Abstract
Background:
Patients with obesity-associated Hashimoto’s thyroiditis (HT) have been prevalent in clinical practice. Obesity is a risk factor for HT as it promotes pro-inflammatory processes and influences the balance of immune cell subsets. Traditional Chinese medicine (TCM) is characterized by its multi-component and multi-target approach and shows potential in treating HT. Specifically, TCM can reduce thyroid antibody levels and alleviate clinical symptoms without impairing thyroid function. Moreover, TCM offers significant benefits in regulating lipid metabolism and decreasing systemic inflammation.
Methods:
Targets of five high-frequency herbs (Hedysarum multijugum Maxim, Radix Bupleuri, Prunella vulgaris, Fritillaria thunbergii Bulbus, and Angelicae sinensis Radix) were obtained from the TCMSP and Swiss Target Prediction databases. Targets associated with obesity-associated HT were collected from the GeneCards, OMIM, and DisGeNET databases. Subsequently, we employed KEGG signaling pathway enrichment and GO biological process enrichment analyses to investigate the potential mechanisms by which the active ingredients of these herbs treat obesity-associated HT. Then, STRING database networks and Cytoscape software were used to construct the protein-protein interaction network and screen for key targets. Finally, molecular docking was performed to predict the binding interactions between the targets.
Results:
Efferocytosis emerged as the key mechanism in the context of five herbs and obesity-associated HT. Quercetin was identified as the primary active ingredient responsible for efferocytosis, and it bound well with efferocytosis-related targets.
Conclusion:
This study’s key finding is that five high-frequency prescribed herbs may treat obesity-associated HT through efferocytosis. This provides new evidence to support the use of TCM in treating obesity-associated HT.
1 Introduction
Hashimoto’s thyroiditis (HT), also known as chronic lymphocytic thyroiditis, is one of the common autoimmune diseases (ADs) in clinical treatment. Modern medicine believes that the etiology of HT is associated with genes, environment, and epigenetic factors (1). Patients with HT and normal thyroid function are mainly treated by nutritional therapy and lifestyle intervention (2–5). If patients suffer hypothyroidism or subclinical hypothyroidism, doctors usually apply levothyroxine alternative therapy (6). TCM has the potential to reduce thyroid antibody levels and improve clinical symptoms without affecting thyroid function and has been evidenced in clinical efficacy and safety (7, 8). To date, antioxidant, immune regulation, and anti-inflammatory properties are the main mechanisms of active ingredients of herbs for HT (6, 9–13).
Obesity serves as a risk factor for HT (14). Clinical studies have shown that it can impact thyroid function and antibody levels in patients with HT (15, 16). In addition, obesity promotes pro-inflammatory processes and influences the balance of immune cell subsets (14). Research on both obesity and HT has largely focused on the perspective of inflammation and immune dysfunction (14, 17).
Efferocytosis is a process in which macrophages specifically recognize and engulf apoptotic cells (18). The process of efferocytosis is divided into three steps: recognition of dying cells, engulfment of dying cells, and digestion of dying cells (18–21). At the same time, efferocytosis can also produce anti-inflammatory factors (20). Recent studies demonstrated that atherosclerosis, aging, cancer, obesity, diabetes, and some ADs are related to efferocytosis (20, 22–25). However, HT has not been mentioned. There were also targeted clinical trials for efferocytosis targets (AXL, MERTK, CD47, Tim4, and Tim3) (20).
Quercetin is a flavonoid compound in many fruits and vegetables (26). Studies have found that quercetin has the effect of regulating immunity (27), promoting apoptosis (28), and upregulating PPAR-γ signaling (29). In addition, quercetin inhibits macrophage M1 polarization and promotes M2 polarization (30), and M2 macrophages are associated with efferocytosis (31).
The objective of this study was to explore the potential mechanisms of five herbs in treating obesity-associated HT by using network pharmacology and molecular docking.
2 Materials and methods
2.1 Collection of herb and disease targets
The targets of Hedysarum multijugum Maxim (HMM), Radix Bupleuri (RB), Prunella vulgaris (PV), Fritillaria thunbergii Bulbus (FTB), and Angelicae Sinensis Radix (ASR) were identified by searching the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database, PubChem database, and Swiss Target Prediction database. Obesity-related targets were searched from the GeneCards database, OMIM database, and DisGeNET database using “Obesity” as the search term. The GeneCards and OMIM databases were used to search for targets using “Hashimoto thyroiditis” as the search term. Finally, three obesity disease datasets and two HT disease datasets were merged to obtain the obesity-associated HT disease genes.
2.2 GO and KEGG pathway enrichment analyses
KEGG pathway enrichment and GO biological process enrichment (including biological processes, molecular functions, and cellular components) were performed on five single herbs and four herb pairs (PV-HMM, PV-FTB, PV-RB, and HMM-ASR) by the Medscape database (p < 0.05). Venn diagrams were employed through the Venn mapping website to analyze the similarities and differences in mechanisms of the enrichment analysis (32).
2.3 Analysis of efferocytosis-related active ingredients
We matched targets of the herb’s active ingredient and herb-related efferocytosis to obtain the most active component. To further verify that the active ingredients are related to efferocytosis, the intersection targets of the compound and disease were matched using the Venn mapping website and then imported into the Metascape database.
2.4 Protein–protein interaction network construction and target protein screening
A total of 38 efferocytosis targets obtained from five herbs were imported into the STRING database to obtain the PPI network of efferocytosis. CytoHubba was used to screen the top 15 key targets. Cytoscape software was used to construct the target network.
2.5 Molecular docking
Molecular docking was performed between the active ingredient and key targets. AXL and MERTK were also included as key targets because they are present in 38 efferocytosis targets and have been clinically validated. The potential proteins were entered into the PDB database to obtain the associated protein structure, downloaded in PDB format, and saved. The AutoDock Vina software was used for molecular docking. The targets were treated by removing water molecules and performing hydrogenation. Receptor was then selected and then exported as a PDBQT file. Quercetin was treated by hydrogenation and selected as the ligand. It was then exported as a PDBQT file. Finally, the interaction strength was obtained by molecular docking of the target protein and quercetin. A molecular docking process is deemed valid when the calculated binding free energy is less than 5.0 kcal/mol, indicating a stable interaction between the ligand and the receptor.
