- 1Projeto Ariranhas/Giant Otter Conservation Fund, Rua Presidente Vargas, Arroio do Meio, Rio Grande do Sul, Brazil
- 2IUCN Species Survival Commission, Otter Specialist Group, Gland, Switzerland
- 3Programa de Pós-graduação em Biologia Animal, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Rio Grande do Sul, Brazil
- 4Instituto de Pesquisas Veterinárias Desidério Finamor (IPVDF), Eldorado do Sul, Rio Grande do Sul, Brazil
- 5Simbios Biotecnologia, Cachoeirinha, Rio Grande do Sul, Brazil
- 6Instituto Federal Farroupilha - IFFAR, Alameda Santiago do Chile, Santa Maria, Rio Grande do Sul, Brazil
The Neotropical otter (Lontra longicaudis) is a semiaquatic mustelid widely distributed across all Brazilian biomes. However, its conservation is increasingly threatened by growing interactions with domestic animals, especially dogs and cats, which create opportunities for pathogen transmission and disease emergence in wild populations. The aim of this study was to assess the exposure of Lontra longicaudis to domestic carnivores and to pathogens associated with these species within a protected area in southern Brazil. For this, we investigated the overlapping occurrence of otters, domestic dogs, and cats in the study area, as well as the exposure to major pathogens commonly associated with dogs (Canine Distemper Virus - CDV, Canine Parvovirus type 2 - CPV, and Neospora caninum) and cats (Toxoplasma gondii and Feline Leukemia Virus - FeLV) in Neotropical otters. Samples from six otters were analyzed. Most of them presented antibodies against CDV (83%), CPV (66%), and T. gondii (66%), while none were seropositive for N. caninum or reagent for FeLV antigen. Direct observation and camera trap monitoring allowed identification of overlapping records of otters, dogs and cats. Our results indicate that Neotropical otters, even in protected areas, are exposed to domestic carnivorans and their pathogens, increasing the likelihood of disease cycles with wildlife.
Introduction
The Neotropical otter (Lontra longicaudis) is a medium-sized mustelid with semiaquatic habits, inhabiting rivers, lagoons, lakes and small water bodies (de Almeida Rodrigues et al., 2013) across all Brazilian biomes (Rheingantz et al., 2014; Rosas-Ribeiro et al., 2017). It is considered a solitary species, despite females can be found with their offspring for several months (de Almeida Rodrigues et al., 2013). It is listed as a “Near Threatened” species at Red List from International Union for Conservation of Nature (IUCN), historically due to the fur trade and currently due to retaliation by fishers and fish farmers, pollution, cattle ranching, urban expansion, hydroelectric network, illegal trade as pets, and diseases (Rheingantz et al., 2022).
Nearly half of the mustelid species are experiencing population declines, with higher risks for some subfamilies, such as Lutrinae (Wright et al., 2022). The high rate of habitat degradation and the increasing proximity between wildlife and human-modified areas pose significant threats to the species conservation (Scanes, 2018). In this context, the exposure of wild animals to domestic dogs and cats have been shown significant impact on the decline of wild carnivore populations (Roelke-Parker et al., 1996). Beyond predation and direct competition, domestic animals may spread a wide range of pathogens to wildlife (Beineke et al., 2015). The detection of viral and protozoan pathogens originating from domestic animals in wild species has increased, and their effects have already been reported to cause disease and mortality in mustelids in various regions of the world (Kimber et al., 2000; De Bosschere et al., 2005; Echenique et al., 2018; Calvo-Mac et al., 2020; Thomas et al., 2020).
The aim of this study was to assess the exposure of Lontra longicaudis to domestic carnivores and pathogens associated with these species within a protected area in southern Brazil. To this end, we investigated the overlapping occurrence of otters, domestic dogs, and cats in the studied area, as well as the exposure to major pathogens commonly associated with dogs (Canine Distemper Virus - CDV, Canine Parvovirus type 2 - CPV, and Neospora caninum) and cats (Toxoplasma gondii and Feline Leukemia Virus - FeLV) in Neotropical otters.
Materials and methods
Study location and sample collection
Our study area was in the Brazilian National Ecological Station (ESEC) Taim (32° 44’ 33” S 52° 34’ 28” W), a conservation unit in southern Brazil included in the list of wetlands of international importance (Ramsar, 2025). The reserve comprises diverse ecosystems with 32,806 ha and is a critical area for conservation in the Pampa, the Brazilian biome with least protected areas and where many endangered species are found (Farias et al., 2023).
In order to investigate the occurrence of pathogens in L. longicaudis, otter samples were obtained through live trapping and carcass collection. Captures were conducted using Tomahawk wire-mesh cages baited with fresh fish and placed along the riverbank. After capture, the animal was anesthetized using a combination of dexmedetomidine (0.01 mg/Kg), ketamine (8 mg/Kg), and methadone (0.2 mg/Kg) and underwent a physical examination by a veterinarian. Blood and rectal swab samples were collected. The animal was identified individually with a subcutaneous microchip. After the full recovery the animal was released at the same site of the capture. All procedures were approved by the ethical committee (CEUA/UFRGS n° 44884) and Brazilian Environmental authorities (SISBIO license number 47357). Fresh carcasses were collected through opportunistic recovery of road-killed otters in the study area, followed by necropsy and biological material sample collection. Following collection, blood and swab samples were kept in a thermal box at 4–8°C for a maximum of 4 hours. Subsequently, whole blood, serum, and swabs were stored at −40°C. At the time of sampling, an aliquot of each sample was preserved in RNAlater. Laboratory analyses were conducted in up to six months after collection. Field expeditions were conducted in the summer-autumn of 2023.
