- Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, Baronissi, Italy
Objective or purpose: To detect the short-term impact of cataract surgery on retinal layers thickness, as the exact mechanism of fundus changes after phacoemulsification has not yet been fully clarified.
Design: A retrospective observational study.
Subjects, participants, and/or controls: Seventy eyes of 70 patients with age ranging from 49 to 92 years, scheduled for cataract surgery, were included.
Methods, intervention, or testing: All subjects underwent a complete ophthalmological examination, including ART-OCT volume with Heidelberg Spectralis before and approximately one month later cataract surgery.
Main outcome measures: The macula was divided into a central foveal region and four parafoveal regions (superior, inferior, nasal, temporal). The scans were then automatically segmented into the different retinal layers and the changes in each layer were assessed.
Results: The results revealed that both the inner retinal layers and the entire retina exhibited a statistically significant thickening in foveal and parafoveal region: IRL (p < 0.001), ONL (p < 0.001), GCL (p = 0.010), RNFL (p = 0.020), and ALL (p < 0.001). Conversely, the outer retinal layers showed a statistically significant reduction in thickness only within the parafoveal regions: ORL (p < 0.001).
Conclusion: This study may provide a pathophysiological explanation for post-phacoemulsification changes affecting the retina.
Introduction
Cataract is the leading cause of treatable vision loss worldwide, affecting approximately 20 million individuals, (1) and cataract extraction is the most frequently performed surgical procedure (2). Any invasive eye surgery, including cataract surgery, initiates a biochemical cascade resulting in an immunological response. Advancements in surgical techniques and pharmacological treatments have effectively reduced this response in recent years, thereby lowering the risk of postoperative complications such as posterior capsule tear, vitreous presence in the anterior chamber, and the development of cystoid macular edema.
Phacoemulsification is the most widely utilized surgical technique, recognized as a well-standardized and safe procedure (3) generally yielding favorable visual outcomes. However, it is not entirely risk-free, as it can lead to side effects impacting various ocular structures, including the retina. Research indicates that cataract surgery can be a contributing factor in the development of posterior segment complications, including cystoid macular edema in pseudophakic patients, potential onset of age-related macular degeneration, and worsening of diabetic retinopathy (4–8). Even uncomplicated cataract surgery can induce retinal changes that are not detectable through ophthalmoscopic examination, often leading to unnoticed damage and subsequent decline in visual function (9–11).
The advent of optical coherence tomography (OCT) has enabled increasingly detailed imaging of retinal and choroidal structures (12, 13). This method is straightforward, safe, non-invasive, and highly precise, allowing for macular measurements with a resolution of 8–10 micrometers (14). Modern OCT equipment also permits precise and detailed segmentation of retinal layers. OCT has become a fundamental tool for identifying subclinical alterations affecting the macula after cataract surgery, leading to a growing number of studies in this area.
Previous research has largely focused on examining modifications across the entire retinal area, with somewhat conflicting results, (15–22) or on changes affecting specific retinal layers involved in common postoperative complications [e.g., outer plexiform/inner nuclear layers in Irvine Gass syndrome; (21–23) retinal nerve fiber layer (RNFL) in elevated IOP (24)].
In 2018, for the first time, Kurt and Kılıç (25) published a study that focused on the segmentation of all retinal layers and individually analyzed the alterations following phacoemulsification. Their findings revealed non-uniform thickening across different retinal layers (25). The present study aims to analyze retinal segmentation thickness before and one month after cataract surgery to detect any short-term surgically induced changes in each layer.
Materials and methods
This research study was conducted in accordance with the ethical guidelines outlined in the Declaration of Helsinki, and the necessary approval from the Institutional Review Board (IRB) (CECS, South Campania Ethics Committee, protocol no. 16544) was secured. All individuals gave a written consent to take part in the study.
Subjects with lens opacities, eligible to cataract surgery at the Ophthalmology Unit of the University of Salerno, which underwent uneventful cataract surgery with in the bag IOL implantation, were recruited for this retrospective observational study.
Patients with corneal leukoma, epiretinal membrane, diabetic retinopathy, advanced hypertensive retinopathy (grade II to IV), age-related macular degeneration, central serous chorioretinopathy, glaucoma, macular hole, previous laser treatment or intravitreal injections, presence of vitreomacular disease, previous ocular surgery, optic neuropathy, uveitis, uncontrolled hypertension with medication, autoimmune diseases, glycated hemoglobin A1c (HbA1c) level > 6.5% and ocular or systemic conditions that could cause retinal alterations were excluded. Only patients with nuclear or cortical opacities that allowed the preoperative OCT examination were recruited.
Therefore, 70 eyes of a total of 70 subjects (42 female and 28 male patients), aged 49 to 92 years (mean age 73 ± 9 years), were selected.
Clinical and instrumental assessment
During the preoperative visit, the participants undertook a complete ophthalmological examination, which involved assessing their clinical history, measuring their uncorrected and corrected visual acuity using the Snellen chart, conducting slit-lamp biomicroscopy and anterior segment evaluation, determining their IOP with a Goldmann applanation tonometer, performing fundus examination, and measuring their axial length using an IOLMaster 500 device (Carl Zeiss Meditec AG, Jena, Germany, version 5.4.4.0006), and spectral domain OCT scanning (Spectralis; Heidelberg Engineering; Heidelberg, Germany, version 6.0) using the “automatic real-time” (ART) volume program. One month later (an interval between 28 and 31 days), the OCT evaluation was reperformed using the device’s follow-up mode.
Surgical technique
Before surgery, a Mydriasert® tablet (Théa Pharma) (containing a combination of tropicamide and phenylephrine hydrochloride, 0.28 mg/5.4 mg) was located in the lower conjunctival fornix to promote mydriasis. Peribulbar anesthesia with 0.75% ropivacaine, followed by eyelids, eyelashes, and conjunctiva disinfection with 5% povidone iodine, and phacoemulsification with Constellation® Vision System (Alcon Laboratories, Inc.) were performed.
All surgeries followed the established protocol, including emulsification of cataractous lenticular nucleus passing through a clear corneal main tunnel of 3 mm on the vertical axis and the “Divide and Conquer” surgical technique, by different surgeons.
