- 1Dermatology Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Milan, Italy
- 3Dermatology Clinic, Maggiore Hospital, University of Trieste, Trieste, Italy
- 4Dermatology Clinic, Department of Medical Sciences, University of Turin, Turin, Italy
- 5Department of Medical Surgical and Health Sciences, University of Trieste, Trieste, Italy
Cutaneous vasculitides encompass a heterogeneous group of clinicopathological entities, which may occur as single-organ vasculitis of the skin or present as skin-limited variant of systemic vasculitis (i.e., skin-limited ANCA-associated vasculitis), and are triggered by various factors, including infections, drugs and vaccines. The COVID-19 pandemic has challenged us with a variety of both disease- and vaccine-associated skin manifestations, including vasculitis. Among the latter, cutaneous small-vessel vasculitis, previously known as leukocytoclastic vasculitis, seems to be the most reported in either scenario, i.e., natural infection and vaccination. Vasculopathy without true vasculitic changes on histology develops in but a minority of cases, mostly severe/critical COVID-19 patients, and appears to be the result of endothelial injury due to pauci-immune thromboembolic mechanisms. Herein, we provide an overview of the available literature on COVID-19-associated and anti-SARS-CoV-2-vaccine-associated cutaneous vasculitis. Although evidence is mostly limited to isolated reports, with a proportion of cases lacking histopathological confirmation, ample overlap with pre-pandemic forms is shown.
Introduction
Cutaneous vasculitides are a heterogeneous group of inflammatory disorders affecting skin blood vessels (1). In 2018 the dermatologic addendum to the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides (D-CHCC) provided a renewed framework and nomenclature for cutaneous vasculitides (CV), devising a classification whereby cutaneous features of systemic vasculitides are discussed and cutaneous single-organ vasculitides that have no systemic counterparts are introduced (2).
Although the D-CHCC substantially furthered our understanding of CV, a wealth of new evidence has become available in the last 5 years. Provisional entities, such as macular lymphocytic arteritis also known as lymphocytic thrombophilic arteritis (LTA) (3) and recurrent cutaneous necrotizing eosinophilic vasculitis (RCNEV) (4), have been characterized more accurately. For instance, LTA has started being recognized as distinct from cutaneous polyarteritis nodosa (cPAN), presenting with a non-infiltrated, asymptomatic, and more widespread pattern of livedo racemosa (3). RCNEV, on the other hand, typically affects middle-aged Asian females, manifesting erythematous to purpuric papuloplaques, angio-oedema on the extremities and peripheral eosinophilia (4). Another provisional entity introduced in the D-CHCC is the so-called immunoglobulin (Ig) M/IgG vasculitis, a form of leukocytoclastic vasculitis involving dermal small-vessels, particularly post-capillary venules. This definition is meant for those cases of skin-limited vasculitis showing IgM/IgG deposits that are not related to cryoglobulinemia, monoclonal gammopathy and connective tissue diseases (2, 5).
The ongoing pandemic has added to the complexity of this scenario, challenging us with a variety of skin manifestations, including cutaneous vasculitis and vasculopathy, either as a direct result of the Coronavirus Disease 2019 (COVID-19) or following vaccination.
Pathogenic mechanisms are not fully understood, although the roles of a hyperactive immune response, complement activation and microvascular injury have been hypothesized.
Herein, we provide an overview of the available evidence on COVID-19-associated and anti-SARS-CoV-2-vaccine-associated CV.
COVID-19-associated cutaneous vasculitis/vasculopathy
COVID-19-associated cutaneous manifestations include six main clinical phenotypes: (i) urticarial, (ii) maculopapular, (iii) papulovesicular, (iv) chilblain-like (6), (v) livedo reticularis/racemosa-like and (vi) purpuric vasculitic-like (7). Latency varies (7–9) and their incidence ranges between 1.8 and 20.4% of COVID-19-patients (9)—though these estimates mainly reflect data from the beginning of the pandemic.
In an Italian multicenter study investigating the clinical spectrum of COVID-19 associated cutaneous manifestations, only 13/200 adult patients presented a purpuric vasculitic pattern, with the latter being a significative risk factor for dyspnea (10)—although no clear relationship was shown with severity. Presentation may vary with livedoid features, retiform purpura and/or acro-ischemic phenomena (Table 1) (10). Systemic corticosteroids (CS) have shown some benefit, but a clear treatment protocol is lacking due to the rarity and incomplete characterization of these forms, as well as their presentation in critically ill patients (11).
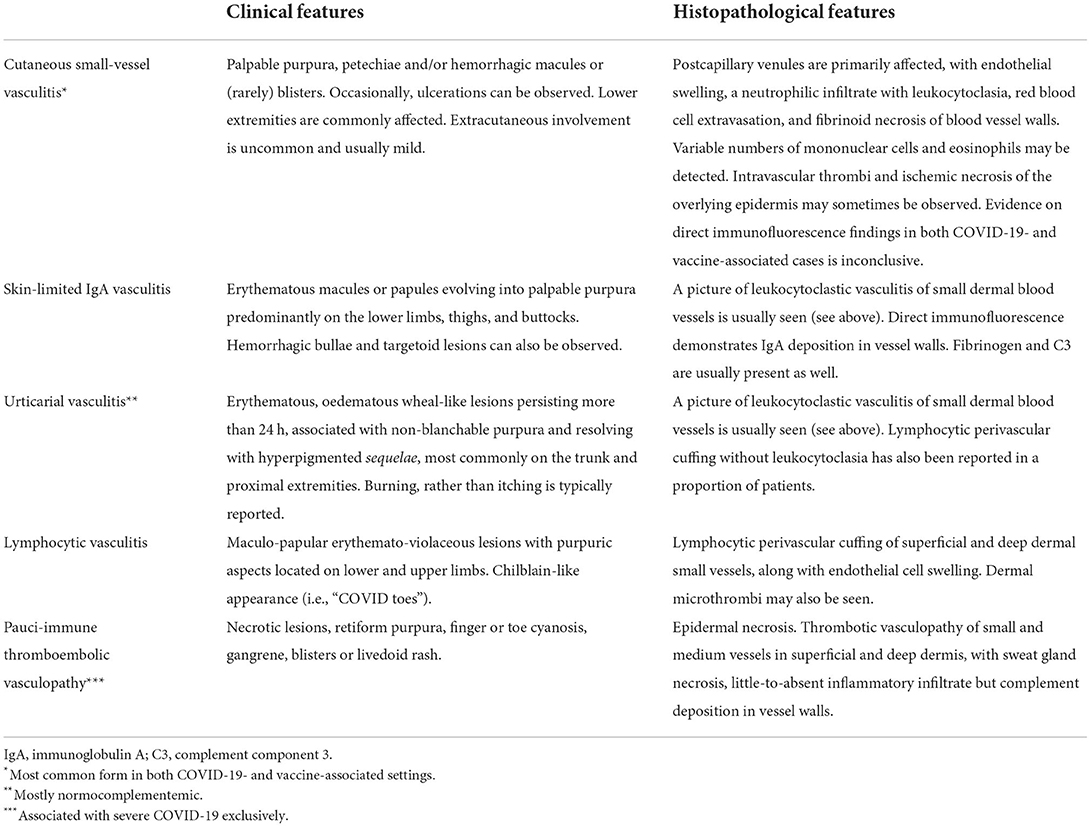
Table 1. Clinical and histopathological features of the main cutaneous vasculitides associated with COVID-19 and/or anti-SARS-CoV-2 vaccination.
