- 1Shenzhen Pingle Orthopedic Hospital (Shenzhen Pingshan Traditional Chinese Medicine Hospital), Shenzhen, Guangdong, China
- 2School of Public Health (Shenzhen), Sun Yat-sen University, Shenzhen, Guangdong, China
Objective: This study aimed to delineate the current trends and hotspots in autophagy research related to metabolic syndrome (MetS) and metabolic dysfunction-associated steatotic liver disease (MASLD), with the aim of guiding future investigations in this area.
Methods: This study extracted research on autophagy in MetS and MASLD from the Web of Science Core Collection (WoSCC) database. Review articles were systematically excluded to focus on original research contributions. A bibliometric analysis and visualization were conducted using VOSviewer 1.6.20, CiteSpace 6.3.R1, and R 4.3.3.
Results: The study included 1,114 articles from 1,220 institutions across 42 countries/regions, demonstrating a significant increase in research output from 2009 to 2024. China led with 506 publications, followed by the USA and Korea. The Egyptian Knowledge Bank constitutes a consortium of institutions operating within the national research framework, with one institution designated as the primary publishing entity. Notably, the journal Nature has emerged as the most frequently cited publication. Singh Rajat received the highest number of citations (3,610), while Marycz Krzysztof was the most prolific author. The most cited article, published in 2009, was titled “Autophagy regulates lipid metabolism.” Keyword trends have shifted from earlier topics such as “phosphorylation” and “gene-expression” to more recent terms like “lipid accumulation” and “mitophagy.” Burst keyword analysis indicated that “liver fibrosis,” “modulation,” “gut microbiota,” and “lipotoxicity” have emerged as significant topics.
Conclusions: This study has elucidated the protective role of autophagy in MASLD and MetS. Future research is anticipated to concentrate on the activation of autophagy in the context of natural product drug discovery, the exploration of underlying molecular mechanisms, the regulation of fatty acid metabolism, and the development of functional nutritional supplements, among other relevant areas.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) currently affects ~30% of the global population, translating to an estimated 314 million individuals (1–4). As the hepatic manifestation of metabolic syndrome (MetS) (5–7), MASLD is intricately linked to insulin resistance, obesity, and dyslipidemia, thereby representing a substantial public health concern (8–10). The disease spectrum ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), fibrosis, and hepatocellular carcinoma, all of which are influenced by factors such as dysregulated lipid metabolism, oxidative stress, inflammation, and mitochondrial dysfunction (9, 10).
Autophagy is a highly conserved cellular degradation process that is pivotal in mitigating the progression of MASLD by facilitating the clearance of lipid droplets, a process referred to as “macrolipophagy,” as well as damaged organelles. Furthermore, autophagy plays a critical role in suppressing oxidative stress and inflammation (11–13). Compromised autophagic flux in MASLD is significantly associated with increased hepatocyte apoptosis, thereby exacerbating the severity of the condition (14). Dysregulation of autophagy exacerbates hepatic lipid accumulation and insulin resistance, thereby contributing to complications associated with MetS (13). Recent advancements underscore the significance of autophagy modulation as a viable therapeutic strategy. Natural products, including polyphenols and saponins, along with pharmacological agents, target key pathways such as AMPK/mTOR, TFEB-mediated lysosomal biogenesis, and PINK1/Parkin-dependent mitophagy (15–21). For instance, quercetin has been shown to activate AMPK, thereby enhancing mitochondrial autophagy, while resveratrol mitigates endoplasmic reticulum (ER) stress via SIRT1 signaling (15–18). Moreover, TFEB agonists, such as formononetin, promote lipophagy, thereby providing valuable insights into the restoration of lipid homeostasis (19–21). Importantly, alongside natural and pharmacological compounds, clinical interventions aimed at promoting weight loss—such as dietary modifications and bariatric surgery—have demonstrated a significant capacity to enhance hepatic autophagic flux in individuals with MASLD (22, 23). Employing mathematical and statistical methodologies, bibliometric analysis yields insights into publication trends, predominant research domains, co-authorship dynamics, keyword frequency, and citation patterns over time. This approach facilitates a comprehensive overview of the academic landscape pertaining to autophagy in MASLD and MetS (24).
Bibliometric analyses elucidate the evolving research trends that underscore the significance of autophagy in MASLD and MetS, while concurrently identifying emerging therapeutic candidates. These investigations reveal an increasing interest in compounds such as berberine (which targets the SIRT1/AMPK axis), vitamin D (which promotes fatty acid β-oxidation), and probiotics (which modulate the gut-liver axis) (25–27). Nevertheless, substantial gaps persist in our comprehension of tissue-specific autophagy regulation and the translational potential of preclinical findings into clinical applications. This review synthesizes 15 years of research to delineate the landscape of MASLD/MetS and autophagy, highlighting pivotal discoveries, unresolved inquiries, and therapeutic innovations. By integrating mechanistic insights with bibliometric trends, we aim to steer future research toward targeted interventions for metabolic liver diseases.
2 Materials and methods
2.1 Data sources and search strategy
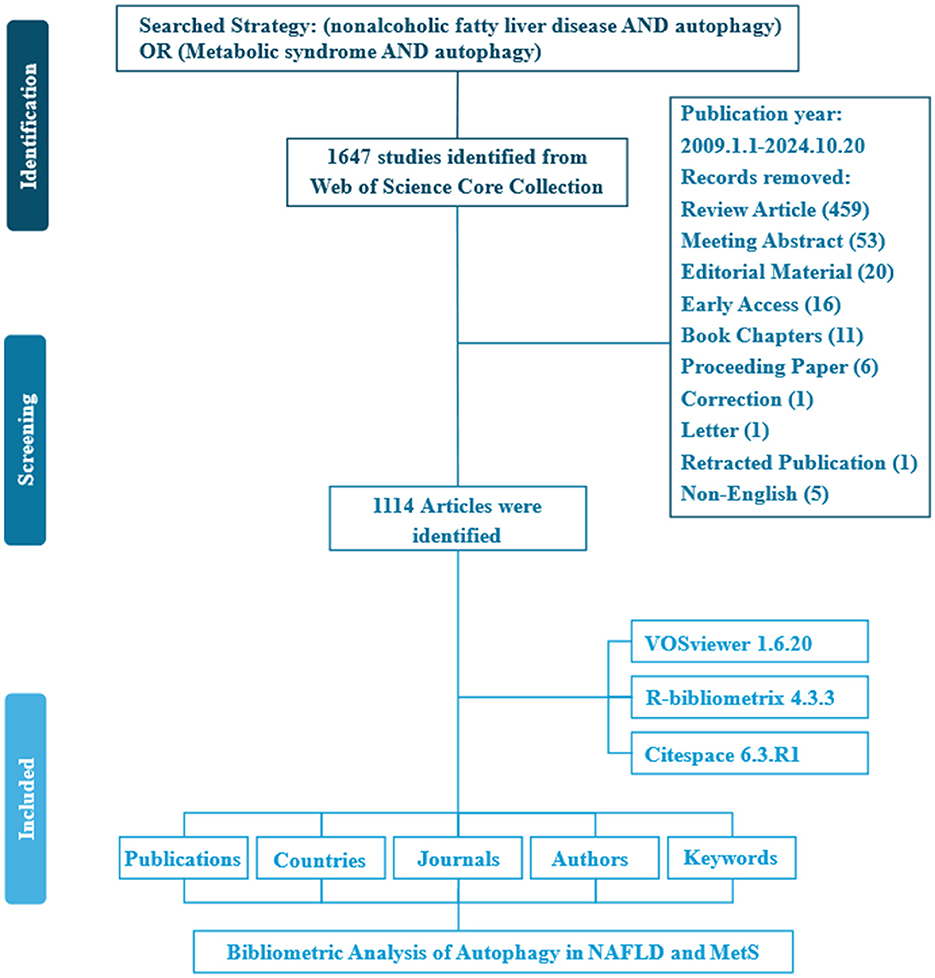
The literature search was conducted to retrieve relevant articles from the inception of the database until October 2024 from the Web of Science Core Collection (WoSCC) (28). The search strategy is delineated in Supplementary Table 1. This study included exclusively “articles” and considered only documents written in English. Given that all data were obtained from a publicly accessible database, ethical declarations or approvals are not necessary.
2.2 Data analysis and visualization
We extracted relevant data from the retrieved article titles and used Microsoft Excel 365 to identify and calculate bibliometric parameters. These metrics cover key aspects of articles, including the number of publications per year, citation frequency, average citation fre-quency, journal title, journal impact factor, country/region of article, publishing institutions, and authors. The visualization and analysis process involved the use of three powerful bibliometric analysis tools to fully analyze the academic data: VOSviewer 1.6.20, CiteSpace 6.3.R1, and R bibliometrix package 4.3.3. VOSviewer is a multifaceted software tool that is instrumental in mapping institutional collaborations, co-authorships, citations, and co-citations (29). It was employed to perform keyword co-occurrence analysis. CiteSpace 6.3.R1 was used for country, institution, and author, the intermediary centrality of keyword emergence detection and co-occurrence analysis (30), with the parameters set to time slicing: from January 2009 to October 2024 (research in this field was originally published in 2009). Network pruning techniques, including pathfinder and clip merge, enhanced the visualizations and improved their interpretability. This refinement enabled the identification of significant shifts in research focus and underscored emerging areas of interest in autophagy related to MetS and MASLD research. Furthermore, the R-based bibliometric package “bibliometrix” was employed for a thorough evaluation of research output, global distributions, and the performance metrics of authors and journals (31). Metrics such as the h-index, g-index, and m-index were calculated to assess the academic impact of authors and institutions (32, 33). Journal performance was evaluated utilizing the most recent 2024 data from Journal Citation Reports (JCR) quartiles and Impact Factors (IF), thereby ensuring timely assessments. These metrics provided critical insights into the academic influence and prestige of journals within the field. By integrating these analytical methodologies, this study presents a systematic overview of research pertaining to autophagy in MetS and MASLD, emphasizing significant contributors, leading institutions, and emerging research trends. The findings offer invaluable guidance for future investigations and promote collaborative opportunities aimed at enhancing the understanding and treatment of MetS and MASLD.
