh et al. found that both Mo. alternatus and Mo. saltuarius have four types (comprising nine subtypes) of antennal sensilla (19). However, there are no reports describing the types and distributions of sensilla on the maxillary and labial palps of adult Mo. saltuarius. In this study, we used scanning electron microscopy to observe the morphology and structural composition of antennae, maxillary palps, and labial palps, as well as the types, numbers, and distributions of sensilla in adult Mo. saltuarius. Differences were identified in these organs and sensilla between males and females, providing a foundation for future work on the internal structures, sensory mechanisms, and host recognition of various sensilla.
2 Materials and methods
2.1 Insects
Adults of Mo. saltuarius were collected from Hexi Village, Shangjiahe Town, Xinbin Manchu Autonomous County, Liaoning Province (41.85°N–41.86°N, 124.37°E–124.38°E) using hanging traps in the forest. The traps were made by Beijing Zhongjie Sifang Biotechnology (Model L002) and contained mainly pinene, L-β-pinene, and (1S)-(+)-3-carene as attractants.
2.2 Sample preparation for scanning electron microscopy
From January to August 2022, the antennae, maxillary palps, and labial palps of male and female Mo. saltuarius were removed under a stereomicroscope (Leica M205A) using a scalpel and forceps, then placed separately into 10.0-mL sterilized centrifuge tubes. The samples were fixed in 2.5% glutaraldehyde for 12 hours at 4°C and then ultrasonicated twice for 50 seconds each in 0.1 M phosphate buffered saline (PBS). The samples were dehydrated in a graded ethanol series (15 minutes each at 30%, 50%, 70%, 85%, and 100%), dried twice for 10 minutes each in 100% acetone, and finally air dried for 24 hours. The samples were affixed to copper stubs using double-sided adhesive tape in both ventral and dorsal orientations. An ion sputtering instrument (model KYKY SBC-12, KYKY Technology) was used to deposit an approximately 20-nm conductive layer of gold. The samples were observed, photographed, and measured using a scanning electron microscope (Thermo Fisher Scientific Apreo C) at 20 kV. Forty body length, six antennae (three dorsal surfaces and three ventral surfaces), six maxillary palps (three dorsal surfaces and three ventral surfaces), and six labial palps (three dorsal surfaces and three ventral surfaces) were observed for males and females.
2.3 Sensillum identification
The sensillum types of the antennae, maxillary palps, and labial palps were classified mainly according to the sensilla naming system established by Dyer and Seabrook (20), Schneider (4), Altner and Prillinger (21), and Zacharuk (22). We also consulted recent literature on the classification of antennae, maxillary palps, and labial palps in adult Coleoptera (23–27).
2.4 Terminology and data analysis
The lengths of each segment of the antennae, maxillary palps, and labial palps, as well as the lengths and basal diameters of the sensilla, were measured using the scale of the SEM. The surface area of each segment was calculated by multiplying the length by the width. Independent sample t-tests performed in SPSS (version 25.0, P<0.05) were used to assess the significance of differences in sizes and areas of antennae, maxillary palps, and labial palps, as well as lengths, widths, basal diameters, densities, and numbers of various sensilla between males and females. Data were expressed as mean±standard error.
3 Results
3.1 Morphological structures of antennae and maxillary and labial palps
3.1.1 General antennal morphology
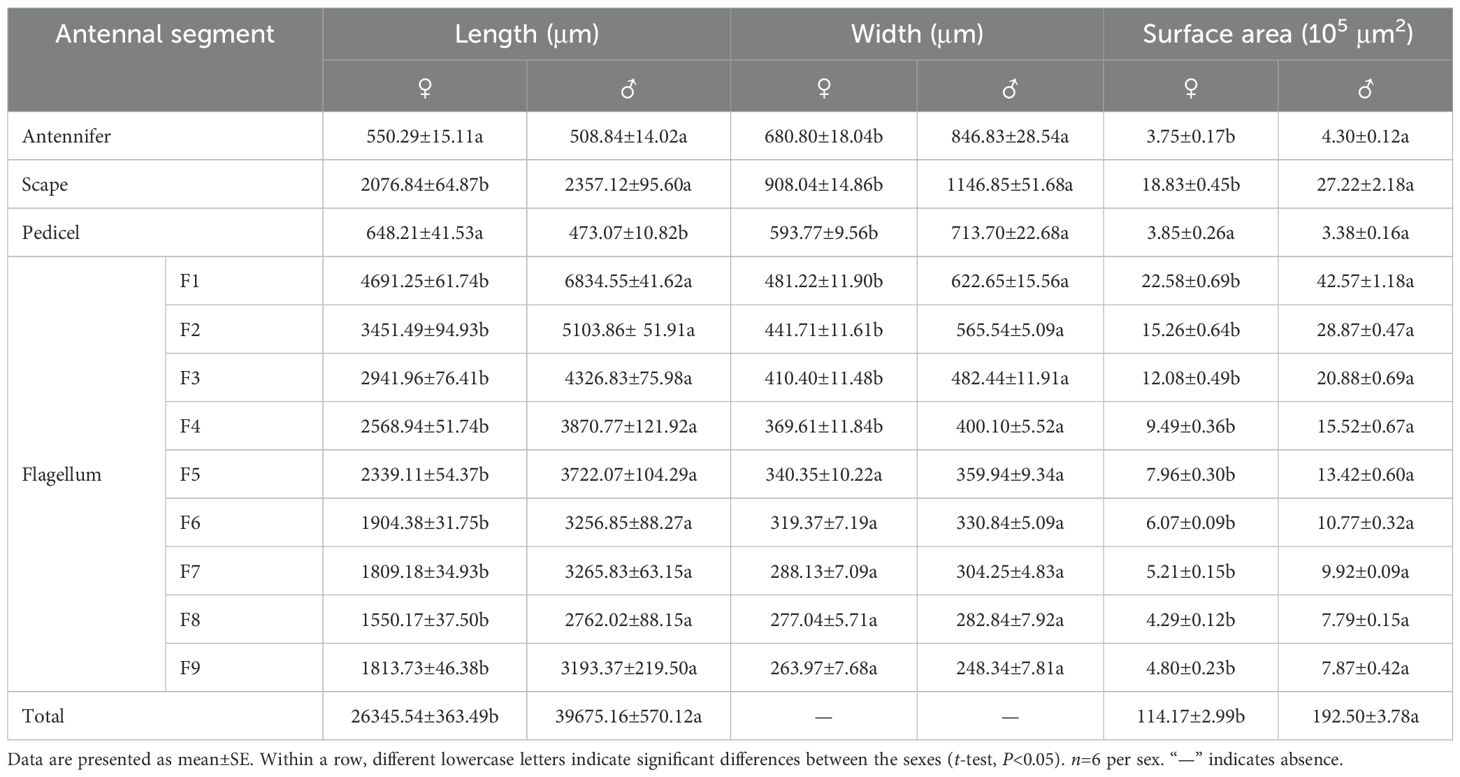
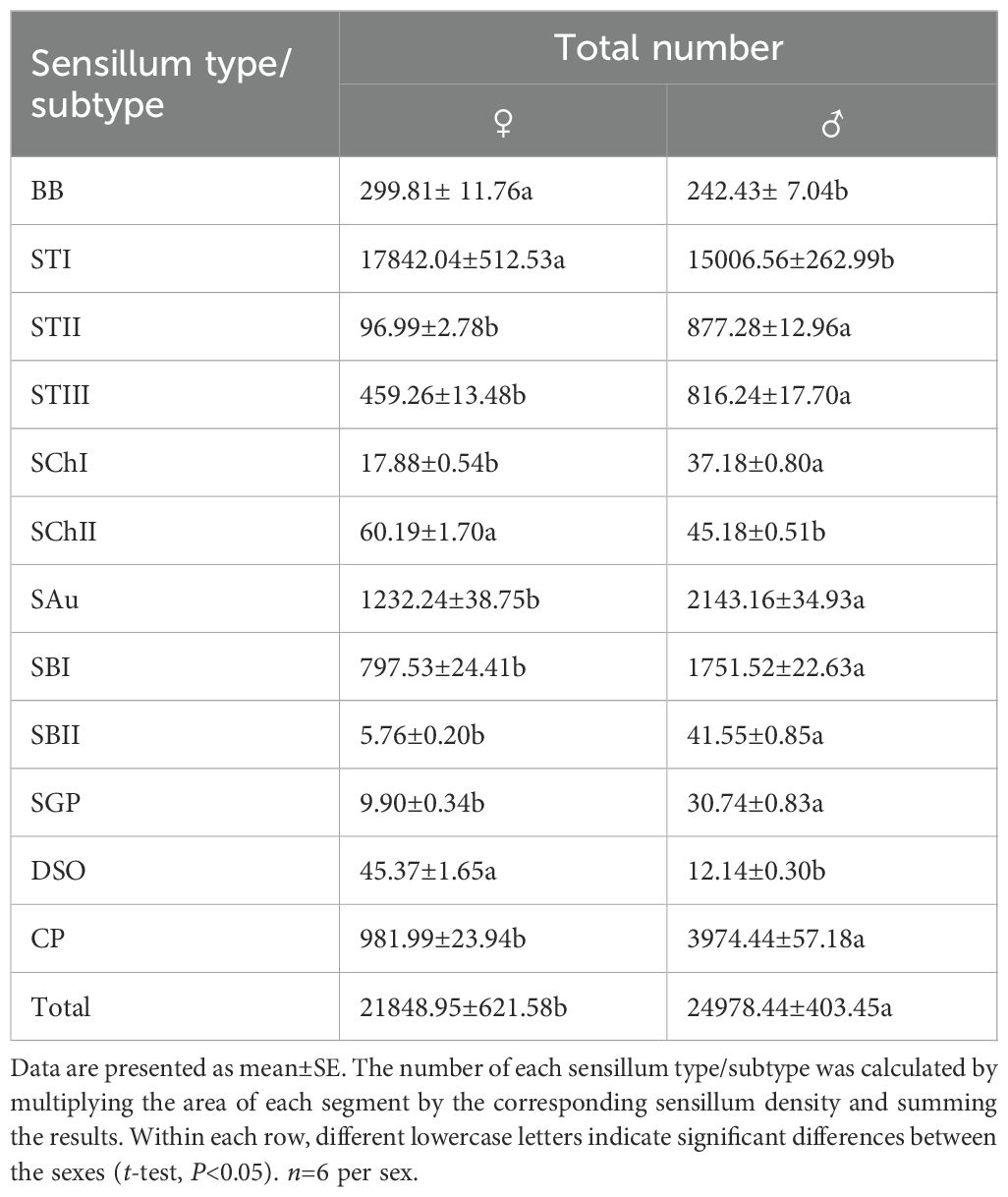
The body length of female Mo. saltuarius was 19.80±0.20 mm (n=40), which was significantly greater (t=2.546, df=78, P=0.013) than that of male Mo. saltuarius (19.11±0.20 mm, n=40). Metastethidium width did not differ between females and males (5.42±0.12 mm vs. 5.41±0.11 mm, t=−0.306, df=78, P=0.760). Like those of most Cerambycidae, the antennae of female and male Mo. saltuarius were the same in shape and structure; both were linear and comprised a radicle (Ra), scape (Sc), pedicel (Pe), and long flagellum (F). The flagellum comprised nine flagellomeres, F1–F9 (Figures 1A, B). The radicle was connected to the acetabular antennal fossa of the head and face, and the segments were connected by joints. Under an optical microscope, the antennae of male Mo. saltuarius were completely black, whereas those of females were black on the bottom, with black and white chequered flagella.
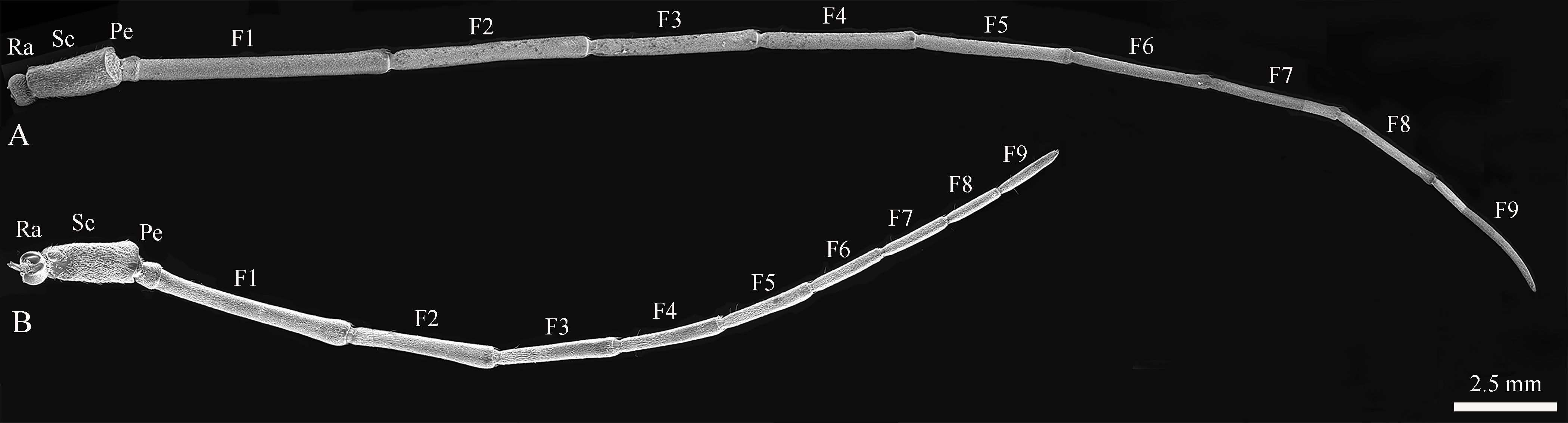
Figure 1. The antennal structures of adult Mo. saltuarius. (A) Male antennae; (B) female antennae. Ra, antennifer/basal radicle; Pe, pedicel; Sc, scape; F1–F9, first to ninth flagellomeres.
The total antennal length in females was 26,345.54±363.49 μm; most of this length was accounted for by the flagellum (~87.57%), followed by the scape (~7.88%), the pedicel (~2.46%), and the radicle (~2.09%) (Table 1). In female Mo. saltuarius, the flagellomeres gradually became shorter from F1 to F8, whereas F9 was longer, about equal to F7 (Table 1). The total antennal length in males was 39,675.16±570.12 μm, significantly longer than that of females (t=19.71, df=10, P=0.000). Again, most of the antennal length was accounted for by the flagellum (~91.58%), followed by the scape (~5.94%), the radicle (~1.28%), and the pedicel (~1.19%) (Table 1). From F1 to F8, the flagellomeres of males gradually became shorter, whereas F9 was longer, about equal to F6 or F7. In general, each flagellomere was significantly longer in males than in females (Table 1), although the antennal pedicel was significantly shorter in males (t=−6.150, df=10, P=0.000).
In females, the scape was the widest antennal segment (908.04±14.86 μm) and was 3.44 times wider than the narrowest segment, F9; the scape was also the widest segment in males (1146.85±51.68 μm) and was 4.62 times wider than F9 (Table 1). The radicle, scape, pedicel, and flagellomeres F1–F4 of male antennae were significantly wider than those of the corresponding female antennae, but the widths of flagellomeres F5–F9 did not differ significantly between males and females (Table 1). The surface area of female antennae (114.17±2.99) ×105 μm2 was significantly smaller than that of male antennae (192.50±3.78) ×105 μm2 (Table 1) (t=−16.255, df=10, P=0.000). There was no significant difference in the area of antennal pedicles between females and males (t=0.424, df=10, P=0.156), whereas the areas of other segments were significantly smaller in females than in males (Table 1).
