Abstract
Introduction:
Cancer is a disease characterized by uncontrolled cell growth that can invade and spread to other body parts. Drugs used for chemotherapy can cause damage to non-cancer cells and lead to a low blood cell count. There are controversial findings regarding the differences in the counts of blood cell types. Studies on the counts of blood cell types before and after chemotherapy are limited. Therefore, this study aimed to address this gap.
Methods:
A retrospective study was conducted on 354 patients from 1 September 2022 to 1 October 2022 to compare blood cell type profiles in pre-and post-operative periods. A chi-squared test and paired t-test were performed to analyze the data.
Results:
Data were collected from 354 patients. The mean age of the respondents was 41.26 (±16.67) years. At the initial diagnosis, 167 patients (47%) were categorized as having stage III cancer, while 159 patients were categorized as having stage IV cancer. Before and after chemotherapy the mean heamoglobin level is 12.95 g/dL and 12.30 g/ dL respectively and the prevalence of anemia among cancer patients before chemotherapy and after chemotherapy was 25.14% (95%:20.88, 29.94) and 35.54% (95%: 30.75; 40.73), respectively. The mean value of neutrophils before and after chemotherapy was 52.72 and 50.82%, respectively. The frequency of neutropenia among cancer patients was 22.32% (95% 18.26; 26.96) before chemotherapy and 27.97% (95%:23.52; 32.88) after chemotherapy, and the mean value of lymphocytes before and after chemotherapy was 37.50 and 34.29%, respectively. Lymphopenia among the study participants was 16.38% (95%:12.87; 20.62) before chemotherapy and 17.51% (95%13.88, 21.84) after chemotherapy. Among patients with stomach, rectal, and bone cancers, there was no significant difference in the counts of all blood cell types before and after chemotherapy. The mean reduction in platelets was 23. 51 × 103cells/mm3 (p = 0.001). For red blood cells (RBCs), the mean decrease was 0.63 × 106cells/mm3 (p = 0.08), and for white blood cells (WBCs), it was 2.49 × 106 cells/mm3 (p = 0.012).
Conclusion and recommendation:
After chemotherapy, all hematological parameters showed a decrease, indicating that chemotherapy significant impacted the levels of these hematological parameters. Therefore, replacement therapy should be considered after chemotherapy.
1 Introduction
Cancer is a disease characterized by uncontrollable cell growth that can invade and spread to other parts of the body. There are numerous potential causes for cancer, including an individual’s genetic, environmental, or constitutional traits (4). Cancer is considered one of the most serious and deadly illnesses of the 21st century (5). The type and location of the cancer, the conventional medical procedures and treatment protocols prevalent in a patient’s culture, and the cancer stage at the time of initial diagnosis influence the treatment a patient receives. Cancer treatment might involve various approaches, including radiation therapy, chemotherapy, and surgery. However, patients are at risk for several adverse effects, and these treatments are typically not curative. As a result, cancer treatments often has many side effects, including anemia, infection, bleeding issues, nausea, vomiting, allergic reactions, pain, soreness, constipation or diarrhea, hair loss, sore mouth, increased energy, and trouble falling asleep (6). Hematological abnormalities are dangerous side effects of chemotherapy. This disease affects the blood and its components, such as red blood cells (RBCs), white blood cells (WBCs), platelets, hemoglobin, and plasma. Hematological abnormalities, particularly anemia, are the most prevalent complications among patients undergoing chemotherapy (7). These conditions can lead to significant health issues, including fatigue, increased infection risk, clotting disorders, and severe bleeding tendencies (8).
According to a study conducted on the effect of chemotherapy and radiotherapy on red blood cells and hemoglobin in cancer patients, there was a difference in the level of red blood cells pre-chemotherapy and post-chemotherapy (9). Administration of drugs for chemotherapy causes damage to non-cancer cells, and this leads to a low count of white blood cells following chemotherapy (10).
Chemotherapy affects the counts of blood cells and biochemical profiles in different ways. It functions as an alkalizing agent and causes the bone marrow’s hematopoietic stem cells to disappear gradually (11). Chemotherapy usually works by blocking the creation of deoxyribonucleic acid (DNA), proteins, and microtubules, which results in the death of cells or the prevention of their proliferation (12). Chemotherapeutic drugs cause (13) deoxyribonucleic acid damage during replication by covalently binding to the deoxyribonucleic acid of bone marrow cells, generating intra-and inter-strand cross-links. Platelets, leukocytes, and hemoglobin levels all drop. A study conducted on the effect of chemotherapy on hemoglobin revealed that there was no significant association between hemoglobin and chemotherapy (14).
Chemotherapy-treated cancer patients have been shown to experience immune system changes and anemia. Frequent, intense chemotherapy has been shown to decrease immune cells and lead to opportunistic infections (15). Chemotherapy side effects are noticeable in the bone marrow, a key source of pluripotent hematopoietic stem cells (16).
Moreover, the use of drugs that are specifically designed to be cytotoxic in the complicated field of cancer treatment invariably has detrimental effects on the blood cell profile. Many of these medications are mostly metabolized in the liver; therefore, while administering chemotherapy, liver-drug interactions need to be taken into consideration (17). Chemotherapy administration poses a challenge to the careful regulation and equilibrium of liver function. Due to the liver’s easy absorption of the majority of chemotherapy medicines, up to 85% of patients experience liver stenosis (17). Chemotherapy administered repeatedly can cause permanent damage to the liver by drawing in inflammatory cells and changing the levels of enzymes, such as lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP), and it can lead to a decrease in erythropoietin (18).
The volume of platelet counts after chemotherapy in patients with cancer showed a decrement due to the effect of chemotherapy. However, a study conducted on ovarian cancer showed that there was an increase in thrombocytes (19).
