- Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, Beijing, China
Background: The advanced lung cancer inflammation index (ALI) is a composite index that combines inflammation and nutritional status, and non-alcoholic fatty liver disease (NAFLD) is associated with inflammation, nutritional status, and obesity. This study aimed to investigate the possible relationship between ALI and NAFLD.
Methods: We extracted cohort datasets from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) for the study. Weighted analyses and multivariate linear regression models were applied to assess the association between ALI and NAFLD. Fitted curves and threshold effects analyses were used to characterize nonlinear relationships.
Results: A total of 6,595 adults aged 18–80 years were included in this study. In multivariate linear regression analysis, there was a significant positive association between ALI and NAFLD [OR: 1.02, 95% CI (1.01, 1.02)]. In subgroup analyses, this positive association was maintained in females [OR: 1.02, 95% CI (1.01, 1.02)] and not in males. In addition, we found that the association between ALI and NAFLD was nonlinear, with an L-shaped relationship and an inflection point of 32.47. ALI showed a U-shaped association with NAFLD in the male population, with an inflection point of 40.65, and an L-shaped association in the female population, with an inflection point of 30.61.
Conclusion: Our study suggests that there is a significant positive association between high ALI levels and NAFLD prevalence in the US adult population. However, more clinical cohort studies are needed to confirm this finding.
1 Introduction
About 30% of the global adult population suffers from non-alcoholic fatty liver disease (NAFLD), one of the most prevalent liver diseases in the world, with the greatest rate of morbidity and mortality among liver-related conditions (1). Except for severe alcohol use or other chronic liver illnesses, NAFLD is defined as steatosis in more than 5% of hepatocytes. The histologic changes in NAFLD include simple steatosis, nonalcoholic fatty liver (NAFL), and non-alcoholic steatohepatitis (NASH), which has a higher degree of inflammation and faster progression of fibrosis (2). In 2020, the European Liver Patient’s Association (ELPA) proposed replacing NAFLD with metabolic dysfunction-associated fatty liver disease (MAFLD), which is defined as hepatic fat deposition associated with obesity, diabetes mellitus, or mixed metabolic disorders (3). However, the change in terminology is hotly debated (4). While MAFLD may better align with metabolic risk factors, its more heterogeneous definition could potentially exclude patients with poorer prognoses (5, 6). Furthermore, since NAFLD better represents the underlying pathophysiological mechanisms of fatty liver disease (7), this study retains the use of the term “NAFLD.”
NAFLD progresses to progressive liver fibrosis and then to irreversible cirrhosis and even to hepatocellular carcinoma, and is becoming an important cause of end-stage liver disease (8). Liver biopsy is the gold standard for the diagnosis and grading of chronic hepatitis and liver fibrosis, however, liver biopsy is invasive, expensive, and has a high-risk factor (9). Therefore, the application of non-invasive indexes for early diagnosis and full management is the key to the prevention and treatment of NAFLD and related end-stage liver diseases. Previous studies have shown that obesity and being overweight are established risk factors for NAFLD (10). Inflammation is a key factor in the progression of liver fibrosis (11), which is driven by the production of inflammatory cytokines including IL-1, IL-6, TNF, and the infiltration of immune cells, such as macrophages, T-lymphocytes, and B-lymphocytes (2, 12, 13). A growing body of research has suggested that many nutritional/inflammatory variables can be valid predictors of NAFLD. For example, indicators of nutritional status, such as body mass index (BMI) and prognostic nutritional index (PNI), have been found to have a positive correlation with the risk of NAFLD (14, 15). The severity of NAFLD also positively correlates with inflammatory indicators, such as the systemic immune inflammation index (SII) and neutrophil-to-lymphocyte ratio (NLR) (16, 17). Therefore, assessing the combined effects of nutrition and inflammation may help to develop clinical intervention strategies to reduce the risk of NAFLD.
The ALI index consists of albumin (ALB), BMI, and NLR (ALI = BMI*ALB/NLR) (18). The ALI combines the nutrition-related index ALB, BMI, and the inflammation-related index NLR to provide a more comprehensive approach to identifying individuals with visceral obesity, metabolic abnormalities, and increased levels of body inflammation (19). The ALI is a comprehensive index that has emerged in recent years as a very promising marker for a variety of diseases including cancer and cardiovascular diseases (20–22). ALI serves as a valuable non-invasive biomarker for early NAFLD detection, offering significant potential in the comprehensive management of NAFLD and its associated end-stage liver complications through nutritional and inflammatory modulation. However, the relationship between ALI and NAFLD remains incompletely understood, so we analyzed extensive data from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) for people aged 18 to 80 years to examine the relationship between ALI and NAFLD in adults.
2 Materials and methods
2.1 Survey description
To evaluate nutrition and health in the country, the National Center for Health Statistics (NCHS) administers the cross-sectional, population-based NHANES countrywide survey. Every NHANES research methodology was approved by the NCHS Research Ethics Review Board and Informed written consent was obtained from all participants before participation. The research design and data for the NHANES are fully detailed at www.cdc.gov/nchs/nhanes/.
2.2 Study population
This study used the US NHANES dataset for consecutive cycles from 2017 to 2020. 3,924 patients with missing ALI data were eliminated. 2,688 subjects with missing CAP data, 42 hepatitis B antigen-positive and 86 hepatitis C antibody-positive or hepatitis C RNA-positive samples, 988 subjects who drank large amounts of alcohol (4, 5, or more drinks per day), and 1,237 subjects under the age of 18 were excluded. In total, 6,595 participants were included in the study, out of 15,560 excluded as ineligible individuals (Figure 1).