3 Results
3.1 Overview of main findings
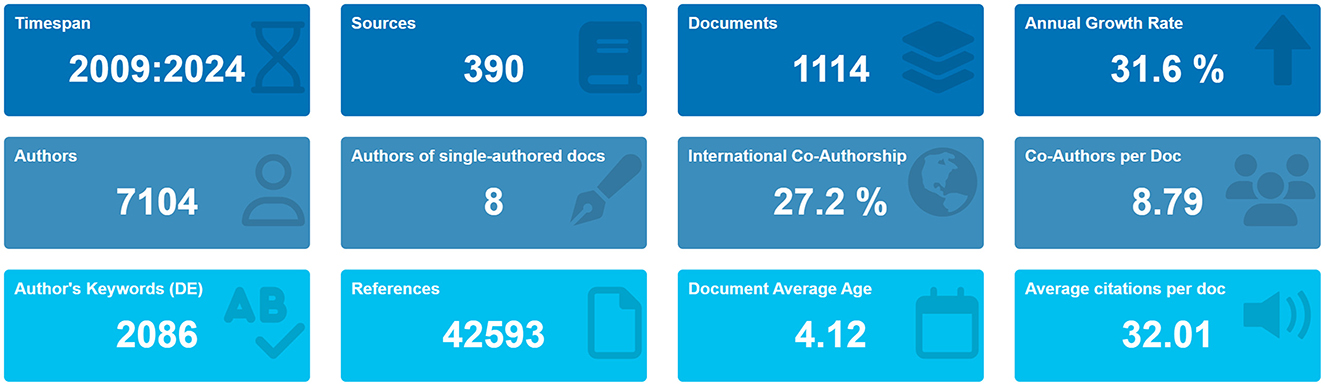
A total of 1,647 studies were retrieved from the WoSCC database. To concentrate on original research contributions, review articles (designated as “review” in WoSCC) were systematically excluded. Consequently, 459 review articles were removed during the screening process. The study flowchart is illustrated in Figure 1. Over the past 15 years, a total of 1,114 articles were contributed by 7,104 authors from 1,220 institutions across 42 countries, and these articles appeared in 390 distinct journals. Collectively, these publications cited 42,593 references, demonstrating a robust engagement with the existing body of literature. The average age of the documents was 4.12 years, with each article receiving an average of 32.01 citations, underscoring the enduring relevance and impact of research in this field (Figure 2).
3.2 Annual publication trends
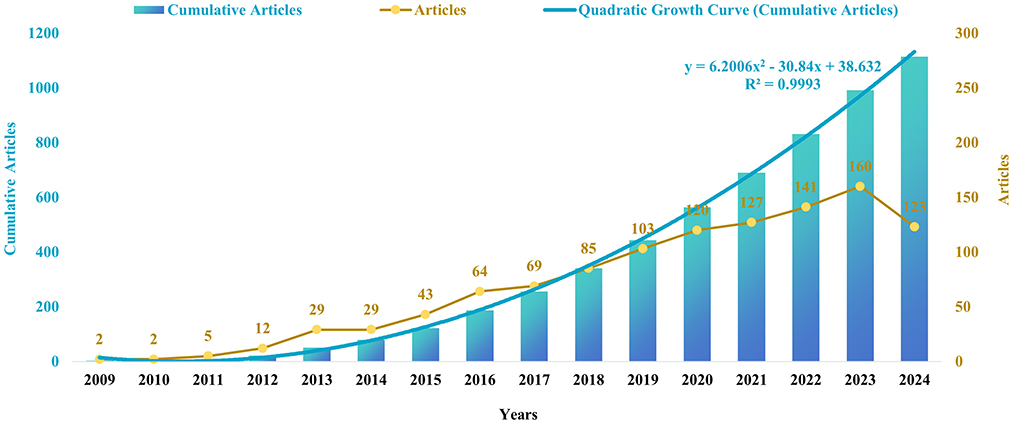
To gain insight into the evolution of research in this field, we conducted an analysis of annual publication trends. Our study period indicated a pronounced upward trajectory in both annual and cumulative publications, particularly since 2010. The increase in cumulative pub-lications over time is consistent with the quadratic growth equation y = 6.2006 x2-30.84 x+38.632, which exhibits a coefficient of determination (R2) of 0.9993 and an annual growth rate of 31.60%. Notably, the year 2023 recorded the highest number of publications, accounting for 14.36% of the total (Figures 2, 3).
3.3 Analysis of countries
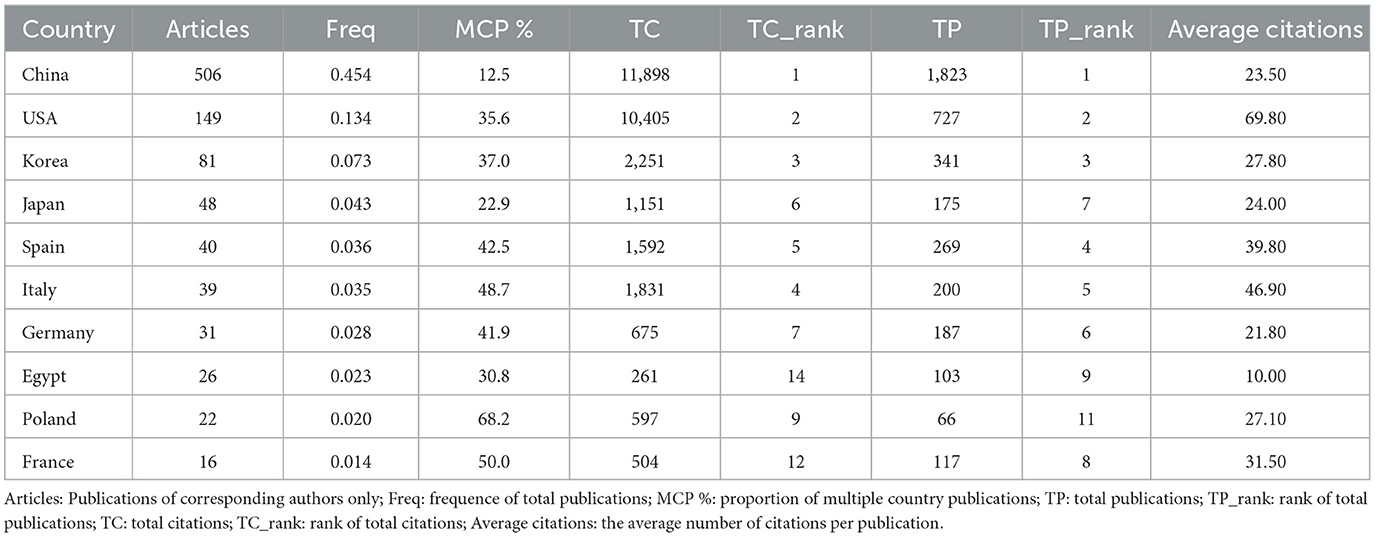
The identified publications originated from 42 countries, with China leading with 506 articles, accounting for 45.40% of all articles. Other significant contributors included the USA (149 articles), Korea (81 articles), Japan (48 articles), Spain (40 articles), and Italy (39 articles; Figure 4A, Table 1). Despite China's dominance in total publication volume, the USA, Canada, and Singapore exhibited the highest average citation rates, with averages of 69.80, 53.50, and 47.90, respectively. Seven countries demonstrated high betweenness centrality (>0.1), ranked in descending order as India, Australia, the USA, Italy, Spain, England, and Brazil (Figures 4C, D). Furthermore, country collaborations were visualized using VOSviewer, indicating that the USA, China, and Italy constituted the most robust international collaboration network (Figure 4B).
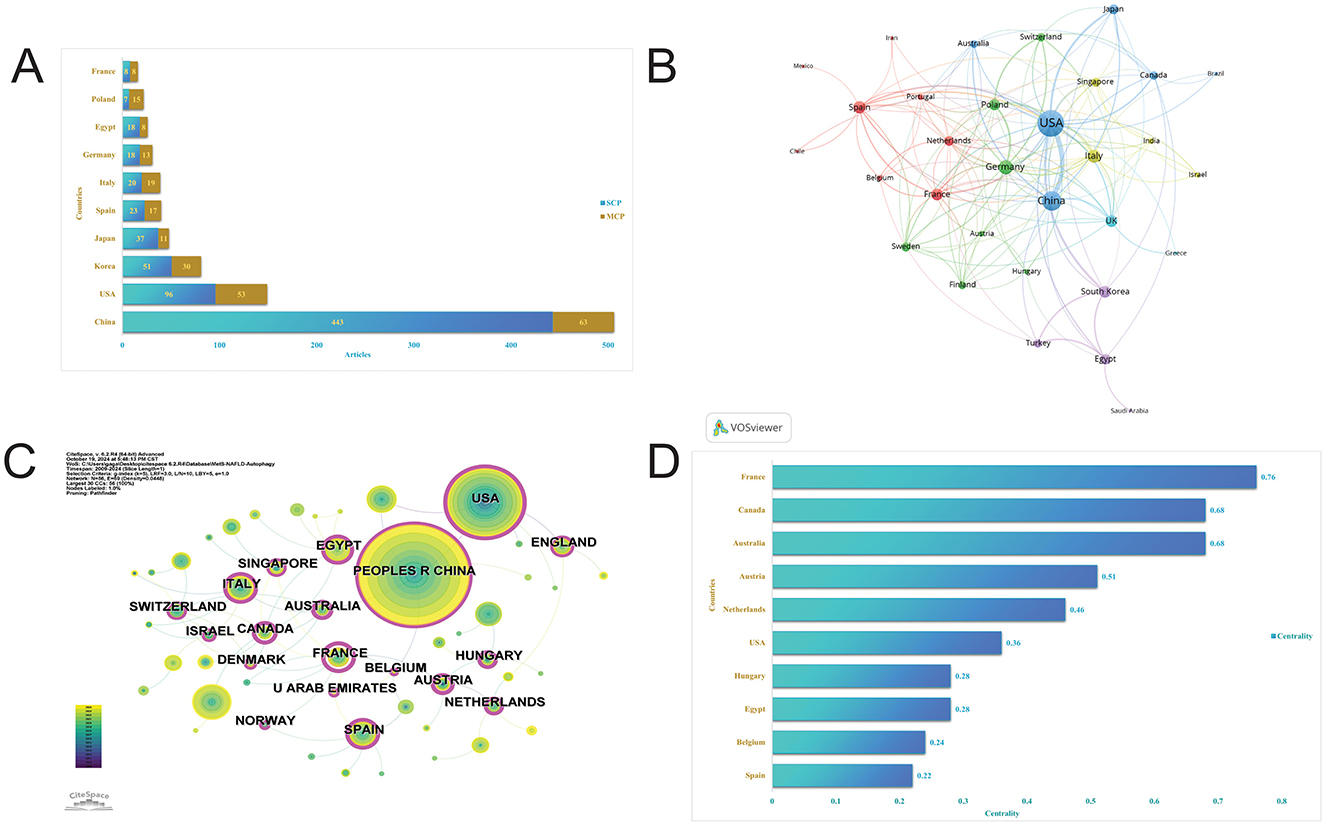
Figure 4. Visualization of countries. (A) Articles by countries. (B) International collaboration network of countries by VOS viewer. (C) International collaboration network of countries by CiteSpace. (D) Intermediary centrality of countries.