3 Results
3.1 Intersections between five high-frequency herb targets and obesity-associated HT targets
A total of 531 HMM targets, 234 FTB targets, 449 RB targets, 334 PV targets, and 94 ASR targets were predicted. A total of 2,296 HT targets were retrieved, with 1,832 from GeneCards and 537 from OMIM. A total of 5,671 obesity targets were acquired, with 5,271 targets from GeneCards, 521 targets from OMIM, and 300 targets from DisGeNET. The targets of obesity and HT were merged, resulting in 6,711 obesity-associated HT targets (Supplementary Table S1).
3.2 The GO enrichment results of five high-frequency herbs and four herb pairs
Through GO analysis, we identified that all five herbs were enriched in response to hormone (GO: 0009725). HMM, RB, PV, and ASR were enriched in the positive regulation of programmed cell death (GO: 0043068) (Figure 1F; Table 1; Supplementary Table S2). Furthermore, HMM, RB, and PV were enriched in six identical biological processes, one of which was the response to oxidative stress (GO: 0006979) (Figure 1F; Supplementary Table S2). The MF results revealed that all five herbs were enriched in transcription factor binding (GO: 0008134), protein domain-specific binding (GO: 0019904), and neurotransmitter receptor activity (GO: 0003707). HMM, RB, PV, and ASR were enriched in oxidoreductase activity (GO: 0016491) (Figure 1G; Table 1; Supplementary Table S2). The cellular component (CC) analysis indicated that these five herbs showed significant enrichment in GO terms related to the receptor complex (GO: 0043235) and organelle outer membrane (GO: 0031968) (Figure 1H; Table 1; Supplementary Table S2). In the biological process (BP) enrichment analysis, HMM and RB were both enriched in response to oxidative stress (GO: 0006979), with a GeneRatio value of 0.91 (Figures 1A,E; Supplementary Table S3). The entry for the positive regulation of programmed cell death (GO: 0043068) was also significantly enriched in PV, with a GeneRatio value of 0.84 (Figure 1D; Supplementary Table S3). In the MF enrichment analysis of the five herbs, HMM, RB, PV, and ASR were all enriched in oxidoreductase activity (GO: 0016491), with a GeneRatio value of 1 (Figures 1A,B,D,E; Supplementary Table S3). The Venn diagram results of the BP enrichment analysis showed that the four herb pairs and their related individual herbs were all enriched in response to hormone (GO: 0009725) (Figure 1I; Supplementary Table S3). In addition, the PV-RB and PV-HMM pairs intersected with their related single herb in response to oxidative stress (GO: 0006979) (Figure 1J; Supplementary Table S3). MF enrichment analysis showed that the HMM-ASR pairs intersected with their related herbs on oxidoreductase activity (GO: 0016491) and hormone binding (GO: 0042562) (Figure 1J; Supplementary Table S3).
Figure 1

(A) GO enrichment analysis of HMM. (B) GO enrichment analysis of ASR. (C) GO enrichment analysis of FTB. (D) GO enrichment analysis of PV. (E) GO enrichment analysis of RB. (F) Venn diagram of five-herb BP analysis. (G) Venn diagram of five-herb MF analysis. (H) Venn diagram of five-herb CC analysis. (I) Venn diagram of four-herb pair BP analysis. (J) Venn diagram of four-herb pair MF analysis. (K) Venn diagram of four-herb pair CC analysis. Bubble figure of five high-frequency herbs and four herb pair KEGG enrichment analysis.
Table 1
| BP | MF | CC | |
|---|---|---|---|
| Five herbs | Response to hormone | Transcription factor binding | Receptor complex |
| Response to xenobiotic stimulus | Protein domain-specific binding | Organelle outer membrane | |
| Regulation of body fluid levels | Neurotransmitter receptor activity | ||
| HMM, RB, PV, and ASR | Positive regulation of programmed cell death | Oxidoreductase activity | |
| HMM, RB, PV, and FTB | Phosphatase binding | ||
| HMM, RB, and PV | Response to oxidative stress |
Venn diagram interaction between five high-frequency herbs.
3.3 The KEGG enrichment analysis of five high-frequency herbs and four herb pairs
KEGG enrichment analysis results showed that all of the herbs were enriched in the pathways of cancer (hsa04270) and efferocytosis (hsa04148) (Figure 2). In addition to efferocytosis, pathways related to inflammation, immunity, hormones, and lipid metabolism were screened for bubble figures. IL-17 signaling pathway (hsa04657) was the most significantly enriched item in KEGG of HMM, with a GeneRatio value of 0.32 (Figure 3C; Table 2). PV was enriched in lipid and atherosclerosis (hsa05417), with a GeneRatio value of 0.208 (Figure 3D; Table 2). The first two enriched pathways of FTB were Th17 cell differentiation (hsa04659) and apoptosis—multiple species (hsa04215), with GeneRatio values of 0.14 and 0.25 (Figure 3B; Table 2). The first item enriched in RB was cellular senescence (hsa04218), with a GeneRatio value of 0.23 (Figure 3E; Table 2). ASR was enriched in the estrogen signaling pathway (hsa04915) (Figure 3A; Supplementary Table S4). The significance of gene ratio is the ratio of active ingredients and pathway intersection genes to total pathway genes. Efferocytosis pathways for HMM, RB, and PV were among the top 20 significantly enriched pathways, and the GeneRatio values of HMM and RB were both 0.18 (Table 2). Venn diagrams and bubble figures indicated that the four herb pairs and five herbs were all significantly enriched in efferocytosis (hsa04148) (Figure 2). In addition, the thyroid hormone signaling pathway (hsa04919) is unique to the PV-HMM herb pair compared to the PV and HMM (Figure 3H; Supplementary Table S4). The AMPK signaling pathway (hsa04152) appeared only in PV-RB and PV-FTB herb pair (Figures 3G,I; Supplementary Table S4). The GO enrichment results of FTB and the CC analysis results of the four drug pairs did not show significant rules (Figures 1C,K).
Figure 2

KEGG enrichment intersections between five herbs and four herb pairs.
Figure 3

(A) KEGG enrichment analysis of ASR. (B) KEGG enrichment analysis of FTB. (C) KEGG enrichment analysis of HMM. (D) KEGG enrichment analysis of PV. (E) KEGG enrichment analysis of RB. (F) KEGG enrichment analysis of HMM-ASR. (G) KEGG enrichment analysis of PV-FTB. (H) KEGG enrichment analysis of PV-HMM. (I) KEGG enrichment analysis of PV-RB.
