- 1Department of Anesthesiology, The Third People’s Hospital of Chengdu (The Affiliated Hospital of Southwest Jiaotong University), College of Medicine, Southwest Jiaotong University, Chengdu, Sichuan, China
- 2Department of Anesthesiology, The Third People’s Hospital of Chengdu, Southwest Jiao Tong University, Chengdu, China
- 3Department of Orthopaedics, The Third People’s Hospital of Chengdu, Southwest Jiao Tong University, Chengdu, China
Background: Ankylosing spondylitis (AS) is a progressive inflammatory disease causing severe kyphosis, which complicates surgical management and increases complication risks. This study aims to analyze the characteristics of severe kyphosis in AS and explore methods to optimize perioperative management and reduce complications.
Methods: We conducted a retrospective analysis of clinical data from five patients with severe kyphosis in AS who underwent surgery between October 2017 and February 2022. The patients had a mean age of 40.20 ± 8.50 years. The analysis included pathophysiological changes in folded patients and perioperative multidisciplinary intervention guidance. It also covered strict preoperative anesthetic evaluations, establishing an optimal fluid pathway during surgery, precise anesthetic monitoring and management, and applying postoperative multimodal analgesia and rehabilitation exercises to optimize perioperative anesthetic management.
Results: Preoperative cardiopulmonary function exercises were required to ensure patients could withstand surgery and anesthesia. Awake fiberoptic tracheal intubation was used to ensure airway safety and anesthesia. Hemodynamic evaluation and management were conducted using PICCO monitoring. Somatosensory evoked potentials (SSEP) and myogenic motor evoked potentials (MMEP) were utilized for neural axis monitoring. Hypothermia was designed to protect the spinal cord. To prevent massive blood loss, controlled hypotension and autotransfusion were implemented.
Conclusion: The correction operation of severe spinal kyphosis is complex and requires a detailed anesthesia plan. Optimizing the management of difficult airways and respiratory regulation, guiding circulation and fluid management through comprehensive monitoring, avoiding factors that aggravate complications, improving postoperative analgesia, and encouraging active rehabilitation exercises are crucial goals for perioperative anesthesia management.
1 Introduction
Ankylosing spondylitis (AS) is a progressive chronic inflammatory disease, primarily affecting the spine and sacroiliac joints and causing fusion and rigidity of the spine (‘bamboo spine’), and eventually developing into cervical rigidity and thoracic and lumbar kyphosis (1). AS is the most common cause of kyphotic deformity, with an incidence rate of approximately 49%, and it affects approximately ~0.1–1.4% of the population (2–4). Kyphosis results from abnormal backward protrusion of the spine, altering the anatomical shape of the spine and its associated tissues. Severe kyphosis often prevents patients from walking upright and looking straight ahead due to disrupted gravity balance, most affected individuals are thin and short in stature (5). Spinal deformity, particularly kyphosis, can lead to restrictive respiratory dysfunction due to thoracic deformity, impacting exercise tolerance. A significant reduction in these volumes of the thoracic and abdominal affects the normal activity space of the heart in the mediastinum, resulting in a smaller cardiac cavity and greatly impacting left ventricular diastolic function (6, 7). Anesthesia and kyphosis surgery are associated with significantly increased morbidity and mortality due to complications such as right ventricular failure, arrhythmia, postoperative hypoxemia and myocardial ischemia (8). The pathophysiology of severe kyphosis, screening for surgical risk factors, preoperative evaluation, comprehensive multidisciplinary planning, intraoperative monitoring and management, and prevention and treatment of postoperative complications pose significant challenges for anesthesiologists.