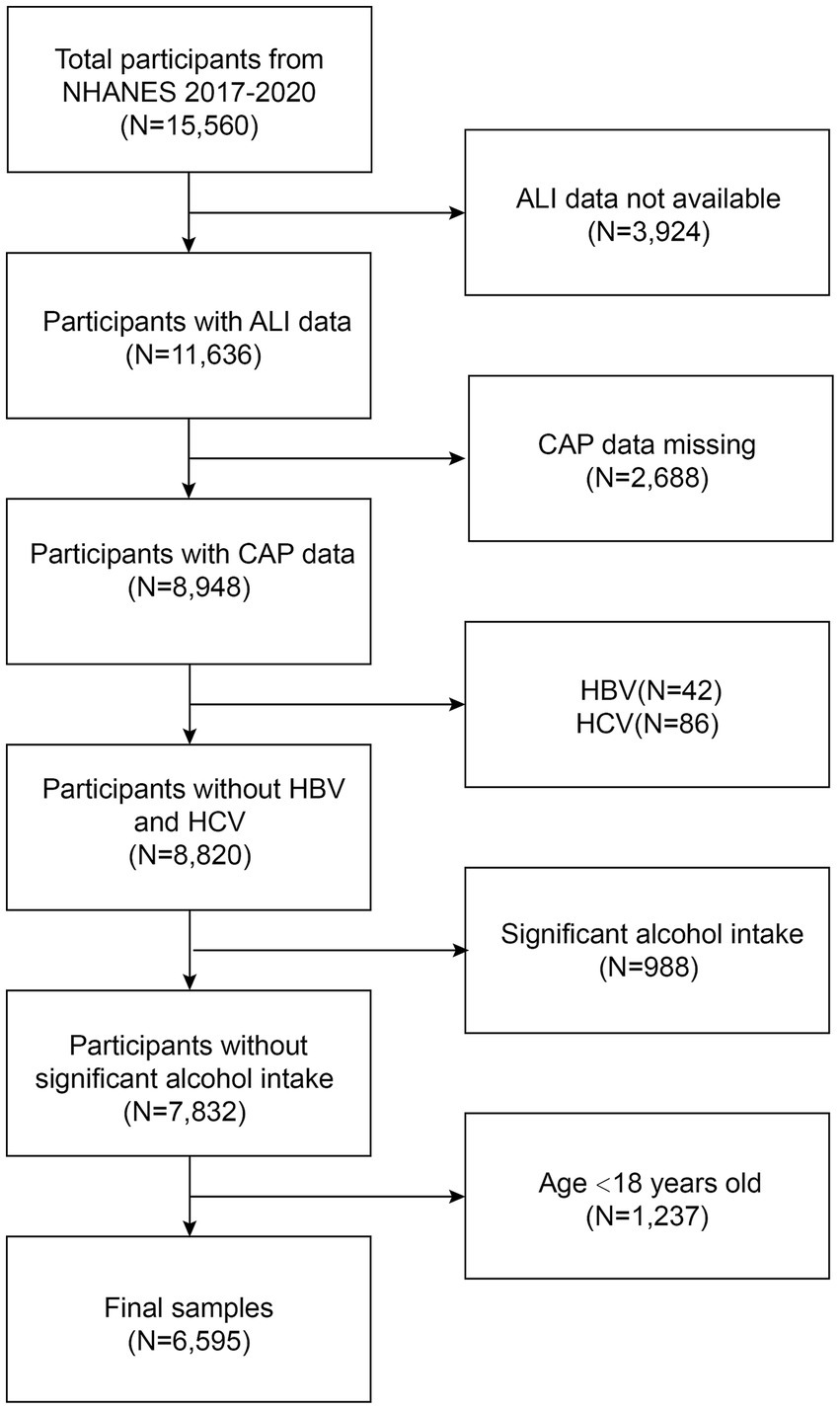
Figure 1. Flowchart of participant selection in NHANES 2017–2020. NHANES, National Health and Nutrition Examination Survey; ALI, advanced lung cancer inflammation index; CAP, controlled attenuation parameter.
2.3 Definition of ALI and NAFLD
The dependent variable in this study is the advanced lung cancer inflammation index, used to assess the patient’s nutritional and inflammation levels. Using the following formula, the ALI was calculated: BMI*ALB/ NLR. BMI was calculated in Kg/m2, and ALB was calculated in g/dL in the formula.
In this study, the Controlled Attenuation Parameter (CAP) was measured using Vibration controlled transient elastography (VCTE) to diagnose NAFLD in adults. CAP threshold ≥248 dB/m indicated the presence of hepatic steatosis in the participants, while a CAP value of less than 248 dB/m indicated the absence of a diagnosis of NAFLD (23).
2.4 Selection of covariates
In the current investigation, covariates included age, gender, race, education level, moderate actives status, smoking status, income-to-poverty ratio, BMI (Kg/m2), waist circumference (cm), total cholesterol (mg/dL), triglyceride (mg/dL), LDL-cholesterol (mg/dL), HDL-cholesterol (mg/dL), albumin (ALB, g/dL), alanine transaminase (ALT, U/L), aspartate aminotransferase (AST, U/L), alkaline phosphatase (ALP, IU/L), serum phosphorus (mg/dL), total calcium (mg/dL), and NLR.
2.5 Statistical analysis
Graphing software R (version: 4.1.3) and EmpowerStats (version: 4.2) were used to conduct the statistical study. The baseline table of the study population was statistically described by the presence or absence of NAFLD, and continuous variables were described using means plus or minus standard deviations (SDs) and weighted linear regression models. To examine the relationship between NAFLD and ALI, ALI levels were categorized into continuous and categorical variables (into quartiles, with the first quartile as the reference), and three models were constructed using multivariate tests: model 1: unadjusted for covariates, model 2: adjusted for gender, age, and race, and model 3: adjusted for all covariates. Multivariate linear regression analyses were performed to