- Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States
“Primary neuroendocrine breast carcinoma (NEBC) is an underdiagnosed subtype of breast cancer, which includes small cell (SCNEC) and large cell neuroendocrine carcinomas (LCNEC). Accurate diagnosis remains challenging given their low incidence; misclassification as invasive breast carcinoma of no special type (IBC-NST), invasive ductal carcinoma (IDC), or a metastatic neuroendocrine carcinoma may occur. Cases with any component of adenocarcinoma and well-differentiated neuroendocrine tumors were excluded. A search of the pathology database (2012–2024) revealed six female patients (27–85 years) with a final pathologic diagnosis of NEBC (stages IA–IV), including four diagnosed with LCNEC and two with SCNEC. Even though most NEBC cases (5 of 6; 83%) were of the luminal subtype, five of six patients (83%) developed distant metastases within 4 years of the initial diagnosis. Molecular profiling of six cases revealed common alterations in the FGF/FGFR and PI3K/AKT/mTOR pathways. In summary, primary neuroendocrine carcinomas of the breast display aggressive behavior. However, they are more likely to harbor certain alterations, such as activating PIK3CA mutations and FGFR1 amplification, which can be of therapeutic value. The Ki-67 index, unlike in the pancreas and gastrointestinal tract, is not suitable for grading neuroendocrine neoplasms (NENs) of the breast. However, it can still serve as a tool for risk stratification, similar to its use in luminal-type breast cancer.
1 Introduction
Primary neuroendocrine breast carcinomas (NEBCs) represent a rare diagnostic entity, with an incidence ranging from 0.1 to 5% (1, 2). Formal diagnostic criteria for NEBCs were first established in 2003 by the WHO as a classification of tumors having >50% neoplastic cells expressing neuroendocrine markers (3). In the 2012 revision, the threshold value of > 50% neuroendocrine marker expression was removed (1). Breast tumors with neuroendocrine differentiation were classified into three groups as follows: well-differentiated neuroendocrine tumor, poorly differentiated/small cell neuroendocrine carcinoma (SCNEC), and invasive breast carcinoma with neuroendocrine differentiation. The third group included invasive breast carcinoma of no special type (IBC-NST) as well as special types, such as solid papillary carcinoma and the hypercellular variant of mucinous carcinoma. Further studies, however, have shown that neuroendocrine differentiation by immunohistochemistry (IHC) is observed in up to 20% of mammary carcinomas (4, 5). Therefore, a key feature of the current revision, the fifth edition of the WHO Classification of Breast Tumors, is the exclusion of specific histologic types, including IBC-NSTs with neuroendocrine differentiation, and the inclusion of large cell neuroendocrine carcinomas (LCNECs), which more accurately reflects the prevalence of NEBCs (6).
2 Materials and methods
Ethical approval and patient consent were not sought for this retrospective study, as the cases used in this case series have been completely de-identified, and no additional tests were performed beyond the diagnostic workup, for which informed consent was obtained from patients for each diagnostic procedure. A search of the pathology database from 2012 to 2024 revealed 17 patients with a final diagnoses of primary neuroendocrine neoplasms (NENs) of the breast, representing 0.18% of all registered breast cancer cases (n = 9,068) during 2012–2024. According to the current WHO criteria (6), the tumors for 7 patients were classified as primary neuroendocrine tumors (NETs) of the breast with low to intermediate-grade morphology, while 10 patients were diagnosed with poorly differentiated NEBCs, corresponding to a prevalence of 0.1%. Well to moderately-differentiated neuroendocrine tumors and invasive breast carcinoma with less than 90% of cells expressing neuroendocrine markers were excluded. Four cases of NEBCs had to be excluded, as histologic specimens could not be retrieved for reevaluation. The remaining six cases had complete clinical follow-up at our institution.
Foundation One test was performed on formalin-fixed paraffin-embedded or whole-blood-driven samples from six patients diagnosed with NEBCs as part of clinical practice. Foundation One, a target-specific next-generation sequencing (NGS)-based device from Foundation Medicine, is capable of detecting 324 molecular alterations, including substitutions, indels, copy number alterations, selected genomic rearrangements, and genomic signatures, such as tumor fraction, blood tumor mutational burden, and microsatellite instability status (7).
A comprehensive literature review was conducted via PubMed search using a combination of keywords: ‘neuroendocrine carcinoma of the breast’, ‘primary neuroendocrine breast carcinoma’, ‘small cell neuroendocrine carcinoma of the breast’, ‘histology’, ‘immunohistochemical profiling’, ‘WHO Classification of neuroendocrine neoplasms of the breast’, ‘management’, ‘prognosis’, and ‘molecular characteristics’. The selected studies were reviewed for clinicopathological characteristics, including TNM staging, therapeutic strategies, prognostic information, immunohistochemical features, and genomic landscape. Well-differentiated neuroendocrine tumors, as well as special types of breast tumors with neuroendocrine differentiation and indolent behavior (i.e., solid papillary carcinoma and hypercellular-subtype mucinous carcinoma), were excluded from the review process.
3 Results
Clinicopathologic characteristics, including molecular characterization of the six cases, are provided in Table 1.
3.1 Case 1
An 85-year-old woman with a 4-year history of hormone receptor (HR)-positive IBC-NST presented with shortness of breath and altered mental status. A computed tomography (CT) scan of the chest revealed multifocal pneumonia distal to increasing bilateral pulmonary nodules, suggestive of progressive disease. The patient was admitted to the intensive care unit with acute hypoxemic respiratory failure and severe sepsis. Shortly after admission, she died from complications of her post-obstructive pneumonia.
Postmortem breast examination revealed a 3 cm, calcified, white-tan mass in the lower outer quadrant of her right breast. Additional relevant findings included numerous pulmonary nodules.
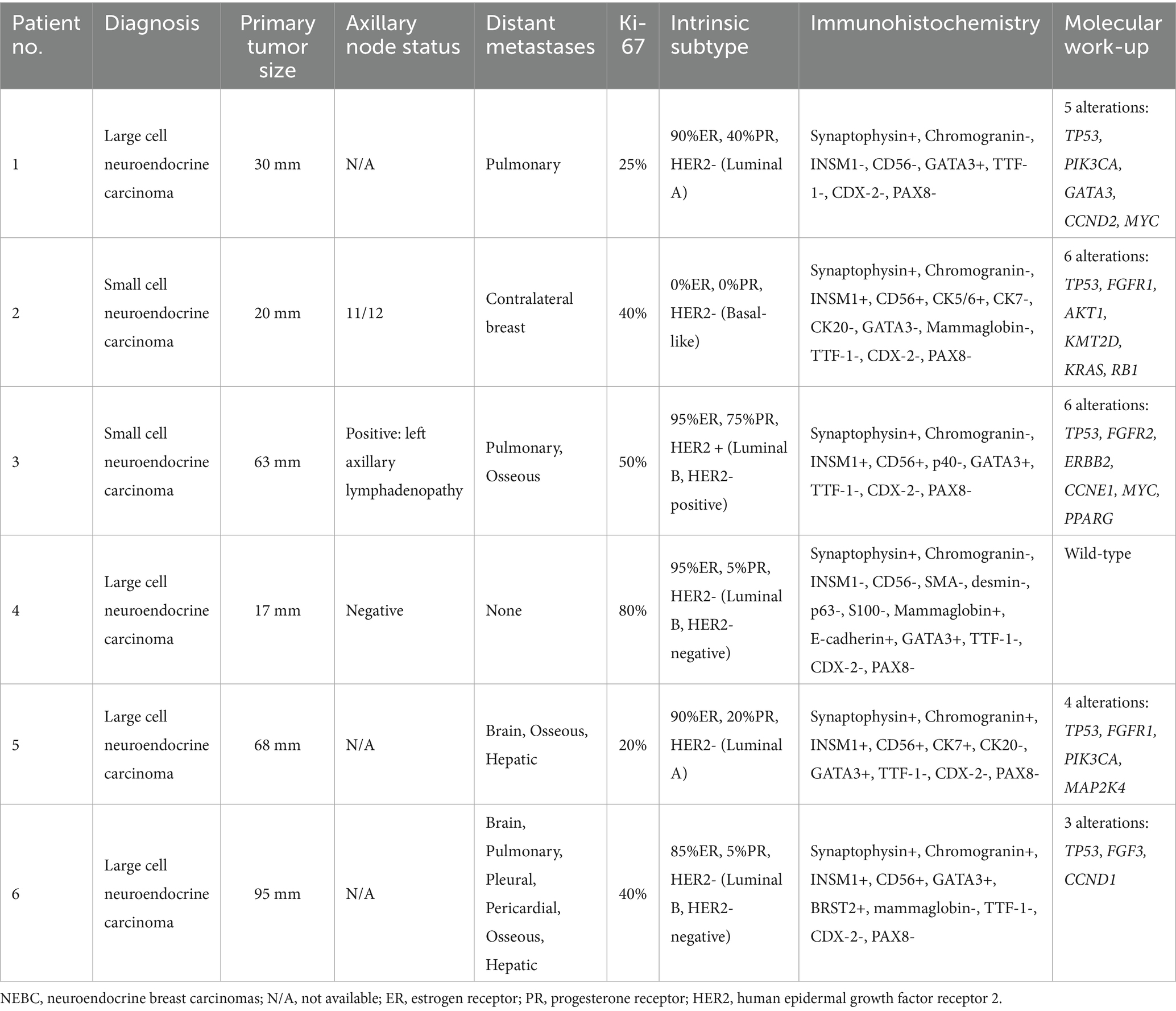
Histological analysis of both the right breast mass and pulmonary nodules showed solid nests and trabeculae of loosely cohesive tumor cells separated by fibrous septa. Associated geographic tumor necrosis was present (Figure 1A). The tumor cells were polygonal with abundant eosinophilic cytoplasm and stippled nuclei with conspicuous nucleoli, reminiscent of LCNEC.