Therefore, we present successful cases of primary surgery for severe kyphosis in ankylosing spondylitis. This study aims to provide a reference for perioperative anesthesia management and treatment of such cases.
2 Methods
2.1 Patient selection
This single-center, retrospective study was granted by the institutional review board of the hospital, with the requirement for written informed consent waived by the ethics committee, all patient data utilized in this study were handled in strict adherence to ethical guidelines. Inclusion criteria were confirmed AS patients with kyphosis as the main deformity, presenting in a folded state (measured Cobb’s angle ≥150°), and complete clinical and auxiliary examination data. Exclusion criteria were primary thoracic deformities, congenital heart disease, history of cardiothoracic surgery, history of spinal trauma or spinal surgery, and inability to undergo the necessary monitoring program (including Psychological or physical limitations; Severe physical illnesses; Inability to undergo specific tests; Inability to follow the follow-up plan; Inability to provide necessary information; Legal or ethical constraints, etc.). A retrospective analysis was conducted on AS patients with severe kyphosis who underwent surgery at the hospital from October 2017 to February 2022. Five patients meeting these criteria were selected.
2.2 Data collection
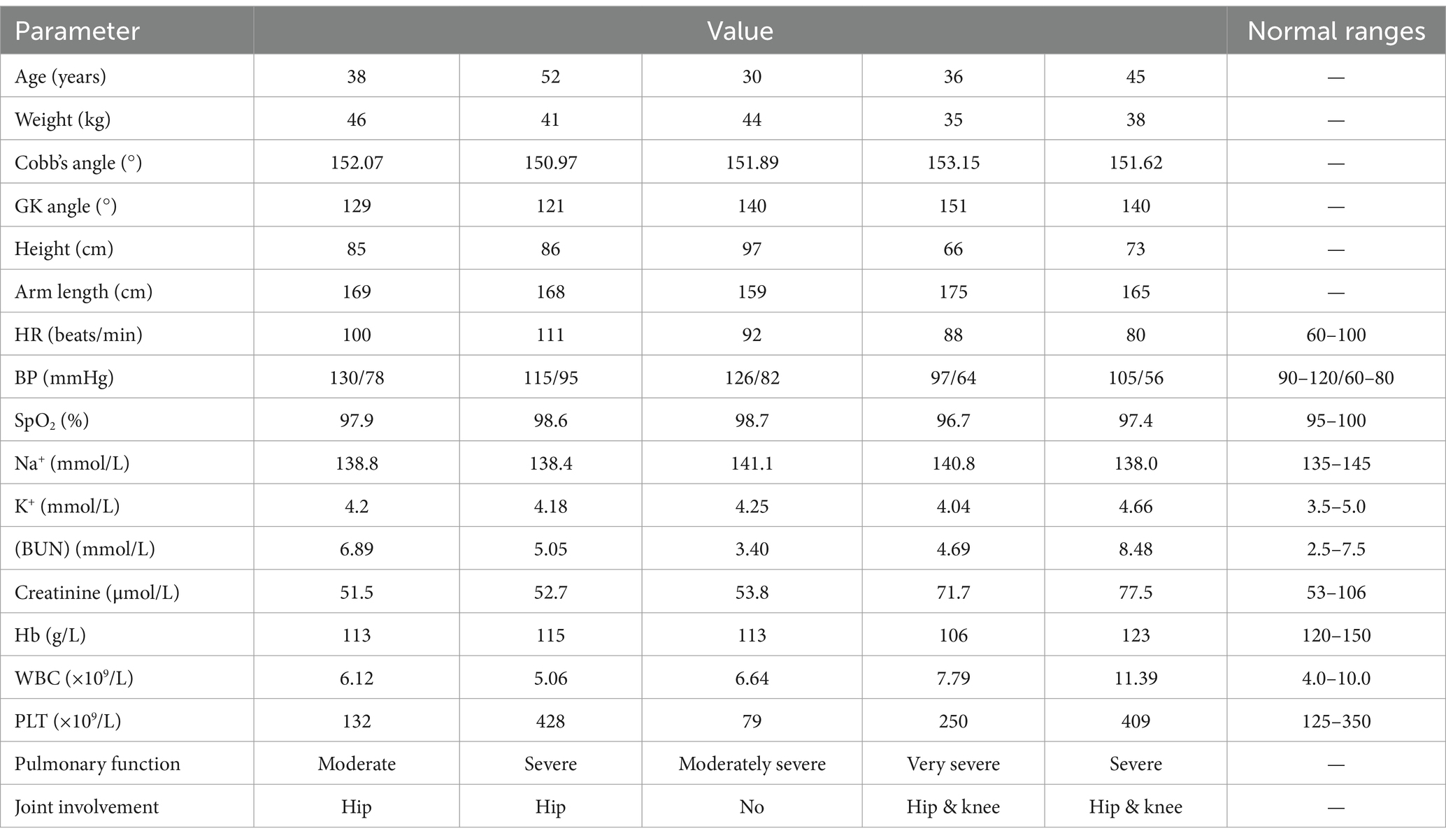
Demographic data such as age, height, weight, and arm length, as well as comorbidities and New York Heart Association (NYHA) cardiac function status before, during, and after kyphosis surgery were collected. Additional data included results from the 6-min walking test, breath-hold test, and other existing comorbidities. Preoperative examinations included pulmonary function tests, cardiac color ultrasound, electrocardiogram, routine blood tests, arterial blood gas analysis, liver and kidney function tests, blood coagulation tests, and neuro electrophysiology. All patients underwent spinal X-ray examination standing, with Cobb’s angle and global kyphosis angle (GK) measured on anteroposterior and lateral films using Surgimap software.
2.3 Statistical analysis
Statistical analysis was performed using SPSS 25.0 software. Measurement data are expressed as mean ± standard deviation (x ± S). A p-value of <0.05 was considered statistically significant.
3 Results
3.1 Basic demographic characteristics of patients with kyphosis underwent surgery in ankylosing spondylitis
All patients with AS were confirmed. Table 1 presents their baseline information and management before kyphosis surgery. Once hemodynamics stabilized post-extubation, all patients were transferred to the general ward for additional treatment.
During the first stage, patients underwent a single level Ponte osteotomy (SPO) in the lateral position. The correction result of the first-stage surgery was assessed utilizing Cobb’s angle, with positive values indicating kyphosis (Figure 1).
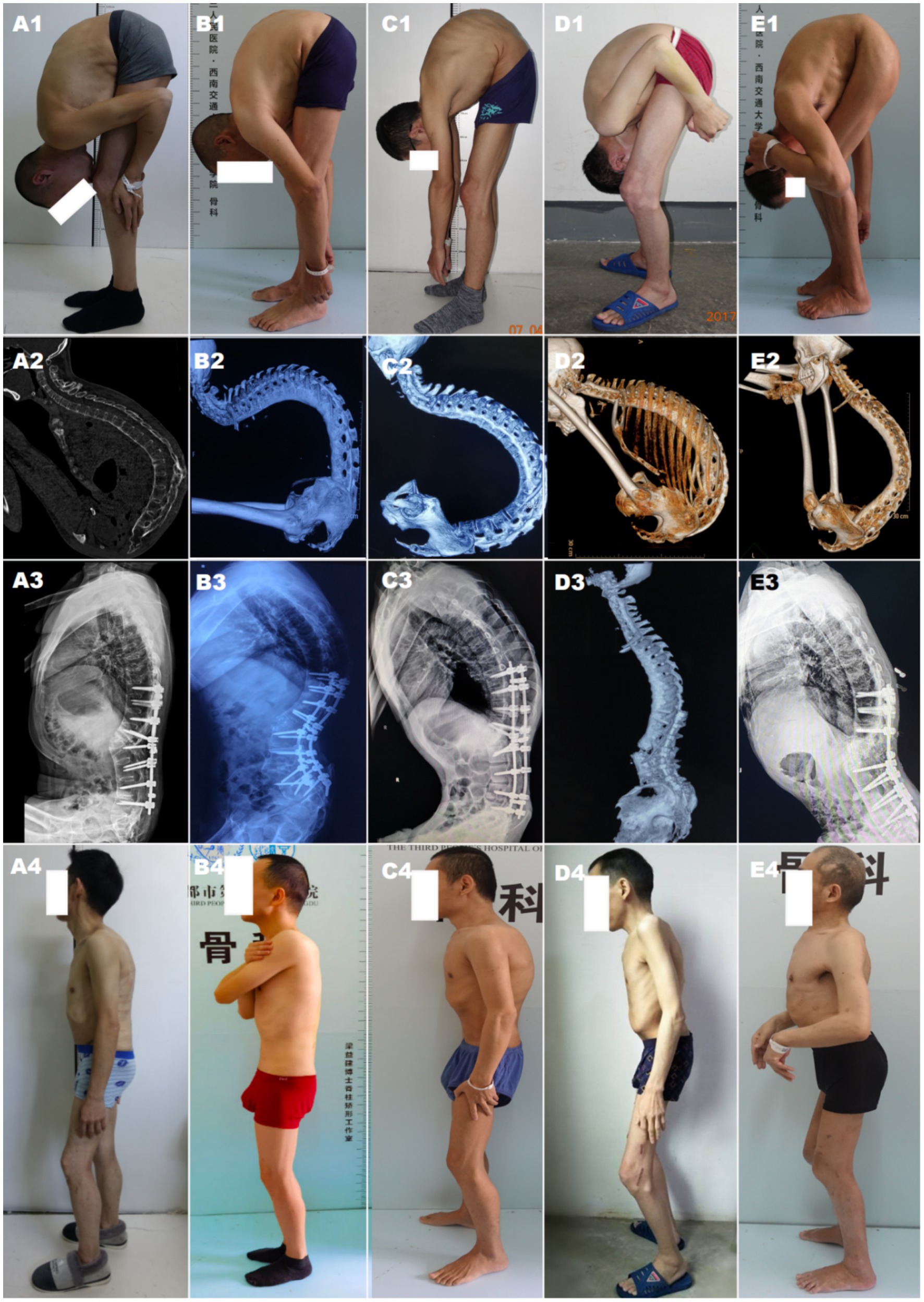
Figure 1. Pre-and postoperative presentation and changes in radiographic spinal level maps. (A–E) represent five patients. (A1–E1) Preoperative standing posture showing thoracolumbar kyphotic deformity; (A2–E2) Preoperative 3D CT reconstruction of the spine; (A3–E3) Postoperative spine alignment shown on X-ray/CT; (A4–E4) Postoperative standing posture changes.
