Abstract
Objective:
IgA nephropathy (IgAN) is the leading cause of end-stage renal disease (ESRD) globally, with its pathological mechanisms closely related to mucosal immune abnormalities and complement activation. Currently, there is no curative treatment. This study aims to systematically evaluate the efficacy differences of existing treatment regimens on clinical remission (CR), 24-h urinary protein excretion (24-h UPE), ESRD or kidney damage (KD) and adverse events (AEs) in IgAN, providing evidence-based support for optimizing stratified treatment strategies.
Methods:
A systematic search was conducted in the PubMed, Web of Science, Embase, and Cochrane Library databases up to February 20, 2025, including 57 randomized controlled trials (RCTs) covering 19 interventions. Pairwise and network meta-analyses were employed to assess binary variable (CR, ESRD or KD, AEs) using risk ratios (RR) and continuous variable (24-h UPE) using standardized mean differences (SMD), with interventions ranked based on the area under the cumulative ranking curve.
Results:
Clinical remission (26 RCTs included in the analysis): The CR for tonsillectomy combined with steroids pulse therapy (TSP) (RR = 8.23, 95% CI 4.11–16.45), anti-APRIL monoclonal antibody sibeprenlimab (RR = 10.00, 1.34–74.48), and steroids combined with renin-angiotensin system inhibitors (STE + RASI) (RR = 5.03, 2.61–9.68) were significantly superior to placebo. Proteinuria control (36 studies assessing 24-h UPE): The BLyS/APRIL dual-target inhibitor telitacicept (SMD = −5.21, −7.55 to −2.87) and STE + RASI (SMD = −1.98, −3.15 to −0.82) significantly reduced 24-h UPE, outperforming the mycophenolate mofetil combined with steroids regimen (SMD = −0.97, −2.74 to 0.80). Renal endpoint events (26 studies analyzing ESKD or KD): STE + RASI reduced the risk of ESKD or KD by 98.1% (optimal SUCRA ranking), followed by the dual endothelin/angiotensin receptor antagonist sparsentan (82.6%). Safety (36 studies reporting adverse events): The complement inhibitor iptacopan (88.4%) and sodium-glucose co-transporter 2 inhibitors (SGLT2i) (85.4%) had the lowest incidence of adverse events, significantly better than immunosuppressive regimens.
Conclusion:
STE + RASI serves as a core therapeutic strategy for IgAN, significantly improving clinical remission rates, reducing the risk of ESRD or KD, and addressing proteinuria. Telitacicept, sparsentan, and TSP can be considered as enhanced options for specific phenotypic patients, while targeted ileal budesonide (Nefecon) has not demonstrated a significant renal protective advantage.
Systematic review registration:
CRD42023494801.
1 Introduction
IgA nephropathy (IgAN), the most prevalent primary glomerulonephritis worldwide, exhibits marked geographic heterogeneity in incidence. Asian populations, particularly in China and Japan, demonstrate a disproportionately high prevalence of IgAN, which ranks among the leading etiologies of glomerular diseases in these regions. The incidence in Asian cohorts significantly exceeds that observed in North America and Africa, a disparity strongly linked to interactions between genetic susceptibility (e.g., HLA-DQB1 polymorphisms) and environmental triggers (e.g., mucosal pathogen exposure) (1, 2). Notably, IgAN predominantly affects individuals under 40 years of age, emerging as a leading cause of kidney failure in young adults. Approximately 40% of patients progress to end-stage renal disease (ESRD) within 20–30 years, imposing substantial healthcare costs and socioeconomic burdens (3–5). Consequently, developing safe and effective disease-modifying therapies represents an urgent global priority.
Recent investigations have elucidated the autoimmune pathogenesis of IgAN. The central mechanism involves defective galactosylation of IgA1 as a key autoantigen (6, 7). Deficiencies in the expression of core 1β1,3-galactosyltransferase (C1GalT1) and its molecular chaperone, Cosmc, within patients’ B cells result in aberrant O-glycosylation of the IgA1 hinge region. The absence of galactose leads to the formation of pathogenic galactose-deficient IgA1 (Gd-IgA1) (8, 9). These abnormally glycosylated IgA1 molecules bind to anti-glycan autoantibodies (IgG/IgA), forming high-molecular-weight immune complexes. These immune complexes deposit in the glomeruli, activating the complement cascade and releasing inflammatory mediators. This process subsequently promotes mesangial cell proliferation and glomerular injury (10–12).
Given the aforementioned mechanisms, immunomodulatory therapies are considered potential intervention strategies, yet their clinical efficacy presents significant discrepancies. Large-scale paired meta-analyses indicate that conventional immunosuppressants, such as cyclophosphamide, although effective in reducing proteinuria, have not demonstrated the ability to decelerate the progression of renal dysfunction (13). Conversely, the TESTING study confirmed that glucocorticoids can reduce the risk of ESRD by 33%, but their long-term adverse effects limit their widespread application (14). The 2024 KDIGO guidelines propose a stratified management strategy, emphasizing the need for comprehensive interventions targeting multiple pathogenic pathways, including the reduction of circulating immune complex levels, inhibition of complement activation, and optimization of supportive care (e.g., the combined use of RASI and SGLT2i) (15). Novel therapies, such as dual endothelin-angiotensin receptor antagonists (sparsentan), have exhibited superior proteinuria-reducing effects compared to traditional RASIs, potentially offering new options for delaying the decline in renal function (16).
However, the current landscape of IgAN treatment still faces three major challenges. First, head-to-head comparisons are lacking for various novel drugs targeting Gd-IgA1 production, immune complex clearance, and complement regulation (e.g., APRIL/BLyS inhibitors, complement factor B antagonists). Secondly, the lack of standardized efficacy assessment criteria across studies, with current research often relying on proteinuria remission as a surrogate endpoint, necessitates further validation of its correlation with long-term renal outcomes. Finally, the optimal approach to precision medicine, based on individual differences such as biomarkers (Gd-IgA1 levels, genetic risk scores), pathological features, and lifestyle factors, remains undefined, leading to challenges in individualized treatment decision-making. Therefore, this study employs network meta-analysis (NMA) to systematically evaluate the efficacy and safety differences of various therapies, integrating both direct and indirect comparative evidence, with the aim of providing dynamically updated decision-making guidance for clinical practice.
2 Materials and methods
Our study followed PRISMA guidelines (17), detailed in the Supplementary materials S1, and is registered with PROSPERO (CRD42023494801).
2.1 Data sources and searches
We systematically searched databases such as PubMed, Web of Science, Embase, and Cochrane Library from inception to February 20, 2025, for randomized controlled trials (RCTs) evaluating the clinical efficacy of various agents in IgAN patients. There were no restrictions on language, publication years, or blinding methods. Our search strategy integrated MeSH terms and free text, including: [(Glomerulonephritis, IgA) OR (Berger’s Disease) OR (IgA Glomerulonephritis) OR (IgA Nephropathy)] AND [(Renin-Angiotensin System Inhibitors) OR (Steroids) OR (Telitacicept) OR (Sparsentan) OR (Mycophenolate Mofetil) OR (Budesonide/Nefecon) OR (Leflunomide) OR (Tacrolimus) OR (Hydroxychloroquine) OR (Tonsillectomy) OR (Rituximab) OR (Mizoribine) OR (Cyclosporin A) OR (Azathioprine) OR (Cyclophosphamide) OR (Atacicept) OR (Iptacopan) OR (Sodium-glucose cotransporter 2 inhibitors) OR (Tonsillectomy with steroid pulse therapy) OR (Sibeprenlimab)] AND (RCTs). We also manually reviewed the literature to ensure comprehensive coverage.