There are controversial findings regarding the differences in the counts of blood cell types across various studies. Most studies have focused on the relationship between specific cancer types and individual blood cell types. There are limited studies on the counts of blood cell types before and after chemotherapy in patients with different cancer conditions in the specified areas. In addition, there are few studies on the abnormalities of hematological parameters in these patients. Therefore, assessing these abnormalities has profound implications for both public health and clinical practice. The findings of this study will support the diagnosis, management, and prevention of complications. Additionally, they will aid in resource allocation, health policy development, and epidemiological surveillance. Understanding the changes in hematological parameters is substantial for implementing appropriate interventions. Therefore, this study aimed to address this gap and provide different scientific bases for various stakeholders.
2 Methods
This study was conducted at Mekele Comprehensive Specialized Hospital in the northern part of Ethiopia. The hospital has two oncology centers: pediatric oncology center and adult oncology center.
2.1 Study design
To compare hematological parameters in pre-and post-chemotherapy cancer patients, an institutional-based retrospective study design was employed from 1 September 2022 to 1 October 2022.
2.2 Populations
All patients with various types of cancer who attended the hospital for chemotherapy were the source population, and patients who started chemotherapy treatment between September 2018 and September 2022 were the study population.
2.3 Inclusion and exclusion criteria
2.3.1 Inclusion criteria
All cancer patients who had undergone chemotherapy and had complete medical records, including demographic information, cancer stage, and various types of blood cell counts, were included in the study.
2.3.2 Exclusion criteria
-
Patients who underwent surgery, radiotherapy, or immunotherapy were excluded from the study
-
Patients with comorbid conditions, such as diabetes mellitus, chronic obstructive pulmonary disease, tuberculosis, HIV/AIDS, chronic kidney disease, congestive heart failure, inflammatory bowel diseases, and viral hepatitis, were excluded as these diseases compromise hematological parameters.
-
Patients who were lost to follow-up before completing the IV cycle of the treatment were excluded from the study
2.4 Sampling method and sample size determination
All samples that met the inclusion criteria were included. The total sample size was 354 patients. The required data were selected from these 354 patients, who were admitted to the oncology unit between September 2018 and September 2022.
2.5 Operational terms
Pre-chemotherapy levels of blood cell types: These were the levels of blood cells at the time of diagnosis and before starting chemotherapy.
Post-chemotherapy levels of blood cell types: These were the levels of blood cells after completing the phase IV treatment of chemotherapy.
Hemoglobin level: The hemoglobin level in female patients was considered low if below 12 g/dL, normal if between 12 g/dL and 16 g/dL, and high if above 16 g/dL. In male patients, it was categorized as low if below 13 g/dL, normal if between 13 g/dL and 18 g/dL, and high if above 18 g/dL (17).
2.6 Variables of the study
2.6.1 Independent variable
Chemotherapy was the independent variable.
2.6.2 Dependent variables
Hematological parameters such as red blood cells, hemoglobin, hematocrits, white blood cells, neutrophils, lymphocytes, and platelets were the dependent variables.
2.6.3 Data collection tools
A data extraction method was used to collect the required information from secondary data sources. Therefore, to collect the necessary information, the register logbook was first reviewed, and the patients’ medical registration numbers were recorded. Redundant medical registration numbers were removed using Excel. Finally, data were collected from SmartCare and individual patient folders.
2.7 Method of data processing and analysis
First, the data were entered and cleaned using EPIDATA (version 4.6). Next, the data were exported to Statistical Package for Social Sciences (SPSS) (version 23) for analysis. Continuous data were analyzed using means, and categorical data were analyzed using Pearson’s chi-squared test to determine the presence of categorical differences before and after chemotherapy. The changes in hematological parameters before and after chemotherapy were compared using a paired t-test. All assumptions of the test were met.
2.8 Data quality control
The data preparation checklist was adapted from different articles. The prepared checklist format was first tested on 50 samples before starting the actual data collection. The purpose of the study was briefly explained to the data collectors. The data collectors included one nurse from the oncology unit, one SmartCare administrator, and one card room worker. A one-day training session was attended by these data collectors. Daily supervision was conducted to ensure proper completion of the checklist.
3 Results
3.1 Sociodemographic characteristics of the patients
Data were collected from 354 patients who had all types of cancer before and after phase IV chemotherapy between September 2018 and September 2022. The number of female and male patients was 215 (60.7%) and 139 (39.3%), respectively. The mean age of the respondents was 41.26 (±16.67) years (Figure 1).
Figure 1

The above bar chart shows the age and sex of patients with cancer at the oncology center of Mekele Comprehensive and Specialized Hospital in 2022.
3.2 Types of cancer in the study participants
Among the total patients, the most common types of cancer were breast cancer (133, 37.6%), lymphoma (89, 25.1%), and sarcoma (36, 10.2%) (Table 1). At the time of first contact, nearly all of them were diagnosed with stage III and stage IV cancer, with 167 (47%) and 159 (45%) cases, respectively (Figure 2).
