Abstract
Background:
The health industry plays a crucial role in improving the quality of life for individuals, continuously driving innovations in health service delivery. Translational research fosters intimate collaboration between scientists and medical professionals. A major obstacle to effective evidence-based treatments is drug adherence, prompting the search for innovative procedures to enhance drug delivery methods.
Objectives:
This study aimed to assess the impact of innovative drug delivery systems (DDS) based on physical stimuli on medication adherence among patients undergoing long-term treatments. The ultimate goal was to establish a framework-based approach to assist in clinical decision-making, enhancing drug absorption efficiency.
Methods:
Two systematic literature reviews (SLRs) was conducted across multiple databases, including PubMed, Scopus, and Web of Science, focusing on DDS activated by physical and biological stimuli. The research process was structured through the Work Breakdown Structure (WBS) methodology, dividing it into five interconnected Work Packages (WPs). Each WP explored specific aspects of the relationship between DDS and the human body.
Results:
The synthesis led to the development of the Triangle Decision-Making Model, a theoretical framework that prioritizes physiological balance to optimize drug delivery. The study underscores the importance of reducing insulin and cortisol levels to minimize inflammation and glycation, promoting an ideal state for drug absorption. The findings highlight the significance of using physical stimuli, such as hyperthermia, ultrasound-triggered drug delivery, and photodynamic therapy, to enhance drug bioavailability and target specificity.
Conclusions:
This research proposes a novel therapeutic intervention, grounded in systematic reviews and focused on improving drug delivery via physical stimuli. Using an open innovation approach, the triangular clinical decision model integrates personalized medicine and nutraceuticals, addressing epigenetics and nutrition's role in medication response. This framework aims to enhance drug absorption, metabolism, and targeted therapies, advancing treatment outcomes. Future studies should refine this model to promote homeostasis and validate its effectiveness across healthcare settings.
1 Introduction
The healthcare industry plays a pivotal role in advancing public health and improving overall quality of life (1). In recent years, the intricate relationship between epigenetics and pharmacology has garnered increasing attention, particularly in the context of chronic diseases and cancer (2–6). This dynamic interplay fosters innovation in healthcare services (1), often realized through a “from bench to bedside and back” approach. This translational process bridges the gap between scientific discovery and clinical application, requiring heightened interdisciplinary awareness and collaboration (7, 8). Its success hinges on the ability of basic scientists and clinical specialists to work together in an environment of mutual understanding and respect, ultimately advancing patient-centered treatments that integrate expert care with medical innovation (9–11).
As healthcare challenges become more complex and patient expectations evolve, the need for continuous innovation grows. One pressing issue in this landscape is medication non-adherence, extensively analyzed by Kardas et al. (12). Non-adherence significantly compromises therapeutic effectiveness, underscoring the urgency of developing innovative strategies to improve medication administration and integration into patients' lives. Kelly and Young (13) emphasized that successful innovation must be both usable and desirable. Given the established influence of epigenetic modifications on gene expression and drug response, integrating these insights into therapeutic strategies presents an opportunity to enhance treatment outcomes (2–6).
Reflecting the concerns raised by Kardas et al. (12), medication adherence remains critical for optimizing evidence-based therapies. Despite over five decades of extensive research and more than 130,000 scientific publications on non-adherence, a definitive solution has yet to be established (12). In this context, drug delivery systems (DDS) have been designed to transport therapeutic agents to their target sites within the body in a controlled and effective manner, improving both efficacy and adherence (14). These systems enhance treatment efficiency, minimize adverse effects, and optimize drug bioavailability, distribution, and release, ensuring maximum therapeutic benefit while mitigating risks associated with conventional administration (14–17). Such advancements hold significant potential for improving adherence to prescribed therapies.
Building on this foundation, the present study evaluates the impact of innovative DDS based on physical stimuli in enhancing medication adherence among patients undergoing long-term treatments. Rather than focusing on the discovery of new drugs, this research aims to optimize the administration of existing medications by improving their delivery to target cells, thereby maximizing therapeutic efficacy. The study explores the potential of physical stimuli to enhance drug bioavailability and effectiveness, linking these mechanisms to physiological balance and optimized cellular transport.
This research aims to determine how physical and/or physiological stimuli enhance pharmacological efficiency by optimizing membrane permeability and systemic biodisponibility, functioning as a biological drug delivery system. Addressing a critical gap in the literature, this study consolidates and analyzes evidence on drug delivery methods that optimize pharmacokinetics, particularly focusing on the role of emerging biological delivery systems. Ultimately, the research seeks to develop innovative solutions through design thinking (18) and open innovation methodologies (19), proposing a structured framework to support clinical decision-making. By refining drug absorption mechanisms, this approach aims to enhance therapeutic efficacy and provide a new perspective on optimizing pharmacological interventions in clinical practice.
2 Methods
To achieve the proposed objectives, this study adopted a Translational Research approach, structured through the Work Breakdown Structure (WBS) methodology, as described by the Project Management Institute (PMI) (2019; 2021) (20, 21). This management framework divided the research process into five interconnected Work Packages (WPs), each exploring specific aspects of the relationship between Drug Delivery Systems (DDS) and human physiology. The goal was to enhance drug absorption efficiency, ensuring a comprehensive and multidisciplinary perspective (Figure 1). Each WP was followed by a detailed qualitative analysis of the results to ensure their relevance and applicability in clinical contexts. The WBS methodology was chosen for its ability to add value to the innovation process, structuring the research step-by-step while maintaining a high scientific standard. This aligns with the principles discussed by Gaspary et al. (22), which emphasize the importance of tailoring research methodologies to specific organizational and scientific contexts to maximize their potential.
Figure 1

WBS methodology applied in this research.
WP1 (Management and Supervision) ensure adherence to the initial objectives and timeline. It aimed to foster team cohesion through Open Innovation (19), continuously refining the WBS as the study progressed while maintaining clear feedback and communication mechanisms across all work packages. Additionally, WP1 assessed the impact of newly developed medical hypotheses. The methodological flow of this study, integrating the WBS framework with systematic reviews, is formally structured in Figure 2, which presents a PRISMA-compliant flowchart detailing the inclusion and exclusion of studies at each stage, ensuring transparency and reproducibility in the research process. Additionally, a specific flowchart for each stage (WP2 and WP4) will be presented separately to provide a detailed breakdown of the screening and selection process in each phase.
Figure 2
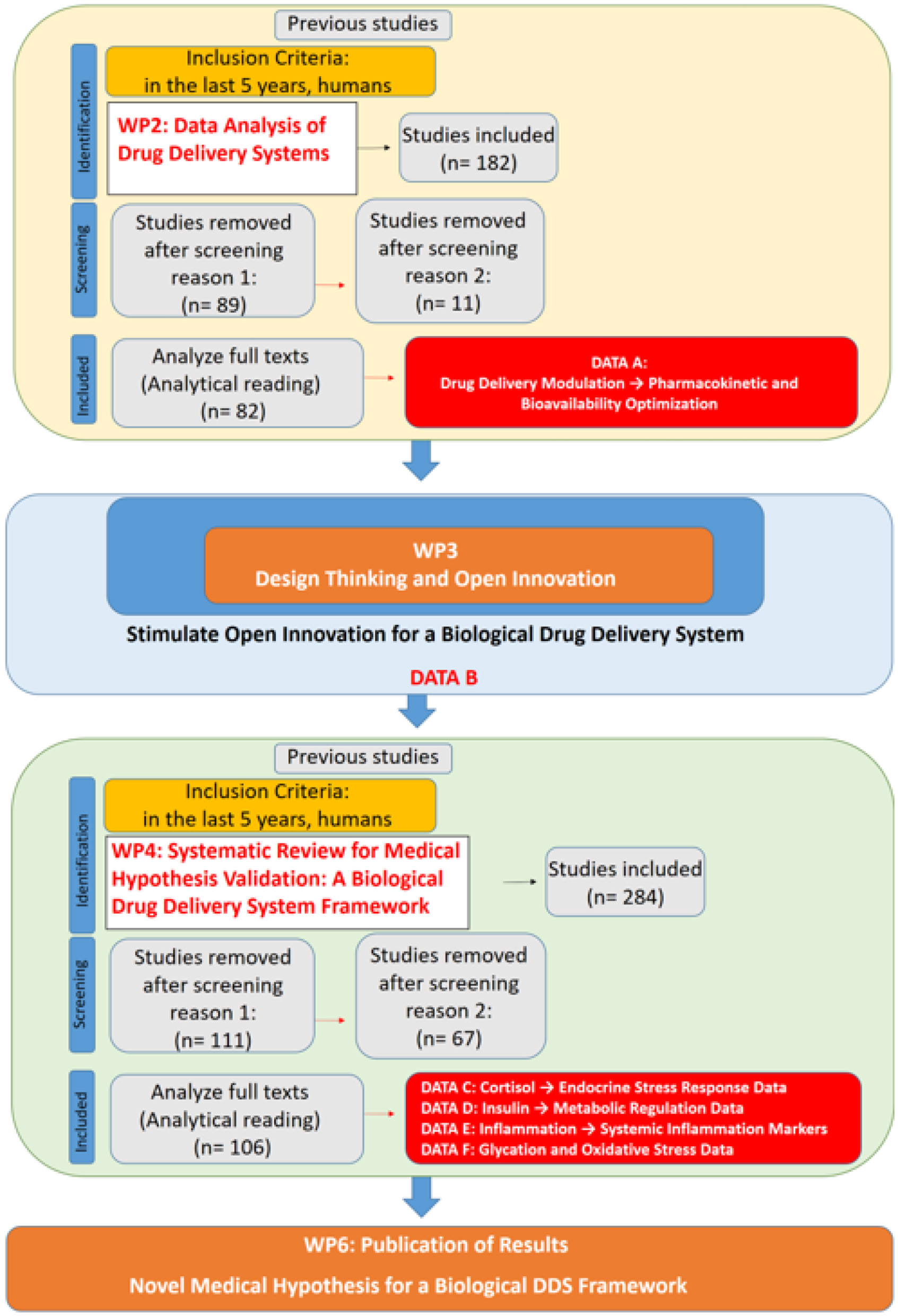
PRISMA-compliant flowchart integrating work breakdown structure (WBS) methodology and systematic reviews for the development of a novel medical hypothesis.
WP2 (Data Analysis of Drug Delivery Systems) sought to identify the primary areas in which physical stimuli influence DDS by employing a multi-criteria decision support methodology based on Bana and Costa et al. (23). A systematic literature review (SLR) was conducted to gather data, applying the eligibility criteria of studies published within the last five years, focused on human subjects, and indexed under the descriptors “Triggered” AND “Drug Delivery System” in English. This search yielded 182 studies, of which 82 were selected for full analysis (Figure 3). The focus of data collection in this stage of the research was to identify parameters that enhance drug performance and systemic bioavailability. This analysis provided the foundation for WP3, which further explored the role of epigenetic changes as a potential enhancer or modulator, influencing biological responses to pharmacological interventions. In this context, epigenetic modifications are not merely a consequence of DDS application but may act as a facilitating mechanism for optimizing drug delivery and systemic distribution.
Figure 3

WP2 SLR flowchart.
WP3 (Design Thinking to Stimulate Open Innovation for a Biological Drug Delivery System) explored how theoretical and empirical data integrated in previous work packages could inform the development of a biological DDS model based on physical stimuli. This phase applied Design Thinking (18) and Open Innovation (19) to establish connections between emerging theoretical insights and practical applications, emphasizing a human-centered approach to problem-solving. Design Thinking is an iterative process that involves empathy, problem definition, ideation, prototyping, and testing, ensuring continuous refinement based on user feedback and experimental validation (24). Open Innovation, in turn, promotes collaboration beyond conventional research environments, facilitating the exchange of ideas, resources, and technologies among researchers, clinicians, and other stakeholders (25). To support the development of this model, an additional literature review was conducted following the criteria proposed by Khan et al. (26). The selection criteria applied in this phase are detailed in Table 1.
Table 1
| Criteria | Variables |
|---|---|
| Database | LILACS; Medline; Web of Science; Scopus; SciELO; Google Scholar; Research Gate; ClinicalTrials.gov; Patentscope; Prospero |
| Timeframe | All studies published until 2024 |
| Languages | English, Portuguese, and Spanish |
| Indexed terms | Descriptors in English were generated from the iterative process. |
| Inclusion criteria for analysis | Broad, with the aim of idea testing |
Article selection criteria in a review for this study based on Khan et al. (26) for WP3.
Design Thinking integrates user needs, technological capabilities, and business constraints, structuring problem-solving through inspiration, ideation, and implementation stages (24, 27–29). The iterative and collaborative nature of this methodology fosters experimentation and continuous refinement, particularly in developing DDS strategies (30–32). Its success in enhancing patient-centered healthcare solutions is well-documented across various clinical applications (29, 33, 34), reinforcing its applicability in designing therapeutic innovations. Open Innovation complements this approach by fostering external collaboration, enhancing knowledge absorption, and broadening the scope of problem-solving strategies (19, 25).
WP4 (Systematic Review for Medical Hypothesis Validation: A Biological Drug Delivery System Framework) aimed to systematically evaluate the primary physiological mechanisms underlying drug absorption, assessing whether the framework developed in WP3 could be supported or refined based on existing evidence. A second systematic literature review was conducted to gather data using the following eligibility criteria: Clinical Trials or Review studies published within the last five years, focused on human subjects, indexed under the descriptors “insulin regulation” or “insulin homeostasis” (yielding 73 articles); “cortisol regulation” or “cortisol homeostasis” (38 articles); “inflammation reduction” (91 articles); and “inhibition of glycation” or “anti-glycation” or “advanced glycation end products inhibition” (82 articles). This process initially identified 284 studies, from which 106 were selected for full analysis (Figure 4).
