- 1Environmental Health and Ecological Sciences, Ifakara Health Institute, Dar es Salaam, Tanzania
- 2Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Allschwil, Switzerland
- 3University of Basel, Basel, Switzerland
- 4Vector Control Product Testing Unit, Environmental Health and Ecological Science, Ifakara Health Institute, Bagamoyo, Tanzania
- 5National Malaria Control Programme, Ministry of Health, Dodoma, Tanzania
- 6President Office, Regional Administration and Local Government, Dodoma, Tanzania
- 7Nelson Mandela African Institute of Science and Technology, Arusha, Tanzania
Background: From 2022 to 2024, a project piloting large-scale larviciding in Tanzania was implemented in Tanga Region. The project used in-country manufactured biolarvicides, Bactivec® and Griselesf®. This study independently assessed the efficacy of both biolarvicide products to ensure that they represented a good option for scaling up.
Methodology: The study was conducted at Ifakara Health Institute (IHI) in Tanzania. Laboratory-based dose–response experiments were performed using Bactivec® and Griselesf® against laboratory-reared early third instar larvae of Anopheles gambiae sensu stricto, Anopheles arabiensis, Anopheles funestus, Aedes aegypti and Culex quinquefasciatus. Larvae were exposed to various concentrations of Bactivec® and Griselesf®. VectoBac® served as a positive control, and distilled water as a negative control. Twelve replicates per concentration, with 25 larvae per replicate, were tested. Larval mortality was recorded at 24 and 48 hours after exposure to Bactivec® and Griselesf®, respectively. Probit regression analysis was used to determine the lethal concentration (LC50 and LC90) values.
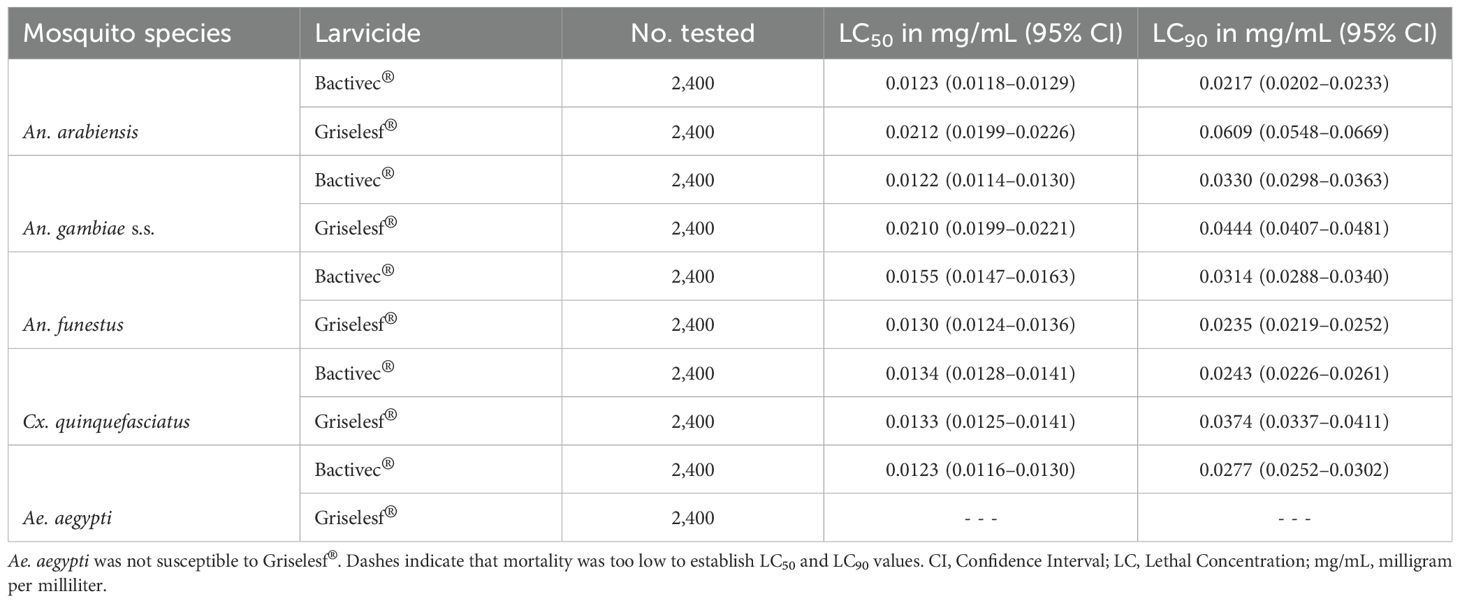
Results: Bactivec® demonstrated similar LC50 values across all species, ranging from 0.0122 mg/mL (95% CI: 0.0114–0.0130) for An. gambiae s.s. to 0.0155 mg/mL (95% CI: 0.0147–0.0163) for An. funestus. LC90 varied slightly, with An. arabiensis being the most susceptible at 0.0217 mg/mL (95% CI: 0.0202–0.0233), and An. gambiae s.s. the least at 0.0330 mg/mL (95% CI: 0.0298–0.0363). Griselesf® showed greater variation, with LC50 ranging from 0.0130 mg/mL (95% CI: 0.0124–0.0136) for An. gambiae s.s. and 0.0212 mg/mL (95% CI: 0.0199–0.0226) for An. arabiensis. Similarly, the LC90 for Griselesf® also varied, being the lowest for An. gambiae s.s., 0.0235 mg/mL (95% CI: 0.0219–0.0252) and the highest for An. arabiensis, 0.0609 mg/mL (95% CI: 0.0548–0.0669). Ae. aegypti was not susceptible to Griselesf® at the concentrations tested. The LC90 observed in this study were below the maximum application rates recommended by the manufacturer but exceeded the minimum application rates for both larvicides.
Conclusion: The Tanzanian-made Bactivec® and Griselesf® demonstrated efficacy against multiple species of mosquitoes, when applied according to the manufacturer’s recommendations except for Ae. aegypti, which was not susceptible to Griselesf®. Field applications should use maximum application rates. As Tanzania prepares to expand larviciding nationwide, the availability of these efficacious biolarvicides within the country will enhance both the feasibility and sustainability of the scale-up effort.
Background
Since 2017, the world malaria reports (World Health Organization, 2017a, 2018, 2019, 2020, 2021a, 2022, 2023, 2024) have indicated that the malaria control progress attained over the past two decades has leveled off, and in some countries, cases of malaria and deaths have recently increased (World Health Organization, 2023). The World Health Organization (WHO) has recommended continued sufficient coverage of insecticide-treated nets (ITNs) and/or indoor residual spraying (IRS) as core mosquito control interventions. In addition, the WHO recommends an addition of secondary interventions such as larval source management (LSM) in areas where optimal ITNs and IRS coverage have been attained, but malaria transmission continues (World Health Organization, 2016a, 2017b, 2021b).
In line with the WHO recommendation, the Government of Tanzania (GoT) has endorsed countrywide implementation of mosquito larviciding using biolarvicides to supplement ITNs or IRS to accelerate progress toward malaria elimination in the country. As a result, mosquito larviciding has become one of the priority interventions in the country (Tanzania MoH, 2016; Tanzania MoH, 2020; Tanzania MoH, 2022).
From 2022 to 2024, a project piloting large-scale larviciding was implemented in Tanga Region (unpublished data). The larviciding project in Tanga Region used two biolarvicide products, namely Bactivec® with the spores of Bacillus thuringiensis var. israelensis (Bti) and Griselesf® with the spores of Bacillus sphaericus (Bs).
Both products are manufactured within the country by the Tanzania Biotech Products Limited (TBPL) (www.tanzaniabiotech.co.tz/), a biotech products plant located at Kibaha in the Coastal Region of Tanzania. The TBPL was established in 2015 as a 100% subsidiary of Tanzania’s National Development Corporation, through a collaboration between the governments of Cuba and Tanzania. The establishment of this biolarvicide factory in Tanzania was driven by a strong political will and commitment by the government to revitalize the country’s malaria control efforts and to produce sufficient larvicides for the country.
The two tested biolarvicides are produced from bacteria species that confer larvicidal impact on mosquito larvae (Lacey, 2007; Silva-Filha et al., 2021). Bacteria-based larvicides produce mosquitocidal toxins that kill mosquito larvae at very low doses, while remaining safe to other non-target organisms and the environment (Rydzanicz and Lonc, 2010; Yaqoob et al., 2016; Belousova et al., 2021; Dang et al., 2021). Evaluations of Bti and Bs formulations manufactured elsewhere and in a variety of settings have demonstrated that these products kill several mosquito vector species and are safe to humans and other non-target organisms (Afrane et al., 2016; Kahindi et al., 2018; Uragayala et al., 2018; Valtierra-de-Luis et al., 2020). A study conducted in 2019 in both laboratory and semi-field settings by the National Institute for Medical Research (NIMR) in Muheza District in Tanzania confirmed that Bactivec® and Griselesf® applied at the label recommended dose were efficacious against multiple species of mosquitoes up to seven days post-application (Derua et al., 2022).