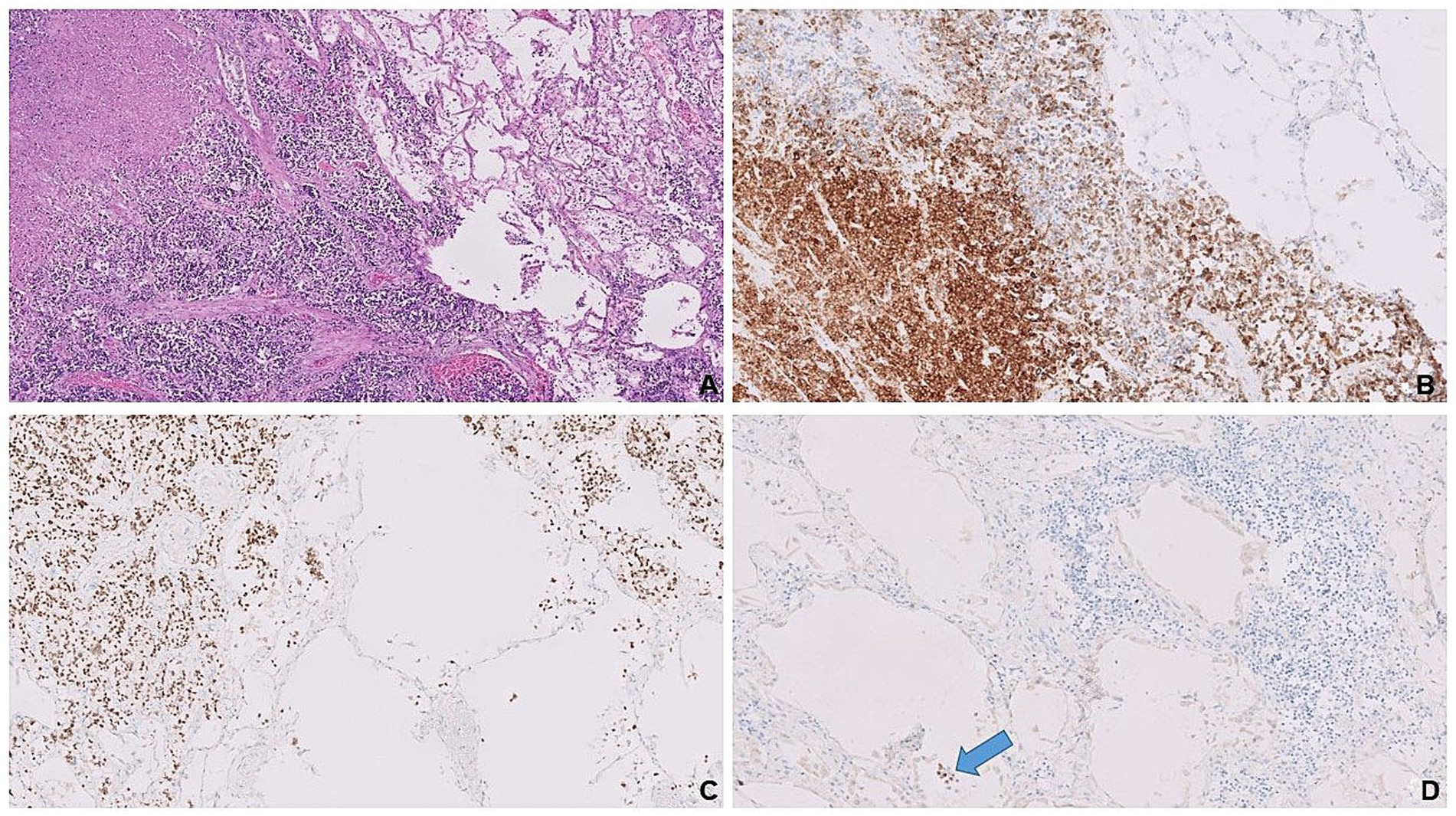
Figure 1. Solid tumor nests adjacent to the benign lung parenchyma on the right. Note geographic necrosis in the left upper corner (A). Tumor cells expressing synaptophysin (B) and nuclear GATA3 (C) vs. lung parenchyma in between tumor clusters shows no staining. Scattered alveolar pneumocytes (blue arrow) expressing TTF-1 (D) as positive internal control vs. no staining in tumor clusters on the right upper corner.
Immunohistochemical analysis demonstrated that the majority of tumor cells expressed synaptophysin (Figure 1B), confirming neuroendocrine differentiation. Since LCNECs commonly arise in the bronchopulmonary or gastroenteropancreatic tracts, immunostaining for TTF-1, CDX2, and PAX8 was performed to rule out metastases from these regions. The tumor cells showed strong nuclear expression for GATA3 (Figure 1C) but no staining for TTF-1 (Figure 1D), CDX2, and PAX8, inferring primary breast origin. Additionally, tumor cells exhibited a luminal A-like phenotype, with positive estrogen receptor (ER) and progesterone receptor (PR) expression and negative human epidermal growth factor receptor 2 (HER2) status.
Molecular testing by targeted NGS revealed clinically significant amplifications of MYC and CCND2 genes, as well as three alterations involving frequently implicated genes in HR-positive breast cancer: PIK3CA, TP53, and GATA3 (8).
3.2 Case 2
A 31-year-old woman with a 4-year history of triple-negative right breast cancer presented with a firm, palpable, left breast mass at the 9 o’clock position. She had previously completed neoadjuvant chemotherapy combined with immunotherapy, followed by a modified radical mastectomy.
A month after the surgery, adjuvant radiotherapy was initiated. After 15 cycles of radiotherapy, the patient was found to have a new anterior chest wall lesion, raising concern for local recurrence. A core needle biopsy of the anterior chest wall lesion revealed SCNEC of unknown primary.
Slides from a previous mastectomy specimen, reported as poorly differentiated invasive ductal carcinoma (IDC), were reviewed with additional ancillary workup, revealing the same immunomorphology as the anterior chest wall lesion. Ipsilateral axillary node involvement (11/12) and the absence of a non-mammary NEC favored the breast as the primary site of the patient’s newly diagnosed SCNEC. She received a four-cycle regimen of carboplatin/etoposide/atezolizumab, the standard therapy for both advanced small-cell lung cancer and extrapulmonary SCNECs. While on maintenance therapy with atezolizumab, a positron emission tomography/computed tomography (PET/CT) in June 2020 revealed resolution of the anterior chest wall lesion but interval development of a left breast mass.
