- 1Department of Nephrology, Taicang Loujiang New City Hospital, Suzhou, China
- 2Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Critical Care Medicine, The Third Affiliated Hospital of Soochow University, Changzhou, China
- 4Department of Nephrology, The Third Affiliated Hospital of Soochow University, Changzhou, China
- 5Blood Purification Centre, The Third Affiliated Hospital of Soochow University, Changzhou, China
Background: Cognitive impairment in hemodialysis patients is higher than that in healthy individuals. However, the potential pathogenesis is not fully clear.
Methods: In this retrospective study, 48 maintenance hemodialysis (MHD) patients of our center who participated in cognitive level test 4 years ago were screened by Montreal Cognitive Assessment (MoCA). We then analyzed relationships between the MoCA-derived cognitive level score, demographic, clinical, and laboratory variables.
Results: The age, dialysis age, Body Mass Index (BMI), and blood calcium levels of MHD patients were significantly abnormal than 4 years ago in this study. Furthermore, we observed that the levels of fibroblast growth factor (FGF-23) in MHD patients with cognitive decline were significantly higher than those in the normal cognitive group. Receiver operating characteristic (ROC) curves demonstrated that FGF-23 was a potential biomarker for diagnosing cognitive decline in hemodialysis patients.
Conclusion: We speculate that dialysis age, increased BMI, decreased blood calcium, and the abnormal level of FGF-23 may be independent risk factors for cognitive impairment in hemodialysis patients. Moreover, BMI and decreased blood calcium can be used as predictive factors of hemodialysis-related cognitive impairment.
Introduction
Chronic kidney disease (CKD) is now recognized as a major global public health problem. The global prevalence of CKD gradually rises, reaching up to 13.4% (1, 2). In CKD patients, the interaction between the kidney and brain can lead to various nervous system diseases, including both functional and organic conditions such as cerebrovascular disease, cognitive impairment, and autonomic and peripheral nervous system diseases (3). Notably, there is a growing focus on the cognitive impairment seen in CKD patients.
Cognition, which refers to the acquisition and application of knowledge, is a fundamental mental process that involves various aspects such as memory, attention, language, time–space orientation, execution, calculation, and judgment. Therefore, cognitive impairment can significantly impact the daily activities of individuals, ranging from mild to severe levels of impairment. It is a crucial function of the brain that can be affected by various factors. One of these factors is chronic kidney disease, which contributes to cognitive impairment through hypertension caused by renal dysfunction, over-activated renin-angiotensin system (RAS), renal anemia, renal osteopathy, and small vessel damage (4). Indeed, the incidence of cognitive impairment in CKD patients receiving maintenance hemodialysis (MHD) has been reported to be as high as 80% (5, 6). Therefore, CKD is considered one of the strongest risk factors for cognitive impairment. This impairment greatly affects MHD patients in terms of decision-making capacity, self-care ability, and treatment compliance, increasing the occurrence of negative emotions and the risk of death (7, 8). However, there is currently a lack of literature that tracks and re-evaluates hemodialysis patients with cognitive impairment, and knowledge about the potential pathogenesis of cognitive impairment in these patients remains limited.
In 2018, a single-center study was conducted to evaluate the cognitive function in 58 CKD patients (9). The results of this study showed that the cognitive function of MHD patients was significantly worse compared to that of healthy controls. Additionally, it was observed that MHD patients with cognitive impairment exhibited significantly lower levels of brain-derived neurotrophic factor (BDNF) and platelet counts (PLT) in comparison with both healthy controls and individuals with normal cognitive function. On the other hand, the levels of tumor necrosis factor (TNF)-α and interleukin 6 (IL-6) were significantly higher in MHD patients with cognitive impairment. These findings suggest that abnormalities in BDNF, TNF-α, IL-6, and PLT counts in serum may be associated with hemodialysis-related cognitive impairment.
In this study, we aimed to investigate the correlation between abnormalities found in patients 4 years ago and cognitive decline. By following up with the participants who were part of the study 4 years ago, we sought to identify the factors that exhibited abnormalities and examine their association with cognitive decline. This investigation may provide valuable insights into potential indicators for early intervention, which could prove beneficial in preventing cognitive decline in MHD patients.
