- 1Department of Child, Adolescent and Women’s Health, Faculty of Medicine and Health Sciences, University of Zimbabwe, Harare, Zimbabwe
- 2SHAZ Program, Faculty of Medicine and Health Sciences, University of Zimbabwe, Harare, Zimbabwe
- 3Department of Paediatric Haematology and Infectious Diseases, School of Medicine, University Teaching Hospitals-CH, University of Zambia, Lusaka, Zambia
- 4SHAZ Program, School of Medicine, University Teaching Hospital-CH, University of Zambia, Lusaka, Zambia
- 5Department of Laboratory Diagnostic and Investigative Sciences, Faculty of Medicine and Health Sciences, University of Zimbabwe, Harare, Zimbabwe
- 6Department of Internal Medicine, Faculty of Medicine and Health Sciences, University of Zimbabwe, Harare, Zimbabwe
- 7Department of Haematology, University Teaching Hospitals-Adult, Lusaka, Zambia
- 8Department of Paediatrics, McMaster University, Hamilton, ON, Canada
- 9McMaster Children’s Hospital, Hamilton, ON, Canada
- 10African Institute of Biomedical Sciences and Technology, Wilkins Hospital, Harare, Zimbabwe
Majority of the 500,000 children born with sickle cell disease (SCD) annually are born in Africa. SCD contributes significantly to morbidity and mortality. This is worsened by the reduced access to therapeutic plus preventive care and limited health outcomes data. To address these challenges, we aim to develop and manage a standardized electronic SCD registry, establish consistent standards of care (SoC) for patients, improve the SCD research and biobanking capacity in Zimbabwe and Zambia. This five-year program employs a multi-pronged approach that include infrastructure and skilled manpower capacity building of SCD clinics, registry, biobanking, cohort and implementation science research studies to improve SCD treatment outcomes. We are collaborating with the SickleInAfrica consortium (Ghana, Mali, Nigeria, Tanzania, Uganda, and South Africa), the African Institute of Biomedical Sciences and Technology (AiBST) and St Jude’s Children Research Hospital. We have established the SCD registry in Zimbabwe and Zambia for children and adult patients enrolling 1796 (45%) of the targeted 4,000 participants as of March 2024. We are participating in SickleInAfrica consortium research activities, training health workers and educating SCD patient communities on SoC. This collaboration with African researchers, policymakers, health workers, and SCD patient communities will improve uptake of SCD SoC and increase our research capacity.
1 Introduction
Sickle Cell Disease (SCD) is a heterogeneous multi-organ disorder characterized by the presence of two abnormal oxygen carriers, one of which must be the hemoglobin S (HbS) (1). Globally, the distribution of the sickle cell gene mirrors the geographical distribution of malaria (2). Population migration has resulted in the spread of SCD beyond tropical regions including Southern Africa which was historically thought to have a low prevalence (3). An estimated 500,000 children are born with the condition in the world every year with 80% of these births occurring in Africa (4). SCD significantly contributes to under-five mortality in Africa and globally (5). It is estimated that 50–90% of children with SCD in Africa die before their 5th birthday (6). Very few children in Africa survive beyond 18 years of age and those who survive into adulthood have premature mortality due to end organ damage (6). Despite the high burden of SCD in Africa, there is limited data on the prevalence and incidence of the disease and its health outcomes. There are limited appropriate and context-specific clinical guidelines and policies for the care of affected individuals in Africa. There is also inadequate uptake of evidence-based preventive and therapeutic practices that have reduced morbidity and mortality in high-income countries (7, 8).
Historically, HbS was considered to be of low prevalence in Southern Africa but the prevalence of SCD has increased over the years due to migration (3). Zimbabwe is estimated to have received close to one million migrants from countries with a high prevalence of SCD and with approximately 100,000 being HbS carriers (3). A 2010 modeling study estimated that the frequency of the HbS allele in Zimbabwe was 0.021 compared to 0.171 in Nigeria, 0.112 in Zambia, 0.074 in Tanzania, and 0.003 in South Africa (9). Another modeling study in 2010 estimated that 534 babies were born with SCD in Zimbabwe with the majority dying undiagnosed early in life (10). An investigation of beta-globin gene haplotypes in bio-banked samples of three cohorts from Malawi, South Africa, and Zimbabwe demonstrated that 12% of 50 healthy unrelated participants from Zimbabwe were carriers of the Hb S gene (11).
In Zambia, SCD is most common in the northern region bordering the Democratic Republic of Congo (DRC) where the sickle cell trait (SCT) rate was reported to be 17.5% (12, 13). A more recent study by Mkushi and Serenje reported a prevalence of 15.5% of SCT and 3.4% of SCD (14). The precise prevalence of SCD in Zambia remains unknown due to limited epidemiological surveys that have been conducted. In 2017, SCD patients accounted for 12% of the all-cause hospital admissions at University Teaching Hospital (UTH) in Zambia (15). SCD was ranked fourth of the top five causes of mortality and was amongst the top 10 reasons for seeking medical attention at the University Teaching Hospitals—Children’s Hospital (UTHs—CH) in Lusaka the capital city of Zambia (Unpublished communication with Dr. Catherine Chunda-Liyoka). Lusaka is in the central part of Zambia and the SCD numbers are likely to be higher in the northern regions of Zambia.
SCD is therefore, a significant cause of morbidity and mortality in children and young adults in Zimbabwe and Zambia. However, there is paucity of data to allow for accurate characterization of the epidemiology and policy formulation accompanied by limited capacity for diagnosis and clinical management for individuals with SCD. There are also limited national policies or programs to guide surveillance, diagnosis, and management of SCD that have been formulated or implemented. Zambia launched its national clinical guidelines and a pilot newborn screening program for SCD in 2020 (16). In addition, there is a need for an SCD registry and research program to inform future practices and policies in the two countries.
