- 1Environmental Health and Ecological Sciences, Ifakara Health Institute, Bagamoyo, Tanzania
- 2School of Life Sciences and Bioengineering, The Nelson Mandela African Institution of Science and Technology (NM-AIST), Arusha, Tanzania
- 3Faculty of Science, University of Basel, Basel, Switzerland
- 4Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Allschwil, Switzerland
- 5Department of Life Sciences, Imperial College of London, London, United Kingdom
Background: In the era of asymptomatic gametocytemia, carriers are scarce but serve as key reservoirs for Plasmodium falciparum gametocytes. Transmission-blocking interventions (TBIs) are gaining attention, considering factors such as artemether–lumefantrine (AL) treatment, mosquito feeding time (day vs. night), and serum replacement, recognized for their potential in influencing direct membrane feeding assay (DMFA) outcomes and reducing assay precision. This study aimed at optimizing DMFA through assessing the following 1) artemether–lumefantrine treatment 2) mosquito feeding time and 3) serum replacement on gametocyte infectiousness to mosquitoes in a low malaria transmission setting
Methods: Six gametocytemic carriers were found to be eligible, from whom 4 mL of venous blood was drawn. This blood was given to female Anopheles gambiae sensu stricto (s.s.) mosquitoes via DMFA under controlled conditions. Oocyst prevalence and intensity were determined on fed mosquitoes: 1) 9 days post-AL treatment, 2) for day feeds versus night feeds, and 3) with and without serum replacement.
Results: Mosquito infection rates declined post-AL treatment, with significantly fewer mosquitoes infected [odds ratio (OR) = 0.20, 95% confidence interval (CI): 0.13–0.31, p = 0.001] compared to day 0. Feeding during the dark cycle time did not significantly affect mosquito infection rates (OR = 0.77, 95% CI: 0.53–1.12, p = 0.175). Lastly, compared to whole blood, serum replacement increased infection rates (OR = 1.73, 95% CI: 1.33–2.25, p = 0.001).
Conclusion: To obtain robust results, we confirm that DMFA should be conducted using blood from gametocytemic carriers without a recent history of AL treatment, using serum replacement to enhance infection success. In this setting, assays could be conducted outside of the mosquitoes’ dark cycle without affecting results.
1 Introduction
The global push toward malaria elimination has intensified efforts to explore novel strategies that target not only asexual stages but also the reduction of human-to-mosquito transmission. Among these strategies are transmission-blocking interventions (TBIs), which target Plasmodium falciparum gametocyte (stage V), the infective parasite stage present among asymptomatic carriers responsible for infecting mosquitoes (Felger et al., 2012; Sumari et al., 2017; Buchwald et al., 2019; Andolina et al., 2021), thereby breaking the transmission cycle. The direct membrane feeding assay (DMFA) is a laboratory-based assay used to assess the infectiousness of blood to Anopheles mosquitoes. It is commonly used to assess the efficacy of TBIs including transmission-blocking drugs (TBDs), transmission-blocking vaccines (TBVs), or vector control tools containing insecticides with transmission-blocking activity. In this assay, freshly drawn blood often from infected participants is placed in a glass feeder covered with a membrane maintained at body temperature, and laboratory-reared mosquitoes are allowed to feed through the membrane. Understanding the sources of variation and bias in DMFA is critical for interpreting results accurately and ensuring reproducibility. Artemisinin-based combination therapies (ACTs) are the foundation of WHO-recommended treatment for uncomplicated P. falciparum malaria and are expected to play a crucial role in future malaria elimination strategies (Grueninger and Hamed, 2013). The ACTs commonly used in Africa include dihydroartemisinin–piperaquine (DHA-PPQ), artesunate–amodiaquine (AS-AQ), and artemether–lumefantrine (AL). However, AL is recommended as a first-line malaria treatment in endemic areas due to its safety profile across different age groups and efficacy against P. falciparum asexual and immature gametocyte stages. However, it has little effect on mature gametocytes, which are crucial for the onward mosquito transmission of the parasite (Makanga and Krudsood, 2009; Bousema et al., 2010; Makanga, 2014; Ngasala et al., 2024). A study in Burkina Faso found a significant reduction in infected mosquitoes and oocyst intensity in participants who had been treated with AL, with only one participant transmitting infection to mosquitoes 7 days post-treatment compared to participants administered with AL and primaquine drug (Gonçalves et al., 2016). Similarly, a trial in Mali reported no mosquito infections when fed 7 days post-AL treatment compared to participants administered with sulfadoxine–pyrimethamine and amodiaquine (Mahamar et al., 2024). In addition, a randomized trial done in Senegal observed rapid clearance and reduction in gametocyte carriage. By day 14, gametocytes had disappeared in the AL group, while in the AS+AQ group, gametocyte disappearance was observed by day 21 post-treatment (Faye et al., 2010). These findings have potential implications for public health. There is a need to conduct more transmission studies using DMFA to critically evaluate TBDs and TBVs to design effective elimination strategies that reduce human-to-mosquito transmission. However, with contradicting effects of gametocyte dynamics, no similar studies have been conducted 9 days post-treatment in a low transmission area in East Africa.
Parasites have developed complex strategies to manipulate their hosts, enhancing transmission and ensuring survival. For instance, Wuchereria bancrofti, the causative agent of lymphatic filariasis, increases its presence in human peripheral circulation at dusk, aligning with the peak feeding time of Culex mosquitoes, thereby increasing the chances of transmission (Hawking, 1967). Similarly, previous studies found that mosquitoes fed with gametocytes at dusk had higher oocyst intensity than those fed at dawn, as this period coincides with increased mosquito susceptibility to malaria parasites (Coulibaly et al., 2017; Habtewold et al., 2019). These adaptive mechanisms are governed by interactions between parasites and their hosts including mosquito metabolism and immunity (Murdock et al., 2013; Rund et al., 2016; Sarah, 2017; Bento et al., 2025). It is possible that feeding mosquitoes during the day as is commonly done for DMFA could affect the results of DMFA as mosquito metabolism and immunity are governed by circadian rhythms (Murdock et al., 2013; Rund et al., 2016; Sarah, 2017).
Besides mosquito immunity, DMFA is affected by human immune factors. Serum replacement is performed as part of DMFA to remove possible host malaria-immune factors, as they can increase mosquito infection rates (Bousema et al., 2012; Bousema et al., 2013; Dos Santos et al., 2024). However, it is difficult to obtain malaria-naive serum in malaria-endemic countries, so it is extremely expensive to conduct serum replacement. As malaria endemicity falls, it is known that immunity is likely to wane.
This study explored potential sources of variation in DMFAs and ways to make them more cost-effective in an area of falling malaria transmission. Specifically, the study assessed 1) if gametocytemic carriers who were initially infectious to mosquitoes remained infectious post-treatment with AL, 2) if the time of conduct of DMFA can be optimized to maximize mosquito infectiousness, and 3) if serum replacement is needed in this area of low malaria endemicity. The information may be very useful especially in areas with low transmission. Therefore, this study was conducted to better understand P. falciparum transmission dynamics in a low-endemic setting by assessing whether gametocytemic carriers who were initially infectious to mosquitoes remained so after treatment with AL. Additionally, the study aimed to evaluate whether the timing of DMFAs could be optimized to enhance the detection of mosquito infection and whether serum replacement was necessary to improve assay sensitivity in this context.
2 Materials and methods
2.1 Mosquito rearing
The A. gambiae s.s. (Ifakara strain) colony was reared at the Bagamoyo Branch Insectary, Kingani. These mosquitoes were maintained under standard conditions in a 12:12-h dark:light cycle, 70% ± 20% humidity, and temperature of 27°C ± 2°C. Larvae were fed on TetraMin® fish flakes (Tetra Ltd., UK) and kept at a density of 200 larvae (from L2 larval stage) per 1 L of distilled water. Adult mosquitoes are kept in 30 cm × 30 cm × 30 cm containers and allowed to feed on a 10% sucrose solution (Hofer et al., 2023). For egg production, adult mosquitoes were given blood through a membrane (Siria et al., 2018). Adult mosquitoes were routinely checked for microsporidia infections, and strict hygiene measures were maintained in the insectary.