3.4 Analysis of institutions
Research publications on autophagy in MASLD and MetS involved a total of 1,220 institutions. The institutions with the highest number of publications were the Egypt Knowledge Bank (Egypt, 93 articles), HuaZhong University of Science and Technology (China, 56 articles), and Yonsei University (South Korea, 53 articles; Figure 5A). The analysis of collaborative networks focused on institutions with a minimum of 10 articles and was visualized using VOSviewer. Clusters were color-coded to reflect the frequency of collaboration among institutions (Figure 5B). Notably, the Chinese Academy of Sciences exhibited the largest node, indicating the highest level of collaboration with other institutions. Furthermore, Figures 5C, D illustrate the significance of institutions operating as intermediaries. The Albert Einstein College of Medicine possesses a betweenness centrality score of 0.34, positioning it as a vital hub for cross-regional collaboration. This status is further augmented by the Centro de Investigación Biomecánica en Red de Diabetes y Enfermedades Metabólicas, which has a betweenness centrality score of 0.25.
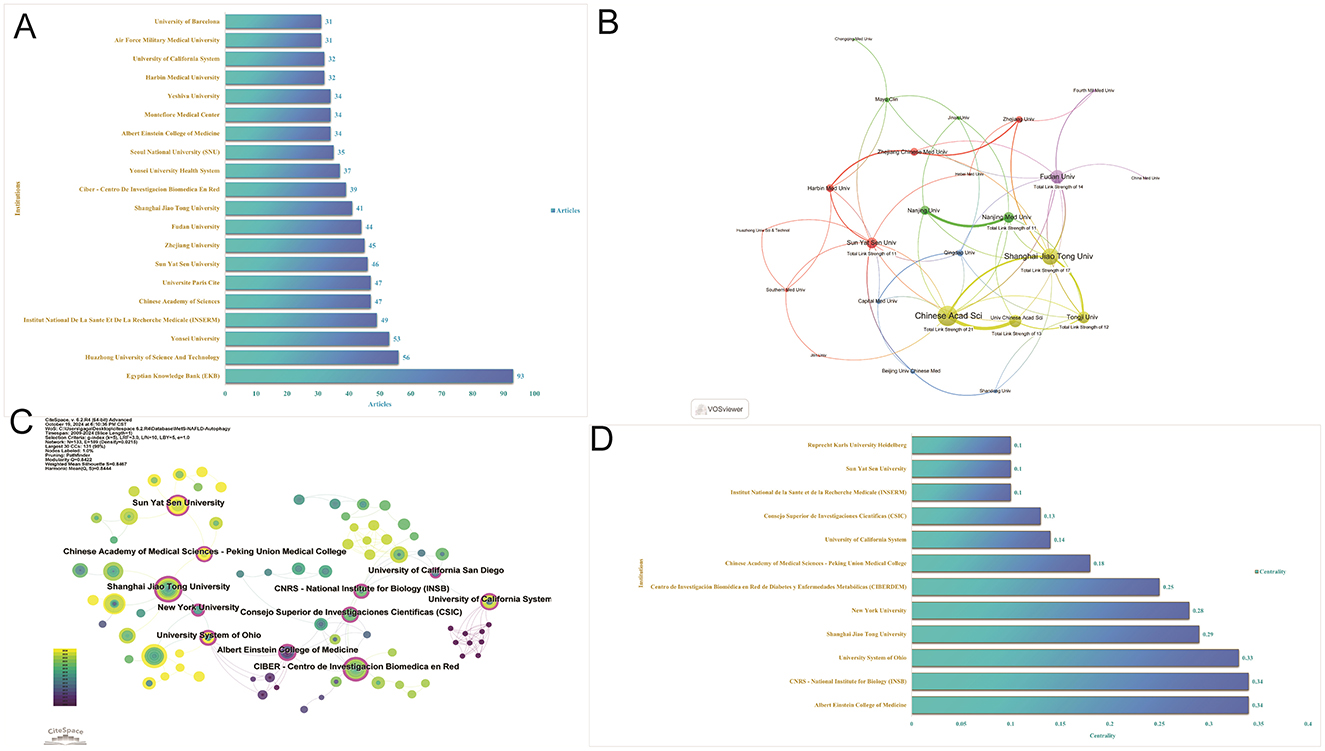
Figure 5. Visualization of institutions. (A) Articles of institutions. (B) Collaborative networks of institutions by VOS viewer. (C) Collaborative networks of institutions by CiteSpace. (D) Intermediary centrality of institutions.
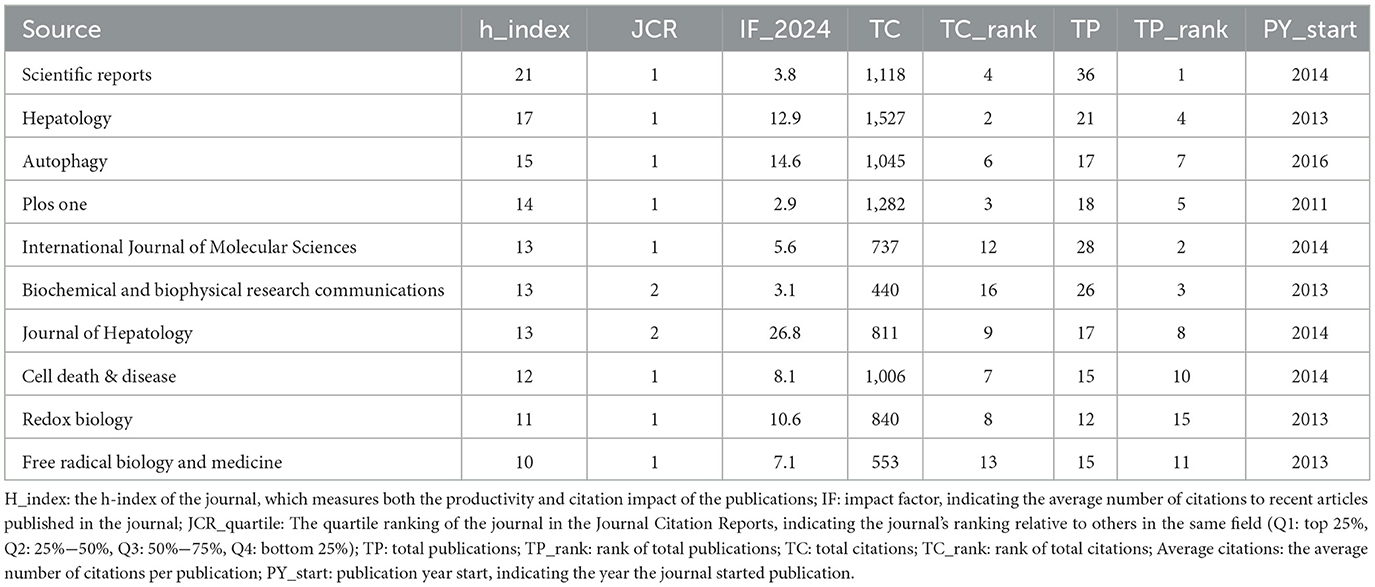
3.5 Analysis of journals
Research on autophagy in MASLD and MetS is significantly represented across 390 academic journals. Scientific Reports leads this body of work with 36 articles, which constitutes 3.23% of the overall total. This is succeeded by the International Journal of Molecular Sciences and Biochemical and Biophysical Research Communications, which have published 28 and 26 articles, respectively, representing 2.51 and 2.33% of the total (Table 2). Co-citation analysis has identified the five key journals with the highest total link strength as follows: Metabolism: Hepatology (118), Cell Death & Disease (114), Autophagy (77), International Journal of Molecular Sciences (77), and Biochemical and Biophysical Research Communications (76) (Figure 6A). Similarly, bibliographic coupling analysis has revealed the five key journals with the highest total link strength: Scientific Reports (7,068), International Journal of Molecular Sciences (5,931), Hepatology (5,535), Autophagy (4,912), and Biochemical and Biophysical Research Communications (4,691; Figure 6B).