Cutaneous small-vessel vasculitis
Cutaneous small-vessel vasculitis, also known as leukocytoclastic vasculitis (LCV), is one of the most common CV reported in COVID-19 patients (Supplementary Table 1) (8, 12–24). Nevertheless, it is rare compared to other COVID-19-related dermatological manifestations. Indeed, according to a case-control study on 198 severe COVID-19 patients, LCV accounts for only 1.8% of all cutaneous findings (25). Clinical appearance spans from classic, bilateral symmetric palpable purpura favoring dependent body sites (Supplementary Figures 1a,b) to vesicobullous, hemorrhagic or targetoid eruptions. Oral or intravenous CS, with or without topical CS, were the mainstay of treatment, whereas intravenous immunoglobulin (IVIg) was employed only in a minority of cases (19, 20, 23). As for the prognosis, most patients experienced complete recovery, except for a few who developed vasculitis-associated gangrene or died due to COVID-19-related complications (12, 19, 20, 24).
IgA vasculitis
IgA vasculitis is a form of small vessel vasculitis characterized by perivascular deposition of hypogalactosylated IgA1 and neutrophil activation, being the most common vasculitis in the pediatric age. Palpable non-thrombocytopenic purpura of lower extremities and buttocks is a characteristic sign of skin-limited IgA vasculitis (IgAV) and Henoch–Schonlein purpura (HSP) (26, 27). Hemorrhagic blisters, as well as targetoid lesions, have been suggested to occur more frequently in skin-limited IgA vasculitis (Supplementary Figure 1c) than cutaneous small vessel vasculitis with IgM/IgG deposits, i.e., LCV (5). Fifteen COVID-19-associated cases (12 males, 3 females), half of which were children, have been reported so far. Palpable purpura (13/15) as well as renal (8/15), gastrointestinal (8/15) and articular (3/15) involvement were documented. In 8 subjects, onset of vasculitis was simultaneous with the infection. All these patients received systemic CS. Biologics and immunosuppressants were administered only in 4 individuals due to concomitant renal impairment (1 rituximab, 2 mycophenolate mofetil, 1 cyclophosphamide), with a favorable response across published reports (28).
Urticarial vasculitis
Urticarial vasculitis (UV) is a rare clinicopathological entity manifesting with indurated wheal-like lesions lasting more than 24 h (Supplementary Figure 1d) and usually leaving post-inflammatory hyperpigmented sequelae upon resolution (29). Although the cause of UV often remains unclear, trigger factors such as drugs, infections, autoimmune diseases, and malignancy have been described (30, 31). Though rare, UV has been described in either symptomatic or asymptomatic COVID-19 patients (32–34). Interestingly, its onset has also been reported a few weeks following recovery from COVID-19 (35). Antihistamines alone or in combination with oral CS were administered in all the subjects (32–35).
Other vasculitides associated with COVID-19
Anti-neutrophil cytoplasmic antibodies (ANCA) associated vasculitis (AAV) can sometimes mimic COVID-19 in terms of pulmonary involvement and COVID-19 may occur simultaneously with AAV. (36). Six patients, 4 of whom were males, were diagnosed with AAV simultaneously or shortly after COVID-19. Three had serum antibodies directed against myeloperoxidase (anti-MPO), while the others had anti-proteinase 3 (anti-PR3) antibody positivity. Fever, respiratory and gastrointestinal symptoms were reported. All patients survived after adequate treatment with immunosuppressive medications (37).
COVID-19-associated cutaneous vasculitides in the pediatric age
According to a recent systematic review by Batu et al., which gathered 36 pediatric patients, the median age of onset of vasculitis was 13 years, with a male predominance (M/F: 2.3). The median time from infection to onset of vasculitis was 17.5 days (range: 2–150). Among those with potential skin involvement, the most frequently reported in that pediatric age included IgAV/HSP (25%) chilblains (19.4%), UV (5.5%), cutaneous leukocytoclastic vasculitis (2.7%), and acute hemorrhagic edema of infancy (AHEI, 2.7%) (38).
Kawasaki disease (KD) is an acute systemic vasculitic syndrome primarily affecting children below the age of 5, that involves small and medium-sized vessels with a predilection for coronary arteries (39). As SARS-CoV-2 infection can lead to endothelial inflammation and dysfunction, it can also trigger the development of KD in certain individuals (40). Moreover, older children (median age of 8 years) can be affected by a similarly severe inflammatory disorder with multisystem involvement (MIS-C), also known as Pediatric Multisystem Inflammatory Syndrome temporally associated with SARS-CoV-2 (PIMS-TS) (41). MIS-C is mainly characterized by systemic vasculitis, mucocutaneous inflammatory signs (rash), multisystem involvement, and hypercoagulation, although thrombotic or embolic events were rare, when compared with adult COVID-19 (42). Although it may present a clinical overlap with KD or toxic shock syndrome, it is regarded as a separate entity (43).
Pathophysiology of COVID-19-associated vasculopathy and vasculitis
Distinct pathomechanisms have been implicated in the genesis of the above-mentioned COVID-19-associated cutaneous findings, depending on the presence/absence of a robust type I interferon signature: (i) transient, true vasculitis in mild cases (e.g., COVID-toes) and (ii) small vessel thromboembolic disease, without true vasculitis (i.e., vasculopathy) in patients with severe disease. A number of other forms may be placed between the two ends of this spectrum (44).
In greater detail, vasculitic changes with lymphocytic perivascular cuffing and infiltration, possibly leading to secondary luminal thrombosis, result from type I interferon responses, akin to familial chilblain lupus or STING-associated vasculopathy with onset in infancy (45, 46).
In contrast, dysfunction of vascular endothelium due to the SARS-CoV-2 infection has been suggested in the pathogenesis of the COVID-19 vasculopathy (47). Initially, it was speculated that endothelial injury was due to direct viral infection (48), but recent evidence demonstrated that endothelial cells present low Angiotensin Converting Enzyme 2 (ACE2) expression and are resistant to SARS-CoV-2 infection, supporting the involvement of an indirect mechanism of endothelial injury in the pathogenesis of COVID-19 vasculopathy (47, 48). This is best exemplified by patient with severe COVID-19 pneumonia. In the lungs, when respiratory and alveolar epithelial cells get infected by SARS-CoV-2 in the setting of defective antiviral interferon signaling, an exacerbated innate inflammatory loop is induced with elevation of Interleukin (IL)-1β, IL-6 and Tumor Necrosis Factor (TNF)α. The subsequent release of several proinflammatory cytokines/chemokines at a systemic level, together with secondary complement activation due to ischemia, leads to indirect endothelial cell injury through loss of their antithrombogenic properties and barrier function (49). This process, known as pulmonary immunothrombosis, possibly followed by pulmonary venous microembolism, may account for the clinico-pathological picture observed in severe COVID-19 cases, with pauci-immune thrombogenic vasculopathy and terminal complement activation in vessel walls (50), but only sporadic SARS-CoV-2 spike protein deposition (44).
Increased levels of galactose deficient IgA1 (gd-IgA1) are necessary for the development of IgA nephritis, with a multi-hit model involving IgA1 and anti-endothelial cell antibodies currently accepted to explain its vasculitic, extrarenal manifestations. Mucosal infections, such as COVID-19, are believed to enhance IL-6 production thereby stimulating poor glycosylation/galactosylation of IgA1 in predisposed subjects. The subsequent formation gd-IgA1 may contribute toward the disease process of IgA vasculitis in a proportion of COVID-19 patients (51).
A humoral response against SARS-CoV-2 antigens, leading to immune-complex formation, could underscore cases of COVID-19-associated urticarial vasculitis and leukocytoclastic vasculitis (52). Indeed, SARS-CoV-2 antigens have been detected in skin biopsies from two UV patients with COVID-19, supporting the existence of a causal link (33). Consistent with a type III hypersensitivity response, circulating immune complexes could act as triggers for classic complement pathway activation, thus promoting neutrophil recruitment, vascular leakage and subsequent vessel wall injury and inflammation (52).
It is noteworthy that anti-phosphatidylserine/prothrombin complex antibodies have been implicated in models of cutaneous vasculitis (53) and that anti-prothrombin antibodies increase after infection with SARS-CoV-2 (54).
