- 1Vector Control Product Testing Unit (VCPTU), Environmental Health and Ecological Science Department, Ifakara Health Institute, Bagamoyo, Tanzania
- 2Vector Biology Unit, Swiss Tropical and Public Health Institute, Allschwil, Switzerland
- 3Faculty of Science, University of Basel, Basel, Switzerland
- 4Medical Research Council (MRC) International Statistics and Epidemiology Group, London School of Hygiene and Tropical Medicine (LSHTM), London, United Kingdom
- 5S. C. Johnson & Son, Inc., Racine, WI, United States
- 6School of Life Sciences and Bio Engineering, The Nelson Mandela, African Institution of Science and Technology, Tengeru, Tanzania
Background: Spatial repellents (SRs) that passively emanate airborne concentrations of an active ingredient within a space disrupt mosquito behaviors to reduce human-vector contact. A clinical trial of SC Johnson’s Mosquito Shield™ (Mosquito Shield) demonstrated a 33% protective efficacy against malaria in Kenya. Mosquito Shield lasts for 1 month, but a longer duration product is needed for malaria control programs. SC Johnson’s Guardian™ (Guardian) is designed to provide longer continuous protection from disease-transmitting mosquitoes.
Methods: We conducted experimental hut trials to i) evaluate the efficacy of Guardian over 12 months (between May 2022 and May 2023) and ii) assess the potential public health utility of Guardian by comparing it to Mosquito Shield over 1 month (midway through the Guardian evaluation in November 2022) against wild pyrethroid-resistant malaria vector mosquitoes. The primary endpoint was the number of blood-fed Anopheles arabiensis, while secondary endpoints were the proportion of dead An. arabiensis at 24 hours and the proportion of blood-fed mosquitoes. For Guardian, the number of mosquito landings was also evaluated by human landing catch, a method routinely used in community or implementation studies.
Results: Over 12 months of continuous use, Guardian reduced the number of An. arabiensis blood-feeding by 82.7% [95% confidence interval (78.5%–86.1%)] and landing by 65.1% (59.4%– 70.0%). Guardian also induced 20.1% mortality (18.4%–21.8%). Guardian was found to be superior to Mosquito Shield in reducing the number of blood-fed An. arabiensis with similar proportions of blood-fed and dead mosquitoes at 24 hours.
Conclusion: Guardian was effective in reducing blood-feeding and landing of wild pyrethroid-resistant malaria vectors for 12 months and shows superior protective efficacy compared to Mosquito Shield in reducing the overall number of blood-feeding mosquitoes. Experimental hut studies are suitable for comparative evaluations of new spatial repellent products because they precisely estimate entomological endpoints elicited by spatial repellents known to significantly impact vectorial capacity and disease transmission.
Introduction
In the last 20 years, the widespread deployment of insecticide-treated nets (ITNs) and the implementation of indoor residual spraying (IRS) have substantially reduced the global malaria burden; yet, progress has stalled, and the World Health Organization (WHO) has emphasized the need for additional control tools (WHO, 2023b). The use of IRS is declining despite its proven effectiveness, primarily due to high implementation costs (van den Berg et al., 2021). ITN use has remained largely unchanged since 2015, with only 56% of young children and pregnant women sleeping under a net in 2022 (WHO, 2023b).
New malaria vector control tools must address the current gaps in protection, global public health funding constraints, insecticide resistance, climate change, and the expanding range of malaria vectors. Several new intervention classes are in the pipeline, including attractive targeted sugar baits, endectocides, gene drive, and spatial repellents (SRs), according to the World Malaria Report 2023 (WHO, 2023a).
Spatial repellents are devices that continuously disperse an active ingredient into the air, sustaining concentrations that disrupt key mosquito behaviors involved in malaria transmission, including host detection, landing, blood-feeding, survival, and reproduction (Bibbs and Kaufman, 2017). Spatial repellent products in the public health space have evolved from consumer-facing products, such as mosquito coils or electricity-powered liquid vaporizers, to passive emanator products (Logan et al., 2022) that work continuously over longer periods of time without the need for daily interaction with the end-user.
The spatial repellent intervention class is currently advancing toward a WHO policy recommendation (WHO, 2023a) and guideline for implementation based on evidence generated on a 1-month duration passive emanator product called SC Johnson Mosquito Shield™ (Mosquito Shield). Several clinical trials have been conducted to demonstrate the impact against disease and thus the public health value of Mosquito Shield in trials conducted in Indonesia (Syafruddin et al., 2020) and Kenya (Ochomo et al., 2025). Additionally, implementation studies on Mosquito Shield in Rwanda and Syria, and on SC Johnson’s Guardian™ (Guardian) in Yemen, Nigeria, and Kenya, have evaluated their effectiveness, coverage, acceptability, distribution strategies, and cost, aiming to address knowledge gaps about their use in hard-to-reach displaced populations (Messenger et al., 2023). Entomological efficacy trials conducted in the laboratory, semi-field, experimental hut (Swai et al., 2023b), and in-home tests (Fongnikin et al., 2024) have shown that Mosquito Shield provides substantial protection from mosquito bites throughout its one-month lifespan.
Whilst the public health value of spatial repellents in general, and Mosquito Shield specifically, as tools against malaria is becoming clearer, the WHO has identified longer-lasting spatial repellents as a research priority (WHO, 2023d). Ideally, a spatial repellent should be effective for 6 months or more, comparable to the duration of IRS (Logan et al., 2022). Guardian was designed to provide continuous protection against malaria vector bites for at least one full malaria transmission season (≥6 months), simplifying programmatic deployment. Should Mosquito Shield be determined by WHO as the first-in-class spatial repellent, then Guardian must demonstrate that it performs better (superior) or no-worse (non-inferior) than the first-in-class product on a primary end-point of choice to join the spatial repellent class (WHO, 2024a).
The WHO requires a comparative analysis to assess the entomological performance of new products against a WHO-prequalified comparator, using data from entomology studies already required for product prequalification (WHO, 2024a). These entomological data provide indirect evidence of public health value using entomological endpoints that are surrogates of clinical efficacy and reassurance that a second-in-class product can offer a similar impact to the first-in-class product with proven epidemiological effects.
This study was designed to evaluate the long-term entomological efficacy of Guardian in reducing mosquito blood-feeding and landing rates. Additionally, we compared Guardian’s performance to a 1-month evaluation of Mosquito Shield in experimental hut trials conducted in the same location (halfway into the Guardian evaluation) against a population of pyrethroid-resistant Anopheles arabiensis mosquitoes in Tanzania.
