- 1Faculté de Medecine et d’Odontostomatologie (FMOS), University of Sciences, Techniques and Technology of Bamako (USTTB), Bamako, Mali
- 2University Clinical Research Center (UCRC), Universite des Sciences, des Techniques et des Technologies de Bamako (USTTB), Bamako, Mali
- 3Mali International Center for Excellence in Malaria Research (Mali-ICEMR), Universite des Sciences, des Techniques et des Technologies de Bamako (USTTB), Bamako, Mali
- 4Infectious Diseases and Medical Entomology Research and Training Center (IDMERTC), Universite des Sciences, des Techniques et des Technologies de Bamako (USTTB), Bamako, Mali
- 5Programme National de Lutte contre le Paludisme du Mali, Programme National de Lutte contre le Paludisme (PNLP), Bamako, Mali
- 6Celia Scott Weatherhead School of Public Health & Tropical Medicine at Tulane University, New Orleans, LA, United States
Background: Urban malaria is an increasing public health issue for Africans in cities experiencing rapid demographic growth. School children bear the high burden of malaria, which affects their health and education; however, they are not covered by control strategies such as seasonal malaria chemoprevention. This study aims to evaluate the school-based prevalence of Plasmodium falciparum infection to inform targeted malaria control strategies in urban and peri-urban settings of Bamako, Mali.
Methods: The city of Bamako in Mali was divided into four ecological strata based on the risk for malaria transmission using GIS tools (urban center, high altitude, riverside, and peripheral neighborhoods). Within each stratum, three schools close to the community health center were chosen to randomly select 200 to 230 children aged 6 to 13 years old per school to collect information on malaria risk factors and test for P. falciparum infection using malaria rapid diagnostic tests (mRTDs) and microscopy.
Results: Overall, the prevalence of P. falciparum infection varied from 0% to 15.5% between study sites. Travel history and fever were not associated with an increased risk of infection (p > 0.05). The odds of malaria infection increased by 2.4 among children with anemia (OR = 2.38, 95% CI = 1.57, 3.61) and 3.8 among children living in an urban site along the Niger River (OR = 3.37, 95% CI = 2.05, 7.45).
Conclusion: This study shows significant spatial variation of P. falciparum infection within Bamako urban settings. The results suggest that, as in rural villages of Mali, school-aged children should be considered a major malaria parasite reservoir in the urban settings of Bamako. Interventions targeting this specific age group could reduce the malaria burden in this area.
1 Background
Despite a decrease in malaria morbidity and mortality following a large scaling-up of malaria control strategies in the past two decades, an increase of five million cases was observed in many sub-Saharan African (SSA) countries between 2021 and 2023 (WHO, 2024). The transmission pattern and burden of the disease differ between urban and rural areas; consequently, the malaria response in urban areas requires data on the determinants that are unique to urban ecosystems and lead to a focal malaria transmission. Urban areas are considered to have a low risk for malaria because of improved housing, socioeconomic status, expanded personal protection, access to effective diagnosis and treatment, and limited breeding sites for Anopheles mosquitoes. While urbanization is expected to lower malaria transmission, many African cities are facing rapid and unplanned urbanization that creates favorable conditions for persisting prevalence and the spread of malaria (Tatem et al., 2013; Kabaria et al., 2017; WHO, 2022; Merga et al., 2025). The World Health Organization (WHO) indicated that 7 out of 10 people in Asia and Africa will be living in urban areas. Bamako is the largest city in Mali, with more than three million inhabitants. Bamako and its vicinity are characterized by urban areas (with higher population density-organized neighborhoods, formal housing, and infrastructure) and the peri-urban areas located at the fringes of the city (a transitional zone that blends rural and urban characteristics, often with informal settlements). Several conditions specific to African cities make urban malaria a challenge for the healthcare system in the context of elimination. These include 1) the growing number of people living in urban areas with approximately 30% and 50% of a country’s inhabitants living in cities, 2) heterogeneity in malaria transmission dynamics across cities and high endemicity in the neighboring rural villages, 3) limited evidence-based control strategies adapted to urban areas in many endemic countries, 4) frequent and continued movement of population from rural to urban areas, and 5) recent invasion of the African continent by the Asian malaria vector Anopheles stephensi adapted to urban environments (WHO, 2022).
In Mali, the extent of urban and peri-urban malaria prevalence is high, particularly among school-age children who bear the highest prevalence and incidence of Plasmodium falciparum malaria but who are not covered by malaria control interventions including seasonal malaria chemoprevention (SMC) and free malaria treatment delivered to children under 5 years old (Toure et al., 2016; Coulibaly et al., 2022; Toure et al., 2022). In contrast to rural settings, population surveys carried out in the past two decades showed a low prevalence of malaria (1%–3%) in Bamako (Programme National de Lutte contre le paludisme au Mali, 2021), while reports from health facility-based data suggest an increase of 53.5% in the annual number of malaria cases from 344,983 cases in 2020 (Enquête Démographique et de Santé (EDS) Mali, 2020) to 529,557 cases in 2023 (Enquête Démographique et de Santé (EDS) Mali, 2023). A high incidence of malaria in the general population (Cissoko et al., 2022) and a two-fold increase in malaria incidence among children 6–59 months between 2018 and 2021 have also been reported (Programme National de Lutte contre le paludisme au Mali, 2021). One limitation of the national malaria surveys, including demographic and health surveys and multiple indicator cluster surveys, is their focus on pregnant women and children under 5 years of age. These surveys do not provide malaria prevalence data on school-age children who increasingly bear a high burden of the disease and represent an essential source of human-to-mosquito transmission of P. falciparum (Nundu et al., 2022). In addition, school-aged clinical malaria episodes have always been associated with lower academic performance, school attendance, behavior, and cognitive development in children (Fernando et al., 2010; Vorasan et al., 2015; Jabir et al., 2025).
Understanding malaria epidemiology in this specific population across urban and peri-urban areas of Bamako is necessary to adapt control interventions and prevention for a better implementation and design of control strategies. With a high school attendance rate in urban settings, a malaria school-based survey provides a more representative estimate of the prevalence of malaria infection in school-aged children, which is a valuable indicator of a community-wide malaria burden and identifies hot spot areas requiring additional focus. School-based surveys are also cost-effective and relatively simple to implement, making them a practical tool for routine malaria surveillance (Brooker et al., 2009; Swana et al., 2018; Sixpence et al., 2024). This study aimed to assess the prevalence and risk factors associated with P. falciparum malaria infection among school-aged children in Bamako and surrounding areas. The underlying hypothesis is that optimal control of urban malaria depends on accurately describing the spatial heterogeneity of the malaria parasite reservoir in Bamako. These findings are intended to inform efficient and targeted malaria control interventions in urban settings of Africa, like Bamako, Mali.
2 Methods
2.1 Study area and site selection
Bamako is the capital city of Mali located on the banks of the Niger River and surrounded by hills at approximately 12.65° N latitude and −7.98° W longitude and has an approximate altitude of 326 m. It has an estimated population of 2.93 million in 2023, representing nearly 14% of Mali’s population, and has one of the highest annual urban growth rates of 4%–5%. The climate is Sudan savanna type, characterized by a dry season from November to May and a rainy season from June to October with an annual precipitation reaching 800–1,000 mm. Bamako is administratively divided into six communes, each having a level 2 district referral hospital (CSRef) covering a dozen community health centers (CSCOM). The CSCOM provides primary care serving an average population of 44,000 inhabitants. The CSCOM is led by a general physician and provides primary care to the community. The city has four level 3 university hospitals and hosts over half of the country’s 1,679 for-profit private facilities. Malaria control efforts are based on the premise of low urban transmission and guided by three main strategies: prompt access to effective anti-malarial ACT for confirmed cases, universal coverage with scale-up of long-lasting insecticide-treated nets (LLINs), and use of intermittent preventive treatment (IPT) by pregnant women. No SMC is currently implemented.
