Abstract
Background:
Endoscopic ultrasound (EUS) is important for diagnosing and staging esophageal cancer. However, substantial variability in the diagnostic and staging accuracy of EUS, especially in early-stage cancers, affects patients’ treatment choices and quality of life.
Aims:
To explore whether conventional endoscopic ultrasonography (EUS-C) combined with submucosal saline injection (EUS-SSI) improves diagnostic accuracy in preoperative T1a and T1b staging in superficial esophageal squamous cell carcinoma (SESCC).
Methods:
Patients with SESCC first underwent EUS-C. Then, they received SSI and underwent a repeat EUS. The diagnostic accuracy of EUS-C and EUS-SSI was evaluated based on the final postoperative pathology results.
Results:
A total of 92 patients with endoscopically diagnosed SESCC were included in the study. Postoperative pathology confirmed superficial SESCC in all patients (T1a stage, n = 77; T1b stage, n = 15). EUS-C correctly identified 54 of 77 patients with T1a cancer and nine of 15 patients with T1b cancer, whereas EUS-SSI identified 68 of 77 patients with T1a cancer and 10 of 15 patients with T1b cancer. EUS-SSI was more accurate than EUS-C in diagnosing T1a and T1b stage SESCC (84.8 and 68.5%, respectively).
Conclusion:
EUS-SSI differentiates between T1a and T1b stages of superficial SESCC with better diagnostic accuracy than EUS-C, thereby reducing the rate of over-staging.
1 Introduction
Superficial esophageal squamous cell carcinoma (SESCC) is a malignant lesion confined to the mucosa or submucosa regardless of the presence or absence of lymph node metastasis (1, 2). Endoscopic submucosal dissection (ESD) is widely used to treat SESCC (3). Indications for ESD have been expanded to cases in which the risk of lymph node metastasis is assumed to be low. Even if the pathological invasion depth is classified as T1b (tumour invasion of the submucosa), ESD can be performed if the invasion is limited to SM1 (submucosal invasion <200 μm from the muscularis mucosa) (4–6), and it is not suitable for submucosal invasion ≥200 μm (7–9).
Therefore, accurate determination of the depth of invasion (T stage) of esophageal cancer is crucial in determining treatment strategies.
Conventional endoscopic ultrasonography (EUS-C) is a technique that can provide clear cross-sectional images of the gastrointestinal (GI) tract wall. It helps diagnose the depth of submucosal tumours or assess cancer invasion, which is why it has been used for T staging of esophageal cancer (10, 11). Although previous studies have shown the clinical efficacy of EUS in T staging of esophageal cancer (12), the results have been largely variable (13). EUS-C cannot satisfactorily distinguish between T1a and T1b stages of esophageal squamous cell carcinoma (11, 14). Specifically, it is difficult to distinguish between T1a (tumour invading the lamina propria and muscularis mucosae) and T1b lesions by EUS because of the thin border between the mucosa and submucosa and overlap in echogenicity (15). Therefore, it is necessary to develop reliable and less invasive methods to distinguish between the T1a and T1b stages of SESCC.
Submucosal saline injection (SSI) is a necessary step in the ESD treatment of early esophageal cancer (16). SSI can increase the thickness of the submucosal layer to prevent gastrointestinal perforation caused by damage to the muscularis propria during treatment (17). And physiological saline is a good transmission medium for sound waves and can be used as an echo contrast enhancer for ultrasound transmission (18, 19).
Therefore, we evaluated whether SSI can be used to improve the accuracy of EUS in distinguishing between T1a and T1b stages of SESCC and analyse potential factors that interfere with staging results.
2 Methods
2.1 Study population
In 92 patients with endoscopically diagnosed SCC, EUS was performed on 92 lesions. All patients underwent EUS-C followed by EUS with SSI (EUS-SSI).
