- 1State Key Laboratory of Wheat Improvement, College of Plant Protection, Shandong Agricultural University, Tai’an, China
- 2Jiaodong Innovation Center, Shandong Institute of Sericulture, Shandong Academy of Agricultural Sciences, Yantai, China
Photoperiod is a critical environmental factor for insect development and physiology, yet little is known about the effects of photoperiodic signals received during photoperiod-sensitive stages on reproductive parameters. The green lacewing, Chrysoperla nipponensis, is a promising candidate for mass rearing in biological control. Photoperiod is the primary environmental factor influencing C. nipponensis reproductive diapause. This study investigates how photoperiodic cues during photoperiod-sensitive stages affect key reproductive parameters such as fecundity, lifespan, oviposition duration, oviposition rate, diapause rate, pre-oviposition period, and lipid content of C. nipponensis. The results showed that short-day conditions (Light:Dark = 9h:15h; L9:D15) during pre-adult stages increase total lipid and triglyceride levels in both third larvae and newly emerged females, thereby enhancing fecundity of female, without reducing lifespan or oviposition. Furthermore, long-day conditions (Light:Dark = 15h:9h; L15:D9) during the pre-adult stage inhibited diapause, while increasing fecundity and extending oviposition duration. Our findings demonstrate that photoperiodic signals during the pre-adult stages significantly affect the reproductive parameters of C. nipponensis, which advances the understanding of photoperiod-dependent reproductive diapause and offers novel insights for optimizing strategies in mass-rearing of natural enemies.
1 Introduction
Green lacewings are widely distributed predatory natural enemies (1–3). Due to their ability to enter diapause, broad prey range, high predation rate and ease of mass-rearing, green lacewings are commercialized and widely used for biological control in various agricultural ecosystems (4–6). To enhance the effectiveness of green lacewings as biological control agents, studies focused on the effects of artificial diets on their reproductive success (7, 8), and others investigated optimal conditions for the cold storage (9, 10). However, the balance between the storage and reproduction in natural enemy insects is crucial for maintaining their supply capacity (4).
The occurrence of diapause provides a natural strategy to facilitate the long-term storage (11). Diapause is a seasonal adaptation in insects, evolved to withstand adverse environmental conditions (12). During diapause, physiological activities such as growth, development, and reproduction are significantly suppressed (13–15). In addition, insects store more nutrients, such as lipids, to prepare for diapause (16, 17). The low metabolism and sufficient nutrient during diapause contributes to extending the lifespan of insects (18, 19).
Photoperiod is a key environmental factor influencing insect diapause (12, 20). Additionally, photoperiod influences the reproductive parameters of insects (21–23). In Holotrichia oblita, as the duration of the dark period increases, the pre-oviposition period shortens, while the oviposition period is extended (24). Otherwise, some insects exhibit the highest fecundity under relatively long photoperiods (21, 25, 26). Therefore, for diapause-capable insects, photoperiod not only directly influences the diapause process but may also affect reproductive parameters.
The green lacewing, Chrysoperla nipponensis, enters diapause as adults, with photoperiod being the primary environmental factor for the induction and maintenance of diapause (27). The third instar larvae, pre-pupae and adult stages of C. nipponensis are all sensitive for photoperiod (28). Reproductive parameters, including fecundity, pre-oviposition period, oviposition period and lifespan, are crucial for developing efficient mass-rearing systems (29, 30). A thorough analysis of C. nipponensis reproductive parameters under varying photoperiods at different developmental stages is essential. Here, we systematically analyzed the effects of long-day and short-day conditions during the larval, pupal and adult stages on C. nipponensis. The results will provide a theoretical basis of enhancing the reproductive potential and developing large-scale artificial breeding for natural enemy insects.
2 Materials and methods
2.1 Insect collection and rearing
The C. nipponensis used in this study was sourced from a stable colony maintained at the Insect Physiology and Ecology Laboratory of Shandong Agricultural University. Eggs were collected by clipping the egg stalks and transferring them to sterile disposable plastic Petri dishes (90 mm × 15 mm). A sufficient quantity of sterilized eggs from Corcyra cephalonica was placed in the dishes as a food source for newly hatched larvae to prevent cannibalism and ensure synchronous development. Then, larvae were individually transferred into cylindrical glass tubes (1 cm in diameter, 7 cm in height). Within these tubes, larvae were fed Megoura japonica reared on broad bean (Vicia faba) plants until pupation. Within 12 hours of adult emergence, male and female adults were paired and housed in canning jars (8 cm in diameter and 10 cm in height). They were fed an artificial diet of yeast powder and powdered sucrose in a 10:8 mass ratio (sucrose ground and sieved through a 60-mesh filter), supplemented with a 10% honey solution. Long-day (LD) conditions were set a 15:9 h light/dark photoperiod, while short-day (SD) conditions were set at a 9:15 h light/dark photoperiod. All rearing conditions were maintained at 25 ± 1 °C and 70 ± 5% relative humidity.
2.2 Quantification of reproductive traits and female lifespan
Newly hatched larvae were divided into six groups (LLL, LLS, LSS, SSS, SSL, and SLL), with larval, pupal, and adult stages exposed to either long-day or short-day conditions in incubators. The treatments are as follows (Figure 1): the LLL group, where the larval, pupal, and adult stages were exposed to long-day conditions; the LLS group, where the larval and pupal stages were exposed to long-day conditions, and the adult stage to short-day conditions; the LSS group, where the larval stage was exposed to a long-day conditions, and the pupal and adult stages to short-day conditions; the SSS group, where the larval, pupal, and the adult stages were exposed to short-day conditions; the SSL group, where the larval and pupal stages were exposed to short-day conditions and the adult stage to long-day conditions; the SLL group, where the larval stage was exposed to short-day conditions, and the pupal and the adult stages to long-day conditions.