Figure 4
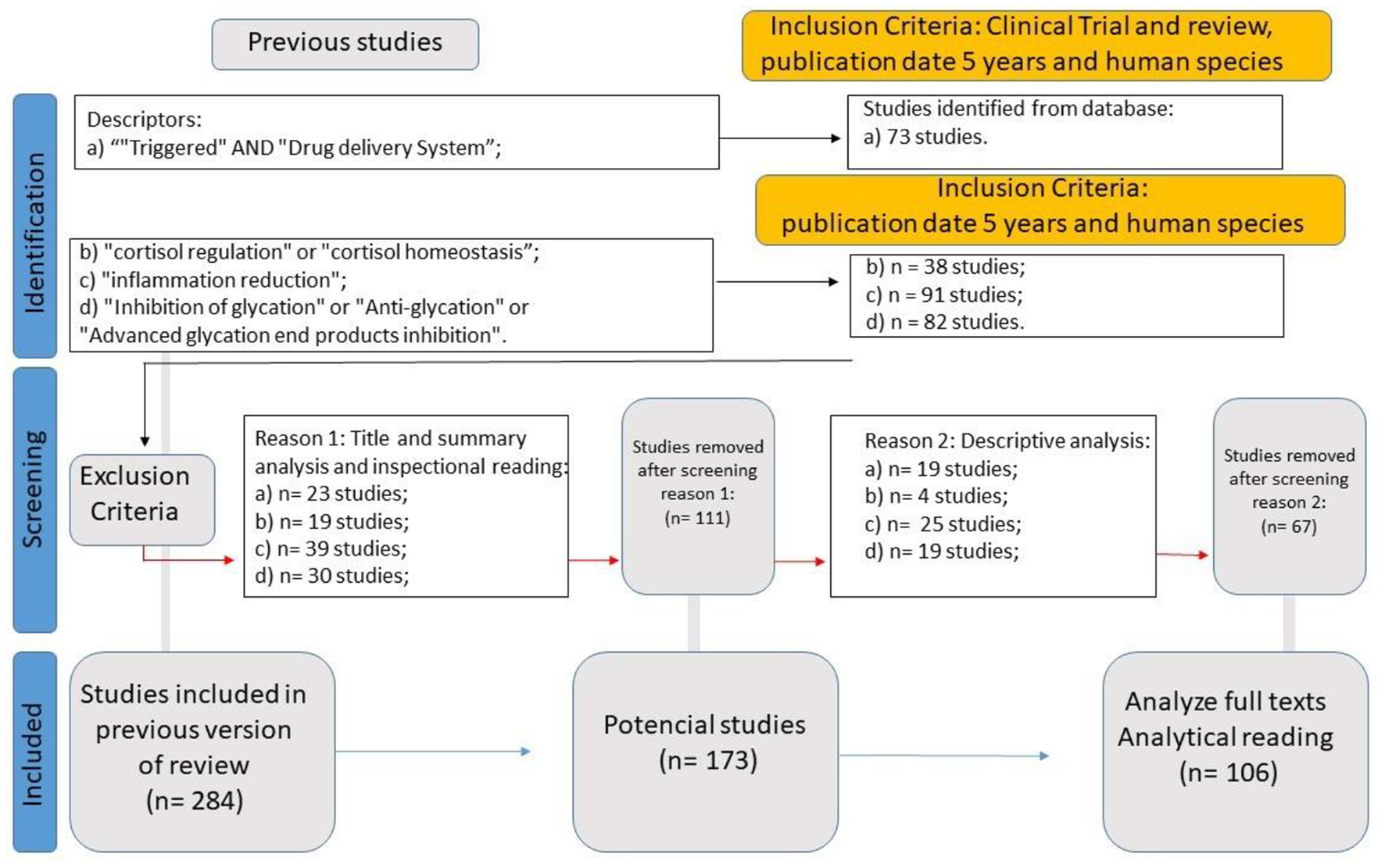
WP4 SLR flowchart.
The methodology employed in WP2 and WP4 was inspired by PRISMA guidelines (35) but tailored to align with the WBS framework, ensuring a structured and systematic approach to literature review and data synthesis. While the reviews were not formally registered, a detailed protocol was followed, outlining the search strategy, inclusion and exclusion criteria, data extraction procedures, and analysis methods. Table 2 summarizes these steps. Future research will consider formal registration to enhance the transparency and replicability of the review process.
Table 2
| PRISMA step | Description |
|---|---|
| Sources and search strategy | Comprehensive search conducted in NIH (80), Scopus (81), and Web of Science (82) to capture key points. |
| Study selection | Two reviewers independently screened titles and abstracts for eligibility. |
| Data collection process | Standardized data extraction form used. Data included study characteristics, methodology, and key findings. The review focused on WP2 objectives: “triggered drug delivery systems” activated by stimuli (e.g., pH, temperature, enzymes, light). These systems are relevant to the Brazilian health system, enhancing treatment effectiveness and control. The initial examination included titles and abstracts, and the process was repeated to achieve the WP4 objectives. |
| Data items | Included definitions, types of biological impacts, and methodologies for measuring these influences for each WP's specific objectives. |
| Risk of bias in individual studies | Assessed methodological quality, measurement rigor, and evidence strength linking WP2 and WP4 objectives. |
| Summary measures and synthesis of results | Synthesized results narratively, highlighting biological influences, their measurement, and impact. |
| Additional analyses | Meta-analysis was not feasible due to study heterogeneity. A multi-criteria methodology was employed to analyze treatment network geometry and potential biases. |
| Exploration of treatment network geometry | Used multi-criteria methodology to visually and analytically represent the evidence network through tables and figures. |
| Identification and mitigation of potential biases | The multi-criteria methodology identified and addressed potential biases, facilitating objective data treatment. Noted limitations include broad inclusion criteria, language bias, and absence of a registered review protocol. These were mitigated through quality assessment and consensus discussions. |
| Exclusion criteria (WP2—drug delivery systems SLR) | Studies were excluded if they did not analyze the role of physical stimuli in DDS, lacked experimental or clinical validation, or focused exclusively on chemical-based delivery mechanisms without correlation to physical stimuli. Reviews without explicit methodological rigor or that did not present clear inclusion criteria were also excluded. |
| Exclusion criteria (WP4—clinical decision model SLR) | Excluded studies were those that lacked relevance to insulin and cortisol regulation, inflammation reduction, or glycation inhibition in a translational context. Studies focusing solely on genetic predisposition, without direct intervention or modulation of physiological responses, were also excluded. Additionally, research without clear quantitative or qualitative assessments of intervention effects was removed. |
| Absence of review registration and considerations for future research | While the systematic reviews in WP2 and WP4 were conducted following PRISMA principles, they were not formally registered in databases such as PROSPERO. This may limit visibility in repositories dedicated to systematic reviews. However, methodological rigor was maintained through structured eligibility criteria, independent reviewer assessment, and adherence to a multi-criteria framework. Future research will consider formal registration to further strengthen transparency and traceability. |
| Evidence base compilation and description | Presented FPVs and CSFs clearly and informatively using a multi-criteria methodology. Ensured a comprehensive description of the evidence base and its contribution to overall analysis. |
SLR methodology inspired by PRISMA guidelines and tailored to the WBS framework.
To ensure methodological rigor, this study was structured according to systematic review principles, incorporating the PICO(S) framework for study design and data synthesis. The population targeted includes individuals with metabolic and inflammatory conditions potentially benefiting from biological drug delivery strategies. The intervention analyzed focuses on systemic physiological optimization to enhance drug bioavailability, particularly through cortisol and insulin regulation, inflammation reduction, and glycation inhibition. The comparison element derives from standard DDS approaches, which prioritize formulation-based mechanisms rather than physiological modulation. The outcomes were assessed through measurable biological markers associated with enhanced pharmacological efficiency. The study designs considered included systematic literature reviews and translational analyses of physiological mechanisms relevant to DDS effectiveness.
In addition to systematic literature analysis, a multi-criteria methodology (23, 36, 37) was applied to rigorously assess study quality. This involved an in-depth examination of study design, methodological rigor, and reported outcomes to identify potential biases or limitations, ensuring that the findings were grounded in high-quality evidence. The multi-criteria decision-making model of Bana e Costa et al. (23), as cited by Gerhardt et al. (37), provided the foundational framework for structuring the review. The information was categorized into three levels: Fundamental Point of View (FPV), Critical Success Factors (CSF), and Key Performance Indicators (KPI) (37).
The FPVs represented the strategic objectives for evaluating physical and biological stimuli in DDS, while CSFs identified essential areas influencing treatment success. KPIs were employed to quantify the effectiveness of different therapeutic interventions. This structured approach allowed for a balanced assessment of evidence, facilitating the development of a robust framework for evaluating DDS innovations (23, 37). The critical role of FPVs in decision-making, as outlined by Negreiros et al. (38), ensured that key strategic priorities were incorporated into the evaluation process. CSFs, following the principles established by Wong and Aspinwall (39), provided a foundation for assessing the practical implementation of DDS strategies. The integration of these elements facilitated a comprehensive evaluation of the treatment network's geometry and its potential applications in clinical practice.
While no meta-analysis was conducted due to heterogeneity in study designs, interventions, and outcome measures, the systematic categorization of findings provided a structured synthesis of existing knowledge. This approach ensured a nuanced understanding of the interplay between DDS strategies and physiological mechanisms, directly supporting the objectives of WP2 and WP4.
WP5 (Publication of Results) focused on evaluating the innovation and applicability of findings using SMART (40) and FINER (41) criteria to assess their potential for clinical implementation. This phase also addressed the methodological limitations and ethical considerations associated with the study, particularly regarding the use of patient data, to ensure transparency and adherence to scientific and ethical standards. By integrating multi-criteria decision-making, systematic literature analysis, and innovative research methodologies, this study sought to establish a framework that enhances the efficacy and adherence of drug delivery systems, providing a structured approach to advancing clinical practice.
3 Results
All results were obtained under the direct monitoring and integration of WP1 actions. As part of WP2, the first SLR included a comprehensive examination of 82 studies, which were assessed for methodological quality and risk of bias. Findings were synthesized narratively due to the heterogeneity of the study designs and outcomes reported. This narrative synthesis highlighted the diversity of physical and biological stimuli used in DDS and their effects on drug bioavailability and efficacy, providing a comprehensive overview of the current state of research in this field.
At this stage of the theoretical framework, the primary objective was to identify the main physical stimuli associated with DDS. Table 3 presents the categorized DDS, selected as Fundamental Points of View (FPVs) to align with this objective. The primary selection criterion was that the system should involve “physical stimulation” applicable in a real-world medical setting, ensuring its translational potential.
Table 3
| FPV | Definition | Recent studies |
|---|---|---|
| Hyperthermia | A therapeutic approach involving the controlled elevation of temperature in specific body regions to enhance drug delivery, improve tissue permeability, and increase the effectiveness of certain treatments, particularly in oncology. | (83–85) |
| Ultrasound-triggered drug delivery | A technique that utilizes ultrasonic waves to enhance drug penetration and control release at targeted locations. When exposed to ultrasound, drug-loaded nanoparticles or microbubbles undergo mechanical vibrations, facilitating localized and efficient drug activation. | (86, 87) |
| Photodynamic therapy | A treatment modality combining a photosensitizing agent with light exposure at a specific wavelength to induce therapeutic effects. This approach is commonly used in oncology and dermatology, where light activation leads to reactive oxygen species (ROS) production, selectively destroying targeted cells. | (88, 89) |
| pH-triggered drug delivery | A system in which drug release is regulated by environmental pH variations. This approach takes advantage of the natural pH differences in various tissues and biological compartments to achieve targeted drug release, enhancing therapeutic precision while minimizing systemic side effects. | (90, 91) |
| Magnetically triggered drug delivery | A delivery system where drug release or activation is modulated by external magnetic fields. This method employs magnetically responsive nanoparticles or carrier systems that are guided or activated by applied magnetic forces, improving site-specific drug accumulation and therapeutic efficacy. | (83, 92, 93) |
Categorization of DDS Involving Physical Stimuli.
FPV, Fundamental Point of View.
Additionally, as part of WP2, Table 4 outlines the parameters deemed critical for the therapeutic success of a drug delivery system. These parameters, classified as Critical Success Factors (CSFs), were systematically analyzed to identify the physiological conditions and mechanisms that directly influence DDS performance.
Table 4
| CSF | Definition | Recent studies |
|---|---|---|
| Complex cellular pathways | These pathways govern drug absorption, distribution, metabolism, and excretion, directly influencing therapeutic efficacy. Understanding these mechanisms allows the development of more precise and efficient DDS strategies. | (94, 95) |
| Cell homeostasis | Maintaining intracellular equilibrium is essential to optimizing drug effectiveness while minimizing side effects. Disruptions in homeostasis can impair drug transport and metabolism. | (96, 97) |
| Oxidative balance | The redox state of cells modulates drug activation and detoxification. Controlling oxidative stress enhances drug efficacy while preventing cellular damage. | (96, 98) |
| pH Regulation | The pH gradient across tissues and organelles affects drug solubility, stability, and targeted release. Designing pH-responsive DDS improves site-specific drug activation. | (90, 99) |
| Electromagnetic effects | External electromagnetic fields can regulate drug transport across membranes, facilitating precise, localized delivery. This strategy enhances drug targeting and minimizes systemic exposure. | (93, 100) |
| Physical stimuli | Light, heat, magnetic fields, and ultrasound are key physical triggers for controlled drug release, offering enhanced spatial and temporal precision in therapy. | (101, 102) |
| Intracellular transport | Optimizing intracellular pathways ensures drugs reach their intended targets within cells, improving bioavailability and therapeutic outcomes. | (103, 104) |
| Physiological cascade | Sequential biochemical events amplify drug responses, creating synergistic therapeutic effects and optimizing DDS efficiency. | (102, 105) |
| Precision medicine | Tailoring drug delivery based on individual genetic, metabolic, and physiological profiles ensures higher treatment efficacy and reduced adverse effects. | (100, 106) |
| Immune response | Understanding the interaction between the immune system and DDS allows for immunomodulatory strategies that enhance drug effectiveness and minimize adverse immune reactions. | (107, 108) |
| Cellular uptake pathway | Optimizing drug penetration across biological barriers enhances site-specific accumulation and therapeutic efficacy while reducing systemic toxicity. | (109) |
| Mitochondrial stimulation | Targeting mitochondria in DDS enables direct intervention in metabolic and degenerative disorders, improving therapeutic precision and cellular energy balance. | (95, 110) |
| Enhancement of zeta potential | Modulating zeta potential improves drug stability, bioavailability, and interaction with target cells, optimizing drug dispersion and cellular uptake. | (111) |
| Antioxidant agents | Incorporating antioxidants into DDS protects drugs and tissues from oxidative damage, stabilizing formulations and enhancing therapeutic benefits. | (112) |
| Tunable photoactivity | Light-activated drug release offers precise spatiotemporal control, reducing systemic exposure and enabling personalized therapeutic approaches. | (113) |
Critical success factors as physiological parameters for therapeutic success in DDS.
