- 1Department of Pharmacy, Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Medical Affairs, Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 3Department of Obstetrics, Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 4Department of Traditional Chinese Medicine, Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 5Department of Good Clinical Practice, Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Antibodies have been widely used globally over the past decade and play an increasingly important role in modern medicine. Notably, significant advancements have been achieved in the realm of gynecology, particularly in gynecological cancers. This study endeavors to present a thorough overview of antibody-related drug clinical studies in gynecology registered on ClinicalTrials.gov, focusing on the basic characteristics of trials, geographical distribution, administration routes, indications, and targets. The analysis indicates a rising prevalence of antibody–drug conjugates (ADCs), bispecific antibodies, and Fc-fusion proteins. This study will help develop new ideas for future research on antibodies in gynecology.
1 Introduction
Antibody therapeutics have emerged as one of the most prominent sectors in the biopharmaceutical industry. Since the approval of the first monoclonal antibody in 1986, the field has experienced rapid and substantial expansion (1). The development of antibodies has evolved from initial mouse-derived forms to chimeric, humanized, and fully human antibodies (2). They have already made a significant impact on the overall sales of biopharmaceutical products in recent years (3).
Antibody-related drugs exhibit high specificity for their target sites and demonstrate superior efficacy compared to conventional drugs, with fewer side effects (4). These agents have been widely applied in gynecology, especially for the treatment of gynecological cancers (5–7). For instance, bevacizumab combined with paclitaxel and carboplatin has been considered first-line therapy for patients with primary peritoneal cancer, fallopian tube cancer, or epithelial ovarian cancer at stage III or IV (8). Furthermore, the results of the KEYNOTE-826 study (9) reveal that pembrolizumab in conjunction with chemotherapy, with or without bevacizumab, is recommended as a category 1 therapy for patients with metastatic, recurrent, or persistent cervical cancer (10). Additionally, an increasing number of antibodies are currently under clinical trial evaluation (11–13). Despite these advancements, there is a lack of research providing a comprehensive overview of clinical trials involving antibody-related drugs in gynecology. To address this gap, registered clinical trials from ClinicalTrials.gov are reviewed in this study to provide new insights for future research into antibodies in gynecology.
2 Materials and methods
2.1 Data sources
The keywords “antibody” and “marketed antibody-related drugs in gynecology” were used to thoroughly search data on ClinicalTrials.gov. This was identified from the databases of the European Medicines Agency (EMA) and the United States Food and Drug Administration (FDA). Subsequently, entries containing “antibody” and specific terms—“Denosumab,” “Bevacizumab,” “Pembrolizumab,” “Romosozumab,” “Dostarlimab,” “Tisotumab vedotin,” or “Mirvetuximab soravtansine”—were retrieved in the field of “other terms.” The search was restricted to studies in “Early Phase 1, Phase 1, Phase 2, Phase 3, and Phase 4” with a study initiation date before 27 May 2024. Following the identification of all the keywords, the data were consolidated and duplicate entries were removed.
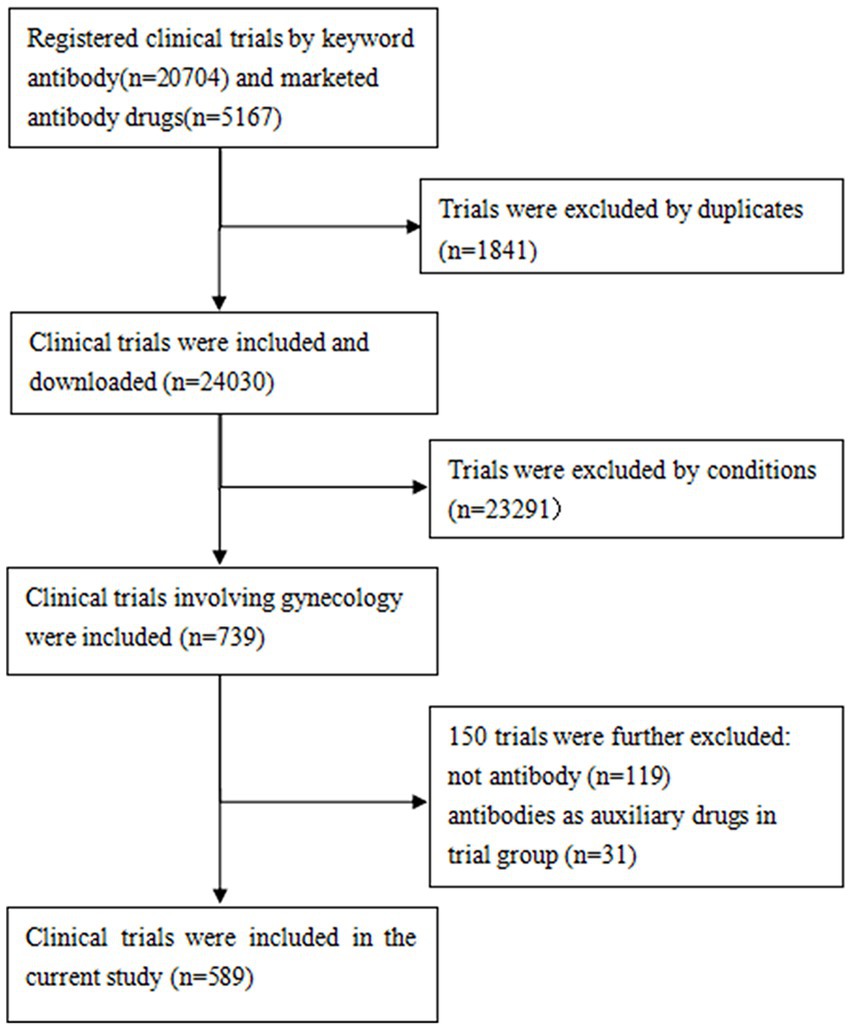
The protocols established in previous investigations of ClinicalTrials.gov were followed (14–16). A comprehensive manual review of all Medical Subject Headings (MeSH) and disease terms cataloged in the National Library of Medicine was conducted to identify those relevant to gynecology. Subsequently, the identified terms were used to extract potential gynecological clinical trials. The study conditions were manually reviewed by a team of trained researchers to select gynecology-related trials. The exclusion criteria were as follows: (1) studies in which antibodies were used as control drugs, and (2) clinical trials in which antibodies were utilized as adjunctive therapies, with a major focus on assessing other drugs rather than the antibodies themselves. Ultimately, a total of 589 clinical trials were included. The data retrieval process is illustrated in Figure 1.
A variety of data were collected for analysis, including study title, condition, status, start date, trial phase, intervention, funder type, location, enrollment, administration route, and drug target. This study was not subjected to review by the institutional review board, since it used publicly available data without involving personal information.
2.2 Statistical analysis
Descriptive analysis was conducted using numerical and percentage formats for categorical variables, while data processing and analysis were performed via Microsoft Excel. Furthermore, network analysis was performed using Gephi to determine the locations and partnerships of the principal research institutions involved in the trials.
3 Results
3.1 Basic characteristics
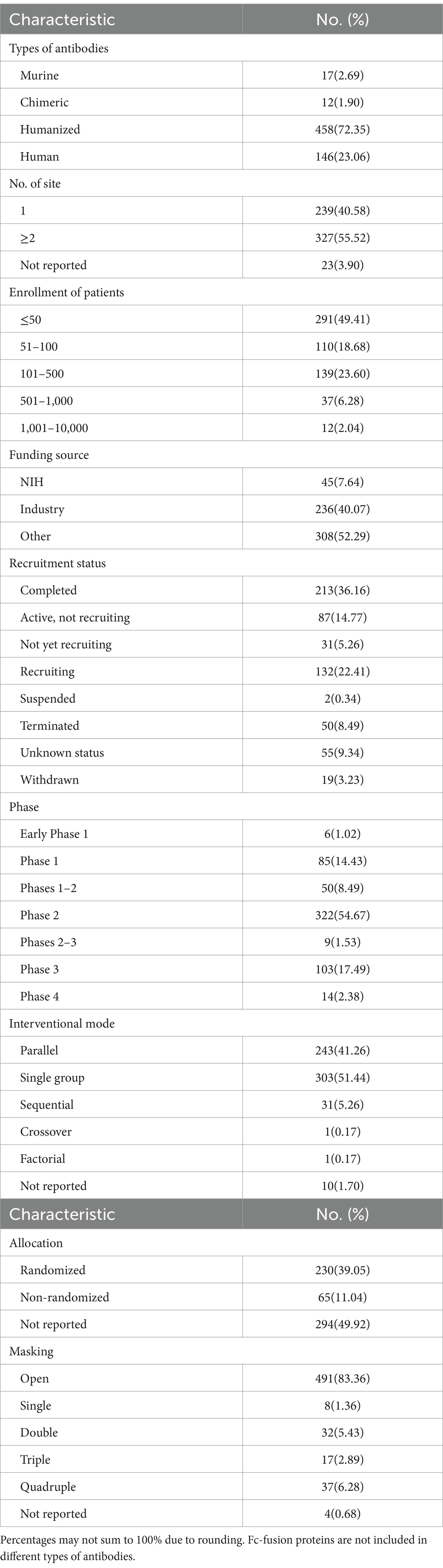
A total of 589 clinical trials involving antibody-related drugs in gynecology were analyzed, including 2 basket trials and 2 umbrella trials, which encompassed 646 antibody drugs. The fundamental characteristics of these trials are presented in Table 1. The antibody-related drugs investigated comprised 17 (2.69%) murine, 12 (1.90%) chimeric, 458 (72.35%) humanized, and 146 (23.06%) human antibodies. It is important to note that Fc-fusion proteins were not included. Approximately half of the studies (291, 49.41%) had planned or actual enrollment of 50 participants or fewer. The majority of antibody drug trials were funded by sources categorized as “other,” comprising 308 studies (52.29%). Meanwhile, 236 studies (40.07%) received funding from industry sources, and 45 studies (7.64%) were funded by the National Institutes of Health (NIH). Furthermore, all antibody-related drugs were classified into five categories: canonical antibody, ADCs, Fc-fusion protein, and bispecific and radiolabeled antibody. Figure 2 depicts the annual distribution of these different antibody-related drug formats, while Figure 3 gives an overview of antibody clinical research.