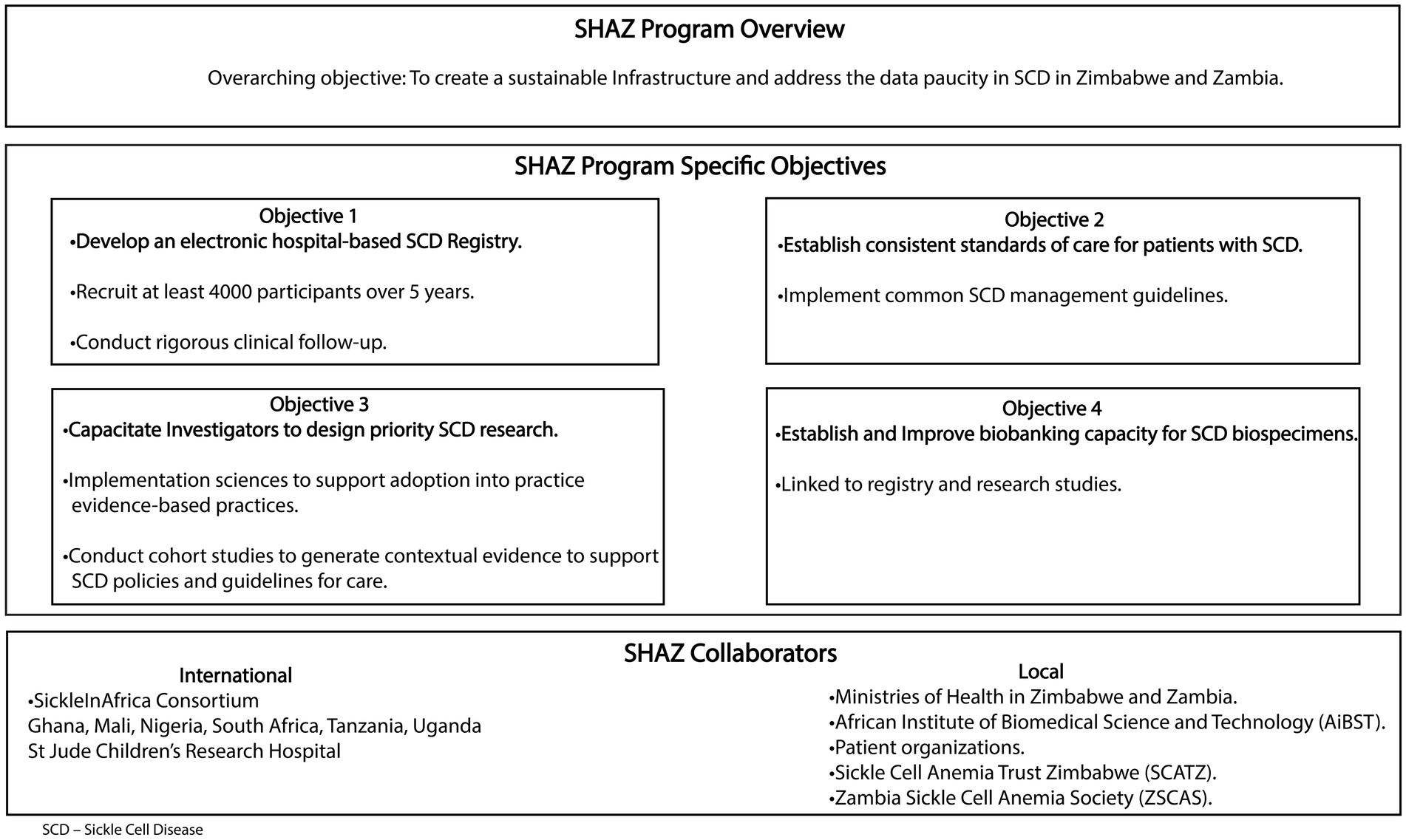
The Sickle Hemoglobinopathy reseArch in Zimbabwe and Zambia (SHAZ) program’s overarching goal is to establish a sustainable infrastructure and operation aimed at addressing the paucity of data that currently exists for SCD in the two countries. In addition, the SHAZ program aims to integrate common SCD management guidelines, develop evidence-based care practices, and bring these practices to the bedside via the conduct of implementation science research (Figure 1). Our collaborators include six countries within the SickleInAfrica consortium (Ghana, Mali, Nigeria, Tanzania, Uganda, and South Africa),1 the African Institute of Biomedical Sciences and Technology (AiBST) and St Jude’s Children Research Hospital in the USA. The SHAZ program specific objectives are to:
i. Develop and manage an electronic SCD registry with standardized and rigorous clinical follow-up within Zimbabwe and Zambia recruiting at least 4,000 participants in 5 years
ii. Establish consistent standards of care for patients with SCD in Zimbabwe and Zambia
iii. Capacitate investigators to design research on priority areas in SCD relevant to Zimbabwe and Zambia.
iv. Establish and improve the biobanking capacity of SCD biospecimens.
We highlight the SHAZ program protocol and lessons learnt in establishing a SCD registry in Zimbabwe and Zambia, two countries within the SickleInAfrica consortium.
2 Materials and methodology
2.1 Study design
The SHAZ program involves mixed methods approaches (qualitative and quantitative), that include infrastructure and skilled manpower capacity building of the SCD clinics, a prospective observational registry, biobank, cohort studies to evaluate the effect of various factors in the treatment and management of SCD, and implementation science studies to improve outcomes of SCD. This is a 5-year program extending from 01 May 2021 to 30 April 2026.
2.2 Study setting
Zimbabwe: There are 6 implementation sites. The main site is Parirenyatwa Teaching Hospital (PTH) SCD clinic which was the first site to implement the SHAZ program in May 2021. PTH is a specialized teaching hospital with a bed capacity of 2000. It offers a comprehensive care package for children and adults with SCD. There are also 5 outreach sites: Bindura Provincial and Mount Darwin District hospitals in Mashonaland Central Province, Murehwa and Mutoko District hospitals in Mashonaland East Province, and Chinhoyi Provincial Hospital in Mashonaland West Province.
Zambia: There is one site at the UTHs SCD center in Lusaka. UTHs is a specialized teaching hospital with a bed capacity of 2000. It offers a comprehensive care package for children and adults with SCD.
The SHAZ sites have been implementing the registry recruitment, the research projects and standards of care for SCD since project inception. All the sites offer inpatient and outpatient services to patients with SCD.
