- 1School of Medicine, Xiamen Eye Center and Eye Institute of Xiamen University, Xiamen, China
- 2Xiamen Clinical Research Center for Eye Diseases, Xiamen, Fujian, China
- 3Xiamen Key Laboratory of Ophthalmology, Xiamen, Fujian, China
- 4Fujian Key Laboratory of Corneal & Ocular Surface Diseases, Xiamen, Fujian, China
- 5Xiamen Key Laboratory of Corneal & Ocular Surface Diseases, Xiamen, Fujian, China
- 6Translational Medicine Institute of Xiamen Eye Center of Xiamen University, Xiamen, Fujian, China
- 7Department of Ophthalmology, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian, China
Postoperative dry eye is a common complication following femtosecond laser-assisted cataract surgery, and the patient interface (PI) used during the procedure may play a significant role in its occurrence. This study, utilizing a meticulous scientific search strategy, identified seven relevant articles through literature search engines. Most of these studies employed contact-type PI during surgeries, while one researcher used a non-contact PI. All studies assessed dry eye symptoms at various postoperative periods using metrics such as the Ocular Surface Disease Index (OSDI), tear Break-Up Time (BUT), Schirmer I test (SIt), and so on. However, the findings were inconsistent. On this basis, this comprehensive review delves into the potential impact of different patient interfaces on corneal nerve damage and conjunctival goblet cell injury, possibly contributing to an increased risk of postoperative dry eye. The review also explores various preventive and solution strategies, including improving PI design, reducing surgical time, and utilizing tear protective agents. The findings highlight the importance of optimizing the PI to minimize the risk of postoperative dry eye in femtosecond laser-assisted cataract surgery.
1 Introduction
Dry eye, a multifactorial disease affecting the ocular surface, has a global prevalence of 5 to 35% (1). Its complex causes and recurrent nature have intensified efforts toward better diagnosis and treatment. Ocular surgeries, notably cataract operations, can trigger or worsen dry eye symptoms, impacting patient satisfaction (2, 3). Due to its ultra-short pulses capable of releasing energy in an extremely brief duration and precisely cutting tissues (4), in recent years, Femtosecond Laser-Assisted Cataract Surgery (FLACS) has gradually become a popular new surgical method (5). Femtosecond laser technology provides highly accurate imaging of the anterior segment, enabling precise capsulorhexis, pre-chopping, clear corneal incision creation, and even limbal relaxing incisions to correct corneal astigmatism. This reduces the risk of capsular rupture during surgery, lowers the required phacoemulsification energy, and further diminishes corneal damage (6). Consequently, this approach not only significantly reduces the difficulty of the procedure but also maximally enhances visual outcomes. FLACS improves postoperative visual quality but does not simultaneously control the incidence of postoperative dry eye. Notably, compared to traditional cataract surgery, studies suggest that patients undergoing FLACS may be more prone to symptoms of dry eye (7). The exploration of dry eye pathogenesis following FLACS is crucial for developing effective prevention and treatment, thereby improving patients’ postoperative visual comfort and quality of life. Current research on this subject is sparse, necessitating a review of the potential mechanisms of dry eye post-FLACS.
2 Epidemiology
Cataract is one of the most common blinding eye diseases worldwide (8), accounting for over 50% of vision loss globally, including 33.4% of blindness and 18.4% of moderate to severe visual impairment (9). In recent years, FLACS has been accepted by more and more patients due to its several advantages, such as less ultrasound energy release and higher surgical success rates (10).
Data indicate that 68.9% of patients report a sensation of foreign body in the eye after FLACS, and 48.3% experience dryness of the eye postoperatively (11). In cases where patients with pre-existing dry eye were excluded before surgery, the incidence of newly diagnosed dry eye 1 week after FLACS could reach as high as 20.9%. This figure drops to 10.82% 1 month postoperatively, and by 3 months after surgery, 1.92% of patients still suffer from surgically induced dry eye (12). Patients with preoperative dry eye symptoms are at a higher likelihood of experiencing various degrees of symptom exacerbation after the surgery, clearly presenting a less than optimistic picture.
We employed a Boolean logic search strategy with a timeframe extending from the database’s inception to October 2023. The search strategy was as follows: (Femtosecond Laser-Assisted Cataract Surgery [Title/Abstract] OR FLACS [Title/Abstract]) AND (Dry Eye Syndrome [Title/Abstract] OR Dry Eye Disease [Title/Abstract] OR Keratoconjunctivitis Sicca [Title/Abstract] OR tear film [Title/Abstract] OR ocular surface [Title/Abstract] OR MGD [Title/Abstract] OR conjunctiva [Title/Abstract]) NOT (LASIK [Title/Abstract] AND SMILE [Title/Abstract]). The search engine used was PubMed, which yielded 11 relevant articles. Upon careful review, we identified that 1 article was a meta-analysis, 1 was focused on adjunctive dry eye medication treatment, 1 involved optical coherence tomography in cataract studies, 2 were clinical controlled trials mentioning postoperative dry eye but lacking specific quantitative data, 1 was a case report, and 1 involved related optical analysis. Consequently, 4 articles were selected. A similar search strategy was employed in China National Knowledge Infrastructure (CNKI) and Wanfang databases, resulting in 3 additional articles meeting the criteria.
We reviewed papers and found that only two studies (13, 14) indicated a reduction in dry eye symptoms after FLACS compared to conventional phacoemulsification surgery (CPS), while other retrieved articles all suggested an exacerbation of dry eye after FLACS (7, 11, 12, 15, 16), upon careful examination of these two studies, we found that FLACS was associated with varying degrees of worsening dry eye symptoms compared to preoperative levels, albeit not as severe as in the CPS group. This may be related to the surgical techniques used during CPS and the stimulation of the ocular surface during the femtosecond laser procedure. In particular, the stimulation of the ocular surface by the patient interface (PI) during FLACS may promote increased secretion of reflex tears postoperatively (17), resulting in better performance in certain dry eye evaluation indices compared to CPS. Additionally, the surgeon’s operative habits are an important factor that cannot be ignored. Different surgical techniques may have a significant impact on the extent of ocular surface damage, thereby affecting the development of dry eye symptoms. The current status of related research both domestically and internationally is detailed in Table 1.