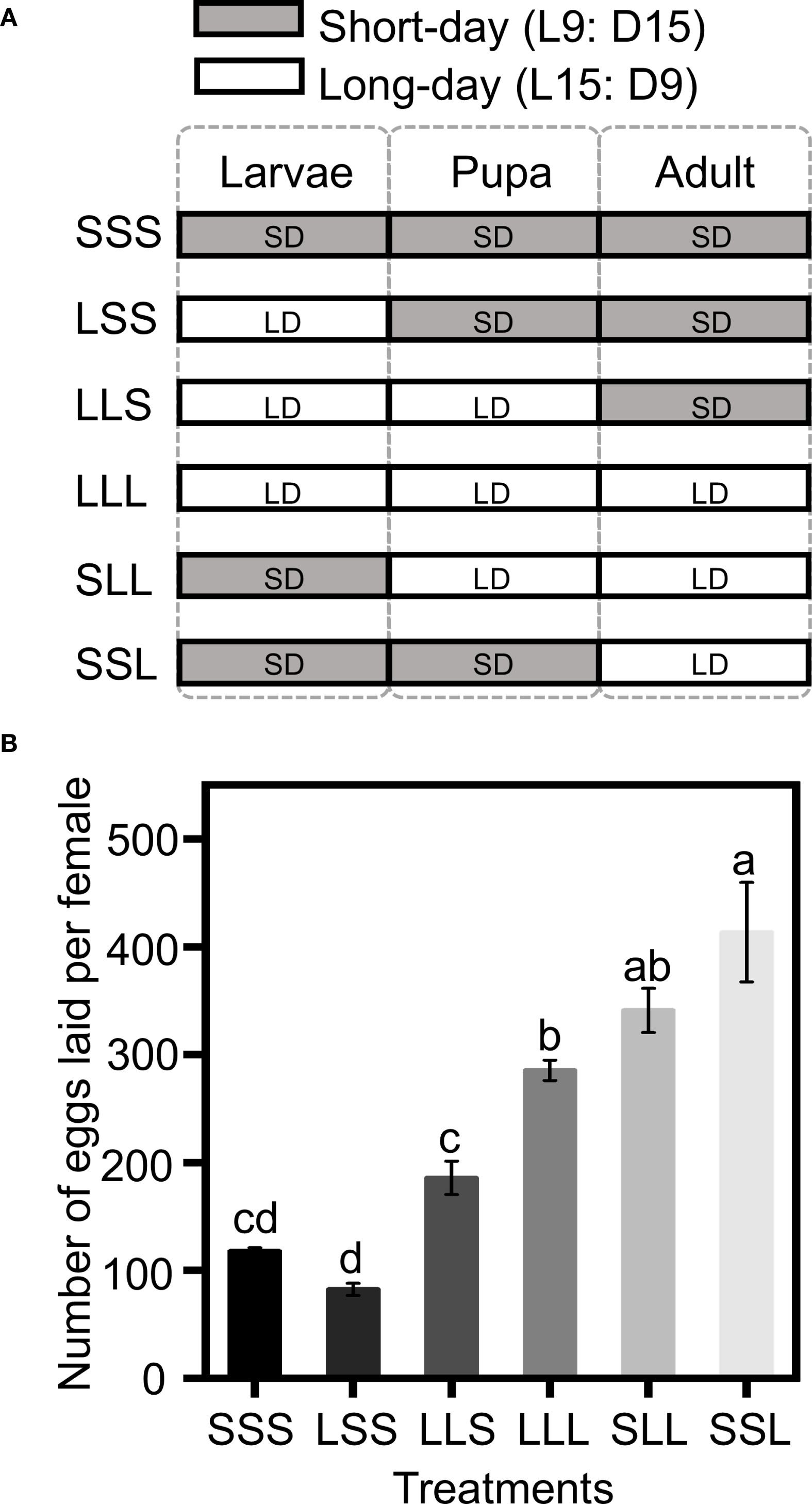
Figure 1. Fecundity with varying photoperiod treatments. (A) Experimental group design diagram. The photoperiod treatments (SSS, SSL, SLL, LLL, LLS, and LSS) were applied to six groups, with each group receiving a specific combination of long-day and short-day conditions for the larval, pupal, and adult stage. The X-axis denotes the developmental stages. The Y-axis represents the photoperiod treatments. SD (short-day condition, L9:D15), LD (long-day condition, L15:D9). (B) Number of eggs laid per female. Data in the figure are presented as Mean ± SEM. Different lowercase letters indicate significant differences among multiple groups according to one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, P < 0.05. n = 30 from 3 independent biological replicates.
To analyze reproductive traits and female lifespan of C. nipponensis under varying photoperiod treatments, the reproductive behavior and developmental progress of female adults were observed and recorded daily until all individuals died. The following parameters were calculated for each treatment: the number of days from adult emergence to the onset of oviposition (pre-oviposition period); the number of days from the beginning to the end of oviposition (oviposition duration); the number of eggs laid by a single female adult (fecundity); and the number of days from female adult emergence to death (lifespan). Each treatment was replicated 3 times, and each replicate included 10 females.
2.3 Measurement of total lipid and triglyceride content
To investigate the mechanisms underlying photoperiod effects on the fecundity of C. nipponensis, total lipid and triglyceride content of third-instar larvae and newly emerged females were measured. The third-instar larvae were reared under constant long-day or short-day conditions from first instar, while newly emerged females were exposed to either long-day or short-day conditions from first instar to eclosion. Each treatment was replicated 4 times, and each replicate included 5 individuals. The total lipid content was determined using the chloroform-methanol extraction method as described by Chen et al. (27). Samples were collected and dried in electrothermal drying oven (GZX-9240MBE, BoXun, China) at 60 °C for 72 h. After drying, dry weight (DW) was measured using the electronic microbalance (A200S, Sartorius, Germany). Meanwhile, C. nipponensis was transferred into a new centrifuge tube, weighed (W1), and then homogenized in 500 μL of chloroform/methanol (2:1, v:v). Samples were centrifuged at 2600 g for 10 min, and the pellets were retained. The process was repeated, and the samples were dried at 60 °C for another 72 h. The dried samples weights (W2) were measured, and the total lipid content was calculated as (W1 − W2)/DW.
