- 1College of Plant Protection, Yangzhou University, Yangzhou, China
- 2College of Fine Arts and Design, Yangzhou University, Yangzhou, China
- 3Yantai Forestry Bureau, Yantai Forest Resources Monitoring and Protection Service Center, Yantai, China
The brown planthopper, Nilaparvata lugens is a major rice pest in Asia, with its high fecundity contributing to recurrent outbreaks. G protein-coupled receptors (GPCRs) are a critical class of transmembrane proteins in insects that sense diverse extracellular and intracellular signals and regulate a wide range of physiological processes. In this study, we characterized GPCR A35 and its function in N. lugens fecundity. Expression profiling revealed that GPCR A35 was highly enriched in female heads and fat bodies, with peak levels in females at 4 days post-eclosion. RNAi-mediated the silencing of GPCR A35 in fifth-instar nymphs by 57–60%, and was effectively delivered to female adults, resulting in a 14.8% reduction in juvenile hormone (JH) titer and marked downregulation of JH biosynthetic and signaling genes, including HMGCR (−60.1%), FPPS (−57.0%), JHAMT (−52.7%), Met (−24.2%), and Kr-h1 (−78.3%). Silencing of GPCR A35 further decreased Vg and VgR expression by 82.1% and 72.9% in females at 4 days post-eclosion, reduced protein contents in fat body and ovaries, and impaired ovarian development with fewer mature oocytes. Consequently, female fecundity declined by 51.3%, oviposition duration shortened by 18.5%, and the F1 population growth index decreased by 46.8%. These results demonstrate that GPCR A35 regulates fecundity in N. lugens by modulating JH-mediated vitellogenesis and oogenesis, providing a novel molecular target for RNAi-based green pest control.
1 Introduction
G protein-coupled receptors (GPCRs) are a critical class of transmembrane proteins in insects that sense diverse extracellular and intracellular signals through their seven-transmembrane helix architecture and transduce these signals into intracellular responses, thereby regulating a wide range of physiological processes (1, 2). As central components of the insect neuroendocrine system and signaling networks, GPCRs are widely distributed across the central nervous system, digestive system, reproductive organs, and sensory structures, where they play essential roles (3). Primarily, GPCRs mediate neurotransmitter and hormonal signaling by recognizing and responding to various neuropeptides, and hormones, and further regulating development, molting, reproduction, and metabolic homeostasis (1, 3–5). Generally, GPCRs are categorized using two widely adopted classification frameworks: the A–F system and the GRAFS system (3). The A–F system categorizes receptors into six groups (A–F) based on sequence homology and functional similarity (6). Among these, Class A, or the “rhodopsin-like” family, is the most abundant; Class B represents the “secretin receptor” family; Class C includes metabotropic glutamate receptors; Class D corresponds to fungal pheromone receptors; Class E encompasses cAMP receptors; and Class F comprises frizzled and smoothened receptors (7–10). By contrast, the GRAFS system was established based on phylogenetic analyses of human GPCRs, dividing them into five families: glutamate (G), rhodopsin (R), adhesion (A), frizzled/taste2 (F), and secretin (S). In insects, classification has primarily followed the A–F scheme (11). A significant breakthrough in insect GPCR research came with the sequencing of the Drosophila melanogaster genome, which provided the first comprehensive receptor inventory in an insect model (12). Since then, genomic data from more than 100 insect species, including Anopheles gambiae, Aedes aegypti, Culex quinquefasciatus, Bombyx mori, Nilaparvata lugens, and others, have been released (13–18). The continued expansion of genomic resources has enabled more accurate annotation and comparative analyses, offering critical insights into the evolutionary diversification of GPCRs and laying a solid foundation for elucidating their diverse roles in insect physiology and molecular biology (19, 20).
Reproduction is a fundamental biological process that underpins insect survival, population persistence, and evolutionary adaptation (21, 22). It is orchestrated by complex neuroendocrine signaling networks that regulate mating behavior, gametogenesis, fertilization, and oviposition in response to internal physiological states and external environmental conditions (23). These reproductive events are closely linked to hormonal control and energy allocation, reflecting the trade-off between reproductive investment and survival under variable ecological conditions (21, 24). Insect reproduction is orchestrated by a complex interplay of endocrine and signaling pathways, including juvenile hormone (JH), ecdysteroids, and insulin/IGF signaling (24, 25). Among these, JH signaling represents a pivotal regulatory signaling, particularly in female reproductive physiology (23, 26). Through its receptor Methoprene-tolerant (Met) and the coactivator Taiman (Tai), JH governs the transcriptional activation of genes involved in vitellogenin synthesis, follicular maturation, and ovarian development (27, 28). Although the relative contribution of these pathways varies across insect orders—for instance, JH acts synergistically with insulin signaling in Coleoptera and coordinates preparatory post-eclosion processes in Diptera—reproduction in Hemiptera is primarily under JH control (27, 29). In this order, JH functions as the principal gonadotropic signal, driving vitellogenesis and oocyte maturation, thereby establishing itself as the central endocrine regulator of female reproduction (30, 31). GPCRs play pivotal roles in insect reproduction, particularly as mediators linking JH signaling to vitellogenin synthesis and uptake (5, 32, 33). In panoistic ovary insects such as Locusta migratoria, systematic RNA interference (RNAi) screening has identified multiple GPCRs essential for vitellogenesis and oocyte maturation; their knockdown markedly impairs yolk protein synthesis and ovarian development (5). In the coleopteran Tribolium castaneum, GPCRs are critical for vitellogenin uptake into oocytes, with receptors such as TcRh2 (Rhodopsin-like) and TcD2R (dopamine D2-like) being indispensable for yolk accumulation and follicle growth. Notably, TcD2R mediates non-genomic JH signaling, as JH treatment in heterologous systems induces dose-dependent changes in intracellular cAMP, suggesting that membrane-associated GPCRs regulate follicular patency and yolk protein uptake (33, 34). Collectively, these findings indicate that JH modulates insect reproduction through classical nuclear receptor pathways and GPCR-mediated membrane signaling (35), and this dual mechanism highlights the GPCRs as promising targets for novel reproductive interference strategies in pest management.
The brown planthopper, N. lugens is a highly destructive pest in rice cropping systems, characterized by strong host specificity, high fecundity, rapid development, and substantial migratory capacity (36–38). Both nymphs and adults feed on rice phloem, causing direct damage such as yellowing and wilting, as well as indirect effects including transmission of rice viruses, collectively leading to significant yield losses (39, 40). Conventional control relies heavily on chemical insecticides. However, widespread pesticide use has resulted in insecticide resistance, disruption of natural enemy populations, and unintended ecological imbalances (36, 41). Consequently, planthopper populations often undergo resurgence, manifesting as acute outbreaks in the first generation following broad-spectrum insecticide applications and as chronic outbreaks in subsequent generations induced by modern low-toxicity pesticides that stimulate reproduction, enhance nutrient availability, and alter gene expression linked to mating and oviposition (41–44). Our previous studies demonstrated that application of the fungicide jinggangmycin (JGM), commonly used to control rice sheath blight, increases glucose levels in rice plants, which in turn stimulates reproduction in N. lugens feeding on JGM-treated rice (41, 45). Transcriptome analysis revealed a significant upregulation of the G protein-coupled receptor GPCR A35, suggesting its potential role in regulating planthopper reproduction. In the present study, RNA interference (RNAi)-mediated silencing of GPCR A35 confirmed that this receptor modulates reproduction by mediating JH synthesis and associated signaling pathways, which further influence ovarian development and vitellogenin accumulation in adults. These findings provide a novel molecular basis for understanding insect reproductive regulation and offer a potential target for developing environmentally friendly strategies to suppress planthopper reproduction.
2 Materials and methods
2.1 Insect culture
Populations of N. lugens were originally sourced from the China National Rice Research Institute (CNRRI, Hangzhou, China). Colonies were continuously maintained on rice plants (Oryza sativa cv. NanGeng 9108) under controlled environmental conditions of 27 ± 2 °C, 70 ± 10% relative humidity, and a 16:8 h light:dark cycle, following the rearing protocol described by Wu et al. (2024) (46).
2.2 The developmental and tissue-specific expression analysis of GPCR A35 in N. lugens
Developmental samples of N. lugens were collected across multiple life stages, including eggs, first to fifth instar nymphs, and both female and male adults (24 and 48 h post-emergence). Each developmental stage was represented by 10–20 individuals, with 3 biological replicates. To investigate the tissue-specific expression pattern of GPCR_A35, six representative tissues, including the head, midgut, fat body, ovary, cuticle, and feet were dissected from 20 female adults (2 day post-eclosion) under a stereomicroscope on ice using sterilized scalpels and forceps. The selection of these tissues was based on their distinct physiological functions and potential relevance to GPCR signaling (47). Each tissue sample was collected in triplicate from 60 females. Samples were immediately frozen in liquid nitrogen and stored at -80 °C until RNA extraction. The PrimeScriptTM 1st Strand cDNA Synthesis Kit (TaKaRa, Beijing, China) produced the first-strand cDNA based on the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was carried out using a CFX96 real-time PCR detection system (Bio-Rad, CA, USA) in 10 μL reaction volumes containing 5 μL of 2× SYBR Premix Ex Taq II (Takara, Tokyo, Japan), 0.4 μL each of forward and reverse primers (10 μM), 1 μL of cDNA template, and 3.6 μL of nuclease-free water. The thermal cycling conditions were: 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, 58 °C for 30 s, and 72 °C for 20 s, with a final extension at 72 °C for 10 min. A melting curve analysis was subsequently performed from 60 to 95 °C to confirm amplification specificity. Primers for GPCR A35 were designed using Primer3Plus (https://www.primer3plus.com/) and are provided in Supplementary Table S1. Nlβ-actin served as the internal reference gene (48), and relative transcript levels were calculated using the 2−ΔΔCT method (45, 49).
