- 1Department of Cardiology, The Second Hospital of Sanming, Sanming, Fujian, China
- 2Department of Critical Care Medicine, The Second Hospital of Sanming, Sanming, Fujian, China
- 3Department of Nephrology, The Second Hospital of Sanming, Sanming, Fujian, China
- 4Department of Pharmacy, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
The pharmacokinetics of meropenem are significantly altered in patients with augmented renal clearance (ARC), resulting in suboptimal plasma concentrations. The objective of this study is to investigate the efficacy of different meropenem regimens in critically ill patients with ARC. To this end, Monte Carlo simulations were conducted. The probability of target attainment (PTA) and the cumulative fraction of response (CFR) were evaluated with consideration of the minimal inhibitory concentration (MIC) breakpoint according to the Clinical and Laboratory Standards Institute (CLSI). The findings of this study demonstrate that meropenem administered at a dosage of 2 g every 8 h (q8 h) 2/3 h to critically ill patients with ARC [creatinine clearance (CrCL) of 140–200 mL/min] results in ≥ 90% PTA (100% fT > MIC) for lower MICs (≤ 2 mg/L). However, for higher MICs (4–8 mg/L), the administration of intensified regimens (2 g q8 h 4/6 h or continuous infusion) was necessary. The CFR analysis confirmed ≥ 90% target attainment for Klebsiella pneumoniae with regimens meropenem 2 g q8 h 2–6 h or continuous infusion, but not for Acinetobacter baumannii or Pseudomonas aeruginosa, regardless of regimen. For resistant Klebsiella pneumoniae (4 < MIC ≤ 8), prolonged (4–6 h) or continuous infusions are recommended. For Acinetobacter baumannii and Pseudomonas aeruginosa, alternative or combination therapies are advised due to insufficient PK/PD target attainment with meropenem monotherapy. The findings emphasize the importance of individualized dosing strategies in ARC patients, considering meropenem’s distinctive PK/PD characteristics, the pathogen’s MIC, and renal function, in order to effectively manage resistant Gram-negative infections while optimizing clinical outcomes.
Introduction
Severe infection stands as a leading cause of intensive care unit (ICU) admission (1), the mortality of patients with such infection remains substantial (2). According to WHO data, infectious disease represents one of the top 10 causes of death worldwide (3). In American, more than 350,000 patients die from serious infections in a year (4). The significance of promptly initiating tailored treatments in critically ill patients with severe infections, particularly sepsis, is a matter of great concern (5). However, the pharmacokinetics (PK) of drugs can be altered by supportive technology and pathological processes in critically ill patients (6, 7). The routine dosage of anti-infective regimens may be insufficient to attain target plasma concentrations (8). Consequently, antimicrobial drug monitoring and dosage optimization are essential to achieve aggressive pharmacodynamics (PD) targets (9).
Gram-negative bacteria (GNB) are the primary pathogens identified in severe infections (10). Meropenem, a broad-spectrum carbapenem antibiotic that prevents the synthesis of essential components of the bacterial cell wall, resulting in the death of the microorganism, is an important treatment for severe GNB infections (11). For meropenem, a β-lactam antibiotic, the duration of time (T) that the unbounded drug concentration above the minimal inhibitory concentration (MIC) is the most important indicator, which is defined as fT > MIC (12). In the context of critically ill patients, the PK/PD target for β-lactam antibiotics is delineated as 100% fT > MIC (or more ambitiously, 100% fT > 4 × MIC), with the objective of enhancing survival rates and mitigating resistance (13, 14). Patients with 100% fT > MIC exhibited significantly higher rates of clinical cure (82%) and bacteriological eradication (97%) in comparison to patients with % fT > MIC less than 100% (15). Consequently, in the present study, 100% fT > MIC were utilized as PK/PD targets for Monte Carlo simulation.
Augmented renal clearance (ARC) is a pathophysiological phenomenon that often occurs in critically ill patients, resulting in enhanced renal function defined as a urinary creatinine clearance of at least 130 mL/min/1.73 m2 (16). The incidence of ARC in critically ill patients ranges from 30% to 65%, and can be as high as 50%–85% in those with sepsis, trauma, and other factors (17, 18). The mechanism of ARC is that the hyperdynamic and hypermetabolic state of critically ill patients increases cardiac output and renal blood flow, leading to increased drug clearance through the kidney (19). It has been reported that patients with ARC are less likely to achieve % fT > MIC with beta-lactam antibiotics (20). Udy et al. (21) also reported that only one third of critically ill patients with sepsis achieved 100% fT > MIC when using piperacillin-tazobactam, owing to elevated drug clearance. These results suggest that ARC promotes drug excretion and leads to inadequate drug exposure, which may compromise clinical efficacy.
In the present study, we aim to explore the alternative dosage regimens of meropenem in critically ill patients with ARC using Monte Carlo simulations. This will provide a potential recommendation for the development of antimicrobial outcomes for such patients.
Materials and methods
Monte Carlo simulations
Monte Carlo simulation was performed using Oracle Crystal Ball 11.1.2.4.850 software embedded in Office Excel 2019. Pharmacokinetic parameters including renal clearance (CL) and volume of distribution (Vd) were assumed to follow a normal distribution, while MIC followed a discrete uniform distribution and free drug fraction (f) followed a uniform distribution. The MIC value was set to a range of 0.125–8 μg/mL. A target value of 100% fT > MIC was set and different creatinine clearance (CrCL) values (140, 160, 180, and 200 mL/min) were tested. The probability of target attainment (PTA) value was then calculated using Monte Carlo simulations run for 10,000 cases for different meropenem dosing regimens as follows:
a: 1 g infused over 0.5 h every 8 h, 1 g q8 h 0.5 h
b: 2 g infused over 2 h every 8 h, 2 g q8 h 2 h
c: 2 g over 3 h every 8 h, 2 g q8 h 3 h
d: 2 g over 4 h every 8 h, 2 g q8 h 4 h
e: 2 g over 6 h every 8 h, 2 g q8 h 6 h
f: 2 g over 8 h every 8 h, continuous infusion.
The results were plotted as PTA-MIC curves.
Equation 1 (22) was used to calculate the values of % fT > MIC for various dosing regimens.