2.2 Selection criteria
The inclusion criteria (Supplementary materials S3) are as follows: (1) study type was RCTs; (2) the study participants were no less than 9 years old, no gender limit, renal biopsy confirmed IgAN; (3) the subjects with proteinuria or 24-h urinary protein excretion (24-h UPE) more than 0.5 g/d and eGFR ≥ 30 mL/min per 1.73 m2 or serum creatinine less than 3.5 mg/dL; (4) interventions for studies should include RASI (renin-angiotensin system inhibitors), STE (steroids), MMF (mycophenolate mofetil), AZA (azathioprine), CsA (Cyclosporin A), Tonsillectomy, RIT (rituximab), TAC (tacrolimus), HCQ (hydroxychloroquine), LEF (leflunomide), MZR (mizoribine), CTX (cyclophosphamide), TSP (tonsillectomy with steroid pulse therapy), nefecon, telitacicept, sparsentan, atacicept, iptacopan and sibeprenlimab; (5) each study reported at least one of the following four indicators: (1) clinical remission (CR; defined as achieving proteinuria <0.3 g/d or a ≥50% reduction in proteinuria), (2) 24-h UPE, (3) ESRD (defined by serum creatinine >707 μmol/L or requirement for maintenance dialysis or kidney transplantation) or kidney damage (KD; defined by either a ≥30% decrease in eGFR from baseline or a doubling of serum creatinine), (4) adverse events (AEs).
Exclusion criteria: (1) clinically confirmed IgAN secondary to systemic diseases such as systemic lupus erythematosus, and allergic purpura; (2) drugs included in the publication were not involved in our study, or publications compared to the same drug in terms of administration route or dosage; (3) articles that had no definitions on clinical remission or 24-h UPE or renal function.
2.3 Data extraction and quality evaluation
We used EndNote software to manage the retrieved literature. After screening the title and abstract, the articles meeting the inclusion criteria were obtained for evaluation and data extraction. In addition, three reviewers (BC, YZ, and YY) independently extracted data through Microsoft Excel. Any disagreements during data extraction were resolved by the fourth reviewer (GX). The data extraction contents included: basic characteristics of the included literature (country, publication year, and first author), study subject information (mean age, sample size, renal function, and baseline of UPE), interventions (different drugs and period of follow-up), and reported outcomes (CR, 24-h UPE, ESRD or KD, and AEs). For information that cannot be directly obtained, we made great efforts to contact the authors via email. The three reviewers (BC, YZ, and YY) independently assessed the risk of bias for all studies according to the Cochrane Risk of Bias tool [Cochrane Handbook for Systematic Reviews of Interventions, version 5.4.0] (18). Each domain can be evaluated as high, low, or unclear risk for the included studies. Any disagreements were resolved by the fourth reviewer (GX).
2.4 Statistical analysis
The data from studies reporting outcomes like clinical remission, ESRD or KD, 24-h UPE, and AEs were extracted. Using a frequentist framework, a random-effects model analyzed the data (19). We calculated the 95% confidence intervals (CI) and relative risk (RR) for dichotomous variables, along with 95% CI and standardized mean difference (SMD) for continuous variables to quantify effect sizes. Statistical analysis was conducted using STATA 17.0, with “mvmeta” and “network” packages for network plots, league tables, publication bias assessment, and treatment ranking probabilities. The Surface Under the Cumulative Ranking curve (SUCRA), which ranges from 0 (worst) to 1 (best) (20), evaluated treatment efficacy, with higher values indicating better strategies. R version 4.2.3, employing “ggplot2” and “gemtc” packages, generated forest plots and regression analyses. For initial values, we executed 50,000 simulations, discarding the first 20,000 as burn-in. Convergence was assessed with Brooks-Gelman-Rubin diagnostic plots. Statistical significance was noted when zero was excluded in the 95% CI for SMD or one for RR. Some heterogeneity was expected; thus, we employed a “design-by-treatment” model (21) for global assessment and node-splitting methods (22) for local analysis, separating direct and indirect comparisons. A p-value above 0.05 indicated no significant inconsistency (23). Following this, a consistency model was applied to analyze remaining statistical data. Heterogeneity was measured using I2, with I2 > 50% indicating significant diversity among RCTs. In our findings, I2 values for AEs, clinical remission, ESRD or KD, and 24-h UPE were all below 12%, reflecting low heterogeneity (Supplementary materials S5). Regression and sensitivity analyses were conducted with R and STATA to investigate potential heterogeneity sources.
2.5 General classification of drugs
We categorized interventions into four efficacy groups based on SUCRA rankings for AEs, clinical remission, ESRD or KD, and 24-h UPE: significant efficacy (SUCRA > 60% for two or more indicators), moderate efficacy (SUCRA 40–60% for two or more indicators), low efficacy (SUCRA < 40% for two or more indicators), and very low efficacy (lowest SUCRA rankings for two indicators), as shown in Supplementary materials S8 and Supplementary Figure 8.
3 Results
3.1 Literature screening process
A total of 1,823 articles were identified. After deduplication using EndNote, 938 articles remained. Excluding animal studies, reviews, case reports, and non-RCTs through title, keyword, and abstract screening, 168 articles were selected. Upon reviewing these 168 full texts, 111 were excluded for reasons such as study design, treatment protocols, subjects, and outcome measures not aligning with our criteria. Ultimately, 57 RCTs (24–80) (including one three-arm RCT and 56 two-arm RCTs) involving 5,123 patients were included. These trials investigated 19 different interventions (excluding combination therapies), such as TSP, MMF, STE, RASI, LEF, CsA, MZR, RIT, HCQ, AZA, TAC, SGLT2i, iptacopan, atacicept, telitacicept, sparsentan, sibeprenlimab, nefecon, and placebo. The literature screening process and results are illustrated in Figure 1.
Figure 1

Flow chart of literature screening process and results. ESRD, end-stage renal disease; KD, kidney damage; 24-h UPE, 24-h urinary protein excretion; randomized controlled trials (RCTs).
3.2 Patient and baseline characteristics
In these 57 RCTs, TSP was used in 4 RCTs involving 221 patients (4 RCTs, 221 patients), MMF (10 RCTs, 2,331 patients), STE (28 RCTs, 1,753 patients), RASI (20 RCTs, 1,464 patients), LEF (4 RCTs, 143 patients), CsA (2 RCTs, 32 patients), MZR (4 RCTs, 141 patients), RIT (1 RCT, 17 patients), HCQ (2 RCTs, 120 patients), AZA (5 RCTs, 202 patients), TAC (1 RCT, 20 patients), SGLT2I (1 RCT, 137 patients), iptacopan (1 RCT, 26 patients), atacicept (1 RCT, 5 patients), telitacicept (1 RCT, 14 patients), sparsentan (1 RCT, 202 patients), sibeprenlimab (1 RCT, 38 patients), nefecon (1 RCT, 182 patients), and STE combined with RASI (3 RCTs, 101 patients). Among these, 26 RCTs reported detailed information regarding clinical remission and ESRD or KD, and 36 RCTs reported baseline and post-treatment 24-h UPE. Additionally, 36 RCTs mentioned different adverse reactions in both the treatment and control groups. The relevant baseline characteristics of the included 57 RCTs can be found in Supplementary materials S2. In this study, all but two RCTs included mentioned randomization. In addition, 18 RCTs (32% of all RCTS) described specific randomization methods, of which 5 RCTs used random number tables, 1 RCT used stratified random sampling, and 12 RCTs used computer-generated randomization, all of which were rated as “low risk” bias in the random sequence generation section. All RCTs that provided complete data and did not selectively report results were rated as “low risk” of bias in the areas of full outcome assessment and selective reporting. However, due to a lack of sufficient information, most RCTs were rated as “uncertain risk” in terms of implementation bias, measurement bias, and other biases. The risk of bias for the eligible studies is presented in Supplementary Figure 1.
3.3 Network structure, consistency, and heterogeneity
The network diagrams for various interventions are presented in Figure 2, illustrating 19 interventions for adverse events, 15 for clinical remission, 13 for ESRD or KD, and 16 for 24-h UPE. Node sizes reflect the sample sizes of the interventions, while line thickness indicates the number of direct comparisons between interventions. The sample sizes and number of RCTs differ across interventions. Diagnostic plots and trace plots confirm satisfactory convergence of this NMA, as detailed in Supplementary Figure 3. Consistency analysis via node-splitting methods (Supplementary Figure 5) shows all p-values exceeding 0.05, indicating strong consistency, except for comparisons involving STE and placebo, STE with RASI, and RASI, STE in ESRD or KD. Heterogeneity analysis (Supplementary Figure 4) reveals significant heterogeneity in comparisons: CsA vs. placebo; STE vs. placebo; RASI vs. placebo; RASI vs. STE; MMF vs. STE in AEs; RASI vs. placebo; STE vs. MMF, RASI, TSP in CR; MMF, STE, STE with AZA vs. placebo; STE vs. STE with AZA, RASI in ESRD or KD; HCQ, MMF, MZR, STE, AZA with AZA vs. placebo; STE vs. LEF, RASI, STE with AZA, STE with RASI; STE with RASI vs. RASI in 24-h UPE. Consequently, a Random-Effects model was chosen for the NMA, with potential heterogeneity sources investigated through regression and sensitivity analyses.
