- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Taipei Medical University Hospital, Taipei City, Taiwan
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei City, Taiwan
- 3Department of Internal Medicine, Taipei Medical University Hospital, Taipei City, Taiwan
- 4Department of Nursing, College of Life Science and Industry, Sunchon National University, Suncheon, South Korea
- 5School of Nursing, National Taipei University of Nursing and Health Sciences, Taipei City, Taiwan
Background: In this study, we aimed to compare the effects of metformin-based dual therapy versus triple therapy on glycemic control and lipid profile changes in Taiwanese patients with type 2 diabetes mellitus (T2DM).
Methods: In total, 60 patients were eligible for participation in this study. Patients received at least 24 months of metformin monotherapy, dual therapy, or triple therapy with metformin plus linagliptin (a dipeptidyl peptidase 4 (DPP-4) inhibitor) or dapagliflozin (a sodium-glucose cotransporter-2 (SGLT2) inhibitor). Blood samples were collected from each patient, followed by evaluation of changes in their blood glucose control and lipid profile-related markers.
Results: A combination of metformin and DPP4 and SGLT2 inhibitor therapy more effectively reduced low-density lipoprotein cholesterol (LDL-C) (p = 0.016) than metformin monotherapy. A combination of metformin and DPP4 and SGLT2 inhibitor therapy more effectively improved total cholesterol (Chol, p = 0.049) and high-density lipoprotein cholesterol (HDL-C) than metformin monotherapy (p = 0.037). Metformin plus linagliptin dual therapy was more effective than metformin monotherapy in reducing glycosylated hemoglobin (HbA1C, p = 0.011). Patients who received a combination of linagliptin and empagliflozin showed a significant reduction in their fasting blood glucose (p = 0.019), HbA1c (p = 0.036), and Chol (p = 0.010) compared with those who received linagliptin dual therapy. Furthermore, patients who received metformin plus dapagliflozin and saxagliptin showed significantly reduced Chol (p = 0.011) and LDL-C (p = 0.035) levels compared with those who received metformin plus dapagliflozin.
Conclusion: In conclusion, dual therapy with metformin and linagliptin yields similar glycemic control ability to triple therapy. Among metformin combination triple therapy, triple therapy of empagliflozin and linagliptin might have a better glycemic control ability than dual therapy of linagliptin. Moreover, Triple therapy of dapagliflozin and saxagliptin might have a better lipid control ability than dual therapy of dapagliflozin.
Introduction
Asia is considered the epicenter of the global epidemic of type 2 diabetes mellitus (T2DM) because of rapid changes in eating habits and lifestyle and the increasing rates of obesity in Asia (1). Many studies have reported the epidemic of T2DM in East Asia and the interethnic differences in genetics, pathophysiology, eating habits, and lifestyle factors between Asian regions and also between Asian and Western countries (2).
Type 2 diabetes mellitus is characterized by chronic hyperglycemia and disturbances of carbohydrate, lipid, and protein metabolism. To date, patients with T2DM who initially achieve glycemic control with a single oral antidiabetic medicine usually require additional agents for maintaining glycemic control due to the progressive nature of the disease (3).
Several studies have verified the correlation between blood glucose levels and serum lipid profiles (4). Thus, use of an appropriate and cost-effective medication for T2DM is recommended. Concomitant use of multiple medicines is often indicated in the management of diseases; however, more medicines might not necessarily be better. Polypharmacy could result in increased healthcare costs and risks of adverse drug events and medication non-adherence (5). Therefore, to compare and confirm the different effects of monotherapy, dual therapy and triple therapy on lipid profiles and glucose control is important under the premise of minimizing side effects.
Metformin is recommended as a first-line oral glucose-lowering medication by the American Diabetes Association (6). It is usually prescribed along with other antidiabetic drugs such as sodium-glucose cotransporter-2 (SGLT2) inhibitors and dipeptidyl peptidase 4 (DPP4) inhibitors to control blood glucose and lipid levels. However, few studies have evaluated the efficiency of different types of metformin-based therapies in glycemic control and lipid profile management and compared them with metformin-based dual (with an SGLT2 inhibitor or a DPP4 inhibitor) and triple therapy (metformin with an SGLT2 inhibitor and a DPP4 inhibitor) in Taiwanese patients. Therefore, in the present study, we aimed to compare the effects of different types of metformin-based therapies on glycemic control and lipid profile changes in Taiwanese patients with T2DM.
Materials and methods
Patients
This study was conducted at Taipei Medical University Hospital, Taiwan. Participants who visited the endocrinology outpatient department between October 2021 and March 2022 were screened for eligibility. Individuals were eligible if they were above 20 years of age; had T2DM; who received at least 24 months of metformin monotherapy or combination therapy with linagliptin (DPP4 inhibitor) or dapagliflozin (SGLT2 inhibitor); and were on regular follow-up for blood tests for glucose, glycosylated hemoglobin (HbA1c), and lipid profile. Patients were excluded if they were receiving insulin injections or lipid-lowering drugs, such as statin. Individuals with renal or hepatic dysfunction or failed blood glucose control were also ineligible.
