Abstract
The incidence of myopia among school children has risen markedly over the last three decades. In urban areas of South and East Asia, as many as 80–90% of young adults are now myopic. This trend is occurring elsewhere around the world. During the COVID-19 lockdowns, children in many countries were confined indoors and spent an undue amount of time exposed to television screens, computers, and mobile devices. This resulted in an acceleration in the incidence and progression of the condition. Myopia is a significant public health issue as it is a leading cause of blindness and other vision problems. Yet the underlying mechanisms that produce the condition remain elusive. Pseudomyopia has recently been proposed as an independent risk factor for myopia. We hypothesize that pseudomyopia induced by prolonged close work, stress, and anxiety combines and is further amplified by chronically low ambient light levels. If time spent outdoors in daylight is restricted, the effects worsen and together may play a significant part in myopia epidemics.
1 Introduction
Myopia results from abnormal axial lengthening and other changes in the eye, causing light to focus in front of the retina rather than directly onto it, which leads to blurred distance vision. Notably, 2.5 billion people are living with myopia globally, and this figure is set to rise to 4.75 billion by 2050 (1). Increasingly, people with moderate myopia are developing high myopia. In such cases, elongation of the eye increases the risk of permanent visual loss through degenerative changes in the eye. Even mild to moderate myopia significantly increases the risk of such complications (2). If unaddressed, the global myopia epidemic will harm the physical (3) and psychological wellbeing (4), and the economic prospects (5) of much of the world's population.
Genetic and environmental factors are involved in myopia, although genetic change does not account for the rapid increases in prevalence during epidemics (6). Intensive education and limited time spent outdoors in daylight are generally recognized as the most critical contributors (7). Evidence for other risk factors investigated is weaker (8), and it is unclear how the mechanisms that may be involved combine to disrupt the normal growth of the eye (8). Spending time outdoors during the day has a protective effect for reasons that are not fully understood (9). The most significant factor appears to be that light outdoors is brighter than indoors and has a broader spectrum (8). Optical differences between indoors and outdoors may also play a part. Reduced accommodation due to more distance viewing outdoors, the pattern of retinal defocus generated outdoors, and a higher spatial frequency may account for some of it (2, 10).
The protective effect of bright light is widely attributed to the neurotransmitter dopamine. The dopamine hypothesis proposes that retinal dopamine release, stimulated by daylight exposure, inhibits axial elongation (11, 12). Blue light is being tested as a potential stimulant for retinal dopamine production (13) and has demonstrated a myopia-inhibiting effect (14). Violet light has done the same to a modest level (15, 16). Also, rather than specific wavelengths, some researchers are investigating the effectiveness of exposure to the entire visible spectrum in myopia treatment (17, 18). Meanwhile, red-light therapy has proven its effectiveness as a short-term treatment for myopia progression (19, 20), but there may be safety issues (21). Also, the long-term effects and safety of pharmacological interventions for myopia progression, such as atropine eye drops, are not clear. Nor are they for some of the contact and spectacle lenses designed to prevent progression (22). Another unresolved issue is whether daylight can slow down or stop myopia from progressing (23–25).
Until the underlying mechanisms are better understood, it will remain a challenge to develop fully effective strategies for myopia control (26). This paper begins by reviewing the background to an earlier hypothesis. This informs the one presented here which is that when both near work and emotional symptoms are present the pseudomyopia induced by them can combine and increase in strength. Furthermore, the effects may be amplified if this union occurs under low light levels. If children's access to daylight outdoors is also limited, the confluence of these risk factors could play a significant part in myopia onset and progression. The paper then examines the roles of pertinent risk factors in the onset and progression of myopia. A visualization of how these may interact and amplify the development of myopia is given in Figure 1.
Figure 1
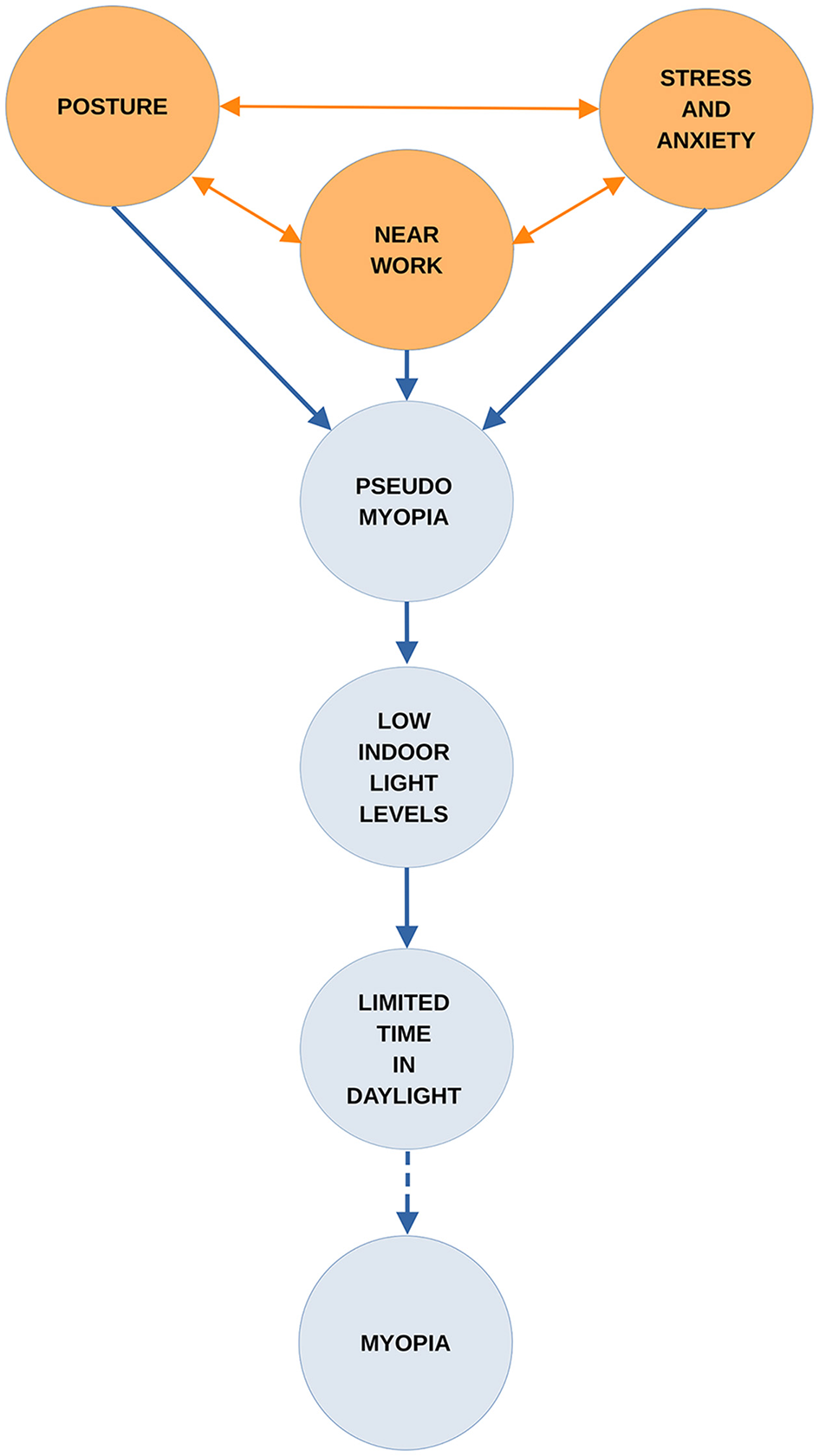
The combined effects of suggested factors leading to myopia.