2.2 Study site, participants, and ethics
This laboratory-controlled study for these assays was conducted at the Malaria Transmission Research Laboratory (MTRL) located at the Ifakara Health Institute (IHI) in Bagamoyo District, Tanzania, between May and August 2024. The target population included male and female individuals aged 6–45 years from the villages of Wamimkoko, Miono, Chamgoi, and Kibindu. The participants included in the study met the following criteria: asymptomatic, provided consent, aged 6–45 years with detectable P. falciparum stage V gametocyte only, and enrolled in DMFA. The exclusion criteria included no consent provided, primaquine use within 4 weeks before this study, fever or history of fever, and evidence of severe illness. Gametocytes were quantified by counting against 500 white blood cells in thick blood smears. Of the 15 participants screened, 6 tested positive for P. falciparum gametocytes, each with counts exceeding 3 gametocytes/500 red blood cell density equivalent to 48 gam/μL of blood. Gametocyte density was calculated based on an assumed leukocyte count of 8,000 cells/µL of blood as indicated in other studies (Kweyamba et al., 2023; Mahamar et al., 2024; Ouattara et al., 2024).
2.3 Screening procedures
A mass test-and-treat (MTAT) approach was used to screen asymptomatic participants. Village workers informed the community members of the date, location, and time of sensitization meetings, where consenting and screening would take place. Screening rounds were performed on participants who consented to participate in the study using the mRDT (SD BIOLONE Malaria Ag P.f/Pan (HRPII/pLDH), Standard Diagnostic, South Korea). Briefly, finger-prick blood samples were collected, and one drop of 50 µL of capillary blood was applied to the test and run with four drops of standard buffer solution. The test was placed on a flat surface for 15 min after which a laboratory technician read the results. For participants who tested mRDT positive, a small amount of finger-prick blood was also taken to make thick smear slides to confirm the presence of asexual and sexual parasites. Slides were taken to the laboratories at IHI and stained for 45 min with 10% Giemsa stain. The slides were then examined for the presence of asexual and sexual stages (gametocyte stage V) of P. falciparum only using light microscopy (LM) (×100 magnification, oil immersion). Asexual parasites (ring stages) were counted per 200 white blood cells (WBCs) and gametocytes per 500 WBCs. The slides that had no P. falciparum parasites were considered negative.
2.4 Mosquito feeding assay and dissection
At the IHI laboratory, the gametocytemic carriers donated 4 mL of venous blood into lithium–heparin-coated vacutainers (Vacuette®, Greiner, Kremsmünster, Austria). The blood was then split into two aliquots, and one aliquot was transferred into prewarmed 1.5 mL Eppendorf tubes (Eppendorf, Germany) and centrifuged for 3 min at 300 relative centrifugal force and 37°C (5702 RH, Eppendorf, Germany). The same amount of autologous serum was replaced with prewarmed malaria-naive AB serum set at 37°C (Blood Transfusion Service, Basel, Switzerland). The malaria-naive AB serum was obtained from European donors with blood type AB who had no prior exposure to malaria (Blood Transfusion Service, Basel, Switzerland). The other portion of the whole blood was directly dispensed to mosquitoes.
Mosquitoes were infected using DMFAs as described elsewhere (Hofer et al., 2024), in which blood was drawn from a gametocytemic carrier and offered to mosquitoes through a preheated glass feeder. In this assay, water-jacketed glass feeders (14 mm Ø, Chemglass, New Jersey, USA) were covered with Parafilm® and connected to a circulating water bath (ELMI, Switzerland) set at 39°C. The water bath was preheated for 15 min before the experiment began.
Eight cups, each with 50 mosquitoes, were used per participant, including four cups for serum replacement and an additional whole blood cup. Each feeder was filled with 300 µL of blood, and mosquitoes were allowed to feed for 15 min. During feeding, the cups were covered with a dark cloth, and the lights in the room were switched off to enhance feeding success. After feeding, the cups were placed in BugDorm plastic cages (30 cm × 30 cm × 30 cm, Megaview Science Co., Ltd., Taiwan) overnight, maintained at 75% ± 2% relative humidity and 27°C ± 1°C temperature under a 12:12-h dark–light cycle in a climatic chamber (S600PLH, AraLab, Lisbon, Portugal). The following morning, unfed mosquitoes were removed using a mouth aspirator, and the remaining mosquitoes were provided with cotton soaked in a 10% sucrose solution. The sugar cotton balls were replaced daily. AL treatment was administered to the participants within 24 h of diagnosis under parental supervision, following Tanzania’s national guidelines (MoHSW, 2006).
Eight-day post-infection mosquitoes were dissected, and midguts were stained for 8 min using a 0.1% mercurochrome solution (Sigma, Switzerland) before examination under a compound microscope at ×40 magnification for the presence of oocysts. The flow of the procedure is shown in Figure 1.
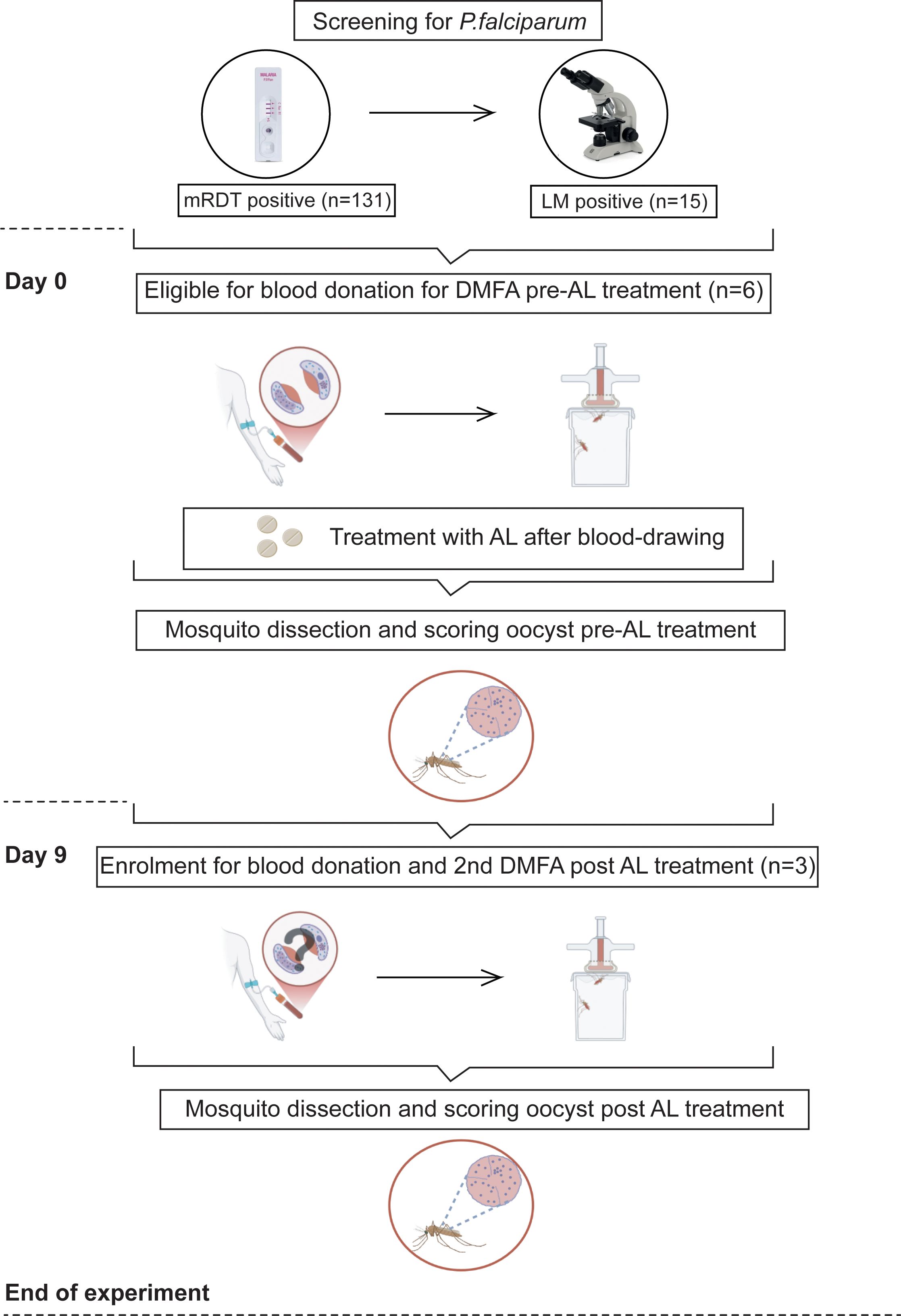
Figure 1. Evaluation for mosquito infectiousness includes drawing blood samples and subsequent DMFA feeding on day 0 and day 9. Participants were screened for Plasmodium infections using mRDT, where (n) is the number of infected participants. Six out of 15 Plasmodium falciparum gametocyte-positive participants confirmed by LM were eligible and enrolled for DMFAs, and their infectiousness was assessed by mosquito dissection and oocyst detection by microscopy 8 days post-infection.
