- 1Australian Research Centre for Human Evolution, Griffith University, Brisbane, Australia
- 2Max Planck Institute of Geoanthropology, Jena, Germany
- 3The Interdisciplinary Center for Archaeology and Evolution of Human Behaviour (ICArEHB), Universidade do Algarve, Faro, Portugal
- 4Department of Coevolution of Land Use and Urbanisation, Max Planck Institute of Geoanthropology, Jena, Germany
- 5Institute of Prehistoric Archaeology, University of Cologne, Cologne, Germany
- 6School of Social Sciences, University of Queensland, Brisbane, Australia
- 7School of Environment and Science, Griffith University, Brisbane, Australia
- 8Department of Classics and Archaeology, University of Malta, Msida, Malta
With its origins in the late 18th and early 19th century, the question of what drove the late Quaternary megafauna extinctions remains one of science’s most enduring and hotly contested debates. Once strictly the domain of archaeologists and paleontologists, the topic has attracted growing interest from other disciplines in recent decades, particularly ecologists and conservation biologists, who view these extinctions as a lens through which to inform contemporary conservation and ecosystem management strategies. Alongside this expansion, the field has seen increasing use of advanced analytical and statistical methods. Yet despite these developments, scientific opinion remains deeply divided over the cause(s) of these extinctions. Each year dozens of papers on the topic are published and along with these review articles that cover the debate or certain aspects of it. However, these reviews tend to reflect the viewpoints of their authors. Recognizing this limitation, the present study aimed to offer a more objective, data-driven overview of the field by conducting a systematic review and analysis of the literature. Specifically, we sought to: (1) trace the development of the megafauna extinction debate to understand how it has evolved over time; (2) identify key thematic and conceptual foci within the literature; and (3) use this synthesis of historical trends and interdisciplinary variation to propose a forward-looking research agenda that encourages greater engagement, discussion, integration, and collaboration across fields. Our analysis reveals strong disciplinary divides, uneven temporal and spatial research coverage, and persistent uncertainty over extinction causes. Despite recent major methodological advances, the field remains fragmented, underscoring the need for a research agenda that fosters interdisciplinary collaboration, expands field and legacy studies, as well as species-specific approaches, and integrates cutting-edge scientific and statistical techniques.
Introduction
The idea that a species could completely disappear from the Earth was first proposed by naturalists in the late 18th and early 19th centuries (for a review on 19th century research into megafauna extinctions see Grayson, 1989), prompting initial efforts to explain the extinction of large mammals like mammoths, mastodons, and giant ground sloths. Early theories included catastrophic events—such as the Biblical flood (Parkinson, 1811; Buckland, 1823) and sudden glaciation (Agassiz, 1837)—as well as more gradual, climate-driven extinctions (Lyell, 1832; Dawkins, 1869). Others rejected climate-based explanations altogether, attributing megafauna losses to intensive overhunting by humans (Turner, 1799; Flemming, 1826). By the late 19th century, the debate had stalled, entering what Monjeau et al., (2017, p. 200) describe as a “phase of conceptual blockage,” as scholars increasingly favored gradual drivers for extinction, whether climatic or anthropogenic, but lacked the precise absolute dating techniques and other advanced scientific methods needed to rigorously test different hypotheses.
The debate was eventually revitalized in the mid 20th century with the development and application of radiocarbon dating (Libby, 1952). In the mid-1960’s, Martin (1966, 1967), drawing on radiocarbon-dated fossils and better-resolved paleoenvironmental data, proposed his influential “overkill” model. Initially applied to North America and later to other continents as well as numerous islands, this hypothesis argued that humans armed with specialized hunting technologies quickly drove many megafauna species to extinction as they spread around the globe. Martin’s work sparked widespread debate (Leakey, 1966, 1967; Martin, 1967; Martin and Wright, 1967; Martin and Klein, 1984), laying the foundation for the modern era of megafauna extinction research. As in the 19th century, most researchers today still fall into one of three broad groups: those attributing the extinctions to climate change, those advocating for human-mediated impacts, and those emphasizing some combination of the two.
The turn of the millennia ushered in a new wave of research, driven in large part by advances in scientific techniques and analytical approaches (Swift et al., 2019). Improvements in radiocarbon dating and the integration of Bayesian modelling have led to significantly refined chronologies (Stuiver et al., 1998; Higham et al., 2006; Jacobi and Higham, 2009), while innovations in luminescence and other radiometric dating techniques extended timelines beyond the limits of reliable 14C dating, ~50 ka (Roberts et al., 2001; Turney et al., 2008; Price et al., 2011). The integration of ancient DNA (aDNA)—now retrievable from both fossils and sediments, and increasingly older materials (van der Valk et al., 2021; Kjær et al., 2022)—has opened new windows into megafauna population dynamics, adaptations, and extinction chronologies (Shapiro et al., 2004; Campos et al., 2010; Lorenzen et al., 2011; Collins et al., 2014; Seersholm et al., 2020; Murchie et al., 2021). Stable isotope analysis has likewise improved and expanded, offering insights into past environments and extinct species’ diets and mobility (Bocherens et al., 2017; Price et al., 2017, 2017; Wooller et al., 2021; McCormack et al., 2022; Koutamanis et al., 2023). Complementing these advances, the rise of open-access databases has enabled large-scale meta-analyses that integrate paleoclimate data, extinction timelines, and archaeological evidence to assess drivers of megafauna extinctions at continental and global scales (Sandom et al., 2014a; Saltré et al., 2016; Berti and Svenning, 2020; Stewart et al., 2021).
These and other advances have coincided with—and sometimes been driven by—a broadening interest in late Quaternary megafauna extinctions. Once largely the pursuit of paleontologists and archaeologists, these extinctions are now studied by researchers working across a range of disciplines. Much new work has focused on understanding the knock-on ecological effects of megafauna extinctions (Johnson, 2009; Gill, 2014; Galetti et al., 2018; Svenning et al., 2024) as well as the potential role of rewilding programs in mitigating these effects (Svenning et al., 2016; Cromsigt et al., 2018; Lundgren et al., 2020). Ultimately, this growing interest reflects scholarly attention toward understanding the deep-time origins and extent of human environmental impacts, aiming to apply these insights to contemporary conservation and restoration efforts (Boivin et al., 2016; Stephens et al., 2019; Ellis et al., 2021). As put by Nagaoka et al., (2018, p. 9684), not only is the subject of the late Quaternary megafauna extinctions used in “defining the boundaries of the Anthropocene and other concepts such as the Sixth Extinction” it is also at the very core of “understanding the nature of the relationship between humans and the environment.” For some scholars, the notion of an abrupt wave of extinction is cited as one thread of evidence for a fundamental shift in human behavior, one that marks the emergence of ‘modern humans’ and sets them apart from their ‘archaic hominin’ predecessors (e.g., Marean, 2015). Others, however, have suggested that human-mediated extinctions may pre-date the emergence of Homo sapiens, pushing back the ecological impacts of our own genus (Faurby et al., 2020; Hauffe et al., 2024; Pereira et al., 2024).
An interesting feature of the modern debate are the stark disciplinary differences in how researchers conceptualize and study megafauna extinctions (Grayson and Meltzer, 2003; Nagaoka, 2012; Nagaoka et al., 2018). A 2018 survey found that archeologists and paleobiologists largely view the cause of extinctions as unresolved, often pointing to a combination of climatic and anthropogenic factors (Nagaoka et al., 2018). In contrast, ecologists tend to consider humans, through overhunting and habitat modification, as the primary driver of extinctions. This divergence highlights the unresolved nature of the megafauna extinction debate, as well as the contrasting views that emerge from diverse disciplinary datasets, preconceptions, and theoretical frameworks. It also underscores the need for greater interdisciplinary engagement, particularly between ecologists and those working in deeper time (Louys et al., 2012; Swanson et al., 2021; Azevedo-Schmidt et al., 2025).
To gain a better understanding of the late Quaternary megafauna extinction debate—its history, evolution, and status—we conducted a quantitative systematic review and analysis of the scientific literature published since the 1950’s. While the debate has been extensively explored through review articles (Barnosky et al., 2004; Wroe and Field, 2006; Louys et al., 2007; Stuart, 2015; Galetti et al., 2018), edited volumes (Martin and Klein, 1984), and popular science books (Kolbert, 2014; MacPhee and Schouten, 2019; Edmeades, 2021; Stuart, 2021), these works tend to reflect the specific viewpoints and perspectives of their authors on what remains a highly contentious topic. Indeed, it has been noted that within the megafauna extinction debate, scholars often advocate strongly for a specific extinction hypothesis while insufficiently engaging with alternative interpretations or the broader body of research (Nagaoka et al., 2018). Recognizing this limitation, the present study sought to provide an objective, data-driven overview of the field. Specifically, we aimed to: (1) trace the development of the megafauna extinction debate to understand how it has evolved over time; (2) identify key thematic and conceptual foci within the literature; and (3) use this synthesis of historical trends and interdisciplinary variation to propose a forward-looking research agenda that encourages greater engagement, discussion, integration, and collaboration across fields.
Methods
To conduct the systematic review, we queried two major academic databases, Web of Science (WoS) and Scopus (accessed November 2021), for articles related to the late Quaternary megafauna extinctions. Terms were searched for within keywords, titles, and abstracts of papers using the following query: “Megafauna” AND “extinction” AND (“Quaternary” OR “Pleistocene” OR “Holocene”). This search returned 596 unique articles published between 1959 and 2021. The metadata from all retrieved articles was compiled into an Excel spreadsheet, the articles randomized, and evenly distributed among three analysts (MS, CP, MZ) for review.
Each article was imported into and coded using MAXQDA (https://www.maxqda.com/), a qualitative data analysis software that enables text highlighting, tagging, and coding. Using these functions, we coded text in each article to answer ten pre-defined questions aimed at capturing how the late Quaternary megafauna extinctions have been conceptualized and studied through time and across different disciplines (Table 1). Articles falling outside the scope of the study were removed based on the exclusion criteria:
1. Article must relate to the Quaternary period (2.6 Ma to present).
2. Article must relate to the extinction of vertebrates.
3. Article must relate to the extinction of megafauna, however defined.
4. Article must focus on megafauna extinctions, or the findings must be related to the extinction of megafauna in some significant way.
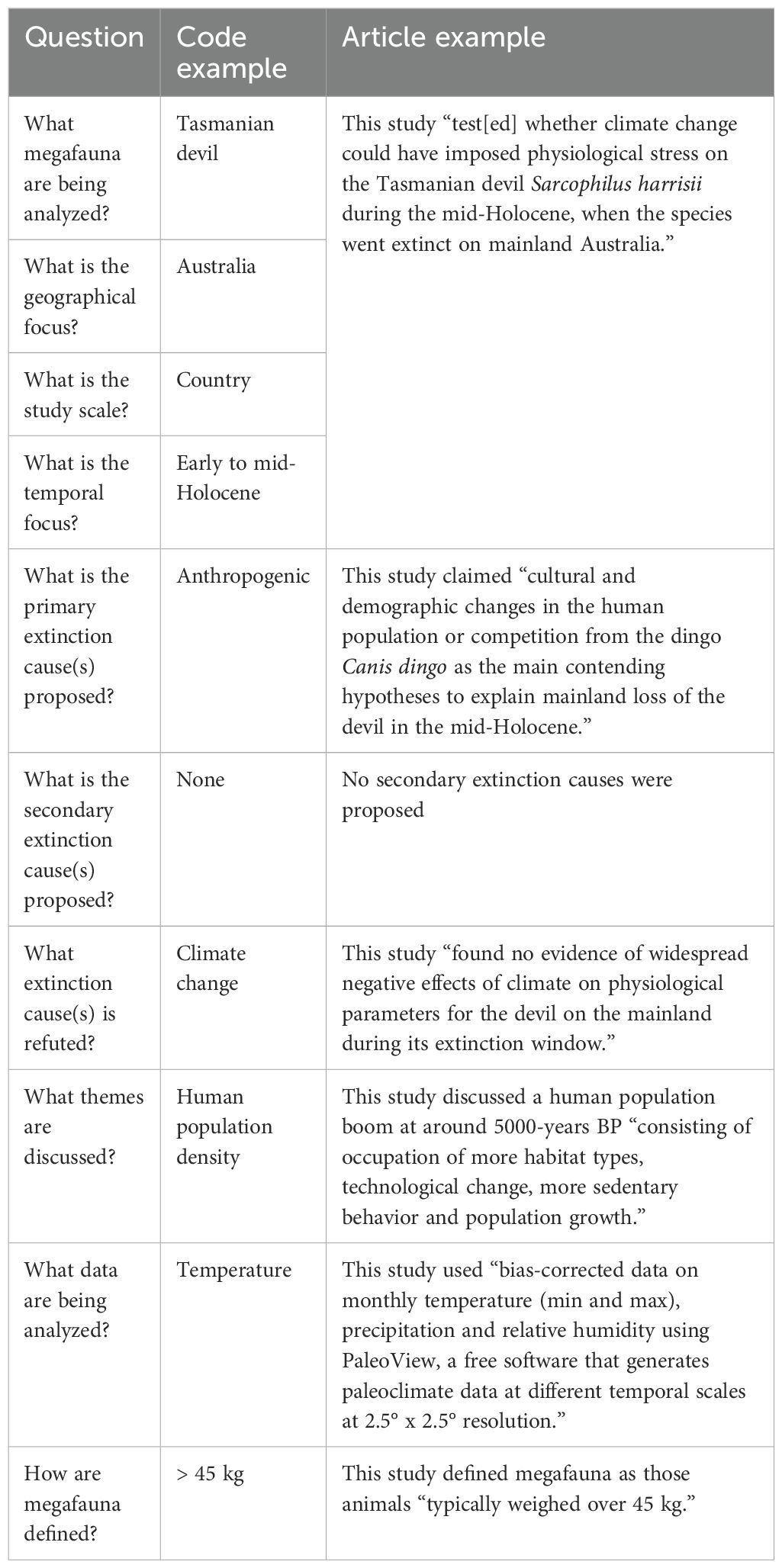
Table 1. The ten questions asked of each article and using Morris et al. (2022) as an example.
Firstly, however, to ensure inter-analyst consistency in data extraction and analysis, a series of “training sets” was conducted. Each analyst was assigned the same set of 20 randomly selected papers from the database. Using the exclusion criteria, they independently screened the papers and coded those deemed relevant until ten papers had been analyzed. This training process was repeated twice more, with new sets of papers each time. After each round, results were compared and discussed to refine the coding approach and improve consistency in paper selection. By the third training set, all analysts consistently selected the same ten papers, demonstrating inter-analyst alignment.
After completing the training set, the analysts independently analyzed 100 articles each, for a total of 300 articles. Given that the initial literature search was conducted in November 2021, it was likely it may not be capturing the most recent trends in the field. To address this imbalance, we conducted a follow-up literature search in WoS using the same search terms but this time focusing on the years 2022–2024. This search yielded an additional 125 articles. From these, 60 articles were analyzed as before, resulting in a total of 360 papers. A full list of the articles included in this study can be found in Appendix 1 (also available at https://github.com/wccarleton/mfreview/blob/main/data/mf_review_papers_final.xlsx).
VOSviewer (version 1.6.20) was used to conduct a series of network cluster analyses based on co-authorship, citations, and keyword co-occurrence using both author and WoS keywords. We also performed a basic hierarchical clustering analysis of our coded themes using MAXQDA’s in-built Code Map tool, which groups codes into clusters based on how similarly (i.e., co-occurrence in documents) codes have been applied in the data. Clusters were generated using the “map position” setting, meaning their arrangement reflects their spatial projection in two-dimensional space rather than from their exact calculated distances. To assess the through-time trend in publication volume, we divided the number of articles published each year in our sample by the total number of articles published each year in the five most common journals in our dataset (data sourced from Scopus).
Following this, we conducted a more formal quantitative analysis combining bibliometric data with codes derived from the literature. Using the NetworkX package in Python, we constructed a directed citation network where each paper is represented by a node and citations by edges. In-degree (the number of edges coming to a node) served as a proxy for internal citation counts, which we validated against broader citation metrics to assess the fidelity of our literature sample. We applied the Leiden network clustering algorithm to automatically determine the number and composition of clusters. These clusters were then quantitatively assessed in relation to three variables:
1. Discipline—whether clusters aligned with disciplinary categories assigned to the relevant papers. Categories were determined based on WoS Subject Categories and journal focus and, where ambiguous, by consulting the article title and abstract.
2. Extinction Cause—whether clusters aligned with the main extinction cause promoted by the relevant papers.
3. Co-authorship—whether clusters were shaped by authors citing close collaborators, leading to overlap between citation and authorship networks.
Finally, we modelled the relationship between the three variables and citation network cluster assignments using a Bayesian logit-softmax model, allowing us to quantify the contribution of each variable. To avoid excessive complexity, we used only the modal co-authorship clusters (i.e., the most frequent cluster represented among the authors of a given paper) as a potential explanatory variable for each paper. While this simplification does not capture all possible co-authorship affiliations, it provides a tractable way to test whether predominant membership in a single co-authorship cluster increases the likelihood of a paper being assigned to a specific citation network cluster.
Results
The literature search returned 1,107 articles (Figure 1). After removing 386 duplicates, we screened 515 records, of which 360 met our inclusion criteria and were assessed. The final sample spans publications from 1959 to 2024, covers 112 journals, includes 1,783 keywords, and contributions from 1,445 authors. The articles reveal a clear growing interest in late Quaternary megafauna extinctions, with a marked surge in publications around the turn of the millennium (Figure 2C).
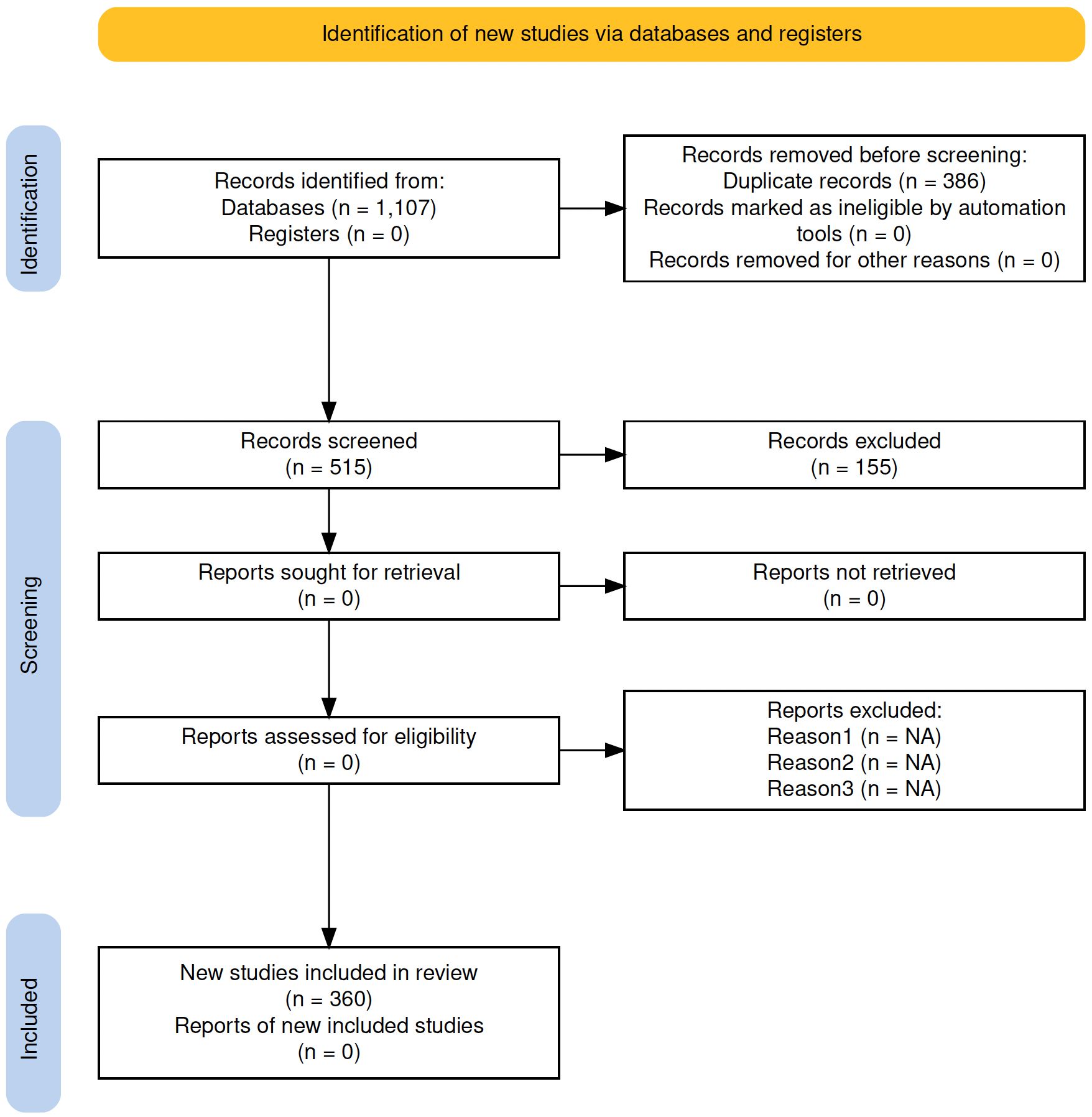
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart (following Moher et al., 2009).
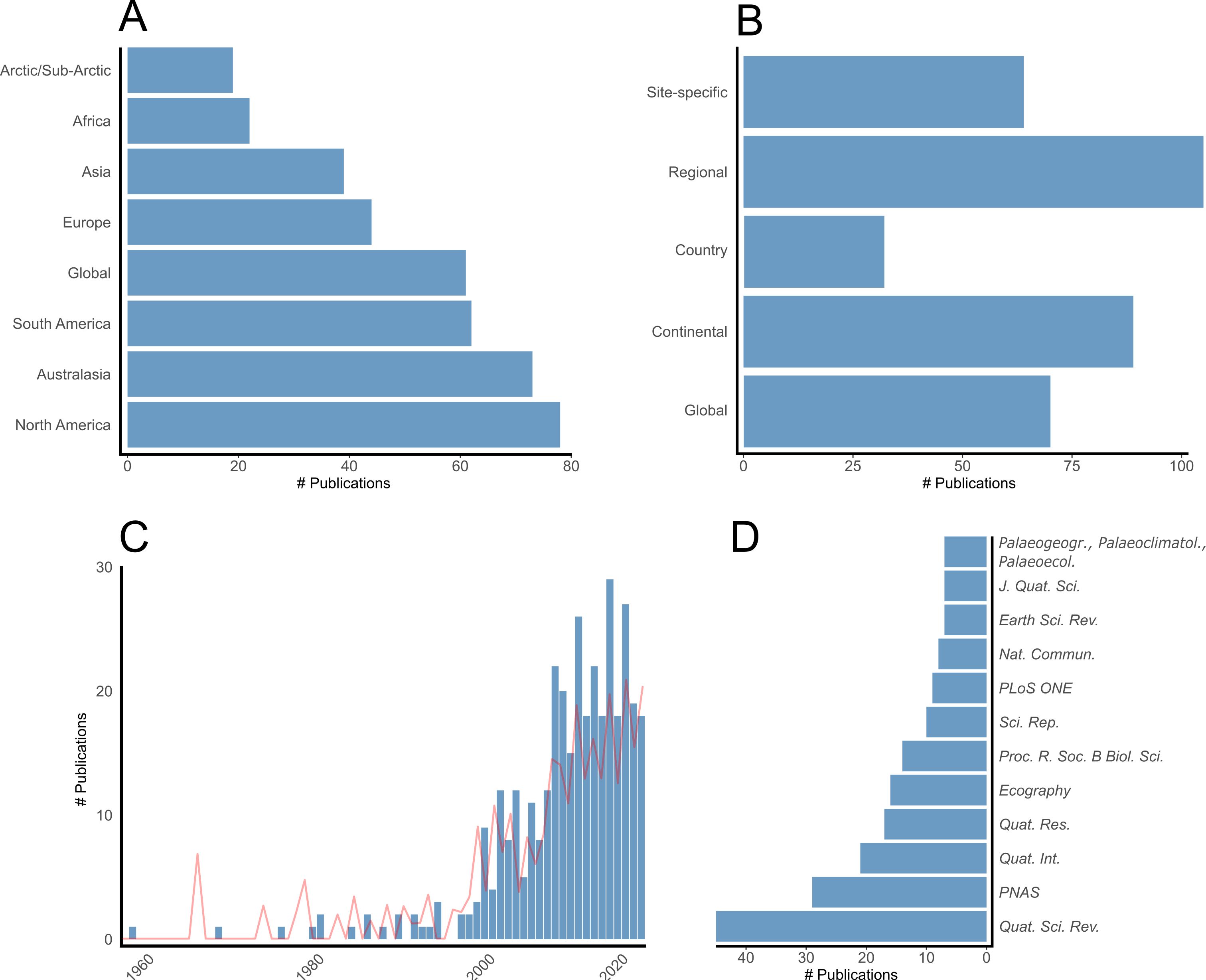
Figure 2. Number of publications by (A) regional focus, (B) study scale, (C) publication year, and (D) journal. Note the pale red line in (C) is number of publications per year corrected for overall publication volume.
