- 1Department of Pharmacy, Chi Mei Medical Center, Chiali, Taiwan
- 2Department of Pharmacy, Chi Mei Medical Center, Tainan, Taiwan
- 3School of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan
- 4Institute of Clinical Pharmacy and Pharmaceutical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5Department of Internal Medicine, Chi Mei Medical Center, Chiali, Taiwan
- 6Department of Nursing, Min-Hwei Junior College of Health Care Management, Tainan, Taiwan
Background: The impact of current inhaled corticosteroid (ICS) therapies on fracture risk in patients with chronic obstructive pulmonary disease (COPD) remains uncertain.
Objective: This study conducts a systematic review and meta-analysis to assess the risk of fractures associated with ICS use over at least 4 years, synthesizing evidence from observational studies conducted in real-world settings among individuals with COPD.
Methods: We systematically searched PubMed, EMBASE, Scopus, and Web of Science from inception to April 21, 2025. Inclusion criteria encompassed studies conducted in COPD patients, evaluating interventions involving ICS-containing treatments compared to alternatives or no ICS use, using cohort or case-control designs, and reporting outcomes related to osteoporosis or fractures. Pooled odds ratios (OR) and hazard ratios (HR) were calculated using random-effects models. Subgroup analyses and meta-regression were performed to explore sources of heterogeneity.
Results: Nine studies (six case-control, three cohort) were included. The pooled OR from case-control studies was 1.03 (95% CI: 0.99–1.08; I2 = 50%), and the pooled HR from cohort studies was 0.95 (95% CI: 0.67–1.33; I2 = 86%). Subgroup analyses indicated a potential increased risk in Asian and European populations but not in North America. Meta-regression revealed that higher oral corticosteroids exposure was significantly associated with increased risk (p = 0.005, R2 = 100%).
Conclusions: Although ICS did not significantly impact osteoporosis or fracture risk, these are common comorbidities in COPD patients. Methodological differences, such as study design, outcome definitions, and oral corticosteroids use, may influence result interpretation and contribute to heterogeneity, limiting study comparability.
Introduction
In individuals with moderate to very severe COPD and frequent exacerbation, a therapy that combines an inhaled corticosteroid (ICS) with a long-acting beta-agonist (LABA) is superior to using either medication by itself. ICS/LABA not only improves respiratory function but also diminishes the frequency of COPD exacerbations. Significant evidence from previous studies show the superiority of combining ICS/LABA over using LABA alone, particularly in patients who have had at least one exacerbation in the preceding year (1–5). For patients with COPD who have experienced previous exacerbations, a Randomized Controlled Trial (RCT) conducted in primary healthcare settings across the United Kingdom revealed that a daily combined therapy of ICS/LABA was linked to a reduction in exacerbation frequency compared to standard treatment, without an increased occurrence of serious adverse effects. The incidence of moderate or severe exacerbations was notably reduced by 8.4% in those receiving ICS/LABA compared to those under usual care (6).
Furthermore, several research findings indicate that the level of blood eosinophils can forecast the effectiveness of adding ICS to ongoing maintenance bronchodilator therapy in averting future exacerbations. The impact of ICS is directly correlated with blood eosinophil levels; at lower eosinophil counts, minimal or no benefits are noted, while the benefits progressively increase with higher eosinophil counts (7–12).
Findings from RCTs on the association between ICS treatment and the risk of reduced bone density and fractures have been inconsistent, possibly because of variations in study methodologies or differences among the ICS formulations (13–17).
Previous meta-analyses have faced limitations, including short follow-up durations and incomplete coverage of all ICS treatments. Additionally, recent observational studies, drawing on national databases from various countries (18–20), have yielded inconsistent findings. As a result, the effects of the ICS treatments currently in use on fracture risk in COPD patients are still not well-defined. Our study systematically reviews the risk of fractures associated with at least 4 years of ICS use, examining evidence from observational studies conducted in real-world settings among individuals with COPD.
Methods
Search strategy
This systematic review was conducted according to the PRISMA 2020 guidelines and registered on PROSPERO (CRD42024520403). Four databases (PubMed, EMBASE, Scopus, Web of Science) were searched from their inception to April 21, 2025. The following medical subject headings terms were used: “Pulmonary Disease, Chronic Obstructive,” “Administration, Inhalation,” “Nebulizers and Vaporizers,” “Adrenal Cortex Hormones,” “Budesonide,” “Fluticasone,” “Beclomethasone,” “Mometasone Furoate,” “Osteoporosis,” “Fractures, Bone,” “bone density,” “skeletal health,” and “bone health.” No language restrictions were imposed. Detailed search strategies are presented in Supplementary Table S1.
Study selection
Inclusion criteria for studies were as follows: (i) patients diagnosed with COPD; (ii) use of ICS-containing treatments for intervention; (iii) comparison with alternative treatments or no ICS usage; (iv) design as a cohort or case-control study; and (v) reported outcomes of osteoporosis or fractures. Exclusion criteria included: (i) studies not reporting relevant outcomes, and (ii) studies on non-human subjects. Additionally, conference abstracts, commentaries, narrative reviews, and case reports were not considered.
This review was structured based on the PICO framework: Population (adults with COPD), Intervention (ICS use), Comparison (no ICS), and Outcomes (osteoporosis or fracture).
Two investigators (SFH and FTH) independently screened the titles and abstracts of the records collected using the aforementioned search strategies to identify and assess potentially eligible studies. Disagreements were resolved by a third investigator (KML). Full-text copies of potentially relevant articles were obtained and reviewed for eligibility.
Data extraction
Data were independently extracted by two investigators (CWH and KML) using a standardized electronic form. Extracted data included: study characteristics (author, year, design, country), population demographics (sample size, sex, age), definitions of COPD and outcomes, ICS types and doses, oral corticosteroid (OCS) and bisphosphonate use, and reported effect estimates with confidence intervals. Disagreements were resolved by a third investigator (SFH).
Quality assessment
Two investigators (CWH and KML) independently assessed the quality for each of the included studies by using the Newcastle–Ottawa Scale (NOS). Disagreements were resolved through discussion and consensus with a third investigator (SFH).
Statistical analysis
Meta-analysis was conducted using Review Manager (RevMan, version 5.4; Nordic Cochrane Center, Copenhagen, Denmark) and R software (version 4.5.0) for meta-regression analysis. For case-control studies, odds ratios (ORs) with 95% confidence intervals (CIs) were pooled using the inverse-variance random-effects model. Hazard ratios (HRs) from cohort studies were likewise combined using the inverse-variance random-effects model. Heterogeneity was assessed using Cochran's Q test and quantified with the I2 statistic, with I2 values interpreted as follows: ≤25% (low), 26–74% (moderate), and ≥75% (high heterogeneity). Subgroup analysis was conducted to explore potential sources of heterogeneity based on (1) outcome definitions (e.g., osteoporosis, any fracture, specific fracture types) and (2) geographic region. Furthermore, meta-regression analysis was performed to examine whether study-level OCS or bisphosphonate exposure influenced the effect estimates. To assess the robustness of the pooled effect estimates and identify influential studies, we conducted a leave-one-out sensitivity analysis.