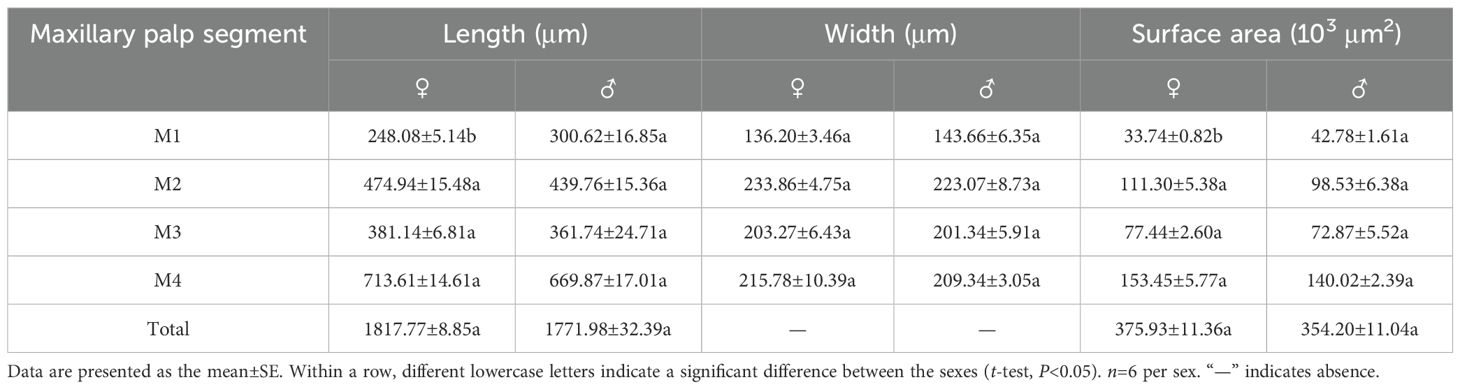
3.1.2 Morphology of maxillary and labial palps
The morphology and structural composition of the maxillary and labial palps were the same in males and females. Under the stereomicroscope, both palps were black, with a bright metallic luster (Figure 2A); under the electron microscope, sensilla could be seen on their surfaces (Figure 2B). The maxillary palps of both males and females comprised 4 segments, M1–M4 (Figure 2C). The length of the maxillary palps in females was 1817.77±8.85 μm, and M4 was the longest segment, accounting for 39.26% of the total length. M2, M3, and M1 accounted for 26.13%, 20.97%, and 13.65% of the total length, respectively (Table 2). The total length of maxillary palps in males was 1771.98±32.39 μm and did not differ significantly from that in females (t=1.363, df=10, P=0.203). M4 was also the longest segment in males, accounting for 37.80% of the total length, and M2, M3, and M1 accounted for 24.82%, 20.41%, and 16.97%, respectively (Table 2). M1 was significantly longer in males than in females (t=2.982, df=10, P=0.014), but none of the other segments differed in length between the sexes (Table 2). M2 was the widest maxillary palp segment in females (233.86±4.75 μm) and was 1.72 times wider than the narrowest segment (M1); M2 was also widest in males (223.07±8.73 μm) and was 1.55 times wider than M1. The widths of corresponding maxillary palp segments did not differ significantly between males and females. The M1 surface area was significantly smaller in females than in males (t=−5.008, df=10, P=0.001), but no other maxillary palp segments differed in surface area between the sexes (Table 2).
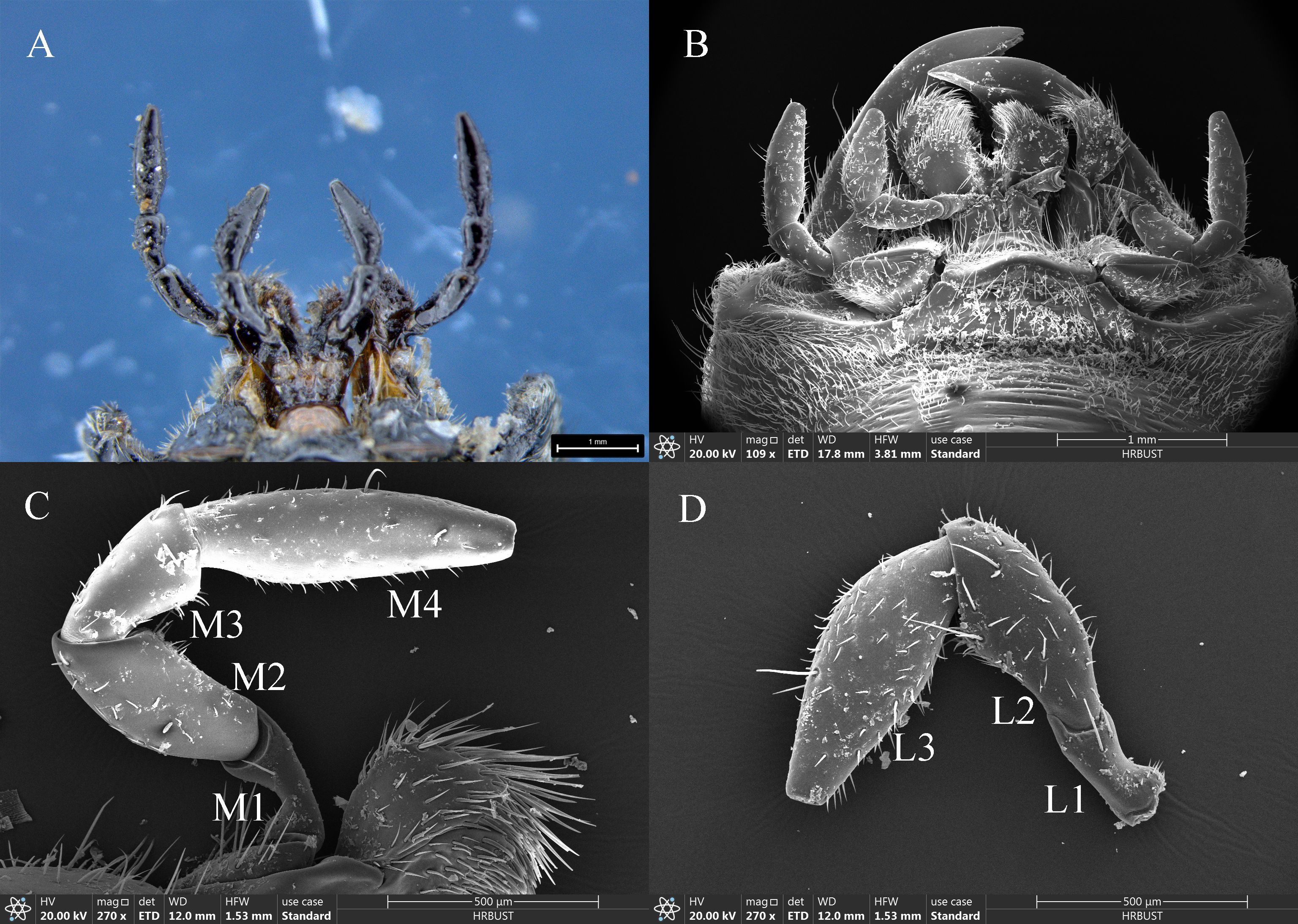
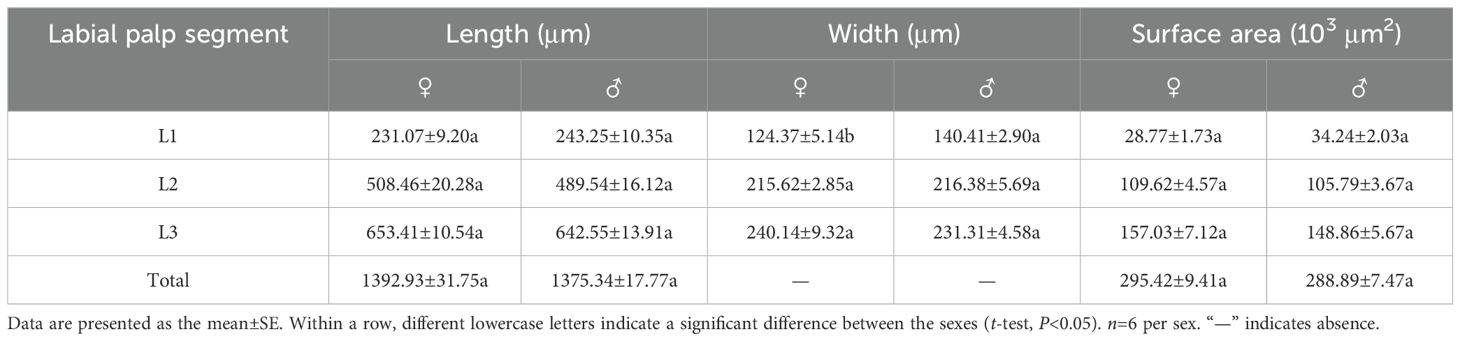
Figure 2. Morphology and structure of the maxillary and labial palps of adult Mo. saltuarius. (A) Morphology of adult maxillary and labial palps observed under an optical microscope; (B) morphology of adult maxillary and labial palps observed by SEM; (C) maxillary palp. Abbreviations: M1–M4, first to fourth segments of the maxillary palp; (D) labial palp. L1–L3, first to third segments of the labial palp.
The labial palps of both males and females comprised 3 segments, L1–L3. The length of female labial palps was 1392.93±31.75 μm; L3 was the longest, accounting for 46.91% of the total length, followed by L2 (36.50%) and L1 (16.59%) (Table 3). The length of male labial palps was 1375.34±17.77 μm; L3 was also the longest, accounting for 46.72% of the total length, followed by L2 (35.59%) and L1 (17.69%) (Table 3). There were no significant differences in the total length or individual segment lengths of labial palps between females and males (Table 3). L3 was the widest segment of female labial palps (240.14±9.32 μm) and was 1.93 times wider than the narrowest segment (L1). L3 was also the widest segment of male labial palps (231.31±4.58 μm) and was 1.65 times wider than L1 (Table 3). L1 was significantly narrower in females than in males (t=−2.717, df=10, P=0.022), but the widths of L2 and L3 did not differ between the sexes (Table 3). There were no significant differences in the total surface area of labial palps or of individual segments between the sexes (Table 3).
3.2 Types and distribution of antennal sensilla
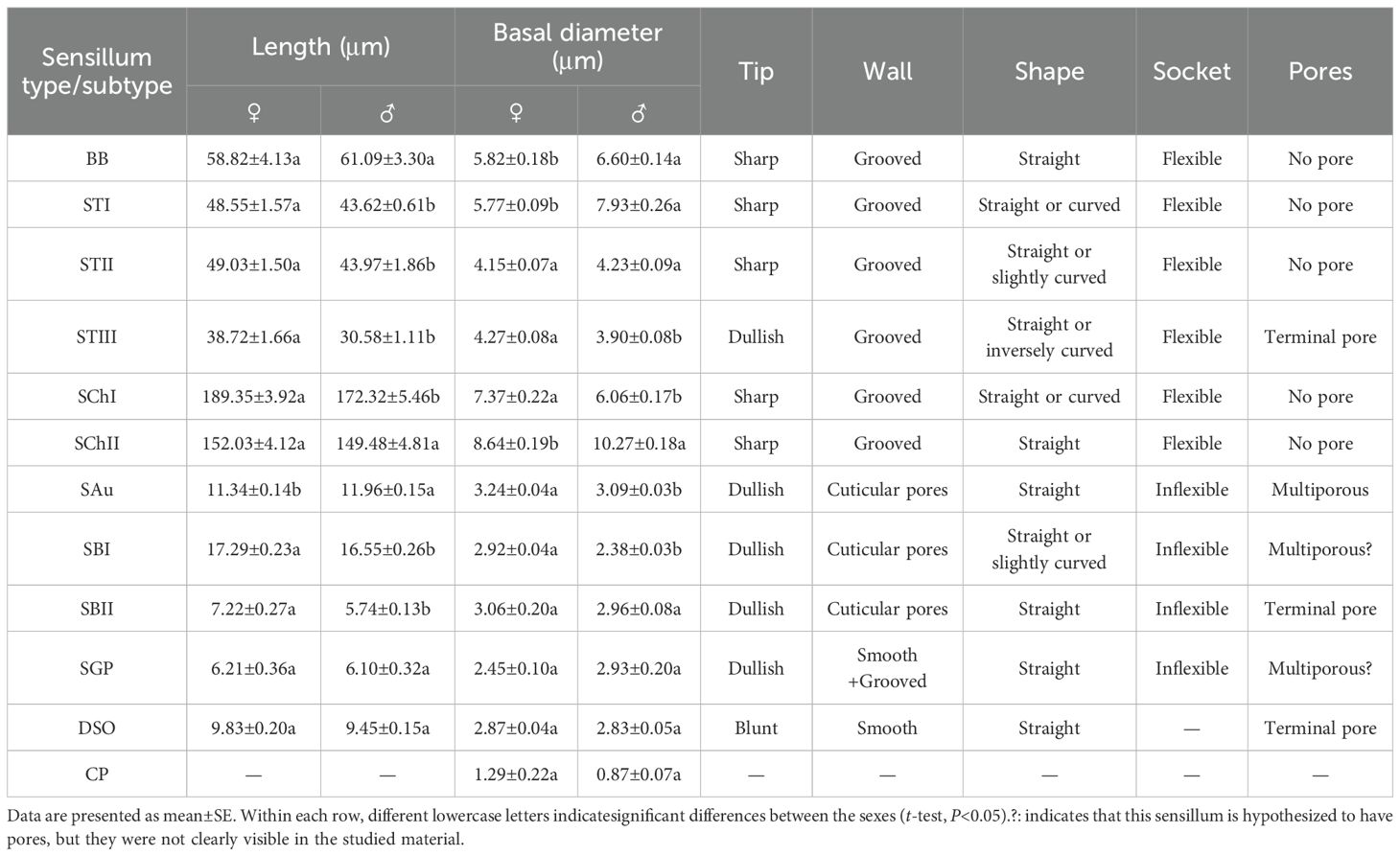
Both sexes had 8 types of sensilla on their antennae: Böhm bristles (BBs), sensilla trichodea (STs, with subtypes STI, STII and STIII), sensilla chaetica (SChs, including SChI and SChII subtypes), sensilla auricillica (SAus), sensilla basiconica (SBs, including SBI and SBII subtypes), sensilla grooved peg (SGPs), dome shaped organs (DSOs), and cuticular pores (CPs). The shapes and distributions of various sensilla are described below.
3.2.1 Böhm bristles
BBs were conical and straight, standing almost perpendicular to the surface of the antennae. They had shallowly grooved hairs and sharp tips; emerged from flexible, shallow cuticular sockets; and lacked visible pores (Figures 3A, B). BBs on the female antennae were 58.82±4.13 μm long (n=100) with a basal diameter of 5.82±0.18 μm (n=100); those on male antennae were 61.09±3.30 μm long (n=100) with a basal diameter of 6.60±0.14 μm (n=100) (Table 4). Clusters of BBs were visible on the radicle and pedicel bases of both sexes (Table 5), and there were no significant differences in BB density between males and females (Table 5).
Figure 3. Ultrastructure of antennal sensilla in adult Mo. saltuarius. (A) BBs on the antennifer; (B) BBs with longitudinal lines on the side wall; (C) STIs and STIIIs on male antennae; (D) STIs and STIIs on male antennae; (E) SChIs and SChIIs; (F) SChIs and STIIIs; (G) SChIIs; (H) STIIIs with longitudinal lines on the side wall; (I) terminal pore of STIII.