Figure 2

Network meta-analysis of eligible comparisons for (A) adverse events, (B) clinical remission, (C) ESRD or KD, (D) 24-h UPE. The width of the lines represents the number of each pairwise comparison. The size of each node is proportional to the number of randomly assigned participants (i.e., sample size). TSP, tonsillectomy with steroid pulse therapy; MMF, mycophenolate mofetil; STE, steroids; RASI, renin-angiotensin system inhibitors; LEF, leflunomide; CsA, Cyclosporin A; MZR, mizoribine; RIT, rituximab; HCQ, hydroxychloroquine; AZA, azathioprine; TAC, Tacrolimus; SGLT2i, sodium glucose cotransporter-2 inhibitor.
3.4 Pairwise meta-analysis
The pairwise meta-analysis results for various agents are in Supplementary materials S6.
3.5 Network meta-analysis results
3.5.1 Adverse events
A total of 36 studies on adverse events involved 3,891 patients across 19 interventions: Placebo (26 RCTs, 1,294 patients), AZA (4 RCTs, 173 patients), atacicept (1 RCT, 5 patients), CsA (2 RCTs, 32 patients), HCQ (1 RCT, 30 patients), iptacopan (1 RCT, 26 patients), LEF (4 RCTs, 143 patients), MMF (7 RCTs, 209 patients), MZR (2 RCTs, 54 patients), MZR + RASI (1 RCT, 30 patients), nefecon (1 RCT, 182 patients), RASI (7 RCTs, 479 patients), SGLT2i (1 RCT, 137 patients), STE (10 RCTs, 425 patients), STE + MMF (1 RCT, 26 patients), sibeprenlimab (1 RCT, 38 patients), sparsentan (1 RCT, 202 patients), TAC (1 RCT, 20 patients), and telitacicept (1 RCT, 14 patients). The network diagram is shown in Figure 2A.
TAC had a higher incidence of adverse reactions compared with all other interventions. The RR of iptacopan, SGLT2i, atacicept, telitacicept, Placebo, MZR, RASI, sparsentan, CsA, sibeprenlimab, MZR + RASI, AZA, STE + MMF, LEF, nefecon, MMF, STE, and HCQ were 41.60 (95%CI: 3.19, 542.94), 25.47 (3.10, 209.60), 25.14 (2.86, 221.14), 18.29 (2.10, 159.52), 16.00 (2.19, 116.88), 17.22 (1.82, 163.06), 15.57 (1.97, 122.90), 14.85 (1.71, 128.87), 13.93 (1.67, 116.38), 13.94 (1.76, 110.19), 11.55 (1.19, 112.53), 10.96 (1.42, 84.44), 10.40 (1.27, 85.35), 10.24 (1.25, 84.06), 8.00 (0.89, 71.70), 8.34 (1.07, 65.25), 8.45 (1.10, 65.12), and 2.67 (0.15, 48.69), respectively (Figure 3).
Figure 3

League table of all comparisons for adverse events and 24-h UPE. Data are RR (95% CI) for adverse events (lower-left quadrant) and SMD (95% CI) for 24-h UPE (upper-right quadrant) in the column-defining treatment compared with the row-defining treatment. RR lower than one favor the column-defining treatment and SMD higher than zero favor the row-defining treatment. Significant results are indicated in bold. SMD, standardized mean difference.
Supplementary materials S4A displays SUCRA values for the interventions, where iptacopan scored 88.4%, SGLT2I 85.4%, Atacicept 83.2%, telitacicept 70.1%, Placebo 66.5%, MZR 66.4%, RASI 63.9%, sparsentan 59.9%, CsA 56.3%, sibeprenlimab 55.9%, MZR + RASI 45.2%, AZA 40.4%, STE + MMF 38.6%, LEF 37.6%, nefecon 27.8%, MMF 25.0%, STE 24.6%, HCQ 12.2%, and TAC 2.5%. Detailed statistical analysis results are illustrated in Figure 4A and Supplementary Figure 2A.
Figure 4

Rankings of SUCRA for (A) adverse events and (B) ESRD or KD. SUCRA, surface under the cumulative ranking curve.
3.5.2 Clinical remission
A total of 26 studies reported the clinical remission outcome, with 1,996 patients involved. The analysis included 15 interventions: placebo (16 RCTs, 466 patients), AZA (1 RCT, 40 patients), CsA (2 RCTs, 32 patients), HCQ (2 RCTs, 120 patients), LEF (3 RCTs, 84 patients), MMF (5 RCTs, 113 patients), MZR (3 RCTs, 72 patients), RASI (6 RCTs, 409 patients), RIT (1 RCT, 17 patients), STE (9 RCTs, 289 patients), STE + MMF (1 RCT, 26 patients), STE + RASI (3 RCTs, 135 patients), sibeprenlimab (1 RCT, 38 patients), sparsentan (1 RCT, 202 patients), and TSP (1 RCT, 49 patients). The network diagram is detailed in Figure 2B.
Except for STE + MMF, AZA, CsA, RIT, and MZR, all other interventions demonstrated superior efficacy in achieving CR compared to placebo. The RR for TSP, sibeprenlimab, STE + RASI, STE, sparsentan, MMF, LEF, RASI, and HCQ are 8.23 (95% CI: 4.11, 16.45), 10.00 (1.34, 74.48), 5.03 (2.61, 9.68), 4.53 (2.38, 8.62), 4.31 (2.29, 8.08), 2.93 (1.77, 4.87), 2.52 (1.38, 4.62), 2.46 (1.34, 4.51), and 1.62 (1.19, 2.21), respectively (Figure 5).
Figure 5
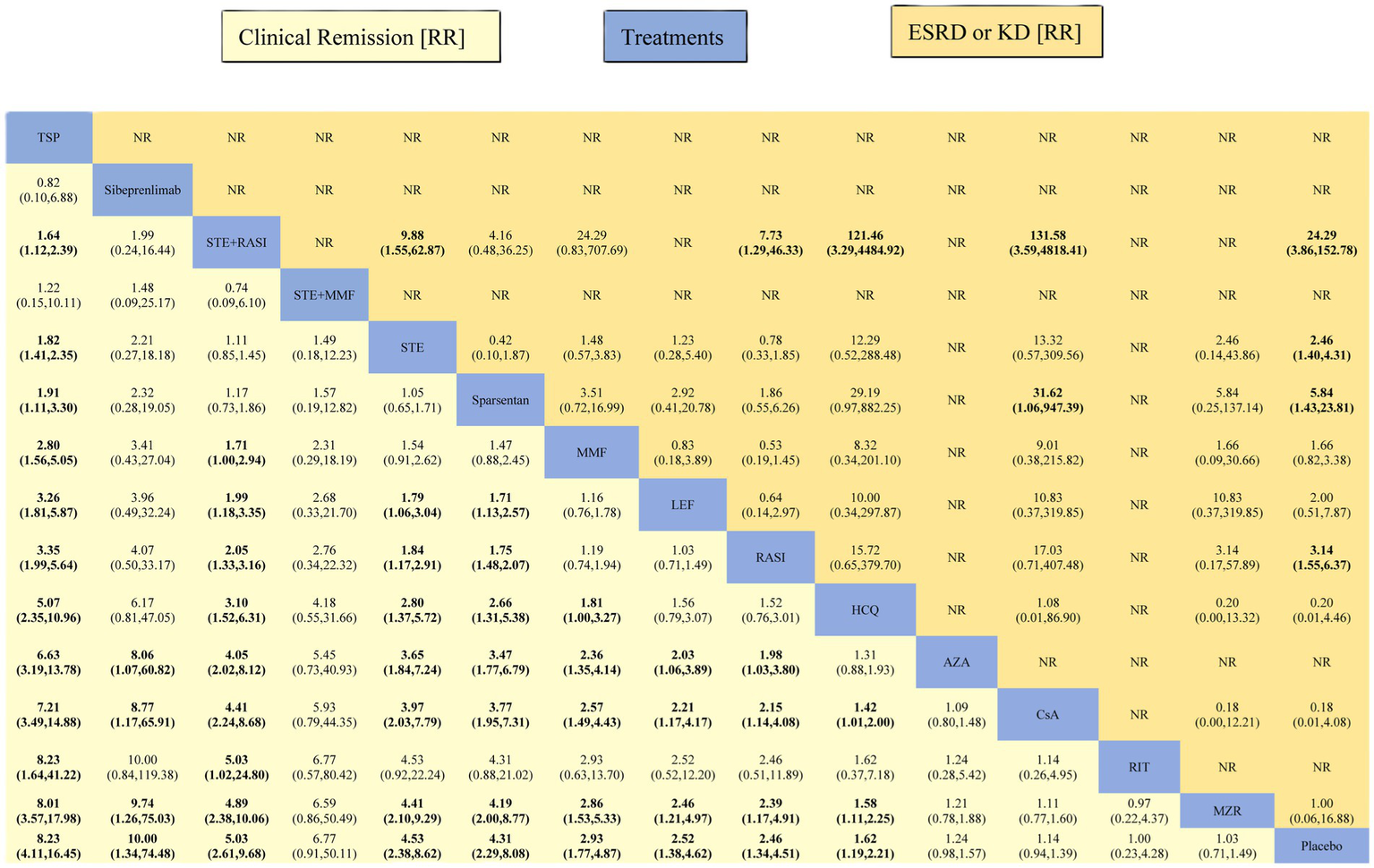
League table of all comparisons for clinical remission and ESRD or KD. Data are RR (95% CI) for clinical remission (lower-left quadrant) and ESRD or KD (upper-right quadrant) in the column-defining treatment compared with the row-defining treatment. RR higher than one favor the column-defining treatment (lower-left quadrant). RR higher than one favor the row-defining treatment (upper-right quadrant). Significant results are indicated in bold. RR, risk ratios; 95% CI, 95% confidence intervals.