The tumour morphology under endoscopic evaluation was classified according to the Paris endoscopic classification for superficial neoplastic lesions (20) as follows: type I (protruded), type 0–IIa (superficial elevated), type 0–IIb (flat), type 0–IIc (superficial depressed), and type III (excavated).
2.2 Procedure and equipment
The SSI protocol involved the injection of saline using a 23G disposable mucosal needle (NM-400 U, Olympus). A 20 MHz ultrasonic probe was used for the EUS examination (P2620-M, Fujifilm, Japan). Around 3–5 mL of normal saline was injected into the submucosa. The puncture point was located 0.5 cm outside the edge of the lesion. The saline injection was stopped when the esophageal mucosa rose by approximately 1 cm. After SSI, the examiner used EUS to determine the depth of the lesion (Figure 1).
Figure 1
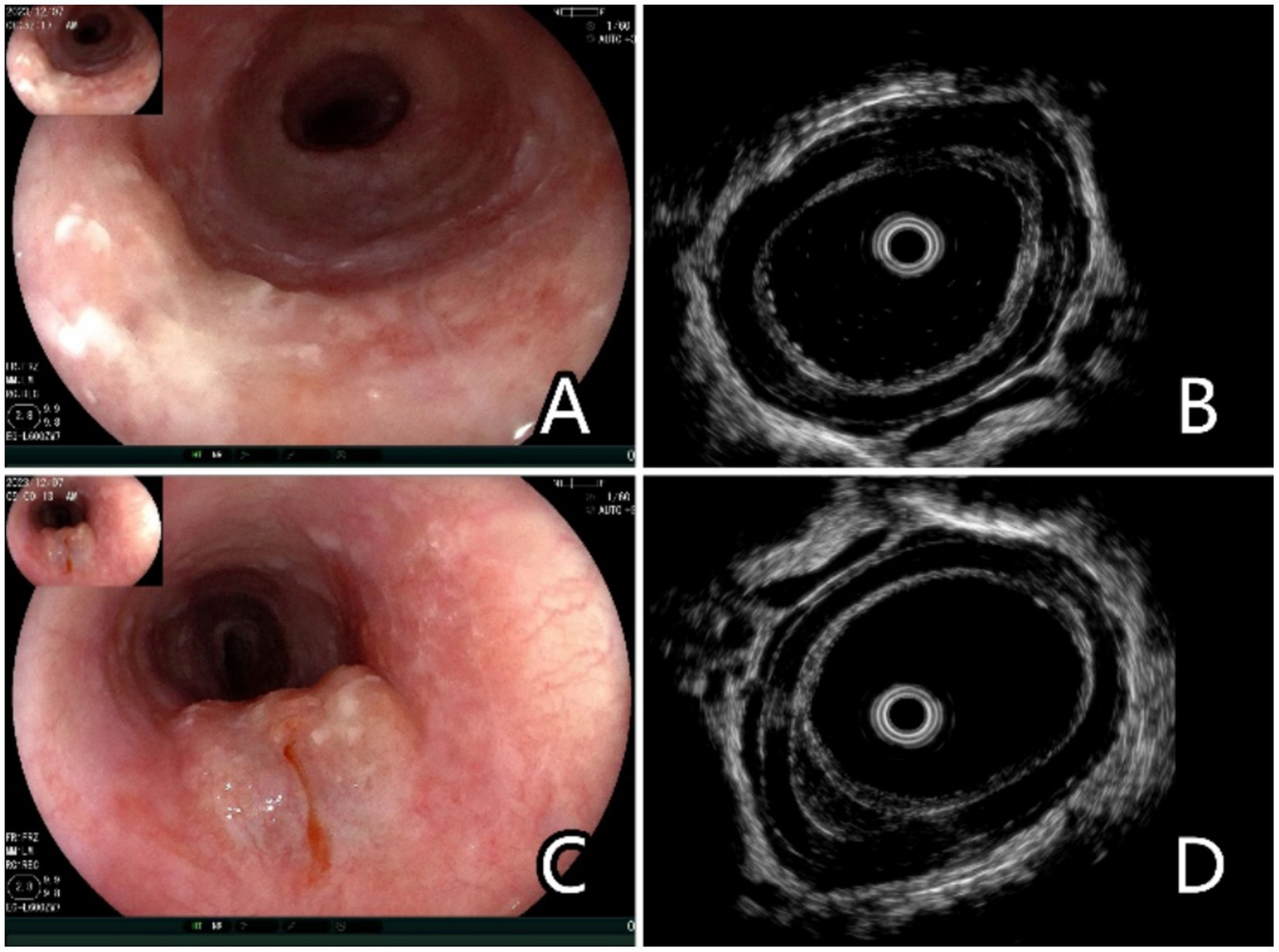
Endoscopic and ultrasonographic images and associated schematic diagrams of T1a superficial esophageal squamous cell carcinoma. (A) Lesions found in the middle oesophagus under white light endoscopy, 0–IIb + IIa, 2 cm × 2 cm. (B) Conventional ultrasonography (EUS-C) revealed that the mucosal layer was thickened and hypoechoic, and it is difficult to differentiate the extent of invasion from the mucosal layer to the submucosal layer. (C) Saline injection can facilitate the lifting of the lesions. (D) Ultrasonography after saline injection (EUS-SSI) shows that the boundary between the mucosa and submucosa is clearly displayed, suggesting that lesions with depth of infiltration limited to the mucosal layer can be easily identified, and the submucosa can be clearly distinguished from the mucosa.
2.3 Outcome assessment
T1a superficial SESCC on EUS was determined based on low-echoic lines of muscularis mucosae that were clearly demarcated from the submucosa. T1b superficial SESCC was determined based on low-echoic line lesions that were not clearly distinguished from the boundary of the submucosal layer. Subsequently, the patients underwent endoscopic or surgical resection within 7 days.
EUS examination and staging were performed simultaneously and were completed by two senior physicians with at least 3 years of EUS experience.
