- 1Vector Control Product Testing Unit, Environmental Health and Ecological Science Department, Ifakara Health Institute, Bagamoyo, Tanzania
- 2Vector Biology Unit, Swiss Tropical and Public Health Institute, Allschwil, Switzerland
- 3Faculty of Science, University of Basel, Basel, Switzerland
- 4The Nelson Mandela, African Institution of Science and Technology, School of Life Sciences and Bio Engineering, Arusha, Tanzania
- 5S. C. Johnson & Son, Inc., Racine, WI, United States
Background: Evaluation of vector control tools follows a phased approach, progressing from laboratory studies to semi-field trials in experimental huts, and finally to small-scale (in-home test) and large-scale (randomized control trials) field evaluations under user conditions. Method selection depends on the specific data objectives.
Methods: We assessed the entomological efficacy of the transfluthrin-based spatial repellent product SC Johnson Mosquito Shield™ in free-flight chambers, semi-field and field experimental hut trials, and an in-home test against Afrotropical malaria vectors. We focused on efficacy endpoints and mosquito collection methods to inform evidence-based evaluation of spatial repellents.
Results: Mosquito Shield reduced number of mosquitoes blood-feeding and landing, and also induced mortality, exophily, and deterrence at different magnitudes across the testing methods. However, not all endpoints were measurable with every method. Landing reductions were measured using human landing catches and remained similar in magnitude across experimental hut tests in the semi-field (71%) and field (70%), as well as in-home tests (66%), but were higher in the free-flight chambers (96%) using a susceptible mosquito strain. Other endpoints (mortality, and exophily) generally showed higher estimates in controlled environments with lab-reared mosquitoes, compared to ambient conditions with wild, free-flying mosquitoes.
Conclusion: This study supports the use of multiple test methods to generate entomological efficacy data required for country registrations, WHO prequalification dossiers, and post-deployment monitoring. The findings highlight the strengths and limitations of free-flight chambers, semi-field systems, experimental huts, and in-home tests in generating efficacy data for new spatial repellent products. These results support integration of Mosquito Shield into malaria vector control programs pending further operational evaluation. Mosquito landing reduction estimated via human landing catches is a reliable metric for monitoring spatial repellent product longevity across efficacy testing methods. The efficacy gradient between controlled and ambient conditions highlights the importance of testing under realistic settings before public health deployment.
Introduction
Vector control remains a crucial component in combating malaria and other mosquito-borne diseases, with historical evidence indicating that well-organized vector control efforts are a key component of successful local elimination. Although insecticide treated nets (ITNs) and indoor residual sprays (IRS) have significantly reduced the malaria burden, they are insufficient for malaria elimination because of funding and operational constraints that limit the delivery of interventions to ensure equity and universal coverage (WHO, 2024; Oxborough et al., 2024) as well as increasing physiological (Suh et al., 2023) and behavioral resistance (Hubbard and Murillo, 2024; Sherrard-Smith et al., 2019) to available insecticides. In addition, climate change is expected to increase the risk of mosquito-borne diseases by creating more favorable conditions for transmission worldwide (Kulkarni et al., 2022; Li and Managi, 2022; Thomson and Stanberry, 2022), further highlighting the need for expanding the vector control toolbox. Spatial repellents, endectocides, gene drive, and attractive targeted sugar baits are intervention classes in the pipeline (WHO, 2023).
Vector control tool evaluations should be tailored to the purpose for which the data is intended, whether for support of country registrations, WHO prequalification, or post-deployment monitoring and evaluation (WHO, 2020; Vontas et al., 2014). Many different methods have been developed to test the efficacy of spatial repellent protypes or products (WHO, 2009, 2013). The WHO guidelines on efficacy testing of spatial repellents, published in 2013, predate the development of a passive spatial repellent product for broad public health use. At that time, robust efficacy data was not available to inform a data-driven discussion on appropriate testing methods. Standardizing and validating efficacy evaluation methods (Lees et al., 2023) would improve data quality and comparability for spatial repellents.
The purpose of this research was to explore the potential roles of different efficacy test methods (free-flight chambers, semi-field systems, experimental huts, and in-home tests) in the assessment of spatial repellent products, using SC Johnson Mosquito Shield™ (Mosquito Shield) as a case study.
Materials and methods
Test item
Mosquito Shield is a multi-layer plastic film dosed with 110 mg transfluthrin that is effective for 30 days post opening. The current manufacturer specified application rate is 1 device per wall (4 total) in regular rooms (9 m2–18 m2), and more in large rooms (2 Mosquito Shields per 9 m2). The same formulation was used across all the studies.
Free-flight chamber tests
Study area
The study was carried out in Free-flight chambers at BioGenus GMbH, in Bergisch Gladbach Germany, where two separate tests were run to determine: 1) the insecticidal effects of Mosquito Shield™ (knock down after 1 h (60 min) and 24-hours mortality) and 2) landing inhibition of Anopheles gambiae mosquitoes. A glossary of endpoints is in Box 1.
Box 1. Definitions of entomological outcomes measured in bioassays of spatial repellents.
Blood feeding inhibition (BFI): reduction in the number of blood-fed mosquitoes caused by sublethal exposure to AIs.
Deterrence: inhibition of entry due to sublethal exposure to AIs i.e., mosquitoes not entering a treated house.
Exophily: (also non-contact irritancy, repellency or non-contact excito-repellency) the directional or non-directional movement of adult female mosquitoes away from treated spaces due to sublethal non-tarsal contact with AIs i.e., mosquitoes exiting a treated house. When exposed to excitorepellent insecticides, mosquitoes tend to move toward light, resulting in their escape from treated houses.
Knock-down (KD): mosquitoes that are moribund (i.e., those unable to fly) after sublethal exposure to AIs.
Landing inhibition (LI): reduction in landings in the treatment arm relative to the control arm, typically measured by human landing catch (HLC).
Mortality: proportion of mosquitoes that are dead after exposure to the AI measured at set timepoints, typically 24 hours after collection.
Protective efficacy (PE): protection elicited by a vector control tool, measured by reduction in landing or reduction in blood-fed mosquitoes in the treatment arm relative to no treatment (negative control).
Study mosquitoes
Sugar-fed, nulliparous Anopheles gambiae mosquitoes (susceptible BioGenius 08 strain) were used for the tests run in free-flight chambers (3-to-4-day old mosquitoes for the insecticidal effects test, and 7-to-14-day old mosquitoes for the landing inhibition test). For detailed procedure on rearing see Supplementary Appendix 1.
Procedures
Insecticidal effects test
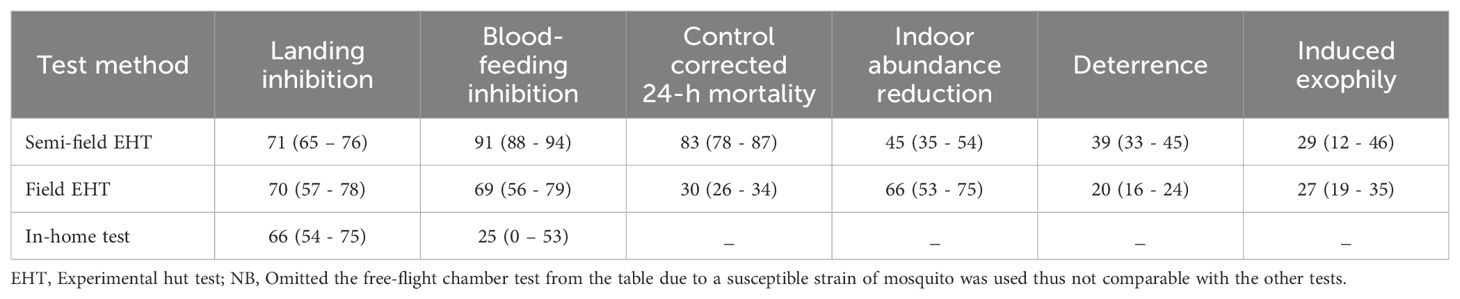
Mosquito Shield was tested for insecticidal effects (knock down and mortality) against free-flying mosquitoes in 30 m3 chambers. Three Mosquito Shield products were placed on the chamber walls at a height of 1.75 m above the ground. Environmental conditions were controlled to be between 25 - 26°C (temperature) and 60 - 70% (relative humidity). To recreate natural air movements found within a home, a 30 cm diameter fan that produced wind speeds of approximately 4.1 m/sec was positioned in the center of the chamber on the floor, tilted upwards in the direction away from the products (Figure 1).