Equation 1
The given equation includes several parameters as follows: The free drug fraction (f), natural logarithm (Ln), infusion rate (R0 = f × dose / T), renal clearance (CL, L/h), volume of distribution (Vd), minimum inhibitory concentration (MIC, μg/mL), intravenous infusion time (T, h), and dosing interval (DI, h). Clinical breakpoints for pathogen susceptibility are defined by the Clinical and Laboratory Standards Institute (CLSI) standards for meropenem (23). For Enterobacterales, MIC ≤ 1 μg/mL is considered susceptible, MIC = 2 μg/mL is considered intermediate, and MIC ≥ 4 μg/mL is considered resistant. For Pseudomonas aeruginosa and Acinetobacter spp., MIC ≤ 2 μg/mL is considered susceptible, MIC = 4 μg/mL is considered intermediate, and MIC ≥ 8 μg/mL is considered resistant (23).
Population pharmacokinetic model and MIC distribution in critically ill patients
Monte Carlo simulations were performed using a population pharmacokinetic (PPK) model published by Gijsen et al. (24). Antimicrobial susceptibility testing of Gram-negative bacteria was derived from a total of 6,520 pathogens detected in critically ill patients in a tertiary care hospital (25). The pathogen-specific MIC distributions, which were used to calculate the cumulative fraction of response (CFR), are shown in Table 1.
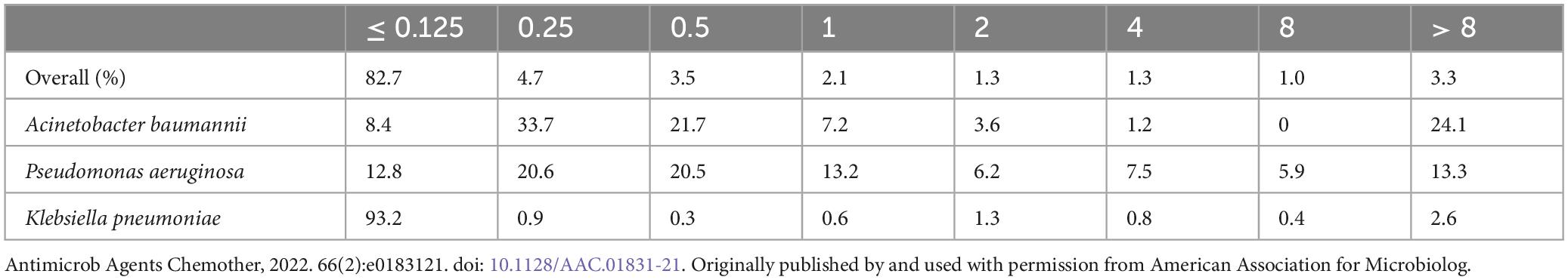
Table 1. Pathogen-specific MIC distributions in critically ill patients (25).
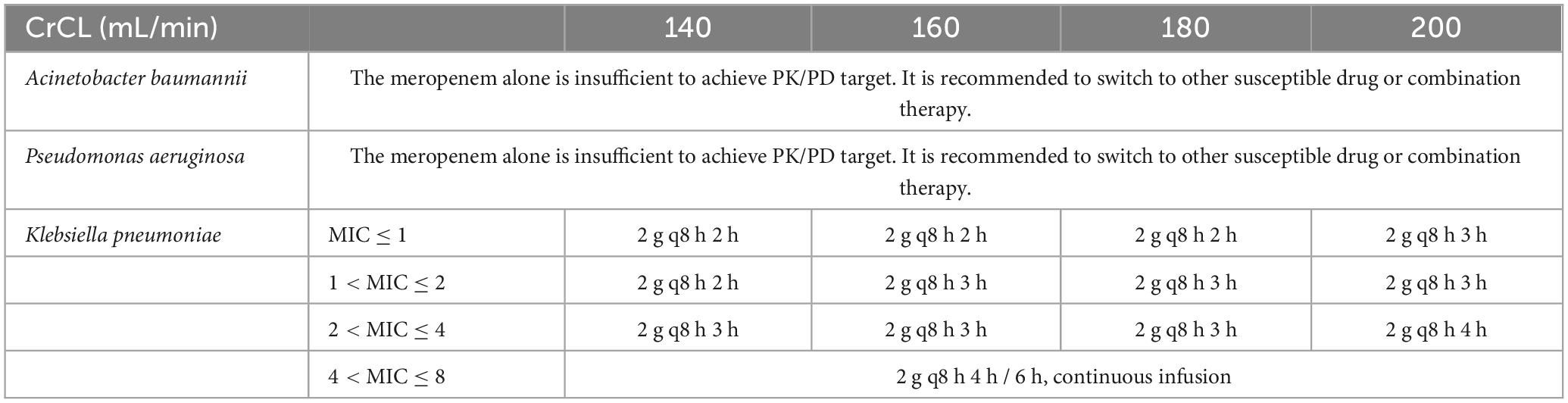
Optimized dosing regimens for critically ill patients with ARC treated with meropenem
Dosage regimens for meropenem were simulated in critically ill patients with CrCL of 140, 160, 180 and 200 mL/min. Suggested regimens included standard therapy, prolonged and continuous infusion. The CFR values were calculated as the weighted summation of the PTA values of each MIC category for a specific dosing regimen and renal clearance. Treatments with a CFR greater than 90% were considered as potential recommendations.
Statistical analysis
Continuous variables are presented as mean (standard deviation) or median (quartiles), while categorical variables are presented as absolute numbers or relative frequencies. Statistical analyses were conducted using SPSS 22.0 software. A p-value of less than 0.05 was considered statistically significant.
Results
PTA of meropenem in critically ill patients with ARC
Based on the present Monte Carlo simulations, for patients with a CrCL of 140 mL/min, the PTA-MIC curves of the meropenem regimens are shown in Figure 1A. For a target of 100% fT > MIC, for pathogens with MIC ≤ 2 mg/L, a PTA of 90% could be achieved with regimen b–f; for pathogens with 2 < MIC ≤ 4 mg/L, a PTA of at least 90% could be achieved with regimen c–f; for pathogens with 4 < MIC ≤ 8 mg/L, a PTA of at least 90% could be achieved with regimen d–f.