Table 2
| Herb | ID | GeneRatio | Description |
|---|---|---|---|
| RB | hsa04215 | 0.34375 | Apoptosis—multiple species |
| HMM | hsa04657 | 0.31578947 | IL-17 signaling pathway |
| HMM | hsa00140 | 0.30645161 | Steroid hormone biosynthesis |
| HMM | hsa04115 | 0.28 | p53 signaling pathway |
| FTB | hsa04215 | 0.25 | Apoptosis—multiple species |
| RB | hsa04218 | 0.22929936 | Cellular senescence |
| HMM | hsa04914 | 0.22522523 | Progesterone-mediated oocyte maturation |
| RB | hsa04913 | 0.21568627 | Ovarian steroidogenesis |
| RB | hsa00140 | 0.20967742 | Steroid hormone biosynthesis |
| PV | hsa00140 | 0.20967742 | Steroid hormone biosynthesis |
| PV | hsa05417 | 0.20833333 | Lipid and atherosclerosis |
| RB | hsa04064 | 0.2 | NF-kappa B signaling pathway |
| HMM | hsa04064 | 0.19047619 | NF-kappa B signaling pathway |
| PV | hsa04923 | 0.18644068 | Regulation of lipolysis in adipocytes |
| RB | hsa05323 | 0.18085106 | Rheumatoid arthritis |
| HMM | hsa04148 | 0.17948718 | Efferocytosis |
| RB | hsa04148 | 0.17948718 | Efferocytosis |
| HMM | hsa04630 | 0.16666667 | JAK–STAT signaling pathway |
| PV | hsa04148 | 0.1474359 | Efferocytosis |
| FTB | hsa04659 | 0.13888889 | Th17 cell differentiation |
Top 20 KEGG enrichment analysis terms for HMM, FTB, RB, PV, and ASR.
3.4 KEGG enrichment analysis of quercetin in efferocytosis
Quercetin was found to be one of the active ingredients by matching the targets of herbs and efferocytosis (Supplementary Table S5). The enrichment results indicated that efferocytosis was one of the pathways for quercetin and obesity-associated HT (Figure 4A). We further performed KEGG enrichment of the intersection of quercetin-HT targets and quercetin-obesity targets (Figures 4B,C). As shown in Figure 4, we observed that in addition to efferocytosis, the IL-17 signaling pathway and NF-κB signaling pathway were also the interaction pathways for the three conditions.
Figure 4

Bubble figure of quercetin KEGG enrichment analysis. (A) Bubble figure of quercetin and obesity-HT. (B) Bubble figure of quercetin and obesity. (C) Bubble figure of quercetin and HT.
3.5 Protein–protein interaction network construction and screening of key targets
A total of 38 proteins and 267 edges were obtained after the 38 efferocytosis targets were imported into the STRING database (Figure 5A), and then, the data were imported into Cytoscape to obtain the PPI network diagram (Figure 5B). The top 15 key nodes screened were CASP3, PPARG, HIF1A, MAPK3, JUN, PTGS2, TGFB1, IL10, CD36, SIRT1, JAK2, MAPK1, PPARA, MAPK14, and PTPN11 (Figure 5C). Reducing PPARG signaling results in defective efferocytosis (20). Other targets do not have direct evidence of relevance to efferocytosis, and even if they bind well with quercetin, further experiments are needed to verify their relationship with efferocytosis.
Figure 5
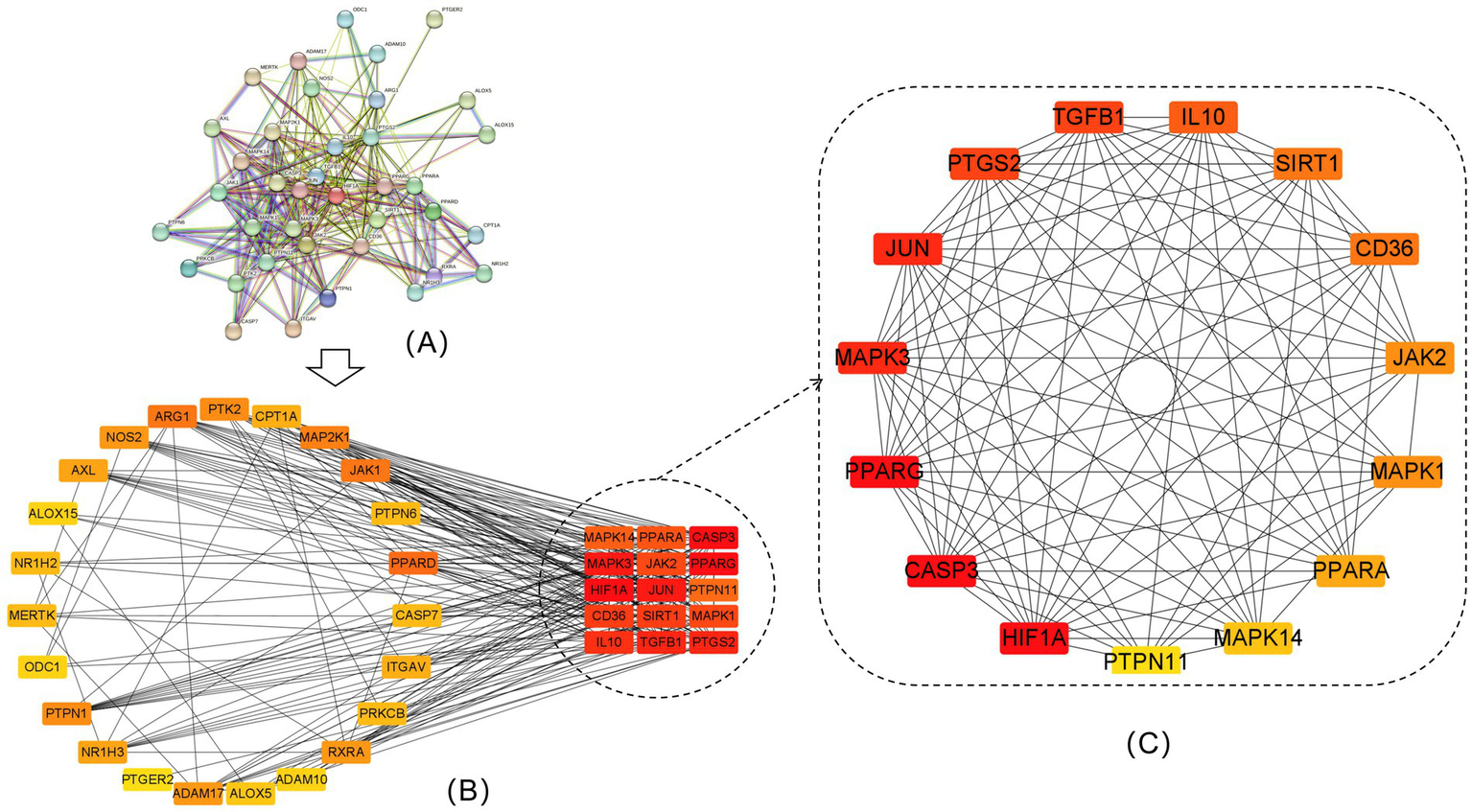
PPI network of intersecting targets and top 15 intersecting targets. (A) PPI network of the 38 efferocytosis targets. (B) Visualization of 38 efferocytosis targets by Cytoscape software. (C) The top 15 core targets for efferocytosis.