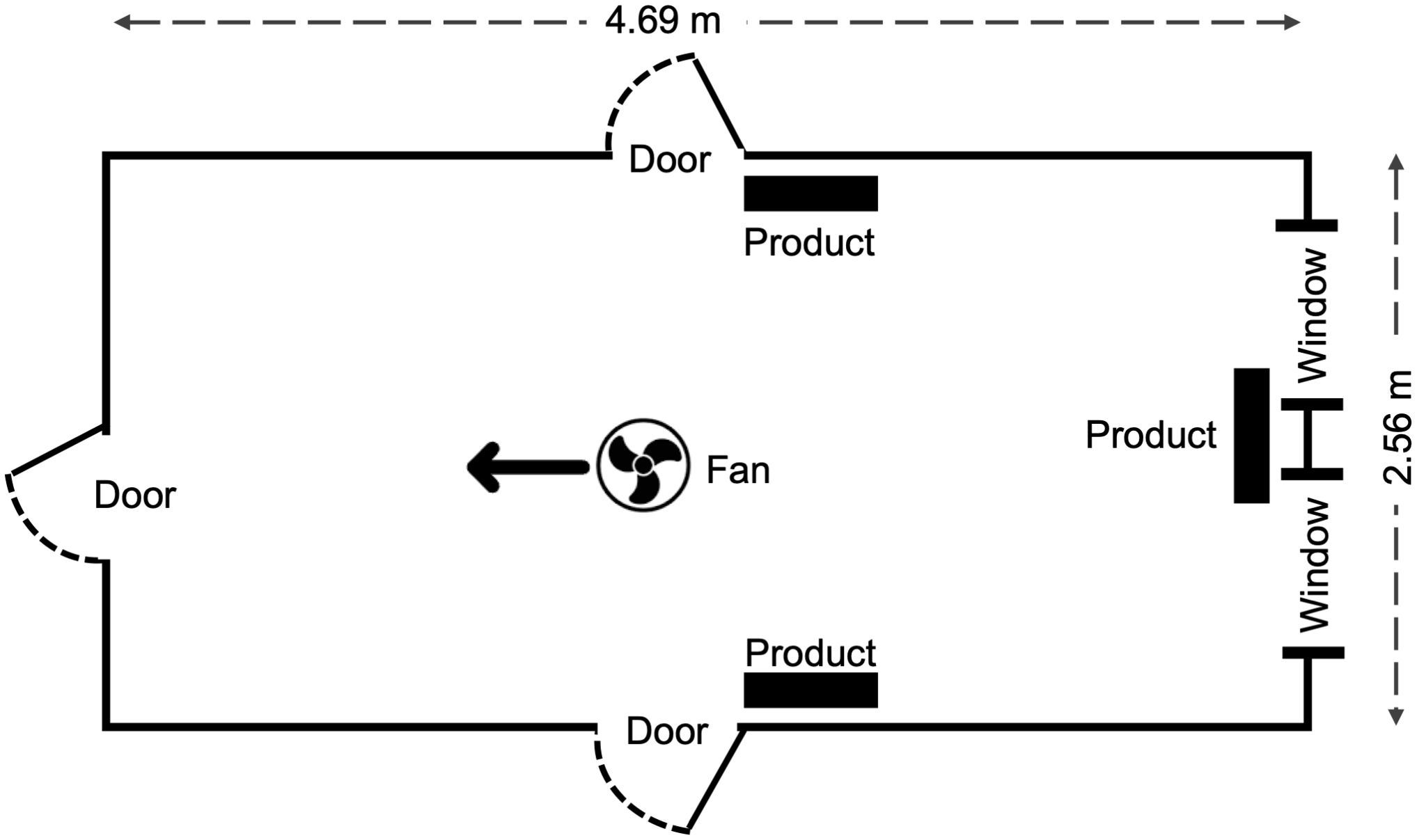
Figure 1. Top view schematic showing product and fan placement in the free flight chambers at BIoGenius GmbH. All doors and windows were closed during experiment runs. Wind speed at each single product was 0 m/sec before start of the tests.
Two hours after the products were opened and placed on the walls to allow transfluthrin evaporation, 50 free-flying mosquitoes were released into the free-flight chamber. Mosquitoes were provided with 10% sugar solution on cellulose swabs during the test. Knockdown was evaluated every 15 minutes up to 2 hours. After 2 hours, mosquitoes were removed from the free-flight chamber, held in a clean environment, and supplied with 10% sugar solution; mortality was observed at 24 hours after test start. Similar procedures were run in the control chambers (those without Mosquito Shield).
The tests were conducted at the following timepoints: freshly-opened, and after 14, 28 and 32 days of aging. Between timepoints, products were removed from the free-flight chambers and stored under similar environmental conditions. At each time point, four replicates of the treatment and control were run simultaneously. The tests were run from May to July 2019.
Landing test
Mosquito Shield was tested for landing inhibition against free-flying mosquitoes in two adjoining 30 m3 free-flight chambers. One hour and fifty-five minutes before starting the tests to allow evaporation of transfluthrin, three Mosquito Shield products were placed in one of the free-flight chambers at the same height and positions as during the insecticidal effect test, and a 30 cm diameter fan was positioned so that it faced one of the corners and was tilted upwards (Figure 2). The temperature and humidity were also controlled as in the insecticidal effects test.