The large-scale larviciding operation in Tanga Region was the first large-scale operation in both urban and rural areas of Africa that used the TBTL products. The only larger-scale larviciding intervention in Tanzania mainland was implemented in the cities of Dar es Salaam (Bang et al., 1975; Fillinger et al., 2008; Chaki et al., 2009; Geissbühler et al., 2009; Worrall and Fillinger, 2011; Maheu-Giroux and Castro, 2013, 2014) and Tanga as part of the city’s Urban Malaria Control Program (UMCP) in the 1990s and used different larvicides. For the country’s rural settings, a few larviciding activities were conducted for research purposes (Mboera et al., 2014; Rahman et al., 2016; Mazigo et al., 2019; Berlin Rubin et al., 2020). As part of a comprehensive evaluation of this project, and before expanding larviciding country-wide, we conducted an independent assessment of the efficacy of both larvicide products, Bactivec® and Griselesf® to ensure that they represented a good option for scaling up.
We evaluated the efficacy of two biolarvicides across different laboratory-reared mosquito species with variable insecticide resistance profiles under controlled laboratory conditions. This approach aimed to assess their potential utility in the settings where both pyrethroid-resistant and non-resistant mosquitoes exist. The primary malaria vector species in the Tanga Region and across Tanzania more broadly include An. gambiae s.s., An. arabiensis, and An. funestus (Kabula et al., 2011; Mwalimu et al., 2024). Numerous insecticide resistance studies have reported widespread pyrethroid resistance across the country (Kabula et al., 2011; Kisinza et al., 2017; Matiya et al., 2019; Matowo et al., 2019, 2021; Tungu et al., 2023). The inclusion of these three malaria vector species in the study was based on their broad geographic distribution in the country. In addition, Ae. aegypti and Culex species, though not malaria vectors, were included due to their wide distribution and role in causing nuisance bites and transmitting arboviral diseases. In the context of malaria vector control, simultaneously targeting of malaria and non-malaria vectors can help reduce both nuisance biting and the risk of malaria and arbovirus transmission. Importantly, targeting multiple mosquito species also enhances community acceptance of vector control interventions, as community members typically do not distinguish between malaria and non-malaria mosquitoes (Takken and Knols, 2009; Dambach et al., 2018; Wilson et al., 2020; Saili et al., 2024). Malaria vector control interventions that fail to reduce populations of non-malaria mosquito species may lead to community perceptions that the interventions are ineffective or lack tangible benefits (Montgomery et al., 2010; Dambach et al., 2018; Magaço et al., 2019).
Materials and methods
Study setting
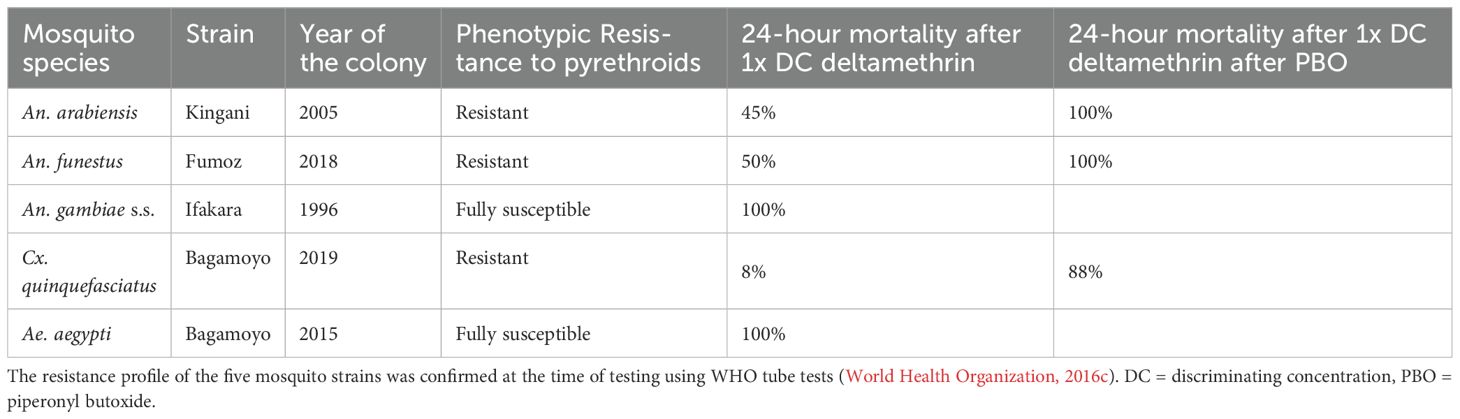
The study was conducted at the Vector Control Product Testing Unit (VCPTU) of the Ifakara Health Institute (IHI) in Bagamoyo, Tanzania, which is accredited with the Good Laboratory Practice certification SANAS OECD G0033 since June 2021. Laboratory-based dose–response experiments were conducted using Bactivec® and Griselesf®. These experiments involved early third instar larvae from five laboratory-reared mosquito species with varying insecticide resistance profiles. Characteristics of tested strains are described further in Table 1.
Mosquito rearing
The mosquito colonies were maintained by feeding larvae with Tetramin® fish food while adult mosquitoes were provided with a 10% sugar solution ad libitum. For egg laying, adults were membrane-fed with heparinized cattle blood between 3 and 6 days after emergence. Temperature and relative humidity within the insectary were maintained at 27 ± 2 °C and 70 ± 25%, respectively, following MR4 guidelines (MR4, 2016).
Bactivec® (investigational product)
Bactivec® is a water-based suspension (WS) biolarvicide made up of the spores and toxic crystals of a bacterium, Bacillus thuringiensis (Bti) var. israelensis (serotype H-14, strain 266/2) with a biopotency above 1,200 international toxic units (ITU)/milligram (mg). The Bactivec® supplied for this study was an aqueous suspension formulation with a concentration of 6 grams per liter (g/L) (www.tanzaniabiotech.co.tz/). The larvicide is indicated to kill third-instar larvae of Ae. aegypti mosquito within 24 to 48 hours, with its efficacy being optimal in clean water (Derua et al., 2022). The manufacturer’s use label rate for Bactivec® is 2–5 mL in 1 square meter (m²) of a water body. The product tested was obtained from the manufacturer (TBPL) and is not pre-qualified by WHO.
Griselesf® (investigational product)
Griselesf® is also a water-based suspension with a concentration of 5 grams per liter (g/L) made up of the spores and toxic crystals of Bacillus sphaericus (serotype H5a5b, strain 2362) with a biopotency of 268 ITU/mg against third instar larvae of Culex pipiens pipiens or Cx. quinquefasciatus larvae. It kills third instar larvae of Cx. pipiens pipiens or Cx. quinquefasciatus mosquito within 24 to 48 hours and with optimal efficacy in polluted water (Derua et al., 2022). The manufacturer’s use label rate for Bactivec® is 5–10 mL in 1 square meter (1m²) of a water body. The product tested was obtained from the manufacturer (TBPL) and is not pre-qualified by WHO.
VectoBac® (positive control)
VectoBac® is a water-dispersible granule (WDG) formulation produced by Valent BioSciences and made up of the endotoxin protein crystals of Bacillus thuringiensis subsp. israelensis (strain AM65-52) for the control of mosquito larvae. It has a biopotency of 3,000 ITU/mg against Ae. aegypti larvae (Djènontin et al., 2014; Sakka et al., 2023). The concentration of the VectoBac® supplied for this study was 5g/L. The manufacturer’s use label rate for VectoBac® is 100 mg in 50 L of water. The product has a WHO pre-qualification (World Health Organization, 2016b).
Storage and handling of larvicides
All larvicides used in the studies were received at the VCPTU in Bagamoyo and stored for one month in a temperature-controlled chemical storage facility before use. The storage temperature was maintained at 25 ± 2 °C, in line with the manufacturer’s recommendation to store the product between 4 and 30 °C (www.tanzaniabiotech.co.tz/). The facility is equipped with an extractor fan to ensure proper ventilation, and access is restricted to authorized personnel.
Preparation of stock solution, serial dilutions/working solutions
Eight serial dilutions of Bactivec® or Griselesf® were prepared and tested in four replicates per day over three consecutive days. Distilled water was used both in the preparation of the 1% stock solution and in subsequent serial dilutions. The prepared dilutions were used within 48 hours after preparation.
Bactivec® stock solution of 1mg/mL was prepared by mixing 83.5 mL of the larvicide with 416.5 mL of distilled water. This stock solution then underwent eight series of dilutions leading to solutions with concentrations of 0.5, 0.25, 0.125, 0.0625, 0.03125, 0.015625, 0.0078125, and 0.00390625 mg/mL.
Griselesf® stock solution of 1mg/mL was prepared by mixing 100 mL of the larvicide with 400 mL of distilled water. This stock solution then underwent a series of eight dilutions leading to solutions with concentrations of 0.5, 0.25, 0.125, 0.0625, 0.03125, 0.015625, 0.0078125, and 0.00390625 mg/mL.