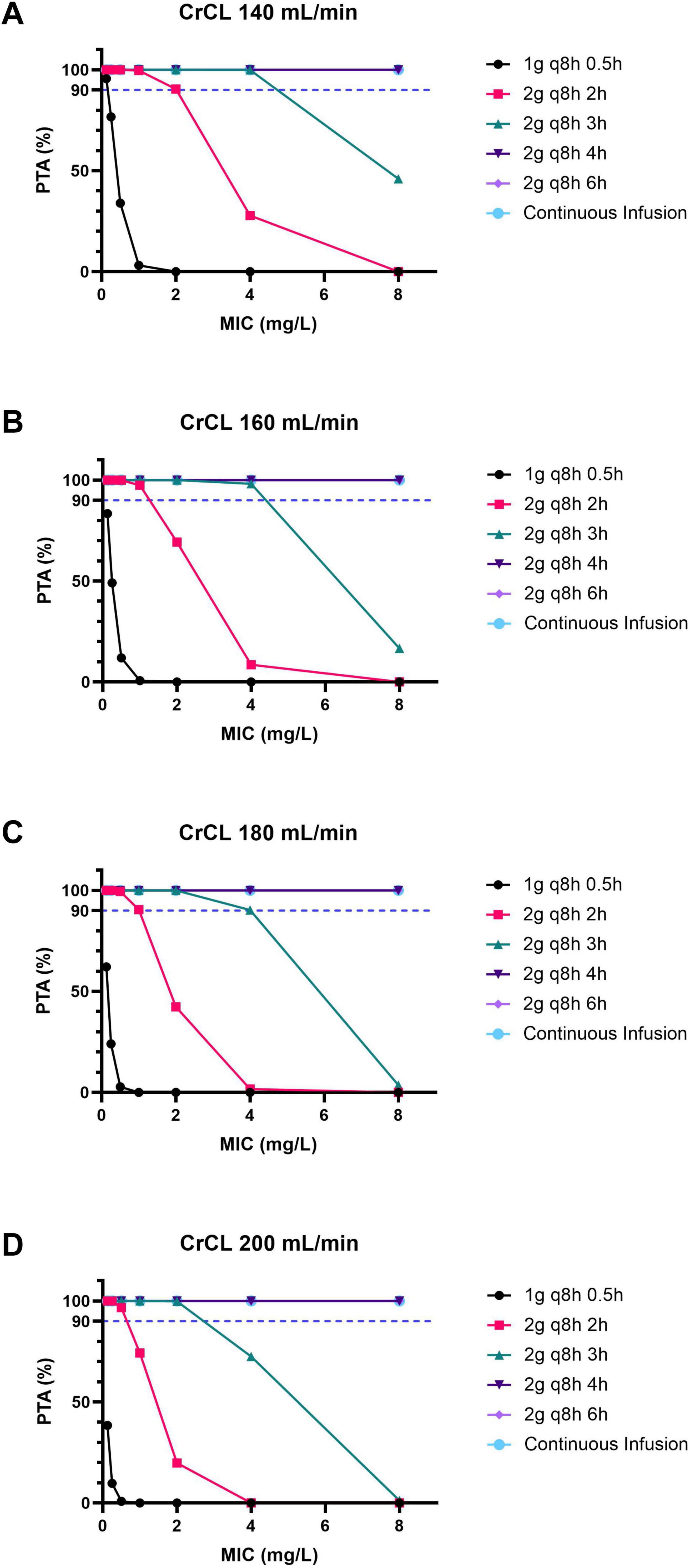
Figure 1. The PTA-MIC curves for patients with ARC under different meropenem dosing regimens. (A) For patients with a CrCL of 140 mL/min. (B) For patients with a CrCL of 160 mL/min. (C) For patients with a CrCL of 180 mL/min. (D) For patients with a CrCL of 200 mL/min. PTA, probability of target attainment; MIC, minimal inhibitory concentration; ARC, augmented renal clearance; CrCL, creatinine clearance.
Likewise, for patients with a CrCL of 160 mL/min, the PTA-MIC curves of the meropenem regimens are shown in Figure 1B. For a target of 100% fT > MIC, for pathogens with MIC ≤ 1 mg/L, a PTA of 90% could be achieved with regimen b–f; for pathogens with 1 < MIC ≤ 4 mg/L, a PTA of at least 90% could be achieved with regimen c–f; for pathogens with 4 < MIC ≤ 8 mg/L, a PTA of at least 90% could be achieved with regimen d–f.
Furthermore, for patients with a CrCL of 180 mL/min, the PTA-MIC curves of the meropenem regimens are shown in Figure 1C. For a target of 100% fT > MIC, for pathogens with MIC ≤ 1 mg/L, a PTA of at least 90% could be achieved with regimen b–f; for pathogens with 1 < MIC ≤ 4 mg/L, a PTA of at least 90% could be achieved with regimen c–f; for pathogens with 4 < MIC ≤ 8 mg/L, a PTA of at least 90% could be achieved with regimen d–f.
Additionally, for patients with a CrCL of 200 mL/min, the PTA-MIC curves of the meropenem regimens are shown in Figure 1D. For a target of 100% fT > MIC, for pathogens with MIC ≤ 2 mg/L, a PTA of 90% could be achieved with regimen c–f; for pathogens with 2 < MIC ≤ 8 mg/L, a PTA of 90% could be achieved with regimen d–f.
CFR of meropenem in critically ill patients with ARC
It is evident that, in order to achieve a target of 100% fT > MIC, a CFR of at least 90% can be attained in critically ill patients with ARC (140 ≤ CrCL ≤ 200 mL/min) when treated with regimens b, c, d, e, f. However, it is unfortunate that all CFR values in cases treated with meropenem 1 g q8 h 0.5 h are less than 90%, as illustrated in Table 2. Furthermore, for infections caused by Acinetobacter baumannii and Pseudomonas aeruginosa, the CFR target could not be achieved in patients with ARC. Furthermore, for infections caused by Klebsiella pneumoniae, the CFR target could be achieved in critically ill patients with ARC with regimens b, c, d, e, f.