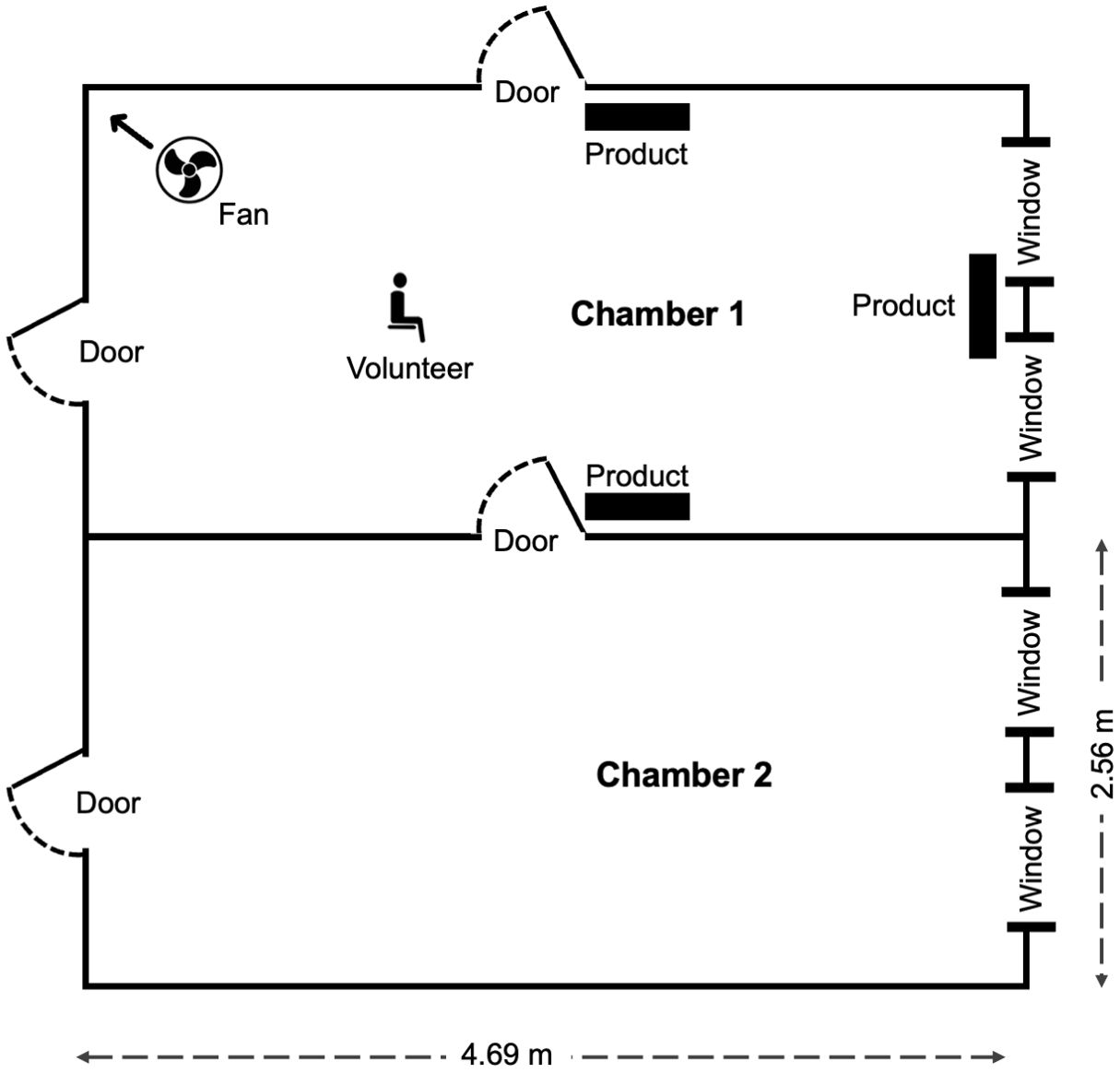
Figure 2. Schematic (top view) showing product and fan placement in the free flight chambers at BIoGenius GmbH. All doors and windows were closed during experiment runs except for the one connecting chamber 1 and 2. Wind speed at each single product was 0 m/sec before start of the tests.
Five minutes before beginning the test, a human volunteer (with bare arms and legs) was seated in the free-flight chamber with the products (chamber 1), 50 mosquitoes were released into the adjacent free-flight chamber without the products (chamber 2), and the door between the free-flight chambers was opened (Figure 2). The door connecting the two chambers remained open for 1 h to allow the volunteer to collect mosquitoes that landed. The number of mosquitoes landing (defined as mosquitoes alighting for longer than 2 sec on the volunteer’s bare arms or legs) on the volunteer were captured, killed, and counted. The number of mosquitoes remaining in the chamber in which they were released was counted at the end of the test to calculate deterrence. Similar procedures were used for the untreated controls.
The tests were conducted at the same timepoints as the insecticidal effects test, with four replicates per timepoint. Untreated controls were for one hour before the start of the tests with Mosquito Shield. The tests were run from January to March 2020.
Semi-field system test
Study area
The semi-field system tests were conducted between February and March 2023 at the Ifakara Health Institute’s Vector Control Product Testing Unit semi-field systems located in Bagamoyo District, Tanzania (Tambwe et al., 2021). The SFS is a large screened enclosure made of two large chambers, 9.6 m × 21 m that are separated by a corridor (Figure 3). The two large chambers were divided into two equal-sized smaller chambers (9.6 m x 10.5 m). Each smaller chamber contained a “new” Ifakara experimental hut (hereinafter hut). The study was conducted in new Ifakara experimental huts (NIEH), modified from the original design by adding a fully sealed plywood partition to create two huts. Each hut measured 3.25 × 3.5 × 2 m with a 0.5 m gabled roof (total volume: 25.59 m³), two windows, and 10 cm-wide eave gaps fitted with baffles to allow mosquito entry but prevent exit (Swai et al., 2023b).
Figure 3. Schematic showing experimental set up in the chambers of the semi-field system in Bagamoyo.
Study mosquitoes
Laboratory-reared, 3-to-5 day old, nulliparous, sugar-starved, pyrethroid-resistant Anopheles arabiensis mosquitoes were used in the semi-field system tests (detailed procedure on rearing in Supplementary Appendix 1). Insecticide susceptibility tests conducted in February 2023 confirmed their resistance (<90% mortality) to pyrethroids with WHO discriminating doses of permethrin, deltamethrin, alphacypermethrin, and lambda-cyhalothrin (Supplementary Table 1).
Procedures
A comparative design with 2 treatment arms (Mosquito Shield or untreated control) replicated twice in four chambers of the semi-field system was used to determine the protective efficacy of Mosquito Shield using two different methods to measure the reduction in number of mosquitoes blood-feeding (blood feeding inhibition) and landing (landing inhibition). In the huts with treatment, four Mosquito Shield products were placed on the walls at a height of 1.75 m above the ground.
A total of eight volunteers were rotated through the two treatments and two methods (blood-feeding test or landing test) over 32 nights. A group of four volunteers were allocated to huts with the same method (blood-feeding test or landing test) for a block of four nights (Figure 3). The volunteers rotated every night for the four nights, then on the fifth night moved to the chambers with the different method (e.g., from blood-feeding test to landing test) where they rotated nightly for another four nights to control for differences in volunteers’ attraction to mosquitoes and ability to conduct human landing catch (HLC). The treatments remained fixed in the same chambers throughout the test to avoid contamination between the arms, but the collection methods were rotated every eight nights to the opposite chambers to control for locational bias. After 32 nights, each volunteer had tested each treatment arm using each method 8 times. The semi-field tests were conducted in February and March 2023. The environmental conditions (median (IQR)) were 280 C (29 – 33) for temperatures and 62% (77 – 82) for relative humidity.
Landing test
Each night from 18:00 h in the evening to 06:00 h the next day morning, four male volunteers sat on chairs in the centre of the huts wearing closed shoes and a net jacket to prevent mosquitoes biting on the feet or above the legs. Using mouth aspirators and torch lights, volunteers caught mosquitoes landing on their exposed limbs placing mosquitoes into paper cups for 50-minutes each hour. In total, 90 mosquitoes were released into each chamber outside of the hut: 15 mosquitoes were released per hour for the first four and last two hours to mimic local mosquito biting patterns. Hourly collections of mosquitoes were transported to a freezer and killed. Huts windows remained open during the landing test, without window traps.
Blood-feeding test
Each night from 18:00 h in the evening to 06:00 h the next day morning, four male volunteers slept under a deliberately too-torn untreated bed net, with six 25×25 cm holes (one on each short side and two on each long side). Window traps were placed on the outsides of all huts. At 18:00 h, 90 mosquitoes were released in each chamber outside the huts. At 06:00, mosquitoes in the huts and exit traps were collected using mouth aspirators and prokopacks, then placed in paper cups and scored according to physiological status (alive unfed, dead fed, alive fed and dead unfed) and location collected (inside the net, hut, and window exit traps). The mosquitoes were then held for 24 h to observe mortality at 260–270 C temperature and 58 – 80% relative humidity.
