- 1Center for Development Research (ZEF), Department of Ecology, University of Bonn, Bonn, Germany
- 2Department of Data Science and Innovation, Health Metrica, Cotonou, Benin
- 3Department of Mathematics, Virginia Tech, Blacksburg, VA, United States
- 4Department of Mathematics, Center for Emerging Zoonotic and Arthrop-borne Pathogens, Virginia Tech, Blacksburg, VA, United States
Introduction: Insecticide-treated bed nets (ITNs) are a cost-efficient prevention method used to prevent malaria, yet their use in poorly urbanized and slum areas remains low. For instance, in these areas in Accra, Ghana, less than 2% of children sleep under fully functional ITNs. Thus, the expected drop in malaria prevalence in Accra and the rest of the country is still much below target. This study deconstructs urban malaria dynamics, revealing the complex interplay of ITNs, spatial heterogeneity, and human behaviors.
Methods: We evaluated urban malaria prevention knowledge, developed a metapopulation framework aligned with empirical findings, and incorporated behavior scenarios to understand urban malaria dynamics better.
Results: Our findings revealed that owning an ITN does not ensure its use, especially in densely populated areas. Limited living space and repurposing are identified as key barriers in Accra, Ghana, with healthcare visits emerging as catalysts for ITN use. Mathematical models incorporating spatial and demographic factors emphasize achieving 60% ITN use in each community patch for epidemic elimination. Our model emphasizes that while ITN use is a crucial intervention in malaria control, it alone may not significantly reduce malaria prevalence without considering spatial, demographic, and behavioral factors.
Discussion: To maximize the effectiveness of ITNs and significantly reduce malaria prevalence, decision-making processes must address the underlying reasons for late or nonadoption of the intervention. Therefore, we strongly recommend prioritizing targeted, one-onone sensitization campaigns, ensuring that barriers to ITN adoption are effectively identified and mitigated.
1 Introduction
The female mosquito, as the primary vector for numerous deadly infectious diseases, is considered the deadliest animal and is responsible for more than a million deaths per year (Prudêncio, 2020). These diseases include Chikungunya, Zika, Dengue, West Nile, Yellow fever, filariasis, and most notably, malaria, which continues to be the leading cause of mortality and morbidity, particularly in sub-Saharan Africa (SSA). For approximately two decades, malaria mortality rates saw a remarkable 67% decrease by the early 2020s (World Health organization, 2020). This achievement was attributed to the implementation of preventive measures such as indoor residual spraying (IRS), distribution of insecticide-treated bed nets (ITNs), and artemisinin-based combination therapy (ACT).
However, the onset of the global COVID-19 pandemic in early 2020 disrupted malaria control programs, notably impacting the distribution of ITNs (Aguma et al., 2023). This disruption had severe consequences for malaria-induced deaths in SSA, paralleling previous observations during the Ebola outbreak in West Africa in 2017 (Sherrard-Smith et al., 2020; World Health Organization, 2020; Aborode et al., 2021). Indeed, the anticipated 627,000 deaths from malaria surpass those linked to SARS-CoV-19 in Sub-Saharan Africa as a result of the suspension of ITN distribution efforts in 2019 (World Health organization, 2020; Ahmed et al., 2022; WHO, 2022). In addition to this risk posed by COVID-19 disruption, the invasion of Anopheles stephensi, described as a more competent vector of malaria-transmitting urban malaria, is magnifying malaria risk, as seen by the expanding evidence of this species invasion in Africa (Ahmed et al., 2022; Hemming-Schroeder and Ahmed, 2023).
ITNs infused with synthetic pyrethroids represent a leading non-pharmaceutical intervention in malaria control, significantly reducing malaria vectors and Plasmodium parasitemia in SSA (Lengeler, 2004; Lim et al., 2011). Since 2007, Ghana has initiated an extensive ITN distribution policy that reached ITN coverage of 85% in 2017 (Awine et al., 2017). Even with their cost efficiency, ITNs remain underutilized: fewer than 2% of children rest under fully operational ITNs (Elder et al., 2011), and in 2011, less than 40% of ITN-owning households in Accra, Ghana, consistently utilized them (Elder et al., 2011; Ahorlu et al., 2019). Although a more recent study indicated a slight rise in the usage rate of ITNs, it remains moderate in Ghana. Specifically, the ITN usage among children under five was only 54%, while the reported rate of ITN utilization in this group was estimated at approximately 61.88% in the Volta Region, Ghana (Orish et al., 2021) and between 21 to 43% nationwide from 2008 to 2021 (Klu et al., 2022). The primary factors contributing to this underuse include rapid physical and chemical degradation and frequent repurposing of ITNs (Ngonghala et al., 2014; Scates et al., 2020). The ramifications of not utilizing ITNs in the context of household net use behavior remain an underexplored facet in malaria research. While numerous studies have delved into the effectiveness on ITNs for malaria prevention, a substantial gap persists in investigating the direct consequences of decisions related to ITN usage and non-usage of the dynamics of malaria. This gap underscores the need for comprehensive research that specifically probes the intricate relationship between household net use behavior and their profound impact on the progression of malaria (Elder et al., 2011; Ahorlu et al., 2019).
Mathematical models have played a pivotal role in understanding disease dynamics and guiding policy decisions (Ross, 1902; Ross, 1911; Macdonald, 1950) and documenting policies for disease management (Heesterbeek et al., 2015). Such models have also been instrumental in designing vector control strategies and vaccination thresholds and assessing the effectiveness of ITNs (Ross, 1902; Ross, 1911; Elder et al., 2011). The basic reproductive number (R0), a metric quantifying the average number of secondary infections produced by single infected individuals in a completely susceptible population, assesses the potential for disease transmission and evaluates the likelihood of an outbreak. Thus, R0 remains a metric for assessing the magnitude of an epidemic. Previous mathematical studies demonstrated the potential for achieving a mosquito population equilibrium below the threshold required for sustained malaria transmission when R0<1 under a constant ITN usage rate (Agusto et al., 2013; Ngonghala et al., 2014; Laxmi et al., 2022; Ngonghala, 2022). However, these studies often assume a constant rate of ITN use, neglecting significant heterogeneity in real-world ITN usage (Killeen and Smith, 2007; Killeen et al., 2007; Gu and Novak, 2009; Govella et al., 2010; Agusto et al., 2013; Briët et al., 2013; Okumu et al., 2013). This simplification overlooks the intricate processes that influence ITN utilization, including risk perception, which can impact malaria transmission dynamics.
Human behavior significantly influences disease dynamics, either facilitating control efforts through awareness or hindering them, such as when vaccine hesitancy exacerbates the spread of infectious diseases (Agusto et al., 2015; Madhumathi et al., 2021). Using an ordinary differential equations (ODE) framework, the persistence of Ebola in West Africa was attributable to communities’ behavior (Agusto et al., 2015). Similarly, we hypothesize that incorporating a proxy quantifying community behavior into an ODE framework will provide insights into malaria progression and provide a better overview of the effectiveness of ITNs.
This study, conducted in James Town and Korle-Dudor, two malaria hotspot areas in Accra, Ghana, offers empirical insights into behaviors affecting malaria control. Using collected data, we employ an epidemiological model that integrates brief community movements (Lagrangian movement) into an extended Susceptible Infected Recovered Susceptible - Susceptible Infected (SIRS-SI) model for a more comprehensive analysis. The model assesses urban malaria dynamics, focusing on changes in the basic reproductive number. Our approach combines empirical data and modeling to understand the impact of community behavior on malaria progression, emphasizing scenarios related to ITN use and physical and chemical degradation of ITNs.