All recruited patients agreed to be enrolled as participants in this clinical trial and provided their informed consent. This study was approved by the Ethics Institutional Review Committee of Cancer Hospital Affiliated with Shandong First Medical University.
2.4 Statistical analysis
All statistical analyses were conducted using SPSS version 22.0 software (SPSS Inc., Chicago, IL). The chi-square test or Fisher’s exact test was used to compare the baseline characteristics of two groups divided by EUS result. In each groups, continuous variables were given as median and interquartile range and categorical variables were given as number and percentage. Predictors associated with T overstaging by EUS in pathologic in the univariable analysis were included in the multivariable logistic regression analysis and probability value less than 0.05 was considered significant.
3 Results
3.1 Baseline characteristics and T staging based on postoperative pathological parameters
All patients showed good tolerance to the endoscopy procedure. There were no adverse events such as serious bleeding, choking, esophageal wall perforation, or anaesthesia-related problems.
The postoperative pathology of 92 patients showed that they all had T1 superficial SESCC (T1a in 77 cases and T1b in 15 cases). No stage T2–T4 tumours were observed. There were no cases of positive lymph nodes assessed via enhanced computed tomography or EUS with or without SSI and postoperative pathology (Table 1).
Table 1
| Variables | Date | p-value |
|---|---|---|
| Age (group) | p = 0.531 | |
| ≤64 yearsa | 43 | |
| >64 years | 49 | |
| Sex | p < 0.01 | |
| M | 81 | |
| F | 11 | |
| Final pathology | p < 0.01 | |
| T1a | 77 | |
| T1b | 15 | |
| T1b-SM1 | 8 | |
| T1b-SM deep | 7 | |
| Differentiation | p = 0.003 | |
| High-grade dysplasia | 8 | |
| Well | 39 | |
| Moderately | 31 | |
| Poor | 14 | |
| Site of neoplasm | p < 0.01 | |
| Upper third | 9 | |
| Middle third | 50 | |
| Lower third | 29 | |
| Endoscopic morphology (EGC type) | p < 0.01 | |
| 0–IIa | 11 | |
| 0–IIb | 66 | |
| 0–IIc | 15 | |
| Size (mm) | p < 0.01 | |
| ≤20 mm | 43 | |
| >20 mm, <50 mm | 36 | |
| ≥50 mm | 13 |
Clinical features and postoperative pathological results of 92 patients with superficial esophageal squamous cell carcinoma.