Results
Search results
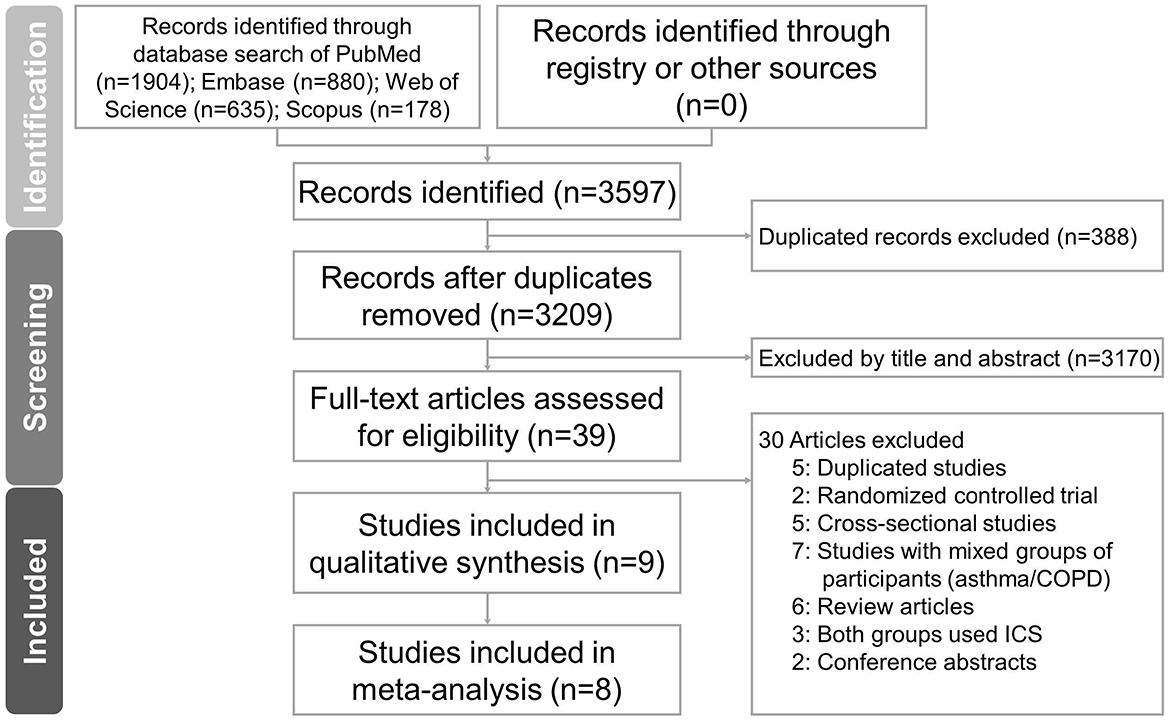
A total of 3,597 records were identified through database searching: PubMed (n = 1,904), Embase (n = 880), Web of Science (n = 635), and Scopus (n = 178). No additional records were retrieved through trial registries or other sources. After removing 388 duplicates, 3,209 records remained for title and abstract screening. Of these, 3,170 records were excluded due to irrelevance. A total of 39 full-text articles were assessed for eligibility. Following full-text review, 9 studies (18–26) met the inclusion criteria and were included in the final analysis. These comprised 6 case-control studies and 3 cohort studies, as shown in Figure 1.
Study characteristics
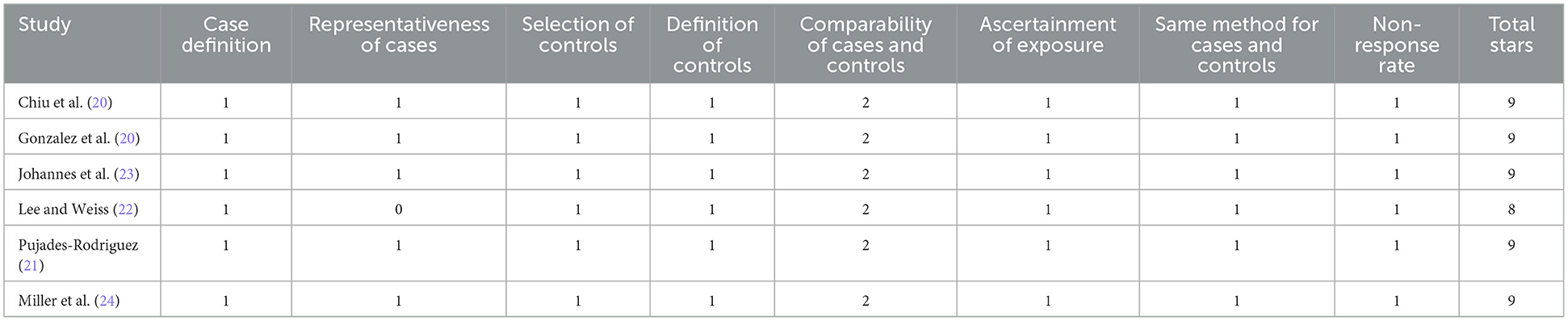
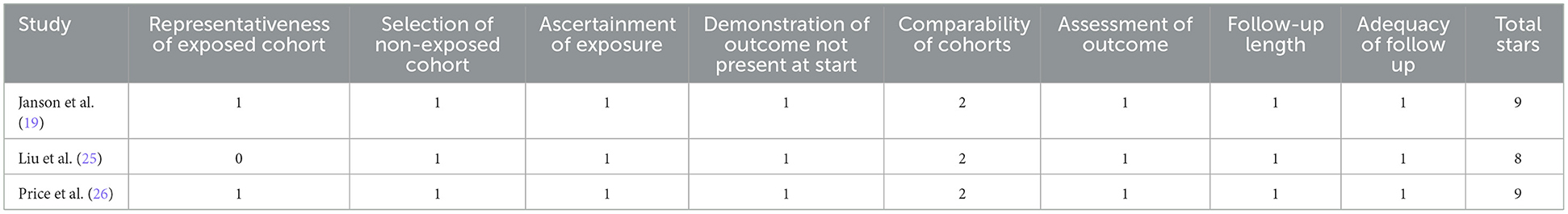
The included studies (18–26) were published between 2004 and 2021 and conducted in Taiwan, the United Kingdom, the United States, Canada, and Sweden. COPD was diagnosed using ICD-9 or ICD-10 codes across all studies. The type of ICS investigated varied and included fluticasone, budesonide, beclomethasone, and fixed-dose combinations (Tables 1, 2). The average patient age ranged from 52.3 to 74.8 years, and the proportion of male participants varied significantly between studies. OCS and bisphosphonate usage rates also differed, ranging from 13.4% to 60.8% and 1.6% to 12.7%, respectively. Study quality, assessed using the NOS, was generally high, with most studies scoring 8 or 9 stars (Tables 3, 4).
