- 1Division of Nephrology, Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Division of Pneumology, Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Division of Nephrology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objectives: To investigate the longitudinal D-dimer trajectories in hospitalized acute kidney injury (AKI) patients and analyze their association with in-hospital mortality risk.
Methods: A retrospective study was conducted using data from AKI patients admitted to Tongji Hospital (July 2012–April 2024). General information, laboratory results, and outcomes were extracted from the medical record system. Patients with at least three D-dimer measurements within 30 days after AKI onset were included. Several latent class trajectory models (LCTMs) were constructed to identify distinct longitudinal dynamic trajectories of D-dimer. Model fit was assessed using Akaike Information Criterion, Bayesian information criterion, entropy, category probability and the optimal model was selected. Logistic regression and Kaplan-Meier survival analysis were employed to evaluate the relationship between D-dimer trajectories and in-hospital mortality. Subgroup analyses were performed to explore potential interactions between D-dimer trajectories and other variables.
Results: Based on LCTMs evaluation, the model fitting indices were comprehensively analyzed, and a two-class model was identified as the optimal LCTM. The dynamic trajectories revealed two distinct patterns: an early peak followed by a gradual decline and a low-level continuous stability after AKI onset. Accordingly, patients were categorized into the high-peak decline group and the sustained low-level group. Logistic regression analysis demonstrated that AKI patients in the high-peak decline group had a significantly increased risk of in-hospital mortality (OR 2.27, 95% CI: 1.94–2.65). Kaplan-Meier survival curves indicated a reduced in-hospital survival rate in the high-peak decline group (p < 0.05). Subgroup analyses showed that, across age, gender, chronic kidney disease, cancer, surgery, myocardial infarction, and cerebral infarction subgroups, the high-peak decline group exhibited a significantly elevated risk of in-hospital mortality (p < 0.05), with no significant interaction effects observed among subgroups (p > 0.05).
Conclusion: Using LCTM analysis, it was determined that D-dimer exhibits two characteristic longitudinal dynamic trajectories following AKI onset: an early peak followed by a gradual decline and a continuous low-level stability. Among these, the trajectory characterized by an early peak followed by a decline in AKI patients was associated with an increased risk of in-hospital mortality and reduced in-hospital survival, independent of age, gender, chronic kidney disease, cancer, surgery, myocardial infarction, or cerebral infarction.
1 Introduction
Acute Kidney Injury (AKI) is a prevalent critical illness associated with high mortality rates among hospitalized patients. The etiology of AKI is multifactorial, influenced by factors such as age, chronic kidney disease (CKD), pulmonary emboli, surgery and cancer. According to epidemiological data, approximately 13.3 million patients are diagnosed with AKI annually, resulting in 1.7 million deaths, with particularly high mortality observed in intensive care unit patients with AKI (1). Multinational and multicenter studies have demonstrated that the in-hospital mortality rate for AKI patients exceeds 60% (2). Moreover, the economic burden imposed by AKI-related mortality far surpasses that of other major diseases, including breast cancer, diabetes, and heart failure (3). Consequently, identifying a biomarker for assessing mortality risk in hospitalized AKI patients holds substantial clinical significance (4).
D-dimer, a soluble degradation product of cross-linked fibrin mediated by plasmin, is an emerging marker of AKI (5). Studies indicate that baseline D-dimer levels are closely associated with the risk of AKI-related mortality. Specifically, an increase in baseline D-dimer levels (D-dimer > 1,108 ng/mL) significantly elevates the risk of death in AKI patients, suggesting its potential as a predictive biomarker for AKI mortality risk (6). Importantly, as an economical, portable, and rapid biomarker, the longitudinal dynamic changes in D-dimer levels offer significant value in evaluating various diseases. For instance, cohort studies on cancer have shown that continuous monitoring of D-dimer dynamics before and after anticoagulant and thrombolytic therapy reveals that 70% of survivors exhibit a decrease in D-dimer levels. Conversely, patients without a reduction in D-dimer levels demonstrate a higher risk of mortality and systemic metastasis (7). However, current studies examining the relationship between D-dimer and AKI prognosis are limited to single-time-point measurements and fixed-threshold grouping, which may miss some key prognostic information.
Latent Class Trajectory Model (LCTM), an advanced statistical method derived from structural equation modeling, fits potential trajectory subgroups within observed data to intuitively describe the developmental trends of D-dimer and uncover its time-dependent patterns. By grouping patients based on distinct trajectories, LCTM addresses the limitations of traditional mean-based standard deviation approaches, which often neglect individual variability (8). This model has demonstrated significant value in various fields, including cognitive impairment (9), dietary intake (10), cancer (11), diabetes (12). Additionally, LCTM has been applied to AKI research, analyzing the relationship between serum creatinine change trajectories and AKI prognosis (13). In a prospective cohort study, the dynamic changes in renal tissue oxygen saturation were analyzed using a latent class model, revealing that patients with a gradual downward trend in renal tissue oxygen saturation exhibited a higher incidence of postoperative AKI, shorter survival, and increased mortality compared to those with continuously high levels (14).
Therefore, this retrospective cohort study introduces LCTM to analyze the dynamic change trajectory of D-dimer following AKI onset. By evaluating D-dimer levels at different time points, identifying potential change trends, and fitting distinct developmental trajectories, we aim to further assess the relationship between D-dimer dynamics and the risk of in-hospital mortality in AKI patients. This analysis provides a theoretical foundation for optimizing AKI treatment strategies.