3.6 Molecular docking results
The docking results showed that 17 targets were well bound to quercetin. MERTK and AXL; the results showed that all proteins bound well with quercetin. The docking scores of the 17 targets are shown in Table 3. Lower docking values indicate stronger binding affinities, with values less than −5.0 kcal/mol indicating potential binding and values less than −7.0 kcal/mol indicating strong binding affinities (33). MERTK, AXL, and PPARG are involved in the process of efferocytosis (20). Other proteins have not been reported to be directly involved in efferocytosis and require further experimental evidence.
Table 3
| Proteins | PDBID | Binding energy |
|---|---|---|
| CASP3 | 5IBP | 7.2 |
| PPARG | 9F7W | 7.6 |
| HIF1A | 2ILM | 8 |
| MAPK3 | 4QTB | 7.7 |
| JUN | 6Y3V | 6.4 |
| PTGS2 | 5F19 | 8.7 |
| TGFB1 | 4KV5 | 7.4 |
| IL10 | 1INR | 6.6 |
| CD36 | 5LGD | 8.5 |
| SIRT1 | 8ANB | 9.2 |
| JAK2 | 3UGC | 7.9 |
| MAPK1 | 5WP1 | 7.5 |
| PPARA | 6KAX | 8.2 |
| MAPK14 | 3LFF | 8.3 |
| PTPN11 | 3ZM0 | 7.4 |
| MERTK | 7AB0 | 6.5 |
| AXL | 4RA0 | 7.4 |
Binding energy of efferocytosis-related proteins.
4 Discussion
KEGG enrichment analysis showed that efferocytosis is the key pathway in obesity-associated HT. We also found that quercetin is the potential ingredient of efferocytosis. However, Cesidio et al. found that quercetin can inhibit the growth and function of normal thyroid cells (34). This toxic result is derived from in vitro cell experiments and animal experiments. In addition, although it has been reported that quercetin has an inhibitory effect on thyroid peroxidase, this conclusion was drawn from in vitro experiments on pig thyroids (35). It is well known that quercetin has a low oral bioavailability (36). It has also been mentioned that at flavonoid concentrations above 20 μM, the effects observed in vitro are hardly applicable to human intake of flavonoids through diet or dietary supplements (37). Plasma concentrations of flavonoids can reach 0.7 to 2.5 μM in subjects who consume large amounts of vegetables, such as onions, or who take dietary supplements (37). The toxic dose of quercetin based on animal experiments was equivalent to approximately 8 mg/kg in the human body (37). Therefore, whether it can reach toxic levels in the human body at normal doses of TCM applications requires further in vivo experiments to confirm. A relevant study has indicated that a daily intake of 1 g of quercetin, when used as a dietary supplement, is considered safe (38). Another study reported that the recommended dose of quercetin was 100–1,200 mg/d, with an intake of up to 2 g/d (37, 39). In addition, a clinical study mentioned that supplementation with quercetin at 500 mg/day and 1,000 mg/day for 12 weeks significantly increased plasma quercetin levels but did not affect innate immune function or inflammatory markers in adult women in the community (40). Therefore, compared with its toxicity, the ability of anti-inflammatory and regulate immunity should be more concerned (36).
Recent studies have shown that quercetin can regulate obesity and obesity-related complications (41–43). Quercetin has anti-inflammatory and anti-oxidative effects (44, 45); it also has been shown to have anti-obesity effects in adipocyte cultures and animal models (46). Jazyra Zynat et al. discovered that quercetin could significantly influence adipose tissue in obese conditions. It achieves this by mitigating intracellular oxidative stress, diminishing chronic low-grade inflammation, and suppressing adipogenesis and lipogenesis (44, 47). Mie Nishimura et al. have shown that quercetin-rich onion powder can reduce HDL cholesterol in obese people (48). In addition, it has been reported that quercetin can prevent obesity by regulating intestinal flora (49–51). It has also been reported that quercetin can intervene in obesity by regulating the expression of liver lipid metabolism-related genes and pro-inflammatory genes (50, 52).
Therefore, it is suggested that quercetin may have a potential intervention effect on efferocytosis. Quercetin has been experimentally demonstrated to treat obesity, and enrichment analysis showed that the efferocytosis was significantly enriched in the intersection genes of quercetin associated with HT. However, the intervention of quercetin on HT still needs further clinical studies.
The regulatory effects of quercetin on some ADs have been demonstrated by in vivo animal experiments, such as experimental osteoarthritis (53). The five herbs mentioned in this study are also frequently found in clinical compound experiments (6, 8), such as Shugan Sanjie Prescription (8), which includes five herbs. Buzhong Yiqi decoction is a traditional formula that contains RB, HMM, and ASR. In a study of the rat model of experimental autoimmune thyroiditis, Buzhong Yiqi granules significantly reduced antibody levels and improved thyroid function in the model group (54). A clinical study has also claimed that BuzhongYiqi decoction can enhance the immunity of the elderly (55). In addition, clinical studies have shown that PV can reduce thyroid antibody levels, improve thyroid function, and reduce thyroid volume (6, 56). HMM can reduce the levels of TPOAb and TgAb and promote the apoptosis of HT mice (6). Saikosaponin D, the active component of RB, could treat HT by regulating macrophage polarization (12).