2.5 Study procedures
Experiment 1: To determine the effects of antimalarial treatment on the infectivity of gametocytes from naturally infected individuals to Anopheles gambiae s.s. mosquitoes
A total of 4,850 female mosquitoes were fed through DMFA on day 0 (3,700) and day 9 (1,150). The DMFA with serum replacement was done on day 0 before the administration of AL treatment. Surviving mosquitoes were dissected for oocysts on day 8. The second DMFA, conducted 9 days post-treatment, was repeated using a different batch of mosquitoes and involved participants whose drawn blood had infected more than 20 mosquitoes in the first DMFA (Figure 1). We proposed this cutoff point because the gametocyte density for these participants was approximately 48 gam/µL on day 0. Consequently, we anticipated that there would be no infection during the second feeding.
Experiment 2: To determine the effect of time of conduct of DMFA on feeding on the infectivity of gametocytes from naturally infected individuals to Anopheles gambiae s.s. mosquitoes
A total of 3,300 female mosquitoes were fed through DMFA with serum replacement with blood obtained from the same participants. In total, 1,750 were fed during the day (13:00–16:00 h) and 1,550 at night (19:00–22:00 h) (Figure 2).
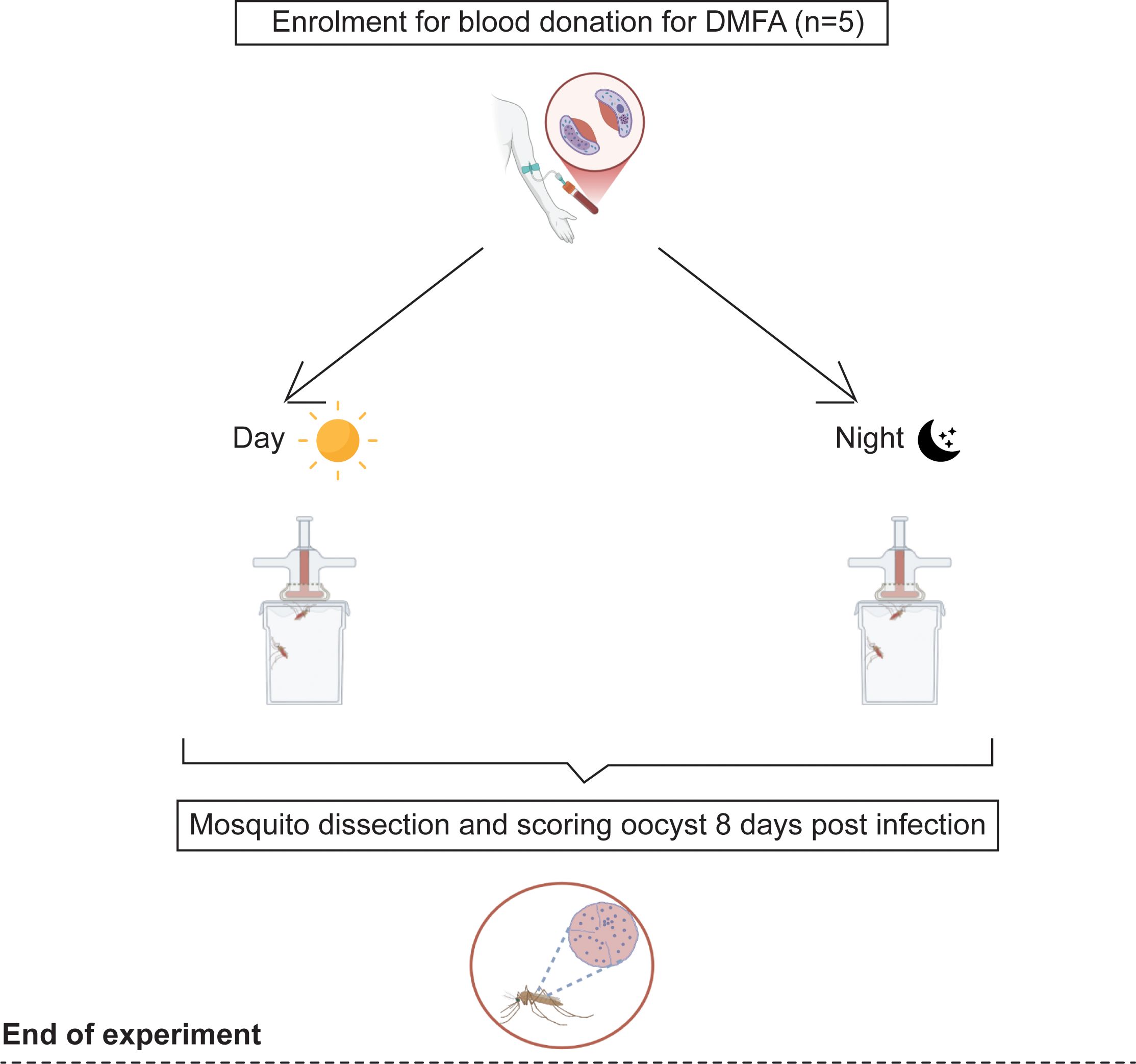
Figure 2. Evaluation for mosquito infectiousness includes drawing blood samples and subsequent DMFA feeding on day 0 (day and night). Five out of five Plasmodium falciparum gametocyte-positive carriers were enrolled for DMFAs, and their infectiousness was assessed by mosquito dissection and oocyst detection by microscopy 8 days post-infection.
Experiment 3: To determine the effect of serum replacement on the infectivity of gametocytes from naturally infected individuals to Anopheles gambiae s.s. mosquitoes
A total of 4,850 female mosquitoes were fed through DMFA with blood obtained from the same participants. In total, 3,250 were fed on blood with serum replacement and 1,600 on whole blood without serum replacement.
2.6 Statistical analysis
Data were entered in Excel (Microsoft 2016) and analyzed using STATA 17 software (StataCorp, College Station, TX, USA). Descriptive statistics were used, whereby the arithmetic mean proportion of infected mosquitoes with a 95% confidence interval (CI). For oocyst intensity, median with minimum and maximum values was presented. Logistic regression was used to determine the prevalence of infected mosquitoes adjusting for days (fixed effect) and participants (random effect): 1) effect of AL, day 9 compared to day 0 (fixed effect); 2) time of DMFA conduct, night compared to day (fixed effect); and 3) effect of serum replacement compared to whole blood (fixed effect). Negative binomial regression with a log link was conducted for oocyst intensity for all experiments with the same fixed and random effects outlined above.
3 Results
3.1 Malaria prevalence among the study participants
A total of 309 participants aged 6–45 years were screened for P. falciparum infection in Chamgoi, Wamimkoko, Miono, and Kibindu villages. Out of 309, 131 (42%) were found to be positive for P. falciparum using mRDT. Of these, 15/131 (11.5%) were found to be positive for P. falciparum gametocytes using LM. Only 40% (6/15) were found to be eligible and enrolled for DMFAs. The mean gametocytemic density among the participants at day 0 was 125 gam/µL.
3.2 Effects of AL on individual infectiousness to mosquitoes
On day 0, six gametocytemic carriers (100%) infected at least one mosquito. Three gametocytemic carriers were invited for the second DMFA as they infected more than 20 mosquitoes from the initial DMFA. On the second DMFA, only one out of three participants (33.3%) remained infectious. A total of 1,612 mosquitoes were dissected across two time points: 1,125 on day 0 pre-treatment and 487 on day 9 post-treatment. The mean proportion of oocyst-infected mosquitoes was lower on day 9 with 0.06 (95% CI: 0.04–0.09), compared to 0.17 (95% CI: 0.15–0.19) on day 0 (OR = 0.20, 95% CI: 0.13–0.30, p = 0.001) (Table 1). There was a significant difference in median oocyst intensity per mosquito between day 9 with 1 (Bousema et al., 2010; Felger et al., 2012; Grueninger and Hamed, 2013; Makanga, 2014; Sumari et al., 2017; Buchwald et al., 2019; Andolina et al., 2021) compared to day 0 with 2 (Hawking, 1967; Makanga and Krudsood, 2009; Bousema et al., 2010; Faye et al., 2010; Felger et al., 2012; Grueninger and Hamed, 2013; Makanga, 2014; Gonçalves et al., 2016; Coulibaly et al., 2017; Sumari et al., 2017; Buchwald et al., 2019; Habtewold et al., 2019; Andolina et al., 2021; Mahamar et al., 2024; Ngasala et al., 2024; Bento et al., 2025) [incidence risk rate (IRR) = 0.46, 95% CI: 0.31–0.68, p = 0.001] (Figures 3A, B; Table 1).