Experimental hut test
Study area
The test was done at the Ifakara Health Institute experimental hut site in Lupiro village Ulanga District, Tanzania from November to December, 2019.
Study mosquitoes
The tests were conducted against a wild, free-flying population of pyrethroid-resistant Anopheles arabiensis mosquitoes (Matowo et al., 2017). Details on insecticide susceptibility of this population during the time of the tests are reported (Supplementary Table 1).
Procedures
The tests were conducted in 24 Ifakara experimental huts: 16 for landing inhibition and 8 for blood-feeding inhibition. Half of the huts in each experiment received Mosquito Shield™ (three per hut at a height of 1.75 m above the ground), while the rest served as controls. Treatments were randomly assigned using a random number generator and remained fixed throughout the study. The design of the huts and mosquito collection methods for the blood-feeding tests and landing tests are as described in the semi-field system test and are detailed in Swai et al., 2023 (Swai et al., 2023b).
In-home test
Study area
The study was carried out in Lupiro village Ulanga District, in Tanzania in June and July 2023.
Study mosquitoes
The dominant malaria vector in Lupiro village is pyrethroid-resistant Anopheles arabiensis (Matowo et al., 2017). Insecticide susceptibility tests conducted in July 2023 with WHO discriminating doses of permethrin, deltamethrin, alphacypermethrin, and lambda-cyhalothrin showed <90% mortality confirming their pyrethroid resistance status (Supplementary Table 1). Their mechanism of pyrethroid resistance is upregulation of mixed-function oxidases (Matowo et al., 2017).
Procedures
More than 40 homes were initially screened for inclusion in the in-home test, with 30 selected. Homes were within a 2 km walking radius, and at least 10 meters apart. Data were collected on housing characteristics including eave openings, roof and wall type, window and door screening, room size and number, presence of ITNs, use of other vector control products, household density (number of individuals living/sleeping in the home), and baseline mosquito density and landing rates. Homes with similar traits were paired, assigning one to the treatment group and the other to the control group, to minimize confounding or effect modification of the outcomes from these household traits. The study had >80% power to detect a 25% reduction in human landing rates with Mosquito Shield similar to Fongnikin et al., 2024 (Fongnikin et al., 2024).
Mosquito Shield was placed at an application rate of 2 per 9m2 in 15 of the homes following product use instructions. The other 15 homes did not receive Mosquito Shield. All households received new ITNs hung one week before the start of the study to ensure all households benefited from participating in the study.
HLC were performed by volunteers at 0-1, 7-8, 14-15, 21-22, 28-29, 31–32 days post placement of Mosquito Shield. Volunteers worked in pairs with two shifts (19:00 to 00:00 h and 00:00 to 0700 h) collecting mosquitoes hourly from one room of each home. Mosquitoes were placed in paper cups, transported to the field laboratory and morphologically identified to species complex. HLC was conducted in each household for a total 12 nights of the 32 study nights (180 trap nights per treatment arm).
On the days between HLC collections, resting mosquitoes were collected from all rooms in each home using prokopack aspirators and transported to the field laboratory, for species identification and physiological assessment. Each room was aspirated for at least 15 min, targeting mosquitoes resting on walls, ceilings, and under furniture. To minimize handling-related mortality, prokopack cups were changed every three minutes during the collections. Prokopack aspirations were conducted in each household for a total of 16 nights of the 32 study nights (240 trap nights per treatment arm).
Analysis
All data was entered directly onto paper data sheets then double-entered into a digital spreadsheet and validated to check for any inconsistencies. Analysis was performed using STATA 18 software (StataCorp LLC, USA). Histograms and measures of variance relative to the mean were used to check data distribution (overdispersion).
Descriptive statistics are presented as means with 95% confidence intervals (95% CI) for numbers of mosquitoes landing or blood-feeding, and as proportions (in percentage) with 95% CI for deterrence, knockdown, and 24-h mortality.
The number landing, number fed, proportion knocked down, and proportion dead at 24 h were modelled as a function of treatment using a multilevel mixed effects regression with a negative binomial distribution and log link for count data (number landing, blood-fed) or with a binomial error and logit link for proportional data (knocked down, 24 h mortality).
Because treatment was fixed in the huts and homes, collinearity between hut/home and treatment was checked and the best fitting model was selected based lowest Akaike Information Criterion (AIC) after controlling for potential confounders in the respective experiments. The final mode for free-flight chamber data included treatment and volunteer effects as fixed factors. The final model used for semi-field system test data had the treatment and volunteer added as fixed factors and experimental night as a random factor. For the in-home test, adjusting for household features like household biomass (number of people), eaves, screened/unscreened windows, roof, wall and floor type did not affect the final model estimates. Thus, the final model had treatment, volunteer, and night as fixed effects; household identification was added as a random effect.
Results
Free-flight chamber test
On average, 100% knock down was achieved within 1 h at all timepoints (0–32 d after opening Mosquito Shield). Mortality was 100% from 0 and 14 d and was 94% at 28 d and 95% at 32 d after opening Mosquito Shield (Figure 4A; Supplementary Table 2).
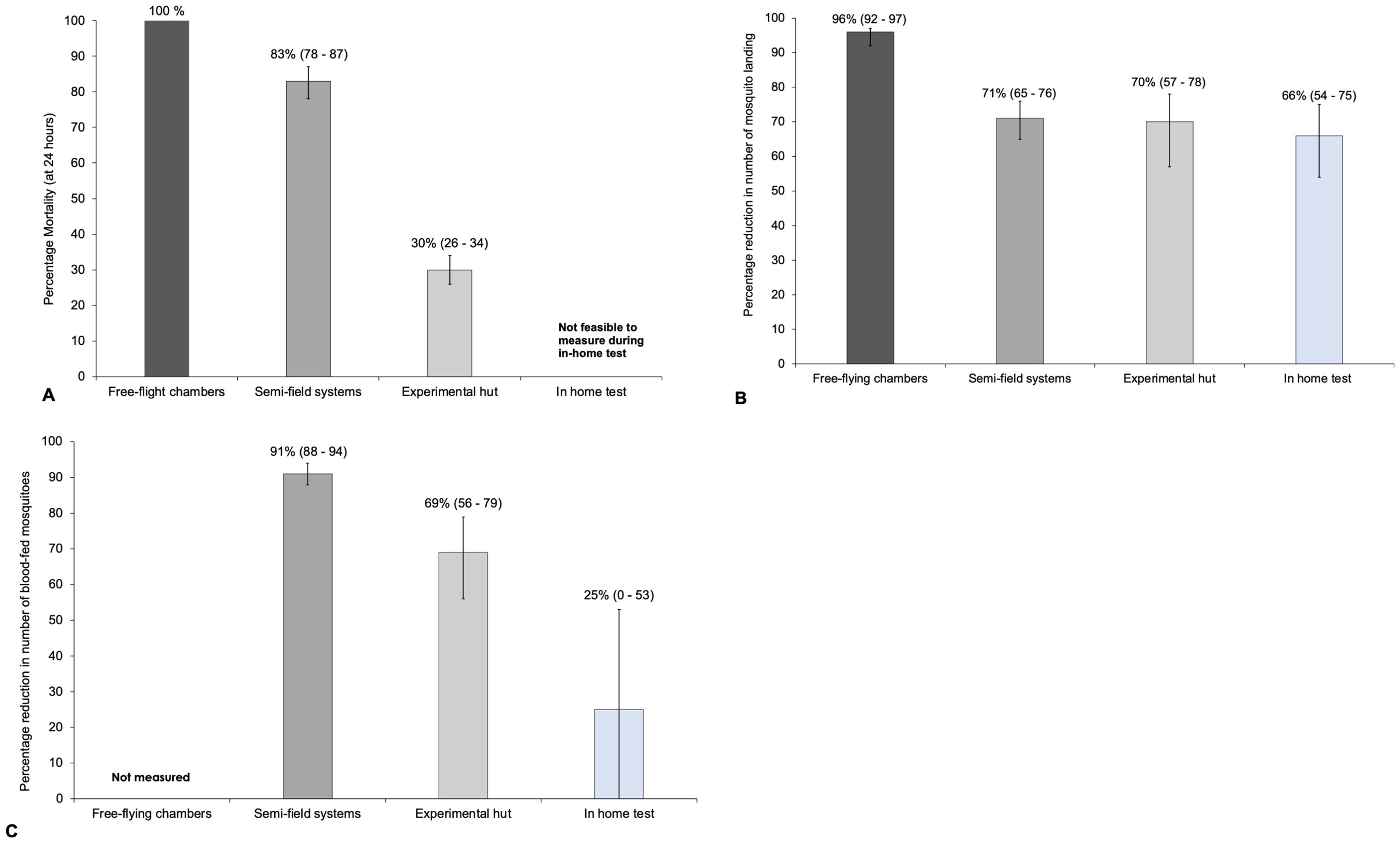
Figure 4. (A) Mortality at 24-hours; (B) Landing inhibition and (C) Blood-feeding inhibition estimates measured in free-flight chambers, semi-field, field and in-home tests. NB: Mosquito used in the free-flight chambers were a susceptible strain and the Mosquito Shield application rate that was higher in the SFS tests (4 per hut) compared to experimental hut test (3 per hut).