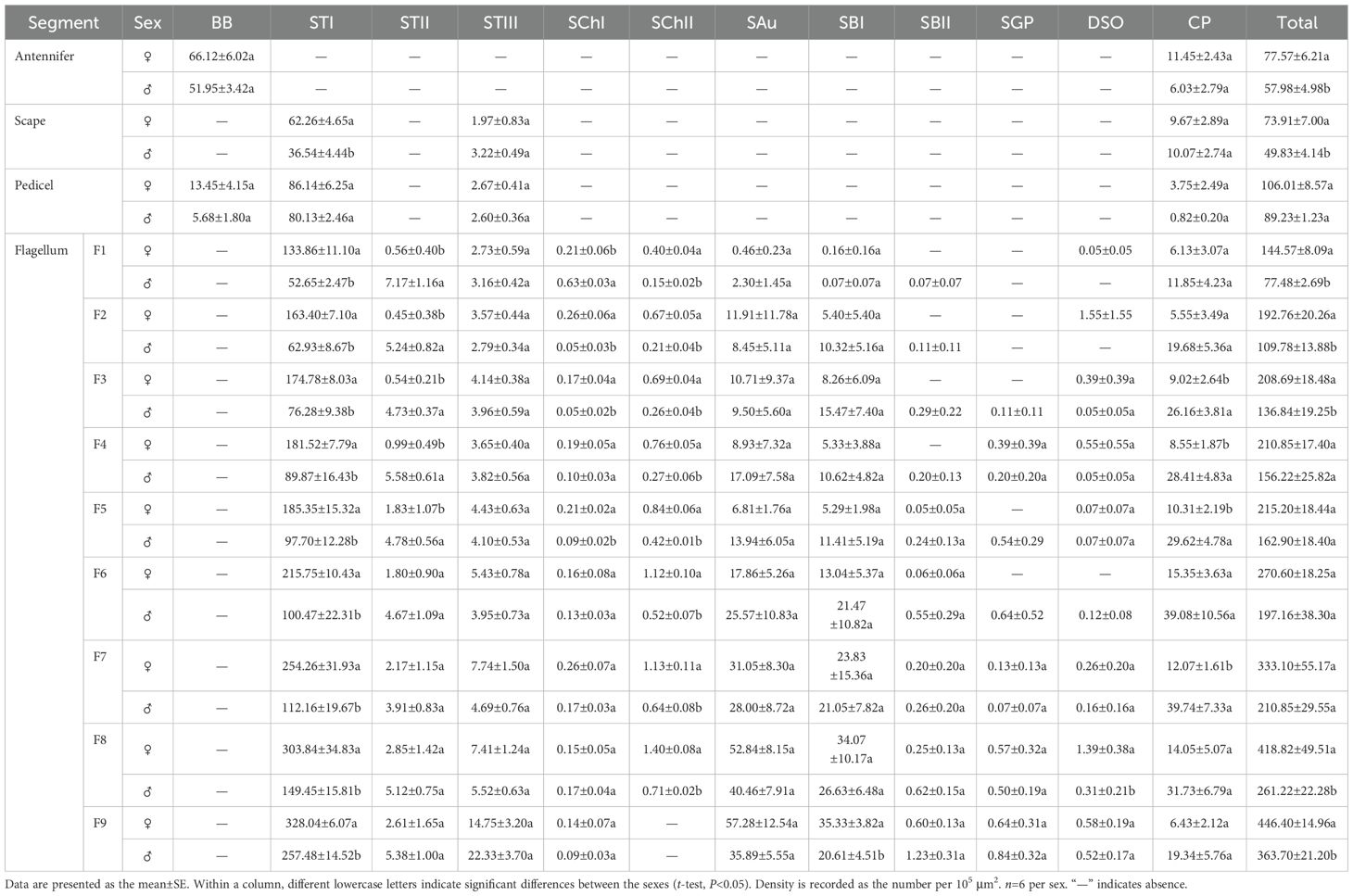
Table 5. Densities and distribution of antennal sensillum types/subtypes in male and female Mo. saltuarius.
3.2.2 Sensilla trichodea
STs were divided into three subtypes, STIs, STIIs and STIIIs. These sensilla lay flat against the surface of the antenna with their tips oriented towards the apex. STIs on female antennae were stout and bristle-like with broad sockets, pointed tips, and grooved walls; they could be either straight or curved, with no wall or apical pores (Figure 3C). STIs on male antennae resembled bamboo shoots, with a thick base that extended uniformly towards the distal end and contracted sharply at the tip, ending in a narrow point (Figure 3D). STIs on female antennae were 48.55±1.57 μm long (n=60) and were significantly longer (t=3.413, df=158, P=0.001) than those on male antennae (43.62±0.61 μm, n=100). The basal diameter of STIs on female antennae was 5.77±0.09 μm (n=60), significantly smaller (t=-6.279, df=158, P=0.000) than that on male antennae (7.93±0.26 μm) (n=100; Table 4). Each STII appeared overall trichoid in shape, with its base attached to the basal socket and standing straight or slightly curved; it exhibited longitudinal or oblique grooves on its surface and terminated in a sharp point at the tip (Figure 3D). STIIs on female antennae were 49.03±1.50 μm long (n=60), significantly longer (t=2.140, df=108, P=0.035) than those on male antennae (43.97±1.86 μm) (n=50; Table 4). The basal diameter of STIIs on female antennae was 4.15±0.07 μm (n=60) and did not differ significantly from that on male antennae (4.23±0.09 μm) (n=50; Table 4). STIIIs were located in slightly raised basal sockets on the antennal surface, oriented perpendicular to the surface and either standing upright or curved inward. Their surfaces exhibited longitudinal grooves, and their slightly blunt apices contained a terminal pore (Figures 3C, H, I). In female antennae, STIIIs measured 38.72±1.66 μm in length (n=60) with a basal diameter of 4.27±0.08 μm (n=60); both values were significantly greater than those of males (t1 = 3.917, df1 = 108, P1 = 0.000; t2 = 3.279, df2 = 108, P2 = 0.001), whole STIIIs measured 30.58±1.11 μm in length (n=50) and 3.90±0.08 μm in basal diameter (n=50; Table 4).
STIs were widely distributed and most densely populated on the antennae of both sexes, where they were present on the scape, pedicel, and all flagellomeres. On female antennae, STI density increased from the base to the tip, with the highest density on the terminal flagellomere. On male antennae, STI density initially increased from the base to the tip, then decreased before increasing again, with a maximum density on F9. STI densities on the scape were 62.26±4.65 per 105 μm2 in females and 36.54±4.44 per 105 μm2 in males, and STI densities on F9 were 328.04±6.07 per 105 μm2 in females and 257.48±14.52 per 105 μm2 in males. STI density on the pedicel did not differ significantly between female and male antennae, but STI densities on the scape and all flagellomeres were significantly greater in females than in males (Table 5). STIIs were found across all flagellomeres in both female and male antennae, and their densities on F1–F5 were significantly lower in females than in males; however, STIIs on flagellomeres F6–F9 did not differ significantly between the sexes (Table 5). STIIIs were present across all antennal segments—scape, pedicel, and flagellum—in both sexes; their densities increased from the base toward the tip and showed no significant differences between corresponding segments of males and females. In female antennae, STIIIs were less abundant on the scape (1.97±0.83 per 105 μm2) and most abundant on F9 (14.75±3.20 per 105 μm2). In males, the lowest density occurred on the pedicel (2.60±0.36 per 105 μm2) and the highest on F9 (22.33±3.70 per 105 μm2; Table 5).
3.2.3 Sensilla chaetica
SChs were classified into two subtypes, SChIs and SChIIs. SChIs were slender and typically curved inward. They arose form sockets and projected almost perpendicularly from the antennal surface, with deeply grooved longitudinal walls and sharply pointed tips (Figures 3E, F). SChIs on female antennae were 189.35±3.92 μm long (n=60) with a basal diameter of 7.37±0.22 μm (n=60); they were significantly longer than SChIs on male antennae (172.32±5.46 μm) (n=50) and had greater basal diameters than those of males (6.06±0.17 μm) (n=50) (t1 = 4.600, df1 = 108, P1 = 0.000; t2 = 2.589, df2 = 108, P2 = 0.011; Table 4). SChIIs had a straight shape and were thicker than SChIs. Each was parallel to the antennal surface, with its base located in a deeper socket; it had deep longitudinal grooves on its surface, along with a sharp tip (Figures 3E, G). SChIIs on female antennae were 152.03±4.12 μm long (n=60) and were not significantly longer (t=0.406, df=108, P=0.686) than those on male antennae (149.48±4.81 μm). The basal diameter of SChIIs on female antennae was 8.64±0.19 μm (n=60) and was significantly smaller (t=−6.093, df=108, P=0.000) than that on male antennae (10.27±0.18 μm, n=50; Table 4).
SChIs were found exclusively on the flagellum of both female and male antennae and were present on flagellomeres F1–F9, albeit in relatively low numbers. Notably, the density of SChIs on F1 was significantly lower in females than in males. Conversely, the densities of SChIs on F2, F3, and F5 were significantly higher in females than in males. There were no significant differences in SChI density between the sexes on other flagellomeres (Table 5). SChIIs were found on F1–F8 of both female and male flagella. SChII density increased progressively from the base to the tip in antennae of both sexes. SChII density on each flagellomere was significantly higher in females than in males. The lowest SChII density recorded in females was 0.40±0.04 per 105 μm2 on F1, and the highest was 1.40±0.08 per 105 μm2 on F8; by contrast, the lowest SChII density in males was 0.15±0.02 per 105 μm2 (F1), and the highest was 0.71±0.02 per 105 μm2 (F8) (Table 5).
3.2.4 Sensilla auricillica
SAus resembled a rabbit’s ear in shape, with a multiporous surface and relatively dull tips (Figures 4A, B). They were cylindrical at the base, with inflexible, shallow sockets, and were almost perpendicular to the antennal surface. SAus on female antennae were 11.34±0.14 μm long (n=60), significantly smaller (t=−3.027, df=108, P=0.003) than those on male antennae (11.96±0.15 μm) (n=50). The basal diameter of SAus on female antennae was 3.24±0.04 μm (n=60), significantly larger (t=3.096, df =108, P=0.002) than that on male antennae (3.09±0.03 μm) (n=50). SAu were found across all flagellomeres in both males and females, and their densities on individual flagellomeres did not differ between the sexes. SAus are mainly found in areas of dense sensilla distribution on each flagellomere (Figures 4C, D). On female antennae, SAu density initially increased from the base toward the tip, then decreased, and finally increased again. SAu density was lowest on F1 (0.46±0.23 per 105 μm2) and highest on F9 (57.28±12.54 per 105 μm2). On male antennae, SAu density initially increased from the base toward the tip, then decreased, increased, and decreased again. SAu density was lowest on F1 (2.30±1.45 per 105 μm2) and highest on F8 (40.46±7.91 per 105 μm2) (Table 5).
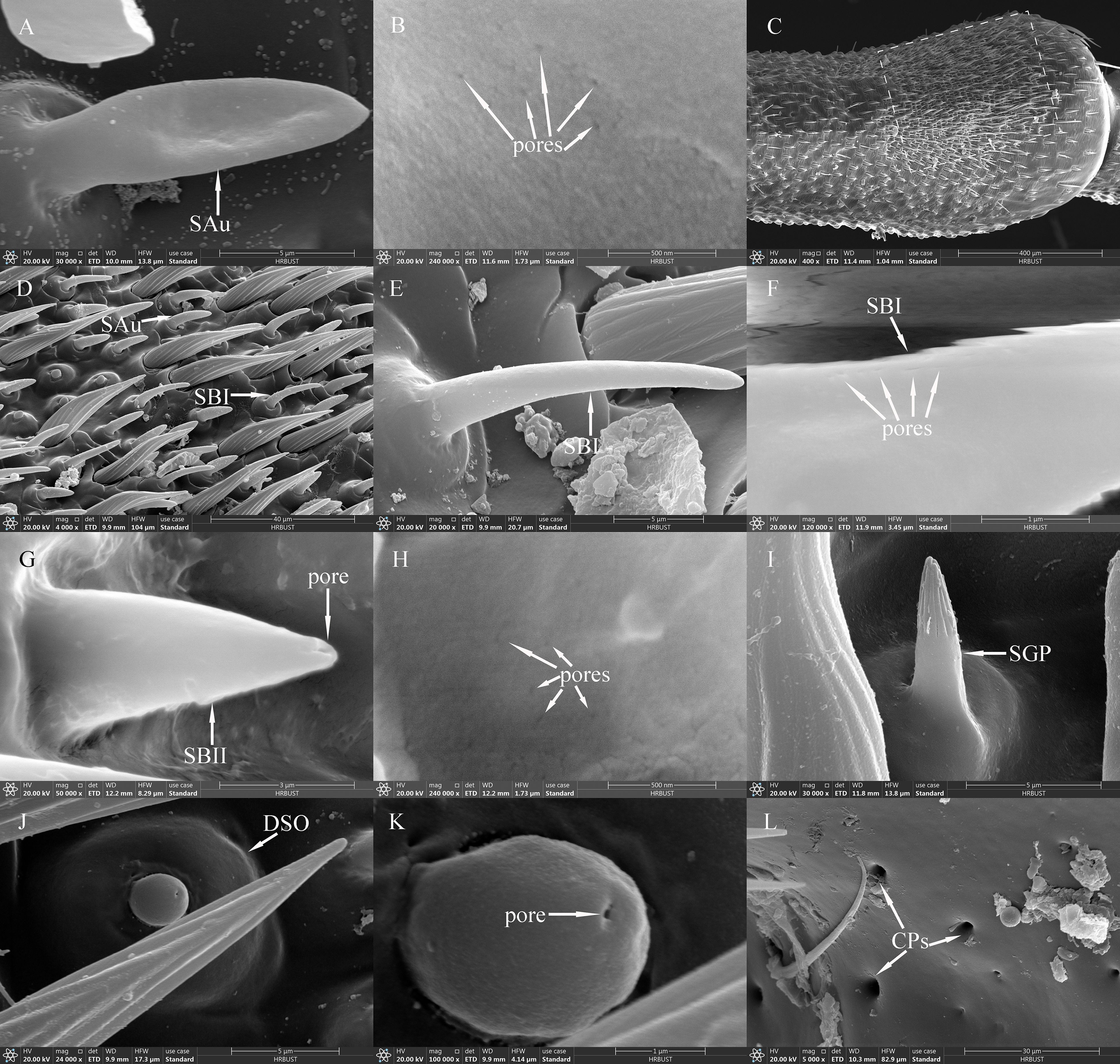
Figure 4. SAus, SBIs, SBIIs, SGPs, DSOs and CPs of adult Mo. saltuarius. (A) SAu; (B) cuticular pores of SAu; (C) the distal end of male antenna F6, with the dashed-line area indicating dense sensilla distribution; (D) area of dense sensilla, with highlighted SAu and SBI; (E) SBI; (F) cuticular pores of SBI; (G) SBII and its terminal pores; (H) cuticular pores of SBII; (I) SGP; (J) DSO; (K) terminal pore of DSO; (L) CPs.
3.2.5 Sensilla basiconica
SBs on Mo. saltuarius antennae were divided into two subtypes, SBIs and SBIIs. SBIs were slender and conical; they lacked a basal socket and curved towards a relatively dull antennal tip; the presence of pores was hypothesized, but they were not clearly visible in the studied material (Figures 4E, F). SBIs on female antennae were 17.29±0.23 μm long (n=60) with a basal diameter of 2.92±0.04 μm (n=60); these values were both significantly larger than those of SBIs on male antennae (16.55±0.26 μm in length and 2.38±0.03 μm in basal diameter, n=50) (Table 4). SBIIs were short and conical with a relatively dull tip that featured a terminal pore; the presence of pores was hypothesized, but they were not clearly visible in the studied material; they stood upright and perpendicular to the antennal surface (Figures 4G, H). SBIIs on female antennae were 6.87±0.39 μm long (n=15), significantly longer than those on male antennae (5.62±0.14 μm) (n=38). However, there was no significant difference in SBII basal diameter between female antennae (3.06±0.15 µm, n=15) and male antennae (2.97±0.09 µm, n=38). SBIs were found on all flagellomeres, and their densities on flagellomeres F1–F8 did not differ among the sexes. However, the SBI density on F9 was significantly greater in females than in males. On female antennae, SBI density initially increased from the base toward the tip, then decreased, and finally increased again; it was lowest on F1 (0.16±0.16 per 105 μm2) and highest on F9 (35.33±3.82 per 105 μm2). On male antennae, SBI density initially increased from the base toward the tip, then decreased, increased, and decreased again; it was lowest on F1 (0.07±0.07 per 105 μm2) and highest on F8 (26.63±6.48 per 105 μm2, Table 5). SBII were found on F5–F9 of female antennae and F1–F9 of male antennae. Their density on F5–F9 did not differ between the sexes. SBII density increased from F5 (0.05±0.05 per 105 μm2) to F9 (0.60±0.13 per 105 μm2) on female antennae and from F1 (0.07±0.07 per 105 μm2) to F9 (1.23±0.31 per 105 μm2; Table 5) on male antennae.
3.2.6 Sensilla grooved peg
SGPs were distinctive double-walled sensilla, smooth basally and grooved distally, that stood upright and perpendicular to the antennal surface; they emerged from a raised cuticular area and had a non-articulating base (Figure 4I). SGPs on female antennae were 6.21±0.36 μm long (n=11) with a basal diameter of 2.45±0.10 μm (n=11), and those on male antennae were 6.10±0.32 μm long (n=12) with a basal diameter of 2.93±0.20 μm (n=12, Table 4); neither their length nor their diameter differed between the sexes (t1 = 0.234, df1 = 21, P=0.817; t2=−2.118, df2 = 21, P=0.052). SGPs were found on F4 and F7–F9 of female antennae, and their density first decreased and then increased across these four flagellomeres. SGP density was lowest on F7 (0.13±0.13 per 105 μm2) and highest on F9 (0.64±0.31 per 105 μm2). SGPs were found on F3–F9 of male antennae. Their density initially increased towards the tip, then decreased, and finally increased again; it was lowest on F7 (0.07±0.07 per 105 μm2), and highest on F9 (0.84±0.32 per 105 μm2; Table 5).
