Abstract
Background:
Recent studies have investigated the relationship between cadmium exposure and kidney stones. Nevertheless, the results remain controversial. Therefore, we performed a comprehensive systematic review and meta-analysis based on the latest evidence to address gaps in the research.
Methods:
Medline, Embase, and the Web of Science databases were searched to identify relevant studies up until 31 July 2024. Characteristics and outcomes of the included studies were extracted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Meta-analyses of Observational Studies in Epidemiology (MOOSE) guidelines. A random effects model was used to determine the association between cadmium exposure and the risk of kidney stones.
Results:
A total of 17 studies involving 159,011 individuals were included in the meta-analysis. When comparing the highest versus lowest cadmium exposure levels, the overall relative risk (RR) for kidney stones was 1.19 [95% confidence interval (CI): 1.10–1.29]. Subgroup analysis showed that urinary (RR = 1.19; 95%CI: 1.08–1.30) and blood (RR = 1.49; 95% CI: 1.10–2.02) cadmium levels were associated with an increased risk of kidney stones. In contrast to non-cadmium-contaminated areas, both blood (RR = 1.08; 95% CI: 1.00–1.15) and urinary (RR = 1.16; 95% CI: 1.05–1.27) cadmium levels were associated with an increased risk of kidney stones in cadmium-contaminated areas. In the dose–response meta-analysis, we observed a consistent linear positive association between cadmium exposure and the risk of kidney stones. The overall RR for every 1.0 μg/L increase in urinary cadmium levels was 1.07 (95% CI: 1.01–1.13).
Conclusion:
Our findings suggest that cadmium exposure is associated with the risk of kidney stones. These findings reinforce the importance of environmental cadmium exposure as a risk factor for kidney stones, extending beyond the influence of conventional risk factors. Efforts to reduce cadmium exposure in the population may help reduce the individual, economic, and societal burdens associated with kidney stones.
Systematic review registration:
Introduction
Kidney stones are a common condition worldwide, with incidence and prevalence increasing among both children and adults (1–3). Approximately 10 ~ 12% of men and 5 ~ 6% of women are affected by kidney stones (4). Kidney stone formation is a complex process resulting from an imbalance in urine between promoters and inhibitors of crystal formation (5, 6). Various risk factors, such as geography, diet, genetics, and occupation, can affect this balance, resulting in the development of kidney stones (7). Among these risk factors, environmental factors are recognized as important contributors to kidney stone formation (8, 9).
Cadmium, a heavy metal, is one of the most toxic industrial and environmental pollutants, which poses a severe threat to human health (10, 11). Cadmium can enter the body through air, water, soil, and food, and it largely accumulates in the kidneys, liver, bones, and other organs, causing irreversible damage to the target organs (12–14). Previous studies have evaluated different types of heavy metals and their concentrations in urinary stones (15, 16). Notably, significantly higher concentrations of 17 elements, including cadmium, were found in all types of stones (15, 16). In addition, cadmium concentrations were higher in calcium phosphate stones, along with other elements (15). Previous studies have reported that cadmium accumulation causes cellular toxicity and damages multiple organs (17). Long-term exposure to cadmium has been linked to a higher calcium excretion rate and tubular impairment with a loss of reabsorptive capacity, which increase the risk of kidney stone formation (18, 19).
Several observational studies have attempted to address the association between cadmium exposure and the risk of kidney stones. However, their results remain controversial. To better understand this issue, we conducted a comprehensive systematic review and meta-analysis of published literature that investigated the correlation between cadmium exposure and the risk of kidney stones.
Methods
Literature search and eligibility criteria
Medline, Embase, and the Web of Science databases were searched up until 31 July 2024. The search terms included “metal exposure OR cadmium” and “kidney stones OR nephrolithiasis OR urolithiasis OR renal stones.” The reference lists of relevant studies were reviewed to identify additional studies. Figure 1 shows the search strategy. Studies were considered eligible if they (1) were published in the English language; (2) had the full text available; (3) evaluated the relationship between cadmium exposure and the risk of kidney stones; (4) provided risk estimates with confidence intervals (CIs) or offered data to calculate these associations; and (5) were case–control, cohort, or cross-sectional studies. The protocol was registered with PROSPERO (CRD42024564167) on 11 July 2024.
Figure 1
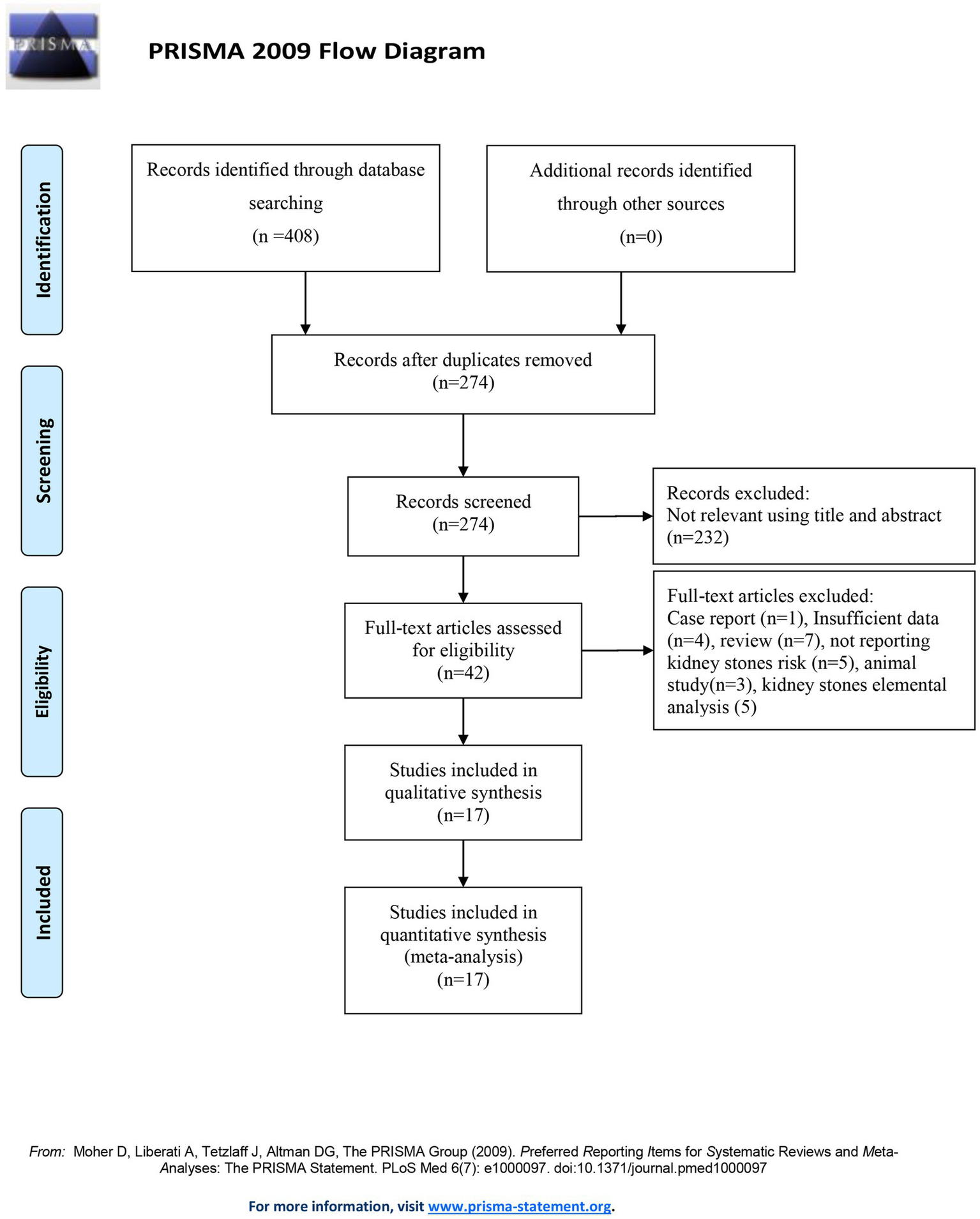
PRISMA flow diagram illustrating the selection of the included studies.