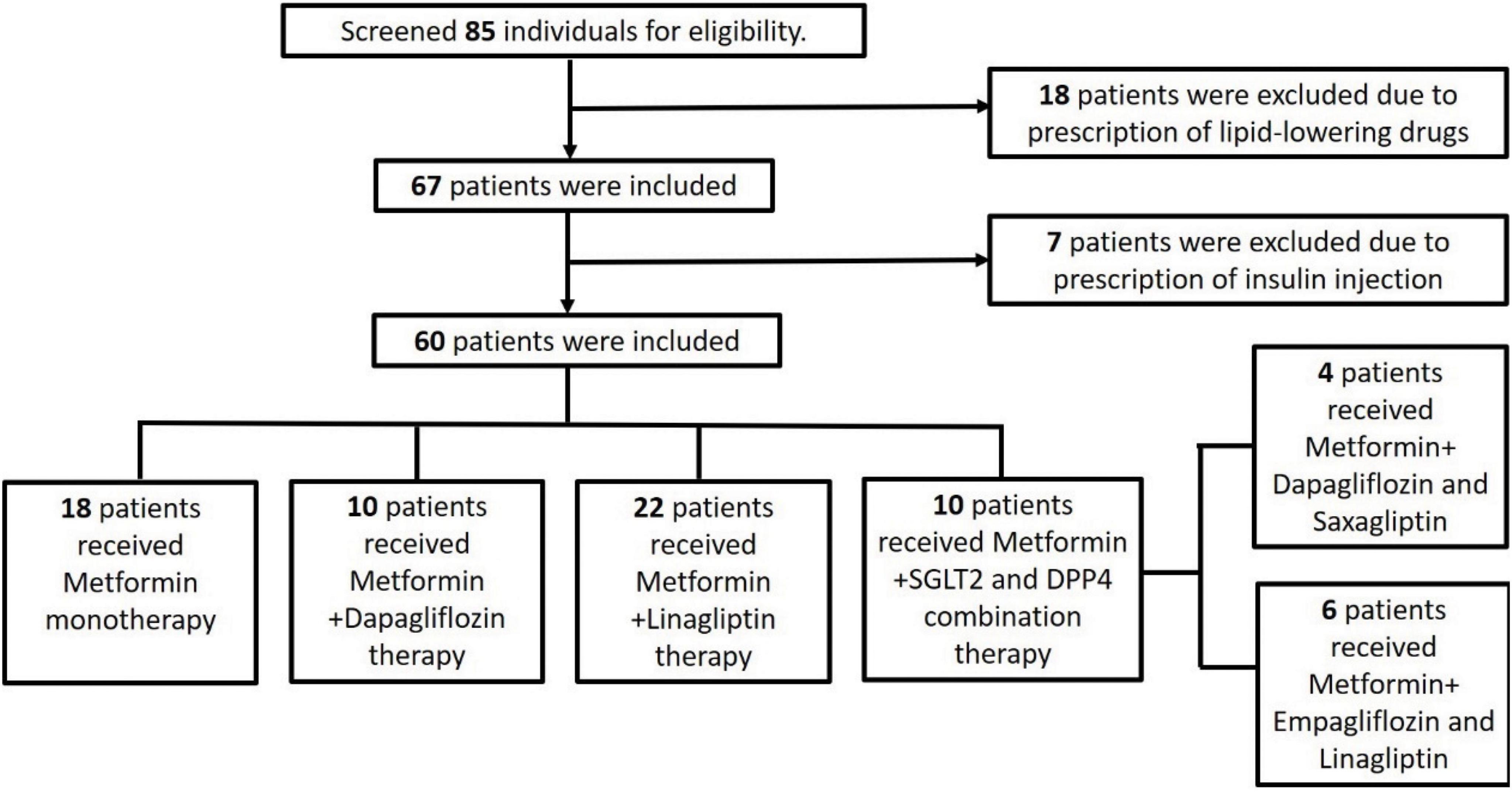
The study screened 85 individuals for eligibility. Of these patients, 18 patients were excluded, because they were prescribed lipid-lowering drugs, and 7 were excluded, because they were prescribed insulin injections. Figure 1 details the number of participants enrolled in the study along with the reason for exclusion of some participants. Finally, the study enrolled 60 participants whose demographic features have been summarized.
All patients provided their written informed consent prior to enrolment. The study protocol was approved by the ethics committee of the Institutional Review Board of Taipei Medical University (Approval No: N202107021). All procedures accorded with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration.
Patient grouping and assignment
Patients were divided into four groups according to the treatment they received: metformin 1,000 mg monotherapy (metformin only group, N = 18); metformin 1,000 mg and linagliptin 5 mg combination therapy (+ DPP4 group, N = 22); metformin 1,000 mg and dapagliflozin 10 mg combination therapy (+ SGLT2 group, N = 10); and either metformin 1,000 mg plus empagliflozin 10 mg and linagliptin 5 mg or dapagliflozin 10 mg and saxagliptin 5 mg combination therapy (+ SGLT2 and DPP4 group, N = 10).