In all cases, the same preloaded hydrophobic intraocular lens Tecnis® Monofocal (PCB00) was implanted in the capsular bag. Injection of 0.1 mL/1 mg of cefuroxime (Ximaract®; Bausch & Lomb UK Ltd.) into the anterior chamber and closure of the main tunnel and two paracentesis were final steps of the surgery. In the postoperative period, the use of dexamethasone and levofloxacin 1 mg/ml + 5 mg/ml (Ducressa; Santen Italia S.r.l.) five times daily for one week, then diclofenac 1 mg/ml (Visunac; Visufarma S.p.A.) three times daily for the following three weeks were prescribed as post-surgical antibacterial and anti-inflammatory prophylaxis.
OCT analysis: retinal parameters
The study involved the use of Spectral-Domain Optical Coherence Tomography (SD-OCT) with the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) to image participants’ retinas. The key details of the imaging process and analysis used are the following:
- Imaging Parameters: Scan Area: 20° × 15° (5.9 × 4.4 mm)
Frames: average of 100 frames using Automatic Real-Time (ART) mode
Sections: 19 horizontal scans spaced at 240 μm intervals
Resolution: 512 A-scans per B-scan
- Image Quality: images with a signal-to-noise ratio (SNR) less than 15 dB were excluded to ensure high-quality data.
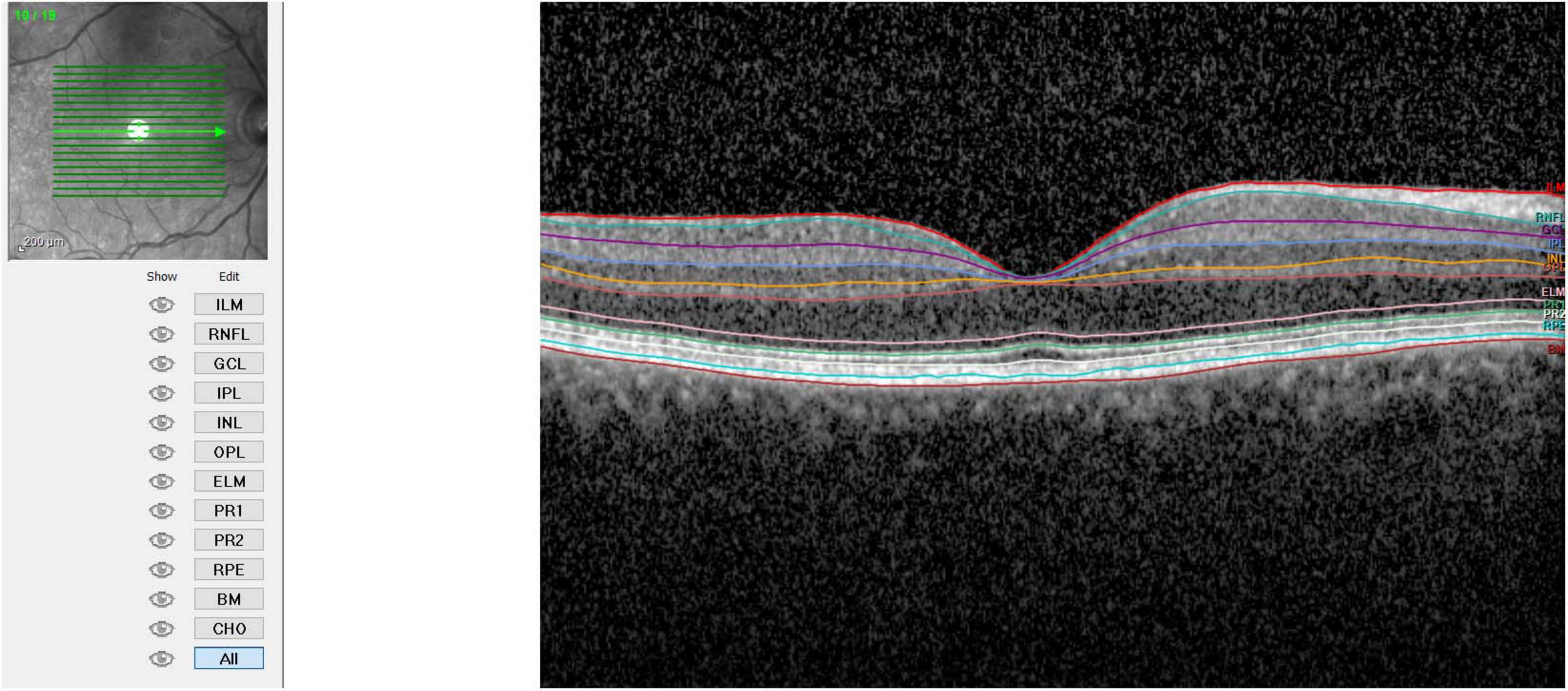
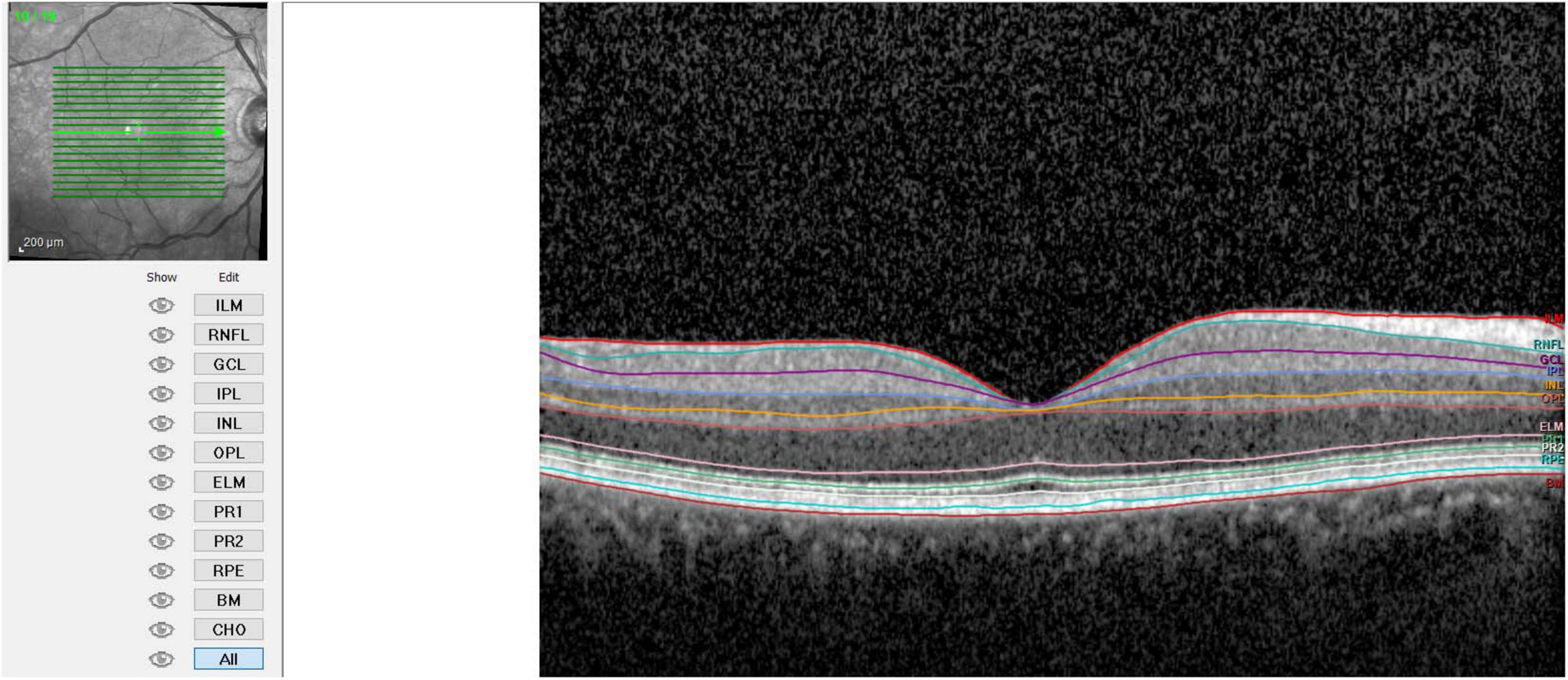
- Retinal Layer Segmentation: the segmentation of retinal layers was performed automatically by the Heidelberg Spectralis software. The system identified 11 optical interfaces to study the 10 distinct retinal layers.