Methods
Participants and study design
This is a retrospective study. In February to May 2017, we selected 58 patients undergoing hemodialysis at the Blood Purification Centre, The Third Affiliated Hospital of Soochow University (9). Out of these patients, 42 were included in this study. The study group consisted of 21 males and 21 females, with an average age of 49.07 ± 7.3 years. The primary diseases observed were chronic glomerulonephritis, type II diabetes, type I diabetes, benign arteriolosclerosis, polycystic kidney disease, nephrotic syndrome, medullary sponge kidney, and systemic lupus erythematosus in 23, 2, 1, 1, 2, 3, 1, and 1 patients, respectively. The remaining eight patients had an unknown cause of CKD. Comparatively, 16 individuals from the original cohort did not participate in the follow-up. Among these, three patients had been transferred, five had died, seven had undergone kidney transplants, and one did not participate due to vision problems.
Exclusion criteria were history of surgery for invasive intracranial pressure monitoring; mental or neurological diseases such as dementia, Alzheimer’s disease and schizophrenia; brain trauma; use of antiepileptic drugs, alcohol, or illicit drugs. Written informed consent was obtained from all participants, and the study was approved by the Ethics Commission of Soochow University (Changzhou, China) and conducted in accordance with the principles of Declaration of Helsinki. This clinical study was registered with the Chinese Clinical Trial Register (Clinical Trial Number: ChiCTR-ROC-16009283).
Treatment
All patients enrolled in this study underwent blood purification three times a week. The treatment regimen was hemodialysis once a week combined with hemodiafiltration twice a week, or hemodialysis twice a week combined with hemodiafiltration once a week. A Fresenius low-throughput polysulfone membrane dialyser (Fresenius FX8, Germany) was used for hemodialysis, and the hemodiafiltration was performed using a Fresenius high-throughput polysulfone membrane dialyser (Fresenius FX80, Germany). Each session was about 4 h, and low-molecular-weight heparin was used for anticoagulation. The dialysis solution was bicarbonate, the average blood flow rate is 200–280 mL/min, and the dialysis flow rate was 500 mL/min. The displacement volume after replacement was about 30% of ultrafiltration flow rate.
Cognitive function tests
Montreal Cognitive Assessment (MoCA) is a test to evaluate the cognitive function of patients. The test concerns eight cognitive domains: concentration, executive function, memory, linguistic ability, the capacity of visual space, abstract thought, computing power, and orientation. For those whose educational level is 12 or fewer years, one point will be added. The total score is 30 points. A score of MoCA ≥26 is considered to indicate normal cognition, and the score < 26 is considered to indicate cognitive impairment.
Laboratory tests
All patients’ blood were examined before hemodialysis within 1 week of cognitive function test. Leukocyte count, red blood cell count, hemoglobin, platelet, and hematocrit were measured using an automated five-class blood cell analyzer electrical impedance device (Sysmex Corporation, Japan). Blood urea nitrogen, creatinine, calcium, phosphorus, and albumin were detected by an automated biochemical analyzer (Leadman Biochemical, China). Parathyroid hormone measurement was achieved using chemiluminescence (Beckman, United States). Test methods and results for serum α-klotho, fibroblast growth factor (FGF-23), IL-6, BDNF, and tumor necrosis factor (TNF)-α levels were derived from the study conducted our years ago (9).
Statistical analysis
Data were expressed as means ± standard error of the mean (S.E.M.). Statistical analyses were performed using SPSS 26.0 (SPSS, Chicago, IL, United States). Comparisons between groups were performed by or χ2 test, Student’s t-test or nonparametric tests. The diagnostic value of test data for diseases was clarified by ROC curve analysis. Survival analysis was used to predict what different levels of the serum biological marker do to the occurrence and development of the disease. A p < 0.05 shows a statistically significant difference.
Results
Cognitive function is worse in hemodialysis patients after 4 years than those 4 years ago
According to the cognitive function test (MoCA) (Figure 1a), the level of cognitive function was evaluated for CKD patients undergoing hemodialysis 4 years ago and after 4 years. Four years ago, the maximum cognitive score was 30 points, with the upper quarter at 28 points, median at 25 points, lower quarter at 24 points, and the minimum at 20 points. In comparison, 4 years later, the maximum cognitive score remained at 30 points, with the upper quarter decreasing to 27 points, median remaining at 25 points, lower quarter decreasing to 23 points, and the minimum decreasing to 18 points. It was observed that on average, the cognitive scores decreased after 4 years when compared to the scores from 4 years ago, indicating a statistically significant difference. The prevalence of cognitive impairment also increased from 61.9% 4 years ago to 69.0% 4 years later. Therefore, there has been an increase in the proportion of hemodialysis patients experiencing cognitive decline, in comparison with 4 years ago (Figure 1b).