3 Mechanisms of postoperative dry eye
3.1 Common mechanisms of CPS and FLACS
Postoperative dry eye can occur to varying degrees after CPS and FLACS, with several common mechanisms identified in the development of dry eye following both procedures. Past research results suggest there are several reasons for dry eye after surgery. First, the creation of corneal incisions, whether using a scalpel or femtosecond laser, leads to the transection of corneal nerves to varying extents. This delays corneal wound healing, reduces corneal sensitivity, and impedes the tear secretion reflex among other phenomena (18). Second, to maintain ocular surface moisture and corneal transparency during surgery, balanced salt solutions are often used to repeatedly rinse the ocular surface, which may cause damage to the corneal epithelium and conjunctival goblet cells (19, 20). Third, an increased postoperative inflammatory response, leading to the recruitment of neutrophils and macrophages as well as the production of free radicals, proteolytic enzymes, and cyclooxygenase, is also considered a key factor in the development of dry eye (21). Fourth, studies have shown that more than 70% of patients experience meibomian gland orifice blockage and lid margin hyperemia 1 month after cataract surgery, leading to a significant reduction in the thickness of the tear film lipid layer and exacerbation of dry eye symptoms (22). This may be related to postoperative inflammatory responses, reduced blink frequency, and frequent use of eye drops. Fifth, prolonged exposure to microscope light is also associated with a shortened tear film break-up time and the exacerbation of dry eye symptoms in the short term (23). Sixth, the use of topical anesthetic agents during surgery can damage structures such as corneal epithelial microvilli, further affecting the normal adhesion of mucins and resulting in decreased stability of the tear film (24).
3.2 Mechanisms of postoperative dry eye after FLACS
3.2.1 More severe postoperative inflammatory response
The PI causes additional damage to ocular surface tissues compared to CPS (16), and FLACS is associated with a more severe inflammatory response in the anterior segment of the eye (25). This alters the ocular surface microenvironment, leading to changes in the concentration of tear cytokines (26). Patients with dry eye often have higher levels of pro-inflammatory cytokines in their tears (27), such as Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Interleukin-8 (IL-8), and MMP-9 (28). Studies have shown that the concentrations of pro-inflammatory cytokines like IL-6 and IL-8 in the eye post-FLACS are significantly higher compared to CPS (29). This exacerbates ocular surface inflammation, affects tear film quality (30), and leads to ocular surface dryness. The dry, oxidative, and hyperosmolar ocular surface environment activates cellular signaling pathways on the ocular surface, such as the Mitogen-Activated Protein Kinases (MAPK) signaling pathway and the Nuclear Factor kB (NF-kB) signaling pathway (31, 32), further stimulating the production of corresponding inflammatory cytokines in a vicious cycle.
3.2.2 Additional damage to corneal
Due to the high precision of femtosecond lasers, the corneal incisions they create are well-cemented. Surgeons often need to employ specific techniques to bluntly separate the completed incisions for further surgical procedures (33, 34). However, this process can be challenging, as unlike the sharp, disposable scalpel used in CPS, which typically completes the incision in one go, the corneal incisions made by femtosecond lasers may sometimes be incomplete or discontinuous and cannot simply be resolved by blunt separation, and may still require the use of a scalpel (35), potentially causing reinjury to the cornea. Additionally, it is worth noting that the precision of the corneal incisions largely depends on the stability of PI, which is crucial for the accurate focus of the femtosecond laser on the cornea. So unstable PI suction could cause unnecessary damage to the cornea (36), affecting the healing of the incision and the overall quality.
3.2.3 Damage to conjunctival goblet cells
Goblet cells are responsible for producing the mucin component of the tear film, which helps the tear film to stably adhere to the ocular surface. However, both contact and non-contact femtosecond laser systems require the application of PI vacuum fixation on the patient’s ocular surface. The negative pressure attraction and compression of PI on the conjunctival tissue can cause partial apoptosis and decreased density of conjunctival goblet cells (37), with contact PI theoretically causing more severe damage. Which is similar to the pathological changes in postoperative dry eye caused by femtosecond laser-assisted in situ keratomileusis (LASIK) (38). Shao et al. also confirmed through research that the conjunctival damage caused by PI during FLACS is greater than that in the CPS group (16). In addition to changes in the number of goblet cells, Chao et al. found that the mucin secretion function of goblet cells was also affected (39), leading to mucin-deficient dry eye. The impact of PI vacuum attraction on the conjunctiva is detailed in Figure 1.
