- 1Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
- 2Coast Area Research Section, BC Ministry of Forests, Nanaimo, BC, Canada
- 3National Wildlife Research Centre, Environment and Climate Change Canada, Carleton University, Ottawa, ON, Canada
- 4Animal Health Center, BC Ministry of Agriculture and Food, Abbotsford, BC, Canada
- 5Department of Zoology, University of Otago, Dunedin, New Zealand
- 6Department of Conservation, Dunedin, New Zealand
Introduction: At the top of aquatic ecosystems, the North American river otter (Lontra canadensis) and mink (Neogale vison) are definitive hosts to a range of parasite species. Despite their ecological importance in riparian habitats, particularly as indicators of ecosystem health, data on their parasite communities remain limited, especially in Western Canada.
Methods: We conducted a systemic literature review of the helminth parasites of river otter and mink across North America, highlighting regional patterns in biodiversity. We also surveyed the helminth communities of river otter and mink in Alberta and British Columbia, comparing our data against historical reports.
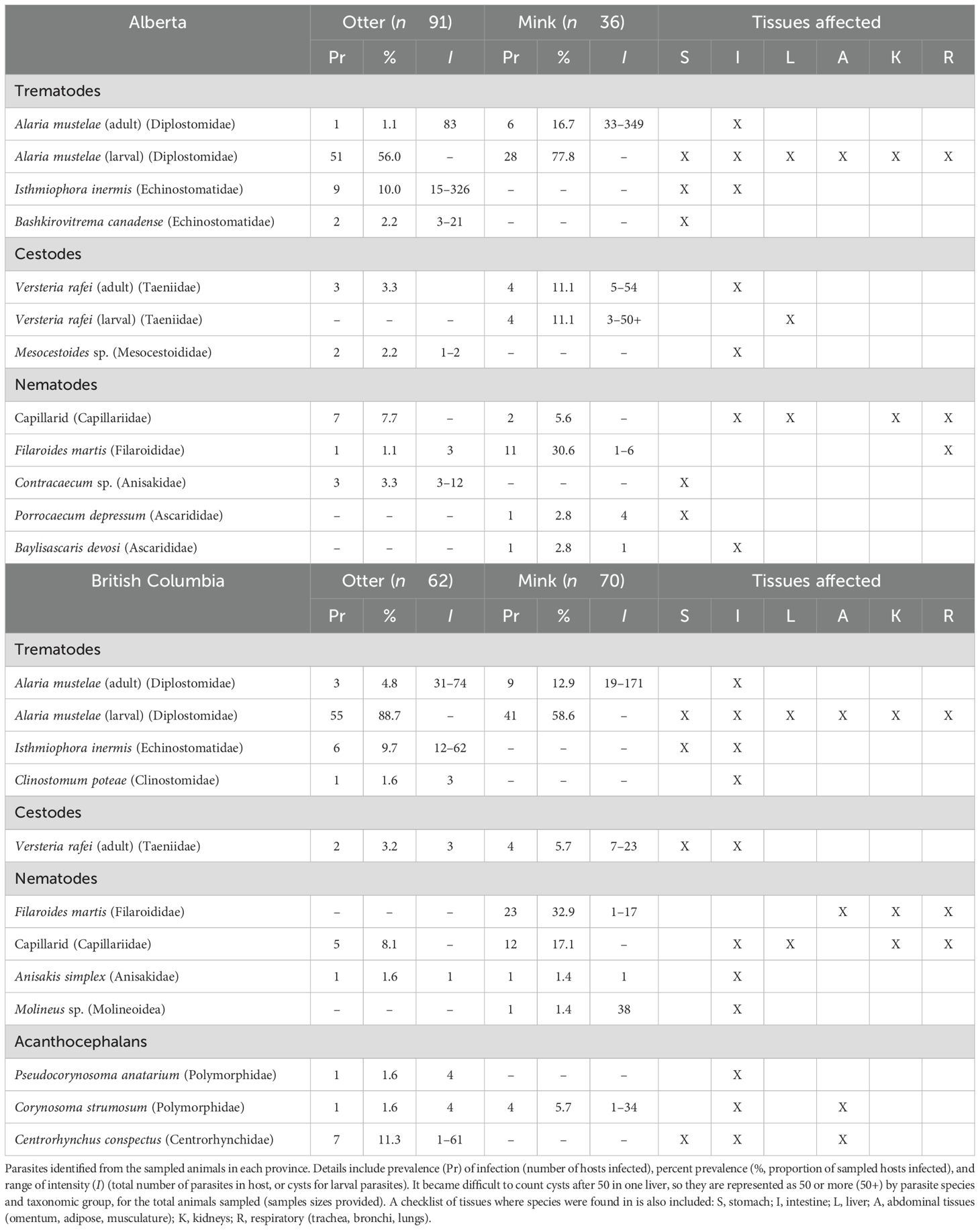
Results: In Alberta and British Columbia, parasite diversity was comparatively low relative to other parts of North America, especially when contrasted with the higher diversity observed in the southern United States. Parasite communities in sampled animals were characterized by four main species (Alaria mustelae, Filaroides martis, Isthmiophora inermis, and Versteria rafei). Larval infections by mesocercariae of A. mustelae were highly prevalent, and larval migrans was associated with inflammation and fibrosis in various tissues. Increasing intensities of infection were significantly related to decreasing nutritional condition.
Discussion: As mesocarnivores that connect aquatic and terrestrial food webs, otter and mink are definitive as well as potential intermediate or paratenic hosts for parasitic species that use aquatic hosts. We observed adult and larval infections of V. rafei and A. mustelae in our animals, which are both of concern for zoonotic transmission. As these associations have not been previously reported in otter or mink, it may represent an emergent disease of concern for these important sentinel species. This research highlights a serious gap in knowledge of helminth communities in Western Canada and the threat of zoonotic infection for vulnerable communities. Research to assess the risk of exposure and infection for First Nation communities and fur trappers who handle these mammals is warranted. Furthermore, research into the distribution of these parasites in other intermediate and definitive hosts is required to understand the range of this threat to wildlife and human communities.
1 Introduction
Important constituents of both riparian and coastal ecosystems, the North American river otter (Lontra canadensis) and mink (Neogale vison) are distributed across a broad swath of North America. These mesocarnivores have a top-down influence on fish and invertebrate species (Larivière and Walton, 1998; Larivière, 1999; Roemer et al., 2009). Once reduced in numbers by the fur trade, river otter have returned to a large portion of their historical range, but are still absent from the southern parts of the Canadian prairie provinces, as well as parts of the central and southwest United States (Larivière and Walton, 1998; Roberts et al., 2020). River otter feed on a broad range of riparian organisms, including fish, frogs, turtles, waterfowl, crustaceans, and other invertebrates, though fish make up most of their diet (Searing, 1979; Larivière and Walton, 1998). Similarly, mink consume fish, frogs, waterfowl, and crustaceans but may add muskrats and small terrestrial mammals to their diets depending on the season and environment (Searing, 1979; Larivière, 1999).
Despite the key role of otter in aquatic ecosystems, their parasites are seldom investigated in North America. Reports are limited to the eastern/southeastern United States during the latter part of the 20th century (Shoop and Corkum, 1981; Flemming et al., 1997; Hoberg et al., 1997; Kollars et al., 1997; Kimber and Kollias, 2000). Parasites of mink have been more frequently recorded, perhaps due to their greater numbers, frequency of trapping, and commercial farming for the fur trade. However, studies included farmed animals and invasive populations found outside of the species’ natural range and may not have represented natural parasite populations. Recent studies have focused on microparasites such as Cryptosporidium sp., Giardia sp (Gaydos et al., 2007), Leptospira sp., Toxoplasma gondii, and viral pathogens, such as parvovirus and canine distemper virus (Sanders et al., 2020; Cotey et al., 2022). Current monitoring is also focused on zoonotic pathogens that cause diseases such as SARS-CoV-2 (COVID-19) and the highly pathogenic avian influenza virus (HPAI) H5N1 (Mok and Qin, 2023).