2 Materials and methods
2.1 Study area
Accra is in the Greater Accra Metropolitan Area (GAMA), which stretches between 5° 28 ‘N to 5° 52’N and 0° 32’W to 0° 02’E in Ghana. It is one of the fastest-growing cities in Western Africa, with an annual population growth rate of 3.1% (GSS, 2013). GAMA is a densely populated area with 1,236 people per km2. Accra is the main administrative and economic center of Ghana, with a contribution of 69.7% private informal workforce employment, 15% private formal, 12.8% public, and 2.2% of the workforce employed in 2008 (GSS, 2008).
Rapid urbanization in Accra has contributed to the increase in housing cost and a affordable accommodation, resulting in 15% of the population of the city residing in informal settlements, such as inadequately managed neighborhoods and slums (UN-Habitat Ghana, 2009). Accra grapples with a significant malaria burden driven by the fast-growing population density in under-resourced communities. Malaria mortality and morbidity remain high in under-resourced communities such as Korle-Dudor and James Town (Fobil et al., 2012; Austin, 2015), which were selected for this study due to their high malaria prevalence, poor housing infrastructure.
James Town is a poorly managed area, designated by the Accra Metropolitan Assembly as a slum (Tutu et al., 2017). Accounting for an average of 8.5 occupants per room, this community is facing, among others, serious problems with sanitation (Awuah et al., 2014; Tutu et al., 2017). Korle-Dudor exhibits some characteristics of a slum, such as self-made drains and the lack of tarred roads (Awuah et al., 2018). Migrants from all parts of Ghana who work mainly in the informal sector dominate the Korle-Dudor community.
2.2 Community survey
We conducted a household survey in James Town and Korle-Dudor, to assess the communities’ perceptions of malaria, with a focus on general knowledge of malaria, care-seeking behavior, and risk factors influencing the use of ITNs. Twenty enumeration areas from a total fifty-three in the two communities were randomly selected. Following the random selection of the enumeration areas, the data collection team performed a detailed survey of households in each of the 20 enumeration areas by selecting 1,200 households for a cross-sectional, in-depth interview. Of the selected households, we obtained a response rate of 85.67%, representing 1,028 households that participated in the survey. Within each household, we applied the Kish criteria (Kish, 1949), considering that any resident of a given household between 18 and 82 years old and who spent at least the last six months had good knowledge of the disease situation. Therefore, regardless of gender, any member of the household older than 17 years with these qualifications had the opportunity to participate in the study on behalf of the household.
2.3 Statistical analysis
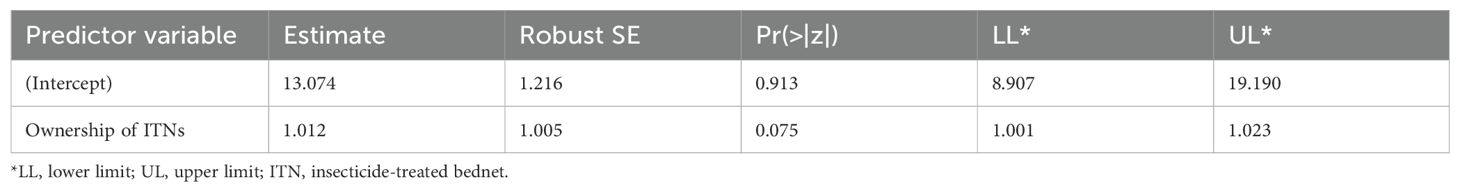
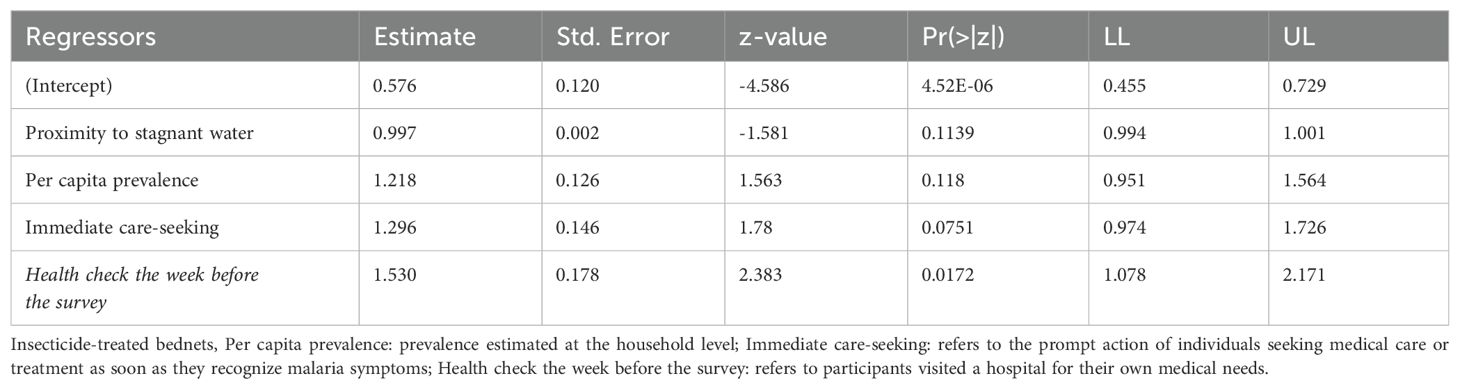
To assess general malaria knowledge and related health-seeking behaviors, descriptive statistics were used. Two generalized linear models were run to investigate: i) the relationship between ITN ownership and use, and ii) the factors influencing ITN use. ITN ownership was defined as the proportion of households possessing at least one ITN, following global ITN ownership indicators (Koenker and Kilian, 2014). A regression analysis was performed to investigate the relationship between ITN use and ownership by regressing the number of households using ITNs against those possessing ITNs. Within a simple Poisson regression framework, 1,000 Monte Carlo permutations were used to estimate probability. To estimate parameters and address overdispersion around the mean, robust standard errors were used (Cameron and Trivedi, 2009). The chi-square test was used to validate the model’s goodness of fit. Logistic regression was similarly used to elucidate the factors that influence ITN use.
2.4 Mathematical model formulation and assumptions
We developed a SIRS-SI model to capture the dynamics of malaria across multiple patches or dwellings (Bichara and Iggidr, 2018; Chen et al., 2020) and age groups, though our presented results are on two patches and two age groups. The choice of this model is the result of non-formal observation of group heterogeneity (higher malaria in children) and spatial heterogeneity (existing hotspots). Even though we only investigated under -resourced communities, we included patches representing more developed urban areas to decipher the progression of urban malaria. By progression of urban malaria we refer to the changes in malaria prevalence over time. The susceptible-infected-recovered-susceptible (SIRS) component describes the dynamics of malaria within the human population located in each patch, while the susceptible-infected (SI) component models the mosquitoes. For the sake of simplicity, we will continue to refer to Anopheles gambiae, which is commonly recognized as the main competent vector of malaria in West Africa (Villena et al., 2022).
The population of humans and mosquitoes is allocated to v patches. Furthermore, the human population has u age groups. The framework accounted for spatial and group heterogeneity (Bichara and Iggidr, 2018). We assumed that the risk of getting infected depends on the location with higher exposure in under -resourced communities (Fobil et al., 2012).
The fraction of human population mkji who travel for a short period of time from patch j to patch k for each age group i is assumed. Each individual belongs to a home patch with a specific risk of infection determined by environmental conditions (Bichara and Iggidr, 2018). This mobility pattern aligns with Lagrangian movement, where origins and destinations always remain intact (Gueron et al., 1996). At time t, the human population in a specific age group j (j=1,…,u) is structured into susceptible (), infected (), and recovered with partial immunity ()while the population of mosquitoes is divided into susceptible () and infected ().
Our model assumes that i) no incubation period before potential transmission, ii) a short lifespan of mosquitoes preventing recovery before death, iii) mosquitoes are confined to their patches (Verdonschot and Besse-Lototskaya, 2014), and iv) humans spending most of their daily time in their home patches, (j=1,…,v).