Table 1
| Type of cancer | Measure of blood cells pre-and post-chemotherapy | Mean | Mean paired difference | 95% CI of mean differences | t-value | Df | p-value | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Breast cancer | Red blood cell pre-chemotherapy | 5.32 | 0.92 | −0.12 | 1.97 | 1.74 | 132 | 0.040* |
| Red blood cell post-chemotherapy | 4.39 | |||||||
| White blood cell pre-chemotherapy | 6.46 | 1.09 | 0.35 | 1.84 | 2.91 | 132 | 0.002* | |
| White blood cell post-chemotherapy | 5.36 | |||||||
| Platelet pre-chemotherapy | 332.9 | 22.7 | 1.75 | 43.66 | 2.14 | 132 | 0.017* | |
| Platelet post-chemotherapy | 310.2 | |||||||
| Colon cancer | White blood cell pre-chemotherapy | 5.87 | 0.73 | −0.11 | 1.57 | 1.79 | 22 | 0.042* |
| White blood cell post-chemotherapy | 5.14 | |||||||
| Red blood cell pre-chemotherapy | 4.70 | 0.12 | 0.12 | 0.56 | 1.0 | 22 | 0.160 | |
| Red blood cell post-chemotherapy | 4.59 | |||||||
| Platelet pre-chemotherapy | 320.78 | 79.56 | 29.12 | 129.93 | 3.27 | 22 | 0.002* | |
| Platelet post-chemotherapy | 241.21 | |||||||
| Leukemia | White blood cell pre-chemotherapy | 32.82 | 18.17 | −3.23 | 39.58 | 2.07 | 6 | 0.041* |
| White blood cell post-chemotherapy | 14.65 | |||||||
| Red blood cell pre-chemotherapy | 2.35 | −0.36 | −1.38 | 0.66 | −0.86 | 6 | 0.790 | |
| Red blood cell post-chemotherapy | 2.71 | |||||||
| Platelet pre-chemotherapy | 57.77 | −110 | −196.4 | −24.30 | −3.13 | 6 | 0.989 | |
| Platelet post-chemotherapy | 168.14 | |||||||
| Lung cancer | White blood cell pre-chemotherapy | 8.53 | 0.85 | −2.26 | 3.98 | 0.64 | 7 | 0.268 |
| White blood cell post-chemotherapy | 7.68 | |||||||
| Red blood cell pre-chemotherapy | 5.27 | 0.48 | 0.07 | 0.89 | 2.74 | 7 | 0.014* | |
| Red blood cell post-chemotherapy | 4.79 | |||||||
| Platelet pre-chemotherapy | 296.50 | −59.75 | −115.36 | −4.13 | −2.54 | 7 | 0.980 | |
| Platelet post-chemotherapy | 356.25 | |||||||
| Lymphoma | White blood cell pre-chemotherapy | 21.30 | 6.02 | −1.39 | 13.44 | 1.61 | 88 | 0.05* |
| White blood cell post-chemotherapy | 15.27 | |||||||
| Red blood cell pre-chemotherapy | 4.37 | 0.23 | 0.07 | 0.38 | 2.85 | 88 | 0.002* | |
| Red blood cell post-chemotherapy | 4.14 | |||||||
| Platelet pre-chemotherapy | 317.00 | 26.53 | −4.44 | 57.53 | 1.70 | 88 | 0.064 | |
| Platelet post-chemotherapy | 290.46 | |||||||
| Others* | White blood cell pre-chemotherapy | 9.60 | 0.27 | −2.93 | 3.48 | 0.17 | 35 | 0.431 |
| White blood cell post-chemotherapy | 9.33 | |||||||
| Red blood cell pre-chemotherapy | 4.56 | 0.54 | 0.25 | 0.82 | 3.85 | 35 | 0.000* | |
| Red blood cell post-chemotherapy | 4.02 | |||||||
| Platelet pre-chemotherapy | 297.69 | 13.94 | −41.74 | 69.63 | 0.50 | 35 | 0.307 | |
| Platelet post-chemotherapy | 283.75 | |||||||
| Rectal cancer | White blood cell pre-chemotherapy | 5.39 | −0.72 | −1.76 | 0.31 | −1.50 | 14 | 0.922 |
| White blood cell post-chemotherapy | 6.12 | |||||||
| Red blood cell pre-chemotherapy | 4.47 | −0.23 | −0.45 | −0.01 | −2.26 | 14 | 0.980 | |
| Red blood cell post-chemotherapy | 4.70 | |||||||
| Platelet pre-chemotherapy | 334.40 | 37.26 | −27.74 | 102.27 | 1.22 | 14 | 0.119 | |
| Platelet post-chemotherapy | 297.13 | |||||||
| Sarcoma | White blood cell pre-chemotherapy | 6.72 | 1.23 | 0.11 | 2.34 | 2.44 | 35 | 0.016* |
| White blood cell post-chemotherapy | 5.49 | |||||||
| Red blood cell pre-chemotherapy | 4.66 | 0.30 | −0.00 | 0.60 | 2.02 | 35 | 0.025* | |
| Red blood cell post-chemotherapy | 4.36 | |||||||
| Platelet pre-chemotherapy | 5.01 | 0.82 | −22.71 | 59.98 | 0.91 | 35 | 0.183 | |
| Platelet post-chemotherapy | 4.19 | |||||||
| Stomach cancer | White blood cell pre-chemotherapy | 5.01 | 0.81 | −2.69 | 4.33 | 0.74 | 3 | 0.256 |
| White blood cell post-chemotherapy | 4.19 | |||||||
| Red blood cell pre-chemotherapy | 3.99 | 0.43 | −1.58 | 2.45 | 0.68 | 3 | 0.270 | |
| Red blood cell post-chemotherapy | 3.56 | |||||||
| Platelet pre-chemotherapy | 348.5 | 71.5 | −141.44 | 284.44 | 1.06 | 3 | 0.181 | |
| Platelet post-chemotherapy | 277 | |||||||
| Bone cancer | White blood cell pre-chemotherapy | 5.27 | 1.63 | −4.33 | 7.59 | 1.17 | 2 | 0.180 |
| White blood cell post-chemotherapy | 3.64 | |||||||
| Red blood cell pre-chemotherapy | 19.34 | 16.17 | −44.54 | 76.89 | 1.14 | 2 | 0.185 | |
| Red blood cell post-chemotherapy | 3.17 | |||||||
| Platelet pre-chemotherapy | 241 | 115.33 | −58.84 | 289.51 | 2.84 | 2 | 0.052 | |
| Platelet post-chemotherapy | 125.66 | |||||||
Difference in the counts of blood cell types before and after chemotherapy by cancer type among patients with cancer at the oncology center of Mekele Comprehensive and Specialized Hospital in 2022.
Red blood cells and white blood cells were measured in units of 106 cells/mm3, while platelets were measured in units of 103 cells/mm3. A p-value marked * indicates the presence of significant paired differences.