2.3 SHAZ registry study population
SCD patients from the selected sites in Zimbabwe and Zambia are eligible for recruitment into the SCD registry. Diagnosis of sickle cell disease in the two countries is based on suggestive clinical findings, complete blood count, peripheral smear examination, sickle cell screening tests (slide or sickle solubility tests and point of care testing with sickle scan available only in Zambia) and confirmatory testing for SCD with hemoglobin electrophoresis and/or Isoelectric focusing. Molecular diagnosis for SCD is not available routinely in both countries. The registry recruits all the age groups across the lifespan. We are including any participant giving their written informed consent, screened and found to have two abnormal copies of the hemoglobin gene, with at least one of the two genes resulting in production of hemoglobin S:
• Hb SS—sickle cell anaemia
• Hb SC
• Hb Sβ0 thalassemia
• Hb Sβ+ thalassemia
• Hb SD
• Hb SE
We are also including participants with sickle cell trait defined as Hb AS. We are excluding participants with no hemoglobin S and those not giving their written informed consent to participate in the registry. Only the participants with SCD contribute to the SickleinAfrica consortium registry while the participants with SCT contribute to the local registry data. The registry aims to recruit at least 4,000 participants with SCD in the 5 years of the study. The 4,000 participants were determined by the SIA consortium’s goal to have an observational registry with a minimum of 24,000 participants as it was deemed necessary to enable the planned implementation science plus cohort sub-studies within the registry.
2.4 Study procedures
Permission was obtained from the Ministries of Health to work with PTH, UTHs, Provincial and District health teams to sensitize health workers and raise awareness of the SHAZ program and SCD registry. The Sickle Cell Anaemia Trust in Zimbabwe (SCATZ) and Zambia Sickle Cell Anaemia Society (ZSCAS) are the patient organizations that assist with community engagement activities raising awareness of the SCD registry project and the SHAZ program. Community awareness campaigns have been done using traditional, digital and visual media as well as community events commemorating SCD. Potential study participants are approached in the general pediatric and adult medical wards; the accident and emergency departments; maternity, antenatal and perinatal service units; surgical wards and the outpatient’s clinics (including the vaccination and growth monitoring units).
2.4.1 Objective 1 and 2
We have set up the SCD center of excellence at PTH and consolidated the one at UTH. We developed the SCD Research Electronic Data Capture, REDCap (RRID: SCR_003445) database (17) by adopting the Sickle Cell Disease Ontology (SCDO)2 and SIA data elements.3 All SCD participants confirmed SCD by their routine health service providers are approached to seek written consent to participate in the SCD registry. Acceptable laboratory confirmatory diagnostic tests performed before recruitment include sickle cell screen, point of care tests, electrophoresis, isoelectric focusing, High-performance liquid chromatography (HPLC) and / or molecular tests. This is an observational longitudinal registry with no interventions planned which collects information on demographics, clinical information, drug use, treatment and outcomes of children and adults with SCD (see Table 1).
The SHAZ team consisting of the coordinator, outreach workers, and data capture clerks work closely with the recruitment site teams to ensure that participant-related activities and data are promptly entered into the database using mobile devices. Strategic health workers’ training in partnership with the Ministry of Health (MOH) at the two hospitals (PTH and UTH) and outreach sites are continuously being carried out by the SHAZ team to raise awareness of the SCD registry program. All SHAZ recruitment sites are being trained on SCD standards of care adopted from the SicklenInAfrica Consortium (18),4 as well as the PEN-Plus program5 in collaboration with policymakers in the ministry of health.
2.4.2 Objectives 3 and 4
Since the inception of the SHAZ project, we have been working to come up with four consortium-wide studies in the areas prioritized by the SIA consortium countries directly funded by the NIH NHLBI awarded grant. There are two cohort studies on pharmacogenomics of pain management and malaria in children with SCD and there are two implementation studies on SCD newborn screening and penicillin/hydroxyurea use being developed. The four protocols will be sent for ethics review separately and implemented by all SIA consortium countries once finalized. We also have been supporting various local site studies in both Zambia and Zimbabwe collaborating with ministry of health, local academic institutions, and other funders. We set up the biorepository for specimen biobanking linked to the proposed cohort and implementation science studies in collaboration with the AiBST in Zimbabwe and the UTH laboratory in Zambia. The biobank has a capacity for up to 50,000 samples which is scalable to at least 100,000 samples and has a robust infrastructure composed of temperature-controlled (−80°C and −20°C) freezers, liquid nitrogen tanks, biosafety hoods, centrifuges and back up power. It uses the LabCollector Laboratory Information Management System (LIMS). The Biobank has well-trained staff of 6 people with expertise in management, information technology, ethics, laboratory technologies, and logistics. The SHAZ program will adopt SOPs for specimen management for each research study linked to the registry project collaborating with SIA consortium DCC. Detailed standard operating procedures (SOPs) will be outlined for each individually developed research protocol.
2.5 Retention of participants in the SCD registry
The following retention strategies are being applied to retain patients in the registry (19, 20):
2.5.1 Barrier-reduction retention methods
• Ensuring investigators and support staff are well trained in research methodology, good clinical practice, and ethics
• Ensuring consistent research staff and hiring staff who can speak the language of the participants as well as understand their cultural values
• Making use of expert SCD patients to mobilize the community and improve community participation.
• Providing SCD education and awareness campaigns and stressing the benefits of participating in long-term cohorts in the communities from which we recruit participants
• Running a pilot test of the registry
• Establishing simple and efficient procedures for data collection. Limiting the number of visits to recruitment and an annual visit for data collection.
• Providing both on-site and telephonic longitudinal data collection to cater to different participants’ preferences. At recruitment, participants are asked to state the method they prefer for communication with the study staff [in person, telephone/cellphone -voice or short message service or social media platforms].
• Ensuring confidentiality and privacy for participants.
• Reimbursing travel costs to participants scheduled for data collection and recognizing the milestones reached in each year of the study.
2.5.2 Community-building retention strategies
• We have engaged the SCD community advocacy groups (SCATZ and ZSCAS) who provided support letters for the grant application. We will continue to work with them as we implement our program. We have also incorporated them into our Advisory committee which advises the principal investigator and research team.
• Providing education and awareness campaigns on SCD for communities guided by our ministries of health policies and regulations.
2.5.3 Follow-up and participant reminder strategies
• Using appointment cards and diaries.
• Using telephone or cellular voice or short message service reminders.
• The integration of important or required annual reviews of SCD patients such as urinalysis, blood pressure check, and transcranial doppler measurement.
2.5.4 Tracing strategies
• Documenting alternative contact details of participants.
• Completion of change of address forms at every visit.