2 Background
Over the last three decades, the prevalence of myopia in urban areas of South and East Asia has increased to the point where 80–90% of children leave school with the condition (27). Other regions of the world are following this trend. When populations move from rural to urban areas, and when the duration and competitiveness of their children's education increases, so does myopia (27). Urbanization is considered to be a potential risk factor (7, 8). Cohn first identified this in the 1860s. The evidence came from the findings of a landmark survey of the eyesight of over 10,000 Prussian school children (28).
Cohn found that children attending schools in towns had more myopia than children in comparable schools in rural areas. He also discovered that the longer children attended school the more likely they were to become myopic. Also, as the educational pressures placed on them increased, myopia became more common and more severe (29). In Prussia's academic High Schools, or Gymnasiums, the percentage of myopia went up steadily from the first year of attendance to the sixth. More than 50% of the children examined in their final year at these schools were myopic (29). Cohn also carried out the first investigation of the effects of classroom lighting on myopia by comparing the amount of daylight in classrooms with the number of myopic children studying in them. He concluded that schools with small windows, or overshadowed by adjacent buildings, had the highest rates. Those situated in the narrowest, darkest streets were the worst affected. Cohn also concluded, as others subsequently did (29), that reading and writing in dimly lit environment was one of the most important factors for myopia onset. Another was leaning forward while doing so. According to Cohn, an upright posture had a protective effect (29).
Cohn recommended good lighting in classrooms and exercise and recreation outdoors in daylight. Education departments arranged their schools for this for many years afterwards (30). In Britain, surveys conducted in London schools during the early 1900s found that girls were more susceptible to myopia than boys (31). This was attributed to the close work girls did in schools, especially needlework, and time spent indoors at home helping with domestic chores. Parents allowed boys to play outdoors in the city's streets but were reluctant to let girls do so (31). This difference in myopia prevalence between the sexes is still found in urban centers in some countries (32).
Other distinguished ophthalmologists, notably Fuchs and then Duke–Elder considered excessive near work to be the most crucial factor in the etiology of myopia (33, 34). They also appreciated the importance of proper illumination and exercise outdoors in daylight in preventing the condition. Fuchs and Duke–Elder also shared Cohn's concerns about the harm done to children by the excessive amount of study many had to undertake, both at school and afterwards at home. However, they were unsure about the mechanisms that caused myopia (33, 34). Cohn had argued that spasm of accommodation was the precursor of chronic myopia (29), but his theory was not accepted; nor does it appear to have been tested during his lifetime. In addition, neither Fuchs, Duke–Elder, nor Cohn directly addressed the influence of academic stress and anxiety on children's eyesight. This came later, in the 1940s, with the introduction of psychosomatic medicine to ophthalmology (35).
Cohn recognized that heredity played a part in the myopia that some children developed. However, in his judgement, this was not the underlying cause in most cases. Heredity did become the prevailing orthodoxy in the 1960s, following the publication of an influential study which asserted that myopia was almost entirely genetic in origin. This was taught to students for many years (36). One consequence was that designing schools for daylight was no longer a priority. Another was that the protective effect of daylight received little research attention (30).
During the COVID-19 pandemic, many countries used nationwide lockdowns as a quarantine measure. Children were confined in their homes and educated online. Exposure time to digital screens for virtual learning and recreation increased while outdoor activities were restricted. There are reports of an acceleration in myopia progression and incidence in children following the pandemic (37–40) and also in the psychological stress they experienced (41, 42). A more positive finding was the identification of pseudomyopia as an independent risk factor for myopia (43). Pseudomyopia, which is also known as transient myopia, ciliary spasm or, as Cohn referred to it, accommodation spasm can follow prolonged near work (44). It can also be caused by head injury and psychological stress, notably fear and anxiety (44, 45). If confirmed as a risk factor in future research, this would explain some of the rapid onset of myopia and its progression during pandemics. It would also assist in understanding how the etiology of myopia relates to daylight exposure, or lack of it.
3 Near work and myopia
Near work has long been regarded as a major risk factor for myopia. Johannes Kepler noted this in students 400 years ago (46). Others have argued the opposite: that near work has no influence on myopia (47) or that there is not enough evidence to prove it (48). Currently, many studies support the connection, but the literature is contradictory (49). For example, in 2021, the results of a study on primary students in Wenzhou, China, found that high levels of outdoor exposure had a marked influence by lowering the risk of myopia onset. Near work had none (50). However, the findings of a pilot study of the effects of learning to read and of sustained intensive near work at a very early age suggest the opposite. They may be strong enough to override the protective effects of time spent outdoors (51). Clinical trials are scarce because participants' adherence to study protocols and the monitoring of this is problematic. Nevertheless, the authors of a recent systematic review and meta-analysis of near work and myopia have concluded there is a statistically significant association, both in infants and adults (52). Meanwhile, some countries with high rates of myopia in children have measures in place to control near work (53).