Triglyceride content was measured using the liquid triglycerides GPO-PAP method. Weigh the samples and add anhydrous ethanol at a ratio of 1:9 (weight (g) to volume (mL)). Homogenize the samples thoroughly on ice using a motorized homogenizer. Centrifuge at 2,500 rpm for 10 min. The supernatant was used to determine triglyceride content using Triglyceride assay kit (code A110-2-1, Nanjing Jiancheng Institute of Bioengineering, China). Measure the absorbance of each tube at a wavelength of 510 nm using a microplate reader (SYNERGYMx, BioTeK, USA). Triglyceride content (μmol/g) = (Sample OD − Blank OD)/(Calibration OD − Blank OD) × Concentration of Sample.
2.4 Adult reproductive status
To define female adults reproductive status under varying photoperiod treatments, oviposition rate, non-oviposition rate, diapause rate and non-diapause rate were assessed 15 days post-eclosion according to the criteria established by Xu et al. (28). Adults that laid eggs and those which did not lay eggs but their ovaries were at developmental stages III or IV were classified as non-diapause. Adults with less developed ovaries were classified as diapause. The photoperiod treatments are divided into six groups (LLL, LLS, LSS, SSS, SSL, and SLL), as described earlier. Each treatment was replicated 3 times, and each replicate included 10 females.
2.5 Statistical analysis
Statistical analysis was performed by GraphPad Prism 8.0 (GraphPad Software, USA). All data are expressed as mean ± standard error of mean (Mean ± SEM). Differences between two groups were assessed using Student’s t-test (two-tailed, * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001). Fecundity, female lifespan, oviposition duration, and pre-oviposition period of C. nipponensis were compared across varying photoperiod treatments using a one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons (P < 0.05).
3 Results
3.1 Effect of photoperiod treatment on female fecundity
Photoperiod treatments at different developmental stages significantly affected the fecundity of C. nipponensis. Groups in which adults were exposed to long-day conditions (LLL, SLL, SSL) had higher fecundity than groups that exposed to short-day conditions (SSS, LSS, LLS) (F = 41.280; df = 5,174; P <0.001) (Figure 1). Fecundity was 285.30 ± 20.19 eggs for LLL, 340.93 ± 23.50 eggs for SLL and 413.53 ± 21.00 eggs for SSL. Notably, SSL exhibited the highest fecundity among LLL, SLL, and SSL, with fecundity increasing as the duration of short-day exposure prolonged during pre-adult stages. Among SSS, LSS and LLS, LLS exhibited the highest fecundity (185.70 ± 28.31 eggs), followed by SSS (117.67 ± 12.83 eggs) and LSS (82.30 ± 10.34 eggs).
3.2 Effect of photoperiod treatment on female lifespan
Photoperiod treatments at different developmental stages significantly influenced the female lifespan of C. nipponensis, with females exposed to short-day conditions (SSS, LSS, LLS) are all longer than those exposed to long-day conditions (LLL, SLL, SSL) (F = 13.602; df = 5,174; P <0.001) (Figure 2A). The female lifespan of SSS, LSS and LLS was 81.23 ± 2.14 days, 93.47 ± 3.83 days, and 84.63 ± 3.78 days, respectively. The female lifespan of LLL, SLL and SSL were 58.13 ± 3.99 days, 65.20 ± 4.49 days, and 66.07 ± 3.56 days, respectively. In the LLL, SLL, and SSL, female lifespan increased with the duration of exposure to short-day conditions during the pre-adult stages, although no significant differences were observed among the three long-day adult groups.
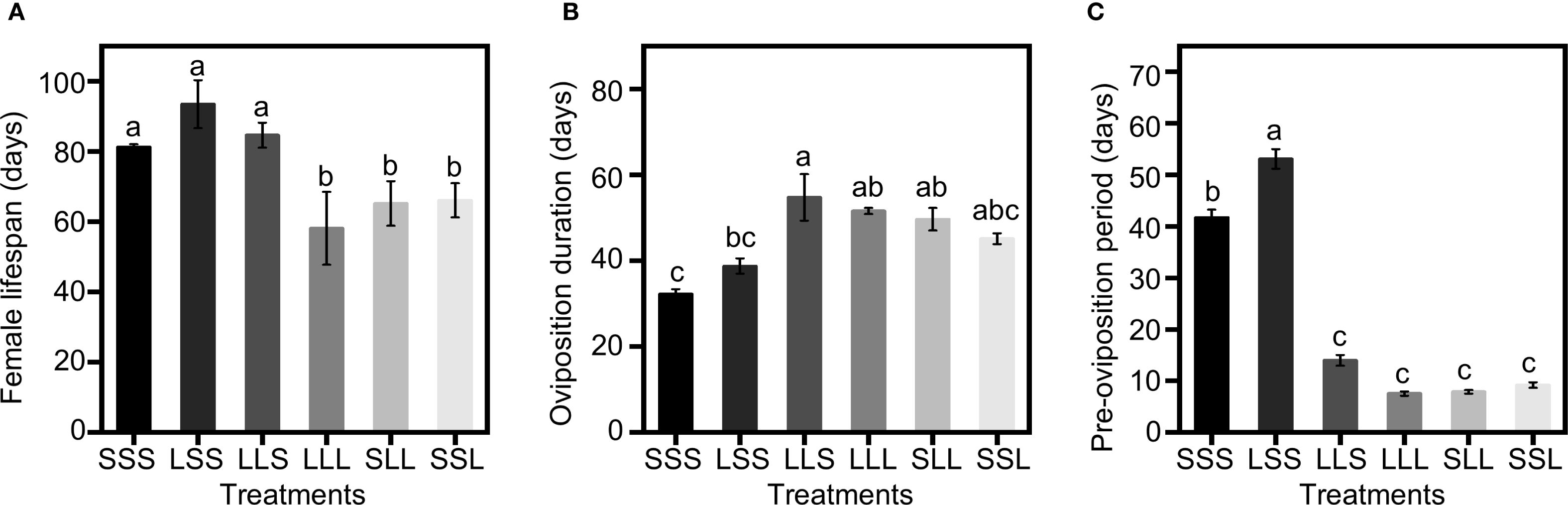
Figure 2. Lifespan, oviposition duration and pre-oviposition period with varying photoperiod treatments. (A) Female lifespan. (B) Oviposition duration. (C) Pre-oviposition period. Data in the figure are presented as Mean ± SEM. Different lowercase letters indicate significant differences among multiple groups according to one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons, P < 0.05. n = 30 from 3 independent biological replicates.