Helminth parasites have received limited attention beyond opportunistically reporting their presence in otter and mink. Infections are generally considered asymptomatic and therefore are assumed non-pathogenic unless the hosts are immunocompromised (Kimber and Kollias, 2000). Exceptions include Paragonimus sp., which may be associated with respiratory illness (Sealander, 1943; Kimber and Kollias, 2000) and Alariasis caused by mesocercariae of Alaria americana (syn. A. canis). To the best of our knowledge, there is only one report that identified mesocercariae of A. americana in the abdominal fat of a single otter from Ontario (Pearson, 1956). In other species, mesocercariae may migrate to the lungs and develop into metacercariae, awaiting transport to another definitive host, or migrate to the host’s trachea, get swallowed, and then develop into adults within the same host (Pearson, 1956). Adults of Alaria mustelae (Diplostomidae) have frequently been reported in mink, although no report of mesocercarial infection could be found. No reports have been published documenting the helminth parasites of river otter and mink in Western Canada, and there is only one report from the western United States (Hoberg et al., 1997).
Baseline data on parasite communities in these mesocarnivores remain limited in Western Canada, and characterizing their parasite assemblages is critical for identifying geographic patterns, understanding host–parasite dynamics, and identifying potential risks to wildlife and human health. The aim of this study is twofold: 1) to conduct a literature review of helminth species reported in otter and mink across North America, thus providing context for our findings and highlighting broader patterns in parasite diversity, and 2) to document the diversity, distribution, and prevalence of helminth parasites of L. canadensis and N. vison in Alberta (AB) and British Columbia (BC), with special emphasis on parasites of zoonotic concern.
2 Materials and methods
2.1 Review of historical accounts
To compare the prevalence and diversity of helminths in river otter and mink in Western Canada with those in the rest of North America, we conducted a systematic literature search for and summarized studies that surveyed parasite communities of otter and mink. The search was based on titles, abstracts, and keywords from the Web of Science and Scopus databases. We ran the following search parameters: (Helminth* Or Parasite* OR Cestod* OR Trematod* OR Digenea* OR Acanthoceph* OR Nematod* OR worm* OR fluke* OR flatworm OR tapeworm OR roundworm*) AND (“river otter” OR mink OR “Lontra canadensis” OR “Neogale vison” OR “Neovison vison”). We only accepted reports from wild-caught North American river otter and mink and excluded any reports that looked at less than five animals or did not report both sample size and prevalence of parasite species, in order to limit biases due to low sample sizes. Resulting studies were used to create a list with the geographic distribution of species, as well as calculate an average prevalence for each (Supplementary A).
2.2 Carcass collection and evaluation
Carcasses of river otter and mink were obtained from licensed fur trappers in Alberta and British Columbia (BC), Canada, during the 2020–2021 and 2021–2022 trapping seasons. Carcasses were frozen after skinning and kept at −20°C until shipping or transport, then necropsied. Gross necropsies were performed on all animals by a wildlife veterinarian or trained biologist according to protocols outlined by the Coast Area Research Section (BC Ministry of Forests) and Environment and Climate Change Canada. Morphometric parameters (body length, tail length, chest girth, and weight) were recorded, and sex was confirmed by visual exam. Age was determined at Matson’s Laboratory (Manhattan, MT, USA) by cementum annuli aging (Stephenson, 1977). General health was assessed by the gross examination of tissues and quantification of fat stores. Abnormalities were recorded and photographed.
The nutritional condition of each animal was determined by quantifying the amount of external and internal fat. Fat stores were first scored qualitatively using a four-class descriptive scale as poor, moderate, good, or excellent. This included external fats: overall coverage, the coverage and thickness of fat on the whole body; intercostal, amount of fat surrounding the intercostal muscles; inguinal, amount of fat in large fat deposits present in the inguinal area; and tail, amount of fat at the base of the tail (only for otter), and internal fat stores: heart, omental, kidney, and internal back (Hatler et al., 2003). Scores were summed to create an internal fat index score (IFIS) and external fat index score (EFIS) and across both to create the overall fat index score (FIS). Qualitative ratings were then numerically transformed—0 (poor), 1 (moderate), 2 (good), and 3 (excellent)—and summed to produce an overall FIS or separately as external (EFIS) and internal (IFIS) scores.
2.3 Parasite identification
During necropsy, animals were examined for parasitic infection by visual assessment and dissection of internal organs. Complete dissections were performed on the trachea, bronchioles, heart, gastrointestinal tract (GIT), kidneys, and bladder. The trachea, bronchioles, and GIT were also flushed with water over a 0.3-mm sieve, and the retained material was examined by a stereo microscope. For the lung and liver, serial, transverse cuts were made through the parenchyma at regular intervals, and the cut surfaces were visually inspected. Gallbladders were opened and examined under a stereo microscope. For the pancreas, striated musculature, omentum, and sinuses, parasites were detected by careful observation and palpation. Parasites were transferred to 70% ethanol for later identification. In the case of animals with individual, scattered, or disseminated punctate white fibrous nodules or plaques, tissue samples were preserved in 70% ethanol and later digested with pepsin. The filtrate was examined for embedded parasites according to standardized protocols (European Commission, 2005). In a subset of these animals, representative samples of gross lesions from various tissues were collected for histopathology by a veterinary pathologist. Due to time and financial constraints, not all animals could be analyzed. Samples were fixed in 10% neutral buffered formalin, trimmed, placed in cassettes, and processed by an automated tissue processor (Tissue-Tek VIP, Sakura, Torrance, CA) through a graded series of alcohols to xylene. Tissues were then embedded in paraffin, sectioned at 5 µm, stained with hematoxylin and eosin (Leica Autostainer XS, ST5010, Concord, ON), and reviewed by light microscopy.
Parasites were identified morphologically by a trained taxonomist based on available descriptions in literature and/or molecularly. For light microscopy, helminths were stained with acetic acid carmine, dehydrated through a graded ethanol series, cleared in clove oil, and mounted permanently in Canada balsam. We molecularly identified all parasite species by sequencing at least one individual per host. For a subset of hosts, we sequenced 10 individuals, when possible, to ensure accurate species delineation, balancing thoroughness with time and budget constraints. DNA was extracted from whole worms or infected tissues using commercially available kits (DNEasy Blood and Tissue, QIAGEN, Germantown, USA). Amplification was conducted in an ABI 2720 Thermal Cycler in a total volume of 25 µL using PCR Master Mix (Invitrogen, Carlsbad, USA) or Taq PCR Kit (New England BioLabs Inc., Ipswich, USA) according to the manufacturer’s instructions. We used primers for various subregions of both ribosomal and mitochondrial DNA suggested by previous reports for specific taxa, following the cycling instructions provided by the authors (Supplementary Table S1). Sequencing was done via the Sanger method by the University of Alberta’s Molecular Biology Service Unit (Edmonton, Canada). Sequences were cleaned and trimmed using MEGA11 (Kumar et al., 2018; Tamura et al., 2021) and compared against those available in GenBank (Clark et al., 2016).