2.1.1 Study site selection
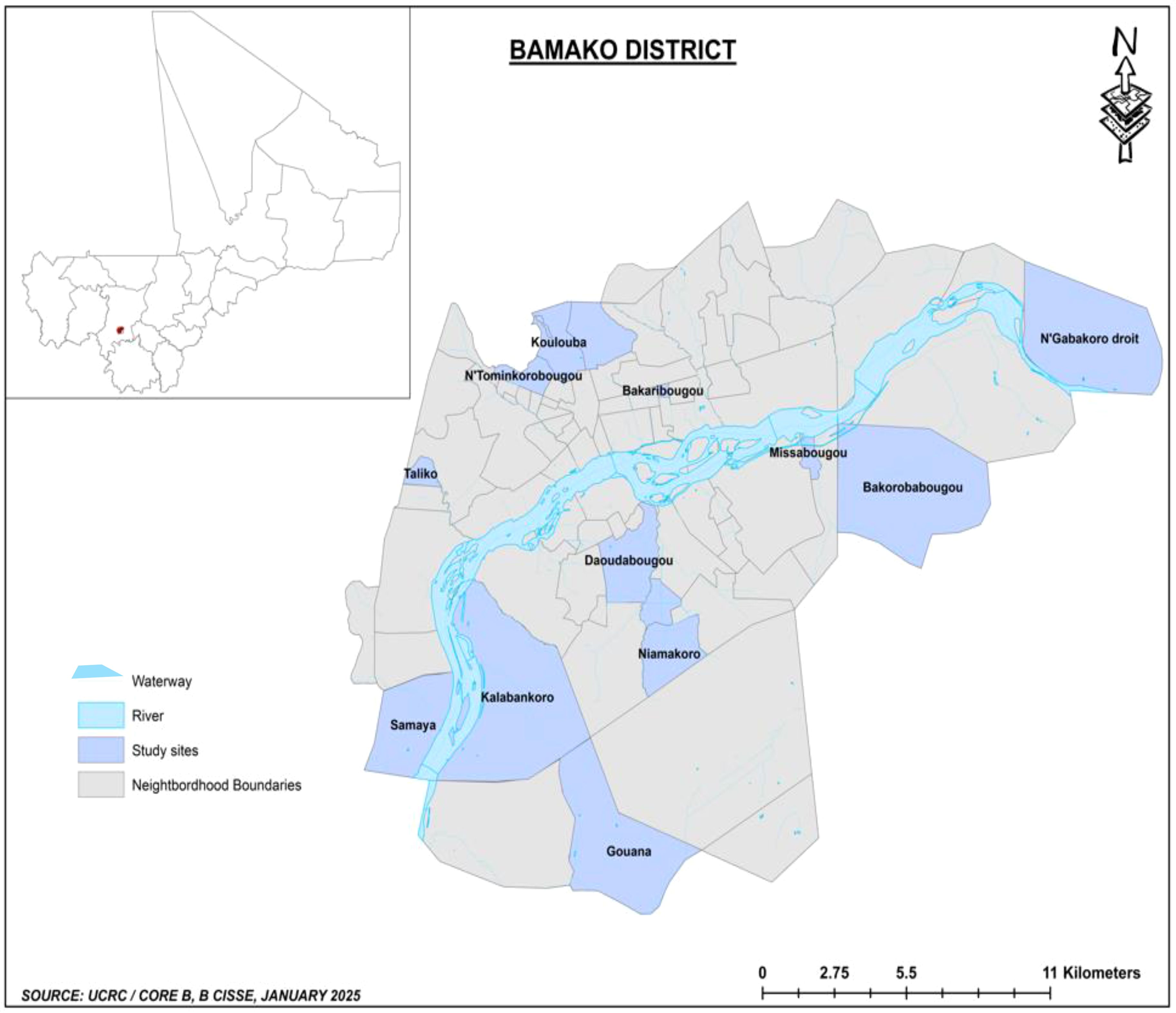
Urban studies across Africa have used different approaches to characterize heterogeneity including population density (persons per square kilometer), socioeconomic factors (housing types, water management, electricity, etc.), environment (the extent of non-agricultural economic activities), and accessibility to services such as health, education, and administration (Vlahov and Galea, 2002; Hay et al., 2005a). For this study, Bamako was divided into four geographic strata based on potential variability in the risk of malaria transmission including the position of the neighborhood in the administrative-defined city limit, the type of neighborhood (informal settlement, old neighborhood, wealthy neighborhood), the influence of altitude, and the proximity to water bodies (e.g., riverside neighborhood) favorable to the proliferation and the persistence of Anopheles mosquito breeding sites (Enquête Démographique et de Santé (EDS) Mali, 2023). The four strata are as follows: 1) urban central district neighborhoods, including administrative and commercial districts; old popular neighborhoods made up of traditional mud brick dwellings, gradually evolving toward semipermanent housing (e.g., Niarela, Medine, Bozola); and modern popular neighborhoods made up of permanent or semipermanent houses (e.g., Lafiabougou, Sogoniko, etc.). These neighborhoods are considered as low-risk transmission areas due to higher population density-organized neighborhoods, formal housing, and infrastructure; 2) urban hill neighborhoods located on hills/elevated areas or at the edge of hills with more vegetation and humidity that are favorable to mosquito populations (e.g., Point G, Koulouba, Sogonafin); 3) urban riverside neighborhoods located along the River Niger exposed to potential year-round mosquito breeding sites related to activities such as fishing or agricultural and gardening (e.g., Badalabougou, Torokorobougou, Moribabougou); and 4) peripheral and peri-urban neighborhoods, formerly called “spontaneous neighborhoods,” initially made of informal settlements and usually located on the outskirts of the city (e.g., Bankoni, Sirakoroni, Talico, Niamakoro, Sabalibougou, etc.). These neighborhoods are a blend of rural and urban characteristics or are partially urbanized with favorable conditions for malaria transmission related to mixed land use with agricultural activities, sporadic housing, and limited infrastructure, and they often lack consistent access to clean water, healthcare, and educational services.
For the selection of the neighborhood for school surveys, 23 neighborhoods were pre-selected within the four classifications at the first stage, based on higher volume of outpatients and completeness of routine malaria surveillance records of the community health centers (CSCOM) and the presence of an elementary school (grades 1 to 9) in the vicinity of the CSCOM. This first stage selection involved six sites in urban central district neighborhoods (Tominkorobougou, Bakaribougou, Sikoroni, Bacodjicoroni, Niamacoro, and Bougouba), five sites in the urban riverside neighborhoods (Kalabancoro, Torokorobougou, Bougouba, Missabougou, Kalabambougou and Ngabakoro Droit), four sites in the urban hill neighborhoods located on top or the flank of a hill (Koulouba, Taliko, Djalakorodji, and Daoudabougou), and eight sites on the peripheral neighborhood of Bamako (Dogodouman, Moribabougou, Bakorobabougou, Samaya, Kabala, Gouana, Senou, and Yirimadio). The second and final stage of selection (Figure 1) involved 12 sites randomly selected to represent the four urban and ecological settings within and around Bamako: 1) central district neighborhood sites (Tominkorobougou, Bakaribougou, and Niamakoro), 2) urban neighborhoods located on top or along the flank of a hill (Koulouba, Taliko, and Daoudabougou), 3) urban riverside neighborhood sites (Kalabancoro, Missabougou, and Ngabakoro Droit), and 4) peripheral and peri-urban neighborhoods (Samaya, Gouana, and Bakorobabougou).
2.2 Study design
A school-based malaria prevalence survey (SMPS) was conducted to assess key malaria indicators among school-aged children, including the prevalence of malaria infection determined through microscopy or rapid diagnostic tests (RDTs) as primary outcomes. Other secondary outcomes include the proportion of children with fever, RDT positivity rate among febrile school children, the proportion of children with anemia, the proportion of households with insecticide-treated nets (ITNs), and the proportion of school children who slept under an ITN the previous night.
In each of the 12 schools selected for the survey, a total of 200 to 230 children were investigated in the study based on the rapid urban malaria appraisal (RUMA) approach (Wang et al., 2005). The study population included school children aged 6 to 13 years who were residents of one of the 12 selected quarters and attending their local school. The study was carried out in December after the end of the rainy season, corresponding to the dry-cold season.
2.2.1 Community engagement procedures
The study preparation encompassed multiple stages from the protocol’s conception and validation of the protocol to the time of data collection (Figure 2). Studies in school settings remain a sensitive topic and require complex levels of responsibility. Thus, a first meeting called “the kickoff meeting” was organized at a hotel in Bamako, and representatives were present from the health and education ministries and the district and local level education and health authorities. In addition, since the participants’ parents were well-organized in Mali, their representatives were also invited to this meeting. Just before the start of the school year in October, the research team organized a meeting with the administration, teachers, representatives of community health workers (CHWs), and the school children’s parents’ association of each school to present the research protocol with its benefits and risks of participation.
Malaria epidemiology was briefly presented, describing the benefits and risks for participants and the importance of the findings for improving current control strategies against malaria. At the end of each meeting, the director of the school provided a list of all students from which we performed a random selection of 200 to 230 children aged between 6 and 13 years old. Informed consent forms were sent to the parents of selected children during the third week of November, with additional information provided to those who requested further clarification about their child’s participation. A home visit by a group composed of one investigator, one school representative, and one local CHW was organized.