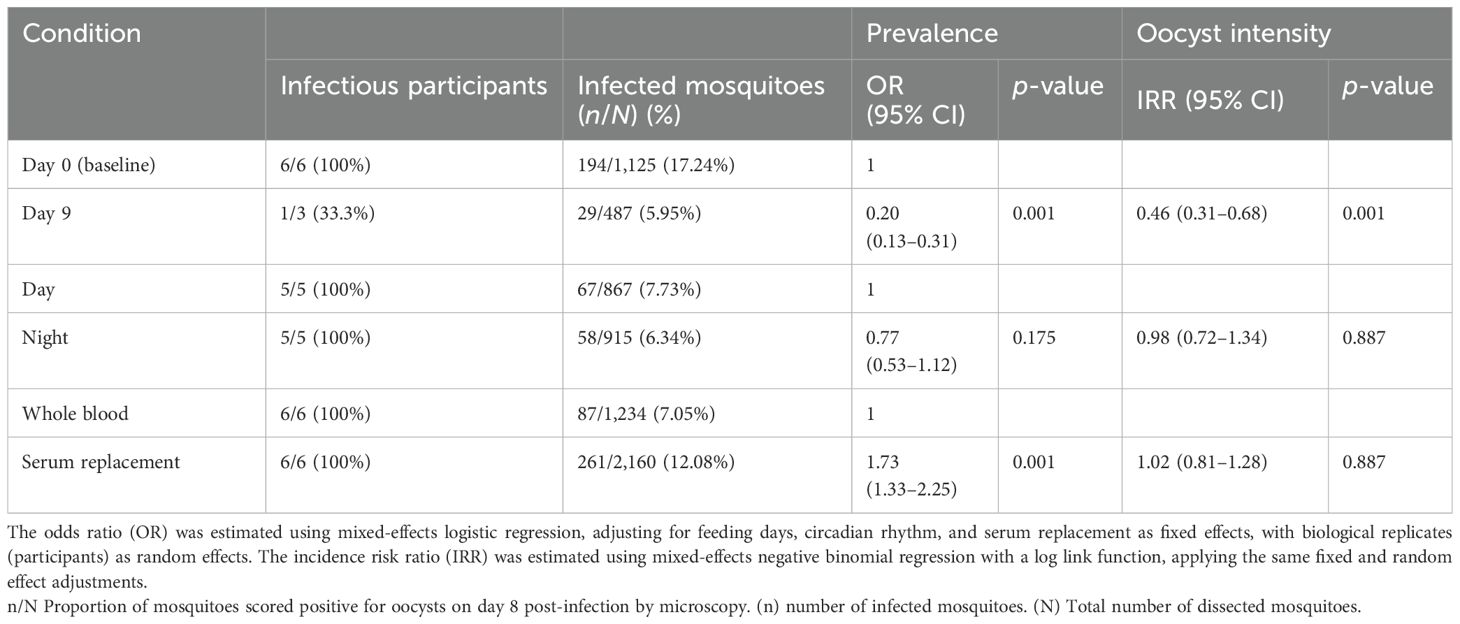
Table 1. Oocyst infection prevalence and infection intensity of Anopheles gambiae s.s. on day 0, day 9, circadian rhythm, and serum replacement.
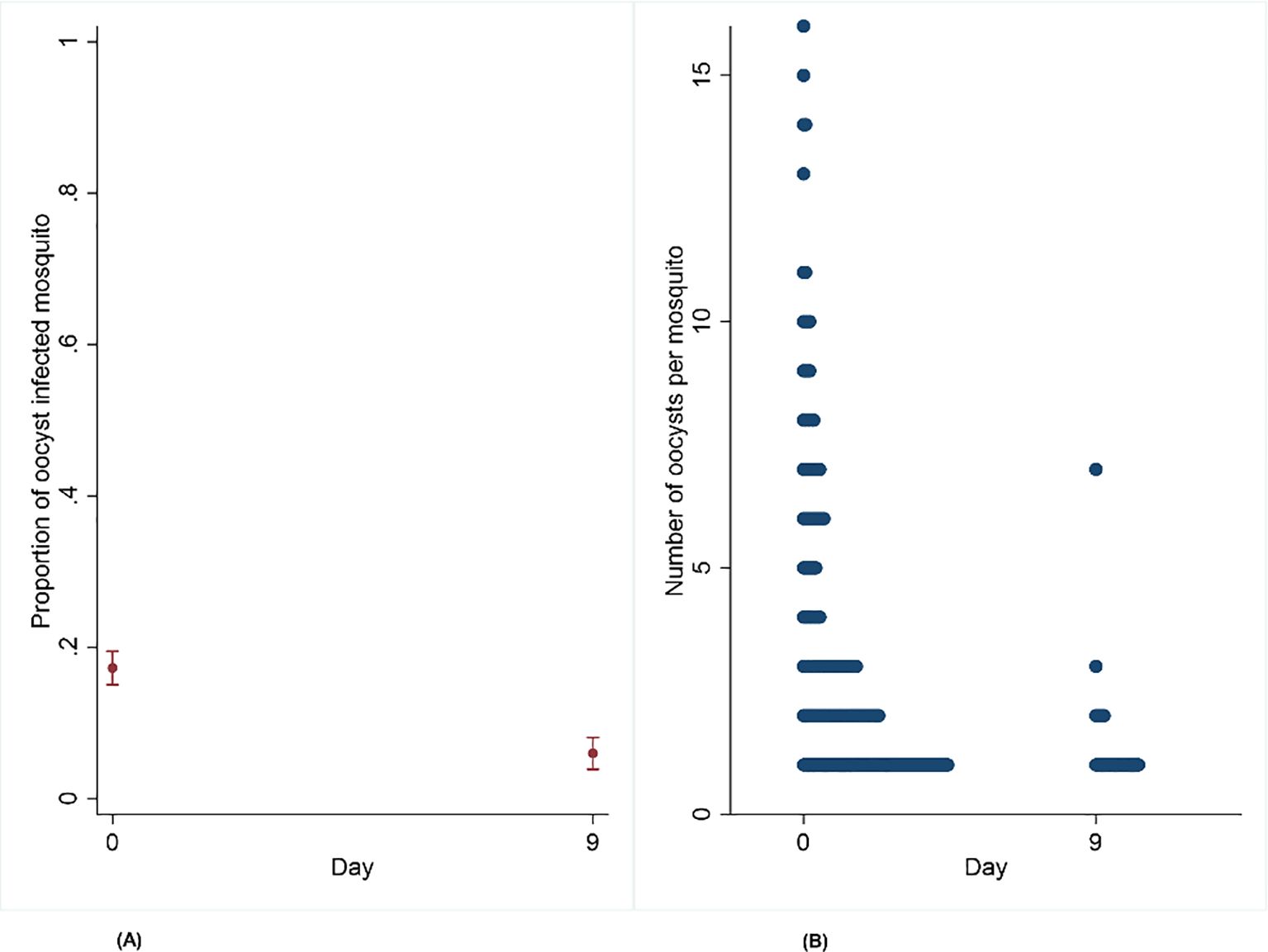
Figure 3. (A, B) Effect of artemether–lumefantrine post-treatment on the proportion of infected mosquitoes and oocyst intensity on Anopheles gambiae s.s. mosquito infectiousness from naturally infected participants on day 9 compared to day 0.
3.3 Effect of time of feeding on the proportion of infected Anopheles gambiae s.s. mosquitoes
A total of 867 mosquitoes were dissected during the day (13:00–16:00 h) and 915 during the night (19:00–22:00 h). The mean proportion of oocyst-infected mosquitoes was very similar: 0.08 (95% CI: 0.06–0.09) from day feeds and 0.06 (95% CI: 0.05–0.07) from night feeds, with a median of 2 oocysts per mosquito ranging from 1 to 16 in both cases. No statistical difference was found for either the prevalence of oocyst-infected mosquitoes (OR = 0.77, 95% CI: 0.53–1.12, p = 0.175) or oocyst intensity within mosquitoes (IRR = 0.98, 95% CI: 0.72–1.34, p = 0.887) between the two groups (Figures 4C, D; Table 1).