2.3 The synthesis of dsRNA and microinjection
Double-stranded RNA (dsRNA) targeting GPCR A35 was generated using primers containing T7 promoter sequences, following the strategy described by Wu et al. (2024) (46). dsRNA targeting green fluorescent protein (GFP) was synthesized as a negative control. All dsRNAs were prepared with the T7 RiboMAX™ Express RNAi System (Promega, Madison, WI, USA). PCR templates for dsRNA synthesis were amplified in a Bio-Rad thermal cycler under the following program: 35 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 45 s, with a final elongation step at 72 °C for 10 min. PCR products were purified using commercial kits (Novozymes Biotechnology, Nanjing, China) and subsequently used for dsRNA transcription. The resulting dsRNAs were stored at −80 °C until further use.
For RNAi assays, the fifth instar nymphs were anesthetized with CO2 and injected with 40, 60, 80, 100, and 120 ng of dsA35 or dsGFP into the mid-thorax using a Nanoject II microinjector (Drummond Scientific, PA, USA) under a stereomicroscope. Injected individuals were maintained in plastic cages (15 × 18 cm) supplied with rice seedlings at the 4-leaf stage (40). Each treatment contained at least 10 individuals and 3 independent biological replicates, and the samples were collected and flash-frozen at 48 h post-injection of dsA35 or dsGFP to determine the efficacy of RNAi.
2.4 Determination of JH titer and expression levels of JH signaling pathway-related genes after silencing of GPCR A35 in female adults
Following adult emergence, female individuals derived from dsRNAs-treated nymphs were collected at 2 days post-eclosion for subsequent analyses of JH titers and the expression of JH signaling-related genes. JH levels were quantified using a commercial Juvenile Hormone ELISA Kit (Qiaodu, Shanghai, China). There were 3 biological replicates, and each replicate consisted of 10 females. Samples were weighed and homogenized in phosphate-buffered saline (PBS) at a ratio of 1 g tissue to 9 mL PBS, followed by centrifugation at 8,000 rpm for 20 min. The supernatants were collected, and the JH titer was determined according to the manufacturer’s protocol. Furthermore, the transcription level of the JH signaling-related genes (3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), farnesyl diphosphate synthase (FPPS), juvenile hormone acid methyltransferase (JHAMT), Tai, Met, and Kr-h1), Vg, and VgR were determined by qRT-PCR. The qRT-PCR was performed following the procedure described above. Reactions were performed in triplicate for each of the three biological replicates, based on three independent RNA samples, and each replicate was composed of 10 individuals. Primers for the aforementioned genes were designed using Primer3Plus (https://www.primer3plus.com/) and are listed in Supplementary Table S1. To assess the amplification efficiency of the aforementioned gene primers. A series of 10-fold dilutions of cDNAs from 2-day-old N. lugens female adults, ranging from 500 ng/µL to 0.05 ng/µL, was used to create the five-point standard curves using a linear regression model. The following equation was used to estimate the qRT-PCR amplification efficiency (E) of all genes: E = (10[−1/slope] − 1) × 100% (50). The efficiencies of all tested primers and the correlation coefficient (R2) for each standard curve are shown in Supplementary Table S1.
2.5 The determination of protein, and the observation of female adult ovaries
After dsRNAs-injected nymphs molted into adults, the soluble proteins were extracted from reproductive tissues (fat body and ovary) of the virgin female adults at 2 days after emergence (n=15, N = 3). The Bradford method was employed to assess the soluble protein content, and BSA (Bovine Serum Albumin) was utilized to build a standard curve to determine the concentration of protein, according to Ge et al. (2020) (45). The ovaries of N. lugens females were isolated and observed at 2 and 4 days post-eclosion. The female adults were anesthetized with CO2 and subsequently dissected in 0.9% saline solution, and the fat bodies around the ovary were stripped cleanly. The isolated ovaries were washed and photographed using a microscope with a digital camera (Olympus, model SZX23, Japan). The count of ovarioles was documented using microscopic examination. There were 9 replicates used for each treatment.
2.6 Determine the reproductive and population parameters of N. lugens
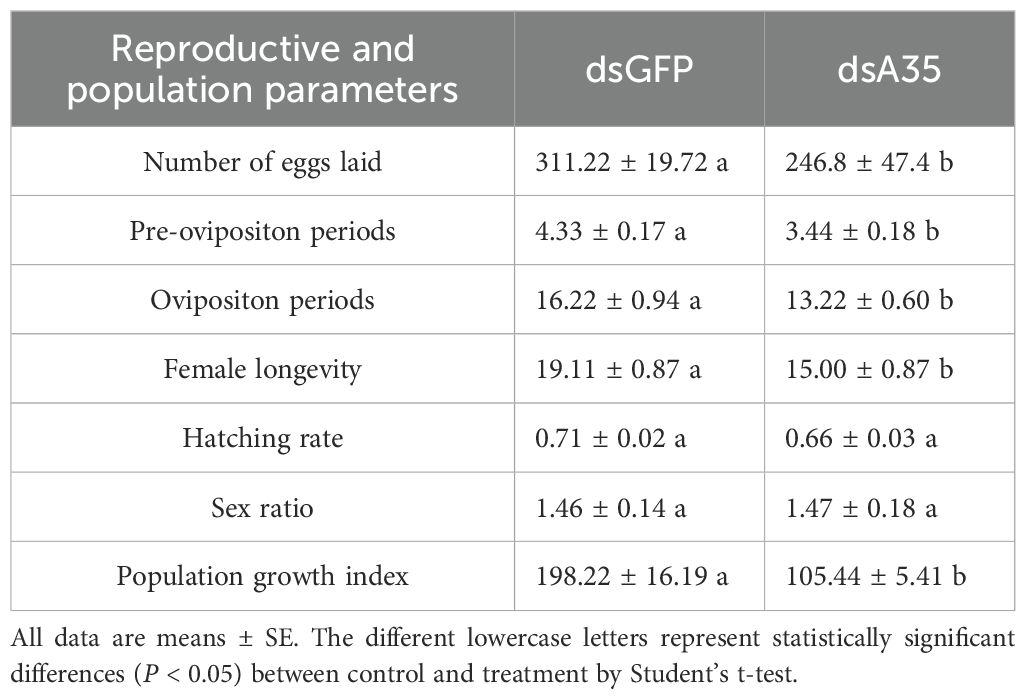
The reproductive parameters of N. lugens were conducted following the procedures of Ge et al. (2020) (45) with minor modifications. After dsRNAs-treated fifth-instar nymphs molted into adults, newly emerged females were paired with untreated males at a 1:2 ratio in glass tubes (2.5 × 15 cm) containing tillering-stage rice stems. Control groups consisted of females injected with dsGFP or PBS. Rice stems were replaced daily during the pre-oviposition phase and every 48 h thereafter throughout the oviposition period until female death. The pre-oviposition period, oviposition duration, and number of eggs laid per female were recorded. Each treatment group included 9 replicates (dsA35♀ × control♂ vs. dsGFP♀ × control♂).
In a parallel experiment, population parameters were evaluated. Treated females were paired with untreated males and transferred onto tillering rice plants enclosed within cylindrical nylon cages (80-mesh, 20 × 80 cm). The number of F1 offspring was determined when the progeny reached the third-instar stage. Nymphs were subsequently reared in glass tubes (2.5 × 15 cm) containing rice stems until adult emergence. Meanwhile, unhatched eggs from the F0 generation were quantified to calculate the hatching rate as: offspring/(offspring + unhatched eggs). The population growth index (PGI) was calculated as F1/F0 (F0 = 4) (45).
2.7 Data analysis
Student’s t-tests were used to compare statistical differences in the gene expression levels and biological parameters of N. lugens between the control and gene-silenced groups. One-way analysis of variance (ANOVA) by the Tukey test was used to compare statistical differences in the gene expression levels of GPCR A35 in different tissues and developmental periods. All statistical tests were conducted in SPSS 22.0 (IBM Inc., Armonk, NY, USA), and plots were generated using Origin 2023 (OriginLab Inc., Northampton, UK).
3 Results
3.1 The spatio-temporal expression profile of GPCR A35 in N. lugens
To investigate the physiological function of GPCR A35, we first analyzed the expression pattern of GPCR A35 in different developmental stages from egg to adult by qRT-PCR. The transcript of GPCR A35 was expressed in the females and had the highest expression at the 4 days post-eclosion, while with only a deficient transcript level in the male adults (Figure 1A). Moreover, the transcript level of GPCR A35 in six tissues (Head, Midgut, Ovary, Feet, Fatbody, and Cuticle) from the 2-day-old female adults was detected by qRT-PCR. The expression of GPCR A35 exhibited significant tissue-specific variation in female adults. Transcript levels were highest in the head, where expression was markedly greater than in other tissues (Figure 1B). Moderate enrichment was observed in the midgut and fat body, whereas only low transcript abundance was detected in the ovary and feet (Figure 1B).