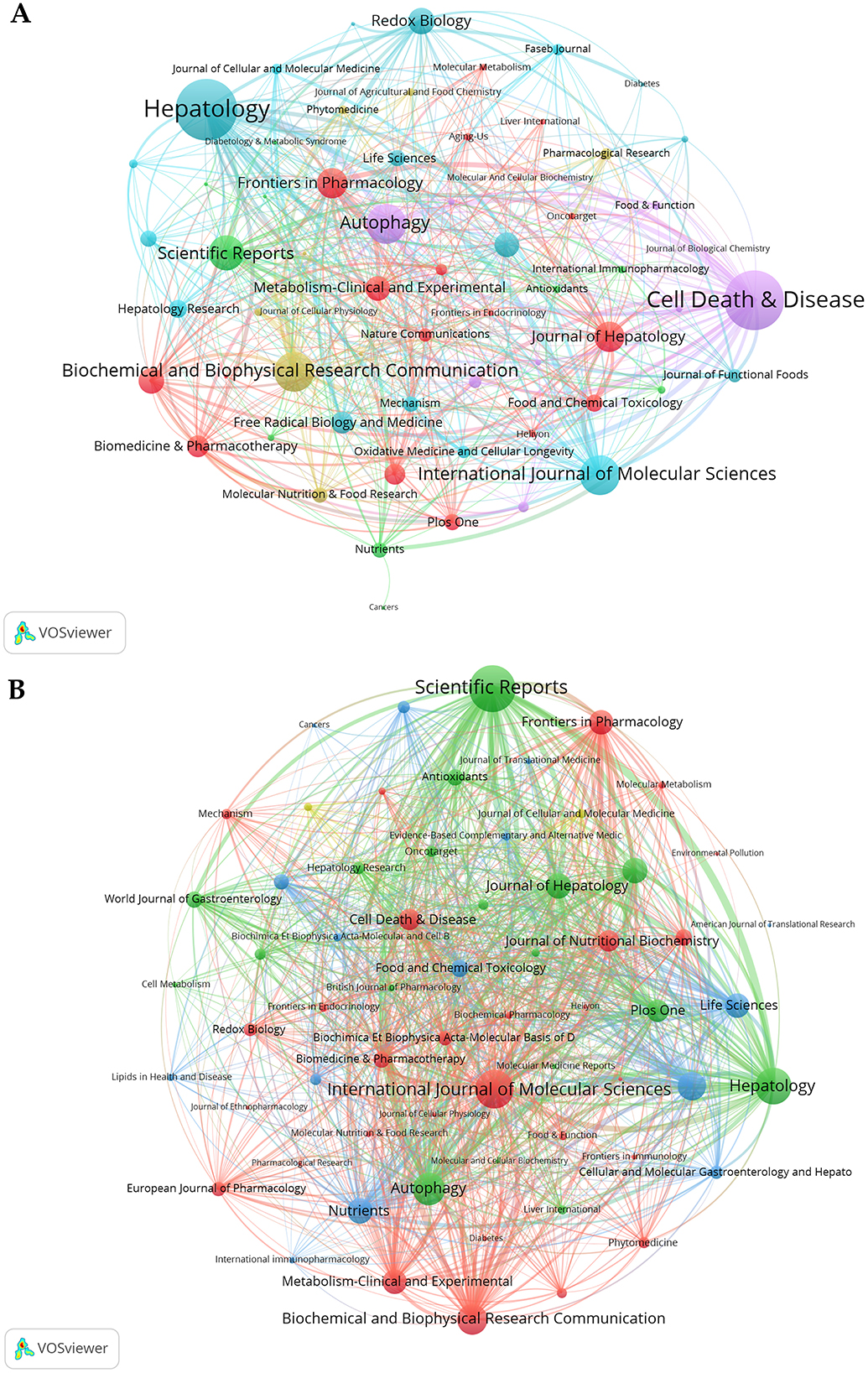
Figure 6. Co-occurrence and bibliographic coupling analysis. (A) The co-occurrence networks of journals. (B) The bibliographic coupling networks of journals.
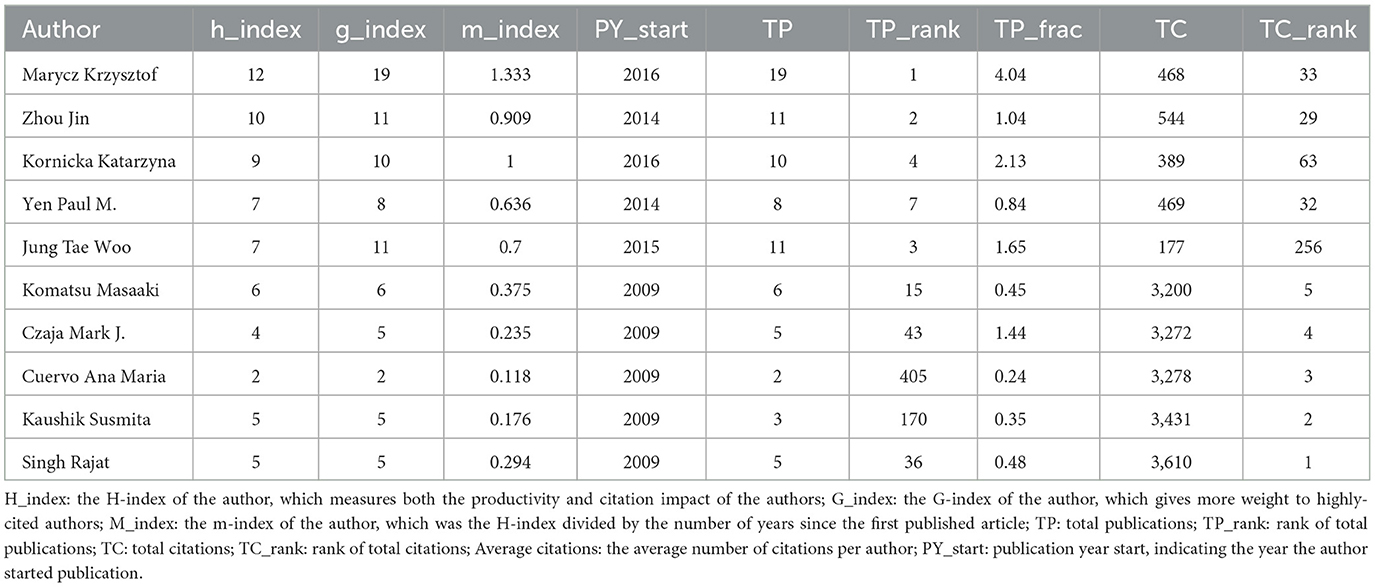
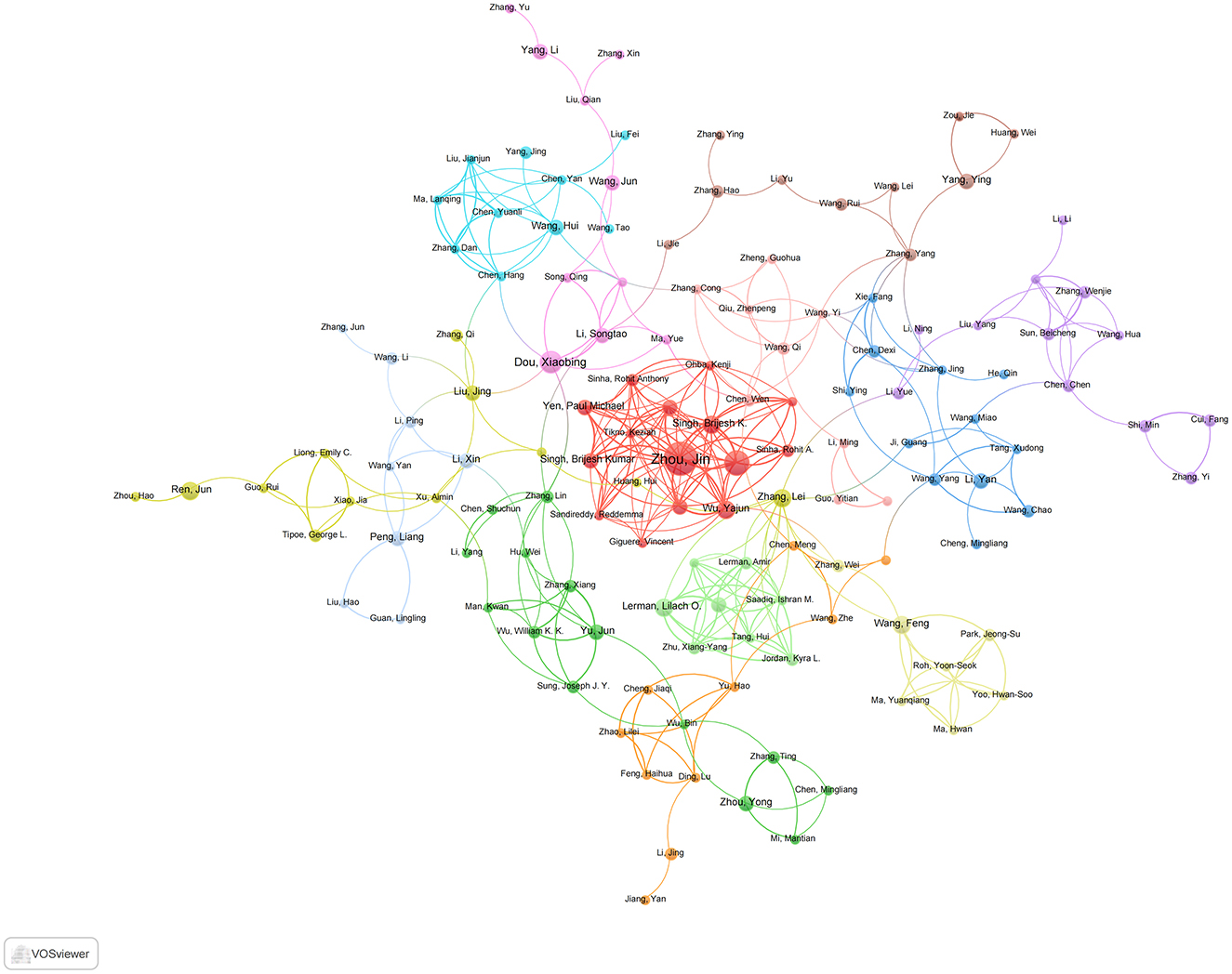
3.6 Analysis of authors and collaborations
A total of 1,114 articles were authored by 8,134 contributors, indicating a broad distribution of authorship and moderate levels of scholarly collaboration. As illustrated in Table 3, authors are ranked according to their h-index (top five) and total citations (bottom five in reverse order). Krzysztof Marycz is identified as the most impactful contributor, with an h-index of 12, closely followed by Jin Zhou, who possesses an h-index of 10. Additionally, Zhou Jin exhibited the highest degree of collaborative engagement in the network analysis, achieving a total link strength of 52, surpassing Yen Paul M. (33) and Singh Brijesh K./Wu Yajun (31) (Figure 7). In terms of citation leadership, Singh Rajat, Kaushik Susmita, Ana María Cuervo, Komatsu Masaaki, and Czaja Mark J. are particularly notable, primarily due to their seminal 2009 Nature paper, “Autophagy regulates lipid metabolism,” which has been cited 3,003 times as of November 2024 and has significantly influenced this research domain. This contrast between productivity metrics (h-index) and citation impact highlights the enduring significance of foundational studies in comparison to contemporary research output.