Data extraction
Data were independently extracted by two investigators using a standardized collection form. The relevant data extracted included the following: first author, publication date, study design, study region, sample size, effect estimates [OR, relative risk (RR), HR, or IRR] with 95% CIs, and the potential confounders used for adjustment. Any discrepancies in the results were resolved through discussion with a third investigator.
Quality assessment
Two investigators used the Newcastle–Ottawa scale (NOS) to conduct quality assessments of case–control and cohort studies (20). A maximum of 9 stars are awarded to each study based on three aspects: 4 stars for the selection of participants, 2 stars for the comparability of groups, and 3 stars for the assessments of outcomes. Scores of 7–9, 4–6, and 0–3 were categorized as high, moderate, and low quality, respectively, for each study. The quality assessment of the cross-sectional studies was conducted following the guidelines provided by the Agency for Healthcare Research and Quality (21). A total of 11 items were included in this self-rating scale, with each item worth one point. The following score categories were used to assess article quality: 0–3 indicated low quality, 4–7 indicated moderate quality, and 8–11 indicated high quality.
Statistical analysis
The primary outcome was the relative risk of kidney stone incidence. Subgroup analyses of the primary outcome were conducted based on sex and whether the area was cadmium-contaminated. For each study, the risk ratio for kidney stones with the corresponding 95% CI was calculated. A random effects model was used to compute the pooled risk ratio. A chi-squared-based Q test and the I2 statistic were performed to evaluate the heterogeneity between studies. If I2 is greater than 50% and the p-value is less than 0.10, heterogeneity was considered statistically significant. A Z-test was performed to assess the significance of the overall RR, and a p-value of < 0.05 was considered statistically significant. We carried out a dose–response meta-analysis of the risk of kidney stones according to the methods proposed by Orisini et al. (22) and Berlin et al. (23). Each category’s mean concentration of cadmium was taken as the corresponding dose. When the upper boundary of the highest category was open-ended, the midpoint was calculated by multiplying the lower boundary by 1.5. We set the lowest category to zero, if it was unavailable. The linear and non-linear models were evaluated based on the null hypothesis, with the spline coefficients set to zero. A sensitivity analysis was conducted to evaluate the stability of the results by excluding one study at a time. Potential publication bias was tested using funnel plots, Begg’s test, and Egger’s test. All statistical analyses were conducted using Stata software (version 14.0) (Stata Corporation, College Station, Texas, United States).
Results
Search results and study characteristics
The systematic search of articles published up to 31 July 2024 identified 408 articles. After screening the titles and abstracts, we obtained 42 studies for a full-text review. After the full-text review, we finally included 17 published studies comprising 159,011 individuals in the analysis (19, 24–39) (Figure 1). Among these, 1 study was a cohort study, 3 were case–control studies, and 13 were cross-sectional studies. Six of these studies were conducted in America, five in Europe, and six in Asia. Furthermore, 14 studies reported cadmium concentrations measured in urine, 5 studies reported cadmium concentrations measured in blood, and 1 study reported cadmium concentrations measured in dietary sources. The articles were published between 1985 and 2024. The detailed characteristics of all included studies are shown in Table 1. The majority of studies were of medium to high quality. One cross-sectional study was of low quality (Table 1).
Table 1
| Author (year) | Country | Study design | Study quality | Sample size | Biological sample type | Measure of effect | RR (kidney stone risk) (95% CI) | Adjustment factors |
|---|---|---|---|---|---|---|---|---|
| Lu et al. (2024) (39) | United States | Cross-sectional study | High | 8,515 | Urine | OR | 1.663 (1.277, 2.167) | Age, sex, ethnicity, education levels, marital status, BMI, hypertension, diabetes, vigorous recreational activities, moderate recreational activities, blood urea nitrogen, creatinine, uric acid, eGFR, and urine creatinine |
| Ye et al. (2023) (36) | United States | Cross-sectional study | High | 9,056 | Urine | OR | 1.51 (1.10, 2.06) | Sex, age, ethnicity, education, alcohol consumption, smoking, family income/poverty, diabetes, BMI, physical activity, cardiovascular disease, and urinary creatinine |