Obesity is a risk factor for HT (14). Song et al. found that obesity increases the risk of overt and subclinical hypothyroidism, thyroid peroxidase antibody (TPOAb) positivity, but not thyroglobulin antibody (TgAb) positivity (16). Yan et al. found that obesity with HT had a higher incidence of subclinical hypothyroidism and TgAb positivity than HT subjects (57). In addition, obese children and adolescents with autoimmune thyroid diseases showed higher thyroid-stimulating hormone (TSH), lower thyroid hormone levels, a higher risk of hypothyroidism, and no association with antibody levels (58). Maria et al. found that overweight or obese women with HT have higher oxidative stress levels compared with women with normal BMI with HT (59). All these research studies suggest that treating obesity may be a valuable therapy for treating HT (Figure 6).
Figure 6

Relationship between HT, obesity, autoimmunity, and efferocytosis.
Obesity is also related to efferocytosis. Li et al. found that efferocytosis was defective in obese mice (60). The mechanism of defective efferocytosis in obesity has also been widely studied. Reduced PPARG signaling has been reported as one of the factors contributing to defective efferocytosis (20). Luo et al. mentioned that PPARG expression levels were reduced in obese mice, which were further found to be associated with defective macrophage erythropoietin signaling (61). Suresh Babu et al. showed that efferocytosis was altered in macrophages due to the shedding of MERTK by ADAM17, which functions as the main protease, using obese and diabetic mice (62, 63). A clinical study on centripetal obesity further confirmed that obesity, particularly when fat is predominantly deposited around the abdominal area, is associated with the cleavage of MERTK by ADAM17 to produce soluble MERTK (64) (Figure 6).
Impaired efferocytosis has been implicated as a pathogenic mechanism in several ADs, and enhancing efferocytosis has emerged as a potential therapeutic strategy for these conditions (65–70). In systemic lupus erythematosus, defective efferocytosis is closely associated with aberrations in PPAR signaling, LXR signaling, ABCA1 expression, and C1q membrane protein deficiency (71, 72). Similarly, macrophages derived from non-obese diabetic (NOD) mice, an established animal model of type 1 diabetes, exhibit defective efferocytosis both in vivo and in vitro (70). In systemic sclerosis, efferocytosis is frequently impaired due to the presence of inhibitory IgG anti-apoptotic cell antibodies and phagocyte dysfunction (69). In osteoarthritis, impaired efferocytosis and elevated levels of apoptotic cells have been observed in synovial tissues, which have been linked to the deficiency of membrane-bound TAM receptors (Tyro3, AXL, and MERTK) (73). In recent years, efferocytosis-informed nanomimetics have demonstrated therapeutic efficacy in murine models of rheumatoid arthritis (74, 75). Regulating macrophage polarization is an effective means to treat inflammatory bowel disease (67), and efferocytosis will promote macrophage M2 polarization (68). In brief, efferocytosis plays a crucial role in several ADs. HT is also a form of AD and is likely to be similarly affected by efferocytosis dysfunction. Therefore, it is reasonable to infer that the promotion of efferocytosis may be one of the effective ways of treating HT (Figure 6).
Since this study relies solely on network pharmacology methods, it inevitably possesses certain limitations. The relationship between efferocytosis and obesity-associated HT has not been supported by experimental and clinical studies. Currently, there is a scarcity of studies on HT as a risk factor for obesity; it still needs further experimental and clinical research. The treatment of quercetin for HT also needs to be confirmed by further clinical studies.
5 Conclusion
The therapeutic effect of five frequently prescribed herbs in treating obesity-associated HT may be achieved through the regulation of efferocytosis. Most of the results from network pharmacology and molecular docking techniques have pointed to efferocytosis as a potential pathway to treat obesity-associated HT. Quercetin is one of the potential ingredients related to the efferocytosis pathway, and it binds well with most of the key efferocytosis-related proteins.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JZ: Data curation, Software, Writing – original draft, Writing – review & editing. TG: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the General Program of the National Natural Science Foundation of China (Grant No. 82174359 and No. 81874441).
Acknowledgments
The author Jiahao Zhou would like to thank singer Shen Zhou. The path ahead may be fraught with uncertainties, yet it is illuminated by hope. The author hopes that the next time she gets to meet singer Shen Zhou, the author has become a braver and stronger person. Finally, the author would like to thank herself and her brave heart.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1538867/full#supplementary-material
SUPPLEMENTARY TABLE S1Information for potential targets of five herbs and obesity-associated HT.
SUPPLEMENTARY TABLE S2Venn diagram data from five-herb GO enrichment analysis.
SUPPLEMENTARY TABLE S3Venn diagram data from four-herb pair GO enrichment analysis.
SUPPLEMENTARY TABLE S4Bubble figure and Venn diagram data from KEGG enrichment analysis.
SUPPLEMENTARY TABLE S5Active ingredient analysis data.
Abbreviations
HT, Hashimoto’s thyroiditis; TCM, Traditional Chinese medicine; ADs, Autoimmune diseases; HMM, Hedysarum multijugum Maxim; RB, Radix Bupleuri; PV, Prunella vulgaris; FTB, Fritillaria thunbergii Bulbus; ASR, Angelicae sinensis Radix; TCMSP, Traditional Chinese Medicine Systems Pharmacology; BP, Biological process; MF, Molecular function; CC, Cellular component.
References
1.
Ralli M Angeletti D Fiore M D'Aguanno V Lambiase A Artico M et al . Hashimoto's thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. (2020) 19:102649. doi: 10.1016/j.autrev.2020.102649
2.
Huwiler VV Maissen-Abgottspon S Stanga Z Mühlebach S Trepp R Bally L et al . Selenium supplementation in patients with Hashimoto thyroiditis: a systematic review and Meta-analysis of randomized clinical trials. Thyroid. (2024) 34:295–313. doi: 10.1089/thy.2023.0556
3.
Rodziewicz A Szewczyk A Bryl E . Gluten-free diet alters the gut microbiome in women with autoimmune thyroiditis. Nutrients. (2024) 16:685. doi: 10.3390/nu16050685
4.
Taheriniya S Arab A Hadi A Fadel A Askari G . Vitamin D and thyroid disorders: a systematic review and Meta-analysis of observational studies. BMC Endocr Disord. (2021) 21:171. doi: 10.1186/s12902-021-00831-5
5.