To obtain samples from domestic carnivorans, ten farms around the reserve were visited. Blood samples and rectal swabs were collected with the prior authorization of the animal owners, through only physical restraint of the animals.
Serological tests (antibody detection)
The presence of antibodies against Toxoplasma gondii was investigated using the Indirect Hemagglutination (IHA) assay, with the multi-species commercial kit Toxotest® (Wiener Lab, Argentina). Samples with titers higher than 1:64 were considered positive for T. gondii. Antibody titers were determined by serial dilutions of the samples.
To detect antibodies against Neospora caninum, a competitive ELISA (cELISA) was performed using the ID Screen® Neospora caninum Multi-species Competition kit (IDVet, Grabels, France). For each sample, the sample-to-negative (S/N) ratio was calculated by dividing the optical density (OD) of the sample (S) by that of the negative control (N). According to the manufacturer’s guidelines, samples with an S/N value below 0.50 (inhibition/blocking above 50%) were positive. The absorbance was measured at 450 nm using a Biochrom EZ Read 2000 spectrophotometer.
The detection of antibodies against Canine Distemper Virus (CDV) and Canine Parvovirus type 2 (CPV) was performed using Enzyme-Linked Immunosorbent Assay (ELISA). Since multi-species ELISA kits for CDV and CPV are not commercially available in Brazil, we employed protein A-based ELISA for antibody detection in otters. This approach has been extensively used and validated for various wildlife species, including for CDV and CPV antibody detection (Muñoz-Hernández et al., 2023; Reck et al., 2025b). For this purpose, we used two indirect ELISA kits (CDV Ab ELISA, BT LAB, Shanghai, China; and CPV-2 ELISA, BT LAB, Shanghai, China), with a modification: the kit’s “anti-dog Peroxidase conjugated” was replaced with the “Protein A - Peroxidase conjugated” (Thermo Fisher, Camarillo, CA, USA) at a 1:10,000 dilution. All other steps followed the manufacturer’s instructions, using reagents supplied in the commercial kit. Serum samples from vaccinated and unvaccinated captive otters, provided by local zoos and rehabilitation centers, served as positive and negative controls for CDV and CPV-2 analyses. Optical density readings were taken at 450 nm using a spectrophotometer (Biochrom EZ Read 2000). The threshold for distinguishing positive from negative samples was based on a calculated cut-off point (mean + four standard deviations of values from negative controls) (Reck et al., 2025b).
Antigen analysis
Blood total samples from domestic cats and otters were tested for the presence of FeLV antigens using a lateral flow chromatographic immunoassay (LFA) with the commercial FIV Ac/FeLV Ag Test Kit (Alere Diagnóstico Veterinário Brasil, São Paulo, SP, Brazil), a branch of Abbott Laboratories (Chicago, IL, USA).
Molecular tests
For all molecular tests, DNA and RNA from biological samples (blood, rectal swab, and brain/spleen tissues in the case of carcasses) were obtained using PREP and PREAmp commercial kits (Newgene, Simbios Biotecnologia, Cachoeirinha, RS, Brazil).
Otter and dog samples were screened for the presence of CDV RNA by reverse transcription quantitative PCR (RT-qPCR) using a commercial kit (NewGene CDVAmp, Simbios Biotecnologia). Otter and dog samples were also investigated for the presence of CPV-2 DNA by RT-qPCR with a commercial kit (NewGene CPV2Amp, Simbios Biotecnologia, Cachoeirinha, RS, Brazil).
Blood samples from otters and domestic cats, or spleen tissues of otter carcasses were screened for the presence of FeLV proviral DNA by qPCR, as previously described by Diesel et al. (2024). All qPCR reactions were performed in a Step One Plus real-time PCR System Thermal Cycler (Applied Biosystems, Norwalk, Connecticut, USA).
Monitoring methods of the target species
Three species were defined as targets: otters, domestic dogs and cats. The linear transect methodology was used for the direct observation of otters, and domestic carnivorans, in the study area. Surveys were conducted from a vehicle traveling along the 16 km road that crosses the reserve, at an average speed of 10 to 20 km/h. The route covered several water bodies on both sides of the road.
Monitoring was carried out four times a day, always during the same periods, during daylight hours, over 10 consecutive days at the beginning sample collection. During each survey, observers recorded the location of otters, dogs, and cats. Individual otters were identified in the field through direct observation, using distinct morphological features such as body size, body shape, and visible scars or injuries. Identification was conducted by same observer during monitoring sessions, and when possible, photographic records were used to support and verify individual recognition.
With the aim to investigate the co-occurrence of otters and domestic animals, camera traps were placed near the 16 km transect route near water bodies and at sites with evidence of otter’s activity (feces and dens) and domestic animal presence. A total of 33 camera traps were deployed at intervals of 1 to 2 km on both sides of the road over a period of four months. Unfortunately, during the study almost half of the cameras were stolen or destroyed by poachers and illegal fishermen.