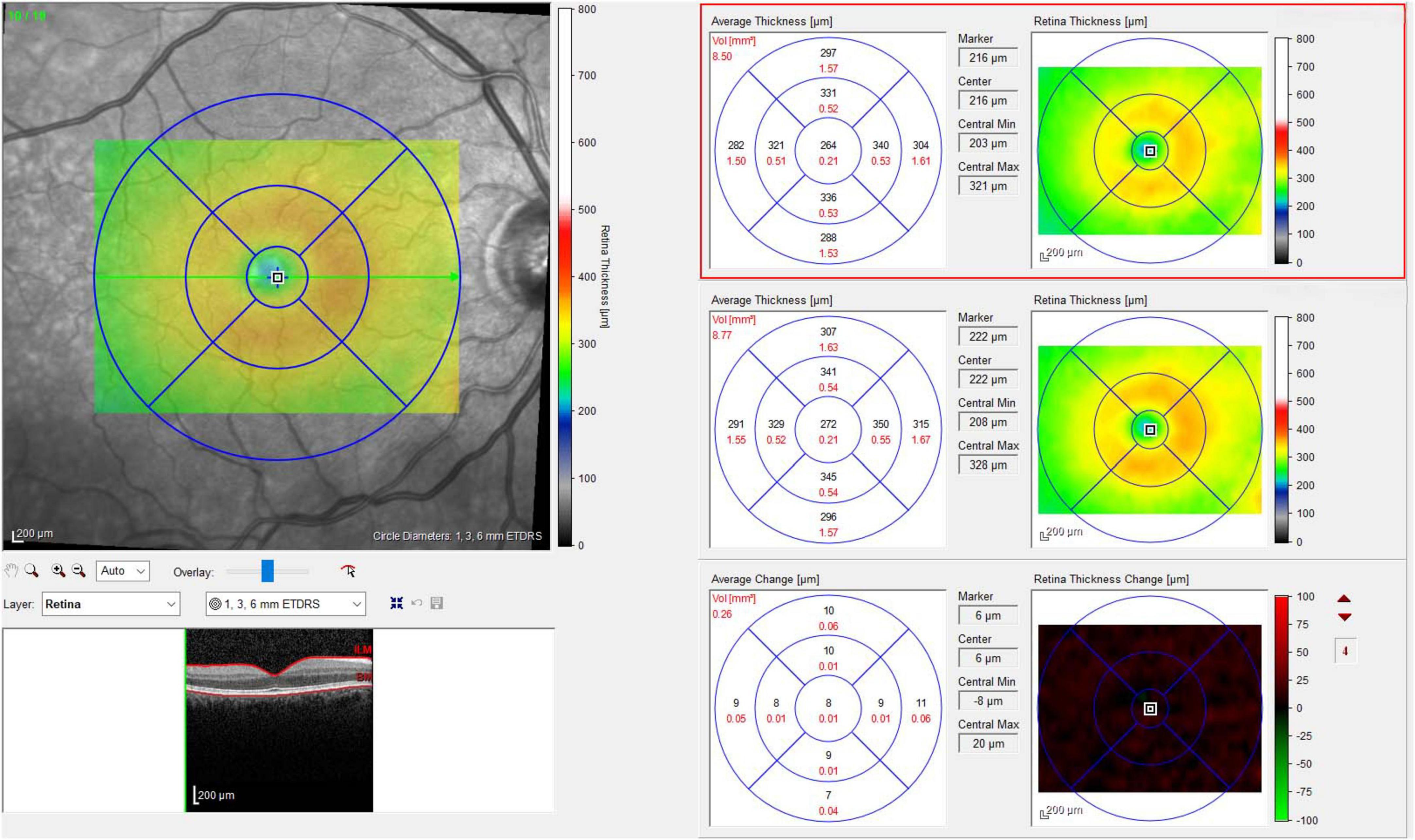
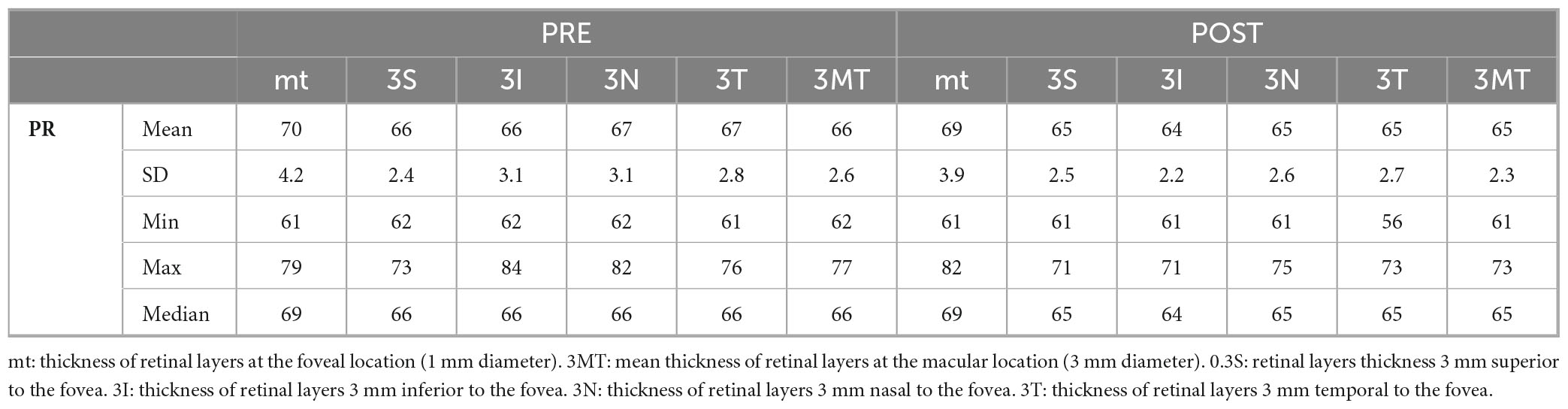
- Thickness Measurement: the standard Early Treatment Diabetic Retinopathy Study (ETDRS) grid was used. Measurements were taken at the following regions: fovea-centered Circle, 1 mm in diameter, and Parafoveal Regions, in superior, nasal, inferior, and temporal regions each with a 3 mm diameter. Finally, the average thickness of the retinal layers was calculated for the fovea (1 mm), parafoveal regions (3 mm each), and the combined average of these five regions (Figures 1–3).