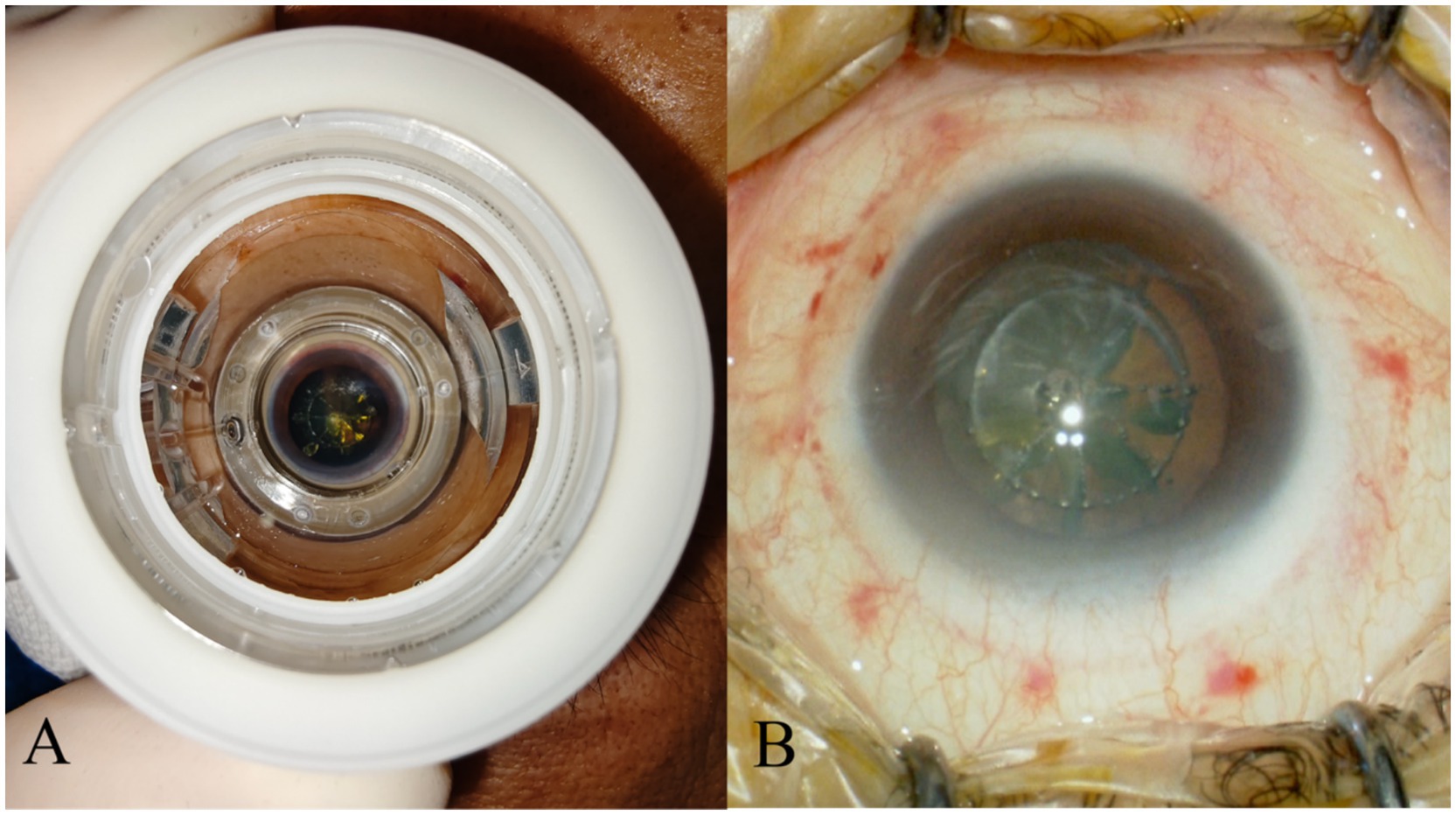
Figure 1. PI left obvious marks on conjunctival tissue. (A) Illustrates the condition when PI is adsorbed onto the patient’s ocular surface. (B) Shows the residual traces of PI on the ocular surface tissues after the negative pressure is released.
3.2.4 Compression effect of PI on the ocular surface
The tear film is fundamental to maintaining the normal structure and function of the ocular surface epithelium, as it moisturizes and protects the cornea and conjunctival epithelium (16). When the integrity of the tear film layers is compromised, tear film break-up occurs, leading to increased tear evaporation. During the femtosecond laser procedure, PI is applied to the patient’s ocular surface. The mechanical compression effect results in a decrease in the regularity of the postoperative ocular surface. This causes discordant interaction between the posterior edge of the eyelids and the corneal surface during blinking, which is not conducive to the tear film uniformly and smoothly covering the ocular surface (11), affecting tear film stability and thereby shortening the tear film break-up time.
3.2.5 Damage to the ocular surface nerves by PI
The ocular surface tissue involves multiple nerves and sensory systems (40), for example: the corneal nerves, which are a major component of the ocular surface nerves responsible for transmitting tactile and pain sensations, and are crucial for maintaining blinking and tear reflexes (41). During FLACS, both the non-contact PI’s negative pressure suction on the ocular surface and the contact PI’s compression (42–44), cause damage to various ocular surface nerves. Pathological changes in corneal nerves are a primary cause of corneal neuropathic pain. And damaged corneal nerves also cause neuroinflammation and sensitization, thereby forming a vicious cycle (45).
3.2.6 Additional surgical time and perioperative medication
Due to the need of operations such as creating clear corneal incisions, capsulorhexis, and pre-chopping of the nucleus on the femtosecond laser platform, there is an additional increase in the time the patient’s ocular surface is exposed and the use of topical anesthetic drugs (46). Studies indicate that the longer the ocular surface tissue is exposed during surgery, the more likely it is to cause damage to the microvilli structure of ocular surface cells and a postoperative decrease in goblet cell density (20). Pupil constriction after femtosecond laser operation is a common complication of FLACS. Some specialists suggest “To reduce the occurrence of pupil constriction after femtosecond laser operation, the preoperative use of non-steroidal anti-inflammatory drugs and increased dosage of mydriatic eye drops is recommended (47).” However, excessive use of non-steroidal anti-inflammatory drugs may lead to slower corneal epithelial healing (48). Despite the well-documented potential to exacerbate dry eye symptoms, the inclusion of the preservative benzalkonium chloride (BAC) in ophthalmic solutions is common practice (49). Compared to traditional phacoemulsification cataract surgery, the use of additional medications in FLACS inevitably increases exposure to preservatives such as BAC. The preservatives in the drugs also cause damage to the ocular surface (50) and reduce the density of conjunctival goblet cells. A schematic diagram illustrating the specific mechanism, created by the authors, is presented in Figure 2.