2.2.2 Recruitment and sample collection
The sample collection was conducted from 3 to 22 December 2024. A meeting was organized with the schoolteachers, representatives of school children’s parents, and the community health officer to explain the study’s procedures, risks, and benefits for the participants and communities. The school administration provided the complete list of all children registered per class a week before the survey for each school. A total of 200 to 230 children were randomly selected proportional to age and gender from this list for this study. Written informed consent was obtained from the parents of each child participant, along with a telephone number to call for questions related to the study if needed. Written parental consent was required in addition to the child’s assent to participate in the study. Children were interviewed with the assistance of schoolteachers regarding their socioeconomic situation and malaria infection histories. Both thin and thick blood films were taken on the same slide and stained with Giemsa stain. Data were collected by well-trained investigators with the help of the schoolteachers and the CHC representative. In total, three teams (four sites per team) composed of one supervisor, one clinician, two biologists, and two surveyors collected the data over 3 to 4 days per school. Data collection, management, and storage were performed using the electronic data capture platform REDCap®. At the end of each day, the data collected were synchronized with the server located at the University Clinical Research Center (UCRC) at Point G, Bamako. Queries and missing data were returned to the field supervisor for revisions or completion before data validation. To ensure confidentiality, only authorized investigators have access to the online data set, and a data sharing plan was developed for the present and future use of data. For each participant, sociodemographic data plus recent current use of individual preventive tools such as long-lasting insecticidal nets (LLINs) and SMC, travel history defined as one more night’s stay in any rural site of Mali the last month, and time spent the last time a child spent a day or more outside their residence were collected. Body temperature was measured using an electronic thermometer during the interview. Malaria rapid diagnostic tests (mRDTs) plus blood smear for microscopy were used to diagnose malaria in each participant. In addition, two dried blood spots were prepared per child for future molecular determination of malaria infection. Malaria-confirmed cases by mRDTs were treated with artemether–lumefantrine (ALU) based on the Mali national malaria control policy.
2.2.2.1 Malaria diagnosis
Each participant was screened for malaria parasite positivity using both mRDT and microscopy. The brand of mRDT used in this study was the SD BIOLINE Malaria Ag P.f/Pan test, a qualitative and differential test for the detection of histidine-rich protein II (HRP-II) antigen of P. falciparum and common Plasmodium lactate dehydrogenase (pLDH) of the Plasmodium species. The blood smear was prepared with a drop of blood on the slide obtained from a fingerprick, which was then air-dried, stained with 2% Giemsa for 30 min, and examined under an optical microscope with a ×100 oil immersion lens. Diagnostic decisions on smear results were made only after reviewing at least 100 fields. Two experienced microscopists independently examined each smear. Results were considered discordant if one microscopist reported a positive result and the other one a negative result, and in such cases, a third microscopist resolved the discrepancy. The use of both mRDT and microscopy was reasonably decided with respect to Mali’s national malaria case management strategies, which require on-site treatment for all confirmed malaria cases during a survey. Thus, treatment was given based on the result of mRDT, while parasite species identification was done using microscopy.
2.2.2.2 Statistical analysis
Continuous variables were reported as means with standard deviations (SD), and categorical variables were presented as counts and percentages. Descriptive analyses were performed to summarize the demographic characteristics of the study participants. Plasmodium falciparum infection prevalence results were stratified or controlled for school and ecogeographic zones. Differences between groups were assessed using chi-square or Fisher’s exact tests for categorical variables. To evaluate predictors of infection, we used multivariable logistic regression models, reporting adjusted odds ratios (aORs) and 95% confidence intervals (CIs). Variables included in the multivariable model were selected a priori based on literature and biological plausibility and included age group, sex, recent travel history to a rural village of Mali, use of LLINs the previous night, fever (T > 37.5°C), anemia (hemoglobin < 11.5 g/dL), recent malaria treatment, and school site location. Additional stratified analyses were performed based on the stratification described above to explore geographic variation in malaria prevalence. Separate logistic regression models were built to identify site-specific malaria risk factors. The results were visualized using forest plots to illustrate adjusted odds ratios and bar charts to compare malaria prevalence across geographic settings. To further assess spatial heterogeneity, we conducted a multilevel mixed-effects logistic regression model with random intercepts for school and ecogeographic site and quantified the proportion of variance in malaria infection attributable to each level of spatial aggregation. The R (Version 2024.12.0 + 467) and Prism GraphPad (Version 10.4.1) applications were used for statistical analysis and output presentation. The type I error threshold for all analyses was set at 5%.
2.2.3 Ethical approval
This study has been approved by the Institutional Review Board (IRB) of the University of Sciences, Techniques and Technologies of Bamako, Mali (USTT-B), under the reference number N°2024/110/CE/USTTB on 3 May 2024. Authorization was sought and obtained at the regional and district education offices for school involvement. The goal, risks, and benefits of the study were explained to the schoolteachers, parents of the children, and participants. Written informed consent was provided by the study participants’ parents for children less than 12 years of age. Consent was obtained from the parents, and assent from the participants aged 12 years and above. The confidentiality of the participants was ensured by using a coded number instead of their names. Patients with positive P. falciparum were treated with ALU, in accordance with Mali’s national malaria treatment guidelines (PNLP - Mali, 2021).
2.2.4 Funding source
The study was funded by the Mali International Center of Excellence in Malaria Research (Mali-ICEMR), sponsored by the US National Institute of Health (NIH) under grant number U19AI129387.
3 Results
3.1 Study flow chart: selection, enrolment, and population included in the analysis
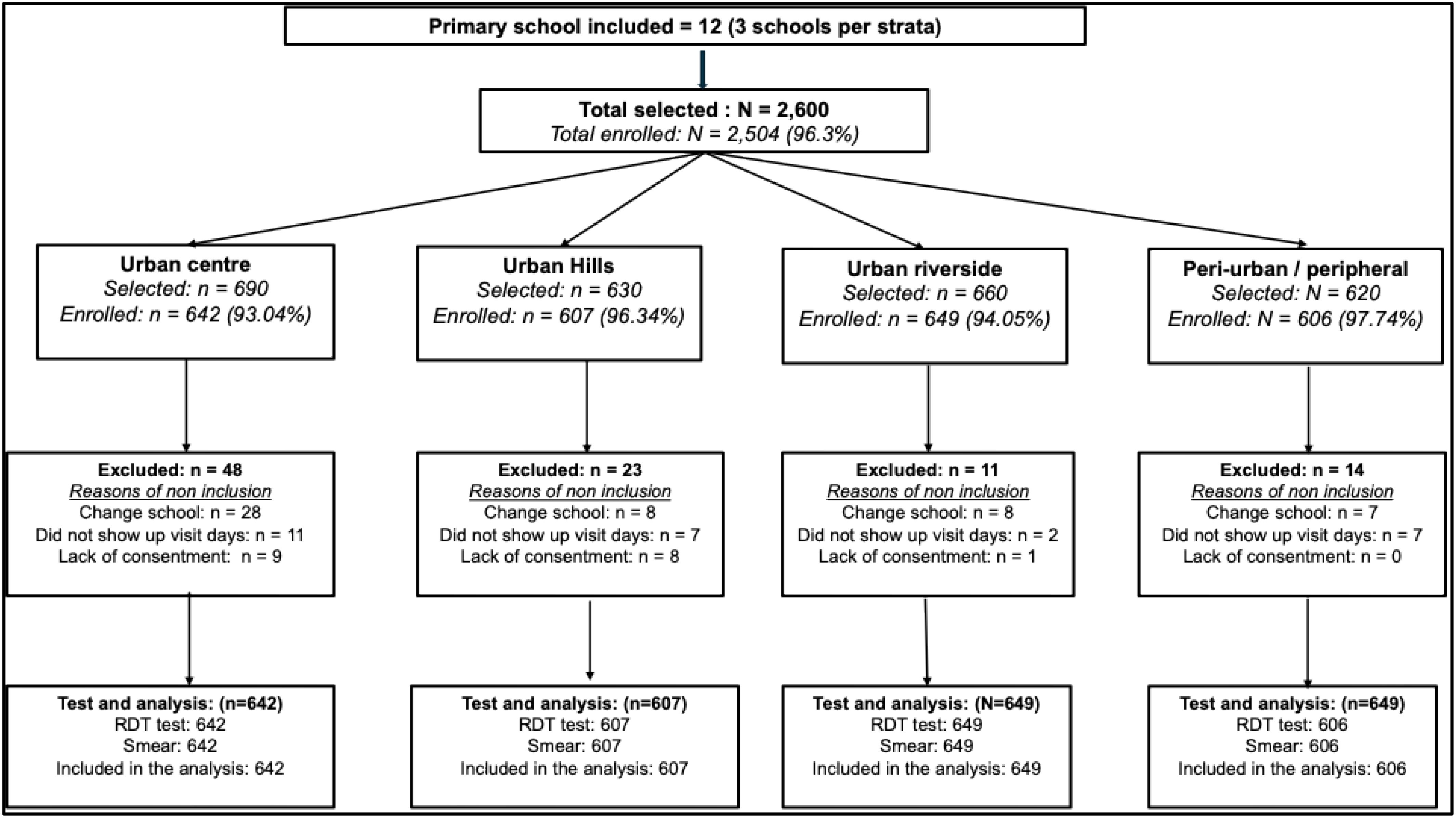
The total number of children randomly selected varied from 620 in peri-urban strata to 690 in the urban center. This variation depends on the variance of the total size of the schools in terms of attendance from the list provided by the administration. In total, 2,504 children were enrolled representing 96.3% of the total selected. All enrolled children were tested for malaria infection using both mRDT and microscopy. Therefore, all 2,504 were included in the analysis presented in this manuscript.