Figure 2
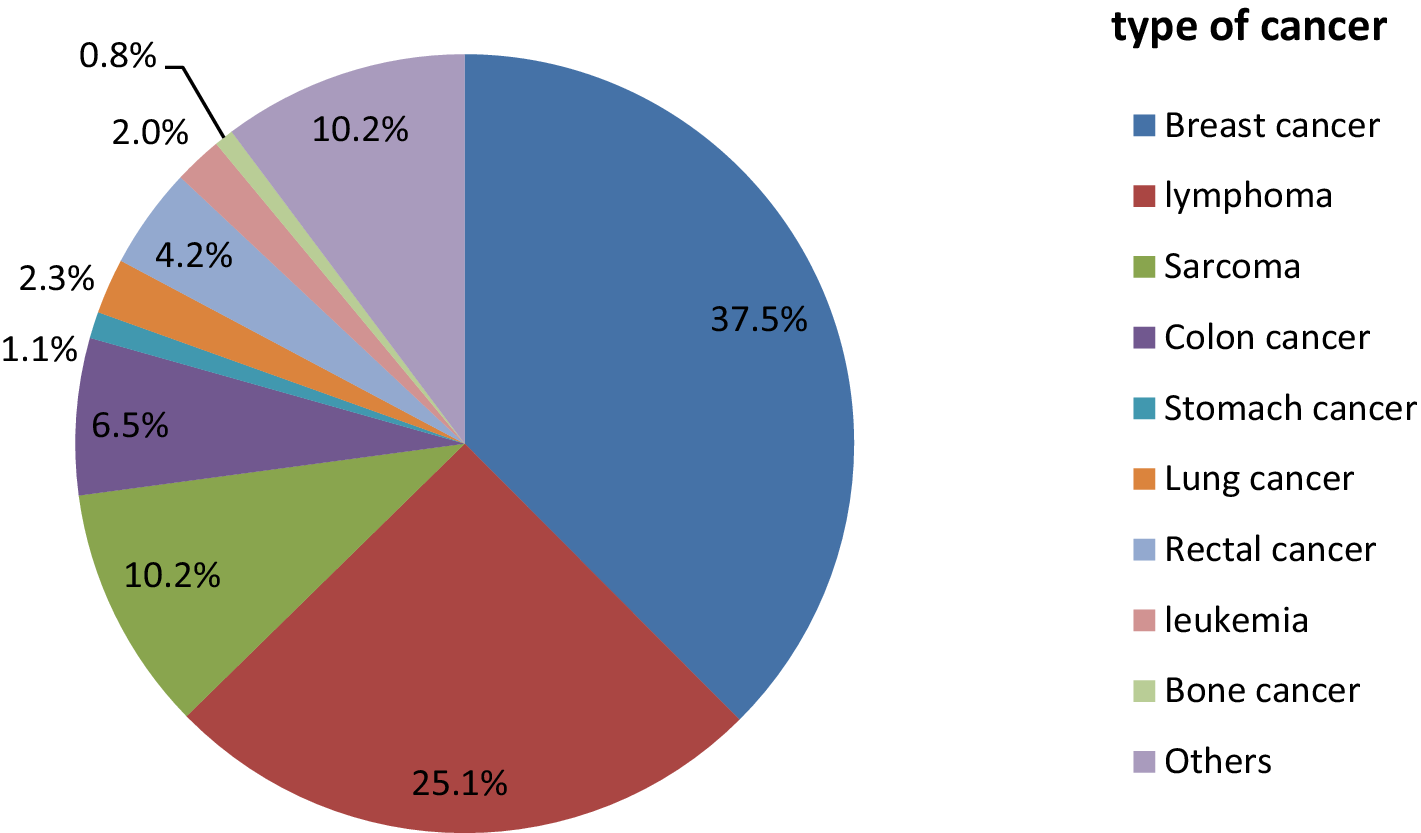
The above pie chart demonstrates the types of cancer among the cancer patients at the oncology center of Mekele Comprehensive and Specialized Hospital in 2022. Others refer to cervical cancer, testicular cancer, uterine cancer, pancreatic cancer, and skin cancer.
3.3 Hematological abnormalities among patients with different types of cancer under chemotherapy
The prevalence of anemia among cancer patients before and after chemotherapy was 25.14% (95%:20.88, 29.94) and 35.54% (95%: 30.75; 40.73), respectively. The frequency of neutropenia among cancer patients was 22.32% (95% 18.26; 26.96) and 27.97% (95%:23.52; 32.88) before and after chemotherapy, respectively. The prevalence of lymphopenia among the study participants was 16.38% (95%:12.87; 20.62) and 17.51% (95% 13.88; 21.84) before and after chemotherapy, respectively (Table 2).