3.2 Preoperative evaluation and management
The preoperative preparation time ranged from 2 weeks to 6 months. Vital capacity was improved through airbag blowing, lip contraction and exhalation exercises, and stair climbing, enhanced cardiopulmonary function. Nutritional enhancement was provided to emaciated patients, and those with reflux esophagitis were treated with proton pump inhibitors (PPIs). All five patients showed obvious lung function impairment during exercise compared to the same age group. Before the operation, one patient (20%) had extremely severe ventilatory dysfunction, with an FVC value less than 40% of the reference value. Three patients (60%) had varying degrees of restrictive ventilatory dysfunction. CT scans revealed ankylosing spondylitis involvement in bilateral hip joints in four patients (80%) and bilateral knee joint involvement in two patients (50%). One patient (20%) had a history of hypertension.
An anesthesiologist evaluated the patients one day before the operation, focusing on several key points (more detailed assessments are shown in Table 2): All patients’ cervical vertebrae were fixed in the anterior flexion position, with limited extension, lateral flexion and rotation; the mouth opening of the patient was slightly limited, with no significant abnormality in the thyromental distance; there’s no severe airway compression or chest deformity was observed. Normal activity in both upper limbs, limited movement in both lower limbs, and a preference for sleeping in the lateral position; patients had difficulty walking upright, and the amount of activities was small. According to ASA clinical practice guidelines (9), three patients were classified as grade III, and two as grade IV, with more severe lung function impairment. Patients needed central venipuncture before the operation, one delayed the operation due to difficulty exposing the bilateral femoral and right internal jugular veins, but the second puncture was completed successfully after one week. The mental state evaluation and psychological counseling of the patient. The diagnosis and treatment plans were discussed preoperatively in a multidisciplinary meeting.
As shown in Figure 2, a typical 45-year-old “folding patient” with severe kyphosis and hip ankylosis was reported. T12–L2 pedicle subtraction osteotomy (PSO) was performed in one stage, along with the anesthetic details, monitoring, patient status.
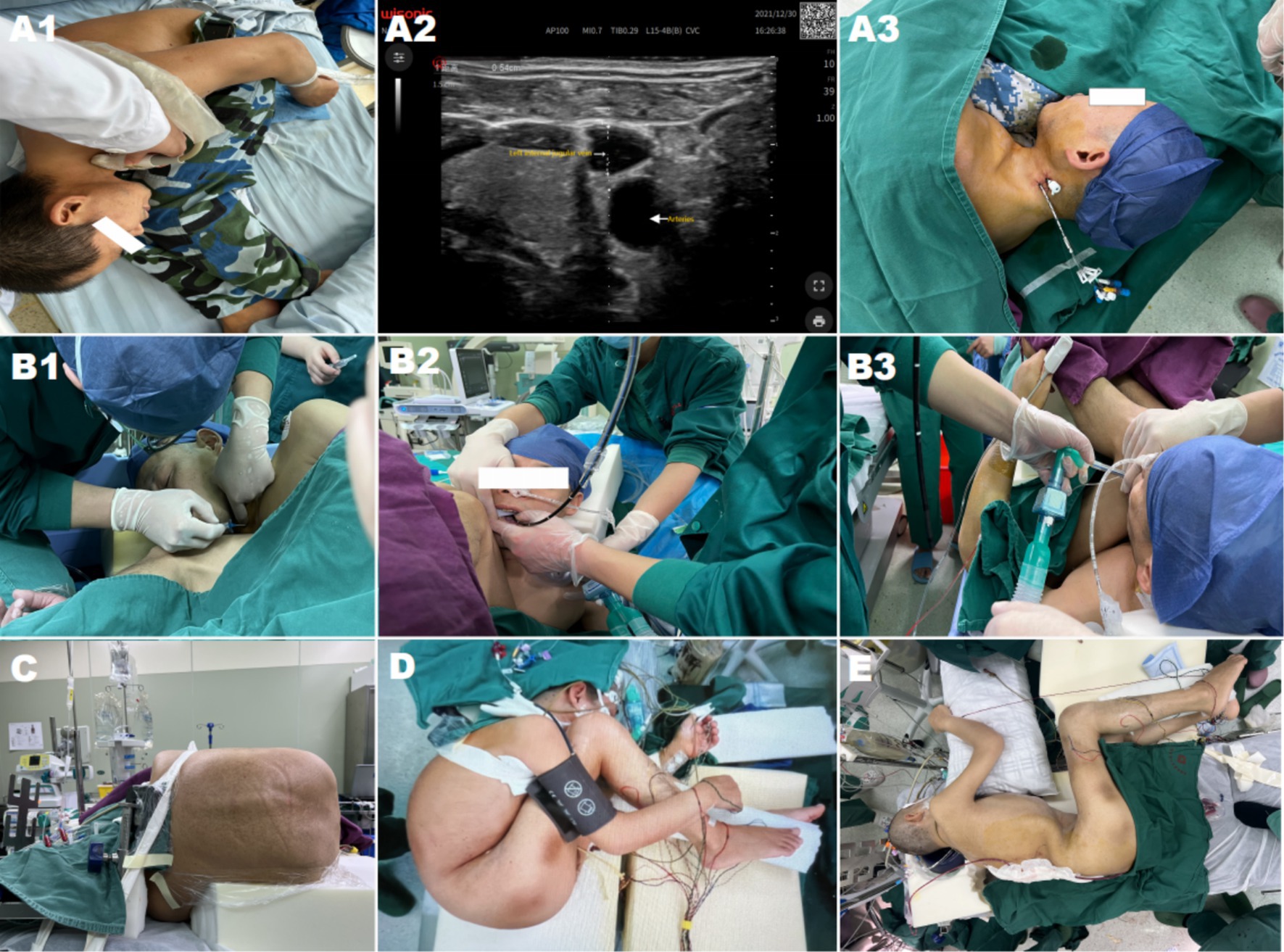
Figure 2. (A1–A3) Display the ultrasonic evaluation of blood vessels and establishment of a central vein. (B1–B3) Exhibit awake endotracheal intubation was performed after ultrasound-guided cricothyroid membrane puncture and tracheal mucosal anesthesia before endotracheal intubation. (C) Shows surgery was performed in a lateral position. (D) Depicts basic intraoperative vital signs and hemodynamic monitoring. (E) Expose the patient corrected the posture after the first-stage surgery.