Microscopic examination of the left breast mass revealed a poorly differentiated neoplasm arranged in solid sheets effacing the breast parenchyma (Figure 2A). Tumor cells were characterized by their high nuclear-to-cytoplasmic (N: C) ratio, molded nuclei with “smudged” chromatin, absence of inconspicuous nucleoli, and frequent mitoses (Figure 2B). Immunohistochemical evaluation confirmed neuroendocrine differentiation by positive expressions of synaptophysin (Figure 2C), CD-56 (Figure 2D), and INSM1. Basal-like subtype was evidenced by HR-/HER2- immunophenotype, accompanied by cytoplasmic staining of tumor cells with the basal cell marker CK5/6. Ki-67 proliferation index was high, up to 40%. Molecular testing by targeted NGS revealed a total of six alterations. Four of these involved frequently implicated genes of SCNECs from various organs (e.g., lung, pancreas, and large bowel): TP53, RB1, KMT2D, and KRAS (9, 10). Additionally, two targetable alterations were detected in AKT1 and FGFR1 genes, both of which have been previously described in NEBCs (11, 12).
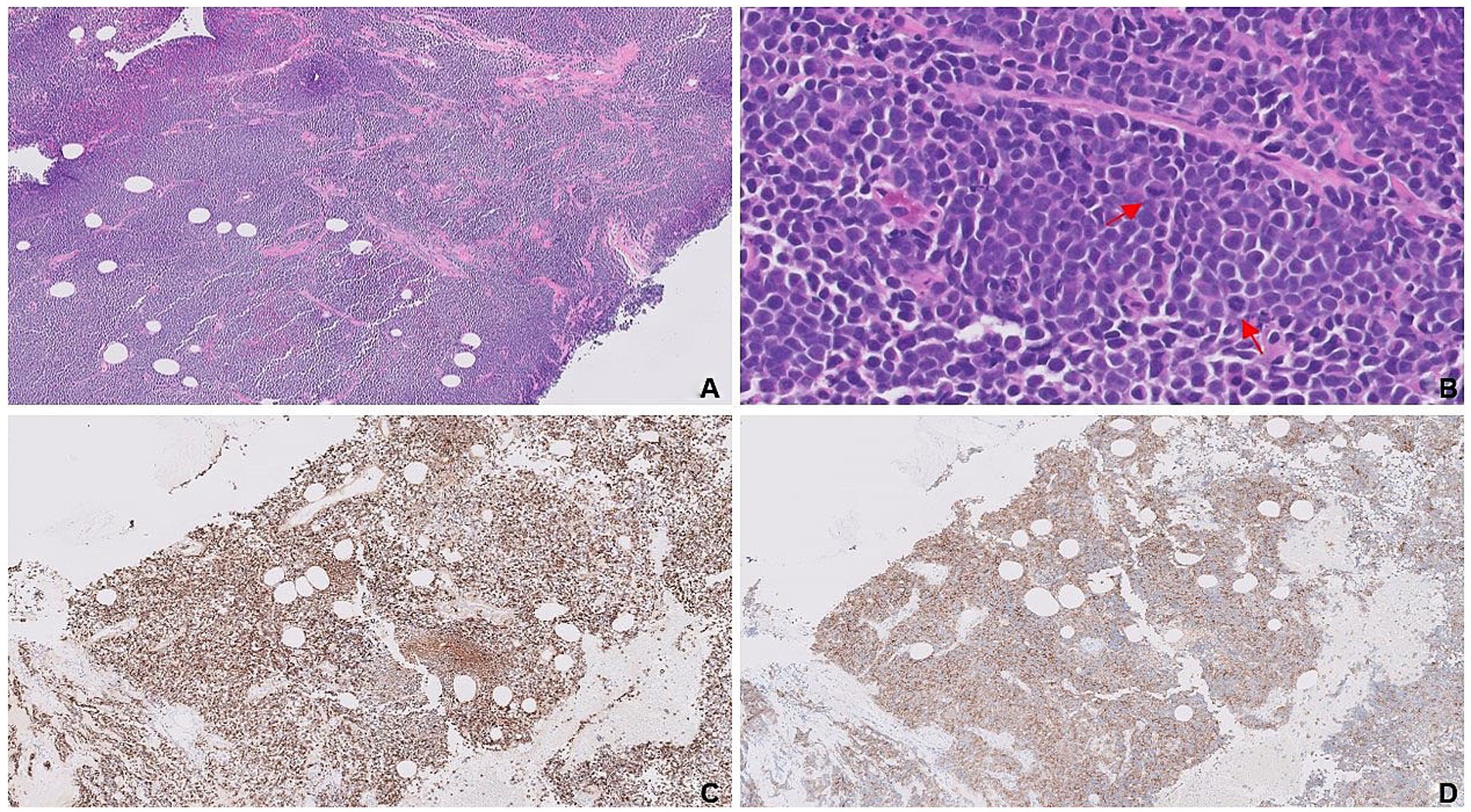
Figure 2. Complete effacement of the breast tissue by sheets of small blue tumor cells (A). Dyscohesive, small, blue round cells with frequent mitotic figures (red arrows; B). Expressions of synaptophysin (C) and CD-56 (D) are confirmatory for neuroendocrine differentiation.
After exhausting the second-line therapy option with lurbinectedin, the patient was referred to a clinical trial of the pan-AKT inhibitor AZD5363. After 5 months of progression-free survival, the patient relapsed, prompting the initiation of hospice care in May 2021.
3.3 Case 3
A 73-year-old woman with a recent diagnosis of breast cancer presented with radicular back pain concerning for metastatic spinal cord compression. In addition to the dominant left breast mass, a recent PET/CT showed innumerable sclerotic/lytic lesions throughout the axial and proximal skeleton, consistent with bony metastases, as well as with mediastinal, hilar, and left axillary lymphadenopathy. The left breast lesion was initially reported as an HR+/HER2+ poorly differentiated IDC, while a recent fine needle aspiration biopsy of mediastinal lymph nodes came positive for SCNEC. A lumbar spine biopsy was performed to determine whether the bony lesions represented metastases from the breast or the lung, given that the majority of SCNECs originate in the lungs.
The tumor was composed of solid nests and trabeculae of small round blue cells exhibiting “crush artifact” (Figure 3A). Cytologically, the tumor cells displayed molded nuclei with stippled chromatin (Figure 3B). A high proliferation rate was evidenced by frequent mitoses and a Ki-67 index up to 50%. Expression of neuroendocrine markers such as synaptophysin (Figure 3C), INSM1, and CD56 confirmed neuroendocrine differentiation, while negative p40 ruled out poorly differentiated squamous cell carcinoma. Diffuse, strong immunoreactivity for GATA3 (Figure 3D), along with negative staining for TTF-1, CDX-2, and PAX8, was consistent with mammary origin. Based on their HR+/HER2+ immunoprofile, the tumor cells belonged to the luminal B-like subtype, consistent with the patient’s breast cancer, which had initially been misclassified as poorly differentiated IDC. Diffuse immunoreactivity for synaptophysin confirmed neuroendocrine differentiation in the primary left breast lesion.