CSF: Critical Success Factors.
The WP2 protocol also identified a set of therapeutic application domains to illustrate the primary diseases and clinical conditions in which DDS employing physical stimuli have shown promising results. Rather than functioning as traditional Key Performance Indicators (KPIs), these domains serve as evidence-based use cases, guiding the translational applicability of the model. These clinical contexts are summarized in Table 5.
Table 5
| Therapeutic application domain | Description | Recent studies |
|---|---|---|
| Cancer | A multifactorial disease driven by genetic mutations, epigenetic alterations, and environmental factors, leading to uncontrolled cellular proliferation, invasion, and metastasis. Effective drug delivery systems are crucial for improving targeted therapy and minimizing systemic toxicity. | (114–116) |
| Infections | Diseases caused by pathogenic microorganisms, including bacteria, viruses, fungi, and parasites. Targeted DDS enhance antimicrobial efficacy, reduce resistance development, and improve patient compliance. | (117, 118) |
| Cardiovascular disease | A broad category encompassing conditions such as coronary artery disease, heart failure, hypertension, and stroke. Drug delivery strategies can optimize therapeutic agents targeting vascular remodeling, inflammation, and oxidative stress. | (119–121) |
| Chronic inflammatory diseases | Persistent inflammatory conditions, such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis, driven by immune dysregulation. Controlled DDS can improve drug targeting to inflamed tissues, reducing systemic adverse effects. | (122, 123) |
| Biomedical applications | The application of biotechnological and medical innovations, such as tissue engineering, regenerative medicine, and advanced diagnostics, to enhance therapeutic precision and efficiency. | (88, 124, 125) |
| Drug resistance | The ability of cancer cells or microorganisms to evade therapeutic effects through genetic and adaptive mechanisms. DDS can enhance drug bioavailability, target resistant populations, and delay resistance onset. | (94, 126, 127) |
| Synergistic therapy | The concurrent application of multiple therapeutic strategies (e.g., chemotherapy and immunotherapy) to enhance efficacy, reduce resistance, and improve clinical outcomes. DDS facilitate controlled release and co-administration of complementary agents. | (128–130) |
| Immunotherapy | A therapeutic approach leveraging the immune system to recognize and eliminate malignant or infected cells, including immune checkpoint inhibitors, monoclonal antibodies, and adoptive cell therapies. DDS improve targeting, reducing off-target effects. | (108, 131) |
| Mitochondrial dysfunction | Deficiencies in mitochondrial bioenergetics and homeostasis implicated in neurodegenerative, metabolic, and inflammatory disorders. Targeted DDS can modulate mitochondrial pathways to restore cellular function. | (95, 110, 132) |
| Oxidative stress | A pathological state resulting from excess reactive oxygen species (ROS) and insufficient antioxidant defenses, contributing to aging, cancer, and cardiovascular diseases. Antioxidant-loaded DDS can mitigate oxidative damage and enhance therapeutic outcomes. | (95, 122) |
Clinical application domains of DDS based on physical stimuli.
The integration of WP2 findings provided the foundation for WP3, which applied Design Thinking to the structured interrelation of FPVs, CSFs, and KPIs. The WP2 review supports the continued investigation of innovative DDS, despite limitations related to study heterogeneity. Notably, approaches focused on optimizing body pH and zeta potential through DDS demonstrated particular promise. Translational research on DDS facilitated the exploration of multiple hypotheses to enhance existing clinical therapies. The hypotheses selected for this study were based on identified physiological response patterns to specific stimuli, aligned with the research objectives.
WP3 specifically analyzed how each FPV and CSF from WP2 influenced acid-base balance, considering cellular, tissue, and systemic pH as reference parameters. Acid-base homeostasis is fundamental to physiological stability, cellular metabolism, and overall function. Dysregulation of plasma pH can lead to significant physiological disturbances, reinforcing the need for precise modulation of this parameter (42). This rationale led to the first formulated Medical Hypothesis (MH A): optimizing body pH is crucial for an effective drug delivery system.
Additionally, zeta potential emerged as a key determinant of therapeutic efficacy. Given that water constitutes ~50–70% of total body weight (42), the stability of colloidal dispersions—characterized by zeta potential—directly impacts molecular interactions in biological systems. The zeta potential quantifies the electrical charge near the interface of colloidal particles, influencing their stability, distribution, and biological interactions (43). While originally described in physicochemical systems, its role in biomedical research is increasingly recognized, particularly concerning nanoparticles and controlled drug release systems (44–46). Thus, in a translational reinterpretation, MH B was formulated: optimization of body zeta potential enhances the therapeutic effects of DDS. Table 6 summarizes the ideation process and initial considerations regarding the direction of future DDS research.
Table 6
| Idea generation | Main associated points of view | Applicable field of medical practice |
|---|---|---|
| Optimizing body pH (MH A) | Hyperthermia Ultrasound-triggered drug delivery Photodynamic therapy pH-triggered drug delivery Magnetically triggered drug delivery |
Orthomolecular (56–58) Integrative (133–135) Complementary (136) |
| Body zeta potential optimization (MH B) |
pH-triggered drug delivery Magnetically triggered drug delivery |
Orthomolecular (58) |
Stages of WP3 design thinking and their correlation with this study.
MH, medical hypothesis.
Following the Design Thinking process, the WP3 actions progressed with the implementation of the Open Innovation methodology, resulting in the construction of a theoretical model for clinical decision-making aimed at optimizing body pH and body potential. This novel approach establishes an optimized body balance by applying the triangular clinical decision model, functioning as a Biological DDS. This framework is structured around three main vertices: Cortisol and Insulin Regulation, Inflammation Reduction, and Glycation Inhibition, as illustrated in Figure 5. As part of the WP3 tasks, the framework underwent its first literature review, as outlined in Table 1, for an initial assessment of its viability. The initial conclusions, summarized in Table 6, motivated the commencement of WP4.
Figure 5

The triangular clinical decision-making model.
The initial conclusions motivating this phase are summarized in Table 7, providing a structured overview of the primary findings that guided the subsequent evaluation steps. WP4 was tasked with evaluating, through a systematic literature review (SLR), whether the model developed in WP3 could function as a viable and biologically integrated DDS. This phase involved reassessing WP2 CSFs from the perspective of the proposed system, systematically analyzing the individual contributions of each vertex in the decision-making model.
Table 7
| Action | Evidence base |
|---|---|
| Cortisol and insulin regulation | The interplay between cortisol and insulin is critical for metabolic homeostasis and overall physiological balance. Cortisol, a glucocorticoid hormone, modulates stress responses, while insulin regulates glucose metabolism. Dysregulation of either hormone contributes to metabolic disorders such as diabetes, obesity, and chronic stress-related pathologies. Studies confirm that interventions aimed at modulating these hormones can enhance metabolic control and improve therapeutic outcomes (137). |
| Inflammation reduction | Chronic inflammation is a key driver of numerous pathologies, including cardiovascular diseases, neurodegeneration, and cancer. Anti-inflammatory strategies, ranging from pharmacological interventions (e.g., NSAIDs, corticosteroids) to lifestyle modifications (e.g., dietary changes, physical activity, and physiotherapy), have been extensively documented. Controlling systemic inflammation is crucial for optimizing the internal environment for drug efficacy and cellular function (5, 138). |
| Glycation inhibition | The accumulation of advanced glycation end products (AGEs) accelerates aging and contributes to metabolic and neurodegenerative diseases. Nutritional and pharmacological interventions aimed at reducing glycation, such as dietary restriction of high-glycemic foods, supplementation with antiglycation agents (e.g., carnosine, pyridoxamine), and the use of AGE inhibitors, have been well-documented as potential strategies for mitigating glycation-related damage (139). |
| Optimization of body zeta potential | Zeta potential plays a crucial role in cellular interactions, nanoparticle stability, and drug delivery. Research suggests that optimizing the body's zeta potential can enhance membrane integrity, improve ion transport, and facilitate controlled drug release. This principle has been increasingly applied in nanomedicine, where electrostatic interactions influence drug stability, bioavailability, and targeted delivery (45, 46, 139–142). |
| Verisimilitude of proposed actions | The triangular clinical decision-making model is scientifically grounded, integrating evidence-based physiological mechanisms with innovative therapeutic applications. The proposed interventions align with existing data on biological drug delivery systems (DDS) that leverage physical and biochemical stimuli to enhance drug absorption and systemic regulation. The framework's validity is supported by established scientific literature, reinforcing its translational potential. |
| Integration and conclusion | The proposed triangular model introduces a novel paradigm for DDS, incorporating body pH optimization, cortisol and insulin regulation, inflammation reduction, glycation inhibition, and zeta potential modulation. By integrating these biological parameters, this approach has the potential to redefine drug delivery methodologies, bridging pharmacological therapy with personalized medicine and systemic health optimization. |
Initial conclusions from the literature review motivating the commencement of WP4.
The first WP4 analysis identified “Complex Cellular Pathways” and “Cell Homeostasis” as the most influential CSFs in insulin regulation. Numerous studies reinforced the central role of intricate cellular signaling networks in maintaining insulin homeostasis. For example, Lecorguillé et al. (47) demonstrated a strong link between glycemic control during pregnancy and DNA methylation patterns in neonatal cord blood, illustrating how cellular homeostasis impacts metabolic regulation. Similarly, Solinas and Becattini (48) highlighted the importance of dietary interventions in modulating insulin response, emphasizing that insulin regulation is contingent upon highly coordinated cellular mechanisms. These findings are summarized in Table 8.
Table 8
| CSF | Description | Recent studies |
|---|---|---|
| Complex cellular pathways | Insulin regulation relies on intricate signaling networks that control glucose uptake, insulin secretion, and cellular responsiveness. Disruptions in these pathways contribute to insulin resistance and metabolic dysregulation. | (48, 68, 79, 143–145) |
| Cell homeostasis | Maintaining a stable intracellular environment is fundamental for insulin signaling, glucose metabolism, and cellular energy balance. Dysregulated homeostasis contributes to metabolic disorders, including diabetes. | (48, 62, 146–149) |
| Oxidative balance | Oxidative stress impairs insulin sensitivity, exacerbating metabolic dysfunction. Maintaining redox balance through antioxidant mechanisms supports insulin action and glucose metabolism. | (48, 68, 143–145, 149–152) |
| Intracellular transport | Insulin and glucose transport mechanisms across cell membranes are critical for maintaining glycemic control and ensuring effective glucose uptake by tissues. | (62, 63) |
| Physiological cascade | The insulin response involves sequential physiological events triggered by metabolic and hormonal signals, ensuring efficient glucose regulation. | (145, 152, 153) |
| Precision medicine | Tailoring diabetes treatment based on genetic predisposition and patient-specific biomarkers enhances therapeutic outcomes and optimizes glycemic control. | (150, 154) |
| Immune response | Inflammatory cytokines interfere with insulin signaling pathways, contributing to insulin resistance. Modulating immune response is crucial for restoring insulin sensitivity and metabolic function. | (62, 63, 151, 155, 156) |
| Mitochondrial stimulation | Mitochondrial efficiency is vital for ATP production and metabolic regulation. Dysfunctional mitochondria impair insulin action, highlighting the importance of mitochondrial stimulation in metabolic health. | (47, 149) |
WP4 analysis 1: interrelationship between insulin regulation and WP2 CSFs.
The second WP4 analysis revealed “Physiological Cascade” and “Intracellular Transport” as pivotal CSF's in cortisol regulation. Studies frequently underscored the importance of intracellular transport mechanisms and regulatory cascades in modulating cortisol balance. For instance, Weiss et al. (49) examined the impact of antenatal corticosteroid exposure on neonatal cortisol regulation, emphasizing the role of intracellular pathways. King et al. (50) explored physiological cascades involved in cortisol production, shedding light on stress-response mechanisms. Bhatt et al. (51) investigated the relationship between PTSD and cortisol regulation, proposing targeted interventions in precision medicine. Table 9 presents a synthesis of these results.
Table 9
| CSF | Description | Recent studies |
|---|---|---|
| Complex cellular pathways | Cortisol regulation is influenced by intricate hormonal interactions and neuroendocrine pathways, particularly in response to stress and maternal mental health. Disruptions in these pathways can lead to long-term metabolic and psychological consequences. | (51, 64, 157) |
| Cell homeostasis | Cortisol plays a fundamental role in maintaining homeostasis, modulating energy metabolism, immune responses, and neurological function. Chronic stress disrupts these mechanisms, leading to systemic dysregulation. | (50, 51) |
| Oxidative balance | Cortisol secretion is closely linked to oxidative stress. Prenatal exposure to tobacco and marijuana has been shown to disrupt oxidative balance, altering cortisol responses and increasing susceptibility to metabolic and neurodevelopmental disorders. | (158) |
| pH regulation | Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, particularly in COVID-19 patients, has been associated with disturbances in systemic pH levels, indicating a link between cortisol homeostasis and acid-base balance. | (159) |
| Physical stimuli | Physical exercise is a potent modulator of cortisol levels, demonstrating how controlled physical stimuli can positively influence the stress response and restore endocrine balance. | (160, 161) |
| Intracellular transport | Cortisol transport across cellular membranes is crucial for its bioavailability and function. Stress and endocrine dysregulation have been shown to impact intracellular cortisol distribution, affecting tissue-specific responses. | (49, 64–67) |
| Physiological cascade | The cortisol response is modulated by a complex cascade of hormonal and metabolic signals. Dysfunctions in this cascade contribute to pathological conditions such as PTSD, metabolic syndrome, and neuropsychiatric disorders. | (49–51, 65, 162–164) |
| Precision medicine | Individualized approaches to cortisol regulation are essential for precision medicine, particularly in psychiatric and metabolic disorders. Genetic predisposition and epigenetic factors influence cortisol sensitivity and therapeutic response. | (162, 165, 166) |
| Immune response | Cortisol exerts immunomodulatory effects, influencing inflammation and immune tolerance. Studies highlight the interaction between maternal cortisol levels and infant stress regulation, demonstrating long-term implications for immune function. | (167) |
WP4 analysis 2: interrelationship between cortisol regulation and WP2 CSFs.