Table 2
| Hematological parameters | Categories | Frequency before chemotherapy | Frequency after chemotherapy | Cutoff points | X2-test | p-value | ||
|---|---|---|---|---|---|---|---|---|
| N (%) | [95% CI] | N (%) | [95% CI] | |||||
| Hemoglobin (female) | Low | 51 (23.72%) | 18.48; 29.90 | 72 (33.49%) | 27.46; 40.09 | <12 g/dL | 85.04 | 0.000 |
| Normal | 157 (73.02%) | 66.66; 78.56 | 142 (66.05%) | 59.42: 72.09 | 12-16 g/dL | |||
| High | 7 (3.25%) | 1.55; 6.69 | 1 (0.47%) | 0.064; 03.26 | >16 g/dL | |||
| Hemoglobin (male) | low | 56 (40.29%) | 32.40; 48.71 | 76 (54.68%) | 46.27; 62.81 | <13 g/dL | 18.79 | 0.001 |
| Normal | 81 (58.27) | 49.84; 66.24 | 44.60 (44.60) | 36.49; 53.01 | 13-18 g/dL | |||
| high | 2 (1.44) | 0.35; 5.63 | 1 (0.72) | 0.09; 5.00 | >18 g/dL | |||
| Hematocrit | Normal | 225 (63.56) | 58.39; 68.42 | 208 (58.76) | 53.53; 63.79 | 36–56% | 68.74 | 0.000 |
| Abnormal | 129 (36.44%) | 31.57; 41.60 | 146 (41.24) | 36.20; 46.46 | <36 and > 56% | |||
| Neutrophils | Low | 79 (22.32%) | 18.26; 26.96 | 99 (27.97%) | 23.52; 32.88 | <40% | 47.29 | 0.000 |
| Normal | 137 (38.70%) | 33.74; 43.89 | 136 (38.41%) | 33.47; 43.61 | 40–60% | |||
| High | 138 (38.98%) | 34.02; 44.18 | 134 (37.85%) | 28.86; 38.71 | >60% | |||
| Lymphocytes | Low | 58 (16.38%) | 12.87; 20.62 | 62 (17.51%) | 13.88; 21.84 | <20% | 26.59 | 0.000 |
| Normal | 185 (52.26%) | 47.03; 57.43 | 189 (53.38%) | 48.43; 58.82 | 20–40% | |||
| High | 111 (31.36%) | 26.72; 36.39 | 103 (29.09%) | 24.31; 33.76 | >40% | |||
| White blood cells | Low | 53 (14.97%) | 11.61; 19.09 | 103 (29.10%) | 24.58; 34.06 | <4,000 | 116.70 | 0.000 |
| Normal | 253 (71.47%) | 66.52; 75.94 | 216 (61.02%) | 55.81; 65.97 | 4,000–11,000 | |||
| High | 48 (13.56%) | 10.36; 17.55 | 35 (9.89%) | 7.17; 13.47 | >11,000 | |||
| Platelets | Low | 32 (9.04%) | 6.45; 12.51 | 41 (11.58%) | 8.63; 15.36 | <150,000 | 66.76 | 0.000 |
| Normal | 273 (77.12%) | 72.43; 81.21 | 292 (82.48%) | 76.93; 85.09 | 150,000-450,000 | |||
| High | 49 (13.84%) | 10.61; 17.86 | 21 (5.93%) | 3.89; 8.93 | >450,000 | |||
Hematological parameters of cancer patients before and after chemotherapy at Mekele Comprehensive and Specialized Hospital in 2022.
White blood cells and platelets were measured by cells/μL.
3.4 Difference in the count of blood cell types pre-and post-chemotherapy among patients with different types of cancer
In patients with breast cancer, all types of blood cell counts showed a significant decrease after chemotherapy. White blood cells and red blood cells were reduced after chemotherapy among sarcoma and lymphoma patients, with paired mean differences of 6.02 × 106 cells/mm3, 0.23 × 106 cells/mm3, 1.23 × 106 cells/mm3, and 0.30 × 106 cells/mm3 (Table 1).
In patients with stomach, rectal, and bone cancers, there was no significant difference in the counts of all blood cell types before and after chemotherapy. This study also revealed that there was an increase in platelets and red blood cells after chemotherapy among leukemia patients, although this increase was not significantly different.
The “Others*” category refers to cervical cancer, testicular cancer, uterine cancer, pancreatic cancer, and skin cancer.
3.5 Difference in the count of blood cell types pre-and post-chemotherapy among all types of cancer patients
The profile of blood cell types before and after chemotherapy for all types of cancer is shown in Table 3. Three types of blood cells—red blood cells, white blood cells, and platelets—were measured at the time of diagnosis and after phase IV chemotherapy. The mean values of red blood cells and white blood cells were measured in cells per mm3 × 106. The mean value of platelets was measured in cell mm3 × 103 (Table 1).
Table 3
| Pairs | Measure of blood cells pre-and post-Chemotherapy | Mean | Mean paired difference | 95% CI of mean differences | t-value | Df | p-value | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Pair1 | Red blood cell pre-chemotherapy | 4.90 | 0.63 | 0.17 | 1.10 | 2.67 | 353 | 0.008 |
| Red blood cell post-chemotherapy | 4.27 | |||||||
| Pair2 | White blood cell pre-chemotherapy | 10.99 | 2.49 | 0.55 | 4.44 | 2.52 | 353 | 0.012 |
| White blood cell post-chemotherapy | 8.50 | |||||||
| Pair3 | Platelet pre-chemotherapy | 316.82 | 23.51 | 9.57 | 37.47 | 3.31 | 353 | 0.001 |
| Platelet post-chemotherapy | 293.30 | |||||||
Comparison of the levels of blood cell types between the pre-and post-chemotherapy periods at Mekele Comprehensive and Specialized Hospital in 2022.
As shown in Table 3, all profiles of blood cell types showed a decrease post-chemotherapy compared to pre-chemotherapy. The mean decrease in platelets was 23.51 × 103 cells/mm3 (p = 0.001), in red blood cells was 0.63 × 106 cells/mm3 (p = 0.08), and in white blood cells was 2.49 × 106 cells/mm3 (p = 0.012).
4 Discussion
Chemotherapy is a treatment with high toxicity to normal cells. Different cancer patients who receive chemotherapy often experience alterations in hematological profiles. Cancer chemotherapy treatments have devastating effects on the patient’s hematopoietic system (16).
Cancer treatment has adverse effects on normal blood cell types. The drugs used in this treatment are cytotoxic to active cells (9, 20, 21). Cancer cells are abnormal and can multiply uncontrollably, while the disadvantage of chemotherapy drugs occurs when these drugs disrupt the normal function and multiplication of healthy cells (1). Cancer patients who undergo chemotherapy face substantially life-threatening complications, such as low hemoglobin levels and a reduction in white blood cells. This leads to anemia and increased susceptibility to various infections (16).