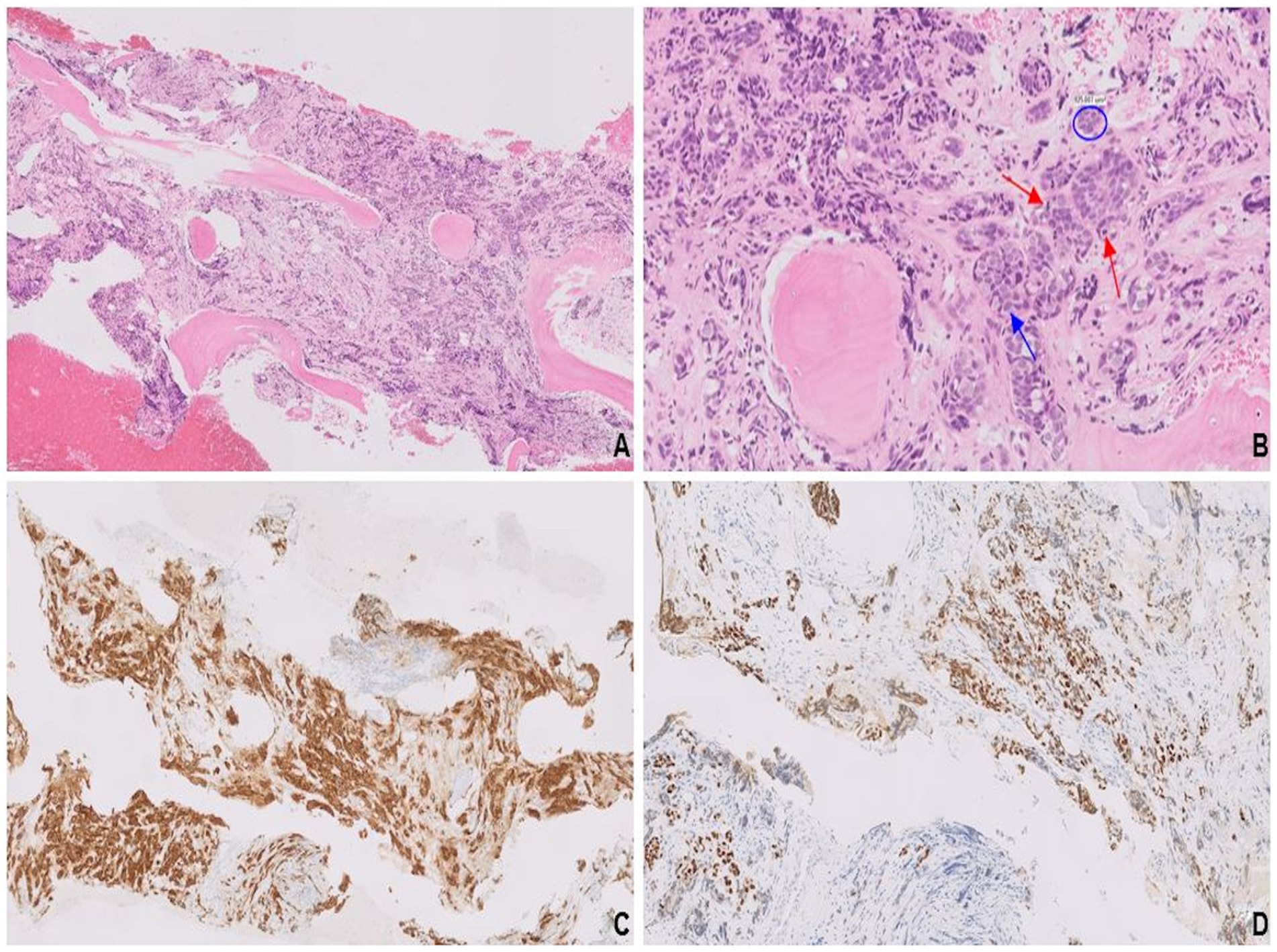
Figure 3. Solid nests and trabeculae of small round blue cells with crush artifact (A). Tumor cells characterized by their scant cytoplasm, nuclear molding (blue circle), finely stippled chromatin (blue arrow), and frequent mitosis (red arrows; B). Synaptophysin expression (C) confirms neuroendocrine differentiation, and diffuse, strong immunoreactivity for GATA3 (D) is consistent with mammary origin.
NGS analysis identified clinically significant amplifications of ERBB2, FGFR2, CCNE1, MYC, and PPARG genes, as well as a clinically significant variant in the TP53 gene.
In January 2019, she began the first-line therapy for HER2 + metastatic breast cancer with a combination of pertuzumab, trastuzumab, and paclitaxel, which was later replaced by endocrine therapy with anastrozole due to paclitaxel-induced peripheral neuropathy. As of April 2024, the patient remained alive, albeit with radiographic progression of osseous metastases and the appearance of new pulmonary nodules.
3.4 Case 4
A 73-year-old woman was diagnosed with triple-negative, poorly differentiated IDC of the right breast at the 2 o’clock position. She underwent a lumpectomy, followed by a course of radiotherapy, completed in September 2012.
In April 2020, a focal right breast asymmetry was detected on annual surveillance mammography. A core needle biopsy showed a high-grade malignancy with areas of extensive necrosis. Diffuse immunoreactivity for synaptophysin was indicative of neuroendocrine differentiation, while negative staining for p63, S100, and SMA/desmin ruled out squamous, melanocytic, and myogenic differentiation, respectively. Positive expressions of GATA3, mammaglobin, and E-cadherin confirmed mammary origin, while negative staining for TTF-1, CDX2, and PAX8 excluded bronchopulmonary, gastrointestinal, and pancreatic primaries, respectively. Further ancillary work-up revealed a HER2-negative, luminal B-like phenotype, characterized by positive ER, low PR, and high Ki-67.
A completion mastectomy specimen showed pT1cN0 LCNEC with an intraductal component (Figures 4A,B), providing compelling histological evidence for primary breast origin. SMA stain (Figure 4C) highlighted the intact myoepithelial cell layer surrounding the intraductal component. Both the invasive and intraductal components showed strong expression of synaptophysin (Figure 4D). Molecular analysis revealed no known clinically actionable alterations.
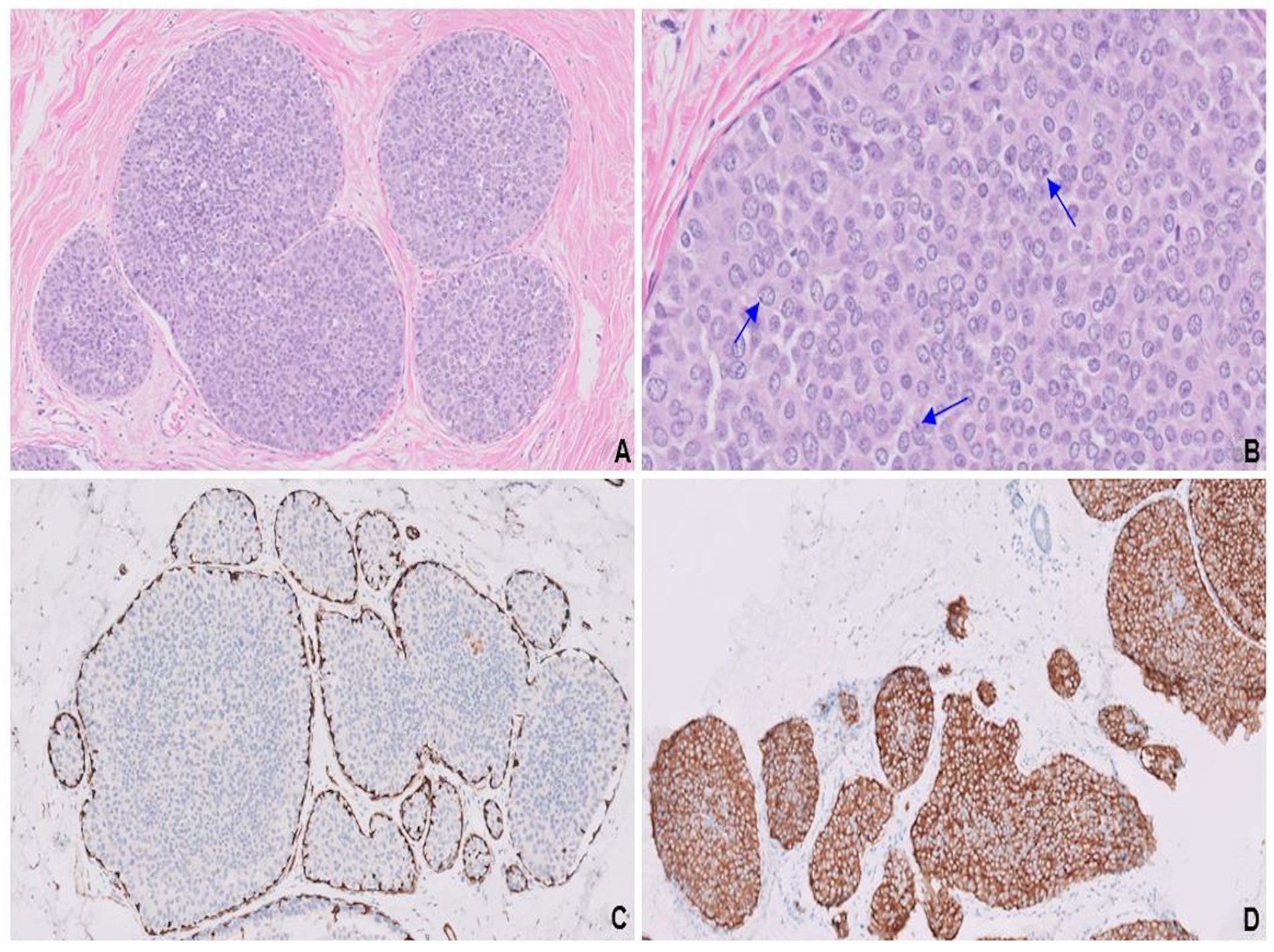
Figure 4. Endocrine ductal carcinoma in situ (E-DCIS) consisting of a duct expanded by the proliferation of monotonous cells (A). Cells with abundant eosinophilic cytoplasm, vesicular nuclei, finely stippled chromatin, and conspicuous nucleoli (blue arrows; B). SMA highlights the intact myoepithelial cell layer (C) surrounding the intraductal component, while synaptophysin is uniformly expressed in both invasive and in situ components (D).