The Supplementary materials S4B presented SUCRA values for 15 interventions concerning CR: TSP 92.8%, sibeprenlimab 85.6%, STE + RASI 79.4%, STE + MMF 77.6%, STE 73.6%, sparsentan 72.2%, MMF 56.5%, LEF 49.0%, RASI 47.6%, HCQ 35.6%, AZA 23.6%, CsA 18.5%, RIT 18.3%, MZR 11.7%, and Placebo 8.0%. Detailed results are shown in Figure 6A and Supplementary Figure 2B.
Figure 6

Rankings of SUCRA for (A) clinical remission and (B) 24-h UPE.
3.5.3 ESRD or KD
A total of 26 studies analyzed this indicator, involving 3,250 patients across 13 interventions: Placebo (20 RCTs, 1,188 patients), CsA (1 RCT, 23 patients), HCQ (1 RCT, 30 patients), LEF (2 RCTs, 80 patients), MMF (5 RCTs, 229 patients), MZR (1 RCT, 21 patients), nefecon (1 RCT, 182 patients), RASI (7 RCTs, 432 patients), SGLT2I (1 RCT, 137 patients), STE (11 RCTs, 662 patients), STE + AZA (3 RCTs, 140 patients), STE + RASI (2 RCTs, 53 patients), and sparsentan (1 RCT, 202 patients). The network visualization is shown in Figure 2C.
All interventions except LEF, nefecon, MMF, MZR, HCQ, and CsA had a lower incidence of ESRD or KD compared to Placebo. The RR for STE + RASI, sparsentan, SGLT2i, RASI, STE, and STE + AZA were recorded as 0.04 (0.01, 0.26), 0.17 (0.04, 0.70), 0.29 (0.09, 0.97), 0.32 (0.16, 0.64), 0.41 (0.23, 0.71), and 0.42 (0.20, 0.86), respectively (Figure 5).
Supplementary materials S4C provides the SUCRA values for 15 ESRD or KD interventions, showing percentages as follows: STE + RASI 98.1%, sparsentan 82.6%, SGLT2I 68.7%, RASI 67.9%, STE 58.0%, STE + AZA 56.5%, LEF 49.7%, nefecon 46.7%, MMF 41.5%, MZR 35.8%, Placebo 21.5%, HCQ 11.6%, CsA 11.2%. Detailed statistical results are in Figure 4B and Supplementary Figure 2C.
3.5.4 24-h UPE
There are 36 studies involving 2,568 patients assessed 24-h UPE across 16 interventions: Placebo (26 RCTs, 911 patients), Atacicept (1 RCT, 5 patients), CsA (2 RCTs, 23 patients), HCQ (2 RCTs, 120 patients), iptacopan (1 RCT, 26 patients), LEF (3 RCTs, 119 patients), MMF (5 RCTs, 103 patients), MZR (2 RCTs, 51 patients), RASI (8 RCTs, 288 patients), RIT (1 RCT, 17 patients), STE (12 RCTs, 630 patients), STE + AZA (4 RCTs, 91 patients), STE + MMF (1 RCT, 26 patients), STE + RASI (3 RCTs, 124 patients), TAC (1 RCT, 20 patients), and telitacicept (1 RCT, 14 patients) (Figure 2D).
All interventions, except for telitacicept, exhibited lower effects on proteinuria reduction. The relative risk (Standardized Mean Difference, SMD) for STE + RASI, STE + MMF, LEF, STE + AZA, STE, iptacopan, HCQ, RASI, Atacicept, MMF, CsA, TAC, RIT, MZR, and Placebo were −3.23 (95% CI: −5.84, −0.61), −4.24 (−7.17, −1.31), −4.33 (−6.93, −1.73), −4.41 (−6.96, −1.86), −4.44 (−6.86, −2.02), −4.40 (−7.32, −1.47), −4.42 (−7.06, −1.79), −4.46 (−6.91, −2.02), −4.49 (−7.64, −1.34), −4.54 (−7.02, −2.05), −4.90 (−7.82, −1.97), −4.97 (−7.91, −2.04), −5.23 (−8.18, −2.28), −5.38 (−8.03, −2.74), and −5.21 (−7.55, −2.87), respectively (Figure 3).
Supplementary materials S4D presents the SUCRA values for the 16 interventions concerning the 24-h UPE, which were 99.9, 87.4, 57.5, 57.1, 53.7, 53.6, 53.1, 53.1, 52.1, 49.4, 48.1, 36.3, 34.2, 27.0, 19.0, and 18.5% for telitacicept, STE + RASI, STE + MMF, LEF, STE + AZA, STE, iptacopan, HCQ, RASI, Atacicept, MMF, CsA, TAC, RIT, MZR, and Placebo, respectively. Detailed statistical analysis results can be found in Figure 6B and Supplementary Figure 2D.
3.6 Meta-regression, publication bias and sensitivity analyses
We performed a subgroup analysis in IgA patients with proteinuria > 1 g/d, and there was a non-significant difference compared with the group with proteinuria > 0.5 g/d. Heterogeneity tests indicated significant differences between subgroups, necessitating further investigation into heterogeneity sources via regression analysis. We adjusted for publication year and sample size as univariate covariates regarding AEs, CR, ESRD or KD, and 24-h UPE. Results indicated that publication year and sample size correlated with heterogeneity in AEs, CR, and ESRD or KD but not in 24-h UPE (Supplementary materials S7). An adjusted funnel plot indicated no significant publication bias (Supplementary Figure 6). Sensitivity analyses showed excluding any single study did not significantly alter the overall effect size, confirming the robustness of our findings (Supplementary Figure 7).