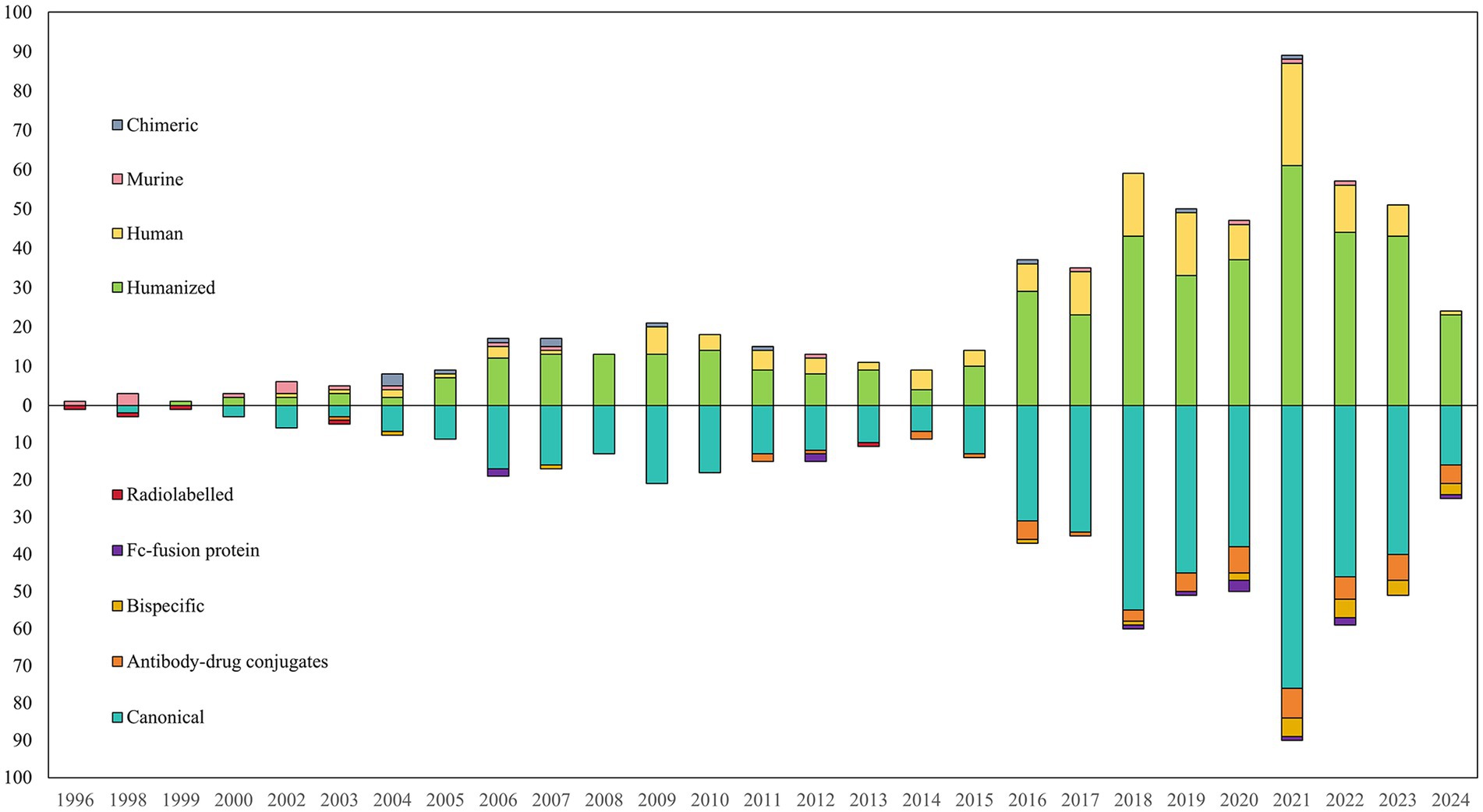
Figure 2. Annual number of different formats of antibody-related drugs in gynecology until May 27, 2024.

Figure 3. Overview of antibody trials. (A) The number of trials in each phase per year from 1996 to 2024; (B) the number of trials in each phase in various antibody types.
3.2 Geographical distribution
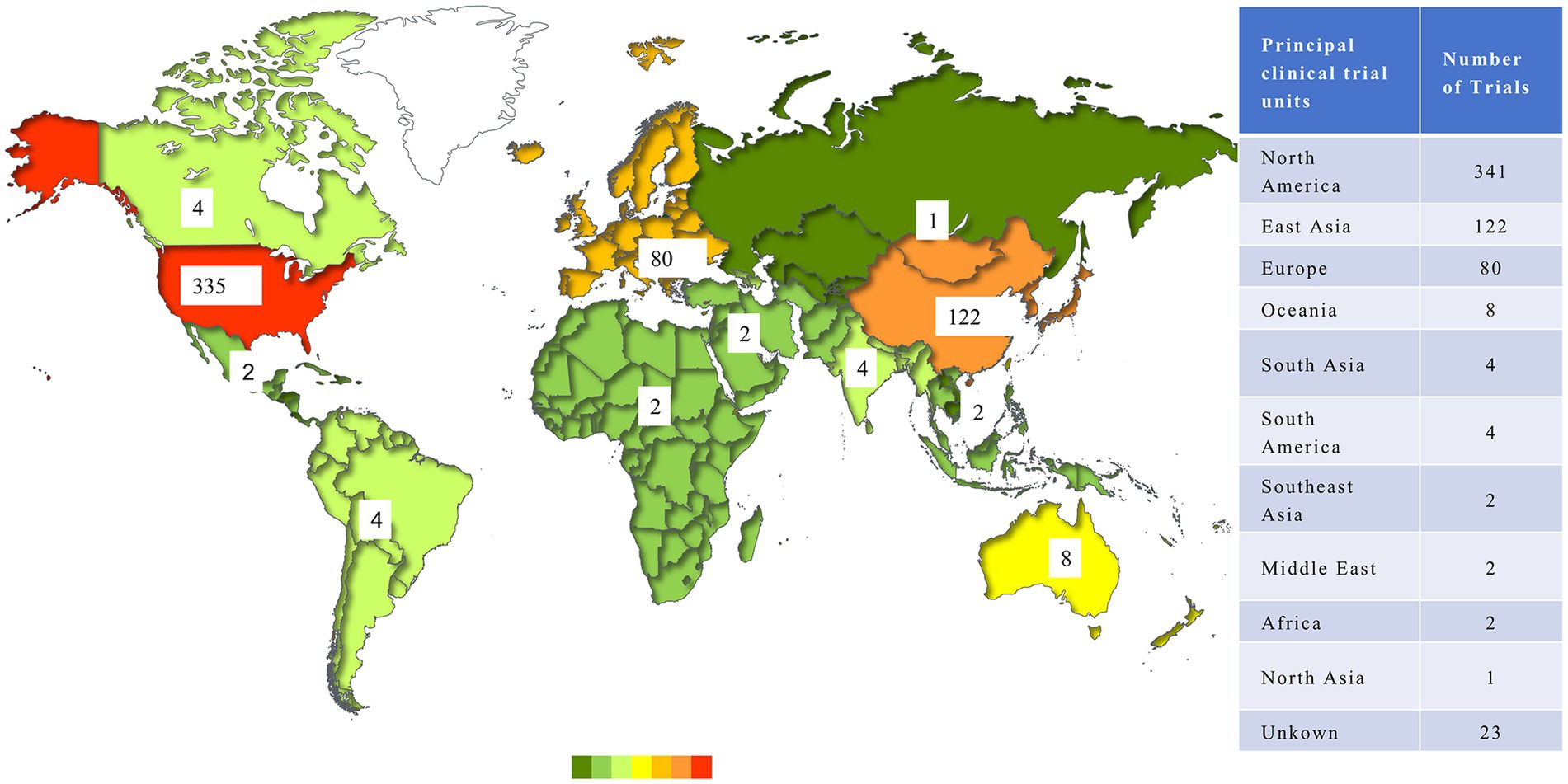
There were 566 trials conducted by 32 countries after the exclusion of trials lacking geographical information. In terms of regional distribution, North America enrolled the largest number of antibody trials (341, 57.89%), followed by East Asia (122, 20.71%) and Europe (80, 13.58%), while other regions had limited trials on this issue. As for countries, the United States possessed an obvious advantage in the development of antibody-related drugs, conducting 335 trials, which accounted for more than half of the world. China represented the most in Asia, with 104 trials. Figure 4 shows the distribution of clinical trials.