As of December 2023, antihormonal therapy was still being continued, and the patient is in good general health, free from tumor recurrence.
3.5 Case 5
A 27-year-old woman with a 2-year history of HR-positive/HER2-negative metastatic breast cancer presented with progressive, right frontal headaches over the past 2 months. Magnetic resonance imaging (MRI) of the brain revealed a large dural-based mass along the right frontal convexity, with an associated leftward shift across the midline. The presence of additional parenchymal lesions raised concern for metastases rather than a primary brain tumor. The right frontal mass was excised to relieve the increased intracranial pressure. Pathology revealed variably sized solid nests and rows, separated by thin fibrovascular septae (Figure 5A). Tumor cells displayed abundant eosinophilic cytoplasm and pleomorphic nuclei with stippled chromatin (Figure 5B), suggestive of LCNEC. Expression of all four neuroendocrine markers [patchy synaptophysin, diffuse and strong chromogranin expression (Figure 5C), and diffuse and strong staining for INSM1 and CD56 (not shown)] was compelling for neuroendocrine differentiation.
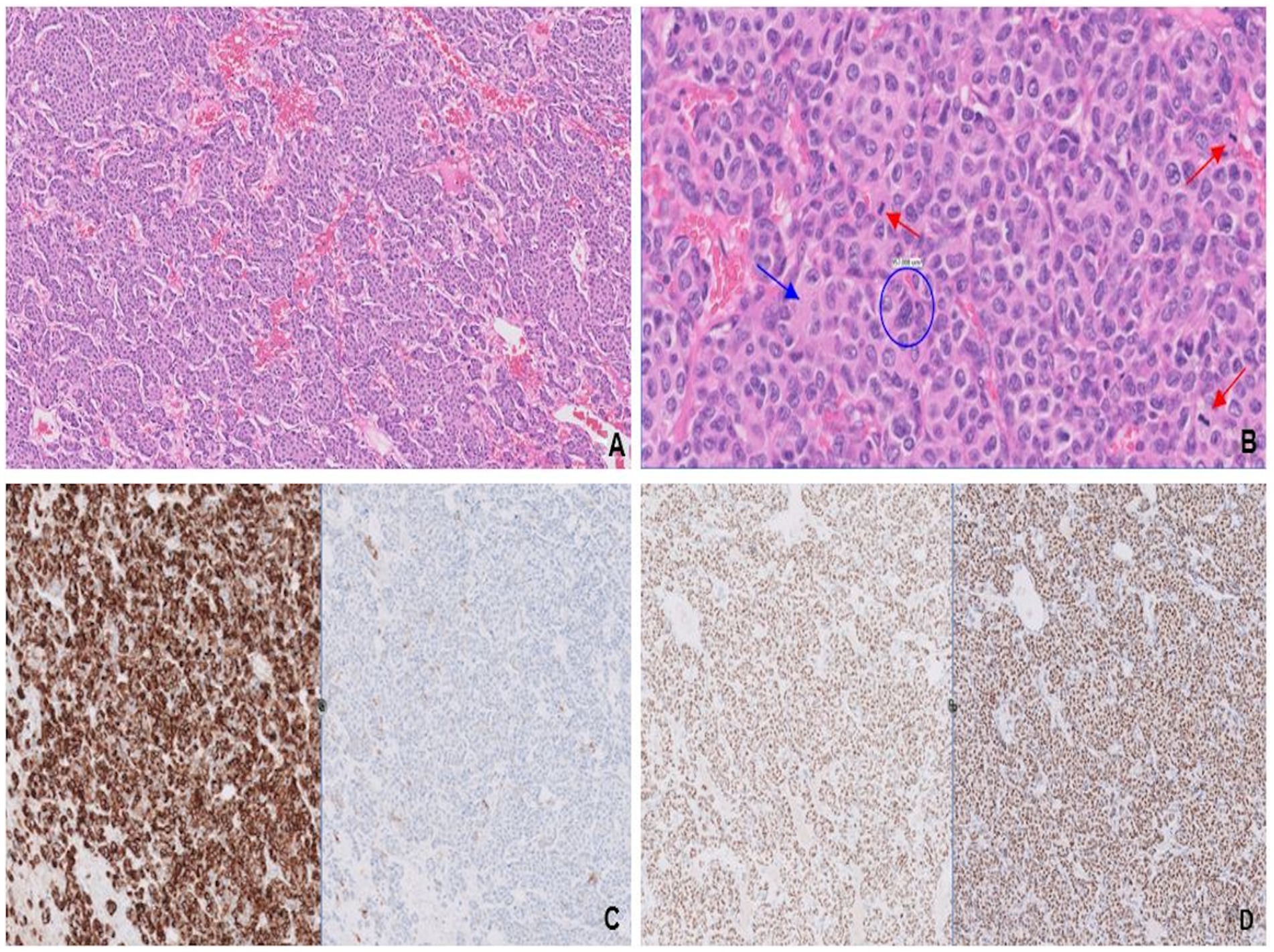
Figure 5. Variably sized solid nests separated by thin fibrovascular septae (A). Tumor cells have abundant eosinophilic cytoplasm (blue arrow), pleomorphic nuclei with “salt-and-pepper” chromatin (blue circle), and variably conspicuous nucleoli, most consistent with large cell neuroendocrine carcinoma. Mitotic figures (red arrows; B) are frequent. Strong chromogranin (left) and patchy synaptophysin (right; C) expression confirms neuroendocrine differentiation, while expressions of ER (left) and GATA-3 (right; D) are consistent with breast origin.
Tumor cells exhibited a CK7+/CK20- profile with a wide differential for the site of origin, including breast, mullerian, lung, thyroid, upper gastrointestinal, and pancreatobiliary tract carcinomas. Considering the past medical history of breast cancer and negative clinical workup for another primary, the breast was considered the most probable primary tumor site. This was supported by uniform expressions of ER and GATA-3 (Figure 5D), as well as high PR expression (20%), favoring a luminal A-like phenotype. The NGS analysis showed three alterations involving the frequently implicated genes of HR-positive breast cancer: PIK3CA, TP53, and MAP2K4 (8). Additionally, a recurring alteration was identified in FGFR1 gene, the amplification of which was recently shown in NEBCs (12).
Given the progression on prior endocrine therapy, palbociclib was added to anti-hormonal therapy with fulvestrant and goserelin. As of May 2020, the patient was still alive with radiographic progression of intracranial metastases, as well as the appearance of new liver lesions.
3.6 Case 6
A 49-year-old woman with no significant history presented after a syncopal fall. Associated signs/symptoms included 3 weeks of progressive weakness, confusion, and lethargy, as well as an unintentional weight loss of 40 pounds over the past 6 months. She denied any personal/family history of malignancy; however, she reported having an abnormal screening mammography of her left breast 4 years ago, which she did not follow up with. MRI of the brain demonstrated a 3.3 cm left frontal cavitary mass with rim enhancement. Numerous subcentimeter metastatic deposits were noted within the bilateral cerebral/cerebellar hemispheres.
A whole-body CT scan showed a heterogeneously enhancing left breast mass extending to the anterior chest wall and skin surface. In addition to the solitary left breast lesion, extensive metastases were identified, involving the pleura/pericardium, bilateral lungs, liver, and spine. Left frontal craniotomy revealed a high-grade malignancy with nested architecture and areas of central comedo-necrosis. (Figure 6A). Salt-and-pepper chromatin with conspicuous nucleoli (Figure 6B) and abundant eosinophilic cytoplasm was suggestive of LCNEC. Diffuse expression of synaptophysin (Figure 6C, left), chromogranin, and CD56, along with patchy but strong staining for INSM1 (Figure 6C, right), confirmed neuroendocrine differentiation. Uniform expressions of ER and GATA3 (Figure 6D, left) as well as patchy staining for BRST2 (Figure 6D, right) were indicative of mammary origin. The lack of staining for TTF-1, CDX2, and PAX8 excluded bronchopulmonary, gastrointestinal, and pancreatic primaries, respectively. Low PR expression (5%) with a high Ki-67 (40%) favored a luminal-B-like phenotype. Staining for membranous HER2 was negative.