M, male; F, female; T1b-SM1, depth of submucosal invasion <200 μm; T1b-SM deep, depth of submucosal invasion DA ≥200 μm.
Median age.
3.2 Comparison of T staging outcomes between the EUS-C and EUS-SSI
EUS-C identified 54 of 77 patients with T1a cancer, whereas EUS-SSI identified 68 of 77 patients with T1a cancer, and EUS-C identified nine of 15 patients with T1b cancer, EUS-SSI identified 10 of 15 patients with T1b cancer. In the present study, EUS-SSI was more accurate than EUS-C in diagnosing T1a and T1b lesions of EGC (84.8 and 68.5%, respectively) (Table 2).
Table 2
| Postoperative pathology stage | |||
|---|---|---|---|
| Postoperative EUS reported stage | T1aa | T1b | |
| EUS-C, n (%) | |||
| T1a | 54 (70.1%)b | 6 (40%) | |
| T1b | 23 (29.9%) | 9 (60%) | |
| EUS-SSI, n (%) | |||
| T1a | 68 (88.3%)b | 5 (33.3%) | |
| T1b | 9 (11.7%) | 10 (66.7%) | |
Preoperative and postoperative staging results for superficial esophageal squamous cell carcinoma with conventional endoscopic ultrasonography and endoscopic ultrasonography after submucosal saline injection.
As preplanned, cases with high-grade dysplasia were classified into T1a; EUS, endoscopic ultrasonography; SSI, submucosal saline injection.
The proportion of T1a detected by EUS-SSI VS. EUS-C (88.3% vs. 70.1%, p < 0.05).
3.3 Comparison of over-staging rate between the EUS-C and EUS-SSI
T1b stage lesions were diagnosed by EUS-C in 32 cases preoperatively, but postoperative pathology results suggested that these were cases of T1a (n = 15) and T1b-SM1 (depth of submucosal invasion <200 μm; n = 8) cancers. T1b stage lesions were diagnosed by EUS-SSI in nine cases preoperatively, but postoperative pathology results suggested that these were cases of T1a (n = 4) and T1b-SM1 (n = 5) cancers. The over-staging rate of EUS-SSI was lower than that of EUS-C (9.8 and 25.0%, respectively) (Table 3).
Table 3
| Group | EUS-C | EUS-SSI | p-value |
|---|---|---|---|
| Overstaging, n (%) | 23 (25.0%) | 9 (9.8%) | p < 0.01 |
| Understaging, n (%) | 6 (6.5%) | 5 (5.4%) | p = 0.77 |
Rate of misdiagnosis for T staging of superficial esophageal squamous cell carcinoma with endoscopic ultrasonography after submucosal saline injection.
EUS, endoscopic ultrasonography; SSI, submucosal saline injection.
3.4 Analysis of risk factors for over-staging based on EUS-SSI
Among the nine patients who were over-staged by EUS-SSI, endoscopic gross classification included one case of 0–IIa, six cases of 0–IIb, and two cases of 0–IIc. Preoperatively, six patients underwent multiple endoscopic biopsy sections in different hospitals. Scars formed by multiple biopsies were seen in the lesion area. The postoperative pathological results showed poorly differentiated SCC in four lesions (Table 4).