Spatial analysis
The location of each studied animal was recorded using a GPS device, and later plotted on maps using ArcGIS 10.5 geographic information system software (ESRI, Redlands, CA, USA). All maps were built using spatial layers from public databases of ESEC Taim. For Kernel analysis, the density interpolation tool within the Spatial Analyst extension was applied to convert otter’s location data into continuous surfaces representing the intensity of otter occurrence per square km. The kernel-density estimates were generated using a bandwidth of 2 km for the bivariate kernel-density function. The resulting heat maps enabled the identification of geographic hotspots for otter activity within the studied population.
Results
During biological sample collection, one adult male otter was live-captured during 110 trap-nights and five freshly road-killed carcasses (2 adult males and 3 adult females) were recovered over a six-month period.
Serological tests detected antibodies against T. gondii in four of the six otters sampled (66%). The antibody titer for T. gondii in otters ranged from 64 to 2048 (geometric mean 181). Using the competitive ELISA, all otters were seronegative for anti-Neospora caninum antibodies. Antibodies against Canine Distemper Virus (CDV) were detected in five of the six otters sampled (83%). Four of the six otters (66%) were considered seropositive for the presence of antibodies for Canine Parvovirus type 2 (CPV).
All domestic dogs and otters tested negative for the presence of CDV RNA and CPV DNA in molecular analyses using real-time PCR. Whereas all otters were negative for FeLV antigen and pro-viral DNA, two domestic cats sampled tested positive for FeLV in both real-time PCR and antigen tests. Table 1 summarizes data regarding pathogen investigation in the six sampled otters.
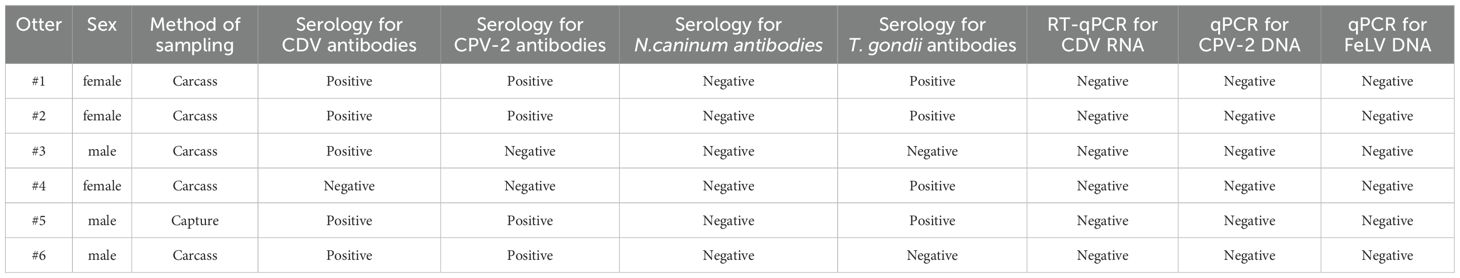
Table 1. Data about sampled otters and the results of the laboratorial tests for the detection of pathogens associated with domestic carnivorans and antibodies against them.
A total of 640 km was surveyed using the linear transect method, which allowed the identification of nine distinct otter individuals based on characteristics such as size, body shape and visible scars, resulting in a total of 22 direct observations. Eleven dogs and five cats were observed inside the reserve along the road that crosses the area. Other domestic species such as cows, horses and pigs were also commonly seen inside the protected area.
A total of 1,019 night-trap sessions were analyzed. Camera trap monitoring detected otters at three of the nineteen successfully monitored sites, with a total of nine recorded events (Table 2). Domestic cats were detected at three sites, totaling eleven events, with four distinct individuals identified. Domestic dogs were recorded at four sites, with a total of 27 detections events and 16 individuals identified. In some cases, dogs were recorded in the company of humans engaged in cattle and horses-related activities. Domestic cats were recorded only at night, while domestic dogs were registered in the day time, mainly at the early morning. Some camera traps recorded the presence of otters and domestic carnivores at the same site within the reserve (Figure 1). Table 2 outlines the data on all records of otters and domestic carnivorans in the studied area.
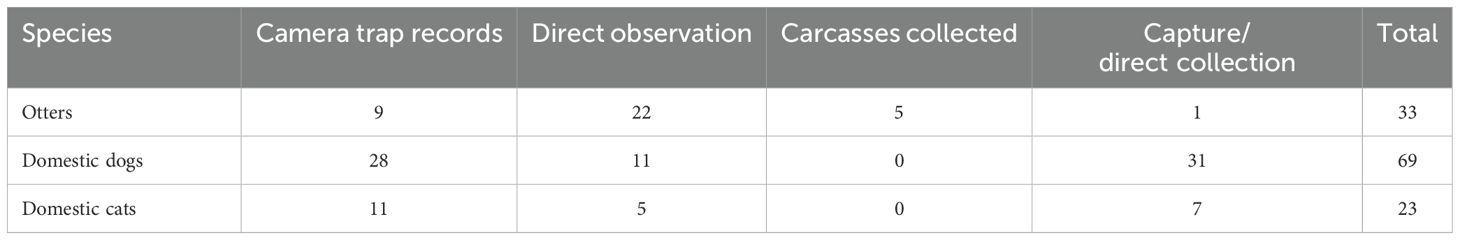
Table 2. Summary of records of otters and domestic carnivorans in the protected area studied according to different methods.

