- 1Department of Anesthesiology, Changde Hospital, Xiangya School of Medicine, Central South University (The First People's Hospital of Changde City), Changde, China
- 2Department of Orthopedic Surgery, Changde Second People's Hospital, Changde, China
- 3Department of Infectious Diseases, Changde Hospital, Xiangya School of Medicine, Central South University (The First People's Hospital of Changde City), Changde, China
Objective: To evaluate the safety and efficacy of remimazolam in hysteroscopic surgery in elderly patients.
Methods: Following hysteroscopic surgery under selected general anesthesia, 60 elderly patients ASA (American Society of Anesthesiologists) class II–III, >65 years old were randomly assigned to one of two groups: the R group (remimazolam) or the C group (propofol), each with 30 patients. Sufentanil 0.1 μg/kg was given 5 min before the operation, remimazolam 0.2 mg/kg intravenously in Group R, then 0.5~1 mg/(kg.h) by pump, propofol 2 mg/kg intravenously in group B, and then 4~8 mg/(kg.h) by pump. Maintain BIS (Bispectral index) 40~70, add remimazolam 0.05 mg/kg or propofol 0.5 mg/kg when the patient is in motion, and stop the administration at the end of the operation. Record the patients' HR, MAP, RR, SpO2, PETCO2, and BIS values at entry (T0), before induction administration (T1), 1 min after administration (T2), 5 min after administration (T3), when stopping administration (T4), when awakening (T5), and 1 min after awakening (T6), as well as the onset time after administration, the awakening time, the success rate of sedation, and the number and dose of additional medications. Reactions are adverse (hypotension, hypertension, respiratory depression incidence, injection pain, nausea and vomiting following surgery, etc.).
Results: The two groups' respective anesthetic success rates were comparable overall. In addition to having a higher BIS value and more extra medications than group C, group R experienced less incidence of respiratory depression, injection pain, and intraoperative hypotension.
Conclusion: Remimazolam, which is equivalent to propofol in terms of safety and efficacy for older patients undergoing hysteroscopic surgery, should be further promoted and used.
1 Introduction
As a minimally invasive procedure offering diagnostic and therapeutic benefits, hysteroscopy has emerged as the foremost method for diagnosing and treating gynecological conditions (1). With the aging demographic, a growing number of elderly individuals are undergoing hysteroscopic surgeries. However, due to age-related declines in organ function and the presence of comorbidities, administering anesthesia to elderly patients presents significant challenges. Currently, propofol intravenous general anesthesia stands as the most prevalent method for hysteroscopic surgeries (2). Nonetheless, its potent respiratory and circulatory depressant effects entail certain risks when utilized in elderly patients (3). The novel benzodiazepine intravenous anesthetic Remimazolam is fast-acting, has a short duration, and help in rapid recovery (4, 5). Metabolized by plasma esterases, it exerts minimal impact on hemodynamics and can be swiftly counteracted by flumazenil. In principle, it represents an ideal anesthetic choice for elderly patients undergoing hysteroscopic surgery (6–9). The aim of the study is to determine whether remimazolam is safe and effective for use during general anesthesia for hysteroscopic surgery in elderly patients. It also advocates for standardized clinical application protocols and provides insights for judicious medication usage in this age group.
2 Materials and methods
2.1 General information
All patients gave their signature on the informed consent form, which was approved by the hospital's ethics committee. Prior to surgery, patients were regularly instructed to abstain from eating and drinking. Additionally, upon arrival in the operating room, patients were given oxygen. A total of 60 elderly patients aged 65 to 80 years undergoing scheduled hysteroscopic surgery from June 2021 to May 2022 were selected. They were classified as ASA II to III, with an expected surgery duration of 10 to 30 min. Exclusion criteria included patients allergic to remimazolam or any of its components, uncontrolled severe hypertension, unstable angina, severe liver, kidney, or respiratory dysfunction, history of prolonged sedative or analgesic drug use, allergic history, patient refusal, and other conditions deemed unsuitable by the researchers. These patients were divided into two groups at random, with 30 cases in each group: the control group (propofol, or C group) and the experimental group (remimazolam, or R group).