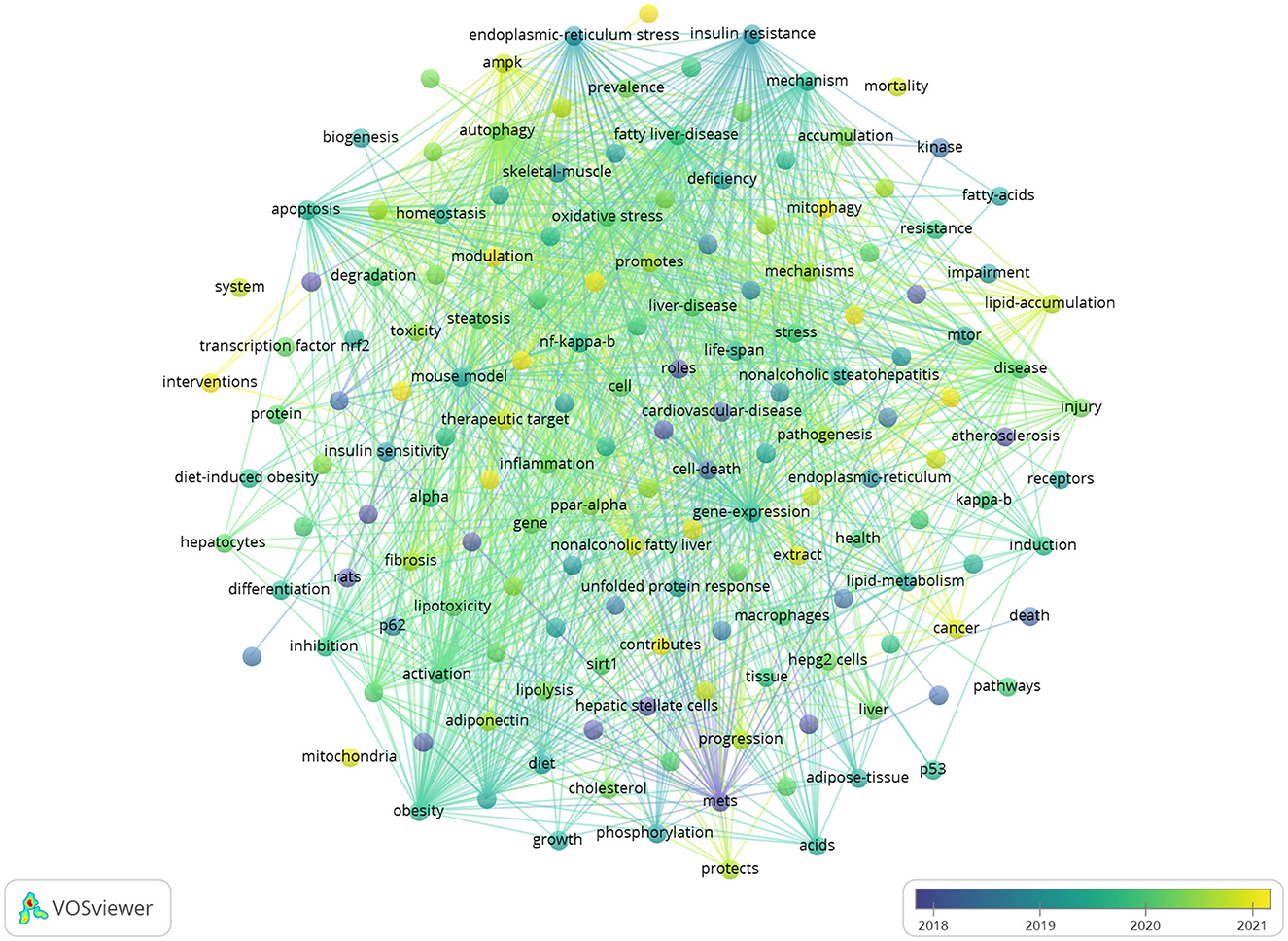
3.7 Analysis of research hotspots and frontiers
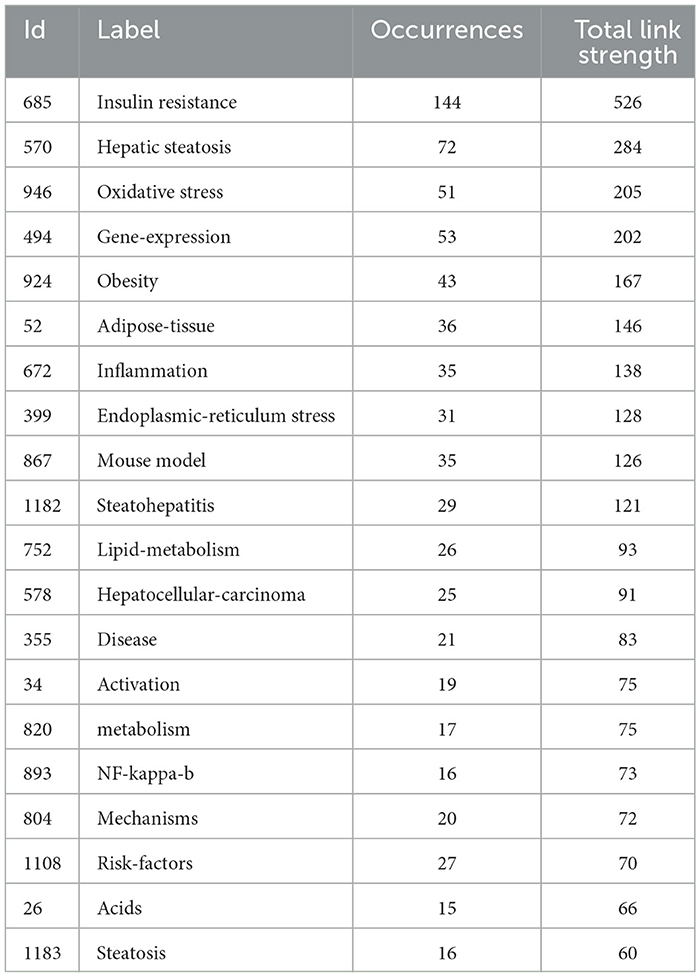
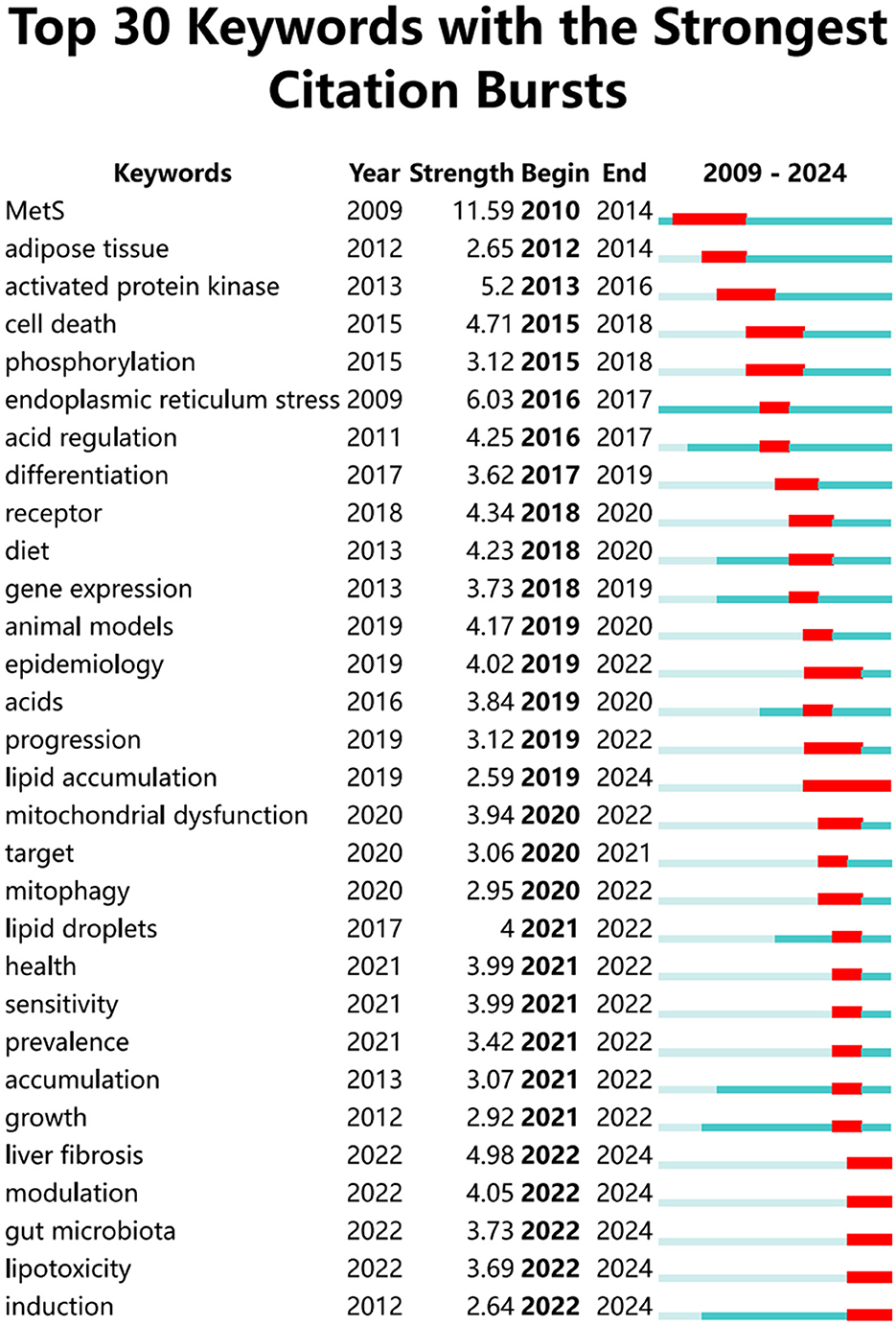
Keywords succinctly encapsulate the fundamental concepts of a paper, outlining the key areas of research interest. A comprehensive keyword analysis on the selected articles was performed using “Author Keywords” from the Biblioshiny application and “Keywords Plus” provided by the VOSviewer application. In total, 130 keywords with a minimum of 10 occurrences were identified in 2,337 keywords. However, upon comparing the results from these two sources, “Keywords Plus” was observed to provide more accurate results, making it the primary data source for the analysis. A network visualization map demonstrating the connections among these keyword co-occurrences was generated using VOSviewer. The sizes of the circles correspond to the frequency of occurrence of the keywords. A co-occurrence word analysis revealed that “insulin resistance,” “hepatic steatosis,” “oxidative stress,” “gene expression,” and “obesity” were the most frequently co-occurring keywords (Figure 8, Table 4). Figure 9 presents the top 30 keywords with the highest burst strengths. The most significant citation burst belongs to”MetS.” Particularly noteworthy is the concentration of keywords such as “Lipid accumulation,” “endoplasmic reticulum stress,” “liver fibrosis,” “modulation,” “gut microbiota,” “lipotoxicity,” “induction,” since 2022, indicating promising developments.