2.4 Statistical analysis
Prevalence, intensity, and abundance were calculated for each parasite genus or species according to Bush et al. (1997), as well as parasite richness (number of parasite species per host). Separate models were run to analyze the effect of parasitic infection on FIS, IFIS, and EFIS (three models in total). We fitted a single generalized linear mixed model (GLMM) with the identity link function for each fat score, using R statistical software 4.4.2 (R Core Team, 2024). The models included the intensity of infection by A. mustelae, total parasite species richness, species (otter or mink), sex (female, male), and age in years as explanatory variables. Infection intensity of A. mustelae was included as it was by far the most prevalent parasite and was categorized as “mild,” “moderate,” or “severe” based on a qualitative assessment of the spread and density of infection. Parasite species richness was also included to account for the possible effect of secondary infections and to avoid zero inflation of the model due to the low prevalence of other species. Host age and sex were included as both may influence the condition. Younger or older animals may be more likely to have poorer body condition irrespective of infection, and females may experience stronger effects due to the burdens of reproduction. Fixed effects in the GLMMs were tested for significance using type II Wald chi-square tests implemented via the “ANOVA” function in the car package. Statistical significance was assessed at p ≤0.05, and model goodness of fit was evaluated using deviance from the model summary.
3 Results
3.1 Literature search
Our literature search produced 27 peer-reviewed reports. Common helminth parasites include echinostomes (Trematoda: Digenea), diplostomids (Trematoda: Digenea), nematodes, and cestodes. Acanthocephalans were also reported but less commonly, with unique populations across North America (Figure 1, Supplementary Tables S2, S3). Documentation of pathogenic species of special interest to researchers included the guinea worm Dranunculis sp (Crichton and Beverley-Burton, 1973; Elsasser et al., 2009; Tumlison and Surf, 2018), nasal worm Skrjabingylus sp (Sealander, 1943; Dorney and Lauerman, 1969; Scherr and Bowman, 2009), heartworm Dirofilaria immitis (Snyder et al., 1989; Zabiega, 1996), and giant kidney worm Dioctophyma renale (Sealander, 1943; Miller and Harkema, 1964; Hoberg et al., 1997). Common parasites also included Filaroides martis, which was reported in mink across Canada and the United States, often at high prevalence (Figure 1, Supplementary Table S3). One study in Ontario (ON, Canada) reported infection in 45% of mink (n = 976; Anderson, 1962). Anderson (1962) identified Filaroides sp. in a single male river otter from ON, which the author asserted was distinct from F. martis and named F. canadensis. However, we could find no further reports of this parasite in otter. Capillarids were also commonly reported, with a prevalence reaching over 70% (Dorney and Lauerman, 1969). Adults of A. mustelae (Diplostomidae) were frequently reported in mink, but not in river otter. Mesocercarial infection of A. americana in the fat of a single otter was reported in Ontario (Pearson, 1956). Other common trematodes included echinostomids such as Baschkirovitrema canadense and Isthmiophora inermis.
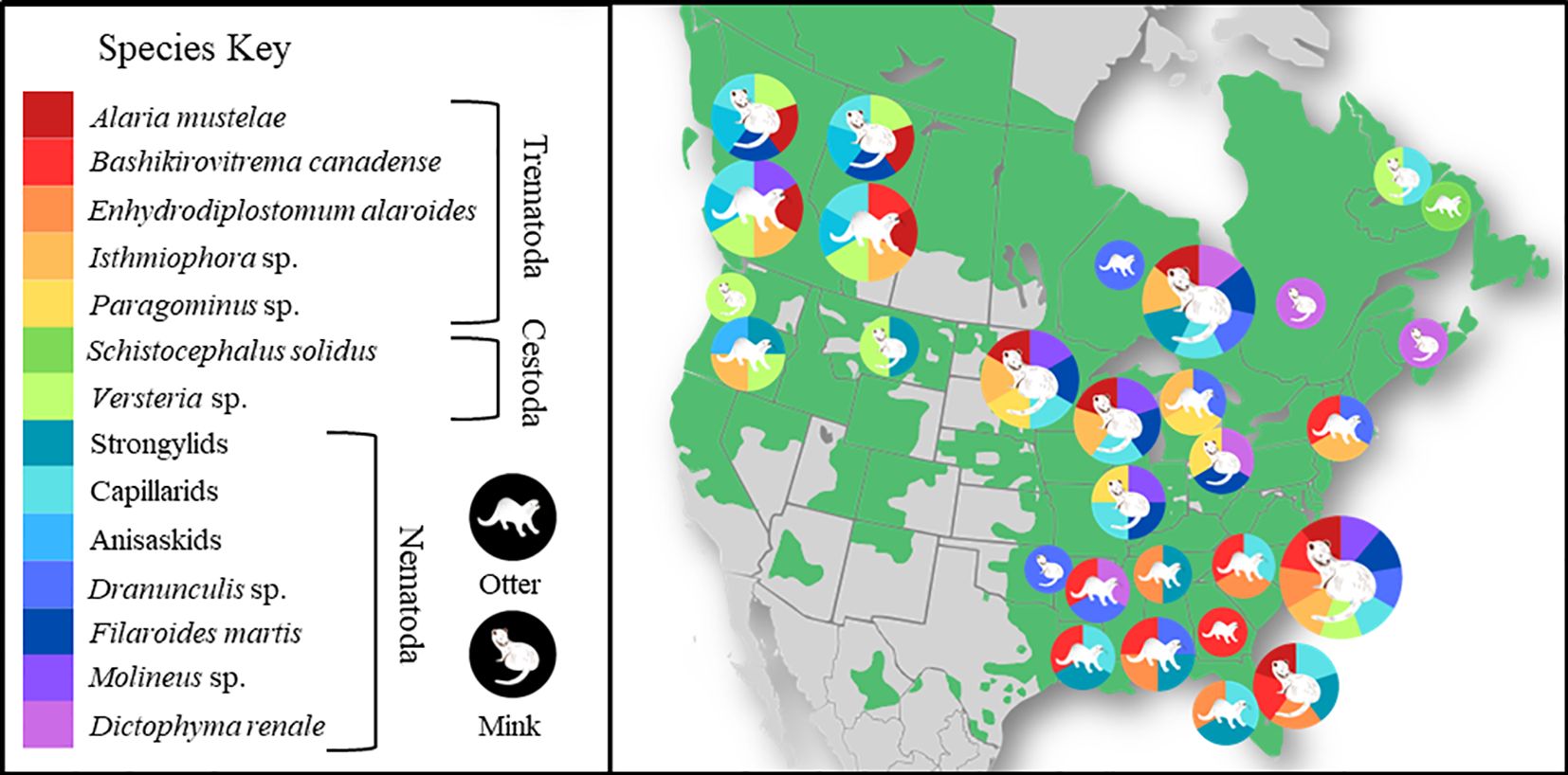
Figure 1. Distribution of parasites of otter and mink. A non-exhaustive distribution of common parasites of otter and mink in the states and provinces where they were reported. Parasite species are represented by color, host species by illustration, circles scaled by the number of parasite species identified in that region. Background color (green) marks the current reported distribution of North American river otter based on reports synthesized by Roberts et al. (2020). Empty spaces include areas where otter are absent or where there is no information. For parasite data, see Supplementary Tables S2, S3. Additional reports of the presence of some parasite species by studies that did not include prevalence and were therefore not included in our systematic search were included from reviews of parasites in mink and otter (Swales, 1933; Kimber and Kollias, 2000). Data for Alberta and British Columbia come from the present study. The figure was made using open-source software and illustrations (canva.com).
3.2 Necropsy results
In total, 153 river otter and 106 mink were included in this study. Animals came from various regions throughout the provinces, with high representation from Vancouver Island in British Columbia (BC) and areas surrounding the Cold Lake and Athabasca oil sands Region in Alberta (AB, Supplementary Figure S1). The sex ratio of the sampled animals was balanced for otter (85 males, 68 females) but biased toward males for mink (82 males, 24 females).