The proportion of children enrolled was always above 93% regardless of the strata. In each stratum, the main reason for non-enrolment was because the child had moved to another school before the survey, followed by those absent during the days of the survey. Except for the peri-urban strata, few parents did not return the signed consent form and therefore their child was excluded: nine in the urban center, eight in urban hills, and one in the urban riverside. The characteristics of the study population and their P. falciparum prevalence levels are shown in Table 1.
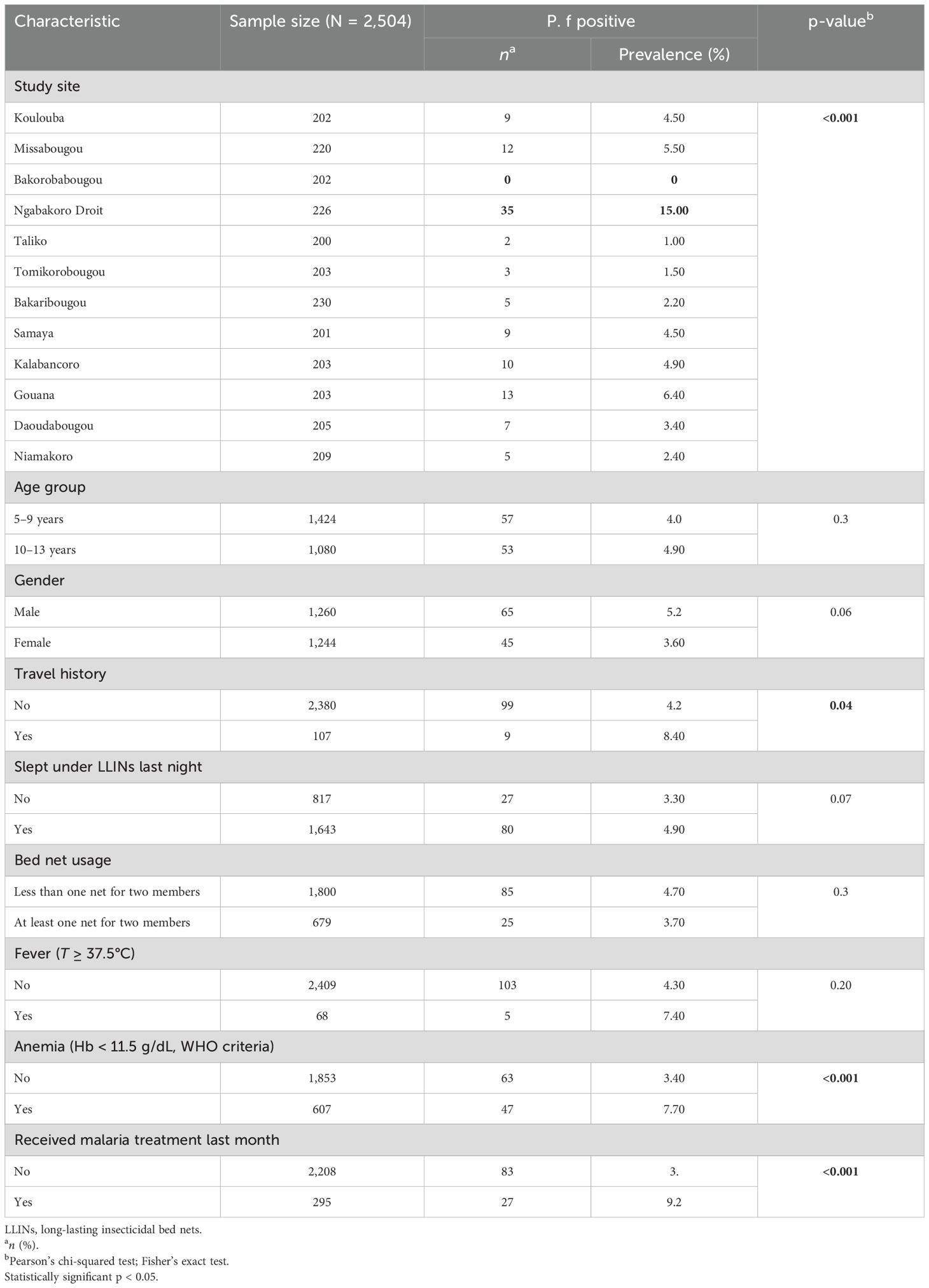
Table 1. Prevalence of Plasmodium falciparum parasitemia in relation to the characteristics of the study participants.
3.2 Demographic characteristics of participants, distribution of Plasmodium falciparum infection, and associated risk factors
A total of 2,504 children were screened for malaria parasitemia in 12 elementary schools representing the four ecological patterns of malaria transmission risks. The number screened per school varied from 200 to 230. The prevalence of P. falciparum infection showed a statistically significant variation between sites from 0% in Bakorobabougou to 15.0% in Ngabakoro Droit (p < 0.001). Children under 10 years of age represented 56.9% of the population with a mean age of 8.99 ± 2.2, and the male-to-female sex ratio was 1.01. Approximately 39.8% of children slept under the net the night before the survey, and approximately 33.05% of the participants lived in households meeting the LLIN universal coverage criteria. The overall prevalence of P. falciparum infection was 4.4% (110/2,504) and varied significantly across study sites (from 0.0% in Bakorobabougou to 15.0% in Ngabakoro Droit; p < 0.001) (Table 1). A higher prevalence was observed among children with a recent travel history in rural areas (p = 0.042), among children with anemia (p < 0.001), and among children who received malaria treatment a month before the survey (p < 0.001).
Between 200 and 230 children were screened in each school, and the number carrying P. falciparum infection varied from 0 in Bakorobabougou to up to 35 in Ngabakoro Droit. The highest number of infected participants resided near the Niger River (Figure 3).