Keyword co-occurrence, citation, and co-authorship network
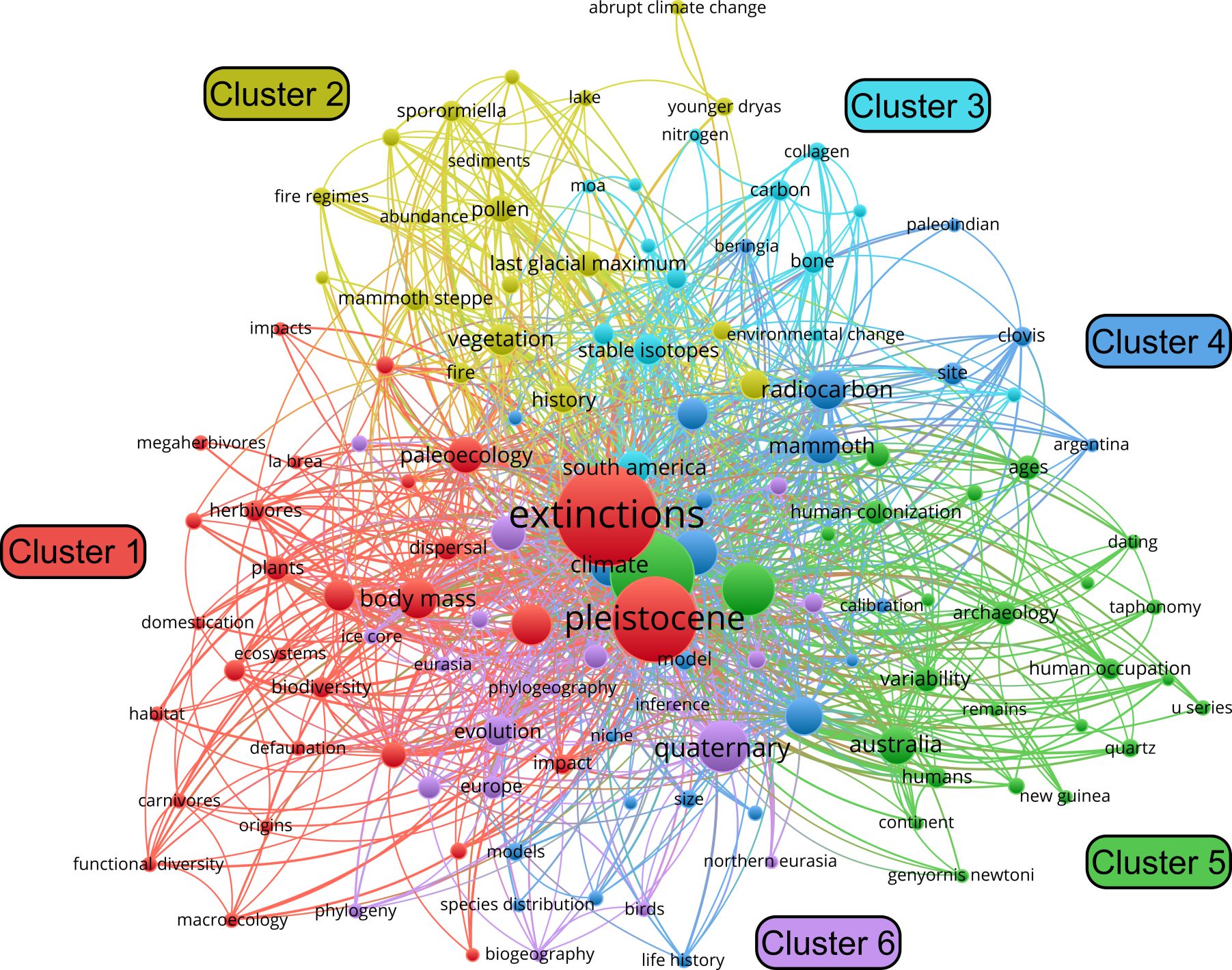
Keywords were drawn from both author-assigned and WoS entries and filtered to include only those appearing at least five times. Of the 1,783 keywords, 130 met this criterion and were grouped into six clusters (Figure 3). Ignoring terms used in the initial search query, the five most commonly occurring keywords were climate change (n = 62), mammals (n = 40), climate (n = 38), paleoecology (n = 31), radiocarbon (n = 35), and body mass (n = 35). More recently emerging keywords include terms common in ecology and conservation biology, such as biodiversity (n = 12), functional diversity (n = 6), communities (n = 6), conservation (n = 17), and consequences (n = 12) (Figure 4).
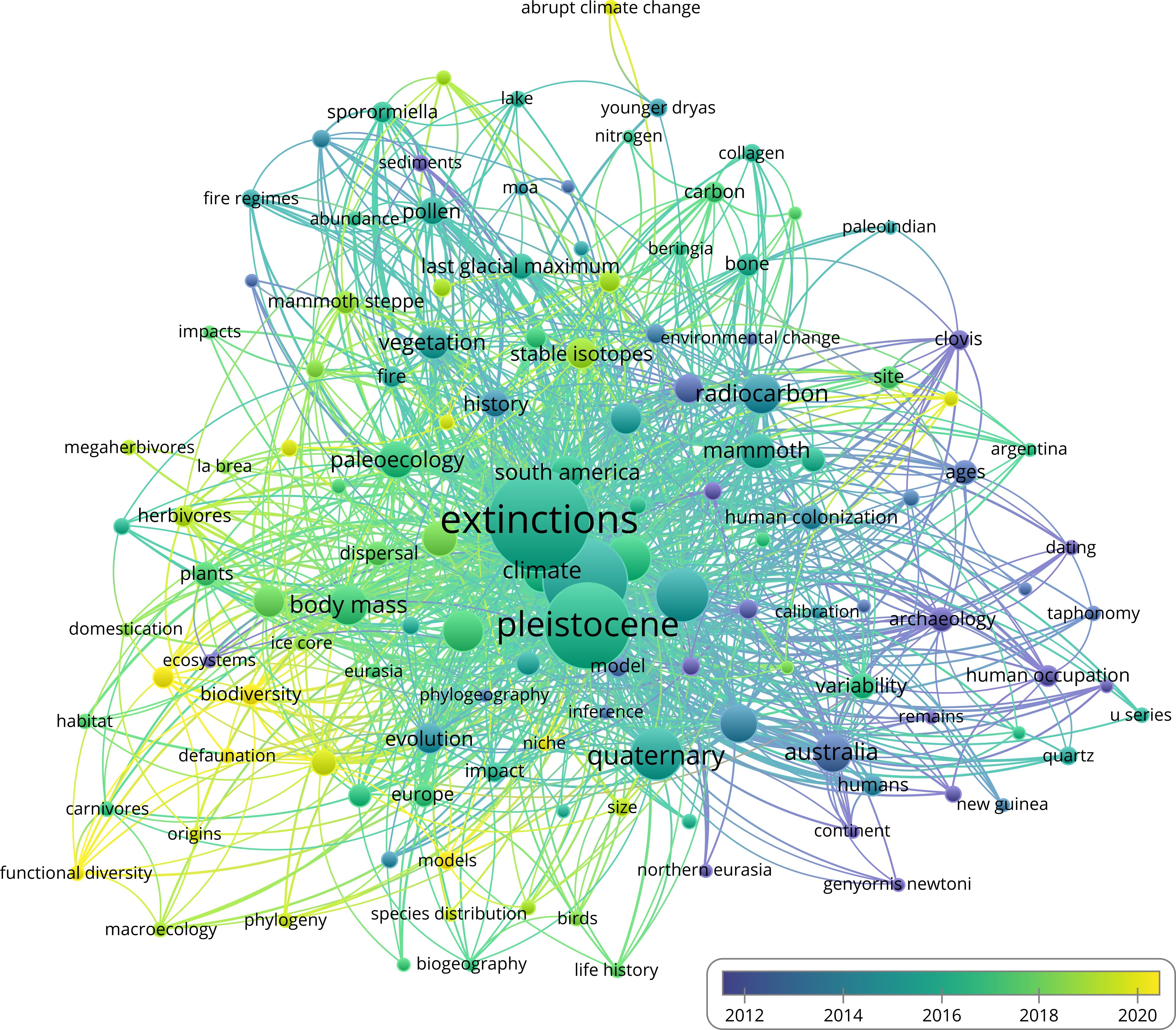
Figure 4. Keyword co-occurrence overlay visualization. Lighter colors denote more recent average years (created using VOSviewer).
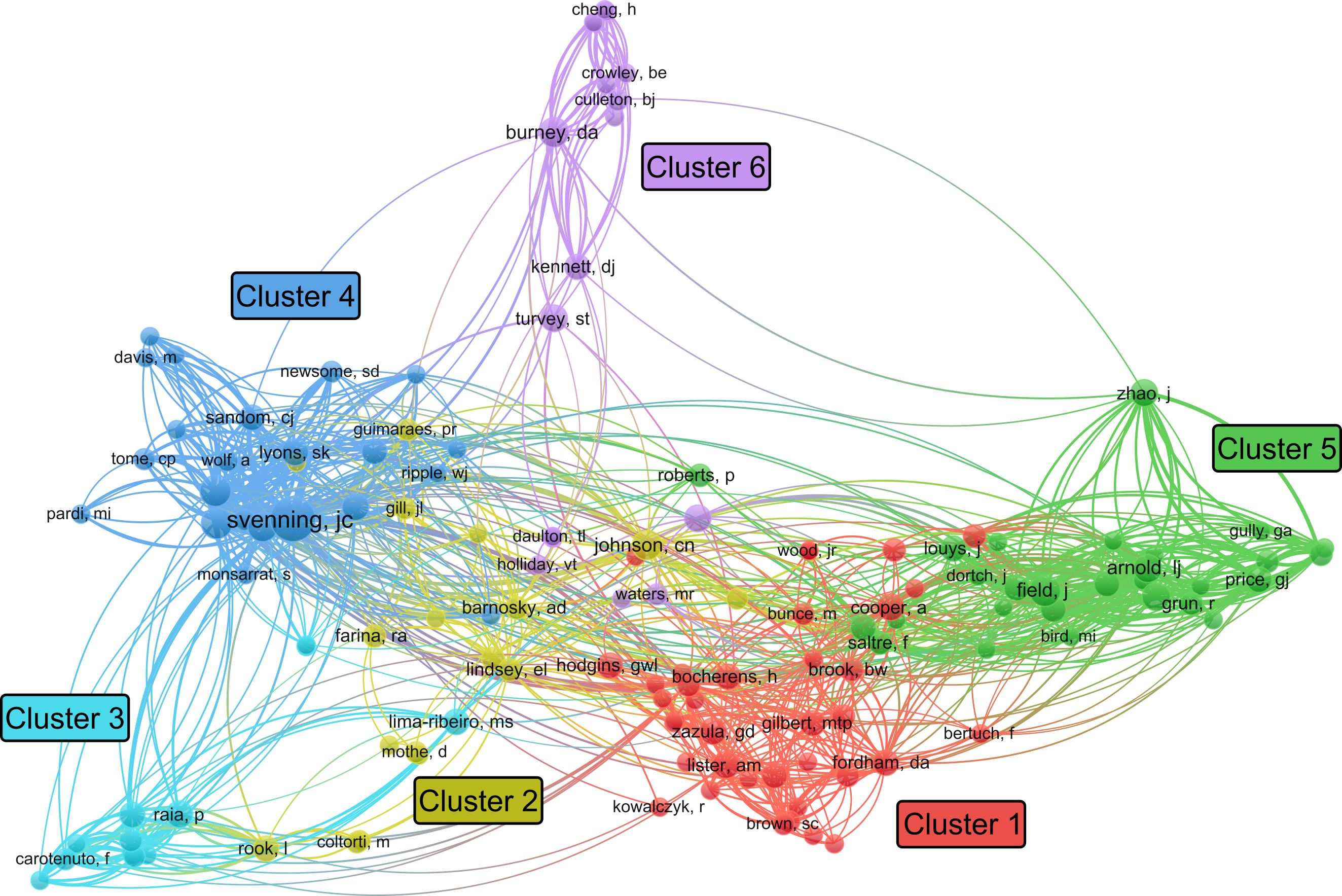
For the author citation network analysis, a threshold of three or more publications per author was applied, resulting in 134 individuals which grouped into six clusters (Figure 5). These clusters broadly align with both research discipline and geographic focus. For instance, cluster 4 consists mostly of ecologists, while clusters 1, 3, and 5 are largely researchers working in Eurasia, Australia, and Madagascar, respectively. Ecologists are responsible for a greater number of articles in recent years, indicated by the brighter colors in Figure 6. A further analysis of the gender of the authors, indicates that 80% (n = 107) of the 134 authors are male, pointing to a strong gender bias within the scientific leadership of megafaunal extinction studies.
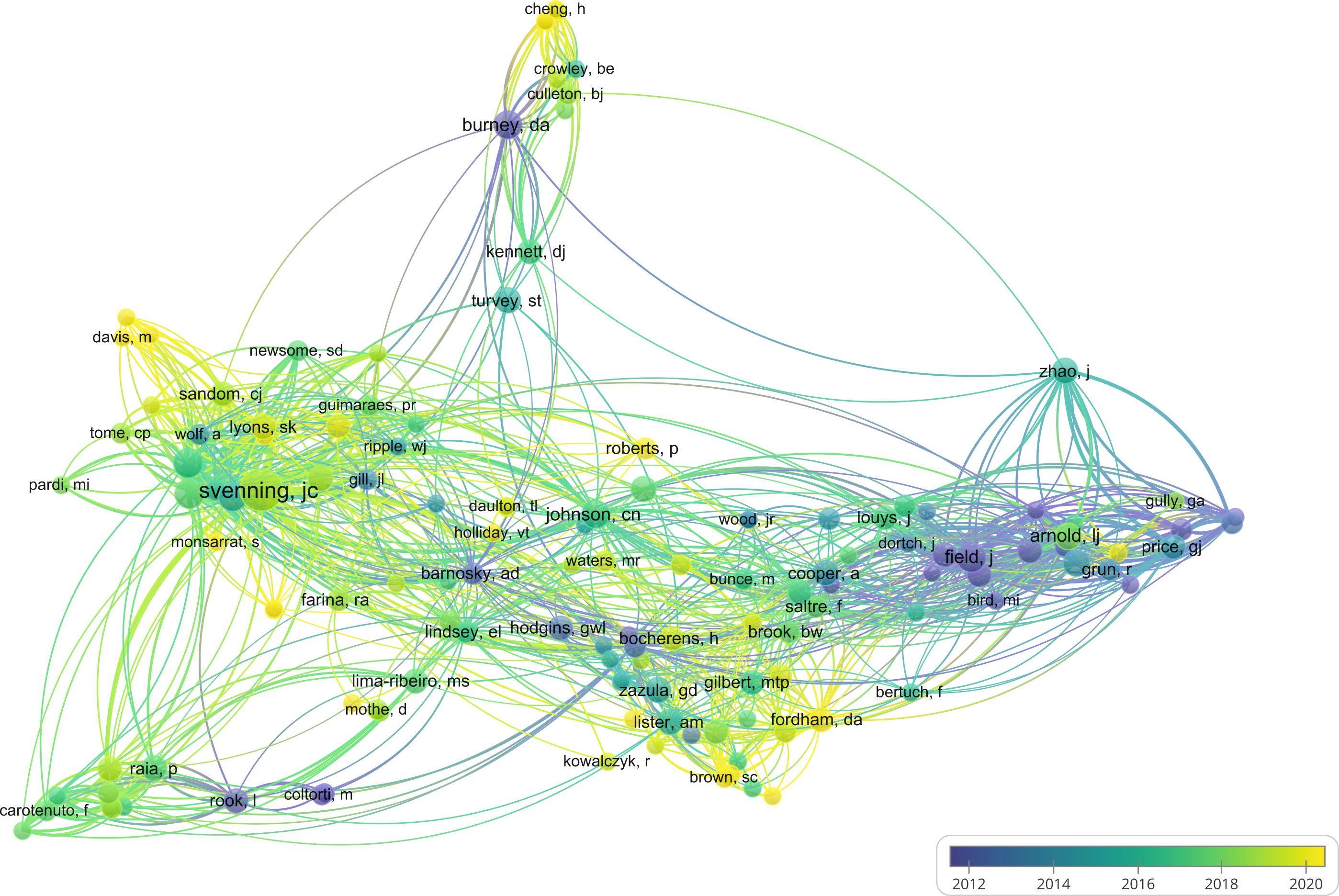
Figure 6. Author citation overlay visualization. Lighter colors denote more recent average publication years (created using VOSviewer).
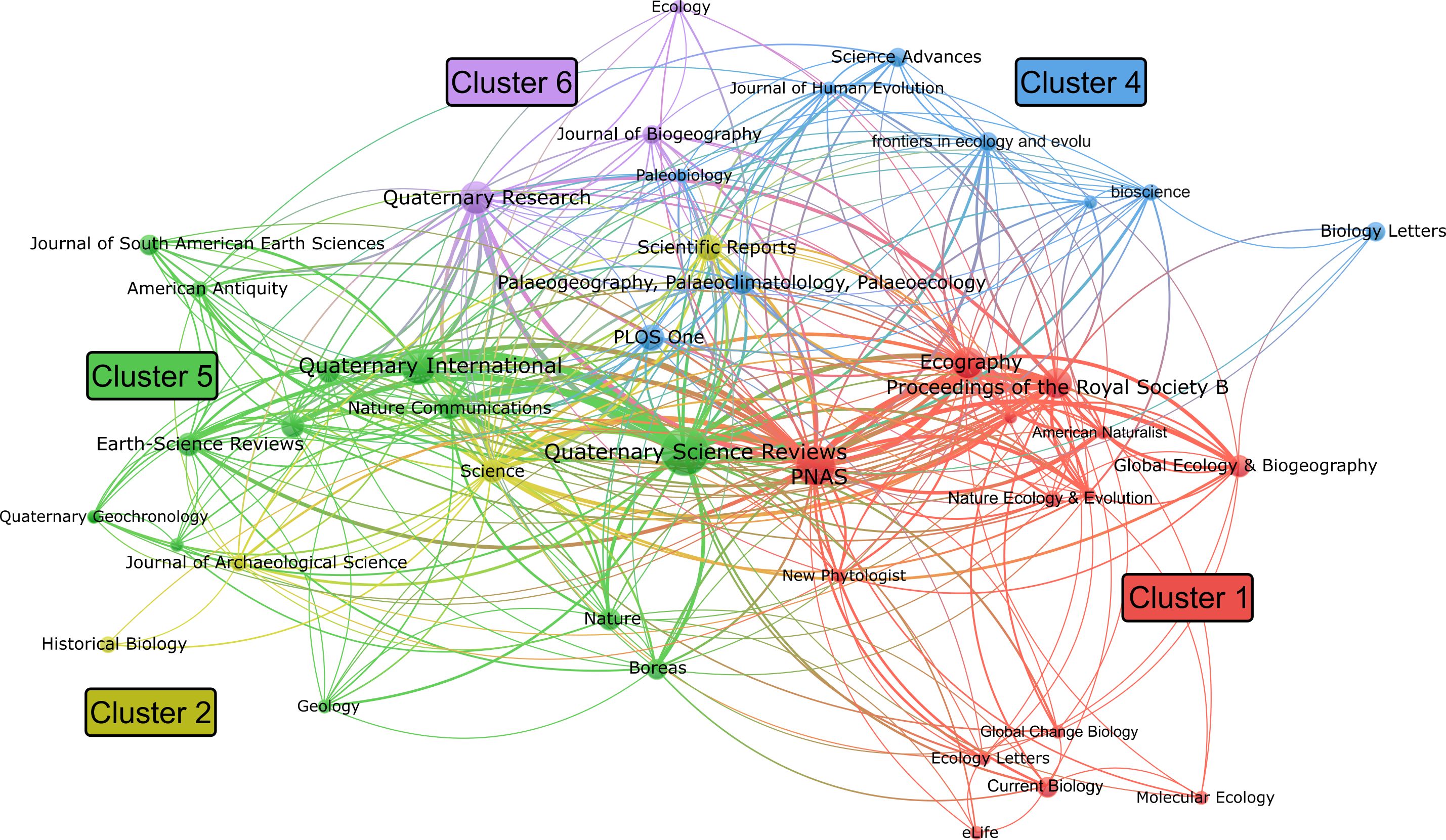
For the journal citation network analysis, a threshold of two or more publications per journal was applied, resulting in 44 journals (Figure 7). The resulting network yielded five distinct clusters, which again largely correspond to disciplinary focus. For example, ecology journals like Ecography, Nature Ecology & Evolution, and Ecology Letters cluster together, as do Quaternary science journals like Quaternary International, Quaternary Geochronology, and Earth-Science Reviews. Two particularly prominent and influential journals in our literature sample are Quaternary Science Reviews and Proceedings of the National Academy of Sciences (PNAS), which contributed 45 and 29 publications, respectively, and exhibit strong citation links with other journals in the network. As shown in Figure 8, ecology journals have become more prominent in recent years.
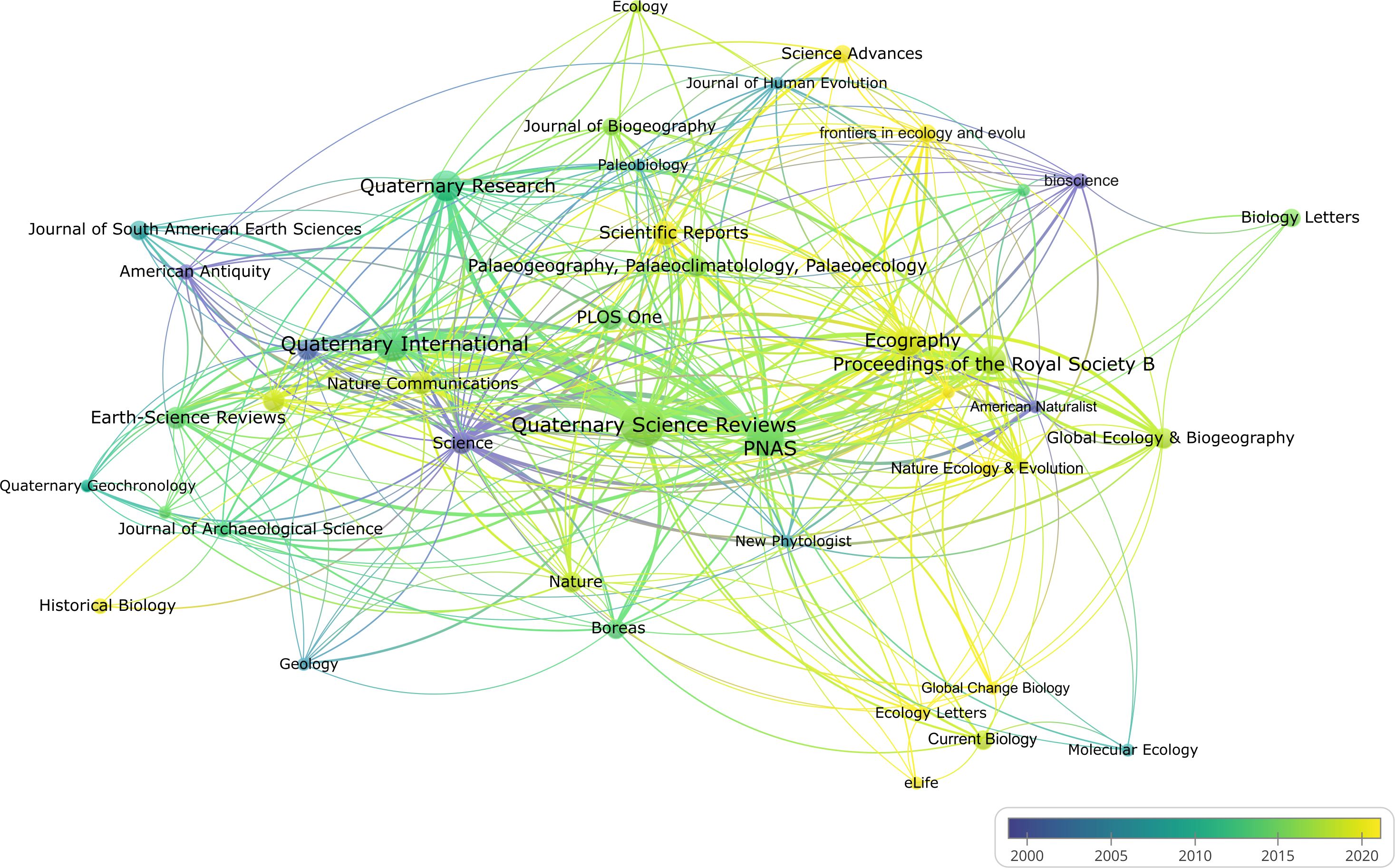
Figure 8. Journal citation overlay visualization. Lighter colors denote more recent average publication years (created using VOSviewer).
