Abstract
Objective:
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease with high incidence and mortality rates. This study aims to identify independent risk factors affecting the mortality risk of COPD patients and construct and validate a nomogram model to provide treatment guidance for COPD patients.
Methods:
Data from COPD patients in the intensive care unit (ICU) were obtained from the Medical Information Mart for Intensive Care-IV (MIMIC-IV) and the eICU Collaborative Research Database (eICU-CRD). The MIMIC-IV dataset was randomly divided into training and testing sets in a 7:3 ratio for model development and evaluation. External validation was performed using the eICU-CRD dataset. Independent prognostic factors were determined using multivariable Cox regression analysis and incorporated into the nomogram. The performance and clinical applicability of the prediction model were evaluated using the concordance index, receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA).
Results:
The MIMIC-IV dataset included 2036 COPD patients, and the eICU-CRD dataset included 13,053 COPD patients. The constructed nomogram model included 7 variables: age, weight, APSIII score, ventilation duration, potassium ion, anion gap, and international normalized ratio. Among these factors, ventilator time was a protective factor, while the remaining six factors were independent risk factors. The nomogram demonstrated good accuracy with C-index values of 0.862, 0.874, and 0.722 in the training set, testing set, and external validation set, respectively. The ROC curve indicated good predictive performance of the nomogram model, and the calibration curve and DCA further confirmed the reliability and clinical utility.
Conclusion:
This study established a simple and effective nomogram model consisting of 7 variables for evaluating the short-term mortality risk of COPD patients. It provides better recommendations for clinical decision-making and improves the short-term survival rate of COPD patients.
1 Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a leading cause of global mortality and disability with large economic burden (1, 2). COPD is a heterogeneous lung disease characterized by chronic airway inflammation, lung tissue damage, and persistent, progressive airflow obstruction (3–5). Due to prolonged exposure to risk factors and changes in the global population age structure, the medical and economic burdens of COPD are expected to surge, with over 200 million COPD patients worldwide in 2019, and projected to become the third leading cause of death by 2030 (6).
Nomogram models have been widely used as predictive tools for various diseases, integrating independent prognostic factors to construct predictive models for assessing patient survival probabilities, and are commonly used in medical research and clinical practice (7). Studies have shown that current nomogram models for COPD primarily assess ICU hospitalization duration, lung function, and disease exacerbations in COPD patients (8–10). Sakamoto et al. (11) collected hospitalization data of COPD patients in Japan to construct a prognostic nomogram, but the included variables were limited, and internal and external validation was not conducted, resulting in limitations in the predictive results of the column chart. Therefore, it is crucial to develop a rapid and accurate column chart model (nomogram) for predicting the mortality risk of COPD patients.
This study conducted a retrospective cohort study using the Critical Care Medical Information Market IV (MIMIC-IV) database, and screened independent prognostic factors for COPD patients through multivariate COX regression analysis. A column chart model was constructed to predict the short-term mortality risk of COPD patients. In addition, we further collected relevant clinical data of COPD patients in the eICU Collaborative Research Database (eICU-CRD), applied the newly developed predictive model to the dataset, and then conducted external validation to evaluate its clinical applicability and value.
This study constructed a nomogram for predicting the short-term mortality risk of COPD patients using clinical data from the MIMIC-IV database, and conducted rigorous internal and external validation, which helps clinicians evaluate the mortality risk of COPD patients and develop personalized treatment strategies.
2 Materials and methods
2.1 Data source
The clinical data of COPD patients included in this study were obtained from the MIMIC-IV database (version 2.2) and eICU-CRD (version 2.0). MIMIC-IV is a large, freely accessible database that includes over 50,000 ICU admissions from Beth Israel Deaconess Medical Center (BIDMC) between 2008 and 2019 (12). The eICU-CRD is a national telemedicine database, containing information from over 200,000 patients admitted to 335 ICUs in 208 hospitals across the United States between 2014 and 2015 (13). Both databases provide extensive clinical data, including demographics, vital signs, laboratory results, medication use, and nursing records. This study involved secondary analysis of publicly available databases, MIMIC-IV and eICU-CRD, therefore, no ethical approval was required. Moreover, as all patient information in the databases has been deidentified, informed consent was not necessary. The first author (Yikun Guo) has completed a training program provided by the collaborating institution and obtained a certificate (number: 62099487) qualifying for access to the databases and retrieval of information.
2.2 Study population
This study included patients diagnosed with COPD based on ICD-9 and ICD-10 codes. The following inclusion require was applied: patients were diagnosed with CODP by the International Classification of Disease. (ICD-9 codes: 491.20, 491.21, 491.2 and 496; ICD-10 codes: J 44, J 44.0, J 44.1 and J 44.9). The following exclusion criteria were applied: (1) age less than 18 or greater than 90 years, (2) ICU stay less than 1 day, and (3) multiple ICU admissions, with only data from the first admission being extracted. Ultimately, a total of 15,089 patients were included in this retrospective study, with 2,036 patients from the MIMIC-IV database and 13,053 patients from the eICU-CRD database. Patients from the MIMIC-IV dataset were randomly allocated to a training set and testing set in a 7:3 ratio, while the patients from the eICU-CRD dataset constituted the external validation set (Figure 1).
Figure 1
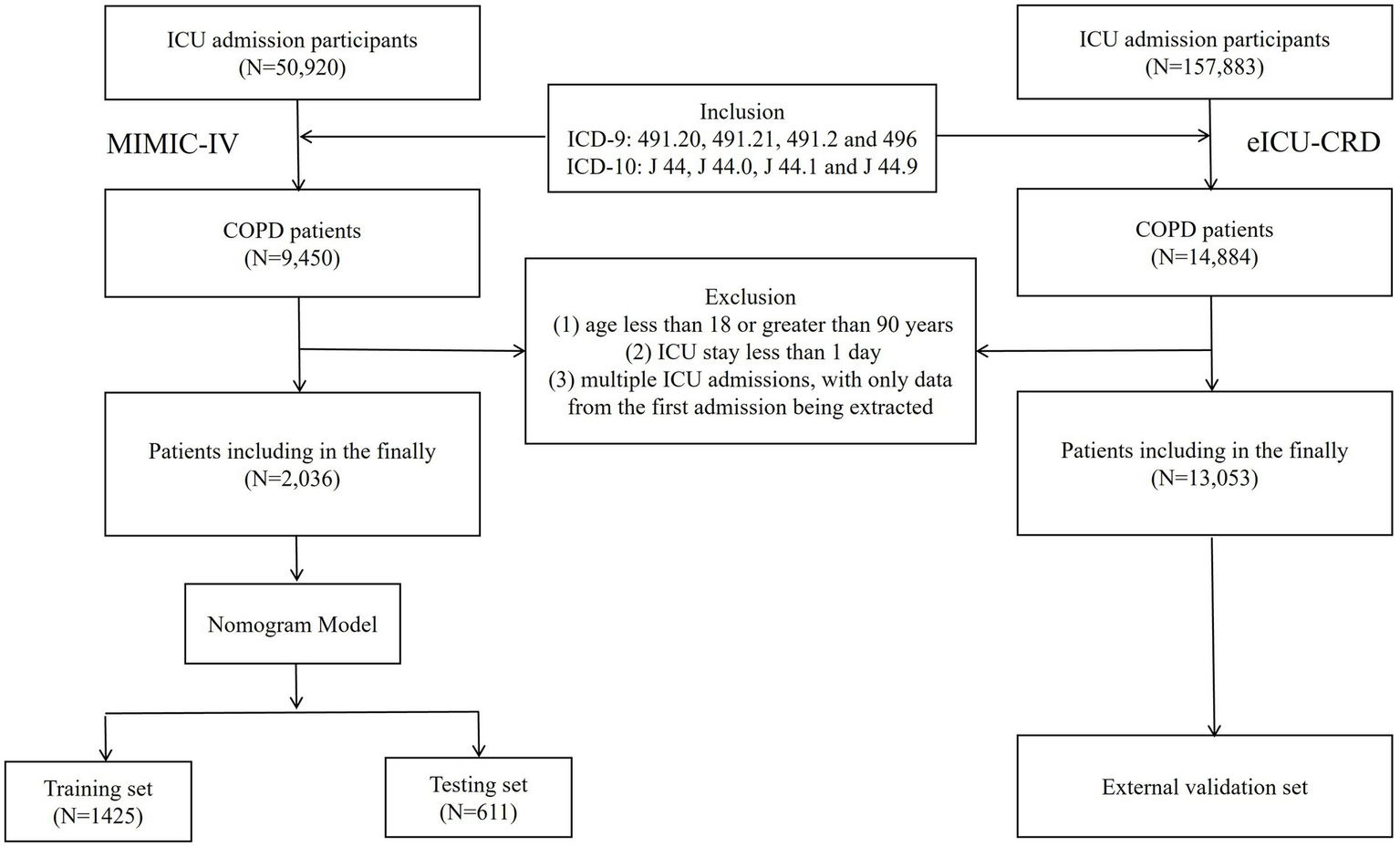
Selecting flowchart.