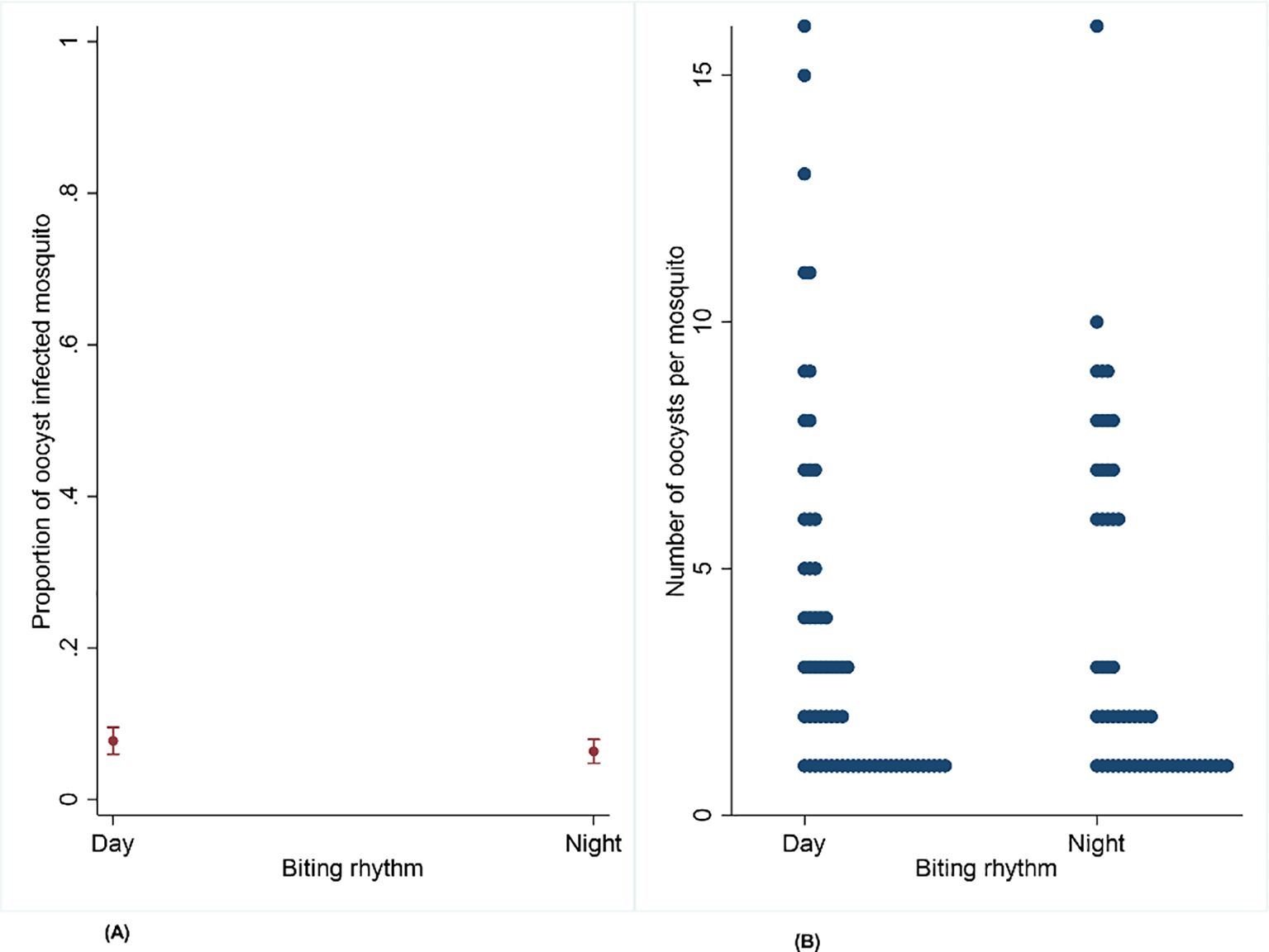
Figure 4. (A, B) Effect of circadian rhythm on the proportion of infected mosquitoes and oocyst intensity on Anopheles gambiae s.s. mosquito infectiousness from naturally infected participants infected during the day and night.
3.4 Effects of serum replacement on infection rate
A total of 3,394 mosquitoes were dissected, with 2,160 from the serum replacement group and 1,234 from the whole blood group. The mean proportion of oocyst-infected mosquitoes was higher in the serum replacement group at 0.12 (95% CI: 0.11–0.13) compared to mosquitoes fed with whole blood at 0.07 (95% CI: 0.06–0.08). There was sufficient evidence to support the claim that serum replacement increases the proportion of infected mosquitoes (OR = 1.73, 95% CI 1.33–2.25, p = 0.001) but not infection intensity (IRR = 1.02, 95% CI: 0.81–1.28, p = 0.887) (Figures 5E, F; Table 1).
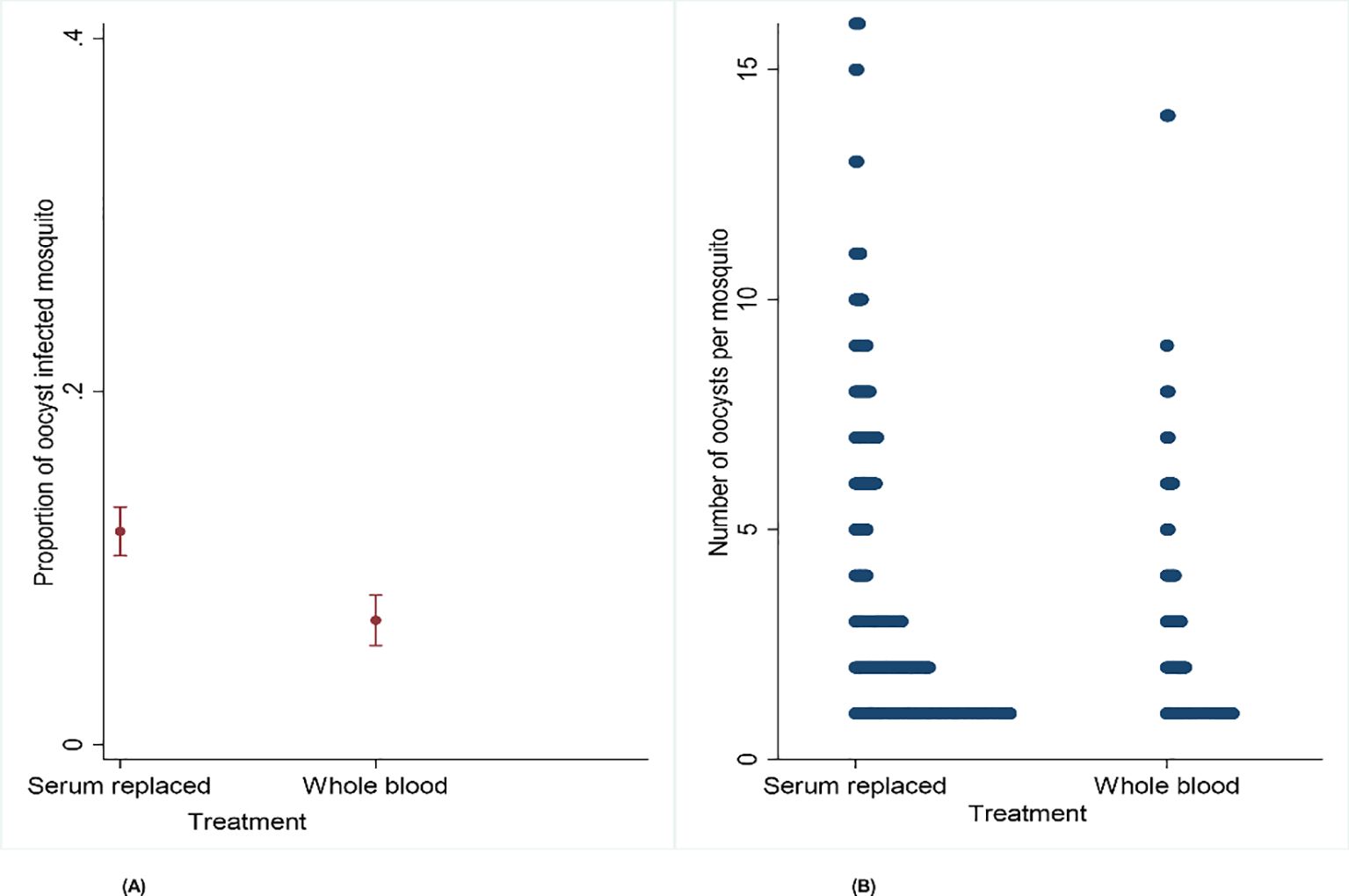
Figure 5. (A, B) Effect of serum replacement on the proportion of infected mosquitoes and oocyst intensity on Anopheles gambiae s.s. mosquito infectiousness from naturally infected participants.