Figure 1. The developmental expression (A) and tissue-specific expression level (B) of GPCR A35 in N. lugens females. All data are reported as means ± SE of three independent biological replications. Lowercase letters represent significant differences in GPCR A35 expression levels among different developmental stages and tissues by the Tukey test, P < 0.05. .
3.2 Effect of silencing GPCR A35 on JH synthesis and JH signaling transduction in N. lugens female adults
Injection of dsA35 at different doses markedly suppressed GPCR A35 expression in fifth-instar nymphs. Transcript levels were reduced by 18.7%, 32.7%, 42.3%, 59.3%, and 60.4% at 48 h after injection with 40–120 ng dsA35, respectively, compared with the dsGFP-injected nymphs (Figure 2A; 40 ng: t = 3.6, P < 0.05; 60 ng: t = 3.9, P < 0.05; 80 ng: t = 11.2, P < 0.001; 100 ng: t = 6.5, P < 0.01; 120 ng: t = 9.0, P < 0.01). The silencing effect increased in a dose-dependent manner, and since 100 ng dsA35 provided robust inhibition, this dose was selected for subsequent experiments. To further investigate the role of GPCR A35 in JH signaling, the dsA35 was injected into 5th N. lugens nymphs. After adult emergence, qRT-PCR was employed to assess the transcript level of GPCR A35 and JH signaling-related genes in N. lugens virgin females at 2 day post-eclosion (Figure 2C). The results showed that the silencing effect of GPCR A35 in fifth-instar nymphs was effectively delivered to female adults, and the expression of GPCR A35 in female adults was significantly downregulated by 57% (Figure 2C). Moreover, the silencing GPCR A35 led to downregulate the JH synthetase, and JH signaling related genes expression levels, including JHAMT, FPPS, HMGCR, Met, and Kr-h1 by 52.7% (t = 8.6, P < 0.01), 57.0% (t = 8.9, P < 0.01), 60.1% (t = 19.3, P < 0.001), 24.2% (t = 4.1, P < 0.05), and 78.3% (t = 11.3, P < 0.001) in comparison with controls at 2 day post-eclosion, respectively (Figure 2C), there was no significant effect on the expression levels of Tai (P > 0.05) which was downstream of the JH signal. Further, the JH titer in females at 2 days post-eclosion was significantly decreased following the knockdown of GPCR A35 in N. lugens nyphmal stage (Figure 2B, t = 4.0, P < 0.05).
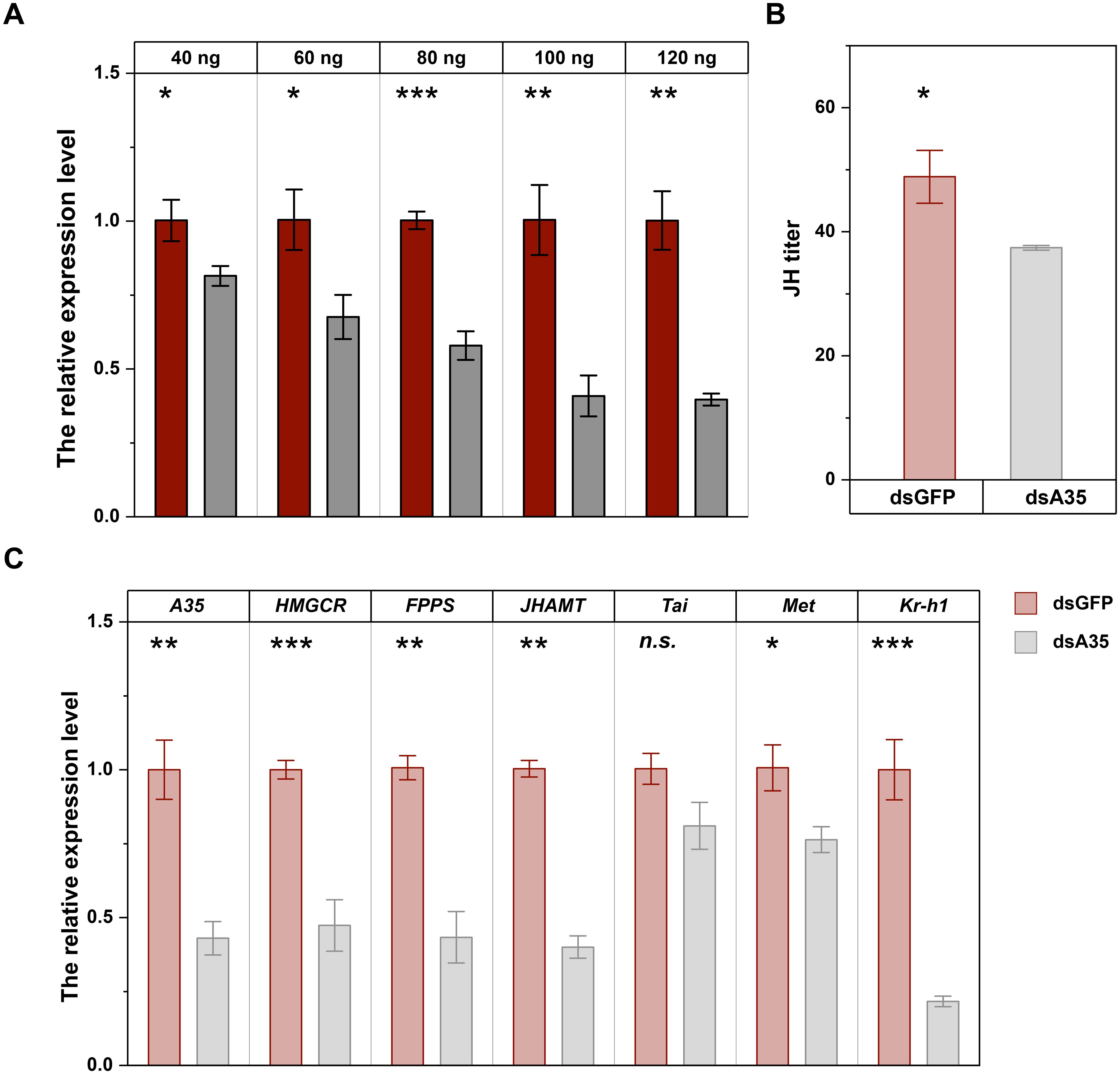
Figure 2. RNAi efficiency of different doses of dsA35 on N. lugens nymphs (A), and the effects of dsA35 on JH titer (B) and expression levels of JH signaling-related genes (C). The asterisks represent no significant differences in expression level between control (dsGFP) and treatment (dsA35) by Student’s t-test: *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant.
3.3 Silencing GPCR A35 in the nymphal stage affects soluble protein contents and the transcript level of Vg and VgR
Silencing of GPCR A35 in the nymphal stage significantly affected protein accumulation in the reproductive tissues of virgin female adults. In the ovary, the soluble protein levels were reduced by 21.4% (t = 10.3, P < 0.01) and 26.6% (t = 6.9, P < 0.01) in females at 2 and 4 days post-eclosion, respectively, compared with dsGFP-treated females (Figure 3A). The protein content in fat bodies also declined, showing reductions of 14.6% (t = 10.3, P < 0.001) at 4 days post-eclosion (Figure 3B). In addition, knockdown of GPCR A35 markedly suppressed the transcription of Vg and VgR. The expression levels decreased by 49.2% (t = 6.9, P < 0.01) and 45.2% (t = 9.8, P < 0.01) at 2 days post-eclosion, and decreased by 82.1% (t = 9.8, P < 0.01) and 72.9% (t = 7.1, P < 0.01) at 4 days post-eclosion, compared to dsGFP-treated females (Figures 3C, D).