In the context of inflammation reduction, “Immune Response” and “Cell Homeostasis” emerged as the most significant CSFs. These elements were consistently identified as critical factors in mitigating inflammation-associated pathologies. Sommer et al. (52) demonstrated that immune modulation effectively reduced inflammation in experimental models, reinforcing the direct relationship between immune balance and cellular homeostasis. Santos (53) examined the regenerative potential of stem cell-based therapies, further illustrating the necessity of maintaining cellular equilibrium in inflammatory responses. Table 10 details the core findings of this analysis.
Table 10
| CSF | Description | Recent studies |
|---|---|---|
| Complex cellular pathways | Inflammation reduction modulates complex cellular pathways by downregulating pro-inflammatory cytokine production and signaling, preserving the integrity of intracellular communication and homeostasis. | (52, 69–73, 168, 169) |
| Cell homeostasis | Controlling inflammation supports cellular homeostasis by preventing metabolic stress, maintaining membrane stability, and ensuring optimal function of biochemical pathways necessary for cellular survival. | (53, 69–73, 168) |
| Oxidative balance | Inflammatory processes contribute to oxidative stress by increasing reactive oxygen species (ROS). Suppressing inflammation restores redox homeostasis, minimizing oxidative damage and enhancing antioxidant defense mechanisms. | (53, 70–74, 168) |
| Physiological cascade | Lowering inflammation stabilizes the systemic physiological cascade by reducing chronic stress, regulating hormone secretion, and preventing excessive immune activation, which impacts metabolic and cardiovascular health. | (70–76, 169, 170) |
| Physical stimuli | Anti-inflammatory interventions improve the body's response to physical stimuli by reducing pain, swelling, and tissue damage, thereby enhancing mobility and musculoskeletal function. | (53, 70–76, 168) |
| Intracellular transport | Inflammation alters intracellular transport by affecting vesicular trafficking and endocytic pathways. Modulating inflammatory mediators improves cellular uptake and drug distribution. | (52, 69–76) |
| Precision medicine | Anti-inflammatory therapies benefit from personalized medicine approaches, allowing tailored treatments based on genetic, metabolic, and immunological profiles to optimize patient outcomes. | (70–76, 169–172) |
| Immune response | Regulating inflammation prevents excessive immune activation, balancing pro- and anti-inflammatory pathways to mitigate autoimmune disorders and chronic inflammatory diseases. | (52, 69–76, 170–172) |
| Antioxidant agents | The inclusion of antioxidants in therapeutic strategies helps reduce inflammation by neutralizing free radicals, protecting cellular components, and mitigating damage associated with chronic oxidative stress. | (70–72, 168) |
| Tunable photoactivity | Light-based modulation of inflammatory processes allows precise targeting of affected tissues, minimizing collateral damage and improving therapeutic outcomes in conditions such as arthritis and dermatological diseases. | (73, 173) |
WP4 analysis 3: interrelationship between inflammation reduction and WP2 CSFs.
Regarding glycation inhibition, multiple CSFs were identified as key contributors to mitigating its pathological effects. These included complex cellular pathways, oxidative balance, intracellular transport, immune response, mitochondrial stimulation, and antioxidant activity. Tang et al. (54) illustrated how advanced glycation end-products (AGEs) disrupt cellular function, while Cheng et al. (55) emphasized the importance of oxidative balance in reducing glycation-induced damage. Although factors such as pH regulation and electromagnetic effects appeared less directly influential, emerging evidence suggests that physical stimuli and tunable photoactivity hold potential for further exploration. Table 11 provides a detailed overview of these findings.
Table 11
| CSF | Description | Recent studies |
|---|---|---|
| Complex cellular pathways | Inhibiting glycation prevents the accumulation of advanced glycation end-products (AGEs), preserving proper cell signaling and maintaining biochemical pathways essential for cellular function. | (54, 55, 174–178) |
| Cell homeostasis | By reducing glycation, cellular homeostasis is preserved, preventing metabolic dysregulation and protein misfolding that can lead to impaired function and cell death. | (54, 55, 179–184) |
| Oxidative balance | Glycation enhances oxidative stress by promoting reactive oxygen species (ROS) production. Its inhibition reduces oxidative damage, sustaining the antioxidant defense system. | (77, 78, 174, 185–188) |
| pH regulation | Glycation inhibition stabilizes cellular pH by preventing the accumulation of acidic by-products that contribute to metabolic acidosis and cellular dysfunction. | (189, 190) |
| Physical stimuli | Preventing glycation preserves tissue elasticity and biomechanical properties, maintaining structural integrity and responsiveness to therapeutic interventions. | (180, 190, 191) |
| Intracellular transport | Inhibiting glycation protects cytoskeletal and transport proteins, ensuring effective intracellular trafficking of biomolecules and drugs. | (54, 55, 174, 178, 179, 181) |
| Physiological cascade | By preserving enzyme activity and receptor function, glycation inhibition maintains regulatory physiological cascades essential for metabolic homeostasis. | (54, 175) |
| Precision medicine | The inhibition of glycation enhances personalized therapeutic strategies by targeting glycation-specific biomarkers, improving tailored interventions. | (192, 193) |
| Immune response | Glycation inhibition mitigates pro-inflammatory AGEs, reducing chronic immune activation and preserving immune system function. | (174, 190, 192, 194, 195) |
| Cellular uptake pathway | Preventing glycation preserves membrane receptor function, optimizing drug absorption and cellular nutrient uptake. | (54, 174, 178, 179, 181, 196) |
| Mitochondrial stimulation | Glycation-induced mitochondrial dysfunction is counteracted, preserving ATP production and cellular energy balance. | (177, 178, 181, 183) |
| Enhancement of zeta potential | Reducing glycation improves zeta potential, optimizing electrostatic interactions critical for cellular communication and homeostasis. | (54) |
| Antioxidant agents | The inhibition of glycation reduces oxidative burden, complementing antioxidant therapies that mitigate AGE-induced damage. | (54, 77, 78, 174, 181, 185–188) |
| Tunable photoactivity | By maintaining protein integrity, glycation inhibition enhances the precision of light-based therapies, improving their efficiency in diagnostics and treatment. | (180, 190) |
WP4 analysis 4: interrelationship between glycation inhibition and WP2 CSFs.
Based on WP4's analysis of 106 reviewed studies, the integration of insulin and cortisol regulation, inflammation reduction, and glycation inhibition as a biological DDS is supported by extensive scientific literature. Modulating these pathways creates a more balanced internal environment, enhancing drug absorption and therapeutic efficacy. This approach aligns with the principles of personalized medicine, suggesting that systemic regulation can significantly improve clinical outcomes.
To achieve these objectives, promoting synergistic metabolic interactions is crucial. Figure 6 presents an evidence-based model for achieving key therapeutic goals. Each component in this framework is supported by scientific literature and aligns with established physiological principles. The strategies outlined reflect a translational approach to optimizing metabolic health and drug delivery efficiency. Additionally, the concept of micronutrient synergy, examined in this study, suggests that specific nutrient combinations may enhance physiological responses, particularly in regulating insulin and cortisol levels, reducing inflammation, and inhibiting glycation (56–59). Table 12 details how micronutrient interactions contribute to these outcomes.
Figure 6

Biological drug delivery system primary goals.
Table 12
| Objective | Description (56–59) |
|---|---|
| Insulin and cortisol regulation | The synergistic effects of specific micronutrients enhance glucose metabolism and modulate the stress response, reducing insulin resistance and stabilizing cortisol levels. Magnesium and chromium improve insulin sensitivity, while vitamin C and pantothenic acid help regulate cortisol secretion, promoting metabolic balance and reducing susceptibility to endocrine disorders. |
| Inflammatory process modulation | Chronic inflammation is a key driver of various pathological conditions. Synergistic micronutrients such as omega-3 fatty acids, vitamin D, selenium, and antioxidants (vitamins C and E, zinc) exhibit strong anti-inflammatory properties. Their combined action strengthens the body's defense against oxidative stress and inflammatory cascades, creating a more stable internal environment conducive to drug therapy efficacy. |
| Glycation inhibition | Glycation leads to the formation of advanced glycation end-products (AGEs), which contribute to aging and chronic disease pathogenesis. Micronutrients such as vitamin B6, alpha-lipoic acid, carnosine, and benfotiamine act synergistically to reduce AGE formation, protecting protein structures and cellular integrity, thereby enhancing longevity and metabolic function. |
Micronutrient synergism and the objectives of the clinical decision model.
The implementation of synergistic micronutrient strategies presents a holistic and scientifically grounded approach to chronic disease management. This conceptual framework acknowledges the complexity of human physiology and the need for multifaceted interventions to optimize therapeutic responses. As part of WP5, the hypothesis—proposing that a well-regulated physiological state, achieved through insulin and cortisol modulation, glycation inhibition, and inflammation control, can function as a biological DDS—was evaluated against SMART (40) and FINER criteria (41), demonstrating its specificity, feasibility, and clinical relevance (Table 13).
Table 13
| Criteria | Description analysis |
|---|---|
| SMART | |
| Specific: | The hypothesis is specific as it outlines three precise clinical interventions: regulation of insulin and cortisol levels, inhibition of glycation, and reduction of inflammation. The goal is clear: to create a well-balanced bodily state that enhances drug delivery efficiency. |
| Measurable: | The hypothesis involves measurable outcomes, such as insulin and cortisol levels, markers of glycation (AGEs), and inflammatory markers. Success can be quantitatively assessed through improvements in these biomarkers and therapeutic outcomes. |
| Achievable: | The hypothesis is achievable with current scientific knowledge and clinical practices. There are existing therapies and interventions targeting each of the specified areas (hormonal regulation, anti-glycation, anti-inflammatory). |
| Relevant: | The hypothesis addresses a significant problem in medicine: the optimization of drug delivery and therapeutic efficacy. It aligns with the growing field of personalized medicine and its emphasis on tailored therapeutic approaches. |
| Time-bound: | The hypothesis implies a time-bound nature as clinical interventions would need to be monitored over specific periods to observe changes and improvements in biomarkers and therapeutic outcomes. Specific timelines for clinical trials and longitudinal studies could be established to evaluate the hypothesis. |
| FINER | |
| Feasible: | The hypothesis is feasible given current technologies and methodologies in endocrinology, pharmacology, and personalized medicine. Research and clinical trials can be designed to test the effects of the proposed interventions. |
| Interesting: | The hypothesis is intriguing as it suggests a novel approach to drug delivery, potentially transforming standard medical practices. It integrates multiple disciplines, which can attract a wide range of scientific and clinical interest. |
| Novel: | The concept of using a well-balanced bodily state as a biological drug delivery system is innovative. It combines traditional therapeutic goals with a new purpose, offering a fresh perspective on optimizing drug efficacy. |
| Ethical: | The interventions proposed (regulation of hormone levels, anti-glycation, and anti-inflammatory treatments) are ethically sound and align with standard clinical practices. The focus on minimizing side effects and maximizing benefits aligns with ethical principles of beneficence and non-maleficence. |
| Relevant: | The hypothesis addresses relevant health issues, such as diabetes, metabolic syndrome, and chronic inflammation. It contributes to the broader goal of improving patient outcomes through personalized and targeted medical approaches. |
WP5 criteria analysis.
4 Discussion
The research process was meticulously structured using the Work Breakdown Structure (WBS) methodology, ensuring a systematic and step-by-step approach that provided detailed insights into each methodological component and its corresponding results. The integration of WBS facilitated the logical segmentation of research processes into interconnected phases, ensuring iterative refinement and cross-disciplinary integration (PMI, 2019; 2021) (20, 21). This methodological rigor was particularly advantageous for integrating diverse fields such as epigenetics, pharmacology, and drug delivery science, as it allowed for the structured incorporation of emerging insights into a coherent decision-making framework. In addition to structuring the study into modular Work Packages (WPs), WBS enabled the sequential identification of Fundamental Points of View (FPVs) and Critical Success Factors (CSFs), ensuring an optimal sequence for the application of Multi-Criteria Decision Analysis (MCDA). By organizing the decision-making process hierarchically, this approach enhances not only reproducibility but also the adaptability of systematic reviews, allowing for continuous refinement as new evidence emerges (35). Moreover, WBS promotes the seamless incorporation of emerging insights from epigenetics, pharmacology, and drug delivery science, while MCDA refines decision-making by categorizing critical evaluation factors into hierarchical components (23). The synergy between these methodologies has been successfully applied in translational healthcare models, particularly in decision-making frameworks that navigate complex biomedical scenarios (22, 60, 61).