2.6 Data management
2.6.1 Data collection
For the SHAZ registry, data is collected and entered directly into an electronic database developed for the study using REDCap (RRID:SCR_003445) data capture software that is password protected to avoid data compromise. REDCap is an online system that has an embedded mobile application that allows offline collection of data and uploads it later using an internet connection. A data dictionary provided by the SIA Data Co-ordinating Center (DCC) was imported into REDCap. Mobile REDCap data collection using tablet computers was chosen for all recruiting sites and the data capturers have a code name to assist the data manager with resolving data queries. Training on the registry protocol and REDCap (RRID:SCR_003445) software preceded any data collection. All enrolled participants are identified using unique Participant Identifiers (PID) in the database and on data collection forms. All information that identifies the participants such as their names, telephone numbers, and addresses are captured on a separate Study Log kept in a secure place accessed by authorized persons only. Data collectors then upload data through an internet connection to the central server and the Data Manager confirms receipt and reviews the data to check for completeness, validity, and accuracy. If data is collected on paper forms, these forms are reviewed for completeness, accuracy and the use of skip logic before data entry directly into the SHAZ Registry database. Paper forms are stored in secure metal lockable cabinets accessed by the Study Coordinator and Principal Investigator only in both Zimbabwe and Zambia.
All registry data is stored on a password protected local server located in a secure commercial data center in Zimbabwe and data backups are kept at the research sites at PGH and UTH with access limited to the Investigators, Study Coordinator, and Data Manager. Data backup is done on a password protected external media and stored securely at a central place with access limited to only authorized personnel. Data capturers in Zimbabwe have no access to data from Zambia and vice versa. Only the principal investigator, co-principal investigators, study coordinator, and data manager have access to data from both sites. A few data points on gender, age, and date of diagnosis are uploaded to the DCC and this is guided by a data sharing agreement signed by Zambia, Zimbabwe and DCC authorized signatories.
Data quality checks begin during data entry as the database is designed with built-in quality control data entry checks to scan for possible errors, missing data and values out of range. To continuously improve the data quality, integrity and validity, the Data Manager does the Quality Control real-time review of every single data entry submitted to the central server daily for accuracy, completeness, timeliness, and logic or skip controls. Queries of any data discrepancy noted are sent back to the data collectors for clarification and resolution. The Data Manager produces data quality reports every week and any trends noted in the quality of data are discussed and re-training done to address recurring data errors. Software malfunction issues that arise are discussed with the DCC and the REDCap software developers.
2.6.2 Data analysis
