- 1Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2National Engineering Research Center for Bioengineering Drugs and the Technologies, Institute of Translational Medicine, Nanchang University, Nanchang, China
- 3Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background: Bowel preparation is necessary for successful colonoscopy, while it can seriously affect intestinal microbial composition and damage the intestinal mucosal barriers in humans.
Methods: To figure out whether probiotics can sustain intestinal homeostasis and guard people's health, the probiotic drug of Bifidobacterium Tetragenous viable Bacteria Tablets (P group, n = 16) or placebo (C group, n = 16) was used for volunteers receiving bowel preparation, and high-throughput sequencing method was applied to monitor their intestinal microbial changes.
Results: The present results suggested that bowel preparation obviously reduced the intestinal microbial diversity, while taking probiotics significantly restored it to normal level. In addition, probiotics sharply reduced the abundance of pathogenic Proteobacteria, and obviously lowered the ratio of Firmicutes/Bacteroidetes compared with control group at phylum level (P < 0.05). And probiotics markedly decreased the abundance of pathogenic Acinetobacter and Streptococcus, while greatly enriched the relative abundance of beneficial bacteria Bacteroides, Roseburia, Faecalibacterium, and Parabacteroides at genus level (P < 0.05).
Conclusion: Probiotic drugs, e.g., Bifidobacterium Tetragenous viable Bacteria Tablets, can be used to restore intestinal dysbacteriosis caused by bowel preparation, and reduce side effects during colonoscopy.
Introduction
Colonoscopy is the preferred method to evaluate the intestinal health of most patients and it is the gold standard for colorectal cancer diagnosis, which can clearly discover intestinal lesions (1). Before colonoscopy, bowel preparation is commonly used to ensure that no residue remains in the intestinal wall to affect the examination process and results via utilizing adjusted diet and related drugs, and the adequacy of bowel preparation can directly affect the final effect of colonoscopy (2). At present, polyethylene glycol (PEG) is widely used for intestinal cleaning before colonoscopy due to its effectiveness and extensive acceptability (3).
As we know, the intestinal microbiota is important to sustain human health. Under physiological conditions, symbiotic physiological anaerobic bacteria, symbiotic conditional pathogenic bacteria and other harmful bacteria co-exist in the intestinal tract in a stable proportion. However, when intestinal microbiota alters or the proportion of intestinal microbiota is out of balance, corresponding pathophysiological changes will occur (4). During the bowel preparation procedure, large quantity of liquid enters the intestinal tract and considerably disrupts the environment of the normal intestinal cavity, and taking laxatives can enhance intestinal dyskinesia and intestinal peristalsis, which makes bacteria cannot adhere to intestinal mucosa (5). Moreover, the large amount of oxygen brought by bowel preparation in intestinal environment heavily reduces the number of anaerobic bacteria, and promotes the growth of aerobic bacteria, resulting in intestinal microbial disorder (6).
Probiotics are “living microorganisms that can have beneficial effects on the host when ingesting sufficient doses” (7), previous studies indicate that probiotics play an active role in a variety of human diseases, including irritable bowel syndrome, inflammatory bowel disease, and colon cancer (8, 9). Our previous studies indicated that probiotic preparations have important effects in reducing inflammatory response after gastrostomy and improving gastrointestinal symptoms in post-operative patients (10), and probiotic preparations also significantly alleviated oral mucosa inflammation caused by radiotherapy in patients with nasopharyngeal carcinoma (11). Although attention has been paid on the side effects of bowel preparation, e.g., imbalance of intestinal microbiota and damages to intestinal mucosa in academic circles, little work is done to reduce side effects of bowel preparation using probiotic supplement.
In the present study, the clinical probiotic drug of Bifidobacterium Tetragenous viable Bacteria Tablets was used to evaluate its effect on volunteers receiving bowel preparation, and high-throughput sequencing was applied to assess whether probiotics had positive effects on intestinal microbiota disorder caused by bowel preparation.
Materials and Methods
Ethics Statement
The present study was approved by the Institutional Review Boards of the Second Affiliated Hospital of Nanchang University (Nanchang, China). Patients provided written informed consent for sample collection. The project has also been registered and approved by the China Clinical Trial Registration Centre (ChiCTR1900022539).
Study Design and Patient Enrolment
The trial was conducted at the Second Affiliated Hospital of Nanchang University in China between December 2018 and November 2019. Thirty-two subjects (29 males, 3 females), with an average age of 51 y (range 30–70 y), height of 1.66 m, weight of 61.78 kg, body mass index (BMI) of 22.39, were enrolled. Five participants had a history of hypertension, four had a history of diabetes, and three had a history of hypertension and diabetes. According to regulations, medication was not discontinued during the study process. Moreover, no participant took antibiotics during the subject, nor had developed infection recently, and no other probiotics and yogurts were taken. None of these volunteers was vegetarian (Table 1).
Trial Protocol
The 32 volunteers were divided into two groups: placebo group (C group, n = 16) and probiotic group (P group, n = 16). Probiotic (Bifidobacterium Tetragenous viable Bacteria Tablets (SiLianKang), Hangzhou Grand Biologic Pharmaceutical Inc., Hangzhou, China. SFDA approval number: S20060010, containing >0.5 × 106 CFU/table Bifidobacterium infantis, >0.5 × 106 CFU/table Lactobacillus acidophilus, >0.5 × 106 CFU/table Enterococcus faecalis, and >0.5 × 105 CFU/table Bacillus cereus). Participants were suggested to eat porridge, noodles and other low-fiber diets the day before bowel preparation, and ate normally diets after colonoscopy. Antibiotics were forbidden during treatment process, as well as drinking and acrimony. All of them started taking placebo or probiotic preparations after colonoscopy for up to 5–7 days (three tablets and three times a day).