3.2.7 Dome shaped organs
DSOs were small, round sensilla that emerged from elevated cuticular domes on the antennal surface. Each DSO had a central terminal pore, sometimes surrounded by a raised cuticular collar (Figures 4J, K). On female antennae, the diameter of the DSO base was 9.83±0.20 μm (n=28), and the diameter of the semi-spherical structure was 2.87±0.04 μm (n=28). These measurements did not differ significantly from those of males, which were 9.45±0.15 μm (n=22) and 2.83±0.05 μm (n=22), respectively (t1 = 1.443, df1 = 48, P1 = 0.155; t2 = 0.640, df2 = 48, P2 = 0.525; Table 4). DSOs were found on the antennal flagellum in both males and females. In females, DSOs were present on F1–F5 and F7–F9 but not on F6. Their density increased, decreased, and increased again from the base to the tip and was highest on F8 (1.55±1.55 per 105 μm2). In males, DSOs were found on F3–F9; their density was similar on F3 and F4 (0.05±0.05 per 105 μm2), then gradually increased from F5 to F9 (0.52±0.17 per 105 μm2). DSO density on F8 was higher in females than in males, but no other flagellomeres showed differences in DSO density between the sexes (Table 5).
3.2.8 Cuticular pores
CPs were visible as small openings in the epidermal surface (Figure 4L); their diameters were 1.29±0.22 μm in females (n=20) and 0.87±0.07 μm in males (n=19), and these values did not differ significantly (t=1.756, df=37, P=0.087; Table 4).CPs were present on the antennifer, scape, pedicel, and all flagellomeres in both females and males. Their densities on the antennifer, scape, pedicel, F1, F2, F6, F8, and F9 did not differ significantly between the sexes, whereas densities on F3, F4, F5, and F7 were significantly lower in females (Table 5).
3.2.9 Distribution of sensilla on the antennae
The total densities of antennal sex in both males and females increased progressively from the base to the tip, with the highest density observed on the most distal flagellomere (Table 5). Both female and male Mo. saltuarius had two types of sensillum on their antennifers: BBs and CPs, which accounted for 85.24% and 14.76% of the sensilla on female antennifers and 89.60% and 10.40% of the sensilla on male antennifers, respectively. Both females and males had three types/subtypes of sensillum on their scapes: STIs, CPs, and STIIIs, which accounted for 84.24%, 13.09%, and 2.67% of the sensilla on female scapes and 73.32%, 20.21%, and 6.47% of the sensilla on male scapes. Both females and males had four types/subtypes of sensillum on their pedicels: STIs, BBs, CPs, and STIIIs, which accounted for 81.26%, 12.68%, 3.54%, and 2.51% of the sensilla on female pedicels and 89.80%, 6.36%, 0.92%, and 2.91% of the sensilla on male pedicels.
In female antennae, there were nine sensillum types/subtypes on F1, F2, and F3: CPs, STIIIs, STIs, STIIs, ChIs, ChIIs, SAus, SBIs, and DSOs. The five most common types/subtypes on F1 were STIs (92.59%), CPs (4.24%), STIIIs (1.89%), STIIs (0.39%), and SAus (0.32%); on F2, they were STIs (84.77%), SAus (6.18%), CPs (2.88%), SBIs (2.80%), and STIIIs (1.85%); and on F3, they were STIs (83.75%), SAus (5.13%), CPs (4.32%), SBIs (3.96%), and STIIIs (1.98%). F4 had one more type of sensillum than F3, namely SGPs; its five most common types/subtypes were STIs (86.09%), SAus (4.23%), CPs (4.05%), SBIs (2.53%), and STIIIs (1.73%). F5 had one more subtype of sensillum than F4, namely SBII, but it lacked SGP; its five most common types/subtypes were STIs (86.13%), CPs (4.79%), SAus (3.16%), SBIs (2.46%), and STIIIs (2.06%). Unlike F5, F6 lacked DSOs; its five most common types/subtypes were STIs (79.73%), SAus (6.60%), CPs (5.67%), SBIs (4.82%), and STIIIs (2.01%). F7 and F8 had two more types of sensillum than F6, namely DSOs and SGPs. The five most common types/subtypes on F7 were STIs (76.33%), SAus (9.32%), SBIs (7.16%), CPs (3.62%), and STIIIs (2.32%); on F8, they were STIs (72.55%), SAus (12.62%), SBIs (8.13%), CPs (3.35%), and STIIIs (1.77%). Unlike F8, F9 lacked SChII; its five most common types/subtypes were STIs (73.48%), SAus (12.83%), SBIs (7.91%), STIIIs (3.30%), and CPs (1.44%).
In male antennae, there were nine types/subtypes of sensillum on F1 and F2: CPs, STIIIs, STIs, STIIs, ChIs, ChIIs, SAus, SBIs, and SBIIs. The five most common types/subtypes on F1 were STIs (67.95%), CPs (15.29%), STIIs (9.26%), STIIIs (4.08%), and SAus (2.97%); on F2, they were STIs (57.32%), CPs (17.93%), SBIs (9.40%), SAus (7.69%), and STIIs (4.78%). There were two additional types of sensillum on F3–F8: DSO and SGP. The five most common types/subtypes on F3 were STIs (55.74%), CPs (19.12%), SBIs (11.30%), SAus (6.94%), and STIIs (3.45%); on F4, they were STIs (57.53%), CPs (18.18%), SAus (10.94%), SBIs (6.80%), and STIIs (3.57%); on F5 they were STIs (59.98%), CPs (18.19%), SAus (8.55%), SBIs (7.00%), and STIIs (2.93%); on F6 they were STIs (50.96%), CPs (19.82%), SAus (12.97%), SBIs (10.89%), and STIIs (2.37%); on F7, they were STIs (53.19%), CPs (18.85%), SAus (13.28%), SBIs (9.98%), and STIIIs (2.23%); and on F8, they were STIs (57.21%), SAus (15.49%), CPs (12.15%), SBIs (10.19%), and STIIIs (2.11%). F9 had one fewer subtype of sensillum than F8, and its five most common types/subtypes were STIs (70.79%), SAus (9.87%), STIIIs (6.14%), SBIs (5.67%), and CPs (5.32%).
The numbers of several antennal sensilla types/subtypes showed significant sexual dimorphism in adult Mo. saltuarius (Table 6). Four types/subtypes—BB, STI, SChII, and DSO—were significantly more numerous in females, whereas eight others—STII, STIII, SChI, SAu, SBI, SBII, SGP, and CP—were significantly less numerous in females than in males (Table 6).
3.3 Types and distribution of sensilla on the labial and maxillary palps
Both male and female Mo. saltuarius had 7 types of sensilla on their labial and maxillary palps: BBs, STs (including STII and STIII subtypes), SChs, SPs, SCos, CPs, and STBs (including the STBI, STBII, STBIII, and STBIV subtypes). The morphologies, numbers, and distribution of these sensilla are described below.
3.3.1 BBs
BBs were present on the maxillary and labial palps of adult Mo. saltuarius and were morphologically similar to those on the adult antennae (Figure 5A). These conical, straight sensilla had sharp tips and smooth-walled hairs; they emerged from shallow flexible cuticular sockets and were nearly perpendicular to the palp surface. BBs on female maxillary palps were 18.47±1.18 μm long (n=13) with a basal diameter of 3.45±0.16 μm (n=13); these values did not differ significantly from those of BBs on male maxillary palps (t1 = 1.736, df1 = 21, P1 = 0.097; t2 = 0.898, df2 = 21, P2 = 0.379; Table 7), which were 15.74±0.92 μm long (n=10) with a basal diameter of 3.25±0.18 μm (n=10). BBs on female labial palps were 19.42±1.22 μm long (n=20) with a basal diameter of 3.83±0.13 μm (n=20); again, these values did not differ significantly from those of BBs on male labial palps (t1=−1.463, df1 = 40, P1 = 0.151; t2=−0.525, df2 = 40, P2 = 0.603), which were 22.18±1.41 μm long (n=22) with a basal diameter of 3.91±0.11 μm (n=22). BBs were found only on M1 and L1 in both males and females, and the number of BBs did not differ between the sexes (Table 8).
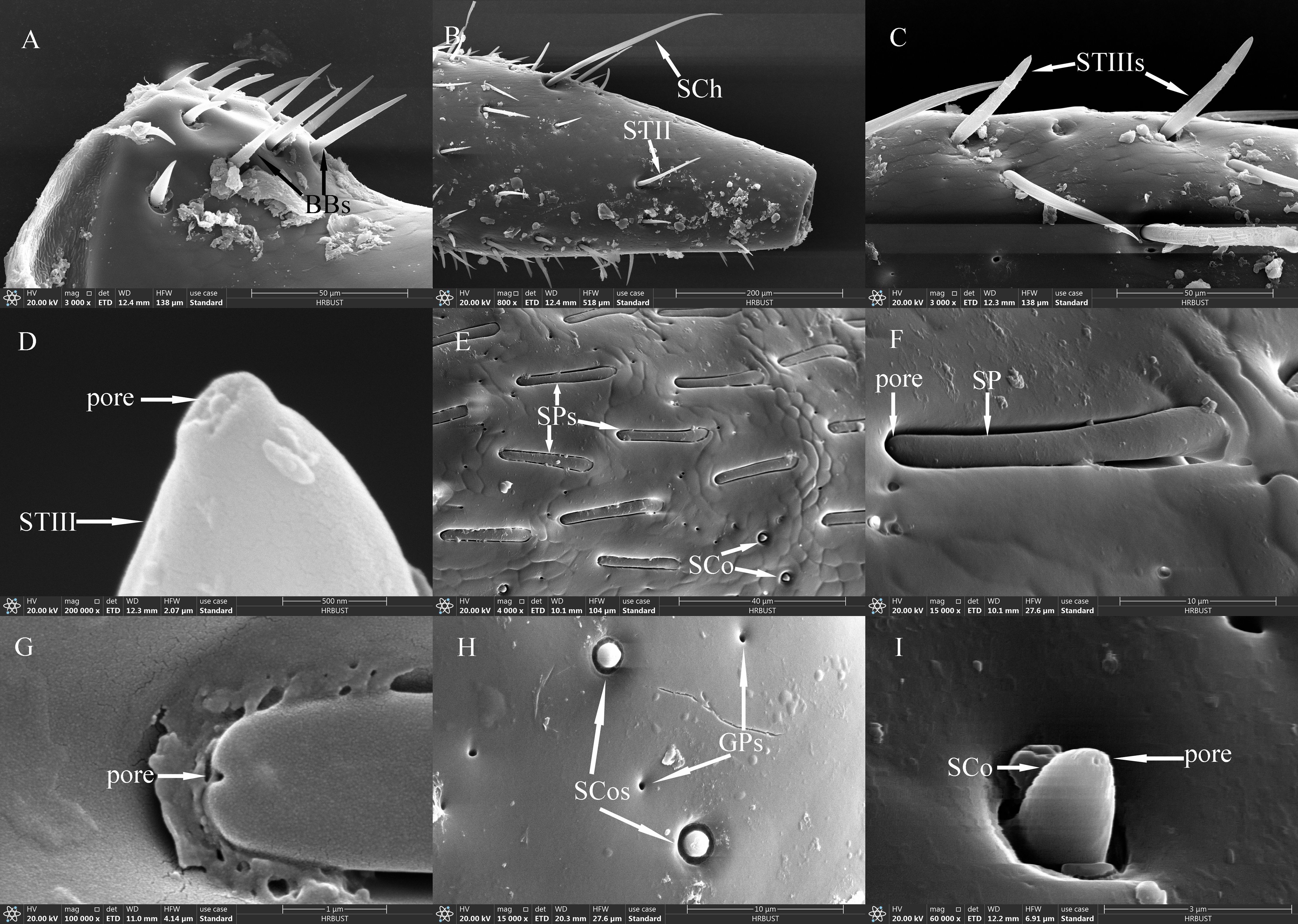
Figure 5. Ultrastructure of sensilla on maxillary and labial palps of adult Mo. saltuarius. (A) BBs on a maxillary palp; (B) STII and SChs; (C) STIIIs; (D) terminal pore of an STIII; (E) morphological characteristics of SPs; (F, G) terminal pores of SPs; (H) SCos and CPs; (I) terminal pore of an SCo.
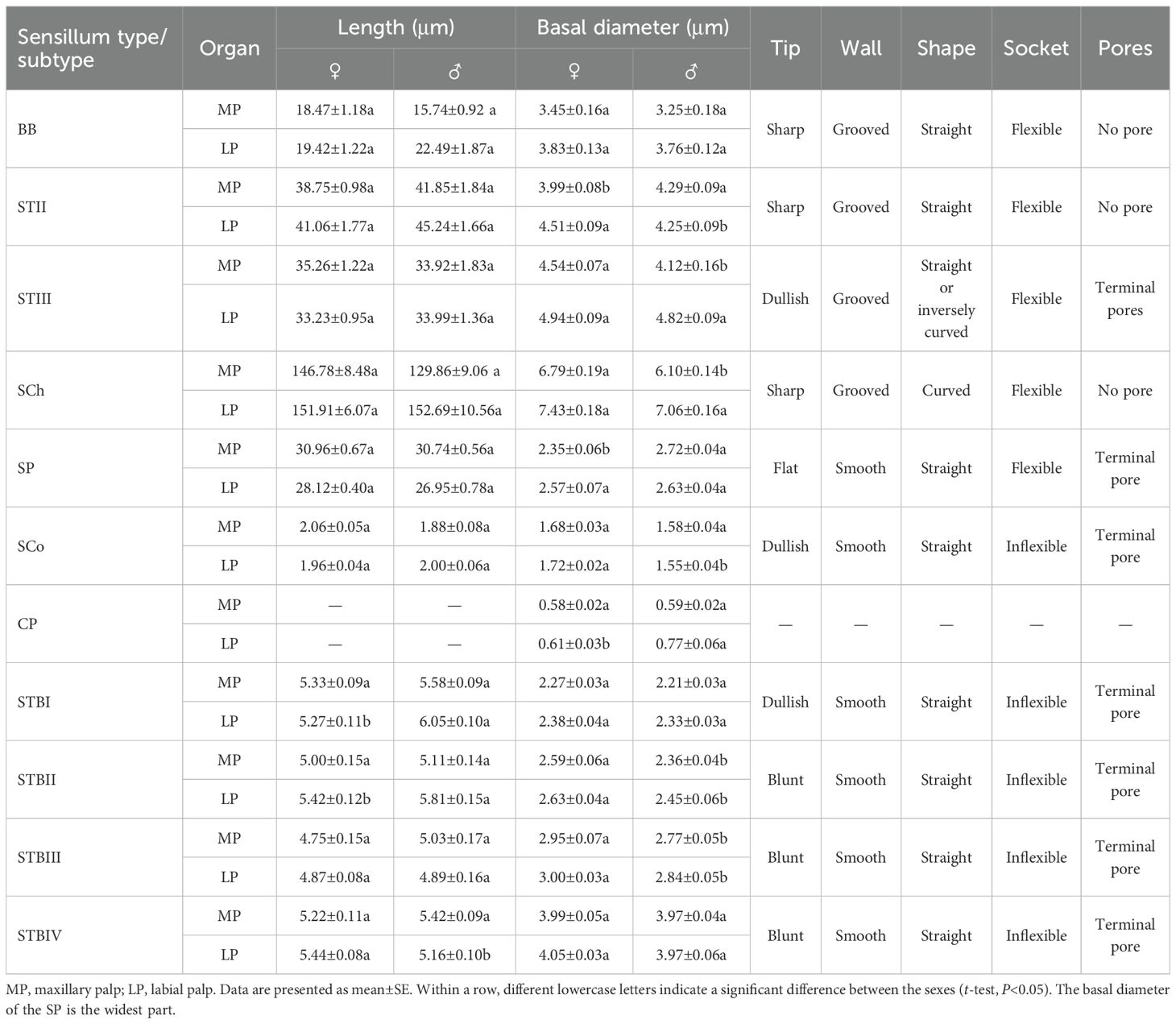
Table 7. Size and morphological characteristics of sensilla on the maxillary and labial palps of adult Mo. saltuarius..