In addition to the aforementioned leading countries, there were 124 trials conducted across multiple countries, which illustrated a network of international cooperation among trial sites. A total of 63 countries participated in these international multi-center trials, of which the United States served as the leading site in 97 instances and collaborated with up to 24 different countries. The primary collaborators with the United States included Australia (17 trials), Canada (16 trials), and Belgium (15 trials). Figure 5 illustrates the interconnection between the participating countries.

Figure 5. International cooperation network of trials. Each node represented a country, and the edge showed the cooperate connection between countries. The size of node represented the frequency of cooperation. Regions were represented by different colors.
3.3 Administration routes
Intravenous (IV) injection constituted the predominant method of antibody-related drug administration, which represented 568 cases (87.93%). Additionally, Subcutaneous (SC) injection was utilized in 72 cases (11.15%), intraperitoneal injection in 3 cases (0.46%), and vaginal administration in 1 case (0.15%). It is worth noting that two clinical trials used dual administration routes: combined IV and SC administration, and combined IV and intraperitoneal administration. Figure 6 illustrates the annual number of registered clinical trials categorized by drug administration.
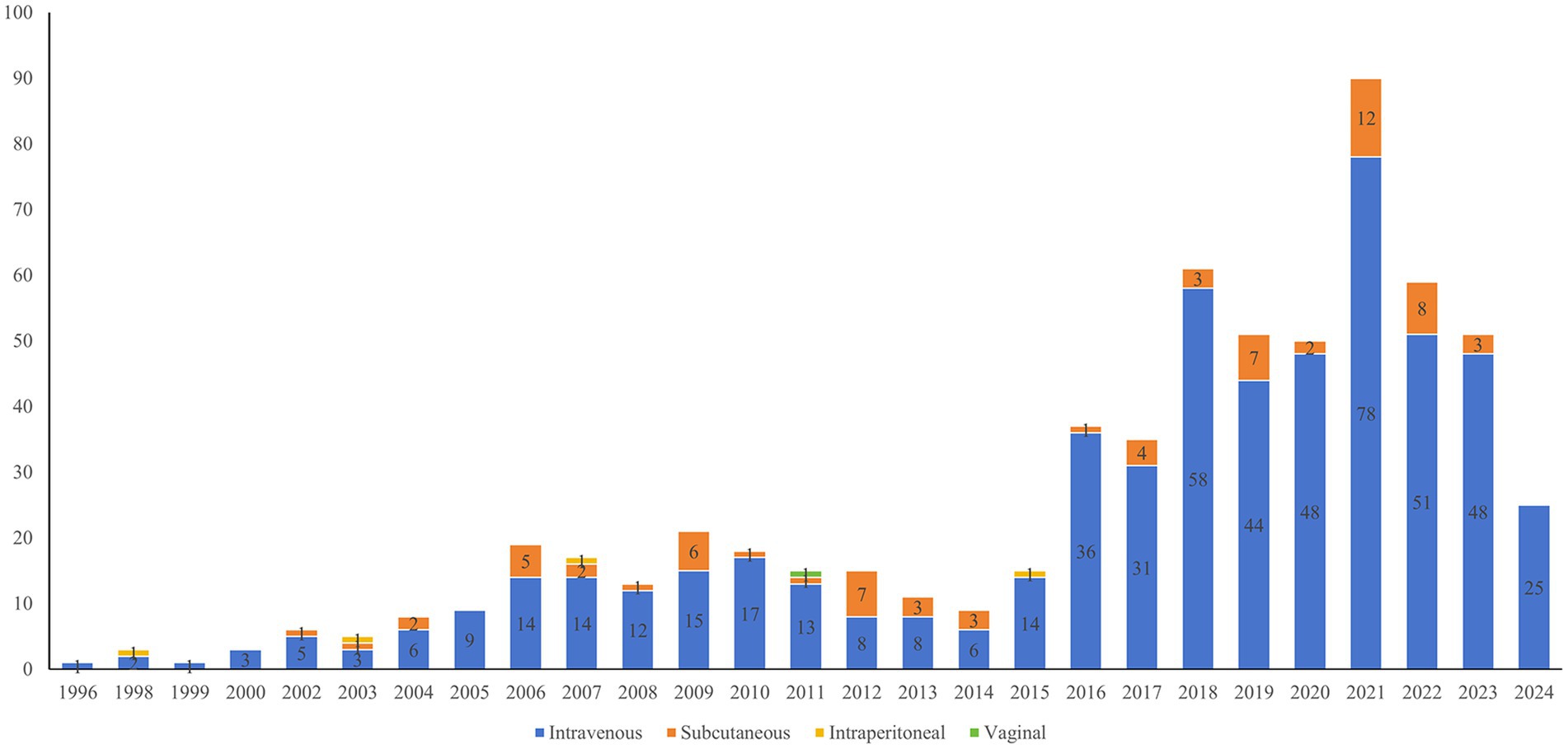
Figure 6. Annual number of antibody drug clinical trials in gynecology by drug administration until May 27, 2024.
3.4 Indications
Antibodies utilized for treating gynecological cancers were predominantly represented in clinical studies. These included ovarian cancer (158, 26.83%), cervical cancer (107, 18.17%), endometrial cancer (66, 11.21%), gestational trophoblastic neoplasm (7, 1.19%), uterine cancer (4, 0.68%), vulvar tumor (4, 0.68%), Paget’s disease of the vulva (1, 0.17%), and multiple cancers (173, 29.37%). Furthermore, antibodies were used in 63 studies (10.70%) that addressed osteoporosis in postmenopausal women. It is noteworthy that a relatively small number of registered clinical trials have explored antibodies for conditions such as endometriosis (3, 0.51%), genital herpes (1, 0.17%), ovarian insufficiency (1, 0.17%), and contraception (1, 0.17%). Figure 7 illustrates the distribution of clinical trials according to indications.
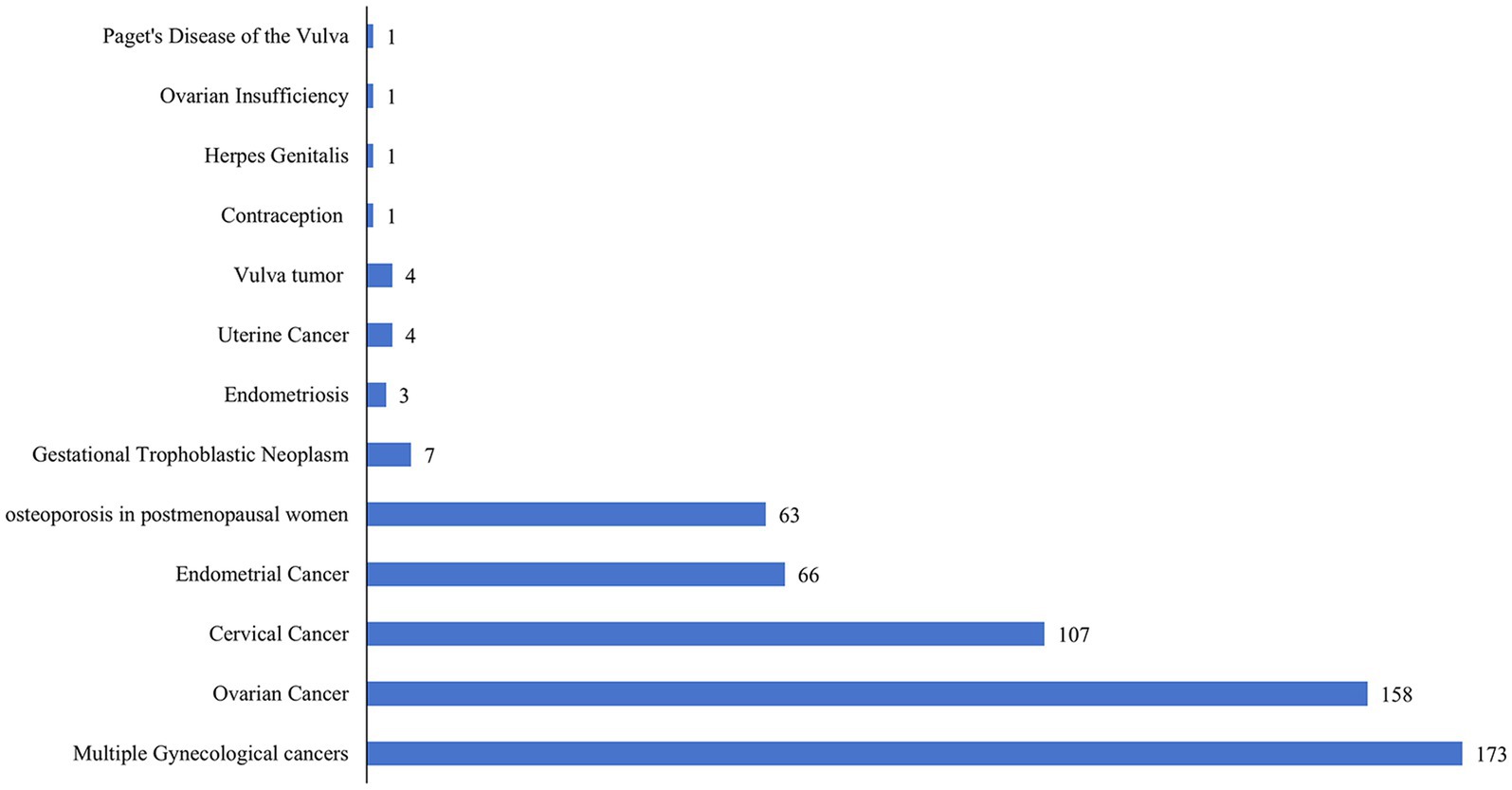
Figure 7. Number of new antibody drug clinical trials in gynecology by indications until May 27, 2024.