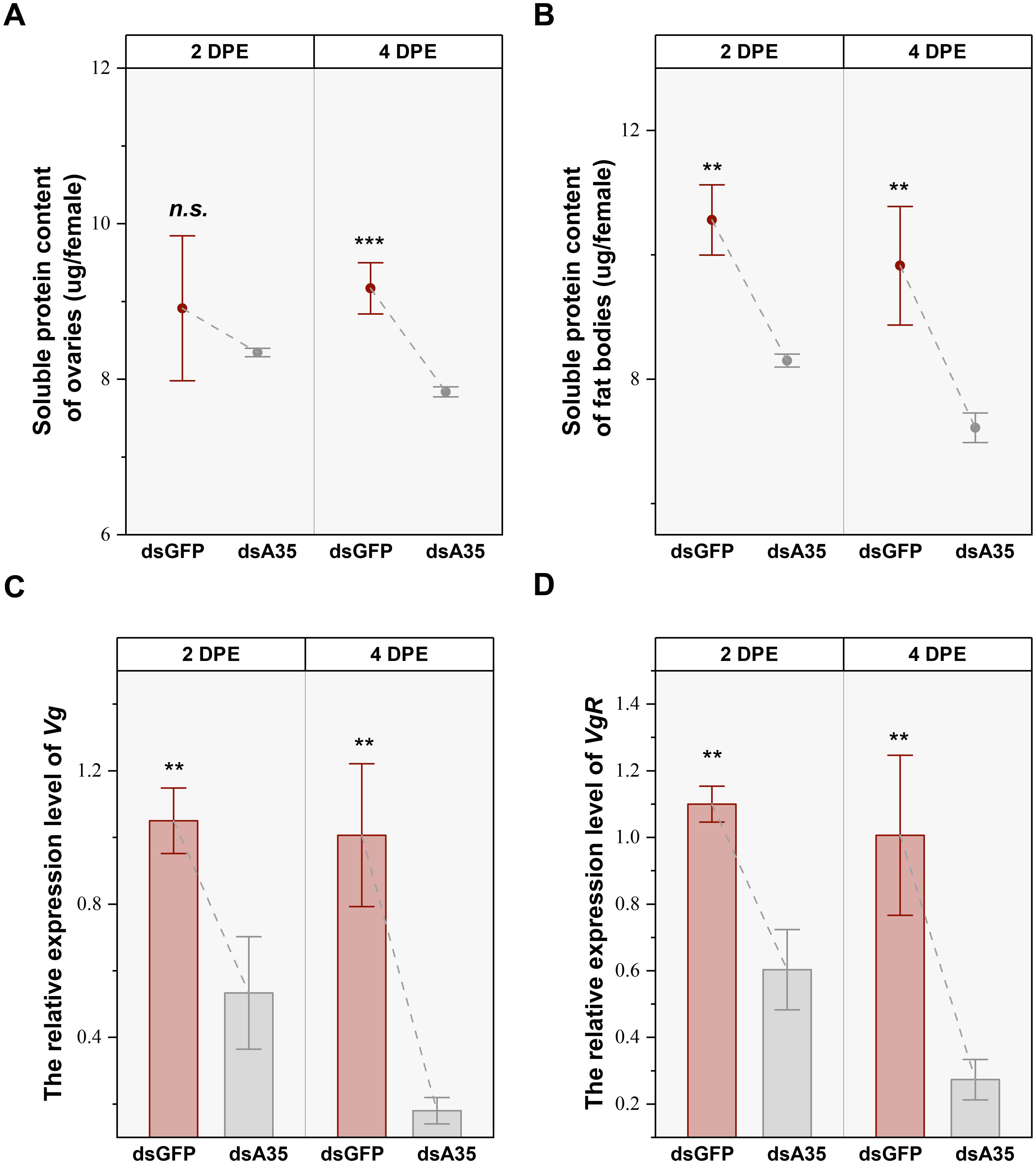
Figure 3. Effects of dsA35 on soluble protein content (A, B), and expression patterns of Vg and VgR (C, D). All data are mean ± SE. The asterisks represent statistical differences between control and treatment: **, P < 0.01; ***, P < 0.001; n.s., not significant. DPE, days post-eclosion.
3.4 Silencing GPCR A35 inhibits ovarian development in female adults
To further elucidate the role of GPCR A35 in the female reproductive process of N. lugens, dsRNAs targeting GPCR A35 or GFP were injected into fifth-instar nymphs, and ovarian morphology was subsequently examined in virgin females at 2 and 4 days post-eclosion (Figure 4C). In control females (dsGFP-injected), ovaries developed normally, displaying abundant, well-formed ovarioles filled with mature eggs at 4 days post-eclosion. In contrast, knockdown of GPCR A35 resulted in markedly impaired ovarian development (Figure 4C). Ovaries from dsA35-treated females exhibited delayed growth, with only a limited number of fully elongated, banana-shaped ovarioles and fewer mature oocytes at 4 days post-eclosion. Quantitative analysis further confirmed these morphological observations: ovarian area and mature oocyte numbers were significantly reduced in dsA35-injected females, with decreases of 9.0% (t = 2.3, P < 0.05) and 26.2% (t = 5.2, P < 0.001) at 2 days post-eclosion, and 15.1% (t = 4.4, P < 0.001) and 18.0% (t = 4.7, P < 0.001) at 4 days post-eclosion, respectively, compared with dsGFP-treated females (Figures 4A, B).
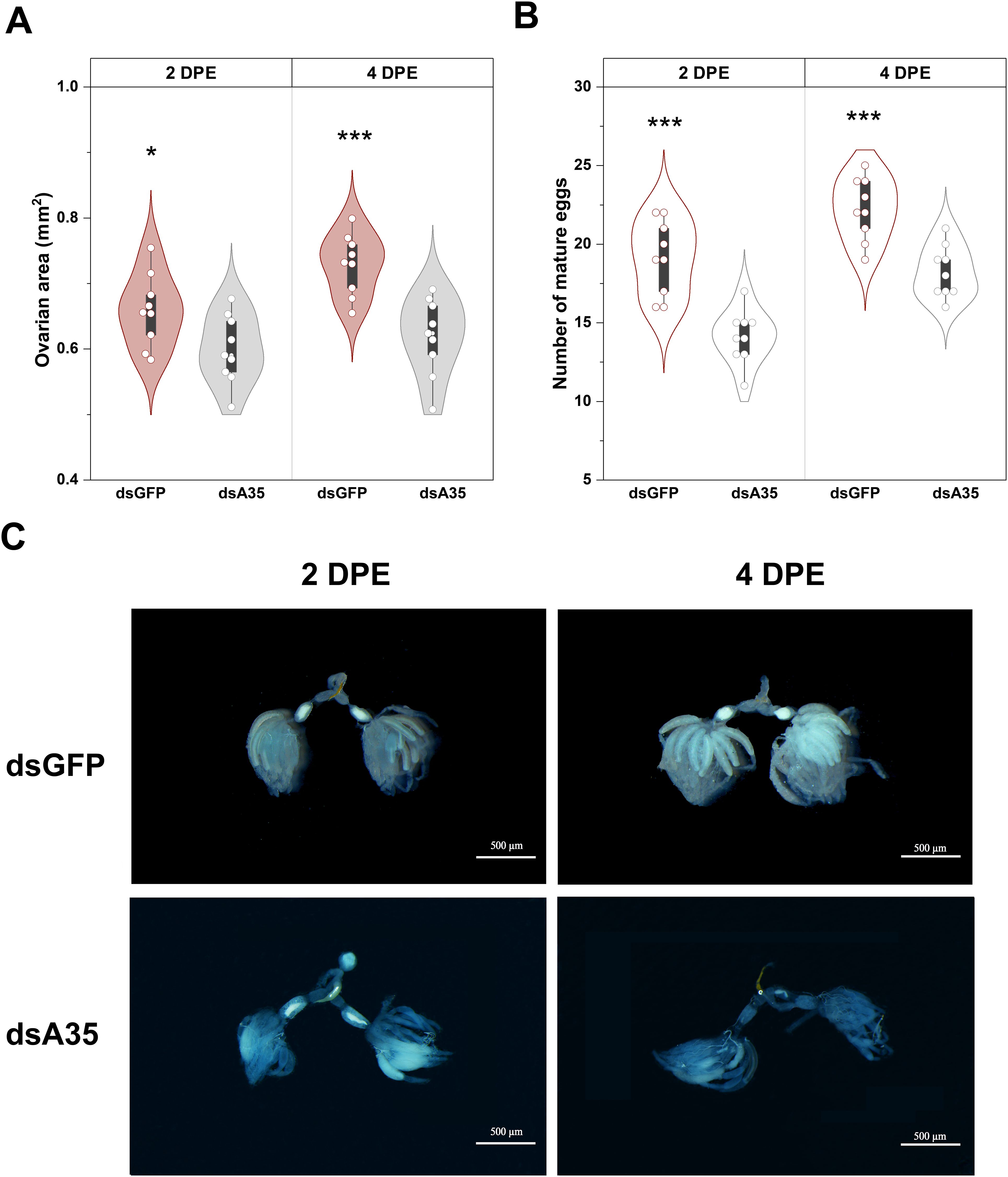
Figure 4. Effects of dsA35 on ovarian area (A), number of mature eggs (B), and ovarian development (C) in females.. All data are means ± SE. The asterisks represent statistically significant differences between control and treatment by Student’s t-test: *, P < 0.05; ***, P < 0.001. DPE, days post-eclosion.
3.5 Silencing of GPCR A35 impairs reproductive performance and population growth in N. lugens
Finally, we assessed the impact of GPCR A35 knockdown on reproductive performance and population parameters of N. lugens (Table 1). The results showed that silencing of GPCR A35 markedly extended the pre-oviposition period (t = 3.7, P < 0.05) and shortened the oviposition duration (t = 2.7, P < 0.05) in female adults, accompanied by a dramatic 51.3% reduction in fecundity compared with dsGFP-injected controls. Moreover, suppression of GPCR A35 during the nymphal stage (F0 generation) significantly impaired population-level traits in the F1 generation. Specifically, the population growth index (PGI) was reduced by 46.8% (t = 5.4, P < 0.05), respectively, relative to controls (Table 1). These findings demonstrate that suppression of GPCR A35 not only compromises female reproductive performance but also exerts a reduction on population expansion.