2.2 Research methods
Prior to surgery, patients were usually fasted, and they were given oxygen when they were admitted to the operating room. Their electrocardiogram and Bispectral Index (BIS) were monitored. Patients received a 0.1 μg/kg intravenous injection of sufentanil during surgical disinfection. Anesthesia induction protocol: The experimental group was given an intravenous injection of remimazolam at a dose of 0.3 mg/kg 1 min prior to surgery. Thereafter, they were continuously infused with 0.6 mg/(kg·h) until the start of the surgery or until BIS < 65. If BIS >65, remimazolam could be supplemented with a 0.1 mg/kg dose once. If BIS remained >65 after supplementation, propofol 0.5 mg/kg could be added and recorded as a failure. The control group was given an intravenous injection of propofol at a dose of 2 mg/kg, and then received a continuous infusion of 4 mg/(kg·h); 0.5 mg/kg of propofol might be added if the patient's BIS was >65. Anesthesia maintenance: Remimazolam 0.1 mg/kg was given as a supplemental dosage to the experimental group if the patient moved or if their BIS > 65, while propofol 0.5 mg/kg was given to the control group. If BIS remained >65 after two supplementations in the experimental group, propofol 0.5 mg/kg could be added and recorded as a failure. The infusion was stopped at the end of the surgery to facilitate a natural waking. Patients with SpO2 < 92% received jaw support, and symptomatic treatment was administered for significant fluctuations in heart rate and blood pressure.
2.3 Observation indicators
Safety indicators were monitored at several points in time, such as the time of admission, before induction, 1 min after drug administration, 5 min after drug administration, at the end of drug administration, upon awakening, and 10 min after awakening. Heart rate (HR), respiratory rate (RR), mean arterial pressure (MAP), and pulse oxygen saturation (SpO2) were among these indications (T0–6). Additionally, awakening time, occurrences of adverse reactions (post-operative nausea and vomiting, dizziness, injection pain), and instances of jaw support were documented. Observer's Assessment of Alertness/Sedation (OAA/S) scores and the Bispectral Index (BIS) were among the effectiveness indicators that were recorded at the same time points as safety indicators. The anesthesia success rate, number of movements, and instances of drug supplementation were also documented. All data were collected by an experienced anesthesiologist blinded to the group assignments.
2.4 Statistical methods
SPSS 26.0 software was utilized to conduct the statistical analysis. Groups were compared using one-way analysis of variance, and normally distributed continuous variables were displayed as mean ± standard deviation. Within-group comparisons were carried out at different times using repeated measures analysis of variance for continuous data. With a significance level of p < 0.05, between-group comparisons involving frequency data expressed as counts were performed using either the chi-square test or Fisher's exact probability test.
3 Results
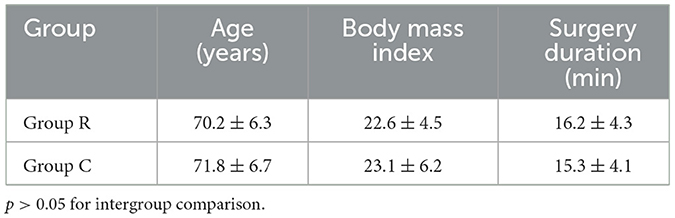
Both groups of patients successfully completed the surgery without any serious complications occurring. The two patient groups' general conditions did not significantly differ from one another (Table 1).
Comparison of HR, MAP, RR, and SpO2 at various time points between the two groups of patients: Compared to T0, HR decreased at T2 − 4 in the experimental group, while in the control group, HR increased at T2 and decreased at T3 − 4, with both groups recovering to preoperative levels at T5. MAP decreased at T2 − 4 in both groups, recovering to preoperative levels at T5. RR in the control group decreased at T2 − 3 and returned to preoperative levels at T4. In the experimental group, RR was higher at T2, MAP was higher at T2 − 4, and HR was lower at T2 than in the control group. These differences in intergroup comparison were statistically significant. For each indicator, there were no statistically significant differences in the intergroup comparison at other time points (Table 2).