Summary descriptive statistics will be employed to describe the participants’ socio-demographic, phenotype, and genotype data as well as the prevalence and incidence of complications as we follow up with the participants throughout the study period. The proposed cohort and implementation science studies outlined will have more detailed descriptions once the full protocols are completed at the time of submission for ethics approval for each protocol.
2.7 Ethics
The research regulatory authorities in Zimbabwe, that is, the Joint Research Ethics Committee (JREC/202/21) and Medical Research Council of Zimbabwe (MRCZ/A/2747) provide ethical oversight of this registry and the proposed research activities. The Ministry of Health and Child Care, clinical directors, and respective provincial medical directors approved the research at the PTH and provinces.
In Zambia, ethical approval was sought and granted by the Excellence Research Ethics and Science Converge, (ERES; 2021-May-092) committee and the National Health Research Authority (NHRA00027/26/09/2023) prior to the commencement of the SHAZ registry program. Permission to conduct the study was also obtained from the ministry of health and the participating institutional leadership.
SHAZ adapted informed consent forms (ICFs) developed by the Clinical Co-ordinating Center (CCC) to conform with the requirements of the local regulatory authorities. Written informed consent in the participant’s preferred language is obtained using ICFs approved by each country’s ethics committee. A data sharing agreement approved by local ethics committees and institutional leaders was implemented between Zimbabwe and Zambia as well as between SHAZ and the Data Co-ordinating Center. This study is embedded in routine care, there are no physical potential risks rendered to the participants in the registry. However, taking part in the registry may result in people knowing the participant has sickle cell disease and there is a risk the participant may experience social discrimination which can result in psychological distress. Psychosocial support for participants is provided by the SCD clinicians and patient support groups. It is emphasized in the ICF that participation in the registry is voluntary, and participants are free to refuse to participate or withdraw from the registry at any time without loss of benefits to care.
2.8 Timeline of the study
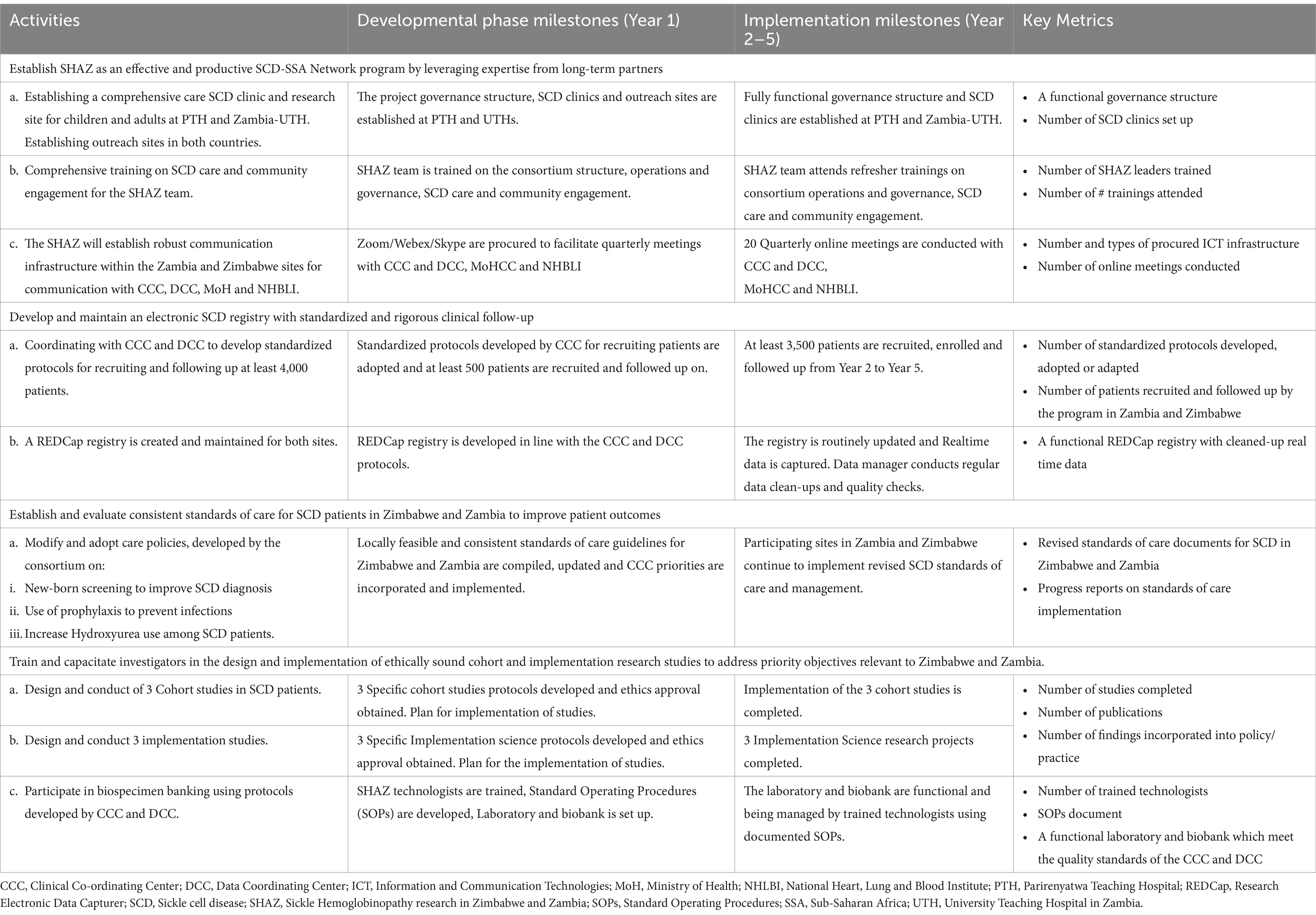
The registry has been recruiting participants as of 1st November 2021 in Zimbabwe and 1st March 2022 in Zambia. A pilot phase was carried out in Zimbabwe from November to December 2021 and any emerging issues were resolved with the DCC. Definitive data collection began in Zimbabwe on 3rd of January 2022 and in Zambia on the 1st of March 2022. Data collection will end on 30th of April 2026 at the end of the funding cycle. The registry project is in the 3rd year of implementation. Table 2 shows the timelines and evaluation metrics for the SHAZ program over 5 years. Year one was a developmental phase of establishing systems for the program linking the two international sites in Zimbabwe and Zambia with the SickleinAfrica consortium already established systems. The registry earnestly began recruitment in the last quarter of year one at both sites and hopefully we will reach 50% or more recruitment by end of year 3.
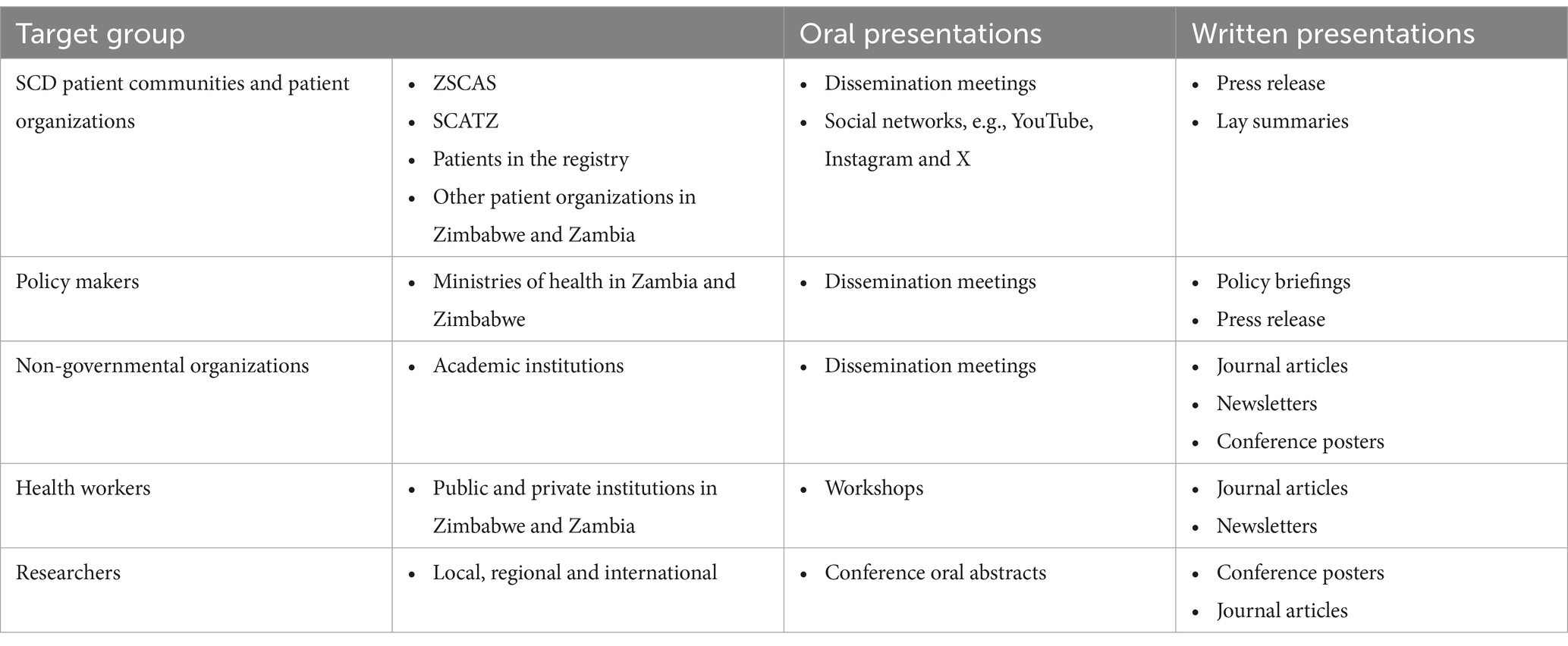
2.9 Plans for dissemination of results
Our target audience includes SCD patient communities, researchers, policy makers, non-governmental organizations and health workers. Table 3 outlines the dissemination plan for the SHAZ Program findings.