The 10 patients who received DPP-4 inhibitor and SGLT-2 inhibitor combination therapy (triple therapy) included 6 patients with empagliflozin 25mg and linagliptin 5 mg and 4 patients with dapagliflozin 10mg and saxagliptin 5mg.
Medicinal compliance
Medication compliance was evaluated by the self-report of remain pill count. When patients come back to follow up, we calculated remain pills from self-report. And remain doses of medicine were record.
Hematological analysis
Routine blood tests were performed at the clinical laboratory. Blood samples were collected from each patient after an overnight fast. Serum uric acid and ketone levels were determined using a one-touch self-metabolic marker monitoring analyzer (FORA MD-6; Fora Care, Taipei, Taiwan). Fasting glucose (glucose AC) was analyzed according to the hexokinase method (ADVIA Chemistry XPT System, Siemens, Berlin, Germany), and HbA1c was determined by high-performance liquid chromatography using an automatic analyzer (Bio-Rad Variant II Turbo 2.0 System, Hercules, California, USA). Serum lactate dehydrogenase (LDH), C- peptide, creatine (Cr), Alanine aminotransferase (ALT), total cholesterol (Chol), triglyceride (Tg), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels were analyzed by enzymatic methods using an automatic analyzer (ADVIA Chemistry XPT). Serum insulin antibody (insulin Ab) was measured by immunoradiometric binding assay in an automatic analyzer (PerkinElmer CSBio, Santa Clara, California, USA).
Modified homeostasis model assessment-insulin resistance index
Homeostasis model assessment-insulin resistance index is a simple and useful method for evaluating insulin resistance. Modified HOMA-IR was calculated using the following equation: 1.5 + fasting blood glucose × fasting C-peptide/2,800 (7, 8). A result above 1.9 indicated early insulin resistance, whereas a result above 2.9 indicated significant insulin resistance (7, 8).
Statistical analysis
Statistical analysis and data management were performed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA). Data are expressed as the mean and median. The Chi-square test was used for results of nominal scale. Data distributions were analyzed by Shapiro-Wilks test for normality. Non-parametric statistics, which are Mann–Whitney U test with Kruskal-Wallis Test were used to determine whether any between-group differences existed in the groups of patients, the post hoc test was using Dunn post hoc test. The Wilcoxon signed-rank test was used to analyze the within-group differences in the paired results of patients. A confidence interval of 95% was employed, and p <0.05 indicated statistical significance.
Results
Baseline characteristics and comparisons between study groups
A total of 60 participants with T2DM who received different medication therapies were included. The statistical results indicated no significant difference between the groups of patients who received different therapies in T2DM duration, body mass index (BMI), age, fasting blood glucose, HbA1c, LDH, and HOMA-IR score and levels of insulin Ab, C-peptide, uric acid, ketone, Cr, and ALT (Table 1).
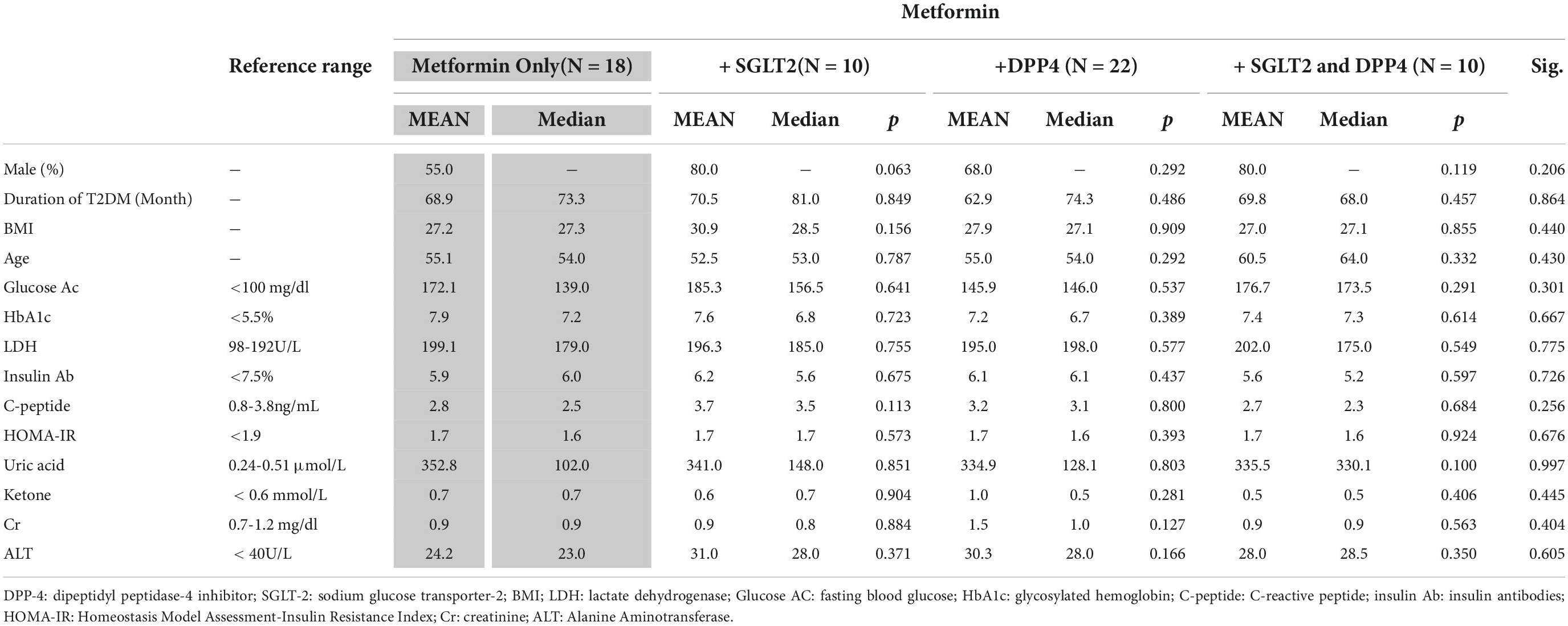
Table 1. Between-group analysis of clinical characteristics of the patients with type 2 diabetes mellitus (T2DM).