Table 4
| No. | Age | Sex | Site | Endoscopy morphology (EGC type) | Size (max, mm) | Biopsies times | Number of biopsies | EUS-C stage | EUS-SSI stage | Pathology stage | Differentiation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | M | Upper third | 0–IIa | 35 | 4 | 6 | T1b | T1b | T1b-SM1 | Poor |
| 2 | 62 | M | Upper third | 0–IIb | 65 | 3 | 5 | T1a | T1b | T1b-SM1 | Moderately |
| 3 | 57 | F | Lower third | 0–IIb | 55 | 3 | 5 | T1b | T1b | T1b-SM1 | Poorly |
| 4 | 59 | M | Middle third | 0–IIc | 25 | 3 | 5 | T1a | T1b | T1a | Well |
| 5 | 63 | M | Middle third | 0–IIb | 45 | 3 | 4 | T1b | T1b | T1a | Poor |
| 6 | 66 | M | Upper third | 0–IIc | 55 | 3 | 4 | T1a | T1b | T1b-SM1 | Moderately |
| 7 | 67 | F | Lower third | 0–IIb | 15 | 2 | 3 | T1b | T1b | T1a | Moderately |
| 8 | 79 | F | Middle third | 0–IIb | 60 | 2 | 3 | T1a | T1b | T1b-SM1 | Poor |
| 9 | 74 | M | Upper third | 0–IIb | 20 | 2 | 3 | T1a | T1b | T1a | Poor |
Clinical features and endoscopic ultrasonography findings before and after submucosal saline injection, and pathological results of nine patients with superficial esophageal squamous cell carcinoma.
M, male; F, female; T1b-SM1, depth of submucosal invasion <200 μm; T1b-SM deep, depth of submucosal invasion DA ≥200 μm.
4 Discussion
Accurate staging is an important basis for selecting treatment options for esophageal cancer (21). Patients with SESCC diagnosed at early stages have a higher survival rate than those diagnosed at advanced stages (22).
With the development of high-definition endoscopy and endoscopic staining technology, the detection rate of early esophageal cancer continues to increase, which is contributing to reducing the mortality rate associated with esophageal cancer (23). Radical esophagectomy and super-minimally invasive resection under digestive endoscopy are the main treatments for early-stage esophageal cancer (24–26). However, the choice of treatment depends on the staging, i.e., T1a or T1b (25, 26). Surgery is usually recommended for T1b cancer cases, whereas ESD is an option for T1a cancer cases (24, 26). Unlike radical esophagectomy, ESD preserves the integrity of organs and physiological structures, and patients have a better quality of life after treatment (27, 28). Therefore, it is crucial to accurately distinguish between T1a and T1b, and EUS is the preferred method for staging.
Although EUS has been used to assess the depth of invasion in early esophageal cancer cases, the reported diagnostic accuracy ranges from 60 to 80% (11, 14). The staging accuracy of EUS-C by two experienced endoscopists was 68.5% in our study, which is consistent with previous reports. However, after SSI, the accuracy of EUS staging for early-stage SESCC increased to 84.8%, suggesting improved staging accuracy for early-stage esophageal cancer.
Some studies have postulated that EUS has no significant advantage over traditional endoscopy and magnification endoscopy in predicting the depth of invasion of early esophageal cancer (29).
The accuracy of EUS is reported to vary greatly depending on the experience of the endoscopist, tumour location, macroscopic type of tumour, tumour size, presence or absence of ulceration, and tumour differentiation type (11).
Lesions with severe inflammation or ulceration or in cases where multiple biopsies have been performed, there is evidence of submucosal fibrosis, and hypoechoic lesions observed on EUS resemble tumour infiltration. For lesions in the upper third of the oesophagus, the accuracy of EUS may be reduced due to differences in esophageal wall thickness, poor esophageal lumen relaxation, and air interference. Furthermore, due to the tubular structure of the oesophagus and frequent peristalsis, it is difficult to fill the oesophagus with degassed water and position the EUS probe near the lesion. In addition, larger tumour size is a risk factor for incorrect judgment of invasion depth (30). Undifferentiated tumour cells may have the potential to undergo micro-infiltration in the submucosa of the esophageal wall, and EUS cannot visualize these micro-invasions, thereby underestimating tumour staging (31).