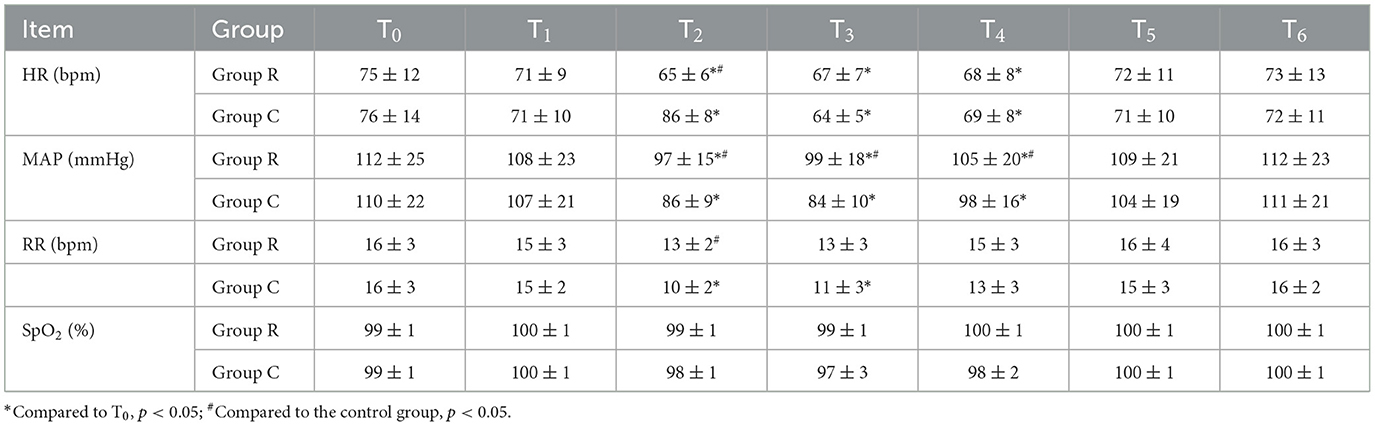
Table 2. Comparison of HR, MAP, RR, and SpO2 at various time points between the two groups of patients (n = 30, ± s).
Comparison of the two patient groups' adverse reactions and postoperative awakening times: There were statistically significant differences in the injection pain and jaw support between the R group and the C group. There were no adverse reactions or statistically significant changes in waking times between the two groups (Table 3).
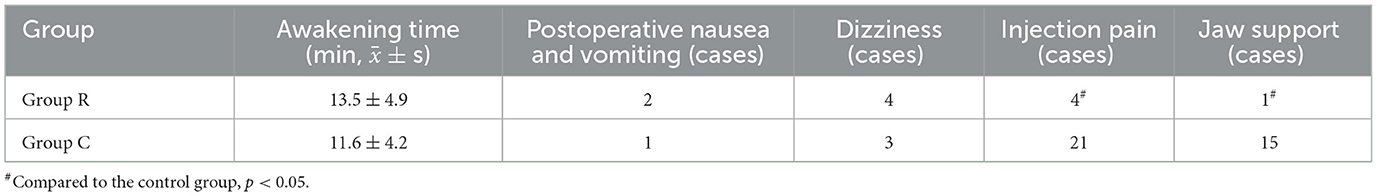
Table 3. Comparison of postoperative awakening time and adverse reactions between the two groups of patients (n = 30).
BIS and OAA/S scores are compared between the two patient groups at different times: Compared to T0, both patients showed a decrease in BIS and OAA/S scores during the T2 − 4 period, with statistically significant differences. During the T2 − 3 period, statistically significant differences were noted in the BIS scores between the R and C groups. At subsequent points, there were not significant differences between the two groups in the other indicators (Table 4).
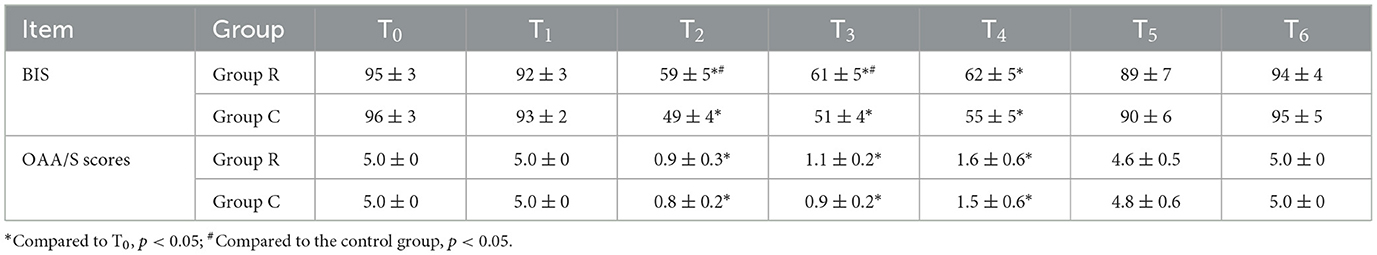
Table 4. Comparison of BIS and OAA/S scores at various time points between the two groups of patients (n = 30, ± s).