Between-group analysis of changes in lipid profiles
A significant difference was observed in the lipid profiles of patients in the metformin only group and the + SGLT2 and DPP4 group in terms of Chol (p = 0.033), HDL-C (p = 0.011), and LDL-C levels (p = 0.015). As indicted in Table 2, significantly different changes were observed in serum Chol (p = 0.010) between the + SGLT2 and DPP4 group and the DPP4 group. Similarly, significantly different changes were observed in Chol (p = 0.002) and LDL-C (p = 0.008) levels between patients treated with + SGLT2 and DPP4, and SGLT2.
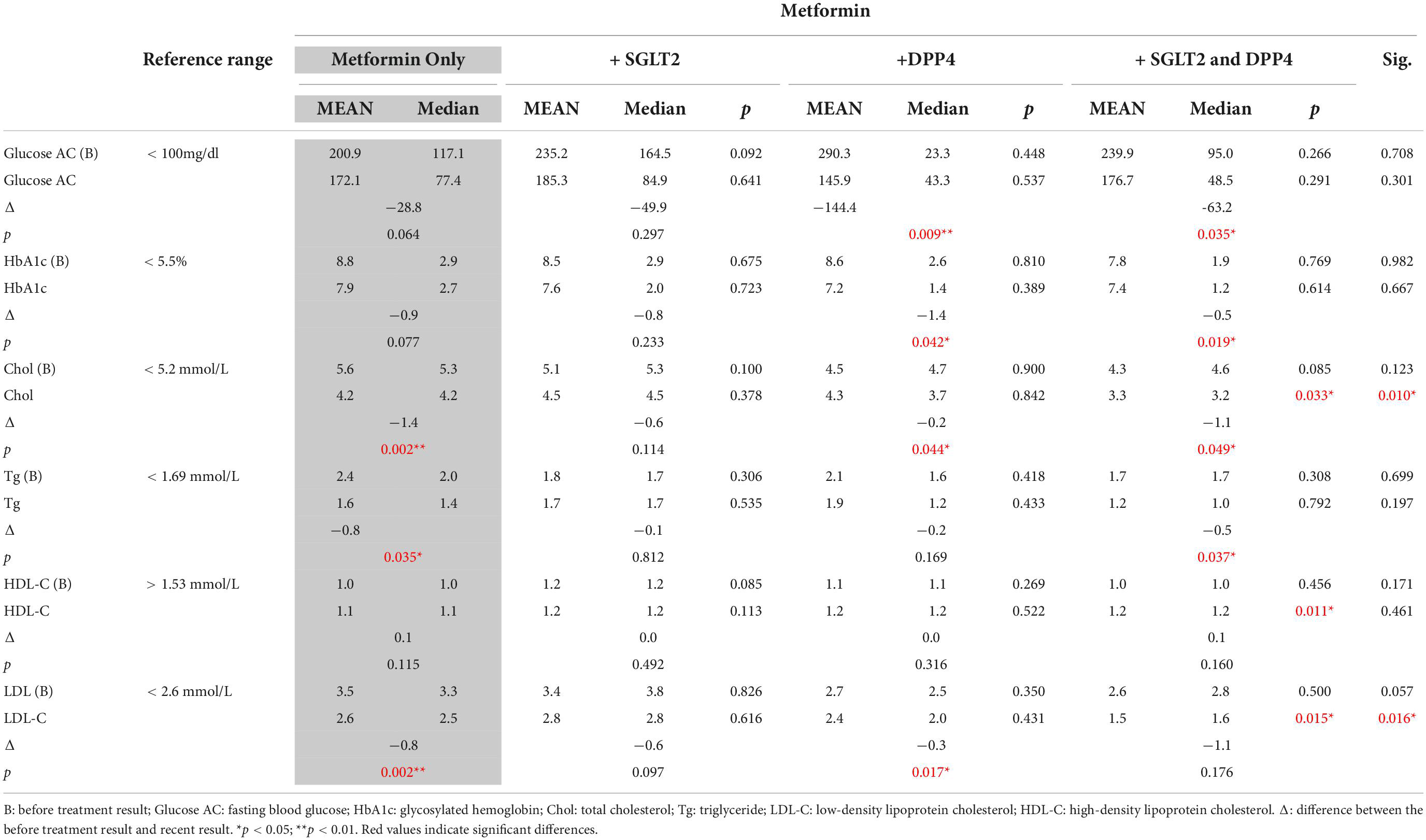
Table 2. Within-group analysis of glycemic control- and lipid profile-related biomarkers before and after treatment in each group.