4 Discussion
This conducted a stratified analysis of 19 interventions based on SUCRA, categorizing drug efficacy into four groups according to the comprehensive rankings of AEs, CR, ESRD or KD, and 24-h UPE. Firstly, the significant efficacy group (SUCRA >60%) includes SGLT2i, telitacicept, sparsentan, and STE + RASI. Their core mechanisms encompass metabolic regulation (SGLT2i), immune complex clearance (telitacicept) (81), and hemodynamic optimization (sparsentan), which significantly reduce the risk of ESRD and 24-h UPE. Secondly, the moderate efficacy group (SUCRA 40–60%) consists of the complement inhibitor iptacopan (C5a antagonist), the anti-APRIL monoclonal antibody sibeprenlimab, and traditional immunotherapy regimens (STE monotherapy/combined with mycophenolate mofetil/azathioprine), indicating that some targeted therapies require adjunctive support to enhance efficacy. Then, the low efficacy group includes nefecon and RIT, potentially related to heterogeneous responses in mucosal immune regulation. Finally, the very low efficacy group comprises HCQ and calcineurin inhibitors (TAC, CsA), reflecting the limitations of nonspecific immunosuppression in IgAN.
Based on KDIGO guidelines (15) and the latest study, treatment strategies for IgAN should prioritize a stratified selection that balances efficacy and safety. Our research demonstrates that STE + RASI, as a classic immunomodulatory regimen, significantly outperforms traditional immunosuppressive therapies (such as STE monotherapy, MMF, LEF, CsA, etc.) in terms of CR, ESRD or KD, and 24-h UPE, with mechanisms involving immune modulation (Th17/IL-23 pathway inhibition) and hemodynamic optimization (reduction of intraglomerular pressure), consistent with finding from Horita et al. (35). Furthermore, previous meta-analyses (82) indicate that immunosuppressive therapy can reduce the long-term risk of ESRD in IgAN patients, although it may increase the risk of long-term adverse events. However, strict monitoring of glucocorticoid-related adverse events is necessary, and it is recommended to use low-dose STE (0.4–0.6 mg/kg/d) for a limited duration (6–9 months), prioritizing high-risk patients with proteinuria ≥1 g/d and eGFR ≥60 mL/min/1.73 m2 (KDIGO 2024 2B recommendation) (15), aligning with the results of the low-dose group in the TESTING study (NEJM 2022) (59).
Although nefecon (targeting budesonide in the ileum) reduced Gd-IgA1 levels by 53% in phase III trials (NEFIGAN study) (62), this meta-analysis indicates its overall efficacy is relatively low (low efficacy group), possibly due to the inability to extract specific data regarding CR and 24-h UPE during the analysis. KDIGO 2024 recommends its use in subgroups with biopsy-confirmed active mesangial proliferation (M1) or C1/C2 (capillary wall lesions) and Gd-IgA1 ≥ 2.5 U/mL, rather than as a broad replacement for traditional regimens. Nevertheless, the unique advantage of nefecon in reducing Gd-IgA1 and IgA immune complex levels should not be overlooked, as it holds immense potential as a drug that can block the progression of IgAN at its source.
For high-risk patients, intensified treatment regimens should be considered, such as the combination of SGLT2i and RASI. In the KDIGO 2024 draft, this combination has been upgraded to first-line support therapy. This combination has been shown to reduce the risk of ESRD and cardiovascular events (83), independent of its hypoglycemic effects. This is particularly suitable for patients with progressive disease with eGFR ≥25 mL/min/1.73 m2. Although the safety of SGLT2 inhibitors in this study was good (SUCRA 85.4%), adverse reactions such as urinary tract infections, worsening renal injury, and blood volume reduction should be noted. On the one hand, our research results show that, compared to RASI, sparsentan, RASI + STE, iptacopan, telitacicept has advantages in reducing 24-h UPE, with fewer side effects and good safety. For patients with persistent nephropathy accompanied by significant proteinuria (UPCR ≥3.5 g/g), telitacicept (BLyS/APRIL inhibitor) (84) combined with RASI can additionally reduce proteinuria by 47% (SMD = −5.21%). On the other hand, this study shows that sparsentan (ETAR/AT1R antagonist) may be superior to traditional RASI in CR and the prevention of renal progression (16, 85). Therefore, sparsentan can be used in patients resistant to RASI, which can effectively slow the rate of eGFR decline, reaching 2.4 mL/min/year (PROTECT study).
Meanwhile, iptacopan (SUCRA 88.4%) performed best in terms of safety. For patients with high complement activation markers (elevated serum C3a/C5a), complement inhibitors (iptacopan) can be used as an alternative for those who are intolerant to hormones. This study also explored the role of tonsillectomy in the treatment of IgAN. The results show that, whether as an adjuvant therapy or as a standalone treatment, tonsillectomy can significantly improve the remission rate of proteinuria and hematuria. This finding is consistent with the results of the Japanese population (86), suggesting that the IgA1 secreted by the tonsil cells may be involved in the pathogenesis of IgAN (87, 88), which aligns with the conclusions drawn from the meta-analysis conducted by Wang (89), Liu (90), and others. Therefore, for patients with recurrent tonsil attacks, tonsillectomy may be an effective alternative or supplementary treatment.
Despite the inclusion of 57 RCTs and 5,123 participants in this study, certain limitations persist. Firstly, heterogeneity in patient baseline characteristics and treatment regimens across different studies may impact the generalizability of the findings. Secondly, long-term efficacy and safety data for some medications remain insufficient, necessitating further validation through larger, multi-center randomized controlled trials. Moreover, future research should strictly adhere to PRISMA guidelines, providing detailed baseline data to support more robust network meta-analyses.
In summary, while Yang (91) and Tan (92) have previously published network meta-analyses on various interventions for IgAN patients, this study provides crucial clinical evidence regarding the efficacy and safety of pharmacological treatments for IgAN. Emerging drugs such as nefecon, telitacicept, and sparsentan have demonstrated significant advantages in reducing proteinuria, preventing ESRD, and improving renal function recovery, while traditional medications like RASI and STE continue to hold an important position (82). However, clinical practice must integrate the Oxford classification (MEST-C), biomarkers (Gd-IgA1, complement activation products), patient comorbidities, cost, and accessibility to formulate optimal treatment strategies. Future research should continue to focus on the long-term efficacy and safety of these medications, providing a more solid evidence base for clinicians and patients.
5 Limitations
The limitations of this study include: (1) Among 57 studies, 39 (68.4%) lacked details on randomization, risking selection bias; (2) Heterogeneity among studies, despite regression and sensitivity analyses; (3) Missing baseline data like proteinuria and GFR changes; (4) Inadequate description of blinding and allocation concealment, risking information bias. Future high-quality, multicenter, large-sample RCTs are needed for more reliable clinical evidence.
6 Conclusion
STE + RASI, as a classic immunomodulatory regimen, demonstrates significant advantages in comprehensive clinical remission (79.4%), ESRD or KD (98.1%), and reduction of 24-h UPE (87.4%); however, its infection and metabolism-related adverse events require close monitoring. Compared to other treatment regimens, sparsentan (82.6%) shows potential superiority in preventing end-stage renal disease; Telitacicept (99.9%) excels in reducing 24-h UPE and may be suitable for patients with persistent proteinuria; iptacopan (88.4%) and SGLT2i (85.4%) provide additional advantages in terms of safety. Additionally, for IgAN patients with recurrent tonsillitis, TSP (92.8%) may be the best option for improving clinical remission rates. Nefecon, as a targeted therapy, has not yet been shown in our studies to be superior to traditional immunosuppressive regimens in delaying eGFR decline and overall safety.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BC: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YZ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. YY: Data curation, Writing – review & editing. GX: Funding acquisition, Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Key Projects of Jiangxi Natural Science Foundation (No. 20224ACB206008), the Key Clinical Research Project of the Second Affiliated Hospital of Nanchang University (No. 2022efyB01), the “Thousand Talents Plan” project of introducing and training high-level talents of innovation and entrepreneurship in Jiangxi Province (No. JXSQ2023201030), and the Jiangxi Province Key Laboratory of Molecular Medicine (No. 2024SSY06231).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1515723/full#supplementary-material
- AEs
Adverse events
- APRIL
A proliferation inducing ligand
- AZA
Azathioprine
- BlyS
B lymphocyte stimulator
- CI
Confidence intervals
- CsA
Cyclosporin A
- CR
Clinical remission
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- Gd-IgA1
Galactose-deficient IgA1
- HCQ
Hydroxychloroquine
- IgAN
IgA nephropathy
- KD
Kidney damage
- KDIGO
Kidney Disease Improving Global Outcomes
- LEF
Leflunomide
- MMF
Mycophenolate mofetil
- MZR
Mizoribine
- NMA
Network meta-analysis
- UPE
Urinary protein excretion
- RASI
Renin-angiotensin system inhibitors
- RCTs
Randomized controlled trials
- RIT
Rituximab
- RR
Risk ratios
- SGLT2i
Sodium glucose cotransporter-2 inhibitor
- SMD
Standardized mean difference
- STE
Steroids
- SUCRA
Surface Under the Cumulative Ranking curve
- TAC
Tacrolimus
- TSP
Tonsillectomy with steroid pulse therapy
Glossary
References
1.