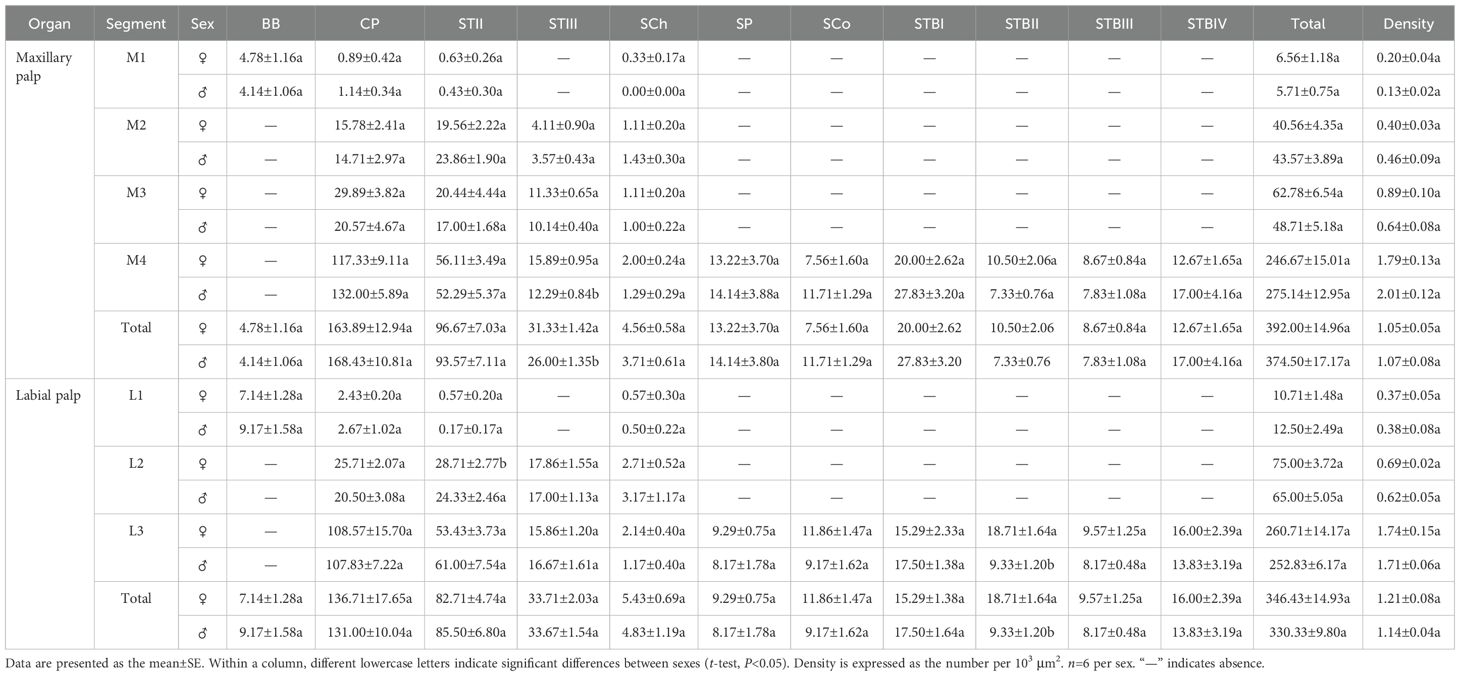
Table 8. Abundance and distribution of different sensillum types/subtypes on the maxillary and labial palps of male and female Mo. saltuarius.
3.3.2 STs
STIIs on the maxillary and labial palps were hair-like sensilla, with their bases located in flexible sockets; they were straight overall, with longitudinal grooves on the surface and sharp tips (Figure 5B). STIIs on female maxillary palps were 38.75±0.98 μm long (n=51) and were not significantly longer (t=−1.574, df=89, P=0.119) than those on male maxillary palps (41.85±1.84 μm, n=40). The basal diameter of STIIs on female maxillary palps was 3.99±0.08 μm (n=51), significantly smaller (t=−2.366, df=89, P=0.020) than that on male maxillary palps (4.29±0.09 μm, n=40). STIIs on female labial palps were 41.06±1.77 μm long (n=50) and were not significantly longer (t=−1.727, df=98, P=0.087) than those on male labial palps (45.24±1.66 μm, n=50). The basal diameter of STIIs on female labial palps was 4.51±0.09 μm (n=50), significantly larger (t=2.106, df=98, P=0.038) than that on male labial palps (4.25±0.09 μm, n=50). STIIIs were located within flexible sockets on the surface of the maxillary and labial palps. They were straight or inversely curved, with longitudinal grooves, and stood at a 60°–90° angle to the palp surface. One to several terminal pores were visible beneath their hat-like tip structures (Figures 5C, D). On female maxillary palps, STIIIs measured 35.26±1.22 μm in length (n=50), which was not significantly greater than in males (33.92±1.83 μm, n=21; t=0.603, df=69, P=0.548; Table 7). However, their basal diameter was significantly larger in females (4.54±0.07 μm, n=50) than in males (4.12±0.16 μm, n=21; t=2.847, df=69, P=0.006; Table 7). On the labial palps, STIIIs in females measured 33.23±0.95 μm in length and 4.94±0.09 μm in basal diameter (n=50), values that did not differ significantly from those in males (33.99±1.36 µm length, 4.82±0.09 μm diameter, n=32; t1=−0.471, df1 = 80, P1 = 0.639; t2 = 0.927, df2 = 80, P2 = 0.357; Table 7).
STIIs were widely distributed and abundant on the maxillary palps of both males and females, increasing in number from the base to the tip. The number of STIIs did not differ significantly between males and females. STIIs were also present on all segments of the labial palps in males and females, increasing in number from the base to the tip. The number of STIIs on L1 and L3, as well as the total number of STIIs on the labial palps, did not differ significantly between the sexes. However, there were significantly fewer STIIs on L2 in females than in males (Table 8). STIIIs were present on M2–M4 of both males and females, with their numbers increasing from the base to the tip. The total number of STIIIs, as well as the number on M4, was significantly greater in females than in males, whereas no sex-based differences were observed on M2 or M3. STIIIs were also detected on L2 and L3 in both sexes, with no significant differences in their numbers (Table 8).
3.3.3 SChs
SChs were slender and elongated, with most curved, and a few standing upright. Each sensillum was set in a flexible socket, had longitudinal grooves along the surface, and ended in a sharp tip (Figure 5B). SChs on female maxillary palps were 146.78±8.48 μm long (n=21) and were not significantly longer (t=1.364, df=38, P=0.181) than those on male maxillary palps (129.86±9.06 μm, n=19). The basal diameter of SChs on female maxillary palps was 6.79±0.19 μm (n=21), significantly larger (t=2.910, df=38, P=0.006) than that on male maxillary palps (6.10±0.14 μm, n=19). SChs on female labial palps were 151.91±6.07 μm long (n=31) with a basal diameter of 7.43±0.18 μm (n=31); these values did not differ significantly (t1=−0.065, df1 = 59, P1 = 0.949; t2 = 1.554, df2 = 59, P2 = 0.126) from those of male labial palps, which were 152.69±10.56 μm long (n=30) with a basal diameter of 7.06±0.16 μm (n=30; Table 7).
On the maxillary palps of females, the number of SChs increased from the base to the tip; it was lowest on M1 (0.33±0.17) and highest on M4 (2.00±0.24). By contrast, on the maxillary palps of males, SCh number initially increased, then decreased from the base to the tip; no SChs were observed on M1, and the largest number were observed on M2 (1.43±0.30). SCh number did not differ between males and females for any segment of the maxillary palps. On both male and female labial palps, SCh number increased and then decreased from the base to the tip; it was highest on L2 for both females (2.71±0.52) and males (3.17±1.17). SCh numbers did not differ significantly between the sexes, either for individual segments or for the labial palps as a whole (Table 8).
3.3.4 Sensilla placodea
SPs were flat with a blunt tip, a smooth surface, and a wide socket. Their upper surface was flush with the surface of the maxillary or labial palp, and they were separated from the organ’s surface on all sides (Figures 5E, F). SPs exhibited different widths at either end, with a pore present at the tip of the narrower end (Figure 5G). SPs on female maxillary palps were 30.96±0.67 μm long (n=18) and were not significantly longer (t=0.248, df=48, P=0.805) than those on males (30.74±0.56 μm). However, SPs on maxillary palps were significantly narrower in females (2.35±0.06 μm, n=18) (t=−5.087, df=48, P=0.000) than in males (2.72±0.04 μm, n=32). By contrast, there were no significant differences (t1 = 1.463, df1 = 62, P1 = 0.149; t2=−0.842, df2 = 45, P2 = 0.404) in the length or width of SPs on the labial palps of females versus males: those of females were 28.12±0.40 μm long (n=39) and 2.57±0.07 μm wide (n=22), and those of males were 26.95±0.78 μm long (n=25) and 2.63±0.04 μm wide (n=25).
SPs were found exclusively on M4 in both males and females, and SP number did not differ significantly between the sexes. Likewise, SPs were found exclusively on L3 in both males and females, and their numbers did not differ between the sexes (Table 8).
3.3.5 Sensilla coeloconica
SCos were conical in shape, situated within a distinct concavity on the basal socket, with a smooth surface and a terminal pore (Figures 5H, I). SCos on female maxillary palps were 2.06±0.05 μm long (n=33) with a basal diameter of 1.68±0.03 μm (n=33); they did not differ significantly (t1 = 1.961, df1 = 46, P=0.056; t2 = 1.616, df2 = 46, P=0.113) from SCos on male maxillary palps, which were 1.88±0.08 μm long (n=15) with a basal diameter of 1.58±0.04 μm (n=15). SCos on female labial palps were 1.96±0.04 μm long (n=36) and did not differ significantly in length (t=−0.606, df=53, P=0.547) from those on male labial palps (2.00±0.06 μm, n=19). The basal diameter of SCos on female labial palps was 1.72±0.02 μm (n=36), significantly larger than that on male labial palps (1.55±0.04 μm, n=19; Table 7). SCos were present on M4 and L3 in both males and females, and their numbers did not differ between the sexes (Table 8).
3.3.6 CPs
CPs appeared as small pores on the surface of the maxillary and labial palps (Figure 5H). CP diameter on female maxillary palps was 0.58±0.02 μm (n=50) and did not differ significantly from that on male maxillary palps (0.59±0.02 μm, n=52) (t=0.422, df=100, P=0.674). CP diameter on female labial palps was 0.61±0.03 μm (n=56), significantly smaller (t=−2.671, df=104, P=0.009) than that on male labial palps (0.77±0.06 μm, n=50; Table 7). CPs were found across all segments of the maxillary palps in both males and females, gradually increasing in number from the base to the tip. CP number did not differ significantly between males and females on any maxillary palp segments. CPs were also found across all segments of the labial palps in both males and females, increasing gradually in number from the base to the tip. Neither the number of CPs in individual segments nor the total number of CPs differed significantly between males and females (Table 8).
3.3.7 Sensilla twig basiconica
STBs on the maxillary palps were densely distributed at the tip of M4 (Figure 6A), whereas those on the labial palps were densely distributed at the tip of L3 (Figure 6B). STBs on the maxillary and labial palps consisted of four subtypes: STBIs, STBIIs, STBIIIs, and STBIVs. STBIs exhibited a conical shape; they were straight, with an inflexible cuticular socket, a smooth wall, and a smaller finger-like projecting tip with a pore (Figures 6C–E). STBIs on female maxillary palps were 5.33±0.09 μm long with a basal diameter of 2.27±0.03 μm; they did not differ significantly from those on male maxillary palps, which were 5.58±0.09 μm long with a basal diameter of 2.21±0.03 μm (Table 7). STBIs were significantly shorter on female labial palps (5.27±0.11 μm) than on male labial palps (6.05±0.10 μm), but they did not differ in basal diameter between females (2.38±0.04 μm) and males (2.33±0.03 μm; Table 7). The number of STBIs on female maxillary palps was 20.00±2.62 and did not differ significantly from that on male maxillary palps (27.83±3.20; Table 8). Likewise, the number of STBIs on female labial palps (15.29±1.38) did not differ significantly from that on male labial palps (17.50±1.64; Table 8).
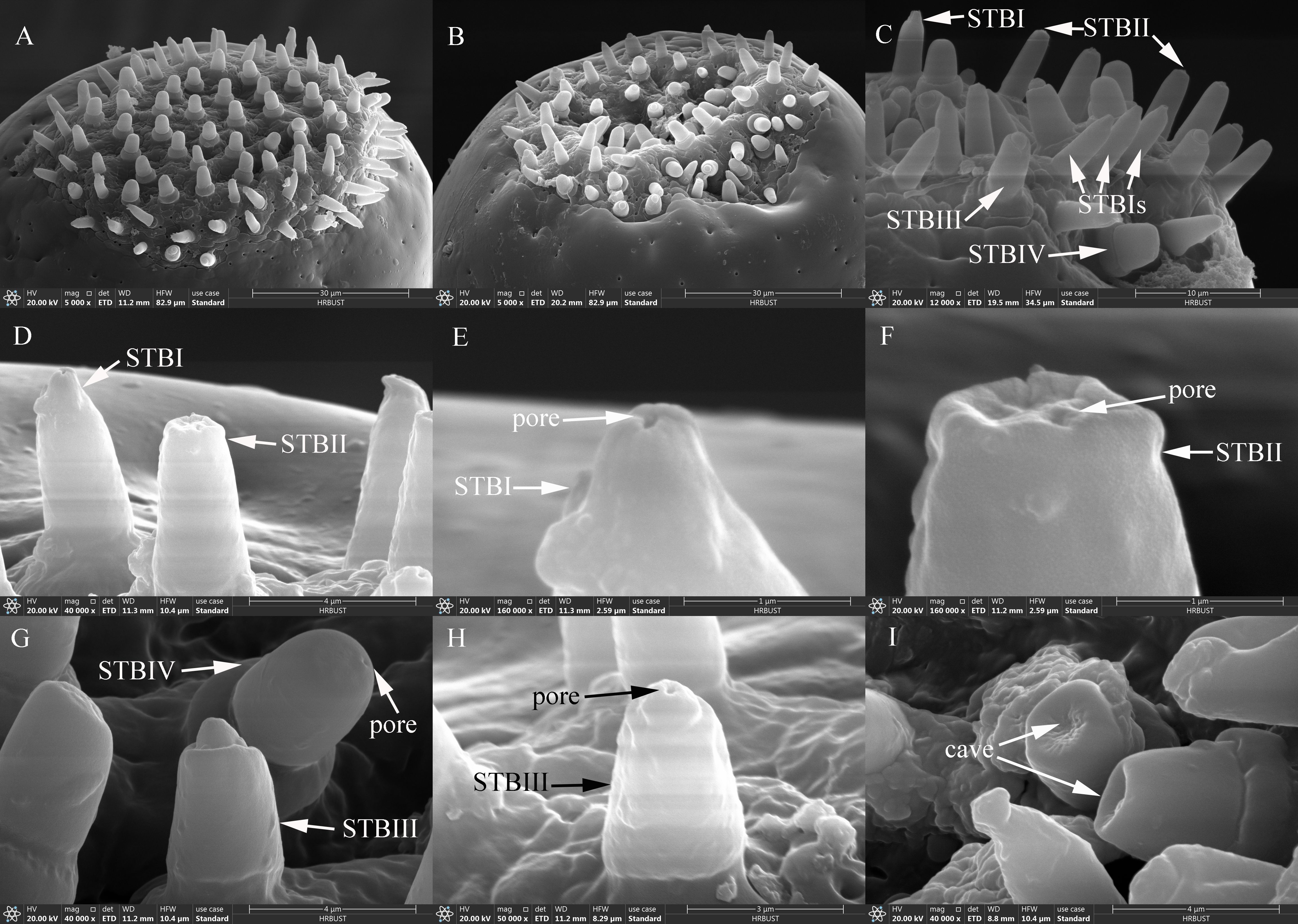
Figure 6. Ultrastructure of STBs on maxillary and labial palps of adult Mo. saltuarius. (A) Sensilla on the maxillary palp tip; (B) sensilla on the tip of the labial palp; (C) ultrastructure of four STB subtypes; (D) STBI and STBII; (E) terminal pore of an STBI; (F) terminal pores of an STBII; (G) STBIII and STBIV; (H) ultrastructure of an STBIII; (I) terminal cave of an STBIV.