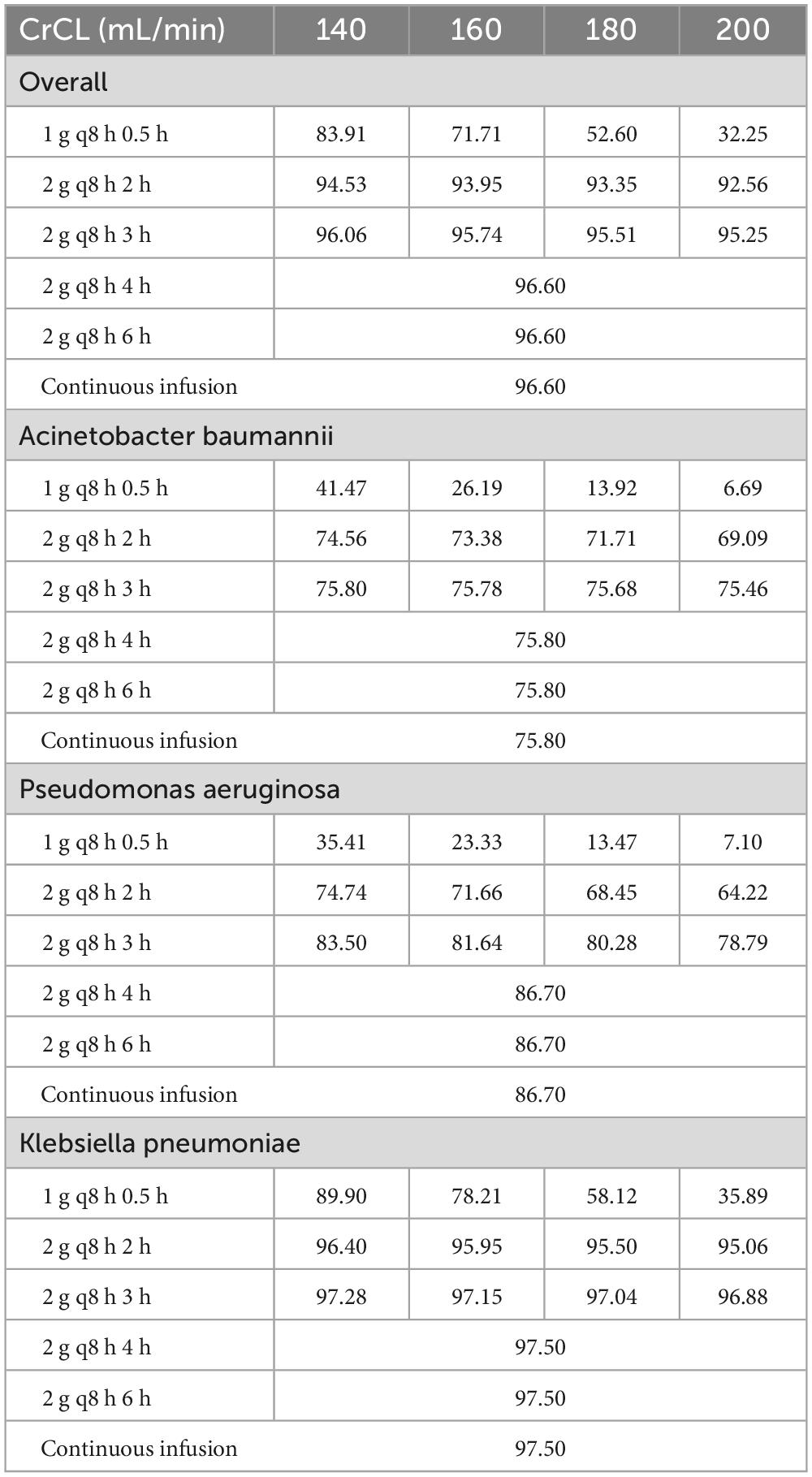
Table 2. The CFR values for different renal functions and dosing regimens achieving a target of 100% fT > MIC.
Dosing regimens and recommendation to real-world settings
The Monte Carlo simulation results indicate that meropenem alone is inadequate for achieving PK/PD targets in patients with ARC (140 ≤ CrCL ≤ 200 mL/min) for infections caused by Acinetobacter baumannii and Pseudomonas aeruginosa. It is recommended that treatment be switched to other susceptible drugs or combination therapy.
For infections caused by Klebsiella pneumoniae, the recommendations were delineated according to the MIC category in accordance with real-world settings (see Table 3). For resistant Klebsiella pneumoniae with an elevated MIC (4 < MIC ≤ 8), the administration of a prolonged infusion (i.e., 4 h, 6 h) or continuous infusion may be advantageous for patients with ARC.
Discussion
In the present study, Monte Carlo simulations demonstrated that meropenem 2 g q8 h 2/3 h, administered to critically ill patients with ARC (CrCL 140–200 mL/min), achieved ≥ 90% PTA (100% fT > MIC) for lower MICs (≤ 2 mg/L). However, for higher MICs (4–8 mg/L), intensified regimens (2 g q8 h 4/6 h or continuous infusion) were required. The CFR analysis confirmed ≥ 90% target attainment for Klebsiella pneumoniae with regimens meropenem 2 g q8 h 2–6 h or continuous infusion, but not for Acinetobacter baumannii or Pseudomonas aeruginosa, regardless of regimen. For resistant Klebsiella pneumoniae (4 < MIC ≤ 8), prolonged (4–6 h) or continuous infusions are recommended. For Acinetobacter baumannii and Pseudomonas aeruginosa, alternative or combination therapies are advised due to insufficient PK/PD target attainment with meropenem monotherapy. These findings underscore the necessity for tailored dosing strategies in ARC patients, contingent on the pathogen’s MIC and renal function.
Sepsis has been defined as an acute, life-threatening condition caused by a dysregulated immune system response to infection (26), affecting millions of individuals annually and resulting in 1/6 ∼1/3 of those afflicted dying as a direct consequence (27). For adults exhibiting signs of septic shock, it is recommended that antimicrobials be administered promptly, ideally within one hour of recognition (28, 29). GNB represent the primary pathogens, accounting for at least 40% of pathogens associated with bloodstream infections (30). Given the alarming global spread of antimicrobial resistance represents a significant threat (5), the lack of appropriate antibiotics for severe infections becomes a crucial issue (31).
Meropenem, a carbapenem antibiotic, has a broad spectrum of antibacterial activity and is widely used in antimicrobial therapy for a variety of bacterial infections, particularly those caused by GNB (32). Meropenem exhibits a time-dependent bactericidal effect, whereby the efficacy of this antimicrobial against pathogens is determined by measuring the percentage of time that the unbounded drug concentration exceeds the MIC between doses. This indicator is also known as % fT > MIC (33). For critically ill patients, it has been established that the PK/PD targets for meropenem should be increased to 100% fT > MIC in order to achieve a higher survival rate and to minimize resistance development (14, 34). Therefore, in the present study, 100% fT > MIC were chosen as PK/PD targets for PTA assessment during Monte Carlo simulation. Nevertheless, Meropenem is a hydrophilic compound that is primarily excreted by the kidneys, which are highly susceptible to alterations in renal function (19). Likewise, a number of pathophysiological alterations can influence the pharmacokinetics of meropenem in critically ill patients, potentially increasing the probability of subtherapeutic levels and affecting the efficacy of therapeutic interventions (34).