For VectoBac®, the positive control, we used the manufacturer’s recommended use label concentration of 100 mg in 50 L of water. Targeting 500 mL of working solution, 1 mg of VectoBac® was mixed with 500 mL of distilled water. This resulted in a solution with a concentration of 0.002 mg/mL, which was used as a testing solution, without further dilution.
Dose–response experiments
On each experimental day, 200 mL cylindrical cups, measuring 6 cm in diameter and 8 cm in height were prepared in a quantity sufficient for four replicates of each of the eight larvicide concentrations and their controls per species. Eight concentrations of each larvicide were tested in four replicates per day, repeated over three consecutive days as per the WHO guidelines (World Health Organization, 2005). This resulted in twelve replicates per concentration per species.
A separate series of assays was conducted for each mosquito species, with each cup containing 25 larvae per species to prevent overcrowding (World Health Organization, 2005). Each cup was filled with 100 mL of distilled water, maintaining a water depth of approximately 5 cm to prevent excess larvae mortality. Using a clean dropper, 25 early third instar mosquito larvae of the target species were transferred into each cup and left to acclimatize for at least 2 hours. The early third instar larvae were selected by targeting larvae that were four days post-hatching (Armstrong and Bransby-Williams, 1961; Benedict et al., 2007; MR4, 2016). After acclimatization, any dead or immobile larvae were removed and replaced with healthy ones. Then the appropriate volumes of larvicide dilution (0.4, 0.9, 1.7, 3.1, 6.3, 12.5, 25, and 50 mL) for each concentration (0.00390625, 0.0078125, 0.015625, 0.03125, 0.0625, 0.125, 0.25, and 0.5 mg/mL, respectively) were introduced into each test cup, starting with the lowest concentration. The investigational larvicides and controls were conducted in parallel. The larvae were kept in contact with Bactivec® for 24 hours and with Griselesf® for 48 hours during the experiments.
Data recording
For Bactivec® (Bti) which is a fast-acting larvicide, larval mortality was recorded 24 hours post-exposure. For Griselesf® (Bs) which is a slower-acting larvicide, larval mortality was recorded after 48 hours. Moribund larvae were counted and added to dead larvae for calculating the percentage mortality. We considered larvae dead if they did not move when probed with a needle in the siphon or the cervical region, and moribund when they were incapable of rising to the surface or not showing the characteristic of diving reaction when the water was disturbed. The mortality data were recorded on paper forms before being double-entered into an Excel spreadsheet. An experiment was considered valid if larval mortality in the control group was less than 5% and pupation was below 10%.
Data analyses
The analysis was conducted with the Stata statistical software (StataCorp, 2021), version 18.0. First, data was checked for inconsistency in day of tests, replicates, and species, and where found, paper forms were consulted. Then, descriptive analysis was conducted, whereby the percentage larval mortality for each larvicide concentration was calculated using pooled data from the twelve replicates. To determine the relationship between larval mortality and larvicide concentrations, a probit regression model was fitted to the data using the log-transformed concentrations. Doses required to kill 50% (LC50) and 90% (LC90) of exposed larvae with their 95% confidence intervals were calculated from the model estimates. The log-transformed concentrations were then exponentiated to obtain the corresponding LC50 and LC90 values in their original units. Dose–response curves were generated to illustrate the observed mortality rates alongside the predicted sigmoid curves.
Results
Larval mortality (%) at various concentrations of the investigational larvicides
The results indicated that an increase in the concentration of both Bactivec® (Figure 1A) and Griselesf® (Figure 1B) led to a higher proportion of mosquito larvae mortality across all tested species, except for Aedes aegypti, which exhibited low mortality (<10%) at all tested Griselesf® concentrations (Figure 1B).
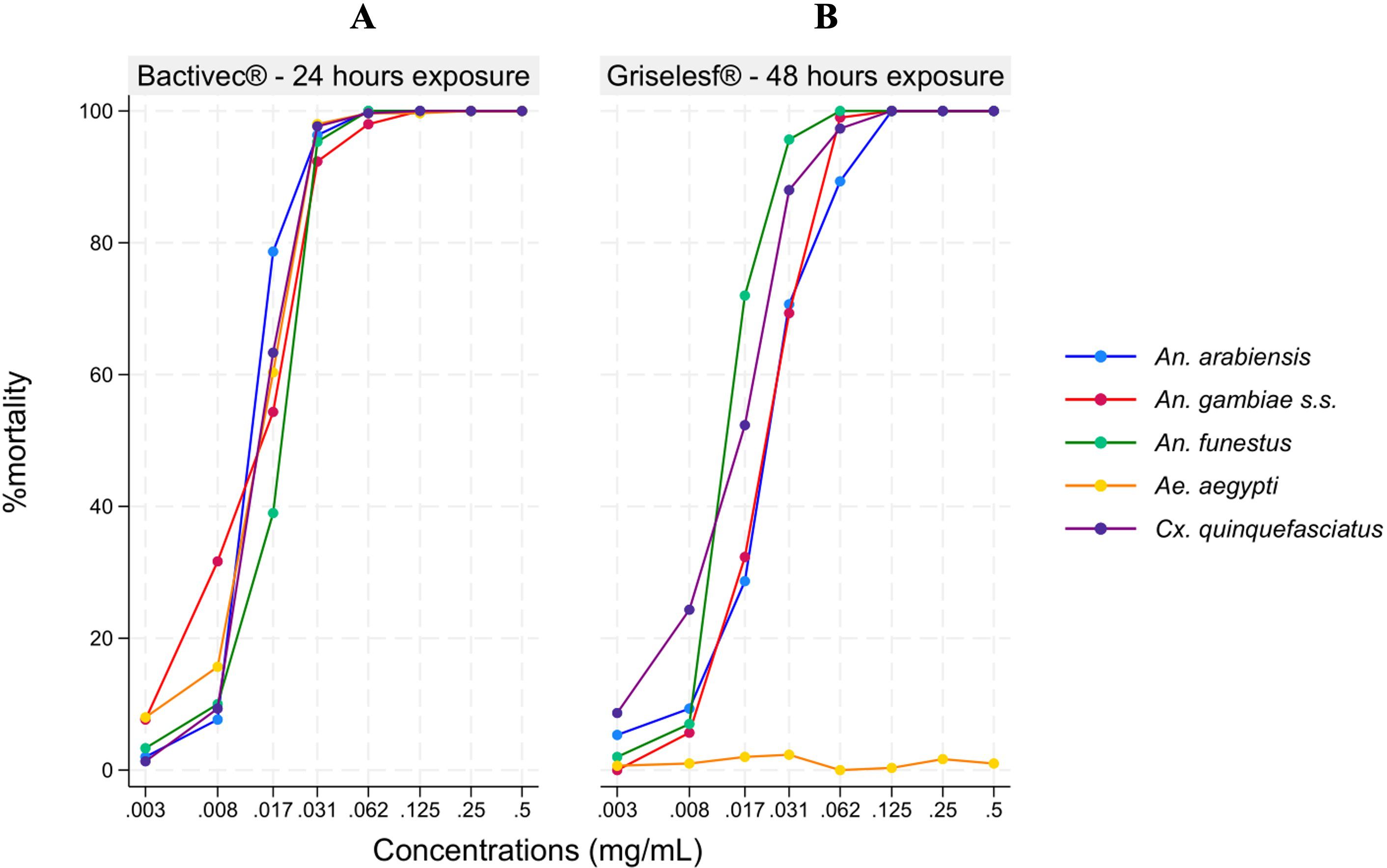
Figure 1. Descriptive results showing percentage of larval mortality across different mosquito species at various larvicide concentrations. (A) Mortality 24 hours after exposure to Bactivec®. (B) Mortality 48 hours after exposure to Griselesf®.
LC50 and LC90 of Bactivec® and Griselesf®
Bactivec®
The lethal concentration of Bactivec® to kill 50% (LC50) and 90% (LC90) after 24 hours of exposure was modeled using a probit distribution. Dose–response curves are presented in Figure 2 for each mosquito species elicited by different concentrations, and the LC50 and LC90 values with 95% CI are presented in Table 2. The LC50 for Bactivec® was similar across all the five species tested (Table 2), but the LC90 varied, ranging from 0.0217 for An. arabiensis (Figure 2A) to 0.0330 for An. gambiae ss (Figure 2B) and 0.0314 for An. funestus (Figure 2C). Cx. quinquefasciatus (Figure 2D) and Aedes aegypti (Figure 2E) had similar dose–response curves and similar LC50 and LC90.