3.2.1 Observed parasitic infections
The prevalence of helminth infection was 90% in river otter and 88% in mink (Table 1). The average parasite species richness was low, generally approximately 1.2 species per animal. Helminths included at least 14 separate species. We found four species of trematodes and six species of nematodes. Multiple capillarid nematodes (Capillariidae) were seen but often too damaged for species identification. Except for one specimen identified as Pseudocapillaria sp., we were unable to successfully sequence capillarid nematode parasites. They were thus nested together as “capillarids.” We also identified two species of cestodes, including a newly described species, Versteria rafei (Taeniidae) (Shanebeck et al., 2024), as well as three species of acanthocephalans (Table 1). Other commonly reported parasites of mink and otter, such as Strongyloides sp., Paragonimus sp., Enhydridiplostomum alaroides, Dracunculus sp., and Dioctophyma renale (Supplementary Tables S2, S3), were not identified in our sampled animals.
Parasites were found throughout various tissues, in both adult and larval forms, with varying prevalence and intensities (Table 1). Some occurrences were associated with gross pathology (Supplementary A, Supplementary Figures S2–S4). For example, in the urinary system, infections by capillarids were associated with distention of the bladder and bloody urine. Adults of V. rafei were found in the intestines of both otter and mink without obvious damage. However, mink were also an intermediate host with larval cysts found in the liver as large as 4 mm. Cysts were associated with liver enlargement when present in large numbers and likely would have negatively affected liver function. In mink, Filaroides martis encysted in the lungs and was associated with yellow tan discoloration of the tissue and emphysema. Parasites within the gastrointestinal tract were rarely associated with gross pathology, except acanthocephalan-induced peritonitis in two juvenile mink. The parasites migrating through the intestinal wall likely led to the death of one juvenile on Vancouver Island (Shanebeck et al., 2022).
3.2.2 Infection of multiple tissue types by mesocercariae of Alaria mustelae
During necropsy, numerous small white nodules were observed in various tissues in the abdominal cavity of many animals (notter = 106 [68.4%]; nmink = 69 [64.5%]) (Figure 2). These lesions were localized most often along the gastrointestinal serosa and pancreatic capsules, with occasional involvement of the liver, spleen, kidney, mesentery, bladder, retroperitoneum, and musculature of the abdominal wall. Host tissue digestion via pepsin produced mesocercaria with morphologic features consistent with descriptions of Alaria sp (Bosma, 1934; Johnson, 1970), though digestion degraded the parasite tegument and compromised staining. Other reports have noted this problem and recommended mechanical digestion of fresh tissue to make use of Alaria’s mobility out of the tissue and inability to swim against gravity (Riehn et al., 2010). Unfortunately, our tissue samples had been frozen and were fixed in 70% ethanol for later analysis, which precluded screening for motile mesocercariae. Collected tissues were also digested, and DNA was extracted for molecular analysis and sequenced with primers specific to digenean parasites. We had a low success rate, obtaining sequences from only 13 samples, consisting of 11 segments of intestine, 1 kidney, and 1 liver. Results from 12 of the analyses matched available GenBank sequences of A. mustelae with over 99% identity. Phylogenetic analysis showed strong clustering of all sequences with exemplars of A. mustelae. Given the phylogenetic distance detected, one sequence from a mink in BC may represent a unique species of Alaria (Supplementary Figure S5).
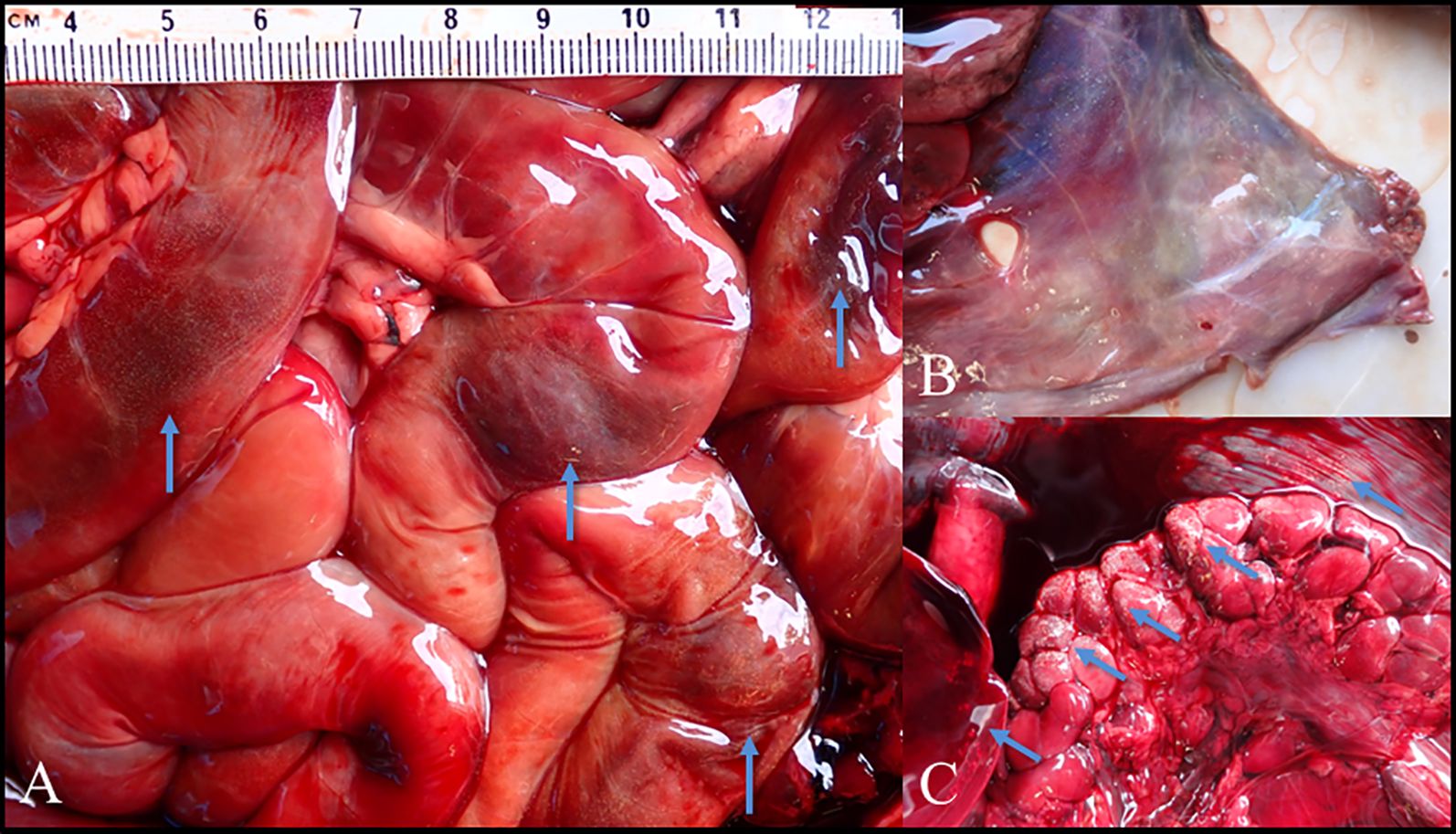
Figure 2. Gross pathology of Alaria infections. Examples of otter tissue infected with mesocercaria of Alaria mustelae. (A) Intestines showing localized areas of hemorrhaging associated with small white nodules. (B) Musculature of the abdominal wall showing diffuse small white nodules. (C) Liver, kidney, and abdominal muscle with localized small white nodules, with enlarged adrenal glands.
Microscopic evaluation of a subset of infected animals, including 3 mink and 18 otter, revealed lesions in a variety of tissues, which would have been sufficiently severe to have resulted in antemortem clinical disease (Supplementary A). In two animals, severe, high-intensity infections were associated with widespread chronic inflammation, characterized by fibrosis within the abdominal cavity and viscera (peritonitis) and kidney damage. The abdominal inflammation would have contributed to pain, impaired gastrointestinal transit of ingesta, and reduced nutrient absorption, whereas the kidney damage would have resulted in increased protein loss within the urine. These processes may have been exacerbated by damage to and scar tissue formation in the pancreas (Supplementary Figure S2), which would have reduced digestive enzyme secretion with subsequent maldigestion and malabsorption of nutrients.
