- 1Department of Radiology, Affiliated Hospital of Zunyi Medical University, Medical Imaging Center of Guizhou Province, Zunyi, China
- 2Department of Graduate School, Zunyi Medical University, Zunyi, China
Diquat (1,1′-ethylene-2,2′-bipyridine), a non-selective herbicide with significant human toxicity, is increasingly used as a substitute for paraquat in weed management practices in China. Diquat intoxication is typified by multiple organ dysfunction syndrome (MODS), predominantly manifesting as acute renal and hepatic injury, and frequently resulting in central nervous system (CNS) impairment with a poor prognosis in severe instances. Despite the rising incidence of diquat poisoning, the imaging characteristics of diquat-induced toxic encephalopathy remain inadequately documented in the literature. In this report, we present a distinctive case involving a female pediatric patient who exhibited MODS affecting the neurologic, renal, hepatic, cardiac, and gastrointestinal systems, in conjunction with rhabdomyolysis. Magnetic resonance imaging (MRI) revealed multiple abnormal signals in the pons, bilateral brachium pontis, thalamus, caudate nucleus, putamen, posterior part of the external capsule, and posterior limb of the right internal capsule. These findings are consistent with the imaging characteristics of osmotic demyelination syndrome (ODS), which is under-recognized but important. After comprehensive systemic treatment, the patient was discharged on the 30th day post-admission.
Introduction
ODS is an acute non-inflammatory demyelinating condition frequently attributed to the rapid correction of hyponatremia (1, 2). The risk of developing ODS is exacerbated by factors such as post-liver transplantation, chronic alcoholism, malnutrition, hypokalemia, and hypophosphatemia. In the past, poisoning has not been recognized as an etiological factor of ODS, although an increasing number of studies have indicated that the MRI characteristics of diquat toxic encephalopathy align with those observed in ODS (3, 4). ODS includes central pontine myelinolysis (CPM) and extrapontine myelinolysis (EPM), and when the pons is involved, the trident or piglet signs are characteristic MRI manifestations of ODS (5, 6). Toxic encephalopathy predominantly induces alterations in cerebral white matter fibers and subcortical gray matter nuclei, potentially involving the cerebellopontine nucleus. However, imaging findings consistent with ODS are infrequent. Consequently, this study aims to elucidate the MRI manifestations of diquat-induced toxic encephalopathy, which may present distinctive yet under recognized features. Crucially, these imaging characteristics can assist clinicians and radiologists in formulating diagnostic hypotheses in instances where the history of toxic exposure is ambiguous, thereby facilitating accurate diagnosis and prompt, effective treatment.