Concerning ANCA-associated vasculitides, NETs overproduction has been described during COVID-19. Prolonged exposure of NETs to proteins as well as their reduced clearance may be key in explaining the onset of ANCA autoimmunity in predisposed subjects infected by SARS-CoV-2 (36).
Main proposed pathomechanisms are summarized in Figure 1.
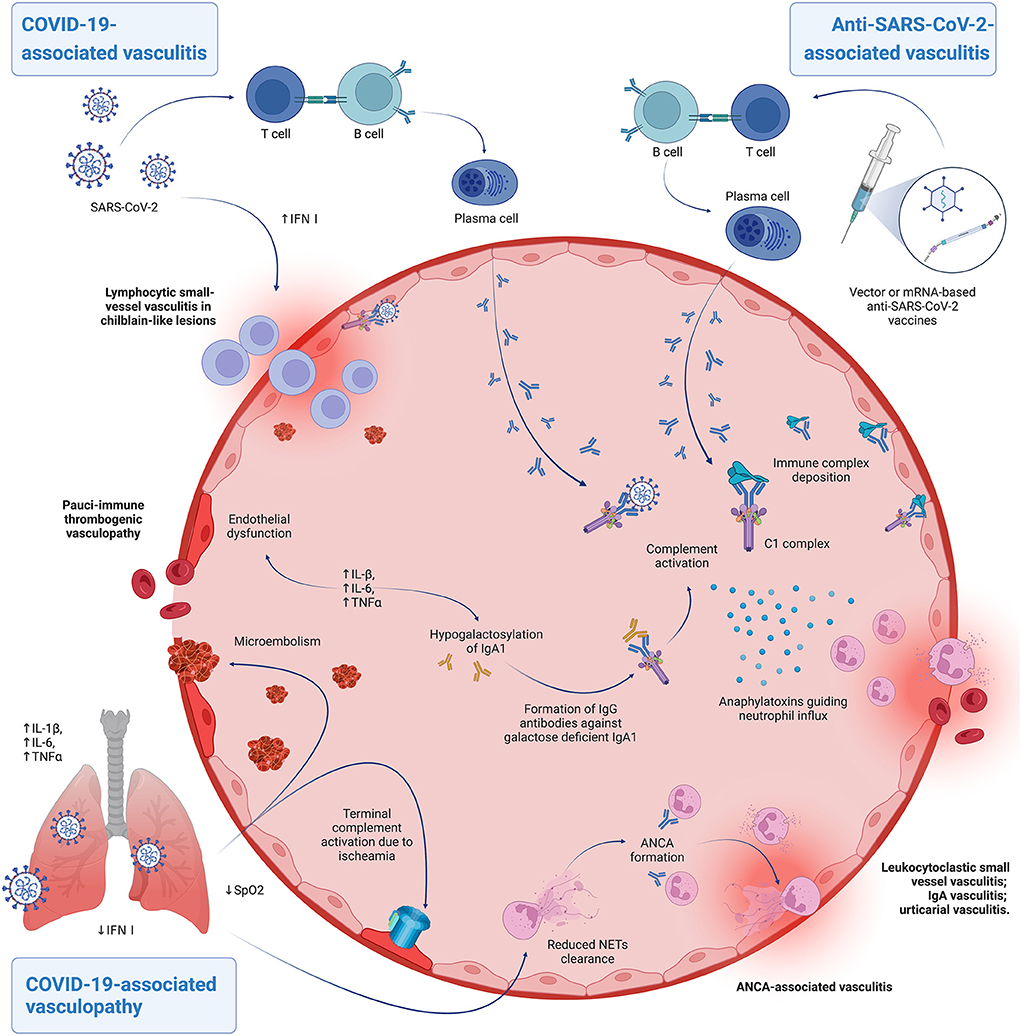
Figure 1. Hypothesized pathomechanisms of COVID-19-associated vasculitis/vasculopathy and anti-SARS-CoV-2-vaccine associated vasculitis. Patients able to mount a robust type I interferon response can develop lymphocytic perivascular cuffing during infection, leading to chilblain-like lesions. The virus can also cause immune complex deposition, with subsequent complement activation and neutrophil recruitment, determining the clinicopathological picture of leukocytoclastic vasculitis. A similar process, possibly with anaphylatoxins inducing exaggerated mastocyte activation, is thought to underscore cases of urticarial vasculitis. Both these forms can occur also in vaccine-related cases. Patients that develop severe COVID-19 due to a defective antiviral response may experience indirect endothelial injury, due to elevated proinflammatory cytokines at a systemic level and ischaemia-induced complement activation. This process is known as pulmonary immunothrombosis and may be complicated by pulmonary venous embolism. Speculatively, the inflammatory milieu brought about by the infection can lead to other forms of cutaneous vasculitides. Elevated IL-6 levels may contribute to hypogalactosylation of IgA1 in predisposed individuals triggering an IgG-mediated response that may result in IgA vasculis. Prolonged exposure and reduced clearance of Neutrophil Extracellular Traps (NETs) in the context of COVID-19 dysregulated inflammatory responses may set the basis for ANCA-associated autoimmunity and vasculitis. Created with BioRender.com.
Anti-SARS-CoV-2 vaccination-induced cutaneous vasculitis
Vasculitides and other vascular affections have also been reported following anti-SARS-CoV-2 vaccination (55).
Cutaneous small-vessel vasculitis
Skin-limited small vessel vasculitis or LCV (56) represents the most common CV reported after anti-SARS-CoV-2 vaccination. It has been observed after the Pfizer-BioNTech mRNA vaccine (BNT16B2b2) (57–65). Moderna mRNA vaccine (mRNA-1273) (66–68). Oxford-AstraZeneca adenoviral vaccine (ChAdOx1 nCoV-19 AZD1222) (69–79). Johnson & Johnson adenoviral vaccine (Ad26.COV2.S) (80–82), and inactivated vaccines [Sinovac CoronaVac (83), Bharat Biotech Covaxin (84), Sinopharm BBiBP-CorV] (85, 86) (Supplementary Table 2).
Almost every case was biopsy-confirmed. Notably, some of these cases were reported as “immunocomplex vasculitides” (65–68). Some patients experienced systemic symptoms such as joint pain (64, 70, 73, 80, 84), and microhematuria (79, 80). In one patient, gastrointestinal involvement with melena and diarrhea was reported (65). In some cases, cryoglobulins were detected on serological analysis (82, 87); notably, the case by Nastro et al. also featured a concomitant atypical herpes zoster of the right leg (59). Among reviewed cases, one had history of SARS-CoV-2 infection (78) and one had a previous diagnosis of leukocytoclastic vasculitis (58).
Treatment was generally represented by oral CS and antihistamines (local corticosteroids, non-steroid anti-inflammatory drugs (NSAIDs), colchicine, antibiotics, analgesics, pentoxifylline, dapsone were also prescribed in a minority of cases). Spontaneous remission was occasionally reported. All patient recovered in 1–8 weeks, except for one individual who developed COVID-19 20 days after vaccination with Oxford-Astrazeneca ChAdOx1 nCoV-19 vaccine (78). This patient presented with cough, myalgia, and fatigue, and developed progressive skin manifestations, including urticarial and purpuric lesions over her upper and lower extremities and abdomen. After 9 days, she developed multiorgan failure and died. In this case it is likely that the SARS-CoV-2 infection rather than the vaccination could have acted as trigger of the vasculitis (78).
IgA vasculitis
IgA vasculitis (88) has been observed after vaccination with Pfizer-BioNTech mRNA vaccine (BNT16B2b2) (57, 89–93), Moderna mRNA vaccine (mRNA-1273) (94, 95), Oxford-AstraZeneca adenoviral vaccine (ChAdOx1 nCoV-19 AZD1222) (96–98), and Sinovac inactivated vaccine (CoronaVac) (99) (Supplementary Table 3). Of note, histology was not available in all cases. Some patients also experienced systemic symptoms such as joint (93, 96–98) or abdominal pain (94), hematuria or renal impairment (90, 94, 96). Treatment of choice was generally represented by oral CS, but spontaneous remission was also occasionally reported. All patients recovered in several weeks. Interestingly, of the reviewed cases, two had history of SARS-CoV-2 infection (97, 99); three had history of previous IgA vasculitis (90, 94) or Henoch-Schönlein purpura (92).