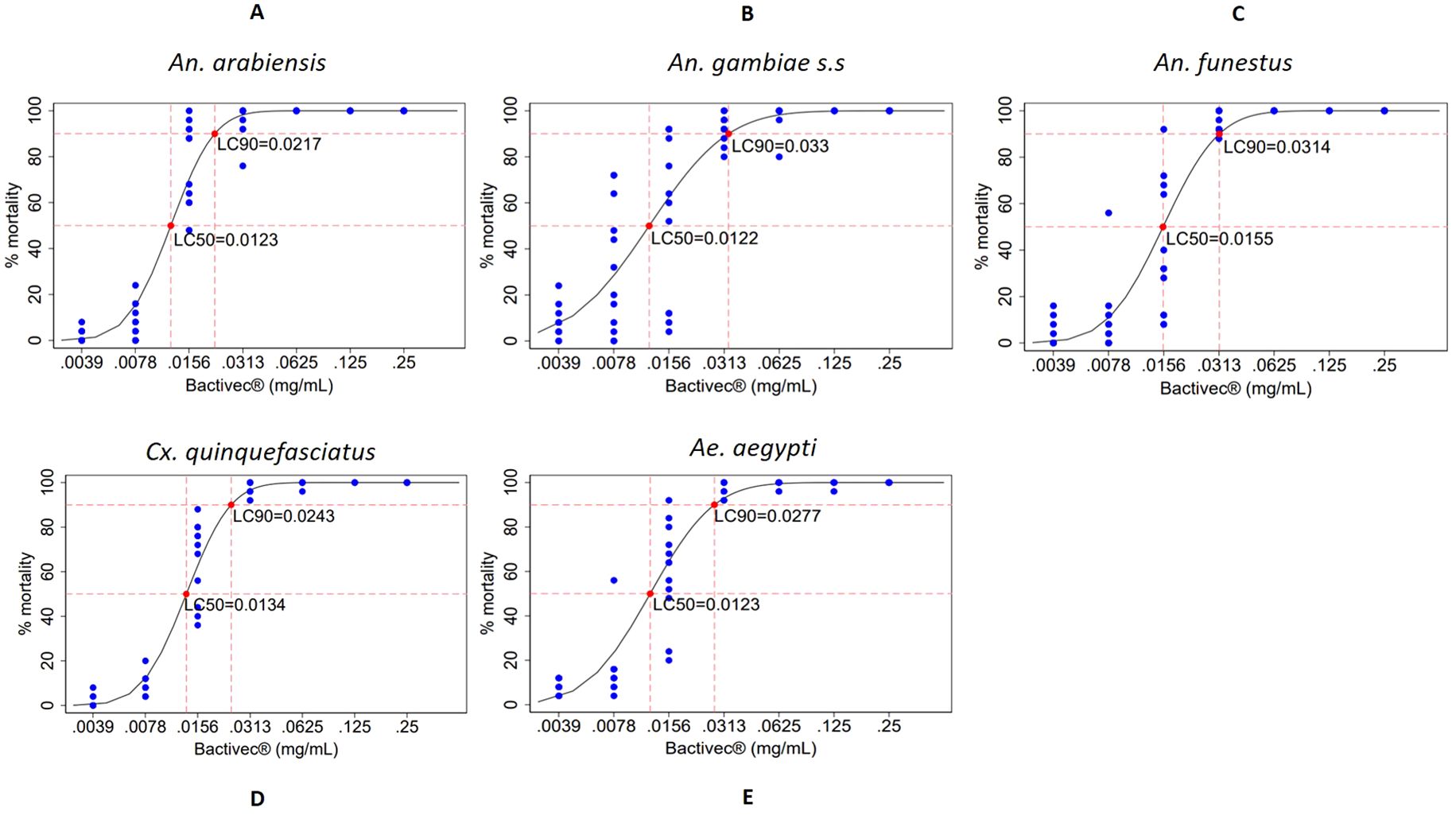
Figure 2. Dose–response curves showing percentage mortality of larvae from different mosquito species across various concentrations of Bactivec®, indicating the points at which LC50 and LC90 were attained. Larval mortality was assessed 24 hours after exposure to the larvicide. Each concentration was tested in 12 replicates of 25 larvae. Blue dots represent the distribution of percentage mortality across replicates, and red dots indicate the estimated LC50 and LC90. (A) An. arabiensis, (B) An. gambiae s.s., (C) An. funestus, (D) Cx. quinquefasciatus, (E) Ae. aegypti, mg/mL, milligram per milliliter.
Griselesf®
The lethal concentration of Griselesf® to kill 50% (LC50) and 90% (LC90) after 48 hours of exposure was modeled using a probit distribution. Dose response curves are presented in Figures 3A–E for each mosquito species elicited by different concentrations, and the LC50 and LC90 values with 95% CI are presented in Table 2. The data for Griselesf® showed greater variability in LC50 and LC90 among different mosquito species compared to Bactivec®. The LC50 was highest for An. arabiensis, 0.0212 (Figure 3A) and An. gambiae s.s., 0.0210 (Figure 3B) but lower for An. funestus, 0.0130 (Figure 3C). Similarly, the LC90 for Griselesf® varied among species, ranging from 0.0235 for An. funestus (Figure 3C) to almost three times higher at 0.0609 for An. arabiensis (Figure 3A). For Cx. quinquefasciatus the LC90 was 0.0374 (Figure 3D). Griselesf® was not efficacious against Ae. aegypti, as shown in Figure 3E, Table 2.
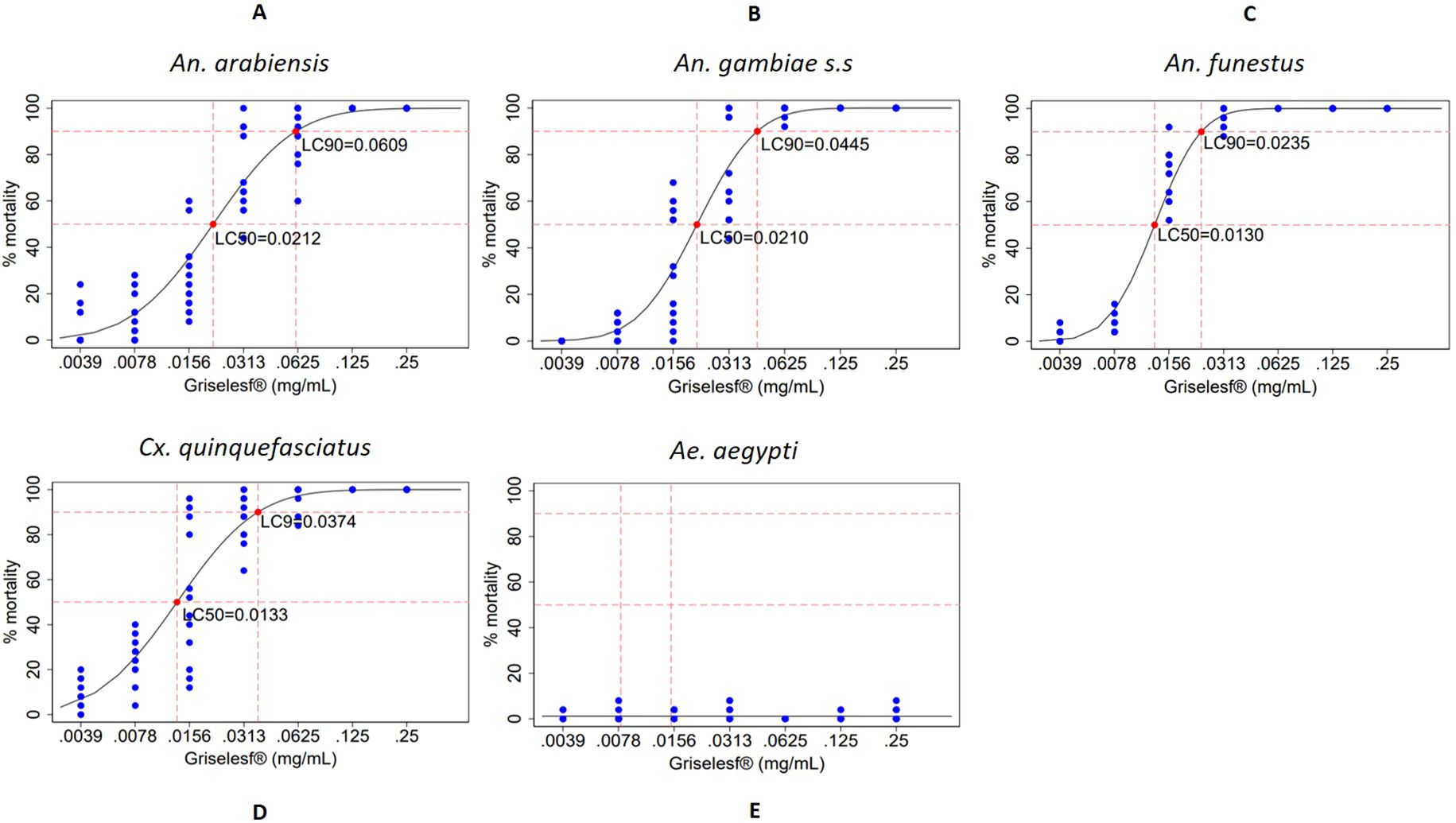
Figure 3. Dose–response curves showing the percentage mortality of larvae from different mosquito species across various larvicide concentrations of Griselesf®, indicating the points at which LC50 and LC90 were attained. Larval mortality was assessed 48 hours after exposure to the larvicide. Each concentration was tested in 12 replicates of 25 larvae. Blue dots represent the distribution of percentage mortality across replicates, and red dots indicate the estimated LC50 and LC90. (A) An. arabiensis, (B) An. gambiae s.s., (C) An. funestus, (D) Cx. quinquefasciatus, (E) Ae. Aegypti, mg/mL, milligram per milliliter.