The triangular clinical decision-making model developed in this study is based on the premise that the regulation of cortisol and insulin levels, inflammation reduction, and glycation inhibition collectively optimize drug absorption and systemic bioavailability. Cortisol and insulin modulation directly influence metabolic stress and transporter efficiency, as dysregulated levels contribute to altered membrane permeability and impaired intracellular transport (48, 49, 62–67). In particular, insulin homeostasis plays a pivotal role in glucose metabolism, which directly impacts the cellular uptake of pharmacological agents by modulating transporter expression and endocytotic pathways. By stabilizing glucose homeostasis and reducing catabolic effects, the model fosters a metabolic environment conducive to efficient molecular transport and cellular uptake, ultimately enhancing drug bioavailability and systemic distribution (62, 68). This metabolic fine-tuning not only improves drug absorption but also minimizes pharmacokinetic variability, ensuring a more predictable therapeutic response. Chronic inflammation, a key driver of endothelial dysfunction, restricts drug permeation by disrupting cellular communication (52, 69–76). Furthermore, glycation inhibition preserves membrane fluidity and zeta potential, reducing biochemical barriers that hinder receptor function and molecular transport (54, 77). The mitigation of advanced glycation end-products (AGEs) decreases oxidative stress, preventing modifications in protein transporters and increasing overall bioavailability of pharmacological agents (55, 78). Together, these three regulatory axes establish a physiological framework that optimizes pharmacokinetics while also mitigating long-term drug resistance, paving the way for more efficient and personalized therapeutic strategies. This synergistic interplay between metabolic, inflammatory, and glycation-related axes is not merely additive but interdependent, forming a dynamic physiological network in which modulation of one vertex reinforces the regulatory effects of the others, collectively enhancing membrane function, transport efficiency, and therapeutic responsiveness.
Crucially, this study conducted an exhaustive integrative analysis demonstrating that the regulation of cortisol and insulin, along with inflammation and glycation control, has the potential to mimic all Critical Success Factors (CSFs) observed in Drug Delivery Systems (DDS) activated by physical stimuli, as identified in Table 4. This mapping was meticulously detailed in Tables 8–11, reinforcing the conceptual validity of the triangular model as a Biological Drug Delivery System (BDDS). This translational approach thus represents a shift in drug delivery science, moving from a purely formulation-based model to a biologically integrated system where metabolic homeostasis itself becomes a determinant of drug absorption and efficacy (50, 63, 79). Unlike conventional DDS, which primarily focus on drug formulation and controlled release, this model emphasizes systemic optimization, preparing the biological environment to enhance drug absorption and therapeutic response.
By leveraging epigenetic modifications, micronutrient synergy, and improved nutrient bioavailability, the model aligns with the principles of translational and precision medicine, moving beyond conventional pharmaceutical modifications (3). Emerging insights into nutrient-epigenome interactions and their impact on drug transporter gene expression have begun to reshape our understanding of bioavailability modulation as an epigenetically regulated process. While classical mechanisms—such as DNA methylation, histone modifications, or non-coding RNA interactions—form the theoretical basis (3, 5, 6), their application here is not mechanistically dissected. Instead, these pathways were integrated into a decision-making model that emphasizes translational applicability. Due to the personalized nature of epigenetic programming and its inherent complexity, a detailed mechanistic mapping would require a level of description disproportionate to the study's scope. Therefore, this model incorporates epigenetic regulation as an operational mechanism—capable of modulating the physiological conditions needed for optimal drug delivery—rather than detailing the molecular steps involved in gene expression control.
This perspective repositions epigenetics from a passive background variable to a central pillar of the integrative therapeutic framework. It conceptualizes epigenetics as an active modulator of drug bioavailability by influencing membrane stability, intracellular signaling, and systemic regulation. As such, epigenetics reinforces the body's intrinsic capacity to optimize therapeutic outcomes and strengthens the foundation of a Biological Drug Delivery System that adapts dynamically to internal cues.
The flexibility of this model distinguishes it from rigid clinical protocols. Instead of defining fixed pharmacological regimens, it functions as a clinical decision-making framework, allowing for real-time adjustments based on patient-specific metabolic responses. This adaptability is particularly relevant in managing chronic diseases, where individualized interventions are crucial due to varying physiological profiles. For example, patients with metabolic syndrome may require different therapeutic adjustments compared to those with autoimmune disorders or psychiatric conditions, emphasizing the need for a tailored, dynamic strategy. The precision and safety of physiological stimuli-based DDS, such as heat therapy, ultrasound triggering, and electromagnetic modulation, depend on real-time biomarker monitoring, metabolic profiling, and continuous reassessment of patient responses. Incorporating AI-driven analytics into this framework could further enhance its clinical utility, enabling predictive modeling and real-time therapeutic adjustments based on patient-specific biomarkers. Ethical considerations are also paramount, ensuring patient autonomy, informed consent, and a thorough risk-benefit analysis in the clinical implementation of biological DDS strategies.
While this model represents an innovative approach to drug delivery, its clinical validation requires further refinement. Future research should focus on the development of AI-driven decision-support tools to assist clinicians in dynamically tailoring interventions, ensuring that physiological modulation strategies are seamlessly integrated into precision medicine rather than applied as generalized treatment algorithms. Additionally, biomarker-based patient stratification studies will be essential to evaluate the model's applicability across different populations, refining the decision-making framework for specific metabolic, inflammatory, and epigenetic profiles. These next steps will be critical in transforming this conceptual model into a clinically validated therapeutic strategy. The effectiveness of this model relies on further research and clinical validation, particularly regarding the previously identified personalized stabilization period, estimated between 10 to 20 weeks. By introducing the concept of a biological drug delivery system, this study expands the conventional understanding of drug delivery by incorporating human physiological responses as an integral component of therapeutic optimization. Rather than solely focusing on pharmaceutical modifications, the model emphasizes the importance of balancing nutrient availability, hormonal regulation, and cell membrane integrity to enhance medication efficacy.
5 Conclusions
This research introduces a novel therapeutic intervention based on two systematic literature reviews, focusing on the role of physical stimuli in enhancing drug delivery. By employing the open innovation method, the triangular clinical decision-making model presents a promising framework aligned with the principles of personalized medicine and nutraceuticals. The model acknowledges the significant influence of epigenetics and nutrition on medication response, integrating these factors into a structured approach aimed at improving drug absorption, metabolism, and targeted therapies. This integrative perspective has the potential to advance treatment efficacy by optimizing biological receptivity to pharmacological interventions.
The effectiveness of this model relies on further research and clinical validation, particularly regarding the previously identified need for a personalized pre-treatment stabilization period, aimed at optimizing metabolic and physiological balance prior to pharmacological interventions. Moreover, the continuity of this physiological modulation during treatment may prove essential to maintain therapeutic responsiveness and minimize pharmacokinetic variability. By introducing the concept of a biological drug delivery system, this study expands the conventional understanding of drug delivery by incorporating human physiological responses as an integral component of therapeutic optimization. Rather than solely focusing on pharmaceutical modifications, the model emphasizes the importance of balancing nutrient availability, hormonal regulation, and cell membrane integrity to enhance medication efficacy.
Although this study presents a theoretical model, its clinical implementation requires additional empirical validation. The proposed framework aligns with precision medicine by emphasizing physiological optimization as a strategy to enhance drug bioavailability, rather than relying exclusively on DDS formulation-based approaches. In this context, biomarker-driven assessments will be essential for defining personalized therapeutic strategies, reinforcing the translational potential of this model. Future research should focus on establishing structured clinical protocols, ensuring that proposed interventions can be tailored to patient-specific needs while maintaining therapeutic efficacy.
The theoretical-methodological framework proposed in this research serves as a reference for future translational studies, encouraging the integration of complementary approaches and reinforcing the value of orthomolecular medical practices. Additionally, the model highlights the critical role of personalized therapeutic strategies in clinical settings, ensuring that interventions are adapted to individual physiological needs. Future investigations should further explore and refine this approach to enhance body homeostasis and therapeutic responses, ultimately validating its efficacy and expanding its application across diverse healthcare contexts.
Statements
Data availability statement
The original contributions presented in the study are included in the article further inquiries can be directed to the corresponding author/s.
Author contributions
JG: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. AC: Conceptualization, Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Nassani AA Javed A Rosak-Szyrocka J Pilar L Yousaf Z Haffar M et al . Major Determinants of Innovation Performance in the Context of Healthcare Sector. Int J Environ Res Public Health. (2023) 20:5007. 10.3390/ijerph20065007
2.
Church D . Genie in Your Genes: Epigenetic Medicine and the New Biology of Intention. Carlsbad: Hay House Inc. (2018).
3.
Keating ST El-Osta A . Epigenetics and metabolism. Circ Res. (2015) 116:715–36. 10.1161/CIRCRESAHA.116.303936
4.
Jones PA Ohtani H Chakravarthy A De Carvalho DD . Epigenetic therapy in immune-oncology. Nat Rev Cancer. (2019) 19:151–61. 10.1038/s41568-019-0109-9
5.
Szarc vel Szic K Ndlovu MN Haegeman G Vanden Berghe W . Nature or nurture: let food be your epigenetic medicine in chronic inflammatory disorders. Biochem Pharmacol. (2010) 80:1816–32. 10.1016/j.bcp.2010.07.029
6.
Vanden Berghe W . Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol Res. (2012) 65:565–76. 10.1016/j.phrs.2012.03.007
7.
Marincola FM . Translational Medicine: A two-way road. J Transl Med. (2003) 1:1–2. 10.1186/1479-5876-1-1
8.
Liebman MN Marincola FM . Expanding the perspective of translational medicine: the value of observational data. J Transl Med. (2012) 27. 10.1186/1479-5876-10-61
9.
Smith SK Selig W Harker M Roberts JN Hesterlee S Leventhal D et al . Patient engagement practices in clinical research among patient groups, industry, and academia in the United States: a survey. PLoS ONE. (2015) 10:e0140232. 10.1371/journal.pone.0140232
10.
Sahs JA Nicasio AV Storey JE Guarnaccia PJ Lewis-Fernández R . Developing research collaborations in an academic clinical setting: challenges and lessons learned. Commun Ment Health J. (2017) 53:647–60. 10.1007/s10597-016-0073-8
11.
Day-Duro E Lubitsh G Smith G . Understanding and investing in healthcare innovation and collaboration. J Health Organ Manag. (2020) 34:469–87. 10.1108/JHOM-07-2019-0206
12.
Kardas P Ágh T Dima A Goetzinger C Potočnjak I Wettermark B et al . Half a century of fragmented research on deviations from advised therapies: is this a good time to call for multidisciplinary medication adherence research centres of excellence?Pharmaceutics. (2023) 15:933. 10.3390/pharmaceutics15030933
13.
Kelly CJ Young AJ . Promoting innovation in healthcare. Future Healthc J. (2017) 4:121–5. 10.7861/futurehosp.4-2-121
14.
Ezike TC Okpala US Onoja UL Nwike CP Ezeako EC Okpara OJ et al . Advances in drug delivery systems, challenges and future directions. Heliyon. (2023) 9:e17488. 10.1016/j.heliyon.2023.e17488
15.
Allen LV Jr. Ansel HC . Ansel's Pharmaceutical Dosage Forms and Drug Delivery Systems.10th Edn. Philadelphia, PA: Wolters Kluwer Health (2014).
16.
Jain KK . Drug Delivery Systems, Vol. 251. Totowa (NJ): Humana Press. (2021). Available online at: http://repo.upertis.ac.id/1886/1/Drug%20Delivery%20System%20In%20Method%20In%20Molecular%20Biology.pdf
17.
Ranade VV Cannon JB . Drug Delivery Systems. Boca Raton: CRC Press (2011).
18.
Brown T . Design thinking. Harv Bus Rev. (2008) 1–10.
19.
Chesbrough H . Open Innovation: The New Imperative for Creating and Profiting from Technology.Boston, MA: Harvard Business School Publishing (2003).
20.
PMI—Project Management Institute . A Guide to the Project Management Body of Knowledge, Seventh Edition. Newtown Square: Project Management Institute (2021)
21.
PMI—Project Management Institute . A Guide to the Project Management Body of Knowledge, Seventh Edition.Newtown Square: Project Management Institute (2021).
22.
Gaspary JFP Edgar L Lopes LFD Rosa CB Siluk JCM . Translational insights into the hormetic potential of carbon dioxide: from physiological mechanisms to innovative adjunct therapeutic potential for cancer. Front Physiol. (2024) 15:1415037. 10.3389/fphys.2024.1415037
23.
Bana e Costa CA Ensslin L Corrêa E. C Vansnick J.-C. (1999). Decision support systems in action: Integrated application in a multicriteria decision aid process. Eur. J. Oper. Res. 113, 315–20. 10.1016/S0377-2217(98)00219-7
24.
Romero V Donaldson H . Human-centred design thinking and public health education: A scoping review. Health Promot J Austr. (2023) 35:688–700. 10.1002/hpja.802
25.
Šlapáková Losová V Dvouletý O . The role of open innovation in addressing resource constraints in healthcare: a systematic literature review. J Health Organ Manag. (2024) 38:150–75. 10.1108/JHOM-06-2023-0203
26.
Khan KS Kunz R Kleijnen J Antes G . Systematic Reviews to Support Evidence-Based Medicine: How to Review and Apply Findings of Healthcare Research. Abingdon: RSM Press (2011).
27.
Kimbell L . Rethinking design thinking: part II. Des Cult. (2011) 3:129–48. 10.2752/175470811X13071166525216
28.
Liedtka J Ogilvie T . Designing for Growth: A Design Thinking Toolkit for Managers. New York: Columbia University Press (2011).
29.
Plattner H Meinel C Leifer L . Design Thinking: Understand—Improve—Apply. Berlin: Springer. (2012).
30.
Smith MA Nigro S . Applying design-thinking principles to practice-based pharmacy research. Ann Pharmacother. (2023) 57:1111–6. 10.1177/10600280221147014
31.
Zhang C Yang L Wan F Bera H Cun D Rantanen J et al . Quality by design thinking in the development of long-acting injectable PLGA/PLA-based microspheres for peptide and protein drug delivery. Int J Pharm. (2020) 585:119441. 10.1016/j.ijpharm.2020.119441
32.
Eleftheriadis GK Genina N Boetker J Rantanen J . Modular design principle based on compartmental drug delivery systems. Adv Drug Deliv Rev. (2021) 178:113921. 10.1016/j.addr.2021.113921
33.
Vagal A Wahab SA Butcher B Zettel N Kemper E Vogel C et al . Human-centered design thinking in radiology. J Am Coll Radiol. (2020) 17:662–7. 10.1016/j.jacr.2019.11.019
34.