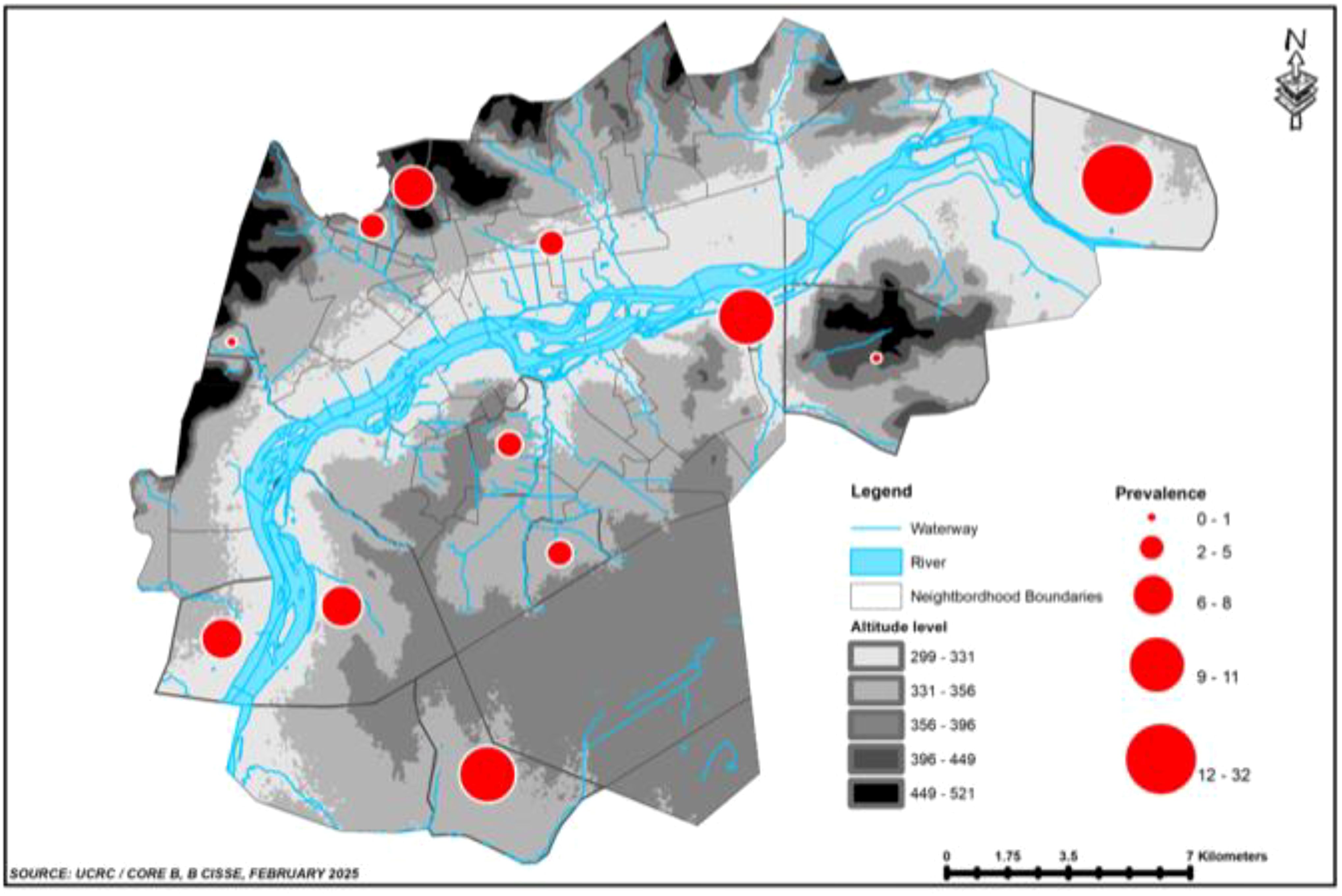
Figure 3. Spatial distribution of the prevalence of asymptomatic P. falciparum infection in December 2004.
Across the four geographical settings, the lowest prevalence of P. falciparum infection was observed in the central urban area (2.0%) and the highest along the Niger River (8.8%). Urban areas along the hills and peri-urban/peripheral sites showed approximately similar prevalence rates with 3.0 and 3.6%, respectively (Figure 4).
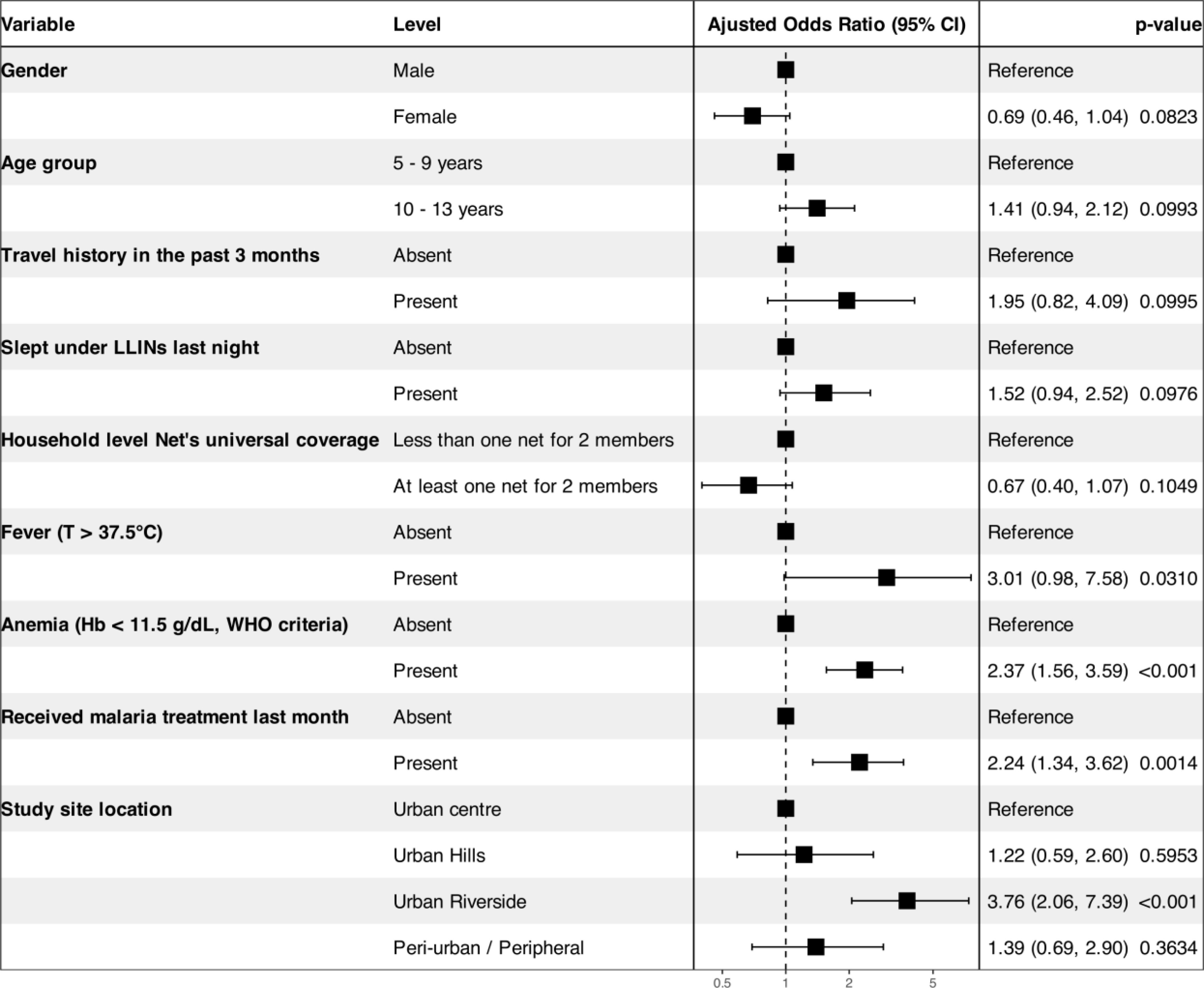
Figure 4. Overall risk factors associated with Plasmodium falciparum infection in Bamako and surrounding areas. Adjustment predictors: gender, age, recent travel to rural areas, LLINs individual use, LLINs universal coverage within households, fever, anemia, recent intake of antimalarial treatment, and location of the study site.
The odds of P. falciparum infection increased by 2.4 in the presence of anemia (aOR = 2.38, 95% CI = 1.57–3.61), by 2.1 among children who were treated for malaria in a 1-month period (aOR = 2.16, 95% CI = 1.29–3.51), and by 3.77 times among children living in urban areas located along the river compared to the sites of the central urban district neighborhoods of Bamako (aOR = 3.77, 95% CI = 2.05–3.25). However, age, gender, axillary temperature ≥ 37.5°C or history of fever within the past 48 h, travel history, sleeping under LLINs the night before the survey, and household meeting the universal coverage criteria did not show any association with P. falciparum infections.
3.3 Strata-specific risk factors associated with Plasmodium falciparum in Bamako, Mali
The evaluation of the association between the risk factors and P. falciparum infection in each of the four ecological settings (urban central district neighborhoods, urban hill neighborhoods, riverside, and peripheral) of Bamako is presented in Table 2. No statistically significant association was observed between P. falciparum infection and any of the selected risk factors in urban central district neighborhood and urban hill neighborhood settings. In urban riverside settings, only the presence of anemia was associated with P. falciparum infection with the risk of infection increasing with anemia (aOR = 2.79, 95% CI = 1.52–5.08). In the peri-urban setting, the odds of carrying malaria parasite increased with age (aOR = 1.76, 95% CI = 1.34–2.42), recent travel history (aOR = 5.57, 95% CI = 1.40–19.88), household not meeting the LLIN universal coverage criteria (aOR = 3.59, 95% CI = 1.11–14.88), anemia (aOR = 3.67, 95% CI = 1.39–10.04), and a recent episode of malaria (aOR = 4.50, 95% CI = 1.53–13.26).