2 Patients and methods
2.1 Patient characteristics
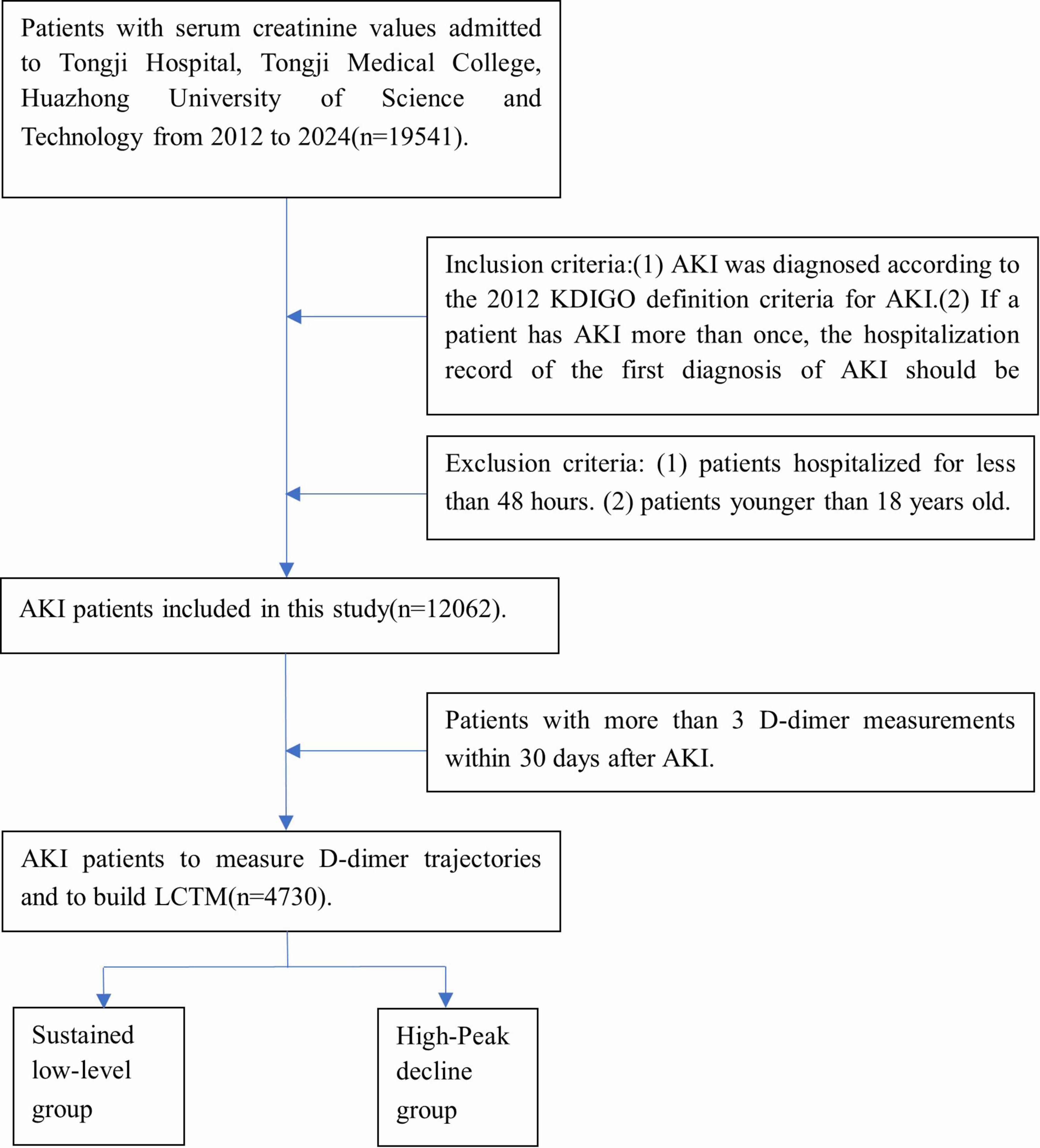
This is a retrospective cohort study. The clinical data of AKI patients admitted to Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology from July 2012 to April 2024 were collected. Informed consent was exempted because of the retrospective nature of this study, and the study protocol was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB202502003). According to the following criteria, AKI patients were screened and a total of 4,730 patients were included.
Inclusion criteria: (1) AKI was diagnosed according to the 2012 KDIGO definition criteria for AKI (15): increased serum creatinine ≥ 0.3 mg/dL (≥ 26.5 μmol/L) within 48 h; An increase in serum creatinine to 1.5 or more times the baseline value, with known or presumed onset within the previous 7 days. (2) If a patient has AKI more than once, the hospitalization record of the first diagnosis of AKI should be retained. (3) Patients with more than 3 D-dimer measurements within 30 days after AKI. Exclusion criteria: patients hospitalized for less than 48 h. (4) Patients younger than 18 years old (Figure 1).
2.2 General information
The medical history data of the patients were collected through the electronic medical record data in the inpatient system. The general information included Length of hospital stay, Age, Sex, In-hospital mortality, comorbidities including Hypertension, Diabetes mellitus, Cancer, CKD, Myocardial Infarction, Cerebral Infarction, Blood disease, Pulmonary emboli, Surgery and Renal Replacement Therapy (RRT). Medication history included Heparin and Warfarin, Antiplatelet Agents, Thrombolytic Agents, Diuretics, and Chemotherapy drugs.
Laboratory parameters included White Blood Cell (WBC) count, Neutrophil count, Lymphocyte count, Hemoglobin, Platelet count, Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Albumin, Cholesterol, Total Bilirubin, Direct Bilirubin, Lactate dehydrogenase (LDH), Blood Urea Nitrogen (BUN), Serum Creatinine, Estimated Glomerular Filtration Rate (eGFR), Bicarbonate ion, and Calcium. Coagulation functions included Prothrombin Time (PT), Prothrombin Activity (PTA), International Normalized Ratio (INR), Activated Partial Thromboplastin Time (APTT), Thrombin Time (TT), Fibrinogen, and D-dimer.
D-dimer was detected by STAGO specific immunoturbidimetric assay, and sodium citrate was used for collection tube anticoagulation [Reference range: 0–0.5 μg/ml FEU (fibrinogen equivalent unit)].