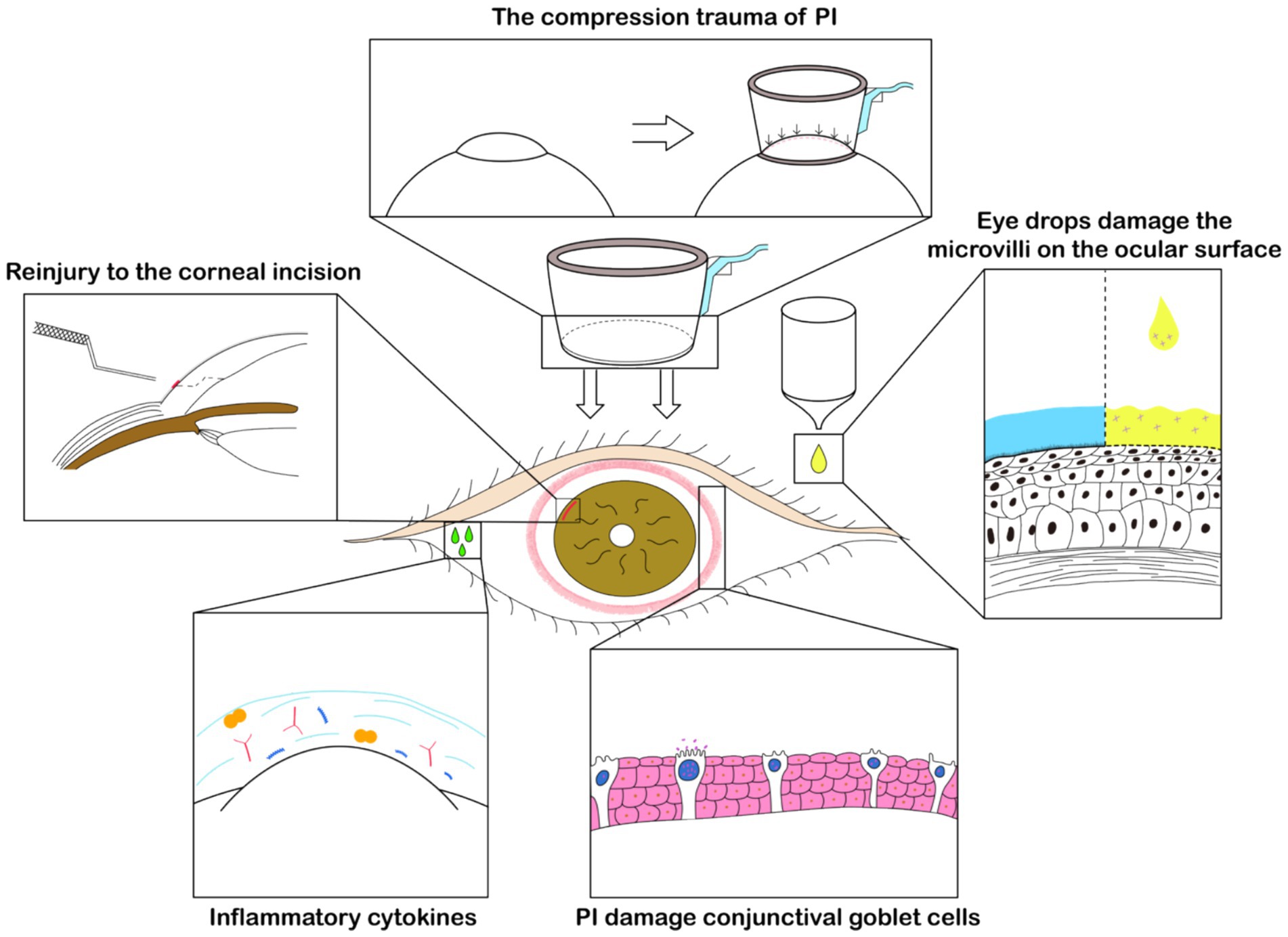
Figure 2. Schematic diagram of FLACS effect on dry eye. The negative pressure suction by the PI left noticeable marks on the conjunctiva, evidencing pressure-induced trauma. This also damages the conjunctival goblet cells, affecting the concentration of inflammatory cytokines on the ocular surface. The use of a specific corneal incision separator may also cause reinjury to the cornea. Moreover, the perioperative increase in the use of eye drops for FLACS such as non-steroidal anti-inflammatory drugs contributes to damage to the eye’s microvilli, playing a significant role in postoperative dry eye.
4 Prevention and treatment measures
4.1 Routine perioperative prevention and treatment of dry eye
It is important to assess dry eye symptoms before surgery. Gupta et al. found that about 80% of patients had abnormal dry eye test results before cataract surgery (51). Clinicians should conduct personalized risk assessments before surgery (52) and, based on the patient’s condition, use artificial tears or perform meibomian gland treatment to relieve dry eye symptoms.
During surgery, to reduce the damage caused by the irrigation of balanced salt solution on the ocular surface, it is recommended to use corneal protectants during surgery (53), or apply a diluted viscoelastic agent to the corneal surface in a certain ratio. This not only effectively maintains corneal moisture but also downregulates the expression of inflammatory factors, further promoting the repair of intraoperative ocular surface damage (54). After surgery, besides routine anti-inflammatory and artificial tear treatments, Intense Pulsed Light (IPL) therapy may be appropriately applied for patients at high risk of dry eye (55). Meanwhile, a corneal bandage lens can reduce mechanical damage to the ocular surface tissues by the eyelid margin, and diminish corneal neuropathy caused by eyelid-related factors (56). It is also suitable for people at high risk of dry eye.
4.2 Targeted prevention and treatment during the perioperative period of FLACS
4.2.1 Femtosecond laser equipment and parameter adjustment
The effectiveness and safety of FLACS are closely related to the settings of femtosecond laser parameters. Surgeons need to finely adjust these parameters according to the individual differences and surgical needs of patients. For patients at risk of dry eye, it may be necessary for the doctor to further reduce the laser’s energy and duration of action to minimize potential damage to the ocular surface tissues (57), reducing the inflammatory response after femtosecond laser operation. Additionally, doctors can more specifically select femtosecond laser equipment and techniques based on the patient’s specific conditions (58). For example, for patients with small eyeballs, due to the greater curvature of the cornea, the use of a contact femtosecond laser device’s PI can cause significant compression on the limbal conjunctival tissue. In such cases, besides using a smaller size of PI model, it is also possible to choose a non-contact femtosecond laser device, if available, to avoid the generation of compression on ocular surface tissues.
4.2.2 Targeted supplementation with artificial tears
To date, in the development of dry eye science, artificial tears that have been developed can simulate one or several components of the tear film, targeting the mucin layer, aqueous layer, and lipid layer for supplementation (59). Preservative-free artificial tears can be used multiple times throughout the day, and it is recommended for patients with dry eye after FLACS to prioritize the use of artificial tears for replacement therapy (60, 61). Using oily artificial tears to alleviate tear film kinetics abnormalities and lipid abnormality type dry eye caused by conjunctival laxity and meibomian gland dysfunction after FLACS can effectively relieve ocular discomfort (62).