4 Near-work-induced transient myopia
Near-work-induced transient myopia (NITM) is a common form of pseudomyopia. It is a short-term myopic shift in distance vision that occurs straight after prolonged near work (54). The shift, or delay in accommodation relaxation, can take place after a few minutes or periods of several hours. The interruption of near tasks can prevent it. People with myopia are more susceptible to NITM than people with normal vision. Also, those with progressing myopia have greater NITM with a longer decay time than people with stable myopia (55). In 2008, pseudomyopia, in the form of NITM, was identified as a possible component of myopia (56). It was suggested that there may be an additive process at work, with residual NITM contributing to the transition to permanent myopia (57). Clinical trials were proposed to investigate whether NITM is involved in the genesis of permanent myopia (57). Such trials do not appear to have been undertaken. One explanation is that there is no experimental proof that accommodation interacts with the emmetropisation process (58). However, it now seems that temporary myopia can become permanent. The study that discovered this began in Shandong province, China, in September 2020. It recruited a cohort of non-myopic children. A 6-month follow-up found that 21.1% of children with pseudomyopic eyes developed myopia. Of non-myopic and non-pseudomyopic, only 3.8% had developed myopia (43). The authors of this study identified pseudomyopia as an independent risk factor for the condition for the first time. But they also noted there is no evidence of a direct path from transient to permanent myopia (43).
5 Low illuminance and myopia
For many years, research into the effects of near work under low illuminance levels received limited attention and the idea that reading in dim light damages eyesight became unfashionable (59). Several studies have since been undertaken to determine how the human eye develops under low levels of light exposure. In 2012, one finding from the Beijing Childhood Eye Study was that low illumination during reading was associated with a higher prevalence and amount of myopia (60). Findings from The Sydney Adolescent Vascular and Eye Study (61) show that 6-year-old children with little exposure to daylight have a 5.2 times greater risk of developing myopia. Significantly, this could rise to as much as 15.9 times if they also perform close-up work. The control group for this longitudinal study, which had a 5-year follow-up, consisted of children who spent significant amounts of time outdoors and little time on near-vision activities. In 2015, a longitudinal study of myopic and non-myopic Australian children grouped them by their daytime light exposure, which was split into three exposure levels: low, medium, and high. There was significantly faster axial eye growth over time among children who experienced the lowest average daytime light exposure, below an average of 459 lux, compared to children who experienced higher levels (62).
Several studies support the theory of an association between light levels typically found indoors and myopia (63–66). Further evidence comes from reports of seasonal differences in myopia progression, with slower progression in summer (67–70). The results of a randomized control trial from 2015 in which the ambient lighting of refurbished classrooms was increased to 558 lux at the desk and 440 lux at the blackboard showed a marked effect. It reduced the prevalence of new-onset myopia in the intervention group to 4% compared to the control group's onset rate of 10%. Decreases in axial growth and refraction were also reported (71).
6 Digital eyestrain, posture, and myopia
Children using digital media during COVID-19 lockdowns may have been susceptible to both myopia and digital eye strain (DES) (72, 73). This is also variously called computer vision syndrome (CVS), visual fatigue (VF), and eye strain, and is part of asthenopia (74). The ocular symptoms of DES include blurred vision from accommodative strain, dry eyes, red eyes, altered blinking characteristics, eye pain, and headache (75–77). Musculoskeletal disorders, such as neck and shoulder pain, are also among the symptoms of DES. These are caused by postural problems, poor ergonomics, and work practices (74). An inadequate posture may also cause myopia, as Cohn argued in his book Hygiene of the Eye in Schools. This states that the adverse effects of a bad posture in children are spinal curvature and shortsight. The latter results from continuous stooping of the head when looking at near objects (29).
Postural changes that activate the neck muscles are known to affect the eye. One study has found that reading with the head tilted forward and the neck at an angle of 45° increases both intraocular pressure and NITM compared to reading with the head held upright (78). Additionally, there is evidence that people with existing neck and shoulder symptoms are more susceptible to eye discomfort when performing near work. This is related to the accommodative demands on their eyes (79). Adopting an upright posture when performing near work reduces the risk of fatiguing the upper trapezius muscle in the neck, which can otherwise lead to neck and shoulder pain (80). However, if there is eye strain, and the eye's ciliary muscle is forced during prolonged near work, this can activate trapezius muscle activity and fatigue it (81, 82). To compound the problem, low-level and acute stress can trigger the trapezius muscle (83, 84). Fortunately, research suggests that in addition to NITM, an upright posture may improve resilience to stress and reduce depression and anxiety (85, 86).
7 Anxiety, psychological stress, and myopia
Depression and psychological stress are associated with several ophthalmic conditions, such as dry eye disease and glaucoma (87, 88). The most common mental health illness associated with pseudomyopia is generalized anxiety disorder (GAD) (89). Pseudomyopia can also manifest following both chronic and acute stress. During World War II, a Medical Officer in the United States Navy reported that ciliary spasms had significantly increased during wartime. This was due to emotional trauma and cost the United States Army and Navy many thousands of man-hours (90). In 1959, the author of a review of 21 cases of pseudomyopia concluded they were brought on by sheer fright. Or, taking on a challenging task in which failure would entail a loss of face (45). Pseudomyopia is also reported to have developed among 30% of residents whose sight was tested following an earthquake in Armenia in 1988 (87, 91).
Increased anxiety levels are common among adolescents and students with myopia. As the degree of myopia increases, so do the anxiety levels (92, 93). Anxiety and stress are also associated with the prolonged use of digital screens (94). A Canadian study has found that the increase in digital media use and TV viewing that occurred during their lockdown was linked to symptoms of anxiety and depression in children and adolescents (95). Prior to COVID-19, anxiety was already common among children and especially those in highly competitive learning environments (96). This is not a new phenomenon. In the 1940s, pseudomyopia was referred to as a relatively common ophthalmic condition, especially among school children, and there was an interaction between near work, stress, and anxiety:
“A typical example of ciliary spasm is seen in the young student who is on the verge of failure in school. Because of his poor scholastic ability he is forced to spend more than the average amount of time in study. His anxiety over his incipient failure causes him to work under more and more pressure until a vicious circle of more reading and greater anxiety results in a spastic myopia of greater or less degree” (97).
8 Educational styles and children's eyesight
A Japanese study published in 2021 suggests that when the academic burden on school children is reduced, it prevents eye damage. Japan has a highly competitive education system. A culture of exam hell is reported to have been problematic in Japanese schools, and in 2002, a less intensive school curriculum was introduced in Japan to try to address this (98).