This detailed protocol ensures consistent and high-quality retinal imaging and analysis, crucial for accurate assessment and study of retinal health and diseases.
Therefore, values were collected for total thickness (ALL), outer retinal layers (ORL), inner retinal layers (IRL), retinal pigment epithelium (RPE), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL), ganglion cell layer (GCL), and nerve fiber layer (NFL). IRL included the sum of the layers between NFL and external limiting membrane (ELM), while ORL included layers from RPE to ELM.
Photoreceptor layer (PRL) measurement resulted from the subtraction of RPE measurements from the ORL.
Then, the same trained ophthalmologist (M.D.B.) checked the automatic retinal layers segmentation, misalignments, decentration or motion artifacts and reviewed data extrapolated from collected OCT scans to avoid measurement biases.
The ETDRS grid was manually aligned with the foveal pit, if not correctly positioned.
All examinations were performed between 12:00 and 2:00 PM. All measurements were made using the Heidelberg Spectralis software (Heidelberg Engineering; Heidelberg, Germany, version 6.0).
Statistical analysis
SPSS software (IBM SPSS Statistics version 25) was used to conduct statistical assessment.
The normality of data distribution was computed with the Kolmogorov–Smirnov test. All parameters of patients and controls between the preoperative and the control visits were compared using a two-tailed t-test, for data distributing in accordance with the Gaussian curve, and the Wilcoxon signed-rank test, for data distributing according to a different trend. Statistical significance was considered for a p-value of less than 0.05. To perform the analysis G*Power software (version 3.1.9.4) was utilized. To maximize the statistical power utilizing a paired t-test, the sample size with α error = 0.05, 1-β error = 0.95 and effect size = 0.437 was set. Non-centrally parameter δ = 3.656, critical t = 1.995, Df = 69, total sample size = 70 and actual power = 0.95 were then calculated. In addition, to maximize the statistical power utilizing Wilcoxon test, the sample size with α error = 0.05, 1-β error = 0.95 and effect size = 0.450 was decided. Non-centrally parameter δ = 3.68, critical t = 1.997, Df = 65.84, total sample size = 70 and actual power = 0.95 were calculated.
Results
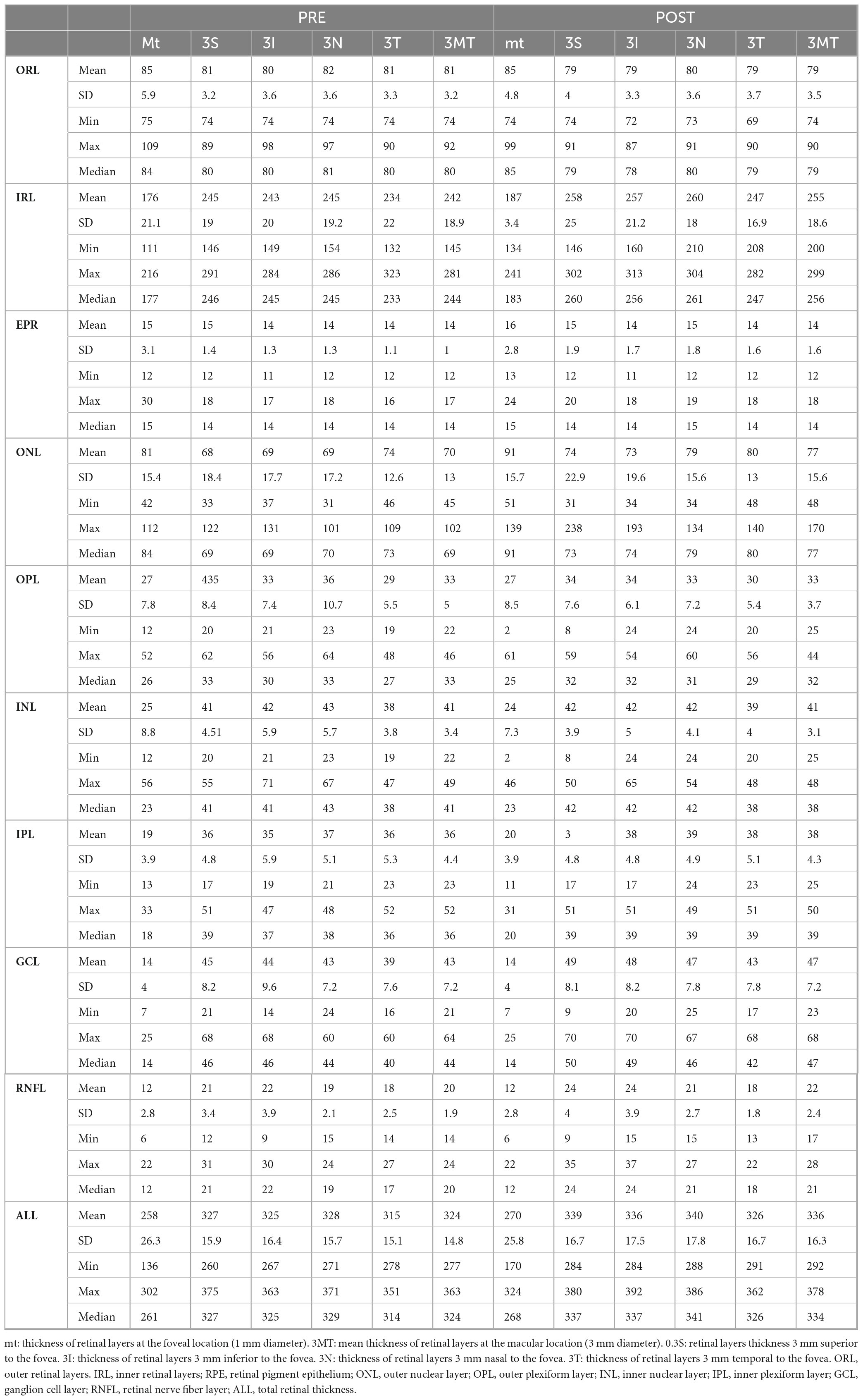
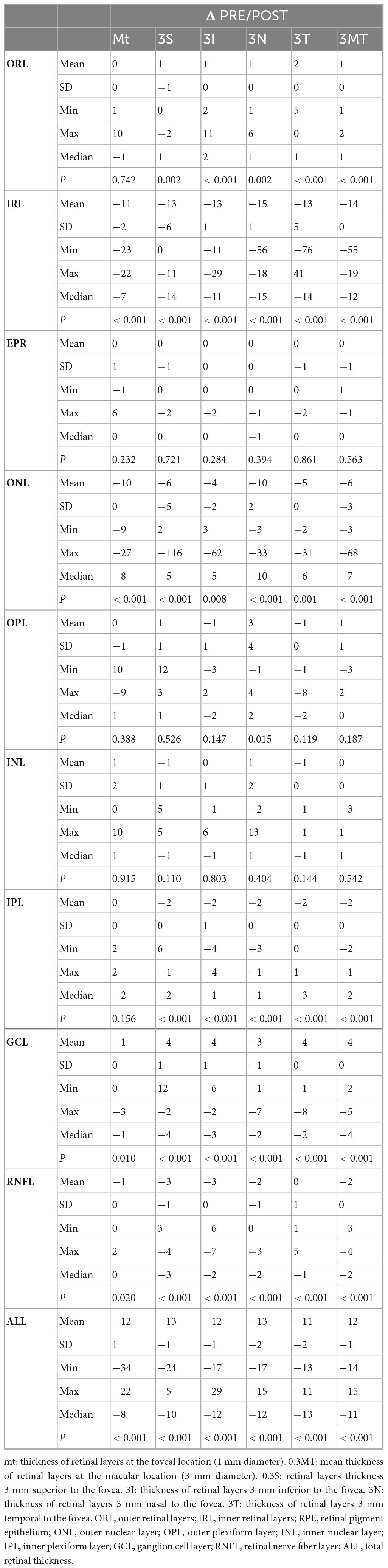