Materials and methods
Study area
The study was conducted from May 2022 to May 2023 at Ifakara Health Institute’s Vector Control and Product Testing Unit (IHI-VCPTU) experimental hut site in Lupiro village (8.385°S, 36.670°E), Ulanga District, southeastern Tanzania. The local primary malaria vectors are An. arabiensis (>99.9% of the An. gambiae complex) and An. funestus (>80% An. funestus s.s.) (Swai et al., 2023b). The dominant vector, An. arabiensis, was resistant (<60% mortality) to alphacypermethrin, deltamethrin, lambda-cyhalothrin, and permethrin at 1x WHO discriminating doses at the time of the conducted trial, but susceptibility was fully restored with pre-exposure to piperonyl butoxide (PBO) (Supplementary Table 1). Their resistance is attributed to CYP450 upregulation (Matowo et al., 2017).
Experimental huts
The study utilized the “New” Ifakara experimental huts (NIEH), which are the same design as the original Ifakara experimental huts (Okumu et al., 2012) but divided with a fully sealed plywood wall to make two huts (Figure 1). The dimensions of the huts are 3.25 m x 3.5 m x 2 m (length x width x height) with a gabled roof of 0.5 m apex and volume of 25.59 m3. Each hut has 10 cm-wide eave gaps on three sides fitted with baffles that allow mosquitoes to enter freely, but they can only exit via two window traps, allowing measurement of endpoints in the majority of mosquitoes that enter the huts (Swai et al., 2023b). Both halves of a single original hut received identical treatments. Temperature and humidity were continuously monitored in one of the huts throughout the study and a median temperature of 25.9°C [interquartile range (IQR) (24.3°C–27.4°C)] and median relative humidity of 71.4% [IQR (64.0 – 77.6)] were recorded.
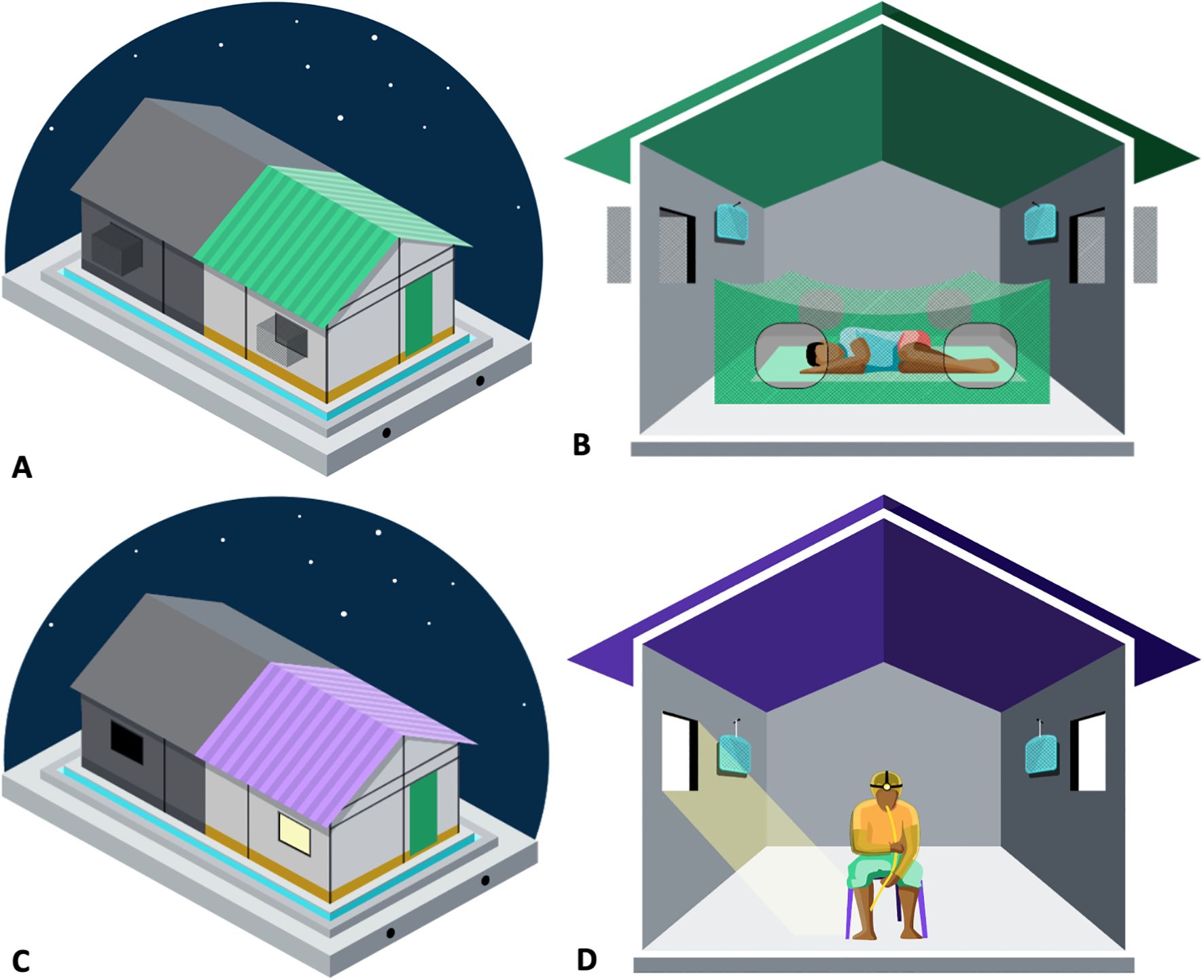
Figure 1. Setup of the huts used for classic experimental hut “feeding experiment” (A, B) and human landing catch “Landing” (C, D), including the placement of Guardian™ (B, D).
Intervention
Guardian is a passive emanator spatial repellent product that contains 2,500 mg of transfluthrin on a mesh substrate within a plastic cage. Two Guardians were placed in each of the huts assigned to the treatment arm at a height of 1.5 m on the length sides of the huts (Figure 1). The Guardian products were installed at 16:00 h on 9 May 2022 and were tested continuously through to 19 May 2023.
Mosquito Shield is a passive emanator spatial repellent product dosed with 110 mg of transfluthrin in a folded 21.6 cm x 26.7 cm sheet of plastic film with a label claim of 30 days. Four Mosquito Shield products were placed according to manufacturer specifications at a height of 1.8 m from the ground and at the center length of each wall in each hut: one on each of the walls. The Mosquito Shield products were installed at 16:00 h on 26 November 2022 and were removed after 32 days.
Both spatial repellent products are manufactured by SC Johnson & Son, Racine, WI, USA.
Study design
Guardian: The study employed a partially randomized block time-series design with two treatment groups (Guardian and no product as control) across eight huts per arm (16 huts in total). The huts were divided into four sets of four huts (two treatment, two control), within which volunteers rotated every night in a 4x4 Latin square design to address varying mosquito attractiveness between individuals. Treatments were assigned to huts sequentially to minimize spatial bias and remained in the same huts throughout the study. The primary endpoint was the number of blood-fed mosquitoes; secondary endpoints were the proportion of blood-fed and dead mosquitoes at 24 hours post-exposure. In addition, the number of mosquitoes landing was measured in a parallel experiment as human landing can be measured operationally, whereas mosquito blood feeding and mortality cannot (Swai et al., 2023b). The efficacy of Guardian was evaluated for 12 months, using the following two methods in the same huts: 1) a feeding experiment measuring mosquito blood feeding and mortality, and 2) a landing experiment measuring mosquito landings on volunteers. At the start of every month, the feeding experiment was run first followed by the landing experiment, each lasting eight consecutive nights.