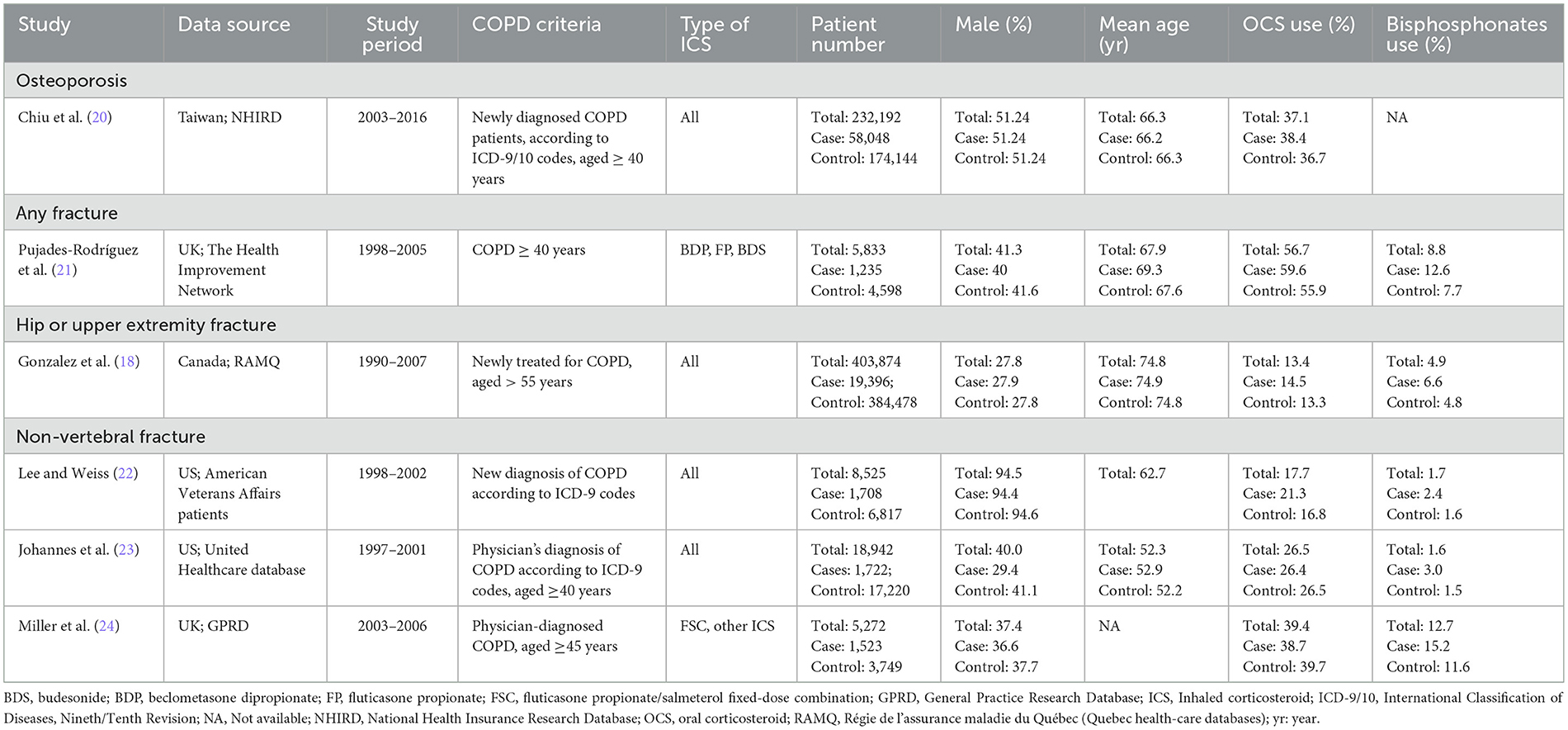
Table 1. Characteristics of case-control studies of inhaled corticosteroids (ICS) and fractures or osteoporosis.
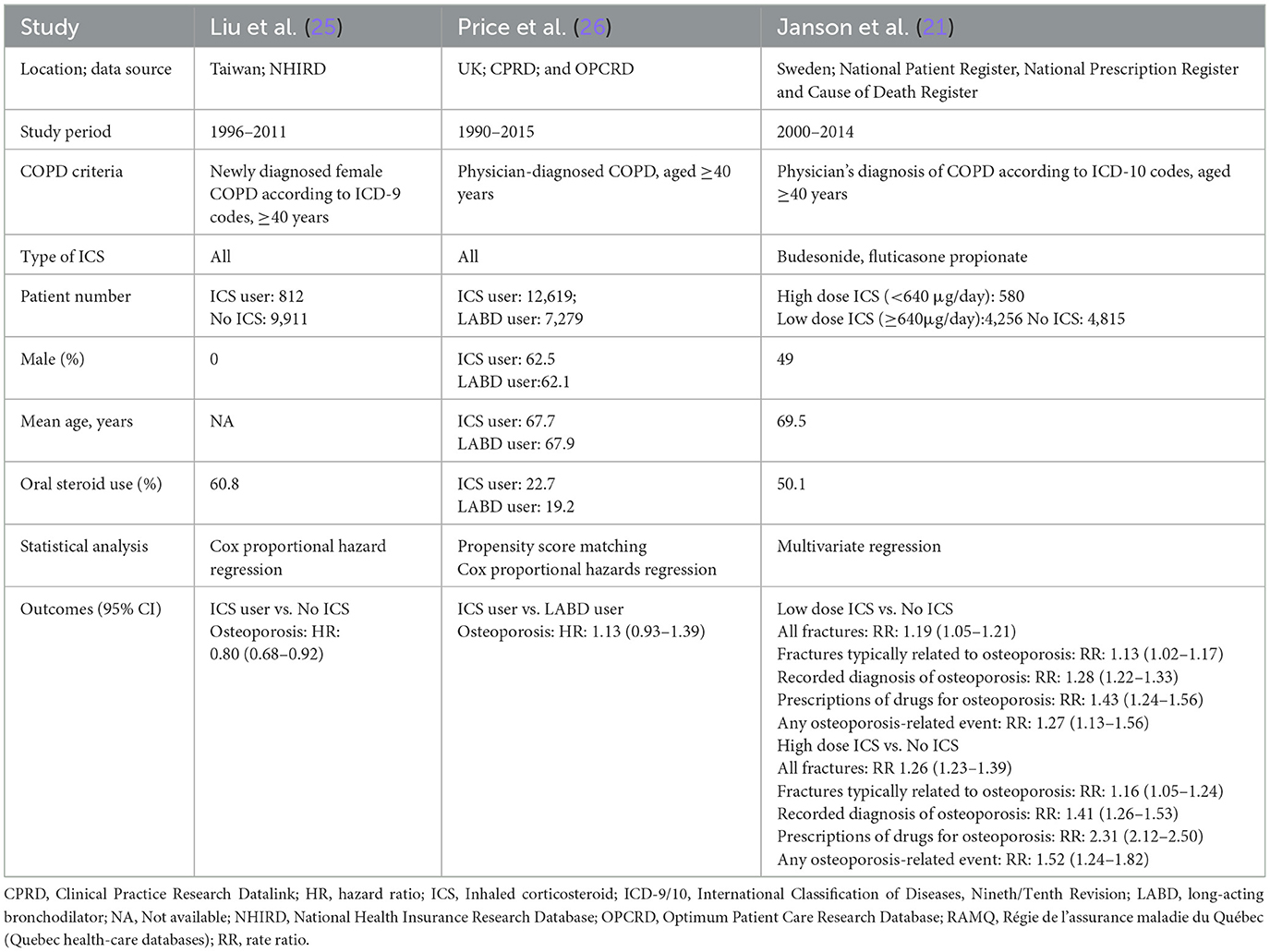
Table 2. Characteristics of cohort studies of inhaled corticosteroids (ICS) and fractures or osteoporosis.