2.3 Data extraction
Utilizing PostgreSQL software (version 13.7.2) and PgAdmin4, relevant clinical data readily accessible from the MIMIC-IV database and eICU-CRD database were extracted to ensure the practicality of the study. All data in this study were extracted using Structured Query Language (SQL). The extracted data encompassed six categories: (1) Demographics: age, gender, and weight. (2) Vital signs: heart rate (HR), mean blood pressure (MBP), systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate (RR), temperature, and oxygen saturation (SpO2). (3) Laboratory indicators: white blood cell count (WBC), red blood cell count (RBC), platelet count (PLT), hemoglobin (HGB), chloride (Cl), potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), anion gap (AG), creatinine (Cr), blood urea nitrogen (BUN), international normalized ratio (INR), activated partial thromboplastin time (APTT), pH, partial pressure of carbon dioxide (PCO2), and partial pressure of oxygen (PO2). (4) Complications: systemic inflammatory response syndrome (SIRS), hypertension, diabetes, heart failure (HF), myocardial infarction, cancer, pneumonia, stroke, atrial fibrillation, kidney failure, and coronary heart disease. (5) Scoring systems: sequential organ failure assessment (SOFA) score, simplified acute physiology score II (SAPSII), Acute Physiology Score III (APS III), Charlson comorbidity index (CCI). (6) Treatment information included mechanical ventilation, mechanical ventilation duration, and the use of antibiotic. (7) Outcome indicators: ICU survival status, ICU length of stay (LOS).
2.4 Statistical analysis
We used the Kolmogorov–Smirnov test to assess the normality of continuous variables. Normally distributed continuous variables were described as mean ± standard deviation and compared between groups using the t-test. Non-normally distributed continuous variables were expressed as interquartile ranges (IQR) and compared between groups using the Wilcoxon rank-sum test. Categorical variables were presented as numbers and percentages, and differences between groups were compared using the chi-square test or Fisher’s exact probability.
This study employed multivariable Cox regression analysis to identify independent prognostic factors in COPD patients. The variance inflation factor (VIF) was calculated to assess the collinearity between variables. Based on the selected variables, a nomogram model for short-term prognosis in COPD patients was developed. The performance of the model was further evaluated using a testing set, with the C-index and receiver operating characteristic (ROC) curve used to assess the predictive ability of the nomogram. The accuracy of the model was assessed using calibration curves. Decision curve analysis (DCA) was performed to evaluate the clinical utility of the nomogram. External validation was conducted using the eICU-CRD dataset.
To ensure the reliability of the analysis, variables with more than 20% missing values in vital signs and laboratory indicators were excluded, and other missing variables were handled using imputation. Each statistical test was conducted with a two-tailed design. R version 4.2.2 was used for statistical analysis, and p-values≤0.05 were considered statistically significant.
3 Results
A total of 2,036 participants were recruited from the MIMIC-IV database based on inclusion and exclusion criteria, including 1,089 (53.5%) males and 947 (46.5%) females, with an average age of 71.8 years. During the ICU stay, 186 patients died, resulting in an ICU mortality rate of 9.13% and an average ICU stay of 4.8 days.
3.1 Baseline characteristics
Table 1 presents the clinical characteristics and baseline data of the participants. The 2,036 participants were randomly allocated to training and testing sets at a 7:3 ratio, with 1,425 in the training set and 611 in the testing set, over half of whom were older adult males. Regarding comorbidities, only 0.8% of patients did not have systemic inflammatory response syndrome (SIRS), and over one-third of patients had cardiovascular and endocrine diseases, such as heart failure (43%), coronary heart disease (39.7%), hypertension (37.9%), and diabetes (33.8%); the prevalence of cancer (19.7%) and stroke (8.9%) as comorbidities was lower. Based on the four included scoring systems, COPD patients likely had sepsis and unstable physiological conditions, indicating a high risk of death. Laboratory indicators suggested possible infections and anemia in the patients. In terms of treatment, most patients received antibiotics (80.5%) and mechanical ventilation (86.3%), with an average duration of mechanical ventilation of 75.9 h. There were no significant baseline characteristic differences between the training set (N = 1,425) and the testing set (N = 611), indicating that the two groups were comparable (p > 0.05).
Table 1
| Characteristics | Training set (N = 1,425) | Testing set (N = 611) | Total (N = 2036) | P-value |
|---|---|---|---|---|
| Demography | ||||
| Gender, n (%) | 0.870 | |||
| Female | 665 (46.7) | 282 (46.2) | 947 (46.5) | |
| Male | 760 (53.3) | 329 (53.8) | 1,089 (53.5) | |
| Age (year) | 71.8 [60.9, 82.7] | 71.7 [60.5, 82.9] | 71.8 [60.8, 82.8] | 0.857 |
| Weight (kg) | 82.4 [57.7, 107.1] | 81.5 [57, 106] | 82.1 [57.4, 106.8] | 0.469 |
| Vital signs | ||||
| HR (times/min) | 89.1 [68.9, 109.3] | 88.1 [68.8, 107.4] | 88.8 [68.8, 108.8] | 0.292 |
| RR (times/min) | 20.1 [13.5, 26.7] | 19.9 [13.2, 26.6] | 20 [13.4, 26.6] | 0.485 |
| Temperature (°C) | 36.8 [35.8, 37.8] | 36.8 [36.2, 37.4] | 36.8 [35.9, 37.7] | 0.854 |
| SBP (mmHg) | 121 [96.3, 145.7] | 121 [96.7, 145.3] | 121 [96.4, 145.6] | 0.998 |