Figure 1. Compiled records of a camera trap in the study area. Twelve records of the target species (otters, domestic dogs and cats) were obtained at the same site. At the upper left side of each picture, the species common name was indicated.
The spatial distribution maps of otter, domestic dog, and domestic cat records, according to the methodologies employed, show a concentration pattern at the extremes of the reserve (Figure 2). Overlapping detection of otters and domestic carnivorans was observed at the same site in three of the camera traps (Figure 2A). Furthermore, two otter hotspots were evident through direct observation, reinforcing the overlap of domestic dogs and cats over the hotspots of otters (Figure 2B) Finally, Figure 2C showed the location of the collection sites.
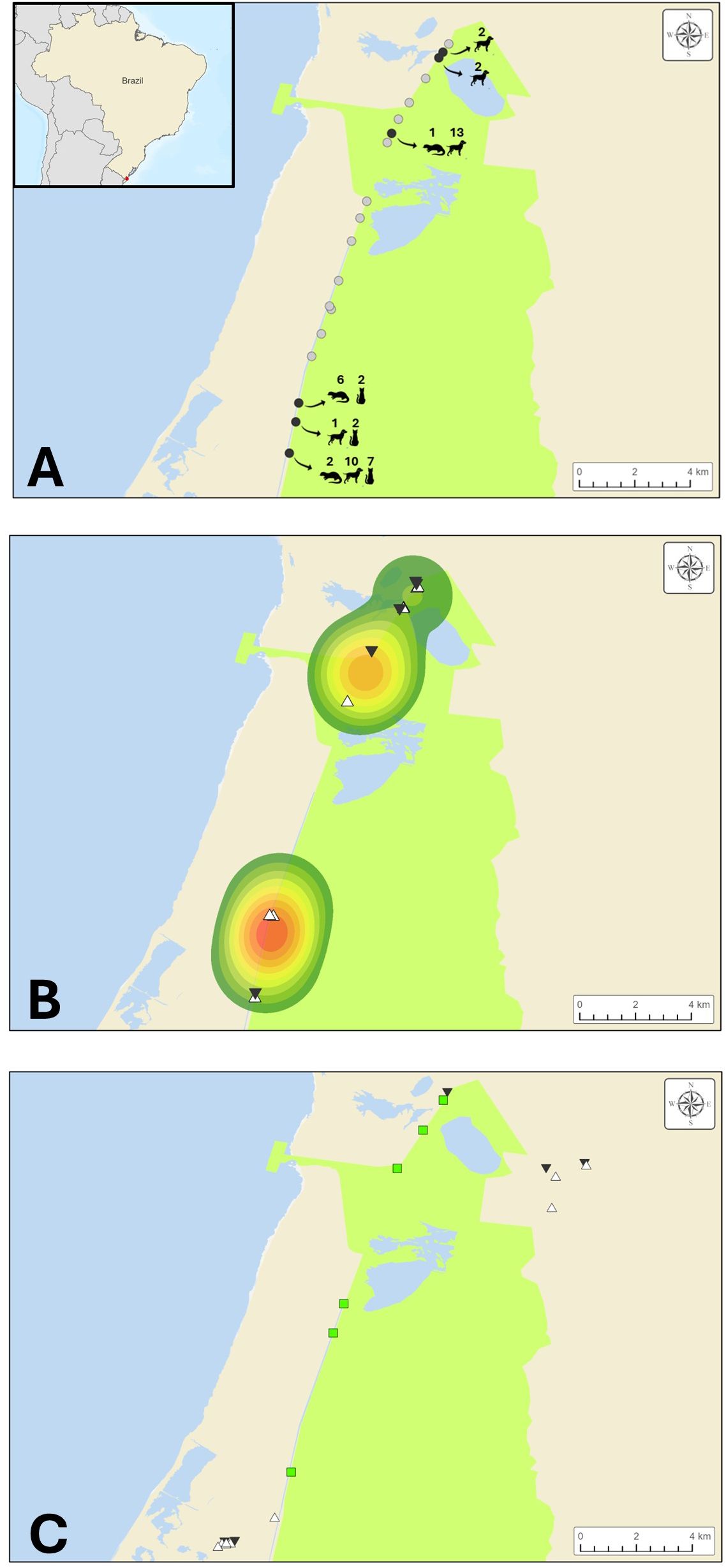
Figure 2. Set of maps representing the study area and location of records of otters, domestic dogs and cats. In all panels, Taim reserve was represented by the green area. Major water bodies were represented in blue. (A) locations of camera traps deployed across the study area. Each circle represents a camera trap location: grey circles indicate no detection of target species (otters, dogs, or cats), while black circles indicate detection of one or more target species. Species icons show which animals were recorded at each site, and the numbers above indicate the number of photographic events. Insert at the upper left side: location of the study area. Red dot on the Brazilian map represents the ESEC Taim reserve. (B) records obtained through linear transect surveys. Kernel density areas (hot colors) indicate regions with the highest frequency of otter sightings. White triangles represent locations where domestic dogs were observed, and black inverted triangles indicate domestic cat occurrences. (C) sites of biological sample collection. Green squares represent sampling sites for otters, white triangles indicate sites where domestic dog samples were collected, and black inverted triangles show where samples from domestic cats were obtained.