4 Discussion
GPCRs defined by their conserved seven-transmembrane (7TM) architecture, represent the largest and most versatile family of cell surface receptors (51). Despite their structural homogeneity, GPCRs exhibit remarkable ligand diversity, being able to detect a wide spectrum of extracellular signals, including photons, odorants, neurotransmitters, and hormones (52). This functional plasticity allows GPCRs to orchestrate a broad range of physiological and biochemical processes, such as sensory perception (vision, olfaction, and taste), regulation of behavior and mood, modulation of immune responses, and control of cell growth and proliferation (53–55). Studies have revealed specific GPCR genes and their potential biological functions that may impact insects physiology (4, 20, 56, 57), including their reproduction (33, 58, 59), and regulating their growth and development (57, 60–62), as well as their stress responses (63–66), their feeding patterns (67, 68), their other behaviors (69–71), and many other physiological processes (47, 56). Moreover, increasing evidence suggests that these receptors play pivotal roles in reproductive regulation and are even implicated in gametogenesis, fertilization, and oviposition, underscoring their broad and multifaceted biological significance. In oviparous insects, reproduction is a complex process involving multiple sequential stages, including previtellogenesis, vitellogenesis, chorion formation, oocyte maturation, and egg hatching (72). Sexual reproduction is essential for insect survival and evolutionary success, as it generates genetic diversity and ensures population continuity. Recent study has highlighted that insect GPCRs also play crucial roles in modulating endocrine signaling (47). The neuropeptide GPCRs, such as allatotropin and allatostatin receptors, are expressed in the corpora allata and regulate JH production through G protein–mediated second messenger pathways, including cAMP and Ca²+ signaling cascades, suggesting that membrane-bound GPCRs can influence hormonal homeostasis either by activating intracellular signaling that affects JH synthesis or by indirectly modulating the downstream JH receptor complex (3, 35, 47, 73, 74).
N. lugens is a major rice pest characterized by its high fecundity and overlapping generations, enabling rapid population development within a single cropping season (75, 76). A previous study in N. lugens identified 48 neuropeptide precursors and about 57 candidate GPCRs (77). Most of these receptors belong to typical rhodopsin- and secretin-like GPCR families, but several unusual receptor types have also been uncovered, suggesting a complex neuroendocrine system in this species. Expression profiling revealed pronounced stage- and tissue-specific patterns, implicating these receptors in diverse physiological functions including development, reproduction, feeding, and stress responses (77, 78). Functional studies showed that elevenin receptor NlA42 regulates cuticular pigmentation, and several octopamine receptors are associated with reproductive regulation and stress adaptation (79). Nevertheless, the majority of N. lugens GPCRs remain functionally uncharacterized, and their downstream signaling pathways are still poorly understood. Our previous studies demonstrated that spraying the fungicide JGM increases glucose content in rice plants, which in turn stimulates reproduction in N. lugens (41, 45). Transcriptome analysis revealed a significant upregulation of the GPCR A35, suggesting its potential role in reproduction. In this study, GPCR A35 was identified in N. lugens, and it is highly expressed in females at 4 days post-eclosion, with enrichment in the head and fat body, while it is less expressed in the ovary and feet. The predominant expression of GPCR A35 in the head, rather than in the ovaries, suggests that it may regulate reproduction indirectly through neuroendocrine pathways rather than by acting directly within ovarian tissues. Considering that JH is synthesized in the corpora allata (23, 31) located near the brain, the head-enriched expression of GPCR A35 is consistent with its potential role in modulating JH biosynthesis and signaling. Similar distribution patterns have been reported in other insects such as Drosophila, Bombyx mori, Solenopsis invicta, and T. castaneum, indicating that GPCRs are predominantly distributed in the insect brain and central nervous system because they serve as primary receptors for neuropeptides and biogenic amines, coordinating neuroendocrine and behavioral functions. (17, 34, 58, 59, 80–82). Their enriched expression underscores their essential roles in regulating behavior, reproduction, feeding, stress responses, and other vital physiological functions, highlighting the centrality of GPCR-mediated signaling to insect development and adaptation (47). The insect fat body is a key metabolic and endocrine organ that synthesizes and stores nutrients, vitellogenin, and hormones, thereby providing the essential energy reserves and signaling molecules required for oocyte maturation and successful reproduction (83). Enrichment of GPCR A35 in the fat body suggests its potential involvement in the reproductive processes of the N. lugens.
In most insects, vitellogenesis, which is a central event of female reproduction, involves the production and secretion of vitellogenin (Vg) and other yolk protein precursors (YPPs) by the fatbody, followed by internalization of YPPs by maturing oocytes through receptor-mediated endocytosis (24, 84). Reproduction in hemimetabolous and Hemiptera is governed by JH (29, 85, 86). JH is a key insect steroid hormone that plays vital roles in female reproduction, particularly in regulating oogenesis and embryogenesis. During oogenesis, JH promotes vitellogenin synthesis in the fat body and facilitates its uptake into oocytes via vitellogenin receptors, thereby supporting yolk accumulation and oocyte maturation (30, 32, 87). In this study, RNAi-mediated silencing of GPCR A35 in fifth-instar nymphs was effectively delivered to female adults, leading to altered expression of JH synthase and a consequent reduction in JH titer. This disruption further affected the downstream JH signaling pathway by downregulating Met and Kr-h1 expression, ultimately decreasing protein content in the female reproductive tissues and suppressing the expression of Vg and VgR. As a result, reproductive performance and the number of offspring were significantly reduced in N. lugens. JH is a key endocrine regulator that controls insect reproduction by modulating vitellogenesis, oocyte maturation, and reproductive organ development. Increasing evidence suggests that GPCRs participate in the modulation of JH biosynthesis and signal transduction, thereby influencing reproductive processes through neuroendocrine regulation and cross-talk with the JH pathway. A neuropeptide GPCR was significantly overexpressed in the corpora cardiaca and brain of B. mori, indicating the potential involvement of JH biosynthesis processes (88). The overexpression of an allatotropin GPCR receptor (AeATr) gene was characterized in the nervous system and corpora alata-corpara cardiac complex of Aedes aegypti. Blood feeding depressed the transcript level of AeATr, and was associated with JH biosynthesis in mosquitoes (74). In the bumblebee, Bombus terrestris, an allatotropin GPCR has been identified that is overexpressed in the male bumblebee accessory glands, predicting its potential involvement in JH biosynthesis (73). A-type allatostatin neuropeptides and GPCRs have been discovered in JH biosynthesis in many insect species, including Drosophila, cockroaches, crickets, and termites (8). In L. migratoria, among the 22 GPCRs identified in the ovarian transcriptome, LGR4, OR-A1, OR-A2, Mthl1, Mthl5, and Smo showed the highest expression in the ovary. RNAi screening revealed that silencing six GPCRs caused defective phenotypes characterized by disrupted vitellogenin accumulation in developing oocytes, arrested ovarian development, and impaired oocyte maturation. Specifically, LGR4 and Oct/TyrR appeared to regulate Vg synthesis in the fat body, whereas OR-A1, OR-A2, mAChR-C, and CirlL were involved in Vg transport and uptake. These results provide important insights into the regulatory roles of GPCRs in JH-mediated reproductive processes in insects (5).
The JH plays a pivotal role in regulating insect metamorphosis and reproduction (89). JH exerts its reproductive control through its receptor Met, which forms a heterodimer with the bHLH-PAS protein Tai. Within the nucleus, the Met–Tai complex binds to specific promoter regions known as JH response elements (JHREs) to regulate target gene transcription (21, 24). Although Tai does not directly bind JH, the JH-Met interaction facilitates their dimerization and subsequent nuclear translocation of Met, a key step in JH signaling (28, 90). Following the identification of Met as the JH receptor, the JH–Met–Kr-h1 regulatory model was established, in which Kr-h1 acts downstream of Met to mediate JH-dependent reproductive functions (91). In this study, suppression of GPCR A35 further downregulated JH signaling pathway by inhibiting Met and Kr-h1 expression. In Helicoverpa armigera, RNAi-mediated silencing of GPCRs downregulated the expression of Kr-h1, which further affected larval growth and development, and GPCRs are also involved in JH III-induced broad isoform 7 (BrZ7) phosphorylation (92, 93). In L. migratoria, JH activated multiple intracellular signaling pathways involving GPCR, RTK, PLC, and IP3R, which phosphorylate the Na+/K+-ATPase subunit at amino acid residue Ser8, consequently activating Na+/K+-ATPase for the induction of patency in vitellogenic follicular epithelium (94). Additionally, JH triggers a cascade comprising GPCR, PLC, extracellular Ca²+, and PKC, leading to VgR phosphorylation and facilitating Vg endocytosis (25). Another JH-induced pathway involves GPCR, which showed that JH acts via the GPCR-Cdc42-aPKC signaling cascade that triggers the phosphorylation of Par3, a critical scaffold protein of zonula adherens. JH-dependent Par3 phosphorylation results in its dissociation from the β-Catenin/E-Cadherin complex, consequently leading to the opening of patency for Vg transport (26). In T. castaneum, dopamine GPCR-mediated JH signaling promotes Vg accumulation and elevates cAMP levels in oocytes (33). Moreover, numerous studies have demonstrated that the silencing of Met, Tai, and Kr-h1 caused downregulation of Vg, inhibition of ovarian maturation, and a decrease in the offspring population and egg hatching rate (31, 95–98). In N. lugens, Met and Kr-h1 were critical in ovarian development and oocyte maturation (95, 99). In the current study, suppression of GPCR A35 downregulated the expression of Kr-h1, which might affect Vg transcription and arrest ovarian development in N. lugens females. Finally, we further found that silencing GPCR A35 led to shortened oviposition durations, decreased fecundity, and a decline in offspring.
