- 1Department of Respiratory and Critical Care Medicine, Beijing Luhe Hospital, Capital Medical University, Beijing, China
- 2Department of Orthopedics, Beijing Luhe Hospital, Capital Medical University, Beijing, China
Although pulmonary infections caused by Schizophyllum commune (S. commune) are still relatively rare in clinical practice, they are increasingly familiar and valued by clinicians. The clinical symptoms and imaging of pulmonary infection with S. commune are not typical, thus diagnosis needs to be confirmed by clinical etiology. S. commune is one of the main fungal species causing allergic bronchopulmonary mycosis. The overall prognosis of S. commune pulmonary infection is good. This paper describes a case of a woman with no underlying disease who had pulmonary infection with S. commune. This report aims to improve clinicians’ awareness of the disease.
Introduction
Schizophyllum commune (S. commune), which belongs to the Phylum Basidiomycota, order Agaricales, Family Schizophyllaceae, and genus Schizophyllum, is a conditional pathogenic fungus. While S. commune is ubiquitously distributed in nature, predominantly colonizing decaying wood and plant debris, its role in human disease remains uncommon. Globally, reported cases exhibit distinct geographical clustering, with the majority documented in Japan and other Asian countries, likely attributable to heightened clinical awareness and advanced diagnostic practices in these regions (1–3). Sporadic cases have also been reported in Europe, North America, and India, though at substantially lower frequencies (4, 5). Pulmonary S. commune infections are a rare form pulmonary mycosis. For instance, Chowdhary et al. (6) analyzed clinical data of 143 cases of ABPM infected by fungi other than aspergillus, and found that the commonest etiologic agent was Candida albicans (60%), followed by Bipolaris species (13%), S. commune (11%). This low incidence may reflect both diagnostic challenges — due to its morphological resemblance to contaminant fungi and the requirement for specialized culture techniques — and potential underreporting in resource-limited settings. In this paper, we summarize the clinical manifestations, imaging characteristics, bronchoscopic manifestations and therapeutic procedure of pulmonary S. commune infection, with a view to improve clinicians’ level of understanding.
Materials and methods
Conventional testing
Conventional testing included bacterial, mycobacterial, and fungal culture and smears. Furthermore, the detection of 1,3-β-glucan and galactomannan was conducted respectively for Candida and Aspergillus.
Metagenomic next-generation sequencing (mNGS) assay
Following standard procedures (7), bronchoalveolar lavage fluid (BALF) specimens were collected by experienced physicians and utilized for mNGS analysis. BALF samples were sent to WillingMed Technology (Beijing) Co., Ltd, for mNGS analysis. Nucleic acid extraction and purification, library construction and quantitative analysis, high-throughput sequencing and bioinformatics data analysis were performed and pathogen reports were generated according to standard procedures (8, 9).
Hematoxylin and Eosin (H&E) staining
Tissue sections (4 μm) were deparaffinized in xylene, rehydrated through graded ethanol series, and stained with Harris hematoxylin (5 min). After differentiation in 1% acid ethanol and bluing in 0.2% ammonia water, sections were counterstained with eosin Y (1 min), dehydrated, cleared in xylene, and mounted with resinous medium.
Grocott’s Methenamine Silver (GMS) staining
Paraffin-embedded necrotic tissue sections (5 μm) were dewaxed, rehydrated, and oxidized with 1% periodic acid (10 min). After borax pretreatment (1%, 5 min), sections were stained with preheated hexamethylenetetramine-silver nitrate (60°C, 30–60 min) until fungal hyphae displayed black metallic deposits. Unbound silver was removed with 2% sodium thiosulfate (2 min), followed by eosin counterstaining (0.1%, 30 sec). Slides were dehydrated and mounted.