In the experimental group, there was 1 case where remimazolam was supplemented twice intraoperatively, but BIS remained >65. Propofol was added, and BIS dropped to < 60, recorded as one case of anesthesia failure. However, the overall anesthetic success rate did not differ significantly between the two groups. There were noteworthy differences between the R and C groups in terms of the number of intraoperative movements and drug supplementation cases (Table 5).

Table 5. Comparison of anesthesia success rate, number of movements, and instances of drug supplementation between the two groups of patients (n = 30).
4 Discussion
Safety Indicator Analysis: Both groups of patients had similar general conditions. During the T2-T4 period, both groups experienced varying decreases in HR and MAP. However, the control group exhibited a rapid increase in HR at T2, possibly due to increased sympathetic activity after a drop in blood pressure or patient discomfort from injection pain. There were statistically significant intergroup variations in the experimental group's MAP reduction from the control group during the T2-T4 time points; the experimental group's decrease was much less. Additionally, the control group showed a slight decline in SpO2 at T3 and T4 and a considerable decrease in RR at T2. The incidence of injection pain was significantly reduced in the experimental group compared to the control group.
Effectiveness Indicator Analysis: During the T2-T4 period, both groups achieved effective sedation depths based on BIS and OAA/S scores. More specifically, at T2 and T3, BIS scores were higher in the experimental group than in the control group, although OAA/S scores were the same. The experimental group had more instances of movement and drug supplementation than the control group, but most were based on increased BIS prompting additional anesthesia, indicating a more sensitive response to BIS. Overall, the anesthesia success rate was comparable between the two groups.
For hysteroscopy operations, propofol is the most commonly utilized intravenous anesthetic because of its rapid onset, short duration of action, and quick recovery. However, it also has significant individual differences and can cause injection pain, respiratory and circulatory depression, propofol infusion syndrome, and dependency on hepatic and renal metabolism, posing risks, especially in elderly patients (10).
The novel ultra-short-acting benzodiazepine sedative-hypnotic agent, remimazolam tosylate, acts on the GABAA receptor, resulting in sedative-hypnotic effects. It has minimal drug interactions, does not accumulate, and is rapidly metabolized by plasma esterases. Remimazolam has a rapid recovery time and onset and offset of action (11), linear pharmacokinetics independent of body weight, and unaffected half-life by infusion time. It can also be rapidly antagonized by flumazenil.
Comparison between Remimazolam Tosylate and Propofol: Remimazolam tosylate is rapidly metabolized without accumulation, while propofol has a longer half-life and can lead to propofol infusion syndrome. Remimazolam tosylate is a safer drug than propofol as it has a lower risk of injection pain, hypotension, and respiratory depression (12, 13). The results are consistent with a previous study (14), but there are the following differences: (1) Different research subjects, we chose elderly (65–80) gynecological surgery patients to provide a basis for their use; (2) Due to different research plans, we focused on the entire surgical process and expanded the scope of use of Remazolam; (3) More comprehensive observation indicators, including objective indicators, scale scores, and all adverse reactions. It has a comparable general anesthesia success rate to propofol in elderly hysteroscopy surgeries, with rapid onset and recovery (15).
5 Conclusion
The utilization of remimazolam tosylate in general anesthesia for hysteroscopy surgeries among elderly patients demonstrates commendable safety, exhibiting milder respiratory and circulatory depression compared to propofol and fewer occurrences of adverse reactions such as injection pain. This signifies certain clinical advantages over propofol.