Schena FP Nistor I . Epidemiology of IgA nephropathy: a global perspective. Semin Nephrol. (2018) 38:435–42. doi: 10.1016/j.semnephrol.2018.05.013
2.
Zaidi O Du F Tang Z Bhattacharjee S Pareja K . Review on epidemiology, disease burden, and treatment patterns of IgA nephropathy in select APAC countries. BMC Nephrol. (2024) 25:136. doi: 10.1186/s12882-024-03555-5
3.
Yang N Tang J Li X Li D Zhu B He Q et al . A patient report scale research to access the symptom burden in patients with IgA nephropathy. Sci Rep. (2024) 14:22104. doi: 10.1038/s41598-024-59586-3
4.
Pattrapornpisut P Avila-Casado C Reich HN . IgA nephropathy: core curriculum 2021. Am J Kidney Dis Off J Natl Kidney Found. (2021) 78:429–41. doi: 10.1053/j.ajkd.2021.01.024
5.
Okonogi H Kawamura T Joh K Koike K Miyazaki Y Ogura M et al . A grading system that predicts the risk of dialysis induction in IgA nephropathy patients based on the combination of the clinical and histological severity. Clin Exp Nephrol. (2019) 23:16–25. doi: 10.1007/s10157-018-1657-0
6.
Tomana M Novak J Julian BA Matousovic K Konecny K Mestecky J . Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. (1999) 104:73–81. doi: 10.1172/JCI5535
7.
Vaz de Castro PAS Amaral AA Almeida MG Selvaskandan H Barratt J Simões e Silva AC . Examining the association between serum galactose-deficient IgA1 and primary IgA nephropathy: a systematic review and meta-analysis. J Nephrol. (2024) 37:2099–112. doi: 10.1007/s40620-023-01874-8
8.
Zeng J Aryal RP Stavenhagen K Luo C Liu R Wang X et al . Cosmc deficiency causes spontaneous autoimmunity by breaking B cell tolerance. Sci Adv. (2021) 7:eabg9118. doi: 10.1126/sciadv.abg9118
9.
Zachova K Jemelkova J Kosztyu P Ohyama Y Takahashi K Zadrazil J et al . Galactose-deficient IgA1 B cells in the circulation of IgA nephropathy patients carry preferentially lambda light chains and mucosal homing receptors. J Am Soc Nephrol JASN. (2022) 33:908–17. doi: 10.1681/ASN.2021081086
10.
Makita Y Suzuki H Nakano D Yanagawa H Kano T Novak J et al . Glomerular deposition of galactose-deficient IgA1-containing immune complexes via glomerular endothelial cell injuries. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2022) 37:1629–36. doi: 10.1093/ndt/gfac204
11.
Moldoveanu Z Wyatt RJ Lee JY Tomana M Julian BA Mestecky J et al . Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. (2007) 71:1148–54. doi: 10.1038/sj.ki.5002185
12.
Nihei Y Suzuki H Suzuki Y . Current understanding of IgA antibodies in the pathogenesis of IgA nephropathy. Front Immunol. (2023) 14:1165394. doi: 10.3389/fimmu.2023.1165394
13.
Tan Q Xue H Ni X Fan L Du W . Comparative effectiveness and safety for the treatments despite optimized renin-angiotensin system blockade among IgA nephropathy patients at high-risk of disease progression: a network meta-analysis of randomized controlled trials. Eur J Intern Med. (2023) 114:66–73. doi: 10.1016/j.ejim.2023.04.022
14.
Lv J Zhang H Wong MG Jardine MJ Hladunewich M Jha V et al . Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. (2017) 318:432–42. doi: 10.1001/jama.2017.9362
15.
Floege J Rovin BH Barratt J Cook HT Noronha IL Reich HN et al . KDIGO-2024-IgAN-IgAV-guideline-public-review-draft.pdf. (2024) Available online at: https://kdigo.org/wp-content/uploads/2024/08/KDIGO-2024-IgAN-IgAV-Guideline-Public-Review-Draft.pdf (Accessed October 22, 2024).
16.
Rovin BH Barratt J Heerspink HJL Alpers CE Bieler S Chae DW et al . Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet Lond Engl. (2023) 402:2077–90. doi: 10.1016/S0140-6736(23)02302-4
17.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg Lond Engl. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
18.
Higgins JPT Altman DG Gøtzsche PC Jüni P Moher D Oxman AD et al . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
19.
Greco T Edefonti V Biondi-Zoccai G Decarli A Gasparini M Zangrillo A et al . A multilevel approach to network meta-analysis within a frequentist framework. Contemp Clin Trials. (2015) 42:51–9. doi: 10.1016/j.cct.2015.03.005
20.
Salanti G Ades AE Ioannidis JPA . Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
21.
Higgins JPT Jackson D Barrett JK Lu G Ades AE White IR . Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3:98–110. doi: 10.1002/jrsm.1044
22.
Chaimani A Vasiliadis HS Pandis N Schmid CH Welton NJ Salanti G . Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. Int J Epidemiol. (2013) 42:1120–31. doi: 10.1093/ije/dyt074
23.
Yu-Kang T . Node-splitting generalized linear mixed models for evaluation of inconsistency in network meta-analysis. Value Health J Int Soc Pharmacoeconomics Outcomes Res. (2016) 19:957–63. doi: 10.1016/j.jval.2016.07.005
24.
Barratt J Tumlin J Suzuki Y Kao A Aydemir A Pudota K et al . Randomized phase II JANUS study of Atacicept in patients with IgA nephropathy and persistent proteinuria. Kidney Int Rep. (2022) 7:1831–41. doi: 10.1016/j.ekir.2022.05.017
25.
Lai KN Lai FM Li PK Vallance-Owen J . Cyclosporin treatment of IgA nephropathy: a short term controlled trial. BMJ. (1987) 295:1165–8. doi: 10.1136/bmj.295.6607.1165
26.
Locatelli F Pozzi C Vecchio LD Bolasco PG Fogazzi GB Andrulli S et al . Role of proteinuria reduction in the progression of IgA nephropathy. Ren Fail. (2001) 23:495–505. doi: 10.1081/JDI-100104732
27.
Chen X Chen P Cai G Wu J Cui Y Zhang Y et al . A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za Zhi. (2002) 82:796–801. PMID:
28.
Praga M Gutiérrez E González E Morales E Hernández E . Treatment of IgA nephropathy with ACE inhibitors: a randomized and controlled trial. J Am Soc Nephrol JASN. (2003) 14:1578–83. doi: 10.1097/01.asn.0000068460.37369.dc
29.
Maes BD Oyen R Claes K Evenepoel P Kuypers D Vanwalleghem J et al . Mycophenolate mofetil in IgA nephropathy: results of a 3-year prospective placebo-controlled randomized study. Kidney Int. (2004) 65:1842–9. doi: 10.1111/j.1523-1755.2004.00588.x
30.
Pozzi C Andrulli S Del Vecchio L Melis P Fogazzi GB Altieri P et al . Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol JASN. (2004) 15:157–63. doi: 10.1097/01.asn.0000103869.08096.4f
31.
Frisch G Lin J Rosenstock J Markowitz G D’Agati V Radhakrishnan J et al . Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double-blind randomized controlled trial. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2005) 20:2139–45. doi: 10.1093/ndt/gfh974
32.
Tang S Leung JCK Chan LYY Lui YH Tang CSO Kan CH et al . Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney Int. (2005) 68:802–12. doi: 10.1111/j.1523-1755.2005.00460.x
33.
Li PKT Leung CB Chow KM Cheng YL Fung SK Mak SK et al . Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am J Kidney Dis Off J Natl Kidney Found. (2006) 47:751–60. doi: 10.1053/j.ajkd.2006.01.017
34.