The so-called Yutori educational policy reformed Japan's first 9 years of compulsory education. It created a more relaxed learning environment, which dispensed with Saturday classes and provided more opportunities for outdoor play (98). The Yutori policy remained in place until 2012, when a more intensive academic system was reintroduced. A retrospective observational study found that myopia progression and an increased prevalence of high myopia occurred only when high-pressure education was in place and not under the Yutori system (98).
Some countries, such as Australia, have maintained high academic standards with lower rates of myopia among school children than those reported elsewhere (99). In Australia, this has been attributed to the lifestyle and educational system (100). Another notable example is Norway, where a study of 16–19-year-old Norwegians found 13% of them were affected by myopia (101). Why myopia is less common in Norway than in many other countries is unclear at present. One factor is that in Norway, young children are outdoors for long periods. According to a survey of Norwegian kindergartens, children spend more than two-thirds of their time outside during the summer and about a third of the winter semester. Norway's kindergartens are designed for outdoor play, and no targets are set for children as they progress toward readiness for school (102). Preparation for academic learning is limited (103).
9 Discussion
The epidemic of myopia that occurred during COVID-19 is not without precedent. A notable outbreak occurred in Canada during the 1960s when Inuit children were taken from their families and forced to attend boarding schools. There followed an epidemic of myopia, which at the time was believed to be genetic in origin. Research was later published which, proposed the school environment as a significant factor in this epidemic and not genetics (104).
A recent paper has revisited the myopia epidemic, and the authors proposed that the removal of First Nation children from a traditional, open-air way of life to one of enforced near work indoors, under bad lighting, and a lack of time outdoors in daylight contributed to the sudden increase in myopia (105). Another component of the epidemic may have been the extreme psychological and physical distress these children experienced in residential schools (105).
It is tempting to speculate that this epidemic occurred because many of the factors proposed in this paper were present and may have combined to produce it. But it is not evident how in more favorable circumstances daylight outdoors would have made the transient myopia in these children disappear and emmetropia return. To do so it would have to achieve two things simultaneously: alleviate the adverse effects of near work on accommodation as well as those from stress and anxiety.
Some of the protective effects of daylight on vision could have a psychological basis. Daylight can improve mental health (106). A lack of it can disrupt the body's circadian rhythms and lead to sleep disorders, depression, and anxiety. If this disruption occurs, it could also trigger circadian dysregulation in the eye, which is a potential risk factor for myopia (107). In addition, the dopamine that entrains intrinsic retinal circadian rhythms can also affect mood, suggesting an interaction (108). If there is one, this would further support the hypothesis that dopamine is central to daylight's protective effect.
Another possible explanation is that just being outdoors—away from the classroom, playing with friends, viewing distant objects, and maintaining an upright posture—may be sufficient to reduce stress and anxiety levels. This hypothesis would not be difficult to assess. However, the part played by postural alignment in myopia may be. There has been little research on the different effects of slumped and upright postures on emotions (86). Equally, it has been recognized for some time that the relationship between the musculoskeletal system and that of accommodation may be bidirectional (109). Other pathways that lead to myopia may share this characteristic. There may also be interactions between them, which could become disrupted should the demands placed upon them become excessive. Daylight's protective effect may be to instigate processes that prevent these interactions.
While that requires further investigation, research is beginning to identify what occurs when several risk factors for myopia are present and access to daylight is limited. Following COVID-19 lockdowns in China, the cumulative effects of some protective measures were found to have been more significant than those from each one taken individually (110). The findings suggest that having good illumination and an upright posture when reading, resting the eyes, adequate sleep, spending time outdoors, regular exercise, and a nutritious diet can significantly reduce myopia progression and incidence in children. This highlights the importance of examining risk factors in combination rather than in isolation.
Some of the most pressing research questions concern the education of children and students. Finding a way of teaching them both at school and at home without damaging their vision must be a priority. There may be a technological solution to this that waits to be discovered. In the meantime, parents and teachers should be made aware of the measures that can protect children's vision and the importance of doing so. In some cultures, there is a belief that allowing children more time outdoors will adversely affect their education (53). It is noteworthy that the Norwegian education system places a strong emphasis on children's overall wellbeing (103) and, although not designed to do so, protects young children from myopia, which others do not. This should be investigated. Outdoor learning and the oral tradition of teaching may have much to commend them where eyesight is concerned (111). It would also be helpful if some of the terms used in myopia research, such as DES and near work, were more clearly defined.
The hypothesis presented in this paper sets out a pathway through which several mechanisms may combine and increase the risk of myopia onset and progression, via pseudomyopia. There is now some support for key elements of it, such as the transition to myopia from pseudomyopia and the additive nature of risk factors involved. While testing the hypothesis presented here should be possible, identifying which specific aspects of outdoor daylight exposure prevent the onset and progression from pseudomyopia to myopia would be more challenging, particularly because these factors may be interacting simultaneously.
A significant limitation of this study is that, to date, there is not enough scientific evidence in the literature to perform a systematic review and a meta-analysis, which would support or refute our hypothesis. Some of our conclusions are drawn from the observations of historical figures who were working at different times, following different protocols. In several cases, the research on which their observations and recommendations were based was never validated. However, there would appear to be sufficient evidence to suggest this hypothesis merits further investigation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RH: Writing – original draft, Writing – review & editing. MA: Conceptualization, Writing – review & editing. CC: Writing – review & editing. LM: Writing – review & editing. MM: Writing – review & editing. WO: Writing – review & editing. OS: Writing – review & editing. KW: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Daylight Academy (https://daylight.academy/) financially supported the workshops for the interdisciplinary knowledge exchange among the authors but were not involved in the process and had no influence on the content.
Acknowledgments
We would like to thank the Daylight Academy (https://daylight.academy/) for funding the workshops and subsequent interdisciplinary knowledge exchange. The authors would like to thank Professor Manuel Spitschan for reviewing earlier versions of the manuscript and for participating in the DLA workshop meetings.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Holden BA Fricke TR Wilson DA Jong M Naidoo KS Sankaridurg P et al . Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. (2016) 5:1036–42. 10.1016/j.ophtha.2016.01.006
2.
Flitcroft DI . The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. (2012) 6:622–60. 10.1016/j.preteyeres.2012.06.004
3.
Haarman AEG Enthoven CA Tideman JWL Tedja MS Verhoeven VJM Klaver CCW . The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. (2020) 4:49. 10.1167/iovs.61.4.49
4.