Larval mortalities in the controls when Bactivec® was tested
In the tests that involved Bactivec®, the larval mortality in the positive control, using Vectobac® at 0.002mg/mL, 24 hours after exposure, was 100% for all the species except An. funestus for which the mortality was 99%. In the negative control group, where larvae were exposed to distilled water, overall mortality was 0.4% with slight variations among the different species, as indicated in Table 3. The results for the controls confirm that the study was conducted at an acceptable quality.
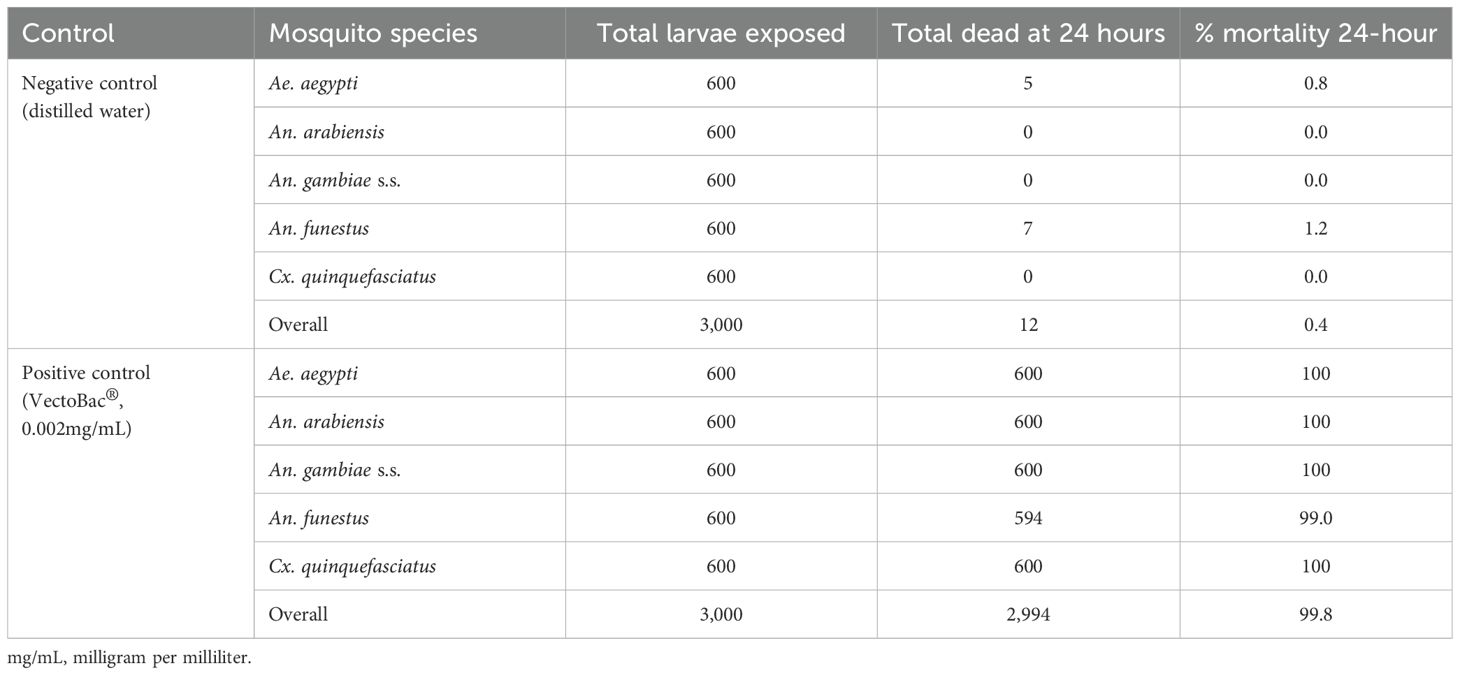
Table 3. Mortality levels in the controls across different species assessed 24 hours after exposure when testing Bactivec®.
Larval mortalities in the controls when Griselesf® was tested
In the experiment involving Griselesf®, the positive control, using Vectobac® at 0.002mg/mL recorded 100% mortality across all the species 48 hours after exposure. In the negative control group, overall mortality was 0.5%, also with slight variations among the different species (Table 4). The results for the controls confirm that the study was conducted at an acceptable quality.
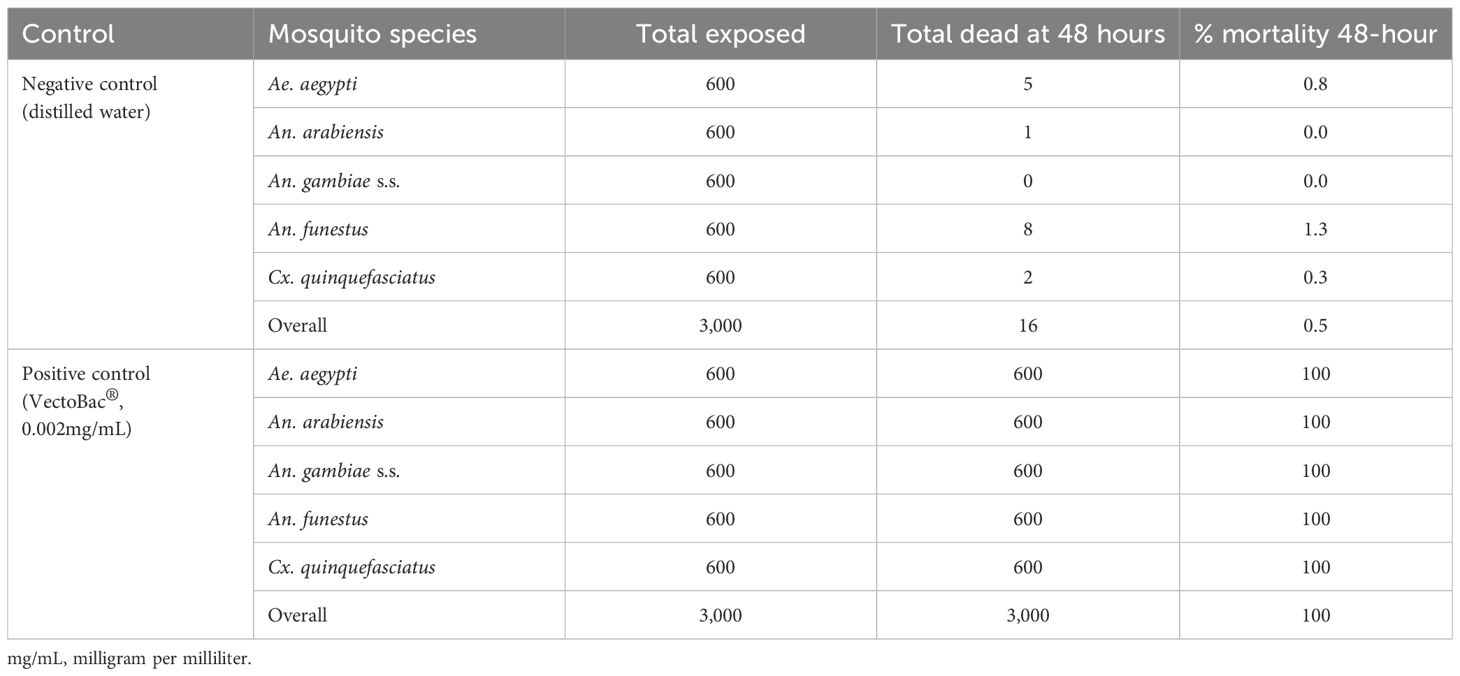
Table 4. Mortality levels in the controls across different species assessed 48 hours after exposure when testing Griselesf®.
Discussion
This laboratory study demonstrated that Bactivec® and Griselesf® were efficacious against multiple mosquito species at lower doses than the manufacturer’s recommended maximum label rate. The manufacturer’s label recommends a larvicide concentration of 6 g/L for Bactivec® and 5 g/L for Griselesf®. Assuming the larvicide spreads evenly over a 1 m² water body, these application rates correspond to concentrations of 0.0125 mg/mL to 0.035 mg/mL for Bactivec® and 0.025 mg/mL to 0.05 mg/mL for Griselesf®. The LC90 observed in this study were below the maximum application rates recommended by the manufacturer but exceeded the minimum application rates for both larvicides. The results of the current study are consistent with those of the 2019 study [22], in which the LC50 among different mosquito species ranged from 0.018 mg/mL to 0.026 mg/mL for Bti and 0.017 mg/mL to 0.029 mg/mL for Bs, while the LC 95 ranged from 0.052 mg/mL to 0.106 mg/mL for Bti and 0.040 mg/mL to 0.086 mg/mL for Bs. However, the product used as positive control (VectoBac®), achieved 100% mortality in all mosquito species and 99% in An. funestus at the lower concentration of 0.002 mg/mL, indicating that the larvicide product was more potent than Bactivec®.