The total population of humans in patch j is and the total Anopheles gambiae (the main malaria vector species in Accra, Ghana) population in the same patch is . The total human population in an isolated patch j (no movement between neighboring patch) at time t is
Similarly, the total population of mosquitoes at this same time t regardless of the connectivity of the human network is
For group , βi is the risk of malaria infection, γi is the recovery rate of the infectious individuals, is the recruitment rate of the susceptible human population, is the natural mortality rate, and be the rate immunity is lost. The remaining parameters of the model are described in Table 1 and the interactions between humans and mosquitoes are represented in the flow diagram (Figure 1).
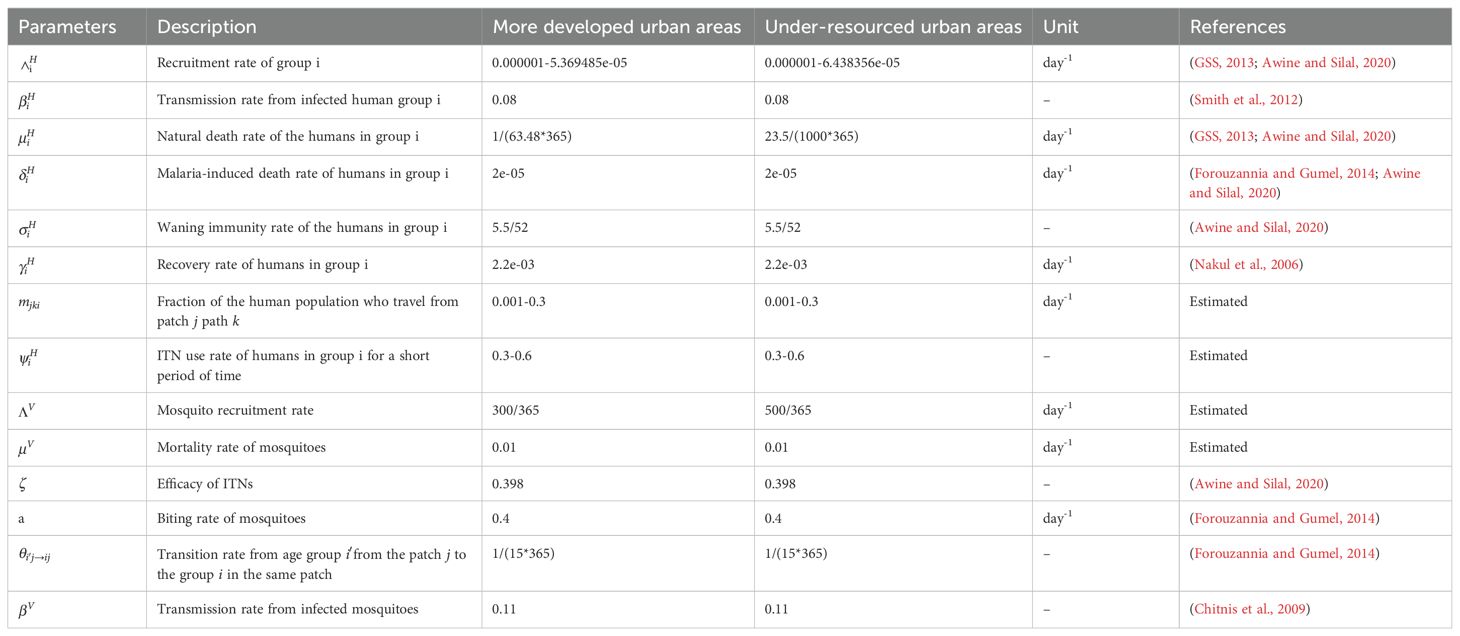
Table 1. Description and values of the range of parameters of the malaria model Equation 1.
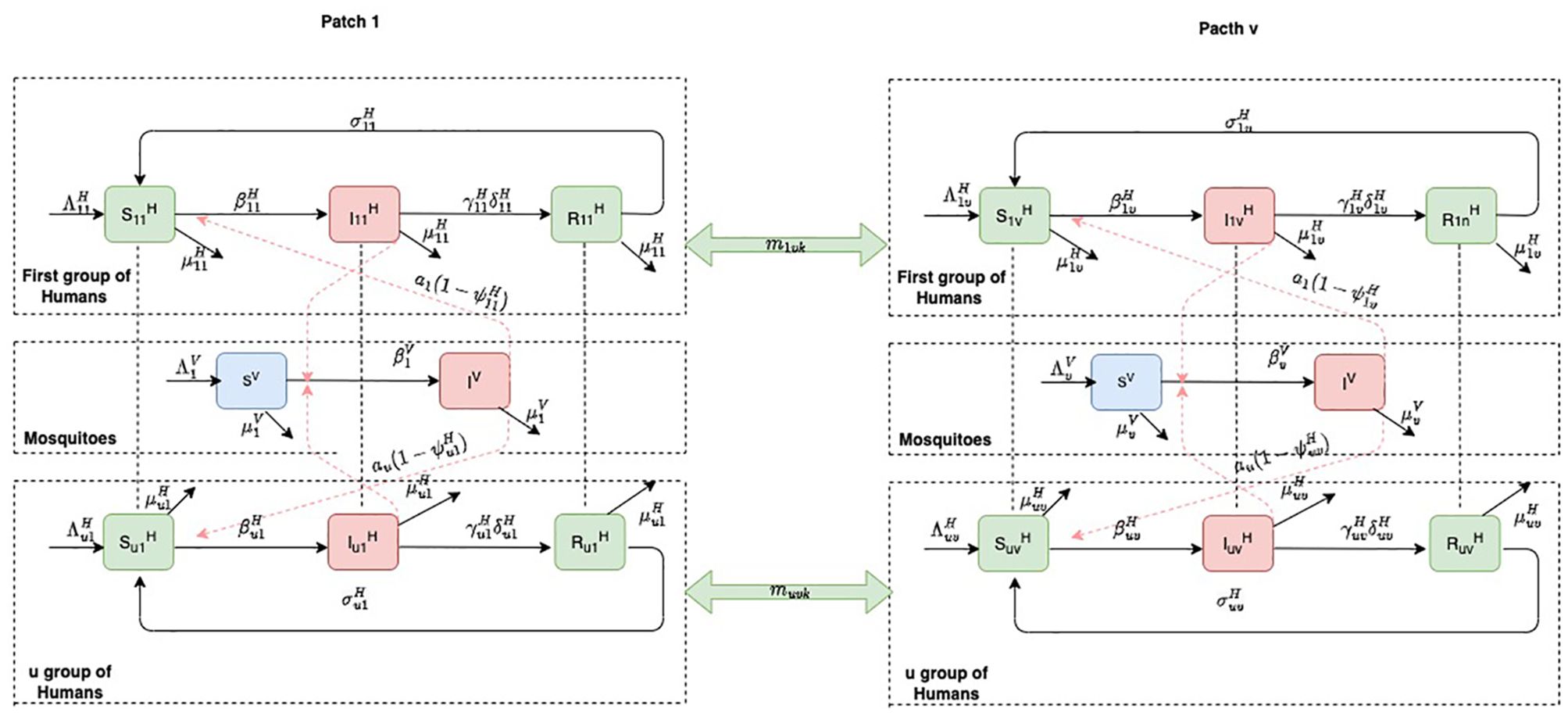
Figure 1. Flow diagram of the malaria patch model. Each box on the right- and left-hand sides represents a patch. The human compartments in green can move from one patch to another. Infected compartments in red are humans and mosquitoes; the susceptible mosquitoes, in blue, move freely within their specific. The dotted red arrows represent the interaction between humans and mosquitoes, while the plain arrows show the flow from one compartment to another.