Table 1 provides a summary of the retinal layers’ measurements, whereas Table 2, illustrates the differences between pre- and post-surgical measurements, which can be summarized as follows:
The central foveal region (1 mm) exhibited a significant thickening in the IRL (p < 0.001), ONL (p < 0.001), GCL (p = 0.010), RNFL (p = 0.020), and ALL (p < 0.001). The other layers showed not statistically significant or negligible variations.
The superior parafoveal region (3 mm) presented a substantial increase in the IRL (p < 0.001), ONL (p < 0.001), IPL (p < 0.001), GCL (p < 0.001), RNFL (p < 0.001), and ALL (p < 0.001).
Additionally, a statistically significant thinning in the ORL (p = 0.002) was present in this region. The other layers showed not statistically significant or negligible variations.
The inferior parafoveal region (3 mm) displayed a statistically significant thickening in the IRL (p < 0.001), ONL (p = 0.008), IPL (p < 0.001), GCL (p < 0.001), RNFL (p < 0.001), and ALL (p < 0.001). A statistically significant reduction in the ORL thickness (p < 0.001) was also demonstrated. The other layers showed not statistically significant or negligible variations.
The nasal parafoveal region (3 mm) showed a statistically significant increase in the IRL (p < 0.001), ONL (p < 0.001), IPL (p < 0.001), GCL (p < 0.001), RNFL (p < 0.001), and ALL (p < 0.001). A meaningful decrease in the ORL (p = 0.002) and OPL (p = 0.015) was also shown.
The other layers showed not statistically significant or negligible variations.
The temporal parafoveal region (3 mm) displayed a statistically significant increase in the IRL (p < 0.001), ONL (p = 0.001), IPL (p < 0.001), GCL (p < 0.001), RNFL (p < 0.001), and ALL (p < 0.001). A statistically significant decrease in the ORL (p < 0.001) was also demonstrated.
The other layers showed not statistically significant or negligible variations.
The average of the five considered regions (foveal and parafoveal) demonstrated a statistically significant thickening of the IRL (p < 0.001), ONL (p < 0.001), IPL (p < 0.001), GCL (p < 0.001), RNFL (p < 0.001), and ALL (p < 0.001). A statistically significant thinning in the ORL layer (p < 0.001) was also displayed. The other layers showed not statistically significant or null variations.
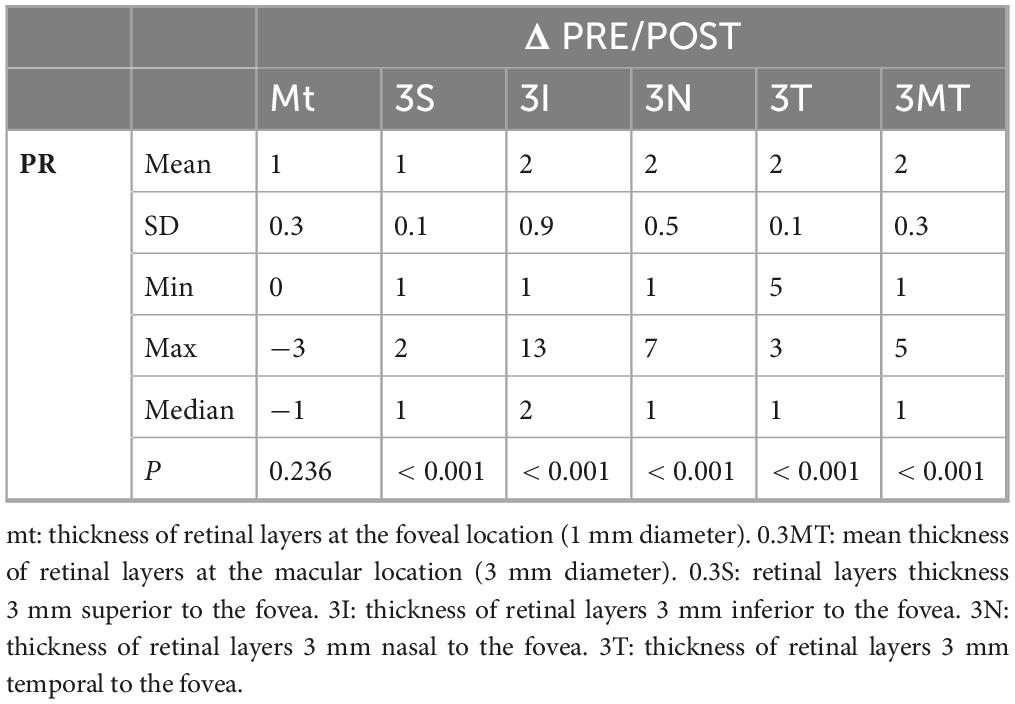
In addition, PR layer showed a statistically significant reduction in the 4 parafoveal regions (p < 0.001), however not in the central foveal region (Tables 3, 4).