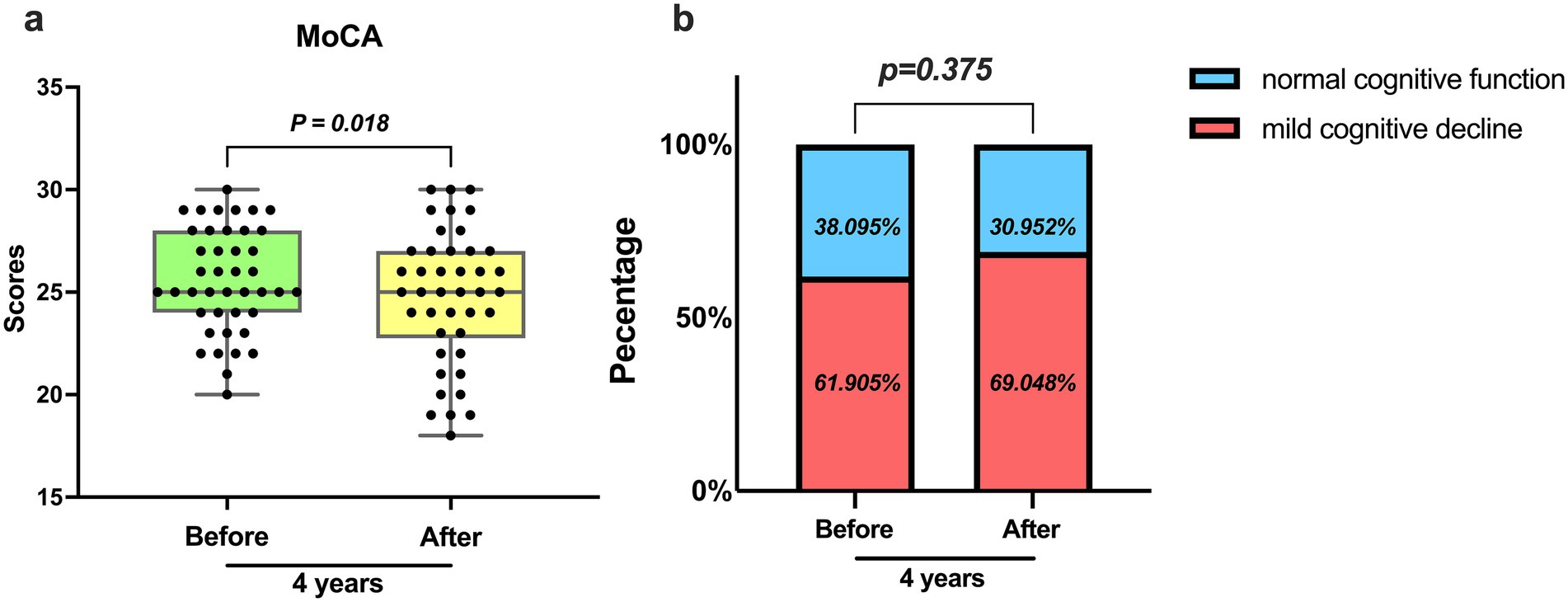
Figure 1. Assessment of cognitive function by MoCA. (a) The average level and degree of variation of MoCA scores 4 years ago and 4 years later (p = 0.018). (b) Occurrence of cognitive impairment 4 years ago and 4 years later (p = 0.375).