| DBP (mmHg) | 69 [50.7, 87.3] | 69.3 [50.2, 88.4] | 69.1 [50.5, 87.7] | 0.792 |
| MBP (mmHg) | 82.7 [64, 101.4] | 92.4 [63.4, 100.8] | 85.6 [63.8, 101.2] | 0.327 |
| SpO2 (%) | 96.2 [91.7, 100.7] | 96.4 [92.8, 100] | 96.2 [92, 100.4] | 0.282 |
| Comorbidities, n (%) | ||||
| SIRS | 0.978 | |||
| 0 | 11 (0.8) | 5 (0.8) | 16 (0.8) | |
| 1 | 174 (12.2) | 69 (11.3) | 243 (11.9) | |
| 2 | 482 (33.8) | 211 (34.5) | 693 (34) | |
| 3 | 603 (42.3) | 257 (42.1) | 860 (42.2) | |
| 4 | 155 (10.9) | 69 (11.3) | 224 (11) | |
| Hypertension | 0.935 | |||
| No | 886 (62.2) | 378 (61.9) | 1,264 (62.1) | |
| Yes | 539 (37.8) | 233 (38.1) | 772 (37.9) | |
| Diabetes mellitus | 0.916 | |||
| No | 945 (66.3) | 403 (66) | 1,348 (66.2) | |
| Yes | 480 (33.7) | 208 (34) | 688 (33.8) | |
| Heart failure | 0.191 | |||
| No | 798 (56) | 362 (59.2) | 1,160 (57) | |
| Yes | 627 (44) | 249 (40.8) | 876 (43) | |
| Myocardial infarction | 0.961 | |||
| No | 1,197 (84) | 512 (83.8) | 1709 (83.9) | |
| Yes | 228 (16) | 99 (16.2) | 327 (16.1) | |
| Cancer | 0.701 | |||
| No | 1,148 (80.6) | 487 (79.7) | 1,635 (80.3) | |
| Yes | 277 (19.4) | 124 (20.3) | 401 (19.7) | |
| Pneumonia | 0.962 | |||
| No | 967 (67.9) | 416 (68.1) | 1,383 (67.9) | |
| Yes | 458 (32.1) | 195 (31.9) | 653 (32.1) | |
| Stroke | 0.757 | |||
| No | 1,296 (90.9) | 559 (91.5) | 1855 (91.1) | |
| Yes | 129 (9.1) | 52 (8.5) | 181 (8.9) | |
| Atrial fibrillation | 0.310 | |||
| No | 848 (59.5) | 379 (62) | 1,227 (60.3) | |
| Yes | 577 (40.5) | 232 (38) | 809 (39.7) | |
| Kidney failure | 0.327 | |||
| No | 977 (68.6) | 433 (70.9) | 1,410 (69.3) | |
| Yes | 448 (31.4) | 178 (29.1) | 626 (30.7) | |
| Coronary heart disease | 0.139 | |||
| No | 844 (59.2) | 384 (62.8) | 1,228 (60.3) | |
| Yes | 581 (40.8) | 227 (37.2) | 808 (39.7) | |
| Scores | ||||
| SOFA | 5.1 [1.5, 8.7] | 5 [1.5, 8.5] | 5.1 [1.5, 8.7] | 0.488 |
| APS III | 45 [25.8, 64.2] | 45.1 [25.7, 64.5] | 45 [25.8, 64.2] | 0.928 |
| SAPS II | 38.7 [25.7, 51.7] | 38.7 [26.2, 51.2] | 38.7 [25.9, 51.5] | 0.997 |
| CCI | 6.7 [4, 9.4] | 6.7 [3.9, 9.5] | 6.7 [3.9, 9.5] | 0.945 |
| Blood gas | ||||
| AG (mmol/L) | 14.6 [11, 18.2] | 14.5 [10.6, 18.4] | 14.6 [10.9,18.3] | 0.821 |
| pH | 7.4 [7.3, 7.5] | 7.4 [7.3,7.5] | 7.4 [7.3,7.5] | 0.564 |
| PCO2 (mmHg) | 46.5 [36.4, 56.6] | 46.8 [37, 56.6] | 46.6 [36.6, 56.6] | 0.487 |
| PO2 (mmHg) | 115.8 [48.3, 183.3] | 114 [50.2, 177.8] | 115.3 [48.9, 181.7] | 0.576 |
| Laboratory tests | ||||
| WBC (K/UL) | 13 [5.8, 20.2] | 13.5 [−2.8, 29.8] | 13.1 [2.3, 23.9] | 0.465 |
| RBC (m/uL) | 3.5 [2.8, 4.2] | 3.5 [2.8, 4.2] | 3.5 [2.8, 4.2] | 0.180 |
| PLT (K/μL) | 199.9 [106.2, 293.6] | 194.3 [106.4, 282.2] | 198.2 [106.2, 290.2] | 0.207 |
| HGB (g/dL) | 10.4 [8.4, 12.4] | 10.3 [8.3, 12.3] | 10.4 [8.4, 12.4] | 0.061 |
| Na (mmol/L) | 138.5 [133.8, 143.2] | 138 [133, 143] | 138.3 [133.5, 143.1] | 0.059 |
| K (mmol/L) | 4.3 [3.7, 4.9] | 4.4 [3.8, 5] | 4.3 [3.7, 4.9] | 0.216 |
| Ca (mg/dL) | 8.4 [7.7, 9.1] | 8.4 [7.8, 9] | 8.4 [7.7, 9.1] | 0.381 |
| Cl (mmol/L) | 102.1 [96.3, 107.9] | 101.5 [95.3, 107.7] | 101.9 [96, 107.8] | 0.069 |
| Mg (mmol/L) | 2.1 [1.6, 2.6] | 2 [1.5, 2.5] | 2 [1.5, 2.5] | 0.181 |
| APTT (s) | 38.6 [19.5, 57.7] | 38.8 [21, 56.6] | 38.7 [20, 57.4] | 0.872 |
| INR | 1.4 [0.8, 2] | 1.4 [0.8, 2] | 1.4 [0.8, 2] | 0.533 |
| BUN (mg/dL) | 27.6 [6.4, 48.8] | 28.4 [5.8, 51] | 27.8 [6.2, 49.4] | 0.436 |
| Cr (mg/dL) | 1.4 [0.3, 2.5] | 1.4 [0, 2.8] | 1.4 [0.2, 2.6] | 0.724 |
| Treatment | ||||
| Mechanical ventilation, n (%) | 0.770 | |||
| No | 192 (13.5) | 86 (14.1) | 278 (13.7) | |
| Yes | 1,233 (86.5) | 525 (85.9) | 1758 (86.3) | |
| Mechanical ventilation duration (hours) | 75.3 [19.2, 169.8] | 77.4 [14.1, 168.9] | 75.9 [17.7, 169.5] | 0.639 |
| Antibiotic, n (%) | 0.595 | |||
| No | 273 (19.2) | 124 (20.3) | 397 (19.5) | |
| Yes | 1,152 (80.8) | 487 (79.7) | 1,639 (80.5) | |
| Outcome | ||||
| LOS in ICU (days) | 4.8 [1.1, 10.7] | 4.8 [0.8, 10.4] | 4.8 [1.0, 10.6] | 0.848 |
| ICU survival status, n (%) | 0.825 | |||
| Alive | 1,293 (90.7) | 557 (91.2) | 1850 (90.9) | |
| Dead | 132 (9.3) | 54 (8.8) | 186 (9.1) | |
Baseline characteristics of COPD patients.
HR: Heart rate; RR: Respiratory rate; DBP: Diastolic blood pressure; SBP: Systolic blood pressure; MBP: Mean blood pressure; SpO2: Blood oxygen saturation; SIRS: systemic inflammatory response syndrome; SOFA: Sequential Organ Failure Assessment; APSIII: Acute Physiology Score III; SAPSII: Simplified Acute Physiology Score II; CCI: Charlson comorbidity index; AG: anion gap; PCO2: partial pressure of carbon dioxide; PO2: partial pressure of oxygen; WBC: white blood cell count; RBC: red blood cell count; PLT: platelet; HGB: hemoglobin; APTT: activated partial thromboplastin time; INR: international normalized ratio; BUN: Blood urea nitrogen; Cr: Creatinine; LOS: length of stay.
3.2 COX regression analysis
Univariable and multivariable Cox regression analyses were conducted on the included variables. Table 2 shows that seven variables were identified as independent prognostic factors in COPD patients. These include age (HR = 1.05, 95%CI: 1.02–1.07, p < 0.001), weight (HR = 1.01, 95%CI: 1.00–1.02, p = 0.036), APSIII (HR = 1.03, 95%CI: 1.02–1.05, p < 0.001), potassium (HR = 1.45, 95%CI: 1.01–2.09, p = 0.045), AG (HR = 1.09, 95%CI: 1.01–1.18, p = 0.021), INR (HR = 1.20, 95%CI: 1.00–1.44, p = 0.048) as independent risk factors for patient prognosis, and ventilator time (HR = 0.99, 95%CI: 0.99–0.99, p < 0.001) as an independent protective factor. Additionally, the VIF values for these variables were all below 4 (age: 1.2852, weight: 1.2148, APSIII: 3.4378, ventilation time: 1.1042, potassium: 1.194, AG: 1.5036, INR: 1.2485), indicating no multicollinearity.