Meta-analysis of case-control studies
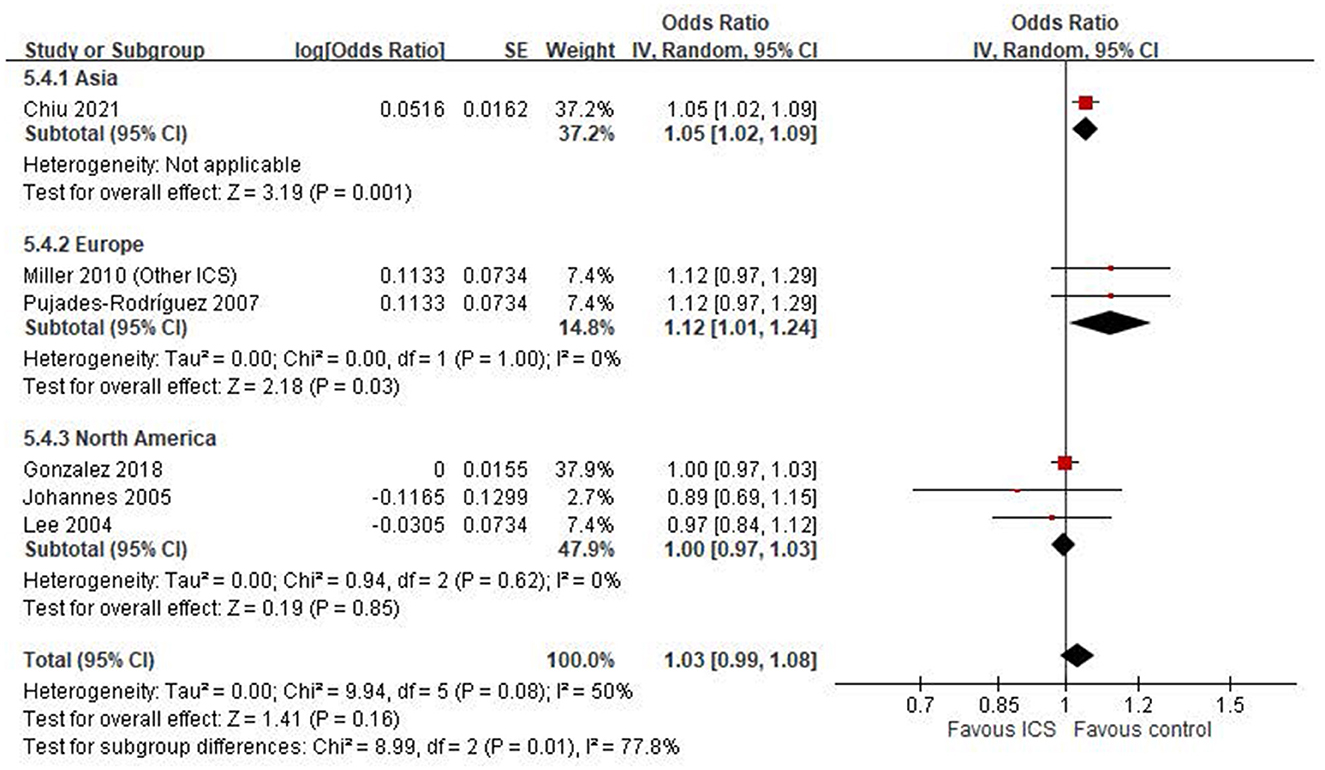
Six case-control studies (18, 20–24) were included in the meta-analysis assessing the association between ICS use and the risk of osteoporosis or fractures. The pooled odds ratio (OR) was 1.03 (95% CI: 0.99–1.08; P = 0.16), indicating a non-significant association between ICS exposure and osteoporosis or fractures risk in COPD patients (Figure 2). Heterogeneity was moderate (I2 = 50%).
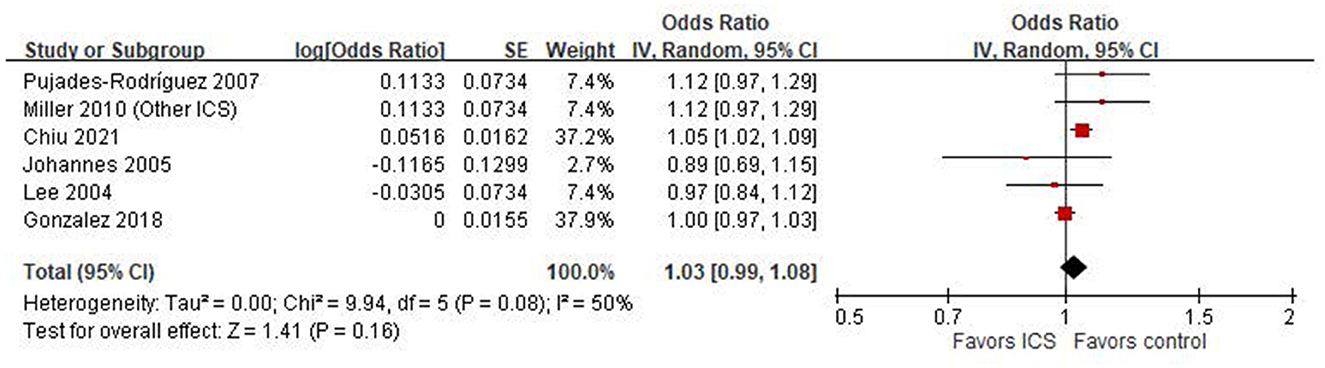
Figure 2. Forest plot of case-control studies examining the association between inhaled corticosteroids (ICS) and the risk of fractures or osteoporosis.
Meta-analysis of cohort studies
Two cohort studies (25, 26) provided HRs examining the association between ICS use and the risk of osteoporosis or fractures. The pooled HR was 0.95 (95% CI: 0.67–1.33; P = 0.75), indicating no significant association between ICS use and osteoporosis or fractures outcomes (Figure 3). However, heterogeneity was high (I2 = 86%, P = 0.008), suggesting substantial variability between studies.
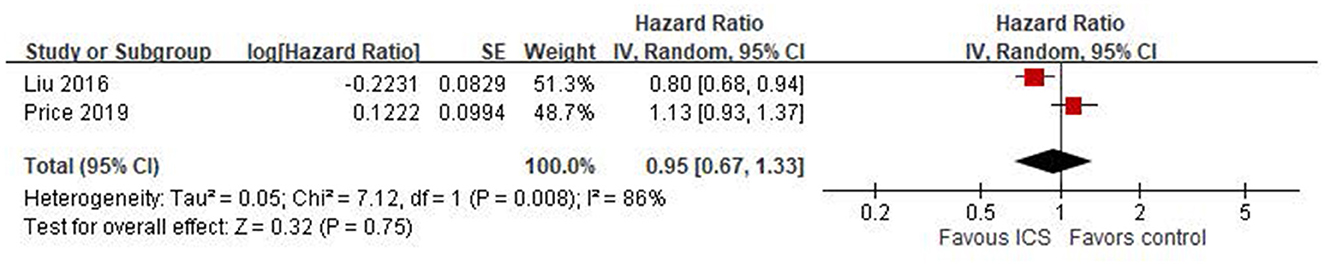
Figure 3. Forest plot of cohort studies using Cox proportional hazard regression assessing ICS-related fracture or osteoporosis risk.