Participants took 2 L polyethylene glycol (PEG, SFDA approval number: H20020031, containing package A: 0.74 g potassium chloride and 1.68 g sodium bicarbonate, package B: 1.46 g sodium chloride and 5.68 g sodium sulfate, package C: 60 g polyethylene glycol 4,000) 4–5 h before colonoscopy and PEG should be completely taken within 1 h. After intravenous anesthesia with 1 ml lidocaine hydrochloride injection (SFDA approval number: H37021309), 1 ml nalbuphine hydrochloride injection (SFDA approval number: H20130127) and 20 ml propofol emulsion injection (SFDA approval number: H20051843), participants underwent colonoscopy. If participants were intolerant during colonoscopy, anesthetics were added as appropriate. Feces in 3 time pints (3 days before, the same day as the bowel preparation just before the colonoscopy, and 7 days after the process) were collected. The collected samples were stored in 50% glycerol (Cat#56-81-5; Sengon Biotech, China) and immediately stored at −80°C for further use.
Total Bacterial Genomic DNA Extraction and High-Throughput Sequencing
A total of 96 fecal samples were collected, and the method of bead blasting combined with genomic DNA kit (Tiangen Biotech Co., Ltd., Beijing, China) was used to extract fecal microbial DNA (12). The concentration and purity of purified DNAs were determined via a spectrophotometer at 230 nm (A 230) and 260 nm (A 260) (NanoDrop; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The V4 region of 16S rDNA gene in each sample was amplified with 515F/806R primer(515F, 5′-GCACCTAAYTGGGYDTAAAGNG-3′; 806R, 5′- TACNVGGGTATCTAATCC-3′), and PCR products were sequenced on IlluminaHiSeq 2000 platform (GenBank accession number PRJNA597277) (13).
Data Analysis
To analyze the high-throughput sequencing data, Cutadapt (version 1.9.1, http://cutadapt.readthedocs.io/en/stable/), UCHIME algorithm http://www.drive5.com/usearch/manual/uchime_algo.html, UPARSE software package (version 7.0.100), QIIME software (version 1.9.1), QIIME software package (version 1.8.0) and SIMCA-P software (version 11.5; Umetrics; Sartorius Stedim Biotech, Malmö, Sweden) were used to determine the α diversity (within samples, indexes of observed OTUs, Chao1, Shannon, Simpson, ACE, and goods coverage) and β diversity (among samples, PCA, PCoA and NMDS) (14, 15).
Data are presented as means ± standard deviation (SD). Statistical analyses were performed using Prism software (version 7.0; GraphPad Software, San Diego, CA, USA) and SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test and F-tests. Error probabilities of P < 0.05 were considered statistically significant.
Results
Patients Baseline Characteristics
Between December 2018 and November 2019, 32 volunteers were enrolled into the placebo group (C group, 16 volunteers) and the probiotics group (P group, 16 volunteers), and their sex, age, BMI, baseline characteristics, past medical history and gastrointestinal reaction before and after bowel preparation were summarized in Table 1. There was no significant difference between C group and P group.
Effect of Bowel Preparation on Intestinal Microbes
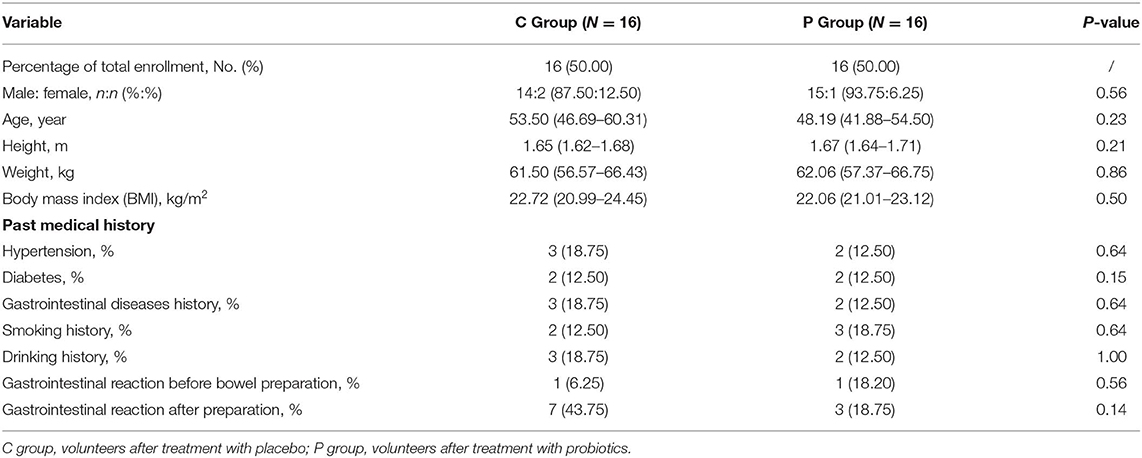
To explore whether bowel preparation can effect intestinal microorganisms, the V4 hypervariable region of bacteria was amplified using the 16S rDNA amplicon sequencing method from feces of 16 volunteers before (CB group), during (CM group) and after bowel preparation (CA group).
In Figures 1A–C, the Shannon index, Simpson index and Observed species indicated that the occurrence of bowel preparation slightly affected the α-diversity of the intestinal microbial community between the CB and CM groups, CB and CA groups, while significantly affected the microbial diversity between CM and CA groups (P < 0.05). And the principal coordinates analysis (PCoA) exhibited that the microbial diversity in CM group and CA group were different compared with that in CB group (Figure 1D). Additionally, the Venn index (Figure 1E) results indicated that there were 2,068, 3,426, and 1,695 OTUs in the CB, CM and CA groups, and their percentage of common OTUs were 27.71% (573/2,068), 16.73% (573/3,426) and 33.81% (573/1,695), respectively.