Case description
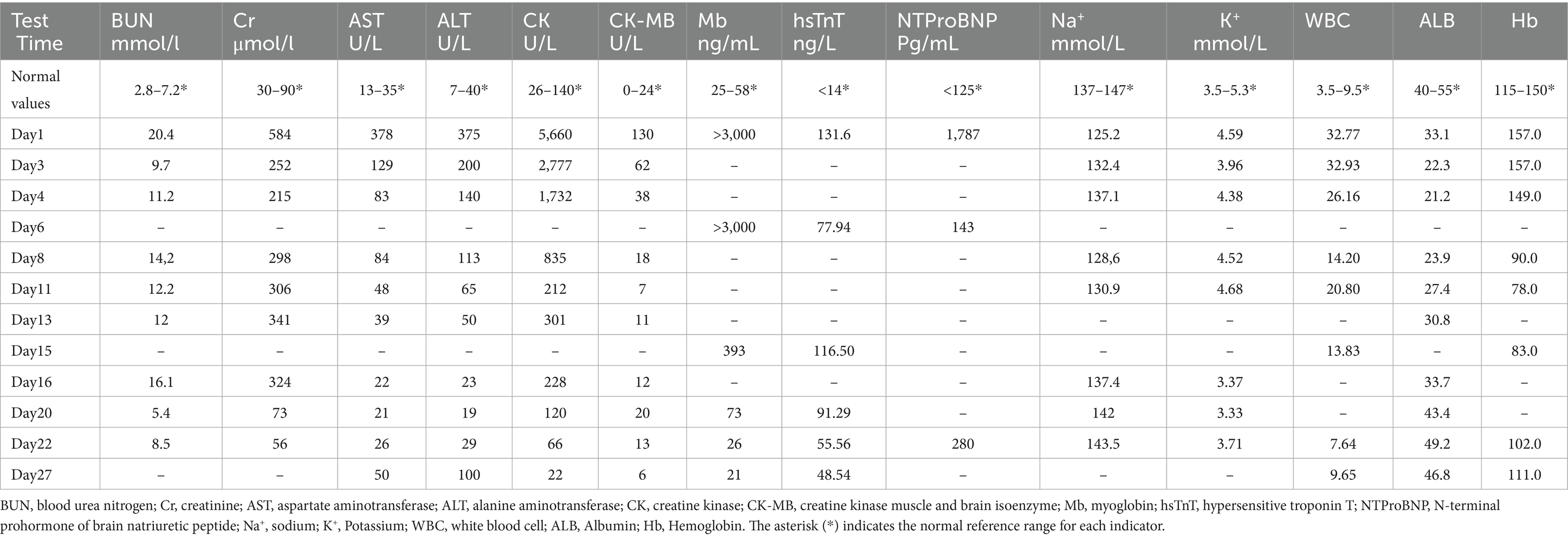
A 13-year-old female patient was admitted to our hospital with anuria and bilateral lower extremity edema lasting for one day. One week prior, the patient experienced abdominal pain and headache without a clear precipitating cause, not associated with vomiting or diarrhea, and was managed with oral medication at a nearby county hospital. Three days before admission, the patient’s symptoms of headache and abdominal pain worsened and were accompanied by vomiting, increased frequency of bowel movements, and fever. The patient was admitted to the local county hospital of traditional Chinese medicine one day prior, presenting with unresolved headache and abdominal pain, as well as the onset of anuria and bilateral lower extremity swelling. Due to the unknown etiology and rapid progression of her condition, she was transferred to our hospital on January 10, 2024, for comprehensive evaluation and management. The patient’s mother has a history of chronic hepatitis B virus (HBV) infection, suggesting that the patient may have acquired HBV through vertical transmission. There is no family history of other genetic or neurological diseases. The patient was previously diagnosed as an HBV carrier, with liver function consistently maintained within the normal range, and did not receive systematic antiviral treatment. Prior to admission, the patient had no significant medical history. Upon admission, the patient was conscious and denied any history of toxic exposure. Vital signs indicated a temperature of 36.8°C, a pulse of 95 beats/min, respiration rate of 20 beats/min, and blood pressure of 118/68 mmHg. Physical examination revealed abdominal diffuse tenderness and nonpitting edema in both lower extremities. Laboratory test results are detailed in Table 1, with electrocardiographic (ECG) findings suggesting T-wave changes. Non-contrast computer tomography (CT) scans of the brain, chest, and abdomen showed normal results.
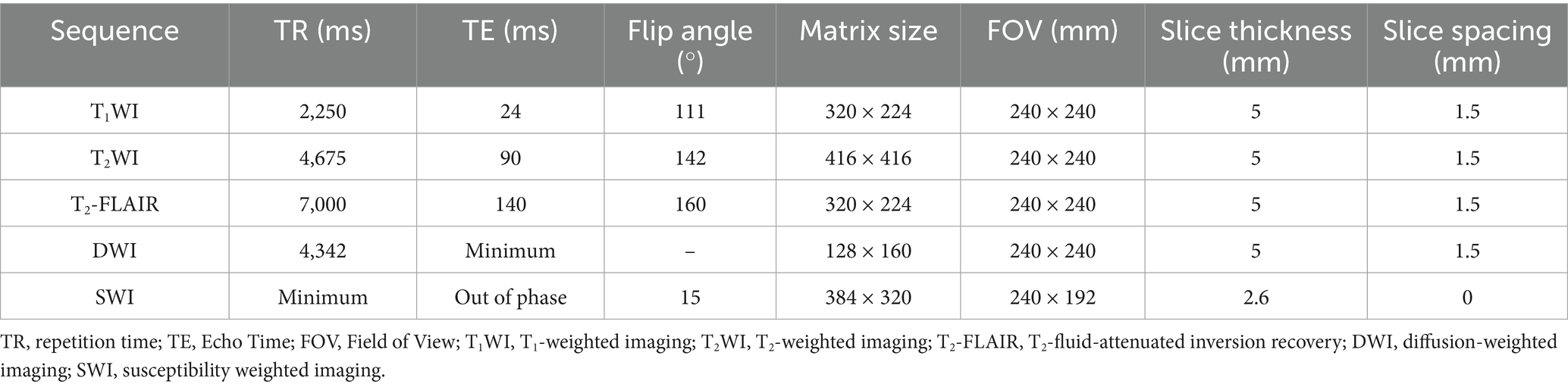
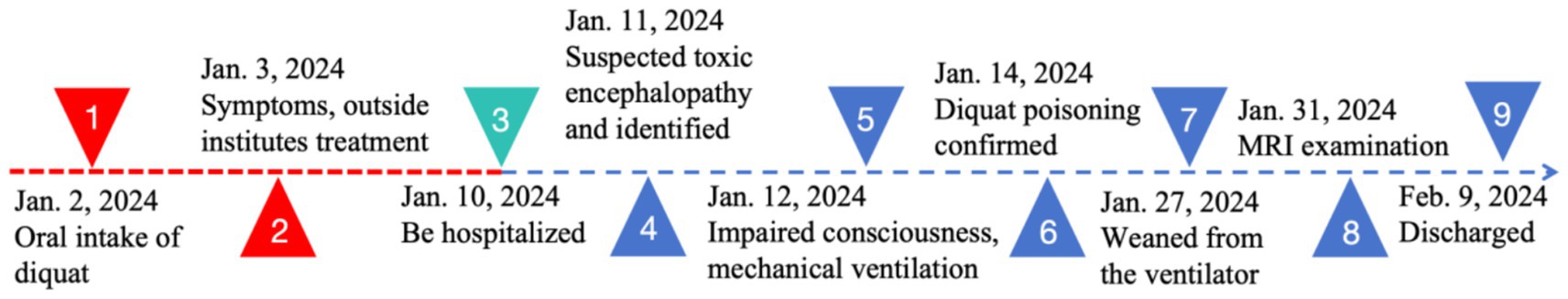
Following admission, the patient received prompt administration of continuous renal replacement therapy, anti-infection treatment, and symptomatic supportive therapy. On the second day of hospitalization, the patient exhibited behavioral symptoms, including heightened speech and disorientation. Physical examination revealed that the patient’s lips exhibited a burning-like appearance, accompanied by significant congestion, fissures, and scab formation. Experienced attending physicians hypothesized