Regression model results showed that both infection intensity (χ2 = 86.85, df = 3, p < 0.001) and species (χ2 = 14.22, df = 1, p < 0.001) were significant predictors of FIS. Infection intensity showed a stronger negative association with IFIS than EFIS (Figure 3), though it was significantly associated with both (Supplementary Table S4). Between EFIS and IFIS, species was only significantly associated with EFIS, and mink had significantly worse EFIS scores than otter. Total parasite species richness, sex, and age were not significantly correlated with fat scores in any of the models (Supplementary Table S4).
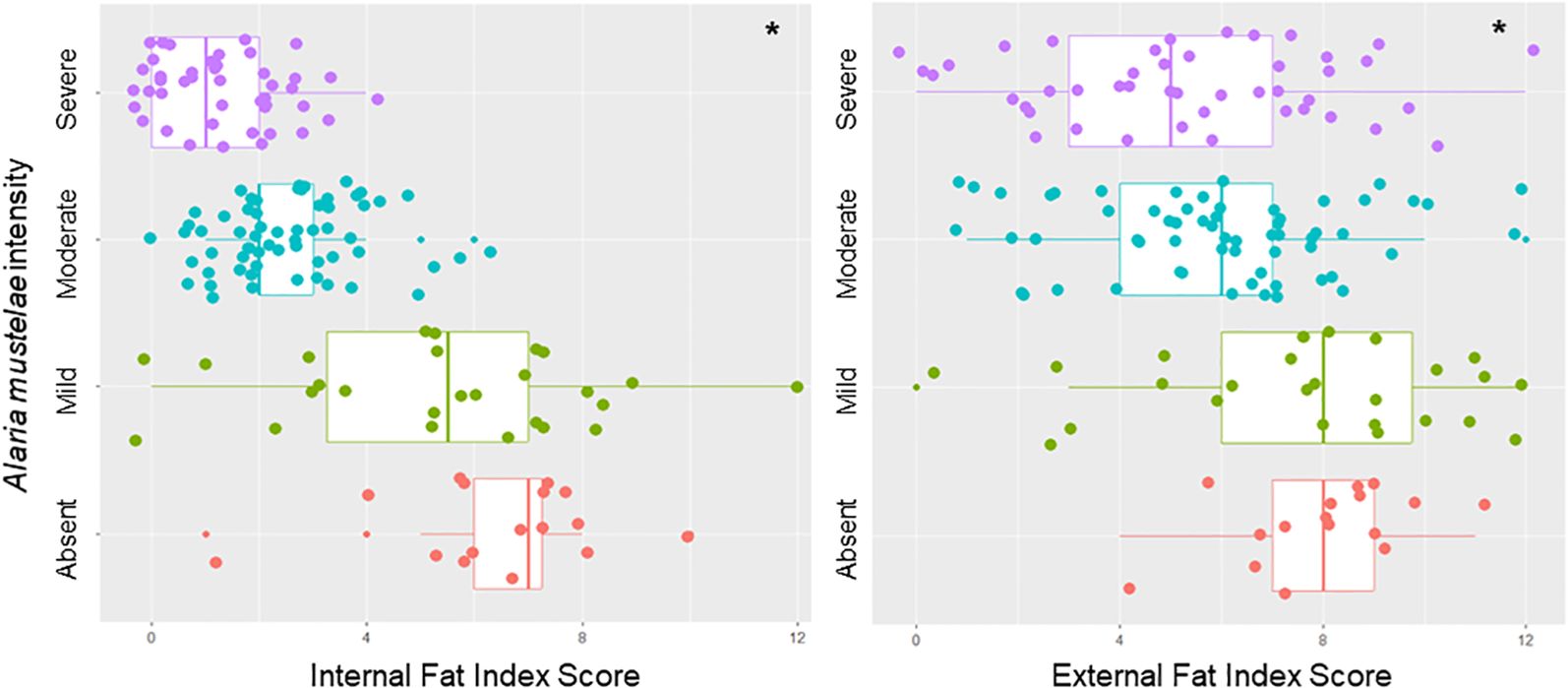
Figure 3. Effect of infection intensity on internal and external fat scores. Differences in nutritional condition based on the internal fat index score (sum of qualitative measures of internal fats like the heart, omentum, and kidney) and external fat index score (sum of qualitative measures of external fats such as inguinal, intercostal, and tail). Categorized by qualitative assessment of infection intensity of Alaria mustelae. *Infection intensity was significantly correlated with both internal fat index score (p < 0.005, df = 3, χ2 = 147.2) and external fat index score (p < 0.005, df = 3, χ2 = 17.5).
4 Discussion
This study investigated the helminth communities of L. canadensis and N. vison in Western Canada, where reports on the diversity or prevalence of helminth parasites in these important aquatic mesocarnivores are scarce. Our data revealed a diverse community of trematodes, nematodes, cestodes, and acanthocephalans. The trematodes were the largest taxonomic group by prevalence, richness, and intensity. These findings differ from the available surveys of otter and mink in other parts of North America that documented a greater prevalence and diversity of nematode species (Supplementary Tables S2, S3). In most cases, A. mustelae was the most prevalent helminth, and both mesocercarial and adult stages were identified. Adult stages in mink occurred at a lower prevalence than the weighted average of previous reports (14% vs. 24%) but were within one standard deviation. Alaria mustelae had not previously been reported in river otter. Filaroides martis infected 33% of mink in British Columbia, which is lower than the 42% weighted average, but again within one standard deviation. Mink in Alberta had a much lower prevalence at 11%.
Infections of Isthmiophora inermis (syn. Euparhyphium inerme; Kostadinova and Gibson, 2002) have only been reported in L. canadensis from the Pacific Northwest, at a similar prevalence to that observed in this study (Hoberg et al., 1997). Isthmiophora melis was described in otter at an unknown prevalence in Massachusetts (Kimber and Kollias, 2000) and from a single animal in Michigan (Beaver, 1941), and I. melis and I. beaveri have been reported in mink from North Carolina, Wisconsin, and Maine (Erickson, 1946; Miller and Harkema, 1964; Dorney and Lauerman, 1969). No Isthmiophora sp. were detected in mink from this study. Hoberg et al. (1997) noted differences in size for I. inermis between North America and Eurasia, but stated that there was insufficient data to separate them as a unique species, including a lack of genetic information. Individuals of I. inermis identified in this study were even larger than those identified by Hoberg et al. (1997) and may indeed represent a unique species in North America. Future molecular and morphological comparisons are needed.
There have been very few reports of acanthocephalan infections in river otter and mink, with limited records of species such as Corynosoma strumosum (Oregon; Hoberg et al., 1997) and Acanthocephalus sp. (Alaska, Tennessee; Flemming et al., 1997; Kollars et al., 1997) in otter and Centrorhynchus conspectus (Miller and Harkema, 1964) and Pseduocorynosoma constrictum (Dorney and Lauerman, 1969) in mink. Except for Acanthocephalus sp., these species were observed in British Columbia, mostly in the mid and southern regions of Vancouver Island. It is unclear why they were not found elsewhere.