2.3 Statistical analysis
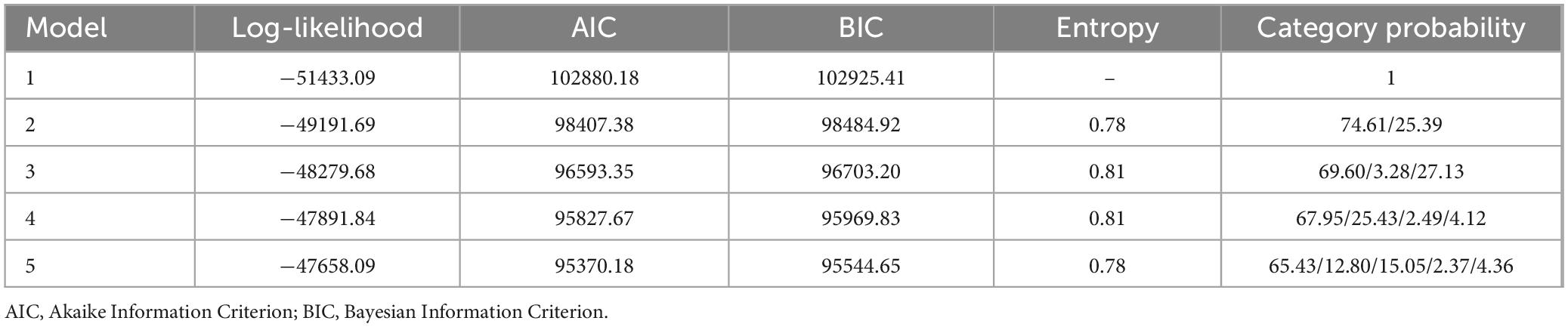
For inpatients with AKI, D-dimer values were measured at least three times within 30 days following the onset of AKI for each patient. Given the varying time intervals between D-dimer measurements among patients, a standardized detection interval of 2 days was established. The mean value of all D-dimer measurements within each 2 days interval was calculated. Using the JMbayes package in R software (version 4.3.2), LCTMs with 1–5 classes were constructed, generating 1–5 subgroups representing distinct dynamic trajectory trends of D-dimer levels (referred to as D-dimer clusters) (Figure 1). These LCTMs were evaluated based on model fit using several indicators, including Log-likelihood value, Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), entropy, and Category probability. A higher log-likelihood value indicates better model-data fit, while lower AIC and BIC values suggest superior model performance. Entropy ranges from 0 to 1, with higher values reflecting better classification accuracy. Category probability represents the likelihood that an individual belongs to a specific D-dimer trajectory subgroup. Typically, the Category probability in each trajectory subgroup should not fall below 5%. Based on these evaluations, the optimal LCTM was selected, and a dynamic trajectory graph was generated. Patients were subsequently categorized according to their D-dimer trajectory patterns. Continuous variables were expressed as medians and quartiles and were analyzed by Kruskal-Wallis H test. Categorical variables were expressed as frequencies and percentages, and the chi-square test was used. Logistic regression analysis and Kaplan-Meier survival curves were employed to investigate the association between different D-dimer trajectory trends and in-hospital mortality risk among AKI patients. Subgroup analyses were conducted to explore the relationships between various covariates and D-dimer trajectory trends. All statistical tests were performed with the use of R software. p < 0.05 was considered statistically significant.
3 Results
3.1 Establishment and grouping of D-dimer longitudinal dynamic trajectory models
Sequentially, 1–5 models were established, generating 1–5 subgroups (clusters) representing the dynamic trajectory trends of D-dimer levels. As the number of clusters increased from 2 to 4, the Log-likelihood value progressively increased, while the AIC and BIC decreased. The highest entropy value reached 0.81, indicating a high degree of classification accuracy. However, when the number of clusters increased from 4 to 5, the increment in the Log-likelihood value and the decrement in AIC and BIC became marginal, accompanied by a decrease in entropy, suggesting that further increasing the number of clusters was not appropriate. Given that statistical indicators serve only as a reference for model selection, the interpretability of the model and the sample size distribution across clusters must also be considered. Notably, in the 3-cluster and 4-cluster models, the probability of certain classes fell below 5%, which may compromise the robustness of these models. After comprehensive calculation and analysis, the 2-cluster model was determined to be the optimal fit for LCTM (Table 1).
In the optimal LCTM, the dynamic trajectory trend of D-dimer is categorized into two clusters, namely “Class 1” and “Class 2.” The figure indicates that in the Class 2 cluster, D-dimer levels peak during the early stages of AKI and subsequently exhibit a gradual downward trend, which is termed the “High-Peak decline Group.” In contrast, in the Class 1 cluster, D-dimer levels remain consistently low and stable, referred to as the “Sustained low-level Group” (Figure 2).
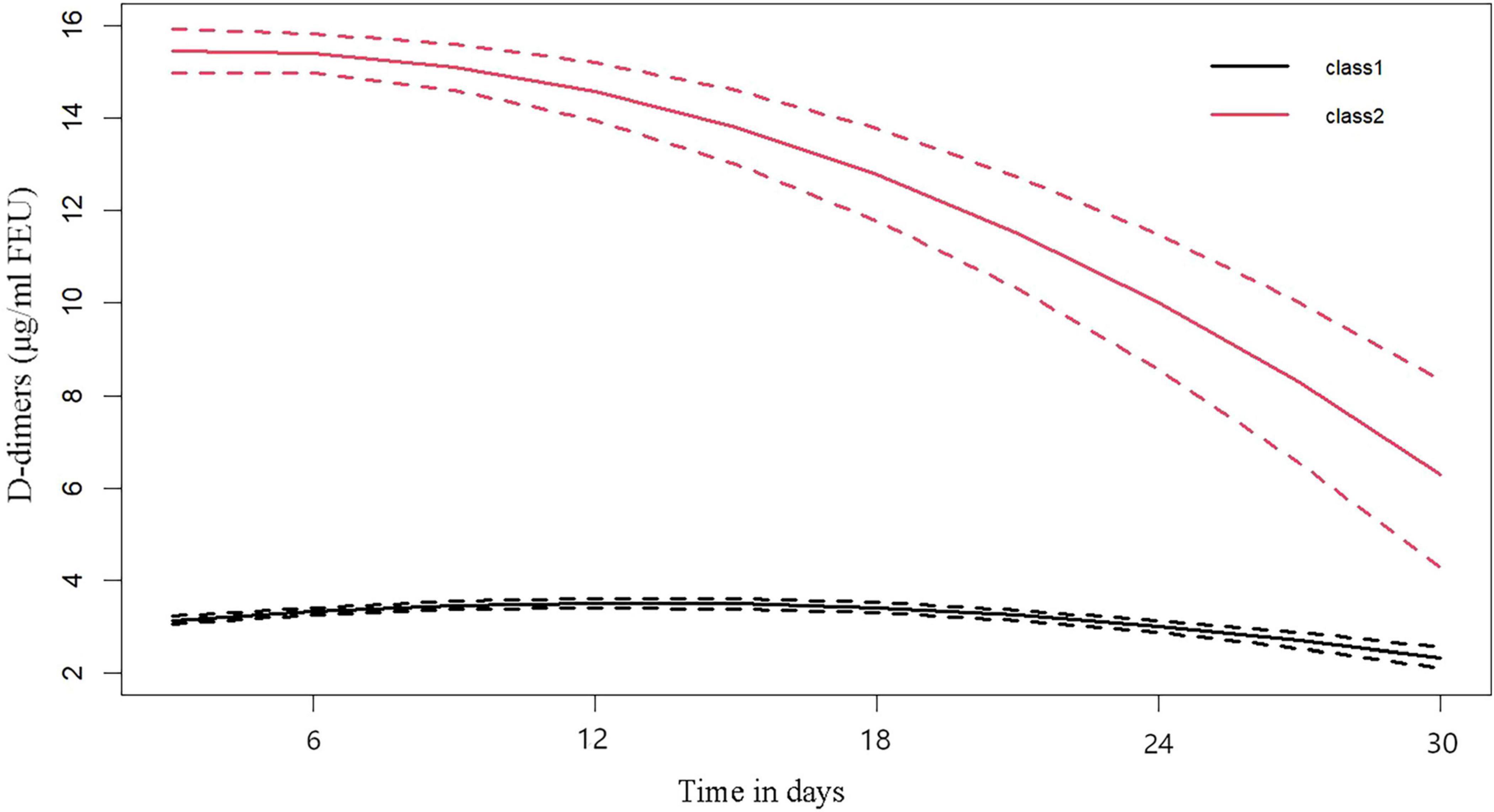
Figure 2. Dimer dynamic trajectories. “class 1” named as the “Sustained low-level group,” “class 2” named as the “high-peak decline group.”