3.5 Targets
All the developing antibody-related drugs that are currently undergoing clinical trials in gynecology were cataloged systematically. The developed antibody-related drugs in gynecological clinical trials are summarized in Table 2. Currently, there are 44 antibodies in gynecological clinical development, but approximately 34% act on just 5 validated and novel targets. Predominantly, mesothelin emerged as the most prevalent (4, 9.09%), followed by programmed cell death protein 1 (PD-1) (3, 6.82%), folate receptor α (FRα) (3, 6.82%), receptor tyrosine-protein kinase erbB-2 (HER2) (3, 6.82%), and T cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif domain (TIGIT) (2, 4.55%).
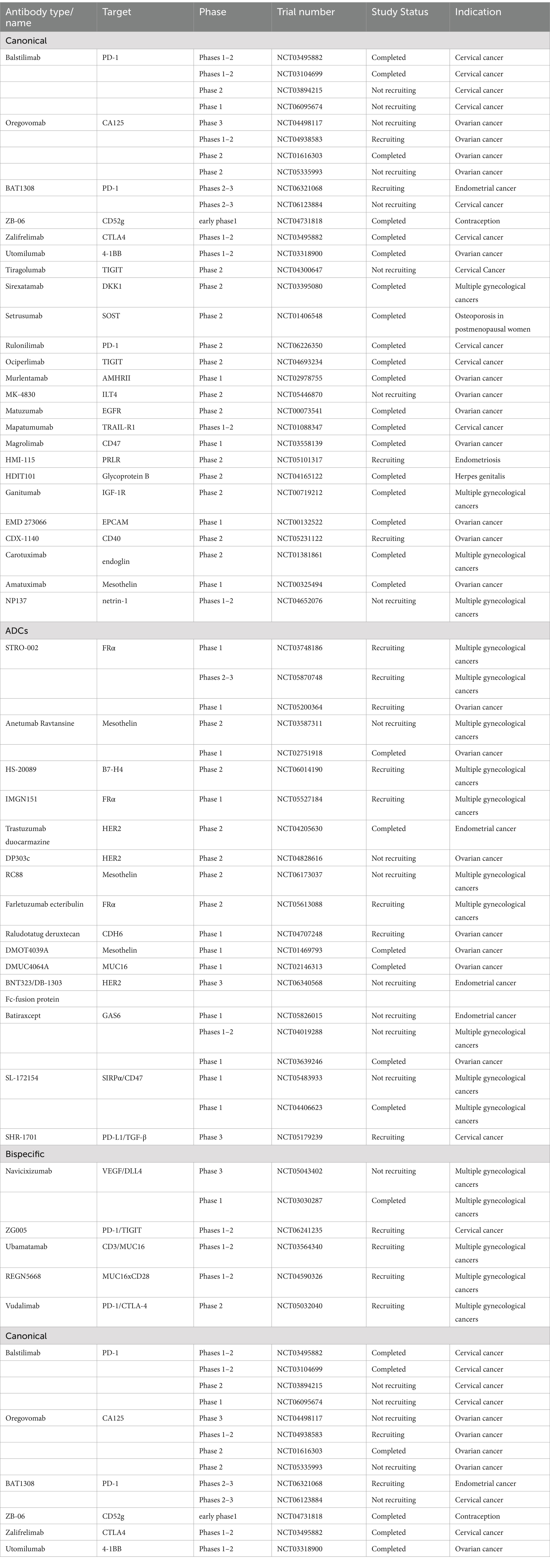
Table 2. Target and indication of developing antibody-related drugs in gynecological clinical trials.
4 Discussion
Since 1996, research into antibodies has significantly expanded in the field of gynecology. Innovative clinical trials, such as umbrella and basket trials, have been implemented to advance the development of antibody-related drugs in the field of gynecology. This study identified two basket trials (NCT04965519 and NCT03827837) and two umbrella trials (NCT03699449 and NCT03574779). Basket trials are characterized by the evaluation of targeted therapy for multiple diseases that share common molecular alterations. Umbrella trials involve the assessment of multiple targeted therapies within a single disease and can be further classified into various subgroups (17). The advantage of basket trials lies in the ability to test smaller sample sizes for a given tumor type, which facilitates research in less common tumor types. By contrast, umbrella trials allow for a more comprehensive analysis of treatment for a specific tumor type (18). The application of these novel clinical trials in gynecology is anticipated to accelerate the evaluation and approval of antibody-related drugs and promote molecular-based individualized treatment strategies (19, 20). In addition, innovative forms, such as ADCs, bispecific antibodies, and Fc-fusion proteins, have gained prominence and are the subjects of extensive investigation. In this review, studies were divided into four distinct groups according to the development of antibody-related drugs.
4.1 Canonical antibodies
Canonical antibodies exhibit a complete antibody structure, which contains two light chains and two full-length heavy chains (21). According to research, these antibodies represent more than 80% of all clinical trials in the field of gynecology.
Since the approval of ipilimumab in 2011, immune checkpoint inhibitors have significantly advanced the treatment of gynecological malignancies (22). In this field, significant inhibitors encompass PD-L1, PD-1, CTLA-4, and LAG-3 (23), among which PD-1/PD-L1 is the most extensively studied (24). Pembrolizumab and dostarlimab have been approved by the FDA to treat gynecological cancers (21). An increasing number of real-world studies contribute to the expanding body of evidence that supports the use of PD-1 inhibitors for recurrent or advanced gynecologic cancers. In a multi-center retrospective cohort study, Korean patients with recurrent endometrial cancer were administered pembrolizumab in combination with lenvatinib for a median duration of 4.5 cycles. During this period, the disease control rate was 76.2% (95% CI, 61.9–88.1), and the best objective response rate was 23.8% (95% CI, 11.9–38.1) (25). The most common treatment-related adverse events included fatigue, hypertension, and hypothyroidism. Additionally, another retrospective study suggested that a lower dosage of pembrolizumab represents a cost-effective and efficacious treatment strategy for patients with refractory gynecologic cancer (26).
The vascular endothelial growth factor (VEGF) serves as a crucial mediator in the process of neovascularization, which is predominantly secreted by cancer cells (27). Bevacizumab is the first anti-angiogenic agent approved for oncological use. Numerous clinical trials have demonstrated the efficacy of bevacizumab in the treatment of gynecologic malignancies (28). There is an increasing body of evidence that supports its efficacy and safety in routine clinical practice. In a retrospective study, bevacizumab was found to be effective and well-tolerated in the real-world treatment of ovarian cancer (29). Furthermore, a multi-institutional retrospective cohort study indicated that bevacizumab maintenance therapy prolonged progression-free survival for metastatic, recurrent, or persistent cervical cancer (30).
In addition, canonical antibodies are also used in the treatment of postmenopausal osteoporosis (31). Notably, denosumab, a fully human antibody targeting RANK-L (32), and romosozumab, a humanized monoclonal antibody targeting sclerostin (SOST) (33), are utilized in this therapeutic context.