poisoning as the underlying cause. Upon further detailed history-taking, it was revealed that the patient had orally ingested approximately 50 mL of diquat (20 g/100 mL) 9 days prior. Subsequently, the blood and dialysate samples were transferred to an external medical facility for analysis of toxic substance composition, revealing diquat concentrations of 12 ng/mL in the blood and 80 ng/mL in the dialysate. The patient exhibited symptoms of impaired consciousness, rapid respiration, elevated heart rate, and unstable oxygen saturation 3 days post-admission, necessitating immediate initiation of invasive ventilator-assisted ventilation. This intervention continued until day 17, at which point the patient’s condition began to improve. On the 21st day post-admission, a brain MRI examination was performed using a 3.0 T magnetic resonance scanner (Discovery MR750w, General Electric, Milwaukee, WI, USA) with a 32-channel dedicated head coil. Specific scan sequence parameters are shown in Table 2. Magnetic resonance imaging revealed multiple abnormal signals in various regions, including the pons, bilateral brachium pontis, thalamus, caudate nucleus, putamen, posterior part of the external capsule, and posterior limb of the right internal capsule. These regions exhibited hypointense signals on T1-weighted imaging (T1WI), hyperintense signals on T2-weighted imaging (T2WI) and T2-fluid-attenuated inversion recovery (T2-FLAIR), and hypointense signals on susceptibility-weighted imaging (SWI). Partial lesions in the pons exhibited hyperintense signals on diffusion-weighted imaging (DWI) and reduced signals on the apparent diffusion coefficient (ADC) map (Figure 1). The central region of the pons exhibited severe damage with hyperintense signals on T2WI, while the corticospinal and corticobulbar tracts were relatively preserved, showing hypointense signals, forming the characteristic “piglet sign” (Figure 1B). These findings were entirely consistent with the diagnosis of ODS. Following systemic treatment, the patient was discharged on the 30th day post-admission. At the time of discharge, the patient was conscious, able to understand medical instructions and produce simple sounds, but unable to answer questions. She was also unable to eat or walk independently and occasionally coughed while drinking. After multiple rehabilitation sessions at a large tertiary hospital in Guangzhou, Guangdong Province, China, as of the time of manuscript preparation (4 months post-discharge), the patient has largely recovered to a level indistinguishable from a healthy individual, although her personality has become extremely irritable compared to her pre-hospitalization state. The timeline of treatment can be seen in Figure 2.