| Wang et al. (2023) (38) | United States | Cross-sectional study | Moderate | 1,244 | Urine | OR | 1.87 (0.80, 4.34) | – |
| Zhao et al. (2023) (24) | United States | Cross-sectional study | Moderate | 7,809 | Urine | OR | 1.85 (1.35, 2.53) | Sex, age, ethnicity, education, household poverty-to-income ratio, marital status, serum cotinine, BMI, urinary creatinine, vitamin C, kidney failure, gout, cancer, activity |
| Li et al. (2022) (32) | China | Case-control | High | 740 | Blood | OR | 1.61 (1.1, 2.34) | Age, sex, BMI, nationality, marital status, occupation, education level, systolic blood pressure, diastolic blood pressure, smoking status, and drinking status, creatinine, urea, and uric acid |
| Liu et al. (2022) (25) | China | Cross-sectional study | Moderate | 5,792 | Urine | OR | 0.52 (0.36, 0.75) | Age, BMI, systolic blood pressure, diastolic blood pressure, serum creatinine, glomerular filtration rate, serum urea, uric acid, urine protein, smoking status, and alcohol intake |
| Huang et al. (2021) (29) | China | Case-control | Moderate | 1,572 | Urine | OR | Male: 1.41 (0.67, 2.98), Female: 1.69 (0.97, 2.93) | Length of residency, BMI, nationality, family income, education level, smoking, alcohol drinking, and hypertension |
| Sun et al. (2019) (37) | United States | Cross-sectional study | High | 29,199 | Urine, blood | OR | Blood*: 1.36 (0.98, 1.87), Urine&: 2.37 (1.12, 5.04) | Age, sex, ethnicity, body mass index, socioeconomic characteristics (including educational level, marital status, and annual family income), smoking, physical activity, total energy intake, and intakes of calcium, phosphate, sodium, potassium, magnesium, total fluid, alcohol, caffeine, vitamins B6, C, and D, and estimated glomerular filtration rate |
| Hara et al. (2016) (30) | Belgium | Cross-sectional study | High | 1,302 | Urine, blood | HR | Blood*: 1.13 (0.93, 1.38), Urine&: 1.23 (0.98, 1.54) | Sex, age, serum magnesium, and 24-h urinary volume and calcium |
| Kaewnate et al. (2012) (19) | Thailand | Case-control | High | 1,085 | Urine | OR | 2.73 (1.16, 6.42) | Sex, age, smoking status, and alcohol consumption |
| Swaddiwudhipong et al. (2011) (26) | Thailand | Cross-sectional study | Moderate | 6,748 | Urine | OR | Male: 1.093 (1.051, 1.138), Female: 1.039 (0.995, 1.084) | Age, alcohol consumption, body mass index, diabetes, hypertension, and urinary cadmium |
| Ferraro et al. (2011) (28) | United States | Cross-sectional study | Moderate | 15,690 | Urine | OR | 1.40 (1.06, 1.86) | Age, ethnicity, body mass index, smoking, region of residence, and daily intake of calcium and sodium |
| Swaddiwudhipong et al. (2010) (33) | Thailand | Cross-sectional study | Moderate | 795 | Urine | OR | 0.99 (0.94, 1.03) | Age, sex, smoking, body mass index, urinary cadmium, diabetes, urinary stone, hypertension, and urinary calcium |
| Järup et al. (1997) (31) | Sweden | Cross-sectional study | Moderate | 46 | Blood | OR | 5.6 (1.03, 30.2) | – |
| Järup et al. (1993) (34) | Sweden | Cross-sectional study | Moderate | 765 | Urine, blood | IRR | Blood*: 3.2 (1.3, 8.3) Urine&: 1.6 (0.5, 5.0) |
– |
| Elinder et al. (1985) (27) | Sweden | Cross-sectional study | Low | 58 | Urine | OR | 8.9 (1.01, 77.9) | – |
| Thomas et al. (2013) (35) | Sweden | Cohort | Moderate | 68,595 | Dietary | HR | Male: 0.97 (0.77, 1.23), Female: 0.99 (0.89, 1.43) | BMI, overweight, obesity, alcohol consumption, cigarette use, and dietary intake of calcium, iron, magnesium, potassium, vitamin B6, and vitamin C |
Study population and exposure characteristics of the studies included in the meta-analyses.
*Blood cadmium levels and kidney stone risk; &Urinary cadmium levels and kidney stone risk; OR, Odds ratio; RR, Relative risk; HR, Hazard ratio; IRR, Incidence rate ratios; BMI, Body mass index; eGFR, Estimated glomerular filtration rate.
Quantitative synthesis
A total of 17 studies involving 159,011 individuals evaluated the association between cadmium exposure and the risk of kidney stones. The results showed that cadmium exposure was associated with an increased risk of kidney stones (RR = 1.19; 95% CI: 1.10–1.29, Figure 2). Significant heterogeneity was observed among the evaluated studies (I2 = 76.4%, p < 0.001). Table 2 shows the results of the subgroup analyses performed to evaluate any potential effects of sex, study design, region, and exposure assessment method on these associations.
Figure 2
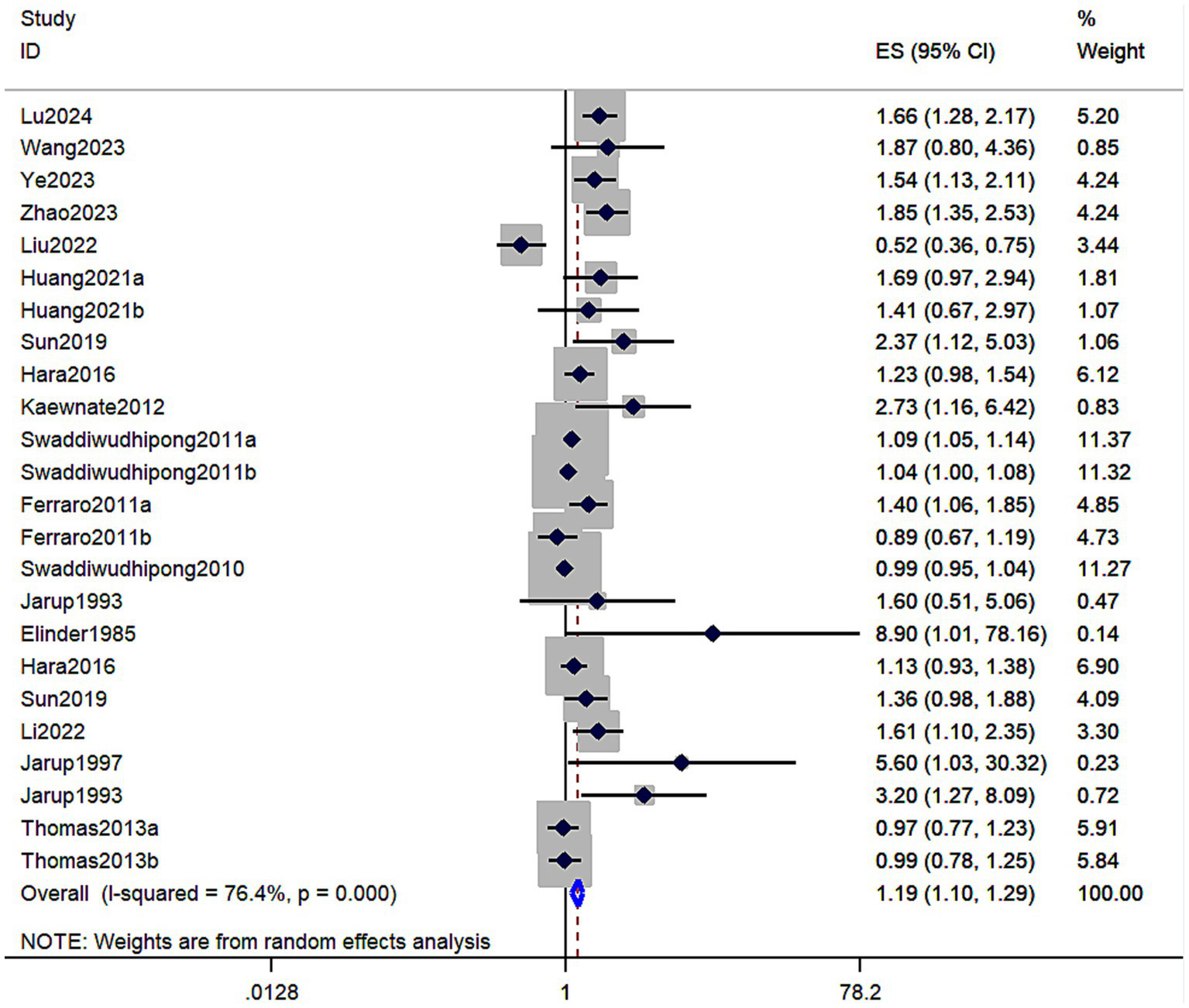
Forest plot showing the association between cadmium exposure and the risk of kidney stones. The results showed that cadmium exposure was associated with an increased risk of kidney stones (RR = 1.19; 95% CI: 1.10–1.29).