Feeding experiment
Each test night involved 16 male volunteers sleeping in huts from 1800 to 0600 h under deliberately-holed “too-torn” SafiNet® bed nets (6 holes of 25 m × 25 cm, making >707 cm2 surface area) (Figures 1A, B). SafiNet® is an untreated polyester net from A to Z Textile Mills, Ltd., Arusha, Tanzania. Mosquitoes inside the bed net or in the window traps were collected in the morning using mouth aspirators and those resting on the walls or knocked down on the floor were collected using Prokopack aspirators. These were then sorted by the location that they were collected from and by physiological status (dead and unfed, dead and fed, alive and unfed, or alive and fed); then held at a 24.7°C (23.7°C –25.4°C) [median (IQR)] with access to 10% sucrose solution for 24 hours. Anopheles species females were morphologically identified to the species level (Coetzee, 2020). A subsample of 105 mosquitoes was submitted to the laboratory for speciation using polymerase chain reaction (PCR) (Scott et al., 1993).
Landing experiment
On each test night, 32 male volunteers conducted human landing catches (HLC) in the 16 huts (Figures 1C, D). Volunteers worked in shift pairs, performing HLC for 5 hours every night, i.e., from either 1800 to 0000 h or 0000 to 0600 h. Volunteers sat at the center of each hut, exposing only their lower legs while wearing closed-toe shoes and net jackets made from untreated netting material to standardize the area that the mosquitoes could land on. Using mouth aspirators and torches, volunteers captured mosquitoes landing on their legs for 50 min of each hour, and took a 10-min refreshment break. Each hour, the captured mosquitoes were taken to the field laboratory and incapacitated in a freezer. The following morning, Anopheles species were morphologically identified to the species level (Coetzee, 2020) and counted. The huts had no window exit-traps and the windows were left open at night to maximize mosquito entry. During the day, the windows were kept closed between 0700 h and 1600 h, leaving the eaves open for airflow like local homes.
Mosquito Shield: An independent experimental hut test of the efficacy of Mosquito Shield was run for its full efficacy duration (32 days) mid-way through the Guardian evaluation, between November and December 2022 (Figure 2). Mosquito collection was done as described in the feeding experiment, except in the Mosquito Shield experiments, SafiNet® bed nets were also deliberately holed with six holes of 25 cm × 25 cm, making >707 cm2 surface area. Data collected from this and the Guardian feeding experiment above were used for the comparison of Guardian and Mosquito Shield.
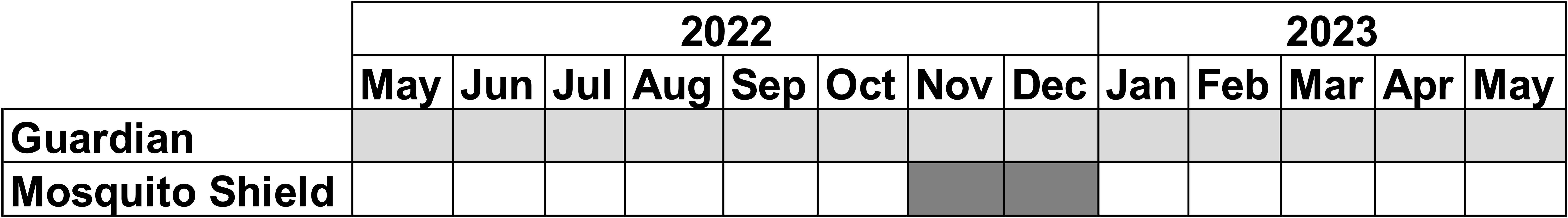
Figure 2. Study timelines for the two independent contemporaneous experimental hut studies used for the comparison of Guardian and Mosquito Shield.
Mosquito Shield was evaluated in a total of eight huts. Half of the huts (four) received no intervention (control) while the remaining (four huts) received the intervention (four Mosquito Shields). The treatments (Mosquito Shield or untreated control) were randomly allocated to huts using a random number generator and remained fixed in the huts for the duration of the study. The primary endpoint was the number of blood-fed mosquitoes and the secondary endpoints were the proportion of blood-fed and dead mosquitoes at 24 hours post-exposure. A total of eight study participants rotated sequentially through the eight huts (four control and four treatment).
Sample size
Sample size calculations were performed using simulation-based power analysis in R statistical software with a significance level of 0.05 for rejecting the null hypothesis. For the evaluation, 1,000 simulations of generalized linear mixed models [6] were run using a Latin square design with volunteers rotating nightly. Variances were set at 0.14 for hut, 0.61 for daily observation, and 0.21 variation in attraction to mosquitoes among volunteers, based on previous observations and an estimated 16 An. arabiensis mosquitoes caught per night. The study was powered to detect a 30% difference in mosquito blood-feeding between the intervention arm and negative control each month, assuming a 60% feeding rate in the control.
The Guardian was run in eight huts per arm for 8 nights per month to give 64 data collection points per month per treatment arm. The Mosquito Shield experiment was run continuously for 32 nights in four huts per treatment arm to give 128 data collection points per treatment arm. Simulations indicated that the independent Guardian and Mosquito Shield studies were powered at 100% [95% confidence interval (CI): 100–100].
A post hoc power analysis with 1,000 simulations of generalized linear mixed models were run using a Latin square design for each experiment. The following estimates observed from the Guardian study were used for the analysis: study variation of log of 1.034, mosquito distribution estimate of log of 0.92, a geometric mean of 15 An. arabiensis mosquitoes caught per night, 13% blood-fed mosquitoes in the intervention arm and 29% blood-fed mosquitoes in the negative control arm. The following estimates observed from the Shield study were used for the analysis: study variation of log of 0.9905, mosquito distribution estimate of log of 0.53, a geometric mean of seven An. arabiensis mosquitoes caught per night, 15% blood-fed mosquitoes in the intervention arm, and 34% blood-fed mosquitoes in the negative control arm. The post hoc power of the Guardian study was 100% (95% CI: 99–100) and that of Mosquito Shield experiment was 98% (95% CI: 97–99).
Analysis
All analyses were performed using STATA® 18 software (StataCorp LLC, USA). We analyzed the Guardian longitudinal entomological efficacy data as follows: data distribution was checked using histograms and measures of variance relative to the mean. Williams means (Alexander, 2012) with 95% CIs were calculated for the number of mosquito landings and blood-feedings. For 24-hour mortality and blood-feeding proportions, arithmetic means with 95% CIs were estimated. Control-corrected 24-hour mortality was not estimated since the mortality in the control arm was <5%. The regression analysis used a mixed effect negative binomial regression model for count outcomes and logistic regression models for proportion outcomes. The models included treatment, volunteer, and night as fixed effects, and hut as a random effect to account for clustering at the level of hut because the interventions were fixed for the duration of the study. Protective efficacy was assessed by computing blood-feeding inhibition and landing inhibition using the formula (1 – IRR/OR) x 100, where IRR and OR represent the incidence rate ratios and odds ratio in the Guardian arm compared to the control arm, respectively.