calculate odds ratios (ORs) and 95% confidence intervals (95% CI) for the three models. Interaction terms were also added to test for heterogeneity of associations between gender, age, BMI, and smoking status subgroups. Smoothed curve fitting was performed by adjusting for variables. The relationship and thresholds between ALI and NAFLD were tested using a threshold effects analysis model. Finally, the same statistical study methods described above were performed for the gender subgroups. p < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of participants
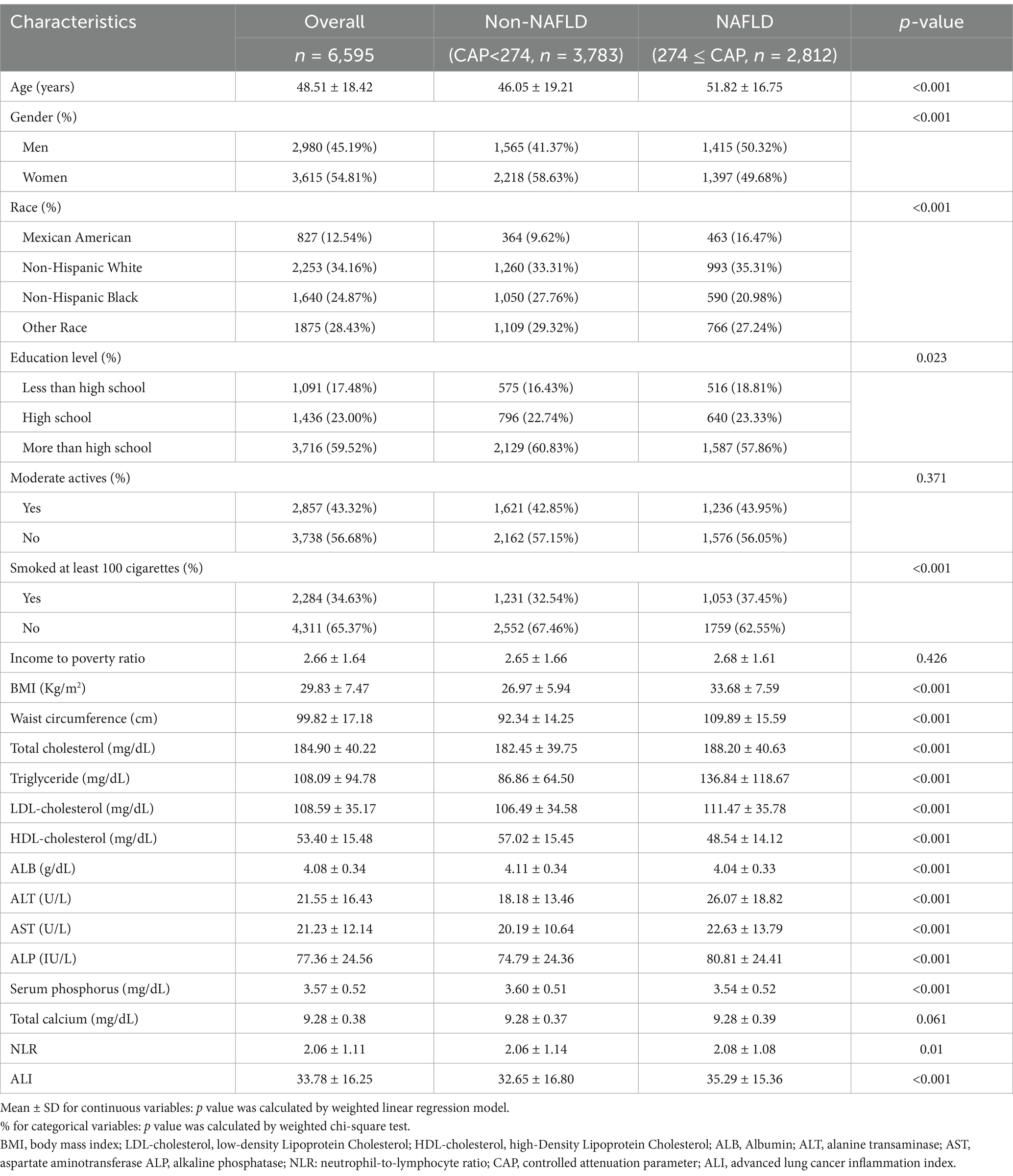
A total of 6,595 adults were included in this study. The mean age of the participants was 48.51 ± 18.42, of which 45.19% were male and 54.81% were female. 12.54% were Mexican American, 34.16% were non-Hispanic White, 24.87% were non-Hispanic Black, and 28.43% were other races. The mean of the ALI was 33.78 ± 16.25. Compared to non-NAFLD participants, the NAFLD group was more likely to be male, older, with a larger proportion of non-Hispanic White, higher smoking status, and higher BMI, larger waist circumference, higher levels of total cholesterol, triglyceride, LDL-C, ALT, AST, ALP, ALI, triglyceride, higher levels of LDL-C, ALT, AST, ALP, ALI, and lower levels of education and HDL-C, ALB, serum phosphorus (Table 1).
3.2 The association between ALI and NAFLD
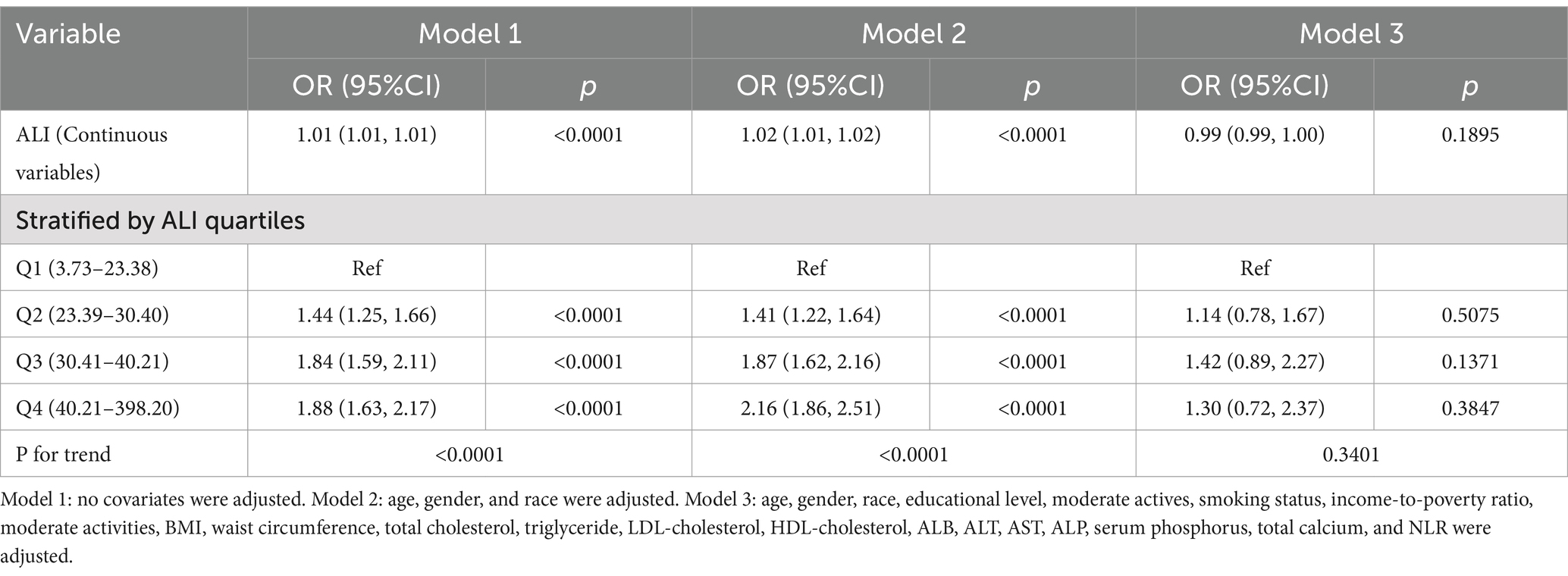
Table 2 provided further insight into the association between ALI and NAFLD through weighted multifactor logistic regression analysis. Continuous variable analysis of ALI showed that ALI and NAFLD were highly correlated in unadjusted models 1 and 2 (adjusted partially). In contrast, in model 3, the relationship between ALI and NAFLD became negative (adjusted for all covariates), although this relationship was not significant. Further analysis of quartiles of ALI showed a significant positive association between higher ALI and NAFLD in un-adjusted model 1 and partially adjusted model 2. In model 2, participants in the highest ALI quartile had a significantly higher risk of NAFLD by 1.16-fold compared with participants in the lowest ALI quartile [OR = 2.16, 95% CI (1.86–2.51), p < 0.0001].