Table 2
| Subgroup | Number of studies | Pooled RR (95% CI) | I 2 statistics (%) | P-value for the heterogeneity Q test |
|---|---|---|---|---|
| Urinary cadmium levels | 14 | 1.19 (1.08, 1.30) | 80.00 | <0.001 |
| Female | 6 | 1.36 (0.94, 1.98) | 71.90 | 0.002 |
| Male | 7 | 1.01 (0.77, 1.33) | 83.50 | <0.001 |
| Mixed* | 10 | 1.66 (1.27, 2.18) | 84.70 | <0.001 |
| Cadmium-contaminated areas | 7 | 1.08 (1.00, 1.15) | 67.80 | 0.002 |
| Non-contaminated areas | 6 | 1.33 (0.97, 1.82) | 83.90 | <0.001 |
| Asian | 5 | 1.03 (0.94, 1.13) | 81.60 | <0.001 |
| Non-Asian | 9 | 1.47 (1.21, 1.77) | 57.10 | 0.013 |
| Cross-sectional studies | 12 | 1.16 (1.06, 1.27) | 81.90 | <0.001 |
| Case–control studies | 2 | 1.78 (1.20, 2.64) | 0.00 | 0.504 |
| Blood cadmium levels | 5 | 1.49 (1.10, 2.02) | 59.00 | 0.045 |
| Female | 1 | 1.57 (1.05, 2.34) | – | – |
| Male | 1 | 1.23 (0.77, 1.97) | – | – |
| Mixed* | 5 | 1.49 (1.10, 2.02) | 59.00 | 0.045 |
| Cadmium-contaminated areas | 4 | 1.69 (1.05, 2.71) | 68.90 | 0.022 |
| Non-contaminated areas | 1 | 1.36 (0.98, 1.88) | – | – |
| Asian | 1 | 1.61 (1.10, 2.35) | – | – |
| Non-Asian | 4 | 1.51 (1.01, 2.25) | 63.60 | 0.041 |
| Cross-sectional studies | 4 | 1.51 (1.01, 2.25) | 63.60 | 0.041 |
| Case–control studies | 1 | 1.61 (1.10, 2.35) | – | – |
| Dietary levels | 1 | 0.98 (0.83, 1.16) | – | – |
| Cohort | 1 | 0.98 (0.83, 1.16) | – | – |
Subgroup analyses of the studies included in the meta-analysis.
*The study population included both male and female individuals; RR, Relative risk.
In addition, 14 studies involving 89,630 individuals in total evaluated the association between urinary cadmium exposure levels and the risk of kidney stones. The results showed that urinary cadmium exposure was associated with an increased risk of kidney stones (RR = 1.19; 95% CI: 1.08–1.30; p < 0.001; Table 2). A high degree of heterogeneity was observed among the evaluated studies (I2 = 80.00%, p < 0.001). The results of the subgroup analyses based on sex showed that cadmium exposure was associated with an increased risk of kidney stones in mixed-sex populations (RR = 1.66; 95% CI: 1.27–2.18, I2 = 84.70%, p < 0.001), compared to women (RR = 1.36; 95% CI: 0.94 to 1.98, I2 = 71.90%, p = 0.002) and men (RR = 1.01; 95% CI: 0.77–1.33, I2 = 83.50%, p < 0.001) (Table 2). Moreover, a statistically significant increased association was observed in cadmium-contaminated areas (RR = 1.08; 95% CI: 1.00–1.15, I2 = 67.8%, p = 0.002), but no association between higher cadmium exposure and the risk of kidney stones was observed in non-contaminated areas (RR = 1.33; 95% CI: 0.97–1.82, I2 = 83.90%, p < 0.001) (Table 2). The subgroup analyses based on study design showed that cadmium exposure was associated with an increased risk of kidney stones in both case–control studies (RR = 1.78; 95% CI: 1.20–2.64, I2 = 0.00%, p = 0.504) and cross-section studies (RR = 1.16; 95% CI: 1.06–1.27, I2 = 81.90%, p < 0.001) (Table 2). The results of the subgroup analyses by region showed that cadmium exposure was associated with an increased risk of kidney stones in non-Asian populations (RR = 1.47; 95% CI: 1.21–1.77, I2 = 57.10%, p < 0.013), whereas no significant association was observed in Asian populations (RR = 1.03; 95% CI: 0.94–1.13, I2 = 58%, p = 0.015) (Table 2). Furthermore, based on the three studies included in the linear dose–response meta-analysis, a significant association was observed between urinary cadmium exposure levels and the risk of kidney stones. Each additional 1 μg/L increase in urinary cadmium was associated with a 7% higher risk of kidney stones (RR = 1.07; 95% CI 1.01–1.13; I2 = 26.4%; three studies; 1,769 cases; range of cadmium level = 0.08–4.225ug/L; Figure 3).
Figure 3
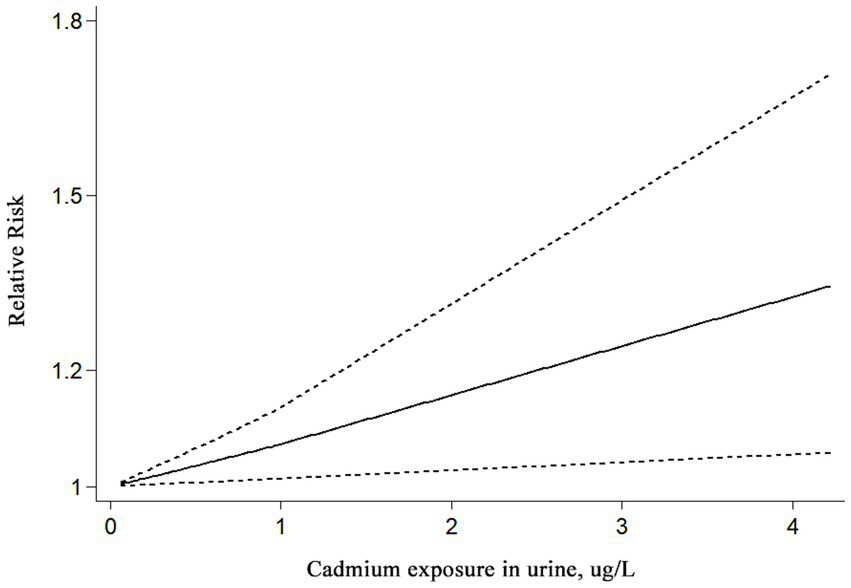
Linear dose–response association between urinary cadmium levels and the risk of kidney stones. Analyses were conducted using a fixed effects model. A significant increase in the risk of kidney stones was observed for each additional 1 μg/L of cadmium in urine.