Li Q Yang J He Y Wang T Zhong L Zhu Z et al . Investigation of the psychological health of first-year high school students with myopia in Guangzhou. Brain Behav. (2020) 4:e01594. 10.1002/brb3.1594
5.
Naidoo KS Fricke TR Frick KD Jong M Naduvilath TJ Resnikoff S et al . Potential Lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. (2019) 3:338–46. 10.1016/j.ophtha.2018.10.029
6.
Morgan IG French AN Ashby RS Guo X Ding X He M et al . The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. (2018) 62:134–49. 10.1016/j.preteyeres.2017.09.004
7.
Morgan IG Wu PC Ostrin LA Tideman JWL Yam JC Lan W et al . IMI Risk factors for myopia. Invest Ophthalmol Vis Sci. (2021) 5:3. 10.1167/iovs.62.5.3
8.
Biswas S El Kareh A Qureshi M Lee DMX Sun CH Lam J et al . The influence of the environment and lifestyle on myopia. J Physiol Anthropol. (2024) 1:7. 10.1186/s40101-024-00354-7
9.
Lingham G Mackey DA Lucas R Yazar S . How does spending time outdoors protect against myopia? A review. Br J Ophthalmol. (2020) 5:593–9. 10.1136/bjophthalmol-2019-314675
10.
Flitcroft DI Harb EN Wildsoet CF . The spatial frequency content of urban and indoor environments as a potential risk factor for myopia development. Invest Ophthalmol Vis Sci. (2020) 11:42. 10.1167/iovs.61.11.42
11.
Brown DM Mazade R Clarkson-Townsend D Hogan K Datta Roy PM Pardue MT . Candidate pathways for retina to scleral signaling in refractive eye growth. Exp Eye Res. (2022) 219:109071. 10.1016/j.exer.2022.109071
12.
Zhou X Pardue MT Iuvone PM Qu J . Dopamine signaling and myopia development: What are the key challenges. Prog Retin Eye Res. (2017) 61:60–71. 10.1016/j.preteyeres.2017.06.003
13.
Carpena-Torres C Schilling T Huete-Toral F Bahmani H Carracedo G . Increased ocular dopamine levels in rabbits after blue light stimulation of the optic nerve head. Exp Eye Res. (2023) 234:109604. 10.1016/j.exer.2023.109604
14.
Thakur S Dhakal R Verkicharla PK . Short-term exposure to blue light shows an inhibitory effect on axial elongation in human eyes independent of defocus. Invest Ophthalmol Vis Sci. (2021) 15:22. 10.1167/iovs.62.15.22
15.
Jeong H Kurihara T Jiang X Kondo S Ueno Y Hayashi Y et al . Suppressive effects of violet light transmission on myopia progression in a mouse model of lens-induced myopia. Exp Eye Res. (2023) 228:109414. 10.1016/j.exer.2023.109414
16.
Torii H Kurihara T Seko Y Negishi K Ohnuma K Inaba T et al . Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. (2017) 15:210–9. 10.1016/j.ebiom.2016.12.007
17.
Muralidharan AR Low SWY Lee YC Barathi VA Saw SM Milea D et al . Recovery From form-deprivation myopia in chicks is dependent upon the fullness and correlated color temperature of the light spectrum. Invest Ophthalmol Vis Sci. (2022) 2:16. 10.1167/iovs.63.2.16
18.
Swiatczak B . Chromatic light therapy for inhibiting myopia progression: human studies. Klin Monbl Augenheilkd. (2024) 241:1126–8. 10.1055/a-2322-9892
19.
Zhou L Tong L Li Y Williams BT Qiu K . Photobiomodulation therapy retarded axial length growth in children with myopia: evidence from a 12-month randomized controlled trial evidence. Sci Rep. (2023) 13:3321. 10.1038/s41598-023-30500-7
20.
Cao K Tian L Ma DL Zhao SQ Li A Jin ZB Jie Y . Daily low-level red light for spherical equivalent error and axial length in children with myopia: a randomized clinical trial. JAMA Ophthalmol. (2024) 6:560–7. 10.1001/jamaophthalmol.2024.0801
21.
Ostrin LA Schill AW . Red light instruments for myopia exceed safety limits. Ophthalmic Physiol Opt. (2024) 2:241–8. 10.1111/opo.13272
22.
Lawrenson JG Shah R Huntjens B Downie LE Virgili G Dhakal R et al . Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database Syst Rev. (2023) 2:CD014758. 10.1002/14651858.CD014758.pub2
23.
Li D Min S Li X . Is spending more time outdoors able to prevent and control myopia in children and adolescents? A meta-analysis. Ophthalmic Res. (2024) 1:393–404. 10.1159/000539229
24.
Kido A Miyake M Watanabe N . Interventions to increase time spent outdoors for preventing incidence and progression of myopia in children. Cochrane Database Syst Rev. (2024) 6:CD013549. 10.1002/14651858.CD013549.pub2
25.
Eppenberger LS Sturm V . The role of time exposed to outdoor light for myopia prevalence and progression: a literature review. Clin Ophthalmol. (2020) 14:1875–90. 10.2147/OPTH.S245192
26.
Muralidharan AR Lança C Biswas S Barathi VA Wan Yu Shermaine L et al . Light and myopia: from epidemiological studies to neurobiological mechanisms. Ther Adv Ophthalmol. (2021) 13:25158414211059246. 10.1177/25158414211059246
27.
Liang J Pu Y Chen J Liu M Ouyang B Jin Z et al . Global prevalence, trend and projection of myopia in children and adolescents from 1990 to 2050: a comprehensive systematic review and meta-analysis. Br J Ophthalmol. (2024) 2024:bjo-2024-325427. 10.1136/bjo-2024-325427
28.
Cohn H . Untersuchungen der Augen von 10,060 Schulkindern nebst Vorschlagen zur Verbesserung der den Augen nachtheiligen Schuleinrichtungen. In:FleischerF, editor. Eine atiologische Studie Leipzig.Leipzig (1867).
29.
Cohn H . Hygiene of the Eye in Schools.London: Simpkin, Marshall and Co. (1886).
30.
Hobday R . Myopia and daylight in schools: a neglected aspect of public health?Perspect Public Health. (2016) 1:50–5. 10.1177/1757913915576679
31.
Harman NB . The effects of school life upon the vision of the child. Proc R Soc Med. (1909) 2:206–16. 10.1177/003591570900201485
32.