In this study, 15 patients with stage T1a cancer were over-staged as T1b in EUS-C examination. Although it was recommended that patients choose diagnostic ESD treatment, some patients received traditional surgical treatment, and their postoperative quality of life was significantly reduced. Subsequent EUS-SSI staging significantly reduced over-staging (25.0% with EUS-C to 9.8% with EUS-SSI). However, there were four patients whose preoperative and postoperative pathologies were incompatible, ranging from T1b to T1a. Further analysis showed that the lesion was located in the upper one-third of the oesophagus. Multiple preoperative endoscopic examinations and moderate-to-poor differentiation are risk factors for EUS-SSI over-staging. Multiple biopsies under endoscopy lead to scar-like hyperplasia and adhesion between the mucosa and submucosa. With EUS visualization, the boundaries can be unclear and hypoechoic changes between the mucosa and submucosa may be observed. These findings affect the judgment of the invasion depth.
Limitations of EUS-SSI include a longer examination time than EUS-C and the possibility of greater discomfort to the patient. These drawbacks can be addressed by using sedatives.
All enrolled patients underwent esophagectomy or ESD within 1 week, and all recovered well. There was no difficulty in endoscopy dissection due to poor submucosal injection lift. All lesions were completely removed. SSI may elicit an inflammatory response and cause fibroplasia in the submucosa, but it is unknown whether SSI makes ESD treatment more difficult if the patient cannot receive treatment soon. More cases and longer follow-ups are needed for further confirmation.
In this study, submucosal injection improved the accuracy of EUS staging of early-stage, i.e., T1a and T1b stages of SESCC. Lesions located in the upper oesophagus and multiple repeated biopsies are risk factors for inaccurate staging. This study has limitations, including its single-center, non-randomized design, which may introduce selection bias and confounding factors. The small sample size might also limit statistical power. Future multicenter RCTs with larger samples are needed to further validate the advantages of EUS-SSI, incorporating broader outcome measures for a more robust evaluation.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MC: Data curation, Methodology, Writing – original draft, Validation. YG: Data curation, Methodology, Validation, Writing – review & editing. JL: Methodology, Conceptualization, Project administration, Software, Writing – original draft. ZL: Conceptualization, Resources, Validation, Writing – review & editing. DW: Conceptualization, Writing – review & editing, Data curation, Funding acquisition, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grant Shandong Medical Science and Technology Development Plan (202303031462).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Shimizu M Zaninotto G Nagata K Graham DY Lauwers GY . Esophageal squamous cell carcinoma with special reference to its early stage. Best Pract Res Clin Gastroenterol. (2013) 27:171–86. doi: 10.1016/j.bpg.2013.03.010
2.
Cho JW Choi SC Jang JY Shin SK Choi KD Lee JH et al . Lymph node metastases in esophageal carcinoma: an endoscopist’s view. Clin Endosc. (2014) 47:523–9. doi: 10.5946/ce.2014.47.6.523
3.
Okubo Y Ishihara R . Endoscopic submucosal dissection for esophageal cancer: current and future. Life. (2023) 13:892. doi: 10.3390/life13040892
4.
Draganov PV Wang AY Othman MO Fukami N . AGA institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. (2019) 17:16–25.e1. doi: 10.1016/j.cgh.2018.07.041
5.
Beaufort IN Frederiks CN Overwater A Brosens LAA Koch AD Pouw RE et al . Endoscopic submucosal dissection for early esophageal squamous cell carcinoma: long-term results from a western cohort. Endoscopy. (2024) 56:325–33. doi: 10.1055/a-2245-7235
6.
Pimentel-Nunes P Libânio D Bastiaansen BAJ Bhandari P Bisschops R Bourke MJ et al . Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2022. Endoscopy. (2022) 54:591–622. doi: 10.1055/a-1811-7025
7.