3.3 Intraoperative details of anesthesia monitoring and management in kyphosis surgery
Routine monitoring of heart rate (HR), non-invasive blood pressure (NIBP), SpO2 and body temperature was conducted upon entering the operating room. An internal jugular vein catheter was placed on the unobstructed side of the patient under ultrasound guidance before anesthesia, allowing for central venous pressure (CVP) monitoring during the operation. Due to the excessive kyphosis, the surgery was performed in the lateral position. During awake tracheal intubation, the patient was instructed to take deep breaths while being preoxygenated with 100% oxygen to increase the end-expiratory oxygen concentration to over 90%, thereby preventing an increase in arterial carbon dioxide concentration and ensuring sufficient oxygenation. This approach provided valuable time for anesthesiologists to perform the intubation. For anesthesia induction, intravenous injections of penehyclidine hydrochloride 0.3 mg, methylprednisolone 40 mg, and oxycodone 3 mg were administered. Simultaneously, dexmedetomidine was pump-infused at a loading dose of 0.5 μg/kg and a maintenance dose of 0.2 μg/kg/h. Blood pressure was monitored via radial artery catheterization, and pulse contour continuous cardiac output (PICCO) monitoring was used for all five patients. After topical anesthesia of the oropharynx with 1% tetracaine and cricothyroid membrane puncture, awake tracheal intubation was performed under fiberoptic bronchoscope guidance. The respiratory rate (RR) was maintained at 18–22 breaths per minute, with a fresh air flow of 2 L/min and a FiO2 of 50%, maintaining the PETCO2 between 35 and 45 mmHg. A lung protective ventilation strategy was employed with a tidal volume (VT) of 5–8 mL/kg, PEEP of 5 cmH2O, periodic lung reexpansion, lung expansion at an interval of 2 h, and lung expansion pressure of 30 cmH2O for 30 s. Ventilation mode and parameters were adjusted based on changes in airway pressure and dynamic lung compliance (Cdyn).
Anesthesia was maintained by a target-controlled infusion of total intravenous anesthesia (TIVA) of propofol, remifentanil and a maintenance dose of dextromethomide. The BIS values were kept between 45 and 60. Intraoperative targeted fluid management, autologous blood transfusion, and intermittent blood sampling were implemented for blood gas analysis. Fluid therapy and vasoactive drug administration were guided by intraoperative blood loss, blood gas indices, arterial pressure (AP), central venous pressure (CVP), pulse pressure variability (PPV) index, systemic vascular resistance index (SVR), and cardiac output measured by PICCO. Four patients developed hypovolemic hypotension (systemic blood pressure < 90/60 mmHg) during the operation and were treated with norepinephrine or epinephrine to maintain hemodynamic stability. The crystalloid to colloid infusion ratio was controlled at 2:1, and red blood cells were supplemented when hemoglobin (Hb) was lower than 90 g/L. Patients were kept warm with a heated blanket, maintaining bladder temperature between 36.0 and 37.0°C.
Continuous monitoring of somatosensory evoked potentials (SSEP) and myogenic motor evoked potentials (MMEP) should be performed in the absence of muscle relaxants and under Total Intravenous Anesthesia to assess for any injury to the neural axis. When the osteotomy was fixed with Lugo’s rod, an intraoperative awakening was required to check for spinal cord injury or compression. Propofol and remifentanil were stopped, and only dexmedetomidine was continued intravenously. Patients were awakened when BIS values exceeded 75, and they accurately moved their lower limbs as instructed, with an awakening time of approximately 15 min. At the end of the operation, four patients returned to spontaneous breathing, with measured tidal volumes reaching the predicted values and their consciousness and muscle tension returning to normal. Patients were monitored for 15 min after extubation before being transferred to the orthopedic ward. One patient required delayed extubation and subsequent transfer to the ICU for further management due to the recovery of only consciousness without adequate respiratory muscle strength. This patient was successfully extubated after achieving adequate tidal volume 17 h later and was transferred back to the orthopedic ward after 2 day of treatment in the ICU.
3.4 Postoperative management and rehabilitation
Postoperative analgesic therapy was administered to all patients, including oral NSAIDs, intravenous analgesics, and analgesic pumps. The postoperative patient-controlled intravenous analgesia (PCIA) regimen included oxycodone 50 mg, tropisetron 10 mg, and 100 mL of normal saline. The PCIA settings were as follows: no background dose, single injection dose of 4 mL, lockout time of 5 min, and a maximum dose of 20 mL in 4 h. None of the patients experienced operation-related neurological impairment. Four patients were successfully extubated while awake. One patient required ventilation with CPAP and SIMV modes in the ICU and was extubated 17 h postoperatively. The patient with delayed extubation continued non-invasive positive pressure ventilation for one day, with no need for a tracheotomy. All patients were required to undergo rehabilitation training to reduce the risk of postoperative complications, improve cardiopulmonary function and physical fitness, strengthen nutritional intake, and prepare for the second-stage operation.
3.5 Postoperative complications
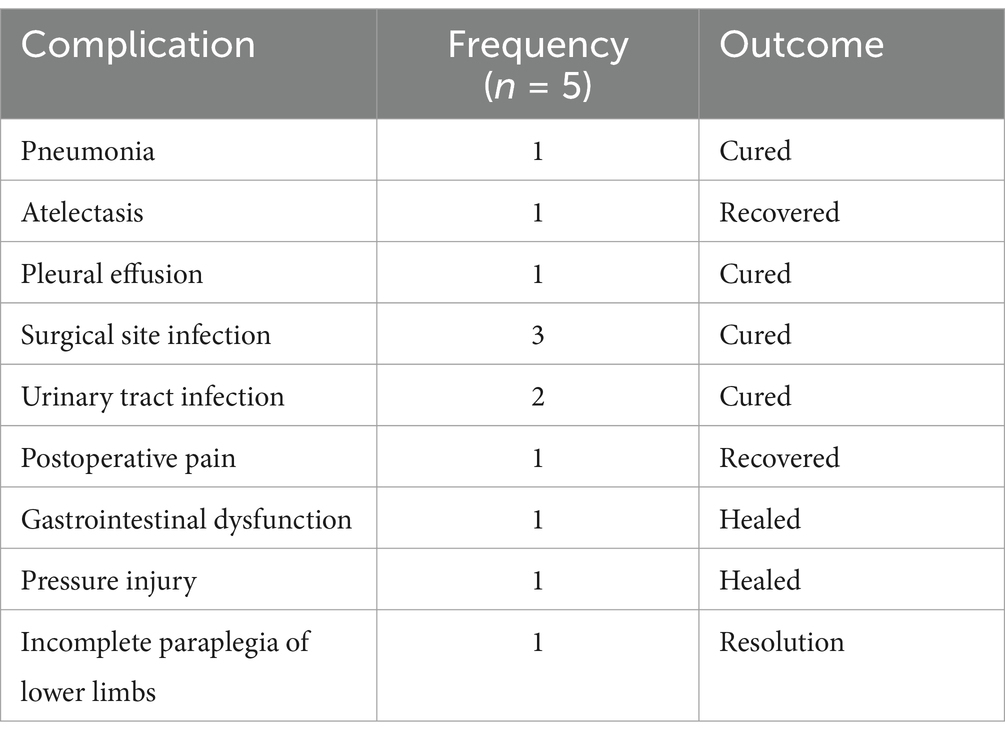
There’s no neurological deficits, vascular injuries or other severe complications were observed. More surgery and anesthesia-related complications are shown in Table 3.