Fleury AL Goldchmit SM Gonzales MA Farias RR Fernandes TL . Innovation in orthopedics: part 1-design thinking. Curr Rev Musculoskelet Med. (2022) 15:143–9. 10.1007/s12178-022-09748-5
35.
Page MJ Moher D Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . MA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:160. 10.1136/bmj.n160
36.
Torres-Carrión PV González-González CS Aciar S Rodríguez-Morales G . Methodology for systematic literature review applied to engineering and education. In: 2018 IEEE Global Engineering Education Conference (EDUCON) (2018). p. 1364–73.
37.
Gerhardt VJ Mairesse Siluk JC Baierle IC Michelin CDF . Theoretical model for identifying market development indicators. Int J Product Perf Manage. (2022) 71:2659–79. 10.1108/IJPPM-05-2020-0259
38.
Negreiros RF Do Carmo BBT Moreira MEP . Multicriteria model of allocation of basic health units: A proposition for midsize city [Modelo multicritério de alocação de unidades básicas de saúde: Uma proposição para cidade de médio porte]. Gest Prod Oper Sist. (2015) 10:13–33. 10.15675/gepros.v10i1.1184
39.
Wong KY Aspinwall E . An empirical study of the important factors for knowledge-management adoption in the SME sector. J Knowl Manag. (2005) 9:64–82. 10.1108/13673270510602773
40.
Drucker PF . (1954). The Practice of Management, Nova Iorque, Harper and Row Traduzido em português: Prática de Administração de Empresas, 2 volumes (1962). Rio de Janeiro: Editora Fundo de Cultura.
41.
Hulley SB Cummings SR Browner WS Grady DG Newman TB . Designing Clinical Research (3rd Edn). Philadelphia, PA: Lippincott Williams and Wilkins (2007).
42.
Gaulton J Crowe B Sherman J . How design thinking and quality improvement can be integrated into a “human-centered quality improvement” approach to solve problems in perinatology. Clin Perinatol. (2023) 50:435–48. 10.1016/j.clp.2023.01.006
43.
Hamm LL Nakhoul N Hering-Smith KS . Acid-base homeostasis. Clin J Am Soc Nephrol. (2015) 10:2232–42. 10.2215/CJN.07400715
44.
Popkin BM D'Anci KE Rosenberg IH . Water, hydration, and health. Nutr. Rev. (2010) 68:439–58. 10.1111/j.1753-4887.2010.00304.x
45.
Hunter R . Zeta potential in colloid science: principles and applications. In:OttewillRHRowellRL, editors. Colloid Sciences Series (2013). Cambridge: Academic Press.
46.
Bondar OV Saifullina DV Shakhmaeva II Mavlyutova II Abdullin TI . Monitoring of the zeta potential of human cells upon reduction in their viability and interaction with polymers. Acta Naturae. (2012) 4:78–81. 10.32607/20758251-2012-4-1-78-81
47.
Lecorguillé M McAuliffe FM Twomey PJ Viljoen K Mehegan J Kelleher CC et al . Maternal glycaemic and insulinemic status and newborn DNA methylation: findings in women with overweight and obesity. J Clin Endocrinol Metab. (2022) 108:85–98. 10.1210/clinem/dgac553
48.
Solinas G Becattini B . An adipoincretin effect links adipostasis with insulin secretion. Trends Endocrinol Metab. (2024) 35:466–77. 10.1016/j.tem.2023.10.009
49.
Weiss SJ Keeton V Richoux S Cooper B Niemann S . Exposure to antenatal corticosteroids and infant cortisol regulation. Psychoneuroendocrinology. (2023) 147:105960. 10.1016/j.psyneuen.2022.105960
50.
King LS Graber MG Colich NL Gotlib IH . Associations of waking cortisol with DHEA and testosterone across the pubertal transition: effects of threat-related early life stress. Psychoneuroendocrinology. (2020) 115:104651. 10.1016/j.psyneuen.2020.104651
51.
Galbally M Watson SJ Lappas M de Kloet ER van Rossum E Wyrwoll C et al . Fetal programming pathway from maternal mental health to infant cortisol functioning: the role of placental 11β-HSD2 mRNA expression. Psychoneuroendocrinology. (2021) 127:105197. 10.1016/j.psyneuen.2021.105197
52.
Sommer C Cohen JN Dehmel S Neuhaus V Schaudien D Braun A et al . Interleukin-2-induced skin inflammation. Eur J Immunol. (2024) 54:e2350580. 10.1002/eji.202350580
53.
Santos L . The impact of nutrition and lifestyle modification on health. Eur J Intern Med. (2022) 97:18–25. 10.1016/j.ejim.2021.09.020
54.
Tang L Guan Q Zhang L Xu M Zhang M Khan MS et al . Synergistic interaction of Cu(II) with caffeic acid and chlorogenic acid in α-glucosidase inhibition. J Sci Food Agric. (2024) 104:518–29. 10.1002/jsfa.12955
55.
Cheng J Li J Xiong RG Wu SX Xu XY Tang GY et al . Effects and mechanisms of anti-diabetic dietary natural products: an updated review. Food Funct. (2024) 15:1758–78. 10.1039/D3FO04505F
56.
Janson M . Orthomolecular medicine: the therapeutic use of dietary supplements for anti-aging. Clin Interv Aging. (2006) 1:261–5. 10.2147/ciia.2006.1.3.261
57.
Williams RJ Kalita DK . A Physician's Handbook on Orthomolecular Medicine. Amsterdam: Elsevier (2016).
58.
Carter S . Orthomolecular medicine. Integr Med (Encinitas). (2019) 18:74.
59.
Townsend JR Kirby TO Marshall TM Church DD Jajtner AR Esposito R et al . Foundational nutrition: implications for human health. Nutrients. (2023) 15:2837. 10.3390/nu15132837
60.
Gaspary JFP Gerhardt VJ de Freitas Michelin C Lopes LFD Rosa CB Siluk JCM . Healthcare can't stop evolving: innovation as the catalyst for unleashing the managerial potential of value-based healthcare by stimulating intangible assets and enhancing organizational resilience. Front Psychol. (2024) 15:1438029. 10.3389/fpsyg.2024.1438029
61.
Gaspary JFP Camara AG Lopes LFD . Translational interdisciplinary research on human chorionic gonadotropin's enhancement of neuroendocrine crosstalk: a novel medical hypothesis for systemic adjunctive treatment of psychiatric disorders. Front Psychiatry. (2025) 16:1537442. 10.3389/fpsyt.2025.1537442
62.
Benchoula K Parhar IS Hwa WE . The molecular mechanism of vgf in appetite, lipids, and insulin regulation. Pharmacol Res. (2021) 172:105855. 10.1016/j.phrs.2021.105855
63.
Soltani A Jafarian A Allameh A . The Predominant microRNAs in β-cell Clusters for Insulin Regulation and Diabetic Control. Curr Drug Targets. (2020) 21:722–34. 10.2174/1389450121666191230145848
64.
Giglberger M Peter HL Kraus E Kreuzpointner L Zänkert S Henze GI et al . Daily life stress and the cortisol awakening response over a 13-months stress period - Findings from the LawSTRESS project. Psychoneuroendocrinology. (2022) 141:105771. 10.1016/j.psyneuen.2022.105771
65.
Epstein CM Houfek JF Rice MJ Weiss SJ . Integrative review of early life adversity and cortisol regulation in pregnancy. J Obstet Gynecol Neonatal Nurs. (2021) 50:242–55. 10.1016/j.jogn.2020.12.006
66.
Khoury JE Beeney J Shiff I Bosquet Enlow M Lyons-Ruth K . Maternal experiences of childhood maltreatment moderate patterns of mother-infant cortisol regulation under stress. Dev Psychobiol. (2021) 63:1309–21. 10.1002/dev.22109
67.
Hibel LC Mercado E Valentino K . Child maltreatment and mother-child transmission of stress physiology. Child Maltreat. (2019) 24:340–52. 10.1177/1077559519826295
68.
Tchéoubi SER Akpovi CD Coppée F Declèves AE Laurent S Agbangla C et al . Molecular and cellular biology of PCSK9: impact on glucose homeostasis. J Drug Target. (2022) 30:948–60. 10.1080/1061186X.2022.2092622
69.
Everett BM MacFadyen JG Thuren T Libby P Glynn RJ Ridker PM et al . Inhibition of interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS Trial. J Am Coll Cardiol. (2020) 76:1660–70. 10.1016/j.jacc.2020.08.011
70.
Stone GW Ali ZA O'Brien SM Rhodes G Genereux P Bangalore S et al . Impact of complete revascularization in the ISCHEMIA trial. J Am Coll Cardiol. (2023) 82:1175–88. 10.1016/j.jacc.2023.06.015
71.
Goudarzi R Thomas P Ryan S . Joint dysfunctionality alleviation along with systemic inflammation reduction following arthrocen treatment in patients with knee osteoarthritis: a randomized double-blind placebo-controlled clinical trial. Medicina (Kaunas). (2022) 58:228. 10.3390/medicina58020228
72.
Boland J Long C . Update on the inflammatory hypothesis of coronary artery disease. Curr Cardiol Rep. (2021) 23. 10.1007/s11886-020-01439-2
73.
Zou H Wang H Zhong Y Zhang Z Wang Z Shang T et al . Prussian blue nanoparticles coated with tumor cell membranes for precise photothermal therapy and subsequent inflammation reduction. Biochem Biophys Res Commun. (2024) 723:150173. 10.1016/j.bbrc.2024.150173
74.
Liu Z Ma X Ilyas I Zheng X Luo S Little PJ et al . Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics. (2021) 11:4502–15. 10.7150/thno.54498
75.
Liberale L Montecucco F Schwarz L Lüscher TF Camici GG . Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc Res. (2021) 117:411–22. 10.1093/cvr/cvaa211
76.
Leyfman Y Erick TK Reddy SS Galwankar S Nanayakkara PWB Di Somma S et al . Potential immunotherapeutic targets for hypoxia due to COVI-flu. Shock. (2020) 54:438–50. 10.1097/SHK.0000000000001627
77.
Osaka N Kushima H Mori Y Saito T Hiromura M Terasaki M et al . Anti-inflammatory and atheroprotective properties of glucagon. Diab Vasc Dis Res. (2020) 17:1479164120965183. 10.1177/1479164120965183
78.
Ohara M Nagaike H Fujikawa T Kohata Y Ogawa M Omachi T et al . Effects of omarigliptin on glucose variability and oxidative stress in type 2 diabetes patients: a prospective study. Diabetes Res Clin Pract. (2021) 179:108999. 10.1016/j.diabres.2021.108999
79.
Dumesic DA Hoyos LR Chazenbalk GD Naik R Padmanabhan V Abbott DH et al . Mechanisms of intergenerational transmission of polycystic ovary syndrome. Reproduction. (2020) 159:R1–R13. 10.1530/REP-19-0197
80.
NIH . PUBMED (2023). Available online at: https://pubmed.ncbi.nlm.nih.gov
81.
Scopus . Scopus—An Overview (2023). Available online at: https://www.scopus.com/home.uri
82.
WOS . Web of Science (2023). Available online at: https://apps.webofknowledge.com
83.
Seynhaeve ALB Amin M Haemmerich D van Rhoon GC Ten Hagen TLM . Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv Drug Deliv Rev. (2020) 163–164:125–44. 10.1016/j.addr.2020.02.004
84.
Wang F Duan H Zhang R Guo H Lin H Chen Y et al . Potentiated cytosolic drug delivery and photonic hyperthermia by 2D free-standing silicene nanosheets for tumor nanomedicine. Nanoscale. (2020) 12:17931–46. 10.1039/D0NR05214K
85.
Sumitha NS Krishna NG Sailaja GS . Multifunctional chitosan ferrogels for targeted cancer therapy by on-demand magnetically triggered drug delivery and hyperthermia. Biomater Adv. (2022) 142:213137. 10.1016/j.bioadv.2022.213137
86.
Fan CH Ho YJ Lin CW Wu N Chiang PH Yeh CK et al . State-of-the-art of ultrasound-triggered drug delivery from ultrasound-responsive drug carriers. Expert Opin Drug Deliv. (2022) 19:997–1009. 10.1080/17425247.2022.2110585
87.
Gharehnazifam Z Dolatabadi R Baniassadi M Shahsavari H Kajbafzadeh AM Abrinia K et al . Multiphysics modeling and experiments on ultrasound-triggered drug delivery from silk fibroin hydrogel for Wilms tumor. Int J Pharm. (2022) 621:121787. 10.1016/j.ijpharm.2022.121787
88.
Xu J Zhang J Zhang F Zhang L . Copolymer-functionalized cellulose nanocrystals as a ph- and nir-triggered drug carrier for simultaneous photothermal therapy and chemotherapy of cancer cells. Biomacromolecules. (2022) 23:4308–17. 10.1021/acs.biomac.2c00770
89.
Li QR Xu HZ Xiao RC Liu B Ma TQ Yu TT et al . Laser-triggered intelligent drug delivery and anti-cancer photodynamic therapy using platelets as the vehicle. Platelets. (2023) 34:2166677. 10.1080/09537104.2023.2166677
90.
Wang L Cai Y An Z Gu W Chen P Cai Q et al . ZnO-functionalized mesoporous inner-empty nanotheranostic platform: upconversion imaging guided chemotherapy with pH-triggered drug delivery. Nanotechnology. (2018) 29:505101. 10.1088/1361-6528/aae0b6
91.
Ding C Wu H Yin ZZ Gao J Wu D Qin Y et al . Disulfide-cleavage- and pH-triggered drug delivery based on a vesicle structured amphiphilic self-assembly. Mater Sci Eng C Mater Biol Appl. (2020) 107:110366. 10.1016/j.msec.2019.110366
92.
Anilkumar TS Shalumon KT Chen JP . Applications of Magnetic Liposomes in Cancer Therapies. Curr Pharm Des. (2019) 25:1490–504. 10.2174/1389203720666190521114936
93.
Santadkha T Skolpap W Thitapakorn V . Diffusion modeling and in vitro release kinetics studies of curcumin-loaded superparamagnetic nanomicelles in cancer drug delivery system. J Pharm Sci. (2022) 111:1690–9. 10.1016/j.xphs.2021.11.015
94.