3.3 Effect of photoperiod treatment on oviposition duration
The oviposition duration was significantly influenced by photoperiod treatments at different developmental stages (F = 4.849; df = 5,174; P <0.001) (Figure 2B). The LLS had the longest oviposition duration among all groups, at 54.77 ± 4.84 days. The oviposition duration of SSS was 32.20 ± 1.89 days and LSS was 38.77 ± 3.11 days, showing a decreased trend with increased exposure to short-day conditions. For the LLL, SLL and SSL, the oviposition durations were 51.60 ± 4.58 days, 49.67 ± 4.25 days, and 45.23 ± 3.75 days, respectively, with no significant differences among these groups.
3.4 Effect of photoperiod treatment on pre-oviposition period
Photoperiod had a significant impact on the pre-oviposition period of C. nipponensis (F = 136.727; df = 5,174; P <0.001) (Figure 2C). Among SSS, LSS and LLS, LLS had a significantly shorter pre-oviposition period than SSS and LSS. Among LLL, SLL and SSL, SSL had the longest pre-oviposition period (9.07 ± 0.21 days), compared to LLL (7.57 ± 0.28 days) and SLL (7.90 ± 0.29 days). Notably, the pre-oviposition period of LLS did not differ significantly from groups that adult stages exposed to long-day conditions (LLL, SLL, SSL).
3.5 Effect of photoperiod treatment on female reproductive status
To further investigate the impact of photoperiod on reproductive status, we assessed the ovarian development and egg-laying behavior of females after varying photoperiod treatments (Table 1). Females exposed to long-day conditions (LLL, SLL, SSL) exhibited 0% diapause rate and 100% oviposition rate. In contrast, females under short -day conditions (SSS, LSS) during the adult stage entered diapause with 100% diapause rate and 0% oviposition rate. Additionally, in LLS, 13.33 ± 0.33% of adults entered diapause, while 86.67 ± 0.33% were non-diapausing. Regarding reproductive behavior, 80.00 ± 0.06% of adults laid eggs, whereas 20.00% did not oviposit.
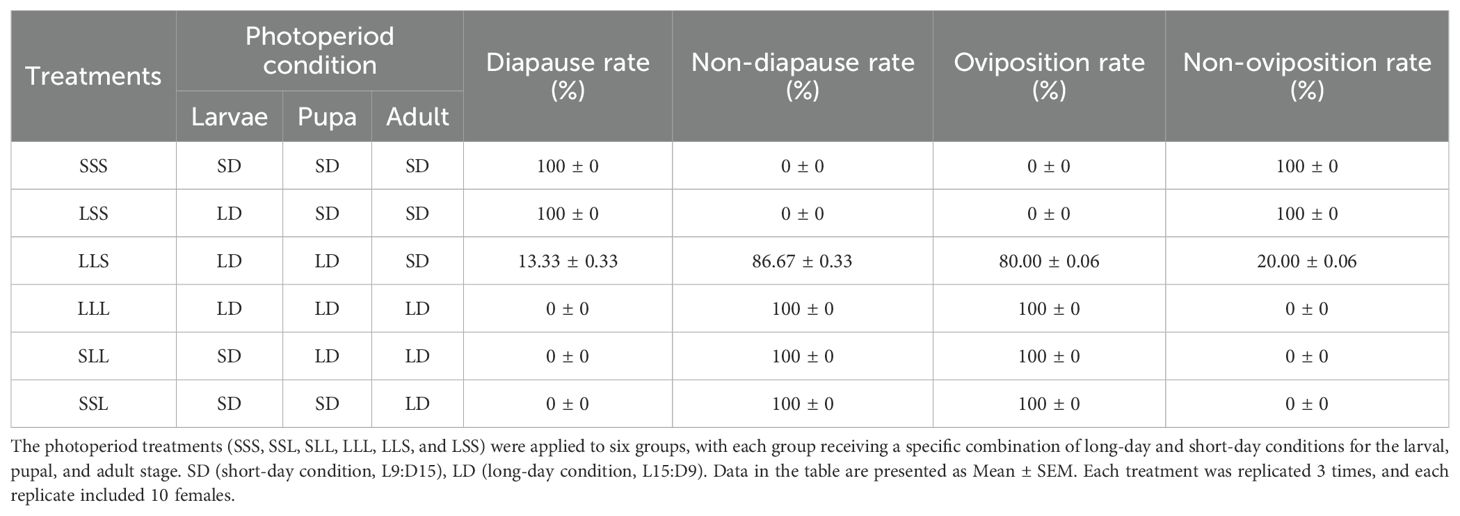
Table 1. Adult reproductive status of C. nipponensis at different developmental stages under long-day or short-day conditions.