Table 2
| Characteristics | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| Demography | ||||||
| Age (year) | 1.03 | 1.01–1.04 | 0.002 | 1.05 | 1.02–1.07 | <0.001 |
| Weight (kg) | 1.01 | 1.00–1.02 | 0.045 | 1.01 | 1.00–1.02 | 0.036 |
| Vital signs | ||||||
| HR (times/min) | 1.01 | 1.00–1.02 | 0.031 | 0.99 | 0.98–1.00 | 0.220 |
| SpO2 (%) | 0.95 | 0.93–0.98 | <0.001 | 0.97 | 0.94–1.01 | 0.146 |
| Comorbidities | ||||||
| Hypertension | ||||||
| No (Reference) | ||||||
| Yes | 0.67 | 0.46–0.98 | 0.037 | 0.99 | 0.59–1.66 | 0.968 |
| Scores | ||||||
| SOFA | 1.15 | 1.11–1.20 | <0.001 | 1.04 | 0.96–1.14 | 0.339 |
| APS III | 1.02 | 1.02–1.03 | <0.001 | 1.03 | 1.02–1.05 | <0.001 |
| SAPS II | 1.04 | 1.03–1.05 | <0.001 | 0.98 | 0.96–1.01 | 0.228 |
| CCI | 1.13 | 1.07–1.20 | <0.001 | 1.06 | 0.96–1.17 | 0.254 |
| Blood gas | ||||||
| AG (mmol/L) | 1.12 | 1.08–1.17 | <0.001 | 1.09 | 1.01–1.18 | 0.021 |
| pH | 0.00 | 0.00–0.01 | <0.001 | 0.70 | 0.01–47.12 | 0.866 |
| Laboratory tests | ||||||
| RBC (m/uL) | 0.69 | 0.54–0.89 | 0.005 | 1.26 | 0.69–2.29 | 0.453 |
| HGB (g/dL) | 0.89 | 0.81–0.97 | 0.007 | 0.84 | 0.67–1.04 | 0.109 |
| K (mmol/L) | 1.63 | 1.23–2.15 | <0.001 | 1.45 | 1.01–2.09 | 0.045 |
| Cl (mmol/L) | 0.74 | 0.57–0.95 | 0.017 | 0.88 | 0.66–1.17 | 0.370 |
| APTT (s) | 1.01 | 1.00–1.02 | 0.006 | 1.01 | 1.00–1.02 | 0.075 |
| INR | 1.29 | 1.16–1.44 | <0.001 | 1.20 | 1.00–1.44 | 0.048 |
| BUN (mg/dL) | 1.01 | 1.01–1.02 | <0.001 | 0.99 | 0.97–1.03 | 0.208 |
| Cr (mg/dL) | 1.27 | 1.15–1.40 | <0.001 | 1.01 | 0.81–1.26 | 0.955 |
| Treatment | ||||||
| Mechanical ventilation | ||||||
| No (Reference) | ||||||
| Yes | 0.39 | 0.23–0.65 | <0.001 | 0.61 | 0.33–1.14 | 0.123 |
| Mechanical ventilation time(hours) | 0.99 | 0.99–0.99 | <0.001 | 0.99 | 0.99–0.99 | <0.001 |
Univariate and multivariate COX regression analysis.
3.3 Construction and validation of nomogram model
Based on the seven risk factors identified from the COX regression analysis, we developed a nomogram to predict the 7-day, 14-day, and 28-day survival probabilities of COPD patients (Figure 2). The total score for each patient was calculated by summing the points corresponding to each variable, with the total score value representing the predicted probability of prognosis for COPD patients. The C-index in the training and testing sets were 0.862 (0.847–0.877) and 0.874 (0.845–0.904), respectively, indicating good model accuracy.
Figure 2
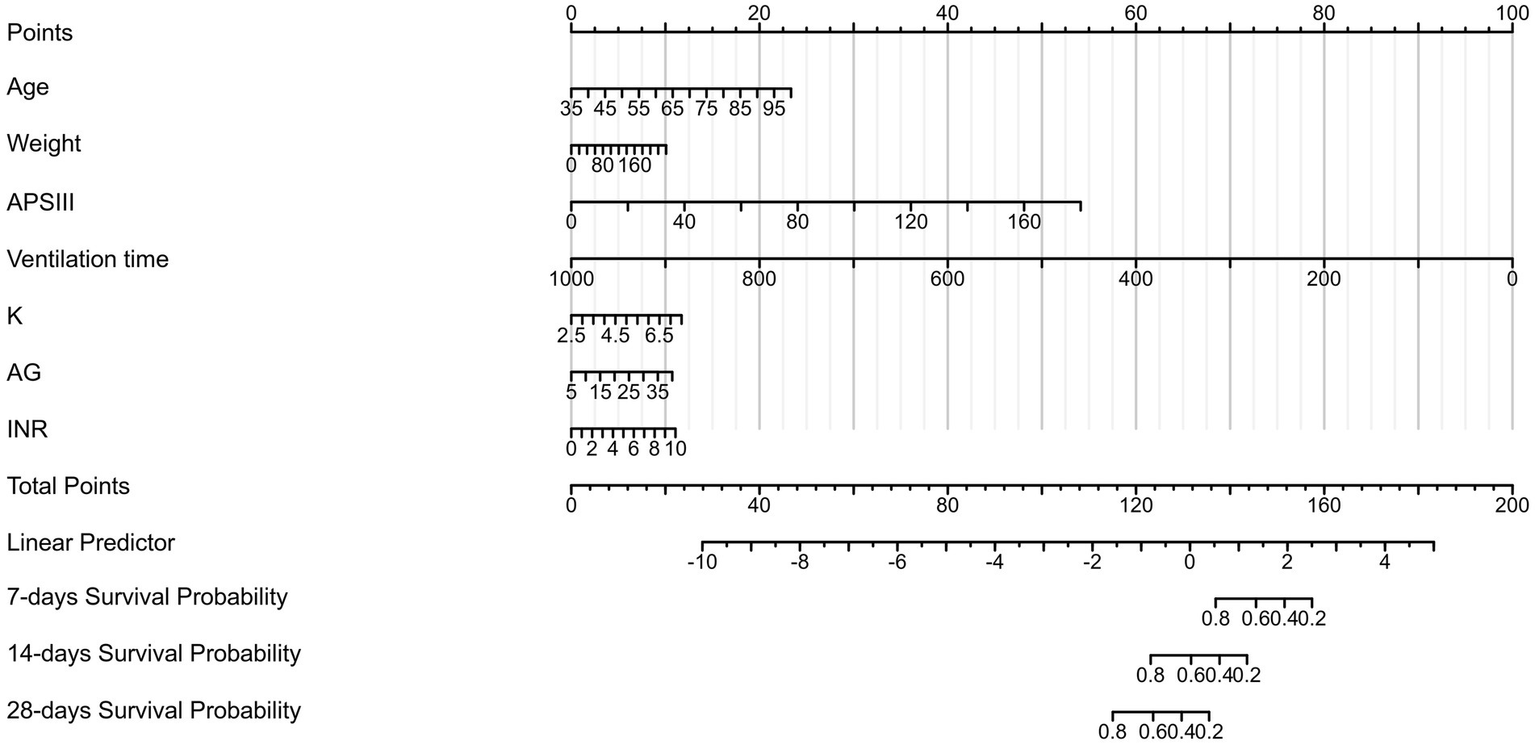
Nomogram for short-term survival probability in COPD patients.
We further validated the accuracy of the nomogram model through ROC curves, calibration curves, and DCA. The ROC curve analysis of the nomogram (Figures 3A,B) showed that the area under the curve (AUC) for 7-day, 14-day, and 28-day mortality risks in the training set were 0.861, 0.862, and 0.976, respectively, and in the testing set were 0.871, 0.956, and 0.959, respectively, indicating good predictive performance of the nomogram. Calibration curves, which more accurately reflect whether the actual outcomes of each nomogram match the predicted outcomes, showed (Figure 4) that the nomograms in both cohorts closely aligned with the diagonal, indicating good fit and consistency with actual prognosis outcomes. DCA evaluates the clinical value of the model by comparing the standardized net benefit and risk threshold probability (14). The green horizontal line represents the benefit when no patients receive intervention, the diagonal red line represents the benefit when all patients receive intervention, and the blue curve represents the benefit when patients receive intervention based on the model’s judgment. The DCA curve (Figure 5) results showed that clinical interventions guided by the nomogram model yielded greater net benefits in both the training and testing sets.