Lymphocytic vasculitis
Lymphocytic vasculitis is a histologic reaction pattern with a dominant lymphocytic inflammatory infiltrate (100). Reported cases of lymphocytic vasculitis followed the inoculation of Pfizer-BioNTech mRNA vaccine (BNT16B2b2) (101), Oxford-AstraZeneca adenoviral vaccine (ChAdOx1 nCoV-19 AZD1222) (102), inactivated vaccine Bharat Biotech Covaxin (103) and mRNA-1273 Moderna vaccine (104) (Supplementary Table 4).
Treatment of choice was generally represented by oral antihistamines or local CS (one case was managed with follow up only). All patients fully recovered in 2 weeks. Notably, one of the patients had SARS-CoV-2 infection before vaccination (101).
Urticarial vasculitis
Cases of UV were reported after Moderna mRNA vaccine (mRNA-1273) (99, 105), Oxford-AstraZeneca adenoviral vaccine (ChAdOx1 nCoV-19 AZD1222) (106), and inactivated vaccine Sinovac CoronaVac (107) (Supplementary Table 5). Treatment of choice was generally represented by oral corticosteroids, but oral antihistamines, dapsone and indomethacin were also used. All patient fully recovered in 1–8 weeks, this being in line with the expected course of drug-induced UV (31).
Other vasculitides: ANCA-associated vasculitis and other forms
Only one case of AAV presenting with cutaneous involvement has been reported following anti-SARS-CoV-2 vaccination (Pfizer-BioNTech mRNA vaccine) (BNT16B2b2) (108). Interestingly, the patient had been taking propylthiouracil for Graves' disease and therefore her condition was identified as a propylthiouracil-induced ANCA-associated vasculitis. In this case, the treatment of choice was represented by oral corticosteroids and the patient recovered after 3 weeks. Of note, there are case reports of AAV with systemic involvement and absence of cutaneous features, triggered by anti-SARS-CoV2 vaccination (80, 109).
Among the unclassifiable forms, we also describe the peculiar vasculitis reported by Nasr et al. The 64-year-old female patient had history of Raynaud's disease, hand arthritis, photosensitivity, Sjogren's syndrome and leukocytoclastic vasculitis; 3 days after receiving the first dose of Pfizer–BioNTech mRNA vaccine she developed fingertip necrosis (caused by a type II cryoglobulinemia) and a new episode of purpuric rash on the lower extremities (likely leukocytoclastic vasculitis). The workup revealed cryoglobulinemia, hypocomplementemia, elevated antinuclear antibodies and IgM antiphospholipid autoantibodies, suggesting a diagnosis of systemic lupus erythematosus and antiphospholipid syndrome (110).
Lastly, multisystem inflammatory syndrome (MIS) after vaccination deserves a brief mention. MIS has been associated with SARS-CoV-2 infection, has a latency of 4–6 weeks, and can be ultimately described as a vasculopathy clinically resembling Kawasaki disease and potentially leading to acute cardiac dysfunction and multiorgan failure (111, 112). It usually occurs in children (MIS-C), but adult forms are also reported (MIS-A) (112). Interestingly, very rare cases of MIS are reported in absence of viral infection, after anti-SARS-CoV-2 vaccination (MIS-V) (113). In children, MIS-V had a frequency of 1.5 cases per million of injected doses, with patients aged 12–20 years, presenting with fever, coagulopathy, mucocutaneous, cardiac, gastrointestinal, and renal involvement. They were treated with systemic CS and/or IGIV and had a favorable outcome (111, 114). Similarly, adult MIS-V forms are also reported, with comparable course, treatment, and outcome (112, 115–118) (Supplementary Table 6).
Pathophysiology of anti-SARS-CoV-2-vaccine-associated vasculopathy and vasculitis
Immune-complex deposition with ensuing complement activation is currently regarded as the plausible pathophysiology for most anti-SARS-CoV-2-vaccination-associated cutaneous vasculitides (119). SARS-CoV-2 vaccine components sharing structural similarities to host proteins may promote a pro-inflammatory state followed by the activation of autoreactive B/T cells, antibody formation, and subsequent immune complex deposition in the small vessels of the skin with potential involvement of internal organs as well (120). Molecular mimicry phenomena may also have a role. An unrelated antigen or an underlying genetic predisposition unmasked, due to the vaccine's immune enhancing properties, should be considered as well.
Conclusions
In conclusion, we reviewed available evidence on COVID-19-associated and anti-SARS-CoV-2-vaccine-associated CV, showing superimposable findings with pre-pandemic cases.
Although IgA immune deposits were prevalent, lack of reporting of immunofluorescence findings in most papers hinders a thorough analysis of above-mentioned cases and calls for further studies, as the very nature of immune deposits in the non-COVID-19/COVID-19-vaccine-associated setting is still debated (5).
Patients referred for purpuric lesions often pose a challenge to dermatologists and many algorithms have been proposed to simplify their differential diagnosis and thereby stratify their prognosis (121). Signs of retiform (i.e., branched) purpura at acral sites or generalized, particularly, have been suggested to portend poor prognosis in patients with complex purpura (121) and, though they may be lacking validation in this specific scenario, they could pose as a useful clue also in COVID-19 patients, to promptly recognize those at a higher risk of immunothrombotic vasculopathy.
Adequately assessing the causal link on an individual case basis along with thorough patient counseling should aim to minimize vaccine hesitancy, as seen in other vaccine-associated dermatological conditions (122).
Despite the wealth of clinical evidence available concerning COVID-19-associated and anti-SARS-CoV-2-vaccine-associated cutaneous vasculitis and vasculopathy, there is a paucity of studies addressing the pathophysiology of these manifestations. Further research is therefore needed to inform pathogenesis-driven treatment.
Author contributions
EZ, GA, CM, MR, and CAM reviewed the pertaining literature and wrote the manuscript. AM, SR, and PQ supervised the draft. CAM and AM edited and approved the final draft. All authors have made substantial contribution to the work and have approved the final version of this article.