Within-group analysis of lipid profiles before and after treatment in each group
The metformin monotherapy group exhibited significantly reduced levels of GPT (p = 0.01), Chol (p = 0.01), and LDL-C (p = 0.001) after treatment. Moreover, the metformin combined with linagliptin (+ DPP4) group exhibited significantly reduced Glucose AC (p = 0.035) and HbA1c (p = 0.019) relative to the pretreatment treatment. Similarly, the + SGLT2 and DPP4 group exhibited significantly reduced fasting blood Glucose (p = 0.035), HbA1c (p = 0.019), GPT (p = 0.007), Chol (p = 0.035), and Tg (p = 0.045) levels relative to the pretreatment values. The changes in lipid profile are presented in Table 2.
Effect of different combination therapies on lipid profile and blood glucose control
To further verify the effect of metformin plus empagliflozin and linagliptin and metformin plus dapagliflozin and saxagliptin in the + SGLT2 and DPP4 group, the groups of patients receiving metformin plus linagliptin and empagliflozin and those receiving metformin plus linagliptin treatment were analyzed. A significant difference was observed in the fasting blood glucose (p = 0.019) and HbA1c (p = 0.036) levels (Table 3) between patients receiving linagliptin and those receiving empagliflozin with linagliptin. Similarly, a significant difference was observed in Chol (p = 0.019) and LDL-C levels (p = 0.035) (Table 4) between patients who received dapagliflozin and saxagliptin.

Table 3. Effects of metformin with linagliptin dual therapy and metformin with linagliptin and empagliflozin triple therapy on lipid profile and blood glucose control of patients with type 2 diabetes mellitus.
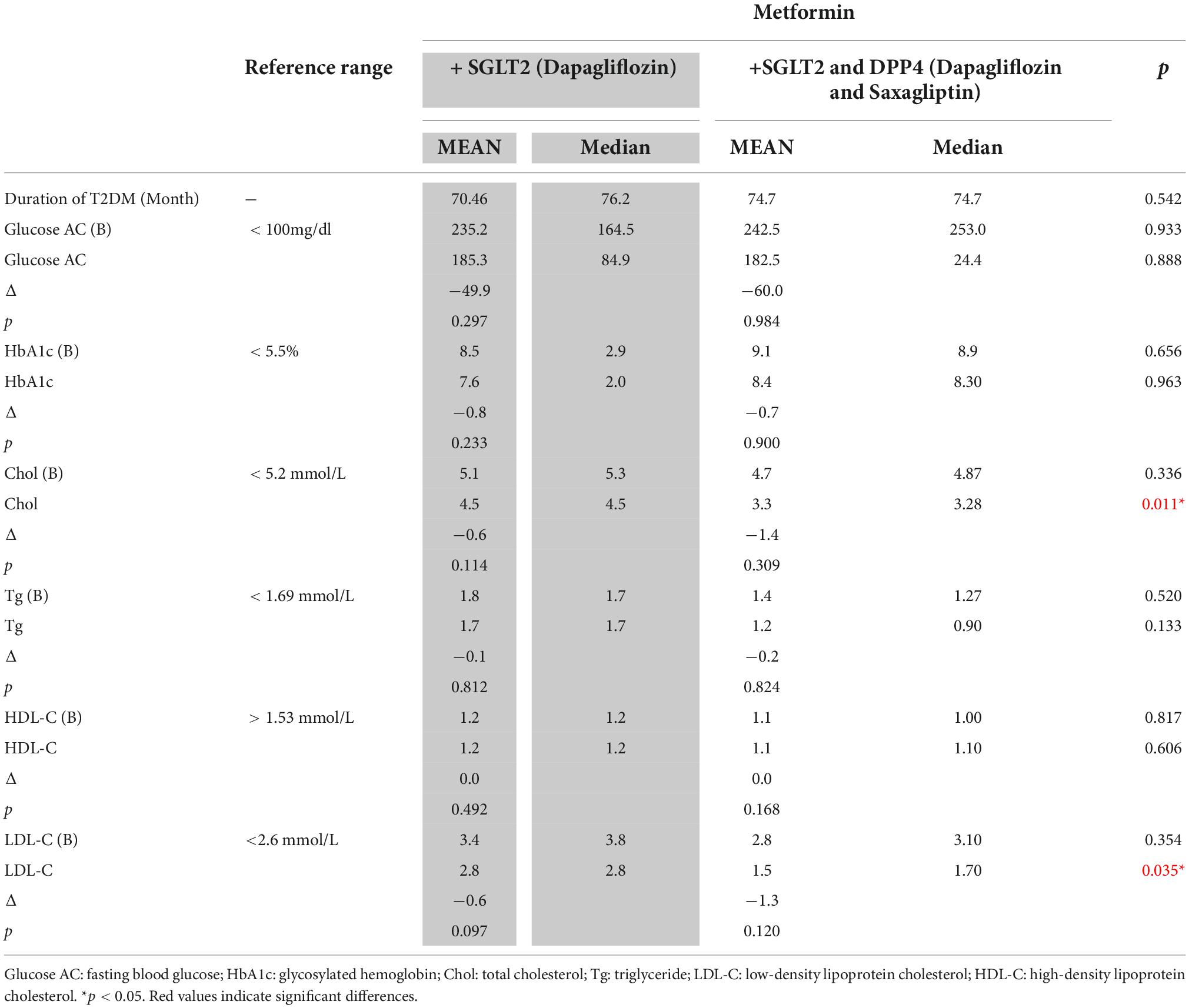
Table 4. Effects of metformin with dapagliflozin dual therapy and metformin with dapagliflozin and saxagliptin triple therapy on lipid profile and blood glucose control in patients with type 2 diabetes mellitus.