Figure 3
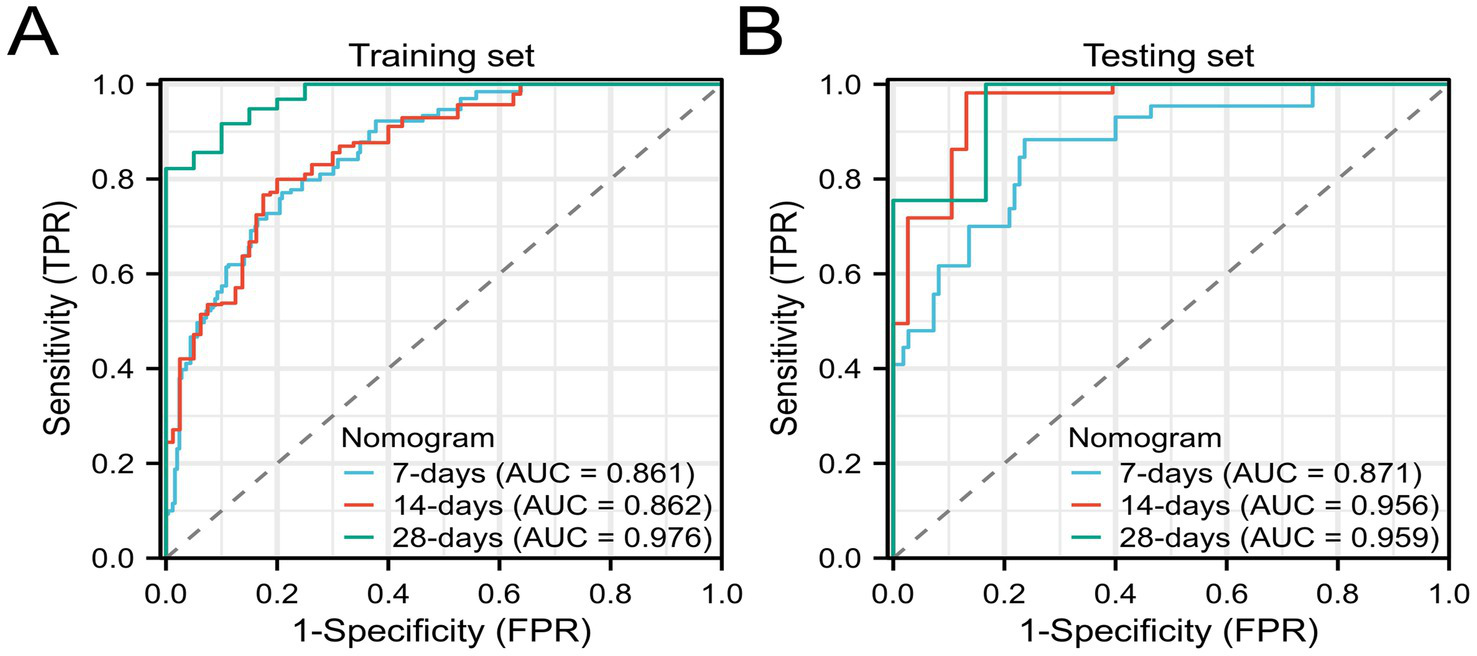
ROC curves of the nomogram model (A) Training set; (B) Testing set.
Figure 4
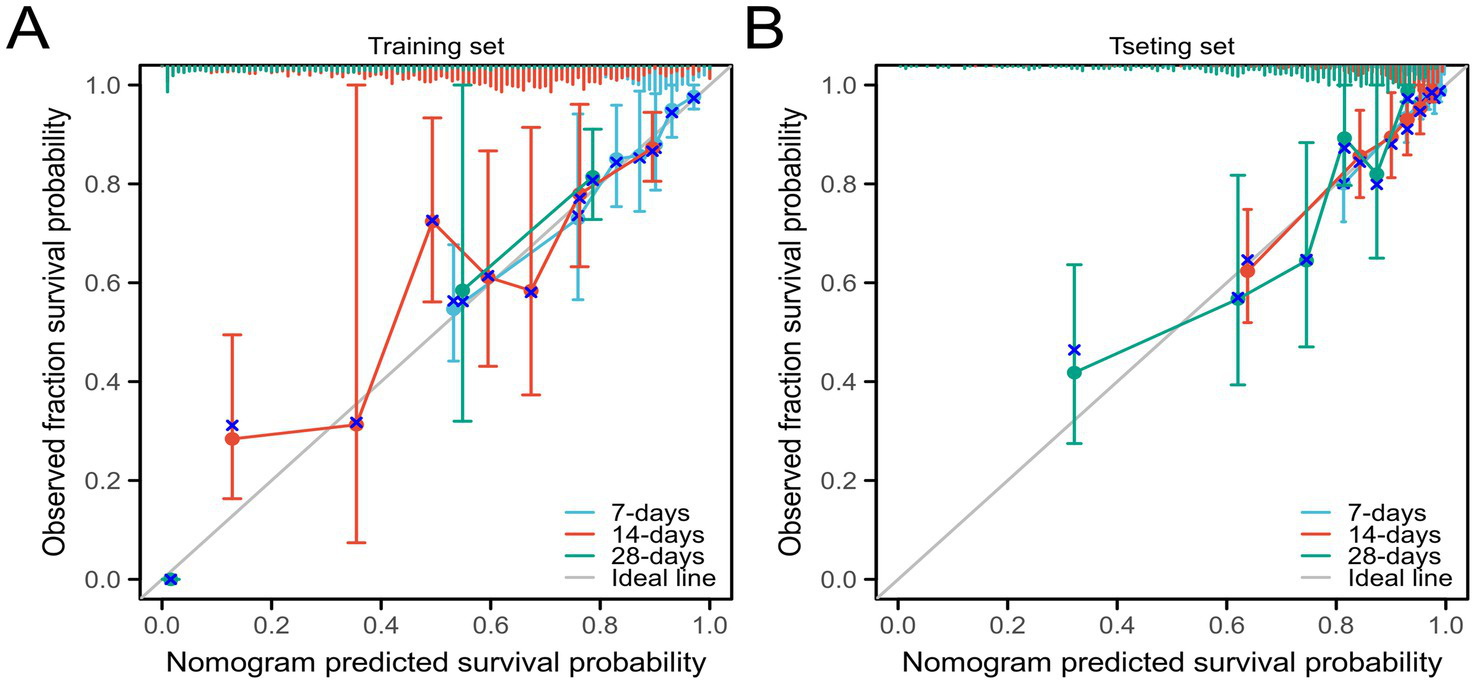
Calibration curves of the nomogram model (A) Training set; (B) Testing set.
Figure 5
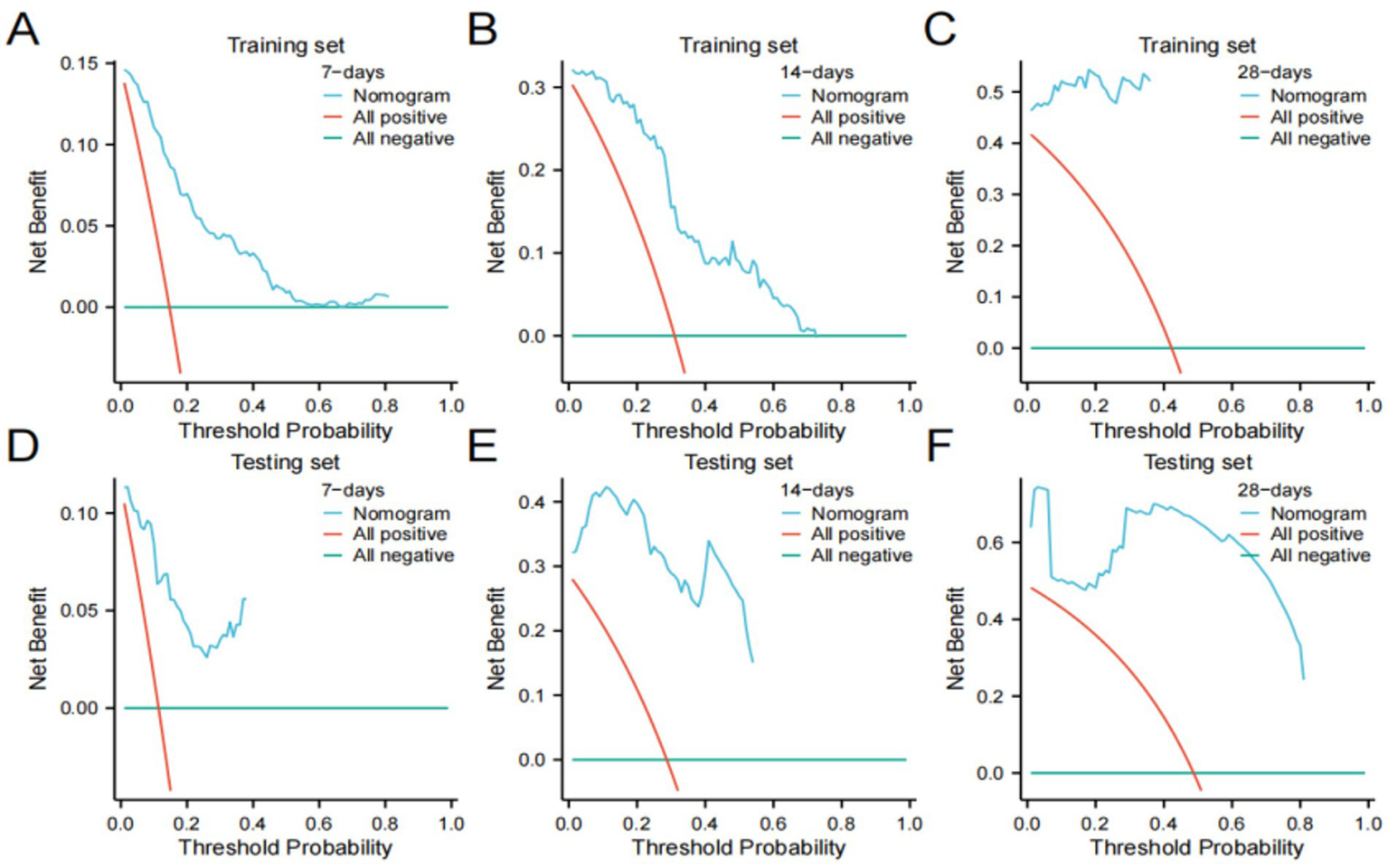
Decision curve analysis (DCA) plots (A–C) Training set; (D-F) Testing set.