For the comparison of Guardian and Mosquito Shield, we chose protective efficacy estimated in terms of the reduction in the number of blood-fed mosquitoes captured in the experimental hut as our primary outcome (Swai et al., 2023b). This is because spatial repellents reduce mosquito house entry and the ability of mosquitoes to blood-feed when they are inside a space (Bibbs and Kaufman, 2017; Ogoma et al., 2014). Secondary outcomes were protective efficacy in terms of reduction in the proportion of blood-fed mosquitoes and increased proportion of dead mosquitoes at 24 hours post-collection from the experimental huts, similar to those measured for ITNs (WHO, 2023c). We examined the effect of Guardian and Mosquito Shield on the number of blood-fed mosquitoes using a mixed effects negative binomial regression. For the secondary outcomes which were proportions, mixed effects logistic regression was used. Intervention, volunteer, and experimental date were included as fixed categorical factors. Hut was added as a random factor to account for clustering of observations because the interventions were fixed for the duration of the study and a dummy variable created to distinguish the Guardian or Mosquito Shield treatments was interacted with the treatment variable.
Results
Confirmatory sub-species identification using PCR showed that 100% (102/102) of the amplified subsample of An. gambiae s.l were An. arabiensis.
In the Guardian feeding experiment, a total of 26,920 An. arabiensis mosquitoes were caught in the control arm and 12,863 in the SR arm. In the Guardian landing experiment, a total of 67,857 An. arabiensis mosquitoes were caught in the control arm and 29,724 in the SR arm. In the Mosquito Shield feeding experiment, a total of 4,557 An. arabiensis mosquitoes were caught in the control arm and 3,205 in the SR arm.
Protective efficacy of Guardian
We observed that over 12 months, compared to the control, Guardian provided 82.7% [95% CI (78.5%–86.1%)] protection from mosquito bites and a 65.1% (59.4%–70.0%) reduction in the number of mosquitoes landing on the human volunteers (Table 1). The blood-feeding and landing protective efficacies of Guardian were statistically significant for the combined 12-month data (Table 1) and for each individual month (Figure 3; Supplementary Tables 2, 3). Each month, the protective efficacy measured by the reduction in the number of blood-feeding mosquitoes was higher than that measured by the reduction in the number of landings (Figure 3). Guardian killed 20.1% (18.4%–21.8%) of the An. arabiensis in the study area, even though this mosquito population is strongly resistant to pyrethroid insecticides. In addition, only 12.7% (11.4%–14.1%) of mosquitoes that entered huts with Guardian blood-fed over the 12-month trial (Table 2). A statistically significant higher mortality rate and lower blood-feeding proportion were also observed in the Guardian arm overall and during each month of the evaluation (Supplementary Tables 4, 5).

Table 1. Protective efficacy of Guardian™ in reducing blood-feeding and human landings of wild pyrethroid-resistant An. arabiensis over 12 months.
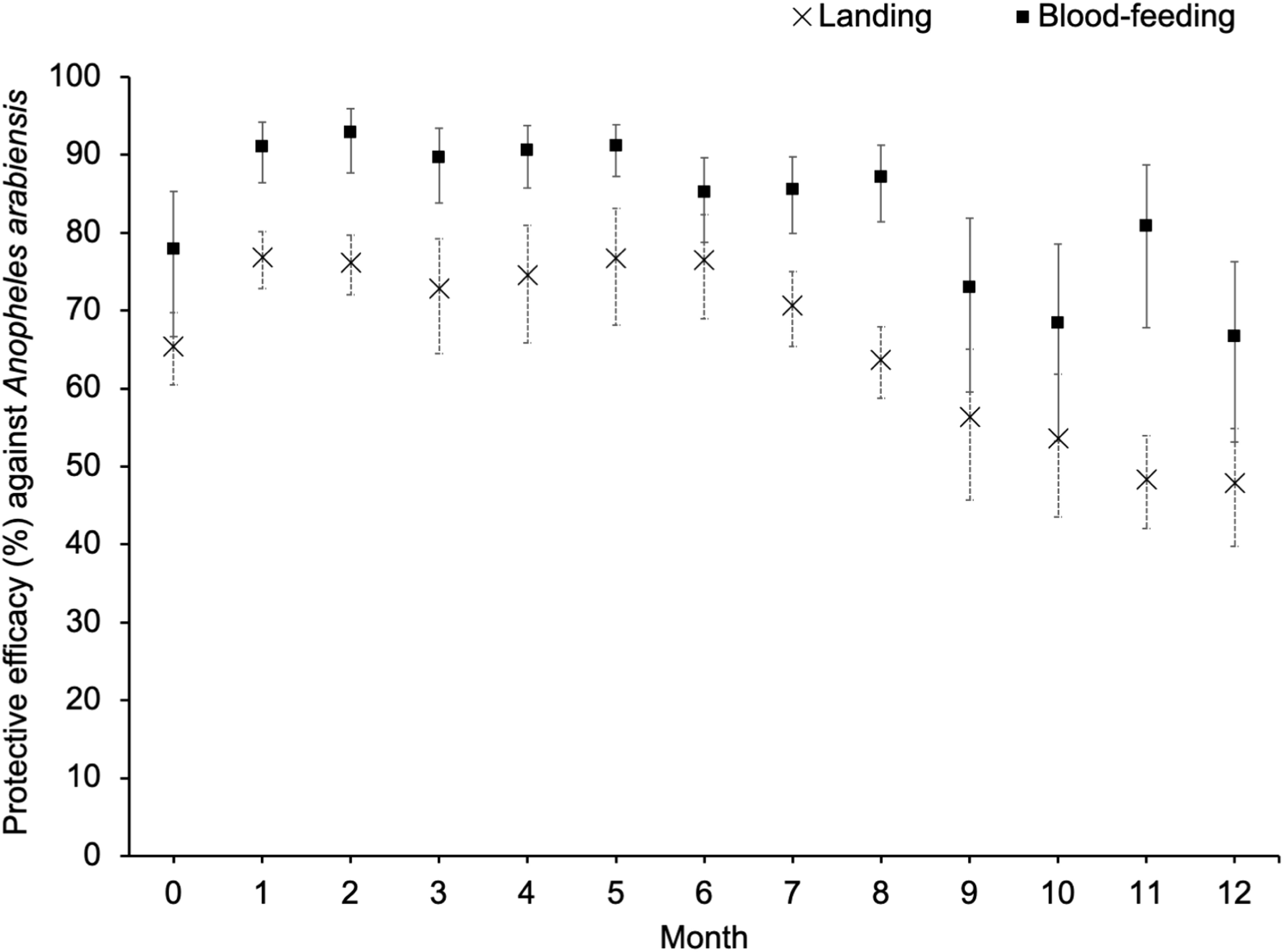
Figure 3. Monthly trend of the landing and blood-feeding inhibition protective efficacy of Guardian against wild pyrethroid-resistant An. arabiensis.