Kim M Kim TJ Kim GH Lee YC Lee H Min BH et al . Outcomes of primary esophagectomy and esophagectomy after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: a propensity-score-matched analysis. Cancers. (2023) 15:5542. doi: 10.3390/cancers15235542
8.
Hatta W Koike T Uno K Asano N Masamune A . Management of superficial esophageal squamous cell carcinoma and early gastric cancer following non-curative endoscopic resection. Cancers. (2022) 14:3757. doi: 10.3390/cancers14153757
9.
Jiang H Tian B Gao Y Bian Y Yu C Xu J et al . Risk and pathological factors of recurrence after endoscopic resection for superficial esophageal squamous cell carcinoma: a systematic review and meta-analysis. Gastrointest Endosc. (2024) 100:1006–1019.e10. doi: 10.1016/j.gie.2024.07.013
10.
Bronswijk M Pérez-Cuadrado-Robles E Van der Merwe S . Endoscopic ultrasound-guided gastrointestinal anastomosis: current status and future perspectives. Dig Endosc. (2023) 35:255–63. doi: 10.1111/den.14381
11.
Choi J Chung H Lee A Kim JL Cho SJ Kim SG . Role of endoscopic ultrasound in selecting superficial esophageal cancers for endoscopic resection. Ann Thorac Surg. (2021) 111:1689–95. doi: 10.1016/j.athoracsur.2020.07.029
12.
Ishihara R Mizusawa J Kushima R Matsuura N Yano T Kataoka T et al . Assessment of the diagnostic performance of endoscopic ultrasonography after conventional endoscopy for the evaluation of esophageal squamous cell carcinoma invasion depth. JAMA Netw Open. (2021) 4:e2125317. doi: 10.1001/jamanetworkopen.2021.25317
13.
Kahlon S Aamar A Butt Z Urayama S . Role of endoscopic ultrasound for pre-intervention evaluation in early esophageal cancer. World J Gastrointest Endosc. (2023) 15:447–57. doi: 10.4253/wjge.v15.i6.447
14.
Dittler HJ Pesarini AC Siewert JR . Endoscopic classification of esophageal cancer: correlation with the T stage. Gastrointest Endosc. (1992) 38:662–8. doi: 10.1016/s0016-5107(92)70561-6
15.
Rampado S Bocus P Battaglia G Ruol A Portale G Ancona E . Endoscopic ultrasound: accuracy in staging superficial carcinomas of the esophagus. Ann Thorac Surg. (2008) 85:251–6. doi: 10.1016/j.athoracsur.2007.08.021
16.
Libânio D Pimentel-Nunes P Bastiaansen B Bisschops R Bourke MJ Deprez PH et al . Endoscopic submucosal dissection techniques and technology: European Society of Gastrointestinal Endoscopy (ESGE) technical review. Endoscopy. (2023) 55:361–89. doi: 10.1055/a-2031-0874
17.
Huai ZY Xian WF Jiang LC Chen XW . Submucosal injection solution for endoscopic resection in gastrointestinal tract: a traditional and network meta-analysis. Gastroenterol Res Pract. (2015) 2015:702768. doi: 10.1155/2015/702768
18.
Johanson JF Cooper G Eisen GM Freeman M Goldstein JL Jensen DM et al . Quality assessment of endoscopic ultrasound. Gastrointest Endosc. (2002) 55:798–801. doi: 10.1016/s0016-5107(02)70406-9
19.
Park JY Jeon TJ . Diagnostic evaluation of endoscopic ultrasonography with submucosal saline injection for differentiating between T1a and T1b early gastric cancer. World J Gastroenterol. (2022) 28:6564–72. doi: 10.3748/wjg.v28.i46.6564
20.
Participants in the Paris Workshop . The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. (2003) 58:S3–S43. doi: 10.1016/s0016-5107(03)02159-x
21.