In conclusion, this study functionally characterized a GPCR A35, in N. lugens. GPCR A35 was predominantly expressed in female heads and fat bodies and proved to be essential for reproduction. RNAi-mediated silencing of GPCR A35 reduced JH biosynthesis and signaling activity, leading to the suppression of Vg and VgR expression, impaired ovarian development, and a marked decline in fecundity. Collectively, these results indicate that GPCR A35 regulates female reproductive capacity by modulating JH-mediated vitellogenesis and oogenesis. This work provides new insights into GPCR-mediated endocrine regulation in hemipteran insects and identifies GPCR A35 as a potential molecular target for RNAi-based green pest management strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MC: Writing – original draft, Formal analysis, Visualization. DD: Validation, Investigation, Writing – original draft. ZM: Writing – review & editing, Validation, Data curation. ZD: Investigation, Writing – review & editing, Validation. YS: Data curation, Writing – review & editing. XZ: Writing – review & editing, Software. LG: Project administration, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the Basic Research Program Natural Science Foundation of Jiangsu Province (grant numbers: BK20250937), Postdoctoral Fellowship Program (Grade C) of China Postdoctoral Science Foundation (grant numbers: GZC20241435), the National Natural Science Foundation of China (grant numbers: 32072415), and the Key Research and Development Plan of Jiangsu Province (grant numbers: BE2022345).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/finsc.2025.1719937/full#supplementary-material
References
1. Wang D, Zhao WL, Cai MJ, Wang JX, and Zhao XF. G-protein-coupled receptor controls steroid hormone signaling in cell membrane. Sci Rep. (2015) 5:8675. doi: 10.1038/srep08675
2. Sharan S and Hill CA eds. Potential of GPCR-targeting insecticides for control of arthropod vectors. Washington, DC: American Chemical Society (2017).
3. Liu N, Wang Y, Li T, and Feng X. G-protein coupled receptors (GPCRs): signaling pathways, characterization, and functions in insect physiology and toxicology. Int J Mol Sci. (2021) 22:1–19. doi: 10.3390/ijms22105260
4. Zhang M, Chen T, Lu X, Lan X, Chen Z, and Lu S. G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery. Signal Transduct Target Ther. (2024) 9:88. doi: 10.1038/s41392-024-01803-6
5. Zheng H, Zeng B, Shang T, and Zhou S. Identification of G protein-coupled receptors required for vitellogenesis and egg development in an insect with panoistic ovary. Insect Sci. (2021) 28:1005–17. doi: 10.1111/1744-7917.12841
6. Hu GM, Mai TL, and Chen CM. Visualizing the GPCR network: classification and evolution. Sci Rep. (2017) 7:15495. doi: 10.1038/s41598-017-15707-9
7. Meeusen T, Mertens I, Clynen E, Baggerman G, Nichols R, Nachman RJ, et al. Identification in Drosophila melanogaster of the invertebrate G protein-coupled FMRFamide receptor. P Natl Acad Sci. (2002) 99:15363–8. doi: 10.1073/pnas.252339599
8. Caers J, Verlinden H, Zels S, Vandersmissen HP, Vuerinckx K, and Schoofs L. More than two decades of research on insect neuropeptide GPCRs: an overview. Front Endocrinol (Lausanne). (2012) 3:151. doi: 10.3389/fendo.2012.00151
9. Vogel KJ, Brown MR, and Strand MR. Phylogenetic investigation of Peptide hormone and growth factor receptors in five dipteran genomes. Front Endocrinol (Lausanne). (2013) 4:193. doi: 10.3389/fendo.2013.00193
10. Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, et al. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. (2005) 25:6145–55. doi: 10.1523/JNEUROSCI.1005-05.2005
11. Schiöth HB and Fredriksson R. The GRAFS classification system of G-protein coupled receptors in comparative perspective. Gen Comp Endocrinol. (2005) 142:94–101. doi: 10.1016/j.ygcen.2004.12.018
12. Hanlon CD and Andrew DJ. Outside-in signaling–a brief review of GPCR signaling with a focus on the Drosophila GPCR family. J Cell Sci. (2015) 128:3533–42. doi: 10.1242/jcs.175158
13. Scott JG, Warren WC, Beukeboom LW, Bopp D, Clark AG, Giers SD, et al. Genome of the house fly, Musca domestica L., a global vector of diseases with adaptations to a septic environment. Genome Biol. (2014) 15:466. doi: 10.1186/s13059-014-0466-3
14. Belmont M, Cazzamali G, Williamson M, Hauser F, and Grimmelikhuijzen CJ. Identification of four evolutionarily related G protein-coupled receptors from the malaria mosquito Anopheles Gambiae. Biochem Biophys Res Commun. (2006) 344:160–5. doi: 10.1016/j.bbrc.2006.03.117
15. Fan Y, Sun P, Wang Y, He X, Deng X, Chen X, et al. The G protein-coupled receptors in the silkworm, Bombyx mori. Insect Biochem Mol Biol. (2010) 40:581–91. doi: 10.1016/j.ibmb.2010.05.005
16. Duan Şahbaz B and Birgül Iyison N. Prediction and expression analysis of G protein-coupled receptors in the laboratory stick insect, Carausius morosus. Turk J Biol. (2019) 43:77–88. doi: 10.3906/biy-1809-27
17. Calkins TL, Tamborindeguy C, and Pietrantonio PV. GPCR annotation, G proteins, and transcriptomics of fire ant (Solenopsis invicta) queen and worker brain: An improved view of signaling in an invasive superorganism. Gen Comp Endocrinol. (2019) 278:89–103. doi: 10.1016/j.ygcen.2018.12.008
18. Veenstra JA, Rombauts S, and Grbić M. In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem Mol Biol. (2012) 42:277–95. doi: 10.1016/j.ibmb.2011.12.009
19. Li F, Zhao X, Li M, He K, Huang C, Zhou Y, et al. Insect genomes: progress and challenges. Insect Mol Biol. (2019) 28:739–58. doi: 10.1111/imb.12599
20. Hill CA, Sharan S, and Watts VJ. Genomics, GPCRs and new targets for the control of insect pests and vectors. Curr Opin Insect Sci. (2018) 30:99–106. doi: 10.1016/j.cois.2018.08.010
21. Khalid MZ, Ahmad S, Ngegba PM, and Zhong G. Role of endocrine system in the regulation of female insect reproduction. Biol (Basel). (2021) 10:614. doi: 10.3390/biology10070614
22. Li HL, Wang XY, Zheng XL, and Lu W. Research progress on oviposition-related genes in insects. J Insect Sci. (2020) 20:1–9. doi: 10.1093/jisesa/ieaa087
23. Santos CG, Humann FC, and Hartfelder K. Juvenile hormone signaling in insect oogenesis. Curr Opin Insect Sci. (2019) 31:43–8. doi: 10.1016/j.cois.2018.07.010
24. Roy S, Saha TT, Zou Z, and Raikhel AS. Regulatory pathways controlling female insect reproduction. Annu Rev Entomol. (2018) 63:489–511. doi: 10.1146/annurev-ento-020117-043258
25. Jing YP, Wen X, Li L, Zhang S, Zhang C, and Zhou S. The vitellogenin receptor functionality of the migratory locust depends on its phosphorylation by juvenile hormone. P Natl Acad Sci. (2021) 118:1–8. doi: 10.1073/pnas.2106908118
26. Zheng H, Wang N, Yun J, Xu H, Yang J, and Zhou S. Juvenile hormone promotes paracellular transport of yolk proteins via remodeling zonula adherens at tricellular junctions in the follicular epithelium. PLoS Genet. (2022) 18:e1010292. doi: 10.1371/journal.pgen.1010292
27. Jindra M, Bellés X, and Shinoda T. Molecular basis of juvenile hormone signaling. Curr Opin Insect Sci. (2015) 11:39–46. doi: 10.1016/j.cois.2015.08.004
28. Jindra M, Palli SR, and Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. (2013) 58:181–204. doi: 10.1146/annurev-ento-120811-153700
29. Gujar H and Palli SR. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci Rep. (2016) 6:35546. doi: 10.1038/srep35546
30. Wu Z, Yang L, He Q, and Zhou S. Regulatory mechanisms of vitellogenesis in insects. Front Cell Dev Biol. (2020) 8:593613. doi: 10.3389/fcell.2020.593613
31. Leyria J, Orchard I, and Lange AB. Impact of JH signaling on reproductive physiology of the classical insect model, Rhodnius prolixus. Int J Mol Sci. (2022) 23:13832. doi: 10.3390/ijms232213832
32. Zhang C, Kim AJ, Rivera-Perez C, Noriega FG, and Kim YJ. The insect somatostatin pathway gates vitellogenesis progression during reproductive maturation and the post-mating response. Nat Commun. (2022) 13:969. doi: 10.1038/s41467-022-28592-2