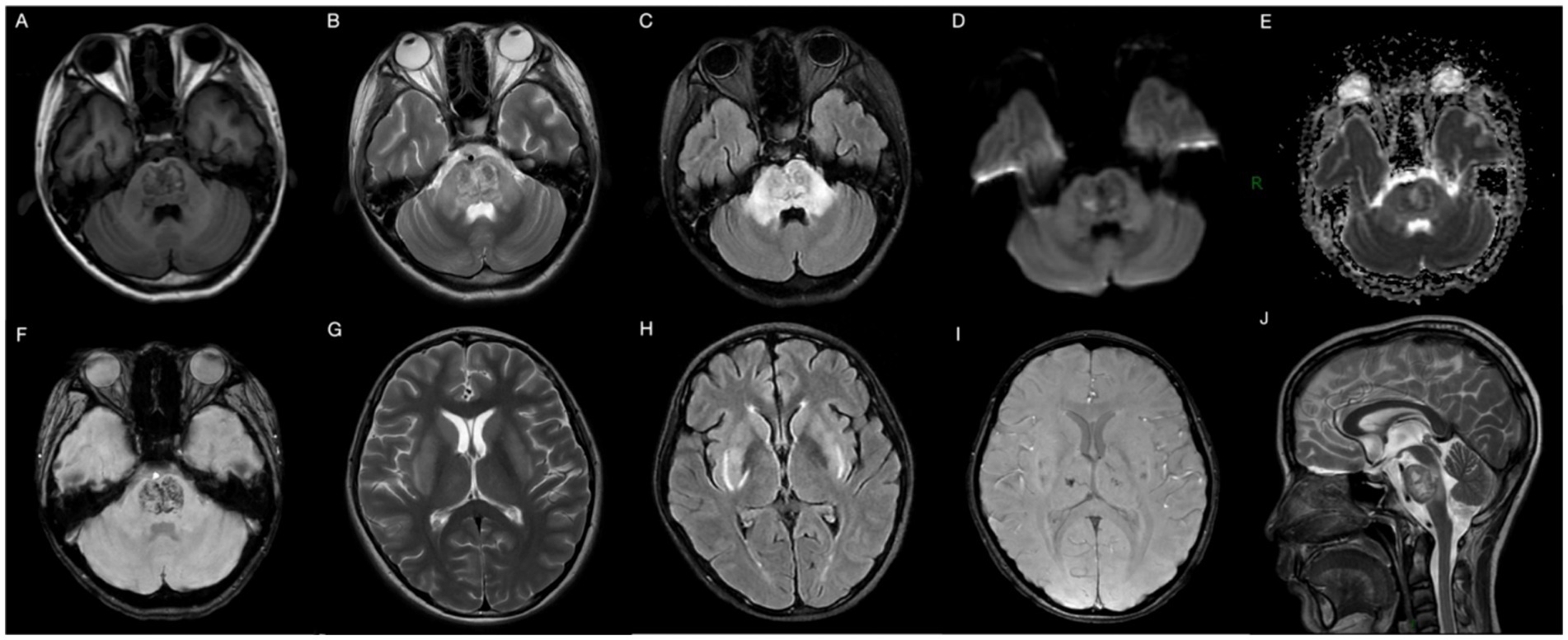
Figure 1. Brain MRI 29 days after ingestion showing abnormal heterogeneous intensities in the pons, bilateral brachium pontis, thalamus, caudate nucleus, putamen, posterior part of the external capsule, and posterior limb of the right internal capsule on T1WI (A), T2WI (B,G,J), T2-FLAIR (C,H), DWI (D), ADC-map (E), and SWI (F,I), in accordance with ODS. T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; T2-FLAIR, T2-fluid-attenuated inversion recovery; DWI, diffusion-weighted imaging; ADC, apparent diffusion coefficient; SWI, susceptibility weighted imaging.
Literature review
To understand the imaging manifestations of diquat toxic encephalopathy, we performed a literature review of all English-language case reports of diquat poisoning that contained MRI manifestations in PubMed, Web of Science, and Ovid databases, dated from the creation of the databases to May 31, 2024. Keywords were used for the search, which included “diquat,” “diquat dibromide,” “magnetic resonance imaging,” “magnetic resonance image,” and “MRI.” The flowchart illustrating the literature screening process is depicted in Figure 3, with a total of four articles encompassing six cases included in the analysis. Each case was meticulously documented, including details such as the first author and country of authorship, year of publication, patient’s sex and age, clinical presentation, timing of MRI performance post-intoxication, lesion site, prognosis, and duration of follow-up (Table 3).

Figure 3. The literature screening process flow chart for diquat toxic encephalopathy with MRI manifestations.