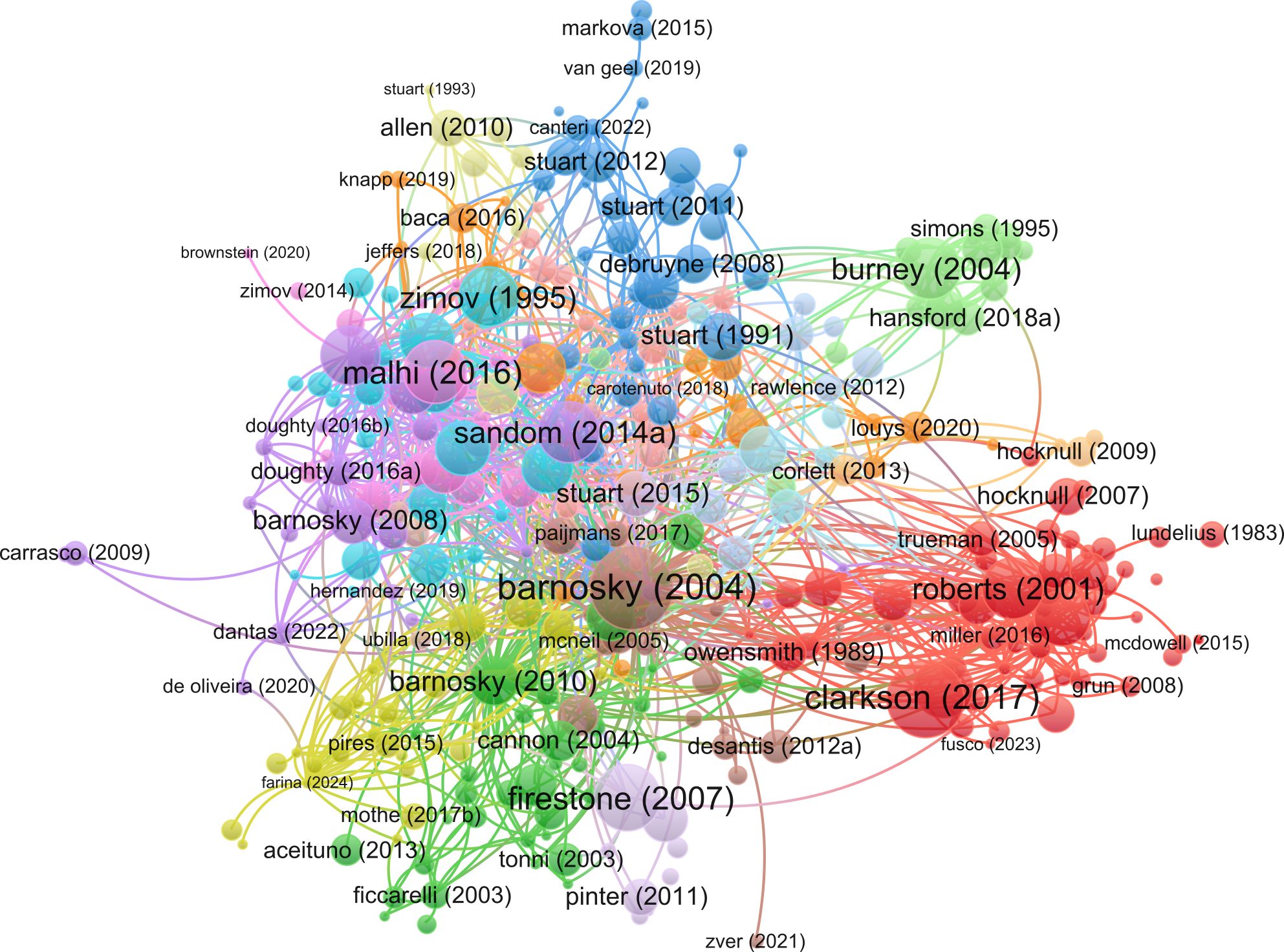
For the publication citation network analysis, 338 articles were analyzed, with 22 excluded due to a lack of connections with other articles. This network yielded 17 distinct clusters, again revealing clear patterns reflecting disciplinary and geographic focus (Figures 9, 2D). Notably, the two review articles by Barnosky et al. (2004) and Koch and Barnosky (2006) stand out as being particularly central and influential in the debate, with a high number of citations and strong links with the broader megafauna extinction literature (Figure 9; Table 2). A comparison with the broader citation network revealed a strong correlation (Supplementary Table S1), indicating that our internal citation metrics reliably reflect the wider influence of papers. The continued publication of megafauna extinction studies in top journals such as Nature, Science, and PNAS highlights the perceived importance and sustained interest in this topic.
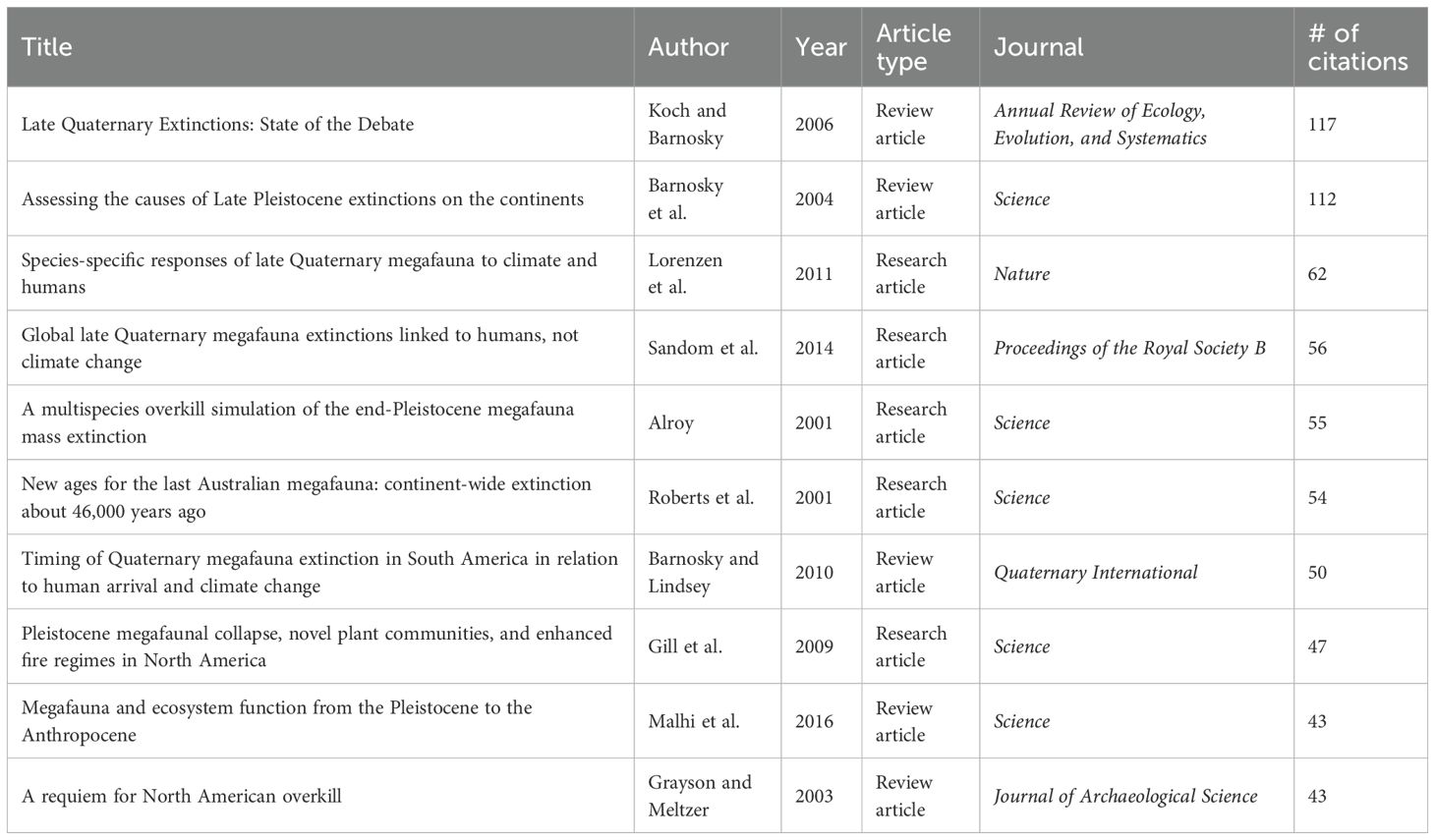
Table 2. Top 10 most cited articles in our literature sample. Note that the citation counts reflect only those from within the internal network, meaning the true citation counts of these articles is much higher.
In our more formal network analysis, we used NetworkX to generate a publication citation network—a visual network of the relationship between articles based on their citations—and applied the Leiden algorithm, which identified seven distinct clusters (Supplementary Figure S1). Some of these have considerable time depth and are densely populated, while others are more recent and contain fewer papers. As with the publication citation network above (Figure 9), citation network clusters reflect disciplinary effects (Supplementary Figure S8). Clustering also reflects proposed extinction causes: some clusters predominantly support human-driven models, others emphasize climate-driven models, and some present a more balanced mix of the three major hypotheses (Supplementary Figure S8).
Applying the Leiden algorithm to the internal co-authorship network identified 80 co-authorship clusters. To extract useable insights and limit the number of potential explanatory variables we selected the top ten clusters for subsequent analysis. These ten clusters account for approximately 80% of the total edges in the internal co-authorship network (Supplementary Figure S5) and, it is reasoned, are representative of the core ideas in the literature sample.
Plotting co-authorship cluster frequency across the internal citation clusters revealed that few co-authorship groups dominate multiple citation clusters in the internal network (Supplementary Figure S6). This suggests that much of the debate about megafauna extinctions—and the corresponding citation clusters in the broader literature—is shaped by a relatively small number of co-author communities. However, the “Other” category, which groups many smaller and less influential author clusters, still plays a major role, dominating three of the citation network clusters.
Lastly, mapping the co-authorship network revealed a complex structure of human social networks (Supplementary Figure S4). Specifically, it exhibits a “small world network” dynamic with high clustering and short distances. In other words, this suggests that the researchers in the megafauna extinction literature are connected, without isolated groups, while still containing distinct clusters of co-authors who form tight-knit communities. The relationship between the three variables and citation network are more formally modelled using a Bayesian logit-softmax model, the results of which are provided in the Supplementary Material. The modelling indicates that citation network cluster membership is predicted by disciplinary affiliation, extinction cause, and co-authorship networks, but the strength of the effects varies by citation cluster.
Summary of the literature
Temporal scope, taxonomic focus, and regional patterns
Most studies in the literature sample focused on the Late Pleistocene (n = 144) and Late Pleistocene–Holocene transition (n = 146). A smaller but significant number of studies focused on Holocene extinctions (n = 42), particularly on islands such as the Caribbean and Mascarene Islands, Madagascar, Mallorca, and New Zealand (Crowley, 2010; Rawlence et al., 2012; Bover et al., 2016; Li et al., 2020). Some Holocene studies reported late survivals on the continents, such as the European wild ass (Equus hydruntinus) in Europe (Crees and Turvey, 2014) and the mid-Holocene extinction of the Tasmanian devil (Sarcophilus harrisii) on mainland Australia (Morris et al., 2022). Few studies reported extinctions that pre-date the Late Pleistocene (Hocknull et al., 2007; Prideaux et al., 2007).
Geographically, research is concentrated in regions that experienced severe megafauna losses, especially Australasia and the Americas, though coverage within these areas is uneven (Figure 2A). In North America, studies are biased toward northern latitudes and iconic sites like the La Brea Tar Pits in California (DeSantis et al., 2012; Jones and Desantis, 2017; Fuller et al., 2020; O’Keefe et al., 2023) and Hall’s Cave in Texas (Smith et al., 2016; Seersholm et al., 2020). South America is dominated by the Pampas region, due to its rich fossil record and purported evidence of butchery (Martínez et al., 2013; Chichkoyan et al., 2017; Lopes et al., 2020; Prates and Perez, 2021; Alberdi et al., 2023; Bellinzoni et al., 2024). In Australia research is concentrated in the southeast and southwest of the continent (Ayliffe et al., 2008; Dortch et al., 2016), with very few studies from the arid interior. Europe and Asia show similar biases, with studies clustered in northern and western Europe and northern and eastern Asia, leaving much of the Eurasian interior unexplored. Mainland Africa has received little research attention (Faith, 2011; Zeller and Göttert, 2021; Kopels and Ullah, 2024), while islands like Madagascar (Burney et al., 2004; Crowley, 2010; Hansford et al., 2021; Hixon et al., 2021b) and New Zealand (Rawlence et al., 2012; Allentoft et al., 2014; Collins et al., 2014; Holdaway et al., 2014; Perry et al., 2014) are notably well represented.
Most analyses were conducted at the regional scale (Figure 2B), often centered on ecologically distinct biomes, such as tropical northern Australia (Hocknull et al., 2007; Bird et al., 2013) or the Pampas of South America (Hubbe et al., 2011; Ubilla et al., 2018). These were followed by continental-scale studies and global syntheses, particularly review articles (Barnosky et al., 2004; Galetti and Dirzo, 2013) and large-scale modelling approaches (Brault et al., 2013; Doughty et al., 2016a). Site-specific studies were less common, often reporting new excavations, revised chronologies, taphonomic analyses, and taxonomic descriptions (Grün et al., 2006; Politis and Messineo, 2008; Dortch et al., 2016; Mather et al., 2022; Otárola-Castillo et al., 2023). Rarest were country centered studies, often focused on island nations (e.g., Madagascar, New Zealand) as well as some country-specific review articles (Hubbe et al., 2013; Jukar et al., 2021).
Most studies focused on megafauna as a broad group (Figure 10), defined here as research on five or more taxa or examining megafauna in general, such as in review articles. Among taxon-specific studies, proboscideans were most investigated, especially mammoths, followed by mastodons and gomphotheres. Large flightless birds were next (e.g., Genyornis, moas, elephant birds), followed by artiodactyls (especially bison), carnivorans (mainly ursids and felids), and perissodactyls (mainly equids and woolly rhinoceros). In Australia, research was heavily biased towards Diprotodon, while other marsupials received limited attention. Reptiles and xenarthrans like sloths (Pilosa) and armadillos (Cingulata) were the focus of relatively few studies.
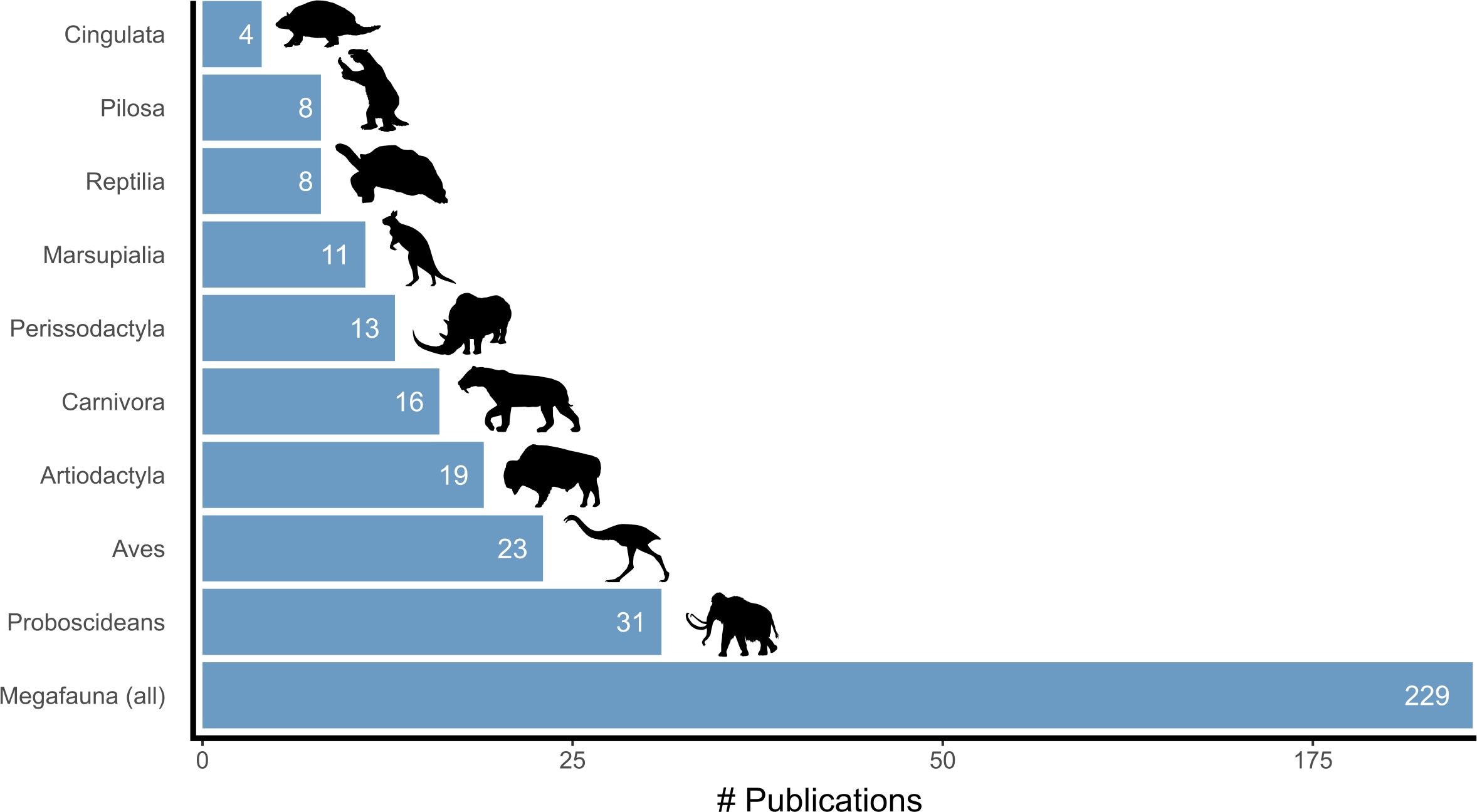
Figure 10. Number of publications by taxonomic focus. Note that an individual paper can include more than one taxon.
Megafauna definitions varied widely. About two thirds of studies offered no formal definitions, while those that did most commonly used weight-based thresholds, typically ≥44 kg (n=59) or ≥45 kg (n=26). Some focused specifically on megaherbivores, typically defined as herbivores ≥1,000 kg. Less frequent thresholds included 10 kg (Sandom et al., 2014a), 40 kg (Adesanya Adeleye et al., 2023), 50 kg (Webb, 2008), and 100 kg (Gill, 2014).
Types of data used in megafauna extinction research
Megafauna extinction research draws on a wide range of data types. Radiocarbon dating appears in about a third of all studies, with alternative dating methods—such as Optically Stimulated Luminescence (OSL), Electron Spin Resonance (ESR), U-series—common in regions like Australia where extinctions occurred near or beyond the limit of radiocarbon dating. Other techniques, such as Uranium-Lead (U-Pb), Thermoluminescence (TL), Amino-Acid Racemization (AAR), and dendrochronology, are less frequent but are occasionally used to refine chronologies when combined with other methods (Newsome et al., 2011; Miller et al., 2016; Seeber et al., 2024; Zhang et al., 2024).
Fossil data remain central to the debate, extending beyond megafauna remains to include studies of coprolites (Rawlence et al., 2012; Murchie et al., 2021), trackways (McNeil et al., 2005; de Carvalho et al., 2020), and small faunal indicators like ostracods (Hixon et al., 2021a), beetles (Sandom et al., 2014b), and eggshells (Newsome et al., 2011). Trait-based analyses are also prominent, especially in modelling studies, focusing on body mass, diet, locomotion, reproduction, and social behavior (Lundgren et al., 2021; Kemp, 2023). This research has been facilitated by the recent development of open-access trait databases such as PHYLCAINE (Faurby et al., 2018), EltonTraits (Wilman et al., 2014), MammalDIET (Kissling et al., 2014), and HerbiTraits (Lundgren et al., 2021). Fossil occurrence and ecological data are also increasingly sourced from repositories like Paleobiology Database, NOW, FosFarBase, IUCN, and the North American Pollen Database (Gill et al., 2012; Carotenuto et al., 2016; Louys et al., 2021).
Contemporary ethological data are frequently used to infer extinct taxa’s behavior and ecological functions, especially for megaherbivores like elephants (Owen-Smith, 1989; Johnson, 2009; Bakker et al., 2016). Ecological responses to megafauna hunting, culling, and conservation pressures are used to parameterize overkill models (Choquenot and Bowman, 1998; Flores, 2014; Novaro and Walker, 2021). Stable isotopes (δ13C, δ18O, δ15N) are widely used to reconstruct diet, mobility, and habitat (Bowman et al., 2010; DeSantis et al., 2017; Hixon et al., 2021b), with newer statistical (e.g., Bayesian mixing models) and analytical techniques (e.g., compound-specific amino acid analysis) now offering greater resolution (Bellinzoni et al., 2024). Other modern proxies include tooth wear and breakage (DeSantis et al., 2012; Van Valkenburgh et al., 2016), taphonomic studies (Garvey et al., 2011; Dortch et al., 2016), and pathologies (McInerney et al., 2022; Zorro-Luján et al., 2023), among many others.
Geological and sedimentological data (e.g., grain size, pH, carbon content) help assess site integrity (Haynes, 2008; Fillios et al., 2010; Lindsey and Lopez, 2015). Plant and fungal remains from cores—especially pollen, charcoal, seeds, phytoliths, and waxes—inform vegetation and fire history (Lopes dos Santos et al., 2013; Perry et al., 2014; Domic et al., 2021). Sporormiella, a dung fungus, is a key proxy for tracking past megafauna populations (Gill et al., 2012; Perrotti, 2018).
Finally, many articles provided substantive syntheses or reviews spanning a range of topics. Some provided broad overviews of the extinction debate (Barnosky et al., 2004; Gill, 2014; Galetti et al., 2018), while others focused on specific continents (Stuart, 1991; Wroe and Field, 2006; Barnosky and Lindsey, 2010; Lubeek and Westaway, 2020; Meltzer, 2020; Fariña and Vizcaíno, 2024), regions (Louys et al., 2007; Bird et al., 2013), or events, such as the Younger Dryas Impact Hypothesis (Pinter et al., 2011; Powell, 2022; Holliday et al., 2023, 2024) and the timing of human arrival in Australasia (O’Connell and Allen, 2004) and the Americas (Fiedel, 2022). Some centered on ecological processes, such as the impact of Aboriginal landscape burning on Australian fauna (Bowman, 1998), while others addressed more practical and methodological issues, such as the interpretation of the Sporormiella record (Fiedel, 2018).
Extinction cause(s)
Overall, the major extinction hypotheses are similarly represented in our literature sample: 82 (23%) articles cite humans as the primary driver, 82 (23%) articles cite climate as the primary driver, 71 (20%) proposed a mixed human-climate cause. A third of articles offered no explicit major driver of extinction, while only a few considered an extraterrestrial cause, such as a solar flare or comet impact (Firestone et al., 2007; LaViolette, 2011; Powell, 2022).
Breaking down extinction cause by region shows that for Australia, North America, and South America, the three extinction models are similarly represented (Figure 11C). In contrast, for Eurasia and studies of the arctic/sub-arctic, climate-based extinction models dominate. The opposite is true for global studies, which overwhelmingly promote human-driven extinction models. Almost all studies on islands advocate for human-driven extinctions, with a few proposing mixed models, particularly in the case of Madagascar.
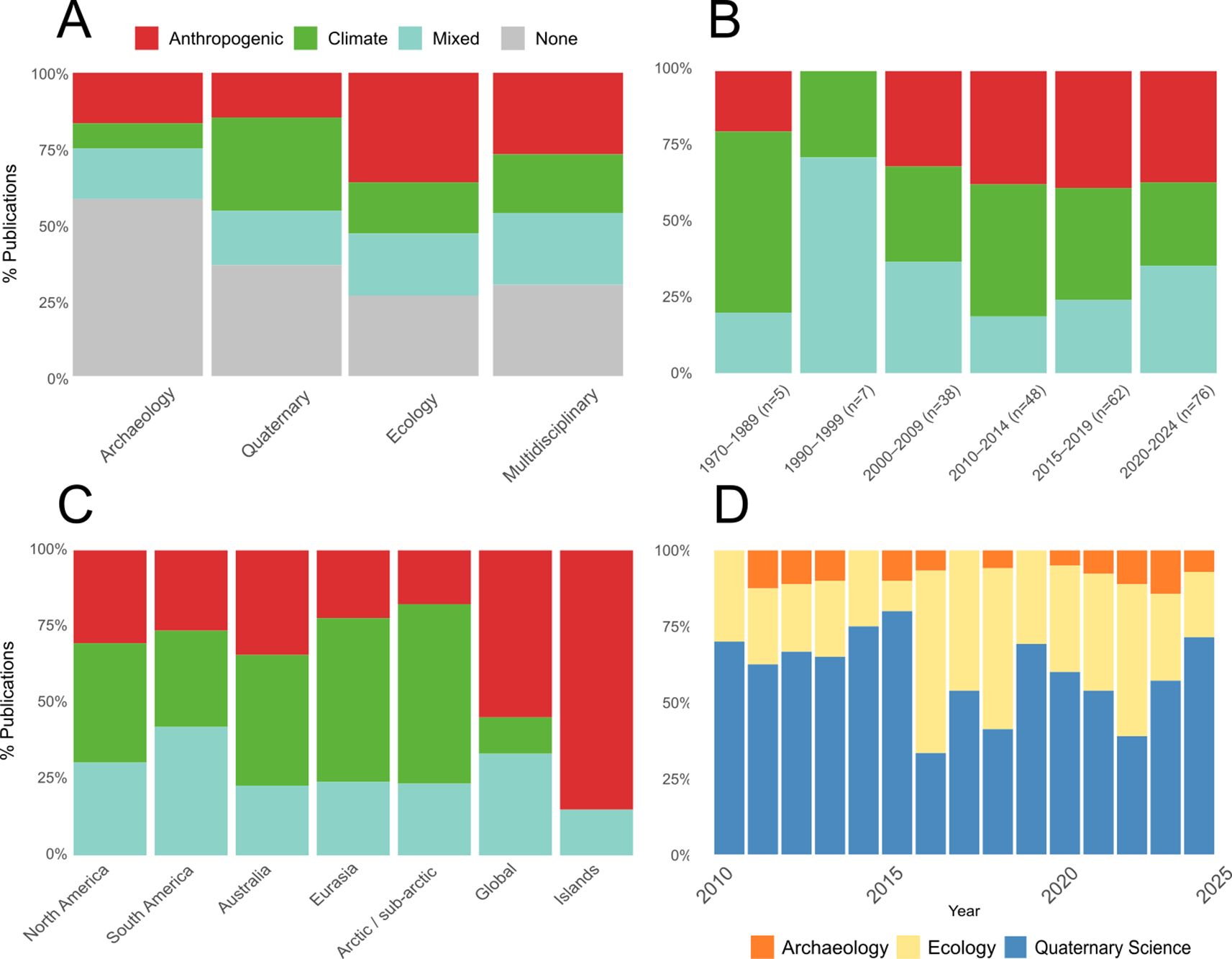
Figure 11. Proportion of the three key extinction hypotheses by discipline (A), time (B), region (C), and proportion of publications by discipline (D). Note for (B) earlier periods have larger time bins due to smaller sample sizes.