The density within patch and age group is defined by the following system of non-linear ordinary differential equations:
As there are patches and age groups, there are equations. These equations, with nonnegative initial conditions and fixed mosquitoes and human population, constitute the SIRS-SI epidemic model. The following result shows that the system is well-posed.
Proposition 1: The nonnegative orthant is positively invariant under the flow of the system (Equation 1) and for all . Furthermore. The solution of the system (Equation 1) is bounded.
2.5 Analysis of the model
Positivity and boundedness of solutions
Lemma 1. The set
is compact and positively invariant for the system (Equation 1).
Proof : Adding the first three equations of the system (Equation 1), representing the compartment of the human population, and the two last equations, which represent the population of mosquitoes, we have the following:
Using the comparison theorem (Lakshmikantham et al., 1989), the region is a positively invariant set is positively invariant; is a compact set.
Disease-free equilibrium
Lemma 2. The disease-free equilibrium (DFE) of the system (Equation 1) is given by () where and where and 0s represent the vectors of infected and recovered humans and infected mosquitoes.
Remark 2.1: In the absence of human mobility, the system (Equation 1) can be assimilated into a single patch. If human communities live in perfect isolation, that is, there is no movement of susceptible or infected individuals between patches, the disease does not spread between patches, since the residence time matrices are null (Bichara and Iggidr, 2018).
To study the basic reproductive number, it is sufficient to consider only the infected compartments (Diekmann et al., 1990). Thus, the essential components of Equation 1 to assess the basic reproductive number are
For the rest of the paper, we will consider the Lagrangian approach used for the two-patch and two-age group metapopulation model.
Then the system (Equation 2) is re-written as for patch
Where and . In steady state, the infectious components of the disease-free equilibrium (DFE) of the model is given by (Lemma 2).
Using the next-generation method (van den Driessche, 2017) we calculate the reproductive number, , which is given by:
where represents the eigenvalues of the . The detailed analytical solution of is given in the Supplemental Material.
2.6 Numerical simulations and scenarios
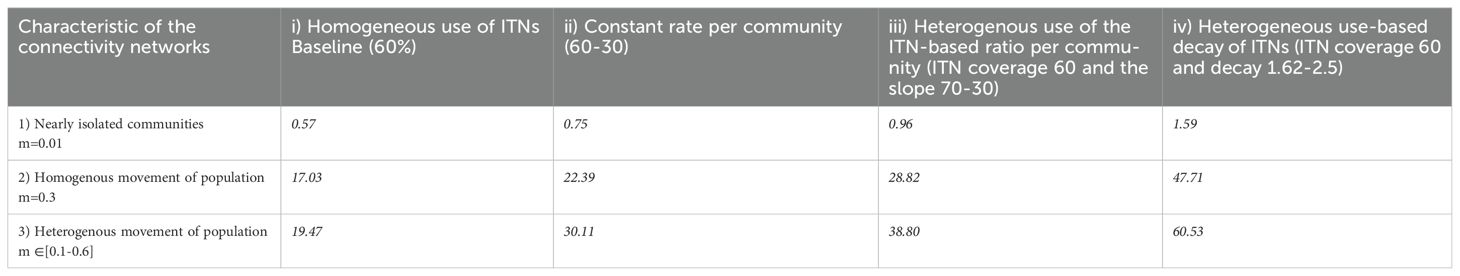
This model was parameterized using a combination of parameters from previous publications, the Ghana population census (2013), and our community survey (Table 1). The community survey was particularly instrumental in estimating the following parameters, namely the fraction of the population who travel between patches, the maximum value of ITN use in each patch, and the mosquito biting rate in under-resourced areas (where environmental conditions and vector control measures differ significantly from other urban settings, which we then extrapolated to more developed urban areas to account for potential variations in transmission dynamics due to differences in infrastructure and living conditions). The model involves two age groups (children and adults) and two patches (under-resourced- and more-developed urban areas). Four scenarios were tested on the uptake of ITNs () in urban areas. Specifically, we denoted the rate of ITN use in the more developed urban areas, and for the rate in the under-resourced areas:
i. The baseline scenario, where ITN use reaches 60% in both under-resource and more developed urban areas with only the human connectivity network ( varying.
ii. The use of ITNs is assimilated to a coverage rate where the ITN uptake ( is constant in both patches. Specifically, the use of ITNs in under-resourced neighborhoods is estimated to be lower than in their more developed urban areas counterparts ().
iii. The use of ITNs depends on the ownership rate. We assumed that there is a linear relationship between the ownership and use of ITNs where the adoption rate (slope of this linear association) is higher in more developed urban areas.
iv. The use of ITNs varies according to the physical or chemical integrity of the nets. We expressed this use rate by an exponential decay function given by f( where the decay rate expressed by is higher in the under-resourced neighborhood.
The scenarios were directly informed by our statistical analysis of the survey data. Specifically, we observed a weak correlation between ITN ownership and actual use, which we interpreted as a potential misrepresentation in policy that high coverage implies high usage. This insight motivated scenaios i) and ii), which test this assumption by modeling ITN use as either equivalent across areas or lower in under-resourced areas, regardless of ownership scenario iii) incorporates the ownership-use relationship identified in the data, with a linear approximation reflecting greater adoption in better-resourced areas. Scenario iv) was developed based on qualitative findings and reported community behavior indicating that ITN use declines over time between ITN distribution’s campaigns, often due to degradation in physical and chemical integrity of the nets; this was implemented via an exponential decay. This approach acknowledges that ITN ownership does not always translate into use, as evidenced by national survey data, and accounts for behavioral and contextual factors influencing utilization (Breakthrough ACTION and VectorWorks). As part of our analysis of community mobility patterns for each scenario, we considered three connectivity networks between under-resourced- and more-developed urban neighborhoods:
1. nearly isolated communities defined by restricted movement (;
2. high and homogeneous connectivity between communities up to 30% of each community travel each day and stay in the neighboring community before returning to their home community; and
3. heterogeneous connectivity network where the mobility of the community is age and community-dependent (.
2.7 Global uncertainty and sensitivity analyses
A sensitivity analysis was performed, using Latin hypercube sampling (LHS) and partial rank correlation coefficient (PRCC) to assess the critical inputs (parameters and initial conditions) of the model and quantify how uncertainty affects the basic reproductive number (Marino et al., 2008). For the LHS, we partitioned the distribution of the parameters into 100 equiprobable subintervals. Prior to the PRCC, we determined a monotonic relationship between each parameter and the .
3 Results
3.1 Empirical evidence of malaria risk
Malaria poses a significant health threat in two surveyed communities, where it is perceived as the primary health risk compared to other diseases (Figure 2A). In James Town, 35% of households identify malaria as their primary health concern, while 25.3% of households in Korle Dudor share this perception.