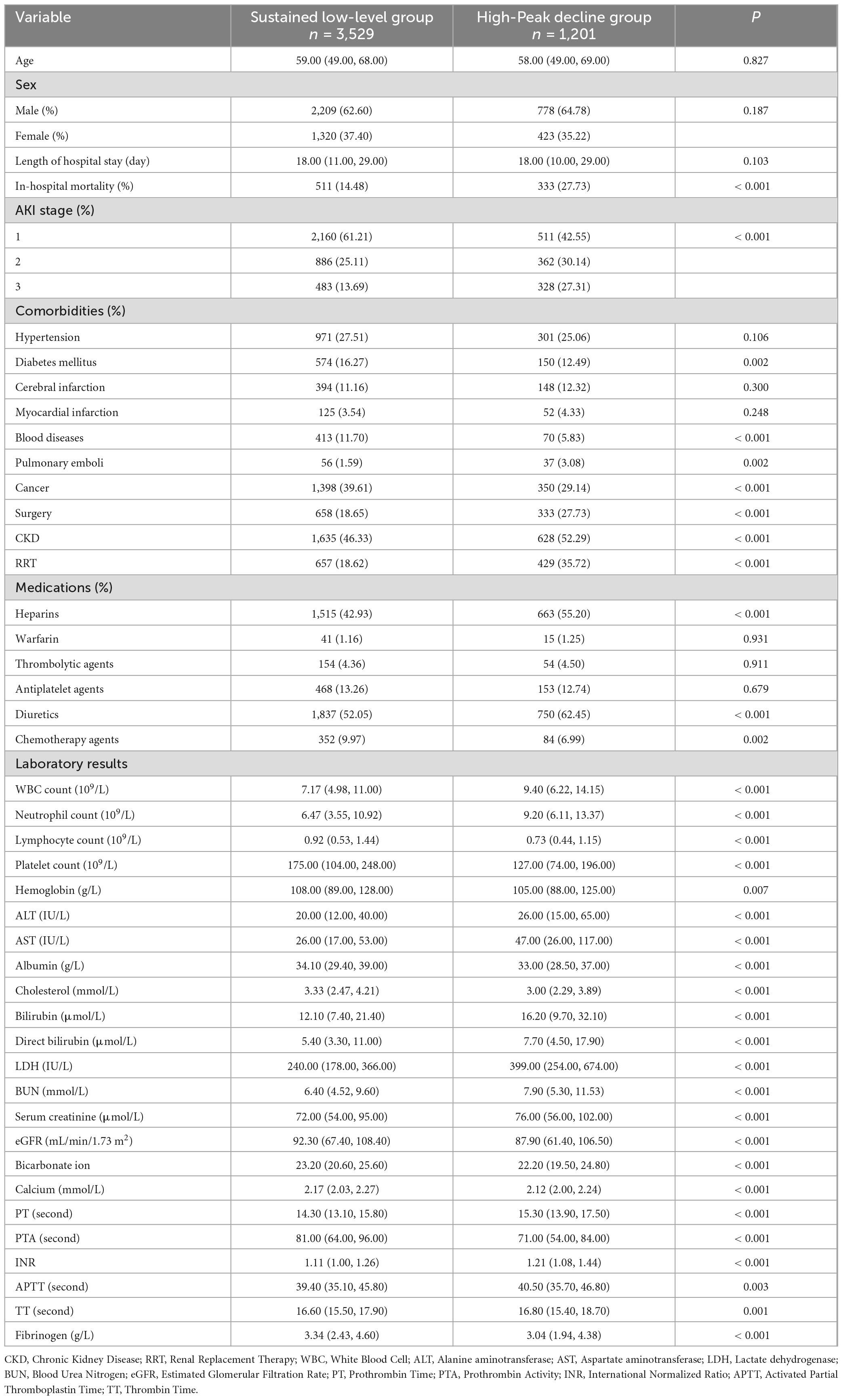
3.2 General characteristics
In-hospital mortality was significantly higher in the high-peak decline group compared to the sustained low-level group. Additionally, during the AKI stage, the proportion of AKI Stage 1 decreased, while the proportions of AKI Stage 2 and AKI Stage 3 increased in the high-peak Decline Group relative to the sustained low-level Group. Furthermore, the high-peak Decline Group exhibited higher proportions of pulmonary emboli, surgical interventions, CKD and RRT. Similarly, the usage rates of medications such as heparin and diuretics were also elevated in this group (Table 2).
3.3 Association of D-dimer with the risk of in-hospital mortality in AKI
By logistic regression analysis, after adjusting for various covariates, we found that in assessing the in-hospital mortality risk of hospitalized AKI patients with different longitudinal dynamic trajectories of D-dimer, compared to the sustained low-level group, the in-hospital mortality risk was significantly higher in the high-peak decline group. Specifically, in the unadjusted model, the risk of in-hospital mortality in the high-peak decline group increased by 2.27 times (95% CI: 1.94–2.65). In Model 2, the important covariates were analyzed by referring to the bidirectional stepwise regression model. After adjustment, the in-hospital mortality risk in the high-peak decline group increased by 1.49 times (95% CI: 1.19–1.86). In Model 3, key covariates were determined based on LASSO regression. After adjustment, the in-hospital mortality risk for AKI patients in the high-peak decline group was 1.78 times (95% CI: 1.48–2.14) higher than that in the sustained low-level group (Table 3).