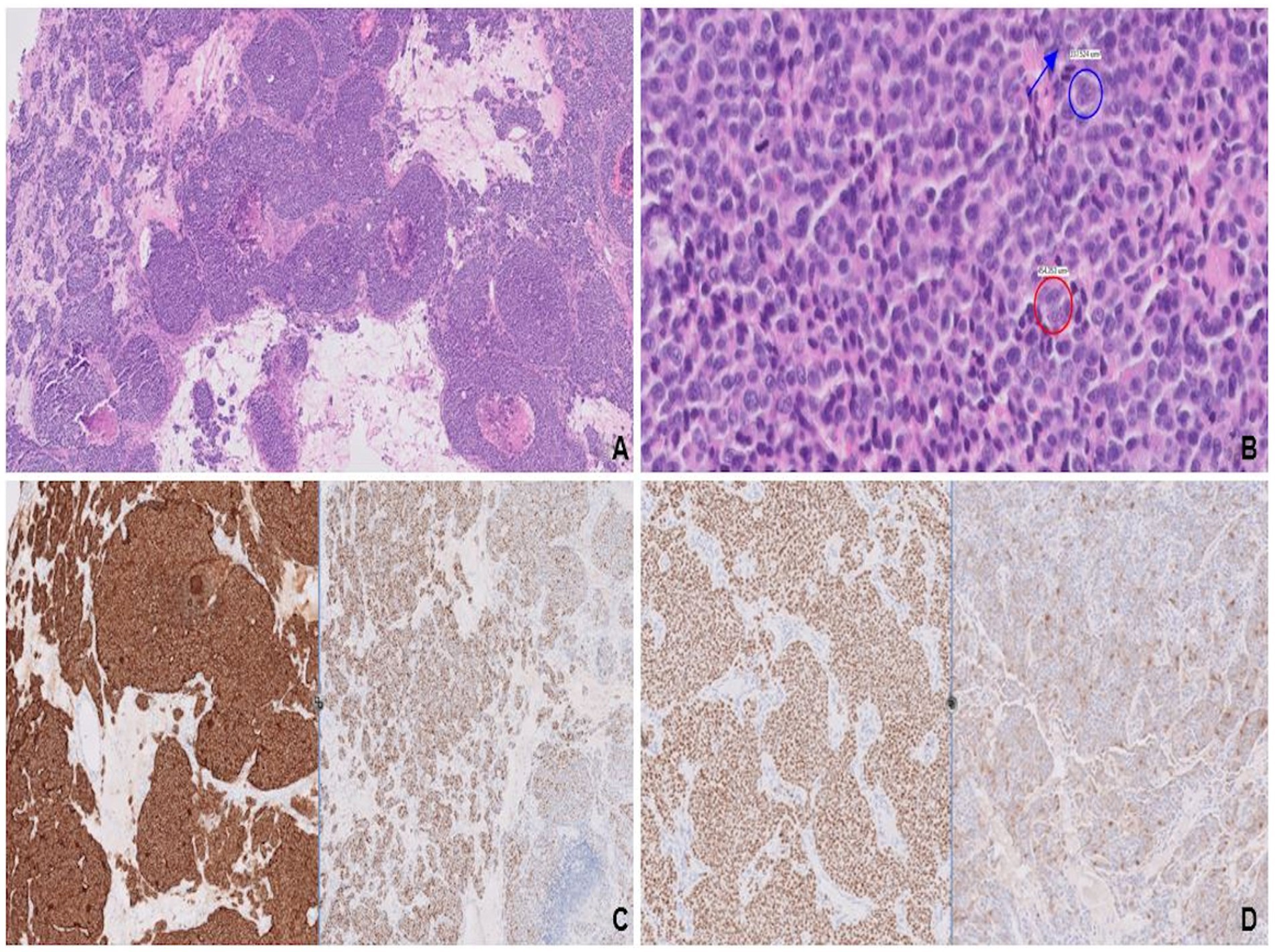
Figure 6. Variably sized solid nests with areas of central comedonecrosis (A). Nuclear features, stippled chromatin (red circle) with conspicuous nucleoli (blue circle and arrow; B), most consistent with large cell neuroendocrine carcinoma. Strong and diffuse expression of synaptophysin (left) along with patchy, strong staining for INSM1 (right; C), confirms neuroendocrine differentiation. Mammary origin is evidenced by diffuse, strong staining for GATA3 (left) and patchy staining for BRST-2 (right; D).
Molecular testing revealed a clinically significant variant in TP53 gene and amplification of the FGF3 and CCND1 genes, the latter of which was previously reported in NEBC (12).
Following left frontal craniotomy, antihormonal therapy with tamoxifen was initiated. However, the patient declined palliative chemotherapy given her baseline poor performance status. Hospitalization was complicated by obstructive pneumonia with severe sepsis, leading to her death a month after her initial diagnosis of widely metastatic NEBC.
4 Discussion
4.1 Current terminology, classification, and epidemiology of primary neuroendocrine neoplasms of the breast
Neuroendocrine differentiation in breast carcinomas was first described in mucinous carcinomas by Feyrter and Hartmann in 1963 (13). The first case series was published in 1977, introducing the term “primary carcinoid of the breast” (14). In third edition of the WHO classification (2003), NEBCs were first recognized as a distinct entity, defined as epithelial tumors morphologically resembling NENs of the gastrointestinal tract and lung, with >50% of the tumor expressing neuroendocrine markers (3).
The 2012 WHO Working Group categorized mammary NENs into two major groups: well-differentiated NETs and poorly-differentiated small cell neuroendocrine carcinomas (SCNECs) (1). By this definition, NEBCs included SCNECs but not LCNECs. A third category encompassed conventional breast carcinomas with neuroendocrine differentiation, as well as special subtypes of breast carcinoma, such as solid papillary carcinoma and the hypercellular variant of mucinous carcinoma. The 2019 WHO classification revised this framework by excluding the latter subtypes and formally including LCNECs (6).
NEBCs are rare, with their true incidence likely underestimated due to frequent misclassification as poorly-differentiated IBC-NST/IDC, other histologic subtypes, or metastatic NEC (15). Their incidence among all types of breast carcinoma ranges from 0.1 to 5%. Based on the 2012 WHO criteria, mammary NENs account for 2–5% of cases (1). However, this representation likely included WDNETs. An analysis of surveillance, epidemiology and end results (SEER) registry data from 2003 to 2009 by Wang et al. identified only 142 NEBC cases, approximating to 0.1% of all breast cancers (2).
4.2 Clinical aspects
NEBCs lack distinctive clinical features, making diagnosis challenging. Compared to IDC, patients more often present with stage II disease with an increased propensity for regional lymph node involvement (16).
NEBCs typically affect white, postmenopausal women in their 60s and 70s, although rare cases occur in premenopausal women (17) and men (18, 19). Compared to IBC-NST, NEBCs present at an older age with larger tumors, higher histologic grade, and more advanced stage. In our series, the mean tumor size was 48 mm (range 17–95 mm), which was more than double the 23 mm mean tumor size reported for IBC-NSTs (2).
Imaging features are non-specific. Gallo et al. described the most common mammographic finding as a hyperdense, irregular, solitary mass (20). To rule out an extra-mammary primary, CT of the chest and abdomen is recommended. Gallium-68 PET/CT may aid in well-differentiated cases, while FDG PET/CT is preferred for poorly differentiated NEBCs.
4.3 Histological diagnosis and ancillary studies
In the latest WHO classification, NEBCs are defined by high-grade morphology resembling their pulmonary counterparts. SCNECs show infiltrative solid sheets of densely packed, hyperchromatic cells with high N:C ratios, scant cytoplasm, and inconspicuous nucleoli. In contrast, LCNECs exhibit abundant cytoplasm and pleomorphic nuclei with prominent nucleoli.