2.10 The current status of the SHAZ program
2.10.1 To establish SHAZ as an effective and productive program in the treatment and management of SCD
The research team engaged and sensitized the policy makers and hospital administrators from both countries on the SHAZ program through an inception meeting and other forums. This has facilitated implementation of the registry, research and standard of care improvement activities in the recruitment sites. Comprehensive care SCD clinics for children and adults with robust outreach programs have been established or improved at the University of Zimbabwe PTH and UTHs in Zambia, respectively. SHAZ research team and patient support groups were recruited. The team has received comprehensive protocol training and appropriate training in laboratory skills, genetic counselling, data management, clinical management of patients with SCD and community engagement. We obtained ethics clearance for the SHAZ registry project in both Zimbabwe (JREC/202/21 and MRCZ/A/2747) and Zambia (ERES 2023-Apr-005 and NHRA000006/19/12/2023). We have signed a data sharing agreement between Zimbabwe and Zambia SHAZ programs as well as with the SIA DCC at University of Cape town. We have established 4 out of the 5 outreach sites (Mutoko, Murehwa, Bindura and Mt. Darwin) in Zimbabwe and are working on establishing the Chinhoyi site before the end of year 3. We participate in the SHAZ meetings: quarterly Advisory committee meetings, monthly executive committee meetings with Zambia, weekly registry data meetings and weekly site administration meetings. We are also participating in biannually SIA consortium meetings,
2.10.2 To develop and maintain an electronic SCD registry with standardized and rigorous clinical follow-up
We adopted the SIA consortium data collection tools to recruit, enroll and follow-up at least 4,000 individuals with SCD. We piloted the registry successfully in year one 3rd quarter and had recruited 48% of participants by February 2024. Zambia has recruited more participants with a ratio of 5 to 1 to Zimbabwean numbers reflecting the difference in epidemiology of SCD in the two countries.
2.10.3 To establish consistent standards of care for patients with SCD in Zimbabwe and Zambia
We engaged ministries of health technical departments in Zambia and Zimbabwe, evaluated existing standards of care for patients with SCD, adapted SIA consortium and PEN-Plus standards of care recommendations. We have trained 27 health workers from both countries in genomics in 2022. The focus in the 5-year period is on implementing SCD newborn screening, improve use of antibiotic prophylaxis for infection prevention and increased hydroxyurea use among SCD patients.
2.10.4 To train and capacity build investigators in the design and implementation of ethically sound cohort and implementation research studies
Our investigators and research staff have participated in training on REDCap (RRID:SCR_003445) software, research methodology for cohort and implementation science research hosted by the CCC, DCC and various partners of the SIA consortium. We are participating in the selection and development of four SickleInAfrica cross consortium studies:
• Two cohort studies in the area of SCD pain management pharmacogenomics and Malaria chemoprophylaxis in children with SCD
• Two implementation studies on SCD newborn screening and Penicillin /Hydroxyurea use
SHAZ is leading the development of the SCD pharmacogenomics of pain management study protocol while other consortium members are leading the finalization and implementation of the other studies. The SHAZ led protocol has been submitted to SIA consortium and NIH for approval. We have also supported site specific research by one Doctoral and five masters’ students in Zimbabwe as well as one research fellow in Zambia.
2.10.5 To Establish a biobank of the SCD biospecimens
Collected and documented in the SCD registry: The SHAZ program has provided additional training to laboratory scientists on biospecimen collection, storage, tracking, shipping and quality control using CCC and DCC protocols. The Biobank has well trained staff made up of 6 people with expertise in management, information technology, ethics, laboratory technologies, and logistics. The biobanking is linked to the research studies. The biobank activities are a collaboration of the SHAZ program, the AiBST and SIA consortium.
3 Discussion
The SHAZ program aims to improve SCD SoC through research collaboration with fellow African researchers, policy makers, health workers and SCD patient community groups. It is supporting innovation through research and teaching. We are on track to meet our specific objectives over a 5-year period. The SHAZ registry protocol was amended in Jan 2022 after discussion at SIA consortium level to include an annual follow up for all recruited participants in the registry. Our research strategy was harmonized with the SIA consortium to streamline funding to support four (4) studies (2 cohort and 2 implementation sciences) which will generate more robust evidence on SCD management in Sub-Saharan Africa instead of the originally planned six (6) small studies. These changes though welcome has put a strain on our financial resources and we have expanded our focus to looking for local and international funders to augment our planned research activities. The SHAZ program has created opportunities to network with other SCD African researchers increasing exchange of ideas and expertise. We look forward to the establishment and signing of the SIA charter which will further cement our participation in the consortium activities and sharing of data. We also have benefitted from the CCC and DCC supporting our registry, SCD SoC and research activities through provision of monetary and non-monetary resources. We have recruited two research fellows one in each country.
3.1 Strengths of the SHAZ program
The SHAZ program is addressing a critical but neglected health issue in a high burden region with limited SCD diagnostic and treatment facilities. The program employs a mixed method approach that uses both quantitative and qualitative research approaches to improve SCD research, SoC and patient outcomes. The SHAZ registry expanded from a pilot SCD registry established in 2018 at the Parirenyatwa Hospital Paediatric Hematology unit, which focused on children under 18 years diagnosed with SCD. The registry now involves two countries, Zimbabwe and Zambia, which share the Zambezi River border and includes both children and adults. Through collaboration with the SickleInAfrica consortium, Zimbabwe and Zambia have the unique opportunity to exchange insights and experiences, enhancing their efforts to addressing SCD. A key advancement of the SHAZ program has been the establishment of an electronic registry, which facilitates the patient socio-demographic plus clinical factors tracking and data management. A biobank has been developed to support research linked to the registry for both countries. This will serve as a resource for future research studies beyond the SHAZ program. Beyond research, the SHAZ program actively engages SCD advocacy community groups and the Ministries of Health in both countries, ensuring patient and family support. The Ministries of Health in Zimbabwe and Zambia are supportive of the SHAZ program.
The SHAZ program also prioritizes capacity building, offering technical training to healthcare professionals to improve SCD awareness, diagnosis and management. By training local researchers and healthcare workers, the program promotes sustainability and long-term impact. The registry’s results will help to guide SCD-related policies and treatment priorities in Zimbabwe and Zambia. Participation in the SIA consortium has enabled the SHAZ program to leverage additional training opportunities that would otherwise be unavailable. These include data management, genetic counselling, grant writing, research methodology and manuscript writing. The Data Coordinating Center has further supported the enrollment of research fellows in Zambia and Zimbabwe. Additionally, the SIA consortium facilitates the development and implementation of multi-country research studies, ensuring alignment with international standards such as REDCap and SCDO data elements enhancing data compatibility and research rigor.
3.2 Limitations of the SHAZ program
Despite its progress the SHAZ program faces several challenges including the limited diagnostic and treatment resources for SCD. Many patients remain undiagnosed due to inadequate facilities, affecting treatment and access to care. There is a need for the research program to find ways of involving other partners to augment funding received and sustain the program. The hospital-based recruitment of the registry facility excludes patients with asymptomatic or mild disease who do not seek care at the recruitment facilities. Expanding outreach efforts is important to improve generalizability. The registry has limited geographic coverage as it operates in specific regions, excluding many SCD patients in non-participating areas. Expanding the program’s reach is a priority. Only 28% (245 of 853) recruited patients in year one returned for the one-year follow-up visit reflecting a trend where patients seek care only when they experience acute or chronic complications. To address this, the SHAZ program is implementing strategies to increase follow up rates. The program has engaged community-based patient advocates, employed SMS reminders and expanded access to point-of-care diagnostics to encourage follow-up.