In this retrospective study, the paired mean values of blood cell types were measured pre-and post-chemotherapy in the patients. The result of this study revealed that the mean count of red blood cells was reduced post-chemotherapy compared to pre-chemotherapy in the patients with breast cancer, lymphoma, lung cancer, and sarcoma. This study is in line with a study conducted on the effect of chemotherapy and radiotherapy on red blood cells and hemoglobin in cancer patients (9, 22). This suppression of red blood cells might be because chemotherapy suppresses the bone marrow, which produces normal and active red blood cells (5, 23). Another explanation for this reduction in red blood cells is that many drugs that are used to treat cancerous cells damage normal red blood cells. Therefore, the reduction in red blood cells leads to a decrease in hemoglobin and hematocrit. Erythropoiesis production may be compromised because of chemotherapy, and this disruption leads to a reduction in red blood cells. Drugs of chemotherapy also induce renal toxicity. Nephrotoxic drugs used in chemotherapy significantly disrupt erythropoietin production. Another reason for this reduction may be a decrease in erythropoietin levels. Chemotherapy has a fragility effect on red blood cells (22). In this study, the prevalence of anemia before and after chemotherapy was 25.14 and 35.59%, respectively. This study is similar to a study conducted in Gondar (24). This similarity may be due to the use of similar chemotherapy cycles to treat patients with cancer in both studies. Another reason for this similarity may be that the regimen of chemotherapy used to treat patients in both study areas is similar. However, the prevalence of anemia among the study participants in the current study was lower than that in a study conducted in northwest Ethiopia (25). This difference might be due to the different chemotherapy regimens used to treat patients. Another reason may be that the number of treatment cycles in the current study is lower compared to the previous study. High numbers of chemotherapy cycles are associated with chemotherapy-induced anemia.
The platelet count in this study showed a significant difference between pre-and post-chemotherapy levels in the patients with cancer. This decrease in platelet counts post-chemotherapy might be due to the destruction of megakaryocyte progenitors during the early stage of differentiation caused by chemotherapy. The difference may be due to the disruption of hormones produced in the kidney and liver that are involved in platelet production by chemotherapy (2, 3). The platelet count post-chemotherapy compared to pre-chemotherapy was low. This study contrasts with a meta-analysis conducted on ovarian cancer (19). This difference may be due to the fact that this study included all types of cancer, while the mentioned study was focused only on ovarian cancer. The results of this study are similar to those of a study conducted in Ethiopia comparing biochemical and hematological profiles, as well as another study on drug-induced neutropenia (26, 27). This similarity may be due to the similar characteristics of the study participants in terms of social, economic, and environmental factors.
According to the result of this study, the white blood cell count decreased after chemotherapy compared to pre-chemotherapy among the patients with breast cancer, colon cancer, leukemia, lymphoma, and sarcoma. This study is consistent with a study conducted on responses to chemotherapy and remission induction therapies (28, 29). This similarity may have occurred because the administered drugs for chemotherapy were similar. Another reason for this consistent result may be that chemotherapy has a cytotoxic effect that disrupts the normal production of white blood cells. In contrast, other studies have revealed a significant increase in white blood cells post-chemotherapy compared to pre-chemotherapy (30). This increase may be due to chemotherapy inducing infections in these patients, which leads to an increase in white blood cells.
In this study, the types of cancer and their frequencies were identified. Breast cancer, lymphoma, and sarcoma were the top three cancer types in this study. This distribution is consistent with trends in global cancer epidemiology, where breast cancer continues to rank among the most commonly diagnosed cancers, especially in women (31). The substantial burden of hematologic and soft tissue cancers in our sample was further suggested by the high frequency of lymphoma and sarcoma. This finding is similar to those of studies conducted in Logos, Nigeria, and Ethiopia (23, 32). This similarity may have occurred because the socio-demographic and biological characteristics of the study participants are similar. Another reason for this may be that they have similar risk factors for these cancer types. The late-stage manifestation of cancer at the time of initial interaction is one of our study’s most alarming findings. There was a significant delay in diagnosis and treatment initiation, as nearly all patients were diagnosed at stages III (47%) and IV (45%). Many factors could be responsible for this delay, such as restricted access to healthcare services, ignorance of early symptoms, and even socioeconomic constraints that impede prompt medical evaluation. Lymphoma was the second most prevalent type of cancer in our study. This study is similar to a study conducted at Ayder Hospital, Ethiopia (27). The consistency of this finding may be due to similarities in socio-demographic characteristics. Another reason for this similarity may be that there are similar determinants for lymphoma cancer. Infections, weakening of the immune system, and exposure to chemicals are risk factors for lymphoma. The study participants in the current study and the other study may have had similar exposure status.
The findings of this study revealed a difference in the measurement of hematological parameters. The level of hemoglobin post-chemotherapy decreased compared to pre-chemotherapy. This observed difference may be related to adverse reactions in patients with normal baseline levels of hemoglobin (33, 34). Chemotherapy-induced bone marrow damage is accompanied by nerve injury to the bone marrow. Another reason for the observed difference might be related to dysfunction in hematopoietic production. This results in chronic bone marrow damage caused by chemotherapy (35, 36).
Lymphocytes, a type of white blood cell involved in immune responses, are specifically sensitive to chemotherapy and often decrease as a result. Their depletion, known as lymphopenia, weakens the adaptive immune system, increasing the patient’s vulnerability to viral infections and reducing overall immune surveillance against malignancies. The current study is similar to other studies that have shown that chemotherapy-induced lymphopenia is associated with poorer overall survival in cancer patients (37, 38).
Neutrophil is a vital component that plays a significant role in fighting microorganisms. In the current study, there was a decrease in the levels of neutrophils pre-chemotherapy compared to post-chemotherapy. This observed difference is in line with previous studies (27, 39). This similarity may be related to the same chemotherapy regimen given to these patients. Another reason for this similarity may be the neutrophil-suppressive effects of chemotherapy in the present study.
In the present study, leukopenia was observed after chemotherapy. However, this finding contrasts with the results of a study on leukocytosis, thrombocytosis, and early mortality among cancer patients initiating chemotherapy (40). This contrasting finding may be due to tissue damage, inflammation, and emotional stress after chemotherapy, which can lead to increased leukocyte production, as observed in the results of the previous study.