4.2 ADCs
ADCs have emerged as one of the most promising therapeutic modalities in oncology due to their ability to provide tumor cells with chemotherapy in a more targeted and safer manner (34, 35). ADCs comprise a highly selective antibody, a cytotoxic payload, and a linker connecting the two components (36). Currently, several ADCs have acquired FDA approval for the treatment of gynecologic cancers, including mirvetuximab soravtansine for ovarian cancer and tisotumab vedotin for cervical cancer (37). Although no ADCs have been approved for endometrial cancer, 8 clinical trials (NCT04205630, NCT06132958, NCT03836157, NCT04251416, NCT06340568, NCT03835819, NCT03832361, and NCT04585958) have been identified to address this unmet critical need. Notably, mirvetuximab soravtansine is currently being evaluated in two phase II clinical trials for endometrial cancer (NCT03835819 and NCT03832361).
Fam-trastuzumab deruxtecan-nxki (T-DXd) contains a topoisomerase I inhibitor payload, a cleavable tetrapeptide linker, and the anti-HER2 antibody trastuzumab (38). On 5 April 2024, the approval of T-DXd was accelerated by the FDA to treat adults with unresectable or metastatic HER2-positive (IHC3+) solid tumors. Previous clinical trials have suggested its potential efficacy in patients with endometrial cancer (39). In the STATICE trial, patients with endometrial cancer expressing HER2 were categorized into HER2-low and HER2-high expression groups. The overall response rate was 70.0% (95% CI, 34.8–93.3) for the HER2-low group and 54.5% (95% CI, 32.2–75.6) for the HER2-high group. These results indicated that T-DXd is effective in patients with endometrial cancer, irrespective of the HER2 expression status (40). Furthermore, the combination of T-DXd and olaparib was assessed in HER2-expressing cancers, including an extension to patients with endometrial cancer (NCT04585958).
Sacituzumab govitecan, ADCs against trophoblast cell surface antigen-2 (Trop-2), has demonstrated its potential efficacy in the treatment of endometrial cancer (41). In a Phase I/II basket study, the objective response rate was 22.2% (95% CI, 6.4–47.6) in the endometrial cancer cohort (42). Currently, a Phase II study of sacituzumab govitecan in patients with persistent or recurrent endometrial carcinoma is ongoing (NCT04251416).
However, in this context, clinical data on other ADCs remain limited.
4.3 Bispecific antibodies
Bispecific antibodies are designed to simultaneously bind to separate antigenic epitopes or distinct antigens (43). Compared with traditional antibodies, bispecific antibodies exhibit greater specificity, which contributes to improved therapeutic efficacy and safety, while reducing the likelihood of adverse reactions (44). This characteristic makes bispecific antibodies a promising strategy in cancer therapy (45). An increasing number of bispecific antibodies with diverse mechanisms are currently under development for oncological applications, including checkpoint inhibitors (CPIs), T-cell engagers (TCEs), natural killer cell engagers (NKCEs), and immune cell engagers (ICEs) (46). In the realm of gynecological cancer, the majority of bispecific antibodies in registered clinical trials are concentrated on checkpoint inhibition. Notably, catumaxomab (NCT00189345, NCT00563836), navicixizumab (NCT05043402, NCT03030287), REGN5668 (NCT04590326), and ubamatamab (NCT03564340) are identified as agents targeting CD3xEpCAM, VEGFxDLL4, MUC16xCD28, and CD3xMUC16, respectively. A selection of representative findings is described in this subsection.
Cadonilimab is a bispecific antibody against PD-1 and CTLA4, which has completed two Phase II studies in patients with metastatic or recurrent cervical cancer (NCT04380805, NCT04868708). The findings of these studies indicated that cadonilimab may provide patients with substantial therapeutic benefits (47, 48). In China, cadonilimab was approved in June 2022 for the treatment of metastatic cervical cancer based on these promising outcomes (49). Furthermore, several clinical studies involving cadonilimab are currently ongoing (NCT05687851, NCT05824494, NCT06066216, NCT04982237, NCT05227651, and NCT05932212).
Navicixizumab is a bispecific agent that targets both DLL4 and VEGF (50). A Phase 1a study of navicixizumab provided preliminary evidence of anti-tumor activity in ovarian cancer, with manageable toxicity (51). A subsequent Phase 1b study combining navicixizumab with paclitaxel (NCT03030287) demonstrated an ORR of 43.2% (95% CI, 28.3–59.0) in patients with platinum-resistant ovarian cancer. Furthermore, 90.9% of patients experienced treatment-related adverse events, including hypertension, fatigue, and headaches (52). A Phase III trial to assess the efficacy and safety of navicixizumab in the treatment of ovarian cancer is currently underway (NCT05043402).
4.4 Fc fusion proteins
Fc fusion proteins contain the Fc domain of an immunoglobulin (IgG) and a desired linked protein (53). Compared to traditional antibodies, Fc fusion proteins exhibit an extended half-life, multiple targeting capabilities, and superior specificity (54). Currently, there are 13 studies on Fc fusion proteins in the field of gynecology. This report highlights the bifunctional fusion proteins Bintrafusp alfa (NCT04246489, NCT04551950), SHR-1701 (NCT05179239), and SL-172154 (NCT05483933, NCT04406623).
Bintrafusp alfa is a bifunctional fusion protein that comprises an extracellular domain of TGF-βreceptor II fused to a human IgG1 monoclonal antibody against PD-L1 (55). This agent has completed both Phase 1 and Phase 2 clinical trials in patients with metastatic or recurrent cervical cancer (NCT04551950, NCT04246489). The findings of these studies have confirmed the safety profile and clinical efficacy of Bintrafusp alfa. Anemia is the most common treatment-related adverse event (56, 57).
SHR-1701 is a bifunctional fusion protein that comprises an extracellular domain of TGF-β II and an antibody targeting PD-L1 (58). Recent data have indicated that SHR-1701 has an ORR of 15.6% in patients with metastatic or recurrent cervical cancer. Additionally, 11 patients (34.4%) experienced treatment-related adverse events of grade 3 or grade 4 severity. Consequently, SHR-1701 may be a potential therapeutic option for patients with cervical cancer (59). Currently, the safety and efficacy of SHR-1701 are being evaluated in a Phase 3 clinical trial (NCT05179239).
SL-172154 is a hexameric fusion protein that comprises SIRPα and CD40L domains connected by an inert Fc linker. This agent has completed a Phase 1 clinical trial in ovarian cancer patients (NCT04406623). Additionally, a subsequent Phase 1 trial is currently underway, which targets patients with platinum-resistant ovarian cancer (NCT05483933).
4.5 Challenges
4.5.1 Subcutaneous delivery of antibodies
Historically, antibody-related drugs are delivered by IV injection due to their inherent physicochemical and biological characteristics (60). However, this method has several disadvantages, including pain, low patient tolerance, and elevated costs. Consequently, there has been a progressive transition in the administration route of antibody-based therapeutics from IV to SC injection. This shift is attributed not only to the reduced administration time but also to the feasibility of home-based administration (61). In recent years, approximately 30% of the approved antibody-related drugs have been administered through SC injection (62). Although several drugs have reached clinical trials, only denosumab and romosozumab have been approved by the EMA and FDA to treat postmenopausal osteoporosis (63).
There were no statistically significant differences in safety and immune response between intramuscular and SC administration routes, according to a Phase 1 clinical trial (NCT00058435) (64). Similarly, the findings of another Phase 1 clinical study (NCT00103545) were also promising (65). However, a subsequent Phase 3 trial (NCT00418574) indicated that patients with ovarian cancer in first remission received no therapeutic benefits from abagovomab (66).
Dalantercept, a recombinant fusion protein designed to target the activin receptor-like kinase 1 (ALK1) receptor, was evaluated in a Phase II clinical trial that comprised 28 patients with endometrial cancer. Patients were administered a dosage of 1.2 mg/kg subcutaneously every 3 weeks (NCT01642082). The majority of participants withdrew from the study primarily due to disease progression. It was concluded that dalantercept had inadequate efficacy as a single-agent treatment (67). Additionally, a separate Phase II trial that evaluated dalantercept in patients with ovarian and fallopian tube cancers failed to exhibit any objective response (68).
Hyaluronidase-zzxf/pertuzumab/trastuzumab is an SC injection that contains a fixed-dose combination of trastuzumab and pertuzumab, which has been approved for treating patients with HER2-positive breast cancer (69). Currently, a Phase 2/3 clinical trial is underway to assess the efficacy of hyaluronidase-zzxf/pertuzumab/trastuzumab in patients with HER2-positive endometrial serous carcinoma (NCT05256225).
Furthermore, a Phase 2 clinical trial is currently underway to assess the efficacy of HMI-115 in patients with endometriosis-related pain. Herein, HMI-115 is administered SC on a bi-weekly basis.