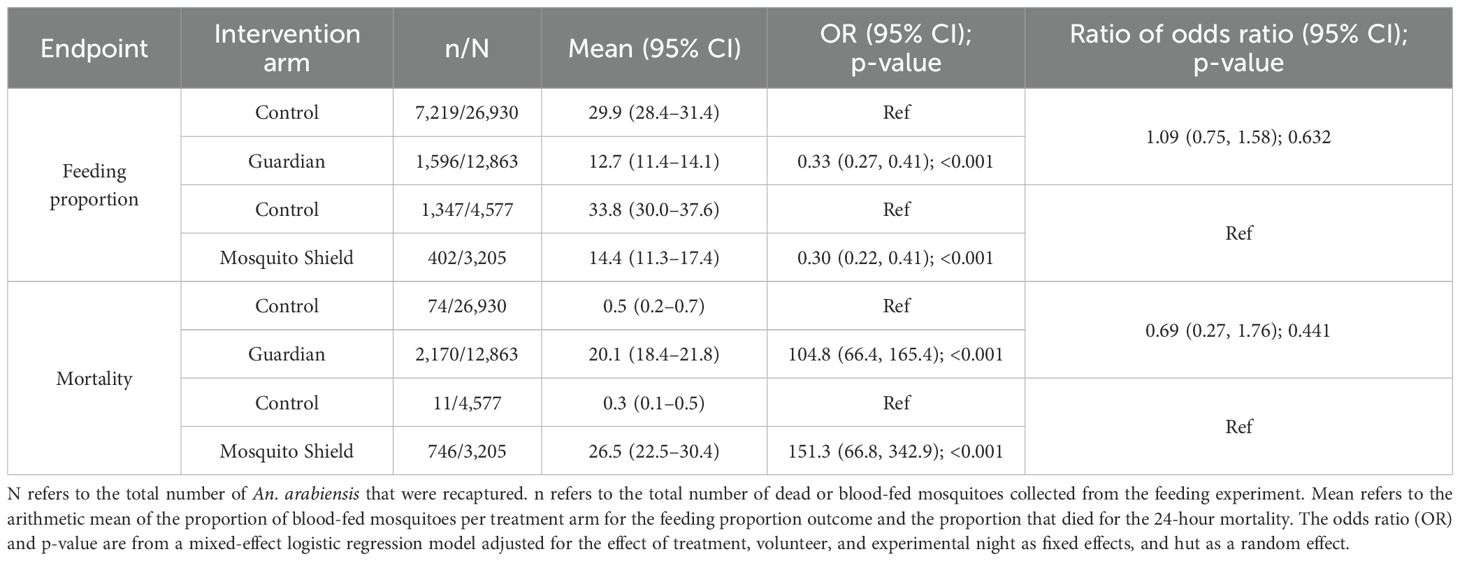
Table 2. Guardian-induced mortality at 24 hours and proportion of blood-fed wild pyrethroid-resistant An. arabiensis (in a percentage) and the comparison with Mosquito Shield™.
Comparison of Guardian and Mosquito Shield
For the primary endpoint of the proportion of blood-fed mosquitoes, the effect of Guardian [0.17 (0.14, 0.21), p<0.001] was larger than the effect of Mosquito Shield [0.29 (0.21, 0.41), p<0.001] (Table 3). The ratio of the rate ratios was 0.60 [(0.40, 0.89), p=0.012] (Table 3), providing evidence that Guardian is superior in reducing the number of blood-fed mosquitoes.
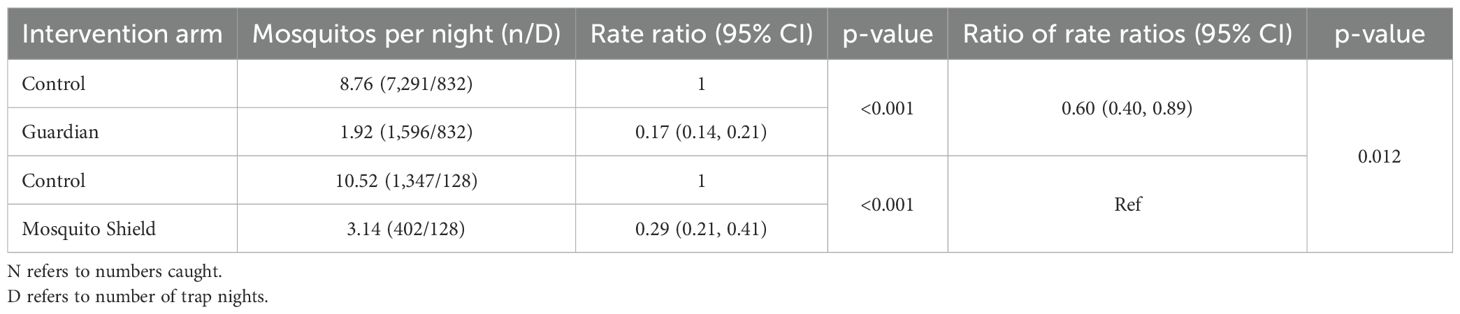
Table 3. Comparison of Guardian™ with Mosquito Shield™ over the duration of life in reducing the number of blood-fed wild pyrethroid-resistant An. arabiensis.
For the secondary endpoints, there was no evidence that Guardian performed differently from Mosquito Shield. The odds ratio for the proportion of blood-fed mosquitoes was 1.09 [(0.75, 1.58), p=0.632] and for induced mortality it was 0.69 [(0.27, 1.76), p=0.441] (Table 2).
Discussion
In this study, we evaluated the efficacy of the Guardian spatial repellent against a pyrethroid-resistant population of An. arabiensis in experimental huts in Tanzania over 12 months. We additionally compared the efficacy of Guardian to Mosquito Shield, which has demonstrated public health benefit in randomized control trials (WHO, 2023a).
Duration of effect and its relevance to public health
The study found that Guardian substantially reduced the number of blood-fed (83%) and landing (65%) pyrethroid-resistant malaria vector mosquitoes for up to 1 year, consistent with the preferred product characteristics for public health use (Logan et al., 2022). Longer-lasting products require less frequent replacement, reducing operational costs and increasing the likelihood of sustained protection. Interventions that offer long-term efficacy with no compliance or maintenance requirements improve user adherence and operational feasibility. Indeed, low user compliance is the major reason that topical repellents (with a duration < 1 day) have not shown public health benefits (Maia et al., 2018). The 1-year duration of Guardian exceeds the observed duration for some IRS formulations currently available (Yukich et al., 2022), although it is shorter than the lifespan of most ITNs (Bertozzi-Villa et al., 2021). Guardian can be installed by household members without technical training and personal protective equipment, and it can be deployed using similar channels to those currently used for ITNs.