4.1 Strengths and limitations of the study
-
Strength: All types of cancer patients were included in the study
4.2 Limitations
-
Since data were collected from a secondary source, some data were not registered
-
Other biochemical profiles were not considered
-
Changes from phase to phase were not compared
5 Conclusion and recommendations
Chemotherapy has a reducing effect on lymphocytes, neutrophils, WBCs, hemoglobin, and platelets. These reductions not only compromise immune function but also increase the risk of infections, bleeding disorders, and treatment delays. Comparative studies have also shown that severe lymphopenia and neutropenia can significantly impact patient prognosis, leading to higher morbidity and mortality if left unmanaged. We also recommend that clinicians implement proactive monitoring and personalized management strategies to balance effective chemotherapy treatment while minimizing hematological toxicity.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Debre Markos University College Medicine and Healthscience ethical review committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
TK: Formal analysis, Writing – original draft, Writing – review & editing. BY: Conceptualization, Data curation, Writing – original draft. GB: Conceptualization, Data curation, Writing – original draft. RS: Conceptualization, Data curation, Writing – original draft. AA: Formal Analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
First, we would like to express our gratitude to the almighty God. Second, we would also like to thank Debre Markos University, the College of Medicine and Health Sciences, and the Research and Community Service Office for their support and technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DNA, deoxyribonucleic acid; LDH, lactate dehydrogenase; RBC, red blood cell; WBC, white blood cells.
References
1.
Noviyani R Indrayathi PA Budiana IG Niruri R Tunas K Adnyani NDD . Effect of paclitaxel-cisplatin chemotherapy towards hemoglobin, platelet, and leukocyte levels in epithelial ovarian cancer patients. J Appl Pharmaceutical Sci. (2019) 9:104–7.
2.
Ferroni P Guadagni F Riondino S Portarena I Mariotti S La Farina F et al . Evaluation of mean platelet volume as a predictive marker for cancer-associated venous thromboembolism during chemotherapy. Haematologica. (2014) 99:1638–44. doi: 10.3324/haematol.2014.109470
3.
Bessman D . Prediction of platelet production during chemotherapy of acute leukemia. Am J Hematol. (1982) 13:219–27. doi: 10.1002/ajh.2830130305 PMID:
4.
Lodish HF . Molecular cell biologyMacmillan (2008).
5.
Gebremeskel G Baye G . Bayu Wondimneh Sathisha Anekere Dasappa Setty 2 Gebrekidan Gebregzabher Asfeha 2 Ezra Belay 2. Cancer Manag Res. (2021) 13:625–32.
6.
Aziz HA Habeeb JM . Study the effect of chemotherapy on some hematological and biochemical parameters of Cancer patients in AL-Muthanna Province, Iraq. Indian J Public Health Res Develop. (2019) 10:813. doi: 10.5958/0976-5506.2019.00395.4
7.
Rodgers GM Becker PS Blinder M Cella D Chanan-Khan A Cleeland C et al . Cancer- and chemotherapy-induced Anemia (2012) 10:628–53. doi: 10.6004/jnccn.2012.0064
8.
Kramer AH Gurka MJ Nathan B Dumont AS Kassell NF . Complications associated with anemia and blood transfusion in patients with aneurysmal subarachnoid hemorrhage. TPJCCM. (2008) 36:2070–5. doi: 10.1097/CCM.0b013e31817c1095
9.
Younis M Iqbal M Shoukat N Nawaz B Watto F Shahzad KA . Effect of chemotherapy and radiotherapy on red blood cells and haemoglobin in cancer patients. Sci Lett. (2014) 2:15–8.
10.
Lowenthal RM Eaton K . Toxicity of chemotherapy. Hematol Oncol Clin. (1996) 10:967–90. doi: 10.1016/S0889-8588(05)70378-6
11.
Banfi A Podesta M Fazzuoli L Roberto Sertoli M Venturini M Santini G et al . High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: a mechanism for post-bone marrow transplantation osteopenia. Cancer. (2001) 92:2419–28. doi: 10.1002/1097-0142(20011101)92:9<2419::AID-CNCR1591>3.0.CO;2-K
12.
Padma R Sundaresan S . Haematological and biochemical changes in pre and post-treatment of buccal mucosa carcinoma patients. Int J Pharm Sci Res. (2016) 7:3002–6. doi: 10.13040/IJPSR.0975-8232.7
13.
Woods D . Chemotherapy induced DNA damage response: convergence of drugs and pathways. Cancer Biol Ther. (2013) 14:379–89. doi: 10.4161/cbt.23761
14.
Wei Y-S Zhou Y-G Wang G-Y Liang Z-H Luo M-R Yang T-A et al . The impact of chemotherapy-associated hemoglobin on prognosis of colorectal cancer patients receiving adjuvant chemotherapy. Cancer Biomark. sage. (2017) 20:627–35. doi: 10.3233/CBM-170601
15.
Medrano-Casique N Tong HY Borobia AM Carcas AJ Frías J Ramírez E . Nonchemotherapy drug-induced agranulocytosis in children detected by a prospective pharmacovigilance program. Pediatr Hematol Oncol. (2016) 33:441–56. doi: 10.1080/08880018.2016.1234523
16.
Siena S Secondino S Giannetta L Carminati O Pedrazzoli P . Optimising management of neutropenia and anaemia in cancer chemotherapy—advances in cytokine therapy. Crit Rev Oncol Hematol. (2003) 48:S39–47. doi: 10.1016/j.critrevonc.2003.05.002
17.
Sobin LH Gospodarowicz MK Wittekind C . TNM classification of malignant tumoursJohn Wiley & Sons (2011).
18.
Network N. Cancer-and induced Anemia. NCCN clinical practice guidelines in oncology (NCCN guidelines®). (2018): 1–51.
19.
Eggemann H Ehricke J Ignatov T Fettke F Semczuk A Costa S et al . Platelet count after chemotherapy is a predictor for outcome for ovarian cancer patients. Cancer Investig. (2015) 33:193–6. doi: 10.3109/07357907.2015.1020384
20.
Nowrousian M Waschke S Bojko P Welt A Schuett P Ebeling P et al . Impact of chemotherapy regimen and hematopoietic growth factor on mobilization and collection of peripheral blood stem cells in cancer patients. Ann Oncol. (2003) 14:i29–36. doi: 10.1093/annonc/mdg706
21.