Case report
A 30-year-old female patient was admitted at our department of respiratory and critical care medicine on January 19, 2022, with cough for more than half a month and fever for 2 weeks. Her main symptoms were cough and a small amount of yellow phlegm accompanied by fever. She also presented with headache as well as chest and back pain. After her symptoms failed to improve following empirical antibiotic therapy (cefazolin and azithromycin), she developed intermittent fever and hemoptysis. She had no prior underlying medical conditions. This patient was a yoga instructor, and used a humidifier for a long time before onset of the aforementioned symptoms. Initial chest x-ray (CXR) revealed left lower lung patch with pleural effusion (Figure 1A). After admission, she was initially diagnosed with community-acquired pneumonia. She was intravenously given moxifloxacin, but still manifested recurrent fever and hemoptysis. The allergen-specific IgE testing of her peripheral blood revealed a total serum IgE level of 2226.00 KU/L, with allergen-specific concentrations as follows: Aspergillus fumigatus (0.62 IU/ml), Dermatophagoides pteronyssinus (2.9 IU/ml), and a combined fungal panel (Candida albicans/Penicillium notatum/Alternaria alternata/Cladosporium herbarum/Aspergillus niger) at 5.0 IU/ml. These values were graded as 1.7, 2.7, and 3.1, respectively, indicating a progressively increasing trend in allergen-specific sensitization levels. Peripheral blood eosinophils account for 10%. The dynamic erythrocyte sedimentation rate was 98 mm/h, while tests for galactomannan and (1, 3)-β-D glucan revealed negative results. Carcinoembryonic antigen was 10.64 U/L, while the fractional exhaled nitric oxide was 42 ppb. A computed tomography (CT) scan of the chest revealed multiple patchy blurred shadows in both lungs, the left of which showed large blurred consolidation shadows, and crescent-shaped liquid density areas in the left thoracic cavity (Figure 1B). Bronchoscopy of the opening in the left lingual lobe revealed a white necrotic material that blocked the lumen. The obstructing material was removed using biopsy forceps, which resulted in a small amount of bleeding and overflow of purulent secretions (Figure 1C). Histopathological Examination staining of the necrotic material revealed inflammatory infiltration predominantly composed of lymphocytes, plasma cells, and eosinophils. The stroma exhibited interstitial edema with focal areas of degeneration and necrosis (Figure 1G). The histopathological examination of necrotic material using GMS staining revealed scattered fungal hyphae (Figure 1H). Metagenomic next-generation sequencing (mNGS) of BALF identified 547 reads of S. commune and 4 reads of human herpesvirus 7 (HHV-7), with the remaining sequences classified as human microbiota. The proportion of eosinophils detected in the BALF was 32%. The treatment plan was subsequently adjusted to voriconazole 200 mg q12h, administered via intravenous infusion, according to the etiology. The patient’s clinical symptoms improved significantly. Repeat bronchoscopy showed that there were no yellow secretions obstructing the left lingual lobe, although purulent secretions could still be aspirated (Figure 1D). Moreover, repeat mNGS of BALF detected no S. commune but only 4 reads of HHV-7, with the remaining sequences classified as human microbiota. Re-examination of the chest, via CT scan, revealed less consolidation of the left lung and left pleural effusion than before (Figure 1E). Follow-up chest CT showed mild bronchiectasis in the right middle lobe and left lingual lobe after 3 months of treatment using voriconazole (Figure 1F).