Discussion
In recent years phacoemulsification for cataract treatment has been gradually improved, becoming a minimally invasive procedure that involves only the anterior segment of the eye.
However, post-operative retinal alterations can have serious consequences, such as cystoid macular edema, or they may present sub-clinically and seem to have no effect on visual outcome.
The precise cause and mechanism behind the fundus changes following emulsification of cataractous lenticular nucleus remain unclear. Several factors have been suggested, including vascular instability, vitreomacular traction, ocular hypotony, and increased light exposure (26).
Some studies indicate that post-operative inflammation may significantly contribute to the evelopment of retinal alterations (27–30).
Numerous factors related to phacoemulsification can affect ocular structures. Ultrasonic energy and fluid dynamics generate mechanical effects, albeit slight, that result in inflammation, compression, and oxygen drop in the surrounding tissues. Each step of this procedure can induce direct tissue changes and immediate pressure variations. Additionally, turbulent fluid flow exerts radiating pressure impacts similar to a shockwave and a small jet, directly affecting the anterior chamber structures and spreading in all directions (9).
Even if asymptomatic, these micro-alterations can be detected with optical coherence tomography (OCT).
Although several studies have been conducted in recent years to verify changes in macular thickness after cataract surgery, their conclusions were not in agreement (14–19, 25, 31, 32).
The present study focuses on changes one month after phacoemulsification, a significant increase in thickness is observed in many retinal layers (IRL, ONL, IPL, GCL, RNFL) both at the central foveal and parafoveal regions, leading to a statistically significant increase in ALL. This finding is consistent with the most recent studies that have examined retinal modifications in terms of thickness following cataract surgery (14–19, 25, 31, 32). However, among these studies, only one segmented the retina and examined the modifications individually.
Nevertheless, this research was limited by the modest number of examined eyes and segmenting the retina into the following layers: RPE, ONL, OPL, INL, IPL, GCL, RNFL (25).
This segmentation excluded the retinal structures between the RPE and ONL, which include photoreceptors and their connections with inner layers (33). Our study introduced further segmentation of the retina into IRL and ORL. The latter group includes all structures between RPE and the ELM, including PRL. Then we obtained the PRL from the ORL, and the results revealed that the ORL, in particular the PRL, is the only segment to show a statistically significant thinning in the 4 parafoveal regions, with no changes in the central foveal region.
This result, observed for the first time in this study, is in contrast with the findings of other studies and may indicate inflammation-induced suffering of the PRL, following phacoemulsification. This structural alteration was not evident during clinical examination, and the sparing of the central foveal region could explain the absence of clinical influence.
It is known that post-operative inflammatory effects can cause free radicals, growth factors and prostaglandins release, which could be leading factors for post-operative retinal alterations (5, 24, 34). By the literature, surgical wounds trigger releasing of prostaglandins into the aqueous humor and blood-aqueous barrier damage, with consequential start of an inflammatory cascade and production of other inflammatory mediators in the aqueous humor and their dispersion into the vitreous cavity. Posterior segment inflammation, consecutive to the anterior segment ones, disrupts both inner and outer blood-retinal barrier (35), inducing choroidal thickness and Vascularity Index increase at the first postoperative month (35–37). Nevertheless, lacking strong evidence, the exact mechanism of retinal structure changes and their impact on the ocular fundus–whether beneficial or harmful–remains unclear.
Menapace et al. (38) published a study involving 120 eyes from 60 patients, with complete follow-up data for 56 patients. The participants were divided into two groups: those undergoing femtosecond laser-assisted cataract surgery and those undergoing manual cataract surgery. The findings indicated similar patterns of macular thickness and volume increase, with no statistically significant differences between the two groups. Our results are consistent with those of the authors, despite our focus on individual retinal layers, to achieve a meticulous assessment of retinal changes (38).
Schwarzenbacher et al. (39) conducted a study involving 112 eyes from 56 patients, also divided into femtosecond laser-assisted and manual cataract surgery groups. They evaluated changes over postoperative periods of 1 week, 3 weeks, 6 weeks, and 18 months, focusing on the inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor (PR) layer, and total retinal thickness. Notably, a significant decrease in PR thickness was observed 1 week post-surgery across all zones. Conversely, the other evaluated layers and the overall retina exhibited an increase in thickness during the initial weeks, remaining significantly elevated 18 months post-surgery in all zones. Our findings align with those of the authors in the early postoperative period; however, our study utilized a larger sample size of 70 eyes from a total of 70 subjects and assessed all individual retinal layers (39).
Großpötzl et al. (40) investigated a cohort of 41 patients who underwent uneventful cataract surgery, with evaluations conducted preoperatively and at 1 day, 1 month, and 3 months postoperatively. The authors focused on all layers of the inner retina, including the OPL, ONL, and total retinal thickness. They reported a decrease in retinal thickness on the first postoperative day, followed by a significant increase at 1 month and a subsequent reduction at 3 months. Our results are consistent with these findings at 1 month post-surgery; however, the strength of our research lies in its comprehensive assessment of all retinal layers. Unlike the authors, we observed a statistically significant decrease in the OPL and PR layer at 1 month post-surgery (40).