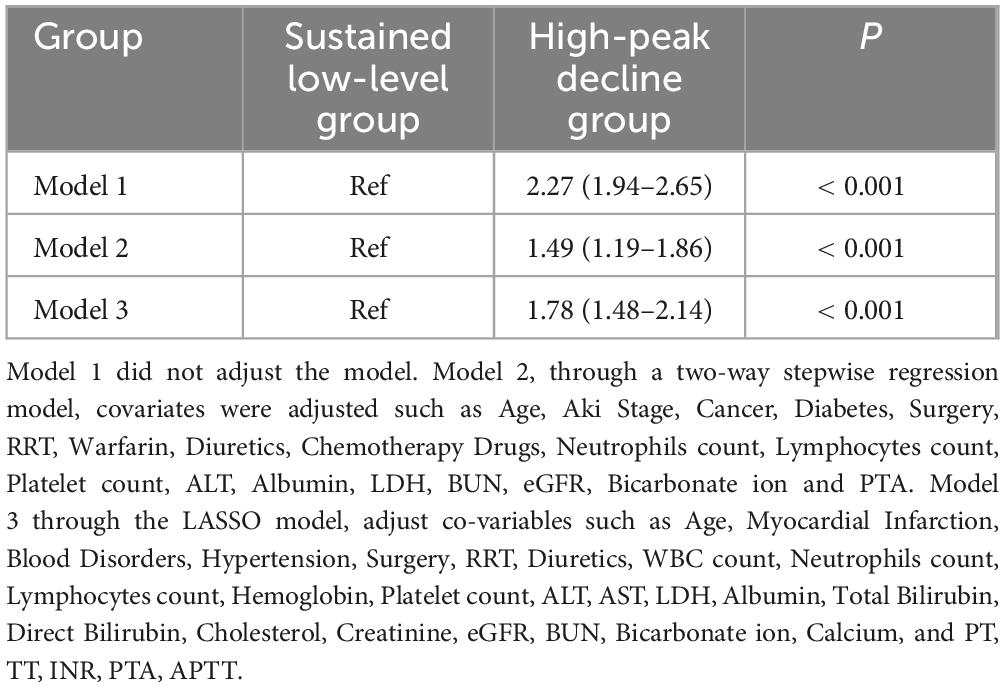
Table 3. Logistics regression analysis of D-dimer dynamic trajectory clusters on the risk of in- in-hospital mortality risk in patients with Acute Kidney Injury (AKI).
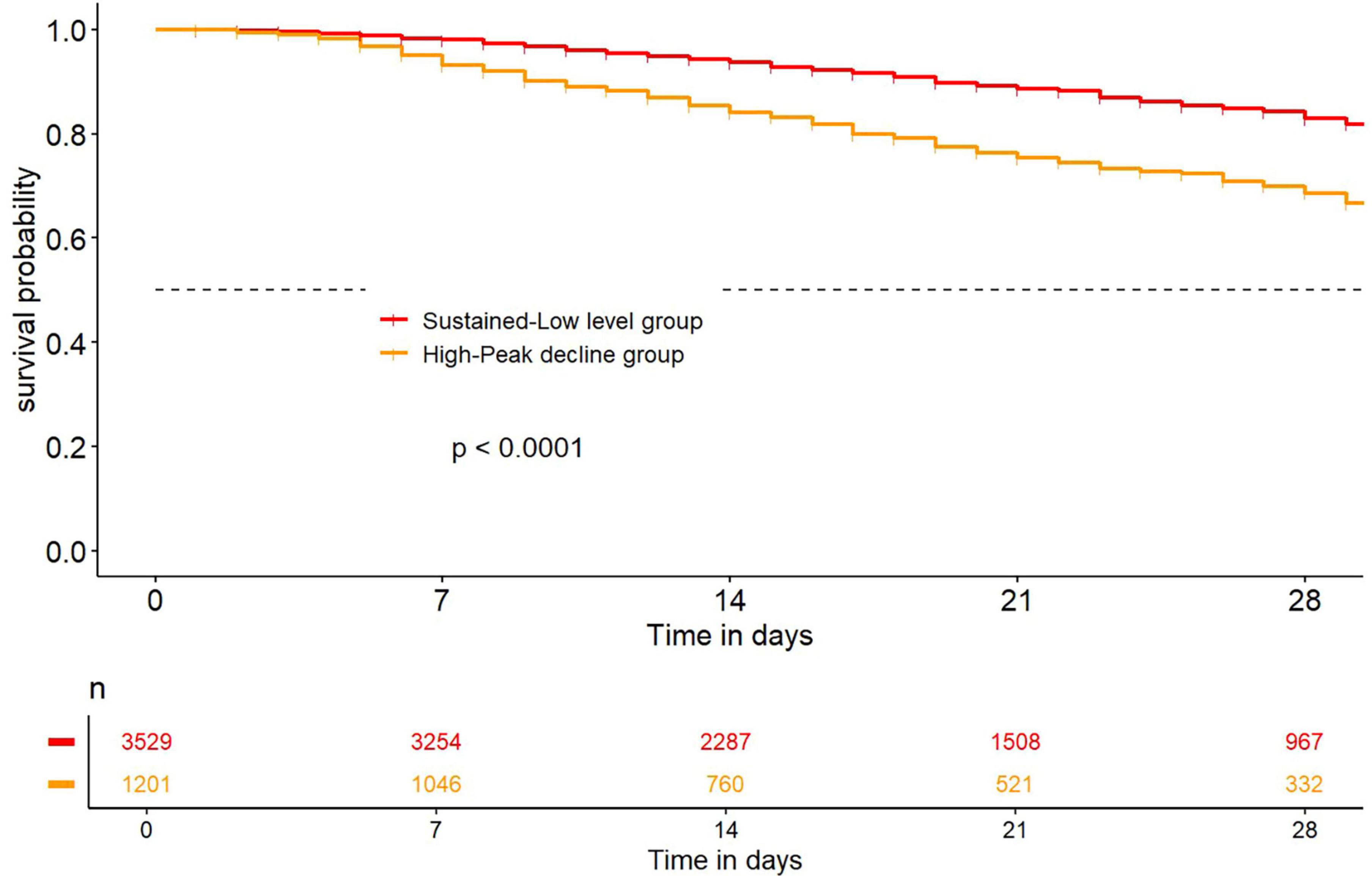
3.4 Kaplan-Meier survival curve
After LCTM analysis, the Kaplan-Meier survival curve showed that the in-hospital survival rate of hospitalized AKI patients in the high-peak decline group was significantly lower than that in the sustained low-level group (p < 0.05), indicating that the in-hospital survival rate was lower when the initial value of D-dimer was high after AKI (Figure 3).