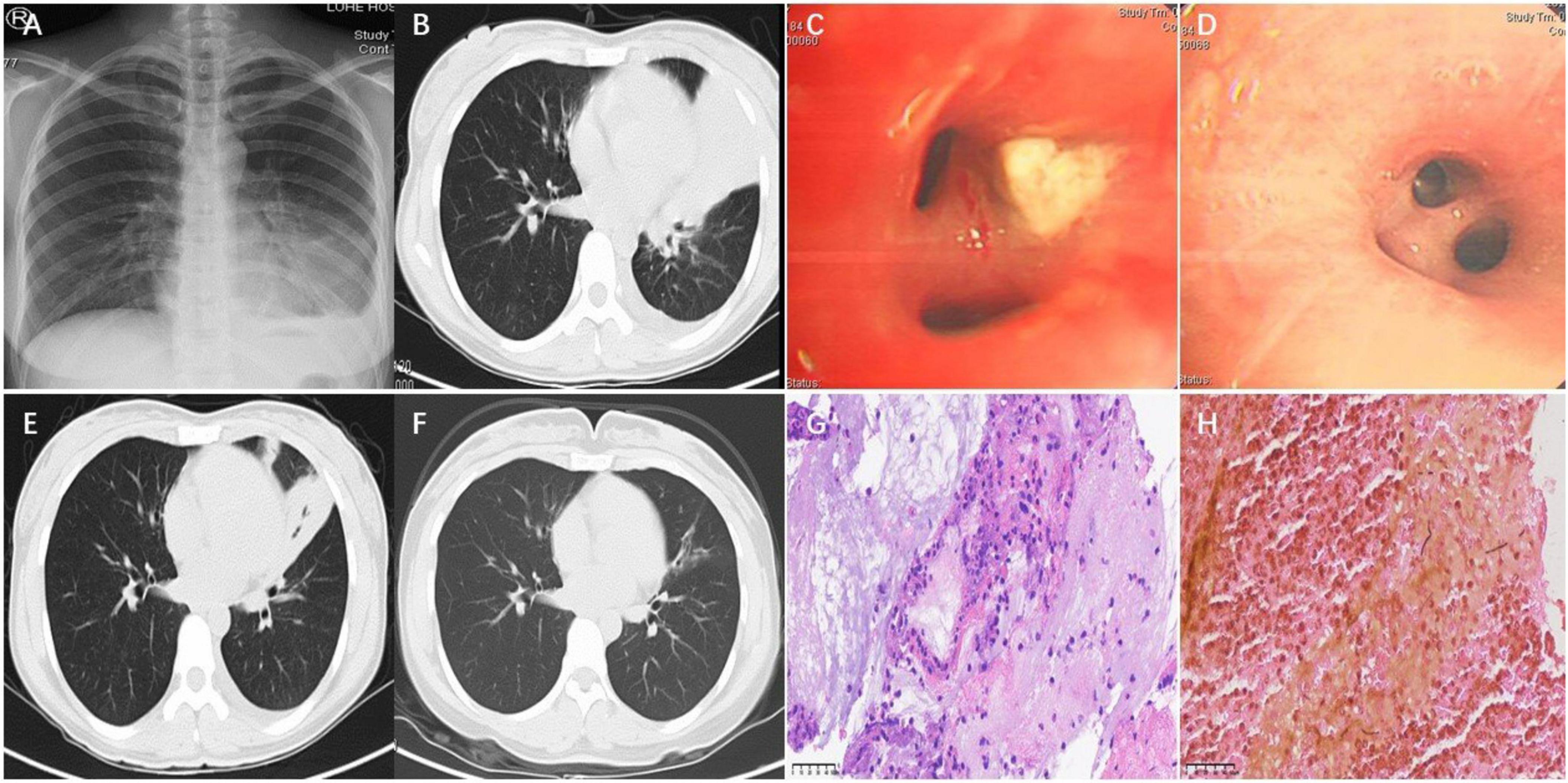
Figure 1. Chest X-ray showed left lower lung patch with pleural effusion (A). Chest CT showed large blurred consolidation shadows in the left lung and crescent-shaped liquid density areas in the left thoracic cavity (B). Bronchoscopy of the opening in the left lingual lobe revealed a white necrotic material that blocked the lumen on January 21 (C). Repeat bronchoscopy showed that there were no yellow secretions obstructing the left lingual lobe on January 26 (D). Re-examination of the chest CT revealed less consolidation of the left lung and left pleural effusion than before on January 29 (E). Chest CT showed mild bronchiectasis in the left lingual lobe on April 24 (F). Histopathological examination (H&E staining, magnification, ×400) (G). Grocott’s Methenamine Silver Staining, magnification, ×400 (H).
Discussion
S. commune is a basidiomycete bracket fungus found commonly in rotting wood, dry logs and fallen trees. Despite its worldwide distribution, this fungus was not considered a human pathogen until 1950 when Kligman isolated it from a case of onychomycosis (10). S. commune was first discovered in Japan in 1994, where it was associated with allergic bronchopulmonary mycosis (ABPM) (1). The associated mycotic infections mainly targets the respiratory tract, owing to the fact that the pathogen releases basidiospores into the atmosphere which are subsequently inhaled (2). Respiratory infections from S. Commune mainly manifest as ABPM by mucoid impaction (3), although in rare cases they may also present as asthma (11), pulmonary fungal ball (5), honeycomb lung (12) and chronic eosinophilic pneumonia (13). It not yet clear which people are susceptible to S. commune, although people without previous underlying diseases and immunodeficiencies can still be affected. Based on previous cases (2), consider vigilance against this fungal infection when the population has a history of exposure to rotting trees or wild fungi. The patient in the present case had normal immune function with no underlying diseases, although it is unclear whether her low body weight and use of a humidifier before disease onset were risk factors. While immunocompetent individuals are rarely affected, prolonged humidifier use may aerosolize environmental fungi, and low body weight could reflect nutritional deficits impacting mucosal immunity—both hypotheses requiring further study. A critical limitation of this etiological investigation lies in the absence of microbial analysis of the patient’s humidifier, which precludes definitive identification of this potential environmental reservoir as the source of fungal exposure. Pulmonary infection caused by S. commune clinically presents with cough, followed by expectoration, and wheezing. Previous physical examinations have revealed that a small number of cases may show no clinical symptoms (4, 12, 14, 15). The patient in the present case had a subacute onset, with cough, sputum, and fever as the main symptoms, and hemoptysis.