Recently, Balog et al. (41) published a study involving 102 eyes from 79 consecutive subjects without any other ocular or systemic diseases who underwent cataract surgery. The authors reported a statistically significant increase in nearly all retinal layers and a decrease in OPL at 7, 30 and 90 days post-phacoemulsification. This finding aligns with our results; we also observed thickening in nearly all retinal layers and thinning of the OPL. The authors suggested that the decrease might be attributed to PR loss. While we cannot confirm this hypothesis, our study specifically focused on the PR layer and found a statistically significant reduction. This decrease may indeed be explained by PR loss; however, our results indicate a reduction in thickness that could also be attributed to structural cellular changes within the PR layer (41).
In conclusion, our study, conducted on 70 eyes of 70 patients scheduled for cataract surgery supports the existing literature, highlighting an increase in retinal thickness following the procedure, and also reveals a previously unobserved reduction in the thickness of the ORL and PRL, observed through a more detailed segmentation of retinal layers than ever before. Due to the retrospective nature of this study, longer follow up visit were not available, because nor morphological signs of retinal changes by OCT neither best corrected visual acuity reduction were present. For the same reason, only the routine pharmacological treatment with NSAID drops was applied to these patients.
The lack of longer follow-up to strengthen our observation represents a limitation of this study. Nevertheless, our results provide a basis for further studies that may provide a pathophysiological explanation for this phenomenon, contributing to an understanding of post-phacoemulsification complications affecting the retina.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Comitato etico “Cometico Campania Sud.” The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MG: Data curation, Writing – original draft. MDB: Validation, Writing – review and editing. AL: Writing – original draft. MDL: Data curation, Writing – original draft. SP: Investigation, Writing – original draft. MA: Methodology, Writing – original draft. AM: Writing – original draft. NR: Supervision, Validation, Writing – review and editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AMD, age-related macular degeneration; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer; IRB, Institutional Review Board; CECS, Cometico Campania Sud; HbA1c, glycated hemoglobin A1c; IOP, intraocular pressure; AL, axial length; ART, automatic real-time; ETDRS, early treatment diabetic retinopathy study; ALL, total thickness; ORL, outer retinal layers; IRL, inner retinal layers; RPE, retinal pigment epithelium; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer; NFL, nerve fiber layer; ELM, external limiting membrane; PR, photoreceptor layer.
References
1. Pascolini D, Mariotti SP. Global estimates of visual impairment. Br J Ophthalmol. (2012) 96:614–8. doi: 10.1136/bjophthalmol-2011-300539
2. Rossi T, Romano M, Iannetta D, Romano V, Gualdi L, D’Agostino I, et al. Cataract surgery practice patterns worldwide: A survey. BMJ Open Ophthalmol. (2021) 6:e000464. doi: 10.1136/bmjophth-2020-000464
3. Gogate P, Kulkarni S, Krishnaiah S, Deshpande R, Joshi S, Palimkar A, et al. Safety and efficacy of phacoemulsification compared with manual small-incision cataract surgery by a randomized controlled clinical trial: Six-week results. Ophthalmology. (2005) 112:869–74. doi: 10.1016/j.ophtha.2004.11.055
4. Biro Z, Balla Z. Foveal and perifoveal retinal thickness measured by OCT in diabetic patients after phacoemulsification cataract surgery. Oftalmologia. (2009) 53:54–60.
5. Pollack A, Marcovich A, Bukelman A, Oliver M. Age-related macular degeneration after extracapsular cataract extraction with intraocular lens implantation. Ophthalmology. (1996) 103:1546–54. doi: 10.1016/s0161-6420(96)30464-8
6. Wang J, Klein R, Smith W, Klein B, Tomany S, Mitchell P. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: Pooled findings from the beaver dam and blue mountains eye studies. Ophthalmology. (2003) 110:1960–7. doi: 10.1016/s0161-6420(03)00816-9
7. Libre P. Intraoperative light toxicity: A possible explanation for the association between cataract surgery and age-related macular degeneration. Am J Ophthalmol. (2003) 136:961. doi: 10.1016/s0002-9394(03)00906-1
8. Cugati S, Mitchell P, Rochtchina E, Tan A, Smith W, Wang J. Cataract surgery and the 10-year incidence of age-related maculopathy: The blue mountains eye study. Ophthalmology. (2006) 113:2020–5. doi: 10.1016/j.ophtha.2006.05.047
9. Pardianto G, Moeloek N, Reveny J, Wage S, Satari I, Sembiring R, et al. Retinal thickness changes after phacoemulsification. Clin Ophthalmol. (2013) 7:2207–14. doi: 10.2147/OPTH.S53223
11. Hee M, Izatt J, Swanson E, Huang D, Schuman J, Lin C, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. (1995) 113:325–32. doi: 10.1001/archopht.1995.01100030081025
12. De Bernardo M, Diana F, Gioia M, De Luca M, Tepedino M, Pellecchia M, et al. The correlation between retinal and choroidal thickness with age-related white matter hyperintensities in progressive supranuclear palsy. J Clin Med. (2023) 12:6671. doi: 10.3390/jcm12206671
13. De Bernardo M, Altieri V, Coppola A, Gioia M, Rosa N. Choroidal evaluation in patients under alpha-lytic therapy. Graefes Arch Clin Exp Ophthalmol. (2020) 258:2729–36. doi: 10.1007/s00417-020-04907-1
14. Georgopoulos G, Papaconstantinou D, Niskopoulou M, Moschos M, Georgalas I, Koutsandrea C. Foveal thickness after phacoemulsification as measured by optical coherence tomography. Clin Ophthalmol. (2008) 2:817–20. doi: 10.2147/opth.s4031
15. Kim S, Belair M, Bressler N, Dunn J, Thorne J, Kedhar S, et al. A method of reporting macular edema after cataract surgery using optical coherence tomography. Retina. (2008) 28:870–6. doi: 10.1097/IAE.0b013e318169d04e