The demonstrated efficacy of these domestically manufactured larvicides against multiple species of malaria vectors highlights the potential of the local production facility to address the challenge of limited access to effective larvicidal products, a key constraint to the scaling up of larviciding programs in Africa (Newby et al., 2025). The results provide evidence and confidence that as Tanzania moves toward nationwide implementation of larviciding, the availability of efficacious larvicides within the country will enhance the feasibility and sustainability of scale-up efforts.
Importantly, the results demonstrated that the two larvicide products were fully efficacious against pyrethroid-resistant strains of mosquitoes, which are becoming more difficult to control using ITNs and IRS (Rubert et al., 2016; Alout et al., 2017; Tokponnon et al., 2019; Lindsay et al., 2021; Suh et al., 2023). The use of biolarvicides is considered a valuable intervention in mitigating mosquito of resistance to insecticides (Kabula et al., 2011; Kisinza et al., 2017; Matiya et al., 2019; Matowo et al., 2019; Bisanzio et al., 2022; Messenger et al., 2023; Tungu et al., 2023). Mosquito resistance to pyrethroids has been reported to be widespread in Tanzania, posing a significant threat to pyrethroid-based vector control interventions (Kisinza et al., 2017; Matiya et al., 2019; Bisanzio et al., 2022; Tungu et al., 2023; Odero et al., 2024). Whether the use of biolarvicides in Tanzania would significantly contribute to mitigation insecticide resistance remains an important question for further investigation.
Similar to the 2019 study (Derua et al., 2022), the current study showed that Ae. aegypti mosquitoes were not susceptible to Griselesf® at the tested concentrations. This is an important finding in view of the control of this vector against the spread of dengue virus. This observation corroborates recommendations from previous studies suggesting that Bs should not be considered for use when targeting Ae. aegypti with larviciding (Rojas-Pinzón and Dussán, 2017; Santana-Martinez et al., 2019; Derua et al., 2022). The reasons for the inefficacy of Bs against Ae.aegypti have been reported by other studies (Rojas-Pinzón and Dussán, 2017; Santana-Martinez et al., 2019). Bs crystal proteins do not bind effectively to the gut receptors of Ae aegypti larvae (and other species in this genus), thereby limiting the larvicidal activity of Bs against this vector (Wirth et al., 2000; Wirth et al., 2001; Nascimento et al., 2020).
Finally, it should be noted that this study was conducted in a controlled laboratory environment, where the real-world factors such as water turbidity, depth, and other environmental conditions, which may influence larvicide effectiveness, were not assessed. Given that larvicide potency is likely to be reduced under field conditions it is recommended in line with findings from a previous study (Derua et al., 2022) that field applications are substantially higher than laboratory-derived LC90 values. Based on these data, maximum field applications should be used.
Conclusion
This study confirmed the efficacy of the Tanzanian-manufactured larvicides, Bactivec® and Griselesf®, against major malaria and dengue vector species, demonstrating their potential as effective larvicidal tools for mosquito control, except for Griselesf®, which did not appear to be efficacious against Ae. aegypti. The demonstrated effectiveness of these locally produced larvicides at maximum application rates supports their potential role in supporting Tanzania’s efforts to scale up larviciding interventions for improved malaria control in the country.
Data availability statement
Data can be made available upon receipt of official reasonable requests, from Vector Control Product and Testing Unit of Ifakara Health Institute and the TEMT project director. Requests to access the datasets should be directed to SM: c21vb3JlQGloaS5vci50eg==, CL: Y2hyaXN0aWFuLmxlbmdlbGVyQHN3aXNzdHBoLmNo.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
TG: Writing – review & editing, Formal analysis, Validation, Data curation, Writing – original draft, Methodology, Supervision, Conceptualization, Visualization, Investigation. DK: Methodology, Data curation, Formal analysis, Writing – review & editing. JM: Writing – review & editing, Validation, Supervision, Methodology. VM: Supervision, Writing – review & editing, Methodology. KS: Methodology, Formal analysis, Writing – review & editing. OO: Writing – review & editing, Formal analysis. FT: Methodology, Writing – review & editing. CD: Writing – review & editing. JB: Writing – review & editing. SL: Writing – review & editing. BY: Writing – review & editing. SK: Writing – review & editing. EK: Writing – review & editing, Formal analysis, Methodology. NK: Writing – review & editing, Methodology. EM: Writing – review & editing, Methodology. PC: Methodology, Investigation, Conceptualization, Writing – review & editing. CL: Writing – review & editing, Methodology, Supervision, Conceptualization, Funding acquisition. SM: Methodology, Investigation, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was funded by a grant from the Swiss Government through the Swiss Embassy in Dar es Salaam to the Swiss Tropical and Public Health Institute (TEMT project). The donor had no influence in the design, implementation, analysis and publication of this work.
Acknowledgments
The authors would like to acknowledge the invaluable support provided by the team of technicians at the VCPTU in Bagamoyo. Our sincere appreciation goes to the insectary team of the VCPTU in Bagamoyo for supplying the mosquito larvae used in this study. We extend our thanks to Jason Moore and the VCPTU management team for providing space for this experiment. We also thank Valent BioSciences for providing VectoBac® we used in this study as positive control.
Conflict of interest
JM, KS, OO, FT, EM, and SM test vector control products for private and public industries and regulatory bodies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Bti, Bacillus thuringiensis israelensis; Bs, Bacillus sphaericus; CI, Confidence Interval; GoT, Government of Tanzania; GTS, Global Technical Strategy; IHI, Ifakara Health Institution; IRS, Indoor Residual Spraying; ITU, International Toxic Units; ITN, Insecticide Treated Nets; LC, Lethal Concentration; LSM, Larval Source Management; TBPL, Tanzania Bio-product Limited; USA, Unites States of America; VCPTU, Vector Control Products Testing Unit; WDG, Water-Dispersible Granules; WHO, World Health Organization; WS, Water-based suspension.
References
Afrane Y. A., Mweresa N. G., Wanjala C. L., Gilbreath Iii T. M., Zhou G., Lee M. C., et al. (2016). Evaluation of long-lasting microbial larvicide for malaria vector control in Kenya. Malar J. 15, 577. doi: 10.1186/s12936-016-1626-6
Alout H., Roche B., Dabiré R. K., and Cohuet A. (2017). Consequences of insecticide resistance on malaria transmission. PLoS Pathog. 13, e1006499. doi: 10.1371/journal.ppat.1006499
Armstrong J. A. and Bransby-Williams W. R. (1961). The maintenance of a colony of Anopheles Gambiae, with observations on the effects of changes in temperature. Bull. World Health Organ 24, 427–435.
Bang Y. H., Sabuni I. B., and Tonn R. J. (1975). Integrated control of urban mosquitoes in Dar es Salaam using community sanitation supplemented by larviciding. East Afr Med. J. 52, 578–588.
Belousova M. E., Malovichko Y. V., Shikov A. E., Nizhnikov A. A., and Antonets K. S. (2021). Dissecting the environmental consequences of bacillus thuringiensis application for natural ecosystems. Toxins (Basel). 13. doi: 10.3390/toxins13050355
Benedict M. Q., Howell P., and Wikins L. (2007). Methods in anopheles research manual, mr4 protocol. Available online at: https://wwwscopuscom/inward/recorduri?eid=2-s20-84893057764&partnerID=40&md5=6cd1f67ec1c3b77530eec8a47e8a0589 (Accessed October 24, 2024).