Nobel T Sihag S . Advances in diagnostic, staging, and restaging evaluation of esophageal and gastric cancer. Surg Oncol Clin N Am. (2024) 33:467–85. doi: 10.1016/j.soc.2024.02.002
22.
Waters JK Reznik SI . Update on management of squamous cell esophageal cancer. Curr Oncol Rep. (2022) 24:375–85. doi: 10.1007/s11912-021-01153-4
23.
Liu M Yang W Guo C Liu Z Li F Liu A et al . Effectiveness of endoscopic screening on esophageal cancer incidence and mortality: a 9-year report of the endoscopic screening for esophageal cancer in China (ESECC) randomized trial. J Clin Oncol. (2024) 42:1655–64. doi: 10.1200/JCO.23.01284
24.
Ajani JA D’Amico TA Bentrem DJ Cooke D Corvera C Das P et al . Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2023) 21:393–422. doi: 10.6004/jnccn.2023.0019
25.
Ishihara R Arima M Iizuka T Oyama T Katada C Kato M et al . Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig Endosc. (2020) 32:452–93. doi: 10.1111/den.13654
26.
Forbes N Elhanafi SE Al-Haddad MA Thosani NC Draganov PV Othman MO et al . American Society for Gastrointestinal Endoscopy guideline on endoscopic submucosal dissection for the management of early esophageal and gastric cancers: summary and recommendations. Gastrointest Endosc. (2023) 98:271–84. doi: 10.1016/j.gie.2023.03.015
27.
Terayama M Okamura A Kuriyama K Takahashi N Tamura M Kanamori J et al . ASO visual abstract: minimally invasive esophagectomy provides better short- and long-term outcomes over open esophagectomy in locally advanced esophageal cancer. Ann Surg Oncol. (2024) 31:5780. doi: 10.1245/s10434-024-15746-3
28.
Kakeji Y Yamamoto H Watanabe M Kono K Ueno H Doki Y et al . Outcome research on esophagectomy analyzed using nationwide databases in Japan: evidences generated from real-world data. Esophagus. (2024) 21:411–8. doi: 10.1007/s10388-024-01080-w
29.
Goda K Tajiri H Ikegami M Yoshida Y Yoshimura N Kato M et al . Magnifying endoscopy with narrow band imaging for predicting the invasion depth of superficial esophageal squamous cell carcinoma. Dis Esophagus. (2009) 22:453–60. doi: 10.1111/j.1442-2050.2009.00942.x
30.
Thosani N Singh H Kapadia A Ochi N Lee JH Ajani J et al . Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. (2012) 75:242–53. doi: 10.1016/j.gie.2011.09.016
31.
Shiratori Y Kanomata N Takagi K Fukuda K . Esophageal basaloid squamous cell carcinoma presenting as a subepithelial lesion. Clin J Gastroenterol. (2021) 14:1324–8. doi: 10.1007/s12328-021-01449-9
Summary
Keywords
endoscopic ultrasonography, superficial esophageal squamous cell carcinoma, submucosal saline injection, tumour stage, treatment options
Citation
Zhang J, Chen M, Gao Y, Liu J, Li Z and Wang D (2025) Endoscopic ultrasound with submucosal saline injection improves the accuracy of T1a and T1b staging in superficial esophageal squamous cell carcinoma. Front. Med. 12:1509628. doi: 10.3389/fmed.2025.1509628
Received
11 October 2024
Accepted
10 April 2025
Published
30 April 2025
Volume
12 - 2025
Edited by
Fu Shen, Naval Medical University, China
Reviewed by
Stavros P. Papadakos, Laiko General Hospital of Athens, Greece
Hui Xing, Houston Methodist Research Institute, United States
Updates
Copyright
© 2025 Zhang, Chen, Gao, Liu, Li and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengjun Li, lizj686@126.comDongyang Wang, dywang@email.sdfmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.