4 Discussion
Understanding factors influencing mosquito infectiousness through DMFA using gametocytes from naturally infected participants is crucial for malaria transmission research and TBI evaluation. This study examined the infectiousness of P. falciparum wild isolates to A. gambiae s.s. mosquitoes pre- and post-AL treatment, the impact of the time of DMFA conduct (day vs. night), and serum replacement versus autologous serum. We demonstrated that, pre-AL treatment, all six participants enrolled for DMFAs (100%) infected mosquitoes, whereas only one in three (33%) infected mosquitoes 9 days post-treatment. A significant reduction in infection prevalence and oocyst intensity on infected mosquitoes was observed on day 9 compared to day 0. To the best of our knowledge, this is the first study to assess the impact of AL 9 days post-treatment, revealing a substantial decline in mosquito infectiousness compared to baseline.
4.1 Effects of AL on individual infectiousness to mosquitoes
This study reveals that the proportion of infected mosquitoes on day 9 was significantly lower than that on day 0, indicating a noteworthy decline in infection prevalence over the nine-day period. The difference may be due to AL’s inability to clear mature gametocytes present at recruitment (Bousema et al., 2006; Makanga, 2014), allowing some participants to remain infectious post-treatment (Sutherland et al., 2005). The research findings align with previous studies showing that AL reduces gametocyte density, mosquito infection rates, and oocyst intensity (Makanga, 2014; Bradley et al., 2019; Mahamar et al., 2024). For example, Sutherland et al. reported that AL decreased not only the rate of gametocyte carriage but also gametocyte carriage time, and the result was parallel to the infectivity of gametocytes to mosquitoes compared to participants administered with chloroquine combined with sulfadoxine–pyrimethamine (CQSP) (Sutherland et al., 2005). Also, Goncalves et al. showed that only one participant transmitted infection to mosquitoes 7 days post-treatment compared to participants administered with AL and primaquine drug (Gonçalves et al., 2016). In a more recent trial in Mali, no infections by day 7 were reported compared to participants administered with sulfadoxine–pyrimethamine and amodiaquine (Mahamar et al., 2024).
Several factors contribute to post-treatment variability, including individual differences in gametocyte clearance (Makanga, 2014; WWARN Gametocyte Study Group, 2016). Additionally, rapid male gametocyte clearance post-treatment may render infections non-transmissible (Roth et al., 2018). Additionally, immature gametocytes (stages I–IV) sequestered in the bone marrow or spleen require more time to mature (Thomson and Robertson, 1935; Smalley et al., 1981). Artemether–lumefantrine targets both asexual and immature gametocyte stages (Chen et al., 1994; Sinclair et al., 2009; Bousema et al., 2010; Smithuis et al., 2010), and lumefantrine further inhibits gametocyte development, which leads to a low mosquito infection rate (van Pelt-Koops et al., 2012). To obtain robust findings, it is essential to optimize assays by using pretreated participants as a source of gametocytes for the evaluation of TBI. Furthermore, this finding has broader implications for other ACTs, as their effects can vary in different settings. In East Africa, including Tanzania, AL is administered to all participants diagnosed with a Plasmodium infection. Although AL has shown effectiveness in reducing gametocyte density and oocyst intensity, some studies have demonstrated that submicroscopic gametocyte densities persist and transmit infections in West Africa (Gonçalves et al., 2016; Mahamar et al., 2024). Thus, addressing the dynamics of gametocyte infectiousness following AL treatment is crucial not only for understanding individual-level transmission potential but also for critically evaluating TBDs and TBVs for designing and informing malaria control strategies, in settings approaching elimination, where reducing the infectious reservoir is critical for interrupting transmission.
4.2 Circadian rhythms and mosquitoes’ infectiousness
We found no significant difference in infection rates between mosquitoes fed during the day and those fed at night. This aligns with previous findings on cultured P. falciparum gametocyte infectivity in A. gambiae susceptible strain (Habtewold et al., 2022) but contrasts with recent findings on sporozoites, where high infection was observed in mice infected during the night rather than during daytime through DSF rather than DMFA (Bento et al., 2025). Additionally, a trial in Mali showed that direct skin feeding (DSF) led to higher infection rates at dusk compared to dawn (Coulibaly et al., 2017). This variation may be due to differences in the experimental setup. For example, in the Mali study, dusk feeding was conducted on day 0, while dawn feeding took place 2 days later (Coulibaly et al., 2017).
The discrepancies on mosquito infectiousness may result from both human and mosquito factors. In mosquitoes, circadian rhythms regulate key behaviors such as mating, host-seeking, sugar foraging, and oviposition (Jones et al., 1967; Das and Dimopoulos, 2008; Sheppard et al., 2017). These rhythms may also influence mosquito immunity to P. falciparum gametocytes, which is affected by gut microbiota composition (Gendrin et al., 2015; Habtewold et al., 2022). Anopheles gambiae midgut microbiota levels fluctuate throughout the day, with the lowest levels recorded at midnight compared to midday (Gendrin et al., 2015). At night, active feeding may trigger mechanisms that reduce normal gut flora, increasing susceptibility to malaria parasites (Habtewold et al., 2022). However, our study used wild malaria isolates with low gametocyte density, likely contributing to the absence of differences in oocyst intensity. In humans, some studies indicate that P. falciparum gametocytes may exhibit time-of-day-dependent patterns in exflagellation readiness and transmission potential, possibly aligning with mosquito feeding behavior to maximize transmission success (Reece et al., 2017; Schneider et al., 2018). These rhythms may be regulated by the parasite’s intrinsic molecular clock or host cues such as melatonin, body temperature, or nutrient availability.
4.3 Impact of serum replacement on mosquito infection rate
As anticipated, a higher proportion of infected mosquitoes was observed in the serum replacement group compared to the whole blood group. This aligns with previous systematic studies, which found that serum replacement enhances mosquito infectivity across 212 membrane-feeding experiments (Bousema et al., 2012). This effect is likely due to the removal of transmission-blocking attributes such as antibodies, drug metabolites, and other factors known to reduce the mosquitoes’ infectivity. Contrary to previous studies, we found no significant difference in oocyst intensity between the two groups (Bousema et al., 2012). Bousema et al. observed that oocyst intensity was found to be higher in serum-replaced blood than in whole blood. Variations in oocyst intensity from our study findings may result from participants being from low-endemic settings and a low transmission season. This reduced the impact of serum replacement on the gametocytes’ infectiousness. However, it has been reported that the mosquito infection rate does not have a direct linear correlation with gametocyte density in human blood (Bousema and Drakeley, 2011; Da et al., 2015; Tadesse et al., 2018). The effect of serum replacement highlighted by this finding informs researchers that as malaria endemicity decreases, immunity is likely to wane. Therefore, performing serum replacement, with or without it, is unlikely to have any effect on oocyst intensity.
In addition to antibodies, various host-derived factors found in whole blood can hinder malaria transmission to mosquitoes. These factors include complement proteins, pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-6 (Naotunne et al., 1993), and acute-phase reactants, all of which may decrease gametocyte viability or disrupt gamete fertilization and ookinete development in the mosquito midgut. Residual antimalarial drugs and their metabolites, particularly long-acting compounds like lumefantrine and piperaquine, slow down the rate of parasite development after ingestion (Peatey et al., 2009; Sinden et al., 2012). Furthermore, oxidative stress markers and alterations in red blood cell membranes may negatively affect gametocyte function and infectivity (Percário et al., 2012). Replacing serum with malaria-naive or pooled control serum likely reduces the influence of these inhibitory components, creating a more conducive and standardized environment for evaluating transmission potential.
4.4 Study limitations
This study has several limitations. First, thick smears for microscopy were not collected on day 9 to confirm the presence of gametocytes, making it difficult to assess gametocyte dynamics and clearance rates. Second, the small sample size of gametocytemic donors, particularly in the AL post-treatment group, where a decline in infectivity was observed, limits the findings. Notably, at least one individual remained infectious during the circadian rhythm experiments. While the study provides valuable insights to enhance bioassays for evaluating transmission-blocking drugs and vaccines among gametocytemic participants, larger sample sizes are necessary to confirm these findings. Third, there was a lack of molecular quantification regarding the persistence of submicroscopic gametocytes. Future studies should aim to assess the drug susceptibility of parasite isolates used in DMFAs alongside targeted sequencing approaches to detect resistance-associated mutations. This will help better understand the interactions between the drugs and different parasite genotypes. Additionally, studies on feeding time points should evaluate both pyrethroid-resistant mosquitoes to provide more comprehensive insights.