Currently, ARC is defined as a urinary creatinine clearance of at least 130 mL/min/1.73 m2. The mechanism of ARC may be attributable to altered physiological processes in critically ill patients, leading to hyperdynamic and hypermetabolic states that increase cardiac output and renal blood flow. This, in turn, results in enhanced drug clearance through the kidneys (19). Furthermore, there is evidence that ARC development is associated with inflammatory stress response, fluid resuscitation, and the use of vasoactive drugs in critically ill patients (35). It has been demonstrated by research that neutropenia accompanied by fever is also a contributing factor to ARC (19). It has been observed that subtherapeutic levels of renally cleared drugs are present in patients who are undergoing ARC, for instance, the β-lactam antibiotics, aminoglycoside and vancomycin (35, 36). In such instances, a lack of sufficient therapeutic antibiotic concentration for patients with ARC has been linked to an increased incidence of treatment failure and the selection of more resistant pathogens (16, 37). Carlier et al. reported that the average % fT > MIC for piperacillin/tazobactam or meropenem was 61% for patients with ARC and 94% for non-ARC patients (20). Liebchen et al. reported that the patient with ARC exhibited inadequate serum trough levels despite meropenem infusion at the maximum approved dose (2 g every 8 h) (38). Therefore, therapeutic drug monitoring (TDM)-guided antibiotic dosing will hopefully maximize antibiotic exposure and reduce bacterial resistance (39), improving clinical outcomes of patients with ARC (38, 40, 41).
Standard dosing of antibiotics in intensive-care-unit (ICU) patients runs the risk of low serum concentrations due to altered physiological conditions such as ARC and increased volume of distribution (3). Low serum-concentrations in combination with multiresistant bacteria at a higher MIC lead to subtherapeutic antibiotic exposure, with the consequence of treatment failure and the selection of more resistant pathogens. As such, standard dosing would be an inadequate strategy in this setting (4). There are limited treatment options for MDR A. baumannii infections and inappropriate initial therapy is associated with increased mortality. Novel antibiotics and combination therapy of existing drugs are deemed necessary in this context (5).
Furthermore, for adults with severe infections, optimizing dosing strategies should be conducted in accordance with PK/PD principles and the specific pharmacological properties of the drug in question (29). A number of studies have been conducted to investigate the PPK of meropenem in critically ill patients (24, 42, 43). In the present study, we employed the PPK model proposed by Gijsen et al. and conducted Monte Carlo simulations to determine the optimal dosage of meropenem in critically ill patients with ARC (24). The PTA and CFR calculations have established the regimen recommendation.
In order to increase the percentage of fT > MIC, the efficacy and safety of prolonged and continuous infusion of meropenem and other β-lactams in critically ill patients, regardless of ARC, have been assessed (44). In a study of neutropenic children with ARC treated by meropenem or piperacillin, continuous infusion was found to reduce the inadequate antimicrobial exposure rate (8% vs. 85%) in comparison with intermittent infusion (45). The BLING-III randomized controlled trial demonstrated that the continuous infusion of meropenem is clinically superior to intermittent infusion in critically ill patients with sepsis (46). Abdul-Aziz et al. (47) have reported that prolonged infusion of β-lactam antibiotics yields reduced risks of 90-day and ICU mortality with increased clinical cures compared to intermittent infusions in patients with GNB infections. Dosing simulations suggest that using continuous infusion regimens may enhance bacterial killing (48). Furthermore, continuous infusion for critical orthotopic liver transplant recipients has been shown to minimize the risk of 30-day resistance (49). A comparative analysis of adverse event incidences, including neurotoxicity, cytopenias, and diarrhea, revealed no significant disparities between prolonged and intermittent infusion regimens (50). The total daily antibiotic dose for the continuous therapy was equivalent to those recommended for intermittent therapy (51). Conversely, a randomized clinical trial revealed that continuous administration of meropenem did not significantly decrease the all-cause mortality and emergence of pandrug-resistant or extensively drug-resistant bacteria at day 28 (52). The findings indicate a persistent uncertainty surrounding the efficacy of prolonged infusions of β-lactam antibiotics in enhancing clinical outcomes in critically ill adults with sepsis (53). The need for further research in this area is underscored by the necessity for large-scale prospective studies that can provide more definitive answers.
Not only does ARC, but also hypoalbuminemia, have the capacity to significantly alter the pharmacokinetics of antibiotics in critically ill patients (54, 55). An increase in the free fraction of drug resulting from hypoalbuminemia will lead to an increase in the Vd and a consequent increase in the rate of renal drug elimination (37). Hypoalbuminemia exerts a significant influence on highly albumin-bound (>90%) and predominantly renal eliminated antibiotics, such as ceftriaxone and ertapenem (56). The lower unbound fraction of vancomycin, along with lower binding antimicrobials, has been observed to be induced by ARC and hypoalbuminemia (57, 58). Consequently, alterations in septic shock, encompassing fluid overload, augmented cardiac output, ARC, and hypoalbuminemia, may result in subtherapeutic concentrations of antimicrobials, thereby influencing treatment efficacy and patient outcomes (59).
In light of that the predominant manifestation of bacterial infections occurs in extra-vascular tissues, the therapeutic effect of antibiotic treatment is contingent on the concentration of free antibiotic in target tissues (60, 61). For instance, respiratory tissue penetration of meropenem was reported to be 40% in the lung (62), and 37.5% of target site concentrations were below the EUCAST clinical breakpoint (63). This relatively low concentration in lung tissue may explain why achieving 50% fT > MIC does not necessarily improve clinical outcomes (64). Conversely, the plasma azithromycin concentrations were only approximately 10% and 1% those of bronchial fluid and lung tissue, respectively (65). Therefore, the free antibiotic concentrations in the tissues are responsible for the antibacterial activity and are more suitable for the determination of the clinical efficacy than the plasma concentration (66).
However, it should be noted that this study is subject to certain limitations. Firstly, the clinical trial is lacking, which means that there is no evidence to assess the efficacy of the regimen’s recommendation. Secondly, only one PPK model has been used in this study, which may not be the most appropriate model for Monte Carlo stimulation in critically ill patients with ARC. Therefore, further Monte Carlo stimulations and clinical studies are warranted.