Trends over time reveal some subtle but potentially meaningful shifts (Figure 11B). Climate-based explanations rose after the turn of the millennium, peaking at 44% of papers between 2010–2014, before declining to a low of 28% in recent years. Anthropogenic-based explanations have steadily grown, from 32% in 2000–2004 to around 38% since 2010. Mixed models were high around the turn of the millennium, followed by a low of 19% between 2010–2014 and a steady rise to 35% in recent years.
Disciplinary differences also emerge (Figure 11A). Quaternary science tends to favor climate-based explanations (31%) over human (15%) or mixed causes (18%). In contrast, ecology journals lean more toward human-driven explanations (36%), with less support for climate (17%) or mixed causes (20%). Most archaeology papers (58%) do not propose a primary extinction cause, indicating a degree of caution or uncertainty within the field. Multidisciplinary journals, meanwhile, show a more balanced representation of extinction hypotheses.
Within each of the major hypotheses, scholars have proposed a range of mechanisms. Anthropogenic causes include general overhunting as well as more specified overkill models such as selective predation of juveniles in slow-reproducing species (McNeil et al., 2005; Brook and Johnson, 2006) and harvesting of large bird eggs (Miller et al., 2016). More recently, attention has shifted towards more indirect human pressures, especially habitat destruction and fragmentation, fire regimes, and competition with livestock (Holdaway et al., 2014; Hansford et al., 2021; Iijima et al., 2022; Adesanya Adeleye et al., 2023; O’Keefe et al., 2023).
Climate-based models typically emphasize habitat loss and vegetation change linked to glacial-interglacial cycles and abrupt climate events like the Last Glacial Maximum, Younger-Dryas, and Bølling-Allerød (Gallo et al., 2013; Huntley et al., 2013; Baca et al., 2016; Villavicencio and Werdelin, 2018; van Geel et al., 2019; Vachula et al., 2020). Transitions between forest and grassland (Field et al., 2001; Long and Yahnke, 2011; Zazula et al., 2014; Gilmour et al., 2015; Benfield et al., 2023), prolonged droughts (Hocknull et al., 2007; DeSantis et al., 2017; Kemp et al., 2019; Lewis et al., 2020; Louys and Roberts, 2020), and wetland expansion (Mann et al., 2013; Puzachenko et al., 2021) are also cited. Less commonly cited are changes in atmospheric CO2 concentration, sea level rise, and volcanic eruptions (Louys et al., 2007; Faith and O’Connell, 2011; Gonzalez et al., 2014).
Many papers propose a synergistic effect, with human activity amplifying existing climate stress (Prescott et al., 2012; Gill, 2014; Mothé et al., 2017; Seersholm et al., 2020; Pilowsky et al., 2023; Fariña and Vizcaíno, 2024; Robu et al., 2024). In some instances, indirect pressures such competition, livestock introduction, and landscape modification are implicated (Hixon et al., 2021b; O’Keefe et al., 2023); in others, humans are seen as delivering a final blow—or coup de grâce—to already declining megafauna populations (Lima-Ribeiro et al., 2012; Cantalapiedra et al., 2021; Fordham et al., 2024).
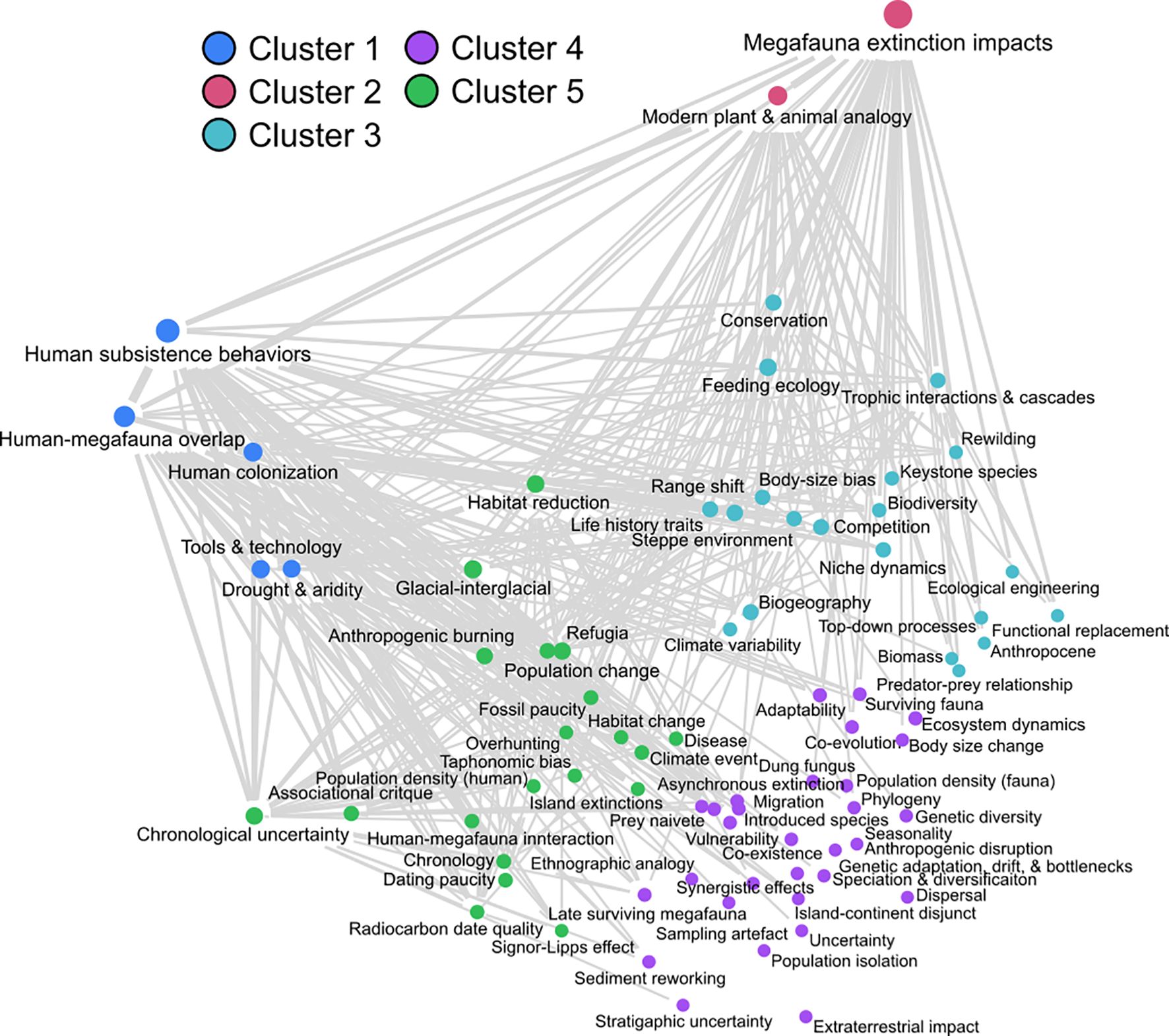
Thematic clusters
Hierarchical clustering of themes appearing in at least 5% of articles returned a total of 78 codes, which we place into five distinct clusters (Figure 12). While this clustering is somewhat arbitrary as the number of clusters is manually selected, it does capture some of the major themes being discussed in the megafauna literature, both historically and more recently. Below, we outline some of the main features of these clusters, drawing only from articles within our literature sample.
Cluster 1: Colonization, subsistence strategies, tool use, and the human role in megafauna extinctions
This cluster explores the deep-time human dimensions of megafauna extinctions, focusing on the timing and nature of human-megafauna overlap and interaction. While once difficult to demonstrate, there is now unequivocal evidence of human-megafauna coexistence across North and South America, Australia, and on several islands (Field et al., 2008; Politis and Messineo, 2008; Meltzer, 2020; Louys et al., 2021). What remains heavily debated in the literature, however, is the duration and extent of these overlaps. In Australia, for example, many researchers place the megafauna extinction “window” between 50–40 ka, coinciding with the spread of people across the continent (Roberts et al., 2001; Lopes dos Santos et al., 2013; Saltré et al., 2016, 2019). Others, however, argue the megafauna record is too patchy to support a discrete extinction window, noting that some species have last appearance dates well before this, others lack direct dates entirely, and some persisted for tens-of-thousands of years after human arrival in some regions (Wroe and Field, 2006; Faith and O’Connell, 2011; Dortch et al., 2016; Westaway et al., 2017; Price et al., 2021). Similar debates persist for the Americas (Waguespack, 2007; Villavicencio and Werdelin, 2018; Meltzer, 2020) and Eurasia (Németh et al., 2017; Wan and Zhang, 2017), as well as for more recently colonized islands (Louys et al., 2021), including even those that have seen considerably research, such as Madagascar (Hansford et al., 2021; Hixon et al., 2021b).
These debates are closely tied to questions about early human subsistence behavior, namely whether early hunter-gatherers were specialized megafauna hunters or pursued broader, more generalized diets. Advancements in hunting technologies suggests that preferential predation of megafauna may have been the direct result of a need for high-caloric fats to mitigate protein metabolism constraints in early humans (Ben-Dor and Barkai, 2024). Further, ethnographic evidence shows that hunting large animals is often motivated by complex factors beyond caloric needs (Brook and Bowman, 2002; Wroe et al., 2004; Cannon and Meltzer, 2008; Nikolskiy and Pitulko, 2013; Carotenuto et al., 2018), while zooarchaeological data indicate that early hunter-gatherers often had diverse, flexible diets that included plants, small game, and aquatic resources, with considerable variation across time and space (Wroe et al., 2004; Aceituno et al., 2013; Carotenuto et al., 2018; DeAngelis and Lyman, 2018; Louys et al., 2021).
A related theme is the stone tool record. Forms such as Clovis and Fishtail points are commonly viewed as specialized tools for hunting megafauna (Carotenuto et al., 2018; Prates and Perez, 2021; Moore et al., 2023; Yaworsky et al., 2023), supported by evidence such as butchered remains (Waters et al., 2015; Papa et al., 2024), weapon trauma (Nikolskiy and Pitulko, 2013; Carlini et al., 2022), biomolecule (e.g., blood) residues (Moore et al., 2023), and spatial associations with megafauna (Prates and Perez, 2021). However, spatial associations remain highly contested at many sites, and some researchers question whether these tool types were explicitly designed for big-game hunting or instead were used within broad-spectrum subsistence strategies (Field et al., 2008; Eren et al., 2022).
Cluster 2: Megafauna extinction impacts and modern ecological analogies
This cluster explores the ecological consequences of megafauna extinctions (Bakker et al., 2016; Berzaghi et al., 2023). While impacts are discussed for a range of megafauna, including birds and reptiles (Pedrono et al., 2013; Joos et al., 2022; Kemp, 2023), megaherbivores have received particular attention owing to their role as ecosystem engineers with the ability to modify, shape, and create habitats (Owen-Smith, 1989; Johnson, 2009; Doughty, 2013; Gill, 2014; Bakker et al., 2016; Doughty et al., 2016b; Bocherens et al., 2017; Bocherens, 2018; Galetti et al., 2018; Pires et al., 2018; Pires, 2024).
Three ecological impacts appear most frequently in the literature: seed dispersal, nutrient redistribution, and fire regulation. Megaherbivores disperse more and larger seeds over longer distances and enhance germination through digestion (Kistler et al., 2015; Berti and Svenning, 2020); redistribute nutrients via feces, carcasses, and soil disturbance (Doughty et al., 2016c, 2020); and reduce fuel loads and fire intensity through browsing and trampling (Owen-Smith, 1989; Webb, 2008; Corlett, 2013; Pedrono et al., 2013; Bakker et al., 2016; Raczka et al., 2018). Their extinction has been linked to reduced plant ranges, population fragmentation, and genetic loss (Kistler et al., 2015; Doughty et al., 2016b; Pires et al., 2018; Berti and Svenning, 2020), major declines in nutrient cycling (Doughty et al., 2016c, 2020), and increased fire frequencies (Owen-Smith, 1989; Webb, 2008; Corlett, 2013; Pedrono et al., 2013; Bakker et al., 2016; Raczka et al., 2018). Other discussed impacts of megafauna extinctions include co-extinctions (Corlett, 2013; Galetti et al., 2018), reduced carbon storage (Doughty et al., 2016b; Berzaghi et al., 2023), atmospheric and climatic changes (Zimov and Zimov, 2014), and reduced microbe and pathogen dispersal (Galetti et al., 2018; Doughty et al., 2020).
Cluster 3: Megafauna ecology, behavior, and implications for modern conservation strategies
This cluster examines how late Quaternary extinctions contribute to modern conservation and restoration strategies. Although such connections have been discussed for decades (Emslie, 1987; Owen-Smith, 1989; Bowman, 1998; Choquenot and Bowman, 1998; Barnosky et al., 2004; Wroe et al., 2004), there has been a notable rise in recent years. Perhaps the most prominent example is the use of fossil record to guide rewilding efforts (Corlett, 2013; Bakker et al., 2016; Bocherens et al., 2017; Berti and Svenning, 2020; Zeller and Göttert, 2021; Morris et al., 2022; Davoli et al., 2024) with a prominent case being Pleistocene Park in Siberia, where species like bison and muskox have been reintroduced in attempt to restore the steppe ecosystem (Zimov and Zimov, 2014).
As part of rewilding efforts, there has been an increasing attention on trait-based rewilding, focused on restoring ecological rather than species-specific functions (Lundgren et al., 2020). This shift aligns with a growing recognition of the role of keystone species and their cascading effects on ecosystem structure and function. Compared to species-centered approaches, trait-based rewilding offers greater flexibility, allowing for the selection of ecologically suitable analogues under contemporary and future climate scenarios, and enhancing functional redundancy and ecosystem resilience (Kemp, 2023).
In response to these recent trends, Moleón et al. (2020) propose two new function-oriented megafauna concepts: ‘keystone megafauna’ and ‘functional megafauna’. Here, the term keystone megafauna refers to the megafauna species that have the strongest influence on the structure and functioning of the ecosystem they inhabit. Functional megafauna, on the other hand, is defined as “the subset of largest species of a given clade or guild that have distinctive functional traits.” A further subcategory, “apex megafauna”, refers to species so large that they were only subject to anthropogenic predation.
Cluster 4: Ecological resilience and adaptive responses among megafauna and human populations amid changing climates and environments
This cluster examines species’ adaptability, vulnerability, and resilience in the face of climatic and anthropogenic pressures. Many studies highlight physiological and behavioral adaptations to arid, cold, or otherwise challenging environments (Wroe and Field, 2006; Larmon et al., 2019; Mann et al., 2019; Lord et al., 2020; Novaro and Walker, 2021; Dembitzer et al., 2022; Alberdi et al., 2023), as well as to specific environments and dietary niches (Webb, 2008; Martínez et al., 2013; Lanoë et al., 2017; Amir et al., 2022; Smith et al., 2022; Bellinzoni et al., 2024; Hardy and Rowland, 2024; Zhang et al., 2024). Some studies attribute extinctions to limited adaptability to environmental change (Zhang et al., 2024), while others reveal that certain species were more ecologically flexible than previously assumed (Hardy and Rowland, 2024).
Advances in aDNA have transformed understanding of extinction dynamics, revealing genetic adaptations (e.g., cold-tolerant TPRA1 variants in woolly rhinoceros) and reconstructing population histories (Lord et al., 2020; Meltzer, 2020; Fiedel, 2022). While some species, like New Zealand’s moa, showed demographic stability until rapid extinction (Allentoft et al., 2014), others, like the Wrangel Island mammoths, experienced sharp declines in genetic diversity prior to extinction, perhaps related to isolation (Nyström et al., 2012).
Human adaptability is also central, with research linking dietary flexibility and technological innovation to shifting ecological conditions. Some studies suggest that specialized megafauna hunting was a response to human physiological needs (Ben-Dor and Barkai, 2024), while others link megafauna disappearances to broadening of diets and technological innovations (Dembitzer et al., 2022).
Lastly, co-evolutionary dynamics and prey naivete are frequently cited to explain geographic variation in extinction severity (Sandom et al., 2014a). In Africa and Eurasia, long-term co-existence with humans has been linked to the evolution of anti-predator behaviors in megafauna (Jukar et al., 2021). Some surviving species may have passed through an “extinction filter”, helping explain their persistence in certain human-disturbed tropical ecosystems (Amir et al., 2022).
Cluster 5: Challenges in reconstructing the timing and drivers of megafaunal extinction
This final cluster highlights the significant biases, uncertainties, and data gaps that continue to complicate the study of late Quaternary megafauna extinctions. Frequently cited issues include the poor quality and resolution of radiocarbon dates (Meltzer and Mead, 1983; Brook and Bowman, 2002; Cupper and Duncan, 2006; Dortch et al., 2016; Kemp et al., 2019; Jukar et al., 2021), a general scarcity of well-dated fossils (Gillespie et al., 1978; Faith and O’Connell, 2011; Gill et al., 2012; Cooke et al., 2017; Fiedel, 2018; Kemp et al., 2019; Hocknull et al., 2020; Price et al., 2021), poorly resolved and spatially biased paleoclimate records (Gill et al., 2012; Kemp et al., 2019; Plint et al., 2019; Hocknull et al., 2020; Seersholm et al., 2020; Adesanya Adeleye et al., 2023), and taphonomic and stratigraphic biases, such as the Signor-Lipps effect and sediment reworking (Field, 2006; Field et al., 2008, 2013). Concerns over radiometric date reliability—particularly of pre-AMS radiocarbon dates—are particularly prevalent and have led to the development of quality ranking systems to screen for reliability (Meltzer and Mead, 1983; Burney et al., 2004; Barnosky and Lindsey, 2010; Saltré et al., 2016).
A central debate concerns the scarcity of butchery sites—the so-called “associational critique” (Cannon and Meltzer, 2008; Surovell and Grund, 2012; Aceituno et al., 2013; Jukar et al., 2019; Wolfe and Broughton, 2020; Bampi et al., 2022; Alberdi et al., 2023). On the one hand, critics argue that the lack of direct evidence of hunting undermines claims of widespread or intensive megafauna hunting (Meltzer, 2020). On the other hand, defenders argue that, considering taphonomic loss, short extinction windows, sparse human populations, and number of butchery sites for extant species, the number of butchery sites for extinct species is as expected (Surovell and Grund, 2012; Wolfe and Broughton, 2020).
Finally, this cluster explores the roles of climate and human land-use in megafauna extinctions. Many studies link glacial-interglacial shifts and altered precipitation to habitat fragmentation and megafauna loss (Reed, 1970; Webb, 2008; Stuart and Lister, 2011; Huntley et al., 2013; Mann et al., 2013; Lima-Ribeiro et al., 2014; Markova et al., 2015; Rabanus-Wallace et al., 2017; Louys and Roberts, 2020; Araújo et al., 2021; Mondanaro et al., 2021). Human practices such as burning—especially “firestick farming” in Australia and New Zealand—are implicated in major vegetation changes that may have contributed to extinctions (Bowman, 1998; Bird et al., 2013; Doughty, 2013; Holdaway et al., 2014; Perry et al., 2014, 2014; Faurby and Svenning, 2015; Westaway et al., 2017; Domic et al., 2021; Adesanya Adeleye et al., 2023). On islands like Madagascar, megafauna extinctions are often tied to agricultural expansion, forest clearance, and livestock introduction (Li et al., 2020; Hixon et al., 2021a).
Discussion
In recent years, ecologists have become increasingly prominent voices in the megafauna extinction debate, bringing with them a growing emphasis on leveraging these extinctions to inform contemporary ecosystem management and conservation strategies. Their contributions have introduced innovative new approaches, methodologies, and research that is more global in scope, providing novel insights not easily reachable through more traditional means. The differences in focus and emphasis are understandable given the distinct histories, training, and research priorities of different disciplines. What is less easily explained, however, is the persistent divide between ecologists and researchers in Quaternary science and archaeology over the primary drivers of late Quaternary megafauna extinctions.
This divide probably relates to the broader structural and disciplinary fragmentation within late Quaternary megafauna extinction research. Our analysis revealed a rich and expanding field, but one that remains fragmented along disciplinary lines. Ecologists, Quaternary scientists, and archaeologists tend to form research clusters, publish in discipline-specific journals, and engage primarily with their own discipline’s literature. This is not wholly unsurprising given the nature of academia. However, we argue that this siloing has likely contributed to the persistent divergent interpretations and limited cross-disciplinary dialogue, despite the overlapping questions and complementary datasets.
These disagreements are not geographically or temporally uniform. For instance, our analysis revealed a broad consensus that human activity played a central role in more recent megafauna extinctions on islands like Madagascar and New Zealand, although debates persist over the precise nature and tempo of these extinctions, which has important implications for understanding the immediate human impacts on ‘pristine’ island ecosystems. In contrast, scientific opinion remains deeply divided in regions with the longest research histories, such as Australia and the Americas, despite recent claims to the contrary (Svenning et al., 2024). At the continental and global scale, recent research has been increasingly shaped by ecologists, whose use of meta-analyses seeks to model the relative contributions of climate change and human activity to megafauna extinctions. Overwhelmingly these studies find little support for climate-based extinction models but strong support for human ones (Sandom et al., 2014a; Saltré et al., 2016; Lemoine et al., 2023), which explains the growing prevalence of anthropogenic extinction models over the past decade.
This approach has drawn criticism from Quaternary scientists and archaeologists (Grayson and Meltzer, 2003; Price et al., 2018). A poorly resolved fossil record and significant dating uncertainties are seen to seriously undermine meta-analyses, while insufficient scrutiny of fossil data has led to unfortunate errors in the datasets used in models (see Price et al., 2018 for critique). In our literature sample most archaeology papers (58%) and, to a lesser extent, Quaternary science (37%) papers offer no primary extinction cause, and while this may reflect disciplinary differences in research aims, it may also reflect a greater appreciation and understanding of the biases and limitations inherent in the fossil and archaeological records.
This is perhaps best exemplified by the longstanding “associational critique”, a common debate in our literature sample, but one that has largely taken place within archaeology journals (Nagaoka et al., 2018). Despite decades of consideration, involving the development of innovative proxies to estimate through-time fossil loss, as well as the application of novel probabilistic and theoretical frameworks to assess the expected frequency of preserved kill sites in the fossil record, there remains little consensus on the seemingly straightforward question of how many kill sites are needed to support the overkill hypothesis (Surovell and Grund, 2012; Wolfe and Broughton, 2020; Grayson et al., 2021).
This debate underscores the complex interplay of geological, temporal, demographic, and behavioral forces that influence fossil preservation and determine what is ultimately recovered and studied by paleontologists and archaeologists, one of the core themes of our literature sample. These challenges are further compounded by significant spatial and temporal gaps in the Quaternary fossil and archaeological records. As our analysis revealed, much of the Eurasian and Australian interiors remain unexplored, research in North America has been largely concentrated in the southwest and northeast, and in South America research has focused on the fossil-rich Pampas region. Several of North America’s (Meltzer, 2020) and around one third of Australia’s (Price et al., 2018) extinct megafauna species remain undated, while the timing of human arrival to the continents, as well as even more recently settled islands like Madagascar, remains debated.
Other thematic clusters that emerged reflect the growing influence of ecological research, particularly around the ecological ramifications of megafauna extinctions and their relevance for contemporary conservation. A key focus has been the use of late Quaternary extinctions to inform rewilding efforts, initially through the reintroduction of extirpated native species, and more recently through the introduction of non-natives as functional analogues. This research has potentially huge benefits for conservation and biodiversity restoration. However, much of it rests on assumptions of overkill and may be ignoring Quaternary science and archaeological research that could help make more accurate inferences regarding species’ ecological tolerances and resilience in the face of long-term climate and human pressures.