Wojtas N Wadolowska L Bandurska-Stankiewicz E . Evaluation of qualitative dietary protocol (Diet4Hashi) application in dietary counseling in Hashimoto thyroiditis: study protocol of a randomized controlled trial. Int J Environ Res Public Health. (2019) 16:841. doi: 10.3390/ijerph16234841
6.
Huang S Ziros PG Chartoumpekis DV Psarias G Duntas L Zuo X et al . Traditional Chinese medicine for Hashimoto's thyroiditis: focus on selenium and antioxidant phytochemicals. Antioxidants. (2024) 13:868. doi: 10.3390/antiox13070868
7.
Zhou L Luo JL Sun A Yang HY Lin YQ Han L . Clinical efficacy and molecular mechanism of Chinese medicine in the treatment of autoimmune thyroiditis. J Ethnopharmacol. (2024) 323:117689. doi: 10.1016/j.jep.2023.117689
8.
Luo J Zhou L Sun A Yang H Zhang P Liu K et al . Herbal medicine for Hashimoto's thyroiditis: a systematic review and network meta-analysis. J Ethnopharmacol. (2024) 323:117663. doi: 10.1016/j.jep.2023.117663
9.
Wei M Ma W Zhang W Yin D Tang Y Jia W et al . Efficacy and safety of Ophiocordyceps sinensis in the treatment of Hashimoto's thyroiditis: a systematic review and meta-analysis. Front Pharmacol. (2023) 14:1272124. doi: 10.3389/fphar.2023.1272124
10.
Ge JC Qian Q Gao YH Zhang YF Li YX Wang X et al . Toxic effects of Tripterygium glycoside tablets on the reproductive system of male rats by metabolomics, cytotoxicity, and molecular docking. Phytomedicine. (2023) 114:154813. doi: 10.1016/j.phymed.2023.154813
11.
Zhao W Duan C Liu Y Lu G Lyu Q Liu X et al . Modulating effects of Astragalus polysaccharide on immune disorders via gut microbiota and the TLR4/NF-κB pathway in rats with syndrome of dampness stagnancy due to spleen deficiency. J Zhejiang Univ Sci B. (2023) 24:650–62. doi: 10.1631/jzus.B2200491
12.
Du P Xu J Jiang Y Zhao J Gao C Fang Y et al . Saikosaponin-d attenuates Hashimoto's thyroiditis by regulating macrophage polarization. J Immunol Res. (2022) 2022:7455494. doi: 10.1155/2022/7455494
13.
Chen J Feng X Huang Q . Modulation of T-bet and GATA-3 expression in experimental autoimmune thyroiditis rats through Ginsenoside treatment. Endocr Res. (2016) 41:28–33. doi: 10.3109/07435800.2015.1066800
14.
Tsigalou C Vallianou N Dalamaga M . Autoantibody production in obesity: is there evidence for a link between obesity and autoimmunity?Curr Obes Rep. (2020) 9:245–54. doi: 10.1007/s13679-020-00397-8
15.
Ostrowska L Gier D Zyśk B . The influence of reducing diets on changes in thyroid parameters in women suffering from obesity and Hashimoto's disease. Nutrients. (2021) 13:862. doi: 10.3390/nu13030862
16.
Song RH Wang B Yao QM Li Q Jia X Zhang JA . The impact of obesity on thyroid autoimmunity and dysfunction: a systematic review and Meta-analysis. Front Immunol. (2019) 10:2349. doi: 10.3389/fimmu.2019.02349
17.
Schleh MW Caslin HL Garcia JN Mashayekhi M Srivastava G Bradley AB et al . Metaflammation in obesity and its therapeutic targeting. Sci Transl Med. (2023) 15:eadf9382. doi: 10.1126/scitranslmed.adf9382
18.
Boada-Romero E Martinez J Heckmann BL Green DR . The clearance of dead cells by efferocytosis. Nat Rev Mol Cell Biol. (2020) 21:398–414. doi: 10.1038/s41580-020-0232-1
19.
Doran AC Yurdagul A Jr Tabas I . Efferocytosis in health and disease. Nat Rev Immunol. (2020) 20:254–67. doi: 10.1038/s41577-019-0240-6
20.
Mehrotra P Ravichandran KS . Drugging the efferocytosis process: concepts and opportunities. Nat Rev Drug Discov. (2022) 21:601–20. doi: 10.1038/s41573-022-00470-y
21.
Trzeciak A Wang YT Perry JSA . First we eat, then we do everything else: the dynamic metabolic regulation of efferocytosis. Cell Metab. (2021) 33:2126–41. doi: 10.1016/j.cmet.2021.08.001
22.
Adkar SS Leeper NJ . Efferocytosis in atherosclerosis. Nat Rev Cardiol. (2024) 21:762–79. doi: 10.1038/s41569-024-01037-7
23.
Zhang M Wei J Sun Y He C Ma S Pan X et al . The efferocytosis process in aging: supporting evidence, mechanisms, and therapeutic prospects for age-related diseases. J Adv Res. (2024) 69:31–49. doi: 10.1016/j.jare.2024.03.008
24.
Abdolmaleki F Farahani N Gheibi Hayat SM Pirro M Bianconi V Barreto GE et al . The role of Efferocytosis in autoimmune diseases. Front Immunol. (2018) 9:1645. doi: 10.3389/fimmu.2018.01645
25.
Kimani SG Geng K Kasikara C Kumar S Sriram G Wu Y et al . Contribution of defective PS recognition and Efferocytosis to chronic inflammation and autoimmunity. Front Immunol. (2014) 5:566. doi: 10.3389/fimmu.2014.00566
26.
Liu RH . Health-promoting components of fruits and vegetables in the diet. Adv Nutr. (2013) 4:384s–92s. doi: 10.3945/an.112.003517
27.
Cialdella-Kam L Nieman D Knab A Shanely R Meaney M Jin F et al . A mixed flavonoid-fish oil supplement induces immune-enhancing and anti-inflammatory transcriptomic changes in adult obese and overweight women-a randomized controlled trial. Nutrients. (2016) 8:277. doi: 10.3390/nu8050277
28.
Srivastava S Somasagara RR Hegde M Nishana M Tadi SK Srivastava M et al . Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. (2016) 6:24049. doi: 10.1038/srep24049
29.