Berlin Rubin N., Mboera L. E. G., Lesser A., Miranda M. L., and Kramer R. (2020). Process evaluation of a community-based microbial larviciding intervention for malaria control in Rural Tanzania. Int. J. Environ. Res. Public Health 17. doi: 10.3390/ijerph17197309
Bisanzio D., Ally M., Ali A. S., Kitojo C., Serbantez N., Kisinza W. N., et al. (2022). Modelling insecticide resistance of malaria vector populations in Tanzania. Am. J. Trop. Med. Hyg. 107, 308–314. doi: 10.4269/ajtmh.21-0262
Chaki P. P., Govella N. J., Shoo B., Hemed A., Tanner M., Fillinger U., et al. (2009). Achieving high coverage of larval-stage mosquito surveillance: challenges for a community-based mosquito control programme in urban Dar es Salaam, Tanzania. Malar J. 8, 311. doi: 10.1186/1475-2875-8-311
Dambach P., Jorge M. M., Traoré I., Phalkey R., Sawadogo H., Zabré P., et al. (2018). A qualitative study of community perception and acceptance of biological larviciding for malaria mosquito control in rural Burkina Faso. BMC Public Health 18, 399. doi: 10.1186/s12889-018-5299-7
Dang C., Zhou X., Sun C., Wang F., Peng Y., and Ye G. (2021). Impacts of Bt rice on non-target organisms assessed by the hazard quotient (HQ). Ecotoxicol Environ. Saf. 207, 111214. doi: 10.1016/j.ecoenv.2020.111214
Derua Y. A., Tungu P. K., Malima R. C., Mwingira V., Kimambo A. G., Batengana B. M., et al. (2022). Laboratory and semi-field evaluation of the efficacy of Bacillus thuringiensis var. Israelensis (Bactivec®) and Bacillus sphaericus (Griselesf®) for control of mosquito vectors in northeastern Tanzania. Curr. Res. Parasitol. Vector Borne Dis. 2, 100089. doi: 10.1016/j.crpvbd.2022.100089
Djènontin A., Pennetier C., Zogo B., Soukou K. B., Ole-Sangba M., Akogbéto M., et al. (2014). Field efficacy of Vectobac GR as a mosquito larvicide for the control of anopheline and culicine mosquitoes in natural habitats in Benin, West Africa. PLoS One 9, e87934. doi: 10.1371/journal.pone.0087934
Fillinger U., Kannady K., William G., Vanek M. J., Dongus S., Nyika D., et al. (2008). A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malar J. 7, 20. doi: 10.1186/1475-2875-7-20
Geissbühler Y., Kannady K., Chaki P. P., Emidi B., Govella N. J., Mayagaya V., et al. (2009). Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS One 4, e5107. doi: 10.1371/journal.pone.0005107
Kabula B., Derua Y. A., Tungui P., Massue D. J., Sambu E., Stanley G., et al. (2011). Malaria entomological profile in Tanzania from 1950 to 2010: a review of mosquito distribution, vectorial capacity and insecticide resistance. Tanzan J. Health Res. 13, 319–331. doi: 10.4314/thrb.v13i5.2
Kahindi S. C., Muriu S., Derua Y. A., Wang X., Zhou G., Lee M. C., et al. (2018). Efficacy and persistence of long-lasting microbial larvicides against malaria vectors in western Kenya highlands. Parasit Vectors. 11, 438. doi: 10.1186/s13071-018-3009-z
Kisinza W. N., Nkya T. E., Kabula B., Overgaard H. J., Massue D. J., Mageni Z., et al. (2017). Multiple insecticide resistance in Anopheles Gambiae from Tanzania: a major concern for malaria vector control. Malar J. 16, 439. doi: 10.1186/s12936-017-2087-2
Lacey L. A. (2007). Bacillus thuringiensis serovariety Israelensis and Bacillus sphaericus for mosquito control. J. Am. Mosq. Control Assoc. 23, 133–163. doi: 10.2987/8756-971x(2007)23[133:Btsiab]2.0.Co;2
Lindsay S. W., Thomas M. B., and Kleinschmidt I. (2021). Threats to the effectiveness of insecticide-treated bednets for malaria control: thinking beyond insecticide resistance. Lancet Glob Health 9, e1325–e1e31. doi: 10.1016/s2214-109x(21)00216-3
Magaço A., Botão C., Nhassengo P., Saide M., Ubisse A., Chicumbe S., et al. (2019). Community knowledge and acceptance of indoor residual spraying for malaria prevention in Mozambique: a qualitative study. Malar J. 18, 27. doi: 10.1186/s12936-019-2653-x
Maheu-Giroux M. and Castro M. C. (2013). Impact of community-based larviciding on the prevalence of malaria infection in Dar es Salaam, Tanzania. PLoS One 8, e71638. doi: 10.1371/journal.pone.0071638
Maheu-Giroux M. and Castro M. C. (2014). Cost-effectiveness of larviciding for urban malaria control in Tanzania. Malar J. 13, 477. doi: 10.1186/1475-2875-13-477
Matiya D. J., Philbert A. B., Kidima W., and Matowo J. J. (2019). Dynamics and monitoring of insecticide resistance in malaria vectors across mainland Tanzania from 1997 to 2017: a systematic review. Malar J. 18, 102. doi: 10.1186/s12936-019-2738-6
Matowo N. S., Abbasi S., Munhenga G., Tanner M., Mapua S. A., Oullo D., et al. (2019). Fine-scale spatial and temporal variations in insecticide resistance in Culex pipiens complex mosquitoes in rural south-eastern Tanzania. Parasit Vectors. 12, 413. doi: 10.1186/s13071-019-3676-4
Matowo N. S., Martin J., Kulkarni M. A., Mosha J. F., Lukole E., Isaya G., et al. (2021). An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone, Tanzania. Sci. Rep. 11, 13457. doi: 10.1038/s41598-021-92741-8
Mazigo H. D., Massawe I. S., Rumisha S. F., Kweka E. J., and Mboera L. E. G. (2019). Rice farmers’ perceptions and acceptability in the use of a combination of biolarvicide (Bacillus thuringiensis var. Israeliensis) and fertilizers application for malaria control and increase rice productivity in a rural district of central Tanzania. Malar J. 18, 71. doi: 10.1186/s12936-019-2697-y
Mboera L. E., Kramer R. A., Miranda M. L., Kilima S. P., Shayo E. H., and Lesser A. (2014). Community knowledge and acceptance of larviciding for malaria control in a rural district of east-central Tanzania. Int. J. Environ. Res. Public Health 11, 5137–5154. doi: 10.3390/ijerph110505137
Messenger L. A., Matowo N. S., Cross C. L., Jumanne M., Portwood N. M., Martin J., et al. (2023). Effects of next-generation, dual-active-ingredient, long-lasting insecticidal net deployment on insecticide resistance in malaria vectors in Tanzania: an analysis of a 3-year, cluster-randomised controlled trial. Lancet Planet Health 7, e673–ee83. doi: 10.1016/s2542-5196(23)00137-7
Montgomery C. M., MunGuambe K., and Pool R. (2010). Group-based citizenship in the acceptance of indoor residual spraying (IRS) for malaria control in Mozambique. Soc. Sci. Med. 70, 1648–1655. doi: 10.1016/j.socscimed.2010.01.020
MR4 (2016). Methods in Anopheles Research Manual. Available online at: https://wwwbeiresourcesorg/Portals/2/VectorResources/2016%20Methods%20in%20Anopheles%20Research%20full%20manualpdf (Accessed October 24, 2024).
Mwalimu C. D., Kiware S., Nshama R., Derua Y., Machafuko P., Gitanya P., et al. (2024). Dynamics of malaria vector composition and Plasmodium falciparum infection in mainland Tanzania: 2017–2021 data from the national malaria vector entomological surveillance. Malar J. 23, 29. doi: 10.1186/s12936-024-04849-7
Nascimento N. A., Torres-Quintero M. C., Molina S. L., Pacheco S., Romão T. P., Pereira-Neves A., et al. (2020). Functional Bacillus thuringiensis Cyt1Aa Is Necessary To Synergize Lysinibacillus sphaericus Binary Toxin (Bin) against Bin-Resistant and -Refractory Mosquito Species. Appl. Environ. Microbiol. 86. doi: 10.1128/aem.02770-19
Newby G., Chaki P., Latham M., Marrenjo D., Ochomo E., Nimmo D., et al. (2025). Larviciding for malaria control and elimination in Africa. Malar J. 24, 16. doi: 10.1186/s12936-024-05236-y
Odero J. O., Nambunga I. H., Masalu J. P., Mkandawile G., Bwanary H., Hape E. E., et al. (2024). Genetic markers associated with the widespread insecticide resistance in malaria vector Anopheles funestus populations across Tanzania. Parasit Vectors. 17, 230. doi: 10.1186/s13071-024-06315-4
Rahman R., Lesser A., Mboera L., and Kramer R. (2016). Cost of microbial larviciding for malaria control in rural Tanzania. Trop. Med. Int. Health 21, 1468–1475. doi: 10.1111/tmi.12767
Rojas-Pinzón P. A. and Dussán J. (2017). Efficacy of the vegetative cells of Lysinibacillus sphaericus for biological control of insecticide-resistant Aedes aEgypti. Parasit Vectors. 10, 231. doi: 10.1186/s13071-017-2171-z
Rubert A., Guillon-Grammatico L., Chandenier J., Dimier-Poisson I., and Desoubeaux G. (2016). Insecticide resistance in Anopheles mosquitoes: additional obstacles in the battle against malaria. Med. Sante Trop. 26, 423–431. doi: 10.1684/mst.2016.0634
Rydzanicz K. and Lonc E. (2010). Ecological safety of mosquitocidal biocides based on Bacillus thuringiensis Israelensis. Wiad Parazytol. 56, 305–314.