Guo W Deng L Yu J Chen Z Woo Y Liu H et al . Sericin nanomicelles with enhanced cellular uptake and pH-triggered release of doxorubicin reverse cancer drug resistance. Drug Deliv. (2018) 25:1103–16. 10.1080/10717544.2018.1469686
95.
Hejazian SM Hosseiniyan Khatibi SM Barzegari A Pavon-Djavid G Razi Soofiyani S Hassannejhad S et al . Nrf-2 as a therapeutic target in acute kidney injury. Life Sci. (2021) 264:118581. 10.1016/j.lfs.2020.118581
96.
Kaur K Carrazzone RJ Matson JB . The benefits of macromolecular/supramolecular approaches in hydrogen sulfide delivery: a review of polymeric and self-assembled hydrogen sulfide donors. Antioxid Redox Signal. (2020) 32:79–95. 10.1089/ars.2019.7864
97.
Park J Jo S Lee YM Saravanakumar G Lee J Park D et al . Enzyme-triggered disassembly of polymeric micelles by controlled depolymerization via cascade cyclization for anticancer drug delivery. ACS Appl Mater Interfaces. (2021) 13:8060–70. 10.1021/acsami.0c22644
98.
Men W Zhu P Dong S Liu W Zhou K Bai Y et al . Fabrication of dual pH/redox-responsive lipid-polymer hybrid nanoparticles for anticancer drug delivery and controlled release. Int J Nanomedicine. (2019) 14:8001–11. 10.2147/IJN.S226798
99.
Wu M Chen J Huang W Yan B Peng Q Liu J et al . Injectable and self-healing nanocomposite hydrogels with ultrasensitive ph-responsiveness and tunable mechanical properties: implications for controlled drug delivery. Biomacromolecules. (2020) 21:2409–20. 10.1021/acs.biomac.0c00347
100.
Tomitaka A Arami H Ahmadivand A Pala N McGoron AJ Takemura Y et al . Magneto-plasmonic nanostars for image-guided and NIR-triggered drug delivery. Sci Rep. (2020) 10:10115. 10.1038/s41598-020-66706-2
101.
Sun T Dasgupta A Zhao Z Nurunnabi M Mitragotri S . Physical triggering strategies for drug delivery. Adv Drug Deliv Rev. (2020) 158:36–62. 10.1016/j.addr.2020.06.010
102.
Zhu JQ Wu H Li ZL Xu XF Xing H Wang MD et al . Responsive Hydrogels Based on Triggered Click Reactions for Liver Cancer. Adv Mater. (2022) 34:e2201651. 10.1002/adma.202201651
103.
Jia HR Zhu YX Xu KF Liu X Wu FG . Plasma membrane-anchorable photosensitizing nanomicelles for lipid raft-responsive and light-controllable intracellular drug delivery. J Control Release. (2018) 286:103–13. 10.1016/j.jconrel.2018.07.027
104.
Lu K Qu Y Lin Y Li L Wu Y Zou Y et al . Photothermal nanoplatform with sugar-triggered cleaning ability for high-efficiency intracellular delivery. ACS Appl Mater Interfaces. (2022) 14:2618–28. 10.1021/acsami.1c21670
105.
Wang S Yu G Yang W Wang Z Jacobson O Tian R et al . Photodynamic-chemodynamic cascade reactions for efficient drug delivery and enhanced combination therapy. Adv Sci. (2021) 8:2002927. 10.1002/advs.202002927
106.
Halamoda-Kenzaoui B Bremer-Hoffmann S . Main trends of immune effects triggered by nanomedicines in preclinical studies. Int J Nanomedicine. (2018) 13:5419–31. 10.2147/IJN.S168808
107.
Sou K Le DL Sato H . Nanocapsules for programmed neurotransmitter release: toward artificial extracellular synaptic vesicles. Small. (2019) 15:e1900132. 10.1002/smll.201900132
108.
Varshney D Qiu SY Graf TP McHugh KJ . Employing drug delivery strategies to overcome challenges using TLR7/8 agonists for cancer immunotherapy. AAPS J. (2021) 23:90. 10.1208/s12248-021-00620-x
109.
Patzelt A Lademann J . Recent advances in follicular drug delivery of nanoparticles. Expert Opin Drug Deliv. (2020) 17:49–60. 10.1080/17425247.2020.1700226
110.
Cho H Cho YY Shim MS Lee JY Lee HS Kang HC et al . Mitochondria-targeted drug delivery in cancers. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165808. 10.1016/j.bbadis.2020.165808
111.
Baker A Khan MS Iqbal MZ Khan MS . Tumor-targeted drug delivery by nanocomposites. Curr Drug Metab. (2020) 21:599–613. 10.2174/1389200221666200520092333
112.
Wu H Peng B Mohammed FS Gao X Qin Z Sheth KN et al . Brain targeting, antioxidant polymeric nanoparticles for stroke drug delivery and therapy. Small. (2022) 18:e2107126. 10.1002/smll.202107126
113.
Zheng Y Li Z Chen H Gao Y . Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur J Pharm Sci. (2020) 144:105213. 10.1016/j.ejps.2020.105213
114.
Lai X Liu XL Pan H Zhu MH Long M Yuan Y et al . Light-triggered efficient sequential drug delivery of biomimetic nanosystem for multimodal chemo-, antiangiogenic, and anti-MDSC therapy in melanoma. Adv Mater. (2022) 34:e2106682. 10.1002/adma.202106682
115.
Katmerlikaya TG Dag A Ozgen PSO Ersen BC . Dual-drug conjugated glyco-nanoassemblies for tumor-triggered targeting and synergistic cancer therapy. ACS Appl Bio Mater. (2022) 5:5356–64. 10.1021/acsabm.2c00749
116.
Zhong ZX Li XZ Liu JT Qin N Duan HQ Duan XC et al . Disulfide bond-based SN38 prodrug nanoassemblies with high drug loading and reduction-triggered drug release for pancreatic cancer therapy. Int J Nanomedicine. (2023) 18:1281–98. 10.2147/IJN.S404848
117.
Santos Ramos MA Santos KC Silva PB Toledo LG Marena GD Rodero CF et al . Nanotechnological strategies for systemic microbial infections treatment: a review. Int J Pharm. (2020) 589:119780. 10.1016/j.ijpharm.2020.119780
118.
Zhou Q Si Z Wang K Li K Hong W Zhang Y et al . Enzyme-triggered smart antimicrobial drug release systems against bacterial infections. J Control Release. (2022) 352:507–26. 10.1016/j.jconrel.2022.10.038
119.
Yang C Li Y Du M Chen Z . Recent advances in ultrasound-triggered therapy. J Drug Target. (2019) 27:33–50. 10.1080/1061186X.2018.1464012
120.
Song Z Song K Xiao Y Guo H Zhu Y Wang X et al . Biologically responsive nanosystems targeting cardiovascular diseases. Curr Drug Deliv. (2021) 18:892–913. 10.2174/1567201818666210127093743
121.
Zhou S Zhao W Hu J Mao C Zhou M . Application of nanotechnology in thrombus therapy. Adv Healthc Mater. (2023) 12:e2202578. 10.1002/adhm.202202578
122.
Deng Z Liu S . Inflammation-responsive delivery systems for the treatment of chronic inflammatory diseases. Drug Deliv Transl Res. (2021) 11:1475–97. 10.1007/s13346-021-00977-8
123.
Helmecke T Hahn D Matzke N Ferdinand L Franke L Kühn S et al . Inflammation-controlled anti-inflammatory hydrogels. Adv Sci. (2023) 10:e2206412. 10.1002/advs.202206412
124.
Massiot J Rosilio V Makky A . Photo-triggerable liposomal drug delivery systems: from simple porphyrin insertion in the lipid bilayer towards supramolecular assemblies of lipid-porphyrin conjugates. J Mater Chem B. (2019) 7:1805–23. 10.1039/C9TB00015A
125.
Pearce AK Anane-Adjei AB Cavanagh RJ Monteiro PF Bennett TM Taresco V et al . Effects of polymer 3D architecture, size, and chemistry on biological transport and drug delivery in vitro and in orthotopic triple negative breast cancer models. Adv Healthc Mater. (2020) 9:e2000892. 10.1002/adhm.202000892
126.
Gordillo-Galeano A Ospina-Giraldo LF Mora-Huertas CE . Lipid nanoparticles with improved biopharmaceutical attributes for tuberculosis treatment. Int J Pharm. (2021) 596:120321. 10.1016/j.ijpharm.2021.120321
127.
Singh P Waghambare P Khan TA Omri A . Colorectal cancer management: strategies in drug delivery. Expert Opin Drug Deliv. (2022) 19:653–70. 10.1080/17425247.2022.2084531
128.
Bao Y Yin L Liu L Chen L . Acid-sensitive ROS-triggered dextran-based drug delivery system for advanced chemo-photodynamic synergistic therapy. J Biomed Mater Res A. (2020) 108:148–56. 10.1002/jbm.a.36800
129.
Yi H Lu W Liu F Zhang G Xie F Liu W et al . ROS-responsive liposomes with NIR light-triggered doxorubicin release for combinatorial therapy of breast cancer. J Nanobiotechnology. (2021) 19:134. 10.1186/s12951-021-00877-6
130.
Zhang X He C Xiang G . Engineering nanomedicines to inhibit hypoxia-inducible Factor-1 for cancer therapy. Cancer Lett. (2022) 530:110–27. 10.1016/j.canlet.2022.01.012
131.
Heshmati Aghda N Dabbaghianamiri M Tunnell JW Betancourt T . Design of smart nanomedicines for effective cancer treatment. Int J Pharm. (2022) 621:121791. 10.1016/j.ijpharm.2022.121791
132.
Guo X Mei J Zhang C . Development of drug dual-carriers delivery system with mitochondria-targeted and pH/heat responsive capacity for synergistic photothermal-chemotherapy of ovarian cancer. Int J Nanomedicine. (2020) 15:301–13. 10.1007/978-981-13-9374-7
133.
O'Brien K Ried K Binjemain T Sali A . Integrative approaches to the treatment of cancer. Cancers. (2022) 14:5933. 10.3390/cancers14235933
134.
Pitcher MH Edwards E Langevin HM Rusch HL Shurtleff D . Complementary and integrative health therapies in whole person resilience research. Stress Health. (2023) 39:55–61. 10.1002/smi.3276
135.
Kleef R Dank M Herold M Agoston EI Lohinszky J Martinek E et al . Comparison of the effectiveness of integrative immunomodulatory treatments and conventional therapies on the survival of selected gastrointestinal cancer patients. Sci Rep. (2023) 13:20360 (Erratum in: Sci Rep. (2024) 14:1129. 10.1038/s41598-023-47802-5
136.
Zollman C Vickers A . What is complementary medicine?BMJ. (1999) 319:693–6. 10.1136/bmj.319.7211.693
137.
Keenan DM Veldhuis JD Basu A Basu RA . novel measure of glucose homeostasis (or loss thereof) comprising the joint dynamics of glucose, insulin, glucagon, and cortisol. Am J Physiol Endocrinol Metab. (2019) 316:E998–E1011. 10.1152/ajpendo.00078.2018
138.
Furman D Campisi J Verdin E Carrera-Bastos P Targ S Franceschi C et al . Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25:1822–32. 10.1038/s41591-019-0675-0
139.
Jagdale AD Patil RS Tupe RS . Attenuation of albumin glycation and oxidative stress by minerals and vitamins: An in vitro perspective of dual-purpose therapy. Vitam Horm. (2024) 125:231–50. 10.1016/bs.vh.2023.12.003
140.
Mendivil-Alvarado H Limon-Miro AT Carvajal-Millan E Lizardi-Mendoza J Mercado-Lara A Coronado-Alvarado CD et al . Extracellular vesicles and their zeta potential as future markers associated with nutrition and molecular biomarkers in breast cancer. Int J Mol Sci. (2023) 24:6810. 10.3390/ijms24076810
141.
Lin G Wang J Yang YG Zhang Y Sun T . Advances in dendritic cell targeting nano-delivery systems for induction of immune tolerance. Front Bioeng Biotechnol. (2023) 11:1242126. 10.3389/fbioe.2023.1242126
142.
Beech JA . Bioelectric potential gradients may initiate cell cycling: ELF and zeta potential gradients may mimic this effect. Bioelectromagnetics. (1997) 18:341–8.
143.
Lewis GF Carpentier AC Pereira S Hahn M Giacca A . Direct and indirect control of hepatic glucose production by insulin. Cell Metab. (2021) 33:709–20. 10.1016/j.cmet.2021.03.007
144.
Hnilicova P Kantorova E Sutovsky S Grofik M Zelenak K Kurca E et al . Imaging methods applicable in the diagnostics of Alzheimer's Disease, considering the involvement of insulin resistance. Int J Mol Sci. (2023) 24:3325. 10.3390/ijms24043325
145.
Zhao H Sun J Ren Z . Incretins beyond the pancreas: exploring the impact of GIP and GLP-1/2 on bone remodeling. Discov Med. (2024) 36:655–65. 10.24976/Discov.Med.202436183.62
146.
Uehara K Santoleri D Whitlock AEG Titchenell PM . Insulin regulation of hepatic lipid homeostasis. Compr Physiol. (2023) 13:4785–809. 10.1002/cphy.c220015
147.
Ebadi SA Sharifi L Rashidi E Ebadi SS Khalili S Sadeghi S et al . Supplementation with vitamin D and insulin homeostasis in healthy overweight and obese adults: a randomized clinical trial. Obes Res Clin Pract. (2021) 15:256–61. 10.1016/j.orcp.2021.03.004
148.
Arjunan A Song J . Pharmacological and physiological roles of adipokines and myokines in metabolic-related dementia. Biomed Pharmacother. (2023) 163:114847. 10.1016/j.biopha.2023.114847
149.