In conclusion, Monte Carlo simulation analysis revealed that in critically ill patients with ARC (CrCL 140–200 mL/min), meropenem 2 g q8 h (2–3 h infusion) achieved optimal PTA for pathogens with MIC ≤ 2 mg/L. However, for more resistant organisms (MIC 4–8 mg/L), extended infusions (4–6 h) or continuous administration were necessary to maintain therapeutic efficacy. While these extended regimens proved effective against Klebsiella pneumoniae, they failed to achieve adequate coverage for Acinetobacter baumannii or Pseudomonas aeruginosa infections, highlighting the need for alternative antimicrobial agents or combination therapy approaches in such cases. These findings emphasize the importance of implementing individualized dosing strategies in ARC patients, taking into account meropenem’s unique PK/PD characteristics, including its time-dependent bactericidal activity and predominant renal elimination, to effectively manage resistant Gram-negative infections while optimizing clinical outcomes.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JLu: Writing – original draft. JLi: Writing – original draft. HL: Writing – review and editing. YY: Writing – original draft. CC: Writing – original draft. JC: Writing – original draft. HZ: Data curation, Formal Analysis, Methodology, Writing – original draft. SZ: Project administration, Supervision, Writing – review and editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sabo S, Venkatramanan A, Shorr A. At the intersection of critical care and infectious diseases: The year in review. Biomedicines. (2024) 12:562. doi: 10.3390/biomedicines12030562
2. Fan Y, Moser J, van Meurs M, Kiers D, Sand J, Leeming D, et al. Neo-epitope detection identifies extracellular matrix turnover in systemic inflammation and sepsis: An exploratory study. Crit Care. (2024) 28:120. doi: 10.1186/s13054-024-04904-4
4. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP). Sepsis. Available online at: https://www.cdc.gov/sepsis/what-is-sepsis.html (accessed April 15, 2024) (2023).
5. Cusack R, Little E, Martin-Loeches I. Practical lessons on antimicrobial therapy for critically Ill patients. Antibiotics (Basel). (2024) 13:162. doi: 10.3390/antibiotics13020162
6. Ackerman M, Ahrens T, Kelly J, Pontillo A. Sepsis. Crit Care Nurs Clin North Am. (2021) 33:407–18. doi: 10.1016/j.cnc.2021.08.003
7. Jamal J, Roger C, Roberts J. Understanding the impact of pathophysiological alterations during critical illness on drug pharmacokinetics. Anaesth Crit Care Pain Med. (2018) 37:515–7. doi: 10.1016/j.accpm.2018.10.006
8. Bilbao-Meseguer I, Barrasa H, Rodríguez-Gascón A, Asín-Prieto E, Maynar J, Sánchez-Izquierdo JÁ, et al. Optimization of levetiracetam dosing regimen in critically ill patients with augmented renal clearance: A Monte Carlo simulation study. J Intensive Care. (2022) 10:21. doi: 10.1186/s40560-022-00611-w
9. Lewis S, Mueller B. Antibiotic dosing recommendations in critically ill patients receiving new innovative kidney replacement therapy. BMC Nephrol. (2024) 25:73. doi: 10.1186/s12882-024-03469-2
10. Cui C, Ma L, Qi X. Analysis of the associated factors in postoperative wound infection following open reduction and internal fixation for elbow fracture. Int Wound J. (2024) 21:e14825. doi: 10.1111/iwj.14825
11. Gobezie M, Hassen M, Tesfaye N, Solomon T, Demessie M, Kassa T, et al. Prevalence of meropenem-resistant Pseudomonas Aeruginosa in Ethiopia: A systematic review and meta-analysis. Antimicrob Resist Infect Control. (2024) 13:37. doi: 10.1186/s13756-024-01389-2
12. Hyatt J, McKinnon P, Zimmer G, Schentag J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. (1995) 28:143–60. doi: 10.2165/00003088-199528020-00005
13. Horstink M, Geel D, Uil C, Deetman P, Endeman H, Abdulla A, et al. Standard versus double dosing of beta-lactam antibiotics in critically ill patients with sepsis: The BULLSEYE study protocol for a multicenter randomized controlled trial. BMC Infect Dis. (2025) 25:392. doi: 10.1186/s12879-025-10747-3
14. Smekal A, Furebring M, Eliasson E, Lipcsey M. Low attainment to PK/PD-targets for β-lactams in a multi-center study on the first 72 h of treatment in ICU patients. Sci Rep. (2022) 12:21891. doi: 10.1038/s41598-022-25967-9
15. McKinnon P, Paladino J, Schentag J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. (2008) 31:345–51. doi: 10.1016/j.ijantimicag.2007.12.009
16. Deenen S, Fransen L, Jaspers T, Workum J. Treatment implications of augmented renal clearance in a critically ill COVID-19 patient: A case report. Fundam Clin Pharmacol. (2023) 37:1011–5. doi: 10.1111/fcp.12916
17. Udy A, Baptista J, Lim N, Joynt G, Jarrett P, Wockner L, et al. Augmented renal clearance in the ICU: Results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations*. Crit Care Med. (2014) 42:520–7. doi: 10.1097/CCM.0000000000000029
18. Hobbs A, Shea K, Roberts K, Daley M. Implications of augmented renal clearance on drug dosing in critically Ill patients: A focus on antibiotics. Pharmacotherapy. (2015) 35:1063–75. doi: 10.1002/phar.1653
19. Hirai K, Ishii H, Shimoshikiryo T, Shimomura T, Tsuji D, Inoue K, et al. Augmented renal clearance in patients with febrile neutropenia is associated with increased risk for subtherapeutic concentrations of vancomycin. Ther Drug Monit. (2016) 38:706–10. doi: 10.1097/FTD.0000000000000346
20. Carlier M, Carrette S, Roberts J, Stove V, Verstraete A, Hoste E, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: Does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care. (2013) 17:R84. doi: 10.1186/cc12705
21. Udy A, Lipman J, Jarrett P, Klein K, Wallis S, Patel K, et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit Care. (2015) 19:28. doi: 10.1186/s13054-015-0750-y