Previous studies have shown that S. commune is one of the main causes of ABPM (6). When pulmonary S. commune infection manifests as ABPM, there is need to distinguish it from ABPA due to their similar clinical manifestations, chest imaging features and bronchoscopy findings. There is currently no standard treatment for pulmonary S. commune infection, with the present clinical treatment modalities mainly involving drug and physical therapy. The commonly used drugs include antifungals, glucocorticoids and expectorants. There is a challenge on which type of antifungal drugs to be selected, and the length of the course of treatment. At the moment, no standard modality exists for treatment of this condition. In vitro susceptibility studies demonstrate that itraconazole, voriconazole, posaconazole, and amphotericin B exhibit potent activity against S. commune (2). Clinical case reports indicate that itraconazole and voriconazole are the most commonly prescribed agents. While prednisone monotherapy has been reported to achieve lesion resolution in isolated cases (13), the absence of long-term follow-up data precludes definitive conclusions regarding its efficacy in eradicating S. commune. Furthermore, although inhaled corticosteroids (e.g., budesonide) may transiently alleviate symptoms, mycological clearance is not achieved, as evidenced by persistent positive cultures in treated patients (11). Physical therapy mainly includes bronchoscopy to remove phlegm plugs (16) and surgical resection of the lesions. Additional research evidence has shown that the overall prognosis of patients with pulmonary S. commune infection is good, with only one death (which was related to occurrence of serious complications and the patient’s resistance to voriconazole) reported so far (17). Reports have also shown that effective anti-infective treatment improves symptoms within 1–6 months, in most cases, the lesions may be completely absorbed, and some lesions will leave bronchiectasis (18, 19). We did not detect S. commune in the second BALF, and her condition improved after voriconazole treatment, showing bronchiectasis on imaging. While conventional clinical diagnosis for etiological identification primarily relies on microbial culture combined with microscopic examination, the absence of fungal colony isolation from the BALF specimen in the present case represents a notable diagnostic limitation. Pulmonary S. commune infection is a rare pulmonary fungal disease, and the clinical understanding of the disease is still lacking. Therefore, there is a need to actively improve bronchoscopy and etiological examination. The findings in the present case provides valuable insights on etiological detection of S. commune, and are expected to guide clinicians during diagnosis and treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CX: Writing – original draft, Writing – review and editing, Investigation. JtS: Writing – original draft, Writing – review and editing, Investigation. JS: Data curation, Writing – review and editing. JY: Data curation, Writing – review and editing. SZ: Validation, Writing – review and editing. JW: Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kamei K, Unno H, Nagao K, Kuriyama T, Nishimura K, Miyaji M. Allergic bronchopulmonary mycosis caused by the basidiomycetous fungus Schizophyllum commune. Clin Infect Dis. (1994) 18:305–9. doi: 10.1093/clinids/18.3.305
2. Chowdhary A, Randhawa H, Gaur S, Agarwal K, Kathuria S, Roy P, et al. Schizophyllum commune as an emerging fungal pathogen: A review and report of two cases. Mycoses. (2013) 56:1–10. doi: 10.1111/j.1439-0507.2012.02190.x
3. Tian L, Mu Y, Zhang H, Su X, Yang C, Shu X, et al. First report on cutaneous infectious granuloma caused by Schizophyllum commune. BMC Infect Dis. (2018) 18:286. doi: 10.1186/s12879-018-3187-5