5 Conclusion
Our study confirms that AL substantially decreases human infectiousness to mosquitoes, underlining the need to collect blood from gametocytemic participants who were not treated with AL previously. Feeding mosquitoes at night does not enhance infection rates; thus, evaluations can be done at daytime or at night. Meanwhile, serum replacement leads to a higher proportion of infected mosquitoes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Ifakara Health Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
DM: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. PK: Writing – review & editing. FM: Writing – review & editing. MM: Writing – review & editing. NM: Formal Analysis, Writing – review & editing. UK: Formal Analysis, Writing – review & editing. TH: Writing – review & editing. JS: Writing – review & editing. LH: Writing – review & editing. SM: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. MT: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The author(s) declare that this study received financial support from the VCPTU as part of a capacity-building initiative designed to enhance the skills and expertise of MSc students. This funding not only facilitated the research but also contributed to the professional development of the students involved. Additionally, scientists from VCPTU played a crucial role in assisting with the study's design and interpreting the results, ensuring that the research adhered to high scientific standards and addressed relevant questions in the field.
Acknowledgments
The authors thank all children, parents, and teachers from Kibindu, Chamgoi, Miono, and Wamimkoko villages for their voluntary participation in the study. We thank Isaack Rutha and Salum Hassan for their support in performing dissection of the potentially infected mosquitoes. We thank Mohammed S. Chabo and Gilbert Maganga for conducting screening rounds in the field. We thank Mwanaidi Hassan for maintaining the rearing of the Anopheles gambiae s.s. colony.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1609614/full#supplementary-material
Abbreviations
TBIs, transmission-blocking interventions; TBVs, transmission-blocking vaccines; TBDs, transmission-blocking drugs; MTRL, Malaria Transmission Research Laboratory; DMFAs, direct membrane feeding assays; DSF, direct skin feeding; LM, light microscopy; mRDT, malaria rapid diagnostic test; AL, artemether–lumefantrine; IHI, Ifakara Health Institute; IRB, Institutional Review Board; WBCs, white blood cells; pLDH, parasite lactate dehydrogenase; CI, confidence interval; OR, odds ratio; IRR, incidence risk rate.
References
Andolina C., Rek J. C., Briggs J., Okoth J., Musiime A., Ramjith J., et al. (2021). Sources of persistent malaria transmission in a setting with effective malaria control in eastern Uganda: a longitudinal, observational cohort study. Lancet Infect. Dis. 21, 1568–1578. doi: 10.1016/S1473-3099(21)00072-4
Bento I., Parrington B. A., Pascual R., Goldberg A. S., Wang E., Liu H., et al. (2025). Parasite and vector circadian clocks mediate efficient malaria transmission. Nat. Microbiol. 10, 882–896. doi: 10.1038/s41564-025-01949-1
Bousema T., Churcher T. S., Morlais I., and Dinglasan R. R. (2013). Can field-based mosquito feeding assays be used for evaluating transmission-blocking interventions? Trends Parasitol. 29, 53–59. doi: 10.1016/j.pt.2012.11.004
Bousema T., Dinglasan R. R., Morlais I., Gouagna L. C., van Warmerdam T., Awono-Ambene P. H., et al. (2012). Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PloS One 7, e42821. doi: 10.1371/journal.pone.0042821
Bousema T. and Drakeley C. (2011). Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin. Microbiol. Rev. 24, 377–410. doi: 10.1128/CMR.00051-10
Bousema T., Okell L., Shekalaghe S., Griffin J. T., Omar S., Sawa P., et al. (2010). Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 9, 136. doi: 10.1186/1475-2875-9-136
Bousema T., Schneider P., Gouagna L. C., Drakeley C. J., Tostmann A., Houben R., et al. (2006). Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J. Infect. Dis. 193, 1151–1159. doi: 10.1086/503051
Bradley J., Soumare H., Mahamar A., Diawara H., Roh M., Delves M., et al. (2019). Transmission-blocking effects of primaquine and methylene blue suggest P. falciparum gametocyte sterilisation rather than effects on sex ratio. Clin. Infect. Diseases. 69, 1436–1439. doi: 10.1093/cid/ciz134
Buchwald A. G., Sorkin J. D., Sixpence A., Chimenya M., Damson M., Wilson M. L., et al. (2019). Association between age and plasmodium falciparum infection dynamics. Am. J. Epidemiol. 188, 169–176. doi: 10.1093/aje/kwy213
Chen P. Q., Li G. Q., Guo X. B., He K. R., Fu Y. X., Fu L. C., et al. (1994). The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin. Med. J. (Engl). 107, 709–711.
Coulibaly M. B., Gabriel E. E., Sinaba Y., Sylla D., Sacko A., Sylla L., et al. (2017). Optimizing direct membrane and direct skin feeding assays for plasmodium falciparum transmission-blocking vaccine trials in bancoumana, Mali. Am. J. Trop. Med. Hyg. 97, 719–725. doi: 10.4269/ajtmh.16-1000
Da D. F., Churcher T. S., Yerbanga R. S., Yaméogo B., Sangaré I., Ouedraogo J. B., et al. (2015). Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Exp. Parasitology. 149, 74–83. doi: 10.1016/j.exppara.2014.12.010
Das S. and Dimopoulos G. (2008). Molecular analysis of photic inhibition of blood-feeding in Anopheles Gambiae. BMC Physiol. 8, 23. doi: 10.1186/1472-6793-8-23
Dos Santos N. A. C., Bastos A da S., Araújo J. E., Pontual J. D. C., Medeiros J. F., Vinetz J. M., et al. (2024). Case Report: Plasmodium vivax Sporozoite Melanization in the Midgut and Salivary Gland of the Malaria Vector Anopheles darlingi. Am. J. Trop. Med. Hyg. 110, 444–447. doi: 10.4269/ajtmh.23-0349
Faye B., Offianan A. T., Ndiaye J. L., Tine R. C., Touré W., Djoman K., et al. (2010). Efficacy and tolerability of artesunate-amodiaquine (Camoquin plus) versus artemether-lumefantrine (Coartem) against uncomplicated Plasmodium falciparum malaria: multisite trial in Senegal and Ivory Coast. Trop. Med. Int. Health 15, 608–613. doi: 10.1111/j.1365-3156.2010.02487
Felger I., Maire M., Bretscher M. T., Falk N., Tiaden A., Sama W., et al. (2012). The dynamics of natural Plasmodium falciparum infections. PloS One 7, e45542. doi: 10.1371/journal.pone.0045542
Gendrin M., Rodgers F. H., Yerbanga R. S., Ouédraogo J. B., Basáñez M. G., Cohuet A., et al. (2015). Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat. Commun. 6, 5921. doi: 10.1038/ncomms6921
Gonçalves B. P., Tiono A. B., Ouédraogo A., Guelbéogo W. M., Bradley J., Nebie I., et al. (2016). Single low dose primaquine to reduce gametocyte carriage and Plasmodium falciparum transmission after artemether-lumefantrine in children with asymptomatic infection: a randomised, double-blind, placebo-controlled trial. BMC Med. 14, 40. doi: 10.1186/s12916-016-0581-y
Grueninger H. and Hamed K. (2013). Transitioning from malaria control to elimination: the vital role of ACTs. Trends Parasitol. 29, 60–64. doi: 10.1016/j.pt.2012.11.002
Habtewold T., Tapanelli S., Masters E. K. G., Hoermann A., Windbichler N., and Christophides G. K. (2019). Streamlined SMFA and mosquito dark-feeding regime significantly improve malaria transmission-blocking assay robustness and sensitivity. Malar J. 18, 24. doi: 10.1186/s12936-019-2663-8
Habtewold T., Tapanelli S., Masters E. K., Windbichler N., and Christophides G. K. (2022). The circadian clock modulates Anopheles Gambiae infection with Plasmodium falciparum. PloS One 17, e0278484. doi: 10.1371/journal.pone.0278484
Hawking F. (1967). The 24-hour periodicity of microfilariae: biological mechanisms responsible for 449 its production and control. Proc. R. Soc. London. Ser. B.Biological 450 Sci. 169, 59–76. doi: 10.1098/rspb.1967.0079
Hofer L. M., Kweyamba P. A., Sayi R. M., Chabo M. S., Maitra S. L., Moore S. J., et al. (2023). Malaria rapid diagnostic tests reliably detect asymptomatic Plasmodium falciparum infections in school-aged children that are infectious to mosquitoes. Parasit Vectors. 16, 217. doi: 10.1186/s13071-023-05761-w
Hofer L. M., Kweyamba P. A., Sayi R. M., Chabo M. S., Mwanga R., Maitra S. L., et al. (2024). Additional blood meals increase sporozoite infection in Anopheles mosquitoes but not Plasmodium falciparum genetic diversity. Sci. Rep. 14, 17467. doi: 10.1038/s41598-024-67990-y
Jones M. D. R., Hill M., and Hope A. M. (1967). The circadian flight activity of the mosquito, anopheles Gambiae: phase setting by the light regime. J. Exp. Biol. 47, 503–511. doi: 10.1242/jeb.47.3.503
Kweyamba P. A., Hofer L. M., Kibondo U. A., Mwanga R. Y., Sayi R. M., Matwewe F., et al. (2023). Sub-lethal exposure to chlorfenapyr reduces the probability of developing Plasmodium falciparum parasites in surviving Anopheles mosquitoes. Parasites Vectors. 16, 342. doi: 10.1186/s13071-023-05963-2
Mahamar A., Smit M. J., Sanogo K., Sinaba Y., Niambele S. M., Sacko A., et al. (2024). Artemether-lumefantrine with or without single-dose primaquine and sulfadoxine-pyrimethamine plus amodiaquine with or without single-dose tafenoquine to reduce Plasmodium falciparum transmission: a phase 2, single-blind, randomised clinical trial in Ouelessebougou, Mali. Lancet Microbe 5, 633–644. doi: 10.1016/S2666-5247(24)00023-5
Makanga M. (2014). A review of the effects of artemether-lumefantrine on gametocyte carriage and disease transmission. Malar J. 28, 13:291. doi: 10.1186/1475-2875-13-291
Makanga M. and Krudsood S. (2009). The clinical efficacy of artemether/lumefantrine (Coartem®). Malar J. 8, S5. doi: 10.1186/1475-2875-8-S1-S5
MoHSW (2006). National guidelines for diagnosis and treatment of malaria (Dar es Salaam: Ministry of Health and Social Welfare).