Our results suggest distinct parasite communities between the Pacific Northwest and Eastern United States and Canadian populations of mink and otter (Figure 1). While some species, such as F. martis and A. mustelae, span North America, many frequently reported parasite species, such as Enhydrodiplostomum alaroides, Dracunculus sp., Paragonimus sp., Skrjabingylus sp., and Dioctophyme renale, were not found in this study or by Hoberg et al. (1997) in Oregon and Washington. Although B. canadense was identified in Alberta, the prevalence was 1%. This prevalence is less than in previous reports from the Southeastern USA, where the weighted average of prevalence between five studies was 47% (Supplementary Table S2). Moreover, no reports of I. inermis in L. canadensis were identified outside of the Pacific Northwest, which suggests that the parasite may be unique to otter of this region. The differences in parasite composition and distribution may be attributed to a variety of factors, but we believe the most likely is the slow recovery of otter after their extirpation from much of North America. The central prairies were free of otter until recent reintroduction efforts were initiated to close the gap (Roberts et al., 2020). Due to this isolation, especially for the coastal mountain regions of British Columbia, WA, and OR, and differences in climate and ecosystem, it is reasonable that northwestern populations of otter and mink feature unique parasite communities.
4.1 Mesocercarial infection by Alaria mustelae
Alaria mustelae is a generalist parasite with a complex life cycle that can include up to five hosts, and is very host-unspecific among vertebrates (Mohl et al., 2009; Bosma, 1931). When the second intermediate or paratenic host is consumed by a competent third intermediate host, the parasites migrate to and transform into metacercaria in the lung parenchyma or striated muscle, such as the diaphragm (Bosma, 1934). Definitive hosts can include a variety of mustelids, canids, and other carnivores (Bosma, 1931).
This study also confirmed both gravid adults and mesocercariae in river otter and mink, which suggests that they may act as both intermediate and definitive hosts. This is the first time that infections of A. mustelae mesocercariae were reported in river otter in North America. Bosma (1934) noted that mink could be infected with metacercariae, but no recent reports were identified in the literature review. The closely related A. alata can infect European mink (Mustela lutreola) as adults and mesocercariae (Tabaran et al., 2013). Both otter and mink are preyed upon or scavenged by wolves (Canis lupus), coyotes (Canis latrans), and wolverines (Gulo gulo) (Melquist et al., 2003). Mink are also consumed by red foxes (Vulpes vulpes), bobcats (Lynx rufus), and even river otter (Larivière, 1999), all of which are suitable definitive hosts for A. mustelae (Mohl et al., 2009). Alaria americana can be transmitted by autoinfection from larvae traveling up the trachea, then swallowed (Pearson, 1956). If A. mustelae exhibits a similar behavior, this may also explain dual infections of mesocercarial and adult parasites.
In this case series, infection by A. mustelae was associated with chronic peritonitis due to migration of mesocercariae into the abdominal cavity (Supplementary A) and was significantly correlated with lower nutritional condition, likely due to generalized debilitation and inappetence associated with chronic disease. The gross and microscopic lesions were consistent with European mink infected by A. alata and identified by white nodules in striated muscle and subcutaneous tissues. The distribution of granulomas and fibrous encapsulation was likely due to the ectopic migration of mesocercariae through tissues (Mathison and Pritt, 2018), but when encysted in muscle tissue, no immune response was evoked (Tabaran et al., 2013). Upon entering a paratenic host, mesocercariae can migrate to the skeletal muscle, diaphragm, adipose tissue, or lungs; encyst; and potentially develop into metacercariae (Bosma, 1934; Johnson, 1979). The extent of this migration, freeze-thaw artifact, and small size of the nodules may account for our difficulty in finding mesocercariae or successfully sequencing samples from intestinal, hepatic, pancreatic, and renal tissues in this case series; with the exception of a few animals, white nodules were not apparent at necropsy. If lesions were collected, encysted mesocercariae would likely have been detected in greater numbers. Based on the nature and extent of the inflammation in the examined tissues, malnutrition was likely a sequela to parasite migration, associated inflammation, and scar tissue formation.
Both the observed pattern of lesions and histopathology were consistent with the migration of mesocercariae through the gastrointestinal wall (Supplementary A, Supplementary Figure S2), en route to striated musculature or adipose tissue to encyst and develop into metacercariae. The observed white nodules likely represent encapsulated viable or degenerate parasites and, less likely, past and resolving migration tracks. Due to the chronic nature of the nodules and the lack of identifiable parasite elements, the lesions were not necessarily indicative of the parasite’s presence at the time of necropsy. Artificial digestion of tissues produced mesocercariae consistent with Alaria sp., though definitive speciation was not possible due to degradation of the tegument by the pepsin. Because of this and our inability to definitively identify trematodes as the etiological agent of the observed fibrosis and inflammation within the peritoneum, it is possible that not all observed lesions were due to A. mustelae. Some infections may have been related to another species, such as A. americana or Diplostomid, as well as disseminated bacterial or fungal infections. Nevertheless, in our study cohort, based on the lack of discernible pathogens (fungal elements or bacteria) and molecular characterization of the co1 mitochondrial region, there were 13 confirmed A. mustelae infections. Due to the similarity in postmortem lesions, detection of mesocercariae morphologically consistent with Alaria sp. in animals presenting with disseminated white nodules, and detection of A. mustelae adults in both river otter and mink, it is likely that the parasites of unconfirmed species in other mink and otter were predominantly A. mustelae. Future research is warranted to further investigate and characterize the lung, diaphragm, adipose tissue, and musculature of animals presenting with the disseminated white nodules to identify the mesocercariae and determine if they can mature into metacercaria in otter and mink.
5 Conclusions
A survey of helminth populations of L. canadensis and N. vison in Alberta and British Columbia identified many of the previously reported parasite species. However, notable species of concern, such as Dracunculus sp. and Dioctophyme renale, were not identified. As with previous reports in mink and otter, overall species richness was low (Sealander, 1943; Forrester, 1992; Hoberg et al., 1997; Foster et al., 2007) and comprised four main species (A. mustelae, F. martis, I. inermis, and V. rafei). Most significantly, otter and mink were infected at high prevalences (up to 81% in L. canadensis from Alberta) with A. mustelae mesocercariae. These infections were associated with inflammation and fibrosis, which likely contributed to the suboptimal nutritional conditions observed in more heavily infected animals. Nutritional condition significantly negatively correlated with increasing infection burdens.
This is the first report of A. mustelae infection in river otter in North America and is of concern for both wildlife management and human health. With moderate to severe pulmonary or ocular infections and potential anaphylaxis, human alariasis poses a serious health concern. Fatal infections have been reported in healthy young individuals (Mohl et al., 2009; Portier et al., 2014; Freeman et al., 1976). The complete life cycle of A. mustelae is unclear, and they may be very unspecific in their paratenic hosts (Mohl et al., 2009; Portier et al., 2014). Diplostomids often infect a variety of fish species as intermediate hosts and can encyst in the eye, striated muscle, or internal viscera (Kohn et al., 1995; Ribeiro et al., 2019). Alaria mustelae may also use frogs and rodents as intermediate hosts and have been reported in muskrats (Dunagan, 1957; Johnson, 1979). If fish are used by A. mustelae as intermediate hosts, infection may be of particular concern for First Nation communities as well as rural populations and sports fishers in Western Canada. Muskrats are also a traditional food source for First Nation peoples and may be another source of infection (Turner et al., 2018). Further research is needed to determine the role of intermediate or paratenic hosts for potential human, mink, and otter alariasis. Medical providers should consider alariasis as a potential differential diagnosis for respiratory and ocular diseases.
As this phenomenon had not previously been reported in mink and otter, let alone at such high prevalence, alariasis may potentially be an emergent disease of concern. Aquatic mammals are essential as advanced warning systems for threats to ecosystems and human communities (Bosart, 2011), and the mesocercarial infections of a zoonotic parasite in otter and mink outlined in this research are cause for concern in Western Canada for at-risk groups.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was approved by the Animal Care and Use Committee, University of Alberta. The study was conducted in accordance with the local legislation and institutional requirements. In accordance with the Canadian Council on Animal Care guidelines, this research was exempt from Animal Research Ethics Board review in Canada because all samples were collected from animals previously harvested for non-research purposes.