4.2.3 Mucin secretagogue treatment
The mucin layer is an essential component of the tear film, with functions that include providing lubrication to the ocular surface, facilitating tear distribution, maintaining tear film stability, and aiding in ocular surface repair. Currently, mucin secretagogue medications primarily include Diquafosol sodium eye drops (63) and Rebamipide (64). Through the analysis of the mechanisms behind post-FLACS dry eye, it is known that dry eye following FLACS is significantly related to the destruction of the mucin layer, therefore, Diquafosol sodium eye drops and Rebamipide can produce beneficial effects (65).
4.2.4 Promotive repair treatment
Due to the possibility of more severe conjunctival epithelial damage and ocular surface nerve injury after FLACS, in addition to artificial tears, postoperative local promotive repair treatments can be administered, such as human epidermal growth factor eye drops, deproteinized calf blood extract eye drops, autologous serum, and so on. Human autologous serum contains components such as nerve growth factor, epidermal growth factor, and fibronectin, which help in the regeneration of nerves and epithelial cells. Thus, for patients with severe dry eye or those who do not respond to artificial tears treatment, the use of autologous serum can be considered (66). It is necessary to pay attention to the storage environment of autologous serum and avoid contamination (67).
4.2.5 Anti-inflammatory treatment
Post-FLACS dry eye is often closely associated with inflammatory responses. The use of low-dose corticosteroid anti-inflammatory drugs can help alleviate local tissue inflammation and stabilize the tear film, which is of significant importance for the stability of the ocular surface post-FLACS (68). Beyond that, cyclosporine A, as an immunosuppressive agent, possesses unique capabilities to improve goblet cell density and anti-apoptotic properties (69). Research has confirmed that cyclosporine A significantly improves dry eye induced by cataract surgery (70). Therefore, the use of corticosteroid anti-inflammatory drugs and immunosuppressive medications is critically important for managing post-FLACS dry eye and maintaining the stability of the ocular surface.
5 Conclusion and prospects
The advent of FLACS undoubtedly represents a significant technological breakthrough in the field of ophthalmology (71). At the same time, we have also focused on the occurrence of postoperative dry eye. Currently, the clinical treatment of FLACS-induced dry eye often involves only symptomatic treatment with artificial tears to alleviate dry eye symptoms and signs, without precise etiological treatment. This is also the reason why some clinical patients have recurrent dry eye symptoms and prolonged illness. Personalized treatment of dry eye is a major trend at present. Only by gaining a deeper understanding of the various impacts that FLACS may have on the eye can we develop more effective prevention and treatment.
Through the discussion in this article, we have found that post-FLACS dry eye is related to multiple factors, such as changes in tear cytokines, corneal damage, inflammatory responses, and the pressure exerted by the PI on the eyelids and ocular surface tissues. Therefore, before and after surgery, we need to take a series of targeted preventive and therapeutic measures, such as preoperative assessment of the patient’s dry eye symptoms, optimization of femtosecond laser parameters, anti-inflammatory treatment, targeted supplementation of artificial tears, and promotion of mucin secretion, to reduce the risk of postoperative dry eye and alleviate postoperative dry eye symptoms. At the same time, we found that the damage caused by the PI to the ocular surface tissues cannot be ignored. Jonathan et al. have previously compared the safety and effectiveness of contact and non-contact PIs, finding that reducing the contact area between the PI and the ocular surface not only reduces damage to the ocular surface but also improves the efficacy of the femtosecond laser (42). We believe this could be a direction for future innovation and development of PIs, namely, achieving the same or even better suction effects with less contact with the ocular surface or softer and more comfortable contact materials, which will be beneficial for the repair of postoperative ocular surface tissues. Of course, this requires further clinical trials for validation.
Author contributions
BL: Writing – original draft. D-kL: Writing – original draft. LZ: Writing – original draft. L-lC: Writing – original draft. Y-yG: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Xiamen Municipal Bureau of Science and Technology (3502Z20244ZD1192) and the Fujian Provincial Natural Science Foundation of China (2024J011323).
Acknowledgments
Thanks to Jing Tang for their help in literatures collection in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FLACS, Femtosecond Laser-Assisted Cataract Surgery; CPS, Conventional Phacoemulsification Surgery; PI, Patient Interface; OSDI, Ocular Surface Disease Index; BUT, Break-Up Time; MMP-9, Matrix Metalloproteinase-9; IL-1β, Interleukin-1 beta; TNF-α, Tumor Necrosis Factor-alpha; SIt, Schirmer I test.