Saili K., de Jager C., Masaninga F., Chisanga B., Sinyolo A., Chiwaula J., et al. (2024). Community perceptions, acceptability, and the durability of house screening interventions against exposure to malaria vectors in Nyimba district, Zambia. BMC Public Health 24, 285. doi: 10.1186/s12889-024-17750-4
Sakka M. K., Ioannou C. S., Papadopoulos N. T., and Athanassiou C. G. (2023). Residual efficacy of selected larvicides against Culex pipiens pipiens (Diptera: Culicidae) under laboratory and semi-field conditions. Environ. Sci. pollut. Res. Int. 30, 40931–40941. doi: 10.1007/s11356-022-24654-6
Santana-Martinez J. C., Silva J. J., and Dussan J. (2019). Efficacy of Lysinibacillus sphaericus against mixed-cultures of field-collected and laboratory larvae of Aedes aEgypti and Culex quinquefasciatus. Bull. Entomol Res. 109, 111–118. doi: 10.1017/s0007485318000342
Silva-Filha M., Romao T. P., Rezende T. M. T., Carvalho K. D. S., Gouveia de Menezes H. S., Alexandre do Nascimento N., et al. (2021). Bacterial toxins active against mosquitoes: mode of action and resistance. Toxins (Basel) 13. doi: 10.3390/toxins13080523
Suh P. F., Elanga-Ndille E., Tchouakui M., Sandeu M. M., Tagne D., Wondji C., et al. (2023). Impact of insecticide resistance on malaria vector competence: a literature review. Malar J. 22, 19. doi: 10.1186/s12936-023-04444-2
Takken W. and Knols B. G. (2009). Malaria vector control: current and future strategies. Trends Parasitol. 25, 101–104. doi: 10.1016/j.pt.2008.12.002
Tanzania MoH (2016). National Guideline for Integrated Malaria Vector Control. Available online at: http://api-hidlafyagotz/uploads/library-documents/1621438892-H1A9nEDvpdf2014 (Accessed October 24, 2024).
Tanzania MoH (2020). National Malaria Strategic Plan 2021-2025. Available online at: http://api-hidlafyagotz/uploads/library-documents/1641210939-jH9mKCtzpdf (Accessed October 24, 2024).
Tanzania MoH (2022). Integrated Vector Management Guidelines and Standard Operating Procedures. Available online at: https://wwwnmcpgotz/storage/app/uploads/public/643/90b/5e3/64390b5e3acaf302809977pdf (Accessed October 24, 2024).
Tokponnon F. T., Sissinto Y., Ogouyémi A. H., Adéothy A. A., Adechoubou A., Houansou T., et al. (2019). Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: evidence from health facility data from Benin. Malar J. 18, 37. doi: 10.1186/s12936-019-2656-7
Tungu P., Kabula B., Nkya T., Machafuko P., Sambu E., Batengana B., et al. (2023). Trends of insecticide resistance monitoring in mainland Tanzania, 2004-2020. Malar J. 22, 100. doi: 10.1186/s12936-023-04508-3
Uragayala S., Kamaraju R., Tiwari S., Ghosh S. K., and Valecha N. (2018). Field testing & evaluation of the efficacy & duration of effectiveness of a biolarvicide, Bactivec(®) SC (Bacillus thuringiensis var. Israelensis SH-14) in Bengaluru, India. Indian J. Med. Res. 147, 299–307. doi: 10.4103/ijmr.IJMR_1631_16
Valtierra-de-Luis D., Villanueva M., Berry C., and Caballero P. (2020). Potential for bacillus thuringiensis and other bacterial toxins as biological control agents to combat dipteran pests of medical and agronomic importance. Toxins (Basel). 12. doi: 10.3390/toxins12120773
Wilson A. L., Courtenay O., Kelly-Hope L. A., Scott T. W., Takken W., Torr S. J., et al. (2020). The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 14, e0007831. doi: 10.1371/journal.pntd.0007831
Wirth M. C., Delécluse A., and Walton W. E. (2001). Cyt1Ab1 and Cyt2Ba1 from Bacillus thuringiensis subsp. medellin and B. thuringiensis subsp. Israelensis Synergize Bacillus sphaericus against Aedes aEgypti and resistant Culex quinquefasciatus (Diptera: Culicidae). Appl. Environ. Microbiol. 67, 3280–3284. doi: 10.1128/aem.67.7.3280-3284.2001
Wirth M. C., Federici B. A., and Walton W. E. (2000). Cyt1A from Bacillus thuringiensis synergizes activity of Bacillus sphaericus against Aedes aEgypti (Diptera: Culicidae). Appl. Environ. Microbiol. 66, 1093–1097. doi: 10.1128/aem.66.3.1093-1097.2000
World Health Organization (2005). Guidelines for laboratory and field testing of mosquito larvicides. Available online at: https://appswhoint/iris/handle/10665/69101 (Accessed October 24, 2024).
World Health Organization (2016a). High burden to high impact: A targeted malaria response. Available online at: https://wwwwhoint/publications/i/item/WHO-CDS-GMP-201825 (Accessed October 24, 2024).
World Health Organization (2016b). Report of the nineteenth WHOPES working group meeting (Geneva: WHO/HQ). Available online at: https://iris.who.int/bitstream/handle/10665/205588/9789241510400_eng.pdf?sequence=1. 8–11 February 2016: review of Veeralin LN, VectoMax GR, Bactivec SC (Accessed Accessed October 24, 2024).
World Health Organization (2016c). Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Available online at: https://iriswhoint/bitstream/handle/10665/250677/9789241511575-engpdf?sequence=1 (Accessed October 24, 2024).
World Health Organization (2017a). World Malaria Report 2017. Available online at: https://wwwwhoint/teams/global-malaria-programme/reports/world-malaria-report-2017 (Accessed October 24, 2024).
World Health Organization (2017b). Global vector control response 2017–2030. Available online at: https://wwwwhoint/publications/i/item/9789241512978 (Accessed October 24, 2024).
World Health Organization (2018). World Malaria Report 2018. Available online at: https://wwwwhoint/teams/global-malaria-programme/reports/world-malaria-report-2018 (Accessed October 24, 2024).
World Health Organization (2019). World Malaria Report 2019. Available online at: https://wwwwhoint/teams/global-malaria-programme/reports/world-malaria-report-2019 (Accessed October 24, 2024).
World Health Organization (2020). World Malaria Report 2020. Available online at: https://wwwwhoint/teams/global-malaria-programme/reports/world-malaria-report-2020 (Accessed October 24, 2024).
World Health Organization (2021a). World malaria report 2021. Available online at: https://wwwwhoint/teams/global-malaria-programme/reports/world-malaria-report-2021 (Accessed October 24, 2024).
World Health Organization (2021b). Global technical strategy for malaria 2016–2030, 2021 update. Available online at: https://wwwwhoint/publications/i/item/9789240031357 (Accessed October 24, 2024).
World Health Organization (2022). World Malaria Report 2022. Available online at: https://wwwwhoint/publications/i/item/9789240064898 (Accessed October 24, 2024).
World Health Organization (2023). World malaria report 2023. Available online at: https://wwwwhoint/teams/global-malaria-programme/reports/world-malaria-report-2023 (Accessed October 24, 2024).
World Health Organization (2024). World malaria report 2024. Available online at: https://wwwwhoint/publications/i/item/9789240104440 (Accessed October 24, 2024).
Worrall E. and Fillinger U. (2011). Large-scale use of mosquito larval source management for malaria control in Africa: a cost analysis. Malar J. 10, 338. doi: 10.1186/1475-2875-10-338
Keywords: Bactivec®, Griselesf®, VectoBac®, Larvicide, efficacy, LC50 and LC90, Anopheles
Citation: Gavana T, Kailembo D, Machange J, Michael V, Swai K, Odufuwa OG, Tenywa F, Dismas Mwalimu C, Bernard J, Lazaro S, Yoram B, Kajange S, Kasagama E, Kisoka N, Mbuba E, Chaki P, Lengeler C and Moore SJ (2025) Laboratory efficacy of Bactivec® and Griselesf® biolarvicides used for large-scale larviciding in Tanzania. Front. Malar. 3:1614476. doi: 10.3389/fmala.2025.1614476
Received: 18 April 2025; Accepted: 30 June 2025;
Published: 21 July 2025.
Edited by:
Diego Alonso, São Paulo State University, BrazilReviewed by:
Koama Bayili, Institut de Recherche en Sciences de la Santé (IRSS), Burkina FasoPaul O. Mireji, Kenya Agricultural and Livestock Research Organization, Kenya
Copyright © 2025 Gavana, Kailembo, Machange, Michael, Swai, Odufuwa, Tenywa, Dismas Mwalimu, Bernard, Lazaro, Yoram, Kajange, Kasagama, Kisoka, Mbuba, Chaki, Lengeler and Moore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tegemeo Gavana, dGdhdmFuYUBpaGkub3IudHo=
 Tegemeo Gavana
Tegemeo Gavana Denis Kailembo2,3
Denis Kailembo2,3 Jane Machange
Jane Machange Kyeba Swai
Kyeba Swai Olukayode G. Odufuwa
Olukayode G. Odufuwa Jubilate Bernard
Jubilate Bernard Emmanuel Mbuba
Emmanuel Mbuba Prosper Chaki
Prosper Chaki Sarah J. Moore
Sarah J. Moore