Castrejón-Tellez V Rodríguez-Pérez J Pérez-Torres I Pérez-Hernández N Cruz-Lagunas A Guarner-Lans V et al . The effect of resveratrol and quercetin treatment on PPAR mediated uncoupling protein (UCP-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int J Mol Sci. (2016) 17:1069. doi: 10.3390/ijms17071069
30.
Tsai CF Chen GW Chen YC Shen CK Lu DY Yang LY et al . Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/Antioxidative balance. Nutrients. (2021) 14:67. doi: 10.3390/nu14010067
31.
Myers KV Amend SR Pienta KJ . Targeting Tyro3, Axl and MerTK (TAM receptors): implications for macrophages in the tumor microenvironment. Mol Cancer. (2019) 18:94. doi: 10.1186/s12943-019-1022-2
32.
Bardou P Mariette J Escudié F Djemiel C Klopp C . jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. (2014) 15:293. doi: 10.1186/1471-2105-15-293
33.
Li C Wen R Liu DW Yan LP Gong Q Yu H . Assessment of the potential of Sarcandra glabra (Thunb.) Nakai. In treating ethanol-induced gastric ulcer in rats based on metabolomics and network analysis. Front Pharmacol. (2022) 13:810344. doi: 10.3389/fphar.2022.810344
34.
Giuliani C di Dalmazi G Bucci I Napolitano G . Quercetin and thyroid. Antioxidants. (2024) 13:10:202. doi: 10.3390/antiox13101202
35.
Habza-Kowalska E Kaczor AA Żuk J Matosiuk D Gawlik-Dziki U . Thyroid peroxidase activity is inhibited by phenolic compounds-impact of interaction. Molecules. (2019) 24:766. doi: 10.3390/molecules24152766
36.
Li Y Yao J Han C Yang J Chaudhry M Wang S et al . Quercetin, inflammation and immunity. Nutrients. (2016) 8:167. doi: 10.3390/nu8030167
37.
Di Dalmazi G Giuliani C . Plant constituents and thyroid: a revision of the main phytochemicals that interfere with thyroid function. Food Chem Toxicol. (2021) 152:112158. doi: 10.1016/j.fct.2021.112158
38.
Deepika D Maurya PK . Health benefits of quercetin in age-related diseases. Molecules. (2022) 27:2498. doi: 10.3390/molecules27082498
39.
Andres S Pevny S Ziegenhagen R Bakhiya N Schäfer B Hirsch-Ernst KI et al . Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. (2018) 62:447. doi: 10.1002/mnfr.201700447
40.
Heinz SA Henson DA Nieman DC Austin MD Jin F . A 12-week supplementation with quercetin does not affect natural killer cell activity, granulocyte oxidative burst activity or granulocyte phagocytosis in female human subjects. Br J Nutr. (2010) 104:849–57. doi: 10.1017/S000711451000156X
41.
Hosseini A Razavi BM Banach M Hosseinzadeh H . Quercetin and metabolic syndrome: a review. Phytother Res. (2021) 35:5352–64. doi: 10.1002/ptr.7144
42.
Leiherer A Stoemmer K Muendlein A Saely C Kinz E Brandtner E et al . Quercetin impacts expression of metabolism- and obesity-associated genes in SGBS adipocytes. Nutrients. (2016) 8:282. doi: 10.3390/nu8050282
43.
Le NH Kim CS Park T Park JH Sung MK Lee DG et al . Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat Inflamm. (2014) 2014:834294. doi: 10.1155/2014/834294
44.
Pérez-Torres I Castrejón-Téllez V Soto ME Rubio-Ruiz ME Manzano-Pech L Guarner-Lans V . Oxidative stress, plant natural antioxidants, and obesity. Int J Mol Sci. (2021) 22:786. doi: 10.3390/ijms22041786
45.
Wang Y Li Z He J Zhao Y . Quercetin regulates lipid metabolism and fat accumulation by regulating inflammatory responses and Glycometabolism pathways: a review. Nutrients. (2024) 16:102. doi: 10.3390/nu16081102
46.
Carrasco-Pozo C Cires MJ Gotteland M . Quercetin and epigallocatechin Gallate in the prevention and treatment of obesity: from molecular to clinical studies. J Med Food. (2019) 22:753–70. doi: 10.1089/jmf.2018.0193
47.
Zhao Y Chen B Shen J Wan L Zhu Y Yi T et al . The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxidative Med Cell Longev. (2017) 2017:1459497. doi: 10.1155/2017/1459497
48.
Nishimura M Muro T Kobori M Nishihira J . Effect of daily ingestion of quercetin-rich onion powder for 12 weeks on visceral fat: a randomised, double-blind, placebo-controlled, parallel-group study. Nutrients. (2019) 12:91. doi: 10.3390/nu12010091
49.
Shabbir U Rubab M Daliri EBM Chelliah R Javed A Oh DH . Curcumin, quercetin, Catechins and metabolic diseases: the role of gut microbiota. Nutrients. (2021) 13:206. doi: 10.3390/nu13010206
50.
Jung CH Cho I Ahn J Jeon TI Ha TY . Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother Res. (2013) 27:139–43. doi: 10.1002/ptr.4687
51.
Zhao L Zhu X Xia M Li J Guo AY Zhu Y et al . Quercetin ameliorates gut microbiota Dysbiosis that drives hypothalamic damage and hepatic lipogenesis in monosodium glutamate-induced abdominal obesity. Front Nutr. (2021) 8:671353. doi: 10.3389/fnut.2021.671353
52.
Barrios-Nolasco A Domínguez-López A Miliar-García A Cornejo-Garrido J Jaramillo-Flores ME . Anti-inflammatory effect of Ethanolic extract from Tabebuia rosea (Bertol.) DC., quercetin, and anti-obesity drugs in adipose tissue in Wistar rats with diet-induced obesity. Molecules. (2023) 28:3801. doi: 10.3390/molecules28093801
53.
Mamani-Matsuda M Kauss T al-Kharrat A Rambert J Fawaz F Thiolat D et al . Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem Pharmacol. (2006) 72:1304–10. doi: 10.1016/j.bcp.2006.08.001
54.
Yuezhu W Yuyang Z Jiajun Q Yuyuan LU Zhongyuan X . Protective effect of thyroid and restores of ovarian function of Buzhong Yiqi granule on experimental autoimmune thyroiditis in female rats. J Tradit Chin Med. (2024) 44:315–23. doi: 10.19852/j.cnki.jtcm.20240203.008
55.