33. Bai H and Palli SR. Identification of G protein-coupled receptors required for vitellogenin uptake into the oocytes of the red flour beetle, Tribolium castaneum. Sci Rep. (2016) 6:1–10. doi: 10.1038/srep27648
34. Verlinden H, Vleugels R, Verdonck R, Urlacher E, Vanden Broeck J, and Mercer A. Pharmacological and signalling properties of a D2-like dopamine receptor (Dop3) in Tribolium castaneum. Insect Biochem Mol Biol. (2015) 56:9–20. doi: 10.1016/j.ibmb.2014.11.002
35. Liu P, Peng HJ, and Zhu J. Juvenile hormone-activated phospholipase C pathway enhances transcriptional activation by the methoprene-tolerant protein. P Natl Acad Sci. (2015) 112:1871–9. doi: 10.1073/pnas.1423204112
36. Wu J, Ge L, Liu F, Song Q, and Stanley D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu Rev Entomol. (2020) 65:409–29. doi: 10.1146/annurev-ento-011019-025215
37. Sun D, Wang H, Zeng J, Xu Q, Wang M, Yu X, et al. The life-history trait trade-offs mediated by reproduction and immunity in the brown planthopper, Nilaparvata lugens Stål. J Integr Agr. (2024) 23:2018–32. doi: 10.1016/j.jia.2024.03.062
38. Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Gonthier P, Jaques Miret JA, et al. Pest categorisation of Nilaparvata lugens. Efsa J. (2023) 21:e07999. doi: 10.2903/j.efsa.2023.7999
39. Zhang H, He B, Xing J, and Lu M. Spatial and temporal patterns of rice planthopper populations in South and Southwest China. Comput Electron Agriculture. (2022) 194:106750. doi: 10.1016/j.compag.2022.106750
40. Zhu H, Ahmad S, Duan Z, Shi J, Tang X, Dong Q, et al. The Jinggangmycin-induced Mthl2 gene regulates the development and stress resistance in Nilaparvata lugens Stal (Hemiptera: Delphacidae). Pestic Biochem Physiol. (2023) 196:105630. doi: 10.1016/j.pestbp.2023.105630
41. Zhao X, Ding Y, Duan Z, Deng D, Mao Z, Zhu Y, et al. The fungicide jinggangmycin stimulates fecundity of Nilaparvata lugens Stål via ILP/Foxo signaling. Pestic Biochem Physiol. (2025) 214:106580. doi: 10.1016/j.pestbp.2025.106580
42. Gao Y, Su SC, Xing JY, Liu ZY, Nässel DR, Bass C, et al. Pesticide-induced resurgence in brown planthopper is mediated by action on a suite of genes that promote juvenile hormone biosynthesis and female fecundity. eLife. (2025) 12:RP91774. doi: 10.7554/eLife.91774
43. Wei Z, Hu W, Lin Q, Cheng X, Tong M, Zhu L, et al. Understanding rice plant resistance to the Brown Planthopper (Nilaparvata lugens): a proteomic approach. Proteomics. (2009) 9:2798–808. doi: 10.1002/pmic.200800840
44. Li J, Xu Y, Wan Y, Yan T, Li J, He S, et al. Nlgalectin mediates insecticide susceptibility in Nilaparvata lugens via modulation of bacterial symbiont. Pestic Biochem Physiol. (2025) 214:106622. doi: 10.1016/j.pestbp.2025.106622
45. Ge L, Zhou Z, Sun K, Huang B, Stanley D, and Song QS. The antibiotic jinggangmycin increases brown planthopper (BPH) fecundity by enhancing rice plant sugar concentrations and BPH insulin-like signaling. Chemosphere. (2020) 249:126463. doi: 10.1016/j.chemosphere.2020.126463
46. Wu T, Dong QQ, Tang XY, Zhu XH, Deng D, Ding YT, et al. CYP303A1 regulates molting and metamorphosis through 20E signaling in Nilaparvata lugens Stål (Hemiptera: Delphacidae). Int J Biol Macromol. (2024) 281:136234. doi: 10.1016/j.ijbiomac.2024.136234
47. Liu N, Li T, Wang Y, and Liu S. G-Protein coupled receptors (GPCRs) in insects-a potential target for new insecticide development. Molecules. (2021) 26:2993. doi: 10.3390/molecules26102993
48. Lü J, Yang C, Zhang Y, and Pan H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insects: A systematic review. Front Physiol. (2018) 9:1560. doi: 10.3389/fphys.2018.01560
49. Livak KJ and Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
50. Zhao XD, Geng YS, Hu TY, Zhao YA, Yang SL, and Hao DJ. Evaluation of Optimal Reference Genes for qRT-PCR Analysis in Hyphantria cunea (Drury). Insects. (2022) 13:1–14. doi: 10.3390/insects13010097
51. Audsley N and Down RE. G protein coupled receptors as targets for next generation pesticides. Insect Biochem Mol Biol. (2015) 67:27–37. doi: 10.1016/j.ibmb.2015.07.014
52. Sonnabend A, Spahn V, Stech M, Zemella A, Stein C, and Kubick S. Production of G protein-coupled receptors in an insect-based cell-free system. Biotechnol Bioeng. (2017) 114:2328–38. doi: 10.1002/bit.26346
53. Gao H, Li Y, Zhang X, Zhang H, Tian Y, and Li B. Unraveling the G protein-coupled receptor superfamily in aphids: Contractions and duplications linked to phloem feeding. Gen Comp Endocrinol. (2024) 347:114435. doi: 10.1016/j.ygcen.2023.114435
54. Zeng Z, Mukherjee A, Varghese AP, Yang XL, Chen S, and Zhang H. Roles of G protein-coupled receptors in inflammatory bowel disease. World J Gastroenterol. (2020) 26:1242–61. doi: 10.3748/wjg.v26.i12.1242
55. Jones EM, Jajoo R, Cancilla D, Lubock NB, Wang J, Satyadi M, et al. A scalable, multiplexed assay for decoding GPCR-ligand interactions with RNA sequencing. Cell Syst. (2019) 8:254–60.e6. doi: 10.1016/j.cels.2019.02.009
56. Birgül Iyison N, Shahraki A, Kahveci K, Düzgün MB, and Gün G. Are insect GPCRs ideal next-generation pesticides: opportunities and challenges. FEBS J. (2021) 288:2727–45. doi: 10.1111/febs.15708
57. Zhao XF. G protein-coupled receptors function as cell membrane receptors for the steroid hormone 20-hydroxyecdysone. Cell Commun Signalig. (2020) 18:146. doi: 10.1186/s12964-020-00620-y
58. Jiang H, Lkhagva A, Daubnerová I, Chae HS, Šimo L, Jung SH, et al. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. P Natl Acad Sci. (2013) 110:E3526–34. doi: 10.1073/pnas.1310676110
59. Nagai C, Mabashi-Asazuma H, Nagasawa H, and Nagata S. Identification and characterization of receptors for ion transport peptide (ITP) and ITP-like (ITPL) in the silkworm Bombyx mori. J Biol Chem. (2014) 289:32166–77. doi: 10.1074/jbc.M114.590646
60. Iga M, Nakaoka T, Suzuki Y, and Kataoka H. Pigment dispersing factor regulates ecdysone biosynthesis via bombyx neuropeptide G protein coupled receptor-B2 in the prothoracic glands of Bombyx mori. PLoS One. (2014) 9:e103239. doi: 10.1371/journal.pone.0103239
61. Regna K, Kurshan PT, Harwood BN, Jenkins AM, Lai CQ, Muskavitch MA, et al. A critical role for the Drosophila dopamine D1-like receptor Dop1R2 at the onset of metamorphosis. BMC Dev Biol. (2016) 16:15. doi: 10.1186/s12861-016-0115-z
62. Kang XL, Zhang JY, Wang D, Zhao YM, Han XL, Wang JX, et al. The steroid hormone 20-hydroxyecdysone binds to dopamine receptor to repress lepidopteran insect feeding and promote pupation. PLoS Genet. (2019) 15:e1008331. doi: 10.1371/journal.pgen.1008331
63. Li T, Liu L, Zhang L, and Liu N. Role of G-protein-coupled receptor-related genes in insecticide resistance of the mosquito, Culex quinquefasciatus. Sci Rep. (2014) 4:6474. doi: 10.1038/srep06474
64. Li T, Cao C, Yang T, Zhang L, He L, Xi Z, et al. A G-protein-coupled receptor regulation pathway in cytochrome P450-mediated permethrin-resistance in mosquitoes, Culex quinquefasciatus. Sci Rep. (2015) 5:17772. doi: 10.1038/srep17772
65. Shen Z, Jiang X, Yan L, Chen Y, Wang W, Shi Y, et al. Structural basis for the interaction of diapause hormone with its receptor in the silkworm, Bombyx mori. FASEB J. (2018) 32:1338–53. doi: 10.1096/fj.201700931R
66. Petruccelli E, Lark A, Mrkvicka JA, and Kitamoto T. Significance of DopEcR, a G-protein coupled dopamine/ecdysteroid receptor, in physiological and behavioral response to stressors. J Neurogenet. (2020) 34:55–68. doi: 10.1080/01677063.2019.1710144
67. Lin F, Hossain MA, Post S, Karashchuk G, Tatar M, De Meyts P, et al. Total solid-phase synthesis of biologically active drosophila insulin-like peptide 2 (DILP2). Aust J Chem. (2017) 70:208–12. doi: 10.1071/CH16626
68. Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, and Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. (2005) 123:145–56. doi: 10.1016/j.cell.2005.07.029