Funding
Publication costs were partially funded by Grant Ricerca Corrente / Finalizzata, Italian Ministry of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.996288/full#supplementary-material
References
1. Marzano AV, Vezzoli P, Berti E. Skin involvement in cutaneous and systemic vasculitis. Autoimmun Rev. (2013) 12:467–76. doi: 10.1016/j.autrev.2012.08.005
2. Sunderkötter CH, Zelger B, Chen KR, Requena L, Piette W, Carlson J, et al. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol. (2018) 70:171–84. doi: 10.1002/art.40375
3. Kelly RI, Wee E, Balta S, Williams RA. Lymphocytic thrombophilic arteritis and cutaneous polyarteritis nodosa: clinicopathologic comparison with blinded histologic assessment. J Am Acad Dermatol. (2020) 83:501–8. doi: 10.1016/j.jaad.2019.10.068
4. Lin TL, Yang CS, Chen YJ. Recurrent cutaneous necrotising eosinophilic vasculitis. Australas J Dermatol. (2021) 62:e102–6. doi: 10.1111/ajd.13451
5. Marzano AV, Genovese G, Tavecchio S, Germiniasi F, Fanoni D, Caproni M, et al. Clinical and immunopathologic features of idiopathic cutaneous immunoglobulin M/G vasculitis versus idiopathic skin-limited immunoglobulin A vasculitis. J Am Acad Dermatol. (2021) 84:175–8. doi: 10.1016/j.jaad.2020.04.060
6. Ramondetta A, Panzone M, Dapavo P, Ortoncelli M, Giura MT, Licciardello M, et al. Chilblain acral lesions in the COVID-19 era. Are they marker of infection in asymptomatic patients? J Eur Acad Dermatol Venereol. (2020) 34:e440–1. doi: 10.1111/jdv.16636
7. Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID-19: current knowledge and future perspectives. Dermatology. (2021) 237:1–12. doi: 10.1159/000512932
8. Kumar G, Pillai S, Norwick P, Bukulmez H. Leucocytoclastic vasculitis secondary to COVID-19 infection in a young child. BMJ Case Rep. (2021) 14:e242192. doi: 10.1136/bcr-2021-242192
9. Kaya G, Kaya A, Saurat JH. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology. (2020) 7:3–16. doi: 10.3390/dermatopathology7010002
10. Marzano AV, Genovese G, Moltrasio C, et al. The clinical spectrum of COVID-19-associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. (2021) 84:1356–63. doi: 10.1016/j.jaad.2021.01.023
11. Wong K, Farooq Alam Shah MU, Khurshid M, Ullah I, Tahir MJ, Yousaf Z. COVID-19 associated vasculitis: a systematic review of case reports and case series. Ann Med Surg. (2022) 74:103249. doi: 10.1016/j.amsu.2022.103249
12. Negrini S, Guadagno A, Greco M, Parodi A, Burlando M. An unusual case of bullous haemorrhagic vasculitis in a COVID-19 patient. J Eur Acad Dermatol Venereol. (2020) 34:e675–6. doi: 10.1111/jdv.16760
13. Caputo V, Schroeder J, Rongioletti F. A generalized purpuric eruption with histopathologic features of leucocytoclastic vasculitis in a patient severely ill with COVID-19. J Eur Acad Dermatol Venereol. (2020) 34:e579–81. doi: 10.1111/jdv.16737
14. Camprodon Gómez M, González-Cruz C, Ferrer B, Barberá MJ. Leukocytoclastic vasculitis in a patient with COVID-19 with positive SARS-CoV-2 PCR in skin biopsy. BMJ Case Rep. (2020) 13:e238039. doi: 10.1136/bcr-2020-238039
15. Tahir A, Sohail Z, Nasim B, Parmar NV. Widespread cutaneous small vessel vasculitis secondary to COVID-19 infection. Int J Dermatol. (2020) 59:1278–9. doi: 10.1111/ijd.15106
16. Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, Moreno-Garcia Del Real C, Burgos-Blasco P, Suarez-Valle A. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19). J Eur Acad Dermatol Venereol. (2020) 34:e536–7. doi: 10.1111/jdv.16663
17. Mayor-Ibarguren A, Feito-Rodriguez M, Quintana Castanedo L, Ruiz-Bravo E, Montero Vega D, Herranz-Pinto P. Cutaneous small vessel vasculitis secondary to COVID-19 infection: a case report. J Eur Acad Dermatol Venereol. (2020) 34:e541–2. doi: 10.1111/jdv.16670
18. Iraji F, Galehdari H, Siadat AH, Bokaei Jazi S. Cutaneous leukocytoclastic vasculitis secondary to COVID-19 infection: A case report. Clin Case Rep. (2020) 9:830–834. doi: 10.1002/ccr3.3596
19. Alattar KO, Subhi FN, Saif Alshamsi AH, Eisa N, Shaikh NA, Mobushar JA, et al. COVID-19-associated leukocytoclastic vasculitis leading to gangrene and amputation. IDCases. (2021) 24:e01117. doi: 10.1016/j.idcr.2021.e01117
20. Zavattaro E, Cammarata E, Tarantino V, Soddu D, Gironi LC, Savoia P. Successful treatment of a bullous vasculitis with intravenous immunoglobulins in a COVID-19 patient. Dermatol Ther. (2021) 34:e14853. doi: 10.1111/dth.14853
21. Gouveia PADC, Cipriano IC, de Melo MAZ, da Silva HTA, Amorim MAO, de Sá Leitão CC, et al. Exuberant bullous vasculitis associated with SARS-CoV-2 infection. IDCases. (2021) 23:e01047. doi: 10.1016/j.idcr.2021.e01047
22. Yildirim Bay E, Moustafa E, Semiz Y, Gündogdu O, Oguz Topal I, Yalçin Ö. Leukocytoclastic vasculitis secondary to COVID-19 infection presenting with inclusion bodies: a histopathological correlation. J Cosmet Dermatol. (2022) 21:27–9. doi: 10.1111/jocd.14637
23. Nassani N, Sweiss N, Berry JT, Calhoun C, Polick A, Trivedi I. Leukocytoclastic vasculitis in cutaneous crohn disease in the setting of COVID-19. Inflamm Bowel Dis. (2021) 27:e74–5. doi: 10.1093/ibd/izab045
24. Capoferri G, Daikeler T, Mühleisen B, Trendelenburg M, Müller S. Cutaneous leukocytoclastic vasculitis secondary to COVID-19 infection leading to extensive skin necrosis. Clin Dermatol. (in press). doi: 10.1016/j.clindermatol.2022.02.013. [Epub ahead of print].
25. Kutlu Ö, Ögüt ND, Erbagci E, Metin A. Dermatologic comorbidities of the patients with severe COVID-19: a case-control study. Dermatol Ther. (2021) 34:e14731. doi: 10.1111/dth.14731
26. M Gardner-Medwin P., Dolezalova C., Cummins T.R. Southwood, Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. (2002) 360:1197–202. doi: 10.1016/S0140-6736(02)11279-7
27. Messova A, Pivina L, Muzdubayeva Z, Sanbayev D, Urazalina Z, Adams A. COVID-19 and new onset IgA vasculitis: a systematic review of case reports. J Emerg Nurs. (2022) 48:348–65. doi: 10.1016/j.jen.2022.05.002
28. Valero C, Baldivieso-Achá JP, Uriarte M, Vicente-Rabaneda EF, Castañeda S, García-Vicuña R. Vasculitis flare after COVID-19: report of two cases in patients with preexistent controlled IgA vasculitis and review of the literature. Rheumatol Int. (2022) 42:1643–52. doi: 10.1007/s00296-022-05153-w
29. Loricera J, Calvo-Río V, Mata C, Ortiz-Sanjuán F, González-López MA, Alvarez L, et al. Urticarial vasculitis in northern Spain: clinical study of 21 cases. Medicine. (2014) 93:53–60. doi: 10.1097/MD.0000000000000013
30. Gu SL, Jorizzo JL. Urticarial vasculitis. Int J Womens Dermatol. (2021) 7:290–7. doi: 10.1016/j.ijwd.2021.01.021
31. Marzano AV, Maronese CA, Genovese G, Ferrucci S, Moltrasio C, Asero R, et al. Urticarial vasculitis: clinical and laboratory findings with a particular emphasis on differential diagnosis. J Allergy Clin Immunol. (2022) 149:1137–49. doi: 10.1016/j.jaci.2022.02.007