Overall, a total of 557 susceptible An. gambiae landed on the volunteers during the control experiments while 25 landed on the volunteers when Mosquito Shield was present, with an average of 35 and 2 lands per hour, respectively. The percentage landing inhibition due to Mosquito Shield was estimated to be 96% (92 – 97%); p<0.0001 (Table 1; Figure 4B). In the control 26.3% (n=210) of mosquitoes did not enter the chamber with the volunteer compared to 52.5% (n=420) when Mosquito Shield was present, showing evidence of mosquito deterrence from the chamber with Mosquito Shield (Supplementary Table 3).
Semi-field system tests
In the landing test, a total of 2,705 pyrethroid-resistant An. arabiensis mosquitoes were recaptured in the control huts and 816 in the Mosquito Shield huts over 32 nights, averaging 42.3 (38.0 – 46.4) mosquitoes per hut per night in the control and 12.8 (10.5 – 15.0) in the Mosquito Shield huts (Supplementary Table 3). Landing inhibition was estimated to be 71% (65 – 76%); p<0.0001) (Table 1; Figure 4B).
In the blood-feeding test, a total of 2,219 mosquitoes were recaptured in the control and 1,311 in the Mosquito Shield arm. Of these, 1,810 (80.6%; 95% CI: 77.1–84.1%) mosquitoes in the control huts were blood-fed, compared to 168 (10.9%; 95% CI: 7.7–14.1%) in the Mosquito Shield huts (Supplementary Table 3). Blood-feeding inhibition was estimated to be 91% (95% CI 88 – 94%); p<0.0001 (Table 1; Figure 4C).
The 24-h mortality of mosquitoes in the Mosquito Shield arm was 85.6% (95% CI 81.3 - 89.9%) compared to 14.0% (95% CI 12.0 – 16.0%) in the control arm. The control-corrected mortality at 24 hours (mortality due to the intervention after factoring in control mosquito mortality) was calculated to be 82.6% (95% CI 78.4 – 86.8%) (Table 1; Figure 4A). In addition, in the huts with the Mosquito Shield there was 28.5% induced exophily (95% CI 1.5 – 45.5%) [OR 1.26 (95% CI 1.07 – 1.48); p<0.007] and overall reduced entry (deterrence) 38.9% (95% CI 33.4 – 44.5%) [IRR=0.58 (95% CI 0.52 - 0.65); p<0.001] compared to the control huts.
Experimental hut test
Landing inhibition was estimated to be 70% (57–78%) [IRR 0.30 (95% CI 0.22–0.43); p < 0.0001] and blood-feeding inhibition was estimated to be 69% (56–79%) [IRR 0.31 (95% CI 0.21–0.44; p < 0.0001]. In addition, Mosquito Shield caused 30% mortality (95% CI 26–34%) [OR 3.0 (95% CI 2.3–3.9); p < 0.0001], and 27% induced exophily (95% CI 19–35%) [OR 3.0 (95% CI 2.2–4.3); p < 0.0001]. Overall reduced entry (deterrence) was 11% (95% CI 0–35%) but was not statistically significant [IRR=0.89 (95% CI 0.65,1.23); p=0.495] (Swai et al., 2023b).
In-home test
A total of 4,281 An. arabiensis were collected by HLC in the control homes and 1,872 in homes with Mosquito Shield over the 16 nights of data collection spanning 32 nights. Overall, landing inhibition was estimated to be 66% (54 – 75%) (Table 1; Figure 4B) with evidence of a difference (p<0.001) between households in the control and Mosquito Shield arms (Supplementary Table 6).
A total of 373 An. arabiensis mosquitoes were collected by prokopack aspiration in the control homes, of which 81 were blood-fed; 89 of 508 collected mosquitoes were bloodfed in homes with Mosquito Shield. Although this corresponded to a 25% (0 – 53%) (Table 1; Figure 4C) reduction in the overall proportion of blood-fed mosquitoes in homes with Mosquito Shield, there was no evidence of a difference between control homes and those with Mosquito Shield (p=0.237) (Supplementary Table 7).
Discussion
This study assessed several different entomological efficacy test methods using a single spatial repellent product, Mosquito Shield, as a case study to determine their potential utility in generating efficacy data to satisfy different data requirements including country registrations, WHO prequalification dossiers, and post-deployment monitoring and evaluation. Multiple different entomological endpoints were measured using the test methods and were compared when possible. Landing reductions were measured using each method via human landing catches and remained similar in magnitude across semi-field, experimental hut, and in-home tests, but were higher in the free-flight chambers. For blood-feeding, and mortality endpoints measured there was a trend toward higher estimates for tests with more artificial environmental conditions and lab-reared mosquitoes specifically in free-flight chambers, versus tests with ambient environmental conditions and wild, free-flying mosquitoes. It should also be considered that the higher estimates observed may have been due to i) use of a susceptible strain of mosquitoes in the free-flight chambers and ii) higher Mosquito Shield application rates in the SFS - 4 spatial repellents per hut compared to experimental hut test that had 3 SR per hut. However, a separate experimental hut evaluation conducted at the test site with 4 Shield SR per hut gave similar results to those observed in this evaluation: 71% (59 – 79) feeding inhibition and 26% (22 - 30) 24-hour mortality (unpublished data).
The test methods compared in this study represent different degrees of artificiality regarding the mosquitoes used in the test and the physical environmental conditions under which the test is performed. While it is important to evaluate the performance against local wild populations of mosquitoes under a range of natural conditions that represent scenarios where a product would be deployed, there is equal value in understanding the technical performance of a product and the duration of protective efficacy under well-controlled and standardized conditions (Table 2).