Sodhi RK Singh R Bansal Y Bishnoi M Parhar I Kuhad A et al . Intersections in neuropsychiatric and metabolic disorders: Possible Role of TRPA1 Channels. Front Endocrinol. (2021) 12:771575. 10.3389/fendo.2021.771575
150.
Mohammadi-Motlagh HR Sadeghalvad M Yavari N Primavera R Soltani S Chetty S et al . β cell and autophagy: what do we know? Biomolecules. (2023) 13:649. 10.3390/biom13040649
151.
Dreisbach C Morgan H Cochran C Gyamfi A Henderson WA Prescott S et al . Metabolic and microbial changes associated with diet and obesity during pregnancy: what can we learn from animal studies?Front Cell Infect Microbiol. (2022) 11:795924. 10.3389/fcimb.2021.795924
152.
Song WY Wang Y Hou XM Tian CC Wu L Ma XS et al . Different expression and localization of aquaporin 7 and aquaporin 9 in granulosa cells, oocytes, and embryos of patients with polycystic ovary syndrome and the negatively correlated relationship with insulin regulation. Fertil Steril. (2021) 115:463–73. 10.1016/j.fertnstert.2020.08.015
153.
Levine JA Sarrafan-Chaharsoughi Z Patel TP Brady SM Chivukula KK Miller E et al . Effects of colchicine on lipolysis and adipose tissue inflammation in adults with obesity and metabolic syndrome. Obesity (Silver Spring). (2022) 30:358–68. 10.1002/oby.23341
154.
Sanchez Caballero L Gorgogietas V Arroyo MN Igoillo-Esteve M . Molecular mechanisms of β-cell dysfunction and death in monogenic forms of diabetes. Int Rev Cell Mol Biol. (2021) 359:139–256. 10.1016/bs.ircmb.2021.02.005
155.
Semova I Levenson AE Krawczyk J Bullock K Gearing ME Ling AV et al . Insulin Prevents Hypercholesterolemia by Suppressing 12α-Hydroxylated Bile Acids. Circulation. (2022) 145:969–82. 10.1161/CIRCULATIONAHA.120.045373
156.
Nam JS Ahn CW Park HJ Kim YS . Semaphorin 3 C is a novel adipokine representing exercise-induced improvements of metabolism in metabolically healthy obese young males. Sci Rep. (2020) 10:10005. 10.1038/s41598-020-67004-7
157.
Hamidovic A Karapetyan K Serdarevic F Choi SH Eisenlohr-Moul T Pinna G et al . Higher circulating cortisol in the follicular vs. luteal phase of the menstrual cycle: a meta-analysis. Front Endocrinol (Lausanne). (2020) 11:311. 10.3389/fendo.2020.00311
158.
Stroud LR Papandonatos GD Jao NC Vergara-Lopez C Huestis MA Salisbury AL et al . Prenatal tobacco and marijuana co-use: sex-specific influences on infant cortisol stress response. Neurotoxicol Teratol. (2020) 79:106882. 10.1016/j.ntt.2020.106882
159.
Jensterle M Herman R JaneŽ A Mahmeed WA Al-Rasadi K Al-Alawi K et al . The relationship between COVID-19 and hypothalamic-pituitary-adrenal axis: a large spectrum from glucocorticoid insufficiency to excess-the CAPISCO international expert panel. Int J Mol Sci. (2022) 23:7326. 10.3390/ijms23137326
160.
Moyers SA Hagger MS . Physical activity and cortisol regulation: a meta-analysis. Biol Psychol. (2023) 179:108548. 10.1016/j.biopsycho.2023.108548
161.
De Nys L Anderson K Ofosu EF Ryde GC Connelly J Whittaker AC et al . The effects of physical activity on cortisol and sleep: a systematic review and meta-analysis. Psychoneuroendocrinology. (2022) 143:105843. 10.1016/j.psyneuen.2022.105843
162.
Bhatt S Hillmer AT Rusowicz A Nabulsi N Matuskey D Angarita GA et al . Imaging brain cortisol regulation in PTSD with a target for 11β-hydroxysteroid dehydrogenase type 1. J Clin Invest. (2021) 131:e150452. 10.1172/JCI150452
163.
Perrone L Frost A Kuzava S Nissim G Vaccaro S Rodriguez M et al . Indicators of deprivation predict diurnal cortisol regulation during infancy. Dev Psychol. (2021) 57:200–10. 10.1037/dev0000966
164.
Galbally M van Rossum EFC Watson SJ de Kloet ER Lewis AJ . Trans-generational stress regulation: Mother-infant cortisol and maternal mental health across the perinatal period. Psychoneuroendocrinology. (2019) 109:104374. 10.1016/j.psyneuen.2019.104374
165.
Sopi D Amin S Ron S Satish T Carnahan P . Proportional-derivative control of cortisol for treatment of PTSD. Annu Int Conf IEEE Eng Med Biol Soc. (2023) 2023:1–4. 10.1109/EMBC40787.2023.10340805
166.
Garnett M Bernard K Hoye J Zajac L Dozier M . Parental sensitivity mediates the sustained effect of Attachment and Biobehavioral Catch-up on cortisol in middle childhood: a randomized clinical trial. Psychoneuroendocrinology. (2020) 121:104809. 10.1016/j.psyneuen.2020.104809
167.
Nazzari S Fearon P Rice F Molteni M Frigerio A . Maternal caregiving moderates the impact of antenatal maternal cortisol on infant stress regulation. J Child Psychol Psychiatry. (2022) 63:871–80. 10.1111/jcpp.13532
168.
Sohrab SS Raj R Nagar A Hawthorne S Paiva-Santos AC Kamal MA et al . Chronic inflammation's transformation to cancer: a nanotherapeutic paradigm. Molecules. (2023) 28:4413. 10.3390/molecules28114413
169.
Ajala ON Everett BM . Targeting inflammation to reduce residual cardiovascular risk. Curr Atheroscler Rep. (2020) 22. 10.1007/s11883-020-00883-3
170.
Ridker PM MacFadyen JG Glynn RJ Bradwin G Hasan AA Rifai N et al . Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur Heart J. (2020) 41:2952–61. 10.1093/eurheartj/ehaa160
171.
Alwi I . Targeting inflammation and immune system in acute myocardial infarction. Acta Med Indones. (2019) 51:287–9.
172.
Bach RR Rudquist RR . Gulf war illness inflammation reduction trial: A phase 2 randomized controlled trial of low-dose prednisone chronotherapy, effects on health-related quality of life. PLoS ONE. (2023) 18:e0286817. 10.1371/journal.pone.0286817
173.
Liu C Yan P Xu X Zhou W Prakash DR Wang S et al . In vivo kidney allograft endothelial specific scavengers for on-site inflammation reduction under antibody-mediated rejection. Small. (2022) 18:e2106746. 10.1002/smll.202106746
174.
Gao Q Ma R Shi L Wang S Liang Y Zhang Z et al . Anti-glycation and anti-inflammatory activities of anthocyanins from purple vegetables. Food Funct. (2023) 14:2034–44. 10.1039/D2FO03645B
175.
Dube G Tiamiou A Bizet M Boumahd Y Gasmi I Crake R et al . Methylglyoxal: a novel upstream regulator of DNA methylation. J Exp Clin Cancer Res. (2023) 42. 10.1186/s13046-023-02637-w
176.
Sun ME Zheng Q . The Tale of DJ-1 (PARK7): A swiss army knife in biomedical and psychological research. Int J Mol Sci. (2023) 24:7409. 10.3390/ijms24087409
177.
Sharma H Kim DY Shim KH Sharma N An SSA . Multi-targeting neuroprotective effects of syzygium aromaticum bud extracts and their key phytocompounds against neurodegenerative diseases. Int J Mol Sci. (2023) 24:8148. 10.3390/ijms24098148
178.
Ali MY Park SE Seong SH Zamponi GW Jung HA Choi JS et al . Ursonic acid from Artemisia montana exerts anti-diabetic effects through anti-glycating properties, and by inhibiting PTP1B and activating the PI3K/Akt signaling pathway in insulin-resistant C2C12 cells. Chem Biol Interact. (2023) 376:110452. 10.1016/j.cbi.2023.110452
179.
Ali M Barakat A El-Faham A Al-Rasheed HH Dahlous K Al-Majid AM et al . Synthesis and characterisation of thiobarbituric acid enamine derivatives, and evaluation of their α-glucosidase inhibitory and anti-glycation activity. J Enzyme Inhib Med Chem. (2020) 35:692–701. 10.1080/14756366.2020.1737045
180.
Shin S Lee J Yoon SH Park D Hwang JS Jung E et al . Anti-glycation activities of methyl gallate in vitro and in human explants. J Cosmet Dermatol. (2022) 21:2602–9. 10.1111/jocd.14406
181.
Knoblich C Dunckelmann K Krüger A Küper T Blatt T Weise JM et al . N-acetyl-L-hydroxyproline—a potent skin anti-ageing active preventing advanced glycation end-product formation in vitro and ex vivo. Int J Cosmet Sci. (2024) 46:297–306. 10.1111/ics.12930
182.
Wongwad E Jadsadajerm S Mungmai L Wisetsai A . Antioxidant, cytotoxic, anti-glycation, and anti-tyrosinase compounds from the leaves of uvaria siamensis. Chem Biodivers. (2024) 21:e202400319. 10.1002/cbdv.202400319
183.
Anaga N Lekshmy K Purushothaman J . (+)-Catechin mitigates impairment in insulin secretion and beta cell damage in methylglyoxal-induced pancreatic beta cells. Mol Biol Rep. (2024) 51:434. 10.1007/s11033-024-09338-3
184.
Wang L Jiang Y Zhao C . The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp Dermatol. (2024) 33:e15065. 10.1111/exd.15065
185.
Wang S Yang Y Xiao D Zheng X Ai B Zheng L et al . Polysaccharides from banana (Musa spp.) blossoms: Isolation, identification and anti-glycation effects. Int J Biol Macromol. (2023) 236:123957. 10.1016/j.ijbiomac.2023.123957
186.
Wani MJ Salman KA Moin S Arif A . Effect of crocin on glycated human low-density lipoprotein: a protective and mechanistic approach. Spectrochim Acta A Mol Biomol Spectrosc. (2023) 286:121958. 10.1016/j.saa.2022.121958
187.
Liao X Brock AA Jackson BT Greenspan P Pegg RB . The cellular antioxidant and anti-glycation capacities of phenolics from Georgia peaches. Food Chem. (2020) 316:126234. 10.1016/j.foodchem.2020.126234
188.
Thakur MR Tupe RS . Protective effect of colchicine on albumin glycation and cellular oxidative stress: Insights into diabetic cardiomyopathy. J Biochem Mol Toxicol. (2024) 38:e23664. 10.1002/jbt.23664
189.
Liu JJ Wang ZY Jiang BB Gao SQ Lin YW . Protective effect of thymoquinone on glycation of human myoglobin induced by d-ribose. Int J Biol Macromol. (2023) 253:127016. 10.1016/j.ijbiomac.2023.127016
190.
Zhou H Zhou L Li B Yue R . Anti-cyclooxygenase, anti-glycation, and anti-skin aging effect of Dendrobium officinale flowers' aqueous extract and its phytochemical validation in aging. Front Immunol. (2023) 14:1095848. 10.3389/fimmu.2023.1095848
191.
Anjum S Khan AK Qamar A Fatima N Drouet S Renouard S et al . Light tailoring: impact of UV-C irradiation on biosynthesis, physiognomies, and clinical activities of morus macroura-mediated monometallic (Ag and ZnO) and bimetallic (Ag-ZnO) nanoparticles. Int J Mol Sci. (2021) 22:11294. 10.3390/ijms222011294
192.
Mori Y Hiromura M Terasaki M Kushima H Ohara M Fukui T et al . Very rare case of Graves' disease with resistance to methimazole: a case report and literature review. J Int Med Res. (2021) 49:300060521996192. 10.1177/0300060521996192
193.
Takehana N Fukui T Mori Y Hiromura M Terasaki M Ohara M et al . Comparison of positive rates between glutamic acid decarboxylase antibodies and ElisaRSR™ 3 Screen ICA™ in recently obtained sera from patients who had been previously diagnosed with slowly progressive type 1 diabetes. J Diabetes Investig. (2023) 14:856–63. 10.1111/jdi.14016
194.
Khan MWA Al Otaibi A Sherwani S Khan WA Alshammari EM Al-Zahrani SA et al . Glycation and Oxidative Stress Increase Autoantibodies in the Elderly. Molecules. (2020) 25:3675. 10.3390/molecules25163675
195.
Khan MWA Otaibi AA Alsukaibi AKD Alshammari EM Al-Zahrani SA Sherwani S et al . Biophysical, biochemical, and molecular docking investigations of anti-glycating, antioxidant, and protein structural stability potential of garlic. Molecules. (2022) 27:1868. 10.3390/molecules27061868
196.
Mehmood H Haroon M Akhtar T Woodward S Haq S Alshehri M et al . Synthesis, anti-diabetic profiling and molecular docking studies of 2-(2-arylidenehydrazinyl)thiazol-4(5H)-ones. Future Med Chem. (2024) 16:1255–66. 10.1080/17568919.2024.2342700
Summary
Keywords
biological drug delivery system, translational research, personalized medicine, epigenetics, nutraceuticals, physical stimuli, medication adherence, inflammation reduction
Citation
Gaspary JFP, Lopes LFD and Camara AG (2025) Bridging epigenetics and pharmacology through systematic reviews tailored to WBS methodology: the triangle decision-making model as a pioneering translational biological drug delivery system. Front. Med. 12:1552904. doi: 10.3389/fmed.2025.1552904
Received
29 December 2024
Accepted
16 April 2025
Published
20 May 2025
Volume
12 - 2025
Edited by
Zili Xie, Icahn School of Medicine at Mount Sinai, United States
Reviewed by
Anca Lucia Pop, Carol Davila University of Medicine and Pharmacy, Romania
Guiyang Zhang, Anhui Medical University, China
Updates
Copyright
© 2025 Gaspary, Lopes and Camara.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Felipe Dias Lopes lflopes67@yahoo.com.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.