Endpoints measured
Throughout the study, blood-feeding inhibition was consistently higher than landing inhibition. This finding has also been observed in other studies of transfluthrin-based hessian emanators (Tambwe et al., 2023; Fairbanks et al., 2024). Pyrethroids also reduce mosquito blood feeding in the presence of human hosts under damaged bed nets (Irish, 2014) or near pyrethroid-treated nets (Lines et al., 1987) by affecting their olfactory neurons, which inhibits their ability to locate the host and acquire a blood meal (Andreazza et al., 2023). Ignoring the secondary impacts of pyrethroid-based spatial repellents likely underestimates their impact on vectorial capacity (Fairbanks et al., 2024). While HLC does expose humans to the risk of disease vectors, it is currently the gold standard means of operationally evaluating the impact of spatial emanators on mosquito populations, as light traps are ineffective (Swai et al., 2023a). However, there are emerging exposure-free methods, such as the miniaturized double net trap (Limwagu et al., 2024) and human-baited double net (Gao et al., 2018), that could potentially be used after testing their suitability for evaluating the efficacy of spatial repellents.
Resistance
In this area, as in much of sub-Saharan Africa, pyrethroid resistance is present. Even so, Guardian killed 20% of An. arabiensis mosquitoes over the duration of 12 months, a rate similar to that of pyrethroid bed nets tested in this location (Kibondo et al., 2022). Transfluthrin, the active ingredient in Guardian, is structurally divergent from the majority of pyrethroids as it has a polyfluorobenzyl moiety and remains active in mosquitoes with upregulated P450 levels that can metabolically detoxify most other pyrethroids (Horstmann and Sonneck, 2016) and was found to be resilient to mechanisms that drive resistance in An. funestus (Nolden et al., 2023): a highly efficient malaria vector known to be resistant to other pyrethroids used in public health (Wangrawa et al., 2024). This supports the use of transfluthrin-based spatial repellents in areas of known pyrethroid resistance, although mosquito mortality will be dependent on both dose and mosquito resistance profile (Fairbanks et al., 2024).
Policy
Comparative evaluations of second-in-class ITNs and IRS with their respective first-in-class equivalents, for which there is epidemiological evidence of public health benefits, are carried out in experimental hut trials (WHO, 2024a). We compared Guardian’s efficacy to Mosquito Shield, finding Guardian superior in reducing the number of blood-fed An. arabiensis. If the WHO recommends spatial repellents, such as Mosquito Shield, as a product class for use in malaria control, our data suggest that Guardian will have a significant epidemiological impact and could also join the same product class. This study adapted ITN experimental hut methods to evaluate indoor passive spatial repellents such as Guardian, considering the hut as a random effect due to fixed treatments. Experimental huts simulate residential settings, allowing us to evaluate vector control tools under standardized conditions in which wild free-flying mosquitoes enter human habitation and interact with a human in the presence of an intervention. They also enable the direct measurement of several endpoints elicited in mosquitoes by spatial repellents, including reduced blood-feeding and induced mortality, which significantly impact vectorial capacity (Brady et al., 2016) and are linked to epidemiological outcomes (Sherrard-Smith et al., 2022). These outcomes are more difficult to measure operationally, unless population-level effects are accurately measured (Magesa et al., 1991).
Limitations
The scope of this study was limited to measuring the entomological efficacy of Guardian on An. arabiensis mosquitoes in Tanzania. In entomological field studies, full blinding is often challenging, and volunteer-related bias in mosquito collection between treatment arms may occur, particularly in human landing catches.
Future directions
Future studies on Guardian should consider measuring its impact on other malaria vectors in different geographical areas and in different contexts of insecticide resistance. An evaluation of Guardian’s operational effectiveness when deployed alone or in combination with core malaria control interventions (ITNs and IRS) as part of integrated vector management will be critical, as it is likely to have an additive effect when combined with other interventions (West et al., 2015). Monitoring the continued effectiveness post-deployment, especially through developing cost-effective chemical and laboratory assays analogous to those used for the operational monitoring of ITNs (WHO, 2011), is a research priority. In addition, mathematical modeling of the impact of spatial repellents in different epidemiological contexts with different intervention mixes could help inform national strategic plans and sub-national tailoring by county or district malaria control programs. Further operational research is ongoing to evaluate the impact that Guardian may have for people at risk of malaria, dengue, and leishmaniasis who are in need of humanitarian assistance and living in temporary shelters (The Mentor Initiative, 2022/2023). Additional information from ongoing and future deployments and operational research on Guardian will inform its distribution strategies, cost of implementation, and operational effectiveness against vector-borne disease.
Conclusion
This study demonstrated that Guardian was efficacious in reducing the number of blood-fed wild pyrethroid-resistant An. arabiensis mosquitoes in Tanzania for up to 1 year. This study advocates for transfluthrin-based spatial repellents as additional vector control tools, offering easily transported, compliance-free protection. This is a major advance in the field of public health as low compliance is the main reason that repellents have previously not shown disease reduction in trials. Comparative efficacy data suggest that Guardian can be expected to provide a similar public health benefit as Mosquito Shield, which has been demonstrated to reduce malaria transmission in East Africa (WHO, 2024b) and Aedes-borne virus transmission in Peru (Morrison et al., 2022). The study also outlines a methodology that was used to measure the efficacy of spatial repellent products that aligns with that used for ITNs and IRSs seeking WHO prequalification. The design enables a precise estimation of blood-feeding reduction and mortality endpoints that both substantially impact the vectorial capacity of, and ultimately disease transmission by, mosquito vectors.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: OSF Repository (https://osf.io/), accession G573C.
Author contributions
JS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. WN: Data curation, Project administration, Supervision, Writing – review & editing. EM: Data curation, Methodology, Resources, Supervision, Writing – review & editing. HN: Data curation, Investigation, Writing – review & editing. NM: Data curation, Investigation, Writing – review & editing. AP: Data curation, Investigation, Writing – review & editing. JB: Formal analysis, Validation, Writing – review & editing. MC: Writing – review & editing, Conceptualization. TM: Writing – review & editing, Conceptualization. SM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors express their sincere thanks and appreciation to the study volunteers, who worked tirelessly over the duration of the experiment. Furthermore, we thank the village leaders and the community that surrounds the Ifakara Health Institute experimental hut site in Lupiro for allowing us to continually run our experiments with minimal interruptions. A special thanks to the Vector Control and Product Testing Unit (VCPTU) management, administrators, and colleagues who helped in organizing logistics and materials, allowing for the smooth execution of the study.