STBIIs exhibited a conical shape with an inflexible cuticular socket and a smooth wall; they had a blunt, rounded tip with a central radial groove and one to several terminal pores (Figures 6C–D, F). STBIIs on female maxillary palps were 5.00±0.15 μm long and did not differ significantly in length from those on male maxillary palps (5.11±0.14 μm). However, the basal diameter of STBIIs was significantly greater on maxillary palps of females (2.59±0.06 μm) than of males (2.36±0.04 μm; Table 7). STBIIs on female labial palps were 5.42±0.12 μm long, significantly shorter than those on males (5.81±0.15 μm), whereas STBII basal diameter was significantly larger on female (2.63±0.04 μm) than on male labial palps (2.45±0.06 μm; Table 7). The number of STBIIs on maxillary palps did not differ between females (10.50±2.06) and males (7.33±0.76; Table 8), but the number of STBIIs on labial palps was significantly higher in females (18.71±1.64) than in males (9.33±1.20; Table 8).
STBIIIs exhibited a two-tiered morphology; the lower tier was robust and cylindrical, whereas the upper tier had a smaller conical shape with a distinct terminal pore (Figures 6C, G–H). The length of STBIIIs on the maxillary palps did not differ between females (4.75±0.15 μm) and males (5.03±0.17 μm; Table 7), but their basal diameter was significantly larger in females (2.95±0.07 μm) than in males (2.77±0.05 μm; Table 7). Likewise, the length of STBIIIs on the labial palps did not differ significantly between females (4.87±0.08 μm) and males (4.89±0.16 μm; Table 7), but their basal diameter was significantly greater in females (3.00±0.03 μm) than in males (2.84±0.05 μm; Table 7). There were also no differences between the sexes in STBIII numbers on the maxillary palps (8.67±0.84 in females, 7.83±1.08 in males; Table 8) or labial palps (9.57±1.25 in females, 8.17±0.48 in males; Table 8).
STBIVs had a wide socket, a blunt tip, and smooth lateral walls; an apical pore or cave was visible (Figures 6G, I). However, a longitudinal section of a similar sensillum shaft in Xylotrechus grayii showed micro apical pores on the tip (25), and the presence of such pores cannot be ruled out in Mo. saltuarius. STBIVs were commonly located in a central position on the tips of maxillary and labial palps (Figures 6A, B). On female maxillary palps, STBIVs were 5.22±0.11 μm long with a basal diameter of 3.99±0.05 μm; these measurements did not differ significantly from those in males (length 5.42±0.09 μm; basal diameter 3.97±0.04 μm; Table 7). STBIVs on female labial palps were 5.44±0.08 μm long, significantly longer than those of males (5.16±0.10 μm). The basal diameter of female labial palps was 4.05±0.03 μm and did not differ significantly from that of males (3.97±0.06 μm; Table 7). Numbers of STBIVs on the maxillary and labial palps did not differ between females (12.67±1.65 and 16.00±2.39) and males (17.00±4.16 and 13.83±3.19; Table 8).
3.3.8 Distribution of sensilla on the maxillary palps
The density and number of sensilla increased gradually from the base to the tip of the maxillary palps in both females and males. The total number and density of sensilla on M1–M4 did not differ significantly between the sexes (Table 8). M1 segments of females contained 4 sensillum types/subtypes: BBs (72.87%), CPs (13.57%), STIIs (9.60%), and SChs (5.03%). By contrast, M1 segments of males contained only 3 sensillum types/subtypes: BBs (72.50%), CPs (19.96%), and STIIs (7.53%). M2 segments of males and females contained four sensillum types/subtypes: STIIs (48.22% in females and 54.75% in males), CPs (38.90% and 33.77%), STIIIs (10.14% and 8.20%) and SChs (2.74% and 3.28%). M3 segments contained the same four sensillum types/subtypes as M2s in both sexes: CPs (47.61% in females and 42.23% in males), STIIs (32.57% and 34.90%), STIIIs (18.05% and 20.82%), and SChs (1.77% and 2.05%). M4 segments contained 10 sensillum types/subtypes in both females and males; in addition to those found on the M3s, M4s also contained SCos, SPs, STBIs, STBIIs, STBIIIs, and STBIVs.
3.3.9 Distribution of sensilla on the labial palps
The total number and density of sensilla on the labial palps increased gradually from the base to the tip in both males and females (Table 8), and there were no significant differences between the sexes in the number or density of sensilla on corresponding segments. L1 segments contained four sensillum types/subtypes in both sexes: BBs (66.67% in females and 73.33% in males), CPs (22.67% and 21.33%), STIIs (5.33% and 1.33%), and SChs (5.33% and 4.00%). L2 segments also contained four sensillum types/subtypes in both sexes: STIIs (38.29% in females and 37.44% in males), CPs (34.29% and 31.54%), STIIIs (23.81% and 26.15%), and SChs (3.62% and 4.87%). L3 segments contained 10 sensillum types/subtypes in both sexes; in addition to those found on the L2s, L3s also contained SCos, SPs, STBIs, STBIIs, STBIIIs, and STBIVs.
4 Discussion
Insect sensilla are classified on the basis of multiple criteria: the shapes of outer cuticular structures such as hairs and plates, the thickness of the cuticular wall, the flexibility of the cuticular socket, the presence and types of cuticular pores, the dendritic branching patterns, and the numbers of innervating sensory neurons (21). Sensilla that function as mechanoreceptors typically lack pores, as do thermo- or hygrosensilla (3, 21, 28). The presence of pores typically indicates that a sensillum functions in chemoperception. A single apical pore is characteristic of gustatory sensilla, whereas multiple pores are typically present on olfactory sensilla (22), a difference that likely reflects the different functions of these sensillum types: olfactory sensilla detect pheromones and plant volatiles at a distance (3, 29, 30), whereas gustatory sensilla detect non-volatile phytochemicals and pheromones through direct contact (3, 31, 32).
The antennae, maxillary palps, labial palps, and associated sensilla of insects are thought to reflect long-term adaptation to specific environments (33, 34). The presence of similar sensillum types in insects also indicates that they are evolutionarily related (26, 35, 36). We can therefore refer to information on other longhorn beetles from the same family or subfamily to gain insight into the types and functions of sensilla in Mo. saltuarius. Longhorn beetles of the Cerambycidae family have up to 13 types of antennal sensilla (24 subtypes), all of which are present in the Cerambycinae subfamily (37–41). By contrast, the Lamiinae subfamily has 9 types (16 subtypes) (42–44), the Lepturinae subfamily has 6 types (9 subtypes) (45), and the Aseminae subfamily has 6 types (10 subtypes) (46).
The Cerambycidae genera Xylotrechus and Massicus exhibit the highest diversity of antennal sensilla, with each genus possessing over 20 distinct subtypes (37, 47). Although the types of antennal sensilla differ among genera and species, some (such as STs, SChs, SBs, SAus, SGPs, DSOs, and BBs) are found in most longhorn beetles (48, 49), and STs, SChs, and SBs are present in nearly all longhorn beetle species (43). Xylotrechus and Massicus (Cerambycinae) (37, 47) and Saperda and Monochamus (Lamiinae) (20, 42, 49) have SAus, SGPs, and DSOs, but none of these sensilla are found in Nadezhdiella and Phoracantha (Cerambycidae) or Coscinesthes (Lamiinae). SGP and DSO sensilla are found in both Lepturinae and Aseminae, but SAus are not present in either subfamily (45, 46).
BBs function in proprioception and are found on all arthropod antennae (3, 4). Here, BBs were observed on the antennae, maxillary palps, and labial palps of Mo. saltuarius and were similar to BBs described in Glenea cantor (50), Aromia bungii (40), Xylotrechus grayii (51), Xylotrechus quadripes (26), and Pharsalia antennata (52). BBs are widely considered to function as mechanoreceptors for gravity perception. Mechanoreceptors on the antennae respond to touch, vibration, and airflow by striking the surface of the antennal flagellum ventrally. BBs occur at the radicle, at the scape–pedicel junction, and at the bases of M1 and L1, consistent with a role in the perception of antennal position and movement (53–55); these locations may provide an optimal angle for sensing antennal position and obtaining precise positioning signals (56).
STIs were most numerous and widely distributed on the antennae of Mo. saltuarius, and their densities on antennae were higher in females than in males. The distribution of Mo. saltuarius STIs was similar to that of sensilla described previously under various names: SBLIs in Monochamus alternatus (23), SC1s in X. quadripes (26), ST-IVs and ST-Vs in Pharsalia antennata (52), STIIs in Rhaphuma horsfieldi (41), and stout SCs in Monochamus notatus, Monochamus scutellatus, and Monochamus galloprovincialis (20, 57). Gland openings or clusters of pores immediately adjacent to STI insertions have been reported previously (20, 57). Because STIs lack pores and show limited innervation, they are unlikely to function in chemoperception (20, 46, 58). Álvarez et al. (57) were unable to record action potentials from STIs (which they termed stout SCs), suggesting that they may be mechanoreceptors. Although STIs are frequently classified as a single subtype, some species have multiple STI subtypes that differ in size and morphology, depending on their antennal distribution (38, 51, 57). STIs on the antennae of male Mo. saltuarius are similar to the ‘bottle-like sensilla’ described in Mo. alternatus (59) and the ‘male peg SCs’ described in Mo. galloprovincialis (57).
The distribution of Mo. saltuarius STIIs was similar to that of sensilla described in other species under a variety of names: SC3s in X. quadripes (26), STIs in Pha. antennata (52), and STIs in R. horsfieldi (41). STIIs also have the general characteristics of a mechanoreceptor.
The morphology of STIIIs in Mo. saltuarius resembled that of SRLs in Mo. alternatus (23), STr2s in Mo. alternatus and Mo. saltuarius (19), STs in Mo. notatus and Mo. scutellatus (20), ST1s and ST2s in Chlorophorus caragana (60), uniporous sensillum chaeticum in Phoracantha recurva (38), finger sensilla 2 in X. rusticus (37), ST1s in X. quadripes (26), and ST2s in Allotraeus asiaticus (61). STIIIs of Mo. saltuarius exhibited longitudinal grooves on their surfaces and one to several terminal pores, classifying them as groove-type chemoreceptors. This sensillum subtype contains an apical pore and is innervated by an average of 7–8 neurons/hair in Mo. notatus and Mo. scutellatus, suggesting a role in contact chemoperception. Here, STIIIs were found on the antennae as well as the maxillary and labial palps of adult Mo. saltuarius. We infer that STIIIs likely serve as the main contact chemoreceptors, with functions in both mechanical and chemical stimulus perception.
SChI morphology in Mo. saltuarius resembled that of sensilla described previously in G. cantor (50). These sensilla have been termed SC1s in Tetropium fuscum (46), SC2s in X. grayii (51), SChIVs in Pha. antennata (52), SChIIs in R. horsfieldi (41), SCIs in X. quadripes (26), and long SCs in Mo. notatus, Mo. scutellatus, and Mo. galloprovincialis (20, 57). Transmission electron microscopy observations of SChIs in Mo. notatus and Mo. scutellatus revealed that these sensilla are innervated by a single sensory neuron (20). In Hymenopteran wasps, SChs serve as tactile mechanoreceptors that sense relative antennal position (62), and some studies have suggested that SChs are involved in host exploration and identification (63, 64). The SChIs on the antennae of Mo. saltuarius are oriented almost perpendicularly to the antennal surface and are much longer than other sensilla, enabling them to make contact with objects first and suggesting that they are also mechanoreceptors.
The morphology of SChIIs resembled that of SCs in Massicus raddei (39), SChIIIs in G. cantor (50), SSTIs in Mo. alternatus (23), SC4s in X. quadripes (26), ChIs in Plagionotus pulcher (65), SChIIIs in Pha. antennata (52), SChIs in R. horsfieldi (41), and distal SCs in Mo. notatus and Mo. scutellatus, as well as large SCs in Mo. galloprovincialis (57). SChIIs are aporous and much longer than other nearby sensillum types. As with other longhorn beetles, Mo. saltuarius had SChIIs at junctions where one flagellomere overlapped with the proximal portion of the next flagellomere (3). SChIIs, like SChIs, are innervated by one sensory neuron (20), and research in Mo. galloprovincialis demonstrated that their neurons produced action potentials in response to movement (57). These characteristics suggest that SChIIs are proprioceptors that detect the positioning of antennal segments.
The SAu of Mo. saltuarius was a spoon- or ear-shaped structure with a relatively flat or concave distal surface and was found mainly on the antennal flagellum. The morphology of SAus resembled that of SBIIs in G. cantor (50), SBIIIs in Mo. alternatus (23), SAus in Mo. alternatus (58), stout SBs in Mo. notatus (20), SB3s in X. grayii (51), SBIs in Leptura arcuata (45), Aus in Ch. caragana (60), SAs in X. quadripes (26), SBIs in Pha. antennata (52), and SBIs and SBIIs in R. horsfieldi (41). SAus in Mo. alternatus had thin walls, abundant cuticular pores, and fewer than five dendritic branches (58). These features suggest that they have an olfactory function, and many studies have proposed that this sensillum may sense the stimulation of host volatiles (54, 66).
The morphology of SBIs in Mo. saltuarius resembled that of SBIs in G. cantor (50), STs in Mo. alternatus (58), SBIIs in Pho. recurva (38), SBIIs in L. arcuata (45), SBIIs in Leptura aethiops (45), SB2s in X. quadripes (26), B1s in Pl. pulcher (65), SBIIs in Pha. antennata (52), and SB1s in R. horsfieldi (41). SBIs in Mo. alternatus (their ‘sensilla trichodea’) had somewhat thicker walls with numerous pores (but fewer than SAus) and were innervated by 1–8 dendrites (58); these SB subtypes also had multiporous walls and were innervated by multiple dendritic branches in T. fuscum, indicative of an olfactory function (46). SBIIs in Mo. saltuarius resembled SB1s in X. grayii (51), SBIVs in L. arcuata and L. aethiops (45 SC2s in Mo. alternatus (58), and SBIIIs in Pha. antennata (52). SBIIs are generally believed to be taste chemoreceptors used for host recognition (21, 67, 68), and taste function (63, 64, 69).
SBIs and SBIIs in Mo. saltuarius were found mainly on the antennal flagellum; they increased gradually in number from the base to the tip and were often found in dense patches on each flagellomere (Figure 4C). The concentration of SBs (Figure 4D and the dashed-line area in Figure 4C) and the differences in their numbers between males and females (both SBIs and SBIIs were higher in males than in females, Table 6) are shown in Mo. saltuarius. This distribution pattern—characterized by a dense sensory region— is similar to that of SBs in Pl. pulcher (65), Mo. notatus, and Mo. scutellatus (20) and is believed to be beneficial for the detection of odor molecules and the perception of sexual information released by conspecifics in X. grayii (51), X. rusticus (37), Phoracantha semipunctata (38), and Pho. recurva (38). Different SB subtypes may selectively recognize external chemical information (37, 38).
The morphology of SGPs resembled that of grooved peg sensilla in Aedes aegypti (70), X. grayii (51) and X. quadripes (26), SGPs in X. rusticus (37) and T. fuscum (46), Stys in Mo. alternatus (58) and Ch. caragana (60), SBIIIs in L. arcuata (45) and L. aethiops (45), and B6s in Pl. pulcher (65). Pores on the proximal smooth cuticle were few and shallow, and no pores were present on the grooved portion of the sensillum (58, 60). However, pore channels run along the grooves of these sensilla in A. aegypti with approximately 38 pore openings per groove, totaling about 456 openings per peg (70). In Pho. semipunctata, each sensillum is innervated by 4–5 bipolar neurons (71). Based on their ultrastructure, SGPs are generally thought to possibly have a dual role as both thermal and chemical sensilla (21, 63, 70, 72).
The morphology of DSOs resembled that of SCas in Callidiellum villosulum (61), Cas in X. quadripes (26), dome organs (campaniform) in Mo. scutellatus (20), SCos in Mo. alternatus and Mo. saltuarius (19), SCs in Pl. pulcher (65), and SCas in Pha. antennata (52). These sensilla in Mo. scutellatus are innervated by at least one neuron per sensillum (20). Electrophysiological evidence in Pterostichus aethiops (73) and Pt. oblongopunctatus (74) suggests that they are thermo- and hygroreceptors.