As this analysis shows, the late Quaternary megafauna extinction debate is a rich, evolving, and methodologically diverse field. Researchers draw on an impressive range of data, from traditional zooarchaeological and paleontological records to emerging biomolecular and geochemical techniques. In many cases, megafauna researchers are not merely adopting new approaches but driving forward methodological and statistical innovations (e.g., Stewart et al., 2021; Kjær et al., 2022; Paterson et al., 2025). Yet, despite these innovations, and more than five decades of intense research, the field remains divided in its conceptual foundations and empirical base. In the following section we outline a forward-looking research agenda built around five research priorities.
Future directions
Fostering interdisciplinary collaboration to bridge disciplinary divides
A persistent lack of integration between the ecological sciences and the paleosciences has been previously noted, rooted in differences in language, conceptual frameworks, methodologies, research agendas, and publications patterns, as well as institutional barriers set in academic training and departmental structures (Rull, 2010; Louys et al., 2012; Plotnick, 2012; Hsieh and Plotnick, 2020; Lister, 2021; Azevedo-Schmidt et al., 2025). Despite the overlapping research questions and complementary datasets, our analysis indicates that this division clearly extends to the late Quaternary megafauna extinction research, which has expanded in recent years to include a greater number of researchers from across a broader range of disciplinary backgrounds.
This disconnect has tangible consequences. For example, ecologists have been noted to overlook limitations in the fossil and archaeological records (Nagaoka et al., 2018), and some meta-analyses contain errors that closer collaboration with archaeologists or Quaternary scientists could have easily prevented (Price et al., 2018). Meanwhile, archaeologists and Quaternary scientists may underestimate ecological and behavioral flexibility, leading to overly simplistic interpretations of their study species and ecosystems. Even commonly used terms like “community” vary across disciplines (Louys et al., 2012), complicating data integration, while core concepts in the megafauna extinction literature—such as “prey naïveté” and “habitat disturbance”—are often invoked without clear definitions, empirical support, or engagement with the relevant records.
Even how megafauna are defined varies considerably in the literature, with body mass definitions ranging from 10 kg to over 100 kg (and over 1000 kg in the case of megaherbivores). Given the dichotomy between the need to establish a standard body-mass threshold and functional definitions of megafauna based on ecological roles (see Moleón et al., 2020), an expanded concept of megafauna may be necessary when comparing taxa from different geographical contexts (e.g., islands, continents) despite providing similar ecological functions (Hansen and Galetti, 2009).
To address these challenges, we call for greater communication, integration, and, perhaps most importantly, collaboration. Dedicated interdisciplinary events—such as conferences, workshops, and edited volumes—should be established and seek to engage ecologists, conservation biologists, Quaternary scientists, archaeologists, and policymakers. Importantly, a common language needs to be established, which will perhaps be best served by archaeologists and Quaternary scientists better situating their research within existing ecological frameworks (Rull, 2010). At the institutional level, universities and research centers should support cross-disciplinary initiatives on late Quaternary megafauna extinctions, promoting knowledge transfer and broadening researchers’ exposure to different methods and perspectives. Rather than attempting to solve the debate, these efforts should seek to reframe research priorities around more integrative and actionable research questions that seek to understand the ecosystem-level effects of megafauna extinctions.
Expanding geographical and temporal coverage through fieldwork and legacy collections
Our review highlights major geographic and temporal biases in megafauna extinction research, biases that can only be addressed by renewed investment in field-based research. While Eurasia and North America benefit from relatively well-developed radiocarbon chronologies, regions such as Africa, Australia, and South America often lack comparable resolution (Bird et al., 2022); though efforts to build high-fidelity, open-access radiocarbon databases are gaining traction (Peters et al., 2019; Iminjili et al., 2025).
This shortage of dated material stems in part from limited fieldwork in certain regions. Unfortunately, support for fieldwork in both paleontology (Reisz and Sues, 2015; Maidment and Butler, 2025; Wang et al., 2025) and ecology (Ríos-Saldaña et al., 2018; Engel et al., 2021; Soga and Gaston, 2025) has declined in recent decades. This has been attributed to: (1) limited funding for fieldwork, which is often perceived as high risk; (2) pressure to publish quickly and in high-impact journals, which is at odds with fieldwork-based research that is often considered low impact and can take years to materialize; and (3) growing societal and environmental concerns, such as the need to reduce the ecological footprint of research and avoid helicopter or parachute science.
These pressures have led to a growing prominence of remote sensing and large-scale modelling studies, which are fast to implement, data-intensive, global in scope, and perceived as more impactful (Ríos-Saldaña et al., 2018). While such “big data” studies have yielded valuable insights into megafauna demographics and extinction chronologies, they ultimately rely on primary data that many archaeologists and paleontologists consider insufficient and heavily biased (Price et al., 2018; Meltzer, 2020).
Recent recommendations from ecologists to support field-based research are equally relevant to the paleosciences (Rafiq et al., 2024; Soga and Gaston, 2025). These include: (1) increasing and diversifying funding opportunities for long-term research projects; (2) encouraging the publication of field-based research in high-impact journals; (3) requiring meta-analyses to cite all contributing papers in the main bibliography to increase visibility and recognition of primary fieldwork; (4) broadening evaluation metrics in grant, award, and hiring decisions to better recognize field-based contributions; and (5) increasing support through institutional policies and resources through, for example, financial and regulatory backing, assisting remote teaching, and field safety and harassment training.
While more fieldwork is critical, significant gains can be made elsewhere. Many museums house large legacy collections that can now be reappraised using revised taxonomies and modern scientific techniques (Allmon et al., 2018; Parry and Eichenberg, 2024). Radiocarbon dating previously untested sites or re-dating specimens analyzed prior to the advent of high-precision AMS methods can greatly improve extinction chronologies. To highlight just one example, Knell and Lee (2020) re-dated megafauna remains from the Lamb Spring site in Colorado and found the new ages to be significantly older than those originally reported from the 1980’s. Even bone fragments previously considered unidentifiable—which fill the draws and storerooms of many museums—now have the potential to be taxonomically identified using quick, cost-effective palaeoproteomics (see below).
Many legacy collections are at risk of becoming forgotten, inaccessible, or “orphaned” with unclear ownership (Parry and Eichenberg, 2024). Efforts to identify, document, inventory, and digitize these collections are urgently needed. Globally, many museums are now engaged in efforts to digitize their massive collections, in what has been described as paleontology’s “second digital revolution”. Digitization efforts at nine North American institutions revealed that their collections contain roughly 23 times more Cenozoic marine invertebrate fossil localities than currently represented in the Paleobiology Database (PBDB), suggesting that globally, perhaps only 2-3% of recorded fossil localities are publicly available (Marshall et al., 2018). Even if late Quaternary megafauna sites are better represented in such public databases, the number of undocumented sites and fossils in museums is still likely to be significant.
Prioritizing species-specific approaches
Our analysis shows that much of the literature approaches megafauna extinction at a broad taxonomic scale, with species-specific studies lacking and disproportionately focused on a few charismatic taxa like mammoths and Diprotodon, while most other species receive limited attention. Although global syntheses can reveal general trends, recent work highlights the quantity and fidelity of associated dates are largely insufficient to discern patterns and possible causes of extinction (Stuart, 2015; Price et al., 2018). Instead, there is a growing recognition among many Quaternary scientists and archaeologists that extinction dynamics must be examined at the species level, within their specific environmental, climatic, and archaeological contexts.
Recent studies have highlighted the value of species-specific approaches. In their influential study, Lorenzen et al. (2011) combined aDNA, species distribution models, and the human fossil record to show that arctic and sub-arctic megafauna species each responded differently to climate change and human activity. Tejada et al. (2021) used nitrogen isotopes to show that instead of being an obligate herbivore like living sloths, the extinct Darwin’s ground sloth (Mylodon darwinii) was an opportunistic scavenger, overturning previous assumptions regarding its feeding behavior. Species-specific studies of extant fauna are equally important for generating more accurate inferences regarding the fossil record. For instance, long-term elephant exclosure experiments in Africa can provide insights into the effects of herbivory on plant dynamics (Kimuyu et al., 2014), data that is critical for understanding the ecological ramifications of megaherbivore extinctions in the past. Though, as the study of Darwin’s ground sloth highlights, care must be taking when drawing comparisons between species no matter how closely related. Even members of the same species may have exhibited different behaviors in the past. For instance, a recent study using strontium isotopes (87Sr/86Sr) showed that many of eastern Africa’s iconic migratory mammals (e.g., blue wildebeest) have not always undertaken long distance migrations (O’Brien et al., 2024).
By bringing the full weight of modern ecological and biological knowledge to species-specific research, combined with archaeological and paleontological knowledge of the limits of the fossil and cultural records, we can better uncover the complex human–animal–environment interactions that shaped past extinctions, generating data more directly relevant to conservation and restoration planning (Lister, 2021; Pardi and DeSantis, 2022).
Integrating emerging scientific methods
Our analysis shows a clear increase in the use of innovative scientific and statistical approaches in late Quaternary megafauna extinction research. Improved dating methods involving single amino acid radiocarbon dating and XAD-2 resin pretreatment can help with issues of contamination and yield more accurate ages (Gillespie et al., 2015; Zazula et al., 2017; Deviese et al., 2018; Kosintsev et al., 2019). For instance, Zazula et al. (2017) used single amino acid radiocarbon dating of hydroxyproline to demonstrate that previously published dates for western camel (Camelops hesternus) were erroneously young by several tens of thousands of years, likely due to carbon contamination from glue or varnish used in fossil preparation and conservation.
Multi-isotope studies and the incorporation of less commonly used isotopes such as sulfur and zinc are leading to more refined reconstructions of megafauna diets, niche partitioning, and mobility (Bourgon et al., 2020; McCormack et al., 2022; Britton et al., 2023; Pederzani et al., 2024; Heddell-Stevens et al., 2024). Compound-specific isotope analysis (CSIA), which distinguishes isotopic values across individual amino acids, has clarified the trophic position of the abovementioned Darwin’s ground sloth (Tejada et al., 2021) and refined dietary insights for the short-faced bear (Kubiak et al., 2023). Despite the greater resolution provided by these approaches, their use in late Quaternary megafauna extinction does remain limited, with only a small number of articles in our literature sample using these (Fuller et al., 2020; Bellinzoni et al., 2024).
Recent years have seen a drastic increase in the application of palaeoproteomics in paleontology. In the absence of aDNA, shotgun proteomics offers an alternative means for phylogenetic placement of extinct species. Megafauna proteins older than 20 million years have now been recovered (Paterson et al., 2025) and proteomic phylogenies have helped resolved the evolutionary relationships of Genyornis (Demarchi et al., 2022), rhinocerotids (Welker et al., 2017; Cappellini et al., 2019; Paterson et al., 2025), sloths (Buckley et al., 2015; Presslee et al., 2019), and other megafauna. Additionally, individual sex determination can now be achieved using enamel proteins (Cappellini et al., 2019; Armaroli et al., 2025; Rey-Iglesia et al., 2025), while proteomic analysis of gut tissue samples provides direct evidence of megafauna diets (Cucina et al., 2021).
Related is Zooarchaeology by Mass Spectrometry (ZooMS), a proteomics method that allows taxonomic identification of otherwise unidentifiable bone fragments. It is high-throughput and cost effective, meaning hundreds and even thousands of specimens can be analyzed in a single study (Buckley et al., 2017b; Brown et al., 2021). ZooMS has proven valuable for expanding taxonomic resolution and identifying material suitable for further stable isotope, aDNA, and radiocarbon analysis (Buckley et al., 2017a, Buckley et al., 2017b; Antonosyan et al., 2024; Peters et al., 2025). Despite its growing global adoption, ZooMS has so far seen little direct application to late Quaternary megafauna extinctions; none of the studies in our sample employed this widely used technique. Nonetheless, its potential is evident. For example, Antonosyan et al. (2024) recently successfully applied it to North America megafauna from museum legacy collections, while Peters et al. (2023, 2025) demonstrated protein survival beyond 50,000 years in subtropical Australia and developed peptide markers for three extinct Australian megafauna species (see also Buckley et al., 2017a).
Though still in its early stages, lipidomics holds potential as well. This technique has been used to detect hormones like testosterone in mammoth tusks (Cherney et al., 2023) and hair (Koren et al., 2018), offering unique insights into physiology and behavior. Collectively, biomolecular approaches can greatly enhance our ability to reconstruct species’ biology, ecology, and evolutionary history, and are especially powerful when applied to poorly preserved or fragmentary remains that tend to dominate Quaternary fossil assemblages. Their wider integration into extinction studies could therefore substantially advance our understanding of megafauna extinctions.
Recent advancements in CT scanning and related imaging techniques (e.g., neutron scanning) has opened new avenues for non-destructive assessments of fossils (Smilg and Berger, 2015; Ziegler et al., 2020; Smith et al., 2021), though recent research cautions that micro-CT scanning may cause radiation damage in biomolecules which may impact downstream analyses (e.g., radiocarbon dating, aDNA) (Duval et al., 2025). Still, applying scanning methods such as these can be used to detect fossils encased in sedimentary material (e.g., breccia), elucidate the taphonomic and depositional histories of fossil assemblages, and minimize damage during fossil extraction (Smith et al., 2021).
On larger scales, drone-mounted LiDAR is significantly enhancing the efficiency and cost-effectiveness of field surveys (Maidment and Butler, 2025). Likewise, the integration of remote sensing with Geographical Information Systems (GIS) modelling has proven valuable for predicting the distribution of fossil-bearing sites, resulting in shorter survey times, lower costs, and substantially higher fossil recovery rates (Block et al., 2016; Maidment and Butler, 2025). Even smartphones are now fitted with 3D modelling and LiDAR software enabling rapid in-the-field scanning of sites and individual fossils and artefacts.
Better management of modelling and uncertainty
Bringing all of these lines of evidence together will require advanced data science approaches. Answering questions about causality, for instance, requires causal modelling (McElreath, 2020), and time will be an important dimension in such models (Granger, 1980). Managing the uncertainties associated with time is paramount and previous approaches that neglect those uncertainties can no longer be considered sufficient (Carleton and Groucutt, 2021).
New Bayesian modelling approaches allow for the integration of uncertainties across datasets and can be used to account for, and propagate, measurement uncertainties and sampling uncertainties all the way through an analytical pipeline. As has been shown, simple regression models involving summed probability density functions (SPDs) and mean climate time series, can be deeply misleading and fail to account for surprisingly large uncertainties that can be associated with both kinds of data (Carleton and Groucutt, 2021; Stewart et al., 2021, 2022; Crema, 2022).
As new approaches, like palaeoproteomics and aDNA, are added into the mix, the laboratory and sampling uncertainties associated with these approaches will have to be carefully considered. The uncertainties must be built into the models that are then used to test hypotheses about megafauna population and evolutionary dynamics. Ideally, these models will start with theoretical framing that guides modelling choices and frames models as expressions of theoretical processes (Shmueli, 2010), as opposed to data dredging and pattern recognition (hunting for correlations) that can easily distort our impressions of past ecological and human-faunal dynamics.
Study limitations
We acknowledge several important limitations and biases that characterize our study. First, by including only English-language articles, we likely overlook important research published in other languages. Indeed, a recent survey found that much of the research on South American megafauna is published in low-reach gray literature (e.g., technical reports, working papers, government documents, academic theses) and local journals written in Latin languages (Bampi et al., 2024). This survey also revealed that more megafauna kill sites are reported in these sources than in English-language articles, suggesting that the perceived scarcity of kill sites in South America compared to North America is due to a language bias, not a lack of data. Similar biases are likely present elsewhere, such as in China and Russia, where it has been noted that few native-language archaeological publications are translated into English (Hein, 2016; Rouse et al., 2024). Consequently, our literature sample may have missed important themes and data in the megafauna extinction debate, particularly from regions like South America and China, where a significant body of research exists in native languages.
Another limitation is the narrow scope of our literature search, which was restricted to just WoS and Scopus. While these two databases are excellent for capturing peer-reviewed journal articles, they do not cover much of the literature found in global policy documents, such as reports, working papers, policy briefs, and other gray literature from think tanks, governmental agencies, and international organizations. So, while this review sought to provide an objective and comprehensive overview of the state of the field, it inevitably misses perspectives on late Quaternary extinctions from outside academia. A quick search on Policy Commons using our original search terms returned 557 publications, including 447 documents, 64 articles, nine theses, and seven books published in English, Spanish, Portuguese, German, and Welsh.
A third limitation of our study concerns the poor coverage of older articles in databases like WoS and Scopus. One of the goals of our review was to trace changes in how late Quaternary megafauna extinctions have been perceived and studied over time. However, our literature sample included very few articles published before the turn of the millennium. More effort could have been made to locate older publications to offer a more complete view of the history of the debate.
Data availability statement
The Python code used to prepare and analyze the data and conduct the network and citation analyses are available at https://github.com/wccarleton/mfreview.
Author contributions
MS: Visualization, Data curation, Investigation, Formal analysis, Conceptualization, Project administration, Writing – review & editing, Writing – original draft, Methodology. CP: Conceptualization, Investigation, Writing – review & editing, Writing – original draft, Formal analysis. MZ: Investigation, Conceptualization, Writing – review & editing, Formal analysis. WC: Writing – review & editing, Formal analysis. PR: Writing – review & editing. NB: Writing – review & editing. HG: Writing – review & editing, Resources.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. The authors thank the Max Planck Society for funding.
Acknowledgments
The authors acknowledge the Max Planck Society for funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmamm.2025.1678231/full#supplementary-material
References
Aceituno F. J., Loaiza N., Delgado-Burbano M. E., and Barrientos G. (2013). The initial human settlement of Northwest South America during the Pleistocene/Holocene transition: Synthesis and perspectives. Quat. Int. 301, 23–33. doi: 10.1016/j.quaint.2012.05.017
Adesanya Adeleye M., Charles Andrew S., Gallagher R., van der Kaars S., De Deckker P., Hua Q., et al. (2023). On the timing of megafaunal extinction and associated floristic consequences in Australia through the lens of functional palaeoecology. Quat. Sci. Rev. 316, 108263. doi: 10.1016/j.quascirev.2023.108263
Agassiz L. (1837). Discours prononce a l’ouverture des seances de la Societe Helvetique des Sciences Naturelles, a Neuchatel le 24 Juillet 1837 (Societe Helvetique des Sciences Naturelles, Actes, 22me session: V-XXX).