References
1. Stapleton, F, Alves, M, Bunya, VY, Jalbert, I, Lekhanont, K, Malet, F, et al. TFOS DEWS II epidemiology report. Ocul Surf. (2017) 15:334–65. doi: 10.1016/j.jtos.2017.05.003
2. Gomes, JAP, Azar, DT, Baudouin, C, Efron, N, Hirayama, M, Horwath-Winter, J, et al. Tfos dews ii iatrogenic report. Ocul Surf. (2017) 15:511–38. doi: 10.1016/j.jtos.2017.05.004
3. Miura, M, Inomata, T, Nakamura, M, Sung, J, Nagino, K, Midorikawa-Inomata, A, et al. Prevalence and characteristics of dry eye disease after cataract surgery: a systematic review and meta-analysis. Ophthalmol Therapy. (2022) 11:1309–32. doi: 10.1007/s40123-022-00513-y
4. Mastropasqua, L, and Nubile, M. Femtosecond laser-assisted penetrating and lamellar Keratoplasty. Cornea. (2015) 6:39–53. doi: 10.1159/000381491
5. Chang, E, and Zhang, A. Femtosecond laser-assisted cataract surgery. Adv Ophthalmol Optom. (2022) 7:177–86. doi: 10.1016/j.yaoo.2022.04.002
6. Narayan, A, Evans, JR, O'Brart, D, Bunce, C, Gore, DM, and Day, AC. Laser-assisted cataract surgery versus standard ultrasound phacoemulsification cataract surgery. Cochrane Database Syst Rev. (2023) 2023:CD010735. doi: 10.1002/14651858.CD010735.pub3
7. Chen, W-T, Chen, Y-Y, and Hung, M-C. Dry eye following femtosecond laser-assisted cataract surgery: a Meta-analysis. J Clin Med. (2022) 11:6228. doi: 10.3390/jcm11216228
8. Hashemi, H, Pakzad, R, Yekta, A, Aghamirsalim, M, Pakbin, M, Ramin, S, et al. Global and regional prevalence of age-related cataract: a comprehensive systematic review and meta-analysis. Eye. (2020) 34:1357–70. doi: 10.1038/s41433-020-0806-3
9. Khairallah, M, Kahloun, R, Bourne, R, Limburg, H, Flaxman, SR, Jonas, JB, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. (2015) 56:6762–9. doi: 10.1167/iovs.15-17201
10. Roberts, TV, Lawless, M, Bali, SJ, Hodge, C, and Sutton, G. Surgical outcomes and safety of femtosecond laser cataract surgery: a prospective study of 1500 consecutive cases. Ophthalmology. (2013) 120:227–33. doi: 10.1016/j.ophtha.2012.10.026
11. Ju, RH, Chen, Y, Chen, HS, Zhou, WJ, Yang, W, Lin, ZD, et al. Changes in ocular surface status and dry eye symptoms following femtosecond laser-assisted cataract surgery. Int J Ophthalmol. (2019) 12:1122–6. doi: 10.18240/ijo.2019.07.11
12. Xu, R, Zhao, SZ, Zeng, QY, Chen, D, and Chang, XK. Risk factor analysis of dry eye after femtosecond laser-assisted cataract surgery. Adv. Ophthalm. (2021) 41:1149–53. doi: 10.13389/j.cnki.rao.2021.0240
13. Chen, BH, Dai, J, Ke, Y, He, TT, Guo, QT, and Si, MJ. Clinical study on the effect of femtosecond laser-assisted cataract surgery on tear film quality. J Clinical Ophthalm. (2018) 26:426–9. doi: 10.3969/j.issn.1006-8422.2018.05.011
14. Zhou, YL, and Zhang, H. Changes in tear film and corneal sensation after femtosecond laser-assisted cataract phacoemulsification surgery. Chin J Exper Ophthalm. (2018) 36:222–6. doi: 10.3760/cma.j.issn.2095-0160.2018.03.013
15. Yu, Y, Hua, H, Wu, M, Yu, Y, Yu, W, Lai, K, et al. Evaluation of dry eye after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. (2015) 41:2614–23. doi: 10.1016/j.jcrs.2015.06.036
16. Shao, D, Zhu, X, Sun, W, Cheng, P, Chen, W, and Wang, H. Effects of femtosecond laser-assisted cataract surgery on dry eye. Exp Ther Med. (2018) 16:5073–8. doi: 10.3892/etm.2018.6862
17. Schargus, M, Ivanova, S, Stute, G, Dick, HB, and Joachim, SC. Comparable effects on tear film parameters after femtosecond laser-assisted and conventional cataract surgery. Int Ophthalmol. (2020) 40:3097–104. doi: 10.1007/s10792-020-01532-z
18. Chen, W, and Sun, YN. Attention to dry eye after cataract surgery. Chin J Optom Ophthalmol Vis Sci. (2021) 23:486–90. doi: 10.3760/cma.j.cn115909-20201020-00401
19. Arshinoff, SA, and Khoury, E. HsS versus a balanced salt solution as a corneal wetting agent during routine cataract extraction and lens implantation. J Cataract Refract Surg. (1997) 23:1221–5. doi: 10.1016/s0886-3350(97)80320-3
20. Oh, T, Jung, Y, Chang, D, Kim, J, and Kim, H. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. (2012) 56:113–8. doi: 10.1007/s10384-012-0117-8
21. Sutu, C, Fukuoka, H, and Afshari, NA. Mechanisms and management of dry eye in cataract surgery patients. Curr Opin Ophthalmol. (2016) 27:24–30. doi: 10.1097/ICU.0000000000000227
22. Han, KE, Yoon, SC, Ahn, JM, Nam, SM, Stulting, RD, Kim, EK, et al. Evaluation of dry eye and meibomian gland dysfunction after cataract surgery. Am J Ophthalmol. (2014) 157:1144–1150 e1. doi: 10.1016/j.ajo.2014.02.036
23. Cho, YK, and Kim, MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. (2009) 23:65–73. doi: 10.3341/kjo.2009.23.2.65
24. Dry Eye Association of Asia China Branch, Cross-Strait Medicine and Health Exchange Association Ophthalmology Professional Committee Ocular Surface and Tear Disease Group, China Medical Association Ophthalmologist Branch Ocular Surface and Dry Eye Group. Chinese expert consensus on dry eye related to eye surgery (2021). Chin J Optom Ophthalmol. (2021) 57, 8:564–72. doi: 10.3760/cma.j.cn112142-20210429-00196