The morphology of CPs resembled that of CPs in X. quadripes (26) and X. rusticus (37) and of the ms in Ma. raddei (39). CPs have been described as similar sensory organs in various families of Coleoptera, including Carabidae, Chrysomelidae, Meloidae, Paussidae, Pselaphidae, Silphidae, and Staphylinidae. CPs were once thought to be pheromone glands, kairomones, or lubricants for antennae and sensilla (75). In Coccinella septempunctata, CPs were described as sensilla ampucellaceous (Ams) with potential chemical and/or thermal sensory functions (76). In moth antennae, CPs may have enzymatic functions that prevent the accumulation of non-active pheromones and plant volatiles (77).
The morphology of SPs resembled that of sensilla placodea in Ca. villosulum (27), digitiform sensilla in Mo. alternatus (78) and Ch. caragana (24), and sensilla digitiformia in X. grayii (25). SPs were not found on the antennae of Mo. saltuarius but were present on the dorsal tip of the last segment of the maxillary (M4) and labial palps (L3). There was a pore at the top of one side of the SP, which is usually considered to function in chemoreception and mechanoreception or to sense changes in CO2, temperature, and humidity (79–81). The SPs of Coleoptera sense contact-vibration stimuli that may be related to the movement of insects in tunnels (82). The distribution of SPs on adult Mo. saltuarius at the dorsal distal segments of the maxillary and labialpalps may enable them to contact the inner wall of the tunnel, thus sensing the temperature, humidity, and vibrations caused by feeding of other similar insects. This enables them to adjust their feeding path and avoid tunnel overlap, and it may also help them to search for the most suitable feeding sites and summering places.
The morphology of SCos on the maxillary and labial palps of Mo. saltuarius resembled that of sensilla pit basiconica in Anoplophora glabripennis and Anoplophora chinensis (43), S.tb.5s in Ch. caragana (24), SCas in Ca. villosulum (27), short sensilla styloconica in Philus antennatus (83), and recessed peg sensilla in Cicindela sexguttata (Cicindelidae). Faucheux considered that their main function was as sensors for CO2, temperature, and humidity (84).
The morphology of STBIs on the maxillary and labial palps of Mo. saltuarius resembled that of Stb1s in Mo. alternatus (78), S.tb.3s in Ch. caragana (24), Sty2s in X. grayii (25), s.b.1s in Siagona europaea (85), and SBIVs in Ca. villosulum (27). These sensilla are innervated by 2–6 dendrites in X. grayii and have a thick dendritic sheath surrounded by tormogen and trichogen cells below the cuticle level (25). STBIs resemble uniporous sensilla that perceive odors through taste or contact (22, 86) and may function as contact chemoreceptors, detecting mechanical and chemical stimuli while performing gustatory and olfactory roles.
The morphology of STBIIs on the maxillary and labial palps of Mo. saltuarius resembled that of SBIIIs in Ca. villosulum (27), Stb2s in Mo. alternatus (78), S.tb.2s in Ch. caragana (24), S.t.b.2s in larval An. glabripennis (87), and S2s in larval Melolontha melolontha (88). These sensilla were thought to be contact gustatory sensilla in Mo. alternatus and larval Me. melolontha (78, 88).
The morphology of STBIIIs on the maxillary and labial palps of Mo. saltuarius resembled that of Stb3s in Mo. alternatus (78), S.tb.4s in the labial palps of Ch. caragana (24), Sty4s in X. grayii (25), S.t.b.6s in larval An. glabripennis (87), and sensilla styloconica in larval An. glabripennis (89). In X. grayii, these sensilla have 3 dendrites and are thought to sense chemical stimuli (25); they have also been identified as chemosensilla in larval An. glabripennis (87) and proposed to function as chemo-, thermo-, and hygroreceptors (25, 87, 90).
The morphology of STBIVs on the maxillary and labial palps of Mo. saltuarius resembled that of Stb4s in Mo. alternatus (78) and Sty3s in X. grayii (25). These sensilla are innervated by 3 dendrites surrounded by tormogen and trichogen cells in X. grayii; the dendrites are suspended under a thin dendritic sheath in the inner lymphatic cavity and extend into the shaft lumen (25). Sty3s in X. grayii have thin walls and small apical pores, which are typical of olfactory and taste sensilla (25). The STBIVs in Mo. saltuarius exhibited a cave on the tip surface, suggesting that the tip wall may be soft and thin; this structure probably contributes to the diffusion of odorants into the shaft. The location of the micro-apical pores may also indicate a gustatory function.
STBs were located on the apices of the maxillary and labial palps of adult Mo. saltuarius and may therefore play a role in adult feeding habits. They may help adults select good, fresh, and nourishing foods while avoiding harmful substances and may also be involved in detecting host-tree chemical cues, monitoring food texture, and evaluating food quality.
The STBs on the tips of the maxillary and labial palps are highly sensitive, because the olfactory sensillum dendrites are divided into many branches and the tips of the conical sensilla have many small pores that admit gas molecules and accept more molecules diffused from the host (91). The olfactory sensilla of both maxillary and labial palps can accept molecular stimuli that have diffused into the air from the host and do not require host contact, enabling the insects to detect their host tree; thus, these sensilla play a role in long-distance host selection. By contrast, taste sensilla can only confirm the presence of the host upon direct contact and are stimulated by dissolved molecules (91, 92).
The antennal composition and flagellomere number of male and female Mo. saltuarius were the same as those of X. rusticus (37), Ar. bungii (40), G. cantor (50), and R. horsfieldi (41). There were no significant differences between males and females in the density of olfactory sensilla on the antennae. However, antennal length and surface area were slightly greater in males than in females. Therefore, the number of chemoreceptors (STIIIs, SAus, SBIs, SBIIs, SGPs and CPs) and the total number of sensilla on the antennae were significantly greater in males (Table 6). There were no significant differences between males and females in the number of sensilla on the maxillary and labial palps.
5 Conclusion
We observed sexual dimorphism in the number, distribution, and morphology of sensilla on the antennae, maxillary palps, and labial palps of adult Mo. saltuarius. Both sexes contained the same sensillum types, which included putative chemoreceptors and/or mechanoreceptors. There were 8 types on the antennae and 7 types on the maxillary and labial palps. The antennal BBs, STIs, STIIs, SChIs, and SChIIs and the labial BBs, STIIs, and SChs may function as mechanoreceptors for proprioception, tactile perception, air-movement detection, and host location and exploration. The antennal SAus, SBIs, SBIIs, and SGPs may be olfactory chemoreceptors used during host searching, or mating. The STIIIs, SPs, STBIs, STBIIs, STBIIIs and STBIVs may be taste chemoreceptors used during feeding. The DSOs, and SCos may be receptors for water, and temperature. The types and densities of sensilla on the antennae of adult Mo. saltuarius increased from the base to the tip, and sensilla with chemical-sensing functions were concentrated mainly on the flagellum. By characterizing the morphology, number, and distribution of different sensillum types, we can better understand the olfactory receptive mechanisms that enable intraspecific and interspecific chemical communication in Mo. saltuarius. In future work, we hope to identify genes related to olfactory sensilla and characterize their roles in host location, mating, oviposition, and other processes in order to limit the damage caused by Mo. saltuarius through genetic manipulation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
JJW: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing, Investigation, Project administration. JGW: Data curation, Investigation, Software, Visualization, Writing – review & editing. JXW: Data curation, Formal Analysis, Methodology, Validation, Writing – review & editing. XJ: Data curation, Investigation, Project administration, Validation, Writing – review & editing. SR: Investigation, Software, Supervision, Validation, Writing – review & editing. CC: Conceptualization, Methodology, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the Presidential Foundation of the Liaoning Academy of Agricultural Sciences (Project 2025HQ1305, 2025XKJS8565) and the Natural Science Foundation of Liaoning Province (Project 2025-MS-319).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lopes O, Marques PC, and Araújo J. The role of antennae in mate recognition in Phoracantha semipunctata (Coleoptera: Cerambycidae). J Insect Behav. (2005) 18:243–57. doi: 10.1007/s10905-005-0478-7
2. Mitchell RF, Hughes DT, Luetje CW, Millar JG, Soriano-Agatón F, Hanks LM, et al. Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae. Insect Biochem Mol Biol. (2012) 42:499–505. doi: 10.1016/j.ibmb.2012.03.007
3. Haddad S, Clarke DJ, Jeong SH, Mitchell RF, and McKenna DD. Antennal sensilla in longhorn beetles (Coleoptera: Cerambycidae). Ann Entomological Soc America. (2023) 116:83–113. doi: 10.1093/aesa/saac026
4. Schneider D. Insect antennae. Annu Rev Entomol. (1964) 9:103–22. doi: 10.1146/annurev.en.09.010164.000535
5. Hallberg E and Hansson BS. Arthropod sensilla: morphology and phylogenetic considerations. Microscopy Res Technique. (1999) 47:428–39. doi: 10.1002/(SICI)1097-0029(19991215)47:6<428::AID-JEMT6>3.0.CO;2-P
6. Ray AM, Ginzel MD, and Hanks LM. Male Megacyllene robiniae (Coleoptera: Cerambycidae) use multiple tactics when aggressively competing for mates. Environ Entomol. (2009) 38:425–32. doi: 10.1603/022.038.0216
7. Hanks LM, Millar JG, and Paine TD. Mating behavior of the Eucalyptus longhorn borer (Coleoptera: Cerambycidae) and the adaptive significance of long ‘horns’. J Insect Behav. (1996) 9:383–93. doi: 10.1007/BF02214017
8. Liu CT and Tong X. Morphological comparison of the sensilla on the maxillary and labial palps between male and female adults of Moechotypa diphysis (Coleoptera: Cerambycidae). Microsc Res Tech. (2023) 86(9):1079–1090. doi: 10.1002/jemt.24303
10. Wang ZC. Monographia of original colored longicorn beetles of China. Beijing: Scientific and Technical Documentation Press (2014) p. 847–8.
11. Li M, Li H, Sheng RC, Sun H, Sun SH, and Chen FM. The first record of Monochamus saltuarius (Coleoptera; Cerambycidae) as vector of Bursaphelenchus xylophilus and its new potential hosts in China. Insects. (2020) 11:636. doi: 10.3390/insects11090636
12. Li M, Dai Y, Wang Y, Wang LC, Sun SH, and Chen FM. New insights into the life history of Monochamus saltuarius (Cerambycidae: Coleoptera) can enhance surveillance strategies for pine wilt disease. J Forestry Res. (2021) 32:2699–707. doi: 10.1007/s11676-021-01296-x
13. Gao RH, Liu L, Li RJ, Fan SM, Dong JH, and Zhao LJ. Predicting potential distributions of Monochamus saltuarius, a novel insect vector of pine wilt disease in China. Front Forests Global Change. (2023) 6:1243996. doi: 10.3389/ffgc.2023.1243996
14. Fan LC, Wang J, Wang WT, and Zheng YN. Larval instars and adult flight period of Monochamus saltuarius (Coleoptera: Cerambycidae). Forests. (2022) 13:910. doi: 10.3390/f13060910
15. Sato H, Takeshi S, and Kobayashi M. Transmission of Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle (Nematoda, Aphelenchoididae) by Monochamus saltuarius (Gebler) (Coleoptera, Cerambycidae). J Phys Soc Japan. (1987) 69:492–6.
16. Jikumaru S and Togashi K. A weak deleterious effect of the avirulent pinewood nematode, Bursaphelenchus mucronatus (Nematoda: Aphelenchoididae), on the longevity of its vector, Monochamus saltuarius (Coleoptera: Cerambycidae). Appl Entomol Zool. (1995) 30:9–16. doi: 10.1303/aez.30.9
17. Kim MK, Kim JS, Han JH, and Kim YJ. Mating behavior of pine sawyer, Monochamus saltuarius Gebler (Coleoptera: Cerambycidae). J Asia-Pacific Entomol. (2006) 9:275–80. doi: 10.1016/S1226-8615(08)60303-9
18. Han JH, Yoon C, Shin SC, and Kim GH. Seasonal occurrence and morphological measurements of pine sawyer, Monochamus saltuarius adults (Coleoptera: Cerambycidae). J Asia-Pacific Entomol. (2007) 10:63–7. doi: 10.1016/S1226-8615(08)60332-5
19. Huh MJ, Park JH, Roh GH, and Park IK. Morphology and distribution of antennal sensilla in the pine wood nematode insect vectors Monochamus alternatus Hope and Monochamus saltuarius Gebler (Coleoptera: Cerambycidae). J Asia-Pacific Entomol. (2023) 26:102024. doi: 10.1016/j.aspen.2022.102024
20. Dyer LJ and Seabrook WD. Sensilla on the antennal flagellum of the sawyer beetles Monochamus notatus (Drury) and Monochamus scutellatus (say) (Coleoptera: Cerambycidae). J Morphol. (1975) 146:513–31. doi: 10.1002/jmor.1051460407
21. Altenr H and Prillinger L. Ultrastructure of invertebrate chemo, thermo-, and hygroreceptors and its functional significance. Int Rev Cytol. (1980) 67:69–139. doi: 10.1016/S0074-7696(08)62427-4
22. Zacharuk RY. Ultrastructure and function of insect chemosensilla. Annu Rev Entomol. (1980) 25:27–47. doi: 10.1146/annurev.en.25.010180.000331
23. Ali S, Ali SAI, Wagan TA, Waris MI, and Wang MQ. Morphology and ultrastructure of the antennal sensilla of Japanese sawyer beetle Monochamus alternatus Hope (Coleoptera: Cerambycidae). J Kansas Entomological Soc. (2017) 90:241–51. doi: 10.2317/0022-8567-90.3.241
24. Zhang YR, Ren LL, Zhang L, Wang R, Yu Y, Lu PF, et al. Ultrastructure and distribution of sensilla on the maxillary and labial palps of Chlorophorus caragana (Coleoptera: Cerambycidae). J Morphol. (2018) 279:574–88. doi: 10.1002/jmor.20791
25. Chen JM, Zhu X, Qiao HL, Liu S, Xu CQ, Xu R, et al. Ultrastructure of sensilla on the maxillary and labial palps of the adult Xylotrechus grayii (Coleoptera: Cerambycidae). Microscopy Res Technique. (2018) 81:669–80. doi: 10.1002/jemt.23022
26. Yang Y, Shan Y, Liu A, Li Y, Liu X, Cao J, et al. Morphology and distribution of antennal sensilla in adults of Xylotrechus quadripes. Microscopy Res Technique. (2022) 85:1146–59. doi: 10.1002/jemt.23983
27. Wu GX, Dong ZS, Zheng XL, Lu W, and Wang XY. Scanning electron microscopy of sensilla on the labial and maxillary palps of adult Callidiellum villosulum Fairmaire (Coleoptera: Cerambycidae). Microscopy Res Technique. (2022) 85:1311–9. doi: 10.1002/jemt.23997
28. Steinbrecht RA. Pore structures in insect olfactory sensilla: a review of data and concepts. Int J Insect Morphol Embryol. (1997) 26:229–45. doi: 10.1016/s0020-7322(97)00024-x
29. Pophof B, Stange G, and Abrell L. Volatile organic compounds as signals in a plant–herbivore system: electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem Senses. (2005) 30:51–68. doi: 10.1093/chemse/bji001
30. Wee SL, Oh HW, and Park KC. Antennal sensillum morphology and electrophysiological responses of olfactory receptor neurons in trichoid sensilla of the diamondback moth (Lepidoptera: Plutellidae). Florida Entomologist. (2016) 99:146–58. doi: 10.1653/024.099.sp118
31. Chapman RF. Contact chemoreception in feeding by phytophagous insects. Annu Rev Entomol. (2003) 48:455–84. doi: 10.1146/annurev.ento.48.091801.112629
32. Fleischer J and Krieger J. Insect pheromone receptors – key elements in sensing intraspecific chemical signals. Front Cell Neurosci. (2018) 12:425. doi: 10.3389/fncel.2018.00425
33. Baaren JV, Boivin G, Bourdais D, and Roux O. Antennal sensilla of hymenopteran parasitic wasps: Variations linked to host exploitation behavior. In: Modern Research and Educational Topics in Microscopy. Badajoz: Formatex Elsevier (2007). p. 345–52.