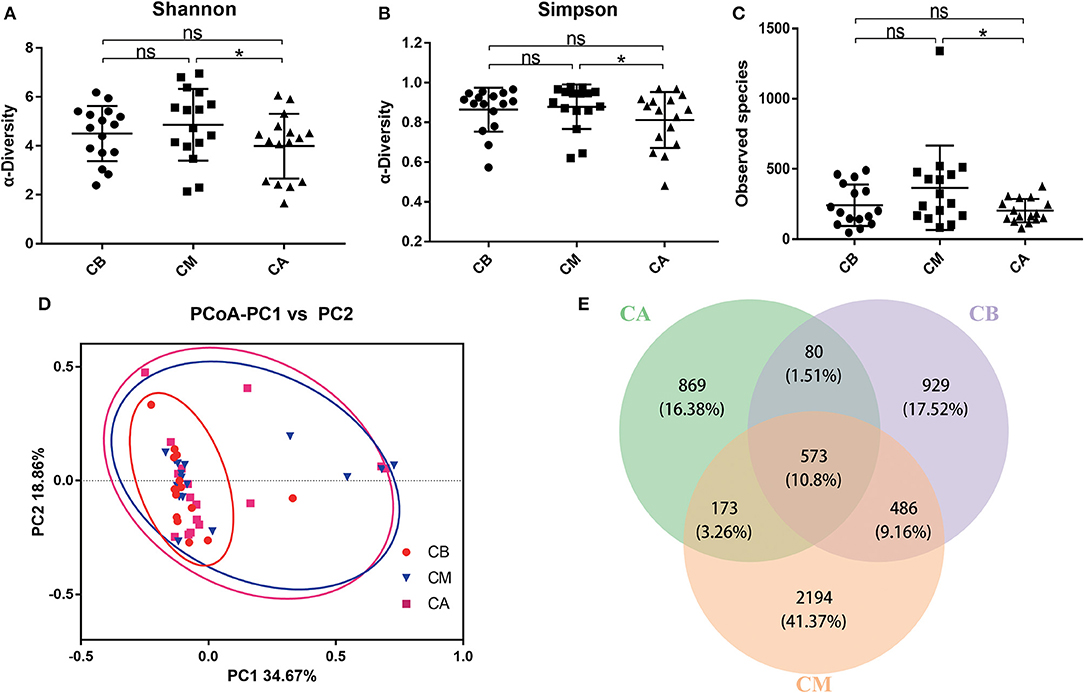
Figure 1. Effect of bowel preparation on intestinal microbiota. (A), Shannon index; (B), Simpson index; (C), Observed species; (D), PCoA of β diversity index; (E), scalar-Venn representation. CB, control group 3 days before bowel preparation (n = 16); CM, control group during bowel preparation (n = 16); CA, control group 5–7 days after bowel preparation (n = 16). Data are presented as means ± SD. ns, P > 0.05; *P < 0.05.
Furthermore, we further analyzed the dominant bacteria at the phylum level (Figures 2A–E), and found that Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria were the predominant phyla in these 3 groups. The results revealed that bowel preparation increased the relative abundance of Proteobacteria (0.266 vs. 0.410) while decreased the relative abundance of Actinobacteria (0.044 vs. 0.029), and had slightly effect on the relative abundance of Firmicutes (0.462 vs. 0.408) and Bacteroidetes (0.194 vs. 0.136) compared with the CB group. Seven days after bowel preparation, the relative abundance of Proteobacteria decreased from 0.410 to 0.335. Strangely, the relative abundance of Actinobacteria increased from 0.029 to 0.119, while the relative abundance of Firmicutes (0.409 vs. 0.389) and Bacteroidetes (0.136 vs. 0.126) still showed a decreasing trend compared with the CM group. At the genus level (Figures 2F–J), during the bowel preparation, it was observed that the relative abundance of Bacteroides and Acinetobacter were dominant bacteria, the relative abundance of Acinetobacter (0.042 vs. 0.176) was significantly increased, and the relative abundance of Streptococcus (0.020 vs. 0.008), Bifidobacterium (0.036 vs. 0.022) and Faecalibacterium (0.075 vs. 0.065) was slightly altered compared with the CB group. Seven days after bowel preparation, however, there was a significant increase of the percentage of Streptococcus (0.007 vs. 0.068) and Bifidobacterium (0.022 vs. 0.109), and a decrease of the relative abundance of Faecalibacterium (0.065 vs. 0.031) and Acinetobacter (0.176 vs. 0.136) compared with the CM group.
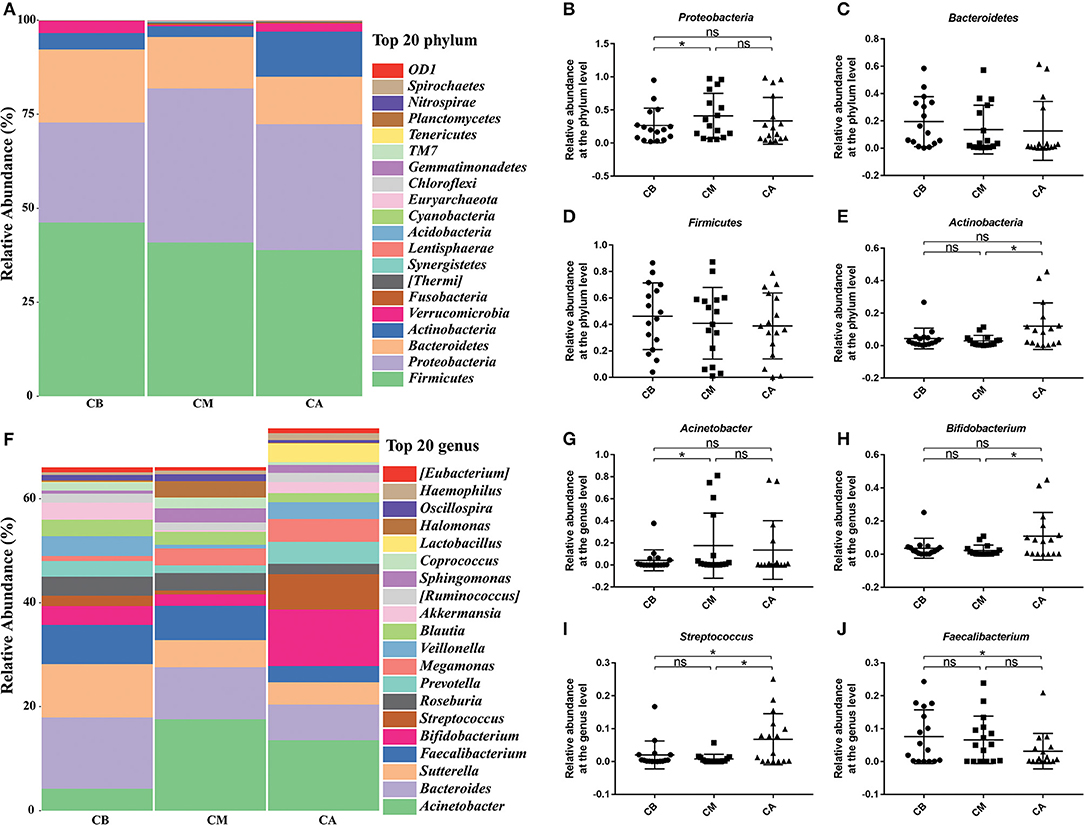
Figure 2. Effect of bowel preparation on microbial composition at phylum and genus levels. (A), the relative abundance of intestinal microbiota at the phylum level; (B), Proteobacteria; (C), Bacteroidetes; (D), Firmicutes; (E), Actinobacteria. (F), the relative abundance of intestinal microbiota at the genus level; (G), Acinetobacter; (H), Bifidobacterium; (I), Streptococcus; (J), Faecalibacterium. CB, control group 3 days before bowel preparation (n = 16); CM, control group during bowel preparation (n = 16); CA, control group 5–7 days after bowel preparation (n = 16). Data are presented as means ± SD. ns, P > 0.05; *P < 0.05.