25. Chen, H, Lin, H, Zheng, D, Liu, Y, Chen, W, and Liu, Y. Expression of cytokines, Chmokines and growth factors in patients undergoing cataract surgery with femtosecond laser pretreatment. PLoS One. (2015) 10:e0137227. doi: 10.1371/journal.pone.0137227
26. Wong, AH, Cheung, RK, Kua, WN, Shih, KC, Chan, TC, and Wan, KH. Dry eyes after SMILE. Asia Pac J Ophthalmol. (2019) 8:397. doi: 10.1097/01.apo.0000580136.80338.d0
27. Mochizuki, M, Sugita, S, and Kamoi, K. Immunological homeostasis of the eye. Prog Retin Eye Res. (2013) 33:10–27. doi: 10.1016/j.preteyeres.2012.10.002
28. Roda, M, Corazza, I, Bacchi Reggiani, ML, Pellegrini, M, Taroni, L, Giannaccare, G, et al. Dry eye disease and tear cytokine levels—a meta-analysis. Int J Mol Sci. (2020) 21:3111. doi: 10.3390/ijms21093111
29. Favuzza, E, Becatti, M, Gori, AM, and Mencucci, R. Cytokines, chemokines, and flare in the anterior chamber after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. (2019) 45:910–4. doi: 10.1016/j.jcrs.2019.01.040
30. Ling, J, Chan, BC, Tsang, MS, Gao, X, Leung, PC, Lam, CW, et al. Current advances in mechanisms and treatment of dry eye disease: toward anti-inflammatory and immunomodulatory therapy and traditional Chinese medicine. Front Med. (2021) 8:815075. doi: 10.3389/fmed.2021.815075
31. Chen, M, Hu, DN, Pan, Z, Lu, CW, Xue, CY, and Aass, I. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. (2010) 90:437–43. doi: 10.1016/j.exer.2009.12.004
32. Seo, MJ, Kim, JM, Lee, MJ, Sohn, YS, Kang, KK, and Yoo, M. The therapeutic effect of DA-6034 on ocular inflammation via suppression of MMP-9 and inflammatory cytokines and activation of the MAPK signaling pathway in an experimental dry eye model. Curr Eye Res. (2010) 35:165–75. doi: 10.3109/02713680903453494
33. Nagy, ZZ. New technology update: femtosecond laser in cataract surgery. Clin Ophthalmol. (2014) 8:1157–67. doi: 10.2147/OPTH.S36040
34. Wang, X, Zhang, Z, Li, X, Xie, L, Zhang, H, Koch, DD, et al. Evaluation of femtosecond laser versus manual clear corneal incisions in cataract surgery using spectral-domain optical coherence tomography. J Refract Surg. (2018) 34:17–22. doi: 10.3928/1081597X-20171109-01
35. Roberts, HW, Day, AC, and O'Brart, DP. Femtosecond laser-assisted cataract surgery: a review. Eur J Ophthalmol. (2020) 30:417–29. doi: 10.1177/1120672119893291
36. Agarwal, K, and Hatch, K. Femtosecond laser assisted cataract surgery: a review. Semin Ophthalmol. (2021) 36:618–27. doi: 10.1080/08820538.2021.1890792
37. Rodriguez, AE, Rodriguez-Prats, JL, Hamdi, IM, Galal, A, Awadalla, M, and Alio, JL. Comparison of goblet cell density after femtosecond laser and mechanical microkeratome in LASIK. Invest Ophthalmol Vis Sci. (2007) 48:2570–5. doi: 10.1167/iovs.06-1259
38. Rodriguez-Prats, JL, Hamdi, IM, Rodriguez, AE, Galal, A, and Alio, JL. Effect of suction ring application during LASIK on goblet cell density. J Refract Surg. (2007) 23:559–62. doi: 10.3928/1081-597X-20070601-04
39. Chao, C, Golebiowski, B, and Stapleton, F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. (2014) 12:32–45. doi: 10.1016/j.jtos.2013.09.001
40. Li, W, and He, X. Ocular surface microenvironment and dry eye diagnosis and treatment. Chin J Optom Ophthalmol. (2022) 58:155–60. doi: 10.3760/cma.j.cn112142-20211122-00552
41. Labetoulle, M, Baudouin, C, Calonge, M, Merayo-Lloves, J, Boboridis, KG, Akova, YA, et al. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. (2019) 97:137–45. doi: 10.1111/aos.13844
42. Talamo, JH, Gooding, P, Angeley, D, Culbertson, WW, Schuele, G, Andersen, D, et al. Optical patient interface in femtosecond laser-assisted cataract surgery: contact corneal applanation versus liquid immersion. J Cataract Refract Surg. (2013) 39:501–10. doi: 10.1016/j.jcrs.2013.01.021
43. Kohnen, T. Interface for femtosecond laser-assisted lens surgery. J Cataract Refract Surg. (2013) 39:491–2. doi: 10.1016/j.jcrs.2013.02.033
44. Nagy, ZZ, Takacs, AI, Filkorn, T, Kranitz, K, Gyenes, A, Juhasz, E, et al. Complications of femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. (2014) 40:20–8. doi: 10.1016/j.jcrs.2013.08.046
45. Rosenthal, P, and Borsook, D. Ocular neuropathic pain. Br J Ophthalmol. (2015) 100:128–34. doi: 10.1136/bjophthalmol-2014-306280
46. Naderi, K, Gormley, J, and O'Brart, D. Cataract surgery and dry eye disease: a review. Eur J Ophthalmol. (2020) 30:840–55. doi: 10.1177/1120672120929958
47. Cataract and Intraocular Lens Group of the Ophthalmology Branch of the Chinese Medical Association. Expert consensus on standards for femtosecond laser-assisted cataract extraction surgery in China (2018). Chin J Ophthalmol. (2018) 54:328–33. doi: 10.3760/cma.j.issn.0412-4081.2018.05.003
48. Khalifa, YM, and Mifflin, MD. Keratitis and corneal melt with ketorolac tromethamine after conductive keratoplasty. Cornea. (2011) 30:477–8. doi: 10.1097/ICO.0b013e3181ef6ec7
49. Clouzeau, C, Godefroy, D, Riancho, L, Rostene, W, Baudouin, C, and Brignole-Baudouin, F. Hyperosmolarity potentiates toxic effects of benzalkonium chloride on conjunctival epithelial cells in vitro. Mol Vis. (2012) 18:851–63.