4 Discussion
4.1 Overview publications analysis
Since 2009, studies on autophagy between MASLD and MetS have experienced rapid growth, particularly after 2014, driven by their pivotal biological roles in metabolic regulation mechanism research. It is evident that autophagy have gradually emerged as a hotspot in MASLD and MetS, indicated by an average citation of 32.01 per article. Additionally, the number of articles on autophagy in MASLD and MetS has steadily increased, with an annual growth rate of 31.60%.
4.2 Country, institution, journal, literature analysis
The countries with the highest publication volumes are primarily China, the USA, and Korea. China ranks first in total publications, while the USA and Canada exhibit the highest average citation rates, all exceeding 50. France demonstrates the greatest intermediary centrality, underscoring its active and prominent role in this field. Despite China possessing the highest total citations, it faces a relatively low average citation frequency of 23.5, suggesting a need for enhanced research quality, influence, and academic exchange among Chinese authors, thereby emphasizing the importance of publishing high-quality research. Notably, the three leading institutions contributing to publication volume are the Egyptian Knowledge Bank, Huazhong University of Science and Technology, and Yonsei University, indicating their pioneering roles in advancing autophagy-related research in MASLD and MetS. Albert Einstein College of Medicine and CNRS-National Institute for Biology (INSB) exhibit the highest intermediary centrality, serving as crucial contributors to fundamental autophagy research within these areas. The three most cited articles received 2,987, 733, and 729 citations, respectively, and were published in Nature (IF: 50.5), Comprehensive Physiology (IF: 4.9), and Nature Cell Biology (IF: 17.3) (13, 34, 35). All three articles focused on the mechanisms by which autophagy reg-ulates MASLD, highlighting the critical need for a mechanistic analysis of this metabolic dis-ease.
4.3 Key words time series analysis
Between 2009 and 2020, research keywords associated with autophagy in MASLD and MetS predominantly focused on terms such as “adipose tissue,” “activated protein kinase,” “cell death,” “phosphorylation,” “endoplasmic reticulum stress,” “acid regulation,” “receptor,” “differentiation,” “gene expression,” “animal models,” “epidemiology,” “progression,” and “lipid accumulation.” This trend signifies that comprehensive investigations have been con-ducted from multiple perspectives, incorporating intracellular mechanisms—such as signal transduction and cellular fate—as well three various research methodologies, including animal models, population studies, and the regulation of gene expression.
Since 2020, research on keywords related to autophagy in MASLD and MetS has in-creasingly centered on terms such as “health,” “mitochondrial dysfunction,” “target,” “mitophagy,” “lipid droplets,” “sensitivity,” “prevalence,” “accumulation,” “growth,” “liver fibrosis,” “modulation,” “gut microbiota,” “lipotoxicity,” and “induction.” This shift indicates a deeper exploration of cellular and subcellular structures, disease progression, autophagy regulation and intervention, as well as the relationship between gut microbiota and autophagy, including its induction. Moreover, keywords such as “lipid accumulation,” “liver fibrosis,” “modulation,” “gut microbiota,” “lipotoxicity,” and “induction” continue to gain prominence through 2024, underscoring the critical public health need for a more profound understanding of the mechanisms of autophagy in MASLD and MetS.
From 2010 to 2019, the keywords pertinent to this research encompassed intracellular mechanisms, research methodologies, gene expression regulation, and foundational multidi-mensional explorations of disease manifestation. In contrast, from 2020 to 2024, the focus has shifted to more nuanced areas, including cellular architecture, disease progression, autophagy regulation, gut microbiota, and disease susceptibility, alongside an increased emphasis on clinical applications. These findings suggest that autophagy may be closely linked to the search for new therapeutic targets and treatments, such as halting disease progression through the regulation of autophagy or improving disease conditions by indirectly influencing autophagy through the modulation of gut flora. Consequently, based on this bibliometric analysis, studies of autophagy in MASLD and MetS are likely to continue enhancing our understanding of their roles in the development of metabolic diseases and their potential as therapeutic targets.
4.4 Summary of autophagy in MASLD and MetS
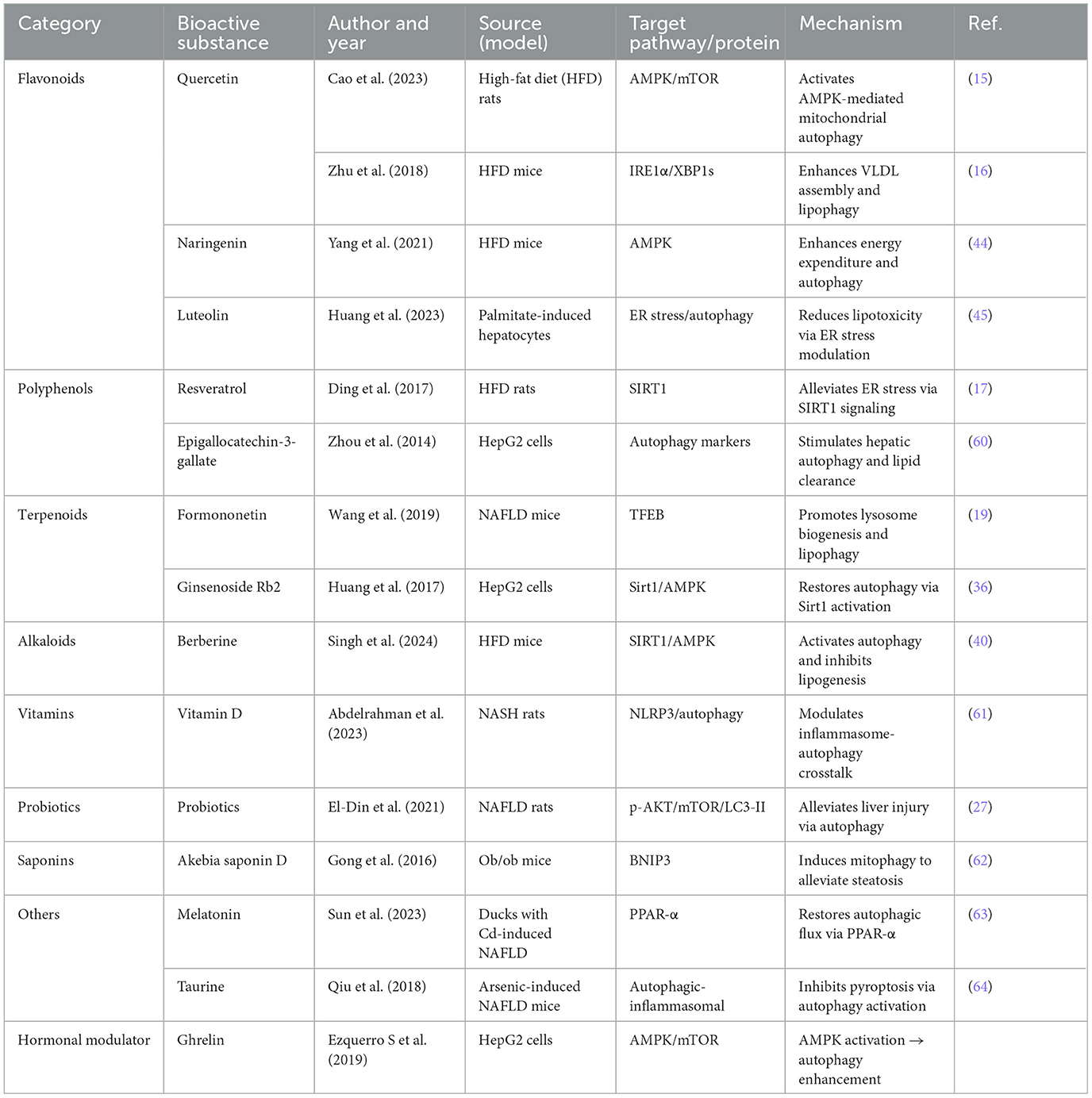
MASLD encompasses a spectrum of liver disorders, ranging from steatosis to fibrosis and hepatocellular carcinoma. The pathogenesis of MASLD is primarily attributed to dysregulated lipid metabolism, oxidative stress, inflammation, and impaired autophagy (3, 8). Novel therapeutic approaches are being developed to restore autophagy and mitochondrial function while simultaneously addressing inflammatory and metabolic pathways. Natural products, including polyphenols [e.g., quercetin (15, 16), resveratrol (17, 18)], have been shown to promote AMPK-mediated autophagy and TFEB-driven lipophagy (19–21). Additionally, saponins [e.g., ginsenosides (36–39)] and terpenoids [e.g., andrographolide (40), cimigenol (41)] modulate SIRT1/AMPK signaling and enhance mitochondrial quality. Alkaloids [e.g., berberine (25, 42)] and flavonoids [e.g., naringin (43, 44), apigenin (45–47)] attenuate lipogenesis through AMPK activation and the modulation of endoplasmic reticulum (ER) stress. Moreover, additional agents such as probiotics (27), omega-3 polyunsaturated fatty acids (PUFAs) (48), and vitamin D (26) contribute to the amelioration of gut-liver axis dysregulation and oxidative stress. Key therapeutic targets identified in the literature include AMPK/mTOR, which serve as critical regulators of autophagy (18, 25, 49–54); TFEB, involved in lysosomal biogenesis (55, 56); the NLRP3 inflammasome, which is implicated in inflammatory processes (57); and PPARs, which play a pivotal role in lipid metabolism (58, 59). The gut hormone ghrelin enhances hepatic autophagy flux through the activation of AMPK signaling, thereby mitigating lipid accumulation and hepatocyte apoptosis in MASLD (14). This pathway presents a promising endogenous mechanism for the restoration of metabolic homeostasis. These findings highlight the potential of bioactive compounds that enhance autophagy and specifically target various pathways to mitigate the progression of MASLD (Table 5).