3.4 External validation
In addition to using the MIMIC-IV dataset as the training and internal testing sets, data from the eICU-CRD database were collected as the external validation set. The external validation set included 13,053 COPD patients and was used to independently validate the newly developed prediction model.
The C-index of the external validation set was 0.722 (0.713–0.731). The AUC for 7-day, 14-day, and 28-day mortality risks were 0.699, 0.683, and 0.667, respectively (Figure 6A), indicating that the model exhibited good clinical predictive ability. Calibration curves (Figure 6B) and DCA results (Figures 6C–E) demonstrated good calibration, clinical utility, and net benefit of the model.
Figure 6
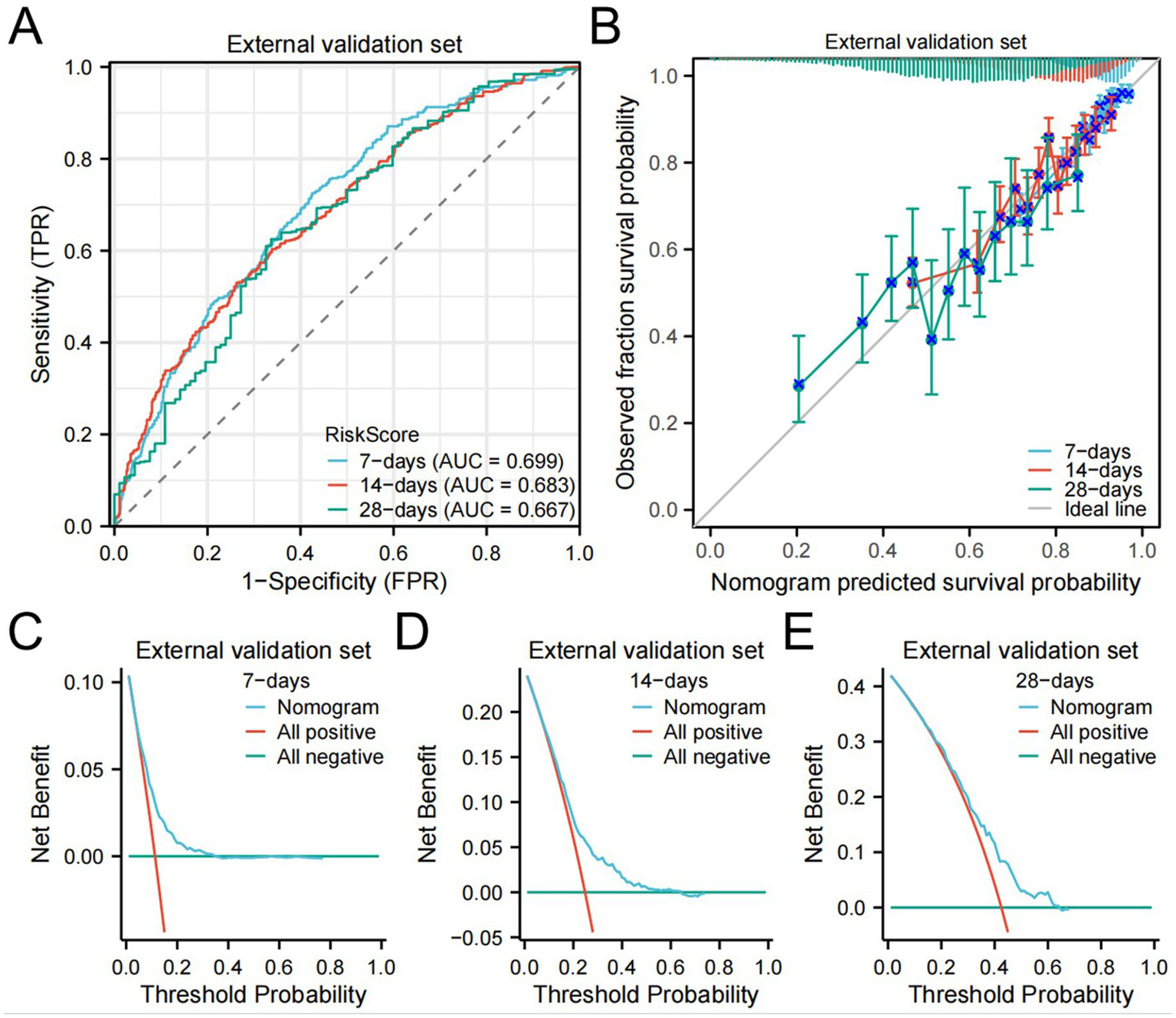
eICU dataset validation results.
4 Discussion
Previous studies have shown that COPD patients requiring hospitalization for treatment generally have poorer prognosis, with a mortality rate of approximately 7% (15). The mortality rate of the included COPD patients in this study was 9.1%, which is consistent with epidemiological survey results. This study utilized clinical data of COPD patients extracted from the MIMIC-IV and eICU-CRD databases and employed Cox regression analysis to identify seven independent risk factors associated with the mortality risk of COPD patients. Subsequently, a convenient and effective nomogram model was constructed and validated to predict the short-term survival probability of COPD patients. This model can effectively strengthen the management of COPD patients, as it demonstrates good predictive ability and clinical utility. For instance, if the 7-day survival rate of the patient is high while the 14-day and 28-day survival rates are low, intervention should be carried out as early as possible. It is necessary to closely monitor the changes in the patient’s symptoms and vital signs and enter the ICU for treatment as soon as possible.
A total of 2,036 COPD patients from the MIMIC-IV database were included in this study. The nomogram model developed identified seven predictive factors, including age, weight, APSIII score, ventilator time, potassium, AG, and INR. Among these factors, ventilator time was a protective factor, while the remaining six factors were independent risk factors. Internal and external validation using different data sources demonstrated the robustness and generalizability of our model. Internal validation can evaluate the degree of model fitting, while external validation can assess the generalization ability of the model, reflect the actual clinical application, and enhance the credibility of the model. The C-index and AUC in external validation is lower than internal. The lower performance in external validation may be related to some potential reasons, such as the differences in patient populations, data quality, between the two databases. In patient populations aspects, there may be significant demographic differences between the two databases, such as age, gender, and racial distribution. The severity of the disease, the proportion of comorbidities, and the treatment methods or levels may have differences. For data quality aspect, the MIMIC-IV database is single-center data of the BIDMC which is one of the nation’s preeminent academic medical centers between 2008 and 2019, while the eICU-CRD database is multi-center data of 208 hospitals which are distributed in different region between 2014 and 2015. The data recording methods, inspection and examination equipment, and approaches may vary among different hospitals. BIDMC might have adopted the new technology earlier, while other hospitals might still be using the old ones.
Studies have shown that age is a significant predictor of mortality across various diseases. As individuals age, their quality of life and the physical and functional status of their organs decline, making the older adult more susceptible to various diseases and gradually increasing their mortality rate (16). Epidemiological surveys indicate that the mortality rate among COPD patients increases with age (17). A non-interventional observational study found that the hospitalization and mortality rates of COPD patients over 40 increase with age, possibly due to the increased susceptibility to infections and the risk of lung function deterioration in older adult patients (18, 19).
Multivariate Cox regression analysis revealed an association between body weight and the risk of death in COPD patients. Several studies have confirmed that obesity is an independent risk factor for chronic respiratory diseases, particularly asthma and COPD (20, 21), which aligns with our findings. A Mendelian randomization study indicated that obesity also increases the likelihood of developing chronic obstructive pulmonary disease (Body mass index (BMI): OR = 1.429; waist circumference: OR = 1.591) (22). Obesity results from the excessive accumulation of adipose tissue, which is an active endocrine organ secreting various cytokines and hormones (23). The pathogenesis of COPD is closely related to inflammatory responses. Obese patients often experience persistent low-grade systemic inflammation (24), and increased peripheral blood leukocytes in obese individuals lead to enhanced inflammation and the production of more pro-inflammatory mediators, promoting the progression and exacerbation of COPD (25). Interestingly, some studies suggest that COPD patients often experience weight loss, and a lower BMI is associated with higher hospital mortality rates, as low weight often reflects malnutrition and reduced muscle mass, which may lead to diminished immunity, decreased physical strength, and impaired survival (26), contrary to our findings. However, it is important to note that body weight itself may not be a direct risk or protective factor but is associated with the overall health and nutritional status of the patient.
