- 1Department of Hematology, Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
- 2Division of Laboratory Medicine, Central Hospital of Dalian University of Technology, Dalian, China
- 3Scientific Research Division, The First People’s Hospital of Baiyin, Baiyin, China
- 4Third Affiliated Hospital of Gansu University of Chinese Medicine, Lanzhou, China
With advancements in medical care and improved public health in China, the incidence of hookworm infections has significantly decreased, particularly in first-tier cities. We report a case of severe microcytic hypochromic anaemia caused by hookworm disease. The patient received multiple blood transfusions for unexplained anaemia, with negative faecal smear results. GI endoscopic examination revealed hookworms in the pyloric ring, antrum, and duodenum, which were removed using biopsy forceps. Morphological analysis identified the worms as Ancylostoma duodenale. The patient was treated with a single dose of 400 mg albendazole and hematinics. Follow-up haemoglobin testing 3 months later showed an improvement to 126 g/L (115–150 g/L). This case highlights the importance of GI endoscopy diagnostics in identifying a typical presentations of hookworm disease, particularly in first-tier cities. Timely and accurate diagnosis of hookworm infections is essential for preventing long-term health consequences and reducing associated healthcare costs.
Introduction
Soil-transmitted helminths remain a significant public health concern, particularly in developing countries, with an estimated 1.5 billion people infected at least once in their lifetime worldwide (1). Despite extensive advancements in medical management, neglected tropical diseases persist in China, likely due to inadequate hygiene practices, particularly in rural areas (2).
Historically, soil-transmitted helminth infections were widespread in China during the 1950s–1960s but have been successfully controlled through significant governmental efforts (2). Consequently, hookworm infections have become extremely rare (3), and many medical practitioners are now unfamiliar with the disease.
The primary species responsible for hookworm infections include Necator americanus, Ancylostoma duodenale, and Ancylostoma ceylanicum, which are commonly found in tropical and subtropical regions (4). While hookworm infections were historically prevalent in China, improvements in healthcare and sanitation have significantly reduced their occurrence, particularly in urban areas. As of 2019, the national infection rate was 0.85%, with a weighted prevalence of 0.66%. However, some provinces, such as Sichuan (4.75%), Chongqing (2.54%), and Hainan (2.44%), still report relatively high infection rates (3).
Factors influencing infection rates include age, sex, sanitation, and lifestyle (5). The severity of hookworm infection depends on worm burden as well as the host’s nutritional status, immunity, and overall health (6). Clinical manifestations are often non-specific, leading to misdiagnoses and delays in treatment.
Due to the rarity of hookworm infection in modern clinical practice, particularly in first-tier cities, most physicians are unfamiliar with its presentation. In the case described here, physicians at the Ninth People’s Hospital opted for an endoscopic examination after other diagnostic methods failed to identify the cause. Although gastrointestinal endoscopy is not a standard routine diagnostic tool for hookworm infection, this case underscores the need for increased awareness among medical practitioners. By presenting this case, we aim to highlight the importance of considering hookworm infection in patients with unexplained iron deficiency anaemia, particularly in individuals from endemic regions.
Case report
A 60-year-old female presented with severe iron deficiency anaemia of unknown origin, reporting fatigue, malaise, and loss of appetite for 6 months. She had recently been living in rural Sichuan, where she had received multiple red blood cell transfusions at a local hospital for persistent anaemia before being referred to our hospital in Shanghai. Physical examination revealed extreme pallor without other notable findings. The patient initially received medical care at Guang’an District People’s Hospital, Guang’an City, Sichuan Province. However, due to uncertainty regarding the underlying condition, she was referred to the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, for further evaluation.
Initial laboratory tests revealed the following results: red blood cell count, 2.84 × 1012/L (normal: 3.8–5.1 × 1012/L); haemoglobin, 38 g/L (normal: 115–150 g/L); haematocrit, 20.7% (normal: 35–45%); mean corpuscular volume (MCV), 59.6 fL (normal: 82.9–98 fL); mean corpuscular haemoglobin (MCH), 15.1 pg (normal: 27–34 pg); and mean corpuscular haemoglobin concentration (MCHC), 253 g/L (normal: 316–354 g/L). Eosinophilia (27.1%; 1.49 × 109/L) (normal: 0.4–8%; 0.02–0.52 × 109/L) suggested a parasitic or allergic process (Supplementary Table 1).