3.6 Effect of photoperiod treatment on lipid content
Photoperiod significantly affected total lipid and triglyceride content in both third-instar larvae and newly emerged females (Figure 3). In third-instar larvae, short-day conditions significantly increased total lipid content (t = 4.28, df = 6, P = 0.005), from 656.82 ± 6.93 μg/mg under long-day conditions to 852.70 ± 45.23 μg/mg under short-day conditions (Figure 3A). Similarly, triglyceride content also significantly increased from 47.66 ± 2.16 μmol/mg under long-day conditions to 79.23 ± 0.88 μmol/mg under short-day conditions in third-instar larvae (t = 13.528, df = 6, P < 0.0001) (Figure 3A). In newly emerged females, total lipid content significantly increased from 261.00 ± 8.23 μg/mg under long-day conditions to 370.67 ± 13.10 μg/mg under short-day conditions (t = 7.090, df = 6, P = 0.0004) (Figure 3B). Similarly, triglyceride content significantly increased from 16.24 ± 0.49 μmol/mg under long-day conditions to 21.24 ± 0.48 μmol/mg under short-day conditions (t = 7.294, df = 6, P = 0.0003) (Figure 3B).
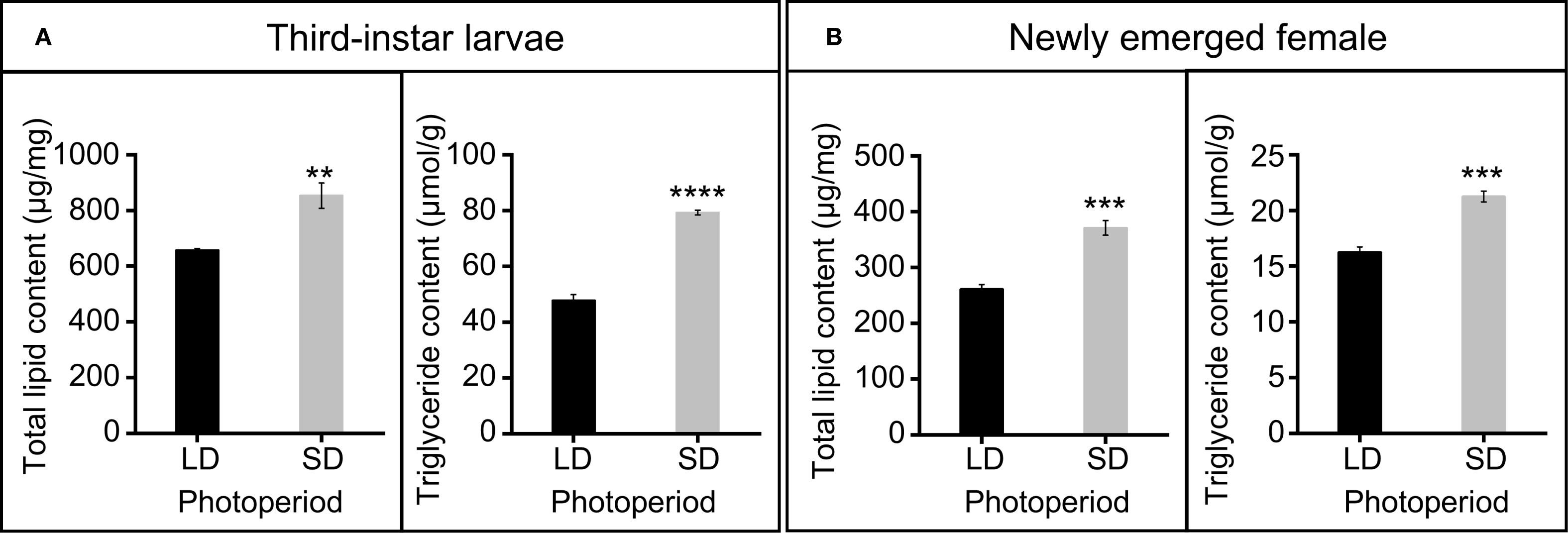
Figure 3. Total lipid and triglyceride content under long-day and short-day conditions. (A) Total lipid and triglyceride content of third larvae. (B) Total lipid and triglyceride content of newly emerged female. Data in the figure are presented as Mean ± SEM. Differences between two groups were analyzed using Student’s t-test (In A, **P = 0.005, ****P < 0.0001, respectively; In B, ***P = 0.0004, ***P = 0.0003, respectively). SD (short-day condition, L9:D15), LD (long-day condition, L15:D9). n = 4 independent biological replicates.
4 Discussion
Insects typically show higher fecundity under long-day conditions than short-day conditions (31–33). For instance, extending the photoperiod beyond 12:12 (light:dark) improves progeny yield in Telenomus remus (34). Our study confirms that females exposed to long-day conditions during the adult stage exhibited higher fecundity than those under short-day conditions, regardless of the photoperiod during the pre-adult stages (Figure 1). High fecundity is typically at the expense of the lifespan (35). In C. nipponensis, we observed shorter lifespan in adult exposed to long-day conditions (LLL, SLL, SSL) than those exposed to short-day conditions (SSS, LSS, LLS). However, among LLL, SLL and SSL, short-day conditions during pre-adult stages significantly increased fecundity (Figure 1), without shorten the lifespan and oviposition duration (Figure 2). These findings could be utilized to improve fecundity of enemy insects.
Lipid accumulation is a physiological response in insects to diapause-inducing signals (27, 36). Lipid reserves primarily accumulate as triglycerides (37), which are crucial for oogenesis, embryogenesis, and egg maturation (38). The fecundity of insects is associated with lipid content (39–41). Larval and pupal stages of C. nipponensis response short photoperiod then increase the lipid content (Figure 3), which may be a critical factor contributing to enhanced fecundity. Short-day conditions also significantly affect the expression of genes involved in lipid synthesis and metabolism in C. nipponensis larvae (42), providing molecular support for our findings.