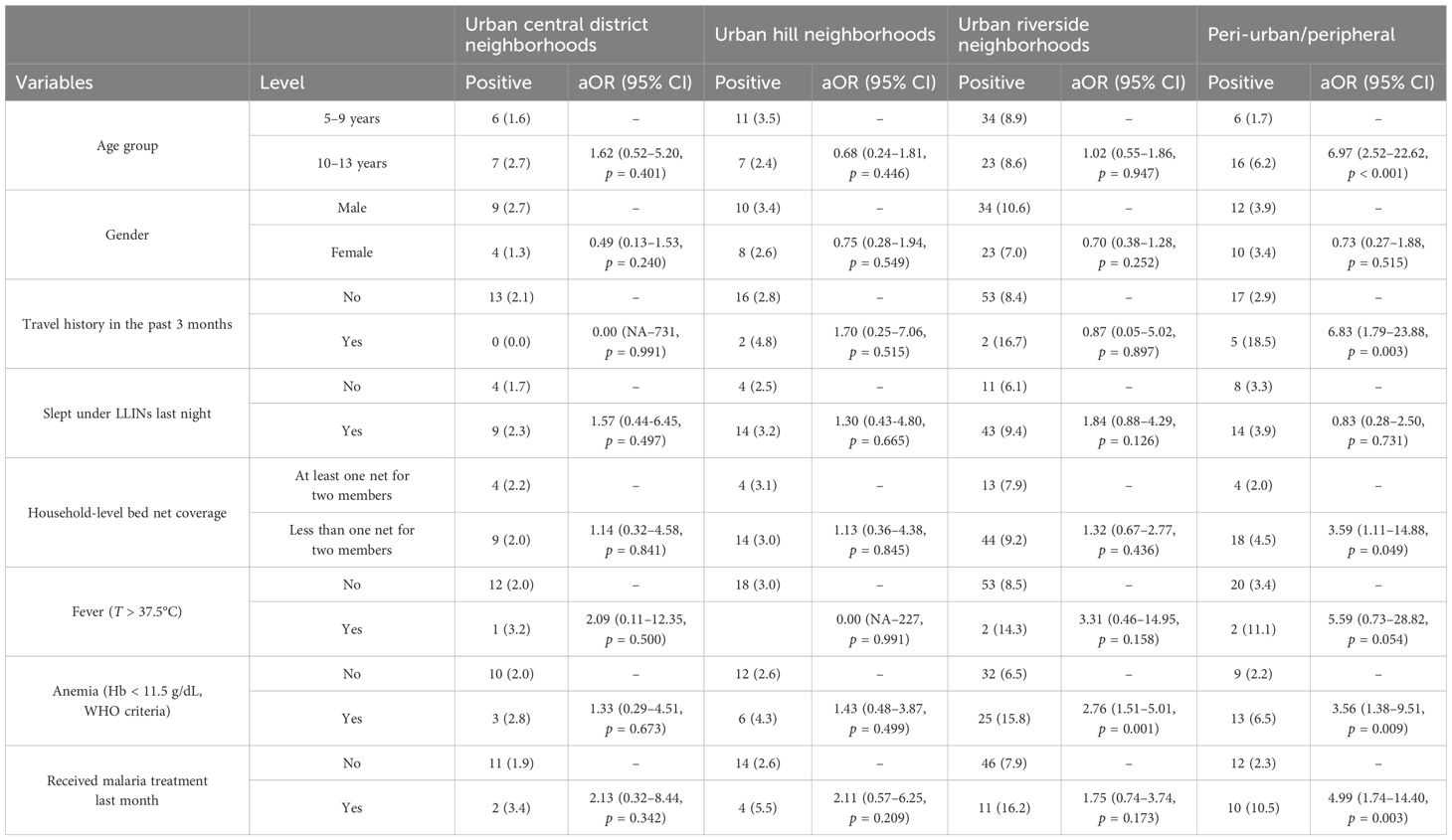
Table 2. Association between Plasmodium falciparum infection and the risk factors in different geographic areas of Bamako.
We fitted a mixed-effects logistic regression model with school nested within an ecogeographic site as a random intercept to evaluate the contribution of spatial heterogeneity in P. falciparum infection prevalence. The variance component for schools nested within ecogeographic sites was 0.561 (SD: 0.75), while that for ecogeographic site was 0.089 (SD: 0.30). The intraclass correlation coefficient (ICC) was 16.5%, suggesting a moderate clustering of malaria infection at the school level, independent of the ecogeographic site (Supplementary Figure S1).
4 Discussion
This study shows that P. falciparum malaria is highly prevalent among school-aged children in urban settings of Bamako, including during seasons traditionally associated with low transmission. Overall, the study shows that in Bamako, Mali, the prevalence of P. falciparum in school-aged children has a spatial signature varying from 0.0% to 15.5%. On average, the lowest prevalence was observed in sites located in the city’s central zone with approximately 2.03%, followed by the sites located on the hills or elevated areas with 2.96% and peri-urban sites with 3.63%. Sites along the Niger River showed the highest prevalence with 8.5%. Variance attributable to schools within geographical areas was 0.56, compared to 0.09 at the site level. Overall, moderate clustering and substantial heterogeneity at the school level were observed: ICC = 0.165 (see Supplementary Figure S1). These findings supported the idea of the geographical variability of the underlying factors that determine the association between urbanization and malaria as described across the country (Cissoko et al., 2022). The high prevalence in sites close to the river is not surprising and is consistent with the studies reporting data from the dry season in Dangassa, Mali, showing a high malaria vector density in houses near the river (Sogoba et al., 2007) and a clustering of malaria cases in parts of the village along the river (Shaffer et al., 2020). It was not surprising that the peripheral sites had the second greatest prevalence (3.63%) because similar observations were made elsewhere across Africa. The primary risk factors associated with this include housing type, agricultural activities, and the proximity of human habitats to mosquito breeding sites (Hay et al., 2005a; Robert et al., 2003; Machault et al., 2010).
Determining the intensity and the heterogeneity of malaria in urban and peri-urban areas of Bamako could have three major implications for community and control programs: the growing risk of uncomplicated and severe malaria in urban cities across the SSA, the uncontrolled and rapid urbanization leading to an environment favorable to malaria vectors as well as population movement between rural villages where the disease is endemic and Bamako, and finally the need to rethink and update the control strategies to the changes observed in malaria epidemiology over the past decades which concern both human, parasites, and vectors (Hay et al., 2005a; Robert et al., 2003; Machault et al., 2010).
4.1 Spatial variation of Plasmodium falciparum malaria infection prevalence across Bamako
Malaria infection prevalence varied significantly between geographic locations of the study sites with a lower prevalence observed in more urbanized sites. Similar observations were made across Africa between urban, neighboring peri-urban, riverside, and semi-rural areas (Hay et al., 2005b; Omumbo et al., 2005; Wilson et al., 2015). Malaria transmission in urban areas is complex and influenced by diverse environmental and socioeconomic factors. Our study revealed active malaria cases, suggesting the occurrence of transmission in the metropolitan areas studied. This finding is consistent with that of Robert et al (Robert et al., 2003), who, in their meta-analysis, highlighted the occurrence of transmission in most urban settings in sub-Saharan Africa, albeit at generally lower levels than in rural areas.
4.2 Localization of malaria parasite reservoir across Bamako
Identifying the areas of asymptomatic and symptomatic malaria cases among school-aged children, known as the most affected age group population in the urban settings of Bamako (Doumbia et al., 2022; Toure et al., 2022), could help better design focal interventions against malaria infection. Thus, these findings demonstrate that asymptomatic infected persons are found throughout the city during the low transmission season regardless of environmental or ecological conditions. Additionally, there is evidence that specific areas, such as urban sites along the river, are more affected. This phenomenon might be explained by the permanent presence of water bodies suitable for Anopheles mosquito breeding sites throughout all seasons, contributing to continuous transmission around these sites. Thus, entomological investigations are needed to determine how sites with this microclimate allow mosquito survival, reproduction, and dispersion, thereby ensuring transmission in the urban environments of Bamako (Cator et al., 2013; Wilson et al., 2015).
4.3 Risk factors associated with Plasmodium falciparum malaria infection in Bamako and surrounding sites
The overall risk assessment analysis showed that the risk of P. falciparum infection increased significantly among anemic children. These findings are in line with those made elsewhere in Africa, with more than half of malaria cases in high and low endemic areas of India also having anemia specifically in children and women (Shankar et al., 2022).
Another important risk factor observed was the location of the participants’ houses or residences. Thus, when comparing the three other settings (urban hill neighborhoods, riverside, and peri-urban–peripheral) to the urban center sites, living along the Niger River was significantly associated with a four-fold increase in the risk of carrying P. falciparum parasites. Similar observations were made in rural and urban settings of Mali, where mosquito breeding sites were located along the river, contributing to maintaining malaria transmission during the dry season with the presence of infection among both vectors and humans (Sogoba et al., 2007; Amadou Tapily et al., 2024).