A total of five studies involving 4,425 individuals in total evaluated the association between blood cadmium exposure levels and the risk of kidney stones. A significant association was found between blood cadmium exposure levels and the risk of kidney stones (RR = 1.49; 95%CI: 1.10–2.02, I2 = 59%, p = 0.045, Table 2) The results of the subgroup analyses by sex showed that cadmium exposure was associated with an increased risk of kidney stone disease in female individuals (RR = 1.57; 95% CI: 1.05–2.34) and mixed-sex populations (RR = 1.49; 95% CI: 1.10–2.02, I2 = 59%, p = 0.045) compared to male individuals (RR = 1.23; 95% CI: 0.77–1.97) (Table 2). In the subgroup analyses using a random effects model, there were significant associations between subgroups based on study design and region (Table 2). Moreover, there was a statistically significant increased risk in cadmium-contaminated areas (RR = 1.69; 95% CI: 1.05–2.71, I2 = 68.9%, p = 0.022), while no association was observed between higher cadmium exposure and the risk of kidney stones in non-contaminated areas (RR = 1.36; 95% CI: 0.98–1.88) (Table 2).
One cohort study examined the association between higher cadmium exposure in dietary sources and the risk of kidney stones, and the results showed no significant association (RR = 0.98; 95% CI: 0.83–1.16) (Table 2).
Sensitivity analysis and publication bias
A sensitivity analysis was conducted to assess the risk of kidney stones by excluding individual studies one at a time, and the results showed that no individual study influenced the overall RRs (Figure 4), indicating that the results of this meta-analysis are relatively stable. Publication bias was observed in the results based on the Egger’s test and funnel plots (Table 3; Figure 5).
Figure 4
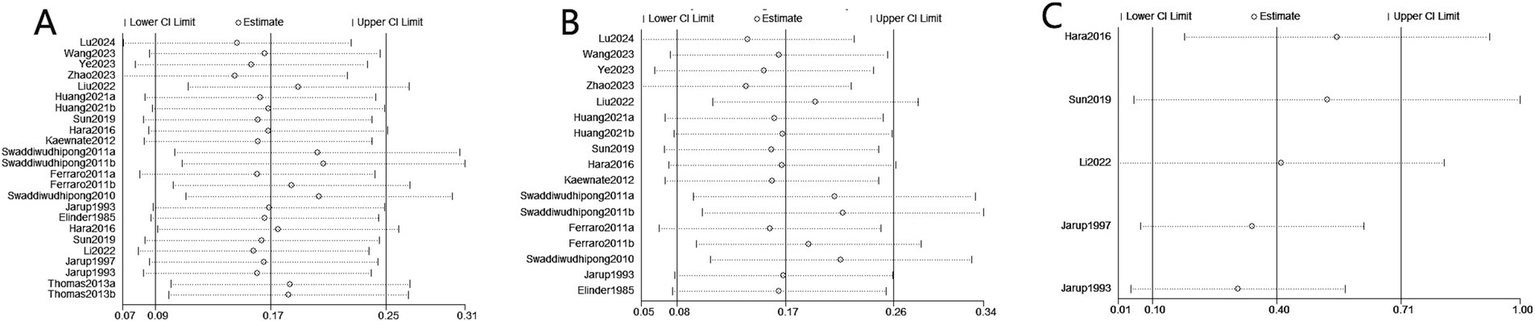
Sensitivity analysis diagrams for the studies assessing the association between cadmium exposure and the risk of kidney stones. (A) Cadmium exposure and the risk of kidney stones; (B) Urinary cadmium exposure levels and the risk of kidney stones; (C) Blood cadmium levels and the risk of kidney stones.
Table 3
| Exposure | Egger’s test | Begg’s test | ||
|---|---|---|---|---|
| Coefficient | P | 95% CI | ||
| Cadmium | 1.441 | 0.003 | 0.540–2.343 | 0.130 |
| Urinary cadmium levels | 1.459 | 0.025 | 0.208–2.709 | 0.773 |
| Blood cadmium levels | 2.532 | 0.005 | 1.447–3.617 | 0.086 |
Publication bias test for the association between cadmium exposure and the risk of kidney stones.
Figure 5
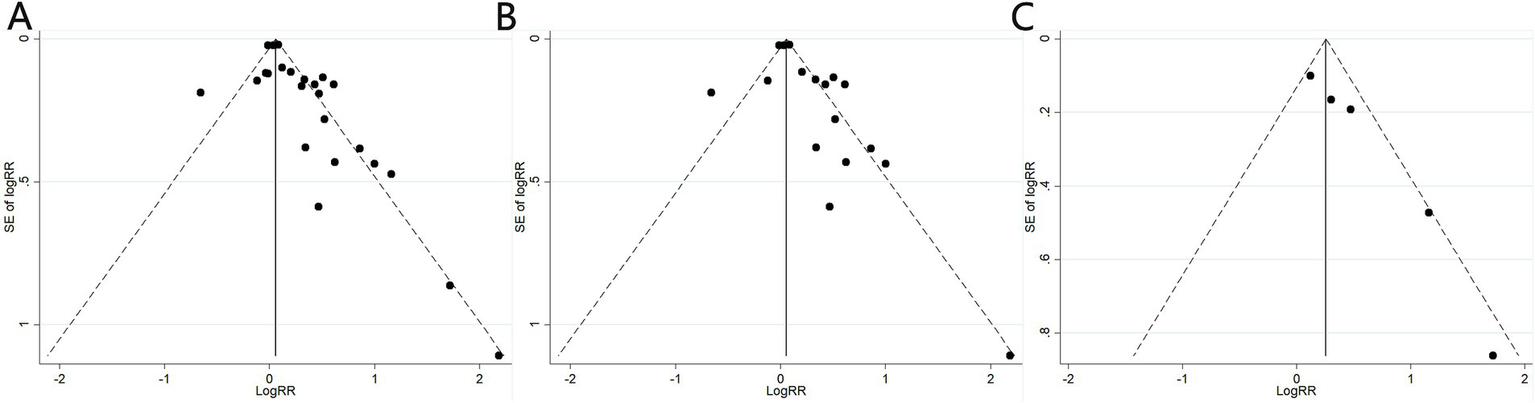
Funnel plots of the studies assessing the association between cadmium exposure and the risk of kidney stones. (A) Cadmium exposure and the risk of kidney stones; (B) Urinary cadmium exposure levels and the risk of kidney stones; (C) Blood cadmium levels and the risk of kidney stones.