The cost of hydroxyurea and penicillin V are relatively high for unemployed or rural patients. This results in some patients defaulting treatment. The program is actively engaging policymakers and other partners to improve the affordability and availability of SCD these medications. There is a need for collaborative initiatives with patients and policymakers to lobby for universal treatment for SCD similar to tuberculosis and HIV/AIDS programs since SCD is an important non-communicable disease affecting many patients in Sub-Saharan Africa. The SHAZ program is dependent on external funding threatening the long term sustainability. However, collaborations with Ministries of Health policymakers, the PEN-Plus program and the private sector partners are being pursued to integrate the SHAZ registry into national health systems. In Zambia, the Ministry of Health has partnered with an international pharmaceutical company to provide subsidized hydroxyurea. There is also ongoing advocacy for local production of hydroxyurea to improve access for SCD patients in both countries. We hope that engaging the policymakers and other local partners will have a spillover effect with adoption of policies that will benefit all SCD patients in the two countries.
4 Conclusion
The SHAZ program in Zimbabwe and Zambia is making significant efforts in policy engagement, community involvement and improving research infrastructure. By the end of the 5 year implementation period, the program will generate hospital based evidence of the SCD burden in the two countries, paving way for larger, more representative epidemiological studies. The SHAZ registry is an important infrastructure which will support basic science, translational and implementation science research. Its impact extends beyond data collection, actively contributing to SCD awareness, patient advocacy and improved SoC in both countries. With continued collaboration and policy integration, the SHAZ program holds serious potential for shaping the future of SCD management in Zimbabwe and Zambia.
Ethics statement
The studies involving humans were approved by (1) The Joint Research Ethics Committee (JREC) affiliated to the University of Zimbabwe and Parirenyatwa Group of Hospitals. (2) The Medical Research Council of Zimbabwe (MRCZ) affiliated with the Ministry of Health and Child Care. (3) The Excellence Research Ethics andScience Converge (ERES) affiliated to Ministry of Health in Zambia. (4) National Health Research Authority (NHRA) affiliated to the Ministry of Health in Zambia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
PK: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. GK: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. CC-L: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. IR: Conceptualization, Investigation, Methodology, Writing – review & editing. PS: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. PG-C: Conceptualization, Investigation, Methodology, Writing – review & editing. HM: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. TMt: Investigation, Project administration, Software, Validation, Writing – review & editing. CP: Investigation, Project administration, Validation, Writing – review & editing. LC: Investigation, Writing – review & editing. NK: Investigation, Writing – review & editing. EC: Data curation, Investigation, Software, Writing – review & editing. TMa: Conceptualization, Methodology, Writing – review & editing. JN: Conceptualization, Investigation, Methodology, Writing – review & editing. UA: Conceptualization, Methodology, Writing – review & editing. CM: Conceptualization, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research reported in this publication is funded by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number 501HL156943 to PK (https://reporter.nih.gov/project-details/10684680). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
We would like to acknowledge the following people: (i) UZ PETRA secretariat: Antony Matsika, Thokozile Mashaah, Nhauro Mupanguri, Felix Madya, Miriro Muvoti, Caroline Tazvivinga and Tendayi Maunganidze (https://petra.org.zw) and James Gita Hakim (posthumously) for the excellent mentorship of the SHAZ research team during the SHAZ protocol and grant writing. (ii) The SHAZ advisory committee: Inam Chitsike, Midion Chidzonga, Jonathan Matenga, Nickhill Bhakta, Uma Athale, Tsungai Chipato, Molyn Chima from SCATZ and Ketty Chunga from ZSCAS our SCD community advocates for guiding us through the process of developing and implementing the SHAZ program. (iii) The SHAZ research administrator Blessing Murangadzva and Data Capturer Nunurai Sphandla and Driver Jackson Mwepeta for working tirelessly to ensure a smooth implementation of the SHAZ program. (iv) The SIA Africa consortium for supporting the SHAZ program participation in the consortium. (v) Pauline Kazembe for editing our manuscript language.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://ZSCAS.sickleinafrica.org/
2. ^https://scdontology.h3abionet.org/
3. ^https://ZSCAS.sickleinafrica.org/SIA_data_elements
4. ^https://sadacc.org/sites/default/files/SPARCo_SoC_SCD_GuidelinesR_Aug2023.pdf
References
1. Kanter, J, and Kruse-Jarres, R. Management of sickle cell disease from childhood through adulthood. Blood Rev. (2013) 27:279–87. doi: 10.1016/j.blre.2013.09.001
2. Piel, FB, Patil, AP, Howes, RE, Nyangiri, OA, Gething, PW, Williams, TN, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun. (2010) 1:1104. doi: 10.1038/ncomms1104
3. Piel, FB, Tatem, AJ, Huang, Z, Gupta, S, Williams, TN, and Weatherall, DJ. Global migration and the changing distribution of sickle haemoglobin: a quantitative study of temporal trends between 1960 and 2000. Lancet Glob Health. (2014) 2:e80–9. doi: 10.1016/S2214-109X(13)70150-5
4. Thomson, AM, McHugh, TA, Oron, AP, Teply, C, Lonberg, N, Vilchis Tella, V, et al. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the global burden of disease study 2021. Lancet Haematol. (2023) 10:e585–99. doi: 10.1016/S2352-3026(23)00118-7
5. Wastnedge, E, Waters, D, Patel, S, Morrison, K, Goh, MY, Adeloye, D, et al. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J Glob Health. (2018) 8:1103. doi: 10.7189/jogh.08.021103
6. Wonkam, A, and Makani, J. Sickle cell disease in Africa: an urgent need for longitudinal cohort studies. Lancet Glob Health. (2019) 7:e1310–1. doi: 10.1016/S2214-109X(19)30364-X
7. 60th Session Regional Committee for Africa. Sickle-cell disease: a strategy for the who African region. Geneva: WHO (2010).