4.4 Risk factors associated with Plasmodium falciparum malaria infection within each of the four geographic settings across Bamako
In urban central district neighborhoods and urban hill sites, regardless of the risk factor, there was no evidence of an association with an increase or decrease in the risk of being infected with P. falciparum. However, in urban riverside neighborhoods, the risk of malaria infection increased three-fold in children with hemoglobin concentration less than 11 g/dL. This finding may be attributable to the fact that the ecological and environmental conditions are both favorable in these sites to maintain continuous exposure to malaria infection, as well as the possible presence of other water-borne diseases such as schistosomiasis, hookworms, dysentery, and diarrhea also causing anemia among children. These results are like those observed in Sélingué, Mali (Toure et al., 2016), and Dangassa (Keita et al., 2024) and call for multiple approaches targeting childhood diseases in areas where conditions are favorable to the spread of water-related infectious diseases, undernutrition, and parasitic diseases in children and adults. In addition, it has been well-established that as malaria transmission intensity increases, repeated malaria infections among young children are associated with chronic anemia. Thus, severe hemolytic anemia is one of the diagnostic criteria for severe malaria (White, 2018).
5 Strengths and limitations
This study represents P. falciparum malaria prevalence both spatially and in terms of sample size. This study also concerns a specific group of the population: the school-aged children, who have already been identified as the main reservoir for the parasite contributing to residual transmission in low transmission settings.
Limitations could be the time of the survey (in December) corresponding to the low transmission season with fewer malaria cases and the fact that the prevalence was only measured using RDT and microscopy, which could lead to an underestimation of the infection compared to the use of polymerase chain reaction (PCR) in this context.
6 Challenges
The step-by-step organization before and during the study implementation helped to anticipate several challenges related to community-based research in SSA urban areas. The main challenge encountered was the time to complete the survey. In Mali, school activities are planned from Monday to Friday, and only the afternoons of Wednesday and Thursday are free. Thus, we had to organize the sample collection according to the availability of the children, which extended the study period.
7 Conclusion
This study shows a significant spatial variation in the prevalence of P. falciparum infection in Bamako City among school-aged children, with a primary association between the risk of infection, anemia, and fever. The study reveals that P. falciparum malaria remains during the low transmission season, with a spatial heterogeneity to consider when planning control and prevention strategies. The parasite reservoir remains significant to keep an ongoing transmission and is mainly clustered in sites located near the Niger River and a few on the peripheral sites. Thus, school-based interventions against malaria such as promoting LLINs through school programs, the use of repellent against mosquito bites in school, the extension of SMC, and malaria vaccine to school-aged children could contribute considerably to reduce the disease burden by reducing the parasite reservoir in low- and high-transmission settings. Further quantitative and qualitative studies covering different malaria transmission seasons in this setting are needed to inform implementation research in urban and peri-urban areas.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of the Faculties of Medicine, Dentistry and Pharmacy of the Mali USTTB. Reference: N°2024/110/CE/USTTB of 3 May 2024. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MT: Resources, Writing – original draft, Investigation, Funding acquisition, Visualization, Validation, Conceptualization, Data curation, Writing – review & editing, Methodology, Supervision. FK: Data curation, Writing – review & editing, Visualization, Software, Formal Analysis, Conceptualization. DS: Investigation, Writing – review & editing, Supervision, Conceptualization. SKe: Investigation, Writing – review & editing, Supervision, Visualization. BK: Supervision, Investigation, Writing – review & editing. AA: Supervision, Investigation, Writing – review & editing. MK: Writing – review & editing, Supervision, Investigation, Conceptualization. DK: Supervision, Data curation, Writing – review & editing, Investigation, Methodology. SD: Writing – review & editing, Conceptualization, Supervision, Investigation. AY: Visualization, Writing – review & editing, Data curation, Methodology. AK: Writing – review & editing, Resources, Supervision. MM: Resources, Writing – review & editing, Supervision. SKa: Methodology, Investigation, Supervision, Writing – review & editing. CT: Supervision, Writing – review & editing, Project administration. BC: Software, Writing – review & editing, Visualization, Formal Analysis, Data curation. SMT: Supervision, Project administration, Writing – review & editing. KT: Data curation, Investigation, Supervision, Conceptualization, Writing – review & editing. HC: Software, Writing – review & editing, Conceptualization, Data curation, Visualization. ST: Supervision, Project administration, Writing – review & editing. AT: Writing – review & editing. JS: Data curation, Visualization, Writing – review & editing, Investigation, Formal Analysis, Software. NS: Validation, Conceptualization, Writing – review & editing, Funding acquisition, Methodology, Resources. MD: Validation, Methodology, Conceptualization, Project administration, Writing – review & editing, Funding acquisition, Resources. SD: Methodology, Conceptualization, Funding acquisition, Writing – review & editing, Visualization, Project administration, Resources, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the Mali International Center of Excellence in Malaria Research (Mali-ICEMR), sponsored by the US National Institute of Health (NIH) under grant number U19AI129387.
Acknowledgments
We thank the Mali Ministries of Health and Education, the local school authorities, and community health workers for facilitating this work. This study was also supported by the National Malaria Control Program of Mali (NMCP) and its partners, including the USAID Presidential Malaria Initiative Office of Bamako (PMI–USAID, Mali) and the NGO Musso Health based in Bakorobabougou on the study site.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1596496/full#supplementary-material
Supplementary Figure 1 | Random effects of school on malaria infection. Note: Random effects from the multilevel logistic regression model showing heterogeneity in Plasmodium falciparum infection prevalence across eco-geographic sites and nested schools. Variance attributable to schools within sites was 0.56 and, 0.09 at the site level. ICC = 0.165: moderate clustering and substantial heterogeneity at the school level.
Abbreviations
AIC, Akaike information criterion; aOR, adjusted odds ratio; CHC, community health center; CHW, community health worker; CI, confidence interval; ICC, interclass correlation coefficient; IRB, Institutional Review Board; LLINs, long-lasting insecticidal nets; NMCP, National Malaria Control Program; mRTDs, malaria rapid diagnostic tests; P. falciparum, Plasmodium falciparum; SD, standard deviation; SMC, seasonal malaria chemoprevention; SSA, Sub-Saharan Africa; WHO, World Health Organization.
References
Amadou Tapily I. S., Machault V., Niare S., Konate S., Diarra M., Doumtabe D., et al. (2024). Variation spatio-temporelle de la population Culicidae et de la transmission du paludisme en milieu urbain du district de Bamako au Mali. Int. J. Biol. Chem. Sci. 18, 1522–1540. doi: 10.4314/ijbcs.v18i4.24
Brooker S., Kolaczinski J. H., Gitonga C. W., Noor A. M., and Snow R. W. (2009). The use of schools for malaria surveillance and programme evaluation in Africa. Malar. J. 8, 231. doi: 10.1186/1475-2875-8-231
Cator L. J., Thomas S., Paaijmans K. P., Ravishankaran S., Justin J. A., Mathai M. T., et al. (2013). Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malar. J. 12, 84. doi: 10.1186/1475-2875-12-84
Cissoko M., Magassa M., Sanogo V., Ouologuem A., Sangare L., Diarra M., et al. (2022). Stratification at the health district level for targeting malaria control interventions in Mali. Sci. Rep. 12, 8271. doi: 10.1038/s41598-022-11974-3
Coulibaly D., Kone A. K., Kane B., Guindo B., Tangara B., Sissoko M., et al. (2022). Shifts in the clinical epidemiology of severe malaria after scaling up control strategies in Mali. Front. Neurol. 13, 988960. doi: 10.3389/fneur.2022.988960
Doumbia S., Toure M., Sogoba N., Alifrangis M., Diakite M., Diarra A., et al. (2022). The west africa ICEMR partnerships for guiding policy to improve the malaria prevention and control. Am. J. Trop. Med. Hyg. 107, 84–89. doi: 10.4269/ajtmh.21-1330
Enquête Démographique et de Santé (EDS) Mali N. M. C. P. (2020). Activity Report Summary Template. Reston, VA: ICF International.
Enquête Démographique et de Santé (EDS) Mali N. M. C. P. (2023). Activity Report Summary Template. Reston, VA: ICF International.