3.3 Subgroup analysis
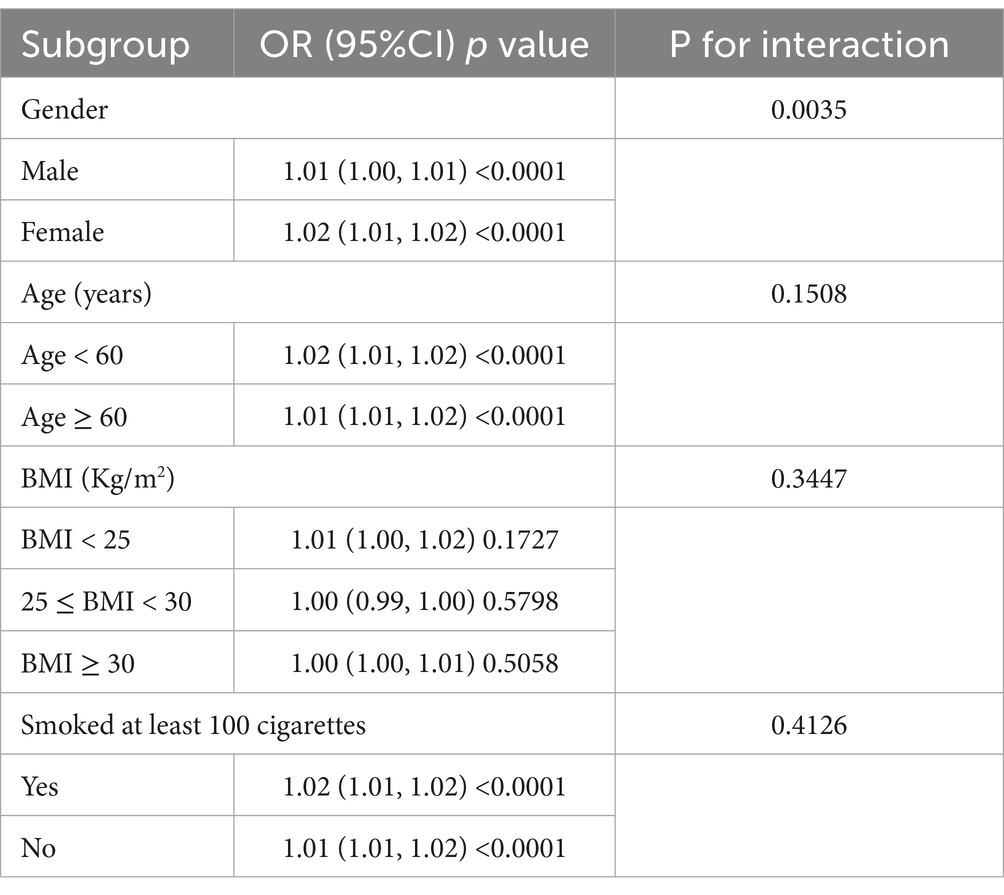
Participants were analysed in model 2 (adjusted partially) stratified by gender, age, BMI, and smoking status to further explore the relationship between ALI and NAFLD. The results showed that gender stratification significantly altered the relationship between ALI and NAFLD (p = 0.0035) (Table 3).
3.4 Nonlinear relationship and threshold effect analysis between ALI and NAFLD
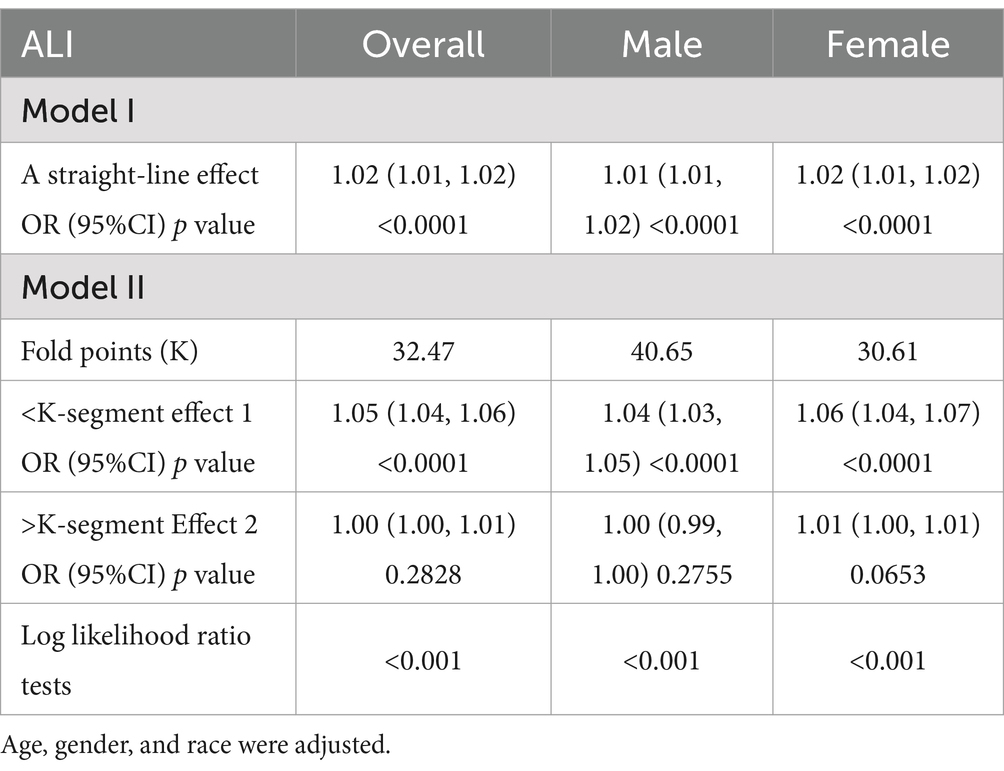
Smoothed curve fitting was used to investigate the nonlinear relationship between ALI and NAFLD in order to identify any threshold or saturation effects. The results showed a nonlinear relationship between ALI and NAFLD [OR = 1.02, 95% CI (1.01–1.02), p < 0.0001] (Table 4). Threshold effect analysis showed that at 32.47, the positive correlation between ALI and NAFLD changed, showing an L-shaped curve. After stratifying the analysis by gender, females still had an L-shaped curve, with higher levels of ALI positively associated with the risk of NAFLD prevalence. A U-shaped curve existed in males, with a negative association between ALI and NAFLD when ALI was >40.65 (Figure 2; Table 4).