Lou T Wang C Chen Z Shi C Tang H Liu X et al . Randomised controlled trial of leflunomide in the treatment of immunoglobulin a nephropathy. Nephrol Carlton Vic. (2006) 11:113–6. doi: 10.1111/j.1440-1797.2006.00547.x
35.
Horita Y Tadokoro M Taura K Ashida R Hiu M Taguchi T et al . Prednisolone co-administered with losartan confers renoprotection in patients with IgA nephropathy. Ren Fail. (2007) 29:441–6. doi: 10.1080/08860220701260511
36.
Woo KT Lau YK Zhao Y Liu FE Tan HB Tan EK et al . Disease progression, response to ACEI/ATRA therapy and influence of ACE gene in IgA nephritis. Cell Mol Immunol. (2007) 4:227–32. PMID:
37.
Lv J Zhang H Chen Y Li G Jiang L Singh AK et al . Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis Off J Natl Kidney Found. (2009) 53:26–32. doi: 10.1053/j.ajkd.2008.07.029
38.
Liu XW Li DM Xu GS Sun SR . Comparison of the therapeutic effects of leflunomide and mycophenolate mofetil in the treatment of immunoglobulin a nephropathy manifesting with nephrotic syndrome. Int J Clin Pharmacol Ther. (2010) 48:509–13. doi: 10.5414/cpp48509
39.
Pozzi C Andrulli S Pani A Scaini P del Vecchio L Fogazzi G et al . Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol JASN. (2010) 21:1783–90. doi: 10.1681/ASN.2010010117
40.
Tang SCW Tang AWC Wong SSH Leung JCK Ho YW Lai KN . Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. (2010) 77:543–9. doi: 10.1038/ki.2009.499
41.
Stangou M Ekonomidou D Giamalis P Liakou H Tsiantoulas A Pantzaki A et al . Steroids and azathioprine in the treatment of IgA nephropathy. Clin Exp Nephrol. (2011) 15:373–80. doi: 10.1007/s10157-011-0415-3
42.
Xie Y Huang S Wang L Miao L Zhang A Li Y et al . Efficacy and safety of mizoribine combined with losartan in the treatment of IgA nephropathy: a multicenter, randomized, controlled study. Am J Med Sci. (2011) 341:367–72. doi: 10.1097/MAJ.0b013e318207e02d
43.
Kim YC Chin HJ Koo HS Kim S . Tacrolimus decreases albuminuria in patients with IgA nephropathy and normal blood pressure: a double-blind randomized controlled trial of efficacy of tacrolimus on IgA nephropathy. PLoS One. (2013) 8:e71545. doi: 10.1371/journal.pone.0071545
44.
Pozzi C Andrulli S Pani A Scaini P Roccatello D Fogazzi G et al . IgA nephropathy with severe chronic renal failure: a randomized controlled trial of corticosteroids and azathioprine. J Nephrol. (2013) 26:86–93. doi: 10.5301/jn.5000110
45.
Kawamura T Yoshimura M Miyazaki Y Okamoto H Kimura K Hirano K et al . A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin a nephropathy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2014) 29:1546–53. doi: 10.1093/ndt/gfu020
46.
Liu H Xu X Fang Y Ji J Zhang X Yuan M et al . Comparison of glucocorticoids alone and combined with cyclosporine a in patients with IgA nephropathy: a prospective randomized controlled trial. Intern Med Tokyo Jpn. (2014) 53:675–81. doi: 10.2169/internalmedicine.53.1136
47.
Masutani K Tsuchimoto A Yamada T Hirakawa M Mitsuiki K Katafuchi R et al . Comparison of steroid-pulse therapy and combined with mizoribine in IgA nephropathy: a randomized controlled trial. Clin Exp Nephrol. (2016) 20:896–903. doi: 10.1007/s10157-016-1226-3
48.
Kaneko T Arai M Ikeda M Morita M Watanabe Y Hirama A et al . Comparison of immunosuppressive therapies for IgA nephropathy after tonsillectomy: three-course versus one-course steroid pulse combined with mizoribine. Int Urol Nephrol. (2015) 47:1823–30. doi: 10.1007/s11255-015-1118-6
49.
Rauen T Eitner F Fitzner C Sommerer C Zeier M Otte B et al . Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. (2015) 373:2225–36. doi: 10.1056/NEJMoa1415463
50.
Lafayette RA Canetta PA Rovin BH Appel GB Novak J Nath KA et al . A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol JASN. (2017) 28:1306–13. doi: 10.1681/ASN.2016060640
51.
Yang D He L Peng X Liu H Peng Y Yuan S et al . The efficacy of tonsillectomy on clinical remission and relapse in patients with IgA nephropathy: a randomized controlled trial. Ren Fail. (2016) 38:242–8. doi: 10.3109/0886022X.2015.1128251
52.
Hirai K Ookawara S Kitano T Miyazawa H Ito K Ueda Y et al . Efficacy and safety of adding mizoribine to standard treatment in patients with immunoglobulin a nephropathy: a randomized controlled trial. Kidney Res Clin Pract. (2017) 36:159–66. doi: 10.23876/j.krcp.2017.36.2.159
53.
Hou JH Le WB Chen N Wang WM Liu ZS Liu D et al . Mycophenolate Mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am J Kidney Dis Off J Natl Kidney Found. (2017) 69:788–95. doi: 10.1053/j.ajkd.2016.11.027
54.
Rauen T Fitzner C Eitner F Sommerer C Zeier M Otte B et al . Effects of two immunosuppressive treatment protocols for IgA nephropathy. J Am Soc Nephrol JASN. (2018) 29:317–25. doi: 10.1681/ASN.2017060713
55.
Kohagura K Arima H Miyasato H Chang TH Yamazato M Kobori H et al . Add-on effect of angiotensin receptor blockade (candesartan) on clinical remission in active IgA nephropathy patients treated with steroid pulse therapy and tonsillectomy: a randomized, parallel-group comparison trial. Kidney Blood Press Res. (2018) 43:780–92. doi: 10.1159/000489914
56.
Liu LJ Yang YZ Shi SF Bao YF Yang C Zhu SN et al . Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis Off J Natl Kidney Found. (2019) 74:15–22. doi: 10.1053/j.ajkd.2019.01.026
57.
Ni Z Zhang Z Yu Z Lu F Mei C Ding X et al . Leflunomide plus low-dose prednisone in patients with progressive IgA nephropathy: a multicenter, prospective, randomized, open-labeled, and controlled trial. Ren Fail. (2021) 43:1214–21. doi: 10.1080/0886022X.2021.1963775
58.
Han SY Jung CY Lee SH Lee DW Lee S Kim CD et al . A multicenter, randomized, open-label, comparative, phase IV study to evaluate the efficacy and safety of combined treatment with mycophenolate mofetil and corticosteroids in advanced immunoglobulin a nephropathy. Kidney Res Clin Pract. (2022) 41:452–61. doi: 10.23876/j.krcp.21.146
59.
Lv J Wong MG Hladunewich MA Jha V Hooi LS Monaghan H et al . Effect of oral methylprednisolone on decline in kidney function or kidney failure in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. (2022) 327:1888–98. doi: 10.1001/jama.2022.5368
60.
Heerspink HJL Radhakrishnan J Alpers CE Barratt J Bieler S Diva U et al . Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet Lond Engl. (2023) 401:1584–94. doi: 10.1016/S0140-6736(23)00569-X
61.
Hou FF Xie D Wang J Xu X Yang X Ai J et al . Effectiveness of mycophenolate Mofetil among patients with progressive IgA nephropathy: a randomized clinical trial. JAMA Netw Open. (2023) 6:e2254054. doi: 10.1001/jamanetworkopen.2022.54054
62.
Lafayette R Kristensen J Stone A Floege J Tesař V Trimarchi H et al . Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet Lond Engl. (2023) 402:859–70. doi: 10.1016/S0140-6736(23)01554-4
63.
Lv J Liu L Hao C Li G Fu P Xing G et al . Randomized phase 2 trial of Telitacicept in patients with IgA nephropathy with persistent proteinuria. Kidney Int Rep. (2023) 8:499–506. doi: 10.1016/j.ekir.2022.12.014
64.
Pozzi C Bolasco PG Fogazzi GB Andrulli S Altieri P Ponticelli C et al . Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet Lond Engl. (1999) 353:883–7. doi: 10.1016/s0140-6736(98)03563-6
65.