Author contributions
KS: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MT: Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing. PT: Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing. SR: Investigation, Writing – review & editing, Formal analysis. BP: Methodology, Validation, Investigation, Resources, Visualization, Writing – review & editing. SG: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. CL: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the Government of Canada's New Frontiers in Research Fund (NFRF), [NFRFT-2020-00073], by the ACA Grants in Biodiversity (supported by the Alberta Conservation Association) [030‐00‐90‐140/1090], by the BC Ministry of Forest's Coast Area Research Section, and by Environment and Climate Change Canada and the Canada-Alberta Oil Sands Monitoring program (OSM). While this work was funded under the Oil Sands Monitoring Program and is a contribution to the Program, it does not necessarily reflect the position of the Program.
Acknowledgments
We would like to acknowledge and thank the First Nations and Métis Nations communities, whose traditional lands provided the river otter and mink used for our study. Many thanks to the Alberta and British Columbia Trappers Associations for their logistical support in collections and the individual trappers who submitted carcasses for this study. A special thanks to Cait Nelson at the BC Wildlife Health Program for her hard work and support for this project, as well as Dr. Caeley Thacker, Maeve Winchester, Shari Willmott, and Paige Monteiro for their assistance during necropsies in BC. Many thanks to our undergraduate assistants Anika Mamun and Sydney Storvold who provided invaluable help during necropsies in Alberta.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The reviewer CM is currently organizing a Research Topic with the author KS.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmamm.2025.1498904/full#supplementary-material
References
Anderson R. (1962). The systematics and transmission of new and previously described metastrongyles (Nematoda: Metastrongylidae) from Mustela vison. Can. J. Zool. 40, 893–920. doi: 10.1139/z62-081
Beaver P. C. (1941). Studies on the life history of Euparyphium melis (Trematoda: Echinostomidae). J. Parasitol. 27, 35–44. doi: 10.2307/3272884
Bosart GD (2011). Marine Mammals as Sentinel Species for Oceans and Human Health. Vet Pathol 48, 676–690.
Bosma N. J. (1931). Alaria mustelae sp. Nov., a trematode requiring four hosts. Science 74, 521–522. doi: 10.1126/science.74.1925.521.b
Bosma N. J. (1934). The life history of the trematode alaria mustelae, Bosma 1931. Trans. Am. Microsc. 53, 116–153. doi: 10.2307/3222088
Bush AO, Lafferty KD, Lotz JM, and Shostak AW (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83, 575–583.
Clark K., Karsch-Mizrachi I., Lipman D. J., Ostell J., and Sayers E. W. (2016). GenBank. Nucleic Acids Res. 44, D67–D72. doi: 10.1093/nar/gkv1276
Cotey S. R., Scimeca R., Chang L., Carpenter A. L., Will E. E., Ott-Conn C., et al. (2022). Toxoplasma gondii prevalence, partial genotypes, and spatial variation in North American river otters (Lontra canadensis) in the upper peninsula of MI, USA. J. Wildl. Dis. 58, 869–881. doi: 10.7589/JWD-D-22-00021
Crichton V. F. J. and Beverley-Burton M. (1973). Distribution and prevalence of Dracunculus spp. (Nematoda: Dracunculoidea) in mammals in ON. Can. J. Zool. 52, 163–167. doi: 10.1139/z74-019
Dorney R. S. and Lauerman L. H. (1969). A helminthological survey of wild mink in WI. Bul. Wildl. Dis. Ass. 5, 35–36. doi: 10.7589/0090-3558-5.1.35
Dunagan T. T. (1957). Helminth parasites of Alaskan muskrats. Trans. Am. Miscros. Soc 76, 318–320. doi: 10.2307/3223897
Elsasser S. C., Floyd R., Hebert P. D., and Schulte-Hostedde A. I. (2009). Species identification of North American Guinea worms (Nematoda: Dracunculus) with DNA barcoding. Mol. Ecol. Resour. 9, 707–712. doi: 10.1111/j.1755-0998.2008.02393.x
Erickson A. B. (1946). Incidence of worm parasites in MN mustelidae and host lists and keys to North American species. Am. Midl. Nat. 36, 494. doi: 10.2307/2421517
European Comission (2005). Commission Regulation (EC) No.2075/2005 of 5th December 2005 laying down specific rules onofficial controls for Trichinella in meat. Off. J. Eur. Union L. 338, 60–82.
Flemming W. J., Dixon C. F., and Lovett J. W. (1997). Helminth parasites of river otters (Lutra canadensis) from southeastern AL. J. Helminthol. 44, 131–135.
Forrester D. (1992). Parasites and diseases of wild mammals in FL (Gainesville: University Press of FL).
Foster G. W., Cunningham M. W., Kinsella J. M., and Owen M. (2007). Parasitic helminths of free-ranging mink (Neovison vison mink) from southern FL. J. Parasitol. 93, 945–946. doi: 10.1645/GE-1172R.1
Freeman R. S., Stuart P. F., Cullen J. B., Ritchie A. C., Mildon A., Fernandes B. J., et al. (1976). Fatal human infection with mesocercariae of the trematode Alaria americana. Am. J. Trop. Med. Hyg. 25, 803–807. doi: 10.4269/ajtmh.1976.25.803
Gaydos J. K., Miller W. A., Gilardi K. V. K., Melli A., Schwant H., Engelstoft C., et al. (2007). Cryptosporidium and giardia in marine-foraging river otters (Lontra canadensis) from the puget sound Georgia basin ecosystem. J. Parasitol. 93, 198–202. doi: 10.1645/GE-928R.1
Hatler D., Blood D., and Beal A. (2003). BC Furbearer Management Guidelines, Marten (Martes americana) (Wildeor Wildlife Research and Consulting for the BC Ministry of Water, Land and Air Protection, the Habitat Conservation Trust Fund, and the British Columbia Trappers Association).
Hoberg E. P., Henny C. J., Hedstrom O. R., and Grove R. A. (1997). Intestinal helminths of river otters (Lutra canadensis) from the Pacific Northwest. J. Parasitol. 83, 105–110. doi: 10.2307/3284324
Johnson A. D. (1970). Alaria mustelae: description of mesocercaria and key to related species. Trans. Am. Microsc. 89, 250–253. doi: 10.2307/3224381
Johnson A. D. (1979). Morphology and life history of alaria mustelae bosma 1931 (Trematoda: diplostomatidae) from MN mustelids. J. Parasitol. 65, 154–160. doi: 10.2307/3280221
Kimber K. R. and Kollias G. V. I. (2000). Infectious and parasitic diseases and contaminant-related problems of North American river otters (Lontra canadensis): a review. J. Zoo Wildl. Med. 31, 452–472. doi: 10.1638/1042-7260(2000)031[0452:IAPDAC]2.0.CO;2
Kohn A., Fernandes B., and Baptista-Farias M. (1995). Metacercariae of Diplostomum (Austrodiplostomum) compactum (Trematoda, Diplostomidae) in the eyes of Plagioscion squamosissimus (Teleostei, Sciaenidae) from the Reservoir of the hydroelectric power station of Itaipu, Brazil. Mem. Inst. Oswaldo. Cruz. 90, 341–344. doi: 10.1590/S0074-02761995000300005
Kollars T. M., Lizotte R. E., and Wilhelm W. E. (1997). Gastrointestinal helminths in the river otter (Lutra canadensis) in tennessee. J. Parasitol. 83, 158–160. doi: 10.2307/3284338
Kostadinova A. and Gibson D. I. (2002). Isthmiophora lühe 1909 and Euparyphium Dietz 1909 (Digenea: Echinostomatidae) re-defined, with comments on their nominal species. Syst. Parasitol. 52, 205–217. doi: 10.1023/A:1015789703396
Kumar S., Stecher G., Li M., Knyaz C., and Tamura K. (2018). Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Larivière S. and Walton L. R. (1998). Lontra canadensis. Mamm. Species 587, 1–8. doi: 10.2307/3504417
Mathison B. A. and Pritt B. S. (2018). A systematic overview of zoonotic helminth infections in North America. Lab. Med. 11, e61–e93. doi: 10.1093/labmed/lmy029
Melquist W. E., Polechla P. J., and Toweill D. (2003). “River Otter,” in Wild Mammals of North America. Eds. Felhamer G. A., Thompson B. C., and Chapman J. A. (The Johns Hopkins University Press, Baltimore and London).