4 Discussion
The underlying condition of rigid kyphosis (hunchback) in AS is characterized by extensive heterotopic bone formation in inflamed spinal ligaments, the increased incidence of compression fractures caused by AS osteoporosis further aggravates this problem (1). Kyphosis causes the center of gravity in the sagittal plane to move forward and backward. When ankylosis occurs, only the movable lower limb joints can compensate for the shift in the center of gravity, and stability is maintained by knee flexion and ankle base flexion (10). In the first stage of surgery, all patients underwent pedicle osteotomy and orthopedic bone graft fusion. Orthopedic osteotomy is considered an effective method for treating kyphosis in AS (11).
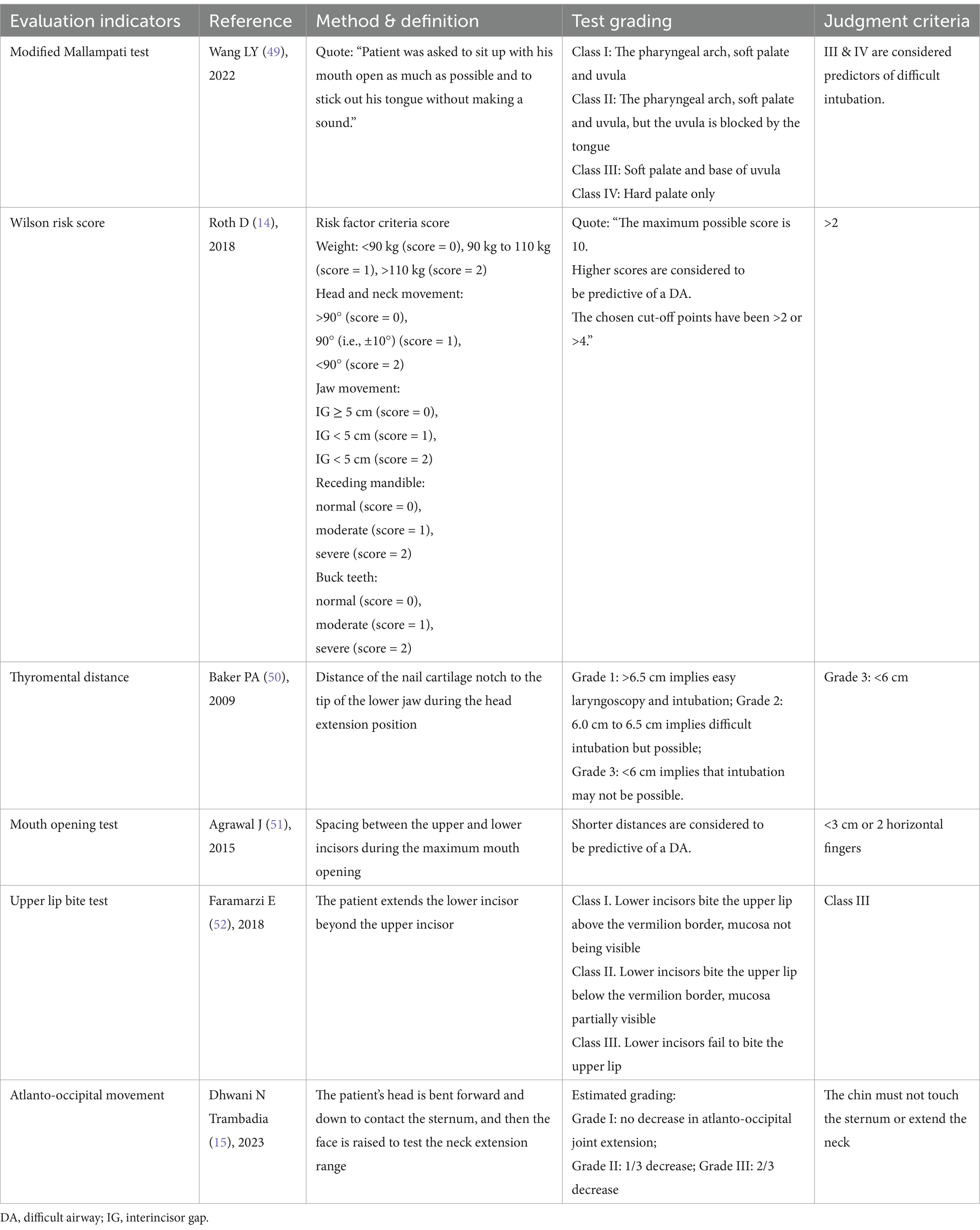
Strict preoperative evaluation before surgery is necessary. Patients with severe kyphosis often present a predictably difficult airway. A difficult airway event involves one or more sequential steps in upper airway management that are challenging or impossible to complete (12). There is no reference standard for a difficult airway, but it encompasses difficult mask ventilation, difficult laryngoscopy, difficult supraglottic airway ventilation, difficult or failed endotracheal intubation and extubation, failed airway establishment, and inadequate ventilation according to the 2022 American Society of Anesthesiologists Practice Guideline for Management of the Difficult Airway (13). Simple and direct assessment of patients with foreseeable, difficult airways is important in reducing adverse anesthetic events due to airway failure. The implementation of the modified Mallampati test, Wilson risk score, thyromental distance measurement, mouth opening test, upper lip bite test, and atlanto-occipital movement assessment by the anesthesiologist helps to understand the patient’s airway condition before surgery and prepare for unexpected intubation failure (14, 15).
Ultrasound, as a new airway assessment tool, is a better predictor of difficult airway. Ultrasound-based measurements can help assess airway compression or deformity (16, 17). Due to their severe deformities, these patients often cannot undergo conventional video laryngoscopy intubation or mask ventilation. Therefore, optimizing the patient’s oxygenation, airway management, and tracheal intubation is crucial, with particular emphasis on preoxygenation and apneic oxygenation. In such cases, performing a cricothyrotomy in the awake state before anesthesia induction is recommended as a reliable backup for failed tracheal intubation (18).
Fiberoptic bronchoscope conscious endotracheal intubation (FBI) is a feasible option for these patients. Awake supraglottic airway-guided FBI (SAGFBI) offers advantages over conventional awake FBI or awake video laryngoscopy. It reduces patient intolerance and associated complications and provides the benefits of “awake test insertion” of the supraglottic airway device (SAD), “awake observation” of the periglottic area, and “awake test ventilation” (19). Research has shown that for patients who cannot lie supine, the face-to-face intubation technique is an effective alternative (20). This technique takes less time in patients with predicted respiratory difficulties than video laryngoscopy. It also causes less damage to the pharyngeal wall or soft tissue and is less likely to result in intubation trauma since it enters along the base of the tongue (21). Compared with a direct laryngoscope, optical intubation tends to cause less hemodynamic instability (22).