22. Wang H, Zhang B, Ni Y, Kuti J, Chen B, Chen M, et al. Pharmacodynamic target attainment of seven antimicrobials against Gram-negative bacteria collected from China in 2003 and 2004. Int J Antimicrob Agents. (2007) 30:452–7. doi: 10.1016/j.ijantimicag.2007.06.005
23. CLSI M100. Performance Standards for Antimicrobial Susceptibility Testing. 34th edn. Available online at: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed April 17, 2024);(2024)
24. Gijsen M, Elkayal O, Annaert P, Van Daele R, Meersseman P, Debaveye Y, et al. Meropenem target attainment and population pharmacokinetics in critically Ill septic patients with preserved or increased renal function. Infect Drug Resist. (2022) 15:53–62. doi: 10.2147/IDR.S343264
25. Liebchen U, Weinelt F, Scharf C, Schroeder I, Paal M, Zoller M, et al. Combination of pharmacokinetic and pathogen susceptibility information to optimize meropenem treatment of gram-negative infections in critically Ill patients. Antimicrob Agents Chemother. (2022) 66:e0183121. doi: 10.1128/AAC.01831-21
26. Schlapbach L, Watson R, Sorce L, Argent A, Menon K, Hall M, et al. International consensus criteria for pediatric sepsis and septic shock. JAMA. (2024) 331:665–74. doi: 10.1001/jama.2024.0179
27. Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd K, Schlattmann P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. (2020) 46:1552–62. doi: 10.1007/s00134-020-06151-x
28. Nve E, Badia J, Amillo-Zaragüeta M, Juvany M, Mourelo-Fariña M, Jorba R. Early management of severe biliary infection in the era of the tokyo guidelines. J Clin Med. (2023) 12:4711. doi: 10.3390/jcm12144711
29. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith C, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med. (2021) 49:e1063–143. doi: 10.1097/CCM.0000000000005337
30. Muñoz P, Cruz A, Rodríguez-Créixems M, Bouza E. Gram-negative bloodstream infections. Int J Antimicrob Agents. (2008) 32:S10–4. doi: 10.1016/j.ijantimicag.2008.06.015
31. Nakashima H, Miyazaki M, Kuwamura T, Oda K, Haga Y, Imakyure O. Relationship between target time above minimum inhibitory concentration achievement rate of meropenem using monte carlo simulation and in-hospital survival in patients with Pseudomonas aeruginosa Bacteremia. Antibiotics (Basel). (2024) 13:219. doi: 10.3390/antibiotics13030219
32. Baldwin C, Lyseng-Williamson K, Keam S. Meropenem: A review of its use in the treatment of serious bacterial infections. Drugs. (2008) 68:803–38. doi: 10.2165/00003495-200868060-00006
33. Ambrose P, Bhavnani S, Rubino C, Louie A, Gumbo T, Forrest A, et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin Infect Dis. (2007) 44:79–86. doi: 10.1086/510079
34. Boonpeng A, Jaruratanasirikul S, Jullangkoon M, Samaeng M, Wattanavijitkul T, Bhurayanontachai R, et al. Population pharmacokinetics/pharmacodynamics and clinical outcomes of meropenem in critically Ill patients. Antimicrob Agents Chemother. (2022) 66:e0084522. doi: 10.1128/aac.00845-22
35. Avedissian S, Rohani R, Bradley J, Le J, Rhodes N. Optimizing aminoglycoside dosing regimens for critically Ill pediatric patients with augmented renal clearance: A convergence of parametric and nonparametric population approaches. Antimicrob Agents Chemother. (2021) 65:e2629–2620. doi: 10.1128/AAC.02629-20
36. Minkutë R, Briedis V, Steponavièiûtë R, Vitkauskienë A, Maèiulaitis R. Augmented renal clearance–an evolving risk factor to consider during the treatment with vancomycin. J Clin Pharm Ther. (2013) 38:462–7. doi: 10.1111/jcpt.12088
37. Udy A, Roberts J, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. (2013) 39:2070–82. doi: 10.1007/s00134-013-3088-4
38. Liebchen U, Paal M, Jung J, Schroeder I, Frey L, Zoller M, et al. Therapeutic drug monitoring-guided high dose meropenem therapy of a multidrug resistant Acinetobacter baumannii - A case report. Respir Med Case Rep. (2020) 29:100966. doi: 10.1016/j.rmcr.2019.100966
39. Vitiello A, Sabbatucci M, Salzano A, Zovi A. The importance of antibiotic treatment duration in antimicrobial resistance. Eur J Clin Microbiol Infect Dis. (2024) 43:1673–5. doi: 10.1007/s10096-024-04867-y
40. Sime F, Roberts M, Peake S, Lipman J, Roberts J. Does beta-lactam pharmacokinetic variability in critically Ill patients justify therapeutic drug monitoring? A systematic review. Ann Intensive Care. (2012) 2:35. doi: 10.1186/2110-5820-2-35
41. Kumta N, Heffernan A, Cotta M, Wallis S, Livermore A, Starr T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of meropenem in neurocritical care patients: A prospective two-center study. Antimicrob Agents Chemother. (2022) 66:e0014222. doi: 10.1128/aac.00142-22
42. Charoensareerat T, Chaijamorn W, Kerdnimith P, Kosumwisaisakul N, Teeranaew P, Rungkitwattanakul D, et al. Optimal meropenem dosing regimens in patients undergoing continuous renal replacement therapy: Systematic review and monte carlo simulations. Blood Purif. (2023) 52:503–15. doi: 10.1159/000529694
43. Murínová I, Švidrnoch M, Guckı T, Øezáè D, Hlaváè J, Slanaø O, et al. Meropenem population pharmacokinetics and model-based dosing optimisation in patients with serious bacterial infection. Eur J Hosp Pharm. (2022) 31:253–8. doi: 10.1136/ejhpharm-2022-003535
44. Tilanus A, Shields R, Lodise T, Drusano G. Translating PK-PD principles into improved methodology for clinical trials which compare intermittent with prolonged infusion of beta-lactam antibiotics. Clin Infect Dis. (2025): doi: 10.1093/cid/ciaf038 Online ahead of print
45. André P, Diezi L, Dao K, Crisinel P, Rothuizen L, Chtioui H, et al. Ensuring sufficient trough plasma concentrations for broad-spectrum beta-lactam antibiotics in children with malignancies: Beware of augmented renal clearance! Front Pediatr. (2021) 9:768438. doi: 10.3389/fped.2021.768438