Conflict of interest
The authors JS, WN, EM, HN, NM, AP, and SM conduct product evaluations for companies that produce vector control products, including S.C. Johnson & Sons, Inc. Authors MC and TM were employed by the company S.C. Johnson & Son, Inc, Racine, Wisconsin.
The authors declare that this study received funding from S.C. Johnson & Son, Inc, Racine, Wisconsin. The funder had the following involvement in the study: discussion of study data requirements, editing of the manuscript. The funder was not involved in the study design, collection, analysis, interpretation of data, or the decision to submit it for publication.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1570480/full#supplementary-material
References
Alexander N. (2012). Review: analysis of parasite and other skewed counts. Trop. Med. Int. Health 17, 684–693. doi: 10.1111/j.1365-3156.2012.02987.x
Andreazza F., Valbon W., and Dong K. (2023). Transfluthrin enhances odorant receptor-mediated spatial repellency in Aedes aEgypti. Pestic Biochem. Physiol. 192, 105387. doi: 10.1016/j.pestbp.2023.105387
Bertozzi-Villa A., Bever C. A., Koenker H., Weiss D. J., Vargas-Ruiz C., Nandi A. K., et al. (2021). Maps and metrics of insecticide-treated net access, use, and nets-per-capita in Africa from 2000-2020. Nat. Commun. 12, 3589. doi: 10.1038/s41467-021-23707-7
Bibbs C. S. and Kaufman P. E. (2017). Volatile pyrethroids as a potential mosquito abatement tool: A review of pyrethroid-containing spatial repellents. J. Integrated Pest Manage. 8, 21–21. doi: 10.1093/jipm/pmx016
Brady O. J., Godfray H. C., Tatem A. J., Gething P. W., Cohen J. M., McKenzie F. E., et al. (2016). Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans. R Soc. Trop. Med. Hyg 110, 107–117. doi: 10.1093/trstmh/trv113
Coetzee M. (2020). Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 19, 70. doi: 10.1186/s12936-020-3144-9
Fairbanks E. L., Tambwe M. M., Moore J., Mpelepele A., Lobo N. F., Mashauri R., et al. (2024). Evaluating human landing catches as a measure of mosquito biting and the importance of considering additional modes of action’. Sci. Rep. 14, 11476. doi: 10.1038/s41598-024-61116-0
Fongnikin A., Ahoga J., Ndombidje B., Hueha C., de Souza E., Oti-Tossou R., et al. (2024). Mosquito Shield™, a transfluthrin passive emanator, protects against pyrethroid-resistant Anopheles Gambiae sensu lato in central Benin’. Malaria J. 23, 225. doi: 10.1186/s12936-024-05043-5
Gao Q., Wang F., Lv X., Cao H., Zhou J., Su F., et al. (2018). ‘Comparison of the human-baited double net trap with the human landing catch for Aedes albopictus monitoring in Shanghai, China’. Parasites Vectors 11, 1–12. doi: 10.1186/s13071-018-3053-8
Horstmann S. and Sonneck R. (2016). Contact bioassays with phenoxybenzyl and tetrafluorobenzyl pyrethroids against target-site and metabolic resistant mosquitoes’. PloS One 11, e0149738. doi: 10.1371/journal.pone.0149738
Irish S. R. (2014). ‘The behaviour of mosquitoes in relation to humans under holed bednets: the evidence from experimental huts’. Mem Inst Oswaldo Cruz 109, 905–911. doi: 10.1590/0074-0276140159
Kibondo U. A., Odufuwa O. G., Ngonyani S. H., Mpelepele A. B., Matanilla I., Ngonyani H., et al. (2022). ‘Influence of testing modality on bioefficacy for the evaluation of Interceptor((R)) G2 mosquito nets to combat malaria mosquitoes in Tanzania’. Parasit Vectors 15, 124. doi: 10.1186/s13071-022-05207-9
Limwagu A. J., Msugupakulya B. J., Kilalangongono M. M., Mwalugelo Y. A., Okumu F. O., Lyimo I. N., et al. (2024). Evaluation of the DN-Mini (miniaturized double net) trap for sampling host-seeking Anopheles mosquitoes in malaria-endemic villages of southern Tanzania. PloS One 19, e0294192. doi: 10.1371/journal.pone.0294192
Lines J. D., Myamba J., and Curtis C. F. (1987). ‘Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania’. Med. Vet. Entomol 1, 37–51. doi: 10.1111/j.1365-2915.1987.tb00321.x
Logan J., Chen-Hussey V., O’Halloran L., Greaves C., Due C., Macdonald M., et al. (2022). An expert review of spatial repellents for mosquito control. IVCC, Arctec. Available online at: https://www.ivcc.com/wp-content/uploads/2020/08/An-Expert-Review-of-Spatial-Repellents-for-Mosquito-Control.pdf.
Magesa S. M., Wilkes T. J., Mnzava A. E., Njunwa K. J., Myamba J., Kivuyo M. D., et al. (1991). ‘Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population’. Acta Trop. 49, 97–108. doi: 10.1016/0001-706X(91)90057-Q
Maia M. F., Kliner M., Richardson M., Lengeler C., and Moore S. J. (2018). Mosquito repellents for malaria prevention. Cochrane Database Syst. Rev. 2, CD011595. doi: 10.1002/14651858.CD011595.pub2
Matowo N. S., Munhenga G., Tanner M., Coetzee M., Feringa W. F., Ngowo H. S., et al. (2017). Fine-scale spatial and temporal heterogeneities in insecticide resistance profiles of the malaria vector, Anopheles arabiensis in rural south-eastern Tanzania. Wellcome Open Res. 2, 96. doi: 10.12688/wellcomeopenres
Messenger L. A., Furnival-Adams J., Chan K., Pelloquin B., Paris L., and Rowland M. (2023). Vector control for malaria prevention during humanitarian emergencies: a systematic review and meta-analysis. Lancet Glob Health 11, e534–e545. doi: 10.1016/S2214-109X(23)00044-X
Morrison A. C., Reiner R. C. Jr., Elson W. H., Astete H., Guevara C., Del Aguila C., et al. (2022). ‘Efficacy of a spatial repellent for control of Aedes-borne virus transmission: A cluster-randomized trial in Iquitos, Peru’. Proc. Natl. Acad. Sci. 119, e2118283119. doi: 10.1073/pnas.2118283119
Nolden M., Velten R., Paine M. J. I., and Nauen R. (2023). ‘Resilience of transfluthrin to oxidative attack by duplicated CYP6P9 variants known to confer pyrethroid resistance in the major malaria mosquito Anopheles funestus’. Pestic Biochem. Physiol. 191, 105356. doi: 10.1016/j.pestbp.2023.105356
Ochomo E. O., Gimnig J. E., Awori Q., Abong’o B., Oria P., Ashitiba N. K., et al. (2025). Effect of a spatial repellent on malaria incidence in an area of western Kenya characterised by high malaria transmission, insecticide resistance, and universal coverage of insecticide treated nets (part of the AEGIS Consortium): a cluster-randomised, controlled trial. Lancet. 405 (10473), 147–156. doi: 10.1016/S0140-6736(24)02253-0