34. Meng J, Bu WJ, Xiao JH, and Huang DW. Morphological characteristics and evolutionary adaptation analysis of the antennal sensilla of fig wasps from China. Acta Entomologica Sin. (2015) 58:800–10. doi: 10.16380/j.kcxb.2015.07.013
35. Heraty JM, Burks RA, Gruaud A, Gibson GAP, Liljeblad J, Munro J, et al. A phylogenetic analysis of the megadiverse Chalcidoidea (Hymenoptera). Cladistics. (2013) 29:466–542. doi: 10.1111/cla.12006
36. Diakova AV, Makarova AA, and Polilov AA. Between extreme simplification and ideal optimization: antennal sensilla morphology of miniaturized Megaphragma wasps (hymenoptera: Trichogrammatidae). PeerJ. (2018) 6:e6005. doi: 10.7717/peerj.6005
37. Cheng H, Yan SC, Xu B, Li J, and Peng L. The ultrastructure and distribution of main antennal sensilla of Xylotrechus rusticus. Chin Bulletion Entomol. (2008) 45:223–32. doi: 10.3969/j.issn.0452-8255.2008.02.011
38. Faucheux MJ. Antennal sensilla of the yellow longicorn beetle Phoracantha recurva Newman 1840: Distribution and comparison with Phoracantha semipunctata (Fabricius 1775) (Coleoptera: cerambycidae). Bull l'Institut Scientifique. (2011) 33:19–29.
39. Wei JR, Ding BF, Tang YL, and Li XM. Morphology and distribution of the antennal sensilla of adult Massicus raddei (Blessig) (Coleoptera: Cerambycidae). Chin J Appl Entomol. (2013) 50:1289–94. doi: 10.7679/j.issn.2095-1353.2013.177
40. Di Palma A, Pistillo M, Griffo R, Garonna AP, and Germinara GS. Scanning electron microscopy of the antennal sensilla and their secretion analysis in adults of Aromia bungii (Faldermann 1835) (Coleoptera, cerambycidae). Insects. (2019) 10:88. doi: 10.3390/insects10040088
41. Yin NN, Zhao N, and Liu NY. Morphological characteristics of Rhaphuma horsfieldi and ultrastructure of its antennae and tarsi. J Southwest Forestry University. (2019) 39:132–40. doi: 10.11929/j.swfu.201903104
42. Li YF. Mating factors and characteristics of head appendages in Saperda populnea. Shanxi, China: Shanxi Agricultural University (2015).
43. Xu LL. Sensilla on antenna, maxillary and labial palps of seven Lamiinae longhorned beetle species at different life stages. Beijing, China: Beijing Forestry University (2016).
44. Crook DJ, Higgins RA, and Ramaswamy SB. Antennal morphology of the soybean stemborer Dectes texanus texanus LeConte (Coleoptera: Cerambycidae). Journal of the Kansas Entomological Society. (2003) 76(3):397–405. Available online at: https://www.jstor.org/stable/25086127.
45. Zhang J. Taxonomic study on the Cerambycidae in Jilin province. Jilin, China: Northeast Normal University (2011).
46. Mackay CA, Sweeney JD, and Kirk Hillier N. Morphology of antennal sensilla of the brown spruce longhorn beetle, Tetropium fuscum (Fabr.) (Coleoptera: Cerambycidae). Arthropod Structure Dev. (2014) 43:469–75. doi: 10.1016/j.asd.2014.04.005
47. Li Y, Meng QF, Zong X, and Gao WT. Ultrastructural observations on antennal sensilla of Massicus raddei Blessig (Coleoptera: Cerambycidae). J Beijing Forestry Univ. (2013) 35:80–6. doi: 10.13332/j.1000-1522.2013.06.005
48. Liu XH, Luo YQ, Cao CJ, and Zong SX. Scanning electron microscopy of antennal sensible of Anoplistes halodendri halodendri and Anoplistes halodendri ephippium (Coleoptera: Cerambycidae). Microscopy Res Technique. (2012) 75:367–73. doi: 10.1002/jemt.21065
49. Zhang XJ, Sun W, Zhang J, Zuo TT, Wang ZQ, and Zhao HW. Research progress of Coleopteran insect species antennal sensilla. J Anhui Agric Sci. (2013) 41:2932–5. doi: 10.13989/j.cnki.0517-6611.2013.07.105
50. Dong ZS, Yang YB, Dou FG, Zhang YJ, Huang HX, Zheng XL, et al. Observations on the ultrastructure of antennal sensilla of adult Glenea cantor (Cerambycidae: Lamiinae). J Insect Sci. (2020) 20:7. doi: 10.1093/jisesa/ieaa013
51. Chen JM, Qiao HL, Chen J, Xu CQ, Liu S, Lian ZM, et al. Observation of antennal sensilla in Xylotrechus grayii (Coleoptera: Cerambycidae) with scanning electron microscopy. Microscopy Res Technique. (2014) 77:264–73. doi: 10.1002/jemt.22338
52. Yin NN, Zhao YJ, Zhao N, and Liu NY. Morphological characteristics of Pharsalia antennata and its sensilla ultrastructure. Plant Prot. (2020) 46:30–40. doi: 10.16688/j.zwbh.2019493
53. Dweck HKM. Antennal sensory receptors of Pteromalus puparum female (Hymenoptera: Pteromalidae), a gregarious pupal endoparasitoid of Pieris rapae. Micron. (2009) 40:769–74. doi: 10.1016/j.micron.2009.07.012
54. Zhou H, Wu WJ, Zhang FP, and Fu YG. Scanning electron microscopy studies of the antennal sensilla of Metaphycus parasaissetiae Zhang and Huang (Hymenoptera: Encyrtidae). Neotropical Entomol. (2013) 42:278–87. doi: 10.1007/s13744-013-0113-9
55. Silva IM, Pereira KS, Spranghers T, Zanuncio JC, and Serrão JE. Antennal sensilla and sexual dimorphism of the parasitoid Trichospilus pupivorus (Hymenoptera: Eulophidae). Microscopy Microanalysis. (2016) 22:913–21. doi: 10.1017/S1431927616011314
56. Merivee E, Ploomi A, Rahi M, Bresciani J, Ravn HP, Luik A, et al. Antennal sensilla of the ground beetle Bembidion properans Steph. (Coleoptera, Carabidae). Micron. (2002) 33:429–40. doi: 10.1016/s0968-4328(02)00003-3
57. Álvarez G, Ammagarahalli B, Hall DR, Pajares JA, and Gemeno C. Smoke, pheromone and kairomone olfactory receptor neurons in males and females of the pine sawyer Monochamus galloprovincialis (Olivier) (Coleoptera: Cerambycidae). J Insect Physiol. (2015) 82:46–55. doi: 10.1016/j.jinsphys.2015.08.004
58. Sun F, Li XR, Liu XX, and Zhang QW. Ultrastructure of six types of antennal sensilla in Monochamus alternatus. Chin Bulletion Entomol. (2010) 47:347–54. doi: 10.7679/j.issn.2095-1353.2010.062
59. Wang SB, Zhou HC, Miao XX, Fan MZ, Li ZZ, Si SL, et al. Scanning electron microscopic observations of Monochamus alternatus antennal sensilla and their electroantennographic responses. Chin J Appl Entomol. (2005) 16:317–22. doi: 10.3321/j.issn:1001-9332.2005.02.024
60. Zhang YR, Ren LL, Zhang L, and Luo YQ. Ultrastructure of antennal and posterior abdominal sensilla in Chlorophorus caragana females. Micron. (2015) 75:45–57. doi: 10.1016/j.micron.2015.04.014
61. Dong ZS, Dou FG, Yang YB, Wickham JD, Tang R, Zhang YJ, et al. First description and comparison of the morphological and ultramicro characteristics of the antennal sensilla of two fir longhorn beetles. PloS One. (2020) 15:e0241115. doi: 10.1371/journal.pone.0241115
62. Ochieng SA, Park KC, Zhu JW, and Baker TC. Functional morphology of antennal chemoreceptors of the parasitoid Microplitis croceipes (Hymenoptera: Braconidae). Arthropod Structure Dev. (2000) 29:231–40. doi: 10.1016/s1467-8039(01)00008-1
63. Isidoro N, Bin F, Colazza S, and Vinson SB. Morphology of antennal gustatory sensilla and glands in some parasitoid Hymenoptera with hypothesis on their role in sex and host recognition. J Hymenoptera Res. (1996) 5:206–39. doi: 10.5962/BHL.PART.28120
64. Onagbola EO and Fadamiro HY. Scanning electron microscopy studies of antennal sensilla of Pteromalus cerealellae (Hymenoptera: Pteromalidae). Micron. (2008) 39:526–35. doi: 10.1016/j.micron.2007.08.001
65. Shang JY, Xu WC, Meng QF, Zhao HR, Liu SD, and Li Y. Antennal sensilla of adult Plagionotus pulcher (Coleoptera: Cerambycidae) observed with scanning electron microscope. J Nanjing Forestry (Natural Sci Edition). (2021) 45:195–200. doi: 10.3969/j.issn.1000-2006.202004059
66. Roh GH, Lee YJ, Zhu JJ, and Park CG. Morphology and distribution of antennal sensilla in egg parasitoid, Ooencyrtus nezarae (Hymenoptera: Encyrtidae). Microscopy Res Technique. (2019) 82:972–82. doi: 10.1002/jemt.23244
67. Pettersson EM, Hallberg E, and Birgersson G. Evidence for the importance of odour-perception in the parasitoid Rhopalicus tutela (Walker) (Hym., Pteromalidae). J Appl Entomol. (2001) 125:293–301. doi: 10.1046/j.1439-0418.2001.00550.x
68. Roux O, van Baaren J, Gers C, Arvanitakis L, and Legal L. Antennal structure and oviposition behavior of the Plutella xylostella specialist parasitoid: Cotesia plutellae. Microscopy Res Technique. (2005) 68:36–44. doi: 10.1002/jemt.20220
69. Gao Y, Luo LZ, and Hammond A. Antennal morphology, structure and sensilla distribution in Microplitis pallidipes (Hymenoptera: Braconidae). Micron. (2007) 38:684–93. doi: 10.1016/j.micron.2006.09.004
70. Cribb BW and Jones MK. Reappraisal of the pore channel system in the grooved pegs of Aedes aEgypti. Tissue Cell. (1995) 27:47–53. doi: 10.1016/s0040-8166(95)80008-5
71. Lopes O. Estudo ultrastructural dos sistemas sensorial e glandular de Phoracantha semipunctata (Coleoptera: Cerambycidae). Universidade de Evora, Portugal: Implicados na Comunicacao Quımica, Tese de doutoramento (1999).
72. Altner H, Routil C, and Loftus R. The structure of bimodal chemo-, thermo-, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res. (1981) 215:289–308. doi: 10.1007/BF00239116
73. Merivee E, Vanatoa A, Luik A, Rahi M, Sammelselg V, and Ploomi A. Electrophysiological identification of cold receptors on the antennae of the ground beetle Pterostichus aethiops. Physiol Entomol. (2003) 28:88–96. doi: 10.1046/j.1365-3032.2003.00320.x
74. Merivee E, Must A, Luik A, and Williams I. Electrophysiological identification of hygroreceptor neurons from the antennal dome shaped sensilla in the ground beetle Pterostichus oblongopunctatus. J Insect Physiol. (2010) 56:1671–8. doi: 10.1016/j.jinsphys.2010.06.017
75. Bin F, Colazza S, Isidoro N, and Vinson SB. Antennal chemosensilla and glands, and their possible meaning in the reproductive behaviour of Trissolcus basalis (Woll.) (Hym.: Scelionidae). Entomologica. (1989) 24:33–97. doi: 10.15162/0425-1016/623
76. Srivastava S and Omkar. Scanning electron microscopy of antennae of Coccinella septempunctata (Coccinellidae: Coleoptera). Entomologia Sin. (2003) 10:271–9. doi: 10.1111/j.1744-7917.2003.tb00392.x
77. Vogt RG and Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. (1981) 293:161–3. doi: 10.1038/293161a0
78. Ren LL. Electrophysiological and behavioral responses of Monochamus alternatus and parasitoid Dastarcus helophoroides to semiochemicals of several tree species. Beijing, China: Beijing Forestry University (2014).
79. Honomichl K and Guse GW. Digitiform sensilla on the maxillar palp of Coleoptera. Acta Zoologica. (1981) 62:17–25. doi: 10.1111/j.1463-6395.1981.tb00613.x
80. Keil TA. Functional morphology of insect mechanoreceptors. Microscopy Res Technique. (1997) 39:506–31. doi: 10.1002/(SICI)1097-0029(19971215)39:6<506::AID-JEMT5>3.0.CO;2-B
81. Ortloff A, Zanetti N, Centeno N, Silva R, Bustamante F, and Olave A. Ultramorphological characteristics of mature larvae of Nitidula carnaria (Schaller 1783) (Coleoptera: Nitidulidae), a beetle species of forensic importance. Forensic Sci Int. (2014) 239:e1–9. doi: 10.1016/J.FORSCIINT.2014.03.010
82. Zacharuk RY and Shields VD. Sensilla of inmmature insects. Annu Rev Entomol. (1991) 36:331–54. doi: 10.1146/annurev.en.36.010191.001555
83. Yin XM. Chemoreceptors on the maxillary and the labial palpi of cerambycoid (Philus antennatus) adults. J Southwest Agric Univ. (1994) 03):270–2. doi: CNKI:SUN:XNND.0.1994-03-022
84. Faucheux MJ. Sensilla digitiformia and campaniformia on the tip of the piercing ovipositor of Dyseriocrania subpurpurella (Haworth 1828) (Lepidoptera: Eriocraniidae). Annales la Societe Entomologique France. (2008) 44:311–4. doi: 10.1080/00379271.2008.10697568
85. Giglio A, Ferrero EA, Perrotta E, Talarico FF, and Brandmayr TZ. Sensory structures involved in prey detection on the labial palp of the ant-hunting beetle Siagona europaea Dejean 1826 (Coleoptera, Carabidae). Acta Zoologica. (2010) 91:328–34. doi: 10.1111/j.1463-6395.2009.00414.x
86. Faucheux MJ. Biodiversity and unity of sensory organs in Lepidopterous Insects. Nantes, editor. Societe des Sciences Naturelles de l’Ouest de la France (1999). p. 296.
87. Xu LL, Zhang L, Yang YC, Ren LL, Wang T, and Zong SX. Morphology of antennal, maxillary palp and labial palp sensilla in different larval instars of the Asian long–horned beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae). Acta Zoologica. (2017) 98:20–31. doi: 10.1111/azo.12146
88. Eilers EJ, Talarico G, Hansson BS, Hilker M, and Reinecke A. Sensing the underground – ultrastructure and function of sensory organs in root-feeding Melolontha melolontha (Coleoptera: Scarabaeinae) larvae. PloS One. (2012) 7:e41357. doi: 10.1371/journal.pone.0041357
89. Yang YC, Xu LL, and Ren LL. Comparative morphology of sensilla on antenna, maxillary palp and labial palp of larvae of white-spotted and yellow-spotted Asian long-horned beetle, Anoplophora glabripennis Motschulsky (Coleoptera: Cerambycidae). Entomological Res. (2017) 47:3–10. doi: 10.1111/1748-5967.12185
90. Cao YK and Huang M. A SEM study of the antenna and mouthparts of Omosita colon (Linnaeus) (Coleoptera: Nitidulidae). Microscopy Res Technique. (2016) 79:1152–64. doi: 10.1002/jemt.22770
91. Li XG and Zhang KB. Chemoreceptors on the maxillary and labial palps of the adult Anoplophora nobilis. Entomological Knowledge. (1991) 06):357–358, 385. doi: CNKI:SUN:KCZS.0.1991-06-015
Keywords: antennae, maxillary and labial palps, sensilla, Monochamus saltuarius Gebler, scanning electron microscopy (SEM), chemoreceptors
Citation: Wang J, Wang J, Wang J, Jiang X, Ren S and Cao C (2025) Morphology and distribution of sensilla on the antennae and mouthparts of adult Monochamus saltuarius Gebler (Coleoptera: Cerambycidae). Front. Insect Sci. 5:1675406. doi: 10.3389/finsc.2025.1675406
Received: 29 July 2025; Accepted: 30 September 2025;
Published: 21 October 2025.
Edited by:
Anita Giglio, University of Calabria, ItalyReviewed by:
Songqing Wu, Fujian Agriculture and Forestry University, ChinaJolanta Brożek, University of Silesia in Katowice, Poland
Copyright © 2025 Wang, Wang, Wang, Jiang, Ren and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Wang, amp3NjUzNzIzQDEyNi5jb20=
 Jianjun Wang
Jianjun Wang Jianguo Wang1
Jianguo Wang1 Xu Jiang
Xu Jiang Chuanwang Cao
Chuanwang Cao