Discussion
We investigated the exposure of L. longicaudis to domestic carnivorans and their pathogens inside a protected area using different methodologies. This issue is an emerging topic for otter conservation worldwide (Thomas et al., 2020; Calvo-Mac et al., 2020).
Although the capture rate of otters may be considered lower to similar methodologies or studies using different trap types, the captured individual showed no injuries related to trapping, different to what is frequently reported for other types of traps (Rutter et al., 2020). Carcass collection method proved to be an effective approach for obtaining biological samples from L. longicaudis in this reserve, which might be a potential way to obtain samples in other regions where wetlands are intersected by roads. This finding aligns with previous studies reporting that several species, particularly semi-aquatic ones, are susceptible of roadkill in this area (Bager and Rosa, 2010).
Serological results showed that four out of six otters tested positive for antibodies against T. gondii, indicating past exposure to the pathogen. The high seroprevalence, combined with frequent cat detections (recorded through multiple methodologies) suggests widespread fecal contamination of aquatic ecosystems with oocysts. Toxoplasma gondii is a globally distributed zoonotic pathogen known to cause significant morbidity and mortality in both humans and animals (Hill et al., 2005). To the best of our knowledge, this is the first report of free-ranging L. longicaudis with positive serology for T. gondii; since the occurrence of this pathogen in Neotropical otters was only recorded previously in captive animals (Alvarado-Esquivel et al., 2013; Silva et al., 2018). However, T. gondii has been frequently reported in several otter species, such as marine otter (Lontra felina), Eurasian otter (Lutra lutra), and particularly in sea otters (Enhydra lutris) with lethal outbreaks (Miller et al., 2004, 2008; Conrad et al., 2005; Chadwick et al., 2013; Calvo-Mac et al., 2020; Dubey et al., 2020).
Although all otter tested negative in serological tests for N. caninum, the parasite is known to be widespread in areas where domestic dogs and cattle are present (Wouda et al., 1999), as supported by camera trap records in the reserve. Previous studies have also reported N. caninum in wild carnivores, including mustelids (Almería, 2013). Although N. caninum has already been detected in the brain of a free-ranging L. lutra (Stuart et al., 2013), its pathogenic potential and conservation risk for otters have not yet been elucidated. Therefore, continued investigation into the presence of this parasite in otter populations is needed.
The presence of antibodies against CDV and CPV, alongside with the absence of viral RNA/DNA in molecular analyses, suggests that the sampled otters were exposed to these viruses in the past but were not actively shedding them at the time of sampling. The high seroprevalence of these viruses, typically associated with domestic dogs, supports the hypothesis of widespread exposure among otters in this region. This is further corroborated by frequent dog sightings recorded during the study. It also suggests that non-lethal exposure to these viruses occurs in Neotropical otters. Both CDV and CPV have caused significant past outbreaks and mortality in wild animals, including reported cases in L. longicaudis and other otter species (Roelke-Parker et al., 1996; Kimber et al., 2000; Tamukai et al,. 2021; De Bosschere et al., 2005; Echenique et al., 2018; Reck et al., 2025b). Indeed, Gras et al. (2018) assessing the role of landscape structures in disease spreading among wildlife found that areas in direct proximity to water bodies were identified as high-risk areas for CDV transmission. It may have a significant impact for semi-aquatic wild animals, such as otters.
Negative qPCR results in domestic dogs do not necessarily indicate an absence of viral infection in dogs roaming in the reserve but suggest that, at the time of sampling, it seems that these animals were not shedding the virus. This is an expected result, as the animals appeared healthy. Notably, during visits to surrounding farms, most of residents stated that they did not regularly vaccinate their dogs and cats (data not shown). Thus, considering that there are several vaccines against CDV and CPV available, the vaccination of dogs and cats from households near to protected areas may be considered also as a critical component of conservation strategies for wild carnivores.
Reports of FeLV infection in free-ranging and captive wild felids have been documented worldwide; however, the virus was only classically associated with felid species (Sacristán et al., 2021; Guimaraes et al., 2009; Reck et al., 2025a). For Lontra canadensis (North American river otter), there was one documented case showing that an individual with clinical signs tested positive for FeLV antibodies, raising the possibility of broader host susceptibility among other carnivoran species (Dalton et al., 1989). Although all L. longicaudis individuals in the present study tested negative for the FeLV, two domestic cats were positive, by both molecular and antigen tests. It may be hypothesized that the potential involvement of another retrovirus leading to false-positive FeLV antibody results in the L. canadensis cannot be ruled out. This finding underscores the need for further studies on retroviral infections in otters.
Camera trap footage revealed a high frequency of domestic dogs and cats moving within the reserve, a protected area where the presence of such animals is prohibited by law (Brasil, 1998). Some of the dogs recorded by the cameras were also observed on nearby farms where domestic animal samples were collected, indicating that these were free-ranging owned animals capable of moving long distances, such as between farms and inside the reserve. The occurrence of dogs and cats in protected areas has been increasingly reported across Brazil, raising concerns due to their negative impacts on wildlife, including predation, competition, disease transmission, and behavioral disturbance (Lessa et al., 2016). Our findings support this concern, given the serological evidence of high exposure to canine-associated pathogens in otters.