32. de Perosanz-Lobo D, Fernandez-Nieto D, Burgos-Blasco P, Selda-Enriquez G, Carretero I, Moreno C, et al. Urticarial vasculitis in COVID-19 infection: a vasculopathy-related symptom? J Eur Acad Dermatol Venereol. (2020) 34:e566–8. doi: 10.1111/jdv.16713
33. Criado PR, Criado RFJ, Gianotti R, Abdalla BAZ, Pincelli TPH, Michalany AO, et al. Urticarial vasculitis revealing immunolabelled nucleocapsid protein of SARS-CoV-2 in two Brazilian asymptomatic patients: the tip of the COVID-19 hidden iceberg? J Eur Acad Dermatol Venereol. (2021) 35:e563–6. doi: 10.1111/jdv.17391
34. Shahidi Dadras M, Rakhshan A, Diab R, Abdollahimajd F. SARS-CoV-2 infection as a potential triggering factor for urticarial vasculitis during pregnancy: a case report. Clin Case Rep. (2021) 9:e04323. doi: 10.1002/ccr3.4323
35. Nasiri S, Dadkhahfar S, Abasifar H, Mortazavi N, Gheisari M. Urticarial vasculitis in a COVID-19 recovered patient. Int J Dermatol. (2020) 59:1285–6. doi: 10.1111/ijd.15112
36. Duran TI, Turkmen E, Dilek M, Sayarlioglu H, Arik N. ANCA-associated vasculitis after COVID-19. Rheumatol Int. (2021) 41:1523–9. doi: 10.1007/s00296-021-04914-3
37. Giryes S, Bragazzi NL, Bridgewood C, De Marco G, McGonagle D. COVID-19 Vasculitis and vasculopathy-Distinct immunopathology emerging from the close juxtaposition of Type II Pneumocytes and Pulmonary Endothelial Cells. Semin Immunopathol. (2022) 44:375–90. doi: 10.1007/s00281-022-00928-6
38. Batu ED, Sener S, Ozen S. COVID-19 associated pediatric vasculitis: a systematic review and detailed analysis of the pathogenesis. Semin Arthritis Rheum. (2022) 55:152047. doi: 10.1016/j.semarthrit.2022.152047
39. Medaglia AA, Siracusa L, Gioè C, Giordano S, Cascio A, Colomba C. Kawasaki disease recurrence in the COVID-19 era: a systematic review of the literature. Ital J Pediatr. (2021) 47:95. doi: 10.1186/s13052-021-01041-4
40. Marzano AV, Cassano N, Moltrasio C, Verdoni L, Genovese G, Vena GA. Multisystem inflammatory syndrome in children associated with COVID-19: a review with an emphasis on mucocutaneous and Kawasaki disease-like findings. Dermatology. (2022) 238:35–43. doi: 10.1159/000515449
41. Yilmaz Ciftdogan D, Ekemen Keles Y, Cetin BS, et al. COVID-19 associated multisystemic inflammatory syndrome in 614 children with and without overlap with Kawasaki disease-Turk MIS-C study group. Eur J Pediatr. (2022) 181:2031–43. doi: 10.1007/s00431-022-04390-2
42. Fontelo P, Bastola MM, Zheng Z, Baik SH. A review of thromboembolic events in hospitalized COVID-19 patients. Thromb J. (2021) 19:47. doi: 10.1186/s12959-021-00298-3
43. Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. (2021) 180:2019–34. doi: 10.1007/s00431-021-03993-5
44. McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics. Lancet Rheumatol. (2021) 3:e224–33. doi: 10.1016/S2665-9913(20)30420-3
45. Frumholtz L, Bouaziz JD, Battistella M, Hadjadj J, Chocron R, Bengoufa D, et al. Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. (2021) 185:1176–85. doi: 10.1111/bjd.20707
46. Hubiche T, Cardot-Leccia N, Le Duff F, Seitz-Polski B, Giordana P, Chiaverini C, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. (2021) 157:202–6. doi: 10.1001/jamadermatol.2020.4324
47. Nicosia RF, Ligresti G, Caporarello N, Akilesh S, Ribatti D. COVID-19 Vasculopathy: mounting evidence for an indirect mechanism of endothelial injury. Am J Pathol. (2021) 191:1374–84. doi: 10.1016/j.ajpath.2021.05.007
48. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
49. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. (2020) 41:3038–44. doi: 10.1093/eurheartj/ehaa623
50. Magro C, Mulvey JJ, Berlin D, Salvatore S, Harp J, Baxter-Stoltzfus A, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220:1–13. doi: 10.1016/j.trsl.2020.04.007
51. Farooq H, Aemaz Ur Rehman M, Asmar A, Asif S, Mushtaq A, Qureshi MA. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: a systematic review. J Taibah Univ Med Sci. (2022) 17:1–13. doi: 10.1016/j.jtumed.2021.08.012
52. Magro C, Nuovo G, Mulvey JJ, Laurence J, Harp J, Crowson AN. The skin as a critical window in unveiling the pathophysiologic principles of COVID-19. Clin Dermatol. (2021) 39:934–65. doi: 10.1016/j.clindermatol.2021.07.001
53. Kawakami T, Okiyama N, Kodera M, Seishima M, Yamaguchi Y. The relationship between anti-phosphatidylserine/prothrombin complex IgM antibodies and cutaneous ulcers in patients with cutaneous vasculitis. J Dermatol. (2021) 48:1457–8. doi: 10.1111/1346-8138.16014
54. Emmenegger M, Kumar SS, Emmenegger V, Malinauskas T, Buettner T, Rose L, et al. Anti-prothrombin autoantibodies enriched after infection with SARS-CoV-2 and influenced by strength of antibody response against SARS-CoV-2 proteins. PLoS Pathog. (2022) 18:e1010289. doi: 10.1371/journal.ppat.1010289
55. Avallone G, Quaglino P, Cavallo F, Roccuzzo G, Ribero S, Zalaudek I, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. (2022) 9:10.1111/ijd.16063. doi: 10.1111/ijd.16063
56. Yap BJM, Lai-Foenander AS, Goh BH, Ong YS, Duangjai A, Saokaew S, et al. Unraveling the immunopathogenesis and genetic variants in vasculitis toward development of personalized medicine. Front Cardiovasc Med. (2021) 8:732369. doi: 10.3389/fcvm.2021.732369
57. Abdelmaksoud A, Wollina U, Temiz SA, Hasan A. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. (2022) 35:e15458. doi: 10.1111/dth.15458
58. Cohen SR, Prussick L, Kahn JS, Gao DX, Radfar A, Rosmarin D. Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int J Dermatol. (2021) 60:1032–3. doi: 10.1111/ijd.15623
59. Nastro F, Fabbrocini G, di Vico F, Marasca C. Small vessel vasculitis related to varicella-zoster virus after Pfizer-BioNTech COVID-19 vaccine. J Eur Acad Dermatol Venereol. (2021) 35:e745–7. doi: 10.1111/jdv.17550
60. Bostan E, Zaid F, Akdogan N, Gokoz O. Possible case of mRNA COVID-19 vaccine-induced small-vessel vasculitis. J Cosmet Dermatol. (2022) 21:51–3. doi: 10.1111/jocd.14568
61. Dicks AB, Gray BH. Images in vascular medicine: leukocytoclastic vasculitis after COVID-19 vaccine booster. Vasc Med. (2022) 27:100–1. doi: 10.1177/1358863X211055507
62. Erler A, Fiedler J, Koch A, Heldmann F, Schütz A. Leukocytoclastic vasculitis after vaccination with a SARS-CoV-2 vaccine. Arthritis Rheumatol. (2021) 73:2188. doi: 10.1002/art.41910
63. Colia R, Rotondo C, Corrado A, Cantatore FP. Cutaneous vasculitis after severe acute respiratory syndrome coronavirus 2 vaccine. Rheumatol Adv Pract. (2021) 5:rkab050. doi: 10.1093/rap/rkab050
64. Altun E, Kuzucular E. Leukocytoclastic vasculitis after COVID-19 vaccination. Dermatol Ther. (2022) 35:e15279. doi: 10.1111/dth.15279
65. Mücke VT, Knop V, Mücke MM, Ochsendorf F, Zeuzem S. First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report. BMC Infect Dis. (2021) 21:958. doi: 10.1186/s12879-021-06655-x
66. Ireifej B, Weingarten M, Dhamrah U, Weingarten M, Hadi S. Leukocytoclastic vasculitic rash following second dose of Moderna COVID-19 vaccine. J Investig Med High Impact Case Rep. (in press). doi: 10.1177/23247096211066283. [Epub ahead of print].