Kuroiwa A Liou S' Yan H Eshita A Naitoh S Nagayama A . Effect of a traditional Japanese herbal medicine, hochu-ekki-to (Bu-Zhong-Yi-qi Tang), on immunity in elderly persons. Int Immunopharmacol. (2004) 4:317–24. doi: 10.1016/j.intimp.2003.12.004
56.
Zhang YL Hu RX Zhao H Yang W Yu DD Li HM et al . Systematic review and trail sequential analysis of preparation of Xiakucao for Hashimoto's thyroiditis. Zhongguo Zhong Yao Za Zhi. (2020) 45:5777–88. doi: 10.19540/j.cnki.cjcmm.20200909.501
57.
Zynat J Li S Ma Y Han L Ma F Zhang Y et al . Impact of abdominal obesity on thyroid auto-antibody positivity: abdominal obesity can enhance the risk of thyroid autoimmunity in men. Int J Endocrinol. (2020) 2020:6816198. doi: 10.1155/2020/6816198
58.
González-Mereles AP Arguinzoniz-Valenzuela SL López-López AP Maqueda-Tenorio SE González-Baqué I . Overweight and obesity in children and adolescents with chronic autoimmune thyroiditis. Bol Med Hosp Infant Mex. (2021) 78:424–31. doi: 10.24875/BMHIM.20000292
59.
Giannakou M Saltiki K Mantzou E Loukari E Philippou G Terzidis K et al . The effect of obesity and dietary habits on oxidative stress in Hashimoto’s thyroiditis. Endocr Connect. (2018) 7:990–7. doi: 10.1530/EC-18-0272
60.
Li S Sun Y Liang CP Thorp EB Han S Jehle AW et al . Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of Ob/Ob mice and reversal by a fish oil diet. Circ Res. (2009) 105:1072–82. doi: 10.1161/CIRCRESAHA.109.199570
61.
Luo B Wang Z Zhang Z Shen Z Zhang Z . The deficiency of macrophage erythropoietin signaling contributes to delayed acute inflammation resolution in diet-induced obese mice. Biochim Biophys Acta Mol basis Dis. (2019) 1865:339–49. doi: 10.1016/j.bbadis.2018.10.005
62.
Suresh Babu S Thandavarayan RA Joladarashi D Jeyabal P Krishnamurthy S Bhimaraj A et al . MicroRNA-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes. Sci Rep. (2016) 6:36207. doi: 10.1038/srep36207
63.
Cai B Thorp EB Doran AC Sansbury BE Daemen MJAP Dorweiler B et al . MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. (2017) 127:564–8. doi: 10.1172/JCI90520
64.
Purnama CA Meiliana A Defi IR Amalia R Sartika CR Wijaya A et al . The important role of phosphatidylserine, ADAM17, TNF-alpha, and soluble MER on Efferocytosis activity in central obesity. J Obes. (2024) 2024:1424404. doi: 10.1155/2024/1424404
65.
Baumann I Kolowos W Voll RE Manger B Gaipl U Neuhuber WL et al . Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. (2002) 46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K
66.
Schneider K Arandjelovic S . Apoptotic cell clearance components in inflammatory arthritis. Immunol Rev. (2023) 319:142–50. doi: 10.1111/imr.13256
67.
Du Y Rong L Cong Y Shen L Zhang N Wang B . Macrophage polarization: an effective approach to targeted therapy of inflammatory bowel disease. Expert Opin Ther Targets. (2021) 25:191–209. doi: 10.1080/14728222.2021.1901079
68.
Angsana J Chen J Liu L Haller CA Chaikof EL . Efferocytosis as a regulator of macrophage chemokine receptor expression and polarization. Eur J Immunol. (2016) 46:1592–9. doi: 10.1002/eji.201546262
69.
Manoussakis MN Fragoulis GE Vakrakou AG Moutsopoulos HM . Impaired clearance of early apoptotic cells mediated by inhibitory IgG antibodies in patients with primary Sjögren's syndrome. PLoS One. (2014) 9:e112100. doi: 10.1371/journal.pone.0112100
70.
O'Brien BA Geng X Orteu CH Huang Y Ghoreishi M Zhang YQ et al . A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. (2006) 26:104–15. doi: 10.1016/j.jaut.2005.11.006
71.
Kidani Y Bensinger SJ . Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol Rev. (2012) 249:72–83. doi: 10.1111/j.1600-065X.2012.01153.x
72.
Walport MJ Davies KA Botto M . C1q and systemic lupus erythematosus. Immunobiology. (1998) 199:265–85. doi: 10.1016/S0171-2985(98)80032-6
73.
Li Q Liu H Yin G Xie Q . Efferocytosis: current status and future prospects in the treatment of autoimmune diseases. Heliyon. (2024) 10:e28399. doi: 10.1016/j.heliyon.2024.e28399
74.
Yuan S Chai Y Xu J Wang Y Jiang L Lu N et al . Engineering Efferocytosis-mimicking Nanovesicles to regulate joint anti-inflammation and peripheral immunosuppression for rheumatoid arthritis therapy. Adv Sci. (2024) 11:e2404198. doi: 10.1002/advs.202404198
75.
Zhang S Liu Y Jing W Chai Q Tang C Li Z et al . Remodeling articular immune homeostasis with an efferocytosis-informed nanoimitator mitigates rheumatoid arthritis in mice. Nat Commun. (2023) 14:817. doi: 10.1038/s41467-023-36468-2
Summary
Keywords
hashimoto’s thyroiditis, obesity, quercetin, efferocytosis, network pharmacology, molecular docking
Citation
Zhou J and Gao T (2025) Therapeutic potential of five frequently prescribed herbs in obesity-associated Hashimoto’s thyroiditis: insights from efferocytosis regulation. Front. Med. 12:1538867. doi: 10.3389/fmed.2025.1538867
Received
03 December 2024
Accepted
05 May 2025
Published
19 May 2025
Volume
12 - 2025
Edited by
Alice Chen, Consultant, Potomac, MD, United States
Reviewed by
Fazil Ahmad, Imam Abdulrahman Bin Faisal University, Saudi Arabia
Tainan Lin, Fujian Provincial Governmental Hospital, China
Updates
Copyright
© 2025 Zhou and Gao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianshu Gao, gaotianshu67@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.