Spatial analyses highlighted the overlap of otter habitat with areas frequented by domestic dogs and cats. Kernel data showed that areas with the highest frequency of otter sightings had a higher frequency of domestic animals. This overlap suggests that domestic dogs and cats are moving inside key areas used by otters. The presence of domestic animals at the edges of the reserve is likely associated with their closer proximity to human settlements. In the case of otters, their higher occurrence in these areas warrants further investigation. When considered together with the camera trap data and the serological findings showing a high number of otters with antibodies against pathogens commonly associated with domestic animals, it reinforces the potential risk of inter-species pathogen transmission. The abundance of domestic carnivores on nearby farms pointed out the potential risk of their incursion into protected areas, as demonstrated by the camera trap results.
Our results support the hypothesis that otter’s exposure to CDV, CPV and T. gondii may result from spillover events originating from dogs and cats that roam near or inside the reserve. Although at this time we cannot definitely prove this hypothesis, further studies may help to solve this issue by conducting long-term monitoring and pathogen genetic characterization in otters. Nevertheless, the results made clear that Neotropical otters, even in protected areas, are certainly exposed to domestic carnivorans and their pathogens. It also reinforces the need for more effective strategies to prevent the presence of domestic animals in protected areas. As a conservation strategy, collaborative efforts with local communities may begin by sharing the results of studies like this, followed by the implementation of free or low-cost vaccination and sterilization campaigns for domestic animals. Moreover, it highlights the potential of the Neotropical otter as a sentinel species for health risk assessment, contributing to the monitoring of pathogen spillover and overall environmental quality in conservation areas.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Comissão de Ética no Uso de Animais da Universidade Federal do Rio Grande do Sul (44884). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GG: Formal analysis, Data curation, Writing – review & editing, Conceptualization, Methodology, Writing – original draft, Investigation. JR: Formal analysis, Writing – review & editing, Methodology, Investigation, Funding acquisition, Resources. LM: Formal analysis, Methodology, Writing – review & editing. VS: Writing – review & editing, Formal analysis, Methodology. VL: Writing – review & editing, Formal analysis, Resources, Methodology, Data curation. CL: Resources, Writing – review & editing, Funding acquisition, Project administration, Supervision. TF: Writing – review & editing, Resources, Funding acquisition, Project administration, Conceptualization, Supervision, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We acknowledge the financial support from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Programa INOVA, Programa de Pesquisa em Saúde Única (Edital FAPERGS 13/2022 – REDE SAÚDE-RS), Edital FAPERGS 06/2024, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Edital INCT, Giant Otter Conservation Group (Projeto Ariranhas).
Acknowledgments
We would like to thank the staff of ESEC Taim for the support in the field expeditions, and to the farmers which allowed the collection of samples from domestic dogs and cats. We thank Luciana for kindly providing meals for the field team, and Luiz Carlos for his support in preparing the capture materials. We acknowledge the financial support from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Programa INOVA, Programa de Pesquisa em Saúde Única (Edital FAPERGS 13/2022 – REDE SAÚDE-RS), Edital FAPERGS 06/2024, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Edital INCT, Giant Otter Conservation Group (Projeto Ariranhas).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almería S. (2013). Neospora caninum and wildlife. Int. Sch. Res. Notices 2013, 947347. doi: 10.5402/2013/947347
Alvarado-Esquivel C., Gayosso-Dominguez E. A., Villena I., and Dubey J. P. (2013). Seroprevalence of Toxoplasma gondii infection in captive mammals in three zoos in Mexico City, Mexico. J. Zoo Wildl. Med. 44, 803–806. doi: 10.1638/2013-0032.1
Bager A. and Rosa C. A. D. (2010). Priority ranking of road sites for mitigating wildlife roadkill. Biota Neotrop. 10, 149–153. doi: 10.1590/S1676-06032010000400020
Beineke A., Baumgärtner W., and Wohlsein P. (2015). Cross-species transmission of canine distemper virus-an update. One Health. 1, 49–59.
Brasil (1998). Lei n° 9.605, de 12 de fevereiro de 1998. Dispõe sobre as sanções penais e administrativas derivadas de condutas e atividades lesivas ao meio ambiente (Brasília, DF: Diário Oficial da União).