As the depth of diapause induction increases, energy is stored and gradually consumed to sustain diapause until termination (43, 44). Adults exposed to short-day conditions have a longer lifespan than those under long-day conditions (Figure 2), which benefits the shelf-life of natural enemies, but their fecundity is reduced (Figure 1). Diapause development depletes nutrients, thereby reducing the energy available for reproduction (45). Notably, the LLS, exposed to long-day conditions during the pre-adult stage, had a longer oviposition period (Figure 2) and higher fecundity compared to SSS (Figure 1). Additionally, the LSS, which under long-day conditions during the larval stage, exhibited a longer lifespan than the SSS group (Figure 2). However, the optimal photoperiod transition to enhance fecundity and extend the shelf-life of natural enemies remains to be determined.
Diapause is a clear advantage in improve shelf-life and long-distance shipment in biological control (9, 46). The accumulation of diapause stimuli during sensitive stages maximizes the diapause response in Anastatus japonicus and Tetrastichus septentrionalis (47, 48). In C. nipponensis the photoperiod during the adult stage is the most critical factor determining reproductive diapause (28). Although the third-larval and pupal stages are also photoperiod-sensitive, short-day conditions during these stages alone do not induce diapause but extend the pre-oviposition period (Figure 2). Moreover, short-day conditions during the pre-adult stage promote diapause whereas long-day conditions reduce diapause incidence (Table 1). Similar phenomena have been observed in Laodelphax striatellus and Chrysopa downesi (49, 50). Therefore, photoperiod signals during pre-adult stages accumulate into the adult stage, influencing reproduction and diapause in C. nipponensis. Our findings demonstrated that complete diapause does not necessarily require short photoperiods throughout the entire developmental period. Diapause induction that begins in pupal stage is sufficient to induce 100% diapause of adults in C. nipponensis.
In summary, photoperiodic signals during pre-adult stages accumulate and influence reproductive and diapause biology of C. nipponensis. Most notably, short-day conditions during the pre-adult stage promote lipid accumulation and enhance fecundity of females. Specifically, the accumulation of short-day conditions stimuli during pupal stage is essential to maximize the diapause response. As is common, the occurrence of diapause facilitates the storage of natural enemy insect products, while balancing reproduction and storage is crucial for commercial applications. Our findings provide insights for advancing fundamental research on reproduction and supporting the large-scale production and application of lacewings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
XK: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MX: Investigation, Methodology, Writing – review & editing. HL: Formal analysis, Visualization, Writing – review & editing. SZ: Validation, Writing – review & editing. DL: Visualization, Writing – review & editing. YX: Funding acquisition, Resources, Supervision, Writing – review & editing. ZC: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2023YFD1700405), the National Science Foundation of Shandong Province (ZR2022MC179) and the National Natural Science Foundation of China (315015904).
Acknowledgments
We are grateful to Jeremiah Joe Kabissa for editing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang NW, Zang LS, Wang S, Guo JY, Xu HX, Zhang F, et al. Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol Control. (2014) 68:92–102. doi: 10.1016/j.biocontrol.2013.06.012
2. Alcalá Herrera R, Cotes B, Agustí N, Tasin M, and Porcel M. Using flower strips to promote green lacewings to control cabbage insect pests. J Pest Sci. (2022) 95:669–83. doi: 10.1007/s10340-021-01419-7
3. Wang XY, Ma H, Zhao YC, Gao Y, and Wu KM. Abundance and seasonal migration patterns of green lacewings (Neuroptera: Chrysopidae) across the Bohai Strait in Eastern Asia. Insects. (2024) 15:321. doi: 10.3390/insects15050321
4. Tauber MJ, Tauber CA, Daane KM, and Hagen KS. Commercialization of predators: recent lessons from green lacewings (Neuroptera: Chrysopidae : Chrosoperla). Am Entomol. (2000) 46:26–38. doi: 10.1093/ae/46.1.26
5. Dami BG, Dos Santos JA, Barbosa EP, Rodriguez-Saona C, and Vacari AM. Functional response of 3 green lacewing species (Neuroptera: Chrysopidae) to Leucoptera coffeella (Lepidoptera: Lyonetiidae). J Insect Sci. (2023) 23:15. doi: 10.1093/jisesa/iead038
6. Berteloot OH, Peusens G, Beliën T, Van Leeuwen T, and De Clercq P. Predation efficacy of Chrysoperla carnea on two economically important stink bugs. Biol Control. (2024) 196:105586. doi: 10.1016/j.biocontrol.2024.105586
7. Gonzalez D, Nave A, Gonçalves F, Nunes FM, Campos M, and Torres L. Effects of ten naturally occurring sugars on the reproductive success of the green lacewing. Chrysoperla carnea Biocontrol. (2016) 61:57–67. doi: 10.1007/s10526-015-9694-z
8. Ye JW, Li J, Li ZG, and Han SC. Rearing of Mallada basalis (Neuroptera: Chrysopidae) on modified artificial diets. PloS One. (2017) 12:e0185223. doi: 10.1371/journal.pone.0185223