This study shows that the availability of antibody-related drugs for SC administration is limited in the field of gynecology. This is because this route of administration is hindered by challenges such as immunogenicity, high viscosity, and protein aggregation (70, 71). To enhance the SC delivery of antibodies, various strategies have been developed, including the combination of proprietary excipients and proteins to address the issues related to the ionic strength and hydrophobic regions of antibodies (60).
Overall, the demand for SC antibodies is high on the market. However, significant obstacles persist and the SC administration is making ongoing advancements.
4.5.2 Potential targets
According to the results, the hottest and most potential targets for developing antibody-related drugs in gynecology are mesothelin, PD-1, FRα, HER2, and TIGIT. Mesothelin is a membrane-bound surface glycoprotein that is highly expressed in ovarian cancer, pancreatic adenocarcinoma, mesothelioma, and several other malignancies. However, its expression is limited in normal tissues (72). Furthermore, mesothelin plays an important role in cell adhesion, drug resistance, and tumor metastasis, which makes it a potential therapeutic target for ovarian cancer (73). Examples are amatuximab (NCT00325494), anetumab ravtansine (NCT03587311, NCT02751918), and RC88 (NCT06173037).
PD-1 is a checkpoint protein that belongs to the CD28 family. It is predominantly expressed in activated CD4 + T cells, CD8 + T cells, and peripheral B cells (74). The PD-1 signaling pathway plays a significant role in the tumor microenvironment of various malignancies and contributes to T cell inactivation and depletion, which facilitates the evasion of antitumor immunity (75). Consequently, PD-1 is a promising target for therapeutic strategies. Examples are balstilimab (NCT03495882, NCT03104699, NCT03894215, NCT06095674), BAT1308 (NCT06321068, NCT06123884), and rulonilimab (NCT06226350).
FRα is a glycoprotein attached to the membrane and is encoded by the FOLR1 gene (76). It is critically involved in cell proliferation, DNA synthesis, and intracellular signaling, which are fundamental to tumorigenesis. The receptor is a promising target for the development of anticancer drugs due to its overexpression in various solid tumors, such as ovarian, lung, and breast cancers (77). Examples are STRO-002 (NCT03748186, NCT05870748, and NCT05200364), IMGN151 (NCT05527184), and farletuzumab ecteribulin (NCT05613088).
HER2 is a constituent of the EGFR family of receptor tyrosine kinases (78). Aberrations of the HER2 gene, including mutations, deletions, and amplifications, have been identified in ovarian, cervical, and endometrial cancers (79). Examples are trastuzumab duocarmazine (NCT04205630), DP303c (NCT04828616), and BNT323/DB-1303 (NCT06340568).
TIGIT is a newly recognized immune checkpoint. It is predominantly expressed in natural killer cells, regulatory T cells, CD4 + T cells, CD8 + T cells, and tumor-infiltrating lymphocytes (80). TIGIT is associated with the exhaustion of NK cells in vivo and is observed in individuals with various types of cancer (81). Examples are tiragolumab (NCT04300647) and ociperlimab (NCT04693234).
5 Conclusion
This study provides a thorough review of clinical trials involving antibody-related drugs in the field of gynecology. The analysis revealed a growing prevalence of ADCs, bispecific antibodies, and Fc-fusion proteins. It has been found that these therapeutic modalities play a crucial role in advancing gynecological oncology. Meanwhile, it also explores the challenges associated with SC administration. The findings of this review may provide valuable insights and new ideas for future research on antibodies in gynecology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XZ: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. LX: Conceptualization, Supervision, Writing – review & editing. FL: Data curation, Funding acquisition, Project administration, Supervision, Writing – review & editing. WC: Data curation, Methodology, Writing – review & editing. CZ: Supervision, Writing – review & editing. YD: Investigation, Supervision, Writing – review & editing. TW: Data curation, Writing – review & editing. SX: Supervision, Validation, Writing – review & editing. HD: Supervision, Validation, Writing – review & editing. MT: Supervision, Validation, Writing – review & editing. WG: Supervision, Validation, Writing – review & editing. EL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Nanchong Social Science Planning Project North Sichuan Health Humanities Research Projects (NC25CB05), Sichuan Provincial Department of Science and Technology (2023YFG0277), the Foundation of Chengdu Medical Scientific Research project (2023004), Yingcai Scheme, Chengdu Women's and Children's Central Hospital (YC2023010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shapiro, MA. Regulatory considerations in the design, development and quality of monoclonal antibodies and related products for the diagnosis and treatment of cancer. Front Oncol. (2024) 14:1379738. doi: 10.3389/fonc.2024.1379738
2. Alejandra, WP, Miriam Irene, JP, Fabio Antonio, GS, Patricia, RR, Elizabeth, TA, Aleman-Aguilar, JP, et al. Production of monoclonal antibodies for therapeutic purposes: a review. Int Immunopharmacol. (2023) 120:110376. doi: 10.1016/j.intimp.2023.110376
3. Kinch, MS, Kraft, Z, and Schwartz, T. Monoclonal antibodies: trends in therapeutic success and commercial focus. Drug Discov Today. (2023) 28:103415. doi: 10.1016/j.drudis.2022.103415
4. Chi, X-K, Xu, X-L, Chen, B-Y, Su, J, and Du, Y-Z. Combining nanotechnology with monoclonal antibody drugs for rheumatoid arthritis treatments. J Nanobiotechnol. (2023) 21:105. doi: 10.1186/s12951-023-01857-8
5. Martín-Sabroso, C, Lozza, I, Torres-Suárez, AI, and Fraguas-Sánchez, AI. Antibody-antineoplastic conjugates in gynecological malignancies: current status and future perspectives. Pharmaceutics. (2021) 13:1705. doi: 10.3390/pharmaceutics13101705
6. Cohen, AC, Roane, BM, and Leath, CA 3rd. Novel therapeutics for recurrent cervical Cancer: moving towards personalized therapy. Drugs. (2020) 80:217–27. doi: 10.1007/s40265-019-01249-z
7. Richardson, DL, Eskander, RN, and O'Malley, DM. Advances in ovarian Cancer care and unmet treatment needs for patients with platinum resistance: a narrative review. JAMA Oncol. (2023) 9:851–9. doi: 10.1001/jamaoncol.2023.0197
8. Perren, TJ, Swart, AM, Pfisterer, J, Ledermann, JA, Pujade-Lauraine, E, Kristensen, G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. (2011) 365:2484–96. doi: 10.1056/NEJMoa1103799
9. Colombo, N, Dubot, C, Lorusso, D, Caceres, MV, Hasegawa, K, Shapira-Frommer, R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical Cancer. N Engl J Med. (2021) 385:1856–67. doi: 10.1056/NEJMoa2112435
10. Abu-Rustum, NR, Yashar, CM, Arend, R, Barber, E, Bradley, K, Brooks, R, et al. NCCN guidelines® insights: cervical Cancer, version 1.2024. J Natl Compr Cancer Netw. (2023) 21:1224–33. doi: 10.6004/jnccn.2023.0062
11. Wang, Q, Peng, H, Qi, X, Wu, M, and Zhao, X. Targeted therapies in gynecological cancers: a comprehensive review of clinical evidence. Signal Transduct Target Ther. (2020) 5:137. doi: 10.1038/s41392-020-0199-6
12. Peng, H, He, X, and Wang, Q. Immune checkpoint blockades in gynecological cancers: a review of clinical trials. Acta Obstet Gynecol Scand. (2022) 101:941–51. doi: 10.1111/aogs.14412
13. Tymon-Rosario, J, Zeybek, B, and Santin, AD. Novel antibody-drug conjugates: current and future roles in gynecologic oncology. Curr Opin Obstet Gynecol. (2021) 33:26–33. doi: 10.1097/GCO.0000000000000642