Calvo-Mac C., Gutleb A. C., Contal S., Ilukewitsch V., Muñoz-Zanzi C., and Medina-Vogel G. (2020). Exposure to Toxoplasma gondii in marine otters (Lontra felina) and domestic cats (Felis catus) in an arid environment in Chile. J. Wildl. Dis. 56, 962–964. doi: 10.7589/2019-10-269
Chadwick E. A., Cable J., Chinchen A., Francis J., Guy E., Kean E. F., et al. (2013). Seroprevalence of Toxoplasma gondii in the Eurasian otter (Lutra lutra) in England and Wales. Parasites Vectors 6, 1–5. doi: 10.1186/1756-3305-6-75
Conrad P. A., Miller M. A., Kreuder C., James E. R., Mazet J., Dabritz H., et al. (2005). Transmission of Toxoplasma: clues from the study of sea otters as sentinels of Toxoplasma gondii flow into the marine environment. Int. J. Parasitol. 35, 1155–1168. doi: 10.1016/j.ijpara.2005.07.002
Dalton L. M., Hines R. S., and Wigdahl D. C. (1989). Feline Leukemia Virus in a North American river otter (Lutra canadensis). Int. Assoc. Aquat. Anim. Med. Available at: https://www.vin.com/apputil/content/defaultadv1.aspx?pId=11257&catId=32470&id=3863796.
de Almeida Rodrigues L., Leuchtenberger C., Kasper C., Junior O. C., and da Silva V. C. F. (2013). Avaliação do risco de extinção da lontra neotropical Lontra longicaudis (Olfers 1818) no Brasil. Biodivers. Bras. 3, 216–227. doi: 10.37002/biodiversidadebrasileira.v3i1.389
De Bosschere H., Roels S., Lemmens N., and Vanopdenbosch E. (2005). Canine distemper virus in Asian clawless otter (Aonyx cinereus) littermates in captivity. Vlaams Diergeneeskd. Tijdschr. 74, 0–0. doi: 10.21825/vdt.89088
Diesel L. P., de Mello L. S., de Oliveira Santana W., Ikuta N., Fonseca A. S. K., Kipper D., et al. (2024). Epidemiological insights into feline leukemia virus infections in an urban cat (Felis catus) population from Brazil. Anim. (Basel) 14, 1051. doi: 10.3390/ani14071051
Dubey J. P., Murata F. H. A., Cerqueira-Cézar C. K., Kwok O. C. H., and Grigg M. E. (2020). Recent epidemiologic and clinical importance of Toxoplasma gondii infections in marine mammals: 2009-2020. Vet Parasitol. 288, 109296.
Echenique J. V., Soares M. P., Mascarenhas C. S., Bandarra P. M., Quadros P., Driemeier D., et al. (2018). Lontra longicaudis infected with canine parvovirus and parasitized by Dioctophyma renale. Pesq. Vet. Bras. 38, 1844–1848. doi: 10.1590/1678-5150-pvb-5744
Farias F. M., Melo C. A. R. D., Pellegrini D. D. C. P., Hasenack H., and Scherer M. D. F. (2023). O cerne do Pampa: Conhecendo o mais austral dos biomas brasileiros. Ciec. e Cultura 75, 01–08. doi: 10.5935/2317-6660.20230049
Gras P., Knuth S., Börner K., Marescot L., Benhaiem S., Aue A., et al. (2018). Landscape structures affect risk of canine distemper in urban wildlife. Front. Ecol. Evol. 6, 136. doi: 10.3389/fevo.2018.00136
Guimaraes A. M., Brandão P. E., de Moraes W., Cubas Z. S., Santos L. C., Villarreal L. Y., et al. (2009). Survey of feline leukemia virus and feline coronaviruses in captive neotropical wild felids from Southern Brazil. J. Zoo Wildl. Med. 40, 360–364. doi: 10.1638/2008-0067.1
Hill D. E., Chirukandoth S., and Dubey J. P. (2005). Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 6, 41–61. doi: 10.1079/AHR2005100
Kimber K. R., Kollias G. V., and Dubovi E. J. (2000). Serologic survey of selected viral agents in recently captured wild North American river otters (Lontra canadensis). J. Zoo Wildl. Med. 31, 168–175. doi: 10.1638/1042-7260(2000)031[0168:SSOSVA]2.0.CO;2
Lessa I., Guimarães T. C. S., de Godoy Bergallo H., Cunha A., and Vieira E. M. (2016). Domestic dogs in protected areas: a threat to Brazilian mammals?. Natureza & Conservação, 14, 46–56.
Miller M., Conrad P., James E. R., Packham A., Toy-Choutka S., Murray M. J., et al. (2008). Transplacental toxoplasmosis in a wild southern sea otter (Enhydra lutris nereis). Vet. Parasitol. 153, 12–18. doi: 10.1016/j.vetpar.2008.01.015
Miller M. A., Grigg M. E., Kreuder C., James E. R., Melli A. C., Crosbie P. R., et al. (2004). An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. Int. J. Parasitol. 34, 275–284. doi: 10.1016/j.ijpara.2003.12.008
Muñoz-Hernández C., Wipf A., Ortega N., Barberá G. G., Salinas J., Gonzálvez M., et al. (2023). Serological and molecular survey of canine distemper virus in red foxes (Vulpes vulpes): Exploring cut-off values and the use of protein A in ELISA tests. Prev. Vet. Med. 221, 106075. doi: 10.1016/j.prevetmed.2023.106075
Ramsar (2025). The list of Wetlands of International Importance. Available online at: https://www.ramsar.org/sites/default/files/2023-08/sitelist.pdf (Accessed April 22, 2025).