50. Chen, W, Li, Z, Hu, J, Zhang, Z, Chen, L, Chen, Y, et al. Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One. (2011) 6:e26103. doi: 10.1371/journal.pone.0026103
51. Gupta, PK, Drinkwater, OJ, VanDusen, KW, Brissette, AR, and Starr, CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. (2018) 44:1090–6. doi: 10.1016/j.jcrs.2018.06.026
52. Villani, E, Marelli, L, Bonsignore, F, Lucentini, S, Luccarelli, S, Sacchi, M, et al. The ocular surface frailty index as a predictor of ocular surface symptom onset after cataract surgery. Ophthalmology. (2020) 127:866–73. doi: 10.1016/j.ophtha.2019.12.012
53. Yu, XY, Wang, DD, Chang, PJ, Xie, JL, Li, ZL, Lian, HL, et al. The effect of corneal protectants on dry eye after cataract surgery. Chin J Optom Ophthalmol Vis Sci. (2017) 4:236–42. doi: 10.3760/cma.j.issn.1674-845X.2017.04.008
54. Zhong, J, Deng, Y, Tian, B, Wang, B, Sun, Y, Huang, H, et al. Hyaluronate acid-dependent protection and enhanced corneal wound healing against oxidative damage in corneal epithelial cells. J Ophthalmol. (2016) 2016:1–10. doi: 10.1155/2016/6538051
55. Suwal, A, Hao, J-l, Zhou, D-d, Liu, X-f, Suwal, R, and Lu, C-w. Use of intense pulsed light to mitigate meibomian gland dysfunction for dry eye disease. Int J Med Sci. (2020) 17:1385–92. doi: 10.7150/ijms.44288
56. Dixon, P, Ghosh, T, Mondal, K, Konar, A, Chauhan, A, and Hazra, S. Controlled delivery of pirfenidone through vitamin E-loaded contact lens ameliorates corneal inflammation. Drug Deliv Transl Res. (2018) 8:1114–26. doi: 10.1007/s13346-018-0541-5
57. Guo, L, Liang, XJ, Zhang, XQ, Xu, YX, and Lin, YJ. Efficacy of femtosecond laser cataract surgery combined with PanOptix trifocal intraocular lens implantation. Int Eye Sci. (2023) 23:312–5. doi: 10.3980/j.issn.1672-5123.2023.2.25
58. Jones, M, Hovanesian, JA, and Keyser, A. Accuracy of the LaserArcs femtosecond cataract surgery arcuate incision nomogram in patients undergoing cataract surgery and astigmatism reduction. Clin Ophthalmol. (2023) 17:681–9. doi: 10.2147/OPTH.S398334
59. Li, Z, Choi, JH, Oh, HJ, Park, SH, Lee, JB, and Yoon, KC. Effects of eye drops containing a mixture of omega-3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress-induced murine dry eye. Curr Eye Res. (2014) 39:871–8. doi: 10.3109/02713683.2014.884595
60. Miyake, K, and Yokoi, N. Influence on ocular surface after cataract surgery and effect of topical diquafosol on postoperative dry eye: a multicenter prospective randomized study. Clin Ophthalmol. (2017) 11:529–40. doi: 10.2147/OPTH.S129178
61. Hsiao, C, Ferko, N, Ainslie-Garcia, M, Pan, S, and Cheng, H. PSU1 an umbrella review comparing the safety and effectiveness of femtosecond laser-assisted cataract surgery with manual cataract surgery. Value Health. (2021) 24:S224. doi: 10.1016/j.jval.2021.04.1121
62. Mencucci, R, De Vitto, C, Cennamo, M, Vignapiano, R, Buzzi, M, and Favuzza, E. Femtosecond laser-assisted cataract surgery in eyes with shallow anterior chamber depth: comparison with conventional phacoemulsification. J Cataract Refract Surg. (2020) 46:1604–10. doi: 10.1097/j.jcrs.0000000000000341
63. Takamura, E, Tsubota, K, Watanabe, H, and Ohashi, YGroup DOSPS. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. (2012) 96:1310–5. doi: 10.1136/bjophthalmol-2011-301448
64. Kinoshita, S, Awamura, S, Oshiden, K, Nakamichi, N, Suzuki, H, Yokoi, N, et al. Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology. (2012) 119:2471–8. doi: 10.1016/j.ophtha.2012.06.052
65. Holland, EJ, Darvish, M, Nichols, KK, Jones, L, and Karpecki, PM. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: a systematic literature review. Ocul Surf. (2019) 17:412–23. doi: 10.1016/j.jtos.2019.02.012
66. Akcam, HT, Unlu, M, Karaca, EE, Yazici, H, Aydin, B, and Hondur, AM. Autologous serum eye-drops and enhanced epithelial healing time after photorefractive keratectomy. Clin Exp Optom. (2018) 101:34–7. doi: 10.1111/cxo.12574
67. Buzzi, M, Versura, P, Grigolo, B, Cavallo, C, Terzi, A, Pellegrini, M, et al. Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus Apher Sci. (2018) 57:549–55. doi: 10.1016/j.transci.2018.06.001
68. Jee, D, Park, M, Lee, HJ, Kim, MS, and Kim, EC. Comparison of treatment with preservative-free versus preserved sodium hyaluronate 0.1% and fluorometholone 0.1% eyedrops after cataract surgery in patients with preexisting dry-eye syndrome. J Cataract Refract Surg. (2015) 41:756–63. doi: 10.1016/j.jcrs.2014.11.034
69. Periman, LM, Mah, FS, and Karpecki, PM. A review of the mechanism of action of cyclosporine a: the role of cyclosporine a in dry eye disease and recent formulation developments. Clin Ophthalmol. (2020) 14:4187–200. doi: 10.2147/OPTH.S279051
70. Lee, JH, Song, IS, Kim, KL, and Yoon, SY. Effectiveness and optical quality of topical 3.0% diquafosol versus 0.05% cyclosporine a in dry eye patients following cataract surgery. J Ophthalmol. (2016) 2016:1–7. doi: 10.1155/2016/8150757
Keywords: femtosecond laser, cataract, dry eye, pathogenesis, patient interface
Citation: Lin B, Li D-k, Zhang L, Chen L-l and Gao Y-y (2024) Postoperative dry eye following femtosecond laser-assisted cataract surgery: insights and preventive strategies. Front. Med. 11:1443769. doi: 10.3389/fmed.2024.1443769
Edited by:
Panpan Ye, Zhejiang University, ChinaReviewed by:
Stephanie C. Joachim, Ruhr University Bochum, GermanyAlfredo Domínguez López, Universidad de Valladolid, Spain
Copyright © 2024 Lin, Li, Zhang, Chen and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-ying Gao, Z2FveWluZ3lpbmcxOTY4QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Bin Lin
Bin Lin Dong-kan Li
Dong-kan Li Ling Zhang7
Ling Zhang7 Ying-ying Gao
Ying-ying Gao