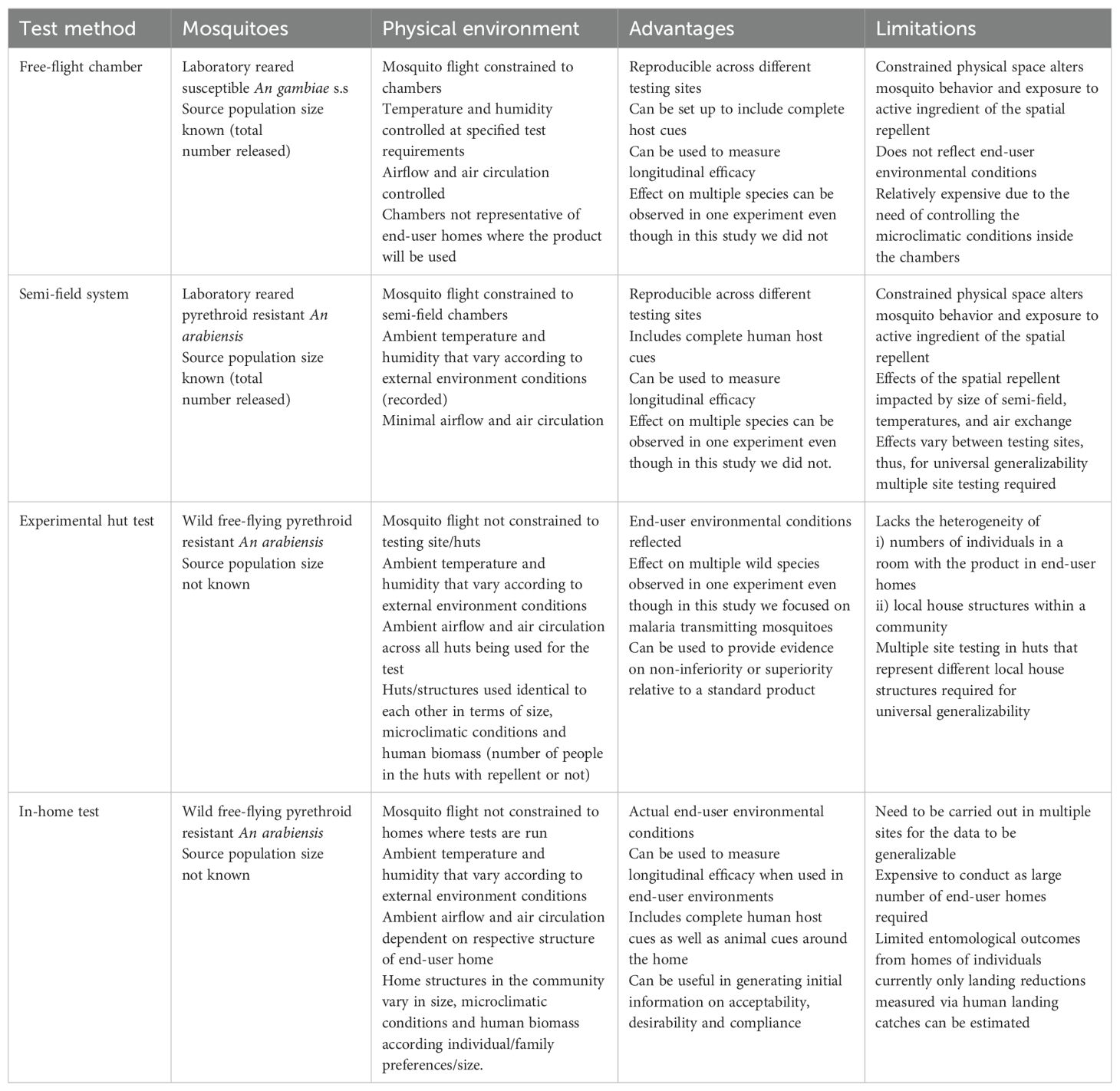
Table 2. Features advantages and limitations of Free-flight chambers, semi-field system, Experimental hut tests and In-home tests used in evaluating spatial repellents.
Tests conducted in free-flight chambers evaluate products against lab-reared mosquitoes in a controlled physical environment and represent both the most artificial and most reproduceable of the tests evaluated in this study. Free-flight chambers offer a robust method to demonstrate the technical performance of the product over its length of life, and offers a high-through-put system to identify the primary toxicological and behavioral effects (Vontas et al., 2014). It allows mosquitoes to interact with human hosts in a laboratory setting which is important because volatile pyrethroids elicit spatial repellency through interference with mosquito odor receptors in addition to sodium gated channels (Bohbot et al., 2011; Zhang et al., 2024; Liu et al., 2021). Mosquito strains and environmental conditions can be aligned across different laboratories to generate reproducible data. While the endpoints measured in free-flight chambers in this study were limited to knockdown, mortality, reductions in landings, and deterrence, with some minor changes to protocol, additional endpoints could be measured including blood-feeding inhibition and post-exposure sublethal effects. One limitation to using free-flight chambers to generate efficacy data is that the environmental conditions selected for the chamber test (temperature, humidity, air movement, air exchange) may not be representative of the conditions in end-user homes, which could impact estimates of efficacy. Additionally, the constrained physical space may artificially alter mosquito behaviors and exposure to the active ingredient (Colucci and Muller, 2018; Barnard et al., 1998).
Tests in semi-field systems, similar to those done in free-flight chambers, involve the release of lab-reared mosquitoes into a confined space under non-ambient environmental conditions. However, unlike free-flight chambers where the environmental conditions are actively controlled, conditions within a semi-field system are uncontrolled (yielding higher temperature, and lower airflow and air exchange). As a result, estimates of efficacy from tests may vary between sites using semi-field systems due to environmental differences (Vajda É et al., 2024). A key advantage of using semi-field systems is the ability to measure multiple endpoints with different lab mosquito strains with known insecticide susceptibilities while also allowing the estimation other post-exposure sub-lethal effects beyond those measured in this study (Fairbanks et al., 2024). Limitations of semi-field systems include restricted air movement and air-exchange, temperatures that are elevated relative to the outdoor environment, and a physical space that constricts movement of mosquitoes (Burton et al., 2023).
Tests in experimental huts measure spatial repellent efficacy against a wild population of mosquitoes under ambient environmental conditions. The structures in which the products are placed are standardized and to some degree relatable to houses in the local context in certain key aspects (e.g. size, mosquito entry points), and therefore generate data that is both locally relevant but without the heterogeneity of local structures in a community (Silver and Service, 2008). With different configurations, experimental huts can measure all endpoints within the scope of this study. One limitation of the experimental hut is that the mosquito population size is not defined as it is for free-flight chamber and semi-field system tests. This leads to greater heterogeneity of data and requires larger sample sizes to precisely evaluate product performance (Johnson et al., 2014) compared to free-flight chambers or semi-field evaluations (Kipingu et al., 2025). Also, as the structures are standardized, they may not be fully representative of product performance across the range of local housing styles.
In-home tests can be used to measure the efficacy of spatial repellents against wild populations of mosquitoes when products are deployed within homes in a community. In-home tests present an option for generating locally-relevant efficacy data if other testing infrastructure is not available and also can be used to measure the efficacy of products when deployed as part of a broader implementation study where feasibility, distribution, coverage, and acceptability also are measured. A limitation of in-home tests is that only a few entomological endpoints can be measured, including landing reduction, blood-feeding reduction measured by prokopack aspirator, and indoor abundance. However, in this study blood-feeding reduction and indoor abundance were not statistically significant potentially due to low numbers captured. Efficacy estimates from in-home tests also may be heterogeneous due to the range of different structures that the products are deployed in and their indoor environmental conditions, so larger numbers of homes may need to be enrolled or careful attention should be paid to ensuring balance of arms considering housing characteristics and baseline entomology data.