67. Gázquez Aguilera EM, Rodríguez García M, Cantón Yebra MT. Cutaneous vasculitis due to COVID-19 vaccination. Med Clin. (2022) 158:493–4. doi: 10.1016/j.medcle.2021.09.019
68. Hočevar A, Simonović Z, Rotar Ž, Tomšič M. Vasculitis as temporally associated with COVID-19 infection or vaccination: a single-center experience. J Rheumatol. (2022) 49:232–3. doi: 10.3899/jrheum.210788
69. Fritzen M, Funchal GDG, Luiz MO, Durigon GS. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. (2022) 97:118–21. doi: 10.1016/j.abd.2021.09.003
70. Sandhu S, Bhatnagar A, Kumar H, Dixit PK, Paliwal G, Suhag DK, et al. Leukocytoclastic vasculitis as a cutaneous manifestation of ChAdOx1 nCoV-19 corona virus vaccine (recombinant). Dermatol Ther. (2021) 34:e15141. doi: 10.1111/dth.15141
71. Jin WJ, Ahn SW, Jang SH, Hong SM, Seol JE, Kim H. Leukocytoclastic vasculitis after coronavirus disease 2019 vaccination. J Dermatol. (2022) 49:e34–5. doi: 10.1111/1346-8138.16212
72. Cavalli G, Colafrancesco S, De Luca G, Rizzo N, Priori R, Conti F, et al. Cutaneous vasculitis following COVID-19 vaccination. Lancet Rheumatol. (2021) 3:e743–4. doi: 10.1016/S2665-9913(21)00309-X
73. Liang I, Swaminathan S, Lee AYS. Emergence of de novo cutaneous vasculitis post coronavirus disease (COVID-19) vaccination. Clin Rheumatol. (2022) 41:1611–2. doi: 10.1007/s10067-021-05948-5
74. Guzmán-Pérez L, Puerta-Peña M, Falkenhain-López D, Montero-Menárguez J, Gutiérrez-Collar C, Rodríguez-Peralto JL, et al. Small-vessel vasculitis following Oxford-AstraZeneca vaccination against SARS-CoV-2. J Eur Acad Dermatol Venereol. (2021) 35:e741–3. doi: 10.1111/jdv.17547
75. Shahrigharahkoshan S, Gagnon LP, Mathieu S. Cutaneous leukocytoclastic vasculitis induction following ChAdOx1 nCoV-19 vaccine. Cureus. (2021) 13:e19005. doi: 10.7759/cureus.19005
76. Fiorillo G, Pancetti S, Cortese A, Toso F, Manara S, Costanzo A, et al. Leukocytoclastic vasculitis (cutaneous small-vessel vasculitis) after COVID-19 vaccination. J Autoimmun. (2022) 127:102783. doi: 10.1016/j.jaut.2021.102783
77. Criado PR, Giordani LP, Yoshimoto TA, Vieira IC, Landman G, Pincelli TP. Vasculitis in the setting of COVID-19: from the disease to the vaccine. Report of a case of cutaneous vasculitis after immunization. Dermatol Ther. (2022) 35:e15367. doi: 10.1111/dth.15367
78. Bozorgmehr R, Enteshari K, Khameneh Bagheri A, Jafarzadeh Esfehani R, Abdollahimajd F. Cutaneous vasculitis following COVID-19 vaccination: a case-based review. Front Emerg Med. (2022) 6:e41. doi: 10.18502/fem.v6i3.9403
79. Uh JA, Lee SK, Kim JH, Lee JH, Kim MS, Lee UH. Cutaneous small-vessel vasculitis after ChAdOx1 COVID-19 vaccination: a report of five cases. Int J Low Extrem Wounds. (2022) 21:193–6. doi: 10.1177/15347346221078734
80. Ball-Burack MR, Kosowsky JM. A case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. (in press). doi: 10.1016/j.jemermed.2021.10.005. [Epub ahead of print].
81. Berry CT, Eliliwi M, Gallagher S, Panaccione S, Klein WM, Healy AL, et al. Cutaneous small vessel vasculitis following single-dose Janssen Ad26.COV2.S vaccination. JAAD Case Rep. (2021) 15:11–4. doi: 10.1016/j.jdcr.2021.07.002
82. Dordević Betetto L, Luzar B, Pipan Tkalec Ž, Ponorac S. Cutaneous leukocytoclastic vasculitis following COVID-19 vaccination with Ad26.COV2.S vaccine: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. (2022) 31:83–7. doi: 10.15570/actaapa.2022.12
83. Bencharattanaphakhi R, Rerknimitr PS. COVID-19 vaccine-induced cutaneous leukocytoclastic vasculitis. JAAD Case Rep. (2021) 18:1–3. doi: 10.1016/j.jdcr.2021.10.002
84. Kar BR, Singh BS, Mohapatra L, Agrawal I. Cutaneous small-vessel vasculitis following COVID-19 vaccine. J Cosmet Dermatol. (2021) 20:3382–3. doi: 10.1111/jocd.14452
85. Shakoei S, Kalantari Y, Nasimi M, Tootoonchi N, Ansari MS, Razavi Z, et al. Cutaneous manifestations following COVID-19 vaccination: a report of 25 cases. Dermatol Ther. (2022) 18:e15651. doi: 10.1111/dth.15651
86. Azzazi Y, Abdelkader HA, Khedr H, El-Komy MHM. Extensive cutaneous leukocytoclastic vasculitis after Sinopharm vaccine: case report and review of the literature. J Cutan Pathol. (2022) 49:736–42. doi: 10.1111/cup.14235
87. Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J Cutan Pathol. (2022) 49:34–41. doi: 10.1111/cup.14104
88. Pillebout E, Sunderkötter C. IgA vasculitis. Semin Immunopathol. (2021) 43:729–38. doi: 10.1007/s00281-021-00874-9
89. Hines AM, Murphy N, Mullin C, Barillas J, Barrientos JC. Henoch-Schönlein purpura presenting post COVID-19 vaccination. Vaccine. (2021) 39:4571–2. doi: 10.1016/j.vaccine.2021.06.079
90. Maye JA, Chong HP, Rajagopal V, Petchey W. Reactivation of IgA vasculitis following COVID-19 vaccination. BMJ Case Rep. (2021) 14:e247188. doi: 10.1136/bcr-2021-247188
91. Iwata H, Kamiya K, Kado S, Nakaya T, Kawata H, Komine M, et al. Case of immunoglobulin A vasculitis following coronavirus disease 2019 vaccination. J Dermatol. (2021) 48:e598–9. doi: 10.1111/1346-8138.16167
92. Kondo M, Yamanaka K. Possible HSP reactivation post-COVID-19 vaccination and booster. Clin Case Rep. (2021) 9:e05032. doi: 10.1002/ccr3.5032
93. Ogura Y, Morimoto H, Otsuka M, Tokura Y. Development of IgA vasculitis after SARS-CoV-2 vaccination. J Cutan Immunol Allergy. (in press). doi: 10.1002/cia2.12242. [Epub ahead of print].