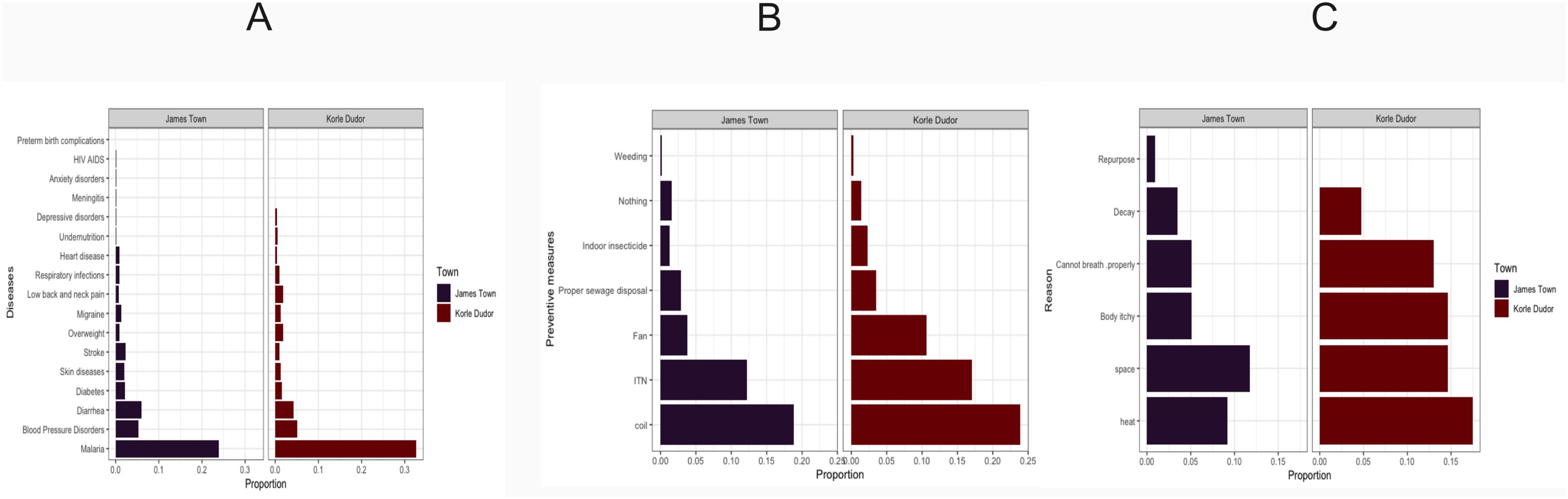
Figure 2. Community Perceptions and Practices Related to Malaria Prevention in James Town and Korle Dudor. (A) Perceived health threats in the surveyed communities of James Town (left) and Korle Dudor (right). (B) Preferred malaria prevention method in the surveyed communities of James Town (left) and Korle Dudor (right). (C) Reasons for ITN non-usage in the surveyed communities of James Town (left) and Korle Dudor (right).
Residents in James Town and Korle-Dudor rely predominantly on repellent coils for malaria prevention, followed by ITNs and other alternatives (Figure 2B). In James Town, 18% of households use repellent coils as their preferred malaria prevention method, while in Korle Dudor, this figure rises to 24%. The prevalent use of ITNs implies a community awareness of their effectiveness, suggesting access to relevant health information. We found that ITN ownership does not statistically explain its use (residual deviance = 17.75675; df = 18 and p-value = 0.472, Table 2). This underscores the influence of other determinants on ITN use within communities.
Logistic regression analysis identifies a statistically significant variable: “Health check week prior to the survey” referring to participants visited a hospital for their own medical needs. (Table 3). A unit increase in the number of times households checked their health status in the week before the survey increased the odds of using ITN by a factor of 1.53. Despite the ownership of ITNs, less than 40% of participants who live in a household that owns at least 1 ITN use them regularly. The key reasons for ITN non-usage include heat generation, limited space, itchiness, breathing difficulties, perceived loss of physical/chemical integrity, and repurposing (protecting seedlings, gardening purposes, and fishing) (Figure 2C).
3.2 Scenario analysis of the progression of urban malaria
We used our mathematical model to test several scenarios aiming to get insight into the dynamics that better approximate the current urban malaria progression. In the first scenario, we examined whether 60% ITN coverage is sufficient to reduce the basic reproductive number (). This scenario and the subsequent ones encompass three distinct cases: first, the fraction of human population who move between communities () is close to zero, i.e., nearly isolated communities; second, the communities exhibit an equal rate of movement from their origin to the destination; and third, one community travels more than the other.
The second scenario explored different rates of ITN coverage to gauge their impact on urban malaria. The third scenario evaluated different thresholds of ITN use, providing insights into the relationship between coverage and use of ITNs and how this relationship impacts . In the fourth and final scenario, we examined the impact of the decay rate of ITNs on disease dynamics. This assessment approximated community behavior based on the increase in reluctance rate as the ITNs lost their physical or chemical integrity, quantified by the decay rate.
Our results showed that when there is limited movement between communities, is low, suggesting that the epidemic will likely die out regardless of ITN use except in the case of decay of physical or chemical integrity of the insecticide bed-net, where reaches 1.59 (Table 4). Moreover, when the coverage of ITNs reaches 60% (baseline scenario), remains low regardless of the mobility pattern displayed by the two communities only in the case of nearly isolated communities (R0 = 0.57) (Table 4).
In the second scenario, we found that the disease persists, as Ro>1, when the rate of ITN use is 60% and 30% in well- and under-resourced-urbanized neighborhoods, respectively. This implies that the spatial heterogeneity in ITN use affects the R0 and subsequently induces persistence in malaria prevalence. For homogeneous and heterogeneous movement, R0 increases significantly to 22.39 and 30.11, respectively (Table 4). When the use of ITNs is modeled as a linear relationship between use and ownership (in the context of simple linear regression ), the disease persists when communities are not isolated (Ro>1). In this case, Ro is higher than in the previous scenario in which ITN use is assimilated into its coverage rate. We observe R0 values of 0.96 for isolated communities, 28.82 for homogeneous movement, and 38.80 for heterogeneous movement (Table 4).
The fourth scenario revealed that when the decay rate of ITNs is incorporated into the model as a proxy to quantify the decision-making of communities (human behavior), the basic reproductive number is even higher. Specifically, R0 reaches 1.59 under isolation, 47.71 with homogeneous movement, and up to 60.53 under heterogeneous movement, indicating a major potential for an outbreak in urban settings despite average ITN coverage of 60% (Table 4).
3.3 Sensitivity analysis
Based on the results of Latin hypercube sampling (LHS) combined with the partial rank correlation coefficient (PRCC) conducted on the model parameters using Ro as the output metric, we conclude that the transition rate () is positively related to the increase in malaria infectivity regardless of the mobility pattern or spatial heterogeneity of ITN use by communities and appears to have more significant impact on Ro than other parameters (Supplementary Figure S1).
4 Discussion
During the last two decades, significant progress has been made in reducing malaria deaths in endemic areas through pharmaceutical (e.g., ACT-based co-therapy) and nonpharmaceutical control measures. While ITNs have proven effective in reducing malaria cases and fatalities in Ghana (Scates et al., 2020), our study reveals that ITN ownership does not always translate to its use.
Our findings challenge two common assumptions: first, that coverage rates can be interpreted as usage rates; and second, that ownership necessarily leads to usage - for example, assuming a proportional relationship such as usage = coefficient × ownership (Lengeler, 2004). Previous models that assumed uniform ITN distribution may have resulted in inaccurate predictions. Notably, our empirical findings are consistent with previous research indicating higher ITN use in low-density households (Abotsi, 2009). Living space was identified as a significant barrier to ITN use in densely populated areas such as James Town and Korle-Dudor (Danso-Wiredu, 2018). Despite their proven efficacy, ITNs face practical challenges in such communities due to space constraints. Because of the immediate pay-offs driven by socioeconomic conditions, repurposing ITNs for agriculture or fishing is common (Laxmi et al., 2022).
Previous studies highlight the complexity of ITN use beyond mere ownership, with discrepancies between possession and actual usage. For example, while 92% of households in one study owned at least one ITN, only 72% reported using them (Kateera et al., 2015). Key factors influencing use include gender (males are 0.4 times less likely to use nets than females), socioeconomic status (lower usage in low-SES households), net availability (reduced use with fewer than two nets), and sleeping arrangements (those sleeping on the floor are less likely to use nets). These findings reinforce the importance of targeted strategies addressing specific barriers to ITN adoption. Similarly, our study underscores the need for localized interventions, as malaria education, particularly after visits to healthcare facilities, significantly increases ITN use, consistent with previous research (Krezanoski et al., 2010; Amoran et al., 2012; Deribew et al., 2012).