Mathur M Barratt J Chacko B Chan TM Kooienga L Oh KH et al . A phase 2 trial of Sibeprenlimab in patients with IgA nephropathy. N Engl J Med. (2024) 390:20–31. doi: 10.1056/NEJMoa2305635
66.
Zhang H Rizk DV Perkovic V Maes B Kashihara N Rovin B et al . Results of a randomized double-blind placebo-controlled phase 2 study propose iptacopan as an alternative complement pathway inhibitor for IgA nephropathy. Kidney Int. (2024) 105:189–99. doi: 10.1016/j.kint.2023.09.027
67.
Sun L Zi X Wang Z Zhang X . The clinical efficacy of fluticasone propionate combined with ACEI/ARB in the treatment of immunoglobulin a nephropathy. BMC Nephrol. (2023) 24:63. doi: 10.1186/s12882-023-03106-4
68.
Wheeler DC Toto RD Stefánsson BV Jongs N Chertow GM Greene T et al . A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. (2021) 100:215–24. doi: 10.1016/j.kint.2021.03.033
69.
Manno C Torres DD Rossini M Pesce F Schena FP . Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2009) 24:3694–701. doi: 10.1093/ndt/gfp356
70.
Coppo R Peruzzi L Amore A Piccoli A Cochat P Stone R et al . IgACE: a placebo-controlled, randomized trial of angiotensin-converting enzyme inhibitors in children and young people with IgA nephropathy and moderate proteinuria. J Am Soc Nephrol JASN. (2007) 18:1880–8. doi: 10.1681/ASN.2006040347
71.
Yang YZ Liu LJ Shi SF Wang JW Chen YQ Lv JC et al . Effects of hydroxychloroquine on proteinuria in immunoglobulin a nephropathy. Am J Nephrol. (2018) 47:145–52. doi: 10.1159/000487330
72.
Nakamura T Ushiyama C Suzuki S Hara M Shimada N Sekizuka K et al . Effects of angiotensin-converting enzyme inhibitor, angiotensin II receptor antagonist and calcium antagonist on urinary podocytes in patients with IgA nephropathy. Am J Nephrol. (2000) 20:373–9. doi: 10.1159/000013619
73.
Yoshikawa N Honda M Iijima K Awazu M Hattori S Nakanishi K et al . Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clin J Am Soc Nephrol CJASN. (2006) 1:511–7. doi: 10.2215/CJN.01120905
74.
Ballardie FW Roberts ISD . Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol JASN. (2002) 13:142–8. doi: 10.1681/ASN.V131142
75.
Hogg RJ Bay RC Jennette JC Sibley R Kumar S Fervenza FC et al . Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. Am J Kidney Dis Off J Natl Kidney Found. (2015) 66:783–91. doi: 10.1053/j.ajkd.2015.06.013
76.
Horita Y Tadokoro M Taura K Suyama N Taguchi T Miyazaki M et al . Low-dose combination therapy with temocapril and losartan reduces proteinuria in normotensive patients with immunoglobulin a nephropathy. Hypertens Res Off J Jpn Soc Hypertens. (2004) 27:963–70. doi: 10.1291/hypres.27.963
77.
Katafuchi R Ikeda K Mizumasa T Tanaka H Ando T Yanase T et al . Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low-dose prednisolone therapy. Am J Kidney Dis Off J Natl Kidney Found. (2003) 41:972–83. doi: 10.1016/s0272-6386(03)00194-x
78.
Kobayashi Y Hiki Y Kokubo T Horii A Tateno S . Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. (1996) 72:237–42. doi: 10.1159/000188848
79.
Koike M Takei T Uchida K Honda K Moriyama T Horita S et al . Clinical assessment of low-dose steroid therapy for patients with IgA nephropathy: a prospective study in a single center. Clin Exp Nephrol. (2008) 12:250–5. doi: 10.1007/s10157-008-0036-7
80.
Min L Wang Q Cao L Zhou W Yuan J Zhang M et al . Comparison of combined leflunomide and low-dose corticosteroid therapy with full-dose corticosteroid monotherapy for progressive IgA nephropathy. Oncotarget. (2017) 8:48375–84. doi: 10.18632/oncotarget.16468
81.
Zan J Liu L Li G Zheng H Chen N Wang C et al . Effect of Telitacicept on circulating Gd-IgA1 and IgA-containing immune complexes in IgA nephropathy. Kidney Int Rep. (2024) 9:1067–71. doi: 10.1016/j.ekir.2024.01.003
82.
Yu J Luo J Zhu H Sui Z Liu H Li L et al . Quantitative comparison of the clinical efficacy of 6 classes drugs for IgA nephropathy: a model-based meta-analysis of drugs for clinical treatments. Front Immunol. (2022) 13:825677. doi: 10.3389/fimmu.2022.825677
83.
Wheeler DC Stefánsson BV Jongs N Chertow GM Greene T Hou FF et al . Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. (2021) 9:22–31. doi: 10.1016/S2213-8587(20)30369-7
84.
Wu L Du X Lu X . Role of telitacicept in the treatment of IgA nephropathy. Eur J Med Res. (2023) 28:369. doi: 10.1186/s40001-023-01320-2
85.
Kohan DE Bedard PW Jenkinson C Hendry B Komers R . Mechanism of protective actions of sparsentan in the kidney: lessons from studies in models of chronic kidney disease. Clin Sci Lond Engl 1979. (2024) 138:645–62. doi: 10.1042/CS20240249
86.
Filippone EJ Gulati R Farber JL . Contemporary review of IgA nephropathy. Front Immunol. (2024) 15:1436923. doi: 10.3389/fimmu.2024.1436923
87.
Yamada K Huang ZQ Reily C Green TJ Suzuki H Novak J et al . LIF/JAK2/STAT1 signaling enhances production of galactose-deficient IgA1 by IgA1-producing cell lines derived from tonsils of patients with IgA nephropathy. Kidney Int Rep. (2024) 9:423–35. doi: 10.1016/j.ekir.2023.11.003
88.
Kano T Suzuki H Makita Y Nihei Y Fukao Y Nakayama M et al . Mucosal immune system dysregulation in the pathogenesis of IgA nephropathy. Biomedicines. (2022) 10:3027. doi: 10.3390/biomedicines10123027
89.
Wang Y Chen J Wang Y Chen Y Wang L Lv Y . A meta-analysis of the clinical remission rate and long-term efficacy of tonsillectomy in patients with IgA nephropathy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2011) 26:1923–31. doi: 10.1093/ndt/gfq674
90.
Liu L Wang L Jiang Y Yao L Dong L Li Z et al . Tonsillectomy for IgA nephropathy: a meta-analysis. Am J Kidney Dis Off J Natl Kidney Found. (2015) 65:80–7. doi: 10.1053/j.ajkd.2014.06.036
91.
Yang P Wang Q Xie C Xu G Wu Q . Efficacy and safety of agents in Iga nephropathy: an update network meta-analysis. Kidney Blood Press Res. (2018) 43:1890–7. doi: 10.1159/000496000
92.
Tan J Dong L Ye D Tang Y Hu T Zhong Z et al . The efficacy and safety of immunosuppressive therapies in the treatment of IgA nephropathy: a network meta-analysis. Sci Rep. (2020) 10:6062. doi: 10.1038/s41598-020-63170-w
Summary
Keywords
IgA nephropathy, sodium-glucose cotransporter 2 inhibitors, telitacicept, nefecon, sparsentan
Citation
Chen B, Zhu Y, Yang Y and Xu G (2025) Efficacy and safety of agents for IgA nephropathy: a network meta-analysis of randomized controlled trials. Front. Med. 12:1515723. doi: 10.3389/fmed.2025.1515723
Received
30 October 2024
Accepted
03 April 2025
Published
18 June 2025
Volume
12 - 2025
Edited by
Hitoshi Suzuki, Juntendo University Urayasu Hospital, Japan
Reviewed by
Jiri Mestecky, University of Alabama at Birmingham, United States
Zeynep Kendi Çelebi, Başkent University, Türkiye
Todd J. Green, University of Alabama at Birmingham, United States
Updates
Copyright
© 2025 Chen, Zhu, Yang and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaosi Xu, gaosixu@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.