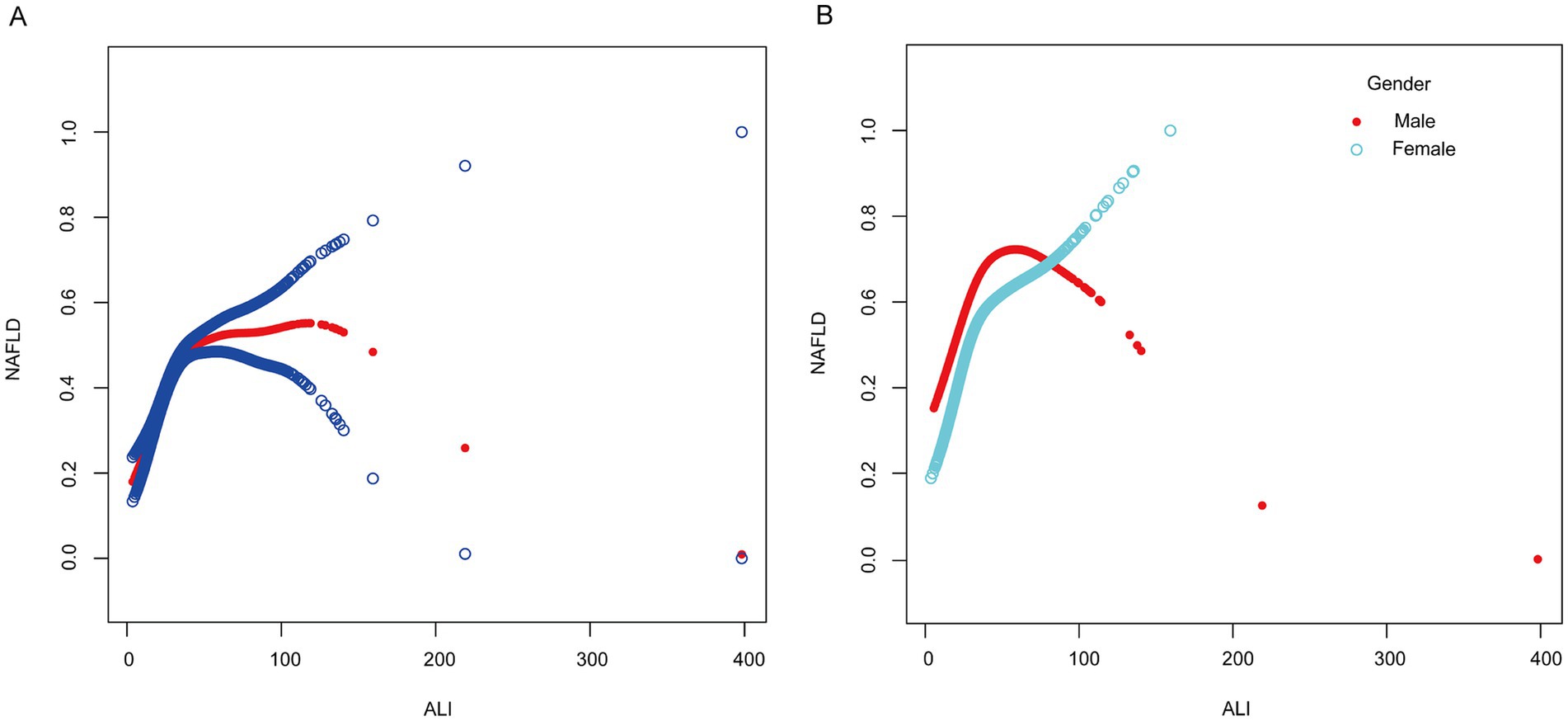
Figure 2. Smooth curve fitting between ALI and NAFLD. (A) The solid red line represents the smooth curve fit between the overall population variables. Blue bands represent the 95% confidence interval from the fit. (B) The red and blue bands, respectively, represent smooth curve fits between ALI and NAFLD in males and females.
4 Discussion
In this cross-sectional study involving 6,595 adults, a positive association between high ALI and the risk of NAFLD was observed. This association remained significant even after adjusting for gender, age, and race. Findings showed that each one-unit increase in ALI was associated with a 2% increase in the risk of NAFLD prevalence [OR: 1.02, 95% CI (1.01, 1.02)], and participants in the highest quartile of ALI had a 1.16-fold increase in the risk of NAFLD compared with the lowest quartile (p < 0.001).
The advanced lung cancer inflammation index was calculated as BMI*ALB/NLR. The concept of ALI was first introduced in 2013 to predict survival and inflammation levels in patients with metastatic non-small cell lung cancer (24). Subsequent studies have shown that ALI can significantly predict the prognosis of malignant diseases such as colorectal, gastric, and liver cancers (25–28). A previous study collected clinical data on 1,654 postoperative patients and found that higher ALI levels were associated with improved 1-, 3-, and 5-year survival rates, highlighting its utility in predicting post-hepatectomy outcomes (21). Beyond its applications in oncology, ALI has also shown significant predictive value for cardiovascular diseases and chronic inflammatory conditions such as rheumatoid arthritis (29, 30).
The pathogenesis of NAFLD remains incompletely understood. One prominent theory posits that overnutrition drives fat accumulation in adipose tissue and ectopic sites, triggering macrophage infiltration, chronic inflammation, and insulin resistance, which disrupts the balance between lipid catabolism and synthesis (31). This metabolic dysfunction may lead to lipotoxicity, oxidative stress, and cell death (32, 33), as well as the subsequent fibrotic process (34). Another idea contends that liver-organ connections and dependencies may cause liver metabolism to become dysregulated, which would increase inflammation and aid in the progression of NAFLD (35–37). Some data suggest that gut microorganisms are significantly altered in patients with NAFLD compared to normal subjects, with a reversal of the ratio of Thickettsia to Mycobacterium and an increase in Aspergillus phylum (38). Meanwhile, bile acids play an important role in regulating the gut microbiome, which may have an impact on the degree of hepatic inflammation and fibrotic progression in NAFLD (39), however, more studies are needed to clarify this conclusion.