Our research shows that in modeled urban areas reaching 60% ITN use without accounting for spatial heterogeneity can eliminate malaria. Incorporating spatial factors, on the other hand, results in persistent malaria, emphasizing the importance of local heterogeneity. In this case, 60% ITN use in each community rather than as an average for the entire urban area is required to eliminate the epidemic. The persistence of urban malaria despite high ITN coverage is explained by heterogeneous spatial patches, a proxy for heterogeneous social behavior.
National-level data from DHS and MIS (Breakthrough ACTION and VectorWorks) suggest that ITN use is often higher among lower-income groups due to targeted distribution efforts, and that ownership does not always align with wealth status. However, different patterns may emerge at the community level, as demonstrated by our study, which focuses on two specific urban areas where ITN usage does not necessarily correspond with access. This underscores the necessity of incorporating localized behavioral and contextual factors into the adoption of ITNs, as opposed to relying on broad generalizations.
Recognizing the difficulty of modeling human behavior, our proxy-based approach focuses on ITN non-use due to integrity loss. While it does not fully capture human behavior, it does shed light on urban malaria persistence, with ITN coverage exceeding 60%.
Our findings show that heterogeneous ITN use is influenced by spatially diverse human behavior. While communities recognize the benefits of ITNs, they are concerned about net decay. To increase uptake, we recommend shorter ITN distribution intervals combined with regular malaria education and follow-ups (Ngonghala, 2022). Integrating group and spatial heterogeneity into compartmental models improves understanding of urban malaria persistence.
This study notable limitation centered around the reliance on the basic reproductive number (Ro) as a crucial indicator of disease persistence. By exclusively using Ro, we overlooked scenarios involving backward bifurcation, where the disease can persist despite having Ro < 1 (Mamo, 2023). However, the utilization of this proxy and scenario-based approach underscored the significance of human behavior. This emphasizes a pivotal realization: the mere distribution of ITNs is insufficient to eradicate malaria. Instead, the focus should extend to community education, encouraging adoption, and promoting strict adherence to ITN use. This nuanced perspective sheds light on the intricate interplay between human behavior and disease dynamics.
Furthermore, we did not investigate the rivalry amongst competent urban malaria vectors in our study for simplicity. A growing body of startling evidence indicates that An. stephensi, a versatile species better suited to urban environments, outcompetes An. gambiae (Samarasekera, 2022). There is proof that An. stephensi can grow in harsh man-made environments, like bottles of water, polluted water, during the dry season (Surendran et al., 2018; Yared et al., 2023). Furthermore, An. stephensi’s opportunistic feeding and resting habits is propelling its spread throughout Africa’s cities. Although An. stephensi is not within the purview of our study, there is evidence that human behavior may contribute to the resurgence or persistence of urban malaria, particularly in the context of our demonstration that the community’s movement away from areas of varying exposure risk and the reluctance to use ITNs together account for the most likely explanation of the current progression of urban malaria. Despite the resistance of An. stephensi to pyrethroid (Hancock et al., 2020; Moyes et al., 2020), the primary ingredient in ITNs, which serve as a physical barrier that keeps mosquitoes and humans apart, frequent use of ITNs should still contribute to reducing the growing risk of malaria cases in African cities.
Another limitation of our study pertains to the indicator used for measuring ITN ownership. Specifically, this indicator fails to account for the actual usability of the nets owned by households. For instance, consider a household that possesses only one net but is possess of six members. This household would be classified with the same level of ITN ownership as another household that owns three nets but also has six members. Both households meet the basic criterion of owning at least one ITN; however, the practical implications for net usage among the members of each household are different. The household with a single net faces the challenge the distribution of that net among six individuals raising questions about accessibility and equitable use among members. In contrast, the household with three nets has a greater capacity to provide adequate protection for its members, thereby enhancing the likelihood of effective malaria prevention. Thus, the ITN ownership metric alone cannot comprehensively capture the nuances of ITN access and availability within different household contexts. While it is true that the ITN ownership indicator has limitations, it is essential to recognize its value in providing a basic measure of net access within households and providing context to analyze the dynamics of urban malaria.
5 Conclusion
For the programmatic planning of ITN distribution, we strongly recommend incorporating estimates that account for human behavior. Doing so will enhance the accuracy of the model outcomes. Additionally, we propose considering stochasticity in the model estimates to enable more versatile and informed decision-making processes. Furthermore, we advocate for an exploration of the backward bifurcation of the system using a similar model. This approach empowers policymakers to assess the effectiveness of existing non-pharmaceutical measures and gain a more precise understanding of the progression of malaria. Lastly, we emphasize the importance of allocating additional efforts and resources towards community education. This initiative aims to encourage communities to prioritize the use of ITNs and other malaria preventive measures. In the current state, the lack of space for ITNs, the use of other alternative like the use of insect spray, and the coils, especially in less privileged communities act as strong counterarguments inhibiting daily use of ITNs. By fostering greater awareness and adherence for this targeted audience utilizing socio-media for example, we anticipate a substantial reduction in malaria prevalence, potentially paving the way for malaria elimination in sub-Saharan Africa. However, challenges in implementing these recommendations should be acknowledged. The complexity of human behavior, socioeconomic constraints, and the need for consistent community engagement may hinder the widespread adoption of these measures. Addressing these obstacles will require ongoing effort, collaboration, and adaptation to local contexts to ensure the success of malaria control initiatives.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by both the ethics committees of ZEF and the Institute of Statistical, Social, and Economic Research (ISSER), University of Ghana, on 28 September 2018 and 04 February 2020, respectively. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. LC: Conceptualization, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing. CB: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing, Conceptualization, Investigation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Ministry of Culture and Science of North Rhine-Westphalia, Germany, through grant no. 9000055 offered to the University of Bonn’s One Health Graduate School.
Acknowledgments
We thank the One Health Graduate School, University of Bonn (Germany) for supporting this study and express our appreciation to Dr. Henri Tonnang for his advice and suggestions to improve this document.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1571912/full#supplementary-material
References
Aborode A. T., David K. B., Uwishema O., Nathaniel A. L., Imisioluwa J. O., Onigbinde S. B., et al. (2021). Fighting COVID-19 at the expense of malaria in africa: the consequences and policy options. Am. J. Trop. Med. Hyg 104, 26–29. doi: 10.4269/ajtmh.20-1181
Abotsi A. K. (2009). The Determinants of Ownership and Usage of Insecticide Treated Nets in the Upper East Region and its Impacts in the Control of Malaria. Available online at: https://papers.ssrn.com/abstract=2322398 (Accessed November 5, 2022).