Imagama et al. (23) showed for the first time that kyphosis, sagittal imbalance, increased daily oral medication and decreased back muscle strength are important risk factors for the development of GERD symptoms. In patients with a stiff spine and Cobb’s angle greater than 150°, preoperative multidisciplinary consultation considers gastroesophageal reflux caused by increased intra-abdominal pressure due to long-term compression of abdominal organs. Therefore, PPIs are administered to treat gastroesophageal reflux disease during the perioperative period. According to the Enhanced Recovery After Surgery (ERAS) program in Tong Y’s study (24), the digestive organs of these patients have been chronically compressed, leading to difficulties in eating and reduced nutritional intake, which in turn has caused cachexia. Therefore, nutritional support was provided during the perioperative period to optimize their preoperative and postoperative conditions.
Previous studies have consistently supported the beneficial effects of exercise on AS, backed by substantial mixed-quality evidence (25). Considering the moderate-to-severe restrictive ventilatory dysfunction and limited lower limb mobility, the preoperative exercise regimen included increasing the duration of flat-ground walking, balloon blowing exercises, and stair climbing. Preoperative pulmonary function tests revealed one patient with very severe respiratory dysfunction and four with moderate-to-severe restrictive ventilatory impairment, with no reports of pulmonary hypertension. These cardiopulmonary abnormalities increased the risk of anesthesia. After admission, patients underwent cardiopulmonary exercises for 1–6 months to improve exercise tolerance.
General anesthesia was selected. Etomidate combined with opioids was used during anesthesia induction to minimize effects on myocardial contractility and SVR, thereby reducing the risk of myocardial depression, decreased venous return, or exacerbation of hypoxia or hypercapni (26, 27). BIS monitoring was used to guide the depth of anesthesia to avoid circulatory inhibition caused by excessive depth of anesthesia. Maintaining an adequate MAP during the operation is crucial to ensure good spinal cord perfusion pressure (28). However, kyphosis correction often involves significant blood loss, and controlled hypotension techniques are employed to reduce intraoperative bleeding (29, 30). Consequently, somatosensory evoked potentials (SSEP) and myogenic motor evoked potentials (MMEP) should be monitored during the operation to ensure that the surgery or hypotension does not compromise spinal cord function (31, 32). Additionally, intraoperative monitoring of regional cerebral oxygen saturation (rSO2) is also beneficial to the management of hemodynamic parameters, early detection of hypoxia in the brain, spinal cord, kidney and other important organs, and formulation of organ protection strategies (33). Target-controlled infusion total intravenous anesthesia (TIVA) was used to maintain anesthesia, providing good controllability, comfort, and safety for intraoperative awakening. It is also essential to monitor blood gas, body temperature, and ventilation to avoid intraoperative arousal delay or difficulty caused by hypothermia, hypoxia, hypercapnia, acidosis, low hemoglobin (Hb), or hypovolemic blood volume.
In patients with kyphosis, thoracic deformities decrease lung compliance, lung volume, total lung capacity, vital capacity, and functional residual capacity (34–36). Impaired lung function can cause exercise intolerance (37, 38). A lung protective ventilation strategy is essential to manage increased airway pressure caused by thoracic and abdominal compression, impaired respiratory function, and prolonged operation time. Low tidal volumes can reduce the incidence of ventilator-associated lung injury (VALI) (39). However, decreased alveolar gas content can lead to alveolar collapse and ventilator-associated pneumonia. Appropriate PEEP and pulmonary resuscitation techniques can prevent atelectasis and alveolar collapse (40).
The PICCO system was used to evaluate hemodynamic parameters, such as continuous and dynamic cardiac index, extravascular lung water index, end-diastolic volume index, and systemic vascular resistance index during and after operation. It is increasingly used in critical operations and intensive care units (41), providing substantial help in guiding perioperative goal-oriented fluid therapy and follow-up treatment (42). Vasoactive drugs such as epinephrine and norepinephrine may be used to maintain or restore normal physiological function. Intraoperative autologous blood transfusion reduces the demand for allogeneic blood, lowers the incidence of transfusion reactions, and helps maintain hemodynamic stability (43). All operations were guided by target-directed fluid therapy under continuous monitoring and volume response evaluation of the lung protective ventilation strategy. The internal environment was corrected through intermittent arterial blood gas analysis (44). The intraoperative ratio of crystalloid to colloid infusion was 2:1, reducing the impact of large volumes of crystalloids on alveolar wall diffusion function and decreasing the incidence of pulmonary edema (45).
The key points of postoperative management include multi-mode pain management, early mobilization through rehabilitation exercises, and a nutritional support program (46). Both NSAIDs and selective COX 2 inhibitors, either alone or in combination, are effective in reducing postoperative pain (47). Additionally, IV-PCA can eliminate the gap between pain and analgesic administration, leading to better recovery and fewer side effects. This method is widely accepted for managing mild to severe postoperative pain (48). All patients need to perform long-term respiratory function exercises to adapt to postoperative respiratory changes. Early rehabilitation and physical exercise are essential for recovery.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Third People’s Hospital of Chengdu Ethical Review Committee. The Ethics Committee’s decision to grant a waiver of patients’ written informed consent was based on the retrospective design of the study and the implementation of stringent measures to protect patient privacy.
Author contributions
LP: Data curation, Formal analysis, Investigation, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. QL: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. LZ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. DZ: Data curation, Project administration, Resources, Writing – review & editing. QF: Funding acquisition, Project administration, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the project of Health and Family Planning Commission of Chengdu (No. 2022167).