Fernando S. D., Rodrigo C., and Rajapakse S. (2010). The ‘hidden’ burden of malaria: cognitive impairment following infection. Malar. J. 9, 366. doi: 10.1186/1475-2875-9-366
Hay S. I., Guerra C. A., Tatem A. J., Atkinson P. M., and Snow R. W. (2005a). Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 3, 81–90. doi: 10.1038/nrmicro1069
Hay S. I., Shanks G. D., Stern D. I., Snow R. W., Randolph S. E., and Rogers D. J. (2005b). Climate variability and malaria epidemics in the highlands of East Africa. Trends Parasitol. 21, 52–53. doi: 10.1016/j.pt.2004.11.007
Jabir M., Panigrahi D. K., Baig M. M., Balakrishnan V., Panda P. K., Kumar A., et al. (2025). Malaria and missed school days: exploring school absenteeism patterns and local strategies in Odisha, India. Front. Public Health 13, 1502247. doi: 10.3389/fpubh.2025.1502247
Kabaria C. W., Gilbert M., Noor A. M., Snow R. W., and Linard C. (2017). The impact of urbanization and population density on childhood Plasmodium falciparum parasite prevalence rates in Africa. Malar. J. 16, 49. doi: 10.1186/s12936-017-1694-2
Keita S., Thiero O., Toure M., Kane F., Keita M., Sanogo I., et al. (2024). Prognostics of multiple malaria episodes and nutritional status in children aged 6 to 59 months from 2013 to 2017 in Dangassa, Koulikoro region, Mali. Malar. J. 23, 186. doi: 10.1186/s12936-024-04999-8
Machault V., Vignolles C., Pages F., Gadiaga L., Gaye A., Sokhna C., et al. (2010). Spatial heterogeneity and temporal evolution of malaria transmission risk in Dakar, Senegal, according to remotely sensed environmental data. Malar. J. 9, 252. doi: 10.1186/1475-2875-9-252
Merga H., Degefa T., Birhanu Z., Tadele A., Lee M. C., Yan G., et al. (2025). Urban malaria in sub-Saharan Africa: a scoping review of epidemiologic studies. Malar. J. 24, 131. doi: 10.1186/s12936-025-05368-9
Nundu S. S., Simpson S. V., Arima H., Muyembe J. J., Mita T., Ahuka S., et al. (2022). It is time to strengthen the malaria control policy of the Democratic Republic of Congo and include schools and school-age children in malaria control measures. Pathogens 11 (7), 729. doi: 10.3390/pathogens11070729
Omumbo J. A., Hay S. I., Snow R. W., Tatem A. J., and Rogers D. J. (2005). Modelling malaria risk in East Africa at high-spatial resolution. Trop. Med. Int. Health 10, 557–566. doi: 10.1111/j.1365-3156.2005.01424.x
PNLP - Mali. (2021). Stratégie nationale de lutte contre le Paludisme. Mali: Programme National de Lutte contre le paludisme au Mali.
Programme National de Lutte contre le paludisme au Mali (2021). Enquête sur les Indicateurs du Paludisme au Mali (EIP Mali) 2021: Indicateurs Clés. Mali: Programme National de Lutte contre le paludisme au Mali.
Robert V., Macintyre K., Keating J., Trape J. F., Duchemin J. B., Warren M., et al. (2003). Malaria transmission in urban sub-Saharan Africa. Am. J. Trop. Med. Hyg. 68, 169–176. doi: 10.4269/ajtmh.2003.68.169
Shaffer J. G., Toure M. B., Sogoba N., Doumbia S. O., Gomis J. F., Ndiaye M., et al. (2020). Clustering of asymptomatic Plasmodium falciparum infection and the effectiveness of targeted malaria control measures. Malar. J. 19, 33. doi: 10.1186/s12936-019-3063-9
Shankar H., Singh M. P., Hussain S. S. A., Phookan S., Singh K., and Mishra N. (2022). Epidemiology of malaria and anemia in high and low malaria-endemic North-Eastern districts of India. Front. Public Health 10, 940898. doi: 10.3389/fpubh.2022.940898
Sixpence A., Vokhiwa M., Kumalakwaanthu W., Pitchford N. J., Seydel K. B., Magder L. S., et al. (2024). Comparing approaches for chemoprevention for school-based malaria control in Malawi: an open label, randomized, controlled clinical trial. EClinicalMedicine 76, 102832. doi: 10.1016/j.eclinm.2024.102832
Sogoba N., Doumbia S., Vounatsou P., Baber I., Keita M., Maiga M., et al. (2007). Monitoring of larval habitats and mosquito densities in the Sudan savanna of Mali: implications for malaria vector control. Am. J. Trop. Med. Hyg. 77, 82–88. doi: 10.4269/ajtmh.2007.77.82
Swana E. K., Yav T. I., Ngwej L. M., Mupemba B. N., Suprianto, Mukeng C. K., et al. (2018). School-based malaria prevalence: informative systematic surveillance measure to assess epidemiological impact of malaria control interventions in the Democratic Republic of the Congo. Malar. J. 17, 141. doi: 10.1186/s12936-018-2297-2
Tatem A. J., Gething P. W., Smith D. L., and Hay S. I. (2013). Urbanization and the global malaria recession. Malar. J. 12, 133. doi: 10.1186/1475-2875-12-133
Toure M., Keita M., Kane F., Sanogo D., Kante S., Konate D., et al. (2022). Trends in malaria epidemiological factors following the implementation of current control strategies in Dangassa, Mali. Malar. J. 21, 65. doi: 10.1186/s12936-022-04058-0
Toure M., Sanogo D., Dembele S., Diawara S. I., Oppfeldt K., Schioler K. L., et al. (2016). Seasonality and shift in age-specific malaria prevalence and incidence in Binko and Carriere villages close to the lake in Selingue, Mali. Malar. J. 15, 219. doi: 10.1186/s12936-016-1251-4
Vlahov D. and Galea S. (2002). Urbanization, urbanicity, and health. J. Urban Health 79, S1–S12. doi: 10.1093/jurban/79.suppl_1.S1
Vorasan N., Pan-Ngum W., Jittamala P., Maneeboonyang W., Rukmanee P., and Lawpoolsri S. (2015). Long-term impact of childhood malaria infection on school performance among school children in a malaria endemic area along the Thai-Myanmar border. Malar. J. 14, 401. doi: 10.1186/s12936-015-0917-7
Wang S. J., Lengeler C., Smith T. A., Vounatsou P., Diadie D. A., Pritroipa X., et al. (2005). Rapid urban malaria appraisal (RUMA) I: epidemiology of urban malaria in Ouagadougou. Malar. J. 4, 43. doi: 10.1186/1475-2875-4-43
WHO (2022). World malaria report 2022: Tracking progress and gaps in the global response to malaria. Geneva: World Health Organization.
WHO (2024). World malaria report 2024: Addressing inequity in the global malaria response. Geneva: World Health Organization.
Keywords: malaria, P. falciparum infection, school-aged children, urban, peri-urban, Bamako
Citation: Toure M, Kane F, Sanogo D, Keita S, Keita B, Aro AZ, Keita M, Konate D, Diawara SI, Yaro AS, Kone A, Magassa M, Kante S, Tangara CO, Tangara K, Coulibaly H, Cisse B, Thiam SM’B, Traore AS, Shaffer JG, Sogoba N, Diakite M and Doumbia S (2025) Prevalence of Plasmodium falciparum infection in school-aged children in urban and peri-urban schools of Bamako, Mali. Front. Malar. 3:1596496. doi: 10.3389/fmala.2025.1596496
Received: 19 March 2025; Accepted: 06 June 2025;
Published: 27 June 2025.
Edited by:
Christian Nsanzabana, Swiss Tropical and Public Health Institute (Swiss TPH), SwitzerlandReviewed by:
Adilson José DePINA, CCS-SIDA/MoH, Cabo VerdeGeofrey Makenga, National Institute of Medical Research (Tanzania), Tanzania
Lauren Cohee, Liverpool School of Tropical Medicine, United Kingdom
Copyright © 2025 Toure, Kane, Sanogo, Keita, Keita, Aro, Keita, Konate, Diawara, Yaro, Kone, Magassa, Kante, Tangara, Tangara, Coulibaly, Cisse, Thiam, Traore, Shaffer, Sogoba, Diakite and Doumbia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahamoudou Toure, bWFoLnRvdXJlQGdtYWlsLmNvbQ==
 Mahamoudou Toure
Mahamoudou Toure Fousseyni Kane
Fousseyni Kane Daouda Sanogo1,2,3
Daouda Sanogo1,2,3 Drissa Konate
Drissa Konate Jeffrey G. Shaffer
Jeffrey G. Shaffer Nafomon Sogoba
Nafomon Sogoba Mahamadou Diakite
Mahamadou Diakite Seydou Doumbia
Seydou Doumbia