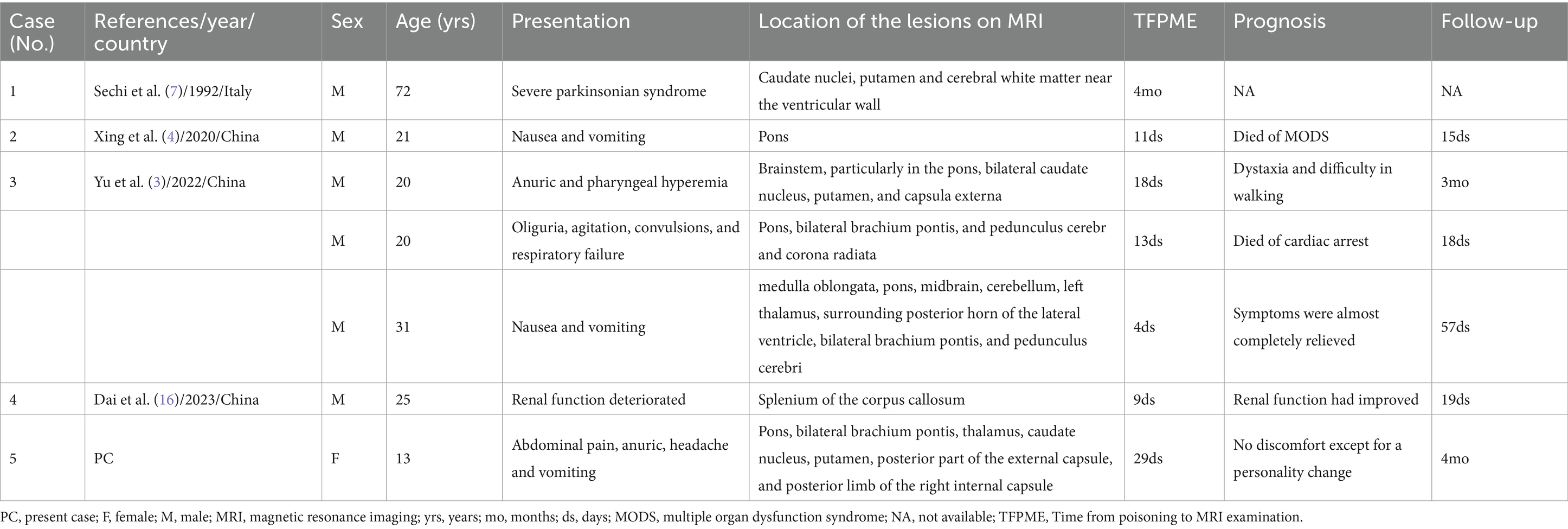
Table 3. The cases of diquat poisoning resulting in toxic encephalopathy with MRI manifestations from the literature review.
Based on the literature, only six cases of diquat toxic encephalopathy, as described in four articles, have been reported to include MRI features. The findings indicated that prior cases exclusively involved males aged between 20 and 72 years, whereas our case represented the youngest individual and the sole female. The patient’s clinical presentation primarily stemmed from gastrointestinal and genitourinary dysfunctions, including symptoms such as nausea, vomiting, renal dysfunction, and anuria. Brain lesions may manifest as either singular or multiple, with the latter typically exhibiting symmetrical distribution. Predominant anatomical locations for lesion involvement include the brainstem, specifically the pons, as well as the bilateral basal ganglia and thalamus. Significantly, 71% (5/7, including our case) of the cases had brain MRI manifestations consistent with ODS, given its specific imaging features. In this cohort, the shortest interval between diquat ingestion and MRI evaluation was 4 days. Unfortunately, two patients died of MODS and cardiac arrest, respectively, while three patients experienced differing degrees of neurological deficits post-discharge, suggesting a poor prognosis for this disease.