Effect of Probiotic Preparation on Intestinal Microbial Balance
To evaluate the effects of probiotics on intestinal microbiota of volunteers receiving bowel preparation, feces were collected before (PB group), during (PM group) and after bowel preparation (PA group) for 7 days (have taken probiotic preparation for 5–7 days).
We observed that the bowel preparation had markedly affected the α-diversity on the Shannon index (Figure 3A) and Simpson index (Figure 3B, P < 0.05) of the microbial community between the PB and PM groups. Interestingly, the Observed species received an obvious increase after bowel preparation but an obvious reduction 7 days after the treatment (Figure 3C). Moreover, PCoA results indicated that taking probiotics greatly restored the disturbed microbiota to normal level in PM group and PA group (Figure 3D), and the common OTUs occupied 28.68% (508/1,771), 23.67% (508/2,146) and 36.92% (508/1,376) of the total OTUs in PB, PM and PA groups, respectively.
Figure 3. Effect of probiotics on intestinal microbiota. (A), Shannon index; (B), Simpson index; (C), Observed species; (D), PCoA of β diversity index; (E), scalar-Venn representation. PB, probiotic group 3 days before bowel preparation (n = 16); PM, control group during bowel preparation (n = 16); PA, probiotic group 5–7 days after bowel preparation (Supplement the Bifidobacterium Tetragenous viable Bacteria Tablets after colonoscopy for up to 5–7 days, three tablets and three times a day) (n = 16). Data are presented as means ± SD. ns, P > 0.05; *P < 0.05.
Then, we further evaluated the effects of probiotic intervention on microbial composition, and found supplementation of probiotics significantly reduced Proteobacteria (0.515 vs. 0.173) and sharply increased the relative abundance of Bacteroides (0.166 vs. 0.338) in PM group compared with PA group at phylum level (P < 0.05). At the genus level, the supplemented probiotics had obviously reduced the percentage of Acinetobacter (0.204 vs. 0.071) in PM group compared with PA group, and significantly enhanced the percentage of Bifidobacterium (0.017 vs. 0.110), Bacteroides (0.095 vs. 0.155) and Faecalibacterium (0.028 vs. 0.060) in PM group compared with PA group (Figures 4F–J).
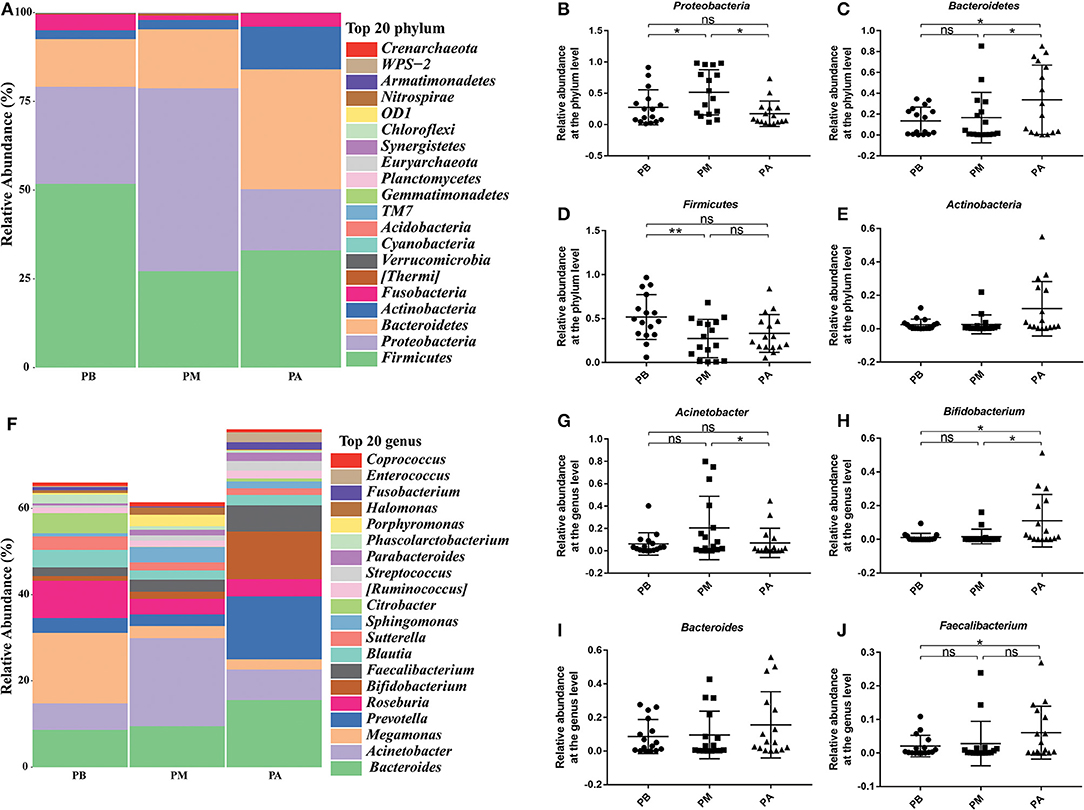
Figure 4. Effect of probiotics on microbial composition at phylum and genus levels. (A), the relative abundance of intestinal microbiota at the phylum level; (B), Proteobacteria; (C), Bacteroidetes; (D), Firmicutes; (E), Actinobacteria. (F), the relative abundance of intestinal microbiota at the genus level; (G), Acinetobacter; (H), Bifidobacterium; (I), Bacteroides; (J), Faecalibacterium. PB, probiotic group 3 days before bowel preparation (n = 16); PM, control group during bowel preparation (n = 16); PA, probiotic group 5–7 days after bowel preparation (Supplement the Bifidobacterium Tetragenous viable Bacteria Tablets after colonoscopy for up to 5–7 days, three tablets and three times a day) (n = 16). Data are presented as means ± SD. ns, P > 0.05; *P < 0.05; **P < 0.01.