14. Turner, B, Rajeshuni, N, Tran, EM, Ludwig, CA, Tauqeer, Z, Weeks, B, et al. Characteristics of ophthalmology trials registered in ClinicalTrials.gov, 2007-2018. Am J Ophthalmol. (2020) 211:132–41. doi: 10.1016/j.ajo.2019.11.004
15. Steinberg, JR, Magnani, CJ, Turner, BE, Weeks, BT, Young, AMP, Lu, CF, et al. Early discontinuation, results reporting, and publication of gynecology clinical trials from 2007 to 2020. Obstet Gynecol. (2022) 139:821–31. doi: 10.1097/AOG.0000000000004735
16. Steinberg, JR, Weeks, BT, Reyes, GA, Conway Fitzgerald, A, Zhang, WY, Lindsay, SE, et al. The obstetrical research landscape: a cross-sectional analysis of clinical trials from 2007-2020. Am J Obstet Gynecol MFM. (2021) 3:100253. doi: 10.1016/j.ajogmf.2020.100253
17. Park, JJH, Hsu, G, Siden, EG, Thorlund, K, and Mills, EJ. An overview of precision oncology basket and umbrella trials for clinicians. CA Cancer J Clin. (2020) 70:125–37. doi: 10.3322/caac.21600
18. Haslam, A, Olivier, T, Tuia, J, and Prasad, V. A systematic review of basket and umbrella trials in oncology: the importance of tissue of origin and molecular target. Eur J Cancer. (2023) 178:227–33. doi: 10.1016/j.ejca.2022.10.027
19. Billingham, L, Brown, L, Framke, T, Greystoke, A, Hovig, E, Mathur, S, et al. Histology independent drug development—is this the future for cancer drugs? Cancer Treat Rev. (2024) 123:102674. doi: 10.1016/j.ctrv.2023.102674
20. Kraus, FBT, Sultova, E, Heinrich, K, Jung, A, Westphalen, CB, Tauber, CV, et al. Genetics and beyond: precision medicine real-world data for patients with cervical, vaginal or vulvar Cancer in a tertiary Cancer center. Int J Mol Sci. (2024) 25:2345. doi: 10.3390/ijms25042345
21. Lyu, X, Zhao, Q, Hui, J, Wang, T, Lin, M, Wang, K, et al. The global landscape of approved antibody therapies. Antibody Ther. (2022) 5:233–57. doi: 10.1093/abt/tbac021
22. Zou, Y, Xu, Y, Chen, X, and Zheng, L. Advances in the application of immune checkpoint inhibitors in gynecological tumors. Int Immunopharmacol. (2023) 117:109774. doi: 10.1016/j.intimp.2023.109774
23. Sun, Q, Hong, Z, Zhang, C, Wang, L, Han, Z, and Ma, D. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct Target Ther. (2023) 8:320. doi: 10.1038/s41392-023-01522-4
24. Mullard, A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov. (2021) 20:491–5. doi: 10.1038/d41573-021-00079-7
25. Kim, J, Noh, JJ, Lee, TK, Kim, SI, Lee, JY, Lee, JW, et al. Real-world experience of pembrolizumab and lenvatinib in recurrent endometrial cancer: a multicenter study in Korea. Gynecol Oncol. (2022) 165:369–75. doi: 10.1016/j.ygyno.2022.02.020
26. Kao, CH, Lin, H, Liu, CT, Ou, YC, Fu, HC, Wu, CC, et al. Real-world efficacy and safety of low-dose pembrolizumab in patients with advanced and refractory gynecologic cancers. J Formos Med Assoc. (2024) 123:487–95. doi: 10.1016/j.jfma.2023.09.020
27. Shaw, P, Dwivedi, SKD, Bhattacharya, R, Mukherjee, P, and Rao, G. VEGF signaling: role in angiogenesis and beyond. Biochim Biophys Acta Rev Cancer. (2024) 1879:189079. doi: 10.1016/j.bbcan.2024.189079
28. Yetkin-Arik, B, Kastelein, AW, Klaassen, I, Jansen, C, Latul, YP, Vittori, M, et al. Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy. Biochim Biophys Acta Rev Cancer. (2021) 1875:188446. doi: 10.1016/j.bbcan.2020.188446
29. Zhang, N, Zheng, H, Gao, Y, Shu, T, and Wang, H. Real-world study of bevacizumab treatment in patients with ovarian cancer: a Chinese single-institution study of 155 patients. BMC Womens Health. (2023) 23:178. doi: 10.1186/s12905-023-02329-9
30. Kotaka, S, Kondo, E, Kawai, Y, Okamoto, K, Kishigami, Y, Yamawaki, T, et al. Real-world efficacy and safety of bevacizumab single-maintenance therapy following platinum-paclitaxel chemotherapy plus bevacizumab in patients with advanced cervical cancer. J Gynecol Oncol. (2023) 34:e60. doi: 10.3802/jgo.2023.34.e60
31. Lewiecki, EM. Monoclonal antibodies for the treatment of osteoporosis. Expert Opin Biol Ther. (2013) 13:183–96. doi: 10.1517/14712598.2012.740006
32. Deeks, ED. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging. (2018) 35:163–73. doi: 10.1007/s40266-018-0525-7
33. Wu, D, Li, L, Wen, Z, and Wang, G. Romosozumab in osteoporosis: yesterday, today and tomorrow. J Transl Med. (2023) 21:668. doi: 10.1186/s12967-023-04563-z
34. Sasso, JM, Tenchov, R, Bird, R, Iyer, KA, Ralhan, K, Rodriguez, Y, et al. The evolving landscape of antibody-drug conjugates: in depth analysis of recent research Progress. Bioconjug Chem. (2023) 34:1951–2000. doi: 10.1021/acs.bioconjchem.3c00374
35. Dumontet, C, Reichert, JM, Senter, PD, Lambert, JM, and Beck, A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. (2023) 22:641–61. doi: 10.1038/s41573-023-00709-2
36. Ruan, DY, Wu, HX, Meng, Q, and Xu, RH. Development of antibody-drug conjugates in cancer: overview and prospects. Cancer Commun (Lond). (2024) 44:3–22. doi: 10.1002/cac2.12517
37. Karpel, HC, Powell, SS, and Pothuri, B. Antibody-drug conjugates in gynecologic Cancer. Am Soc Clin Oncol Educ Book. (2023) 43:e390772. doi: 10.1200/EDBK_390772
38. Edoardo, C, and Giuseppe, C. Trastuzumab-deruxtecan in solid tumors with HER2 alterations: from early phase development to the first agnostic approval of an antibody-drug conjugate. Expert Opin Investig Drugs. (2024) 33:851–65. doi: 10.1080/13543784.2024.2376573
39. Meric-Bernstam, F, Makker, V, Oaknin, A, Oh, DY, Banerjee, S, González-Martín, A, et al. Efficacy and safety of Trastuzumab Deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/JCO.23.02005
40. Nishikawa, T, Hasegawa, K, Matsumoto, K, Mori, M, Hirashima, Y, Takehara, K, et al. Trastuzumab Deruxtecan for human epidermal growth factor receptor 2-expressing advanced or recurrent uterine Carcinosarcoma (NCCH1615): the STATICE trial. J Clin Oncol. (2023) 41:2789–99. doi: 10.1200/JCO.22.02558
41. Webster, EM, Zeybek, B, Tymon-Rosario, J, and Santin, AD. Sacituzumab govitecan: a promising antibody-drug conjugate for the treatment of poorly differentiated endometrial cancer. Oncoscience. (2020) 7:68–9. doi: 10.18632/oncoscience.514
42. Bardia, A, Messersmith, WA, Kio, EA, Berlin, JD, Vahdat, L, Masters, GA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. (2021) 32:746–56. doi: 10.1016/j.annonc.2021.03.005
43. Sun, Y, Yu, X, Wang, X, Yuan, K, Wang, G, Hu, L, et al. Bispecific antibodies in cancer therapy: target selection and regulatory requirements. Acta Pharm Sin B. (2023) 13:3583–97. doi: 10.1016/j.apsb.2023.05.023
44. Guo, X, Wu, Y, Xue, Y, Xie, N, and Shen, G. Revolutionizing cancer immunotherapy: unleashing the potential of bispecific antibodies for targeted treatment. Front Immunol. (2023) 14:1291836. doi: 10.3389/fimmu.2023.1291836
45. Wei, J, Yang, Y, Wang, G, and Liu, M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. (2022) 13:1035276. doi: 10.3389/fimmu.2022.1035276
46. Klein, C, Brinkmann, U, Reichert, JM, and Kontermann, RE. The present and future of bispecific antibodies for cancer therapy. Nat Rev Drug Discov. (2024) 23:301–19. doi: 10.1038/s41573-024-00896-6
47. Lou, H, Cai, H, Huang, X, Li, G, Wang, L, Liu, F, et al. Cadonilimab combined with chemotherapy with or without bevacizumab as first-line treatment in recurrent or metastatic cervical Cancer (COMPASSION-13): a phase 2 study. Clin Cancer Res. (2024) 30:1501–8. doi: 10.1158/1078-0432.CCR-23-3162
48. Wu, X, Ji, J, Lou, H, Li, Y, Feng, M, Xu, N, et al. Efficacy and safety of cadonilimab, an anti-PD-1/CTLA4 bi-specific antibody, in previously treated recurrent or metastatic (R/M) cervical cancer: a multicenter, open-label, single-arm, phase II trial (075). Gynecol Oncol. (2022) 166:S47–8. doi: 10.1016/S0090-8258(22)01293-8