Discussion and conclusion
Diquat and paraquat are both non-selective bipyridine herbicides with similar structures. Following the prohibition of paraquat in numerous countries globally in recent years, the utilization and incidents of intoxication involving diquat have been on the rise as a substitute. Diquat can be absorbed through multiple pathways, including ingestion, inhalation, and mucous membranes, and in rare cases, through intramuscular, subcutaneous, and vaginal injections (7, 8). Gastrointestinal ingestion is the most common route of diquat poisoning, and most of them (approximately 90–95%) are excreted in the feces within 24 h, with only about 2% being absorbed into the blood system for distribution throughout the body (9). Following absorption, the kidneys and liver are the organs most commonly affected, with peak concentrations reached within 2 h leading to various toxic effects on multiple organs and tissues (10). Unlike paraquat poisoning, CNS damage from diquat poisoning is more common and severe, while alveolar epithelial injury is typically mild. The mechanisms of toxicity in diquat toxic encephalopathy are complex and remain unclear, generally thought to be associated with cascade reactions and dopaminergic neuron damage induced by oxidative stress, resulting in nerve cell and neuronal axonal degeneration, and ODS (3, 9, 11, 12). Bonneh-Barkay et al. (11) demonstrated through in vitro experiments that diquat can cause severe damage to dopaminergic neurons and a significant reduction in dopamine uptake. During hospitalization, our patient exhibited behavioral abnormalities, such as heightened speech and disorientation, as well as personality changes, including irritability, which are highly consistent with the clinical manifestations of dopaminergic neuron dysfunction. Furthermore, previous literature has reported that patients with diquat poisoning develop severe parkinsonian symptoms (7). Although we currently lack direct objective evidence, based on the literature support and existing clinical evidence, we believe that the hypothesis of “dopaminergic neuron damage” is reasonable. In the past, most studies focused on hepatic and renal injuries, while MRI features of cases with CNS involvement were rarely reported.
The clinical manifestations of diquat poisoning are diverse and non-specific. Oral intake of large quantities (200–300 mL) of diquat will lead to extensive ulcers and even bleeding in the digestive tract (13). Gastrointestinal symptoms are the most prominent early clinical manifestations. Corrosive damage includes burning pain in the mouth, ulcers, mucosal edema, esophageal damage, nausea, vomiting, abdominal pain, and diarrhea. However, abdominal pain caused by diquat poisoning may be related to gastrointestinal lesions (e.g., functional disturbances, mucosal inflammation, or ulcers) or other non-specific factors (e.g., electrolyte imbalances or systemic inflammatory responses). Additionally, CT scans have limited sensitivity for early or functional lesions. Therefore, even if abdominal pain is the earliest and most prominent symptom, non-contrast abdominal CT scans may still yield negative results, as demonstrated in our case. To more accurately assess gastrointestinal pathology, we recommend further imaging studies (e.g., contrast-enhanced CT or MRI) or endoscopic examinations if clinical symptoms persist or worsen. Following absorption, the kidney serves as the primary excretory organ for diquat and is also the primary target organ of injury (14). The severity of renal damage can range from simple proteinuria to acute renal failure. Liver, hematologic, and respiratory injuries can also occur in diquat poisoning, and it is worth noting that when lung injuries are severe, further identification of the toxicant should be performed. Diquat has a poisonous effect on central nervous cells (9). Therefore, CNS symptoms are common, including dizziness, drowsiness, convulsions, coma, excitement, restlessness, and disorientation. In our review, the most frequent symptoms in patients were caused by gastrointestinal and renal damage, and CNS-related symptoms were also common but not prominent at the time of admission, except for one patient who presented with severe Parkinsonian syndrome. In addition, severe diquat poisoning can cause rhabdomyolysis, which may aggravate kidney injury and induce myocardial damage (15).
Electrocardiographic T-wave changes can be either physiological or pathological. In patients with diquat poisoning, electrolyte disturbances, myocardial injury, rhabdomyolysis, and systemic inflammatory responses may all contribute to T-wave changes. However, there are few reported studies on electrocardiographic changes in patients with diquat poisoning. Yu et al. (15) reported a case of an 18-year-old female who developed sinus tachycardia (heart rate of 165 beats per minute) after ingesting approximately 200 mL of diquat (20 g/100 mL) and died of cardiac arrest 29 h after ingestion. In contrast, our patient was fortunate. Although her electrocardiogram showed T-wave changes at admission, after continuous electrocardiographic monitoring, electrolyte correction, supportive care, control of systemic inflammatory response, and management of potential myocardial injury and rhabdomyolysis, a follow-up electrocardiogram on the 20th day of hospitalization revealed a return to normal.