Rai BB Ashby RS French AN Maddess T . Rural-urban differences in myopia prevalence among myopes presenting to Bhutanese retinal clinical services: a 3-year national study. Graefes Arch Clin Exp Ophthalmol. (2021) 259:613–21. 10.1007/s00417-020-04891-6
33.
Fuchs E . The Textbook of Ophthalmology (Second Edition). New York: D Appleton & Company. (1901).
34.
Duke-Elder S . The Practice Refraction (Second Edition) London: J & A Churchill Ltd. (1935).
35.
Hartmann E . Psychosomatic phenomena in ophthalmology. Br J Ophthalmol. (1949) 8:461–76. 10.1136/bjo.33.8.461
36.
Morgan IG Rose KA . Myopia: is the nature-nurture debate finally over?Clin Exp Optom. (2018) 102:3–17. 10.1111/cxo.12845
37.
Laan D Tan ETC Huis In Het V . Myopia progression in children during home confinement in the COVID-19 pandemic: a systematic review and meta-analysis. J Optom. (2024) 1:100493. 10.1016/j.optom.2023.100493
38.
Ma M Xiong S Zhao S Zheng Z Sun T Li C et al . COVID-19 home quarantine accelerated the progression of myopia in children aged 7 to 12 years in China. Invest Ophthalmol Vis Sci. (2021) 10:37. 10.1167/iovs.62.10.37
39.
Yang Z Wang X Zhang S Ye H Chen Y Xia Y . Pediatric myopia progression during the COVID-19 pandemic home quarantine and the risk factors: a systematic review and meta-analysis. Front Public Health. (2022) 10:835449. 10.3389/fpubh.2022.835449
40.
Guo C Li Y Luo L Lin J Qiu K Zhang M . Progression and incidence of myopia among schoolchildren in the post- COVID-19 pandemic period: a prospective cohort study in Shantou, China. BMJ Open. (2023) 8:e074548. 10.1136/bmjopen-2023-074548
41.
Harrison L Carducci B Klein JD Bhutta ZA . Indirect effects of COVID-19 on child and adolescent mental health: an overview of systematic reviews. BMJ Glob Health. (2022) 12:e010713. 10.1136/bmjgh-2022-010713
42.
Panchal U Salazar de Pablo G Franco M Moreno C Parellada M Arango C et al . The impact of COVID-19 lockdown on child and adolescent mental health: systematic review. Eur Child Adolesc Psychiatry. (2023) 7:1151–77. 10.1007/s00787-021-01856-w
43.
Sun W Yu M Wu J . Han X, Han X, Jan C, Song J, et al. Pseudomyopia as an independent risk factor for myopia onset: a prospective cohort study among school-aged children. Br J Ophthalmol. (2024) 6:873–8. 10.1136/bjo-2022-322330
44.
García-Montero M Felipe-Márquez G Arriola-Villalobos P Garzón N . Pseudomyopia: A review. Vision (Basel). (2022) 1:17. 10.3390/vision6010017
45.
Savin LH . Functional spasm of accommodation. Br J Ophthalmol. (1959) 43:3–8. 10.1136/bjo.43.1.3
46.
Mark HH . Johannes Kepler on the eye and vision. Am J Ophthalmol. (1971) 72:869–78. 10.1016/0002-9394(71)91682-5
47.
Sorsby A Benjamin B . Modes of inheritance of errors of refraction. J Med Genet. (1973) 2:161–4. 10.1136/jmg.10.2.161
48.
Mutti DO Zadnik K . Has near work's star fallen?Optom Vis Sci. (2009) 86:76–78. 10.1097/OPX.0b013e31819974ae
49.
Gajjar S Ostrin LA A . systematic review of near work and myopia: measurement, relationships, mechanisms and clinical corollaries. Acta Ophthalmol. (2022) 4:376–87. 10.1111/aos.15043
50.
Jiang D Lin H Li C Liu L Xiao H Lin Y et al . Longitudinal association between myopia and parental myopia and outdoor time among students in Wenzhou: a 25-year longitudinal cohort study. BMC Ophthalmol. (2021) 1:11. 10.1186/s12886-020-01763-9
51.
Gordon-Shaag A Shneor E Doron R Levine J Ostrin LA . Environmental and behavioral factors with refractive error in Israeli boys. Optom Vis Sci. (2021) 8:959–70. 10.1097/OPX.0000000000001755
52.
Dutheil F Oueslati T Delamarre L Castanon J Maurin C Chiambaretta F et al . Myopia and near work: a systematic review and meta-analysis. Int J Environ Res Public Health. (2023) 1:875. 10.3390/ijerph20010875
53.
Jan C Li L Keay L Stafford RS Congdon N Morgan I . Prevention of myopia, China. Bull World Health Organ. (2020) 6:435–7. 10.2471/BLT.19.240903
54.
Ong E Ciuffreda KJ . Nearwork-induced transient myopia. A critical review. Doc Ophthalmol. (1995) 91:57–85. 10.1007/BF01204624
55.
Sivaraman V Rizwana JH Ramani K Price H Calver R Pardhan S et al . Near work- induced transient myopia in Indian subjects. Clin Exp Optom. (2015) 6:541–6. 10.1111/cxo.12306
56.
Vasudevan B Ciuffreda KJ . Additivity of near work–induced transient myopia and its decay characteristics in different refractive groups. Investig Opthalmol Vis Sci. (2008) 49:836–41. 10.1167/iovs.07-0197
57.
Ciuffreda KJ Vasudevan B . Nearwork-induced transient myopia (NITM) and permanent myopia–is there a link?Ophthalmic Physiol Opt. (2008) 2:103–14. 10.1111/j.1475-1313.2008.00550.x
58.
Schaeffel F Swiatczak B . Mechanisms of emmetropization and what might go wrong in myopia. Vision Res. (2024) 220:108402. 10.1016/j.visres.2024.108402
59.
Vreeman RC Carroll AE . Medical myths. BMJ. (2007) 7633:1288–9. 10.1136/bmj.39420.420370.25
60.
You QS Wu LJ Duan JL Luo YX Liu LJ Li X et al . Factors associated with myopia in school children in China: the Beijing childhood eye study. PLOS One. (2012) 7:e52668. 10.1371/journal.pone.0052668
61.
French AN Morgan IG Mitchell P Rose KA . Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. (2013) 10:2100–8. 10.1016/j.ophtha.2013.02.035
62.