9. Li YY, Wang MZ, Gao F, Zhang HZ, Chen H, Wang MQ, et al. Exploiting diapause and cold tolerance to enhance the use of the green lacewing Chrysopa formosa for biological control. Biol Control. (2018) 127:116–26. doi: 10.1016/j.biocontrol.2018.08.024
10. Zhang TT, Zhang GC, Zhang LS, Chen HY, Wang MQ, Liu CX, et al. Effects of cold storage on quality of Chrysopa pallens and recovery of fecundity by insulin. Sci Rep. (2019) 9:5311. doi: 10.1038/s41598-019-41618-y
11. Quinn NF, Robertson RR, and Duan JJ. Effect of storage conditions on host egg suitability and the reproductive fitness of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid of the invasive emerald ash borer (Agrilus planipennis) (Coleoptera: Buprestidae). Environ Entomol. (2024) 53:946–53. doi: 10.1093/ee/nvae081
12. Saunders DS. Dormancy, diapause, and the role of the circadian system in insect photoperiodism. Annu Rev Entomol. (2020) 65:373–89. doi: 10.1146/annurev-ento-011019-025116
13. Denlinger DL and Armbruster PA. Mosquito diapause. Annu Rev Entomol. (2014) 59:73–93. doi: 10.1146/annurev-ento-011613-162023
14. Duan TF, Gao SJ, Wang HC, Li L, Li YY, Tan Y, et al. MicroRNA let-7-5p targets the juvenile hormone primary response gene Krüppel homolog 1 and regulates reproductive diapause in Galeruca daurica. Insect Biochem Mol Biol. (2022) 142:103727. doi: 10.1016/j.ibmb.2022.103727
15. Hand SC, Denlinger DL, Podrabsky JE, and Roy R. Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish. Am J Physiol Regul Integr Comp Physiol. (2016) 310:R1193–1211. doi: 10.1152/ajpregu.00250.2015
16. Short CA and Hahn DA. Fat enough for the winter? Does nutritional status affect diapause? J Insect Physiol. (2023) 145:104488. doi: 10.1016/j.jinsphys.2023.104488
17. Wang X, Xu M, Kong X, Zhong S, Kabissa JJ, Li D, et al. The role of insulin receptor InR in photoperiod-regulated reproductive diapause of Chrysoperla nipponensis. Insect Biochem Mol Biol. (2025) 180:104305. doi: 10.1016/j.ibmb.2025.104305
18. Sim C and Denlinger DL. Insulin signaling and the regulation of insect diapause. Front Physiol. (2013) 4:189. doi: 10.3389/fphys.2013.00189
19. Su XL, Su ZR, and Xu WH. The protease Lon prolongs insect lifespan by responding to reactive oxygen species and degrading mitochondrial transcription factor A. Biochim Biophys Acta-Mol Cell Res. (2024) 1871:119648. doi: 10.1016/j.bbamcr.2023.119648
20. Miki T, Shinohara T, Chafino S, Noji S, and Tomioka K. Photoperiod and temperature separately regulate nymphal development through JH and insulin/TOR signaling pathways in an insect. Proc Natl Acad Sci. (2020) 117:5525–31. doi: 10.1073/pnas.1922747117
21. Greenberg SM, Sappington TW, Adamczyk JJ, Liu TX, and Setamou M. Effects of photoperiod on boll weevil (Coleoptera: Curculionidae) development, survival, and reproduction. Environ Entomol. (2008) 37:1396–402. doi: 10.1603/0046-225X-37.6.1396
22. Luker LA, Hatle JD, and Juliano SA. Reproductive responses to photoperiod by a south Florida population of the grasshopper Romalea microptera (Orthoptera: Romaleidae). Environ Entomol. (2002) 31:702–7. doi: 10.1603/0046-225X-31.4.702
23. Nissinen AI, Pinto-Zevallos DM, Jauhiainen L, and Vänninen I. The effect of photoperiod and light quality on Macrolophus pygmaeus Rambur (Hemiptera: Miridae) nymphal development, fecundity and longevity. Biol Control. (2017) 108:30–9. doi: 10.1016/j.biocontrol.2017.02.001
24. Xie MH, Zhong YZ, Lin LL, Zhang GL, Su WH, Ni WL, et al. Effect of photoperiod on longevity, food consumption, and reproduction of Holotrichia oblita (Coleoptera: Scarabaeidae). Environ Entomol. (2021) 50:1151–7. doi: 10.1093/ee/nvab066
25. Karmakar A, Mobarak SH, Koner A, Mitra P, and Barik A. Effects of photoperiods on demography and population growth of Aulacophora foveicollis Lucas reared on Solena amplexicaulis plant. Int J Trop Insect Sci. (2021) 41:1407–18. doi: 10.1007/s42690-020-00335-0
26. Singh N, Mishra G, and Omkar. Effect of photoperiod on slow and fast developing individuals in aphidophagous ladybirds, Menochilus sexmaculatus and Propylea dissecta (Coleoptera: Coccinellidae). Insect Sci. (2016) 23:117–33. doi: 10.1111/1744-7917.12182
27. Chen ZZ, Wang X, Kong X, Zhao YM, Xu MH, Gao YQ, et al. Quantitative transcriptomic and proteomic analyses reveal the potential maintenance mechanism of female adult reproductive diapause in. Chrysoperla nipponensis Pest Manag Sci. (2023) 79:1897–911. doi: 10.1002/ps.7375
28. Xu YY, Mu JY, Hu C, and Wang HG. Photoperiodic control of adult diapause in Chrysoperla sinica (Tjeder) (Neuroptera: Chrysopidae)—I. Critical photoperiod and sensitive stages of adult diapause induction. Insect Sci. (2004) 11:191–8. doi: 10.1111/j.1744-7917.2004.tb00239.x
29. Gonzalez N, Fournier M, Buitenhuis R, and Lucas E. Artificial adult diet as a new tool for improving a biocontrol program with predatory hoverflies. Agriculture. (2024) 14:527. doi: 10.3390/agriculture14040527
30. Ouattara TY, Fournier M, Gonzalez N, Rojo S, and Lucas E. Reproductive parameters of a new biocontrol agent, Eupeodes americanus (Diptera: Syrphidae) and comparison with the commercialized Aphidoletes aphidimyza (Diptera: Cecidomyiidae). J Econ Entomol. (2024) 117:1760–8. doi: 10.1093/jee/toae133