Imaging such as CT and MRI play an important role in the diagnosis and differential diagnosis of diquat toxic encephalopathy, especially MRI has great advantages due to its radiation-free, high soft-tissue resolution and multiparametric imaging features. Therefore, in this article, we focused on its MRI characteristics. Lesions in the brain can be single (16) or multiple (7), and multiple lesions are often symmetrically distributed. Xing et al. (4) reported the case of a 21-year-old male who ingested 100 mL of diquat (20 g/100 mL) and confirmed as CPM by MRI. In 2022, Yu et al. (3) reported three cases of diquat poisoning with MRI features consistent with ODS. In our case, MRI revealed multiple symmetrical intracranial lesions involving the pons and basal ganglia regions and thalamus, with the pons lesions showing characteristic piglet signs on T2WI and T2-FLAIR, a specific MRI manifestation of ODS (17). Diquat poisoning can also lead to basal ganglia hemorrhage (18), pontine hemorrhage or infarction (19). As an advanced MRI sequence, DWI is useful for cytotoxic edema, where restricted diffusion suggests the presence of acute infarction. SWI is sensitive to detecting paramagnetic (e.g., hemosiderin, deoxyhemoglobin) and antimagnetic substances (e.g., bone minerals, dystrophic calcification). Thus, SWI can be sensitive to detect the hyperintensity presented by microbleeds, as in our case. Based on these results, for patients with neurologic symptoms after diquat poisoning, we recommend a multimodal MRI examination including high-level neurofunctional imaging (e.g., DWI, SWI, etc.), which may be meaningful for the understanding of underlying pathophysiologic mechanisms of diquat toxic encephalopathy and for patient prognosis determination.
Currently, there is no specific antidote for diquat, and when the CNS is involved, there is a high rate of disability and mortality. Fortunately, unlike paraquat, diquat does not accumulate in the lungs and has a better prognosis after intensive care treatment. The clinical treatment protocol is directed at reducing absorption and/or increasing elimination (20). When skin or eyes are exposed to a solution of diquat, remove all the contaminated clothing, and repeated rinsing with water is the first preventive measure. Gastrointestinal tract ingested diquat will not be absorbed quickly, therefore, activated charcoal adsorption, gastric lavage, and catheterization are usually used as early treatment options (18, 21). Measures such as diuresis, hemodialysis, and hemoperfusion help eliminate diquat from the circulation, although they do not remove clinically and toxicologically significant quantities of the herbicide (20).
Overall, acute chemical poisoning is a significant contributor to unplanned hospitalization. Uncertainty history of toxic exposure can lead to delays in treatment, potentially resulting in severe outcomes such as permanent disability or death. Diquat poisoning may be another cause of ODS, although the specific mechanism remains unclear. However, the extant evidence is limited, and further studies that cover more cases are required.
Our case is unique because the patient, a left-behind child, did not disclose her diquat ingestion to her guardian or clinician, delaying optimal treatment. Fortunately, after systematic intervention, she only experienced a personality change. MRI is vital for diagnosing diquat toxic encephalopathy but can be overlooked and misdiagnosed. Thus, if the patient’s condition permits, a timely cranial MRI is essential during clinical treatment.
Patient perspective
The incidence of diaquat toxic encephalopathy is on the rise, yet its imaging characteristics are inadequately documented and acknowledged. Imaging plays a crucial role in the diagnosis and differentiation of CNS disease. Our review indicates that distinctive intracranial imaging presentations may manifest as soon as 4 days following diquat exposure. Consequently, prompt MRI is imperative in instances of suspected diquat poisoning accompanied by CNS symptoms.
In this particular instance, the patient, a left-behind children, initially withheld information regarding a potential diquat poisoning history, resulting in delayed treatment outside of the hospital setting. Fortunately, following comprehensive care at the Affiliated Hospital of Zunyi Medical University, the patient experienced a successful recovery, ultimately providing solace to her family and fostering a profound gratitude for our healthcare team’s specialized expertise and exceptional care.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