Discussion
A total of 17 studies involving 159,011 participants met the inclusion criteria and were eventually included in our systematic review and meta-analysis. Overall, our results indicated that the risk of kidney stones was increased in individuals with higher cadmium exposure. Importantly, we observed an increased risk of kidney stones in individuals living in cadmium-contaminated areas, while no such risk was found in non-contaminated areas. Moreover, the dose–response meta-analysis indicated that an increase of 1ug/L in urinary cadmium was associated with a 7% rise in the risk of developing kidney stones. These findings are of great significance, as they highlight the association between cadmium exposure and the risk of kidney stones and may help in preventing the formation of kidney stones by minimizing exposure to cadmium, especially in cadmium-contaminated areas.
The heavy metal cadmium is one of the most toxic industrial and environmental pollutants, which poses a severe threat to human health (10, 11). Cadmium can enter the body through air, water, soil, and food, and it largely accumulates in the kidneys, liver, bones, and other organs, causing irreversible damage to the target organs (18, 40). Higher cadmium exposure has also been considered a possible risk factor for kidney stones. Similar to our findings, higher cadmium exposure has previously been associated with the risk of kidney stones in several studies utilizing data from the National Health and Nutrition Examination Survey (NHANES) (24, 36, 37). Kaewnate et al. also observed an association between elevated levels of urinary cadmium and urinary stones among 1,085 study residents from 13 cadmium-contaminated villages in Thailand (19). In their results, elevated levels of urinary cadmium appeared to increase the risk of urinary stones, with an adjusted odds ratio (OR) of 2.73 and a 95% confidence interval (CI) of 1.16–6.42, after adjusting for other co-variables (19). A case–control study conducted by Li et al. showed that the ratio of plasma cadmium to kidney stones in the highest quartile was 1.606 (95% CI, 1.100–2.344) compared to the lowest quartile in rural areas of Guangxi, China (32). However, several studies found no association between higher cadmium exposure and the risk of kidney stones. Hara et al. observed that higher levels of blood cadmium and urinary cadmium were not associated with an increased risk of kidney stones (30). Liu et al. reported that higher blood cadmium exposure was not associated with the risk of kidney stones in the Qiandongnan Prefecture, China (25). This difference between studies may be due to the study design, sample size, nationalities, or study regions. Thus, more high-quality studies are needed to further assess the associations.
A meta-analysis conducted by Guo et al., which included six studies with a total of 88,045 participants, found that higher cadmium exposure was significantly associated with an increased risk of urolithiasis, with a 1.32-fold increase in risk (41). Their study focused exclusively on cadmium levels in urine and dietary intake. Compared to the previous meta-analysis, we extended this investigation by including blood cadmium levels as an additional factor. Consequently, we included more studies with a larger sample size and performed multiple subgroup analyses to assess heterogeneity and publication bias. According to the subgroup analysis, urinary and blood cadmium levels were associated with the risk of kidney stones, while dietary cadmium exposure was not significantly associated with the risk of kidney stones. A low exposure dose and limited number of studies may explain the lack of association between dietary cadmium exposure and the risk of kidney stones. In addition, a linear dose–response meta-analysis was conducted, which showed a significant association between cadmium exposure in urine and the risk of kidney stones, with an increased risk observed for every additional 1 μg/L of cadmium in urine. In the subgroup meta-analyses based on study design, we found that higher cadmium exposure was associated with an increased risk of kidney stones in both case–control and cross-sectional studies. We also observed a higher risk of kidney stone disease in individuals living in cadmium-contaminated areas but not in those from non-contaminated areas. One possible explanation for the lack of association in non-contaminated areas is that exposure levels were not high enough to significantly affect the urinary composition. The results of the subgroup analyses based on sex showed that urinary cadmium exposure was associated with an increased risk of kidney stones in mixed-sex populations compared to women and men. Similarly, blood cadmium exposure was not associated with an increased risk of kidney stones in men. The subgroup analyses based on ethnicity showed that urinary cadmium exposure was not associated with an increased risk of kidney stone disease in Asian populations compared to non-Asian populations. This lack of association is likely due to the limited number of studies included in the meta-analysis. Cadmium exposure may lead to renal stone formation, but the exact mechanism is not clear. Cadmium has a long half-life, and once it enters the body, it accumulates irreversibly in the kidneys (42, 43). Cadmium accumulation in the kidneys leads to a higher calcium excretion rate, which can raise the likelihood of developing kidney stones (12, 44). Cadmium can recombine with metallothionein produced by renal tubular epithelial cells, causing significant damage to kidney tubular cells and impairing their reabsorption function (45, 46). Cellular injury in renal tubular epithelial cells induces crystal nucleation and aggregation, and this may result in ineffective crystallization modulators and localized areas of supersaturation in the interstitial space (47). Kidney stones may form as a result of this process. In addition, sexual hormones may play an important role in the development of nephrolithiasis (47, 48). As an endocrine-disrupting chemical, cadmium may contribute to the formation of renal stones by disrupting endocrine functions (49).
For the purpose of reporting our observations, we conducted a comprehensive literature search following the PRISMA guidelines. Our study included 17 studies involving 159,011 participants to evaluate the relationship between cadmium exposure and the risk of kidney stones. The large sample size is an important strength of this study. However, there were several limitations. First, the studies included in the meta-analysis showed a high level of heterogeneity, which persisted even after extensive sensitivity analysis and several subgroup analyses. Second, there were too few studies to draw a definitive conclusion about the risk of kidney stones in people with dietary cadmium exposure. Finally, due to limited data, we could not assess the dose–response relationship between blood cadmium levels and the risk of kidney stones. Similarly, we were unable to assess the effects of age, smoking habits, ethnicity, and study quality on the risk of kidney stones associated with cadmium exposure. Therefore, more prospective cohort studies that evaluate the incidence of kidney stones in relation to cadmium exposure are needed.