Medicinal compliance
When calculated remain pills from self-report (since last 3 months), mean remain dose by drug was not statistically different each group. The remain medicine dose of groups were 1.9 (median: 2.0), 1.0 (median: 1.0), 2.0 (median: 2.5) and 2.4 (median: 2.0) doses (p = 0.132), respectively (data not shown).
Discussion
The main finding of this study is that although a combination of metformin with a DPP4 and an SGLT2 inhibitor more effectively improved Chol and LDL-C than a combination of metformin and linagliptin or dapagliflozin dual therapy did. However, a similar effect also was found on reducing fasting glucose and HbA1C levels with metformin and linagliptin dual therapy.
Although the reasons for high or low medication adherence differ among patients, complicated regimens and a larger number of medications can reduce compliance (9). Compliance to therapy is important point in chronic conditions. Methods of measuring adherence can be either direct (biological marker) or indirect (self-reporting, questionnaires, pill counts) (10). Therefore, we calculated the remain dose of pills when every return visit for ensure homogeneity of study population. Base on the remain pill counts form study population by self-report, we ensured treatment compliance homogeneity of study population in groups.
Metformin is the first-line treatment for individuals with newly diagnosed T2DM. Our study corroborated previous findings that metformin monotherapy considerably improves dyslipidemia in statin-naive individuals with T2DM (11), especially by reducing serum LDL-C via an AMP-activated protein kinase pathway (12). Metformin has been reported to improve insulin sensitivity by increasing it; it also reduces the rate of lipolysis, thereby decreasing the conversion of free fatty acids in the liver (13). A previous meta-analysis showed that metformin reduced body weight and improved the lipid profiles of 60-year-old participants. The results also suggest that metformin treatment may reduce the risk of major coronary events and all-cause mortality in diabetic populations (14).
The mechanism through which DPP4 inhibitors affect the lipid profile in T2DM remains poorly understood. This effect could be explained by glucagon-like peptide-1 receptor. DPP4 inhibitors might inhibit lipid absorption in the gastrointestinal tract. The action of DPP4 inhibitors is based on their prevention of the inactivation of incretin. A previous study reported that these compounds improve glycemic control, both when applied in monotherapy and in combination with other oral hyperglycemic agents. Patients with different levels of glycemic control who received DPP4 inhibitors combined with metformin therapy exhibited reduced fasting blood glucose and HbA1C levels compared with those who received continuous therapy with metformin alone (15). This result is consistent with our findings in presented study.
Previous findings have indicated that individuals from East Asia have lower insulin resistance and greater sensitivity to the incretin effect (16). In particular, the glucose control efficacy of DPP4 inhibitors or incretin receptor agonists has been reported to be greater in Asian populations, especially Japanese (17) and Korean populations (18). The difference in the treatment responses could be ascribed to a different lower insulin secretory function and less insulin resistance in T2DM or the different genetics in these populations. Previous results show that DPP4 inhibitors achieve good glucose control in the Asian population (19). This result is consistent with our findings in a Taiwanese population. However, in our study, the triple therapy group also showed good glucose control and lipid profile effects.
Dyslipidemia is associated with an increased risk of cardiovascular disease in patients with T2DM. Previous studies have also suggested that the lipid-reducing efficiency of linagliptin or metformin may be lower than that of dapagliflozin (20) and a placebo (21). The different effects of monotherapy, dual therapy, and triple therapy with metformin on the lipid profiles of patients with T2DM in this study are largely consistent with the results of previous studies (22–24). In our study, triple therapy reduced the lipid profile as effectively as linagliptin dual therapy did, there were also significant differences when compared with metformin monotherapy. However, the mean Chol, HDL-C, and LDL-C levels significantly differed from baseline to after treatment DPP4 and SGLT2 inhibitor combination therapy (62.9 months on average).
In terms of blood glucose control, early combination treatment with linagliptin and metformin has been reported to improve hyperglycemia, resulting in a significant reduction in fasting glucose relative to that achieved after metformin monotherapy (25). Another study reported that compared with linagliptin monotherapy, linagliptin plus metformin treatment significantly reduced HbA1c levels after 24 weeks relative to the baseline levels (26). According to present results, similar to triple therapy, linagliptin dual therapy could improve blood glucose and HbA1C levels.