NEBCs are often underrecognized due to the absence of classic neuroendocrine features such as “salt-and-pepper” chromatin (21). They may mimic poorly differentiated IDC, invasive lobular carcinoma, or solid DCIS. Accurate diagnosis requires careful morphologic evaluation followed by IHC confirmation. Among neuroendocrine markers, synaptophysin shows the highest sensitivity. Additional markers—INSM1, chromogranin, and CD56—have variable expression. Neuron-specific enolase is non-specific and currently, it is not recommended as part of the diagnostic panel. Recently, a tissue microarray analysis showed that adding chromogranin to synaptophysin detects an extra 4.2% of breast cancer cases with neuroendocrine differentiation, while INSM1 identifies 15% of cases negative for both (22). INSM1 expression has also been associated with improved disease-free survival in luminal breast cancers, supporting its inclusion alongside synaptophysin ± chromogranin in diagnostic panels (23).
Given their rarity, metastatic NECs must first be excluded. The presence of an in situ component confirms breast origin, although it is rarely observed in biopsies. Lineage-specific markers aid in distinction: ER, GATA3, mammaglobin, and GCDFP15 support a mammary origin, while TTF-1, CDX2, and PAX8/Islet 1 help exclude pulmonary, gastrointestinal, and pancreatic primaries, respectively.
Unlike gastrointestinal or pulmonary NENs, mammary NENs are not formally graded by the Ki-67 index, but Ki-67 remains useful for risk stratification, similar to its role in luminal-type breast cancer (24).
4.4 Current management and prognosis
There are no established treatment guidelines for NEBCs and their management largely mirrors that of ductal-type breast cancer. Early-stage disease is treated with surgery +/− radiotherapy based on tumor size and nodal status. Chemotherapy is used for metastatic disease or as neoadjuvant therapy in locally advanced, inoperable cases (25). Combinations of platinum agents and etoposide—as used for pulmonary/extra-pulmonary SCNECs—are commonly administered alongside taxane-based chemotherapy, which is routinely used for breast cancer (26). In our cohort, the patient with triple-negative SCNEC was initially treated with taxane-based chemotherapy, followed by a platinum-etoposide combination.
The majority of NEBCs are HR-positive, supporting the use of endocrine therapy. In cases of endocrine therapy resistance, the addition of CDK4/6 inhibitors (e.g., palbociclib) can improve outcomes in HR-positive disease by targeting cell-cycle pathways (27). All HR-positive patients in our series received endocrine therapy. In one case of endocrine resistance, CDK 4/6 inhibitor palbociclib was administered along with conventional endocrine therapy (i.e., letrozole). Anti-HER2 therapy may be considered in rare HER2-amplified NEBCs, as in our case with HER2 overexpression (28).
Emerging data suggest that somatostatin receptors (SSTR2A and SSTR5) may be expressed in up to 71% of tumors, indicating a potential therapeutic target analogous to other NENs (29).
Prognosis remains controversial, likely due to evolving classification. Earlier WHO definitions included indolent subtypes (e.g., solid papillary and hypercellular mucinous carcinomas), which may have obscured the outcome data. However, recent studies suggest poorer outcomes. An analysis of 142 NEBCs from the SEER database demonstrated worse overall and disease-free survival compared to IBC-NST (2). Multivariate analysis confirmed neuroendocrine differentiation as an independent adverse prognostic factor. NEBCs also show a high risk of recurrence, with distant metastases reported in the liver, bone, lungs, pleura, brain, pancreas, and soft tissues (16, 25, 30). In our series, 83% (5/6) of patients developed distant metastases within 4 years of diagnosis.
Given their aggressive behavior and potential for delayed metastasis years after treatment, long-term radiologic surveillance is recommended.
5 Conclusion
NEBCs are rare tumors that typically exhibit an ER-positive, luminal phenotype. However, unlike IBC-NST, where the luminal phenotype correlates with favorable prognosis, NEBCs are associated with poorer outcomes at similar stages (2, 30).
In this study, molecular profiling of six NEBC cases revealed potentially actionable alterations in three cases: FGFR1 amplifications (cases 2 and 5) and PI3K/AKT/mTOR pathway alterations (cases 1, 2, and 5). Although comprehensive genomic data on NEBCs remain limited, recurrent aberrations in FGF/FGFR and PI3K/AKT/mTOR pathways have been reported (31). The presence of PI3K/AKT/mTOR alterations suggests molecular overlap with IBC-NST (32).
These pathways offer therapeutic opportunities. Everolimus, an mTOR inhibitor, is FDA-approved for use in both pancreatic NENs and HR-positive breast cancer in combination with exemestane (33, 34). Other agents include PI3K inhibitors and the pan-AKT inhibitor AZD5363, which was used in a triple-negative small cell NEBC case in our cohort (Case #2), achieving 5 months of progression-free survival.
The FGF/FGFR pathway also represents a potential target for precision therapy, with selective FGFR inhibitors showing efficacy in tumors with FGFR mutations, amplifications, or gene fusions (35, 36), including single-agent activity in breast carcinoma (37).
Consideration of NEBCs in a differential diagnosis of poorly differentiated breast cancer is essential for timely and accurate diagnosis, which, in turn, can facilitate optimal management, including the application of aforementioned precision oncology approaches.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The requirement for ethical approval was waived by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai for this retrospective study, as all cases were fully de-identified and no additional procedures were performed beyond the routine diagnostic workup, for which informed consent had been obtained at the time of clinical care. Human samples used in this study were acquired as by-products of standard medical care. In accordance with the local legislation and institutional requirements, written informed consent for participation was not required. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author contributions
NA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tan, PH, Schnitt, SJ, van de Vijver, MJ, Ellis, IO, and Lakhani, SR. Papillary and neuroendocrine breast lesions: the WHO stance. Histopathology. (2015) 66:761–70. doi: 10.1111/his.12463
2. Wang, J, Wei, B, Albarracin, CT, Hu, J, Abraham, SC, and Wu, Y. Invasive neuroendocrine carcinoma of the breast: a population-based study from the surveillance, epidemiology and end results (SEER) database. BMC Cancer. (2014) 14:147. doi: 10.1186/1471-2407-14-147
3. Tavassoli, FA, and Devilee, P. Pathology and genetics of tumours of the breast and female genital organs: WHO classification of Tumours series. Lyon, France: IARC Press (2003).
4. Miremadi, A, Pinder, SE, Lee, AH, Bell, JA, Paish, EC, Wencyk, P, et al. Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology. (2002) 40:215–22. doi: 10.1046/j.1365-2559.2002.01336.x
5. Wachter, DL, Hartmann, A, Beckmann, MW, Fasching, PA, Hein, A, Bayer, CM, et al. Expression of neuroendocrine markers in different molecular subtypes of breast carcinoma. Biomed Res Int. (2014) 2014:408459. doi: 10.1155/2014/408459
6. Tan, PH, Ellis, I, and Allison, K. WHO classification of Tumours of the breast. 5th ed. Lyon, France: IARC (2019).
7. FoundationOne Medicine Site. Available online at: https://www.foundationmedicine.it/ (Accessed October, 2021).
8. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. (2012) 490:61–70. doi: 10.1038/nature11412
9. McNamara, MG, Scoazec, JY, and Walter, T. Extrapulmonary poorly differentiated NECs, including molecular and immune aspects. Endocr Relat Cancer. (2020) 27:R219–38. doi: 10.1530/ERC-19-0483
10. Yuan, T, Wang, X, Sun, S, Cao, Z, Feng, X, and Gao, Y. Profiling of 520 candidate genes in 50 surgically treated Chinese small cell lung Cancer patients. Front. Oncologia. (2021) 11:644434. doi: 10.3389/fonc.2021.644434
11. Marchiò, C, Geyer, FC, Ng, CK, Piscuoglio, S, De Filippo, MR, Cupo, M, et al. The genetic landscape of breast carcinomas with neuroendocrine differentiation. J Pathol. (2017) 241:405–19. doi: 10.1002/path.4837
12. Bean, GR, Najjar, S, Shin, SJ, Hosfield, EM, Caswell-Jin, JL, Urisman, A, et al. Correction to: genetic and immunohistochemical profiling of small cell and large cell neuroendocrine carcinomas of the breast. Mod Pathol. (2022) 35:1494–5. doi: 10.1038/s41379-022-01099-3
13. Feyrter, F, and Hartmann, G. On the carcinoid growth form of the carcinoma Mammae, especially the carcinoma Solidum (Gelatinosum) Mammae. Frankf Z Pathol. (1963) 73:24–39.