The results of this meta-analysis strengthen the evidence that higher cadmium exposure is a risk factor for kidney stones. Further detailed research is needed to better understand the mechanisms underlying these associations. Efforts to reduce cadmium exposure in the population may help reduce the individual, economic, and societal burdens of kidney stones.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Z-JR: Conceptualization, Methodology, Writing – original draft. QZ: Data curation, Writing – original draft. N-XT: Data curation, Software, Writing – original draft. Y-DL: Data curation, Investigation, Writing – review & editing. D-LL: Writing – review & editing. A-LL: Methodology, Software, Writing – review & editing. CY: Data curation, Software, Writing – review & editing. FW: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Postdoctoral Science Foundation of Chongqing; Grant ID: CSTB2022NSCQ-BHX0675.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Stamatelou K Goldfarb DS . Epidemiology of kidney stones. Healthcare (Basel, Switzerland). (2023) 11:424. doi: 10.3390/healthcare11030424
2.
Fontenelle LF Sarti TD . Kidney stones: treatment and prevention. Am Fam Physician. (2019) 99:490–6. PMID:
3.
Edvardsson VO Ingvarsdottir SE Palsson R Indridason OS . Incidence of kidney stone disease in Icelandic children and adolescents from 1985 to 2013: results of a nationwide study. Pediatr Nephrol (Berlin, Germany). (2018) 33:1375–84. doi: 10.1007/s00467-018-3947-x
4.
Sigurjonsdottir VK Runolfsdottir HL Indridason OS Palsson R Edvardsson VO . Impact of nephrolithiasis on kidney function. BMC Nephrol. (2015) 16:149. doi: 10.1186/s12882-015-0126-1
5.
Singh P Harris PC Sas DJ Lieske JC . The genetics of kidney stone disease and nephrocalcinosis. Nat Rev Nephrol. (2022) 18:224–40. doi: 10.1038/s41581-021-00513-4
6.
Negri AL Spivacow FR . Kidney stone matrix proteins: role in stone formation. World J Nephrol. (2023) 12:21–8. doi: 10.5527/wjn.v12.i2.21
7.
Kachkoul R Touimi GB El Mouhri G El Habbani R Mohim M Lahrichi A . Urolithiasis: history, epidemiology, aetiologic factors and management. Malays J Pathol. (2023) 45:333–52.
8.
González-Enguita C Bueno-Serrano G López de Alda-González A García-Giménez R . Environmental conditions as determinants of kidney stone formation. ACS Appl Bio Mater. (2023) 6:5030–6. doi: 10.1021/acsabm.3c00722
9.
Ferraro PM Bargagli M Trinchieri A Gambaro G . Risk of kidney stones: influence of dietary factors, dietary patterns, and vegetarian-vegan diets. Nutrients. (2020) 12:779. doi: 10.3390/nu12030779
10.
Gao M Li C Xu M Liu Y Cong M Liu S . LncRNA MT1DP aggravates cadmium-induced oxidative stress by repressing the function of Nrf2 and is dependent on interaction with miR-365. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). (2018) 5:1800087. doi: 10.1002/advs.201800087
11.
Pi H Xu S Reiter RJ Guo P Zhang L Li Y et al . SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy. (2015) 11:1037–51. doi: 10.1080/15548627.2015.1052208
12.
Wang M Chen Z Song W Hong D Huang L Li Y . A review on cadmium exposure in the population and intervention strategies against cadmium toxicity. Bull Environ Contam Toxicol. (2021) 106:65–74. doi: 10.1007/s00128-020-03088-1
13.
Adams SV Newcomb PA Shafer MM Atkinson C Bowles EJ Newton KM et al . Sources of cadmium exposure among healthy premenopausal women. Sci Total Environ. (2011) 409:1632–7. doi: 10.1016/j.scitotenv.2011.01.037
14.
Doccioli C Sera F Francavilla A Cupisti A Biggeri A . Association of cadmium environmental exposure with chronic kidney disease: a systematic review and meta-analysis. Sci Total Environ. (2024) 906:167165. doi: 10.1016/j.scitotenv.2023.167165
15.
Abdel-Gawad M Ali-El-Dein B Elsobky E Mehta S Alsaigh N Knoll T et al . Micro-elemental analysis and characterization of major heavy metals and trace elements in the urinary stones collected from patients living in diverse geographical regions. Environ Sci Pollut Res Int. (2022) 29:68941–9. doi: 10.1007/s11356-022-20732-x
16.
Khalil AA Gondal MA Shemis M Khan IS . Detection of carcinogenic metals in kidney stones using ultraviolet laser-induced breakdown spectroscopy. Appl Opt. (2015) 54:2123–31. doi: 10.1364/ao.54.002123
17.
Templeton DM Liu Y . Multiple roles of cadmium in cell death and survival. Chem Biol Interact. (2010) 188:267–75. doi: 10.1016/j.cbi.2010.03.040
18.
Satarug S Garrett SH Sens MA Sens DA . Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. (2010) 118:182–90. doi: 10.1289/ehp.0901234
19.
Kaewnate Y Niyomtam S Tangvarasittichai O Meemark S Pingmuangkaew P Tangvarasittichai S . Association of elevated urinary cadmium with urinary stone, hypercalciuria and renal tubular dysfunction in the population of cadmium-contaminated area. Bull Environ Contam Toxicol. (2012) 89:1120–4. doi: 10.1007/s00128-012-0856-8
20.
Stang A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
21.
Wu Y Fu Y He Y Gong X Fan H Han Z et al . Prevalence and influencing factors of sleep disorders in patients with CRS: a protocol for systematic review and meta-analysis. BMJ Open. (2023) 13:e078430. doi: 10.1136/bmjopen-2023-078430
22.
Orsini N Bellocco R Greenland S . Generalized least squares for trend estimation of summarized dose–response data. Stata J. (2006) 6:40–57. doi: 10.1177/1536867x0600600103
23.
Berlin JA Longnecker MP Greenland S . Meta-analysis of epidemiologic dose-response data. Epidemiology. (1993) 4:218–28. doi: 10.1097/00001648-199305000-00005
24.
Zhao H Fang L Chen Y Ma Y Xu S Ni J et al . Associations of exposure to heavy metal mixtures with kidney stone among U.S. adults: a cross-sectional study. Environ Sci Pollut Res Int. (2023) 30:96591–603. doi: 10.1007/s11356-023-29318-7
25.
Liu Y Zhang C Qin Z Yang Q Lei J Tang X et al . Analysis of threshold effect of urinary heavy metal elements on the high prevalence of nephrolithiasis in men. Biol Trace Elem Res. (2022) 200:1078–88. doi: 10.1007/s12011-021-02740-z
26.
Swaddiwudhipong W Mahasakpan P Limpatanachote P Krintratun S . An association between urinary cadmium and urinary stone disease in persons living in cadmium-contaminated villages in northwestern Thailand: a population study. Environ Res. (2011) 111:579–83. doi: 10.1016/j.envres.2011.01.007
27.