Our findings suggest that ALI is positively associated with NAFLD prevalence, potentially due to its components: NLR, ALB, and BMI. Elevated NLR reflects systemic inflammation, which is common in NAFLD and its progression to NASH (40, 41). A cross-sectional study of 1,620 U.S. adults also linked higher NLR to increased liver fibrosis scores in NAFLD patients, consistent with our results (42). Additionally, ALB is a key marker of nutritional status and a major prognostic factor in liver disease (43). In patients with advanced cirrhosis, ALB levels decrease by 60–80%, accompanied by multiple post-translational modifications that alter its structure and functional properties (44). Our study found significantly lower ALB levels in NAFLD participants compared to the normal group (p < 0.001). From the above findings, we can infer that the ratio of ALB to NLR was reduced in NAFLD participants. Finally, numerous studies have demonstrated that higher BMI, particularly visceral obesity, contributes to hepatic fat accumulation (45). In obesity, the expansion of white adipose tissue, particularly visceral fat, results in the release of excess non-esterified fatty acids into the portal circulation. According to the portal vein theory, this process drives hepatic lipid accumulation and accelerates the development of steatosis (46). This is consistent with our findings that NAFLD participants had higher BMI values compared to non-NAFLD participants. In light of the aforementioned, we think that BMI is more important in the favorable correlation between ALI and NAFLD.
Our study reveals for the first time that there are gender differences in the association between ALI and NAFLD. In the adult male population, the association between ALI and NAFLD exhibited an inverted U-shaped relationship, with a positive correlation below an ALI threshold of 40.65 and a negative correlation above it. In contrast, females displayed an L-shaped relationship, where ALI was positively associated with NAFLD prevalence below a threshold of 30.61. Above this threshold, the association remained positive but was not statistically significant (p = 0.0653). The results of this study may be related to the secretion of sex hormones. Several studies have shown that the prevalence of NAFLD is reduced in premenopausal females compared to males, while postmenopausal females exhibit similar rates to age-matched males (47, 48). This is similar to our findings that the male population was more predominant in NAFLD participants compared to the non-NAFLD population (p < 0.001), suggesting a protective effect of estrogen. In a previous study, it was shown that estrogen enhances the insulin sensitivity of adipose tissue and reduces adipose tissue catabolism (49). Another study highlighted that estrogen-related receptor alpha regulates the production and secretion of triglyceride-rich very low-density lipoproteins in the liver, preventing excessive accumulation of lipids in the liver and reducing hepatic inflammatory injury (50). In addition, fat distribution may also be another factor for gender differences. In men, adipose is more distributed in the abdomen and viscera, while in women, adipose is more distributed in the hips and lower limbs (51). Compared to abdominal adipose tissue, gluteal and lower limb adipose have a lower lipolytic response to epinephrine and norepinephrine, reducing free fatty acid transport to the liver (52). Although the definitive mechanism for this gender difference is not fully understood, current evidence indicates that persistently elevated ALI levels in women are linked to a higher prevalence of NAFLD and warrant close monitoring. However, in the male population, identifying an ALI inflection point is crucial, as NAFLD prevalence may decline beyond a specific ALI threshold. The clinical treatment and non-invasive prediction of NAFLD may benefit from these insights.
This study has several strengths. First, our research is based on a nationally representative sample of the multi-ethnic diverse adult population in the United States. Second, to our knowledge, while one study initially demonstrated an association between ALI and NAFLD (53), further research in this area remains limited. Having confirmed the positive association, we expanded the scope of investigation through subgroup analyses and threshold effect analyses to fill the relevant research gaps. Additionally, as a composite index combining inflammation and nutritional markers, ALI shows potential as a non-invasive biomarker for NAFLD, aligning with the need for accessible diagnostic tools in hepatology. However, this study still has some limitations. First, this was a cross-sectional study and therefore we were unable to determine a causal relationship between ALI and NAFLD. Second, hepatic steatosis in this study was assessed using VCTE. Although current studies have demonstrated its high accuracy and sensitivity, it remains inferior to the gold standard of liver biopsy (54, 55). Third, while ALI combines BMI, ALB, and NLR, it may not fully capture the multifactorial nature of NAFLD pathogenesis, which involves complex metabolic, inflammatory, and genetic interactions (56). Fourth, due to the incomplete data records on women’s menopause within the NHANES database for 2017–2020, this study was unable to evaluate the potential impact of pre- and post-menopausal age differences on the observed nonlinear associations in the female population. Finally, the U-shaped and L-shaped nonlinear associations identified in this study may increase the complexity of clinical interpretation. Therefore, further prospective studies with larger samples are warranted to validate these findings.
5 Conclusion
This cross-sectional study suggests that there is a significant positive association between high ALI levels and the prevalence of NAFLD in the US adult population. However, more clinical cohort studies are needed to confirm this finding.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by the consent form was signed by participants in the survey, and participants consented to have specimens of their blood stored for future research. The CDC/NCHS Ethics Review Board (ERB) approved. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LC: Conceptualization, Formal analysis, Methodology, Writing – original draft. SL: Funding acquisition, Methodology, Writing – original draft. HL: Data curation, Project administration, Writing – original draft. JY: Project administration, Resources, Writing – original draft. MY: Conceptualization, Funding acquisition, Formal analysis, Data curation, Writing – review & editing. GY: Conceptualization, Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Capital’s Funds for Health Improvement and Research of China (CFH -2-2234) and National Natural Science Foundation of China (82374538).