APSIII score, as part of the APACHE II scoring system is one of the tools used in critical care medicine to assess the severity of a patient’s condition and prognosis, and it is applicable to ICU patients (27). APACHE III covers 12 physiological indicators, including general vital signs, inflammatory markers, and internal environment parameters, providing a more comprehensive quantification of the risk and extent of multi-system damage in patients. APSIII is simpler than the APACHE II score. Therefore, a higher APS score indicates a more unstable physiological state and a more severe condition. The AG is commonly used to evaluate acid–base disturbances and analyze primary metabolic acidosis (28), which is a strong predictor of prognosis in critically ill patients (29). Critically ill COPD patients often develop severe acidemia, leading to myocardial depression, respiratory muscle weakness, increased pro-inflammatory cytokines, and adverse effects on survival prognosis (30). Thus, AG is a risk factor for the prognosis of COPD patients, consistent with our findings.
Potassium ion concentration is an indicator that is often concerned in clinical practice. The Laboratory-Based Intermountain Validated Exacerbation Scores based on laboratory value found that potassium would increase risk of mortality (31), which is consistent with our research results. Moreover, the potassium level of CODP patients with a poor prognosis was significantly lower than that of patients with a good prognosis (32). A retrospective study found that the serum K level of deceased patients with AECODP was significantly lower than that of living patients with AECODP. (33) These are contrary to our findings. This may be related to the fact that CODP patients’ therapeutic drugs (such as β₂ receptor agonists, diuretics or glucocorticoids) and complications (such as heart failure, insufficient renal perfusion) may cause abnormal blood potassium levels. When suffering from CODP, carbon dioxide retention can cause respiratory acidosis. At this time, cells exchange and buffer acidic substances through H+/K+, causing potassium ions to move from the intracellular to the extracellular, which may lead to transient hyperkalemia.
The INR is used to measure the prothrombin time and is crucial for balancing bleeding and coagulation risks (34). COPD patients often exhibit a hypercoagulable state involving changes in various coagulation factors, necessitating anticoagulant therapy, which is monitored through INR levels to assess the effectiveness of the treatment (35). An abnormally high INR may be associated with excessive anticoagulant medication, increasing the risk of bleeding, leading to reduced nutritional status and immunity, and increasing the occurrence of adverse prognostic events, making it a risk factor for patient prognosis. A retrospective clinical study found that the INR in the AECOPD group was significantly higher than in the stable COPD group, indicating that coagulation abnormalities are associated with COPD exacerbations (36). It is noteworthy that while a hypercoagulable state in COPD patients may manifest as a low INR level, the identification of INR as an independent risk factor for COPD prognosis in this study also holds clinical significance.
A retrospective study found that over half (59.3%) of the patients requiring prolonged mechanical ventilation had COPD (37). Another study involving patients on mechanical ventilation for more than 24 h indicated that COPD was one of the most prevalent diseases among the 327 participants (38). As COPD progresses, hypercapnic respiratory failure is one of the causes of death in these patients, with a 1-year mortality rate of 12% (39). Multiple studies have confirmed that long-term non-invasive mechanical ventilation, providing continuous positive airway pressure (CPAP) and supplemental oxygen therapy, can mitigate the negative effects of severe hypercapnia and improve survival rates in COPD patients (40–42).
This study also has some limitations. Firstly, the clinical data we obtained are from different hospitals in different regions, which may introduce some variations in the measurement values. Despite standardization of the data, these differences are inevitable. Secondly, the databases selected for this model are both from hospitals in US and may only reflect some patient information of this country. The use in other countries needs further verification. Thirdly, variables with more than 20% missing values were not included in the study, potentially introducing selection bias and incomplete data, affecting the robustness of the results. Fourthly, as a retrospective cohort study, the nomogram requires further prospective validation before clinical application to increase the reliability of the conclusions. Lastly, the observational nature of the study suggests that unknown confounding factors may influence our results.
5 Conclusion
This study successfully developed and validated a simple and effective nomogram model, demonstrating that age, body weight, APS III score, ventilation time, potassium levels, AG, and INR are independent predictors of prognosis in COPD patients. The nomogram model can be used to predict short-term mortality in COPD patients, aiding physicians in identifying high-risk patients, thereby facilitating the development of personalized treatment plans, enhancing patient management, optimizing resource utilization, and reducing mortality rates of COPD patients in clinical practice.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/ participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
YG: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – original draft. CZ: Conceptualization, Visualization, Writing – original draft. JY: Writing – review & editing, Project administration. CB: Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (81973784), the project of Dongzhimen Hospital, Beijing University of Chinese Medicine (DZMKJCX-2024-012) and the project of Sepsis Research Institute of Dongzhimen Hospital, Beijing University of Chinese Medicine (a university-affiliated Class III institute).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Chen S Kuhn M Prettner K Yu F Yang T Bärnighausen T et al . The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020-50: a health-augmented macroeconomic modelling study. Lancet Glob Health. (2023) 11:e1183–93. doi: 10.1016/s2214-109x(23)00217-6
2.
Bustacchini S Chiatti C Furneri G Lattanzio F Mantovani LG . The economic burden of chronic obstructive pulmonary disease in the elderly: results from a systematic review of the literature. Curr Opin Pulm Med. (2011) 17:S35–41. doi: 10.1097/01.mcp.0000410746.82840.79
3.
Agustí A Celli BR Criner GJ Halpin D Anzueto A Barnes P et al . Global initiative for chronic obstructive lung disease 2023 report: gold executive summary. Eur Respir J. (2023) 61:2300239. doi: 10.1183/13993003.00239-2023
4.
Christenson SA Smith BM Bafadhel M Putcha N . Chronic obstructive pulmonary disease. Lancet. (2022) 399:2227–42. doi: 10.1016/s0140-6736(22)00470-6
5.
GOLD . Global strategy for prevention, diagnosis and management of COPD (2025 report) (2024). Available online at: https://goldcopd.org/2025-gold-report/ (Accessed July 28, 2024).
6.
GBD 2019 Chronic Respiratory Diseases Collaborators . Global burden of chronic respiratory diseases and risk factors, 1990-2019: An update from the global burden of disease study 2019. EClinicalMedicine. (2023) 59:101936. doi: 10.1016/j.eclinm.2023.101936
7.
Li H He Y Huang L Luo H Zhu X . The nomogram model predicting overall survival and guiding clinical decision in patients with glioblastoma based on the seer database. Front Oncol. (2020) 10:1051. doi: 10.3389/fonc.2020.01051
8.
Cheng H Li J Wei F Yang X Yuan S Huang X et al . A risk nomogram for predicting prolonged intensive care unit stays in patients with chronic obstructive pulmonary disease. Front Med. (2023) 10:1177786. doi: 10.3389/fmed.2023.1177786
9.
Lee SC An C Yoo J Park S Shin D Han CH . Development and validation of a nomogram to predict pulmonary function and the presence of chronic obstructive pulmonary disease in a Korean population. BMC Pulm Med. (2021) 21:32. doi: 10.1186/s12890-021-01391-z
10.
Chen X Wang Q Hu Y Zhang L Xiong W Xu Y et al . A nomogram for predicting severe exacerbations in stable Copd patients. Int J Chron Obstruct Pulmon Dis. (2020) 15:379–88. doi: 10.2147/copd.S234241
11.
Sakamoto Y Yamauchi Y Yasunaga H Takeshima H Hasegawa W Jo T et al . Development of a nomogram for predicting in-hospital mortality of patients with exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2017) 12:1605–11. doi: 10.2147/copd.S129714
12.
Johnson AEW Bulgarelli L Shen L Gayles A Shammout A Horng S et al . Mimic-iv, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
13.
Pollard TJ Johnson AEW Raffa JD Celi LA Mark RG Badawi O . The Eicu collaborative research database, a freely available multi-center database for critical care research. Scientific Data. (2018) 5:180178. doi: 10.1038/sdata.2018.178
14.