3.5 Subgroup analysis
The high-peak decline group exhibited a significantly higher risk of in-hospital mortality compared to the sustained low-level group, irrespective of whether AKI patients were older than 60 years, male or female, or had comorbidities such as CKD, cancer, surgery, myocardial infarction, or cerebral infarction. No significant interaction was observed among the subgroups (p > 0.05). Specifically, the risk of mortality for patients with AKI combined with myocardial infarction increased by 3.10 times (95% CI: 1.49–6.42), while the risk of in-hospital mortality for patients with AKI combined with cancer increased by 3.09 times (95% CI: 1.85–5.17). Additionally, no significant correlation was found between the in-hospital mortality risk in the high-peak decline group and the sustained low-level group among AKI patients with pulmonary emboli (OR 1.93, 95% CI: 0.67–5.57) (Table 4).
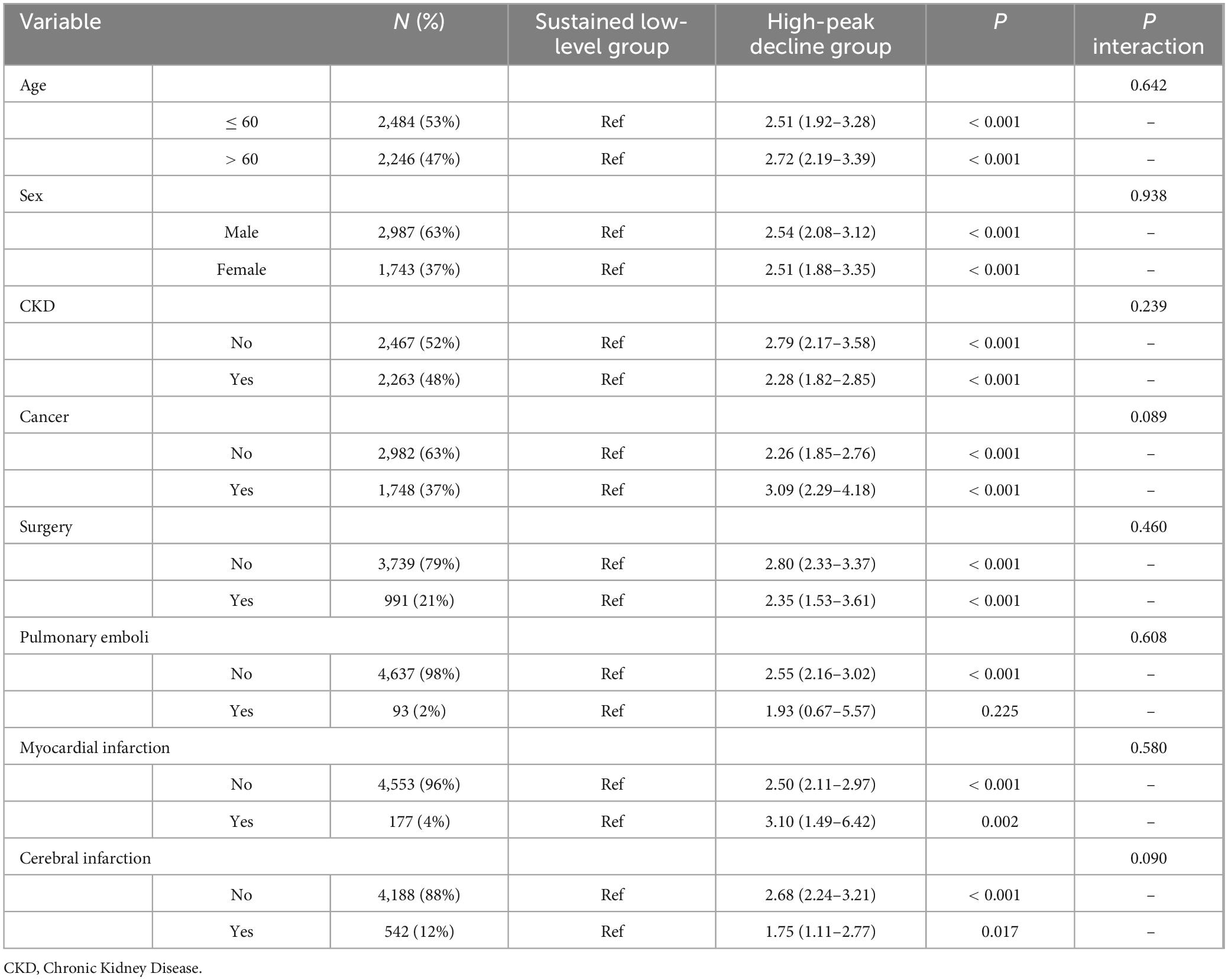
Table 4. Subgroup analysis of the D-dimer dynamic trajectory clusters on the risk of in-hospital mortality risk in patients with Acute Kidney Injury (AKI).
4 Discussion
In this study, 1–5 LCTMs were established. Based on model evaluation indicators such as BIC and AIC, the LCTM in cluster 2 was ultimately selected as the optimal model. The dynamic trajectory diagram revealed that D-dimer levels in the best LCTM exhibited two characteristic evolution trends following AKI onset: the high-peak decline group, characterized by an early peak value followed by a time-dependent gradual decrease, and the sustained low-level group, which maintained a stable state of low values. Logistic regression analysis demonstrated that in-hospital mortality risk in the high-peak decline group was significantly increased (OR 2.27, 95% CI: 1.94–2.65). Kaplan-Meier survival curves further indicated that the short-term hospital survival rate of patients in the high-peak decline group was significantly lower than that in the control group (p < 0.05). Subgroup analysis revealed no significant interaction among subgroups based on age, sex, CKD, cancer, surgery, myocardial infarction, or cerebral infarction (p > 0.05).
Some studies have identified D-dimer as an emerging marker for AKI (5), with its baseline level holding important clinical significance in the occurrence and progression of AKI. Higher baseline D-dimer levels are associated with an increased risk of AKI (16). When the baseline D-dimer level exceeds 2.2 mg/L, it becomes a risk factor for AKI (17). Elevated baseline D-dimer levels can also predict AKI progression, with an Area Under Curve (AUC) of 0.755 (95% CI: 0.718–0.793) (18). Furthermore, D-dimer is strongly associated with mortality risk in AKI patients. Studies have shown that an increase in baseline D-dimer levels (D-dimer > 1,108 ng/mL), particularly when combined with a hypercoagulable state, significantly increases the risk of death in pregnant patients with AKI, with an AUC of 0.828 (95%CI: 0.670–0.986) (6). However, these studies are limited to baseline assessments and suffer from the disadvantage of single-time-point detection or fixed-threshold grouping (19), which fails to reflect the dynamic imbalance between the coagulation system and secondary fibrinolytic system.