Read SA Collins MJ Vincent SJ . Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci. (2015) 11:6779–87. 10.1167/iovs.14-15978
63.
Landis EG Yang V Brown DM Pardue MT Read SA . Dim light exposure and myopia in children. Invest Ophthalmol Vis Sci. (2018) 12:4804–11. 10.1167/iovs.18-24415
64.
Landis EG Park HN Chrenek M He L Sidhu C Chakraborty R et al . Ambient light regulates retinal dopamine signaling and myopia susceptibility. Invest Ophthalmol Vis Sci. (2021) 1:28. 10.1167/iovs.62.1.28
65.
Irigaraya LF Bernateneb J Szepsc A Albertazzid R Cortíneze F Lanca C et al . Children's bedroom illumination while reading at night. Oftalmología Clínica y Experimental. (2024) 4:e516–e522. 10.70313/2718.7446.v17.n04.362
66.
Cohen Y Iribarren R Ben-Eli H Massarwa A Shama-Bakri N Chassid O . Light intensity in nursery schools: a possible factor in refractive development. Asia Pac J Ophthalmol (Phila). (2022) 1:66–71. 10.1097/APO.0000000000000474
67.
Donovan L Sankaridurg P Ho A Chen X Lin Z Thomas V et al . Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci. (2012) 8:1196–202. 10.1097/OPX.0b013e3182640996
68.
Gwiazda J Deng L Manny R Norton TT the CLEERE Study Group . Seasonal variations in the progression of myopia in children enrolled in the correction of myopia evaluation trial. Invest Ophthalmol Vis Sci. (2014) 55:752–8. 10.1167/iovs.13-13029
69.
Cui D Trier K Munk Ribel-Madsen S . Effect of day length on eye growth, myopia progression, and change of corneal power in myopic children. Ophthalmology. (2013) 120:1074–9. 10.1016/j.ophtha.2012.10.022
70.
Rusnak S Salcman V Hecova L Kasl Z . Myopia progression risk: seasonal and lifestyle variations in axial length growth in Czech children. J Ophthalmol. (2018) 2018:5076454. 10.1155/2018/5076454
71.
Hua WJ Jin JX Wu XY Yang JW Jiang X Gao GP et al . Elevated light levels in schools have a protective effect on myopia. Ophthalmic Physiol Opt. (2015) 35:252–62. 10.1111/opo.12207
72.
Ganne P Najeeb S Chaitanya G Sharma A Krishnappa NC . Digital eye strain epidemic amid COVID-19 pandemic – a cross-sectional survey. Ophthalmic Epidemiol. (2021) 28:285–92. 10.1080/09286586.2020.1862243
73.
Lotfy NM Shafik HM Nassief M . Risk factor assessment of digital eye strain during the COVID-19 pandemic: a cross-sectional survey. Med Hypothesis Discov Innov Ophthalmol. (2022) 3:119–28. 10.51329/mehdiophthal1455
74.
Mylona I Glynatsis MN Floros GD Kandarakis S . Spotlight on digital eye strain. Clin Optom (Auckl). (2023) 15:29–36. 10.2147/OPTO.S389114
75.
Rosenfield M . Computer vision syndrome: a review of ocular causes and potential treatments. Ophthalmic Physiol Opt. (2011) 5:502–15. 10.1111/j.1475-1313.2011.00834.x
76.
Sheppard AL Wolffsohn JS . Digital eye strain: prevalence, measurement and amelioration. BMJ Open Ophthalmol. (2018) 3:e000146. 10.1136/bmjophth-2018-000146
77.
Pucker AD Kerr AM Sanderson J Lievens C . Digital eye strain: updated perspectives. Clin Optom (Auckl). (2024) 16:233–246. 10.2147/OPTO.S412382
78.
Liang X Wei S Ming L Zhao S Zhang Y Wang N . The impact of different postures on acute intraocular pressure and accommodation responses during reading. BMC Ophthalmology. (2024) 24:405. 10.1186/s12886-024-03675-4
79.
Zetterberg C Forsman M Richter HO . Neck/shoulder discomfort due to visually demanding experimental near work is influenced by previous neck pain, task duration, astigmatism, internal eye discomfort and accommodation. PLoS ONE. (2017) 8:e0182439. 10.1371/journal.pone.0182439
80.
Lee H Lee Y . Effects of postural changes using a standing desk on the craniovertebral angle, muscle fatigue, work performance, and discomfort in individuals with a forward head posture. Healthcare (Basel). (2024) 23:2436. 10.3390/healthcare12232436
81.
Zetterberg C Forsman M Richter HO . Effects of visually demanding near work on trapezius muscle activity. J Electromyogr Kinesiol. (2013) 23:1190–8. 10.1016/j.jelekin.2013.06.003
82.
Domkin D Forsman M Richter HO . Effect of ciliary-muscle contraction force on trapezius muscle activity during computer mouse work. Eur J Appl Physiol. (2019) 2:389–97. 10.1007/s00421-018-4031-8
83.
Nilsen KB Sand T Stovner LJ Leistad RB Westgaard RH . Autonomic and muscular responses and recovery to one-hour laboratory mental stress in healthy subjects. BMC Musculoskelet Disord. (2007) 8:81. 10.1186/1471-2474-8-81
84.
Marker RJ Campeau S Maluf KS . Psychosocial stress alters the strength of reticulospinal input to the human upper trapezius. J Neurophysiol. (2017) 1:457–66. 10.1152/jn.00448.2016
85.
Wilkes C Kydd R Sagar M Broadbent E . Upright posture improves affect and fatigue in people with depressive symptoms. J Behav Ther Exp Psychiatry. (2017) 54:143–9. 10.1016/j.jbtep.2016.07.015
86.
Nair S Sagar M Sollers J 3rd Consedine N Broadbent E . Do slumped and upright postures affect stress responses? A randomized trial. Health Psychol. (2015) 34:632–41. 10.1037/hea0000146
87.
Sabel BA Wang J Cárdenas-Morales L Faiq M Heim C . Mental stress as consequence and cause of vision loss: the dawn of psychosomatic ophthalmology for preventive and personalized medicine. EPMA J. (2018) 2:133–60. 10.1007/s13167-018-0136-8
88.