Subgroup analysis by outcome definition
We conducted an exploratory subgroup analysis to examine the association between ICS use and specific skeletal outcomes (Figure 4). Among the included studies, only one reported an outcome explicitly defined as osteoporosis (20), which showed a statistically significant association with ICS use (OR: 1.05, 95% CI: 1.02–1.09, P = 0.001). For other fracture-related outcomes, results were inconsistent across individual studies. One study (21) reported an increased risk of any fracture (OR: 1.12, 95% CI: 0.97–1.29), while another (18) found a null association for hip or upper extremity fracture (OR: 1.00, 95% CI: 0.97–1.03). For non-vertebral fractures, a pooled estimate from three studies (22–24) yielded an OR of 1.01 (95% CI: 0.89–1.15, P = 0.85), with moderate heterogeneity (I2 = 37%).
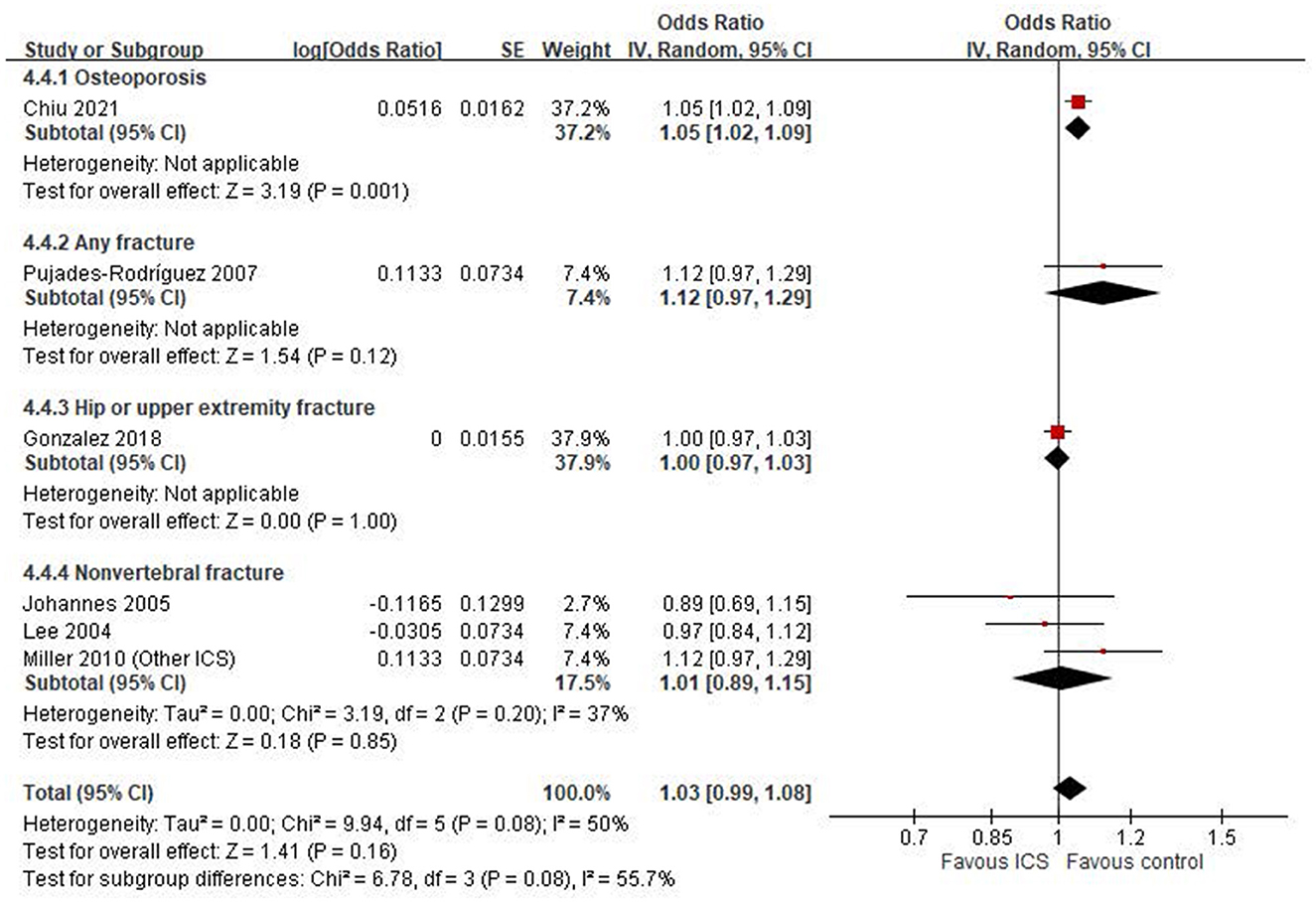
Figure 4. Subgroup analysis by outcome definition: osteoporosis, any fracture, hip or upper extremity fracture and non-vertebral fracture.
Subgroup analysis by geographic region
A subgroup analysis based on study region revealed notable differences in the association between ICS use and fracture or osteoporosis risk (Figure 5). In the Asian subgroup, represented by a single large study (20), ICS use was significantly associated with an increased risk (OR: 1.05, 95% CI: 1.02–1.09, P = 0.001). In contrast, the pooled estimate from European studies (21, 24) also demonstrated a statistically significant increase in risk (OR: 1.12, 95% CI: 1.01–1.24, P = 0.03), with no observed heterogeneity (I2 = 0%). Conversely, studies conducted in North America (18, 22, 23) showed no significant association (OR: 1.00, 95% CI: 0.97–1.03, P = 0.85). Between-region heterogeneity was statistically significant (Chi2 = 8.99, df = 2, P = 0.01; I2 = 77.8%).
Meta-regression analysis
To explore potential sources of heterogeneity, meta-regression analysis was performed using study-level covariates, including the proportion of patients receiving OCS and bisphosphonates. As illustrated in Figure 6, a significant positive association was observed between OCS prevalence and effect size (p = 0.005, R2 = 100.0%). Similarly, bisphosphonate use was also significantly associated with the effect estimates (p = 0.029, R2 = 100.0%), as shown in Figure 7.