94. Obeid M, Fenwick C, Pantaleo G. Reactivation of IgA vasculitis after COVID-19 vaccination. Lancet Rheumatol. (2021) 3:e617. doi: 10.1016/S2665-9913(21)00211-3
95. Grossman ME, Appel G, Little AJ, Ko CJ. Post-COVID-19 vaccination IgA vasculitis in an adult. J Cutan Pathol. (2022) 49:385–7. doi: 10.1111/cup.14168
96. Sirufo MM, Raggiunti M, Magnanimi LM, Ginaldi L, De Martinis M. Henoch-Schönlein purpura following the first dose of COVID-19 viral vector vaccine: a case report. Vaccines. (2021) 9:1078. doi: 10.3390/vaccines9101078
97. Naitlho A, Lahlou W, Bourial A, Rais H, Ismaili N, Abousahfa I, et al. A rare case of Henoch-Schönlein purpura following a COVID-19 vaccine-case report. SN Compr Clin Med. (2021) 3:2618–21. doi: 10.1007/s42399-021-01025-9
98. Badier L, Toledano A, Porel T, Dumond S, Jouglen J, Sailler L, et al. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV-19. Autoimmun Rev. (2021) 20:102951. doi: 10.1016/j.autrev.2021.102951
99. Bostan E, Gulseren D, Gokoz O. New-onset leukocytoclastic vasculitis after COVID-19 vaccine. Int J Dermatol. (2021) 60:1305–6. doi: 10.1111/ijd.15777
100. Carlson JA, Chen KR. Cutaneous vasculitis update: neutrophilic muscular vessel and eosinophilic, granulomatous, and lymphocytic vasculitis syndromes. Am J Dermatopathol. (2007) 29:32–43. doi: 10.1097/01.dad.0000245198.80847.ff
101. Vassallo C, Boveri E, Brazzelli V, Rampino T, Bruno R, Bonometti A, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. (2021) 34:e15076. doi: 10.1111/dth.15076
102. Ungari M, Pezzarossa E. Cutaneous lymphocytic vasculitis after administration of the second dose of AZD1222 (Oxford-AstraZeneca) Severe acute respiratory syndrome coronavirus 2 vaccination: casuality or causality? Am J Dermatopathol. (2022) 44:80–2. doi: 10.1097/DAD.0000000000002104
103. Kharkar V, Vishwanath T, Mahajan S, Joshi R, Gole P. Asymmetrical cutaneous vasculitis following COVID-19 vaccination with unusual eosinophil preponderance. Clin Exp Dermatol. (2021) 46:1596–7. doi: 10.1111/ced.14797
104. Avallone G, Cavallo F, Astrua C, Caldarola G, Conforti C, De Simone C, et al. Cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: a real-life multicentre experience. J Eur Acad Dermatol Venereol. (in press). doi: 10.1111/jdv.18386. [Epub ahead of print].
105. Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. (2022) 35:e15282. doi: 10.1111/dth.15282
106. Baraldi C, Boling LB, Patrizi A, Prodi C, Deleonardi G, Gaspari V, et al. Unique case of urticarial skin eruptions after COVID-19 vaccination. Am J Dermatopathol. (2022) 44:198–200. doi: 10.1097/DAD.0000000000002036
107. Dash S, Behera B, Sethy M, Mishra J, Garg S. COVID-19 vaccine-induced urticarial vasculitis. Dermatol Ther. (2021) 34:e15093. doi: 10.1111/dth.15093
108. Okuda S, Hirooka Y, Sugiyama M. Propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis after COVID-19 vaccination. Vaccines. (2021) 9:842. doi: 10.3390/vaccines9080842
109. Hakroush S, Tampe B. Case Report: ANCA-Associated vasculitis presenting with rhabdomyolysis and Pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol. (2021) 12:762006. doi: 10.3389/fimmu.2021.762006
110. Nasr S, Khalil S, Poiesz BJ, Banki K, Perl A. Pfizer–biontech COVID-19 RNA vaccination induces phosphatidylserine autoantibodies, cryoglobulinemia, and digital necrosis in a patient with pre-existing autoimmunity. Clin Immunol Communic. (2021) 1:1–3. doi: 10.1016/j.clicom.2021.08.001
111. Ouldali N, Bagheri H, Salvo F, Antona D, Pariente A, Leblanc C, et al. Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: a national post-authorization pharmacovigilance study. Lancet Reg Health Eur. (2022) 17:100393. doi: 10.1101/2022.01.17.22269263
112. Nune A, Iyengar KP, Goddard C, Ahmed AE. Multisystem inflammatory syndrome in an adult following the SARS-CoV-2 vaccine (MIS-V). BMJ Case Rep. (2021) 14:e243888. doi: 10.1136/bcr-2021-243888
113. Iyengar KP, Nune A, Ish P, Botchu R, Shashidhara MK, Jain VK. Multisystem inflammatory syndrome after SARS-CoV-2 vaccination (MIS-V), to interpret with caution. Postgrad Med J. (2022) 98:e91. doi: 10.1136/postgradmedj-2021-140869
114. Yousaf AR, Cortese MM, Taylor AW, Broder KR, Oster ME, Wong JM, et al. Reported cases of multisystem inflammatory syndrome in children aged 12–20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: a surveillance investigation. Lancet Child Adolesc Health. (2022) 6:303–12. doi: 10.1101/2022.01.03.22268681
115. Stappers S, Ceuleers B, Van Brusselen D, Willems P, de Tavernier B, Verlinden A. A case of multisystem inflammatory syndrome (MIS-A) in an adult woman 18 days after COVID-19 vaccination. Acta Clin Belg. (2022) 77:772–7. doi: 10.1080/17843286.2021.1977899
116. Park JW, Yu SN, Chang SH, Ahn YH, Jeon MH. Multisystem inflammatory syndrome in an adult after COVID-19 vaccination: a case report and literature review. J Korean Med Sci. (2021) 36:e312. doi: 10.3346/jkms.2021.36.e312
117. Uwaydah AK, Hassan NMM, Abu Ghoush MS, Shahin KMM. Adult multisystem inflammatory syndrome in a patient who recovered from COVID-19 postvaccination. BMJ Case Rep. (2021)14:e242060. doi: 10.1136/bcr-2021-242060
118. Salzman MB, Huang CW, O'Brien CM, Castillo RD. Multisystem inflammatory syndrome after SARS-CoV-2 infection and COVID-19 vaccination. Emerg Infect Dis. (2021) 27:1944–8. doi: 10.3201/eid2707.210594
119. Kim Y, Kang J, Lee SG, Kim GT. COVID-19 vaccination-related small vessel vasculitis with multiorgan involvement. Z Rheumatol. (2022) 19:1–4. doi: 10.1007/s00393-022-01159-8
120. Agmon-Levin N, Paz Z, Israeli E, Shoenfeld Y. Vaccines and autoimmunity. Nat Rev Rheumatol. (2009) 5:648–52. doi: 10.1038/nrrheum.2009.196
121. Gehlhausen JR, Wetter DA, Nelson C, Ramachandran S, McNiff JM, Ko CJ. A detailed analysis of the distribution, morphology, and histopathology of complex purpura in hospitalized patients: a case series of 68 patients. J Am Acad Dermatol. (2021) 84:1188–96. doi: 10.1016/j.jaad.2020.04.149
122. Avallone G, Giordano S, Astrua C, Merli M, Senetta R, Conforti C, et al. Reply to 'The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated?' by Damiani G et al. J Eur Acad Dermatol Venereol. (2022) 36:e433–5. doi: 10.1111/jdv.17959
Keywords: vasculitis, vasculopathy, COVID-19, COVID vaccines, cutaneous manifestation
Citation: Maronese CA, Zelin E, Avallone G, Moltrasio C, Romagnuolo M, Ribero S, Quaglino P and Marzano AV (2022) Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front. Med. 9:996288. doi: 10.3389/fmed.2022.996288
Received: 17 July 2022; Accepted: 04 August 2022;
Published: 23 August 2022.
Edited by:
Erkan Alpsoy, Akdeniz University, TurkeyReviewed by:
Umit Tursen, Mersin University, TurkeyCopyright © 2022 Maronese, Zelin, Avallone, Moltrasio, Romagnuolo, Ribero, Quaglino and Marzano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angelo Valerio Marzano, YW5nZWxvLm1hcnphbm9AdW5pbWkuaXQ=
 Carlo Alberto Maronese
Carlo Alberto Maronese Enrico Zelin
Enrico Zelin Gianluca Avallone
Gianluca Avallone Chiara Moltrasio
Chiara Moltrasio Maurizio Romagnuolo
Maurizio Romagnuolo Simone Ribero
Simone Ribero Pietro Quaglino
Pietro Quaglino Angelo Valerio Marzano
Angelo Valerio Marzano