Alberdi M. T., Bonini R., Bellinzoni J., Gómez G., Steffan P., Lucero N., et al. (2023). New records of Equus and Hippidion (Mammalia, Perissodactyla) from the Late Pleistocene of the Salado River (Buenos Aires province, Argentina). Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen 309, 273–290. doi: 10.1127/njgpa/2023/1162
Allentoft M. E., Heller R., Oskam C. L., Lorenzen E. D., Hale M. L., Gilbert M. T. P., et al. (2014). Extinct New Zealand megafauna were not in decline before human colonization. Proc. Natl. Acad. Sci. U. S. A. 111, 4922–4927. doi: 10.1073/pnas.1314972111
Allmon W. D., Dietl G. P., Hendricks J. R., and Ross R. M. (2018). “Bridging the two fossil records: Paleontology’s ‘big data’ future resides in museum collections,” in Museums at the Forefront of the History and Philosophy of Geology: History Made, History in the Making. Eds. Rosenberg G. D. and Clary R. M. (Colorado, USA: Geological Society of America). doi: 10.1130/2018.2535(03
Amir Z., Moore J. H., Negret P. J., and Luskin M. S. (2022). Megafauna extinctions produce idiosyncratic Anthropocene assemblages. Sci. Adv. 8, eabq2307. doi: 10.1126/sciadv.abq2307
Antonosyan M., Hill E., Jodry M., Amano N., Brown S., Rick T., et al. (2024). A new legacy: potential of zooarchaeology by mass spectrometry in the analysis of North American megafaunal remains. Front. Mamm. Sci. 3. doi: 10.3389/fmamm.2024.1399358
Araújo T., MaChado H., Mothé D., and dos Santos Avilla L. (2021). Species distribution modeling reveals the ecological niche of extinct megafauna from South America. Quat. Res. 104, 151–158. doi: 10.1017/qua.2021.24
Armaroli E., Fontani F., Iacovera R., Cilli E., Latorre A., Luiselli D., et al. (2025). Ecology and demographic structure of an extinct ibex population in Upper Palaeolithic Italian Alps. bioRxiv. doi: 10.1101/2025.04.01.646553
Ayliffe L. K., Prideaux G. J., Bird M. I., Grün R., Roberts R. G., Gully G. A., et al. (2008). Age constraints on Pleistocene megafauna at Tight Entrance Cave in southwestern Australia. Quat. Sci. Rev. 27, 1784–1788. doi: 10.1016/j.quascirev.2008.07.008
Azevedo-Schmidt L., Landrum M., Spoth M. M., Brocchini N. R., Hamley K. M., Mereghetti A., et al. (2025). Advancing terrestrial ecology by improving cross-temporal research and collaboration. BioScience 75, 15–29. doi: 10.1093/biosci/biae108
Baca M., Popović D., Stefaniak K., Marciszak A., Urbanowski M., Nadachowski A., et al. (2016). Retreat and extinction of the Late Pleistocene cave bear (Ursus spelaeus sensu lato). Sci. Nat. 103, 92. doi: 10.1007/s00114-016-1414-8
Bakker E. S., Gill J. L., Johnson C. N., Vera F. W. M., Sandom C. J., Asner G. P., et al. (2016). Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl. Acad. Sci. U. S. A. 113, 847–855. doi: 10.1073/pnas.1502545112
Bampi H., Barberi M., and Lima-Ribeiro M. S. (2022). Megafauna kill sites in South America: A critical review. Quat. Sci. Rev. 298, 107851. doi: 10.1016/j.quascirev.2022.107851
Bampi H., Pires-Oliveira J. C., Loyola-Bartra O., and Lima-Ribeiro M. S. (2024). Language bias, not knowledge shortfall, underestimates the evidence of megafauna kill sites in South America. J. S. Am. Earth Sci. 146, 105078. doi: 10.1016/j.jsames.2024.105078
Barnosky A. D., Koch P. L., Feranec R. S., Wing S. L., and Shabel A. B. (2004). Assessing the causes of Late Pleistocene extinctions on the continents. Science 306, 70–75. doi: 10.1126/science.1101476
Barnosky A. D. and Lindsey E. L. (2010). Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quat. Int. 217, 10–29. doi: 10.1016/j.quaint.2009.11.017
Bellinzoni J. E., Valenzuela L. O., Bonini R. A., Fuchs L., Gómez G. N., Steffan P. G., et al. (2024). An isotopic approach for assessing synergies among extinction drivers on Late Pleistocene megafauna in the Argentine Pampas. J. Archaeol. Sci. Rep. 58, 104687. doi: 10.1016/j.jasrep.2024.104687
Ben-Dor M. and Barkai R. (2024). A matter of fat: Hunting preferences affected Pleistocene megafaunal extinctions and human evolution. Quat. Sci. Rev. 331, 108660. doi: 10.1016/j.quascirev.2024.108660
Benfield A. J., Ivory S. J., Hodelka B. N., Zimmerman S. R. H., and McGlue M. M. (2023). Terrestrial ecosystem transformations in response to rapid climate change during the last deglaciation around Mono Lake, California, USA. Quat. Res. 113, 87–104. doi: 10.1017/qua.2022.70
Berti E. and Svenning J.-C. (2020). Megafauna extinctions have reduced biotic connectivity worldwide. Glob. Ecol. Biogeogr. 29, 2131–2142. doi: 10.1111/geb.13182
Berzaghi F., Bretagnolle F., Durand-Bessart C., and Blake S. (2023). Megaherbivores modify forest structure and increase carbon stocks through multiple pathways. Proc. Natl. Acad. Sci. U. S. A. 120, e2201832120. doi: 10.1073/pnas.2201832120
Bird M. I., Hutley L. B., Lawes M. J., Lloyd J., Luly J. G., Ridd P. V., et al. (2013). Humans, megafauna and environmental change in tropical Australia. J. Quaternary Sci. 28, 439–452. doi: 10.1002/jqs.2639
Bird D., Miranda L., Vander Linden M., Robinson E., Bocinsky R. K., Nicholson C., et al. (2022). p3k14c, a synthetic global database of archaeological radiocarbon dates. Sci. Data 9, 27. doi: 10.1038/s41597-022-01118-7
Block S., Saltré F., Rodríguez-Rey M., Fordham D. A., Unkel I., and Bradshaw C. J. A. (2016). Where to dig for fossils: combining climate-envelope, taphonomy and discovery models. PLoS One 11, e0151090. doi: 10.1371/journal.pone.0151090
Bocherens H. (2018). The rise of the anthroposphere since 50,000 years: an ecological replacement of megaherbivores by humans in terrestrial ecosystems? Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00003
Bocherens H., Cotte M., Bonini R. A., Straccia P., Scian D., Soibelzon L., et al. (2017). Isotopic insight on paleodiet of extinct Pleistocene megafaunal Xenarthrans from Argentina. Gondwana Res. 48, 7–14. doi: 10.1016/j.gr.2017.04.003
Boivin N. L., Zeder M. A., Fuller D. Q., Crowther A., Larson G., Erlandson J. M., et al. (2016). Ecological consequences of human niche construction: Examining long-term anthropogenic shaping of global species distributions. Proc. Natl. Acad. Sci. U. S. A. 113, 6388–6396. doi: 10.1073/pnas.1525200113
Bourgon N., Jaouen K., Bacon A.-M., Jochum K. P., Dufour E., Duringer P., et al. (2020). Zinc isotopes in Late Pleistocene fossil teeth from a Southeast Asian cave setting preserve paleodietary information. Proc. Natl. Acad. Sci. U. S. A. 117, 4675–4681. doi: 10.1073/pnas.1911744117
Bover P., Valenzuela A., Torres E., Cooper A., Pons J., and Alcover J. A. (2016). Closing the gap: New data on the last documented Myotragus and the first human evidence on Mallorca (Balearic Islands, Western Mediterranean Sea). Holocene 26, 1887–1891. doi: 10.1177/0959683616645945
Bowman D. M. J. S. (1998). The impact of Aboriginal landscape burning on the Australian biota. New Phytol. 140, 385–410. doi: 10.1046/j.1469-8137.1998.00289.x
Bowman D. M. J. S., Murphy B. P., and McMahon C. R. (2010). Using carbon isotope analysis of the diet of two introduced Australian megaherbivores to understand Pleistocene megafaunal extinctions. J. Biogeogr. 37, 499–505. doi: 10.1111/j.1365-2699.2009.02206.x
Brault M.-O., Mysak L. A., Matthews H. D., and Simmons C. T. (2013). Assessing the impact of late Pleistocene megafaunal extinctions on global vegetation and climate. Clim. Past 9, 1761–1771. doi: 10.5194/cp-9-1761-2013
Britton K., Jimenez E.-L., Le Corre M., Renou S., Rendu W., Richards M. P., et al. (2023). Multi-isotope analysis of bone collagen of Late Pleistocene ungulates reveals niche partitioning and behavioural plasticity of reindeer during MIS 3. Sci. Rep. 13, 15722. doi: 10.1038/s41598-023-42199-7
Brook B. W. and Bowman D. M. J. S. (2002). Explaining the Pleistocene megafaunal extinctions: Models, chronologies, and assumptions. Proc. Natl. Acad. Sci. U. S. A. 99, 14624–14627. doi: 10.1073/pnas.232126899
Brook B. W. and Johnson C. N. (2006). Selective hunting of juveniles as a cause of the imperceptible overkill of the Australian Pleistocene megafauna. Alcheringa 30, 39–48. doi: 10.1080/03115510609506854
Brown S., Wang N., Oertle A., Kozlikin M. B., Shunkov M. V., Derevianko A. P., et al. (2021). Zooarchaeology through the lens of collagen fingerprinting at Denisova Cave. Sci. Rep. 11, 15457. doi: 10.1038/s41598-021-94731-2
Buckland W. (1823). Reliquiæ diluvianæ; or Observations on the organic remains contained in caves, fissures, and diluvial gravel, and on other geological phenomena, attesting the action of an universal deluge (London: J. Murray). doi: 10.5962/bhl.title.160204
Buckley M., Cosgrove R., Garvey J., and Prideaux G. J. (2017a). Identifying remains of extinct kangaroos in Late Pleistocene deposits using collagen fingerprinting. J. Quat. Sci. 32, 653–660. doi: 10.1002/jqs.2964
Buckley M., Fariña R. A., Lawless C., Tambusso P. S., Varela L., Carlini A. A., et al. (2015). Collagen sequence analysis of the extinct giant ground sloths lestodon and megatherium. PLoS One 10, e0139611. doi: 10.1371/journal.pone.0139611
Buckley M., Harvey V. L., and Chamberlain A. T. (2017b). Species identification and decay assessment of Late Pleistocene fragmentary vertebrate remains from Pin Hole Cave (Creswell Crags, UK) using collagen fingerprinting. Boreas 46, 402–411. doi: 10.1111/bor.12225
Burney D. A., Burney L. P., Godfrey L. R., Jungers W. L., Goodman S. M., Wright H. T., et al. (2004). A chronology for late prehistoric Madagascar. J. Hum. Evol. 47, 25–63. doi: 10.1016/j.jhevol.2004.05.005
Campos P. F., Willerslev E., Sher A., Orlando L., Axelsson E., Tikhonov A., et al. (2010). Ancient DNA analyses exclude humans as the driving force behind late Pleistocene musk ox (Ovibos moschatus) population dynamics. Proc. Natl. Acad. Sci. U. S. A. 107, 5675–5680. doi: 10.1073/pnas.0907189107
Cannon M. D. and Meltzer D. J. (2008). Explaining variability in Early PaleoIndian foraging. Quat. Int. 191, 5–17. doi: 10.1016/j.quaint.2008.03.002
Cantalapiedra J. L., Sanisidro Ó., Zhang H., Alberdi M. T., Prado J. L., Blanco F., et al. (2021). The rise and fall of proboscidean ecological diversity. Nat. Ecol. Evol. 5, 1266–1272. doi: 10.1038/s41559-021-01498-w
Cappellini E., Welker F., Pandolfi L., Ramos-Madrigal J., Samodova D., Rüther P. L., et al. (2019). Early Pleistocene enamel proteome from Dmanisi resolves Stephanorhinus phylogeny. Nature 574, 103–107. doi: 10.1038/s41586-019-1555-y
Carleton W. C. and Groucutt H. S. (2021). Sum things are not what they seem: Problems with point-wise interpretations and quantitative analyses of proxies based on aggregated radiocarbon dates. Holocene 31, 630–643. doi: 10.1177/0959683620981700
Carlini A. A., Carrillo-Briceño J. D., Jaimes A., Aguilera O., Zurita A. E., Iriarte J., et al. (2022). Damaged glyptodontid skulls from Late Pleistocene sites of northwestern Venezuela: evidence of hunting by humans? Swiss J. Palaeontol. 141, 11. doi: 10.1186/s13358-022-00253-3
Carotenuto F., Di Febbraro M., Melchionna M., Castiglione S., Saggese F., Serio C., et al. (2016). The influence of climate on species distribution over time and space during the late Quaternary. Quat. Sci. Rev. 149, 188–199. doi: 10.1016/j.quascirev.2016.07.036
Carotenuto F., Di Febbraro M., Melchionna M., Mondanaro A., Castiglione S., Serio C., et al. (2018). The well-behaved killer: Late Pleistocene humans in Eurasia were significantly associated with living megafauna only. Palaeogeogr. Palaeoclimatol. Palaeoecol. 500, 24–32. doi: 10.1016/j.palaeo.2018.03.036
Cherney M. D., Fisher D. C., Auchus R. J., Rountrey A. N., Selcer P., Shirley E. A., et al. (2023). Testosterone histories from tusks reveal woolly mammoth musth episodes. Nature 617, 533–539. doi: 10.1038/s41586-023-06020-9
Chichkoyan K. V., Martínez-Navarro B., Moigne A.-M., Cioppi E., Belinchón M., and Lanata J. L. (2017). Description and interpretation of Megatherium americanum atlas with evidence of human intervention. Rivista Italiana di Paleontol. e Stratigrafia 123. doi: 10.13130/2039-4942/8019
Choquenot D. and Bowman D. M. J. S. (1998). Marsupial megafauna, Aborigines and the overkill hypothesis: application of predator-prey models to the question of Pleistocene extinction in Australia. Glob. Ecol. Biogeogr. Let. 7, 167–180. doi: 10.1046/j.1466-822X.1998.00285.x
Collins C. J., Rawlence N. J., Prost S., Anderson C. N. K., Knapp M., Scofield R. P., et al. (2014). Extinction and recolonization of coastal megafauna following human arrival in New Zealand. Proc. R. Soc B. 281, 20140097. doi: 10.1098/rspb.2014.0097
Cooke S. B., Dávalos L. M., Mychajliw A. M., Turvey S. T., and Upham N. S. (2017). Anthropogenic extinction dominates holocene declines of west Indian mammals. Annu. Rev. Ecol. Evol. Syst. 48, 301–327. doi: 10.1146/annurev-ecolsys-110316-022754
Corlett R. T. (2013). The shifted baseline: Prehistoric defaunation in the tropics and its consequences for biodiversity conservation. Biol. Conserv. 163, 13–21. doi: 10.1016/j.biocon.2012.11.012
Crees J. J. and Turvey S. T. (2014). Holocene extinction dynamics of Equus hydruntinus, a late-surviving European megafaunal mammal. Quat. Sci. Rev. 91, 16–29. doi: 10.1016/j.quascirev.2014.03.003
Crema E. R. (2022). Statistical inference of prehistoric demography from frequency distributions of radiocarbon dates: A review and a guide for the perplexed. J. Archaeol Method Theory 29, 1387–1418. doi: 10.1007/s10816-022-09559-5
Cromsigt J. P. G. M., te Beest M., Kerley G. I. H., Landman M., le Roux E., and Smith F. A. (2018). Trophic rewilding as a climate change mitigation strategy? Philos. Trans. R. Soc. B: Biol. Sci. 373, 20170440. doi: 10.1098/rstb.2017.0440
Crowley B. E. (2010). A refined chronology of prehistoric Madagascar and the demise of the megafauna. Quat. Sci. Rev. 29, 2591–2603. doi: 10.1016/j.quascirev.2010.06.030
Cucina A., Cunsolo V., Di Francesco A., Saletti R., Zilberstein G., Zilberstein S., et al. (2021). Meta-proteomic analysis of the Shandrin mammoth by EVA technology and high-resolution mass spectrometry: what is its gut microbiota telling us? Amino Acids 53, 1507–1521. doi: 10.1007/s00726-021-03061-0
Cupper M. L. and Duncan J. (2006). Last glacial megafaunal death assemblage and early human occupation at Lake Menindee, southeastern Australia. Quat. Res. 66, 332–341. doi: 10.1016/j.yqres.2006.06.004
Davoli M., Monsarrat S., Pedersen R. Ø., Scussolini P., Karger D. N., Normand S., et al. (2024). Megafauna diversity and functional declines in Europe from the Last Interglacial to the present. Glob. Ecol. Biogeogr. 33, 34–47. doi: 10.1111/geb.13778
Dawkins W. B. (1869). On the distribution of the british postglacial mammals. QJGS 25, 192–217. doi: 10.1144/GSL.JGS.1869.025.01-02.35
DeAngelis J. A. and Lyman R. L. (2018). Evaluation of the Early Paleo-Indian zooarchaeological record as evidence of diet breadth. Archaeol. Anthropol. Sci. 10, 555–570. doi: 10.1007/s12520-016-0377-1
de Carvalho C. N., Figueiredo S., Muniz F., Belo J., Cunha P. P., Baucon A., et al. (2020). Tracking the last elephants in Europe during the Würm Pleniglacial: the importance of the Late Pleistocene aeolianite record in SW Iberia. Ichnos 27, 352–360. doi: 10.1080/10420940.2020.1744586
Demarchi B., Stiller J., Grealy A., Mackie M., Deng Y., Gilbert T., et al. (2022). Ancient proteins resolve controversy over the identity of Genyornis eggshell. Proc. Natl. Acad. Sci. U. S. A. 119, e2109326119. doi: 10.1073/pnas.2109326119
Dembitzer J., Barkai R., Ben-Dor M., and Meiri S. (2022). Levantine overkill: 1.5 million years of hunting down the body size distribution. Quat. Sci. Rev. 276, 107316. doi: 10.1016/j.quascirev.2021.107316
DeSantis L. R. G., Field J. H., Wroe S., and Dodson J. R. (2017). Dietary responses of Sahul (Pleistocene Australia–New Guinea) megafauna to climate and environmental change. Paleobiology 43, 181–195. doi: 10.1017/pab.2016.50
DeSantis L. R. G., Schubert B. W., Scott J. R., and Ungar P. S. (2012). Implications of diet for the extinction of saber-toothed cats and American lions. PLoS One 7, e52453. doi: 10.1371/journal.pone.0052453
Deviese T., Comeskey D., McCullagh J., Bronk Ramsey C., and Higham T. (2018). New protocol for compound-specific radiocarbon analysis of archaeological bones. Rapid Commun. Mass Spectromet. 32, 373–379. doi: 10.1002/rcm.8047
Domic A. I., Hixon S. W., Velez M. I., Ivory S. J., Douglass K. G., Brenner M., et al. (2021). Influence of late holocene climate change and human land use on terrestrial and aquatic ecosystems in Southwest Madagascar. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.688512
Dortch J., Cupper M., Grün R., Harpley B., Lee K., and Field J. (2016). The timing and cause of megafauna mass deaths at Lancefield Swamp, south-eastern Australia. Quat. Sci. Rev. 145, 161–182. doi: 10.1016/j.quascirev.2016.05.042
Doughty C. E. (2013). Preindustrial human impacts on global and regional environment. Annu. Rev. Environ. Resour. 38, 503–527. doi: 10.1146/annurev-environ-032012-095147
Doughty C., Faurby S., and Svenning J.-C. (2016c). The impact of the megafauna extinctions on savanna woody cover in South America. Ecography 39, 213–222. doi: 10.1111/ecog.01593
Doughty C. E., Faurby S., Wolf A., Malhi Y., and Svenning J.-C. (2016a). Changing NPP consumption patterns in the Holocene: From megafauna-’liberated’ NPP to ‘ecological bankruptcy.’. Anthr. Rev. 3, 174–187. doi: 10.1177/2053019616650466
Doughty C. E., Prys-Jones T. O., Faurby S., Abraham A. J., Hepp C., Leshyk V., et al. (2020). Megafauna decline have reduced pathogen dispersal which may have increased emergent infectious diseases. Ecography 43, 1107–1117. doi: 10.1111/ecog.05209
Doughty C. E., Wolf A., Morueta-Holme N., Jørgensen P. M., Sandel B., Violle C., et al. (2016b). Megafauna extinction, tree species range reduction, and carbon storage in Amazonian forests. Ecography 39, 194–203. doi: 10.1111/ecog.01587
Duval M., Martín-Francés L., and Wood R. (2025). On the impact of micro-CT scanning on radiocarbon dating of fossil material: A cautionary note for the palaeoanthropological community and beyond. Radiocarbon 1–10, 709–718. doi: 10.1017/RDC.2025.23
Edmeades B. T. (2021). Megafauna: first victims of the human-caused extinction (Austin, TX: Houndstooth Press).
Ellis E. C., Gauthier N., Klein Goldewijk K., Bliege Bird R., Boivin N., Díaz S., et al. (2021). People have shaped most of terrestrial nature for at least 12,000 years. Proc. Natl. Acad. Sci. U. S. A. 118, e2023483118. doi: 10.1073/pnas.2023483118
Emslie S. D. (1987). Age and diet of fossil california condors in grand canyon, arizona. Science 237, 768–770. doi: 10.1126/science.237.4816.768
Engel M. S., Ceríaco L. M. P., Daniel G. M., Dellapé P. M., Löbl I., Marinov M., et al. (2021). The taxonomic impediment: a shortage of taxonomists, not the lack of technical approaches. Zool. J. Linn. Soc 193, 381–387. doi: 10.1093/zoolinnean/zlab072
Eren M. I., Meltzer D. J., Story B., Buchanan B., Yeager D., and Bebber M. R. (2022). Not just for proboscidean hunting: on the efficacy and functions of Clovis fluted points. Journal of Archaeological Science: Reports 45, 103601. doi: 10.1016/j.jasrep.2022.103601
Faith J. T. (2011). Ungulate community richness, grazer extinctions, and human subsistence behavior in southern Africa’s Cape Floral Region. Palaeogeogr. Palaeoclimatol. Palaeoecol. 306, 219–227. doi: 10.1016/j.palaeo.2011.04.025
Faith J. T. and O’Connell J. F. (2011). Revisiting the late Pleistocene mammal extinction record at Tight Entrance Cave, southwestern Australia. Quat. Res. 76, 397–400. doi: 10.1016/j.yqres.2011.08.001
Fariña R. A. and Vizcaíno S. F. (2024). Giants beasts updated: A review of new knowledge about the South American megafauna. J. Quat. Sci. 39, 1139–1153. doi: 10.1002/jqs.3663
Faurby S., Davis M., Pedersen R. Ø., Schowanek S. D., Antonelli1 A., and Svenning J.-C. (2018). PHYLACINE 1.2: the phylogenetic atlas of mammal macroecology. Ecology 99, 2626–2626. doi: 10.1002/ecy.2443
Faurby S. and Svenning J.-C. (2015). Historic and prehistoric human-driven extinctions have reshaped global mammal diversity patterns. Divers. Distrib. 21, 1155–1166. doi: 10.1111/ddi.12369
Faurby S., Daniele S., Lars W., and Antonelli A. (2020). Brain expansion in early hominins predicts carnivore extinctions in East Africa. Ecology Letters 23, 537–544. doi: 10.1111/ele.13451
Fiedel S. J. (2018). The spore conundrum: Does a dung fungus decline signal humans’ arrival in the Eastern United States? Quat. Int. 466, 247–255. doi: 10.1016/j.quaint.2015.11.130
Fiedel S. J. (2022). Initial human colonization of the Americas, redux. Radiocarbon 64, 845–897. doi: 10.1017/RDC.2021.103
Field J. (2006). Trampling through the pleistocene: does taphonomy matter at cuddie springs? Aust. Archaeol. 63, 9–20. doi: 10.1080/03122417.2006.11681834
Field J., Fillios M., and Wroe S. (2008). Chronological overlap between humans and megafauna in Sahul (Pleistocene Australia–New Guinea): A review of the evidence. Earth-Science Rev. 89, 97–115. doi: 10.1016/j.earscirev.2008.04.006
Field J., Fullagar R., and Lord G. (2001). A large area archaeological excavation at Cuddie Springs. Antiquity 75, 696–702. doi: 10.1017/S0003598X00089195
Field J., Wroe S., Trueman C. N., Garvey J., and Wyatt-Spratt S. (2013). Looking for the archaeological signature in Australian Megafaunal extinctions. Quat. Int. 285, 76–88. doi: 10.1016/j.quaint.2011.04.013
Fillios M., Field J., and Charles B. (2010). Investigating human and megafauna co-occurrence in Australian prehistory: Mode and causality in fossil accumulations at Cuddie Springs. Quat. Int. 211, 123–143. doi: 10.1016/j.quaint.2009.04.003
Firestone R. B., West A., Kennett J. P., Becker L., Bunch T. E., Revay Z. S., et al. (2007). Evidence for an extraterrestrial impact 12,900 years ago that contributed to the megafaunal extinctions and the Younger Dryas cooling. Proc. Natl. Acad. Sci. U. S. A. 104, 16016–16021. doi: 10.1073/pnas.0706977104
Flemming J. (1826). The geological deluge, as interpreted by Baron Cuvier and Professor Buckland, inconsistent with the testimony of Moses and the phenomena of nature. Edinburgh Philos. J. 14, 205–239.
Flores J. C. (2014). Modelling Late Pleistocene megafaunal extinction and critical cases: A simple prey–predator perspective. Ecol. Model. 291, 218–223. doi: 10.1016/j.ecolmodel.2014.08.004
Fordham D. A., Brown S. C., Canteri E., Austin J. J., Lomolino M. V., Haythorne S., et al. (2024). 52,000 years of woolly rhinoceros population dynamics reveal extinction mechanisms. Proc. Natl. Acad. Sci. U. S. A. 121, e2316419121. doi: 10.1073/pnas.2316419121
Fuller B. T., Southon J. R., Fahrni S. M., Farrell A. B., Takeuchi G. T., Nehlich O., et al. (2020). Pleistocene paleoecology and feeding behavior of terrestrial vertebrates recorded in a pre-LGM asphaltic deposit at Rancho La Brea, California. Palaeogeogr. Palaeoclimatol. Palaeoecol. 537, 109383. doi: 10.1016/j.palaeo.2019.109383
Galetti M. and Dirzo R. (2013). Ecological and evolutionary consequences of living in a defaunated world. Biol. Conserv. 163, 1–6. doi: 10.1016/j.biocon.2013.04.020
Galetti M., Moleón M., Jordano P., Pires M. M., Guimarães P. R. Jr., Pape T., et al. (2018). Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. 93, 845–862. doi: 10.1111/brv.12374
Gallo V., Avilla L. S., Pereira R. C. L., and Absolon B. A. (2013). Distributional patterns of herbivore megamammals during the Late Pleistocene of South America. An. Acad. Bras. Ciênc. 85, 533–546. doi: 10.1590/S0001-37652013000200005
Garvey J., Cochrane B., Field J., and Boney C. (2011). Modern emu (Dromaius novaehollandiae) butchery, economic utility and analogues for the Australian archaeological record. Environ. Archaeol. 16, 97–112. doi: 10.1179/174963111X13110803260840
Gill J. L. (2014). Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169. doi: 10.1111/nph.12576
Gill J. L., Williams J. W., Jackson S. T., Donnelly J. P., and Schellinger G. C. (2012). Climatic and megaherbivory controls on late-glacial vegetation dynamics: a new, high-resolution, multi-proxy record from Silver Lake, Ohio. Quat. Sci. Rev. 34, 66–80. doi: 10.1016/j.quascirev.2011.12.008
Gillespie R., Horton D. R., Ladd P., Macumber P. G., Rich T. H., Thorne R., et al. (1978). Lancefield swamp and the extinction of the Australian megafauna. Science 200, 1044–1048. doi: 10.1126/science.200.4345.1044
Gillespie R., Wood R., Fallon S., Stafford T. W. Jr., and Southon J. (2015). New 14C dates for Spring Creek and Mowbray Swamp megafauna: XAD-2 processing. Archaeol. Ocean. 50, 43–48. doi: 10.1002/arco.5045
Gilmour D. M., Butler V. L., O’Connor J. E., Davis E. B., Culleton B. J., Kennett D. J., et al. (2015). Chronology and ecology of late pleistocene megafauna in the Northern Willamette valley, oregon. Quat. Res. 83, 127–136. doi: 10.1016/j.yqres.2014.09.003
Gonzalez S., Huddart D., Israde-Alcántara I., Dominguez-Vazquez G., and Bischoff J. (2014). Tocuila Mammoths, Basin of Mexico: Late Pleistocene–Early Holocene stratigraphy and the geological context of the bone accumulation. Quat. Sci. Rev. 96, 222–239. doi: 10.1016/j.quascirev.2014.02.003
Granger C. W. J. (1980). Testing for causality: A personal viewpoint. J. Econ. Dynamics Control 2, 329–352. doi: 10.1016/0165-1889(80)90069-X
Grayson D. (1989). “Nineteenth-century explanations of pleistocene extinctions: A review and analysis,” in Quaternary extinctions: a prehistoric revolution (University of Arizona press, Tucson (Ariz), 5–39.