Mamtani NH Mamtani HG Chaturvedi SK . Psychiatric aspects of ophthalmic disorders: a narrative review. Indian J Ophthalmol. (2023) 71:1810–5. 10.4103/ijo.IJO_2101_22
89.
Khalid K Padda J Pokhriyal S Hitawala G Khan MS Upadhyay P et al . Pseudomyopia and its association with anxiety. Cureus. (2021) 8:e17411. 10.7759/cureus.17411
90.
Harrington D . Wartime ocular neuroses. J Nerv Ment Dis. (1944) 99:622–30. 10.1097/00005053-194405000-00018
91.
Avetisov ES Gundorova RA Shakarian AA Oganesian AA . Vliianie ostrogo psikhogennogo stressa na sostaianie nekotorykh funktsii zritel'nogo analizatora [Effects of acute psychogenic stress on the state of several functions of the visual analyzer]. Vestn Oftalmol. (1991) 107:17–9.
92.
Nitzan I Shmueli O Safir M . Association of myopia with anxiety and mood disorders in adolescents. Eye (Lond). (2024) 38:3016–8. 10.1038/s41433-024-03170-6
93.
Zhang H Gao H Zhu Y Zhu Y Dang W Wei R et al . Relationship between myopia and other risk factors with anxiety and depression among chinese university freshmen during the COVID-19 pandemic. Front Public Health. (2021) 9:774237. 10.3389/fpubh.2021.774237
94.
Lissak G . Adverse physiological and psychological effects of screen time on children and adolescents: literature review and case study. Environ Res. (2018) 164:149–57. 10.1016/j.envres.2018.01.015
95.
Li X Vanderloo LM Keown-Stoneman CDG Cost KT Charach A Maguire JL et al . Screen use and mental health symptoms in canadian children and youth during the COVID-19 pandemic. JAMA Netw Open. (2021) 4:e2140875. 10.1001/jamanetworkopen.2021.40875
96.
Caldwell DM Davies SR Hetrick SE Palmer JC Caro P López-López JA et al . School-based interventions to prevent anxiety and depression in children and young people: a systematic review and network meta-analysis. Lancet Psychiatry. (2019) 12:1011–20. 10.1016/S2215-0366(19)30403-1
97.
Harrington DO . Psychosomatic interrelationships in ophthalmology. Am J Ophthalmol. (1948) 31:1241–51. 10.1016/0002-9394(48)91014-9
98.
Ishiko S Kagokawa H Nishikawa N Song Y Sugawara K Nakagawa H et al . Impact of the pressure-free Yutori Education Program on myopia in Japan. J Clin Med. (2021) 18:4229. 10.3390/jcm10184229
99.
Fu A Watt KM Junghans B Delaveris A Stapleton F . Prevalence of myopia among disadvantaged Australian schoolchildren: A 5-year cross-sectional study. PLoS ONE. (2020) 8:e0238122. 10.1371/journal.pone.0238122
100.
Junghans BM Crewther SG . Little evidence for an epidemic of myopia in Australian primary school children over the last 30 years. BMC Ophthalmol. (2005) 5:1. 10.1186/1471-2415-5-1
101.
Hagen LA Gjelle JVB Arnegard S Pedersen HR Gilson SJ Baraas RC . Prevalence and possible factors of myopia in Norwegian adolescents. Sci Rep. (2018) 1:13479. 10.1038/s41598-018-31790-y
102.
Moser T Martinsen MT . The outdoor environment in Norwegian kindergartens as pedagogical space for toddlers' play, learning and development. EECERJ. (2010) 4:457–71. 10.1080/1350293X.2010.525931
103.
Lenes R Gonzales CR Størksen I McClelland MM . Children's self-regulation in Norway and the United States: the role of mother's education and child gender across cultural contexts. Front Psychol. (2020) 11:566208. 10.3389/fpsyg.2020.566208
104.
Morgan RW Speakman JS Grimshaw SE . Inuit myopia: an environmentally induced “epidemic”?Can Med Assoc J. (1975) 5:575–7.
105.
Rozema JJ Boulet C Cohen Y Stell WK Iribarren L van Rens GHMB et al . Reappraisal of the historical myopia epidemic in native Arctic communities. Ophthalmic Physiol Opt. (2021) 6:1332–45. 10.1111/opo.12879
106.
Bertani DE De Novellis AMP Farina R Latella E Meloni M Scala C et al . “Shedding light on light”: a review on the effects on mental health of exposure to optical radiation. Int J Environ Res Public Health. (2021) 4:1670. 10.3390/ijerph18041670
107.
Chakraborty R Ostrin LA Nickla DL Iuvone PM Pardue MT Stone RA . Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt. (2018) 3:217–45. 10.1111/opo.12453
108.
Cawley EI Park S aan het Rot M Sancton K Benkelfat C Young SN et al . Dopamine and light: dissecting effects on mood and motivational states in women with subsyndromal seasonal affective disorder. J Psychiatry Neurosci. (2013) 6:388–97. 10.1503/jpn.120181
109.
Richter HO . Neck pain brought into focus. Work. (2014) 47:413–8. 10.3233/WOR-131776
110.
Dong Y Jan C Chen L Ma T Liu J Zhang Y et al . The cumulative effect of multilevel factors on myopia prevalence, incidence, and progression among children and adolescents in china during the COVID-19 pandemic. Transl Vis Sci Technol. (2022) 12:9. 10.1167/tvst.11.12.9
111.
Hobday R . Outdoor Learning and Children's Eyesight. In:JuckerRvon AuJ, editor. High-Quality Outdoor Learning. Cham: Springer. (2022).
Summary
Keywords
pseudomyopia, near work, anxiety, posture, daylight, intensive education
Citation
Hobday R, Aarts M, Cajochen C, Maierova L, Münch M, Osterhaus W, Stefani O and Wulff K (2025) Myopia and daylight—A combination of factors. Front. Med. 12:1481209. doi: 10.3389/fmed.2025.1481209
Received
23 October 2024
Accepted
14 May 2025
Published
02 July 2025
Volume
12 - 2025
Edited by
Majid Moshirfar, University of Utah, United States
Reviewed by
Annegret Dahlmann-Noor, Moorfields Eye Hospital NHS Foundation Trust, United Kingdom
Bhim Bahadur Rai, Australian National University, Australia
Updates
Copyright
© 2025 Hobday, Aarts, Cajochen, Maierova, Münch, Osterhaus, Stefani and Wulff.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard Hobday ra.hobday@pm.me
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.