Aguma H. B., Rukaari M., Nakamatte R., Achii P., Miti J. T., Muhumuza S., et al. (2023). Mass distribution campaign of long-lasting insecticidal nets (LLINs) during the COVID-19 pandemic in Uganda: lessons learned. Malar J. 22, 310. doi: 10.1186/s12936-023-04753-6
Agusto F. B., Del Valle S. Y., Blayneh K. W., Ngonghala C. N., Goncalves M. J., Li N., et al. (2013). The impact of bed-net use on malaria prevalence. J. Theor. Biol. 320, 58–65. doi: 10.1016/j.jtbi.2012.12.007
Agusto F. B., Teboh-Ewungkem M. I., and Gumel A. B. (2015). Mathematical assessment of the effect of traditional beliefs and customs on the transmission dynamics of the 2014 Ebola outbreaks. BMC Med. 13, 96. doi: 10.1186/s12916-015-0318-3
Ahmed A., Abubakr M., Ali Y., Siddig E. E., and Mohamed N. S. (2022). Vector control strategy for Anopheles stephensi in Africa. Lancet Microbe 3, e403. doi: 10.1016/S2666-5247(22)00039-8
Ahorlu C. S., Adongo P., Koenker H., Zigirumugabe S., Sika-Bright S., Koka E., et al. (2019). Understanding the gap between access and use: a qualitative study on barriers and facilitators to insecticide-treated net use in Ghana. Malar J. 18, 417. doi: 10.1186/s12936-019-3051-0
Amoran O. E., Fatugase K. O., Fatugase O. M., and Alausa K. O. (2012). Impact of health education intervention on insecticide treated nets uptake among nursing mothers in rural communities in Nigeria. BMC Res. Notes 5, 444. doi: 10.1186/1756-0500-5-444
Austin K. F. (2015). Dependency, urban slums, and the forgotten plagues. Sociol Perspect. 58, 286–310. doi: 10.1177/0731121414556542
Awine T., Malm K., Bart-Plange C., and Silal S. P. (2017). Towards malaria control and elimination in Ghana: challenges and decision making tools to guide planning. Glob Health Action 10, 1–9. doi: 10.1080/16549716.2017.1381471
Awine T. and Silal S. P. (2020). Accounting for regional transmission variability and the impact of malaria control interventions in Ghana: a population level mathematical modelling approach. Malar J. 19, 1–21. doi: 10.1186/s12936-020-03496-y
Awuah R. B., Anarfi J. K., Agyemang C., Ogedegbe G., and Aikins A.d.-G. (2014). Prevalence, awareness, treatment and control of hypertension in urban poor communities in Accra, Ghana. J. Hypertens. 32, 1203–1210. doi: 10.1097/HJH.0000000000000165
Awuah R. B., Asante P. Y., Sakyi L., Biney A. A. E. E., Kushitor M. K., Agyei F., et al. (2018). Factors associated with treatment-seeking for malaria in urban poor communities in Accra, Ghana. Malar J. 17, 1–8. doi: 10.1186/s12936-018-2311-8
Bichara D. and Iggidr A. (2018). Multi-patch and multi-group epidemic models: a new framework. J. Math Biol. 77, 107–134. doi: 10.1007/s00285-017-1191-9
Breakthrough ACTION and VectorWorks ITN access and use report (Breakthr ACTION Res). Available online at: https://breakthroughactionandresearch.org/resources/itn-use-and-access-report/ (Accessed March 25, 2025).
Briët O. J., Chitnis N., Fuseini G., Schwabe C., Monti F., Slotman M., et al. (2013). Effects of changing mosquito host searching behaviour on the cost effectiveness of a mass distribution of long-lasting, insecticidal nets: a modelling study. Malar J. 12, 1–8. doi: 10.1186/1475-2875-12-215
Cameron A. C. and Trivedi P. K. (2009). Microeconometrics using stata. 1st ed (Texas: STata Press), 680. College Station.
Chen S., Owolabi Y., Li A., Lo E., Robinson P., Janies D., et al. (2020). Patch dynamics modeling framework from pathogens’ perspective: Unified and standardized approach for complicated epidemic systems. PloS One 15, e0238186. doi: 10.1371/journal.pone.0238186
Chitnis N., Smith T., and Steketee R. (2009). A mathematical model for the dynamics of malaria in mosquitoes feeding on a heterogeneous host population. J. Biol. Dyn 2, 259–285. doi: 10.1080/17513750701769857
Danso-Wiredu E. Y. (2018). Housing strategies in low income urban communities in Accra, Ghana. GeoJournal 83, 663–677. doi: 10.1007/s10708-017-9792-9
Deribew A., Birhanu Z., Sena L., Dejene T., Reda A. A., Sudhakar M., et al. (2012). The effect of household heads training on long-lasting insecticide-treated bed nets utilization: a cluster randomized controlled trial in Ethiopia. Malar J. 11, 99. doi: 10.1186/1475-2875-11-99
Diekmann O., Heesterbeek J. A. P., and Metz J. A. J. (1990). On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math Biol. 28, 365–382. doi: 10.1007/BF00178324
Elder J. P., Botwe A. A., Selby R. A., Franklin N., and Shaw W. D. (2011). Community trial of insecticide-treated bed net use promotion in southern Ghana: the Net Use Intervention study. Transl. Behav. Med. 1, 341–349. doi: 10.1007/s13142-011-0032-4
Fobil J. N., Levers C., Lakes T., Loag W., Kraemer A., and May J. (2012). Mapping urban malaria and diarrhea mortality in accra, Ghana: evidence of vulnerabilities and implications for urban health policy. J. Urban Health 89, 977–991. doi: 10.1007/s11524-012-9702-x
Forouzannia F. and Gumel A. B. (2014). Mathematical analysis of an age-structured model for malaria transmission dynamics. Math Biosci 247, 80–94. doi: 10.1016/j.mbs.2013.10.011
Govella N. J., Okumu F. O., and Killeen G. F. (2010). Short report: Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am. J. Trop. Med. Hyg 82, 415–419. doi: 10.4269/ajtmh.2010.09-0579
GSS (2008). Ghana living standards survey report of the fifth round (GLSS 5) (Accra, Ghana: Ghana Statistical Service, GSS), 1–146.
Gu W. and Novak R. J. (2009). Predicting the impact of insecticide-treated bed nets on malaria transmission: The devil is in the detail. Malar J. 8, 1–10. doi: 10.1186/1475-2875-8-256
Gueron S., Levin S. A., and Rubenstein D. I. (1996). The dynamics of herds: from individuals to aggregations. J. Theor. Biol. 182, 85–98. doi: 10.1006/jtbi.1996.0144
Hancock P. A., Hendriks C. J. M., Tangena J.-A., Gibson H., Hemingway J., Coleman M., et al. (2020). Mapping trends in insecticide resistance phenotypes in African malaria vectors. PloS Biol. 18, e3000633. doi: 10.1371/journal.pbio.3000633
Heesterbeek H., Anderson R. M., Andreasen V., Bansal S., De Angelis D., Dye C., et al. (2015). Modeling infectious disease dynamics in the complex landscape of global health. Science 347, aaa4339–aaa4339. doi: 10.1126/science.aaa4339
Hemming-Schroeder E. and Ahmed A. (2023). Anopheles stephensi in Africa: vector control opportunities for cobreeding An. stephensi and Aedes arbovirus vectors. Trends Parasitol 39, 86–90. doi: 10.1016/j.pt.2022.11.011
Kateera F., Ingabire C. M., Hakizimana E., Rulisa A., Karinda P., Grobusch M. P., et al. (2015). Long-lasting insecticidal net source, ownership and use in the context of universal coverage: a household survey in eastern Rwanda. Malar J. 14, 390. doi: 10.1186/s12936-015-0915-9
Killeen G. F. and Smith T. A. (2007). Exploring the contributions of bed nets, cattle, insecticides and excitorepellency to malaria control: a deterministic model of mosquito host-seeking behaviour and mortality. Trans. R Soc. Trop. Med. Hyg 101, 867–880. doi: 10.1016/j.trstmh.2007.04.022
Killeen G. F., Smith T. A., Ferguson H. M., Mshinda H., Abdulla S., Lengeler C., et al. (2007). Preventing childhood malaria in africa by protecting adults from mosquitoes with insecticide-treated nets. PloS Med. 4, e229. doi: 10.1371/journal.pmed.0040229
Kish L. (1949). A procedure for objective respondent selection within the household. J. Am. Stat. Assoc 44, 380–387. doi: 10.2307/2280236
Klu D., Aberese-Ako M., Manyeh A. K., Immurana M., Doegah P., Dalaba M., et al. (2022). Mixed effect analysis of factors influencing the use of insecticides treated bed nets among pregnant women in Ghana: evidence from the 2019 Malaria Indicator Survey. BMC Pregnancy Childbirth 22, 258. doi: 10.1186/s12884-022-04586-2
Koenker H. and Kilian A. (2014). Recalculating the net use gap: A multi-country comparison of ITN use versus ITN access. PloS One 9, e97496. doi: 10.1371/journal.pone.0097496
Krezanoski P. J., Comfort A. B., and Hamer D. H. (2010). Effect of incentives on insecticide-treated bed net use in sub-Saharan Africa: a cluster randomized trial in Madagascar. Malar J. 9, 186. doi: 10.1186/1475-2875-9-186
Lakshmikantham V., Leela S., and Martynyuk A. A. (1989). Stability analysis of nonlinear systems (Heidelberg New York Dordrecht London: Springer International Publishing Switzerland). Taylor&Francis Group LLC.