Liver and renal function tests, urinalysis, and stool analysis revealed no abnormalities, and no eggs or worms were observed in the faeces under microscopy. The faecal specimens were examined three times under a microscope; however, the faecal occult blood test was positive. Additionally, the patient was not immunocompromised and had not received any other medications. Interestingly, no eggs were detected in the faeces, which puzzled the clinicians and prompted an endoscopy. A possible explanation is that both the clinicians and laboratory staff were inexperienced in diagnosing hookworm in a hospital from a first-tier city, despite it being a teaching hospital.
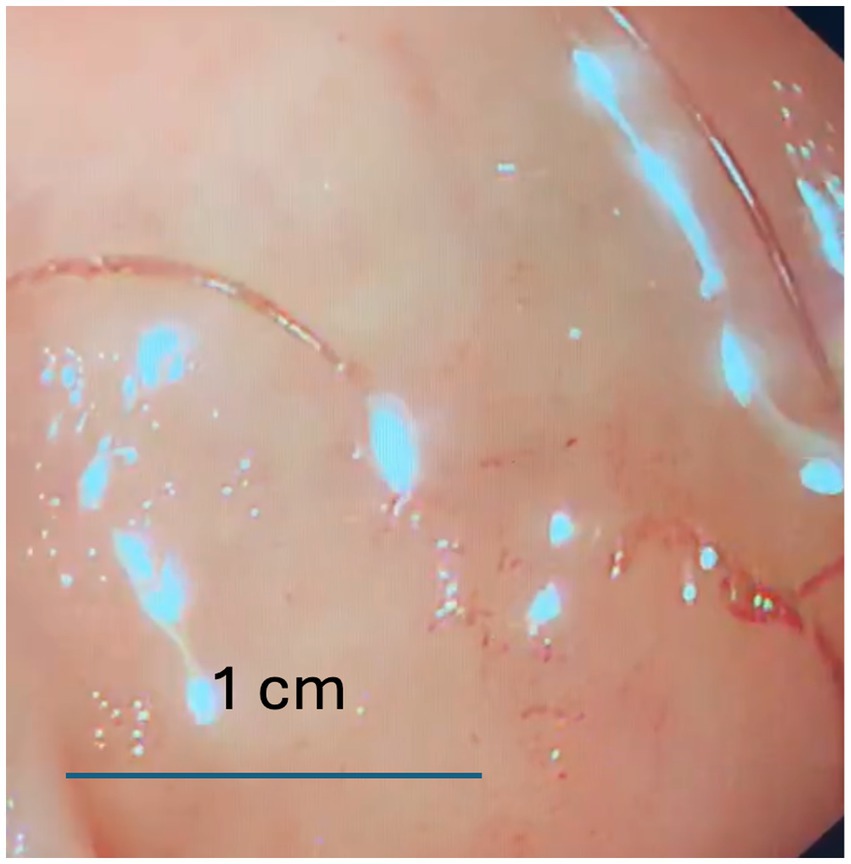
To investigate further, gastroscopy and colonoscopy were performed. While colonoscopy yielded no significant findings, gastroscopy identified living worms in the pyloric ring, antrum, and duodenum, along with areas of active bleeding (Figure 1). The worms were removed using biopsy forceps and were morphologically identified as Ancylostoma duodenale, a finding confirmed by microscopic examination (Figure 2).

Figure 2. Typical hookworm infection was confirmed through microscopic examination of the worm captured via endoscopy.
The patient was treated with a single 400 mg dose of albendazole and hematinics. Her haemoglobin level improved to 85 g/L at discharge and normalized to 126 g/L after 3 months.
Discussion
Hookworm is among the most common soil-transmitted helminths, residing in the host’s small intestine and causing iron deficiency anaemia (IDA) through chronic blood loss (7). Its life cycle begins when third-stage larvae (L3) penetrate human skin from contaminated soil, enter the bloodstream, and travel to the lungs. The larvae subsequently migrate to the pharynx, are swallowed, and mature into adult worms in the small intestine, where they attach to the mucosa, feed on blood, and reproduce, releasing eggs that are expelled through faeces (8).
Timely diagnosis and treatment of hookworm infection are crucial for preventing severe complications. Diagnosis is typically made by detecting characteristic eggs in stool samples via microscopy. However, this method requires technical expertise and is prone to false negatives (9), particularly in low-prevalence areas such as this one. More recently, emerging technologies such as automated digital stool analysers have improved diagnostic accuracy but are not widely available, perhaps due to cost (10). Additionally, gastrointestinal endoscopy is increasingly used as a complementary diagnostic tool, particularly in atypical cases or when stool tests yield negative results (11, 12). However, capsule endoscopy appears to be a more versatile option with fewer potential complications or unintended outcomes (7).