Vickers AJ Holland F . Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J. (2021) 21:1643–8. doi: 10.1016/j.spinee.2021.02.024
15.
Jankowski M Bochenek B Wieczorek J Figurski M Gruszczyńska M Goryński P et al . Epidemiological Characteristics of 101,471 Patients Hospitalized with Chronic Obstructive Pulmonary Disease (Copd) in Poland in 2019: Multimorbidity, Duration of Hospitalization, in-Hospital Mortality. Adv Respir Med. (2023) 91:368–82. doi: 10.3390/arm91050029
16.
Stone RA Lowe D Potter JM Buckingham RJ Roberts CM Pursey NJ . Managing patients with Copd exacerbation: does age matter?Age Ageing. (2012) 41:461–8. doi: 10.1093/ageing/afs039
17.
Guo Y Bai J Zhang X Jin Q Liu Y Yu C . Secular trends of mortality and years of life lost due to chronic obstructive pulmonary disease in Wuhan, China from 2010 to 2019: age-period-cohort analysis. Int J Environ Res Public Health. (2022) 19:685. doi: 10.3390/ijerph191710685
18.
Morena D Izquierdo JL Rodríguez J Cuesta J Benavent M Perralejo A et al . The clinical profile of patients with Copd is conditioned by age. J Clin Med. (2023) 12:595. doi: 10.3390/jcm12247595
19.
Vaz Fragoso CA Gill TM . Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. (2012) 67A:264–75. doi: 10.1093/gerona/glr198
20.
Kisiel MA Arnfelt O Lindberg E Jogi O Malinovschi A Johannessen A et al . Association between abdominal and general obesity and respiratory symptoms, asthma and Copd. Results from the Rhine study. Respir Med. (2023) 211:107213. doi: 10.1016/j.rmed.2023.107213
21.
Ramos-Nino ME MacLean CD Littenberg B . Association between prevalence of obstructive lung disease and obesity: results from the Vermont diabetes information system. Asthma Res Pract. (2021) 7:6. doi: 10.1186/s40733-021-00073-1
22.
Yang W Yang Y Guo Y Guo J Ma M Han B et al . Obesity and Risk for Respiratory Diseases: A Mendelian Randomization Study. Front Endocrinol (Lausanne). (2023) 14:1197730. doi: 10.3389/fendo.2023.1197730
23.
Unamuno X Gómez-Ambrosi J Rodríguez A Becerril S Frühbeck G Catalán V et al . Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur J Clin Invest. (2018) 48:e12997. doi: 10.1111/eci.12997
24.
Kyriakopoulos C Chronis C Papapetrou E Tatsioni A Gartzonika K Tsaousi C et al . Prothrombotic state in patients with stable Copd: An observational study. ERJ Open Res. (2021) 7:00297–2021. doi: 10.1183/23120541.00297-2021
25.
Bantulà M Roca-Ferrer J Arismendi E Picado C . Asthma and obesity: two diseases on the rise and bridged by inflammation. J Clin Med. (2021) 10:69. doi: 10.3390/jcm10020169
26.
Wada H Ikeda A Maruyama K Yamagishi K Barnes PJ Tanigawa T et al . Low Bmi and Weight Loss Aggravate Copd Mortality in Men, Findings from a Large Prospective Cohort: The Jacc Study. Sci Rep. (2021) 11:1531. doi: 10.1038/s41598-020-79860-4
27.
LeGall JR Loirat P Alpérovitch A . APACHE II--a severity of disease classification system. Crit Care Med. (1986) 14:754–5. doi: 10.1097/00003246-198608000-00027
28.
Li R Jin X Ren J Deng G Li J Gao Y et al . Relationship of admission serum anion gap and prognosis of critically ill patients: a large multicenter cohort study. Dis Markers. (2022) 2022:5926049. doi: 10.1155/2022/5926049
29.
Chen X Yang Q Gao L Chen W Gao X Li Y et al . Association between serum anion gap and mortality in critically ill patients with Copd in Icu: data from the Mimic iv database. Int J Chron Obstruct Pulmon Dis. (2024) 19:579–87. doi: 10.2147/copd.S433619
30.
Trudzinski FC Kahnert K Vogelmeier CF Alter P Seiler F Fähndrich S et al . Combined effects of lung function, blood gases and kidney function on the exacerbation risk in stable Copd: results from the Cosyconet cohort. Respir Med. (2019) 154:18–26. doi: 10.1016/j.rmed.2019.06.007
31.
Blagev DP Collingridge DS Rea S Horne BD Press VG Churpek MM et al . The laboratory-based intermountain validated exacerbation (live) score identifies chronic obstructive pulmonary disease patients at high mortality risk. Front Med. (2018) 5:173. doi: 10.3389/fmed.2018.00173
32.
Wang L Yi R Wei L Xiong J . Changes of B2-microglobulin and electrolytes in different stages of Copd and their value in evaluating prognosis. J Med Biochem. (2024) 43:946–54. doi: 10.5937/jomb0-50905
33.
Ogan N Günay E Baha A Çandar T Akpınar EE . The effect of serum electrolyte disturbances and uric acid level on the mortality of patients with acute exacerbation of chronic obstructive pulmonary disease. Turk Thorac J. (2020) 21:322–8. doi: 10.5152/TurkThoracJ.2019.19034
34.
Zaidi SRH Rout P . Interpretation of blood clotting studies and values (Pt, Ptt, Aptt, Inr, anti-factor Xa, D-dimer). StatPearls Publishing LLC: Treasure Island, FL (2024).
35.
Rahaghi FN Pistenmaa CL . Hypercoagulation in Copd: the clot thickens. ERJ Open Res. (2021) 7:00534–2021. doi: 10.1183/23120541.00534-2021
36.
Shi X Li H . Anticoagulation therapy in patients with chronic obstructive pulmonary disease in the acute exacerbation stage. Exp Ther Med. (2013) 5:1367–70. doi: 10.3892/etm.2013.1001
37.
Schönhofer B Euteneuer S Nava S Suchi S Köhler D . Survival of mechanically ventilated patients admitted to a specialised weaning Centre. Intensive Care Med. (2002) 28:908–16. doi: 10.1007/s00134-002-1287-5
38.
Gillespie DJ Marsh HM Divertie MB Meadows JA 3rd . Clinical outcome of respiratory failure in patients requiring prolonged (greater than 24 hours) mechanical ventilation. Chest. (1986) 90:364–9. doi: 10.1378/chest.90.3.364
39.
Köhnlein T Windisch W Köhler D Drabik A Geiseler J Hartl S et al . Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. (2014) 2:698–705. doi: 10.1016/s2213-2600(14)70153-5
40.
Fricke K Tatkov S Domanski U Franke KJ Nilius G Schneider H . Nasal high flow reduces hypercapnia by clearance of anatomical dead space in a Copd patient. Respir Med Case Rep. (2016) 19:115–7. doi: 10.1016/j.rmcr.2016.08.010
41.
Nava S Grassi M Fanfulla F Domenighetti G Carlucci A Perren A et al . Non-invasive ventilation in elderly patients with acute Hypercapnic respiratory failure: a randomised controlled trial. Age Ageing. (2011) 40:444–50. doi: 10.1093/ageing/afr003
42.
Tsolaki V Pastaka C Karetsi E Zygoulis P Koutsokera A Gourgoulianis KI et al . One-year non-invasive ventilation in chronic hypercapnic COPD: effect on quality of life. Respir Med. (2008) 102:904–11. doi: 10.1016/j.rmed.2008.01.003
Summary
Keywords
MIMIC-IV, chronic obstructive pulmonary disease, mortality risk, nomogram, external validation, intensive care unit
Citation
Guo Y, Zuo C, Yan J and Ban C (2025) Nomogram model of mortality risk in patients with chronic obstructive pulmonary disease in intensive care unit: based on MIMIC-IV database and external validation study. Front. Med. 12:1547047. doi: 10.3389/fmed.2025.1547047
Received
18 December 2024
Accepted
27 June 2025
Published
22 July 2025
Volume
12 - 2025
Edited by
Eugenia M. Bastos, Independent Researcher, Sommerville, MA, United States
Reviewed by
My Hanh Bui, Hanoi Medical University, Vietnam
Pengfei Zhu, Merck Sharp and Dohme (China) Ltd., China
Updates
Copyright
© 2025 Guo, Zuo, Yan and Ban.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Yan, y123j123y123j123@163.comChengjun Ban, A3326@bucm.edu.cn
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.