The Microbial Changes Between Groups CA Group and PA Group
To better understand the effect of probiotics on bowel preparation, we compared the microbial diversity between volunteers in groups CA and PA. As shown in Figures 5A–C, supplementation of probiotics had markedly enhanced the Shannon index and Simpson index (P < 0.05), while decreased the observed species. The PCoA results indicated that samples in CA group and PA group scattered far away from each other (Figure 5D). There were 1,695 and 1,376 OTUs in the CA group and PA group, and the common OTU number was 570 (Figure 5E). In addition, the Lefse analysis demonstrated that Bacteroidia (at class), Bacteroidetes (at phylum), Bacteroidaceae (at family), Bacteroides (at genus), Fusobacteriaceae (at family), Porphyromonadaceae (at family), and Parabacteroides (at genus) were significantly higher in the PA group than in the CA group (Figure 5F).
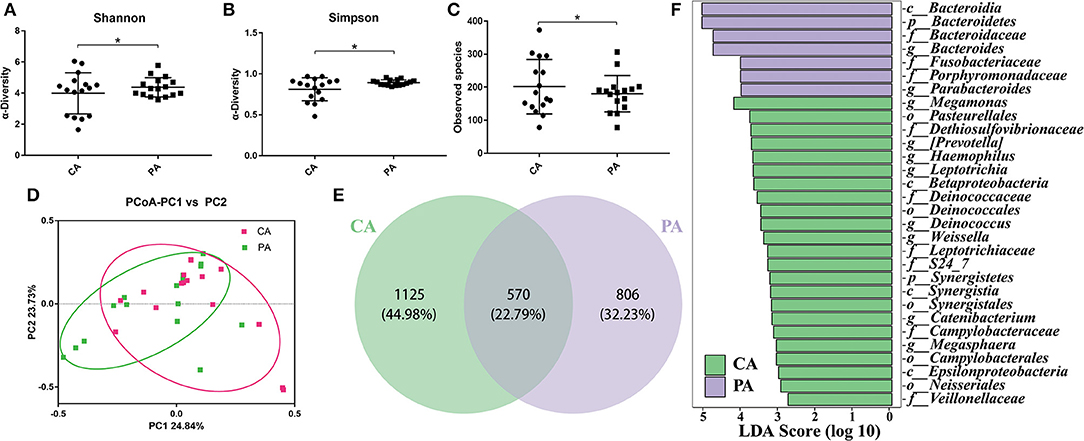
Figure 5. The microbial changes between groups CA and PA. (A), Shannon index; (B), Simpson index; (C), Observed species; (D), PCoA of β diversity index; (E), scalar-Venn representation; (F), Lefse index. CA, control group 5–7 days after bowel preparation (n=16); PA, probiotic group 5–7 days after bowel preparation (Supplement the Bifidobacterium Tetragenous viable Bacteria Tablets after colonoscopy for up to 5–7 days, three tablets and three times a day) (n = 16). Data are presented as means ± SD. *P < 0.05.
Then, specific bacteria in CA group and PA group were compared. Supplementation of probiotics markedly enriched the percentage of Bacteroidetes (0.126 vs. 0.338), while reduced the percentage of Proteobacteria (0.335 vs. 0.173) and Firmicutes (0.389 vs. 0.330) compared with the PA group at the phylum level (P < 0.05). At genus level (Figures 6E–K), the supplementation of probiotics decreased the relative abundance of Acinetobacter (0.136 vs. 0.071) and Streptococcus (0.068 vs. 0.023), while increased the relative abundance of Bacteroides (0.068 vs. 0.155), Roseburia (0.02 vs. 0.04), Faecalibacterium (0.031 vs. 0.060) and Parabacteroides (0.16 vs. 1.92%).