All of the test methods evaluated in this study estimated at least one relevant measure of efficacy over the length of life of the product, and each method may be useful for generating data for at least one of the purposes identified to be in scope: country registrations, WHO prequalification, or post-deployment entomological monitoring. For country registrations, all of the test methods in this study could be relevant options to demonstrate technical performance of a spatial repellent product depending on in-country availability of testing chambers, systems, or huts, with in-home tests being a universal option.
For data generated for dossiers for WHO prequalification, consideration should be given to the intent of the existing WHO guidelines for the evaluation of spatial repellents. Specifically, it is indicated that data should include 1) laboratory studies testing the spatial repellent product to determine the product application rate and the duration of protective efficacy against mosquitoes, and 2) field studies to measure the efficacy against wild free-flying populations of mosquitoes under natural environmental conditions. This study indicates that either data generated in a free-flight chamber or semi-field system could satisfy the expected laboratory data, and that experimental hut or in-home tests could be used to provide efficacy data under natural environmental conditions.
To measure efficacy of spatial repellents post-deployment, in-home tests similar to the one described in this study and by Fongnikin et al., 2024 (Fongnikin et al., 2024) could be used. In fact, an in-home test currently is underway as part of a programmatic deployment of spatial repellents in western Kenya (Unpublished). Landing reductions measured via HLC remain the most relevant endpoint to measure during an in-home test. Programmatically, other methods such as light traps and prokopak aspirators are used to measure indoor densities of mosquitoes, however, these methods may fail to show similar efficacy to HLC (Stevenson et al., 2018; Swai et al., 2023a) and can be biased towards species, sexes or physiological states collected (McDermott and Mullens, 2018).
There are a number of other tools available to capture mosquitoes that could be further explored in the context of spatial repellent efficacy testing. Several exposure-free methods including the mosquito-electrocuting trap (MET) (Tambwe et al., 2021), and miniaturized double-net trap (DN-Mini) (Vajda et al., 2023) have shown some promise for use in evaluating efficacy of spatial repellents. But while they are exposure free, these still rely on humans as the bait and the presence of other individuals within their vicinity impacts the protective efficacy estimates. Further evaluation of exposure-free methods that can be potentially used as alternatives to HLC to evaluate efficacy of spatial repellents are recommended.
Conclusion
This case study supports the use of several entomological efficacy test methods to generate efficacy data to satisfy different data requirements including country registrations, WHO prequalification dossiers, and post-deployment monitoring and evaluation. Since each test method evaluated in the study provided valuable efficacy data for different objectives, we recommend standardizing and validating these methods for broader use in evaluating other spatial repellent products. Additionally, updating the WHO guidelines for efficacy testing of spatial repellents to reflect the current available data would benefit manufacturers and contract research organizations, as these guidelines serve as a key reference.
The findings of these studies further demonstrate that Mosquito Shield elicits multiple distinct effects in Anopheles mosquitoes including landing inhibition, feeding inhibition, knock down and mortality as well as deterrence and exophily. Human landing catches remains the gold standard for efficacy testing and landing reduction is a reliable metric for monitoring product efficacy and longevity across the testing methods.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JS: Data curation, Conceptualization, Formal Analysis, Methodology, Writing – review & editing, Supervision, Investigation, Writing – original draft. WN: Writing – review & editing, Data curation, Project administration, Supervision. HN: Data curation, Supervision, Writing – review & editing. NM: Writing – review & editing, Supervision, Data curation. AM: Data curation, Writing – review & editing, Resources. SN: Supervision, Investigation, Data curation, Writing – review & editing. IK: Data curation, Writing – review & editing, Supervision. AM: Data curation, Investigation, Supervision, Writing – review & editing. JM: Data curation, Methodology, Writing – review & editing, Resources. MC: Conceptualization, Writing – review & editing, Methodology. TM: Writing – review & editing, Conceptualization, Methodology. SM: Data curation, Writing – original draft, Funding acquisition, Resources, Conceptualization, Investigation, Formal Analysis, Methodology, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors express their sincere thanks and appreciation to the BioGenus GMbH, Biology Technologie Park, Campus 1 in Germany where the free-flight chamber test were done. Study volunteers, who worked tirelessly over the duration of the experiment. Village leaders and community around the Ifakara Health Institute experimental hut site in Lupiro, for allowing us to run both our hut and in-home tests with minimal interruptions. A special thanks to the Vector Control and Product Testing Unit (VCPTU) management, administrators and colleagues who helped in organizing logistics and materials allowing smooth performance of the study.
Conflict of interest
The authors JS, WN, HN, NM, AM, SN, IK, AM, JM, and SM conduct product evaluations for companies that produce vector control products including S.C. Johnson & sons, Inc. MC and TM are employed by S.C. Johnson & Son, Inc, Racine, Wisconsin; they had no role in data collection or analysis.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmala.2025.1621965/full#supplementary-material
References
Barnard D. R., Posey K. H., Smith D., and Schreck C. E. (1998). ‘Mosquito density, biting rate and cage size effects on repellent tests.’. Med. Vet. Entomol. 12, 39–45. doi: 10.1046/j.1365-2915.1998.00078.x
Bohbot J. D., Fu L., Le T. C., Chauhan K. R., Cantrell C. L., and Dickens J. C. (2011). ‘Multiple activities of insect repellents on odorant receptors in mosquitoes’. Med. Vet. Entomol. 25, 436–444. doi: 10.1111/j.1365-2915.2011.00949.x
Burton T. A., Kabinga L. H., Simubali L., Hayre Q., Moore S. J., Stevenson J. C., et al. (2023). ‘Semi-field evaluation of a volatile transfluthrin-based intervention reveals efficacy as a spatial repellent and evidence of other modes of action’. PloS One 18, e0285501. doi: 10.1371/journal.pone.0285501
Colucci B. and Muller P. (2018). ‘Evaluation of standard field and laboratory methods to compare protection times of the topical repellents PMD and DEET’. Sci. Rep. 8, 12578. doi: 10.1038/s41598-018-30998-2
Fairbanks E. L., Tambwe M. M., Moore J., Mpelepele A., Lobo N. F., Mashauri R., et al. (2024). ‘Evaluating human landing catches as a measure of mosquito biting and the importance of considering additional modes of action’. Sci. Rep. 14, 11476. doi: 10.1038/s41598-024-61116-0
Fongnikin A., Ahoga J., Ndombidje B., Hueha C., de Souza E., Oti-Tossou R., et al. (2024). ‘Mosquito Shield™, a transfluthrin passive emanator, protects against pyrethroid-resistant Anopheles Gambiae sensu lato in central Benin’. Malaria J. 23, 225. doi: 10.1186/s12936-024-05043-5
Hubbard C. B. and Murillo A. C. (2024). Behavioral resistance to insecticides: current understanding, challenges, and future directions. Curr. Opin. Insect Sci. 63, 101177. doi: 10.1016/j.cois.2024.101177
Johnson P. C. D., Barry S. J. E., Ferguson H. F., and Muller P. (2014). Power analysis for generalized linear mixed models in ecology and evolution. Methods Ecol. Evol. 6, 133–142. doi: 10.1111/2041-210X.12306
Kipingu A. M., Lweitoijera D. W., Ng’habi K. N., Kiware S. S., Viana M., and Johnson P. C. (2025). A power analysis framework to aid the design of robust semi-field vector control experiments, 30 September 2024, PREPRINT (Version 1) (Research Square). doi: 10.21203/rs.3.rs-4970151/v1
Kulkarni M. A., Duguay C., and Ost K. (2022). ‘Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: a scoping review of reviews’. Globalization Health 18, 1–18. doi: 10.1186/s12992-021-00793-2
Lees R. S., Fornadel C., Snetselaar J., Wagman J., and Spiers A. (2023). Insecticides for mosquito control: improving and validating methods to strengthen the evidence base. Insects 14, 116. doi: 10.3390/insects14020116
Li C. and Managi S. (2022). ‘Global malaria infection risk from climate change’. Environ. Res. 214, 114028. doi: 10.1016/j.envres.2022.114028
Liu F., Wang Q., Xu P., Andreazza F., Valbon W. R., Bandason E., et al. (2021). ‘A dual-target molecular mechanism of pyrethrum repellency against mosquitoes’. Nat. Commun. 12, 2553. doi: 10.1038/s41467-021-22847-0
Matowo N. S., Munhenga G., Tanner M., Coetzee M., Feringa W. F., Ngowo H. S., et al. (2017). Fine-scale spatial and temporal heterogeneities in insecticide resistance profiles of the malaria vector, Anopheles arabiensis in rural south-eastern Tanzania. Wellcome Open Res. 2, 96. doi: 10.12688/wellcomeopenres
McDermott E. G. and Mullens B. A. (2018). ‘The dark side of light traps’. J. Med. Entomol. 55, 251–261. doi: 10.1093/jme/tjx207
Oxborough R. M., Chilito K. C. F., Tokponnon F., and Messenger L. A. (2024). ‘Malaria vector control in sub-Saharan Africa: complex trade-offs to combat the growing threat of insecticide resistance’. Lancet Planetary Health 8, e804–e812. doi: 10.1016/S2542-5196(24)00172-4
Sherrard-Smith E., Skarp J. E., Beale A. D., Fornadel C., Norris L. C., Moore S. J., et al. (2019). ‘Mosquito feeding behavior and how it influences residual malaria transmission across Africa’. Proc. Natl. Acad. Sci. 116, 15086–15095. doi: 10.1073/pnas.1820646116
Silver J. B. and Service M. W. (2008). “Chapter 16 Experimental Hut Techniques,” in Mosquito Ecology: Field Sampling Methods (Springer), 1494.