16. Barsam A, Chandra A, Bunce C, Whitefield L. Prospective randomized controlled trial to compare the effect on the macula of AquaLase liquefaction and ultrasound phacoemulsification cataract surgery. J Cataract Refract Surg. (2009) 34:991–5. doi: 10.1016/j.jcrs.2008.02.017
17. Kurz S, Krummenauer F, Thieme H, Dick H. Optical coherence tomography of macular thickness after biaxial vs coaxial microincision clear corneal cataract surgery. Eur J Ophthalmol. (2009) 19:990–7. doi: 10.1177/112067210901900615
18. Cagini C, Fiore T, Iaccheri B, Piccinelli F, Ricci M, Fruttini D. Macular thickness measured by optical coherence tomography in a healthy population before and after uncomplicated cataract phacoemulsification surgery. Curr Eye Res. (2009) 34:1036–41. doi: 10.3109/02713680903288937
19. Ghosh S, Roy I, Biswas P, Maji D, Mondal L, Mukhopadhyay S, et al. Prospective randomized comparative study of macular thickness following phacoemulsification and manual small incision cataract surgery. Acta Ophthalmol. (2010) 88:e102–6. doi: 10.1111/j.1755-3768.2010.01896.x
20. Bellocq D, Mathis T, Voirin N, Bentaleb Z, Sallit R, Denis P, et al. Incidence of irvine gass syndrome after phacoemulsification with spectral-domain optical coherence tomography. Ocul Immunol Inflamm. (2019) 27:1224–31. doi: 10.1080/09273948.2019.1634215
21. Amjad A, Shaheer M, Rafique A. Retinal nerve fiber layer thickness changes after phacoemulsification with intraocular lens implantation. J Coll Physicians Surg Pak. (2018) 28:919–22. doi: 10.29271/jcpsp.2018.12.919
22. Picillo M, Salerno G, Tepedino M, Abate F, Cuoco S, Gioia M, et al. Retinal thinning in progressive supranuclear palsy: Differences with healthy controls and correlation with clinical variables. Neurol Sci. (2022) 43:4803–9. doi: 10.1007/s10072-022-06061-4
23. Runge A, Remlinger J, Abegg M, Ferrazzini T, Brügger D, Weigt-Usinger K, et al. Retinal layer segmentation in a cohort of healthy children via optical coherence tomography. PLoS One. (2022) 17:e0276958. doi: 10.1371/journal.pone.0276958
24. Anderson D, Mullins R, Hageman G, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. (2022) 134:411–31. doi: 10.1016/s0002-9394(02)01624-0
25. Kurt A, Kılıç R. The effects of uncomplicated cataract surgery on retinal layer thickness. J Ophthalmol. (2018) 2018:7218639. doi: 10.1155/2018/7218639
26. Shelsta H, Jampol L. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. (2011) 31:4–12. doi: 10.1097/IAE.0b013e3181fd9740
27. Yılmaz T, Karci A, Yilmaz İ, Yılmaz A, Yıldırım Y, Sakalar YB. Long-term changes in subfoveal choroidal thickness after cataract surgery. Med Sci Monit. (2016) 22:1566–70. doi: 10.12659/msm.898714
28. Pierru A, Carles M, Gastaud P, Baillif S. Measurement of subfoveal choroidal thickness after cataract surgery in enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci. (2014) 55:4967–74. doi: 10.1167/iovs.14-14172
29. Ibrahim A, Elgouhary S, Nassar M, El Batanony A. Changes in choroidal thickness after cataract surgery. Semin Ophthalmol. (2018) 33:664–70. doi: 10.1080/08820538.2017.1416410
30. Lobo C, Faria P, Soares M, Bernardes R, Cunha-Vaz J. Macular alterations after small-incision cataract surgery. J Cataract Refract Surg. (2004) 30:752–60. doi: 10.1016/S0886-3350(03)00582-0
31. von Jagow B, Ohrloff C, Kohnen T. Macular thickness after uneventful cataract surgery determined by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. (2007) 245:1765–71. doi: 10.1007/s00417-007-0605-6
32. Perente I, Utine C, Ozturker C, Cakir M, Kaya V, Eren H, et al. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr Eye Res. (2007) 32:241–7. doi: 10.1080/02713680601160610
33. Bhende M, Shetty S, Parthasarathy M, Ramya S. Optical coherence tomography: A guide to interpretation of common macular diseases. Indian J Ophthalmol. (2018) 66:20–35. doi: 10.4103/ijo.IJO_902_17
34. Kim S, Flach A, Jampol L. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. (2010) 55:108–33. doi: 10.1016/j.survophthal.2009.07.005
35. Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. (2002) 47:S203–18. doi: 10.1016/s0039-6257(02)00294-1
36. Tso M, Shih C. Experimental macular edema after lens extraction. Invest Ophthalmol Vis Sci. (1977) 16:381–92.
37. İçöz M. Evaluation of structural and vascular changes in the choroid after uneventful phacoemulsification surgery. Rom J Ophthalmol. (2023) 67:50–6. doi: 10.22336/rjo.2023.9
38. Menapace R, Schartmüller D, Röggla V, Reiter G, Leydolt C, Schwarzenbacher L. Ultrasound energy consumption and macular changes with manual and femtolaser-assisted high-fluidics cataract surgery: A prospective randomized comparison. Acta Ophthalmol. (2022) 100:e414–22. doi: 10.1111/aos.14983
39. Schwarzenbacher L, Schmidt-Erfurth U, Schartmüller D, Röggla V, Leydolt C, Menapace R, et al. Long-term impact of low-energy femtosecond laser and manual cataract surgery on macular layer thickness: A prospective randomized study. Acta Ophthalmol. (2024) 102:e862–8. doi: 10.1111/aos.16667
40. Großpötzl M, Malle E, Riedl R, Gran J, Djavid D, Posch-Pertl L, et al. Changes of individual retinal layer thickness post-uneventful cataract surgery determined by spectral-domain optical coherence tomography over a 3-months period. Heliyon. (2024) 10:e35096. doi: 10.1016/j.heliyon.2024.e35096
Keywords: cataract surgery, cataract, retina, retinal thickness, OCT, SD-OCT, retinal layers
Citation: Gioia M, De Bernardo M, La Marca A, De Luca M, Pagliarulo S, Avella M, Mignone A and Rosa N (2025) The short term impact of uncomplicated cataract surgery on retinal layers thickness. Front. Med. 12:1537402. doi: 10.3389/fmed.2025.1537402
Received: 30 November 2024; Accepted: 01 July 2025;
Published: 21 July 2025.
Edited by:
Gregor S. Reiter, Medical University of Vienna, AustriaCopyright © 2025 Gioia, De Bernardo, La Marca, De Luca, Pagliarulo, Avella, Mignone and Rosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maddalena De Bernardo, bWRlYmVybmFyZG9AdW5pc2EuaXQ=
 Marco Gioia
Marco Gioia Maddalena De Bernardo
Maddalena De Bernardo Aniello La Marca
Aniello La Marca Martina De Luca
Martina De Luca Sergio Pagliarulo
Sergio Pagliarulo Mariagrazia Avella
Mariagrazia Avella Alfredo Mignone
Alfredo Mignone Nicola Rosa
Nicola Rosa