Acknowledgments
We appreciate the contribution of all patients, their families, the investigators and the medical staff. We are grateful to all authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Woodward, LJ, and Kam, PC. Ankylosing spondylitis: recent developments and anaesthetic implications. Anaesthesia. (2009) 64:540–8. doi: 10.1111/j.1365-2044.2008.05794.x
2. Wang, R, and Ward, MM. Epidemiology of axial spondyloarthritis: an update. Curr Opin Rheumatol. (2018) 30:137–43. doi: 10.1097/BOR.0000000000000475
3. Joaquim, AF, de Oliveira, SA, Appenzeller, S, and Patel, AA. Spine surgery and ankylosing spondylitis: optimizing perioperative management. Clin Spine Surg. (2023) 36:8–14. doi: 10.1097/BSD.0000000000001306
4. López-Medina, C, and Moltó, A. Update on the epidemiology, risk factors, and disease outcomes of axial spondyloarthritis. Best Pract Res Clin Rheumatol. (2018) 32:241–53. doi: 10.1016/j.berh.2018.10.006
5. Diebo, B, Liu, S, Lafage, V, and Schwab, F. Osteotomies in the treatment of spinal deformities: indications, classification, and surgical planning. Eur J Orthop Surg Traumatol. (2014) 24:S11–20. doi: 10.1007/s00590-014-1471-7
6. Çağliyan Türk, A, Arslan, S, Karavelioğlu, Y, Kalcik, M, Özel, S, Musmul, A, et al. Pulmonary function, aerobic capacity and related variables in patients with ankylosing spondylitis. Arch Rheumatol. (2019) 34:317–25. doi: 10.5606/ArchRheumatol.2019.7145
7. Yang, Y, Huang, L, Zhao, G, Xia, J, Tian, X, Liu, C, et al. Influence of kyphosis in ankylosing spondylitis on cardiopulmonary functions. Medicine (Baltimore). (2023) 102:e35592. doi: 10.1097/MD.0000000000035592
8. Li, Q, Zeng, F, Chen, T, Liang, M, Lei, X, Liang, Y, et al. Management of severe scoliosis with pulmonary arterial hypertension: a single-center retrospective case series study. Geriatr Orthop Surg Rehabil. (2022) 13:21514593221080279. doi: 10.1177/21514593221080279
9. Connis, RT, Nickinovich, DG, Caplan, RA, and Apfelbaum, JL. Evaluation and classification of evidence for the ASA clinical practice guidelines In: R Miller, editor. Miller’ s anesthesia. 8th ed. Philadelphia: Elsevier (2015). 3257–70.
10. Uckun, A, and Sezer, I. Ankylosing spondylitis and balance. Eurasian J Med. (2017) 49:207–10. doi: 10.5152/eurasianjmed.2017.17116
11. Kim, KT, Park, DH, Lee, SH, and Lee, JH. Results of corrective osteotomy and treatment strategy for ankylosing spondylitis with kyphotic deformity. Clin Orthop Surg. (2015) 7:330–6. doi: 10.4055/cios.2015.7.3.330
12. Chen, H, Zheng, Y, Fu, Q, and Li, P. A review of the current status and progress in difficult airway assessment research. Eur J Med Res. (2024) 29:172. doi: 10.1186/s40001-024-01759-x
13. Apfelbaum, JL, Hagberg, CA, Connis, RT, Abdelmalak, BB, Agarkar, M, Dutton, RP, et al. 2022 American Society of Anesthesiologists practice guidelines for management of the difficult airway. Anesthesiology. (2022) 136:31–81. doi: 10.1097/ALN.0000000000004002
14. Roth, D, Pace, NL, Lee, A, Hovhannisyan, K, Warenits, AM, Arrich, J, et al. Airway physical examination tests for detection of difficult airway management in apparently normal adult patients. Cochrane Database Syst Rev. (2018) 5:CD008874. doi: 10.1002/14651858.CD008874.pub2
15. Trambadia, DN, Yadav, P, and A, S. Preoperative assessment to predict difficult airway using multiple screening tests. Cureus. (2023) 15:e46868. doi: 10.7759/cureus.46868
16. Waindeskar, V, Padala, SRAN, Jain, S, Kiran, M, Mandal, P, and Pakhare, AP. Prediction of the difficult airway by pre-operative ultrasound-based measurement of airway parameters: a prospective observational study. Indian J Anaesth. (2023) 67:785–90. doi: 10.4103/ija.ija_464_23
17. Giordano, G, Alessandri, F, Zulian, A, Bilotta, F, and Pugliese, F. Pre-operative ultrasound prediction of difficult airway management in adult patients: a systematic review of clinical evidence. Eur J Anaesthesiol. (2023) 40:313–25. doi: 10.1097/EJA.0000000000001805
18. Higgs, A, McGrath, BA, Goddard, C, Rangasami, J, Suntharalingam, G, Gale, R, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. (2018) 120:323–52. doi: 10.1016/j.bja.2017.10.021
19. Lim, WY, and Wong, P. Awake supraglottic airway guided flexible bronchoscopic intubation in patients with anticipated difficult airways: a case series and narrative review. Korean J Anesthesiol. (2019) 72:548–57. doi: 10.4097/kja.19318
20. Jeong, H, Chae, M, Seo, H, Yi, JW, Kang, JM, and Lee, BJ. Face-to-face intubation using a lightwand in a patient with severe thoracolumbar kyphosis: a case report. BMC Anesthesiol. (2018) 18:92. doi: 10.1186/s12871-018-0556-y
21. Yang, KH, Jeong, CH, Song, KC, Song, JY, Song, JH, and Byon, HJ. Comparison between glidescope and lightwand for tracheal intubation in patients with a simulated difficult airway. Korean J Anesthesiol. (2015) 68:22–6. doi: 10.4097/kjae.2015.68.1.22
22. Rhee, KY, Lee, JR, Kim, J, Park, S, Kwon, WK, and Han, S. A comparison of lighted stylet (Surch-lite) and direct laryngoscopic intubation in patients with high Mallampati scores. Anesth Analg. (2009) 108:1215–9. doi: 10.1213/ane.0b013e3181994fba
23. Imagama, S, Hasegawa, Y, Wakao, N, Hirano, K, Hamajima, N, and Ishiguro, N. Influence of lumbar kyphosis and back muscle strength on the symptoms of gastroesophageal reflux disease in middle-aged and elderly people. Eur Spine J. (2012) 21:2149–57. doi: 10.1007/s00586-012-2207-1
24. Tong, Y, Fernandez, L, Bendo, JA, and Spivak, JM. Enhanced recovery after surgery trends in adult spine surgery: a systematic review. Int J Spine Surg. (2020) 14:623–40. doi: 10.14444/7083
25. Dagfinrud, H, Kvien, TK, and Hagen, KB. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. (2008) 2008:CD002822. doi: 10.1002/14651858.CD002822.pub3
26. Valk, BI, and Struys, MMRF. Etomidate and its analogs: a review of pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. (2021) 60:1253–69. doi: 10.1007/s40262-021-01038-6
27. Barakat, H, Al Nawwar, R, Abou Nader, J, Aouad, M, Yazbeck Karam, V, and Gholmieh, L. Opioid-free versus opioid-based anesthesia in major spine surgery: a prospective, randomized, controlled clinical trial. Minerva Anestesiol. (2024) 90:482–90. doi: 10.23736/S0375-9393.24.17962-X