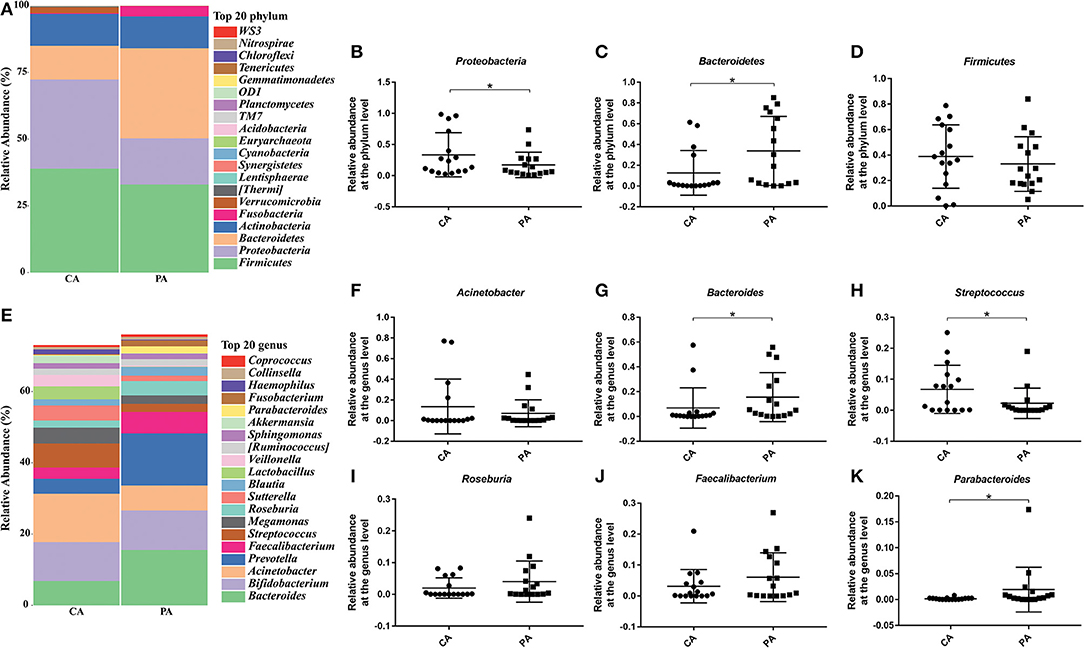
Figure 6. Effect of probiotics on microbial composition between groups CA and PA at phylum and genus levels. (A), the relative abundance of intestinal microbiota at the phylum level; (B), Proteobacteria; (C), Bacteroidetes; (D), Firmicutes. (E), the relative abundance of intestinal microbiota at the genus level; (F), Acinetobacter; (G), Bacteroides; (H), Streptococcus; (I), Roseburia; (J), Faecalibacterium; (K), Parabacteroides. CA, control group 5–7 days after bowel preparation (n = 16); PA, probiotic group 5–7 days after bowel preparation (Supplement the Bifidobacterium Tetragenous viable Bacteria Tablets after colonoscopy for up to 5–7 days, three tablets and three times a day) (n = 16). Data are presented as means ± SD. *P < 0.05.
Discussion
Bowel cleaning is necessary during colonoscopy, and its long-time use of safety makes people ignore its negative impact on intestinal microorganisms (16). As we know, colonic microorganisms are the basis for promoting normal mammalian physiological functions, including angiogenesis, metabolism, digestion and immune system development (17). What's more, various diseases, including obesity, type 2 diabetes, colorectal cancer and inflammatory bowel disease will occur when intestinal microbiota is out of balance (8, 9, 18).
The main components of normal intestinal microecosystem are obligate anaerobes (Bacteroidetes and Firmicutes), and facultative anaerobes (such as Proteobacteria) usually only account for a small proportion, and the imbalance of intestinal microbiota is often caused by increasing number of facultative anaerobes (19). The bowel preparation can bring a large amount of oxygen into the intestinal cavity, damage the anaerobic environment of the intestinal cavity and provide a good growth environment for facultative anaerobic or aerobic bacteria. Previous studies and the present work, likewise, demonstrated that bowel preparation had greatly increased the abundance of Proteobacteria [Figures 2, 3; (19)].
In this study, we found that the bowel preparation significantly decreased phyla Bacteroidetes and Firmicutes (Figure 2), and taking probiotics had little effect on the phylum Firmicutes, while greatly increased the abundance of Bacteroidetes (Figure 4). Previous studies had shown that the increase of the abundance of Firmicutes and the decrease of the abundance of Bacteroidetes were closely related to unhealthy conditions. Therefore, the increase of Firmicutes/Bacteroidetes may pose a potential risk to patient's health (20–22). In the present study, we found that the ratio of Firmicutes/ Bacteroidetes in CA group (3.08) was higher than that in PA group (0.98), suggesting that taking probiotics could reduce the potential disease risks by bowel preparation. In addition, human body usually does not have the ability to degrade most of complex polysaccharides (the main component and main nutrition source of our daily diet) until reaching the colon, and Bacteroidetes play a vital role in degrading complex polysaccharides of cellulose, pectin and xylan, which can help people absorb more energy from the diet (23). Moreover, butyrate produced by Bacteroidetes plays an important role in maintaining the intestinal health of the host, exerting immunity and anti-tumor effect (24).
At genus level, supplementation of probiotics significantly reduced the abundance of Acinetobacter which is a common aerobic and gram-negative bacterium in nature, belonging to a vital pathogen causing hospital infection especially in patients with low immune function (Acinetobacter baumannii) (25). Acinetobacter had been listed as the third commonest human pathogen in the intensive care unit of South Korean hospitals, and its inherent drug resistance to a variety of antibiotics made it obtain determinants of drug resistance to various antibacterial drugs (26). Additionally, bowel preparation significantly reduced the level of Bacteroides, and probiotics obviously recovered its abundance. Studies had revealed that Bacteroides can reduce the intestinal oxygen level to promote the growth of strict anaerobes (23) and some strains of Bifidobacterium have been put into use as probiotics in food and medicine (10, 27). Bacteroides and Bifidobacterium can establish stable and long-term contact with host and benefit health of human body, can degrade dietary fiber into short-chain fatty acids (SCFAs), which provides energy source for cells, promotes barrier function and reduces the occurrence of inflammatory reactions (28, 29).