Aldarouish M Su X Qiao J Gao C Chen Y Dai A et al . Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer. Int J Immunopathol Pharmacol. (2019) 33:2058738419839592. doi: 10.1177/2058738419839592
22.
Khoshbin AR Mohamadabadi F Vafaeian F Babania A Akbarian S Khandozi R et al . The effect of radiotherapy and chemotherapy on osmotic fragility of red blood cells and plasma levels of malondialdehyde in patients with breast cancer. Rep Pract Oncol Radiother. (2015) 20:305–8. doi: 10.1016/j.rpor.2014.11.002
23.
Akinbami A Popoola A Adediran A Dosunmu A Oshinaike O Adebola P et al . Full blood count pattern of pre-chemotherapy breast cancer patients in Lagos, Nigeria. Caspian J Intern Med. (2013) 2012:1–6. doi: 10.1155/2012/352753
24.
Aynalem M Adem N Wendesson F Misganaw B Mintesnot S Godo N et al . Hematological abnormalities before and after initiation of cancer treatment among breast cancer patients attending at the University of Gondar comprehensive specialized hospital cancer treatment center (2022) 17:e0271895. doi: 10.1371/journal.pone.0271895
25.
Wondm SA Dagnew SB Gubae K Tesfaye TC FBJFiM T . Determinants of anemia among patients receiving cancer chemotherapy in Northwest Ethiopia. Front Med (Lausanne). (2024) 11:1415877. doi: 10.3389/fmed.2024.1415877
26.
Moore DCJP . Drug-induced neutropenia: a focus on rituximab-induced late-onset neutropenia. Therapeutics. (2016) 41:765.
27.
Wondimneh B Anekere Dasappa Setty S Gebregzabher Asfeha G Belay E Gebremeskel G GJCM Baye et al . Comparison of hematological and biochemical profile changes in pre-and post-chemotherapy treatment of cancer patients attended at ayder comprehensive specialized hospital, Mekelle, northern Ethiopia 2019: a retrospective cohort study. (2021): 625–632.
28.
Han HS Rybicki LA Thiel K Kalaycio ME Sobecks R Advani A et al . White blood cell count nadir following remission induction chemotherapy is predictive of outcome in older adults with acute myeloid leukemia. Leuk Lymphoma. (2007) 48:1561–8. doi: 10.1080/10428190701474373
29.
Ribeiro R Broniscer A Rivera G Hancock M Raimondi S Sandlund J et al . Philadelphia chromosome-positive acute lymphoblastic leukemia in children: durable responses to chemotherapy associated with low initial white blood cell counts. Leukemia. (1997) 11:1493–6. doi: 10.1038/sj.leu.2400749
30.
Mughal TIJHO . Current and future use of hematopoietic growth factors in cancer medicine. Hematol Oncol. (2004) 22:121–34. doi: 10.1002/hon.736
31.
DJTl P . Epidemiology of cancer: global patterns and trends. Toxicol Lett. (1998) 102-103:227–34. doi: 10.1016/S0378-4274(98)00311-7
32.
Hadgu E Seifu D Tigneh W Bokretsion Y Bekele A Abebe M et al . Breast cancer in Ethiopia: evidence for geographic difference in the distribution of molecular subtypes in Africa. BMC Womens Health. (2018) 18:1–8. doi: 10.1186/s12905-018-0531-2
33.
Wei Q Yuan X Xu Q Li J Chen L JJCM Y et al . Correlation between hemoglobin levels and the prognosis of first-line chemotherapy in patients with advanced gastric cancer. Cancer Manag Res. (2020) 12:7009–19. doi: 10.2147/CMAR.S256074
34.
Park MH Baek B Jin HK Bae J-SJAC . Novel peptides derived from neuropeptide Y prevent chemotherapy-induced bone marrow damage by regulating hematopoietic stem cell microenvironment. Anim Cells Syst (Seoul). (2018) 22:281–8. doi: 10.1080/19768354.2018.1517826
35.
Lucas D Scheiermann C Chow A Kunisaki Y Bruns I Barrick C et al . Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. (2013) 19:695–703. doi: 10.1038/nm.3155
36.
Cavaletti G Marmiroli PJNRN . Chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst. (2010) 6:657–66. doi: 10.1038/nrneurol.2010.160
37.
Vigano S Alatzoglou D Irving M Ménétrier-Caux C Caux C Romero P et al . Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front Immunol. (2019) 10:925. doi: 10.3389/fimmu.2019.00925
38.
Belkaid Y Hand TWJC . Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
39.
Lyman GH Abella E Pettengell RJC . Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. (2014) 90:190–9. doi: 10.1016/j.critrevonc.2013.12.006
40.
Connolly GC Khorana AA Kuderer NM Culakova E Francis CW Lyman GHJTR . Leukocytosis, thrombosis and early mortality in cancer patients initiating chemotherapy. Thromb Res. (2010) 126:113–8. doi: 10.1016/j.thromres.2010.05.012
41.
Organization WH . Guideline on haemoglobin cutoffs to define anaemia in individuals and populations. Geneva, Switzerland: World Health Organization (2024).
Summary
Keywords
blood cell type, chemotherapy, differences, patient with cancer, northern Ethiopia
Citation
Kassie TD, Yimenu BW, Baye Temesgen G, Shimelash RA and Abneh AA (2025) Differences in the count of blood cells pre-and post-chemotherapy in patients with cancer: a retrospective study (2022). Front. Med. 12:1485676. doi: 10.3389/fmed.2025.1485676
Received
30 August 2024
Accepted
25 March 2025
Published
16 April 2025
Volume
12 - 2025
Edited by
Daniele Maria-Ferreira, Instituto de Pesquisa Pelé Pequeno Príncipe, Brazil
Reviewed by
Dereje Mengesha Berta, University of Gondar, Ethiopia
Hadi Kuncoro, Mulawarman University, Indonesia
Abraha Gosh Woldemariam, Aksum University, Ethiopia
Updates
Copyright
© 2025 Kassie, Yimenu, Baye Temesgen, Shimelash and Abneh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadele Derbew Kassie, tadelederbew@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.