Grayson D. K. and Meltzer D. J. (2003). A requiem for North American overkill. J. Archaeol. Sci. 30, 585–593. doi: 10.1016/S0305-4403(02)00205-4
Grayson D. K., Meltzer D. J., and Breslawski R. P. (2021). Overkill and the North American archaeological record—not guilty by association? A comment on Wolfe and Broughton, (2020). J. Archaeol. Sci. 128, 105312. doi: 10.1016/j.jas.2020.105312
Grün R., Eggins S., Wells R. T., Rhodes E., Bestland E., Spooner N., et al. (2006). A cautionary tale from down under: Dating the Black Creek Swamp megafauna site on Kangaroo Island, South Australia. Quat. Geochronol. 1, 142–150. doi: 10.1016/j.quageo.2006.05.029
Hansen D. M. and Galetti M. (2009). The forgotten megafauna. Science 324, 42–43. doi: 10.1126/science.1172393
Hansford J. P., Lister A. M., Weston E. M., and Turvey S. T. (2021). Simultaneous extinction of Madagascar’s megaherbivores correlates with late Holocene human-caused landscape transformation. Quat. Sci. Rev. 263, 106996. doi: 10.1016/j.quascirev.2021.106996
Hardy F. C. and Rowland S. M. (2024). Stable isotopic analysis of fossil Bison tooth enamel indicates flexible dietary ecology across Pleistocene North America. Quat. Sci. Rev. 334, 108741. doi: 10.1016/j.quascirev.2024.108741
Hauffe T., Cantalapiedra J. L., and Silvestro D. (2024). Trait-mediated speciation and human-driven extinctions in proboscideans revealed by unsupervised Bayesian neural networks. Science Advances 10, adl2643. doi: 10.1126/sciadv.adl2643
Haynes C. V. (2008). Younger Dryas “black mats” and the Rancholabrean termination in North America. Proc. Natl. Acad. Sci. U. S. A. 105, 6520–6525. doi: 10.1073/pnas.0800560105
Heddell-Stevens P., Jöris O., Britton K., Matthies T., Lucas M., Scott E., et al. (2024). Multi-isotope reconstruction of Late Pleistocene large-herbivore biogeography and mobility patterns in Central Europe. Commun. Biol. 7, 568. doi: 10.1038/s42003-024-06233-2
Hein A. (2016). The problem of typology in Chinese archaeology. Early China 39, 21–52. doi: 10.1017/eac.2015.18
Higham T. F. G., Jacobi R. M., and Ramsey C. B. (2006). AMS radiocarbon dating of ancient bone using ultrafiltration. Radiocarbon 48, 179–195. doi: 10.1017/S0033822200066388
Hixon S. W., Curtis J. H., Brenner M., Douglass K. G., Domic A. I., Culleton B. J., et al. (2021a). Drought Coincided with, but Does Not Explain, Late Holocene Megafauna Extinctions in SW Madagascar. Climate 9, 138. doi: 10.3390/cli9090138
Hixon S. W., Douglass K. G., Crowley B. E., Rakotozafy L. M. A., Clark G., Anderson A., et al. (2021b). Late Holocene spread of pastoralism coincides with endemic megafaunal extinction on Madagascar. Proc. R. Soc B. 288, 20211204. doi: 10.1098/rspb.2021.1204
Hocknull S. A., Lewis R., Arnold L. J., Pietsch T., Joannes-Boyau R., Price G. J., et al. (2020). Extinction of eastern Sahul megafauna coincides with sustained environmental deterioration. Nat. Commun. 11, 2250. doi: 10.1038/s41467-020-15785-w
Hocknull S. A., Zhao J., Feng Y., and Webb G. E. (2007). Responses of Quaternary rainforest vertebrates to climate change in Australia. Earth Planet. Sci. Lett. 264, 317–331. doi: 10.1016/j.epsl.2007.10.004
Holdaway R. N., Allentoft M. E., Jacomb C., Oskam C. L., Beavan N. R., and Bunce M. (2014). An extremely low-density human population exterminated New Zealand moa. Nat. Commun. 5, 5436. doi: 10.1038/ncomms6436
Holliday V. T., Daulton T. L., Bartlein P. J., Boslough M. B., Breslawski R. P., Fisher A. E., et al. (2023). Comprehensive refutation of the younger dryas impact hypothesis (YDIH). Earth-Science Rev. 247, 104502. doi: 10.1016/j.earscirev.2023.104502
Holliday V. T., Daulton T. L., Bartlein P. J., Boslough M. B., Breslawski R. P., Fisher A. E., et al. (2024). Rebuttal of Sweatman, Powell, and West’s “Rejection of Holliday et al.’s alleged refutation of the Younger Dryas Impact Hypothesis. Earth-Science Rev. 258, 104961. doi: 10.1016/j.earscirev.2024.104961
Hsieh S. and Plotnick R. E. (2020). The representation of animal behaviour in the fossil record. Anim. Behav. 169, 65–80. doi: 10.1016/j.anbehav.2020.09.010
Hubbe A., Hubbe M., Karmann I., Cruz F. W., and Neves W. A. (2011). Insights into Holocene megafauna survival and extinction in southeastern Brazil from new AMS 14C dates. Quat. Res. 79, 152–157. doi: 10.1016/j.yqres.2012.11.009
Hubbe A., Hubbe M., and Neves W. A. (2013). The Brazilian megamastofauna of the Pleistocene/Holocene transition and its relationship with the early human settlement of the continent. Earth-Science Rev. 118, 1–10. doi: 10.1016/j.earscirev.2013.01.003
Huntley B., Allen J. R. M., Collingham Y. C., Hickler T., Lister A. M., Singarayer J., et al. (2013). Millennial climatic fluctuations are key to the structure of last glacial ecosystems. PLoS One 8, e61963. doi: 10.1371/journal.pone.0061963
Iijima M., Qiao Y., Lin W., Peng Y., Yoneda M., and Liu J. (2022). An intermediate crocodylian linking two extant gharials from the Bronze Age of China and its human-induced extinction. Proc. R. Soc B. 289, 20220085. doi: 10.1098/rspb.2022.0085
Iminjili V., Crowther A., Fisher M. T., Kay A., Roberts P., Goldstein S., et al. (2025). A dataset of scientific dates from archaeological sites in eastern Africa spanning 5000 BCE to 1800 CE. Sci. Data 12, 801. doi: 10.1038/s41597-025-05138-x
Jacobi R. M. and Higham T. F. G. (2009). The early Lateglacial re-colonization of Britain: new radiocarbon evidence from Gough’s Cave, southwest England. Quat. Sci. Rev. 28, 1895–1913. doi: 10.1016/j.quascirev.2009.03.006
Johnson C. (2009). Ecological consequences of Late Quaternary extinctions of megafauna. Proc. R. Soc B. 276, 2509–2519. doi: 10.1098/rspb.2008.1921
Jones D. B. and Desantis L. R. G. (2017). Dietary ecology of ungulates from the La Brea tar pits in southern California: A multi-proxy approach. Palaeogeogr. Palaeoclimatol. Palaeoecol. 466, 110–127. doi: 10.1016/j.palaeo.2016.11.019
Joos J., Pimiento C., Miles D. B., and Müller J. (2022). Quaternary megafauna extinctions altered body size distribution in tortoises. Proc. R. Soc B. 289, 20221947. doi: 10.1098/rspb.2022.1947
Jukar A. M., Lyons S. K., Wagner P. J., and Uhen M. D. (2021). Late quaternary extinctions in the Indian subcontinent. Palaeogeogr. Palaeoclimatol. Palaeoecol. 562, 110137. doi: 10.1016/j.palaeo.2020.110137
Jukar A. M., Patnaik R., Chauhan P. R., Li H.-C., and Lin J.-P. (2019). The youngest occurrence of Hexaprotodon Falconer and Cautley 1836 (Hippopotamidae, Mammalia) from South Asia with a discussion on its extinction. Quat. Int. 528, 130–137. doi: 10.1016/j.quaint.2019.01.005
Kemp M. E. (2023). Defaunation and species introductions alter long-term functional trait diversity in insular reptiles. Proc. Natl. Acad. Sci. U. S. A. 120, e2201944119. doi: 10.1073/pnas.2201944119
Kemp C. W., Tibby J., Arnold L. J., and Barr C. (2019). Australian hydroclimate during Marine Isotope Stage 3: A synthesis and review. Quat. Sci. Rev. 204, 94–104. doi: 10.1016/j.quascirev.2018.11.016
Kimuyu D. M., Sensenig R. L., Riginos C., Veblen K. E., and Young T. P. (2014). Native and domestic browsers and grazers reduce fuels, fire temperatures, and acacia ant mortality in an African savanna. Ecol. Appl. 24, 741–749. doi: 10.1890/13-1135.1
Kissling W. D., Dalby L., Fløjgaard C., Lenoir J., Sandel B., Sandom C., et al. (2014). Establishing macroecological trait datasets: digitalization, extrapolation, and validation of diet preferences in terrestrial mammals worldwide. Ecol. Evol. 4, 2913–2930. doi: 10.1002/ece3.1136
Kistler L., Newsom L. A., Ryan T. M., Clarke A. C., Smith B. D., and Perry G. H. (2015). Gourds and squashes (Cucurbita spp.) adapted to megafaunal extinction and ecological anachronism through domestication. Proc. Natl. Acad. Sci. U. S. A. 112, 15107–15112. doi: 10.1073/pnas.1516109112
Kjær K. H., Winther Pedersen M., De Sanctis B., De Cahsan B., Korneliussen T. S., Michelsen C. S., et al. (2022). A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA. Nature 612, 283–291. doi: 10.1038/s41586-022-05453-y
Knell E. J. and Lee C. M. (2020). New radiocarbon dates from the late paleoIndian cody complex component at the lamb spring site, Douglas County, Colorado. PaleoAmerica 6, 374–378. doi: 10.1080/20555563.2020.1762408
Koch P. L. and Barnosky A. D. (2006). Late quaternary extinctions: state of the debate. Annu. Rev. Ecol. Evol. Syst. 37, 215–250. doi: 10.1146/annurev.ecolsys.34.011802.132415
Kolbert E. (2014). The sixth extinction: an unnatural history. 1st ed. (New York: Henry Holt and Company).
Kopels M. C. and Ullah I. I. (2024). Modeling post-Pleistocene megafauna extinctions as complex social-ecological systems. Quat. Res. 121, 1–14. doi: 10.1017/qua.2024.6
Koren L., Matas D., Pečnerová P., Dalén L., Tikhonov A., Gilbert M. T. P., et al. (2018). Testosterone in ancient hair from an extinct species. Palaeontology 61, 797–802. doi: 10.1111/pala.12391
Kosintsev P., Mitchell K. J., Devièse T., van der Plicht J., Kuitems M., Petrova E., et al. (2019). Evolution and extinction of the giant rhinoceros Elasmotherium sibiricum sheds light on late Quaternary megafaunal extinctions. Nat. Ecol. Evol. 3, 31–38. doi: 10.1038/s41559-018-0722-0
Koutamanis D., McCurry M., Tacail T., and Dosseto A. (2023). Reconstructing Pleistocene Australian herbivore megafauna diet using calcium and strontium isotopes. R. Soc Open Sci. 10, 230991. doi: 10.1098/rsos.230991
Kubiak C., Grimes V., Van Biesen G., Keddie G., Buckley M., Macdonald R., et al. (2023). Dietary niche separation of three Late Pleistocene bear species from Vancouver Island, on the Pacific Northwest Coast of North America. J. Quat. Sci. 38, 8–20. doi: 10.1002/jqs.3451
Lanoë F. B., Reuther J. D., Holmes C. E., and Hodgins G. W. L. (2017). Human paleoecological integration in subarctic eastern Beringia. Quat. Sci. Rev. 175, 85–96. doi: 10.1016/j.quascirev.2017.10.003
Larmon J. T., McDonald H. G., Ambrose S., DeSantis L. R. G., and Lucero L. J. (2019). A year in the life of a giant ground sloth during the Last Glacial Maximum in Belize. Sci. Adv. 5, eaau1200. doi: 10.1126/sciadv.aau1200
LaViolette P. A. (2011). Evidence for a solar flare cause of the pleistocene mass extinction. Radiocarbon 53, 303–323. doi: 10.1017/S0033822200056575
Leakey L. S. B. (1966). Africa and pleistocene overkill? Nature 212, 1615–1616. doi: 10.1038/2121615a0
Lemoine R. T., Buitenwerf R., and Svenning J.-C. (2023). Megafauna extinctions in the late-Quaternary are linked to human range expansion, not climate change. Anthropocene 44, 100403. doi: 10.1016/j.ancene.2023.100403
Lewis R. J., Tibby J., Arnold L. J., Barr C., Marshall J., McGregor G., et al. (2020). Insights into subtropical Australian aridity from Welsby Lagoon, north Stradbroke Island, over the past 80,000 years. Quat. Sci. Rev. 234, 106262. doi: 10.1016/j.quascirev.2020.106262
Li H., Sinha A., Anquetil André A., Spötl C., Vonhof H. B., Meunier A., et al. (2020). A multimillennial climatic context for the megafaunal extinctions in Madagascar and Mascarene Islands. Sci. Adv. 6, eabb2459. doi: 10.1126/sciadv.abb2459
Lima-Ribeiro M. S., Hortal J., Varela S., and Diniz-Filho J. A. F. (2014). Constraint envelope analyses of macroecological patterns reveal climatic effects on Pleistocene mammal extinctions. Quat. Res. 82, 260–269. doi: 10.1016/j.yqres.2014.02.003
Lima-Ribeiro M. S., Varela S., Nogués-Bravo D., and Diniz-Filho J. A. F. (2012). Potential suitable areas of giant ground sloths dropped before its extinction in South America: the evidences from bioclimatic envelope modeling. NatCon 10, 145–151. doi: 10.4322/natcon.2012.022
Lindsey E. L. and Lopez E. X. R. (2015). Tanque Loma, a new late-Pleistocene megafaunal tar seep locality from southwest Ecuador. J. S. Am. Earth Sci. 57, 61–82. doi: 10.1016/j.jsames.2014.11.003
Lister A. M. (2021). “Phenotypic plasticity in the fossil record,” in Phenotypic Plasticity & Evolution (Boca Raton, Florida, USA: CRC Press) 31–95.
Long C. A. and Yahnke C. J. (2011). End of the Pleistocene: elk-moose (Cervalces) and caribou (Rangifer) in Wisconsin. J. Mammal. 92, 1127–1135. doi: 10.1644/10-MAMM-A-395.1
Lopes R. P., Pereira J. C., Kerber L., and Dillenburg S. R. (2020). The extinction of the Pleistocene megafauna in the Pampa of southern Brazil. Quat. Sci. Rev. 242, 106428. doi: 10.1016/j.quascirev.2020.106428
Lopes dos Santos R. A., De Deckker P., Hopmans E. C., Magee J. W., Mets A., Sinninghe Damsté J. S., et al. (2013). Abrupt vegetation change after the late quaternary megafaunal extinction in Southeastern Australia. Nat. Geosci. 6, 627–631. doi: 10.1038/ngeo1856
Lord E., Dussex N., Kierczak M., Díez-del-Molino D., Ryder O. A., Stanton D. W. G., et al. (2020). Pre-extinction demographic stability and genomic signatures of adaptation in the woolly rhinoceros. Curr. Biol. 30, 3871–3879.e7. doi: 10.1016/j.cub.2020.07.046
Lorenzen E. D., Nogués-Bravo D., Orlando L., Weinstock J., Binladen J., Marske K. A., et al. (2011). Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–364. doi: 10.1038/nature10574
Louys J., Braje T. J., Chang C.-H., Cosgrove R., Fitzpatrick S. M., Fujita M., et al. (2021). No evidence for widespread island extinctions after Pleistocene hominin arrival. Proc. Natl. Acad. Sci. U. S. A. 118, e2023005118. doi: 10.1073/pnas.2023005118
Louys J., Curnoe D., and Tong H. (2007). Characteristics of Pleistocene megafauna extinctions in Southeast Asia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 243, 152–173. doi: 10.1016/j.palaeo.2006.07.011
Louys J. and Roberts P. (2020). Environmental drivers of megafauna and hominin extinction in Southeast Asia. Nature 586, 402–406. doi: 10.1038/s41586-020-2810-y
Louys J., Wilkinson D. M., and Bishop L. C. (2012). “Ecology needs a paleontological perspective,” in Paleontology in Ecology and Conservation. Ed. Louys J. (Springer, Berlin, Heidelberg), 23–38. doi: 10.1007/978-3-642-25038-5_3
Lubeek J. K. and Westaway K. E. (2020). Megabeasts under the microscope: a closer look at Quaternary extinctions in the Asia-Pacific. Quat. Int. 568, 1–19. doi: 10.1016/j.quaint.2020.08.015
Lundgren E. J., Ramp D., Rowan J., Middleton O., Schowanek S. D., Sanisidro O., et al. (2020). Introduced herbivores restore Late Pleistocene ecological functions. Proc. Natl. Acad. Sci. U. S. A. 117, 7871–7878. doi: 10.1073/pnas.1915769117
Lundgren E. J., Schowanek S. D., Rowan J., Middleton O., Pedersen R. Ø., Wallach A. D., et al. (2021). Functional traits of the world’s late Quaternary large-bodied avian and mammalian herbivores. Sci. Data 8, 17. doi: 10.1038/s41597-020-00788-5
Lyell C. (1832). Principles of Geology: An Attempt to Explain the Former Changes of the Earth’s Surface, by Reference to Causes now in Operation: Volume 2 (Cambridge: Cambridge University Press). doi: 10.1017/CBO9780511701566
MacPhee R. D. E. and Schouten P. (2019). End of the megafauna: the fate of the world’s hugest, fiercest, and strangest animals. 1st ed. (New York: W.W. Norton & Company).
Maidment S. and Butler R. J. (2025). New frontiers in dinosaur exploration. Biol. Lett. 21. doi: 10.1098/rsbl.2025.0045
Mann D. H., Groves P., Gaglioti B. V., and Shapiro B. A. (2019). Climate-driven ecological stability as a globally shared cause of Late Quaternary megafaunal extinctions: the Plaids and Stripes Hypothesis. Biol. Rev. 94, 328–352. doi: 10.1111/brv.12456
Mann D. H., Groves P., Kunz M. L., Reanier R. E., and Gaglioti B. V. (2013). Ice-age megafauna in Arctic Alaska: extinction, invasion, survival. Quat. Sci. Rev. 70, 91–108. doi: 10.1016/j.quascirev.2013.03.015
Marean C. W. (2015). An evolutionary anthropological perspective on modern human origins. Annu. Rev. Anthropol. 44, 533–556. doi: 10.1146/annurev-anthro-102313-025954
Markova A. K., Puzachenko A. Y., van Kolfschoten T., Kosintsev P. A., Kuznetsova T. V., Tikhonov A. N., et al. (2015). Changes in the Eurasian distribution of the musk ox (Ovibos moschatus) and the extinct bison (Bison priscus) during the last 50 ka BP. Quat. Int. 378, 99–110. doi: 10.1016/j.quaint.2015.01.020
Marshall C. R., Finnegan S., Clites E. C., Holroyd P. A., Bonuso N., Cortez C., et al. (2018). Quantifying the dark data in museum fossil collections as palaeontology undergoes a second digital revolution. Biol. Lett. 14, 20180431. doi: 10.1098/rsbl.2018.0431
Martin P. S. and Klein R. G. (1984). Quaternary extinctions: a prehistoric revolution (Tucson (Ariz: University of Arizona Press).
Martin P. S. and Wright H. E. (1967). Pleistocene Extinctions: the Search for a Cause (New Haven, Connecticut: Yale University Press).
Martínez G., Gutiérrez M. A., and Tonni E. P. (2013). Paleoenvironments and faunal extinctions: Analysis of the archaeological assemblages at the Paso Otero locality (Argentina) during the Late Pleistocene–Early Holocene. Quat. Int. 299, 53–63. doi: 10.1016/j.quaint.2012.08.2103
Mather E. K., Lee M. S. Y., and Worthy T. H. (2022). A new look at an old Australian raptor places “Taphaetus” lacertosus de Vis 1905 in the Old World vultures (Accipitridae: Aegypiinae. Zootaxa 5168, 1–23. doi: 10.11646/zootaxa.5168.1.1
McCormack J., Griffiths M. L., Kim S. L., Shimada K., Karnes M., Maisch H., et al. (2022). Trophic position of Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nat. Commun. 13, 2980. doi: 10.1038/s41467-022-30528-9
McElreath R. (2020). Statistical Rethinking: A Bayesian Course with Examples in R and STAN. 2nd (New York: Chapman and Hall/CRC). doi: 10.1201/9780429029608
McInerney P. L., Arnold L. J., Burke C., Camens A. B., and Worthy T. H. (2022). Multiple occurrences of pathologies suggesting a common and severe bone infection in a population of the Australian Pleistocene giant, Genyornis newtoni (Aves, Dromornithidae). Papers Palaeontol. 8, e1415. doi: 10.1002/spp2.1415
McNeil P., Hills L. V., Kooyman B., and Tolman S. M. (2005). Mammoth tracks indicate a declining Late Pleistocene population in southwestern Alberta, Canada. Quat. Sci. Rev. 24, 1253–1259. doi: 10.1016/j.quascirev.2004.08.019
Meltzer D. J. (2020). Overkill, glacial history, and the extinction of North America’s Ice Age megafauna. Proc. Natl. Acad. Sci. U. S. A. 117, 28555–28563. doi: 10.1073/pnas.2015032117
Meltzer D. J. and Mead J. I. (1983). The timing of Late Pleistocene mammalian extinctions in North America. Quat. Res. 19, 130–135. doi: 10.1016/0033-5894(83)90032-7
Miller G., Magee J., Smith M., Spooner N., Baynes A., Lehman S., et al. (2016). Human predation contributed to the extinction of the Australian megafaunal bird Genyornis newtoni ∼47 ka. Nat. Commun. 7, 10496. doi: 10.1038/ncomms10496
Moher D., Liberati A., Tetzlaff J., and Altman D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535. doi: 10.1136/bmj.b2535
Moleón M., Sánchez-Zapata J. A., Donázar J. A., Revilla E., Martín-López B., Gutiérrez-Cánovas C., et al. (2020). Rethinking megafauna. Proc. R. Soc B. 287, 20192643. doi: 10.1098/rspb.2019.2643
Mondanaro A., Di Febbraro M., Melchionna M., Maiorano L., Di Marco M., Edwards N. R., et al. (2021). The role of habitat fragmentation in Pleistocene megafauna extinction in Eurasia. Ecography 44, 1619–1630. doi: 10.1111/ecog.05939
Monjeau J. A., Araujo B., Abramson G., Kuperman M. N., Laguna M. F., and Lanata J. L. (2017). The controversy space on Quaternary megafaunal extinctions. Quat. Int. 431, 194–204. doi: 10.1016/j.quaint.2015.10.022
Moore C. R., Kimball L. R., Goodyear A. C., Brooks M. J., Daniel I. R., West A., et al. (2023). Paleoamerican exploitation of extinct megafauna revealed through immunological blood residue and microwear analysis, North and South Carolina, USA. Sci. Rep. 13, 9464. doi: 10.1038/s41598-023-36617-z
Morris S. D., Kearney M. R., Johnson C. N., and Brook B. W. (2022). Too hot for the devil? Did climate change cause the mid-Holocene extinction of the Tasmanian devil Sarcophilus harrisii from mainland Australia? Ecography 2022. doi: 10.1111/ecog.05799
Mothé D., dos Santos Avilla L., Asevedo L., Borges-Silva L., Rosas M., Labarca-Encina R., et al. (2017). Sixty years after ‘The mastodonts of Brazil’: The state of the art of South American proboscideans (Proboscidea, Gomphotheriidae). Quat. Int. 443, 52–64. doi: 10.1016/j.quaint.2016.08.028
Murchie T. J., Monteath A. J., Mahony M. E., Long G. S., Cocker S., Sadoway T., et al. (2021). Collapse of the mammoth-steppe in central Yukon as revealed by ancient environmental DNA. Nat. Commun. 12, 7120. doi: 10.1038/s41467-021-27439-6
Nagaoka L. (2012). Conservation Biology and Applied Zooarchaeology (Arizona, USA: University of Arizona Press). Conservation Biology and Applied Zooarchaeology edited by Steve Wolverton and R. Lee Lyman. 110–138. doi: 10.2307/j.ctt180r2x3.9
Nagaoka L., Rick T., and Wolverton S. (2018). The overkill model and its impact on environmental research. Ecol. Evol. 8, 9683–9696. doi: 10.1002/ece3.4393
Németh A., Bárány A., Csorba G., Magyari E., Pazonyi P., and Pálfy J. (2017). Holocene mammal extinctions in the Carpathian Basin: a review. Mamm. Rev. 47, 38–52. doi: 10.1111/mam.12075
Newsome S. D., Miller G. H., Magee J. W., and Fogel M. L. (2011). Quaternary record of aridity and mean annual precipitation based on δ15N in ratite and dromornithid eggshells from Lake Eyre, Australia. Oecologia 167, 1151–1162. doi: 10.1007/s00442-011-2046-5
Nikolskiy P. and Pitulko V. (2013). Evidence from the Yana Palaeolithic site, Arctic Siberia, yields clues to the riddle of mammoth hunting. J. Archaeol. Sci. 40, 4189–4197. doi: 10.1016/j.jas.2013.05.020
Novaro A. J. and Walker R. S. (2021). Lessons of 15,000 years of human–wildlife interaction for conservation in patagonia in the 21st century. Diversity 13, 633. doi: 10.3390/d13120633
Nyström V., Humphrey J., Skoglund P., Mckeown N. J., Vartanyan S., Shaw P. W., et al. (2012). Microsatellite genotyping reveals end-Pleistocene decline in mammoth autosomal genetic variation. Mol. Ecol. 21, 3391–3402. doi: 10.1111/j.1365-294X.2012.05525.x
O’Brien K., Podkovyroff K., Fernandez D. P., Tryon C. A., Cerling T. E., Ashioya L., et al. (2024). Limited herbivore migration during the Last Glacial Period of Kenya. Nat. Ecol. Evol. 8, 1191–1198. doi: 10.1038/s41559-024-02413-9
O’Connell J. F. and Allen J. (2004). Dating the colonization of Sahul (Pleistocene Australia–New Guinea): a review of recent research. J. Archaeol. Sci. 31, 835–853. doi: 10.1016/j.jas.2003.11.005
O’Keefe F. R., Dunn R. E., Weitzel E. M., Waters M. R., Martinez L. N., Binder W. J., et al. (2023). Pre–Younger Dryas megafaunal extirpation at Rancho La Brea linked to fire-driven state shift. Science 381, eabo3594. doi: 10.1126/science.abo3594