Ogoma S. B., Ngonyani H., Simfukwe E. T., Mseka A., Moore J., Maia M. F., et al. (2014). The mode of action of spatial repellents and their impact on vectorial capacity of Anopheles Gambiae sensu stricto. PloS One 9, e110433. doi: 10.1371/journal.pone.0110433
Okumu F. O., Moore J., Mbeyela E., Sherlock M., Sangusangu R., Ligamba G., et al. (2012). A modified experimental hut design for studying responses of disease-transmitting mosquitoes to indoor interventions: the Ifakara experimental huts. PloS One 7, e30967. doi: 10.1371/journal.pone.0030967
Scott J. A., Brogdon W. G., and Collins F. H. (1993). Identification of single specimens of the Anopheles Gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. hygiene 49, 520–529. doi: 10.4269/ajtmh.1993.49.520
Sherrard-Smith E., Ngufor C., Sanou A., Guelbeogo M. W., N’Guessan R., Elobolobo E., et al. (2022). ‘Inferring the epidemiological benefit of indoor vector control interventions against malaria from mosquito data’. Nat. Commun. 13, 3862. doi: 10.1038/s41467-022-30700-1
Swai J. K., Kibondo U. A., Ntabaliba W. S., Ngoyani H. A., Makungwa N. O., Mseka A. P., et al. (2023a). CDC light traps underestimate the protective efficacy of an indoor spatial repellent against bites from wild Anopheles arabiensis mosquitoes in Tanzania. Malar J. 22, 141. doi: 10.1186/s12936-023-04568-5
Swai J. K., Soto A. C., Ntabaliba W. S., Kibondo U. A., Ngonyani H. A., Mseka A. P., et al. (2023b). ‘Efficacy of the spatial repellent product Mosquito Shield against wild pyrethroid-resistant Anopheles arabiensis in south-eastern Tanzania’. Malar J. 22, 249. doi: 10.1186/s12936-023-04674-4
Syafruddin D., Asih P. B. S., Rozi I. E., Permana D. H., Nur Hidayati A. P., Syahrani L., et al. (2020). Efficacy of a spatial repellent for control of malaria in Indonesia: A cluster-randomized controlled trial’. Am. J. Trop. Med. Hyg 103, 344–358. doi: 10.4269/ajtmh.19-0554
Tambwe M. M., Kibondo U. A., Odufuwa O. G., Moore J., Mpelepele A., Mashauri R., et al. (2023). Human landing catches provide a useful measure of protective efficacy for the evaluation of volatile pyrethroid spatial repellents’. Parasit Vectors 16, 90. doi: 10.1186/s13071-023-05685-5
The Mentor Initiative (2022/2023). The mentor initiative annual report 2022/2023. (United Kingdom: Burns house, 4th Floor, South, Haywards Heath RH16 1PG).
van den Berg H., da Silva Bezerra H. S., Al-Eryani S., Chanda E., Nagpal B. N., Knox T. B., et al. (2021). Recent trends in global insecticide use for disease vector control and potential implications for resistance management’. Sci. Rep. 11, 23867. doi: 10.1038/s41598-021-03367-9
Wangrawa D. W., Odero J. O., Baldini F., Okumu F., and Badolo A. (2024). ‘Distribution and insecticide resistance profile of the major malaria vector Anopheles funestus group across the African continent’. Med. Vet. Entomol 38, 119–137. doi: 10.1111/mve.12706
West P. A., Protopopoff N., Wright A., Kivaju Z., Tigererwa R., Mosha F. W., et al. (2015). Enhanced protection against malaria by indoor residual spraying in addition to insecticide treated nets: is it dependent on transmission intensity or net usage? PloS One 10, e0115661. doi: 10.1371/journal.pone.0115661
WHO (2011). Guidelines for monitoring the durability of long-lasting insecticidal mosquito nets under operational conditions (Geneva, Switzerland: World Health Organization).
WHO (2023a). Eighteenth meeting of the WHO Vector Control Advisory Group: meeting report, 24–26 April 2023 (Geneva, Switzerland: World Health Organisation).
WHO (2023c). WHO guideline for the prequalification assessment of insecticide- treated nets. (Geneva, Switzerland: World Health Organization).
WHO (2023d). WHO guidelines for malaria (Geneva, Switzerland: World Health Organization). (WHO/UCN/GMP/2023.01). License: CC BY-NC-SA 3.0 IGO.
WHO (2024a). Data requirements and protocol for determining comparative efficacy of vector control products (Geneva, Switzerland: World Health Organization).
WHO (2024b). Twentieth meeting of the WHO Vector Control Advisory Group: meeting report, 25–28 March 2024 (Geneva, Switzerland: World Health Organisation). Licence: CC BY-NC-SA 3.0 IGO.
Yukich J., Digre P., Scates S., Boydens L., Obi E., Moran N., et al. (2022). Incremental cost and cost-effectiveness of the addition of indoor residual spraying with pirimiphos-methyl in sub-Saharan Africa versus standard malaria control: results of data collection and analysis in the Next Generation Indoor Residual Sprays (NgenIRS) project, an economic-evaluation. Malaria J. 21, 185. doi: 10.1186/s12936-022-04160-3
Keywords: long lasting spatial emanators, transfluthrin based spatial emanators, SC Johnson Guardian™, SC Johnson Mosquito Shield™, entomological efficacy, comparative evaluation, pyrethroid resistant Anopheles
Citation: Swai JK, Ntabaliba WS, Mbuba E, Ngoyani HA, Makungwa NO, Mseka AP, Bradley J, Chura MR, Mascari TM and Moore SJ (2025) SC Johnson Guardian™ spatial repellent shows 1-year efficacy against wild pyrethroid-resistant Anopheles arabiensis, with a similar blood-feeding inhibition efficacy to Mosquito Shield™ in a Tanzanian experimental hut trial. Front. Malar. 3:1570480. doi: 10.3389/fmala.2025.1570480
Received: 03 February 2025; Accepted: 26 May 2025;
Published: 23 June 2025.
Edited by:
Olivier Gnankine, University of Ouagadougou, Burkina FasoReviewed by:
Adilson José DePina, CCS-SIDA/MoH, Cabo VerdeSoma Diloma Dieudonné, Nazi Boni University, Burkina Faso
Copyright © 2025 Swai, Ntabaliba, Mbuba, Ngoyani, Makungwa, Mseka, Bradley, Chura, Mascari and Moore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johnson Kyeba Swai, c2t5ZWJhQGloaS5vci50eg==
 Johnson Kyeba Swai
Johnson Kyeba Swai Watson Samuel Ntabaliba1
Watson Samuel Ntabaliba1 Emmanuel Mbuba
Emmanuel Mbuba John Bradley
John Bradley Sarah Jane Moore
Sarah Jane Moore