In the end, we compared the microbial diversity of CA and PA, and found that taking probiotics predominantly enhanced the abundance of beneficial bacteria such as Roseburia (mainly or only produces butyrate, which can reduce the level of inflammation in the whole, especially in the blood, further reduce the degree of atherosclerosis. It remains lower levels in people with cardiovascular diseases) (30, 31), Faecalibacterium (a symbiotic bacterium that widely exists in the gastrointestinal tract of animals and humans. It was significantly reduced in Crohn's patients, and might be used as a probiotic to treat Crohn's disease) (32–34) and Parabacteroides (can resist intestinal inflammation, the main metabolic end products are beneficial acetic acid and succinic acid, which are lower than the normal range in intestinal tract of patients with colitis) (35–37) at the genus level. Nevertheless, the abundance of harmful bacteria such as Streptococcus was strikingly reduced (Figures 5, 6). Streptococcus is a common opportunistic pathogen, including Streptococcus pyogenes, Streptococcus viridans and Streptococcus pneumoniae, which can cause purulent inflammation, endocarditis and septicaemia, further threaten human health and life (38, 39). Haenni et al. indicated that due to the widespread use of Tetracycline, Macrolide, and Lincosamide antibiotics in the global animal sector, the antibiotic resistance of Streptococcus zooepidermidis has emerged, leading to treatment failure (40, 41).
In the present study, we found that oral probiotics did alleviate the intestinal microbial disturbance caused by bowel preparation, greatly reduced the pathogens of Proteobacteria (at the phylum level), Acinetobacter (at the genus level), Streptococcus (at the genus level), and enhanced the probiotics of Bacteroidetes (at the phylum level), Bacteroides (at the genus level), Roseburia (at the genus level), Faecalibacterium (at the genus level) and Parabacteroides (at the genus level). Therefore, we have reasons to believe that supplement of probiotic preparations will accelerate the establishment of intestinal microbial balance after intestinal cleaning, suppress the growth of harmful bacteria and benefit the maintenance of intestinal health.
Data Availability Statement
The datasets generated for this study can be found in the NCBI: GenBank accession number PRJNA597277.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Boards of the Second Affiliated Hospital of Nanchang University (Nanchang, China). The project has also been registered and approved by the China Clinical Trial Registration Center (ChiCTR1900022539). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TC and XD designed the experiments, analyzed the data, and wrote the manuscript. CZ, HT, RY, ZL, YH, and KW performed the experiments. All authors discussed the results and commented on the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81960103 to XD), the Natural Science Foundation of Jiangxi province (Grant No. 20192ACBL20034 to ZL), the Science and Technology Project of Jiangxi (Grant Nos. 20181BBG70028, 20181BCB24003, and 20194BCJ22032 to TC), and double 10-thousand plan of Jiangxi Province to TC (innovation and technology professionals as the high-end talent).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kim MS, Park J, Park JH, Kim HJ, Jang HJ, Joo HR, et al. Does polyethylene glycol. (PEG) plus ascorbic acid induce more mucosal injuries than split-dose 4-L PEG during bowel preparation? Gut Liver. (2016) 10:237–43. doi: 10.5009/gnl14439
2. Schoenfeld P, Dominitz JA. No polyp left behind:defining bowel preparation adequacy to avoid missed polyps. Gastroenterology. (2016) 150:303–6. doi: 10.1053/j.gastro.2015.12.024
3. Parra-Blanco A, Ruiz A, Alvarez-Lobos M, Amoros A, Gana JC, Ibanez P, et al. Achieving the best bowel preparation for colonoscopy. World J Gastroentero. (2014) 20:17709–26. doi: 10.3748/wjg.v20.i47.17709
4. Hofer U. Microbiome:bacterial imbalance in Crohn's disease. Nat Rev Microbiol. (2014) 12:312–3. doi: 10.1038/nrmicro3255
5. Rea K, O'Mahony SM, Dinan TG, Cryan JF. The role of the gastrointestinal microbiota in visceral pain. Handb Exp Pharmacol. (2017) 239:269–87. doi: 10.1007/164_2016_115
6. Jalanka J, Salonen A, Salojarvi J, Ritari J, Immonen O, Marciani L, et al. Effects of bowel cleansing on the intestinal microbiota. Gut. (2015) 64:1562–8. doi: 10.1136/gutjnl-2014-307240
7. Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Brit J Nutr. (2017) 117:93–107. doi: 10.1017/S0007114516004037
8. Tojo R, Suarez A, Clemente MG, de los Reyes-Gavilan CG, Margolles A, Gueimonde M, et al. Intestinal microbiota in health and disease:role of bifidobacteria in gut homeostasis. World J Gastroentero. (2014) 20:15163–76. doi: 10.3748/wjg.v20.i41.15163
9. Nie P, Li Z, Wang Y, Zhang Y, Zhao M, Luo J, et al. Gut microbiome interventions in human health and diseases. Med Res Rev. (2019) 39:2286–313. doi: 10.1002/med.21584
10. Zheng C, Chen T, Wang Y, Gao Y, Kong Y, Liu Z, et al. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J Cancer. (2019) 10:568–76. doi: 10.7150/jca.29072
11. Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. (2019) 125:1081–90. doi: 10.1002/cncr.31907
12. Meng F, Chen T, Wang X, Wang X, Wei H, Tian P, et al. Evaluation of the accuracy and sensitivity of highthroughput sequencing technology using known microbiota. Mol Med Rep. (2018) 17:408–13. doi: 10.3892/mmr.2017.7849
13. Liu Z, Kong Y, Gao Y, Ren Y, Zheng C, Deng X, et al. Revealing the interaction between intrauterine adhesion and vaginal microbiota using highthroughput sequencing. Mol Med Rep. (2019) 19:4167–74. doi: 10.3892/mmr.2019.10092