4. Tullio V, Mandras N, Banche G, Allizond V, Gaido E, Roana J, et al. Schizophyllum commune: An unusual of agent bronchopneumonia in an immunocompromised patient. Med Mycol. (2008) 46:735–8. doi: 10.1080/13693780802256091
5. Sigler L, de la Maza L, Tan G, Egger K, Sherburne R. Diagnostic difficulties caused by a nonclamped Schizophyllum commune isolate in a case of fungus ball of the lung. J Clin Microbiol. (1995) 33:1979–83. doi: 10.1128/jcm.33.8.1979-1983.1995
6. Chowdhary A, Agarwal K, Kathuria S, Gaur S, Randhawa H, Meis J. Allergic bronchopulmonary mycosis due to fungi other than Aspergillus: A global overview. Crit Rev Microbiol. (2014) 40:30–48. doi: 10.3109/1040841X.2012.754401
7. Meyer K, Raghu G, Baughman R, Brown K, Costabel U, du Bois R, et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. (2012) 185:1004–14. doi: 10.1164/rccm.201202-0320ST
8. Chen H, Zheng Y, Zhang X, Liu S, Yin Y, Guo Y, et al. Clinical evaluation of cell-free and cellular metagenomic next-generation sequencing of infected body fluids. J Adv Res. (2024) 55:119–29. doi: 10.1016/j.jare.2023.02.018
9. Huang L, Xu S, Huang Z, Chen Y, Xu N, Xie B. Risk factors associated with Pneumocystis jirovecii pneumonia in non-HIV immunocompromised patients and co-pathogens analysis by metagenomic next-generation sequencing. BMC Pulm Med. (2023) 23:72. doi: 10.1186/s12890-022-02300-8
10. Kligman A. A basidiomycete probably causing onychomycosis. J Invest Dermatol. (1950) 14:67–70. doi: 10.1038/jid.1950.10
11. Ogawa H, Fujimura M, Takeuchi Y, Makimura K. Two cases of Schizophyllum asthma: Is this a new clinical entity or a precursor of ABPM? Pulm Pharmacol Ther. (2011) 24:559–62. doi: 10.1016/j.pupt.2011.04.030
12. Iizasa T, Kamei K, Chiyo M, Suzuki M, Baba M, Toyosaki T, et al. Colonization with Schizophyllum commune of localized honeycomb lung with mucus. Respiration. (2001) 68:201–3. doi: 10.1159/000050493
13. Kawayama T, Fujiki R, Rikimaru T, Aizawa H. Chronic eosinophilic pneumonia associated with Schizophyllum commune. Respirology. (2003) 8:529–31. doi: 10.1046/j.1440-1843.2003.00504.x
14. Bulajic N, Cvijanovic V, Vukojevic J, Tomic D, Johnson E. Schizophyllum commune associated with bronchogenous cyst. Mycoses. (2006) 49:343–5. doi: 10.1111/j.1439-0507.2006.01247.x
15. Roan J, Hsieh H, Tsai H, Wu C, Hsu C, Wu S, et al. Pulmonary nodules caused by Schizophyllum commune after cardiac transplantation. J Infect. (2009) 58:164–7. doi: 10.1016/j.jinf.2008.11.012
16. Ogawa H, Fujimura M, Takeuchi Y, Makimura K, Satoh K. The definitive diagnostic process and successful treatment for ABPM caused by Schizophyllum commune: A report of two cases. Allergol Int. (2012) 61:163–9. doi: 10.2332/allergolint.11-CR-0325
17. Chan J, Teng J, Li I, Wong S, Leung S, Ho P, et al. Fatal empyema thoracis caused by Schizophyllum commune with cross-reactive cryptococcal antigenemia. J Clin Microbiol. (2014) 52:683–7. doi: 10.1128/JCM.02770-13
18. Seki M, Ohno H, Gotoh K, Motooka D, Nakamura S, Iida T, et al. Allergic bronchopulmonary mycosis due to co-infection with Aspergillus fumigatus and Schizophyllum commune. IDCases. (2014) 1:5–8. doi: 10.1016/j.idcr.2014.01.001
Keywords: pulmonary infection, pulmonary mycosis, Schizophyllum commune, S. commune, allergic bronchopulmonary mycosis
Citation: Xue C, Shen J, Song J, Yang J, Zhang S and Wang J (2025) Pulmonary infection caused by Schizophyllum commune: a case study. Front. Med. 12:1475896. doi: 10.3389/fmed.2025.1475896
Received: 04 August 2024; Accepted: 26 May 2025;
Published: 16 June 2025.
Edited by:
Liang Zhao, Dalian University of Technology, ChinaReviewed by:
Mohamed Ismail Abdelwahab Hassan, University Hospital Jena, GermanyKoichi Tomoda, Kawasaki Medical University General Hospital, Japan
Copyright © 2025 Xue, Shen, Song, Yang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinxiang Wang, amlueGlhbmd3YW5nQGNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Chunxue Xue
Chunxue Xue Jiangtao Shen2†
Jiangtao Shen2† Jie Song
Jie Song Jinxiang Wang
Jinxiang Wang