69. Kwon H, Ali Agha M, Smith RC, Nachman RJ, Marion-Poll F, and Pietrantonio PV. Leucokinin mimetic elicits aversive behavior in mosquito Aedes aEgypti (L.) and inhibits the sugar taste neuron. P Natl Acad Sci. (2016) 113:6880–5. doi: 10.1073/pnas.1520404113
70. Grohmann L, Blenau W, Erber J, Ebert PR, Strünker T, and Baumann A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J Neurochem. (2003) 86:725–35. doi: 10.1046/j.1471-4159.2003.01876.x
71. Kwon H and Pietrantonio PV. Calcitonin receptor 1 (AedaeGPCRCAL1) hindgut expression and direct role in myotropic action in females of the mosquito Aedes aEgypti (L.). Insect Biochem Mol Biol. (2013) 43:588–93. doi: 10.1016/j.ibmb.2013.03.005
72. Cheng Y, Li Y, Li W, Song Y, Zeng R, and Lu K. Effect of hepatocyte nuclear factor 4 on the fecundity of Nilaparvata lugens: insights from RNA interference combined with transcriptomic analysis. Genomics. (2020) 112:4585–94. doi: 10.1016/j.ygeno.2020.08.002
73. Verlinden H, Lismont E, Bil M, Urlacher E, Mercer A, Vanden Broeck J, et al. Characterisation of a functional allatotropin receptor in the bumblebee, Bombus terrestris (Hymenoptera, Apidae). Gen Comp Endocrinol. (2013) 193:193–200. doi: 10.1016/j.ygcen.2013.08.006
74. Nouzova M, Brockhoff A, Mayoral JG, Goodwin M, Meyerhof W, and Noriega FG. Functional characterization of an allatotropin receptor expressed in the corpora allata of mosquitoes. Peptides. (2012) 34:201–8. doi: 10.1016/j.peptides.2011.07.025
75. Hu G, Lu M-H, Tuan H, Liu W-C, Xie M-C, McInerney C, et al. Population dynamics of rice planthoppers, Nilaparvata lugens and Sogatella furcifera (Hemiptera, Delphacidae) in Central Vietnam and its effects on their spring migration to China. Bull Entomol Res. (2017) 107:369–81. doi: 10.1017/S0007485316001024
76. Gou F, Zhang D, Chen S, Zhang M, and Chen J. Role of nuclear protein Akirin in the modulation of female reproduction in Nilaparvata lugens (Hemiptera: Delphacidae). Front Physiol. (2024) 15:1415746. doi: 10.3389/fphys.2024.1415746
77. Tanaka Y, Suetsugu Y, Yamamoto K, Noda H, and Shinoda T. Transcriptome analysis of neuropeptides and G-protein coupled receptors (GPCRs) for neuropeptides in the brown planthopper Nilaparvata lugens. Peptides. (2014) 53:125–33. doi: 10.1016/j.peptides.2013.07.027
78. Wu SF, Jv XM, Huang JM, and Gao CF. Molecular features and expression profiles of octopamine receptors in the brown planthopper, Nilaparvata lugens. Pest Manag Sci. (2019) 75:2663–71. doi: 10.1002/ps.5371
79. Uchiyama H, Maehara S, Ohta H, Seki T, and Tanaka Y. Elevenin regulates the body color through a G protein-coupled receptor NlA42 in the brown planthopper Nilaparvata lugens. Gen Comp Endocrinol. (2018) 258:33–8. doi: 10.1016/j.ygcen.2017.07.017
80. Petruccelli E, Li Q, Rao Y, and Kitamoto T. The unique dopamine/ecdysteroid receptor modulates ethanol-induced sedation in drosophila. J Neurosci. (2016) 36:4647–57. doi: 10.1523/JNEUROSCI.3774-15.2016
81. Deng X, Yang H, He X, Liao Y, Zheng C, Zhou Q, et al. Activation of Bombyx neuropeptide G protein-coupled receptor A4 via a Gαi-dependent signaling pathway by direct interaction with neuropeptide F from silkworm, Bombyx mori. Insect Biochem Mol Biol. (2014) 45:77–88. doi: 10.1016/j.ibmb.2013.12.007
82. Stafflinger E, Hansen KK, Hauser F, Schneider M, Cazzamali G, Williamson M, et al. Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects. P Natl Acad Sci. (2008) 105:3262–7. doi: 10.1073/pnas.0710897105
83. Li S, Yu X, and Feng Q. Fat body biology in the last decade. Annu Rev Entomol. (2019) 64:315–33. doi: 10.1146/annurev-ento-011118-112007
84. Mitchell RD 3rd, Sonenshine DE, and Perez de Leon AA. Vitellogenin receptor as a target for tick control: a mini-review. Front Physiol. (2019) 10:618. doi: 10.3389/fphys.2019.00618
85. Lu K, Zhou J, Chen X, Li W, Li Y, Cheng Y, et al. Deficiency of brummer impaires lipid mobilization and JH-mediated vitellogenesis in the brown planthopper, Nilaparvata lugens. Front Physiol. (2018) 9:1535. doi: 10.3389/fphys.2018.01535
86. Smykal V, Bajgar A, Provaznik J, Fexova S, Buricova M, Takaki K, et al. Juvenile hormone signaling during reproduction and development of the linden bug, Pyrrhocoris apterus. Insect Biochem Mol Biol. (2014) 45:69–76. doi: 10.1016/j.ibmb.2013.12.003
87. Leyria J. Endocrine factors modulating vitellogenesis and oogenesis in insects: An update. Mol Cell Endocrinol. (2024) 587:112211. doi: 10.1016/j.mce.2024.112211
88. Yamanaka N, Yamamoto S, Zitnan D, Watanabe K, Kawada T, Satake H, et al. Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS One. (2008) 3:e3048. doi: 10.1371/journal.pone.0003048
89. Zhu S, Liu F, Chen X, Xia S, Wu Y, Tang W, et al. Inter-organelle communication dynamically orchestrates juvenile hormone biosynthesis and female reproduction. Natl Sci Rev. (2025) 12:nwaf022. doi: 10.1093/nsr/nwaf022
90. Wang Z, Yang L, Song J, Kang L, and Zhou S. An isoform of Taiman that contains a PRD-repeat motif is indispensable for transducing the vitellogenic juvenile hormone signal in Locusta migratoria. Insect Biochem Mol Biol. (2017) 82:31–40. doi: 10.1016/j.ibmb.2017.01.009
91. He Q and Zhang Y. Kr-h1, a cornerstone gene in insect life history. Front Physiol. (2022) 13:905441. doi: 10.3389/fphys.2022.905441
92. Li YL, Li YX, Wang XP, Kang XL, Guo KQ, Dong DJ, et al. Identification and functional analysis of G protein-coupled receptors in 20-hydroxyecdysone signaling from the helicoverpa armigera genome. Front Cell Dev Biol. (2021) 9:753787. doi: 10.3389/fcell.2021.753787
93. Li YX, Kang XL, Li YL, Wang XP, Yan Q, Wang JX, et al. Receptor tyrosine kinases CAD96CA and FGFR1 function as the cell membrane receptors of insect juvenile hormone. Elife. (2025) 13:RP97189. doi: 10.7554/eLife.97189
94. Jing YP, An H, Zhang S, Wang N, and Zhou S. Protein kinase C mediates juvenile hormone-dependent phosphorylation of Na(+)/K(+)-ATPase to induce ovarian follicular patency for yolk protein uptake. J Biol Chem. (2018) 293:20112–22. doi: 10.1074/jbc.RA118.005692
95. Mao YW, Li Y, Gao H, and Lin XD. The direct interaction between E93 and Kr-h1 mediated their antagonistic effect on ovary development of the brown planthopper. Int J Mol Sci. (2019) 20:2431. doi: 10.3390/ijms20102431
96. Miao L, Zhang N, Jiang H, Dong F, Yang X, Xu X, et al. Involvement of two paralogous methoprene-tolerant genes in the regulation of Vitellogenin and Vitellogenin receptor expression in the rice stem borer, Chilo suppressalis. Front Genet. (2020) 11:609. doi: 10.3389/fgene.2020.00609
97. Cheng WN, Li XJ, Zhao JJ, and Zhu-Salzman K. Cloning and characterization of Methoprene-tolerant (Met) and Kruppel homolog 1 (Kr-h1) genes in the wheat blossom midge, Sitodiplosis mosellana. Insect Sci. (2020) 27:292–303. doi: 10.1111/1744-7917.12638
98. Dong L, Muramatsu N, Numata H, and Ito C. Functional analysis of a juvenile hormone inducible transcription factor, Kruppel homolog 1, in the bean bug, Riptortus pedestris. Zoolog Sci. (2022) 39:562–9. doi: 10.2108/zs220025
Keywords: G protein-coupled receptors, Nilaparvata lugens, fecundity, juvenile hormone, signal transduction
Citation: Cao M, Deng D, Mao Z, Duan Z, Sun Y, Zhao X and Ge L (2025) The GPCR A35 regulates fecundity of Nilaparvata lugens Stål via juvenile hormone signaling. Front. Insect Sci. 5:1719937. doi: 10.3389/finsc.2025.1719937
Received: 07 October 2025; Accepted: 12 November 2025; Revised: 11 November 2025;
Published: 26 November 2025.
Edited by:
Sourav Roy, The University of Texas at El Paso, United StatesReviewed by:
Kailong Li, Hunan Academy of Agricultural Sciences (CAAS), ChinaXiang Li, Henan Agricultural University, China
Copyright © 2025 Cao, Deng, Mao, Duan, Sun, Zhao and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xudong Zhao, MDA4NjIyQHl6dS5lZHUuY24=; Linquan Ge, bHFnZUB5enUuZWR1LmNu
†These authors have contributed equally to this work
 Minjuan Cao1,2†
Minjuan Cao1,2† Xudong Zhao
Xudong Zhao Linquan Ge
Linquan Ge