Previous studies reported that combination therapy with empagliflozin, linagliptin, and metformin (22) or dapagliflozin, saxagliptin, and metformin (27) produced considerable glucose-lowering effects in patients with T2DM. In a 52-week study, reductions in HbA1c with empagliflozin plus linagliptin therapy were superior to those with the joint use of either empagliflozin or linagliptin with metformin (28). To further verify this effect, we classified patients in the metformin triple therapy group into two different groups and reanalyzed the findings. We found that the addition of empagliflozin to metformin plus linagliptin led to a more effective reduction in fasting glucose, HbA1C l and Chol levels.
Moreover, the addition of saxagliptin to metformin plus dapagliflozin therapy led to a more effective reduction in serum Chol and LDL-C levels (Table 4). The results may indicate that a combination of metformin plus both linagliptin and saxagliptin affects the lipid profile in a manner different from that observed when a combination of metformin plus both empagliflozin and dapagliflozin is used. In other words, our results suggest that empagliflozin might have a better glycemic control ability than dapagliflozin. The results showed improvement in fasting glucose and HbA1C levels similar to that in a previous study (29) and demonstrated that saxagliptin might afford better LDL-C control than linagliptin. In the results of previous cross-sectional study, saxagliptin users had a significantly lower CVD risk than other DPP-4 drug users matched for sex, age, duration of drug use, systolic blood pressure, lipid profile, and fasting glucose (30). However, further large-scale observational studies evaluating the differences among these drugs is in terms of their cardiovascular benefits or glucose control abilities are needed.
This study has some limitations. First, our study was conducted at a single center, and the sample size was relatively small. Second, owing to the retrospective nature of the study, the medication history of the participants was not controlled, and whether the participants had ever been prescribed other antidiabetic medicines with varying drugs was unclear. Therefore, the interaction effects or side effects of the drugs may have been underestimated. Third, because this study only involved Taiwanese people, ethnic differences could not be accounted for. Thus, we followed a strict patient selection protocol to ensure homogeneity between groups. Further studies are needed to apply the results of this study to larger populations.
Conclusion
In conclusion, we report that dual therapy with metformin and linagliptin yields similar glycemic control ability to triple therapy. Among metformin combination triple therapy, triple therapy of empagliflozin and linagliptin might have a better glycemic control ability than dual therapy of linagliptin. Moreover, Triple therapy of dapagliflozin and saxagliptin might have a better lipid control ability than dual therapy of dapagliflozin.
Combination therapy of metformin with an SGLT2 inhibitor and a DPP4 inhibitor may be an effective, but albeit relatively expensive, treatment for patients with T2DM. Thus, based on the results, dual therapy with metformin and linagliptin may be a better option for long-term glycemic control because of the similar glucose control ability to triple therapy. Further studies should investigate the long-term efficacy and cost-effectiveness of each combination therapy. These results could provide a guide for clinical physicians to select a more appropriate prescription from metformin monotherapy, dual therapy, or triple therapy in the future.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of Institutional Review Board of Taipei Medical University (Approval No: N202107021). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Y-SH and Y-YL designed this study, collected and analyzed the data. Y-SH, Y-YL, and S-FW wrote the main manuscript. C-HH, C-LH, and Y-PL revised the manuscript. M-CY and A-YH reviewed the manuscript and provided the recommend in study design. All authors reviewed the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH. Epidemic obesity and type 2 diabetes in Asia. Lancet. (2006) 368:1681–8.
2. Cho YM. Incretin physiology and pathophysiology from an Asian perspective. J Diabetes Invest. (2015) 6:495–507.
3. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the american diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care. (2012) 35:1364–79.
4. Unalacak M, Kara IH, Baltaci D, Erdem O, Bucaktepe PG. Effects of ramadan fasting on biochemical and hematological parameters and cytokines in healthy and obese individuals. Metab Syndr Relat Dis. (2011) 9:157–61.
5. Ruiz B, García M, Aguirre U, Aguirre C. Factors predicting hospital readmissions related to adverse drug reactions. Eur J Clin Pharmacol. (2008) 64:715–22.
6. Association AD. 1. improving care and promoting health in populations: standards of medical care in diabetes—2020. Diabetes Care. (2019) 43:S7–13.
7. Li X, Zhou ZG, Qi HY, Chen XY, Huang G. [Replacement of insulin by fasting C-peptide in modified homeostasis model assessment to evaluate insulin resistance and islet beta cell function]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2004) 29:419–23.
8. Nurullina G, Valeeva F. AB0409 modified homa-ir index in patients with rheumatic diseases receiving glucocorticoid therapy. Ann Rheumat Dis. (2016) 75:1046.
9. Lauffenburger JC, Landon JE, Fischer MA. Effect of combination therapy on adherence among US patients initiating therapy for hypertension: a cohort study. J Gen Intern Med. (2017) 32:619–25.
10. García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. (2013) 4:175–94.
11. Lin SH, Cheng PC, Tu ST, Hsu SR, Cheng YC. Effect of metformin monotherapy on serum lipid profile in statin-naïve individuals with newly diagnosed type 2 diabetes mellitus: a cohort study. PeerJ. (2018) 6:e4578.