31. Carvalho AR, Bueno VHP, Pedroso EC, Kon LI, Diniz AJF, and Silva RJ. Influence of photoperiod on Orius thyestes Herring (Hemiptera: Anthocoridae) reproduction and longevity. Neotrop Entomol. (2006) 35:489–92. doi: 10.1590/S1519-566X2006000400010
32. Fantinou AA, Perdikis DCH, and Zota KF. Reproductive responses to photoperiod and temperature by diapausing and nondiapausing populations of Sesamia nonagrioides Lef. (Lepidoptera–Noctuidae). Physiol Entomol. (2004) 29:169–75. doi: 10.1111/j.1365-3032.2004.00381.x
33. Yu HX, Yuan XJ, Xie ZQ, Zhang QQ, Zheng CY, and Sun LJ. A long photoperiod promoted the development, reproduction, and predation of Harmonia axyridis Pallas (Coleoptera: Coccinellidae) at an average greenhouse temperature during the winter. Insects. (2024) 15:214. doi: 10.3390/insects15040214
34. Chen W, Weng Q, Nie R, Zhang H, Jing X, Wang M, et al. Optimizing photoperiod, exposure time, and host-to-parasitoid ratio for mass-rearing of Telenomus remus, an egg parasitoid of Spodoptera frugiperda, on Spodoptera litura eggs. Insects. (2021) 12:1050. doi: 10.3390/insects12121050
35. Haeler E, Fiedler K, and Grill A. What prolongs a butterfly’s life?: Trade-offs between dormancy, fecundity and body size. PloS One. (2014) 9:e111955. doi: 10.1371/journal.pone.0111955
36. Zhu L, Tian Z, Guo S, Liu W, Zhu F, and Wang XP. Circadian clock genes link photoperiodic signals to lipid accumulation during diapause preparation in the diapause-destined female cabbage beetles. Colaphellus bowringi Insect Biochem Mol Biol. (2019) 104:1–10. doi: 10.1016/j.ibmb.2018.11.001
37. Cedden D, Güney G, and Toprak U. The integral role of de novo lipogenesis in the preparation for seasonal dormancy. Proc Natl Acad Sci. (2024) 121:e2406194121. doi: 10.1073/pnas.2406194121
38. Heier C and Kühnlein RP. Triacylglycerol metabolism in Drosophila melanogaster. Genetics. (2018) 210:1163–84. doi: 10.1534/genetics.118.301583
39. Cao J and Ling L. miRNA-GABA influences female Aedes aEgypti reproduction by modulating midgut homeostasis. Cell Rep. (2025) 44:115776. doi: 10.1016/j.celrep.2025.115776
40. Morales-Ramos JA and Rojas MG. Importance of lipids for queen fecundity and colony growth of Coptotermes formosanus (Isoptera: Rhinotermitidae). Environ Entomol. (2007) 36:1014–7. doi: 10.1603/0046-225X(2007)36[1014:IOLFQF]2.0.CO;2
41. Xu J, Tang Y, Jin Y, Ma T, Zhang C, Lou J, et al. Knockdown of FAS2 impairs fecundity by inhibiting lipid accumulation and increasing glycogen storage in Locusta migratoria. Insects. (2025) 16:120. doi: 10.3390/insects16020120
42. Liu SY, Gao YQ, Shi R, Huang HY, Xu YY, and Chen ZZ. Transcriptomics provide insights into the photoperiodic regulation of reproductive diapause in the green lacewing, Chrysoperla nipponensis (Okamoto) (Neuroptera: Chrysopidae). Insects. (2024) 15:136. doi: 10.3390/insects15020136
43. Kostál V, Tamura M, Tollarová M, and Zahradnícková H. Enzymatic capacity for accumulation of polyol cryoprotectants changes during diapause development in the adult red firebug. Pyrrhocoris apterus Physiol Entomol. (2004) 29:344–55. doi: 10.1111/j.0307-6962.2004.00396.x
44. Kostál V. Eco-physiological phases of insect diapause. J Insect Physiol. (2006) 52:113–27. doi: 10.1016/j.jinsphys.2005.09.008
45. Hahn DA and Denlinger DL. Energetics of insect diapause. Annu Rev Entomol. (2011) 56:103–21. doi: 10.1146/annurev-ento-112408-085436
46. Zhao C, Xia Y, Xiao JJ, Liu ZX, Zhang BX, and Li DS. Advantages of diapause in Anastatus japonicus Ashmead mass production on eggs of the Chinese oak silkworm, Antheraea pernyi. Pest Manag Sci. (2024) 80:756–62. doi: 10.1002/ps.7807
47. Li ZX, Shi JR, Yang LY, Cheng Y, Liu XD, and Sun SH. Diapause induction, color changes, and supercooling point of diapause larvae of Tetrastichus septentrionalis Yang (Hymenoptera: Eulophidae). Insects. (2023) 14:826. doi: 10.3390/insects14100826
48. Zhao C, Guo Y, Liu ZX, Xia Y, Li YY, Song ZW, et al. Temperature and photoperiodic response of diapause induction in Anastatus japonicus, an egg parasitoid of stink bugs. Insects. (2021) 12:872. doi: 10.3390/insects12100872
49. Hou YY, Xu LZ, Wu Y, Wang P, Shi JJ, and Zhai BP. Geographic variation of diapause and sensitive stages of photoperiodic response in Laodelphax striatellus Fallén (Hemiptera: Delphacidae). J Insect Sci. (2016) 16:13. doi: 10.1093/jisesa/iev161
Keywords: photoperiod, reproduction, diapause, green lacewing, fecundity
Citation: Kong X, Xu M, Li H, Zhong S, Li D, Xu Y and Chen Z (2025) Effects of pre-adult photoperiod experience on reproductive parameters of Chrysoperla nipponensis (Tjeder): potential implications for mass-rearing of natural enemies. Front. Insect Sci. 5:1680910. doi: 10.3389/finsc.2025.1680910
Received: 06 August 2025; Accepted: 04 September 2025;
Published: 25 September 2025.
Edited by:
Xiaofeng Xia, Fujian Agriculture and Forestry University, ChinaReviewed by:
Yao Zhichao, Yangzhou University, ChinaGanyu Zhang, Tea Research Institute of Shandong Academy of Agricultural Sciences, China
Copyright © 2025 Kong, Xu, Li, Zhong, Li, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenzhen Chen, Y2hlbnp6MDMyN0AxNjMuY29t
 Xue Kong1
Xue Kong1 Yongyu Xu
Yongyu Xu Zhenzhen Chen
Zhenzhen Chen