By introducing LCTM, this study identified dynamic track changes associated with disease prognosis. Previous studies found that D-dimer exhibited two trends before and after thrombolytic therapy in patients, where patients whose plasma D-dimer levels rapidly increased, peaked, and then rapidly declined to preoperative levels achieved complete recanalization of lower limb arteries (20). Additionally, some studies have shown that COVID-19 patients with stable D-dimer trajectories had hazard ratios (HRs) of 0.29 (95% CI: 0.17–0.49, p < 0.0001) relative to those with increasing D-dimer trajectories for mortality outcomes (21). This study revealed two potential trends in D-dimer dynamics following AKI onset: one where D-dimer peaks early and gradually decreases over time, classified as the high-peak decline group, and another where D-dimer remains consistently low within 30 days post-AKI, classified as the sustained low-level group. Patients with an initial peak D-dimer value exhibit a lower in-hospital survival rate and higher in-hospital mortality risk, suggesting that the early peak in D-dimer during AKI may result from the interaction between enhanced early coagulation function and hyperfibrinolysis or partial dissolution of microthrombi. The subsequent decline could be attributed to therapeutic intervention or compensatory hyperfibrinolysis. Conversely, persistent low-level status indicates minimal coagulation activation and less severe tubular injury. Importantly, this finding remained unaffected by age, sex, CKD, cancer, surgery, myocardial infarction, or cerebral infarction, underscoring the broad universality of the prognostic value of D-dimer dynamic trajectories.
This mechanism aligns with the pathological process of coagulation dysfunction observed in liver cirrhosis with renal injury (22). From a pathophysiological perspective, when the imbalance between coagulation and anticoagulation systems leads to hypercoagulation, the probability of thromboembolism in the renal microcirculation system significantly increases (23). Studies have shown that the hypercoagulable state can cause microvascular fibrin deposition (24), which in turn increases renal microthrombosis (23). The early peak in D-dimer may indicate the formation of renal microthrombi, followed by compensatory activation of the endogenous fibrinolytic system (25). Impaired clearance of microthrombi results in glomerular capillary network ischemia-reperfusion injury. Oxidative stress and inflammatory mediators such as Interleukin- 6 (IL-6) and Tumor Necrosis Factor-α (TNF-α) subsequently promote renal tubular cell apoptosis (26), leading to decreased glomerular filtration rate and renal tubular reabsorption disorder, thereby exacerbating kidney injury (27).
This study has limitations. This is a single-center retrospective cohort study. First, Single-center studies are limited by the geographical location and population distribution of the specific institution. Caution should be exercised when extrapolating the research conclusions to other regions. Second, the retrospective study inherently limits causal inference. Additionally, this retrospective study lacks data on inflammatory markers such as IL-6, C-reactive protein (CRP), and Procalcitonin (PCT), as well as standardized clinical severity scores like SOFA and APACHE II. The absence of these critical variables may have obscured the independent contributions of multi-organ dysfunction and systemic inflammatory responses to in-hospital mortality risk among AKI patients, potentially leading to an overestimation of the predictive value of D-dimer dynamics for mortality risk. Furthermore, AKI diagnosis was based solely on serum creatinine levels without incorporating urine output criteria, which could result in underdiagnosis of early or mild AKI cases. And the lack of follow-up data after discharge also lacks a comprehensive long-term prognosis analysis. Additionally, the study found no significant association between pulmonary emboli and the longitudinal dynamic changes in D-dimer levels. The limited sample size of pulmonary emboli cases in the subgroup analysis may have compromised statistical power, highlighting the need for further investigation with a larger cohort. Future studies should consider adopting a multi-center prospective design, standardizing measurement protocols, systematically collecting urine output data, and integrating multiple dynamic indicators such as coagulation factors and inflammatory markers to strengthen the robustness and clinical relevance of the evidence.
In conclusion, longitudinal dynamic trajectory analysis of D-dimer revealed two distinct trajectories following AKI onset. Compared with the continuously low-level stable trajectory, patients in the early peak value and gradually decreasing trajectory exhibited a lower in-hospital survival rate and a significantly increased risk of in-hospital mortality. These findings were unaffected by age, sex, CKD, cancers, surgery, myocardial infarction, or cerebral infarction. D-dimer offers the advantages of economic and rapid, making it a valuable reference for prognosis evaluation and individualized treatment decisions in AKI patients.