12. Xu T, Brandmaier S, Messias AC, Herder C, Draisma HHM. Effects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetes. Diabetes Care. (2015) 38:1858–67.
14. Solymár M, Ivic I, Pótó L, Hegyi P, Garami A. Metformin induces significant reduction of body weight, total cholesterol and LDL levels in the elderly - a meta-analysis. PLoS One. (2018) 13:e0207947. doi: 10.1371/journal.pone.0207947
15. Ahrén B. Novel combination treatment of type 2 diabetes DPP-4 inhibition + metformin. Vasc Health Risk Manage. (2008) 4:383–94.
16. Møller JB, Pedersen M, Tanaka H, Ohsugi M, Overgaard RV. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. (2014) 37:796–804.
17. Yabe D, Kuroe A, Lee S, Watanabe K, Hyo T. Little enhancement of meal-induced glucagon-like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. J Diabetes Invest. (2010) 1:56–9.
18. Oh TJ, Kim MY, Shin JY, Lee JC, Kim S. The incretin effect in Korean subjects with normal glucose tolerance or type 2 diabetes. Clin Endocrinol. (2014) 80:221–7.
19. Ntuk UE, Gill JM, Mackay DF, Sattar N, Pell JP. Ethnic-specific obesity cutoffs for diabetes risk: cross-sectional study of 490,288 UK biobank participants. Diabetes Care. (2014) 37:2500–7.
20. Cha S-A, Park Y-M, Yun J-S, Lim T-S, Song K-H. A comparison of effects of DPP-4 inhibitor and SGLT2 inhibitor on lipid profile in patients with type 2 diabetes. Lipids Health Dis. (2017) 16:58.
21. Liu J, Sempos C, Donahue RP, Dorn J, Trevisan M. Joint distribution of non-HDL and LDL cholesterol and coronary heart disease risk prediction among individuals with and without diabetes. Diabetes Care. (2005) 28:1916–21.
22. Lingvay I, Beetz N, Sennewald R, Schuler-Metz A, Bertulis J. Triple fixed-dose combination empagliflozin, linagliptin, and metformin for patients with type 2 diabetes. Postgrad Med. (2020) 132:337–45.
23. Tariqi AQ, Naughton CC. Water, health, and environmental justice in California: geospatial analysis of nitrate contamination and thyroid cancer. Environ Eng Sci. (2021) 38:377–88.
24. Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. (2016) 40:201–9.
25. Lv Q, Shen J, Miao L, Ye B, Schepers C. Early combination therapy with linagliptin and metformin in people with type 2 diabetes improves glycemic control to HbA1c≤6.5% without increasing hypoglycemia: pooled analysis of two randomized clinical trials. Diabetes Ther. (2020) 11:1317–30.
26. Ma RC, Del Prato S, Gallwitz B, Shivane VK, Lewis-D’Agostino D. Oral glucose lowering with linagliptin and metformin compared with linagliptin alone as initial treatment in Asian patients with newly diagnosed type 2 diabetes and marked hyperglycemia: subgroup analysis of a randomized clinical trial. J Diabetes Invest. (2017) 9:579–86.
27. Del Prato S, Rosenstock J, Garcia-Sanchez R, Iqbal N, Hansen L. Safety and tolerability of dapagliflozin, saxagliptin and metformin in combination: post-hoc analysis of concomitant add-on versus sequential add-on to metformin and of triple versus dual therapy with metformin. Diabetes Obes Metab. (2018) 20:1542–6.
28. DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. (2015) 38:384–93.
29. Lee PCH, Gu Y, Yeung MY, Fong CHY, Woo YC. Dapagliflozin and empagliflozin ameliorate hepatic dysfunction among chinese subjects with diabetes in part through glycemic improvement: a single-center, retrospective, observational study. Diabetes Ther. (2018) 9:285–95.
Keywords: type 2 diabetes mellitus, metformin, glycemic control, lipid profile, concomitant therapy
Citation: Lin Y-Y, Weng S-F, Hsu C-H, Huang C-L, Lin Y-P, Yeh M-C, Han A-Y and Hsieh Y-S (2022) Effect of metformin monotherapy and dual or triple concomitant therapy with metformin on glycemic control and lipid profile management of patients with type 2 diabetes mellitus. Front. Med. 9:995944. doi: 10.3389/fmed.2022.995944
Received: 16 July 2022; Accepted: 27 September 2022;
Published: 14 October 2022.
Edited by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroReviewed by:
Jelena Kotur-Stevuljević, University of Belgrade, SerbiaEsma R. Isenovic, University of Belgrade, Serbia
Copyright © 2022 Lin, Weng, Hsu, Huang, Lin, Yeh, Han and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Shan Hsieh, eXVzaGFuQG50dW5ocy5lZHUudHc=
†These authors have contributed equally to this work
 Yan-Yu Lin1†
Yan-Yu Lin1† Chen-Ling Huang
Chen-Ling Huang Yu-Shan Hsieh
Yu-Shan Hsieh