Acknowledgments
We acknowledge the NHANES database for providing their platforms and contributors for uploading their meaningful datasets. We thank all the participants included in the present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Younossi, Z, Anstee, QM, Marietti, M, Hardy, T, Henry, L, Eslam, M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
2. Xu, X, Poulsen, KL, Wu, L, Liu, S, Miyata, T, Song, Q, et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct Target Ther. (2022) 7:287. doi: 10.1038/s41392-022-01119-3
3. Eslam, M, Sanyal, AJ, and George, J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
4. Younossi, ZM, Rinella, ME, Sanyal, AJ, Harrison, SA, Brunt, EM, Goodman, Z, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. (2021) 73:1194–8. doi: 10.1002/hep.31420
5. Ye, Q, Zou, B, Yeo, YH, Li, J, Huang, DQ, Wu, Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2020) 5:739–52. doi: 10.1016/s2468-1253(20)30077-7
6. Huang, J, Kumar, R, Wang, M, Zhu, Y, and Lin, S. MAFLD criteria overlooks a number of patients with severe steatosis: is it clinically relevant? J Hepatol. (2020) 73:1265–7. doi: 10.1016/j.jhep.2020.06.016
7. Grander, C, Grabherr, F, and Tilg, H. Non-alcoholic fatty liver disease: pathophysiological concepts and treatment options. Cardiovasc Res. (2023) 119:1787–98. doi: 10.1093/cvr/cvad095
8. Younossi, ZM, Ong, JP, Takahashi, H, Yilmaz, Y, Eguc Hi, Y, El Kassas, M, et al. A global survey of physicians knowledge about nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2022) 20:e1456–68. doi: 10.1016/j.cgh.2021.06.048
9. Younes, R, Caviglia, GP, Govaere, O, Rosso, C, Armandi, A, Sanavia, T, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol. (2021) 75:786–94. doi: 10.1016/j.jhep.2021.05.008
10. Younossi, ZM, Golabi, P, Paik, JM, Henry, A, Van Dongen, C, and Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology (Baltimore, Md). (2023) 77:1335–47. doi: 10.1097/hep.0000000000000004
11. Manzano-Nunez, R, Rivera-Esteban, J, Navarro, J, Bañares, J, Sena, E, Schattenberg, JM, et al. Uncovering the NAFLD burden in people living with HIV from high- and middle-income nations: a meta-analysis with a data gap from Subsaharan Africa. J Int AIDS Soc. (2023) 26:e26072. doi: 10.1002/jia2.26072
12. Song, C, Long, X, He, J, and Huang, Y. Recent evaluation about inflammatory mechanisms in nonalcoholic fatty liver disease. Front Pharmacol. (2023) 14:1081334. doi: 10.3389/fphar.2023.1081334
13. Schuster, S, Cabrera, D, Arrese, M, and Feldstein, AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. (2018) 15:349–64. doi: 10.1038/s41575-018-0009-6
14. Chen, G, Fan, L, Yang, T, Xu, T, Wang, Z, Wang, Y, et al. Prognostic nutritional index (PNI) and risk of non-alcoholic fatty liver disease and advanced liver fibrosis in US adults: evidence from NHANES 2017-2020. Heliyon. (2024) 10:e25660. doi: 10.1016/j.heliyon.2024.e25660
15. Peng, H, Pan, L, Ran, S, Wang, M, Huang, S, Zhao, M, et al. Prediction of MAFLD and NAFLD using different screening indexes: a cross-sectional study in U.S. adults. Front Endocrinol. (2023) 14:1083032. doi: 10.3389/fendo.2023.1083032
16. Zhao, E, Cheng, Y, Yu, C, Li, H, and Fan, X. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann Med. (2023) 55:2197652. doi: 10.1080/07853890.2023.2197652
17. Liu, K, Tang, S, Liu, C, Ma, J, Cao, X, Yang, X, et al. Systemic immune-inflammatory biomarkers (SII, NLR, PLR and LMR) linked to non-alcoholic fatty liver disease risk. Front Immunol. (2024) 15:1337241. doi: 10.3389/fimmu.2024.1337241
18. Chen, X, Hong, C, Guo, Z, Huang, H, and Ye, L. Association between advanced lung cancer inflammation index and all-cause and cardiovascular mortality among stroke patients: NHANES, 1999-2018. Front Public Health. (2024) 12:1370322. doi: 10.3389/fpubh.2024.1370322
19. Song, M, Zhang, Q, Song, C, Liu, T, Zhang, X, Ruan, G, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. (2022) 13:2504–14. doi: 10.1002/jcsm.13032
20. Zhang, Y, Pan, Y, Tu, J, Liao, L, Lin, S, Chen, K, et al. The advanced lung cancer inflammation index predicts long-term outcomes in patients with hypertension: national health and nutrition examination study, 1999-2014. Front Nutr. (2022) 9:989914. doi: 10.3389/fnut.2022.989914
21. Qiu, X, Shen, S, Lu, D, Jiang, N, Feng, Y, Li, J, et al. Predictive efficacy of the advanced lung Cancer inflammation index in hepatocellular carcinoma after hepatectomy. J Inflamm Res. (2024) 17:5197–210. doi: 10.2147/jir.S468215
22. Shi, T, Wang, Y, Peng, Y, Wang, M, Zhou, Y, Gu, W, et al. Advanced lung cancer inflammation index combined with geriatric nutritional risk index predict all-cause mortality in heart failure patients. BMC Cardiovasc Disord. (2023) 23:565. doi: 10.1186/s12872-023-03608-x
23. Xie, R, Xiao, M, Li, L, Ma, N, Liu, M, Huang, X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
24. Jafri, SH, Shi, R, and Mills, G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. (2013) 13:158. doi: 10.1186/1471-2407-13-158
25. Mountzios, G, Samantas, E, Senghas, K, Zervas, E, Krisam, J, Samitas, K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO open. (2021) 6:100254. doi: 10.1016/j.esmoop.2021.100254
26. Horino, T, Tokunaga, R, Miyamoto, Y, Hiyoshi, Y, Akiyama, T, Daitoku, N, et al. The advanced lung cancer inflammation index is a novel independent prognosticator in colorectal cancer patients after curative resection. Ann Gastroenterol Surg. (2022) 6:83–91. doi: 10.1002/ags3.12499
27. Zhang, X, Wang, D, Sun, T, Li, W, and Dang, C. Advanced lung cancer inflammation index (ALI) predicts prognosis of patients with gastric cancer after surgical resection. BMC Cancer. (2022) 22:684. doi: 10.1186/s12885-022-09774-z
28. Li, Q, Ma, F, Tsilimigras, DI, Åberg, F, and Wang, JF. The value of the advanced lung Cancer inflammation index (ALI) in assessing the prognosis of patients with hepatocellular carcinoma treated with camrelizumab: a retrospective cohort study. Ann Transl Med. (2022) 10:1233. doi: 10.21037/atm-22-5099
29. Tian, X, Qu, Z, Sun, Y, and Zhang, B. Association between the advanced lung cancer inflammation index and all-cause and cardiovascular mortality in patients with RA: insights from NHANES data analysis. Heliyon. (2024) 10:e33673. doi: 10.1016/j.heliyon.2024.e33673