WFL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Visualization. HZ: Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lambeck, J, Hieber, M, Dreßing, A, and Niesen, WD. Central pontine myelinosis and osmotic demyelination syndrome. Dtsch Arztebl Int. (2019) 116:600–6. doi: 10.3238/arztebl.2019.0600
2. Singh, TD, Fugate, JE, and Rabinstein, AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol. (2014) 21:1443–50. doi: 10.1111/ene.12571
3. Yu, G, Jian, T, Cui, S, Shi, L, Kan, B, and Jian, X. Acute diquat poisoning resulting in toxic encephalopathy: a report of three cases. Clin Toxicol (Phila). (2022) 60:647–50. doi: 10.1080/15563650.2021.2013495
4. Xing, J, Chu, Z, Han, D, Jiang, X, Zang, X, Liu, Y, et al. Lethal diquat poisoning manifesting as central pontine myelinolysis and acute kidney injury: a case report and literature review. J Int Med Res. (2020) 48:300060520943824. doi: 10.1177/0300060520943824
5. Beh, SC . Temporal evolution of the trident and piglet signs of osmotic demyelination syndrome. J Neurol Sci. (2017) 373:268–73. doi: 10.1016/j.jns.2017.01.024
6. Howard, SA, Barletta, JA, Klufas, RA, Saad, A, and De Girolami, U. Best cases from the AFIP: osmotic demyelination syndrome. Radiographics. (2009) 29:933–8. doi: 10.1148/rg.293085151
7. Sechi, GP, Agnetti, V, Piredda, M, Canu, M, Deserra, F, Omar, HA, et al. Acute and persistent parkinsonism after use of diquat. Neurology. (1992) 42:261–3. doi: 10.1212/wnl.42.1.261
8. Rudez, J, Sepcić, K, and Sepcić, J. Vaginally applied diquat intoxication. J Toxicol Clin Toxicol. (1999) 37:877–9. doi: 10.1081/clt-100102470
9. Ren, Y, Guo, F, and Wang, L. Imaging findings and toxicological mechanisms of nervous system injury caused by diquat. Mol Neurobiol. (2024) 61:9272–83. doi: 10.1007/s12035-024-04172-x
10. Xia, Z, Liu, W, and Liu, L. Clinical and pathological characteristics of diquat poisoning-related acute kidney injury. Ren Fail. (2023) 45:2283590. doi: 10.1080/0886022x.2023.2283590
11. Bonneh-Barkay, D, Langston, WJ, and Di Monte, DA. Toxicity of redox cycling pesticides in primary mesencephalic cultures. Antioxid Redox Signal. (2005) 7:649–53. doi: 10.1089/ars.2005.7.649
12. Kuter, K, Nowak, P, Gołembiowska, K, and Ossowska, K. Increased reactive oxygen species production in the brain after repeated low-dose pesticide paraquat exposure in rats. A comparison with peripheral tissues. Neurochem Res. (2010) 35:1121–30. doi: 10.1007/s11064-010-0163-x
13. McCarthy, LG, and Speth, CP. Diquat intoxication. Ann Emerg Med. (1983) 12:394–6. doi: 10.1016/s0196-0644(83)80474-0
14. Zhang, H, Zhang, J, Li, J, Mao, Z, Qian, J, Zong, C, et al. Multi-omics analyses reveal the mechanisms of early stage kidney toxicity by diquat. Toxics. (2023) 11:184. doi: 10.3390/toxics11020184
15. Yu, G, Wang, J, Jian, T, Shi, L, Zhao, L, Li, Y, et al. Case series: diquat poisoning with acute kidney failure, myocardial damage, and rhabdomyolysis. Front Public Health. (2022) 10:991587. doi: 10.3389/fpubh.2022.991587
16. Dai, P, Sun, J, Yu, Z, Zhang, T, Wen, Z, Jian, T, et al. Case report: reversible splenial lesion syndrome caused by diquat poisoning. Front Neurol. (2023) 14:1178272. doi: 10.3389/fneur.2023.1178272
17. Balcerac, A, Nichelli, L, Demeret, S, and Le Guennec, L. The piglet and the trident sign in osmotic demyelination syndrome. Intensive Care Med. (2021) 47:476–7. doi: 10.1007/s00134-021-06354-w
18. Saeed, SA, Wilks, MF, and Coupe, M. Acute diquat poisoning with intracerebral bleeding. Postgrad Med J. (2001) 77:329–32. doi: 10.1136/pmj.77.907.329
19. Vanholder, R, Colardyn, F, De Reuck, J, Praet, M, Lameire, N, and Ringoir, S. Diquat intoxication: report of two cases and review of the literature. Am J Med. (1981) 70:1267–71. doi: 10.1016/0002-9343(81)90836-6
20. Magalhães, N, Carvalho, F, and Dinis-Oliveira, RJ. Human and experimental toxicology of diquat poisoning: toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum Exp Toxicol. (2018) 37:1131–60. doi: 10.1177/0960327118765330
Keywords: diquat, toxic encephalopathy, osmotic demyelination syndrome, magnetic resonance imaging, case report
Citation: Li W and Zhou H (2025) Diquat poisoning, maybe another cause of osmotic demyelination syndrome: case report and literature review. Front. Med. 12:1465003. doi: 10.3389/fmed.2025.1465003
Edited by:
Victoria Bunik, Lomonosov Moscow State University, RussiaReviewed by:
Yanxia Gao, The First Affiliated Hospital of Zhengzhou University, ChinaGuangcai Yu, Shandong University, China
Copyright © 2025 Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenfu Li, TFdGVVpIQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Wenfu Li
Wenfu Li Hui Zhou
Hui Zhou