Miller G. C. and Harkema R. (1964). Studies on helminths of NC vertebrates. V. Parasites of the mink, mustela vison schreber. J. Parasitol. 50, 717. doi: 10.2307/3276190
Mohl K., Grosse K., Hamedy A., Wuste T., Kabelitz P., and Lucker E. (2009). Biology of Alaria spp. and human exposition risk to Alaria mesocercariae-a review. Parasitol. Res. 105, 1–15. doi: 10.1007/s00436-009-1444-7
Mok C. K. and Qin K. (2023). Mink infection with influenza A viruses: an ignored intermediate host? One Health Adv. 1, 5. doi: 10.1186/s44280-023-00004-0
Pearson J. (1956). Studies on the life cycles and morphology of the larval stages of Alaria arisaemoides Augustine and Uribe 1927 and Alaria canis LaRue and Fallis 1936 (Trematoda: diplostomidae). Can. J. Zool. 34, 295–387. doi: 10.1139/z56-043
Portier J., Vallee I., Lacour S. A., Martin-Schaller R., Ferte H., and Durand B. (2014). Increasing circulation of Alaria alata mesocercaria in wild boar populations of the Rhine valley, France 2007-2011. Vet. Parasitol. 199, 153–159. doi: 10.1016/j.vetpar.2013.09.029
R Core Team (2024). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/.
Ribeiro J. S., Oliveira F. C. R., and Ederli N. B. (2019). First report of Diplostomidae metacercariae (Trematoda: Digenea) in African catfish Clarias gariepinus (Siluriformes: Clariidae) in Brazil. Rev. Bras. Parasitol. Vet. 28, 677–684. doi: 10.1590/s1984-29612019081
Riehn K., Hamedy A., Große K., Zeitler L., and Lücker E. (2010). A novel detection method for Alaria alata mesocercariae in meat. Parasitol. Res. 107, 213–220. doi: 10.1007/s00436-010-1853-7
Roberts N. M., Lovallo M. J., and Crimmins S. M. (2020). River otter status, management, and distribution in the United States: evidence of large-scale population increase and range expansion. J. Fish Wildl. Manage. 11, 279–286. doi: 10.3996/102018-JFWM-093
Roemer G. W., Gompper M. E., and Van Valkenburgh B. (2009). The ecological role of the mammalian mesocarnivore. BioScience 59, 165–173. doi: 10.1525/bio.2009.59.2.9
Sanders C. W., Olfenbuttel C., Pacifici K., Hess G. R., Livingston R. S., and DePerno C. S. (2020). Leptospira, parvovirus, and toxoplasma in the North American river otter (Lontra canadensis) in NC, USA. J. Wildl. Dis. 56, 791–802. doi: 10.7589/2019-05-129
Scherr H. and Bowman J. (2009). A sex-biased effect of parasitism on skull morphology in river otters. Écoscience 16, 119–124. doi: 10.2980/16-1-3203
Sealander J. A. (1943). Notes on some parasites of the mink in southern MI. J. Parasitol. 29, 361–362. doi: 10.2307/3272617
Searing G. F. (1979). Distribution, abundance and habitat associations of beavers, muskrats, mink and river otters in the Aoserp Study Area, Northeast Alberta (Alberta Oil Sands Environmental Research Program).
Shanebeck K. M., Bennett J., Green S. J., Lagrue C., and Presswell B. (2024). A new species of Versteria (Cestoda: Taeniidae) parasitizing Neogale vison and Lontra canadensis (Carnivora: Mustelidae) from Western Canada. J. Helminthol. 98, e4, 1–e4,11. doi: 10.1017/S0022149X23000895
Shanebeck K. M., Thacker C., and Lagrue C. (2022). Corynosoma strumosum (Acanthocephala) infection in marine foraging mink (Neogale vison) and river otter (Lontra canadensis) and associated peritonitis in a juvenile mink. Parasitol. Int. 89, 102579. doi: 10.1016/j.parint.2022.102579
Shoop W. L. and Corkum K. C. (1981). Some trematodes of mammals in LA. Tulane Univ. Stud. Zool. Bot. 22, 109–121.
Snyder D. E., Hamir A. N., Nettles V. F., and Rupprecht C. E. (1989). Dirofilaria immitis in a River Otter (Lutra canadensis) from LA. J. Wildl. Dis. 25, 629. doi: 10.7589/0090-3558-25.4.629
Stephenson A. B. (1977). Age determination and morphological variation of ON otters. Can. J. Zool. 55, 1577–1583. doi: 10.1139/z77-206
Swales W. E. (1933). A review of Canadian helminthology: I. The present status of knowledge of the helminth parasites of domesticated and semidomesticated mammals and economically important birds in Canada, as determined from work published prior to 1933. Can. J. Res. 8, 468–477. doi: 10.1139/cjr33-048
Tabaran F., Sandor A. D., Marinov M., Catoi C., and Mihalca A. D. (2013). Alaria alata Infection in European mink. Emerg. Infect. Dis. 19, 1547–1549. doi: 10.3201/eid1909.130081
Tamura K., Stecher G., and Kumar S. (2021). MEGA 11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027. doi: 10.1093/molbev/msab120
Tumlison R. and Surf A. (2018). Prevalence, structure, and distribution of novel parasite cysts containing dracunculus species in river otters (Lontra canadensis) from arkansas. J. Parasitol. 104, 319–321. doi: 10.1645/17-138
Turner C. K., Lantz T. C., and Gwich’in Tribal Council Department of Cultural Heritage (2018). Springtime in the delta: the socio-cultural importance of muskrats to gwich’in inuvialuit trappers through periods of ecological and socioeconomic change. Hum. Ecol. 46, 601–611. doi: 10.1007/s10745-018-0014-y
Keywords: Alaria, helminths, disease ecology, zoonotic disease, body condition
Citation: Shanebeck KM, Todd M, Thomas PJ, Raverty S, Presswell B, Green S and Lagrue C (2025) Parasite populations of river otter and mink in Western Canada, and the first report of the zoonotic trematode Alaria mustelae in river otter in North America. Front. Mamm. Sci. 4:1498904. doi: 10.3389/fmamm.2025.1498904
Received: 24 September 2024; Accepted: 24 June 2025;
Published: 23 July 2025.
Edited by:
Gonzalo Medina-Vogel, Andres Bello University, ChileReviewed by:
Felipe A. Hernández, Universidad Austral de Chile, ChileO. Alejandro Aleuy, University of Calgary, Canada
Carlos Calvo-Mac, CONSERVACCION, Peru
Copyright © 2025 Shanebeck, Todd, Thomas, Raverty, Presswell, Green and Lagrue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyle M. Shanebeck, a3lsZS5tLnNoYW5lYmVja0BnbWFpbC5jb20=
 Kyle M. Shanebeck
Kyle M. Shanebeck Melissa Todd
Melissa Todd Philippe J. Thomas3
Philippe J. Thomas3 Stephen Raverty
Stephen Raverty Stephanie Green
Stephanie Green