Laxmi, Ngonghala C. N., and Bhattacharyya S. (2022). An evolutionary game model of individual choices and bed net use: elucidating key aspect in malaria elimination strategies. R Soc. Open Sci. 9, 220685. doi: 10.1098/rsos.220685
Lengeler C. (2004). Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2:1465–1858. doi: 10.1002/14651858.CD000363.pub2
Lim S. S., Fullman N., Stokes A., Ravishankar N., Masiye F., Murray C. J. L., et al. (2011). Net benefits: A multicountry analysis of observational data examining associations between insecticide-treated mosquito nets and health outcomes. PloS Med. 8:1–13. doi: 10.1371/journal.pmed.1001091
Macdonald G. (1950). The analysis of infection rates in diseases in which superinfection occurs. Trop. Dis. Bull. 47, 907–915.
Madhumathi J., Sinha R., Veeraraghavan B., and Walia K. (2021). Use of “Social media”—an option for spreading awareness in infection prevention. Curr. Treat Options Infect. Dis. 13, 14–31. doi: 10.1007/s40506-020-00244-3
Mamo T. T. (2023). Bifurcation analysis of hepatitis B virus with non-cytolytic cure process on infected liver and blood cells. Sci. Rep. 13, 7018. doi: 10.1038/s41598-023-27468-9
Marino S., Hogue I. B., Ray C. J., and Kirschner D. E. (2008). A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254, 178–196. doi: 10.1016/j.jtbi.2008.04.011
Moyes C. L., Athinya D. K., Seethaler T., Battle K. E., Sinka M., Hadi M. P., et al. (2020). Evaluating insecticide resistance across African districts to aid malaria control decisions. Proc. Natl. Acad. Sci. 117, 22042–22050. doi: 10.1073/pnas.2006781117
Nakul C., Cushing J. M., and Hyman J. M. (2006). Bifurcation analysis of a mathematical model for malaria transmission. SIAM J. Appl. Math 67, 24–45. doi: 10.1137/050638941
Ngonghala C. N. (2022). Assessing the impact of insecticide-treated nets in the face of insecticide resistance on malaria control. J. Theor. Biol. 555, 111281. doi: 10.1016/j.jtbi.2022.111281
Ngonghala C. N., Del Valle S. Y., Zhao R., and Mohammed-Awel J. (2014). Quantifying the impact of decay in bed-net efficacy on malaria transmission. J. Theor. Biol. 363, 247–261. doi: 10.1016/j.jtbi.2014.08.018
Okumu F. O., Kiware S. S., Moore S. J., and Killeen G. F. (2013). Mathematical evaluation of community level impact of combining bed nets and indoor residual spraying upon malaria transmission in areas where the main vectors are Anopheles arabiensis mosquitoes. Parasit Vectors 6, 1–13. doi: 10.1186/1756-3305-6-17
Orish V. N., Maalman R. S.-E., Donkor O. Y., Ceruantes B. Y. H., Osei E., Amu H., et al. (2021). Assessing health-seeking behaviour and malaria prevention practices among communities in four districts of the Volta Region of Ghana. Malar J. 20, 450. doi: 10.1186/s12936-021-03986-7
Prudêncio M. (2020). In fairness to mosquitoes. Trends Parasitol 36, 876–877. doi: 10.1016/j.pt.2020.08.003
Ross R. (1911). Some quantitative studies in epidemiology. Nature 87, 466–467. doi: 10.1038/087466a0
Samarasekera U. (2022). A missed opportunity? Anopheles stephensi in Africa. Lancet 400, 1914–1915. doi: 10.1016/S0140-6736(22)02483-7
Scates S. S., Finn T. P., Wisniewski J., Dadi D., Mandike R., Khamis M., et al. (2020). Costs of insecticide-treated bed net distribution systems in sub-Saharan Africa. Malar J. 19, 1–18. doi: 10.1186/s12936-020-03164-1
Sherrard-Smith E., Hogan A. B., Hamlet A., Watson O. J., Whittaker C., Winskill P., et al. (2020). The potential public health consequences of COVID-19 on malaria in Africa. Nat. Med. 26, 1411–1416. doi: 10.1038/s41591-020-1025-y
Smith D. L., Battle K. E., Hay S. I., Barker C. M., Scott T. W., and McKenzie F. E. (2012). Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PloS Pathog 8, e1002588. doi: 10.1371/journal.ppat.1002588
Surendran S. N., Sivabalakrishnan K., Gajapathy K., Arthiyan S., Jayadas T. T. P., Karvannan K., et al. (2018). Genotype and biotype of invasive Anopheles stephensi in Mannar Island of Sri Lanka. Parasit Vectors 11, 3. doi: 10.1186/s13071-017-2601-y
Tutu R. A., Boateng J. K., Busingye J. D., and Ameyaw E. (2017). Asymmetry in an uneven place: migrants’ lifestyles, social capital, and self-rated health status in James Town, Accra. GeoJournal 82, 907–921. doi: 10.1007/s10708-016-9723-1
UN-Habitat Ghana (2009). Accra urban profile (Accra, Ghana: Regional and Technical Cooperative Division), 1–36.
van den Driessche P. (2017). Reproduction numbers of infectious disease models. Infect. Dis. Model 2, 288–303. doi: 10.1016/j.idm.2017.06.002
Verdonschot P. F. M. and Besse-Lototskaya A. A. (2014). Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 45, 69–79. doi: 10.1016/J.LIMNO.2013.11.002
Villena O. C., Ryan S. J., Murdock C. C., and Johnson L. R. (2022). Temperature impacts the environmental suitability for malaria transmission by Anopheles Gambiae and Anopheles stephensi. Ecology 103, e3685. doi: 10.1002/ecy.3685
WHO (2022). World malaria report 2021. Available online at: https://www.who.int/publications-detail-redirect/9789240040496 (Accessed March 20, 2024).
World Health organization (2020). World malaria report 2020: 20 years of global progress and challenges (Geneva: World Health Organization).
World Health Organization (2020). The potential impact of health service disruptions on the burden of malaria: A modelling analysis for countries in sub-Saharan Africa (Geneva), 1–44.
Keywords: insecticide-treated bed-net use, ordinary differential equations, urbanization, patch model, Ghana
Citation: Savi MK, Childs LM and Borgemeister C (2025) Beyond insecticide treated bed nets coverage to heterogeneous human behaviors and spatial realities. Front. Malar. 3:1571912. doi: 10.3389/fmala.2025.1571912
Received: 11 February 2025; Accepted: 21 April 2025;
Published: 16 May 2025.
Edited by:
Annette Elizabeth Kaiser, University of Duisburg-Essen, GermanyReviewed by:
Enock Benito, Parul University, IndiaGabrielle Hunter, Johns Hopkins Center for Communication Programs, United States
Ruijun Zhao, Minnesota State University, Mankato, United States
Copyright © 2025 Savi, Childs and Borgemeister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Merveille Koissi Savi, bWVydmVpbGxla29pc3NpLnNhdmlAZ21haWwuY29t
 Merveille Koissi Savi
Merveille Koissi Savi Lauren M. Childs
Lauren M. Childs Christian Borgemeister
Christian Borgemeister