Reck J., Gonchoroski G. Z., de Mello L. S., da Silveira V. P., Lunge V. R., Kasper C. B., et al. (2025a). Feline Leukemia Virus in Free-ranging Neotropical Wild Felids and in Domestic Cats Found Inside Protected Areas within Rio Grande do Sul, Brazil. J. Wildl. Dis. 61, 708–713. doi: 10.7589/JWD-D-24-00136
Reck J., Gonchoroski G. Z., Ogrzewalska M., Fonseca A. S. K., Ikuta N., Lunge V. R., et al. (2025b). Eco-epidemiological Investigation of a Disease Outbreak among Pampas Foxes (Lycalopex gymnocercus) from a Protected Area in Southern Brazil. J. Wildl. Dis. 61, 773–781. doi: 10.7589/JWD-D-25-00004
Rheingantz M. L., de Menezes J. F. S., and de Thoisy B. (2014). Defining Neotropical otter Lontra longicaudis distribution, conservation priorities and ecological frontiers. Trop. Conserv. Sci. 7, 214–229. doi: 10.1177/194008291400700204
Rheingantz M. L., Rosas-Ribeiro P., Gallo-Reynoso J., Fonseca da Silva V. C., Wallace R., Utreras V., et al. (2022). Lontra longicaudis (amended version of 2021 assessment). IUCN Red List Threatened Species 2022, e.T12304A219373698. doi: 10.2305/IUCN.UK.2022-2.RLTS.T12304A219373698.en
Roelke-Parker M. E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., et al. (1996). A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 379, 441–445. doi: 10.1038/379441a0
Rosas-Ribeiro P., Ranulpho R., and Venticinque E. (2017). New records and update on the geographic distribution of Lontra longicaudis (Olfers 1818) (Carnivora: Mustelidae) in Seasonally Dry Tropical Forests of northeastern Brazil. Check List 13, 1–8. doi: 10.15560/13.3.2108
Rutter A. U., Hanrahan A. T., Nielsen C. K., and Schauber E. M. (2020). Functionality of a new live-capture device for river otters. Journal of Fish and Wildlife Management, 11, 238–244.
Sacristán I., Acuña F., Aguilar E., García S., José López M., Cabello J., et al. (2021). Cross-species transmission of retroviruses among domestic and wild felids in human-occupied landscapes in Chile. Evol. Appl. 14, 1070–1082. doi: 10.1111/eva.13181
Scanes C. G. (2018). “Human activity and habitat loss: destruction, fragmentation, and degradation,” in Animals and Human Society (London, UK: Academic Press), 451–482.
Silva M. A., Pena H. F. J., Soares H. S., Aizawa J., Oliveira S., Alves B. F., et al. (2018). Isolation and genetic characterization of Toxoplasma gondii from free-ranging and captive birds and mammals in Pernambuco state, Brazil. Rev. Bras. Parasitol. Vet. 27, 481–487. doi: 10.1590/s1984-296120180059
Stuart P., Zintl A., De Waal T., Mulcahy G., Hawkins C., and Lawton C. (2013). Investigating the role of wild carnivores in the epidemiology of bovine neosporosis. Parasitology 140, 296–302.
Tamukai K., Minami S., Kadekaru S., Mitsui I., Maeda K., and Une Y. (2021). New canine parvovirus 2a infection in an imported Asian small-clawed otter (Aonyx cinereus) in Japan. J. Vet. Med. Sci. 83, 507–511. doi: 10.1292/jvms.20-0480
Thomas N., White C. L., Saliki J., Schuler K., Lynch D., Nielsen O., et al. (2020). Canine distemper virus in the sea otter (Enhydra lutris) population in Washington State, USA. J. Wildl. Dis. 56, 873–883. doi: 10.7589/JWD-D-19-00008
Wouda W., Dijkstra T., Kramer A. M. H., Van Maanen C., and Brinkhof J. M. A. (1999). Seroepidemiological evidence for a relationship between Neospora caninum infections in dogs and cattle. Int. J. Parasitol. 29, 1677–1682. doi: 10.1016/S0020-7519(99)00105-8
Keywords: mustelid, neotropical, otter, dog, cat, distemper, toxoplasmosis, disease
Citation: Gonchoroski GZ, Reck J, Mello LS, Silveira VP, Lunge VR, Leuchtenberger C and Freitas TRO (2025) Exposure of free-living Lontra longicaudis (Neotropical otter) to domestic carnivores and their associated pathogens in a protected area in Brazil. Front. Mamm. Sci. 4:1634247. doi: 10.3389/fmamm.2025.1634247
Received: 23 May 2025; Accepted: 02 July 2025;
Published: 23 July 2025.
Edited by:
Thomas S. Jung, Government of Yukon, CanadaReviewed by:
Juan Pablo Gallo, Council of Science and Technology (CONACYT), MexicoM. Fabiola Corona-Figueroa, Universidad de San Carlos de Guatemala, Guatemala
Copyright © 2025 Gonchoroski, Reck, Mello, Silveira, Lunge, Leuchtenberger and Freitas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Greice Z. Gonchoroski, Z3JlaWNlZ29uY2hvcm9za2l6QGdtYWlsLmNvbQ==
 Greice Z. Gonchoroski
Greice Z. Gonchoroski Jose Reck
Jose Reck Lauren S. Mello5
Lauren S. Mello5 Vagner R. Lunge
Vagner R. Lunge Caroline Leuchtenberger
Caroline Leuchtenberger Thales R. O. Freitas
Thales R. O. Freitas