General clinical characteristics of patients receiving hemodialysis
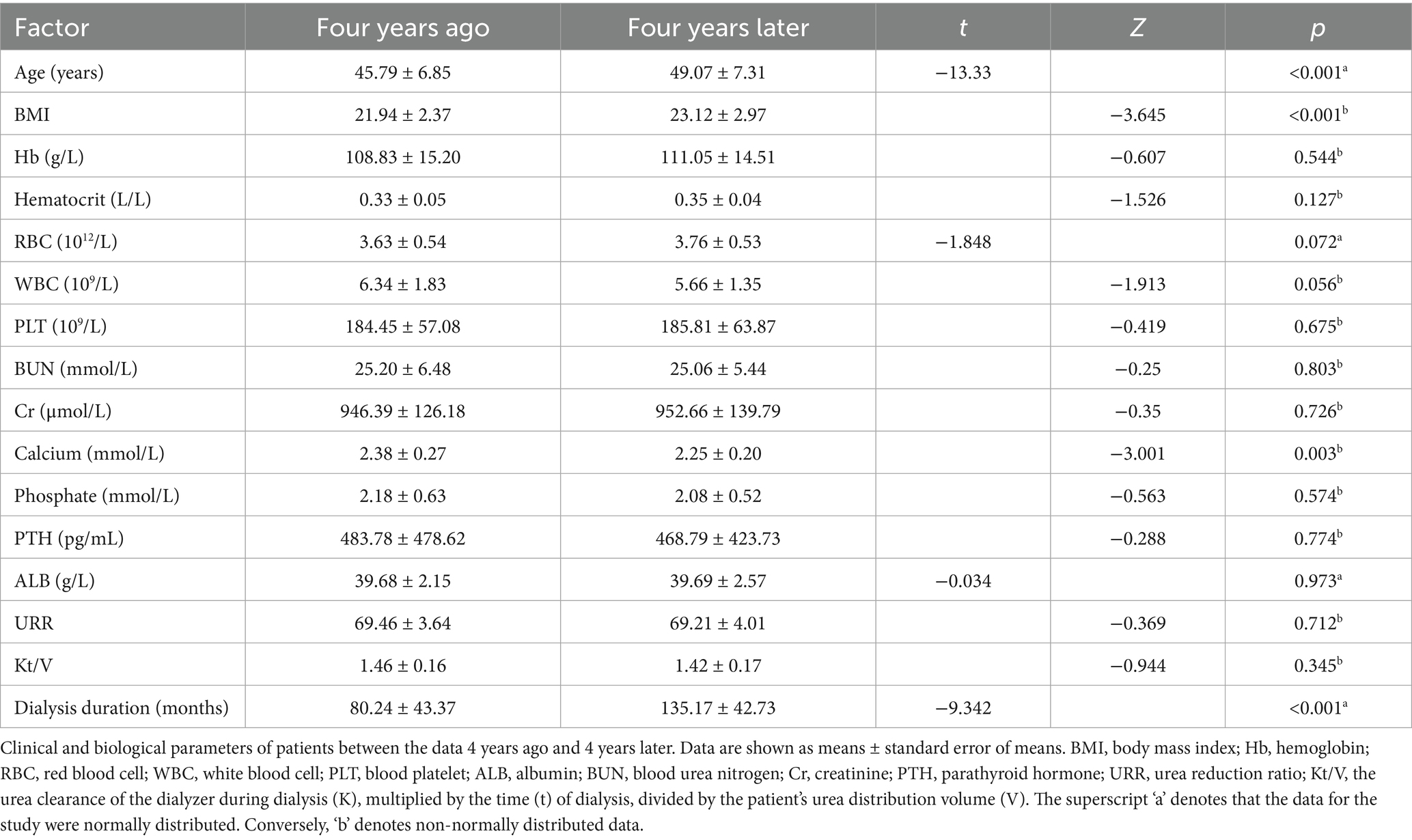
Table 1 presents the general clinical data of the study patients, encompassing age, sex, routine blood test values, and biomedical parameters. Analyzing the data 4 years ago and 4 years later, we found significant differences in age, dialysis duration, Body Mass Index (BMI), and calcium. Conversely, leukocyte count, red blood cell count, hemoglobin, platelet count, hematocrit, blood urea nitrogen, creatinine, phosphorus, albumin, parathyroid hormone, urea reduction ratio (URR), and Kt/V were not found to be different.
Serum biomarker levels
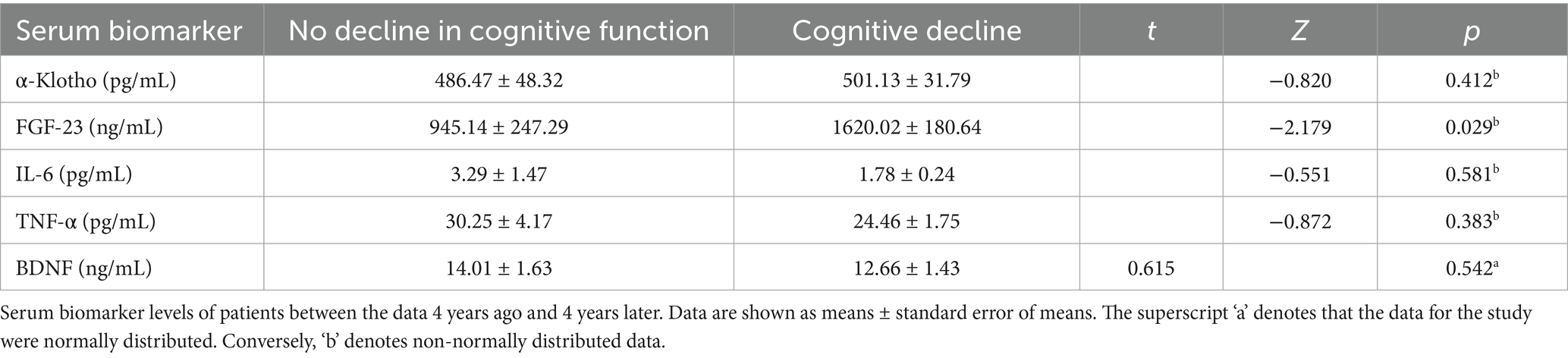
In light of whether their MoCA scores after 4 years had decreased compared to 4 years ago, 42 hemodialysis patients were divided into two groups. The analysis of serum biomarker levels 4 years ago revealed no significant differences in α-klotho, IL6, TNF-α, and BDNF between the two groups. However, a significant difference was observed in FGF-23 (Table 2).