8. Piel, FB, Rees, DC, DeBaun, MR, Nnodu, O, Ranque, B, Thompson, AA, et al. Defining global strategies to improve outcomes in sickle cell disease: a lancet Haematology commission. Lancet Haematol. (2023) 10:e633–86. doi: 10.1016/S2352-3026(23)00096-0
9. Piel, FB, Hay, SI, Gupta, S, Weatherall, DJ, and Williams, TN. Global burden of sickle cell Anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. (2013) 10:e1001484. doi: 10.1371/journal.pmed.1001484
10. Piel, FB, Patil, AP, Howes, RE, Nyangiri, OA, Gething, PW, Dewi, M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. (2013) 381:142–51. doi: 10.1016/S0140-6736(12)61229-X
11. Pule, GD, Chimusa, ER, Mnika, K, Mhandire, K, Kampira, E, Dandara, C, et al. Beta-globin gene haplotypes and selected malaria-associated variants among black southern African populations. Glob Health Epidemiol Genom. (2017) 2:e17. doi: 10.1017/gheg.2017.14
12. Barclay, G. Sickle cell anaemia in Zambia. Trans R Soc Trop Med Hyg. (1971) 65:529–30. doi: 10.1016/0035-9203(71)90166-0
13. Athale, UH, and Chintu, C. The effect of sickle cell anaemia on adolescents and their growth and development: lessons from the sickle cell anaemia clinic. J Trop Pediatr. (1994) 40:246–52. doi: 10.1093/tropej/40.4.246
14. Chindima, N, Nkhoma, P, Sinkala, M, Zulu, M, Kafita, D, Simakando, M, et al. The use of dried blood spots: a potential tool for the introduction of a neonatal screening program for sickle cell Anemia in Zambia. Int J Appl Basic Med Res. (2018) 8:30–2. doi: 10.4103/ijabmr.IJABMR_105_16
15. Ministry of Health Zambia. (2022). The Zambia non-communicable diseases and injuries poverty commission report: Reframing non-Communicable Diseases & Injuries in Zambia [internet]. Available online at: https://static1.squarespace.com/static/55d4de6de4b011a1673a40a6/t/62d0459607789d6c2c9f4b72/1657816491776/Zambia_NCDI+Commission+Report_Final.pdf (Accessed March 21, 2024).
16. Makoni, M. Newborn screening for sickle cell disease in Africa. Lancet Haematol. (2021) 8:e476. doi: 10.1016/S2352-3026(21)00166-6
17. Harris, PA, Taylor, R, Thielke, R, Payne, J, Gonzalez, N, and Conde, JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
18. Paintsil, V, Ally, M, Isa, H, Anie, KA, Mgaya, J, Nkanyemka, M, et al. Development of multi-level standards of care recommendations for sickle cell disease: experience from SickleInAfrica. Front Genet. (2023) 13:13. doi: 10.3389/fgene.2022.1052179
19. Teague, S, Youssef, GJ, Macdonald, JA, Sciberras, E, Shatte, A, Fuller-Tyszkiewicz, M, et al. Retention strategies in longitudinal cohort studies: a systematic review and meta-analysis. BMC Med Res Methodol. (2018) 18:1–22. doi: 10.1186/s12874-018-0586-7
20. Robinson, KA, Dinglas, VD, Sukrithan, V, Yalamanchilli, R, Mendez-Tellez, PA, Dennison-Himmelfarb, C, et al. Updated systematic review identifies substantial number of retention strategies: using more strategies retains more study participants. J Clin Epidemiol. (2015) 68:1481–7. doi: 10.1016/j.jclinepi.2015.04.013
Glossary
AIDS - Acquired Immunodeficiency Syndrome
Hb - Hemoglobin
AiBST - African Institute of Biomedical Sciences and Technology
CCC - Clinical Co-ordinating Center
CH - children’s hospital
DCC - Data Co-ordinating Center
DRC - Democratic Republic of Congo
ERES - Excellence Research Ethics and Science Converge
HIV - Human Immunodeficiency Virus
HPLC - High Performance Liquid Chromatography
ICF - Informed Consent Forms
JREC - Joint Research Ethics Committee
MRCZ - Medical Research Council of Zimbabwe
NHLBI - National Heart Lung and Blood Institute
NHRA - National Health Research Association
NIH - National Institute of Health
PEN-Plus - Package of Essential NCD Interventions—Plus
PID - Participant Identifiers
PTH - Parirenyatwa Teaching Hospital
REDcap - Research Electronic Data Capture
SCATZ - Sickle Cell Anaemia Trust of Zimbabwe
SCD - Sickle cell disease
SCT - Sickle cell trait
SHAZ - Sickle Haemoglobinopathy Research in Zimbabwe and Zambia
SIA - SickleinAfrica
SoC - Standards of Care
USA - United States of America
UTH - University Teaching Hospital
UZ Petra - University of Zimbabwe Partnership for Education Research and Training Advancement
ZSCAS - Zambia Sickle Cell Anemia Society
Keywords: sickle cell disease, Africa, Zimbabwe, Zambia, registry, SickleInAfrica, cohort, standards of care
Citation: Kuona P, Kandawasvika GQ, Chunda-Liyoka C, Ruredzo IM, Sambo PM, Gorejena-Chidawanyika P, Mantina HM, Mtisi TJ, Phiri C, Chikara L, Kaweme NM, Chivige E, Namushi J, Maboreke TC, Athale U and Masimirembwa C (2025) Sickle hemoglobinopathy research in Zimbabwe and Zambia: setting up an international sickle cell disease registry. Front. Med. 12:1484763. doi: 10.3389/fmed.2025.1484763
Edited by:
Eleni Gavriilaki, Aristotle University of Thessaloniki, GreeceReviewed by:
Evans Xorse Amuzu, Komfo Anokye Teaching Hospital, GhanaCharles Antwi-Boasiao, University of Ghana, Ghana
Copyright © 2025 Kuona, Kandawasvika, Chunda-Liyoka, Ruredzo, Sambo, Gorejena-Chidawanyika, Mantina, Mtisi, Phiri, Chikara, Kaweme, Chivige, Namushi, Maboreke, Athale and Masimirembwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patience Kuona, cGF0aWVrdW9uYUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share second authorship
‡These authors share senior authorship
 Patience Kuona
Patience Kuona Gwendoline Q. Kandawasvika
Gwendoline Q. Kandawasvika Catherine Chunda-Liyoka
Catherine Chunda-Liyoka Ian M. Ruredzo2,5
Ian M. Ruredzo2,5 Takudzwa J. Mtisi
Takudzwa J. Mtisi Natasha M. Kaweme
Natasha M. Kaweme Jombo Namushi
Jombo Namushi Uma Athale
Uma Athale