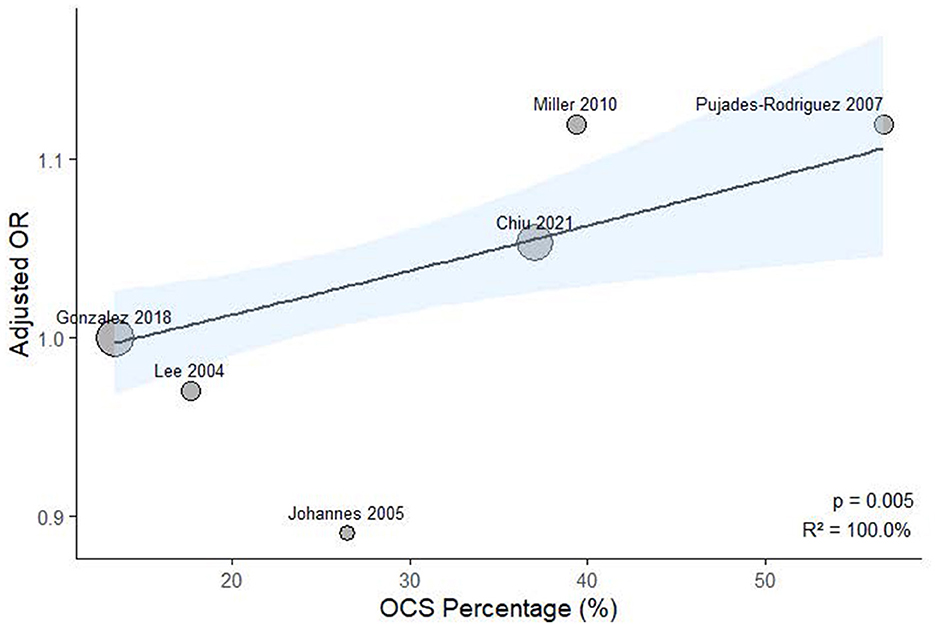
Figure 6. Meta-regression analysis examining the association between study-level oral corticosteroid exposure prevalence and ICS-related fracture or osteoporosis risk.
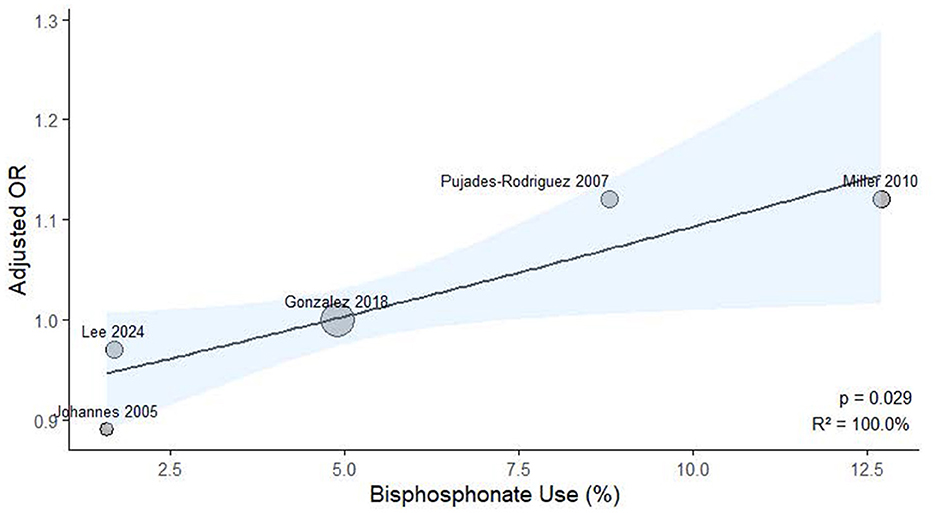
Figure 7. Meta-regression analysis examining the association between study-level bisphosphonate exposure prevalence and ICS-related fracture or osteoporosis risk.
Sensitivity analysis
To evaluate the robustness of the findings, a sensitivity analysis was conducted by excluding Gonzalez et al. (18), which had a very large sample size and a near-null effect estimate. As shown in Supplementary Figure S1, the exclusion led to a slightly higher pooled odds ratio of 1.05 (95% CI: 1.01–1.10, P = 0.01), while heterogeneity was markedly reduced (I2 = 8%). The removal of other studies resulted in minor fluctuations in the effect estimate but did not materially alter the conclusions.
Discussion
Our systematic review examined the impact of ICS on osteoporosis or fractures in patients with COPD. It did not find a statistically significant association between ICS use and osteoporosis or fractures in these patients. Our meta-analyses included both case-control and cohort studies, which inherently differ in study design and susceptibility to bias. The pooled estimate from six case-control studies showed no statistically significant association between ICS use and osteoporosis or fracture risk (OR = 1.03, 95% CI: 0.99–1.08; I2 = 50%), whereas the two cohort studies also showed no significant association (HR = 0.95, 95% CI: 0.67–1.33) but with high heterogeneity (I2 = 86%), indicating substantial variability in study populations, exposure definitions, and follow-up durations. In subgroup analyses, we found differences in effect estimates depending on outcome definitions. Only one study assessing osteoporosis specifically reported a significant association with ICS use (OR = 1.05), while other studies evaluating general fracture outcomes showed mixed results. Moreover, the geographic region appeared to influence the results. Asian and European studies showed a statistically significant increased risk associated with ICS, while North American studies reported null associations. This regional variation may reflect differences in clinical practice, population characteristics, healthcare systems, or confounding control. These methodological and contextual differences likely contributed to the observed heterogeneity and should be considered when interpreting the overall findings.
A previous random-effects meta-analysis that included patients with asthma or COPD assessed the long-term effects of ICS use on bone mineral density. It concluded that prolonged use of ICSs did not significantly alter bone mineral density in these groups (27). Another systematic review and meta-analysis also explored the impact of ICS on fracture risk, bone mineral density, and bone markers in patients with asthma and COPD, and it indicated an increased fracture risk (28).
Considering the distinct nature of COPD and asthma and the fact that COPD patients tend to have more comorbidities, such as cataracts, osteoporosis, coronary artery disease, pneumonia, and airway infections, the results of ICS use might differ between the two groups. In contrast, asthma patients typically exhibit fewer comorbidities, likely due to a younger age profile (29). Therefore, meta-analyses that include both asthma and COPD patients may not accurately represent the effects of ICS on individuals with COPD alone.
A meta-analysis sought to assess the advantages and risks of adding ICS treatment for patients with severe or very severe COPD. The analysis incorporated data from 9 randomized controlled trials and demonstrated that combining an ICS with a LABA reduced exacerbation rates but heightened the risks of pneumonia and oral candidiasis when compared to monotherapy with long-acting bronchodilators. Moreover, the incorporation of ICS markedly improved patient-perceived health and wellbeing (30). However, this study did not explore the potential effects on osteoporosis and fractures.
Previous systematic reviews have indicated that ICSs do not increase fracture risk in patients with COPD. However, these findings are subject to debate due to the limited number and early publication dates of the articles included (31–34).
A systematic review and meta-analysis of 44 randomized controlled trials, involving 87,594 patients, was conducted to determine the impact of inhaled corticosteroids on fracture risk in COPD patients. The analysis revealed that inhaled therapies containing ICSs, such as ICS/LABA combinations and triple therapy, were significantly associated with an increased risk of fractures in COPD patients compared to inhaled treatments without ICSs (35). The analysis only included randomized controlled trials (RCTs) that reported fracture event data. Trials that did not report fracture events, as well as non-randomized controlled trials such as observational studies, case series, and reviews, were excluded. Additionally, the study did not address the outcome of osteoporosis nor consider the use of oral corticosteroids. Simultaneously, another study analyzed parallel-group randomized controlled trials (RCTs) comparing ICS with non-ICS therapies in individuals with COPD, specifically looking at reported adverse events including fractures or osteoporosis. This meta-analysis included 61,380 participants from 26 RCTs and found that ICS did not increase the risk of fractures or osteoporosis (36).