46. Dulhunty J, Brett S, De Waele J, Rajbhandari D, Billot L, Cotta M, et al. Continuous vs Intermittent β-Lactam antibiotic infusions in critically Ill patients with sepsis: The BLING III randomized clinical trial. JAMA. (2024) 332:629–37. doi: 10.1001/jama.2024.9779
47. Abdul-Aziz M, Hammond N, Brett S, Cotta M, De Waele J, Devaux A, et al. Prolonged vs intermittent infusions of β-Lactam antibiotics in adults with sepsis or septic shock: A systematic review and meta-analysis. JAMA. (2024) 332:638–48. doi: 10.1001/jama.2024.9803
48. O’Jeanson A, Nielsen E, Friberg LE. A model-based evaluation of the pharmacokinetics-pharmacodynamics (PKPD) of avibactam in combination with ceftazidime. JAC Antimicrob Resist. (2025) 7:dlaf036. doi: 10.1093/jacamr/dlaf036
49. Gatti M, Rinaldi M, Laici C, Bonazzetti C, Vizioli L, Ambretti S, et al. Impact of attaining an aggressive PK/PD target with continuous infusion beta-lactams on the clinical efficacy of targeted therapy of early post-transplant Gram-negative infections in critically ill OLT recipients. An interim analysis of a 3-year prospective, observational, study. J Infect Dis. (2025): doi: 10.1093/infdis/jiaf048 Online ahead of print
50. Rolain H, Schwartz Z, Jubrail R, Downes K, Hong L, FakhriRavari A, et al. Meta-analysis on safety of standard vs. prolonged infusion of beta-lactams. Int J Antimicrob Agents. (2024) 64:107309. doi: 10.1016/j.ijantimicag.2024.107309
51. Hagiya H. Detailed regimens for the prolonged β-lactam infusion therapy. J Infect Chemother. (2024) 30:1324–6. doi: 10.1016/j.jiac.2024.07.003
52. Monti G, Bradic N, Marzaroli M, Konkayev A, Fominskiy E, Kotani Y, et al. Continuous vs intermittent meropenem administration in critically Ill patients with sepsis: The MERCY randomized clinical trial. JAMA. (2023) 330:141–51. doi: 10.1001/jama.2023.10598
53. Gatti M, Rinaldi M, Laici C, Siniscalchi A, Viale P, Pea F. Role of a real-time TDM-based expert clinical pharmacological advice program in optimizing the early pharmacokinetic/Pharmacodynamic target attainment of continuous infusion beta-lactams among orthotopic liver transplant recipients with documented or suspected gram-negative infections. Antibiotics (Basel). (2023) 12:1599. doi: 10.3390/antibiotics12111599
54. Abdul-Aziz M, McDonald C, McWhinney B, Ungerer J, Lipman J, Roberts J. Low flucloxacillin concentrations in a patient with central nervous system infection: The need for plasma and cerebrospinal fluid drug monitoring in the ICU. Ann Pharmacother. (2014) 48:1380–4. doi: 10.1177/1060028014540610
55. Tsai D, Lipman J, Roberts J. Pharmacokinetic/pharmacodynamic considerations for the optimization of antimicrobial delivery in the critically ill. Curr Opin Crit Care. (2015) 21:412–20. doi: 10.1097/MCC.0000000000000229
56. Roberts J, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. (2013) 52:1–8. doi: 10.1007/s40262-012-0018-5
57. Kees M, Wicha S, Seefeld A, Kees F, Kloft C. Unbound fraction of vancomycin in intensive care unit patients. J Clin Pharmacol. (2014) 54:318–23. doi: 10.1002/jcph.175
58. Alzahrani A, Hakami A, AlAzmi A, Karim S, Ali A, Burzangi A, et al. Augmented renal clearance and hypoalbuminemia-induced low vancomycin trough concentrations in febrile neutropenic patients with hematological malignancies. Cureus. (2022) 14:e29568. doi: 10.7759/cureus.29568
59. Shahbazi F, Shojaei L, Farvadi F, Kadivarian S. Antimicrobial safety considerations in critically ill patients: Part II: Focused on anti-microbial toxicities. Expert Rev Clin Pharmacol. (2022) 15:563–73. doi: 10.1080/17512433.2022.2093716
60. Jager N, van Hest R, Lipman J, Roberts J, Cotta M. Antibiotic exposure at the site of infection: Principles and assessment of tissue penetration. Expert Rev Clin Pharmacol. (2019) 12:623–34. doi: 10.1080/17512433.2019.1621161
61. Liu P, Müller M, Derendorf H. Rational dosing of antibiotics: The use of plasma concentrations versus tissue concentrations. Int J Antimicrob Agents. (2002) 19:285–90. doi: 10.1016/s0924-8579(02)00024-9
62. Craig W. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis. (1997) 24:S266–75. doi: 10.1093/clinids/24.supplement_2.s266
63. Paal M, Scharf C, Denninger A, Ilia L, Kloft C, Kneidinger N, et al. Target site pharmacokinetics of meropenem: Measurement in human explanted lung tissue by bronchoalveolar lavage, microdialysis, and homogenized lung tissue. Antimicrob Agents Chemother. (2021) 65:e0156421. doi: 10.1128/AAC.01564-21
64. Razzazzadeh S, Darazam I, Hajiesmaeili M, Salamzadeh J, Mahboubi A, Sadeghnezhad E, et al. Investigation of pharmacokinetic and clinical outcomes of various meropenem regimens in patients with ventilator-associated pneumonia and augmented renal clearance. Eur J Clin Pharmacol. (2022) 78:823–9. doi: 10.1007/s00228-022-03291-5
65. Danesi R, Lupetti A, Barbara C, Ghelardi E, Chella A, Malizia T, et al. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J Antimicrob Chemother. (2003) 51:939–45. doi: 10.1093/jac/dkg138
Keywords: meropenem, Monte Carlo simulation, augmented renal clearance, pharmacokinetics/pharmacodynamics, sepsis
Citation: Luo J, Liu J, Lin H, Yang Y, Chen C, Chen J, Zhong H and Zhang S (2025) Optimization of meropenem dosing regimens in critically ill patients with augmented renal clearance. Front. Med. 12:1550053. doi: 10.3389/fmed.2025.1550053
Received: 22 December 2024; Accepted: 18 April 2025;
Published: 09 May 2025.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Alessandra Oliva, Sapienza University of Rome, ItalyFrancesco Forfori, University of Pisa, Italy
Copyright © 2025 Luo, Liu, Lin, Yang, Chen, Chen, Zhong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han Zhong, aGFubmFoemhvbmdAMTYzLmNvbQ==; Shipao Zhang, enNwYW9Ac2luYS5jb20=
†These authors have contributed equally to this work and share first authorship
 Jinfeng Luo1†
Jinfeng Luo1† Shipao Zhang
Shipao Zhang