Molecular diagnostics, such as mitochondrial genome sequencing (13), have also proven valuable in accurately identifying hookworm species, which is critical for selecting appropriate anthelmintic treatment and advancing epidemiological research (14, 15). However, cost-effectiveness should be considered, as spontaneous tube sedimentation (STS) diagnostic techniques have demonstrated both accuracy and affordability, especially in the developing countries (16). Accurate identification is particularly important when specimens are incomplete, as different species exhibit variable drug sensitivities.
In this case, substantially delayed diagnosis was attributed to the patient’s relocation from rural Sichuan to Shanghai, which confused doctors in an area where awareness of hookworm infection is limited. This case highlights the importance of thorough diagnostic workups and the need for increased vigilance among urban healthcare providers.
Furthermore, for public health and epidemic control, the WHO recommends administering anthelmintic medications to children (17) and implementing combined treatment strategies for schistosomiasis and soil-transmitted helminthiasis in high-prevalence regions, particularly in Africa (18). Although significant efforts have been made to minimise or control helminthic transmission and infection through large-scale deworming and rebuilding sanitation systems, challenges persist, particularly in the poorest regions of central China, where economic hardship is exacerbated by mountainous and desert terrain (19). Given this case, it may be advisable to extend such preventative measures to adults in rural Chinese regions with a higher parasite prevalence, as recommended by the WHO (18). Despite stool examination is easy and reliable if it is handled by the experienced staff, but our case demonstrated further training is necessary for the medical practitioners, and prepared for any unprepared. More recently, PCR testing has been applied in diagnosis of hookworm infection, improving substantially in sensitivity and specificity (20). Such approach probably will be very useful in hospitals from first-tier cities.
Conclusion
Timely and accurate diagnosis of worm infection is essential for minimizing long-term health consequences and reducing associated healthcare costs. This case highlights the utility of gastrointestinal endoscopy in identifying a typical presentations of hookworm disease. Preventative measures, including deworming programmes for children and potentially adults, remain vital in controlling hookworm infections and mitigating their public health impact.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Human Ethics Committee, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YS: Data curation, Investigation, Writing – original draft. HC: Writing – review & editing. BH: Writing – review & editing. YW: Writing – review & editing. YZ: Writing – review & editing. SB: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Gansu Provincial Science and Technology Program Sponsorship (24RCKD001), China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1541099/full#supplementary-material
References
1. WHO. (2023). Soil-transmitted helminth infections. Available online at: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections. (Accessed January 18, 2023)
2. Qian, MB, Chen, J, Bergquist, R, Li, ZJ, Li, SZ, Xiao, N, et al. Neglected tropical diseases in the People’s Republic of China: progress towards elimination. Infect Dis Poverty. (2019) 8:86. doi: 10.1186/s40249-019-0599-4
3. Zhu, HH, Huang, JL, Chen, YD, Zhou, CH, Zhu, TJ, Qian, MB, et al. National surveillance of hookworm disease in China: a population study. PLoS Negl Trop Dis. (2022) 16:e0010405. doi: 10.1371/journal.pntd.0010405
4. Ilik, V, Schwarz, EM, Noskova, E, and Pafco, B. Hookworm genomics: dusk or dawn? Trends Parasitol. (2024) 40:452–65. doi: 10.1016/j.pt.2024.04.003
5. Jourdan, PM, Lamberton, PHL, Fenwick, A, and Addiss, DG. Soil-transmitted helminth infections. Lancet. (2018) 391:252–65. doi: 10.1016/S0140-6736(17)31930-X