Data availability statement
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (TJ-IRB202502003). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from The clinical data of AKI patients in Tongji Hospital, affiliated with Tongji Medical College of Huazhong University of Science and Technology from July 2012 to April 2024 were retrospectively analyzed. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – original draft. JS: Conceptualization, Formal Analysis, Investigation, Software, Supervision, Writing – review and editing. XZ: Conceptualization, Data curation, Investigation, Software, Supervision, Writing – review and editing. SG: Conceptualization, Formal Analysis, Project administration, Resources, Supervision, Validation, Visualization, Writing – review and editing. NL: Formal Analysis, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank the Ethics Committee of Tongji Hospital for permission for our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ronco C, Bellomo R, Kellum J. Acute kidney injury. Lancet. (2019) 394:1949–64. doi: 10.1016/S0140-6736(19)32563-2
2. Uchino S, Kellum J, Bellomo R, Doig G, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. (2005) 294:813–8. doi: 10.1001/jama.294.7.813
3. Kellum J, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders H. Acute kidney injury. Nat Rev Dis Primers. (2021) 7:52. doi: 10.1038/s41572-021-00284-z
4. Rossiter A, La A, Koyner J, Forni L. New biomarkers in acute kidney injury. Crit Rev Clin Lab Sci. (2024) 61:23–44. doi: 10.1080/10408363.2023.2242481
5. Park J, Kim S, Choi H, Hong S, Chae M. Predictive role of the D-dimer level in acute kidney injury in living donor liver transplantation: A retrospective observational cohort study. J Clin Med. (2022) 11:450. doi: 10.3390/jcm11020450
6. Wang B, Jiang Q, Wu X. Association of D-dimers with acute kidney injury in pregnant women: A retrospective study. J Int Med Res. (2020) 48:300060520966899. doi: 10.1177/0300060520966899
7. Nam K, Kim C, Kim T, An S, Oh K, Mo H, et al. Predictors of 30-day mortality and the risk of recurrent systemic thromboembolism in cancer patients suffering acute ischemic stroke. PLoS One. (2017) 12:e0172793. doi: 10.1371/journal.pone.0172793
8. Lennon H, Kelly S, Sperrin M, Buchan I, Cross A, Leitzmann M, et al. Framework to construct and interpret latent class trajectory modelling. BMJ Open. (2018) 8:e020683. doi: 10.1136/bmjopen-2017-020683
9. Du M, Tao L, Liu M, Liu J. Trajectories of health conditions and their associations with the risk of cognitive impairment among older adults: Insights from a national prospective cohort study. BMC Med. (2024) 22:20. doi: 10.1186/s12916-024-03245-x
10. Zhao C, Wang Y, Jia X, Fan J, Wang N, Yang Y, et al. Associations of dietary diversity trajectories with frailty among Chinese older adults: A latent class trajectory analysis based on a CLHLS cohort. Nutrients. (2024) 16:1445. doi: 10.3390/nu16101445
11. Jensen B, Watson C, Geifman N, Baker J, Badrick E, Renehan A. Weight changes in type 2 diabetes and cancer risk: A latent class trajectory model study. Obes Facts. (2022) 15:150–9. doi: 10.1159/000520200
12. Raghavan S, Liu W, Berkowitz S, Barón A, Plomondon M, Maddox T, et al. Association of glycemic control trajectory with short-term mortality in diabetes patients with high cardiovascular risk: A joint latent class modeling study. J Gen Intern Med. (2020) 35:2266–73. doi: 10.1007/s11606-020-05848-5
13. Takkavatakarn K, Oh W, Chan L, Hofer I, Shawwa K, Kraft M, et al. Machine learning derived serum creatinine trajectories in acute kidney injury in critically ill patients with sepsis. Crit Care. (2024) 28:156. doi: 10.1186/s13054-024-04935-x
14. Liu C, Wang X, Shi W, Yu Y, Sha X, Wang P, et al. The relationship between trajectories of renal oxygen saturation and acute kidney injury: A prospective cohort study with a secondary analysis. Aging Clin Exp Res. (2024) 36:46. doi: 10.1007/s40520-024-02701-1
15. Chawla L, Bellomo R, Bihorac A, Goldstein S, Siew, Bagshaw S, et al. Acute kidney disease and renal recovery: Consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. (2017) 13:241–57. doi: 10.1038/nrneph.2017.2
16. Han H, Li J, Chen D, Zhang F, Wan X, Cao CA. Clinical risk scoring system of acute respiratory distress syndrome-induced acute kidney injury. Med Sci Monit. (2019) 25:5606–12. doi: 10.12659/MSM.915905
17. Liu J, Su Z, Zhang Z. A multiplicity of morbidity and prognostic factors in patients with septic and acute kidney injury. Zhonghua Yi Xue Za Zhi. (2011) 91:1112–4. doi: 10.3760/cma.j.issn.0376-2491.2011.16.009
18. Chen B, Liu J, Li Y, He X, Zhou C, Chen Y, et al. Elevated D-Dimer levels correlate with the development of hepatorenal syndrome and a poor outcome in patients with cirrhosis. Scand J Gastroenterol. (2022) 57:1486–93. doi: 10.1080/00365521.2022.2098051
19. Lu Y, Dong J, Sun Y, Hu L, Liu Y, Chu X, et al. Gender-specific predictive ability for the risk of hypertension incidence related to baseline level or trajectories of adiposity indices: A cohort study of functional community. Int J Obesity. (2005) 46:1036–43. doi: 10.1038/s41366-022-01081-8
20. Liu X, Xie H, Zheng G, Liu Y. The plasma D-dimer trends and their value in acute lower limb ischemia patients treated by catheter directed thrombolysis. Sci Rep. (2021) 11:10388. doi: 10.1038/s41598-021-89905-x
21. Naymagon L, Zubizarreta N, Feld J, van Gerwen M, Alsen M, Thibaud S, et al. Admission D-dimer levels, D-dimer trends, and outcomes in COVID-19. Thromb Res. (2020) 196:99–105. doi: 10.1016/j.thromres.2020.08.032
22. Ciavarella A, Gnocchi D, Custodero C, Lenato G, Fiore G, Sabbà C, et al. Translational insight into prothrombotic state and hypercoagulation in nonalcoholic fatty liver disease. Thromb Res. (2021) 198:139–50. doi: 10.1016/j.thromres.2020.12.002
23. Verma S, Molitoris B. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol. (2015) 35:96–107. doi: 10.1016/j.semnephrol.2015.01.010
24. Malyszko J, Malyszko J, Pawlak D, Pawlak K, Buczko W, Mysliwiec M, et al. Hemostasis, platelet function and serotonin in acute and chronic renal failure. Thrombosis Res. (1996) 83:351–61. doi: 10.1016/0049-3848(96)00145-4
25. Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. (2019) 15:87–108. doi: 10.1038/s41581-018-0098-z
26. Iba T, Helms J, Maier C, Levi M, Scarlatescu E, Levy J. The role of thromboinflammation in acute kidney injury among patients with septic coagulopathy. J Thromb Haemost. (2024) 22:1530–40. doi: 10.1016/j.jtha.2024.02.006
Keywords: acute kidney injury, D-dimer, latent class trajectory model, in-hospital mortality risk, dynamic trajectories
Citation: Rao L, Sun J, Zhao X, Ge S and Li N (2025) Association between D-dimer and in-hospital mortality risk in Acute Kidney Injury based on latent class dynamic trajectory. Front. Med. 12:1554213. doi: 10.3389/fmed.2025.1554213
Received: 01 January 2025; Accepted: 28 April 2025;
Published: 02 June 2025.
Edited by:
Rodrigo Assar, University of Chile, ChileReviewed by:
Lirane Elize Defante Ferreto, Western Paraná State University, BrazilGonzalo Briceño-Mayorga, Hospital San Juan de Dios, Chile
Copyright © 2025 Rao, Sun, Zhao, Ge and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ningxu Li, bGluaW5neHVAaHVzdC5lZHUuY24=; Shuwang Ge, Z2VzaHV3YW5nQHRqaC50am11LmVkdS5jbg==
†These authors have contributed equally to this work and share last authorship
 Lixiang Rao
Lixiang Rao Jiazheng Sun
Jiazheng Sun Xingyang Zhao3
Xingyang Zhao3 Shuwang Ge
Shuwang Ge