14. Bolger AM, Lohse M, Usadel B. Trimmomatic:a flexible trimmer for illumina sequence data. Bioinformatics. (2014) 30:2114–20. doi: 10.1093/bioinformatics/btu170
15. Edgar RC. UPARSE:highly accurate OTU sequences from microbial amplicon reads. Nat Methods. (2013) 10:996–8. doi: 10.1038/nmeth.2604
16. Moon W. Optimal and safe bowel preparation for colonoscopy. Clin Endosc. (2013) 46:219–23. doi: 10.5946/ce.2013.46.3.219
17. Drago L, Toscano M, De Grandi R, Casini V, Pace F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. Eur J Gastroenterol Hepatol. (2016) 28:532–7. doi: 10.1097/MEG.0000000000000581
18. Nelson MH, Diven MA, Huff LW, Paulos CM. Harnessing the microbiome to enhance cancer immunotherapy. J Immunol Res. (2015) 2015:368736–48. doi: 10.1155/2015/368736
19. Litvak Y, Byndloss MX, Tsolis RM, Baumler AJ. Dysbiotic proteobacteria expansion:a microbial signature of epithelial dysfunction. Curr Opin Microbiol. (2017) 39:1–6. doi: 10.1016/j.mib.2017.07.003
20. Forbes JD, Van Domselaar G, Bernstein CN. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. (2016) 7:1081. doi: 10.3389/fmicb.2016.01081
21. Xue B, Xie J, Huang J, Chen L, Gao L, Ou S, et al. Plant polyphenols alter a pathway of energy metabolism by inhibiting fecal bacteroidetes and firmicutes in vitro. Food Funct. (2016) 7:1501–7. doi: 10.1039/C5FO01438G
22. Dwivedi M, Ansarullah, Radichev I, Kemp EH. Alteration of immune-mechanisms by human microbiota and development and prevention of human diseases. J Immunol Res. (2017) 2017:6985256–8. doi: 10.1155/2017/6985256
23. Wexler AG, Goodman AL. An insider's perspective: Bacteroides as a window into the microbiome. Nat Microbiol. (2017) 2:17026–50. doi: 10.1038/nmicrobiol.2017.26
24. Thomas F, Hehemann JH, Rebuffet E, Czjzek M, Michel G. Environmental and gut bacteroidetes:the food connection. Front Microbiol. (2011) 2:93. doi: 10.3389/fmicb.2011.00093
25. Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens:microbiological, clinical, and epidemiological features. Clin Microbiol Rev. (1996) 9:148–65. doi: 10.1128/CMR.9.2.148-165.1996
26. Gurung M, Nam HM, Tamang MD, Chae MH, Jang GC, Jung SC, et al. Prevalence and antimicrobial susceptibility of Acinetobacter from raw bulk tank milk in Korea. J Dairy Sci. (2013) 96:1997–2002. doi: 10.3168/jds.2012-5965
27. Allen AP, Clarke G, Cryan JF, Quigley EMM, Dinan TG. Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. strain specificity, symptoms, and mechanisms. Curr Med Res Opin. (2017) 33:1349–51. doi: 10.1080/03007995.2017.1322571
28. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article:the role of butyrate on colonic function. Aliment Pharm Ther. (2008) 27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x
29. Carlson JL, Erickson JM, Hess JM, Gould TJ, Slavin JL. Prebiotic dietary fiber and gut health:comparing the in vitro fermentations of beta-glucan, inulin and xylooligosaccharide. Nutrients. (2017) 9:1–17. doi: 10.3390/nu9121361
30. Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. (2018) 3:1461–71. doi: 10.1038/s41564-018-0272-x
31. Rohde KH, Dyer DW. Mechanisms of iron acquisition by the human pathogens neisseria meningitidis and neisseria gonorrhoeae. Front Biosci Landmrk. (2003) 8:d1186–218. doi: 10.2741/1133
32. Benevides L, Burman S, Martin R, Robert V, Thomas M, Miquel S, et al. New insights into the diversity of the genus faecalibacterium. Front Microbiol. (2017) 8:1790. doi: 10.3389/fmicb.2017.01790
33. Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. (2009) 11:2574–84. doi: 10.1111/j.1462-2920.2009.01982.x
34. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. (2008) 105:16731–6. doi: 10.1073/pnas.0804812105
35. Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, et al. Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. PLoS ONE. (2016) 11:e0152126. doi: 10.1371/journal.pone.0152126
36. Gao B, Wang R, Peng Y, Li X. Effects of a homogeneous polysaccharide from Sijunzi decoction on human intestinal microbes and short chain fatty acids in vitro. J Ethnopharmacol. (2018) 224:465–73. doi: 10.1016/j.jep.2018.06.006
37. Gomez-Arango LF, Barrett HL, Wilkinson SA, Callaway LK, McIntyre HD, Morrison M, et al. Low dietary fiber intake increases collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes. (2018) 9:189–201. doi: 10.1080/19490976.2017.1406584
38. Nguyen CT, Park SS, Rhee DK. Stress responses in Streptococcus species and their effects on the host. J Microbiol. (2015) 53:741–9. doi: 10.1007/s12275-015-5432-6
39. Turner AG, Ong CY, Walker MJ, Djoko KY, McEwan AG. Transition metal homeostasis in Streptococcus pyogenes and Streptococcus pneumoniae. Adv Microb Physiol. (2017) 70:123–91. doi: 10.1016/bs.ampbs.2017.01.002
40. Baracco GJ. Infections caused by group C and G Streptococcus. (Streptococcus dysgalactiae subsp. equisimilis and others):epidemiological and clinical aspects. Microbiol Spectr. (2019) 7:1–11. doi: 10.1128/microbiolspec.GPP3-0016-2018
Keywords: bowel preparation, probiotics, high-throughput sequencing, Proteobacteria, Firmicutes/Bacteroidetes
Citation: Deng X, Tian H, Yang R, Han Y, Wei K, Zheng C, Liu Z and Chen T (2020) Oral Probiotics Alleviate Intestinal Dysbacteriosis for People Receiving Bowel Preparation. Front. Med. 7:73. doi: 10.3389/fmed.2020.00073
Received: 29 December 2019; Accepted: 19 February 2020;
Published: 28 February 2020.
Edited by:
Zongxin Ling, Zhejiang University, ChinaCopyright © 2020 Deng, Tian, Yang, Han, Wei, Zheng, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingtao Chen, Y2hlbnRpbmd0YW8xOTg0QDE2My5jb20=; Cihua Zheng, emhlbmdjaDA4MTFAMTYzLmNvbQ==
 Xiaorong Deng1
Xiaorong Deng1 Cihua Zheng
Cihua Zheng Tingtao Chen
Tingtao Chen