6. Loukas, A, and Prociv, P. Immune responses in hookworm infections. Clin Microbiol Rev. (2001) 14:689–703. doi: 10.1128/CMR.14.4.689-703.2001
7. Alarcon-Fernandez, O, Baudet, JS, and Sanchez Del Rio, A. Iron-deficiency anemia caused by hookworm infestation. Clin Gastroenterol Hepatol. (2006) 4:A32. doi: 10.1016/j.cgh.2006.01.009
8. Brooker, S, Bethony, J, and Hotez, PJ. Human hookworm infection in the 21st century. Adv Parasitol. (2004) 58:197–288. doi: 10.1016/S0065-308X(04)58004-1
9. Allam, AF, Farag, HF, Lotfy, W, Fawzy, HH, Elhadad, H, and Shehab, AY. Comparison among FLOTAC, Kato-Katz and formalin ether concentration techniques for diagnosis of intestinal parasitic infections in school children in an Egyptian rural setting. Parasitology. (2021) 148:289–94. doi: 10.1017/S0031182020001675
10. Boonyong, S, Hunnangkul, S, Vijit, S, Wattano, S, Tantayapirak, P, Loymek, S, et al. High-throughput detection of parasites and ova in stool using the fully automatic digital feces analyzer, orienter model fa280. Parasit Vectors. (2024) 17:13. doi: 10.1186/s13071-023-06108-1
11. Reddy, SC, and Vega, KJ. Endoscopic diagnosis of chronic severe upper GI bleeding due to helminthic infection. Gastrointest Endosc. (2008) 67:990–2. doi: 10.1016/j.gie.2007.10.001
12. Chen, YY, and Soon, MS. Endoscopic diagnosis of hookworm infection that caused intestinal bleeding. Gastrointest Endosc. (2005) 62:142. doi: 10.1016/S0016-5107(05)00515-8
13. Xu, FF, Niu, YF, Chen, WQ, Liu, SS, Li, JR, Jiang, P, et al. Hookworm infection in central China: morphological and molecular diagnosis. Parasit Vectors. (2021) 14:537. doi: 10.1186/s13071-021-05035-3
14. Bethony, J, Brooker, S, Albonico, M, Geiger, SM, Loukas, A, Diemert, D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. (2006) 367:1521–32. doi: 10.1016/S0140-6736(06)68653-4
15. Becker, SL, Sieto, B, Silue, KD, Adjossan, L, Kone, S, Hatz, C, et al. Diagnosis, clinical features, and self-reported morbidity of strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis. (2011) 5:e1292. doi: 10.1371/journal.pntd.0001292
16. Fenta, A, Kebede, D, Tilahun, A, Mesganaw, B, Adugna, A, Yihunie, W, et al. Evaluation of the diagnostic techniques in the detection of hookworm infestation among school children in Ethiopia: cross-sectional study design. Heliyon. (2024) 10:e39936. doi: 10.1016/j.heliyon.2024.e39936
17. Taylor-Robinson, DC, Maayan, N, Donegan, S, Chaplin, M, and Garner, P. Public health deworming programmes for soil-transmitted helminths in children living in endemic areas. Cochrane Database Syst Rev. (2019) 2019:CD000371. doi: 10.1002/14651858.CD000371.pub7
18. WHO. (2018). WHO data show unprecedented treatment coverage for bilharzia and intestinal worms. Available online at: https://www.who.int/news/item/14-12-2018-who-data-show-unprecedented-treatment-coverage-for-bilharzia-and-intestinal-worms#:~:text=The%20latest%20data%20on%20treatment,making%20it%20technically%20feasible%20to. (Accessed December 14, 2018)
19. Zheng, Q, Chen, Y, Zhang, HB, Chen, JX, and Zhou, XN. The control of hookworm infection in China. Parasit Vectors. (2009) 2:44. doi: 10.1186/1756-3305-2-44
Keywords: hookworm, adult, inconspicuous, endoscopic, metropolitan
Citation: Si Y, Cai H, Hambly BD, Wang Y, Zhang Y and Bao SB (2025) Case Report: Endoscopic examination improves the diagnosis of inconspicuous helminth infections in adults in Shanghai. Front. Med. 12:1541099. doi: 10.3389/fmed.2025.1541099
Edited by:
Changqing Yang, Tongji University School of Medicine, ChinaReviewed by:
Hui Liu, The Affiliated Hospital of Xuzhou Medical University, ChinaLan Zhong, Tongji University School of Medicine, China
Copyright © 2025 Si, Cai, Hambly, Wang, Zhang and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuli Wang, d2FuZ3l1bGkyM0AxNjMuY29t; Yanfang Zhang, emhhbmd5YW5mYW5nMjBAMTYzLmNvbQ==; Shisan (Bob) Bao, cHJvZmJhb0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Yang Si
Yang Si Hongjiao Cai
Hongjiao Cai Brett D. Hambly
Brett D. Hambly Yuli Wang1*
Yuli Wang1* Shisan (Bob) Bao
Shisan (Bob) Bao