In the context of elderly patients undergoing general anesthesia for hysteroscopy surgeries, remimazolam tosylate showcases rapid onset and recovery while maintaining anesthesia success rates (effectiveness) comparable to propofol. This underscores its potential as an innovative solution for elderly patients undergoing such surgeries and suggests its merit for further promotion and application.
However, limitations of this study include a restricted sample size, which hindered the effective delineation of intergroup differences; insufficient evidence from evidence-based medicine regarding the optimal dosage and regimen of remimazolam tosylate; and a short follow-up period, which impeded a comprehensive assessment of long-term patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Ethics Committee of Changde Hospital, Xiangya School of Medicine, Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MX: Writing – original draft, Writing – review & editing. FZ: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. QT: Investigation, Writing – original draft, Writing – review & editing. HD: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. ST: Conceptualization, Methodology, Project administration, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lasmar RB, Lasmar BP. Hysteroscopic management of intrauterine benign diseases. Minim Invasive Ther Allied Technol. (2021) 30:263–71. doi: 10.1080/13645706.2021.1944218
2. Chen C, Tang W, Ye W, Zhong W, Li Y. ED50 of propofol combined with nalbuphine on the sedative effect in painless hysteroscopy. Pain Ther. (2021) 10:1235–43. doi: 10.1007/s40122-021-00280-x
3. Yu J, Xiang B, Song Y, Chen H, Li Y, Liu C. ED50 of propofol in combination with low-dose sufentanil for intravenous anaesthesia in hysteroscopy. Basic Clin Pharmacol Toxicol. (2019) 125:460–5. doi: 10.1111/bcpt.13280
4. Choi JY, Lee HS, Kim JY, Han DW, Yang JY, Kim MJ, et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. (2022) 82:110955. doi: 10.1016/j.jclinane.2022.110955
5. Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. (2021) 127:41–55. doi: 10.1016/j.bja.2021.03.028
6. Dai G, Pei L, Duan F, Liao M, Zhang Y, Zhu M, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. (2021) 87:1073–9. doi: 10.23736/S0375-9393.21.15517-8
7. Rex DK, Bhandari R, Lorch DG, Meyers M, Schippers F, Bernstein D. Safety and efficacy of remimazolam in high risk colonoscopy: a randomized trial. Dig Liver Dis. (2021) 53:94–101. doi: 10.1016/j.dld.2020.10.039
8. Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. (2020) 34:491–501. doi: 10.1007/s00540-020-02776-w
9. Liu X, Ding B, Shi F, Zhang Y, Liu L, Sha Y, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. (2021) 15:4675–85. doi: 10.2147/DDDT.S339535
10. Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. (2021) 21:156. doi: 10.1186/s12871-021-01373-y
11. Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. (2019) 155:137–46. doi: 10.1016/j.chest.2018.09.015
12. Chen S, Wang J, Xu X, Huang Y, Xue S, Wu A, et al. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. Am J Transl Res. (2020) 12:4594–603.
13. Chen SH, Yuan TM, Zhang J, Bai H, Tian M, Pan CX, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. (2021) 36:474–81. doi: 10.1111/jgh.15188
14. Fan S, Zhu Y, Sui C, Li Q, Jiang W, Zhang L. Remimazolam compared to propofol during hysteroscopy: a safety and efficacy analysis. Pain Ther. (2023) 12:695–706. doi: 10.1007/s40122-023-00483-4
Keywords: remimazolam, old age, hysteroscopic surgery, clinical study, general anesthesia
Citation: Xie M, Zeng F, Tian Q, Deng H and Tao S (2024) Clinical study on the safety and efficacy of remimazolam in hysteroscopic surgery under general anesthesia in elderly patients. Front. Med. 11:1409233. doi: 10.3389/fmed.2024.1409233
Received: 29 March 2024; Accepted: 21 October 2024;
Published: 07 November 2024.
Edited by:
Hao Li, Shanghai Jiao Tong University, ChinaCopyright © 2024 Xie, Zeng, Tian, Deng and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiwei Deng, ZGh3XzQ4MjNAMTYzLmNvbQ==; Shanqing Tao, Nzg5MTQ5ODNAcXEuY29t
 Manjie Xie
Manjie Xie Fanrui Zeng
Fanrui Zeng Qiao Tian
Qiao Tian Huiwei Deng
Huiwei Deng Shanqing Tao
Shanqing Tao