Stevenson J. C., Simubali L., Mudenda T., Cardol E., Bernier U. R., Vazquez A. A., et al. (2018). ‘Controlled release spatial repellent devices (CRDs) as novel tools against malaria transmission: a semi-field study in Macha, Zambia’. Malaria J. 17, 1–16. doi: 10.1186/s12936-018-2558-0
Suh P. F., Elanga-Ndille E., Tchouakui M., Sandeu M. M., Tagne D., Wondji C., et al. (2023). ‘Impact of insecticide resistance on malaria vector competence: a literature review’. Malaria J. 22, 19. doi: 10.1186/s12936-023-04444-2
Swai J. K., Kibondo U. A., Ntabaliba W. S., Ngoyani H. A., Makungwa N. O., Mseka A. P., et al. (2023a). ‘CDC light traps underestimate the protective efficacy of an indoor spatial repellent against bites from wild Anopheles arabiensis mosquitoes in Tanzania’. Malaria J. 22, 141. doi: 10.1186/s12936-023-04568-5
Swai J. K., Soto A. C., Ntabaliba W. S., Kibondo U. A., Ngonyani H. A., Mseka A. P., et al. (2023b). ‘Efficacy of the spatial repellent product Mosquito Shield™ against wild pyrethroid-resistant Anopheles arabiensis in south-eastern Tanzania’. Malaria J. 22, 249. doi: 10.1186/s12936-023-04674-4
Tambwe M. M., Saddler A., Kibondo U. A., Mashauri R., Kreppel K. S., Govella N. J., et al. (2021). ‘Semi-field evaluation of the exposure-free mosquito electrocuting trap and BG-Sentinel trap as an alternative to the human landing catch for measuring the efficacy of transfluthrin emanators against Aedes aEgypti’. Parasites Vectors 14, 265. doi: 10.1186/s13071-021-04754-x
Thomson M. C. and Stanberry L. R. (2022). ‘Climate change and vectorborne diseases’. New Engl. J. Med. 387, 1969–1978. doi: 10.1056/NEJMra2200092
Vajda É. A., Saeung M., Ross A., McIver D. J., Tatarsky A., Moore S. J., et al. (2023). ‘A semi-field evaluation in Thailand of the use of human landing catches (HLC) versus human-baited double net trap (HDN) for assessing the impact of a volatile pyrethroid spatial repellent and pyrethroid-treated clothing on Anopheles minimus landing’. Malaria J. 22, 202. doi: 10.1186/s12936-023-04619-x
Vajda É A., Ross A., Saeung M., Pongsiri A., McIver D. J., Tatarsky A., et al. (2024). ‘The effect of novel mosquito bite prevention tools on Anopheles minimus landing and key secondary endpoints: semi-field evaluations in Thailand’. Malar. J. 23, 387. doi: 10.1186/s12936-024-05188-3
Vontas J., Moore S., Kleinschmidt I., Ranson H., Lindsay S., Lengeler C., et al. (2014). Framework for rapid assessment and adoption of new vector control tools. J. Trends Parasitol. 30, 191–204. doi: 10.1016/j.pt.2014.02.005
WHO (2009). Guidelines for efficacy testing of household insecticide products: mosquito coils, vaporizer mats, liquid vaporizers, ambient emanators and aerosols (World Health Organization).
WHO (2013). Guidelines for efficacy testing of spatial repellents. (Geneva, Switzerland: World Health Organization).
WHO (2020). Norms, standards and processes underpinning development of WHO recommendations on vector control (Geneva, Switzerland: World Health Organization).
WHO (2023). Eighteenth meeting of the WHO Vector Control Advisory Group, 24–26 April 2023 (Geneva, Switzerland: World Health Organization).
WHO (2024). World malaria report 2024: addressing inequity in the global malaria response. (Geneva, Switzerland: World Health Organization).
Keywords: free flight chamber tests, semi field system tests, experimental hut tests, in-home tests, spatial repellent efficacy testing, SC Johnson Mosquito Shield
Citation: Swai JK, Ntabaliba WS, Ngonyani HA, Makungwa NO, Mseka AP, Ngoyani SH, Kibwengo IS, Mpelepele AB, Moore JD, Chura MR, Mascari TM and Moore SJ (2025) Efficacy of the spatial repellent SC Johnson Mosquito Shield™ against anophelines in free-flight chambers, semi-field systems, experimental huts, and in-home tests. Front. Malar. 3:1621965. doi: 10.3389/fmala.2025.1621965
Received: 02 May 2025; Accepted: 04 July 2025;
Published: 24 July 2025.
Edited by:
Yousif Elsafi Himeidan, National Center for the Prevention & Control of Plant Pest & Animal Diseases (Weqaa Center), Saudi ArabiaReviewed by:
Adilson José Depina, CCS-SIDA/MoH, Cabo VerdeEliningaya J. Kweka, Catholic University of Health and Allied Sciences (CUHAS), Tanzania
Franklin Mosha, Kilimanjaro Christian Medical University College, Tanzania
Copyright © 2025 Swai, Ntabaliba, Ngonyani, Makungwa, Mseka, Ngoyani, Kibwengo, Mpelepele, Moore, Chura, Mascari and Moore. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johnson K. Swai, c2t5ZWJhQGloaS5vci50eg==
 Johnson K. Swai
Johnson K. Swai Watson S. Ntabaliba1
Watson S. Ntabaliba1 Jason D. Moore
Jason D. Moore Thomas M. Mascari
Thomas M. Mascari Sarah J. Moore
Sarah J. Moore