4.5 Strengths and limitations
This bibliometric analysis represents the inaugural examination of the research status and trends concerning autophagy in MetS and MASLD over the past 15 years. This study offers scholars a comprehensive overview and stimulates new research inquiries, thereby fostering academic advancement within this domain. However, it is essential to acknowledge the limi-tations inherent in this study. Firstly, the analysis is restricted to English-language article sourced from the WoSCC database spanning the years 2009 to 2024, which may lead to potential gaps in the data available for analysis. Secondly, this investigation specifically retrieved publications associated with “autophagy,” MetS and MASLD, which may not capture the en-tirety of research conducted on autophagy in relation to MASLD and MetS. Nevertheless, the WoSCC database is recognized for housing some of the world's most influential and esteemed academic journals. The 1,114 articles analyzed in this study serve to mitigate these biases and provide an effective representation of the global status of research in this area.
5 Conclusion and outlook
This pioneering study provides a comprehensive analysis of the research trajectory and future prospects of autophagy in MASLD and MetS. There has been a significant increase in the number of publications in this area from 2009 to 2024. China has emerged as a leading contributor to this body of research, while France is recognized as the most influential country in the field. Enhanced global collaboration between China and France is essential to facilitate more extensive research. An analysis of journals indicates that autophagy research in MASLD and MetS constitutes a central focus within liver disease research, with prominent journals such as Scientific Reports and Hepatology playing a pivotal role in advancing the field. Keyword co-occurrence analysis reveals that the primary areas of focus include mitophagy, endoplasmic reticulum stress, insulin resistance, and other metabolic disorders. Notably, the interactions among mitophagy, lipid accumulation, oxidative stress, and inflammation are key topics of investigation. Current research on underlying mechanisms primarily revolves around the NF-kB and AMPK pathways, while cutting-edge studies are exploring avenues such as PI3K, NRF2, and mTOR. These findings provide timely insights that may inform therapeutic approaches for managing fatty liver disease and assist researchers in selecting appropriate journals for publi-cation, identifying potential collaborators, and remaining abreast of current trends and devel-opments in the field, thereby advancing overall knowledge.
Author contributions
XLiu: Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. XLi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1525526/full#supplementary-material
References
1. Gu S, Qiao Y, Liu S, Yang S, Cong S, Wang S, et al. Frontiers and hotspots of adipose tissue and NAFLD: a bibliometric analysis from 2002 to 2022. Front Physiol. (2023) 14:1278952. doi: 10.3389/fphys.2023.1278952
2. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. (2018) 69:896–904. doi: 10.1016/j.jhep.2018.05.036
3. Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. (2021) 397:2212–24. doi: 10.1016/S0140-6736(20)32511-3
4. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. (2023) 77:1335–47. doi: 10.1097/HEP.0000000000000004
5. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. (2001) 50:1844–50. doi: 10.2337/diabetes.50.8.1844
6. Yki-Jarvinen H. Fat in the liver and insulin resistance. Ann Med. (2005) 37:347–56. doi: 10.1080/07853890510037383
7. Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. (2008) 14:185–92. doi: 10.3748/wjg.14.185
8. Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. (2014) 2:901–10. doi: 10.1016/S2213-8587(14)70032-4
9. Rinaldi L, Pafundi PC, Galiero R, Caturano A, Morone MV, Silvestri C, et al. Mechanisms of non-alcoholic fatty liver disease in the metabolic syndrome. A narrative review. Antioxidants. (2021) 10:270. doi: 10.3390/antiox10020270
10. Karajamaki AJ, Bloigu R, Kauma H, Kesaniemi YA, Koivurova OP, Perkiomaki J, et al. Non-alcoholic fatty liver disease with and without metabolic syndrome: different long-term outcomes. Metabolism. (2017) 66:55–63. doi: 10.1016/j.metabol.2016.06.009
11. Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. (2007) 8:931–7. doi: 10.1038/nrm2245
12. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. (2009) 43:67–93. doi: 10.1146/annurev-genet-102808-114910
13. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. (2009) 458:1131–5. doi: 10.1038/nature07976
14. Ezquerro S, Mocha F, Fruhbeck G, Guzman-Ruiz R, Valenti V, Mugueta C, et al. Ghrelin reduces TNF-alpha-induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab. (2019) 104:21–37. doi: 10.1210/jc.2018-01171
15. Cao P, Wang Y, Zhang C, Sullivan MA, Chen W, Jing X, et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J Nutr Biochem. (2023) 120:109414. doi: 10.1016/j.jnutbio.2023.109414
16. Zhu X, Xiong T, Liu P, Guo X, Xiao L, Zhou F, et al. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem Toxicol. (2018) 114:52–60. doi: 10.1016/j.fct.2018.02.019
17. Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE. (2017) 12:e0183541. doi: 10.1371/journal.pone.0183541
18. Song J, Huang Y, Zheng W, Yan J, Cheng M, Zhao R, et al. Resveratrol reduces intracellular reactive oxygen species levels by inducing autophagy through the AMPK-mTOR pathway. Front Med. (2018) 12:697–706. doi: 10.1007/s11684-018-0655-7
19. Wang Y, Zhao H, Li X, Wang Q, Yan M, Zhang H, et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J Nutr Biochem. (2019) 73:108214. doi: 10.1016/j.jnutbio.2019.07.005
20. Zhang H, Lu J, Liu H, Guan L, Xu S, Wang Z, et al. Ajugol enhances TFEB-mediated lysosome biogenesis and lipophagy to alleviate non-alcoholic fatty liver disease. Pharmacol Res. (2021) 174:105964. doi: 10.1016/j.phrs.2021.105964
21. Zhou W, Yan X, Zhai Y, Liu H, Guan L, Qiao Y, et al. Phillygenin ameliorates nonalcoholic fatty liver disease via TFEB-mediated lysosome biogenesis and lipophagy. Phytomedicine. (2022) 103:154235. doi: 10.1016/j.phymed.2022.154235
22. Ezquerro S, Mendez-Gimenez L, Becerril S, Moncada R, Valenti V, Catalan V, et al. Acylated and desacyl ghrelin are associated with hepatic lipogenesis, beta-oxidation and autophagy: role in NAFLD amelioration after sleeve gastrectomy in obese rats. Sci Rep. (2016) 6:39942. doi: 10.1038/srep39942
23. Xu C, Markova M, Seebeck N, Loft A, Hornemann S, Gantert T, et al. High-protein diet more effectively reduces hepatic fat than low-protein diet despite lower autophagy and FGF21 levels. Liver Int. (2020) 40:2982–97. doi: 10.1111/liv.14596
24. Yuan ZZ, Fan YZ, Cheng SJ, Wei FJ, Gao J, Wang CX, et al. A bibliometric analysis of hydrogel research in various fields: the trends and evolution of hydrogel application. J Nanobiotechnology. (2025) 23:70. doi: 10.1186/s12951-025-03090-x
25. Sharma A, Anand SK, Singh N, Dwarkanath A, Dwivedi UN, Kakkar P. Berbamine induced activation of the SIRT1/LKB1/AMPK signaling axis attenuates the development of hepatic steatosis in high-fat diet-induced NAFLD rats. Food Funct. (2021) 12:892–909. doi: 10.1039/D0FO02501A
26. Du T, Xiang L, Zhang J, Yang C, Zhao W, Li J, et al. Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and beta-oxidation. Front Endocrinol. (2023) 14:1138078. doi: 10.3389/fendo.2023.1138078
27. Seif El-Din SH, Salem MB, El-Lakkany NM, Hammam OA, Nasr SM, Okasha H, et al. Early intervention with probiotics and metformin alleviates liver injury in NAFLD rats via targeting gut microbiota dysbiosis and p-AKT/mTOR/LC-3II pathways. Hum Exp Toxicol. (2021) 40:1496–509. doi: 10.1177/0960327121999445
28. Wu F, Gao J, Kang J, Wang X, Niu Q, Liu J, et al. Knowledge mapping of exosomes in autoimmune diseases: a bibliometric analysis (2002-2021). Front Immunol. (2022) 13:939433. doi: 10.3389/fimmu.2022.939433
29. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
30. Wu PN, Liu JL, Fang MJ, Fu XS, Wei JL, Wang Y, et al. Global trends in colorectal cancer and metabolic syndrome research: a bibliometric and visualization analysis. Int J Surg. (2024) 110:3723–33. doi: 10.1097/JS9.0000000000001342
31. Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. (2022) 11:173–6. doi: 10.1007/S40037-021-00695-4
32. Bertoli-Barsotti L, Lando T. A theoretical model of the relationship between the h-index and other simple citation indicators. Scientometrics. (2017) 111:1415–48. doi: 10.1007/s11192-017-2351-9
33. Robinson DBT, Hopkins L, Brown C, Abdelrahman T, Powell AG, Egan RJ, et al. Relative value of adapted novel bibliometrics in evaluating surgical academic impact and reach. World J Surg. (2019) 43:967–72. doi: 10.1007/s00268-018-04893-w
34. Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. (2017) 8:1–8. doi: 10.1002/j.2040-4603.2018.tb00008.x
35. Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. (2013) 15:647–58. doi: 10.1038/ncb2718
36. Huang Q, Wang T, Yang L, Wang HY. Ginsenoside Rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of Sirt1 and activation of AMPK. Int J Mol Sci. (2017) 18:1063. doi: 10.3390/ijms18051063
37. Chen X, Xue W, Zhang J, Peng J, Huang W. Ginsenoside Rg1 attenuates the NASH phenotype by regulating the miR-375-3p/ATG2B/PTEN-AKT axis to mediate autophagy and pyroptosis. Lipids Health Dis. (2023) 22:22. doi: 10.1186/s12944-023-01787-2
38. Yue C, Li D, Fan S, Tao F, Yu Y, Lu W, et al. Long-term and liver-selected ginsenoside C-K nanoparticles retard NAFLD progression by restoring lipid homeostasis. Biomaterials. (2023) 301:122291. doi: 10.1016/j.biomaterials.2023.122291
39. Liu J, Zhang T, Zhu J, Ruan S, Li R, Guo B, et al. Honokiol attenuates lipotoxicity in hepatocytes via activating SIRT3-AMPK mediated lipophagy. Chin Med. (2021) 16:115. doi: 10.1186/s13020-021-00528-w
40. Singh A, Ansari A, Gupta J, Singh H, Jagavelu K, Sashidhara KV. Androsin alleviates non-alcoholic fatty liver disease by activating autophagy and attenuating de novo lipogenesis. Phytomedicine. (2024) 129:155702. doi: 10.1016/j.phymed.2024.155702
41. Li S, Ma Y, Chen W. Active ingredients of Erhuang Quzhi Granules for treating non-alcoholic fatty liver disease based on the NF-kappaB/NLRP3 pathway. Fitoterapia. (2023) 171:105704. doi: 10.1016/j.fitote.2023.105704
42. Meng F, Song C, Liu J, Chen F, Zhu Y, Fang X, et al. Chlorogenic acid modulates autophagy by inhibiting the activity of ALKBH5 demethylase, thereby ameliorating hepatic steatosis. J Agric Food Chem. (2023) 71:15073–86. doi: 10.1021/acs.jafc.3c03710
43. Guan L, Guo L, Zhang H, Liu H, Zhou W, Zhai Y, et al. Naringin protects against non-alcoholic fatty liver disease by promoting autophagic flux and lipophagy. Mol Nutr Food Res. (2024) 68:e2200812. doi: 10.1002/mnfr.202200812
44. Yang Y, Wu Y, Zou J, Wang YH, Xu MX, Huang W, et al. Naringenin attenuates non-alcoholic fatty liver disease by enhancing energy expenditure and regulating autophagy via AMPK. Front Pharmacol. (2021) 12:687095. doi: 10.3389/fphar.2021.687095
45. Huang CY, Chen HW, Lo CW, Wang YR, Li CC, Liu KL, et al. Luteolin ameliorates palmitate-induced lipotoxicity in hepatocytes by mediating endoplasmic reticulum stress and autophagy. Food Chem Toxicol. (2023) 171:113554. doi: 10.1016/j.fct.2022.113554
46. Lee HS, Park BS, Kang HM, Kim JH, Shin SH, Kim IR. Role of luteolin-induced apoptosis and autophagy in human glioblastoma cell lines. Medicina. (2021) 57:879. doi: 10.3390/medicina57090879
47. Hsu MC, Guo BC, Chen CH, Hu PA, Lee TS. Apigenin ameliorates hepatic lipid accumulation by activating the autophagy-mitochondria pathway. J Food Drug Anal. (2021) 29:240–54. doi: 10.38212/2224-6614.3269
48. Ramadan NM, Elmasry K, Elsayed HRH, El-Mesery A, Eraky SM. The hepatoprotective effects of n3-polyunsaturated fatty acids against non-alcoholic fatty liver disease in diabetic rats through the FOXO1/PPARalpha/GABARAPL1 signalling pathway. Life Sci. (2022) 311(Pt A):121145. doi: 10.1016/j.lfs.2022.121145
49. Afshari H, Noori S, Zarghi A. Hepatic steatosis alleviated by a novel metformin and quercetin combination activating autophagy through the cAMP/AMPK/SIRT1 pathway. Iran J Pharm Res. (2023) 22:e136952. doi: 10.5812/ijpr-136952
50. Afshari H, Noori S, Zarghi A. A novel combination of metformin and resveratrol alleviates hepatic steatosis by activating autophagy through the cAMP/AMPK/SIRT1 signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. (2023) 396:3135–48. doi: 10.1007/s00210-023-02520-7
51. Marycz K, Kornicka K, Irwin-Houston JM, Weiss C. Combination of resveratrol and 5-azacytydine improves osteogenesis of metabolic syndrome mesenchymal stem cells. J Cell Mol Med. (2018) 22:4771–93. doi: 10.1111/jcmm.13731
52. Kornicka K, Szlapka-Kosarzewska J, Smieszek A, Marycz K. 5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy. J Cell Mol Med. (2019) 23:237–59. doi: 10.1111/jcmm.13914
53. Marycz K, Houston JMI, Weiss C, Rocken M, Kornicka K. 5-azacytidine and resveratrol enhance chondrogenic differentiation of metabolic syndrome-derived mesenchymal stem cells by modulating autophagy. Oxid Med Cell Longev. (2019) 2019:1523140. doi: 10.1155/2019/1523140
54. Wang C, Yang F, Zeng W, Chen X, Qiu Z, Wang Q, et al. Vine tea total flavonoids activate the AMPK/mTOR pathway to amelioration hepatic steatosis in mice fed a high-fat diet. J Food Sci. (2024) 89:3019–36. doi: 10.1111/1750-3841.17025
55. Vasarri M, Barletta E, Degl'Innocenti D. Posidonia oceanica (L) delile extract reduces lipid accumulation through autophagy activation in HepG2 cells. Pharmaceuticals. (2021) 14:969. doi: 10.3390/ph14100969
56. Du X, Di Malta C, Fang Z, Shen T, Niu X, Chen M, et al. Nuciferine protects against high-fat diet-induced hepatic steatosis and insulin resistance via activating TFEB-mediated autophagy-lysosomal pathway. Acta Pharm Sin B. (2022) 12:2869–86. doi: 10.1016/j.apsb.2021.12.012
57. Fu J, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. (2023) 41:301–16. doi: 10.1146/annurev-immunol-081022-021207
58. Yoo J, Jeong IK, Ahn KJ, Chung HY, Hwang YC. Fenofibrate, a PPARalpha agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy. Metabolism. (2021) 120:154798. doi: 10.1016/j.metabol.2021.154798
59. Amer AE, Ghoneim HA, Abdelaziz RR, Shehatou GSG, Suddek GM. Saroglitazar mitigated NASH-associated hepatic injury in dexamethasone-treated rats via modulating autophagy, apoptosis, and necroptosis. Toxicol Appl Pharmacol. (2024) 482:116774. doi: 10.1016/j.taap.2023.116774
60. Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, Bay BH, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE. (2014) 9:e87161. doi: 10.1371/journal.pone.0087161
61. Abdelrahman BA, Hammam OA, El-Khatib AS, Attia YM. The role of vitamin D3 in modulating the interplay between NLRP3 inflammasome and autophagy in NASH. Biochem Biophys Res Commun. (2023) 688:149122. doi: 10.1016/j.bbrc.2023.149122
62. Gong LL, Li GR, Zhang W, Liu H, Lv YL, Han FF, et al. Akebia saponin D decreases hepatic steatosis through autophagy modulation. J Pharmacol Exp Ther. (2016) 359:392–400. doi: 10.1124/jpet.116.236562
63. Sun J, Bian Y, Ma Y, Ali W, Wang T, Yuan Y, et al. Melatonin alleviates cadmium-induced nonalcoholic fatty liver disease in ducks by alleviating autophagic flow arrest via PPAR-alpha and reducing oxidative stress. Poult Sci. (2023) 102:102835. doi: 10.1016/j.psj.2023.102835
Keywords: autophagy, non-alcoholic fatty liver disease, metabolic syndrome, bibliometric analysis, VOSviewer, CiteSpace
Citation: Liu X and Li X (2025) Mapping and visualization of global research progress on autophagy in metabolic dysfunction-associated steatotic liver disease and metabolic syndrome: a bibliometric analysis (2009–2024). Front. Med. 12:1525526. doi: 10.3389/fmed.2025.1525526
Received: 09 November 2024; Accepted: 09 June 2025;
Published: 25 June 2025.
Edited by:
Eshak I. Bahbah, Al-Azhar University, EgyptReviewed by:
Amaia Rodríguez, University of Navarra, SpainKatarzyna Piotrowska, Pomeranian Medical University in Szczecin, Poland
Copyright © 2025 Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Li, bGl4aWFuZzg2QG1haWwyLnN5c3UuZWR1LmNu
†These authors have contributed equally to this work
 Xing Liu
Xing Liu Xiang Li
Xiang Li