50. Fu, S, Corr, BR, Culm-Merdek, K, Mockbee, C, and Youssoufian, H. Navicixizumab plus paclitaxel shows clinical benefit in platinum-resistant ovarian Cancer. Cancer Discov. (2022) 12:OF6. doi: 10.1158/2159-8290.CD-RW2022-073
51. Jimeno, A, Moore, KN, Gordon, M, Chugh, R, Diamond, JR, Aljumaily, R, et al. A first-in-human phase 1a study of the bispecific anti-DLL4/anti-VEGF antibody navicixizumab (OMP-305B83) in patients with previously treated solid tumors. Investig New Drugs. (2019) 37:461–72. doi: 10.1007/s10637-018-0665-y
52. Fu, S, Corr, BR, Culm-Merdek, K, Mockbee, C, Youssoufian, H, Stagg, R, et al. Phase Ib study of Navicixizumab plus paclitaxel in patients with platinum-resistant ovarian, primary peritoneal, or fallopian tube Cancer. J Clin Oncol. (2022) 40:2568–77. doi: 10.1200/JCO.21.01801
53. Duivelshof, BL, Murisier, A, Camperi, J, Fekete, S, Beck, A, Guillarme, D, et al. Therapeutic fc-fusion proteins: current analytical strategies. J Sep Sci. (2021) 44:35–62. doi: 10.1002/jssc.202000765
54. Jafari, R, Zolbanin, NM, Rafatpanah, H, Majidi, J, and Kazemi, T. Fc-fusion proteins in therapy: An updated view. Curr Med Chem. (2017) 24:1228–37. doi: 10.2174/0929867324666170113112759
55. Gameiro, SR, Strauss, J, Gulley, JL, and Schlom, J. Preclinical and clinical studies of bintrafusp alfa, a novel bifunctional anti-PD-L1/TGFβRII agent: current status. Exp Biol Med (Maywood). (2022) 247:1124–34. doi: 10.1177/15353702221089910
56. Oaknin, A, Ghamande, SA, Kasamatsu, Y, Gil-Martin, M, Grau-Bejar, JF, Garcia-Duran, C, et al. Phase I trial of first-line Bintrafusp alfa in patients with locally advanced or persistent/recurrent/metastatic cervical Cancer. Clin Cancer Res. (2024) 30:975–83. doi: 10.1158/1078-0432.CCR-23-1829
57. Birrer, M, Li, G, Yunokawa, M, Lee, JY, Kim, BG, Oppermann, CP, et al. Bintrafusp alfa for recurrent or metastatic cervical Cancer after platinum failure: a nonrandomized controlled trial. JAMA Oncol. (2024) 10:1204–11. doi: 10.1001/jamaoncol.2024.2145
58. Miller, KM, and Friedman, CF. Bifunctional blockade: a novel immunotherapy approach for cervical Cancer. Clin Cancer Res. (2022) 28:5238–40. doi: 10.1158/1078-0432.CCR-22-1779
59. Feng, J, Tang, D, Wang, J, Zhou, Q, Peng, J, Lou, H, et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGFβ, for recurrent or metastatic cervical Cancer: a clinical expansion cohort of a phase I study. Clin Cancer Res. (2022) 28:5297–305. doi: 10.1158/1078-0432.CCR-22-0346
60. Pitiot, A, Heuzé-Vourc'h, N, and Sécher, T. Alternative routes of Administration for Therapeutic Antibodies-State of the art. Antibodies (Basel). (2022) 11:56. doi: 10.3390/antib11030056
61. Viola, M, Sequeira, J, Seiça, R, Veiga, F, Serra, J, Santos, AC, et al. Subcutaneous delivery of monoclonal antibodies: how do we get there? J Control Release. (2018) 286:301–14. doi: 10.1016/j.jconrel.2018.08.001
62. Strickley, RG, and Lambert, WJ. A review of formulations of commercially available antibodies. J Pharm Sci. (2021) 110:2590–608.e56. doi: 10.1016/j.xphs.2021.03.017
63. Kobayakawa, T, Miyazaki, A, Saito, M, Suzuki, T, Takahashi, J, and Nakamura, Y. Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci Rep. (2021) 11:11801. doi: 10.1038/s41598-021-91248-6
64. Schultes, BC, Smith, LM, and Nicodemus, CF. Phase I study of abagovomab in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer. Clin Cancer Res. (2007) 13:4026–7. doi: 10.1158/1078-0432.CCR-06-2664
65. Pfisterer, J, du Bois, A, Sehouli, J, Loibl, S, Reinartz, S, Reuss, A, et al. The anti-idiotypic antibody abagovomab in patients with recurrent ovarian cancer. A phase I trial of the AGO-OVAR. Ann Oncol. (2006) 17:1568–77. doi: 10.1093/annonc/mdl357
66. Sabbatini, P, Harter, P, Scambia, G, Sehouli, J, Meier, W, Wimberger, P, et al. Abagovomab as maintenance therapy in patients with epithelial ovarian Cancer: a phase III trial of the AGO OVAR, COGI, GINECO, and GEICO—the MIMOSA study. J Clin Oncol. (2013) 31:1554–61. doi: 10.1200/JCO.2012.46.4057
67. Makker, V, Filiaci, VL, Chen, LM, Darus, CJ, Kendrick, JE, Sutton, G, et al. Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: an NRG oncology/gynecologic oncology group study 0229N. Gynecol Oncol. (2015) 138:24–9. doi: 10.1016/j.ygyno.2015.04.006
68. Burger, RA, Deng, W, Makker, V, Collins, Y, Gray, H, Debernardo, R, et al. Phase II evaluation of dalantercept in the treatment of persistent or recurrent epithelial ovarian cancer: An NRG oncology/gynecologic oncology group study. Gynecol Oncol. (2018) 150:466–70. doi: 10.1016/j.ygyno.2018.06.017
69. Tan, AR, Im, SA, Mattar, A, Colomer, R, Stroyakovskii, D, Nowecki, Z, et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. (2021) 22:85–97. doi: 10.1016/S1470-2045(20)30536-2
70. Awwad, S, and Angkawinitwong, U. Overview of antibody drug delivery. Pharmaceutics. (2018) 10:83. doi: 10.3390/pharmaceutics10030083
71. Bittner, B, and Schmidt, J. Advancing subcutaneous dosing regimens for biotherapeutics: clinical strategies for expedited market access. BioDrugs. (2024) 38:23–46. doi: 10.1007/s40259-023-00626-1
72. Hassan, R, Bera, T, and Pastan, I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. (2004) 10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801
73. Shen, J, Sun, X, and Zhou, J. Insights into the role of Mesothelin as a diagnostic and therapeutic target in ovarian carcinoma. Front Oncol. (2020) 10:1263. doi: 10.3389/fonc.2020.01263
74. Tang, Q, Chen, Y, Li, X, Long, S, Shi, Y, Yu, Y, et al. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. (2022) 13:964442. doi: 10.3389/fimmu.2022.964442
75. Lin, X, Kang, K, Chen, P, Zeng, Z, Li, G, Xiong, W, et al. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol Cancer. (2024) 23:108. doi: 10.1186/s12943-024-02023-w
76. Gonzalez, T, Muminovic, M, Nano, O, and Vulfovich, M. Folate receptor alpha-a novel approach to Cancer therapy. Int J Mol Sci. (2024) 25:1046. doi: 10.3390/ijms25021046
77. Scaranti, M, Cojocaru, E, Banerjee, S, and Banerji, U. Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol. (2020) 17:349–59. doi: 10.1038/s41571-020-0339-5
78. Zhu, K, Yang, X, Tai, H, Zhong, X, Luo, T, and Zheng, H. HER2-targeted therapies in cancer: a systematic review. Biomark Res. (2024) 12:16. doi: 10.1186/s40364-024-00565-1
79. Oh, DY, and Bang, YJ. HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol. (2020) 17:33–48. doi: 10.1038/s41571-019-0268-3
80. Chauvin, JM, and Zarour, HM. TIGIT in cancer immunotherapy. J Immunother Cancer. (2020) 8:e000957. doi: 10.1136/jitc-2020-000957
Keywords: Clinicaltrials.gov, antibodies, gynecology, antibody–drug conjugates, bispecific antibodies, Fc-fusion proteins, administration route
Citation: Zhou X, Xiao L, Lai F, Chen W, Zhou C, Deng Y, Wang T, Xing S, Diao H, Tang M, Guo W and Luo E (2025) Comprehensive overview of antibody drug-related clinical studies in gynecology: insights from ClinicalTrials.gov. Front. Med. 12:1521587. doi: 10.3389/fmed.2025.1521587
Edited by:
Sarah M. Cohen, Hadassah Medical Center, IsraelReviewed by:
Fabian Bernhard Thaddäus Kraus, Ludwig Maximilian University of Munich, GermanyMarco Cavaco, Universidade de Lisboa, Portugal
Wen-Wei Lin, Kaohsiung Medical University, Taiwan
Copyright © 2025 Zhou, Xiao, Lai, Chen, Zhou, Deng, Wang, Xing, Diao, Tang, Guo and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erdan Luo, bHVvZXJkYW5Ac2luYS5jbg==
 Xiaoling Zhou
Xiaoling Zhou Li Xiao2
Li Xiao2 Shasha Xing
Shasha Xing Haoyang Diao
Haoyang Diao Wenmei Guo
Wenmei Guo Erdan Luo
Erdan Luo