Elinder CG Edling C Lindberg E Kågedal B Vesterberg O . Assessment of renal function in workers previously exposed to cadmium. Br J Ind Med. (1985) 42:754–60. doi: 10.1136/oem.42.11.754
28.
Ferraro PM Bonello M Frigo AC D'Addessi A Sturniolo A Gambaro G . Cadmium exposure and kidney stone formation in the general population--an analysis of the National Health and nutrition examination survey III data. J Endourol. (2011) 25:875–80. doi: 10.1089/end.2010.0572
29.
Huang JL Mo ZY Li ZY Liang GY Liu HL Aschner M et al . Association of lead and cadmium exposure with kidney stone incidence: a study on the non-occupational population in Nandan of China. J Trace Elem Med Biol. (2021) 68:126852. doi: 10.1016/j.jtemb.2021.126852
30.
Hara A Yang WY Petit T Zhang ZY Gu YM Wei FF et al . Incidence of nephrolithiasis in relation to environmental exposure to lead and cadmium in a population study. Environ Res. (2016) 145:1–8. doi: 10.1016/j.envres.2015.11.013
31.
Järup L Persson B Elinder CG . Blood cadmium as an indicator of dose in a long-term follow-up of workers previously exposed to cadmium. Scand J Work Environ Health. (1997) 23:31–6. doi: 10.5271/sjweh.175
32.
Li Y He K Cao L Tang X Gou R Luo T et al . Association between plasma cadmium and renal stone prevalence in adults in rural areas of Guangxi, China: a case-control study. BMC Nephrol. (2022) 23:323. doi: 10.1186/s12882-022-02945-x
33.
Swaddiwudhipong W Limpatanachote P Nishijo M Honda R Mahasakpan P Krintratun S . Cadmium-exposed population in Mae Sot district, Tak province: 3. Associations between urinary cadmium and renal dysfunction, hypertension, diabetes, and urinary stones. J Med Assoc Thai. (2010) 93:231–8. PMID:
34.
Järup L Elinder CG . Incidence of renal stones among cadmium exposed battery workers. Br J Ind Med. (1993) 50:598–602. doi: 10.1136/oem.50.7.598
35.
Thomas LD Elinder CG Tiselius HG Wolk A Akesson A . Dietary cadmium exposure and kidney stone incidence: a population-based prospective cohort study of men & women. Environ Int. (2013) 59:148–51. doi: 10.1016/j.envint.2013.06.008
36.
Ye ZY Chen ZZ Luo JY Xu LJ Fan DP Wang J . National analysis of urinary cadmium concentration and kidney stone: evidence from NHANES (2011-2020). Front Public Health. (2023) 11:1146263. doi: 10.3389/fpubh.2023.1146263
37.
Sun YF Zhou Q Zheng J . Nephrotoxic metals of cadmium, lead, mercury and arsenic and the odds of kidney stones in adults: an exposure-response analysis of NHANES 2007-2016. Environ Int. (2019) 132:105115. doi: 10.1016/j.envint.2019.105115
38.
Wang X Zhang J Ma ZB Yang YY Dang Y Cao ST et al . Association and interactions between mixed exposure to trace elements and the prevalence of kidney stones: a study of NHANES 2017-2018. Front Public Health. (2023) 11:1251637. doi: 10.3389/fpubh.2023.1251637
39.
Lu J Hong D Wu Q Xia Y Chen G Zhou T et al . Association between urinary cobalt exposure and kidney stones in U.S. adult population: results from the National Health and nutrition examination survey. Ren Fail. (2024) 46:2325645. doi: 10.1080/0886022x.2024.2325645
40.
Tinkov AA Aschner M Santamaria A Bogdanov AR Tizabi Y Virgolini MB et al . Dissecting the role of cadmium, lead, arsenic, and mercury in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Environ Res. (2023) 238:117134. doi: 10.1016/j.envres.2023.117134
41.
Guo ZL Wang JY Gong LL Gan S Gu CM Wang SS . Association between cadmium exposure and urolithiasis risk: a systematic review and meta-analysis. Medicine (Baltimore). (2018) 97:e9460. doi: 10.1097/md.0000000000009460
42.
Bautista CJ Arango N Plata C Mitre-Aguilar IB Trujillo J Ramírez V . Mechanism of cadmium-induced nephrotoxicity. Toxicology. (2024) 502:153726. doi: 10.1016/j.tox.2024.153726
43.
Marini HR Bellone F Catalano A Squadrito G Micali A Puzzolo D et al . Nutraceuticals as alternative approach against cadmium-induced kidney damage: a narrative review. Meta. (2023) 13:722. doi: 10.3390/metabo13060722
44.
Wallin M Sallsten G Fabricius-Lagging E Öhrn C Lundh T Barregard L . Kidney cadmium levels and associations with urinary calcium and bone mineral density: a cross-sectional study in Sweden. Environ Health. (2013) 12:22. doi: 10.1186/1476-069x-12-22
45.
Sabolić I Breljak D Skarica M Herak-Kramberger CM . Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals. (2010) 23:897–926. doi: 10.1007/s10534-010-9351-z
46.
Prozialeck WC Edwards JR . Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J Pharmacol Exp Ther. (2012) 343:2–12. doi: 10.1124/jpet.110.166769
47.
Wang Z Zhang Y Zhang J Deng Q Liang H . Recent advances on the mechanisms of kidney stone formation (review). Int J Mol Med. (2021) 48:149. doi: 10.3892/ijmm.2021.4982
48.
Nackeeran S Katz J Ramasamy R Marcovich R . Association between sex hormones and kidney stones: analysis of the National Health and nutrition examination survey. World J Urol. (2021) 39:1269–75. doi: 10.1007/s00345-020-03286-w
49.
Yang O Kim HL Weon JI Seo YR . Endocrine-disrupting chemicals: review of toxicological mechanisms using molecular pathway analysis. J Cancer Prev. (2015) 20:12–24. doi: 10.15430/jcp.2015.20.1.12
Summary
Keywords
kidney stones, cadmium exposure, heavy metal, systematic review, meta-analysis
Citation
Ren Z-J, Zhang Q, Tang N-X, Li Y-D, Lu D-L, Lin A-L, Yang C and Wang F (2025) Environmental cadmium exposure and the risk of kidney stones: a systematic review and dose-response meta-analysis. Front. Med. 12:1555028. doi: 10.3389/fmed.2025.1555028
Received
03 January 2025
Accepted
10 July 2025
Published
30 July 2025
Volume
12 - 2025
Edited by
Fatma Mohamady El-Demerdash, Alexandria University, Egypt
Reviewed by
Chenyu Li, University of Pennsylvania, United States
Sofia Sofia, Syiah Kuala University, Indonesia
Updates
Copyright
© 2025 Ren, Zhang, Tang, Li, Lu, Lin, Yang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wang, wangfeng526@hospital.cqmu.edu.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.