Otárola-Castillo E. R., Torquato M. G., Keevil T. L., May A., Coon S., Stow E. J., et al. (2023). A new approach to the quantitative analysis of bone surface modifications: the bowser road mastodon and implications for the data to understand human-megafauna interactions in North America. J. Archaeol. Method Theory 30, 1028–1063. doi: 10.1007/s10816-022-09583-5
Owen-Smith N. (1989). Megafaunal extinctions: the conservation message from 11,000 years B.P. Conserv. Biol. 3, 405–412. doi: 10.1111/j.1523-1739.1989.tb00246.x
Papa M. D., Reyes M. D. L., Poiré D. G., Rascovan N., Jofré G., and Delgado M. (2024). Anthropic cut marks in extinct megafauna bones from the Pampean region (Argentina) at the last glacial maximum. PLoS One 19, e0304956. doi: 10.1371/journal.pone.0304956
Pardi M. I. and DeSantis L. R. G. (2022). Interpreting spatially explicit variation in dietary proxies through species distribution modeling reveals foraging preferences of mammoth (Mammuthus) and American mastodon (Mammut americanum). Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.1064299
Parkinson J. (1811). Organic remains of a former world: an examination of the mineralized remains of the vegetables and animals of the Antediluvian world; generally termed extraneous fossils (London: M.A. Nattali). doi: 10.5962/bhl.title.131022
Parry L. E. and Eichenberg E. E. (2024). Interdisciplinary approaches to reconciling legacy paleontological collections to advance discovery and improve resource management. Parks Stewardship Forum 40, 257–270. doi: 10.5070/P540162934
Paterson R. S., Mackie M., Capobianco A., Heckeberg N. S., Fraser D., Demarchi B., et al. (2025). Phylogenetically informative proteins from an Early Miocene rhinocerotid. Nature 643, 719–724. doi: 10.1038/s41586-025-09231-4
Pederzani S., Britton K., Trost M., Fewlass H., Bourgon N., McCormack J., et al. (2024). Stable isotopes show Homo sapiens dispersed into cold steppes ~45,000 years ago at Ilsenhöhle in Ranis, Germany. Nat. Ecol. Evol. 8, 578–588. doi: 10.1038/s41559-023-02318-z
Pedrono M., Griffiths O. L., Clausen A., Smith L. L., Griffiths C. J., Wilmé L., et al. (2013). Using a surviving lineage of Madagascar’s vanished megafauna for ecological restoration. Biol. Conserv. 159, 501–506. doi: 10.1016/j.biocon.2012.11.027
Pereira A. G, Antonelli A., Silvestro D., and Faurby S. (2024). Two major extinction events in the evolutionary history of turtles: one caused by an asteroid, the other by hominins. The American Naturalist 203, 644–654. doi: 10.1086/729604
Perrotti A. G. (2018). Pollen and Sporormiella evidence for terminal Pleistocene vegetation change and megafaunal extinction at Page-Ladson, Florida. Quat. Int. 466, 256–268. doi: 10.1016/j.quaint.2017.10.015
Perry G. L. W., Wheeler A. B., Wood J. R., and Wilmshurst J. M. (2014). A high-precision chronology for the rapid extinction of New Zealand moa (Aves, Dinornithiformes). Quat. Sci. Rev. 105, 126–135. doi: 10.1016/j.quascirev.2014.09.025
Peters C., Oertle A., Gillespie R., Boivin N., and Douka K. (2025). Collagen peptide markers for three extinct Australian megafauna species. Front. Mamm. Sci. 4. doi: 10.3389/fmamm.2025.1564287
Peters K. J., Saltré F., Friedrich T., Jacobs Z., Wood R., McDowell M., et al. (2019). FosSahul 2.0, an updated database for the Late Quaternary fossil records of Sahul. Sci. Data 6, 272. doi: 10.1038/s41597-019-0267-3
Peters C., Wang Y., Vakil V., Cramb J., Dortch J., Hocknull S., et al. (2023). Bone collagen from subtropical Australia is preserved for more than 50,000 years. Commun. Earth. Environ. 4, 438. doi: 10.1038/s43247-023-01114-8
Pilowsky J. A., Brown S. C., Llamas B., van Loenen A. L., Kowalczyk R., Hofman-Kamińska E., et al. (2023). Millennial processes of population decline, range contraction and near extinction of the European bison. Proc. R. Soc B. 290, 20231095. doi: 10.1098/rspb.2023.1095
Pinter N., Scott A. C., Daulton T. L., Podoll A., Koeberl C., Anderson R. S., et al. (2011). The Younger Dryas impact hypothesis: A requiem. Earth-Science Rev. 106, 247–264. doi: 10.1016/j.earscirev.2011.02.005
Pires M. M. (2024). The restructuring of ecological networks by the pleistocene extinction. Annu. Rev. Earth Planet. Sci. 52, 133–158. doi: 10.1146/annurev-earth-040722-104845
Pires M. M., Guimarães P. R., Galetti M., and Jordano P. (2018). Pleistocene megafaunal extinctions and the functional loss of long-distance seed-dispersal services. Ecography 41, 153–163. doi: 10.1111/ecog.03163
Plint T., Longstaffe F. J., and Zazula G. (2019). Giant beaver palaeoecology inferred from stable isotopes. Sci. Rep. 9, 7179. doi: 10.1038/s41598-019-43710-9
Plotnick R. E. (2012). Behavioral biology of trace fossils. Paleobiology 38, 459–473. doi: 10.1666/11008.1
Politis G. G. and Messineo P. G. (2008). The Campo Laborde site: New evidence for the Holocene survival of Pleistocene megafauna in the Argentine Pampas. Quat. Int. 191, 98–114. doi: 10.1016/j.quaint.2007.12.003
Powell J. L. (2022). Premature rejection in science: The case of the Younger Dryas Impact Hypothesis. Sci. Prog. 105, 368504211064272. doi: 10.1177/00368504211064272
Prates L. and Perez S. I. (2021). Late Pleistocene South American megafaunal extinctions associated with rise of Fishtail points and human population. Nat. Commun. 12, 2175. doi: 10.1038/s41467-021-22506-4
Prescott G. W., Williams D. R., Balmford A., Green R. E., and Manica A. (2012). Quantitative global analysis of the role of climate and people in explaining late Quaternary megafaunal extinctions. Proc. Natl. Acad. Sci. U. S. A. 109, 4527–4531. doi: 10.1073/pnas.1113875109
Presslee S., Slater G. J., Pujos F., Forasiepi A. M., Fischer R., Molloy K., et al. (2019). Palaeoproteomics resolves sloth relationships. Nat. Ecol. Evol. 3, 1121–1130. doi: 10.1038/s41559-019-0909-z
Price G. J., Ferguson K. J., Webb G. E., Feng Y., Higgins P., Nguyen A. D., et al. (2017). Seasonal migration of marsupial megafauna in Pleistocene Sahul (Australia–New Guinea). Proc. R. Soc B. 284, 20170785. doi: 10.1098/rspb.2017.0785
Price G. J., Fitzsimmons K. E., Nguyen A. D., Zhao J., Feng Y., Sobbe I. H., et al. (2021). New ages of the world’s largest-ever marsupial: Diprotodon optatum from Pleistocene Australia. Quat. Int. 603, 64–73. doi: 10.1016/j.quaint.2021.06.013
Price G. J., Louys J., Faith J. T., Lorenzen E., and Westaway M. C. (2018). Big data little help in megafauna mysteries. Nature 558, 23–25. doi: 10.1038/d41586-018-05330-7
Price G. J., Webb G. E., Zhao J., Feng Y., Murray A. S., Cooke B. N., et al. (2011). Dating megafaunal extinction on the Pleistocene Darling Downs, eastern Australia: the promise and pitfalls of dating as a test of extinction hypotheses. Quat. Sci. Rev. 30, 899–914. doi: 10.1016/j.quascirev.2011.01.011
Prideaux G. J., Long J. A., Ayliffe L. K., Hellstrom J. C., Pillans B., Boles W. E., et al. (2007). An arid-adapted middle Pleistocene vertebrate fauna from south-central Australia. Nature 445, 422–425. doi: 10.1038/nature05471
Puzachenko A. Y., Levchenko V. A., Bertuch F., Zazovskaya E. P., and Kirillova I. V. (2021). Late Pleistocene chronology and environment of woolly rhinoceros (Coelodonta antiquitatis (Blumenbach 1799)) in beringia. Quat. Sci. Rev. 263, 106994. doi: 10.1016/j.quascirev.2021.106994
Rabanus-Wallace M. T., Wooller M. J., Zazula G. D., Shute E., Jahren A. H., Kosintsev P., et al. (2017). Megafaunal isotopes reveal role of increased moisture on rangeland during late Pleistocene extinctions. Nat. Ecol. Evol. 1, 1–5. doi: 10.1038/s41559-017-0125
Raczka M. F., Bush M. B., and Oliveira P. E. D. (2018). The collapse of megafaunal populations in southeastern Brazil. Quat. Res. 89, 103–118. doi: 10.1017/qua.2017.60
Rafiq K., Jordan N. R., McNutt J. W., Neelo J., Attias N., Boersma D., et al. (2024). Removing institutional barriers to long-term fieldwork is critical for advancing ecology. Trends Ecol. Evol. 39, 1059–1062. doi: 10.1016/j.tree.2024.10.003
Rawlence N. J., Metcalf J. L., Wood J. R., Worthy T. H., Austin J. J., and Cooper A. (2012). The effect of climate and environmental change on the megafaunal moa of New Zealand in the absence of humans. Quat. Sci. Rev. 50, 141–153. doi: 10.1016/j.quascirev.2012.07.004
Reed C. A. (1970). Extinction of mammalian megafauna in the old world late quaternary. BioScience 20, 284–288. doi: 10.2307/1295207
Reisz R. R. and Sues H.-D. (2015). The challenges and opportunities for research in paleontology for the next decade. Front. Earth Sci. 3. doi: 10.3389/feart.2015.00009
Rey-Iglesia A., Pryor A. J. E., de Jager D., Wilson T., Teeter M. A., Margaryan A., et al. (2025). Ancient biomolecular analysis of 39 mammoth individuals from Kostenki 11-Ia elucidates Upper Palaeolithic human resource use. Quaternary Environ. Humans 3, 100049. doi: 10.1016/j.qeh.2024.100049
Ríos-Saldaña C. A., Delibes-Mateos M., and Ferreira C. C. (2018). Are fieldwork studies being relegated to second place in conservation science? Glob. Ecol. Conserv. 14, e00389. doi: 10.1016/j.gecco.2018.e00389
Roberts R. G., Flannery T. F., Ayliffe L. K., Yoshida H., Olley J. M., Prideaux G. J., et al. (2001). New ages for the last Australian megafauna: continent-wide extinction about 46,000 years ago. Science 292, 1888–1892. doi: 10.1126/science.1060264
Robu M., Marom N., Mirea I.-C., Faur L.-M., Petculescu A., Kenesz M., et al. (2024). Intraguild interactions among carnivorans of the last glacial: The case of wolves and bears from Muierilor Cave, Romania. Quat. Sci. Rev. 334, 108720. doi: 10.1016/j.quascirev.2024.108720
Rouse L. M., Dupuy P. N. D., Abdykanova A., Brite E. B., Hermes T. R., Kidd F., et al. (2024). Re]Integrating a dispersed agenda: advancing archaeological research in Central Eurasia. Antiquity 98, 1088–1096. doi: 10.15184/aqy.2024.73
Rull V. (2010). Ecology and palaeoecology: two approaches, one objective. Open Ecol. J. 3, 1–5. doi: 10.2174/1874213001003020001
Saltré F., Chadoeuf J., Peters K. J., McDowell M. C., Friedrich T., Timmermann A., et al. (2019). Climate-human interaction associated with southeast Australian megafauna extinction patterns. Nat. Commun. 10, 5311. doi: 10.1038/s41467-019-13277-0
Saltré F., Rodríguez-Rey M., Brook B. W., Johnson C. N., Turney C. S. M., Alroy J., et al. (2016). Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 7, 10511. doi: 10.1038/ncomms10511
Sandom C. J., Ejrnæs R., Hansen M. D. D., and Svenning J.-C. (2014b). High herbivore density associated with vegetation diversity in interglacial ecosystems. Proc. Natl. Acad. Sci. U. S. A. 111, 4162–4167. doi: 10.1073/pnas.1311014111
Sandom C., Faurby S., Sandel B., and Svenning J.-C. (2014a). Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. R. Soc B. 281, 20133254. doi: 10.1098/rspb.2013.3254
Seeber P. A., Batke L., Dvornikov Y., Schmidt A., Wang Y., Stoof-Leichsenring K., et al. (2024). Mitochondrial genomes of Pleistocene megafauna retrieved from recent sediment layers of two Siberian lakes. eLife 12, RP89992. doi: 10.7554/eLife.89992
Seersholm F. V., Werndly D. J., Grealy A., Johnson T., Keenan Early E. M., Lundelius E. L., et al. (2020). Rapid range shifts and megafaunal extinctions associated with late Pleistocene climate change. Nat. Commun. 11, 2770. doi: 10.1038/s41467-020-16502-3
Shapiro B., Drummond A. J., Rambaut A., Wilson M. C., Matheus P. E., Sher A. V., et al. (2004). Rise and fall of the beringian steppe bison. Science 306, 1561–1565. doi: 10.1126/science.1101074
Smilg J. S. and Berger L. R. (2015). Discovering hominins - application of medical computed tomography (CT) to fossil-bearing rocks from the site of malapa, South Africa. PLoS One 10, e0145340. doi: 10.1371/journal.pone.0145340
Smith H. E., Bevitt J. J., Zaim J., Rizal Y., Puspaningrum M. R., Trihascaryo A., et al. (2021). High-resolution high-throughput thermal neutron tomographic imaging of fossiliferous cave breccias from Sumatra. Sci. Rep. 11, 19953. doi: 10.1038/s41598-021-99290-0
Smith F. A., Elliott Smith E. A., Villaseñor A., Tomé C. P., Lyons S. K., and Newsome S. D. (2022). Late Pleistocene megafauna extinction leads to missing pieces of ecological space in a North American mammal community. Proc. Natl. Acad. Sci. U. S. A. 119, e2115015119. doi: 10.1073/pnas.2115015119
Smith F. A., Tomé C. P., Elliott Smith E. A., Lyons S. K., Newsome S. D., and Stafford T. W. (2016). Unraveling the consequences of the terminal Pleistocene megafauna extinction on mammal community assembly. Ecography 39, 223–239. doi: 10.1111/ecog.01779
Soga M. and Gaston K. J. (2025). Extinction of experience among ecologists. Trends Ecol. Evol. 40, 212–215. doi: 10.1016/j.tree.2024.12.010
Stephens L., Fuller D., Boivin N., Rick T., Gauthier N., Kay A., et al. (2019). Archaeological assessment reveals Earth’s early transformation through land use. Science 365, 897–902. doi: 10.1126/science.aax1192
Stewart M., Carleton W. C., and Groucutt H. S. (2021). Climate change, not human population growth, correlates with Late Quaternary megafauna declines in North America. Nat. Commun. 12, 965. doi: 10.1038/s41467-021-21201-8
Stewart M., Carleton W. C., and Groucutt H. S. (2022). Reply to: Accurate population proxies do not exist between 11.7 and 11.5 ka in North America. Nature Communications 12, 4693. doi: 10.1038/s41467-022-32355-4
Stuart A. J. (1991). Mammalian extinctions in the late pleistocene of Northern Eurasia and North America. Biol. Rev. 66, 453–562. doi: 10.1111/j.1469-185X.1991.tb01149.x
Stuart A. J. (2015). Late Quaternary megafaunal extinctions on the continents: a short review. Geol. J. 50, 338–363. doi: 10.1002/gj.2633
Stuart A. J. (2021). Vanished giants: the lost world of the Ice Age (Chicago: The University of Chicago Press).
Stuart A. J. and Lister A. M. (2011). Extinction chronology of the cave lion Panthera spelaea. Quat. Sci. Rev. 30, 2329–2340. doi: 10.1016/j.quascirev.2010.04.023
Stuiver M., Reimer P. J., Bard E., Beck J. W., Burr G. S., Hughen K. A., et al. (1998). INTCAL98 radiocarbon age calibration, 24,000–0 cal BP. Radiocarbon 40, 1041–1083. doi: 10.1017/S0033822200019123
Surovell T. A. and Grund B. S. (2012). The Associational Critique of Quaternary Overkill and why it is Largely Irrelevant to the Extinction Debate. Am. Antiq. 77, 672–687. doi: 10.7183/0002-7316.77.4.672
Svenning J.-C., Lemoine R. T., Bergman J., Buitenwerf R., Le Roux E., Lundgren E., et al. (2024). The late-Quaternary megafauna extinctions: patterns, causes, ecological consequences, and implications for ecosystem management in the Anthropocene. Camb. Prisms Extinction 2, 1–68. doi: 10.1017/ext.2024.4
Svenning J.-C., Pedersen P. B. M., Donlan C. J., Ejrnæs R., Faurby S., Galetti M., et al. (2016). Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc. Natl. Acad. Sci. U. S. A. 113, 898–906. doi: 10.1073/pnas.1502556112
Swanson H. A., Svenning J.-C., Saxena A., Muscarella R., Franklin J., Garbelotto M., et al. (2021). History as grounds for interdisciplinarity: promoting sustainable woodlands via an integrative ecological and socio-cultural perspective. One Earth 4, 226–237. doi: 10.1016/j.oneear.2021.01.006
Swift J. A., Bunce M., Dortch J., Douglass K., Faith J. T., Fellows Yates J. A., et al. (2019). Micro methods for megafauna: novel approaches to late quaternary extinctions and their contributions to faunal conservation in the anthropocene. BioScience 69, 877–887. doi: 10.1093/biosci/biz105
Tejada J. V., Flynn J. J., MacPhee R., O’Connell T. C., Cerling T. E., Bermudez L., et al. (2021). Isotope data from amino acids indicate Darwin’s ground sloth was not an herbivore. Sci. Rep. 11, 18944. doi: 10.1038/s41598-021-97996-9
Turner G. (1799). Memoir on the extraneous fossils, denominated mammoth bones: principally designed to shew, that they are the remains of more than one species of nondescript animal. Trans. Am. Philos. Soc. 4, 510–518. doi: 10.2307/1005126
Turney C. S. M., Flannery T. F., Roberts R. G., Reid C., Fifield L. K., Higham T. F. G., et al. (2008). Late-surviving megafauna in Tasmania, Australia, implicate human involvement in their extinction. Proc. Natl. Acad. Sci. U. S. A. 105, 12150–12153. doi: 10.1073/pnas.0801360105
Ubilla M., Rinderknecht A., Corona A., and Perea D. (2018). Mammals in last 30 to 7 ka interval (Late pleistocene-early holocene) in Southern Uruguay (Santa lucía River Basin): Last occurrences, climate, and biogeography. J. Mammal Evol. 25, 291–300. doi: 10.1007/s10914-017-9380-2
Vachula R. S., Huang Y., Russell J. M., Abbott M. B., Finkenbinder M. S., and O’Donnell J. A. (2020). Sedimentary biomarkers reaffirm human impacts on northern Beringian ecosystems during the Last Glacial period. Boreas 49, 514–525. doi: 10.1111/bor.12449
van der Valk T., Pečnerová P., Díez-del-Molino D., Bergström A., Oppenheimer J., Hartmann S., et al. (2021). Million-year-old DNA sheds light on the genomic history of mammoths. Nature 591, 265–269. doi: 10.1038/s41586-021-03224-9
van Geel B., Langeveld B. W., Mol D., van der Knaap P. W. O., and van Leeuwen J. F. N. (2019). Pollen and spores from molar folds reflect food choice of late Pleistocene and Early Holocene herbivores in The Netherlands and the adjacent North Sea area. Quat. Sci. Rev. 225, 106030. doi: 10.1016/j.quascirev.2019.106030
Van Valkenburgh B., Hayward M. W., Ripple W. J., Meloro C., and Roth V. L. (2016). The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc. Natl. Acad. Sci. U. S. A. 113, 862–867. doi: 10.1073/pnas.1502554112
Villavicencio N. A. and Werdelin L. (2018). The Casa del Diablo cave (Puno, Peru) and the late Pleistocene demise of megafauna in the Andean Altiplano. Quat. Sci. Rev. 195, 21–31. doi: 10.1016/j.quascirev.2018.07.013
Waguespack N. M. (2007). Why we’re still arguing about the Pleistocene occupation of the Americas. Evol. Anthropol. Issues News Rev. 16, 63–74. doi: 10.1002/evan.20124
Wan X. and Zhang Z. (2017). Climate warming and humans played different roles in triggering Late Quaternary extinctions in east and west Eurasia. Proc. R. Soc. B: Biol. Sci. 284, 20162438. doi: 10.1098/rspb.2016.2438
Wang H., Sterli J., Dupret V., Blom H., Berta A., Turner S., et al. (2025). A decade of vertebrate palaeontology research: global taxa distribution, gender dynamics and evolving methodologies. R Soc. Open Sci. 12, 250263. doi: 10.1098/rsos.250263
Waters M. R., Stafford T. W., Kooyman B., and Hills L. V. (2015). Late Pleistocene horse and camel hunting at the southern margin of the ice-free corridor: Reassessing the age of Wally’s Beach, Canada. Proc. Natl. Acad. Sci. U. S. A. 112, 4263–4267. doi: 10.1073/pnas.1420650112
Webb S. (2008). Megafauna demography and late Quaternary climatic change in Australia: A predisposition to extinction. Boreas 37, 329–345. doi: 10.1111/j.1502-3885.2008.00026.x
Welker F., Smith G. M., Hutson J. M., Kindler L., Garcia-Moreno A., Villaluenga A., et al. (2017). Middle Pleistocene protein sequences from the rhinoceros genus Stephanorhinus and the phylogeny of extant and extinct Middle/Late Pleistocene Rhinocerotidae. PeerJ 5, e3033. doi: 10.7717/peerj.3033
Westaway M. C., Olley J., and Grün R. (2017). At least 17,000 years of coexistence: Modern humans and megafauna at the Willandra Lakes, South-Eastern Australia. Quat. Sci. Rev. 157, 206–211. doi: 10.1016/j.quascirev.2016.11.031
Wilman H., Belmaker J., Simpson J., de la Rosa C., Rivadeneira M. M., and Jetz W. (2014). EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 95, 2027–2027. doi: 10.1890/13-1917.1
Wolfe A. L. and Broughton J. M. (2020). A foraging theory perspective on the associational critique of North American Pleistocene overkill. J. Archaeol. Sci. 119, 105162. doi: 10.1016/j.jas.2020.105162
Wooller M. J., Bataille C., Druckenmiller P., Erickson G. M., Groves P., Haubenstock N., et al. (2021). Lifetime mobility of an Arctic woolly mammoth. Science 373, 806–808. doi: 10.1126/science.abg1134
Wroe S. and Field J. (2006). A review of the evidence for a human role in the extinction of Australian megafauna and an alternative interpretation. Quat. Sci. Rev. 25, 2692–2703. doi: 10.1016/j.quascirev.2006.03.005
Wroe S., Field J., Fullagar R., and Jermin L. S. (2004). Megafaunal extinction in the late Quaternary and the global overkill hypothesis. Alcheringa 28, 291–331. doi: 10.1080/03115510408619286
Yaworsky P. M., Hussain S. T., and Riede F. (2023). Climate-driven habitat shifts of high-ranked prey species structure Late Upper Paleolithic hunting. Sci. Rep. 13, 4238. doi: 10.1038/s41598-023-31085-x
Zazula G. D., MacPhee R. D. E., Metcalfe J. Z., Reyes A. V., Brock F., Druckenmiller P. S., et al. (2014). American mastodon extirpation in the Arctic and Subarctic predates human colonization and terminal Pleistocene climate change. Proc. Natl. Acad. Sci. U. S. A. 111, 18460–18465. doi: 10.1073/pnas.1416072111
Zazula G. D., MacPhee R. D. E., Southon J., Nalawade-Chavan S., Reyes A. V., Hewitson S., et al. (2017). A case of early Wisconsinan “over-chill”: New radiocarbon evidence for early extirpation of western camel (Camelops hesternus) in eastern Beringia. Quat. Sci. Rev. 171, 48–57. doi: 10.1016/j.quascirev.2017.06.031
Zeller U. and Göttert T. (2021). Humans, megafauna and landscape structure – Rock engravings from Namibia encourage a comparative approach to central Europe and southern Africa. Vert. Zool. 71, 631–643. doi: 10.3897/vz.71.e72811
Zhang Y., Westaway K. E., Haberle S., Lubeek J. K., Bailey M., Ciochon R., et al. (2024). The demise of the giant ape Gigantopithecus blacki. Nature 625, 535–539. doi: 10.1038/s41586-023-06900-0
Ziegler M. J., Perez V. J., Pirlo J., Narducci R. E., Moran S. M., Selba M. C., et al. (2020). Applications of 3D paleontological data at the Florida museum of natural history. Front. Earth Sci. 8. doi: 10.3389/feart.2020.600696
Zimov S. and Zimov N. (2014). Role of megafauna and frozen soil in the atmospheric CH4 dynamics. PLoS One 9, e93331. doi: 10.1371/journal.pone.0093331
Keywords: megafauna extinctions, Pleistocene, Holocene, conservation, rewilding, biases
Citation: Stewart M, Peters C, Ziegler MJ, Carleton WC, Roberts P, Boivin N and Groucutt HS (2025) The state of the late Quaternary megafauna extinction debate: a systematic review and analysis. Front. Mamm. Sci. 4:1678231. doi: 10.3389/fmamm.2025.1678231
Received: 02 August 2025; Accepted: 23 September 2025;
Published: 16 October 2025.
Edited by:
Dionisios Youlatos, Aristotle University of Thessaloniki, GreeceReviewed by:
Rhys Lemoine, Gothenburg Research Institute, SwedenHugo Bampi, Universidade Federal de Goiás, Brazil
Copyright © 2025 Stewart, Peters, Ziegler, Carleton, Roberts, Boivin and Groucutt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mathew Stewart, bWF0aGV3LnN0ZXdhcnRAZ3JpZmZpdGguZWR1LmF1
 Mathew Stewart
Mathew Stewart Carli Peters
Carli Peters Michael J. Ziegler
Michael J. Ziegler W. Christopher Carleton4
W. Christopher Carleton4 Patrick Roberts
Patrick Roberts Huw S. Groucutt
Huw S. Groucutt