The study faced multiple limitations. Primarily, the meta-analysis included only RCTs, omitting observational studies which could offer valuable information on the long-term impacts of ICS therapy in patients with COPD. Moreover, the majority of these RCTs concentrated on evaluating respiratory outcomes and mortality rates, and did not implement precise methodologies to accurately identify incidents of fractures or osteoporosis. This lack of specificity could significantly heighten the possibility of misclassification or underdiagnosis of these conditions. Additionally, the mean duration of therapy across the included trials was ~1–2 years, a timeframe that may be insufficient to fully capture the side effects associated with ICS, such as fractures and osteoporosis.
Our study included both cohort and case-control studies that reported on the outcomes of osteoporosis or fractures, observing participants for a period exceeding 4 years. Additionally, we considered the use of oral steroids in our analysis. Despite this comprehensive approach, we were unable to demonstrate a statistically significant increase in osteoporosis or fractures among individuals with COPD who received ICS compared to those with COPD who were not exposed to ICS. However, in meta-regression, we did find study-level oral corticosteroid prevalence revealed a significant positive association, indicating that higher background exposure to oral corticosteroids was correlated with a greater risk of osteoporosis or fractures among ICS users.
Our systematic review focused on cohort or case-control studies that assessed the impact of ICS on osteoporosis or fractures in patients with COPD. We found no consistent evidence of a significant adverse relationship between ICS use and specific sites such as the lumbar spine or femur. Additionally, there was insufficient data to establish any dose-response relationship or to evaluate potential differences between various ICS compounds. Our results should be viewed in light of other recent publications. Two systematic reviews specifically investigated fractures or osteoporosis in ICS users with COPD: one found that ICS exposure did not increase the risk of fractures or osteoporosis (36), while other recent meta-analyses have reported a statistically significant increase in the risk of fractures associated with ICS use in these patients (35).
Our research revealed that exposure to ICS did not elevate the risk of fractures or osteoporosis in COPD patients. A meta-regression using continuous study-level oral corticosteroids prevalence identified a statistically significant trend (p = 0.005; R2 = 100%), indicating that higher oral corticosteroids exposure was associated with stronger effect estimates. Additionally, a meta-regression using study-level bisphosphonate use as a covariate revealed a significant positive association with ICS-related risk (p = 0.029; R2 = 100%). These results imply that ICS-associated fracture risk may be influenced by concomitant oral corticosteroid use, and that higher bisphosphonate use may indicate a study population with pre-existing skeletal fragility. We concentrated specifically on the use of ICS in patients with COPD, utilizing cohort or case-control studies, which allowed for longer observation periods compared to randomized controlled trials. To ensure the accuracy of our findings, we employed stringent selection criteria designed to comprehensively include COPD patients and exclude those with asthma, and we conducted our analysis over extended periods in these observational studies.
Oral corticosteroids are commonly used to manage acute exacerbations in COPD. Patients should be counseled to use the lowest effective dose that adequately controls their symptoms and minimizes future risk of osteoporosis, thereby reducing the likelihood of bone-related side effects. Screening for bone density and managing osteoporosis should be prioritized in populations at high risk in patients with COPD. Physicians need to be particularly vigilant about the risk of fractures in patients with COPD using oral corticosteroids over the long term.
Considering the high prevalence of COPD, the often necessary use of systemic steroid and ICS therapy, and the associated increased risks of osteoporosis and fractures, it is essential to develop COPD-specific guidelines for bone protection. These guidelines would serve to inform and educate both clinicians and patients, with the goal of reducing the incidence of preventable osteoporotic fractures.
Limitations
We fully acknowledge that several critical variables, such as cumulative ICS dosage, treatment duration, baseline COPD severity, smoking status, physical activity level, and history of prior fractures were not analyzed in our review. This is primarily due to the lack of consistently reported data across the included studies, which prevented us from conducting further stratified or adjusted analyses.
We acknowledge that the relatively small number of included studies may limit the generalizability of our findings. We emphasize the need for further high-quality studies in diverse populations to validate our conclusions. To better understand the observed heterogeneity, we conducted subgroup analyses based on outcome definitions and geographic region, as well as a meta-regression using oral corticosteroid and bisphosphonate prevalence as a continuous covariate. These additional analyses revealed that differences in clinical definitions, geographic context, and background oral corticosteroid exposure may have contributed significantly to inter-study variability. We have expanded our discussion on the inherent limitations of observational research, particularly the susceptibility to residual confounding. Although we used the NOS to assess study quality, we recognize that unmeasured or uncontrolled confounders (e.g., comorbidities, medication adherence) may still influence the results. As noted, most included studies did not differentiate between ICS monotherapy and ICS combined with oral corticosteroid. While this is a significant limitation, we addressed it analytically by performing a study-level meta-regression using oral corticosteroid prevalence, which revealed a significant trend, suggesting that higher oral corticosteroid exposure may amplify the ICS-associated risk.
Conclusion
Although ICS did not significantly affect the risk of osteoporosis or fractures in our study, these conditions are common comorbidities in patients with COPD. Methodological differences among the included studies, such as study design (cohort vs. case-control), definitions of outcomes, and variations in oral corticosteroid use may influence the interpretation of the results. These differences contribute to clinical and methodological heterogeneity, which may affect effect estimates and limit the comparability across studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
H-FH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. C-WH: Conceptualization, Formal analysis, Investigation, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. F-TH: Conceptualization, Data curation, Investigation, Writing – original draft. K-ML: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1503475/full#supplementary-material
References
1. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. (2007) 356:775–89. doi: 10.1056/NEJMoa063070
2. Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. (2016) 387:1817–26. doi: 10.1016/S0140-6736(16)30069-1
3. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler vs. long- acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2012) 9:CD006829. doi: 10.1002/14651858.CD006829.pub2
4. Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler vs. inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (2013) 8:CD006826. doi: 10.1002/14651858.CD006826.pub2
5. Calverley PMA, Anderson JA, Brook RD, Crim C, Gallot N, Kilbride S, et al. Fluticasone furoate, vilanterol, and lung function decline in patients with moderate chronic obstructive pulmonary disease and heightened cardiovascular risk. Am J Respir Crit Care Med. (2018) 197:47–55. doi: 10.1164/rccm.201610-2086OC
6. Vestbo J, Leather D, Diar Bakerly N, New J, Gibson JM, McCorkindale S, et al. Salford Lung Study Investigators. Effectiveness of fluticasone furoate-vilanterol for COPD in clinical practice. N Engl J Med. (2016) 375:1253–60. doi: 10.1056/NEJMoa1608033
7. Bafadhel M, Peterson S, De Blas MA, Calverley PM, Rennard SI, Richter K, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. (2018) 6:117–26. doi: 10.1016/S2213-2600(18)30006-7
8. Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2015) 192:523–5. doi: 10.1164/rccm.201502-0235LE
9. Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G, et al. Extrafine inhaled triple therapy vs. dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. (2018) 391:1076–84. doi: 10.1016/S0140-6736(18)30206-X
10. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. (2015) 3:435–42. doi: 10.1016/S2213-2600(15)00106-X
11. Vestbo J, Papi A, Corradi M, Blazhko V, Montagna I, Francisco C, et al. Single inhaler extrafine triple therapy vs. long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. (2017) 389:1919–29. doi: 10.1016/S0140-6736(17)30188-5
12. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. Once-daily single-inhaler triple vs. dual therapy in patients with COPD. N Engl J Med. (2018) 378:1671–80. doi: 10.1056/NEJMoa1713901
13. Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European respiratory society study on chronic obstructive pulmonary disease. N Engl J Med. (1999) 340:1948–53. doi: 10.1056/NEJM199906243402503
14. Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol vs. vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. (2013) 1:210–23. doi: 10.1016/S2213-2600(13)70040-7
15. Johnell O, Pauwels R, Lofdahl CG, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. Eur Respir J. (2002) 19:1058–63. doi: 10.1183/09031936.02.00276602
16. Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits LR, et al. Prevalence and progression of osteoporosis in patients with COPD: results from the towards a revolution in COPD health study. Chest. (2009) 136:1456–65. doi: 10.1378/chest.08-3016
17. Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta- analysis of randomised controlled trials and observational studies. Thorax. (2011) 66:699–708. doi: 10.1136/thx.2011.160028
18. Gonzalez AV, Coulombe J, Ernst P, Suissa S. Long-term use of inhaled corticosteroids in COPD and the risk of fracture. Chest. (2018) 153:321–8. doi: 10.1016/j.chest.2017.07.002
19. Janson C, Lisspers K, Ställberg B, Johansson G, Gutzwiller FS, Mezzi K, et al. Osteoporosis and fracture risk associated with inhaled corticosteroid use among Swedish COPD patients: the ARCTIC study. Eur Respir J. (2021) 57:2000515. doi: 10.1183/13993003.00515-2020
20. Chiu KL, Lee CC, Chen CY. Evaluating the association of osteoporosis with inhaled corticosteroid use in chronic obstructive pulmonary disease in Taiwan. Sci Rep. (2021) 11:724. doi: 10.1038/s41598-020-80815-y
21. Pujades-Rodríguez M, Smith CJ, Hubbard RB. Inhaled corticosteroids and the risk of fracture in chronic obstructive pulmonary disease. QJM. (2007) 100:509–17. doi: 10.1093/qjmed/hcm056
22. Lee TA, Weiss KB. Fracture risk associated with inhaled corticosteroid use in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2004) 169:855–9. doi: 10.1164/rccm.200307-926OC
23. Johannes CB, Schneider GA, Dube TJ, Alfredson TD, Davis KJ, Walker AM. The risk of nonvertebral fracture related to inhaled corticosteroid exposure among adults with chronic respiratory disease. Chest. (2005) 127:89–97. doi: 10.1378/chest.127.1.89
24. Miller DP, Watkins SE, Sampson T, Davis KJ. Long-term use of fluticasone propionate/salmeterol fixed-dose combination and incidence of nonvertebral fractures among patients with COPD in the UK General Practice Research Database. Phys Sportsmed. (2010) 38:19–27. doi: 10.3810/psm.2010.12.1821
25. Liu SF, Kuo HC, Liu GH, Ho SC, Chang HC, Huang HT, et al. Inhaled corticosteroids can reduce osteoporosis in female patients with COPD. Int J Chron Obstruct Pulmon Dis. (2016) 11:1607–14. doi: 10.2147/COPD.S106054
26. Price DB, Voorham J, Brusselle G, Clemens A, Kostikas K, Stephens JW, et al. Inhaled corticosteroids in COPD and onset of type 2 diabetes and osteoporosis: matched cohort study. NPJ Prim Care Respir Med. (2019) 29:38. doi: 10.1038/s41533-019-0150-x
27. Halpern MT, Schmier JK, Van Kerkhove MD, Watkins M, Kalberg CJ. Impact of long-term inhaled corticosteroid therapy on bone mineral density: results of a meta-analysis. Ann Allergy Asthma Immunol. (2004) 92:201–7. doi: 10.1016/S1081-1206(10)61548-7
28. Richy F, Bousquet J, Ehrlich GE, Meunier PJ, Israel E, Morii H, et al. Inhaled corticosteroids effects on bone in asthmatic and COPD patients: a quantitative systematic review. Osteoporos Int. (2003) 14:179–90. doi: 10.1007/s00198-003-1398-z
29. Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. (2005) 128:2099–107. doi: 10.1378/chest.128.4.2099
30. Sobieraj DM, White CM, Coleman CI. Benefits and risks of adjunctive inhaled corticosteroids in chronic obstructive pulmonary disease: a meta-analysis. Clin Ther. (2008) 30:1416–25. doi: 10.1016/j.clinthera.2008.08.004
31. Miravitlles M, Auladell-Rispau A, Monteagudo M, Vázquez-Niebla JC, Mohammed J, Nuñez A, et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur Respir Rev. (2021) 30:210075. doi: 10.1183/16000617.0075-2021
32. Drummond MB, Dasenbrook EC, Pitz MW, Murphy DJ, Fan E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. (2008) 300:2407–16. doi: 10.1001/jama.2008.717
33. Chang YP, Lai CH, Lin CY, Chang YC, Lin MC, Chong IW, et al. Mortality and vertebral fracture risk associated with long-term oral steroid use in patients with chronic obstructive pulmonary disease: a systemic review and meta-analysis. Chron Respir Dis. (2019) 16:1479973119838280. doi: 10.1177/1479973119838280
34. Caramori G, Ruggeri P, Arpinelli F, Salvi L, Girbino G. Long-term use of inhaled glucocorticoids in patients with stable chronic obstructive pulmonary disease and risk of bone fractures: a narrative review of the literature. Int J Chron Obstruct Pulmon Dis. (2019) 14:1085–97. doi: 10.2147/COPD.S190215
35. Peng S, Tan C, Du L, Niu Y, Liu X, Wang R. Effect of fracture risk in inhaled corticosteroids in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Pulm Med 23, 304. (2023). doi: 10.1186/s12890-023-02602-5
Keywords: chronic obstructive pulmonary disease, inhaled corticosteroids, meta-analysis, systematic review, osteoporosis, fracture
Citation: Huang H-F, Hsu C-W, Hsieh F-T and Liao K-M (2025) Evaluating inhaled corticosteroids' impact on osteoporosis and fracture risk in COPD patients: a real-world evidence-based systematic review and meta-analysis. Front. Med. 12:1503475. doi: 10.3389/fmed.2025.1503475
Received: 29 September 2024; Accepted: 12 May 2025;
Published: 06 June 2025.
Edited by:
Rodrigo Torres-Castro, University of Chile, ChileReviewed by:
Matías Felipe Otto, Autonomous University of Chile, ChileJeonghoon Ha, The Catholic University of Korea, Republic of Korea
Copyright © 2025 Huang, Hsu, Hsieh and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuang-Ming Liao, YWJjODg3MEB5YWhvby5jb20udHc=
†These authors have contributed equally to this work and share first authorship
 Hsiao-Feng Huang1†
Hsiao-Feng Huang1† Chin-Wei Hsu
Chin-Wei Hsu Fei-Ting Hsieh
Fei-Ting Hsieh Kuang-Ming Liao
Kuang-Ming Liao