28. Singla, D, Agrawal, S, P, TK, Adhikary, AB, and Mangla, M. Comparative efficacy of intraoperative patient state index vs. bi-spectral index in patients undergoing elective spine surgery with neuromonitoring under general anaesthesia: a randomized controlled trial. Turk. J Anaesthesiol Reanim. (2024) 52:154–60. doi: 10.4274/TJAR.2024.241663
29. Dutton, RP. Controlled hypotension for spinal surgery. Eur Spine J. (2004) 13:S66–71. doi: 10.1007/s00586-004-0756-7
30. Ma, RX, Qiao, RQ, Xu, MY, Li, RF, and Hu, YC. Application of controlled hypotension during surgery for spinal metastasis. Technol Cancer Res Treat. (2022) 21:15330338221105718. doi: 10.1177/15330338221105718
31. Lall, RR, Lall, RR, Hauptman, JS, Munoz, C, Cybulski, GR, Koski, T, et al. Intraoperative neurophysiological monitoring in spine surgery: indications, efficacy, and role of the preoperative checklist. Neurosurg Focus. (2012) 33:E10. doi: 10.3171/2012.9.FOCUS12235
32. Scibilia, A, Terranova, C, Rizzo, V, Raffa, G, Morelli, A, Esposito, F, et al. Intraoperative neurophysiological mapping and monitoring in spinal tumor surgery: sirens or indispensable tools? Neurosurg Focus. (2016) 41:E18. doi: 10.3171/2016.5.FOCUS16141
33. Zhang, J, Shen, H, Wang, H, Xiao, F, Deng, L, Chen, X, et al. Intraoperative application of regional cerebral oxygen saturation monitoring for geriatric patients in China: a survey. Front Med (Lausanne). (2023) 10:1165821. doi: 10.3389/fmed.2023.1165821
34. Koumbourlis, AC. Scoliosis and the respiratory system. Paediatr Respir Rev. (2006) 7:152–60. doi: 10.1016/j.prrv.2006.04.009
35. Lin, Y, Shen, J, Chen, L, Yuan, W, Cong, H, Luo, J, et al. Cardiopulmonary function in patients with congenital scoliosis: an observational study. J Bone Joint Surg Am. (2019) 101:1109–18. doi: 10.2106/JBJS.18.00935
36. Lin, Y, Tan, H, Rong, T, Chen, C, Shen, J, Liu, S, et al. Impact of thoracic cage dimension and geometry on cardiopulmonary function inpatients with congenital scoliosis: a prospective study. Spine. (2019) 44:1441–8. doi: 10.1097/BRS.0000000000003178
37. Ozdem Yr, O, Inanici, F, and Hasçelik, Z. Reduced vital capacity leads to exercise intolerance in patients with ankylosing spondylitis. Eur J Phys Rehabil Med. (2011) 47:391–7.
38. Shen, J, Lin, Y, Luo, J, and Xiao, Y. Cardiopulmonary exercise testing in patients with idiopathic scoliosis. J Bone Joint Surg Am. (2016) 98:1614–22. doi: 10.2106/JBJS.15.01403
39. Young, CC, Harris, EM, Vacchiano, C, Bodnar, S, Bukowy, B, Elliott, RRD, et al. Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth. (2019) 123:898–913. doi: 10.1016/j.bja.2019.08.017
40. Deng, QW, Tan, WC, Zhao, BC, Wen, SH, Shen, JT, and Xu, M. Intraoperative ventilation strategies to prevent postoperative pulmonary complications: a network meta-analysis of randomised controlled trials. Br J Anaesth. (2020) 124:324–35. doi: 10.1016/j.bja.2019.10.024
41. Li, C, Wang, S, Wang, H, Wu, Y, Ma, J, Li, W, et al. The effects of hemodynamic monitoring using the PiCCO system on critically ill patients. Am J Transl Res. (2021) 13:10578–85.
42. Kan, CFK, and Skaggs, JD. Current commonly used dynamic parameters and monitoring systems for perioperative goal-directed fluid therapy: a review. Yale J Biol Med. (2023) 96:107–23. doi: 10.59249/JOAP6662
43. Zhou, J. A review of the application of autologous blood transfusion. Braz J Med Biol Res. (2016) 49:e5493. doi: 10.1590/1414-431x20165493
44. Som, A, Maitra, S, Bhattacharjee, S, and Baidya, DK. Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: a meta-analysis and trial sequential analysis of randomized controlled trials. J Anesth. (2017) 31:66–81. doi: 10.1007/s00540-016-2261-7
45. Tyagi, A, Maitra, S, and Bhattacharjee, S. Comparison of colloid and crystalloid using goal-directed fluid therapy protocol in non-cardiac surgery: a meta-analysis of randomized controlled trials. J Anesth. (2020) 34:865–75. doi: 10.1007/s00540-020-02832-5
46. Bansal, T, Sharan, AD, and Garg, B. Enhanced recovery after surgery (ERAS) protocol in spine surgery. J Clin Orthop Trauma. (2022) 31:101944. doi: 10.1016/j.jcot.2022.101944
47. Walker, CT, Gullotti, DM, Prendergast, V, Radosevich, J, Grimm, D, Cole, TS, et al. Implementation of a standardized multimodal postoperative analgesia protocol improves pain control, reduces opioid consumption, and shortens length of hospital stay after posterior lumbar spinal fusion. Neurosurgery. (2020) 87:130–6. doi: 10.1093/neuros/nyz312
48. Motamed, C. Clinical update on patient-controlled analgesia for acute postoperative pain. Pharmacy (Basel). (2022) 10:22. doi: 10.3390/pharmacy10010022
49. Wang, LY, Zhang, KD, Zhang, ZH, Zhang, DX, Wang, HL, and Qi, F. Evaluation of the reliability of the upper lip bite test and the modified mallampati test in predicting difficult intubation under direct laryngoscopy in apparently normal patients: a prospective observational clinical study. BMC Anesthesiol. (2022) 22:314. doi: 10.1186/s12871-022-01855-7
50. Baker, PA, Depuydt, A, and Thompson, JM. Thyromental distance measurement--fingers don't rule. Anaesthesia. (2009) 64:878–82. doi: 10.1111/j.1365-2044.2009.05985.x
51. Agrawal, J, Shenai, PK, Chatra, L, and Kumar, PY. Evaluation of normal range of mouth opening using three finger index: South India perspective study. Indian J Dent Res. (2015) 26:361–5. doi: 10.4103/0970-9290.167638
Keywords: anesthetic management, ankylosing spondylitis, kyphosis surgery, severe kyphosis, hemodynamic monitoring
Citation: Peng L, Li Q, Zheng L, Zhao D and Fu Q (2025) Anesthetic management of folders with severe kyphosis in ankylosing spondylitis: a single-center retrospective case series study. Front. Med. 12:1503912. doi: 10.3389/fmed.2025.1503912
Edited by:
Luca Proietti, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Giuseppe Murdaca, University of Genoa, ItalyLaura Scaramuzzo, Agostino Gemelli University Polyclinic (IRCCS), Italy
Maria Concetta Meluzio, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2025 Peng, Li, Zheng, Zhao and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Fu, ZnVxaWFuZzE4NzhAb3V0bG9vay5jb20=
 Lin Peng
Lin Peng Qiang Li2
Qiang Li2 Qiang Fu
Qiang Fu