14. Cubilla, AL, and Woodruff, JM. Primary carcinoid tumor of the breast: a report of eight patients. Am. J. Surg. Pathol. (1977) 1:283–92. doi: 10.1097/00000478-197712000-00001
15. Sun, H, Dai, S, Xu, J, Liu, L, Yu, J, and Sun, T. Primary neuroendocrine tumor of the breast: current understanding and future perspectives. Front Oncol. (2022) 12:848485. doi: 10.3389/fonc.2022.848485
16. Wei, B, Ding, T, Xing, Y, Wei, W, Tian, Z, Tang, F, et al. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer. (2010) 116:4463–73. doi: 10.1002/cncr.25352
17. Yoshimura, N, Sasada, T, and Yonehara, S. Primary large-cell neuroendocrine carcinoma of the breast occurring in a pre-menopausal woman. Breast Care. (2015) 10:281–3. doi: 10.1159/000431070
18. Jundt, G, Schulz, A, Heitz, PU, and Osborn, M. Small cell neuroendocrine (oat cell) carcinoma of the male breast. Immunocytochemical and ultrastructural investigations. Virchows Arch A Pathol Anat Histopathol. (1984) 404:213–21. doi: 10.1007/BF00704065
19. Jiang, J, Wang, G, Lv, L, Liu, C, Liang, X, and Zhao, H. Primary small-cell neuroendocrine carcinoma of the male breast: a rare case report with review of the literature. Onco Targets Ther. (2014) 7:663–6. doi: 10.2147/OTT.S60782
20. Gallo, M, Campione, S, Di Vito, V, et al. Primary neuroendocrine neoplasms of the breast: still open issues. Front Endocrinol. (2021) 11:610230. doi: 10.3389/fendo.2020.610230
21. Tang, F, Wei, B, Tian, Z, Gilcrease, MZ, Huo, L, Albarracin, CT, et al. Invasive mammary carcinoma with neuroendocrine differentiation: histological features and diagnostic challenges. Histopathology. (2011) 59:106–15. doi: 10.1111/j.1365-2559.2011.03880.x
22. Tsang, JY, and Tse, GM. Breast cancer with neuroendocrine differentiation: an update based on the latest WHO classification. Mod Pathol. (2021) 34:1062–73. doi: 10.1038/s41379-021-00736-7
23. Razvi, H, Tsang, JY, Poon, IK, Chan, SK, Cheung, SY, Shea, KH, et al. INSM1 is a novel prognostic neuroendocrine marker for luminal B breast cancer. Pathology. (2021) 53:170–8. doi: 10.1016/j.pathol.2020.07.004
24. Rindi, G, Klimstra, DS, Abedi-Ardekani, B, Asa, SL, Bosman, FT, Brambilla, E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. (2018) 31:1770–86. doi: 10.1038/s41379-018-0110-y
25. Inno, A, Bogina, G, Turazza, M, Bortesi, L, Duranti, S, Massocco, A, et al. Neuroendocrine carcinoma of the breast: current evidence and future perspectives. Oncologist. (2016) 21:28–32. doi: 10.1634/theoncologist.2015-0309
26. Angarita, FA, Rodríguez, JL, Meek, E, Sánchez, JO, Tawil, M, and Torregrosa, L. Locally-advanced primary neuroendocrine carcinoma of the breast: case report and review of the literature. World J Surg Oncol. (2013) 11:128. doi: 10.1186/1477-7819-11-128
27. Piezzo, M, Chiodini, P, Riemma, M, Cocco, S, Caputo, R, Cianniello, D, et al. Progression-free survival and overall survival of CDK 4/6 inhibitors plus endocrine therapy in metastatic breast Cancer: a systematic review and Meta-analysis. Int J Mol Sci. (2020) 21:6400. doi: 10.3390/ijms21176400
28. Gevorgyan, A, Bregni, G, Galli, G, Zanardi, E, de Braud, F, and di Cosimo, S. HER2-positive neuroendocrine breast Cancer: case report and review of literature. Breast Care. (2016) 11:424–6. doi: 10.1159/000453572
29. Terlević, R, Perić Balja, M, Tomas, D, Skenderi, F, Krušlin, B, Vranic, S, et al. Somatostatin receptor SSTR2A and SSTR5 expression in neuroendocrine breast cancer. Ann Diagn Pathol. (2019) 38:62–6. doi: 10.1016/j.anndiagpath.2018.11.002
30. Lavigne, M, Menet, E, Tille, JC, Lae, M, Fuhrmann, L, Bonneau, C, et al. Comprehensive clinical and molecular analyses of neuroendocrine carcinomas of the breast. Mod Pathol. (2018) 31:68–82. doi: 10.1038/modpathol.2017.107
31. Ang, D, Ballard, M, Beadling, C, Warrick, A, Schilling, A, O’Gara, R, et al. Novel mutations in neuroendocrine carcinoma of the breast: possible therapeutic targets. Appl Immunohistochem Mol Morphol. (2015) 23:97–103. doi: 10.1097/PDM.0b013e3182a40fd1
32. Miricescu, D, Totan, A, Stanescu-Spinu, II, Badoiu, SC, Stefani, C, and Greabu, M. PI3K/AKT/mTOR signaling pathway in breast Cancer: from molecular landscape to clinical aspects. Int J Mol Sci. (2020) 22:173. doi: 10.3390/ijms22010173
33. Yao, JC, Shah, MH, Ito, T, Bohas, CL, Wolin, EM, van Cutsem, E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. (2011) 364:514–23. doi: 10.1056/NEJMoa1009290
34. Burris, HA, Lebrun, F, Rugo, HS, Beck, JT, Piccart, M, Neven, P, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial [published correction appears in Cancer]. Cancer. (2013) 119:1908–15. doi: 10.1002/cncr.28010
35. Nogova, L, Sequist, LV, Perez Garcia, JM, Andre, F, Delord, JP, Hidalgo, M, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol. (2017) 35:157–65. doi: 10.1200/JCO.2016.67.2048
36. Chae, YK, Hong, F, Vaklavas, C, Cheng, HH, Hammerman, P, Mitchell, EP, et al. Phase II study of AZD4547 in patients with tumors harboring aberrations in the FGFR pathway: results from the NCI-MATCH trial (EAY131) subprotocol W. J Clin Oncol. (2020) 38:2407–17. doi: 10.1200/JCO.19.02630
37. André, F, Bachelot, T, Campone, M, Dalenc, F, Perez-Garcia, JM, Hurvitz, SA, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. (2013) 19:3693–702. doi: 10.1158/1078-0432.CCR-13-0190
Glossary
NEBC - neuroendocrine breast carcinomas
NEC - neuroendocrine carcinoma
SCNEC - small cell neuroendocrine carcinomas
LCNEC - large cell neuroendocrine carcinomas
IBC-NST - invasive breast carcinoma of no special type
IDC - invasive ductal carcinoma
HR - hormone receptor
NENs - neuroendocrine neoplasms
IHC - immunohistochemistry
NET - neuroendocrine tumors
CT - computed tomography
PET/CT - positron emission tomography/computed tomography
MRI - magnetic resonance imaging
ER - estrogen receptor
PR - progesterone receptor
HER2 - human epidermal growth factor receptor 2
N: C - nuclear-to-cytoplasmic
NGS - next-generation sequencing
SEER - surveillance, epidemiology and end results
PI3K/AKT/mTOR - phosphoinositide 3 kinase/AKT/mammalian target of rapamycin.
Keywords: neuroendocrine carcinoma, breast neoplasms, neuroendocrine tumors, mTOR inhibitors, synaptophysin
Citation: Albayrak NE (2025) Case Report: Neuroendocrine carcinoma of the breast: a review of the literature and illustration of six cases. Front. Med. 12:1551309. doi: 10.3389/fmed.2025.1551309
Edited by:
Ihsan Ullah, Khyber Medical University, PakistanReviewed by:
Paulo Hilario Nascimento Saldiva, University of So Paulo, BrazilIvan Šoša, University of Rijeka, Croatia
Copyright © 2025 Albayrak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nazire E. Albayrak, bmF6aXJlLmFsYmF5cmFrQHVjc2YuZWR1
†Present addressNazire E. Albayrak, Department of Pathology, University of California, San Francisco, San Francisco, CA, United States
 Nazire E. Albayrak
Nazire E. Albayrak