Murdock C. C., Moller-Jacobs L. L., and Thomas M. B. (2013). Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proc. R Soc. B. 280, 20132030. doi: 10.1098/rspb.2013.2030
Naotunne T. S., Karunaweera N. D., Mendis K. N., and Carter R. (1993). Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology 78, 555–562.
Ngasala B., Chiduo M. G., Bushukatale S., Mmbando B. P., Makene T., Kamugisha E., et al. (2024). Efficacy and safety of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in mainland Tanzania, 2018. Malaria J. 23, 95. doi: 10.1186/s12936-024-04926-x
Ouattara S., Hien D., Nao E., Pare P., Guissou E., Cohuet A., et al. (2024). A simple, field-applicable method to increase the infectivity of wild isolates of Plasmodium falciparum to mosquito vectors. Malaria J. 23, 135. doi: 10.1186/s12936-024-04969-0
Peatey C. L., Skinner-Adams T. S., Dixon M. W. A., McCarthy J. S., Gardiner D. L., and Trenholme K. R. (2009). Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J. Infect. Dis. 15, 1518–1521. doi: 10.1086/644645
Percário S., Moreira D. R., Gomes B. A. Q., Ferreira M. E. S., Gonçalves A. C. M., Laurindo P. S. O. C., et al. (2012). Oxidative stress in malaria. Int. J. Mol. Sci. 13, 16346–16372. doi: 10.3390/ijms131216346
Reece S. E., Prior K. F., and Mideo N. (2017). The life and times of parasites: rhythms in strategies for within-host survival and between-host transmission. J. Biolog. Rhythms 32 (6), 516–533. doi: 10.1177/0748730417718904
Roth J. M., Sawa P., Omweri G., Osoti V., Makio N., Bradley J., et al. (2018). Plasmodium falciparum gametocyte dynamics after pyronaridine–artesunate or artemether–lumefantrine treatment. Malaria J. 17, 1–1134. doi: 10.1186/s12936-018-2373-7
Rund S., O’Donnell A., Gentile J., and Reece S. (2016). Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 7, 14. doi: 10.3390/insects7020014
Sarah E.R. (2017). The life and times of parasites: rhythms in strategies for within-host 464 survival and between-host transmission. J. Biol. Rhythms. 32, 516–533.
Schneider P., Rund S. S. C., Smith N. L., Prior K. F., O’Donnell A J., Reece S. E., et al. (2018). Adaptive periodicity in the infectivity of malaria gametocytes to mosquitoes. Proc. R. Soc. B. 285 (1876), 20181876. doi: 10.1098/rspb.2018.1876
Sheppard A. D., Rund S. S. C., George G. F., Clark E., Acri D. J., and Duffield G. E. (2017). Light manipulation of mosquito behaviour: acute and sustained photic suppression of biting activity in the Anopheles Gambiae malaria mosquito. Parasites Vectors. 10, 255. doi: 10.1186/s13071-017-2196-3
Sinclair D., Zani B., Donegan S., Olliaro P., and Garner P. (2009). Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst. Rev. 2009, CD007483. doi: 10.1002/14651858.CD007483.pub2
Sinden R. E., Carter R., Drakeley C., and Leroy D. (2012). The biology of sexual development of Plasmodium: the design and implementation of transmission-blocking strategies. Malar J. 11, 70. doi: 10.1186/1475-2875-11-70
Siria D. J., Batista E. P. A., Opiyo M. A., Melo E. F., Sumaye R. D., Ngowo H. S., et al. (2018). Evaluation of a simple polytetrafluoroethylene (PTFE)-based membrane for blood-feeding of malaria and dengue fever vectors in the laboratory. Parasites Vectors. 11, 236. doi: 10.1186/s13071-018-2823-7
Smalley M. E., Abdalla S., and Brown J. (1981). The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans. R Soc. Trop. Med. Hyg 75, 103–105. doi: 10.1016/0035-9203(81)90019-5
Smithuis F., Kyaw M. K., Phe O., Win T., Aung P. P., Oo A. P. P., et al. (2010). Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect. Dis. 10, 673–681. doi: 10.1016/S1473-3099(10)70187-0
Sumari D., Mwingira F., Selemani M., Mugasa J., Mugittu K., and Gwakisa P. (2017). Malaria prevalence in asymptomatic and symptomatic children in Kiwangwa, Bagamoyo district, Tanzania. Malaria J. 16, 222. doi: 10.1186/s12936-017-1870-4
Sutherland C. J., Ord R., Dunyo S., Jawara M., Drakeley C. J., Alexander N., et al. (2005). Reduction of malaria transmission to anopheles mosquitoes with a six-dose regimen of co-artemether. PloS Med. 2, e92. doi: 10.1371/journal.pmed.0020092
Tadesse F. G., Slater H. C., Chali W., Teelen K., Lanke K., Belachew M., et al. (2018). The Relative Contribution of Symptomatic and Asymptomatic Plasmodium vivax and Plasmodium falciparum Infections to the Infectious Reservoir in a Low-Endemic Setting in Ethiopia. Clin. Infect. Diseases. 66, 1883–1891. doi: 10.1093/cid/cix1123
Thomson J. G. and Robertson A. (1935). The structure and development of plasmodium falciparum gametocytes in the internal organs and peripheral circulation. Trans. R. Soc. Trop. Med. Hygiene. 1), 31–40. doi: 10.1016/S0035-9203(35)90015-3
van Pelt-Koops J. C., Pett H. E., Graumans W., van der Vegte-Bolmer M., van Gemert G. J., Rottmann M., et al. (2012). The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob. Agents Chemother. 56, 3544–3548. doi: 10.1128/AAC.06377-11
Keywords: DMFA, AL, TBIs, Anopheles gambiae s.s., gametocytes, oocysts, asymptomatic carrier
Citation: Mmasi DJ, Kweyamba P, Matwewe F, Maasayi MS, Mvungi NJ, Kibondo UA, Habtewold T, Stevenson JC, Hofer LM, Moore SJ and Tambwe MM (2025) Impact of artemether–lumefantrine treatment, circadian rhythm, and serum replacement on the infectiousness of wild Plasmodium falciparum gametocytes to Anopheles gambiae sensu stricto mosquitoes. Front. Malar. 3:1609614. doi: 10.3389/fmala.2025.1609614
Received: 10 April 2025; Accepted: 09 June 2025;
Published: 03 July 2025.
Edited by:
Richard Oxborough, University of Nevada, Las Vegas, United StatesReviewed by:
Myat Htut Nyunt, Department of Medical Research, MyanmarAriel H. Magallon-Tejada, Gorgas Memorial Institute of Health Studies, Panama
Copyright © 2025 Mmasi, Kweyamba, Matwewe, Maasayi, Mvungi, Kibondo, Habtewold, Stevenson, Hofer, Moore and Tambwe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorin Joachim Mmasi, ZG1tYXNpQGloaS5vci50eg==
 Dorin Joachim Mmasi
Dorin Joachim Mmasi Prisca Kweyamba1,3,4
Prisca Kweyamba1,3,4 Sarah Jane Moore
Sarah Jane Moore