30. Chen, Y, Guan, M, Wang, R, and Wang, X. Relationship between advanced lung cancer inflammation index and long-term all-cause, cardiovascular, and cancer mortality among type 2 diabetes mellitus patients: NHANES, 1999-2018. Front Endocrinol. (2023) 14:1298345. doi: 10.3389/fendo.2023.1298345
31. Powell, EE, Wong, VW, and Rinella, M. Non-alcoholic fatty liver disease. Lancet (London, England). (2021) 397:2212–24. doi: 10.1016/s0140-6736(20)32511-3
32. Friedman, SL, Neuschwander-Tetri, BA, Rinella, M, and Sanyal, AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. (2018) 24:908–22. doi: 10.1038/s41591-018-0104-9
33. Sanyal, AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. (2019) 16:377–86. doi: 10.1038/s41575-019-0144-8
34. Lefere, S, and Tacke, F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP. (2019) 1:30–43. doi: 10.1016/j.jhepr.2019.02.004
35. Ghorpade, DS, Ozcan, L, Zheng, Z, Nicoloro, SM, Shen, Y, Chen, E, et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. (2018) 555:673–7. doi: 10.1038/nature26138
36. Azzu, V, Vacca, M, Virtue, S, Allison, M, and Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology. (2020) 158:1899–912. doi: 10.1053/j.gastro.2019.12.054
37. Aron-Wisnewsky, J, Warmbrunn, MV, Nieuwdorp, M, and Clément, K. Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology. (2020) 158:1881–98. doi: 10.1053/j.gastro.2020.01.049
38. Caussy, C, Hsu, C, Lo, MT, Liu, A, Bettencourt, R, Ajmera, VH, et al. Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology. (2018) 68:918–32. doi: 10.1002/hep.29892
39. Smirnova, E, Muthiah, MD, Narayan, N, Siddiqui, MS, Puri, P, Luketic, VA, et al. Metabolic reprogramming of the intestinal microbiome with functional bile acid changes underlie the development of NAFLD. Hepatology. (2022) 76:1811–24. doi: 10.1002/hep.32568
40. Radulescu, D, Baleanu, VD, Padureanu, V, Radulescu, PM, Bordu, S, Patrascu, S, et al. Neutrophil/lymphocyte ratio as predictor of anastomotic leak after gastric Cancer surgery. Diagnostics. (2020) 10:799. doi: 10.3390/diagnostics10100799
41. Farrell, GC, Haczeyni, F, and Chitturi, S. Pathogenesis of NASH: how metabolic complications of Overnutrition favour lipotoxicity and pro-inflammatory fatty liver disease. Adv Exp Med Biol. (2018) 1061:19–44. doi: 10.1007/978-981-10-8684-7_3
42. Wang, Y, Guo, S, He, Y, Zhang, Q, Zhou, N, Wang, D, et al. Relationship between neutrophil-to-lymphocyte ratio and liver fibrosis in nonalcoholic fatty liver disease among adults in the United States: data from the National Health and nutrition examination survey 2017-2018. Turk J Gastroenterol. (2024) 35:335–42. doi: 10.5152/tjg.2024.23231
43. Baldassarre, M, Naldi, M, Zaccherini, G, Bartoletti, M, Antognoli, A, Laggetta, M, et al. Determination of effective albumin in patients with decompensated cirrhosis: clinical and prognostic implications. Hepatology. (2021) 74:2058–73. doi: 10.1002/hep.31798
44. Bernardi, M, Angeli, P, Claria, J, Moreau, R, Gines, P, Jalan, R, et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. (2020) 69:1127–38. doi: 10.1136/gutjnl-2019-318843
45. Gao, Y, Zhang, W, Zeng, LQ, Bai, H, Li, J, Zhou, J, et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. (2020) 36:101635. doi: 10.1016/j.redox.2020.101635
46. Ho, CM, Ho, SL, Jeng, YM, Lai, YS, Chen, YH, Lu, SC, et al. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J Inflam. (2019) 16:7. doi: 10.1186/s12950-019-0211-5
47. Morán-Costoya, A, Proenza, AM, Gianotti, M, Lladó, I, and Valle, A. Sex differences in nonalcoholic fatty liver disease: estrogen influence on the liver-adipose tissue crosstalk. Antioxid Redox Signal. (2021) 35:753–74. doi: 10.1089/ars.2021.0044
48. Wei, Y, Liu, J, Wang, G, and Wang, Y. Sex differences in the association between adipose insulin resistance and non-alcoholic fatty liver disease in Chinese adults. Biol Sex Differ. (2023) 14:69. doi: 10.1186/s13293-023-00549-0
49. Cherubini, A, Ostadreza, M, Jamialahmadi, O, Pelusi, S, Rrapaj, E, Casirati, E, et al. Interaction between estrogen receptor-α and PNPLA3 p.I148M variant drives fatty liver disease susceptibility in women. Nat Med. (2023) 29:2643–55. doi: 10.1038/s41591-023-02553-8
50. Yang, M, Liu, Q, Huang, T, Tan, W, Qu, L, Chen, T, et al. Dysfunction of estrogen-related receptor alpha-dependent hepatic VLDL secretion contributes to sex disparity in NAFLD/NASH development. Theranostics. (2020) 10:10874–91. doi: 10.7150/thno.47037
51. Goossens, GH, Jocken, JWE, and Blaak, EE. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat Rev Endocrinol. (2021) 17:47–66. doi: 10.1038/s41574-020-00431-8
52. Kim, D, Cholankeril, G, and Ahmed, A. Association between body fat distribution and nonalcoholic fatty liver disease/fibrosis based on race/ethnicity. J Obesity Meta Syndrome. (2024) 33:326–36. doi: 10.7570/jomes24005
53. Pan, L, Wang, L, Ma, H, and Ding, F. Relevance of combined influence of nutritional and inflammatory status on non-alcoholic fatty liver disease and advanced fibrosis: a mediation analysis of lipid biomarkers. J Gastroenterol Hepatol. (2024) 39:2853–62. doi: 10.1111/jgh.16760
54. Lin, H, Lee, HW, Yip, TC, Tsochatzis, E, Petta, S, Bugianesi, E, et al. Vibration-controlled transient Elastography scores to predict liver-related events in Steatotic liver disease. JAMA. (2024) 331:1287–97. doi: 10.1001/jama.2024.1447
55. Zhou, XD, Yip, TC, Huang, DQ, Muthiah, MD, Noureddin, M, and Zheng, MH. Vibration-controlled transient Elastography in shaping the epidemiology and Management of Steatotic Liver Disease. Clin Mol Hepatol. (2024). doi: 10.3350/cmh.2024.1131
Keywords: non-alcoholic fatty liver disease, advanced lung cancer inflammation index, NHANES, cross-sectional study, positive correlation
Citation: Cheng L, Li S, Li H, You J, Yu M and Yang G (2025) The association of advanced lung cancer inflammation index with non-alcoholic fatty liver disease in NHANES 2017–2020. Front. Med. 12:1516464. doi: 10.3389/fmed.2025.1516464
Edited by:
Dong Hu, Anhui University of Science and Technology, ChinaReviewed by:
Rebeca Escutia Gutiérrez, University of Guadalajara, MexicoMohamed Abdelnabi, University of Calgary, Canada
Copyright © 2025 Cheng, Li, Li, You, Yu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guowang Yang, Z3Vvd2FuZ195YW5nQDE2My5jb20= Mingwei Yu, eXVtaW5nd2VpMTEyMEAxNjMuY29t
 Lin Cheng
Lin Cheng Shumeng Li
Shumeng Li Guowang Yang
Guowang Yang