Evaluation of serum biomarkers, BMI and calcium for the diagnosis of hemodialysis-related cognitive decline using ROC curve analysis
Analysis of all dialysis-induced cognitive decline cases revealed that the area under the ROC curve for α-klotho, FGF-23, IL-6, TNF-α, BDNF, BMI, and calcium were 0.58, 0.55, 0.58, 0.59, 0.70, 0.64, and 0.62, respectively (Figure 2). The best cutoff values for serum α-klotho, FGF-23, IL-6, TNF-α, BDNF, BMI, and calcium levels for the diagnosis of dialysis-induced cognitive decline were 469.5 pg/mL, 1,264.5 ng/mL, 1.8 pg/mL, 21.1 pg/mL, 20.0 ng/mL, 23.9 kg/m2, and 2.52 mmol/L, respectively. In summary, the diagnostic value of serum biomarkers, BMI, and calcium for hemodialysis-related cognitive decline can be summarized.
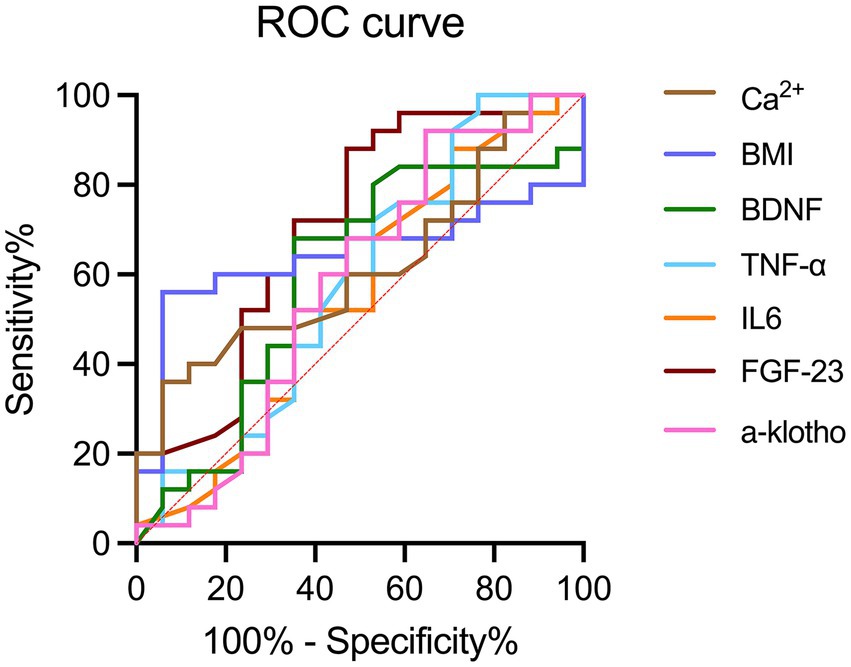
Figure 2. ROC curves of BMI, calcium, and serum biomarkers, including α-klotho, FGF-23, IL-6, TNF-α, and BDNF for the diagnosis of hemodialysis-related cognitive decline. BDNF, brain-derived neurotrophic factor; FGF-23, fibroblast growth factor-23; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; ROC, receiver operating characteristic.
Evaluation of the relativity of serum biomarkers, BMI, age, and calcium to hemodialysis-related cognitive decline using survival analysis
Next, we used survival analysis to predict the effects of different levels of FGF-23 (Figure 3a), BMI (Figure 3b) and calcium (Figure 3c) on the occurrence of cognitive impairment after 4 years. According to the results of multivariate Cox proportional hazard, we found that increasing age was a risk factor for cognitive impairment (Figure 3d).
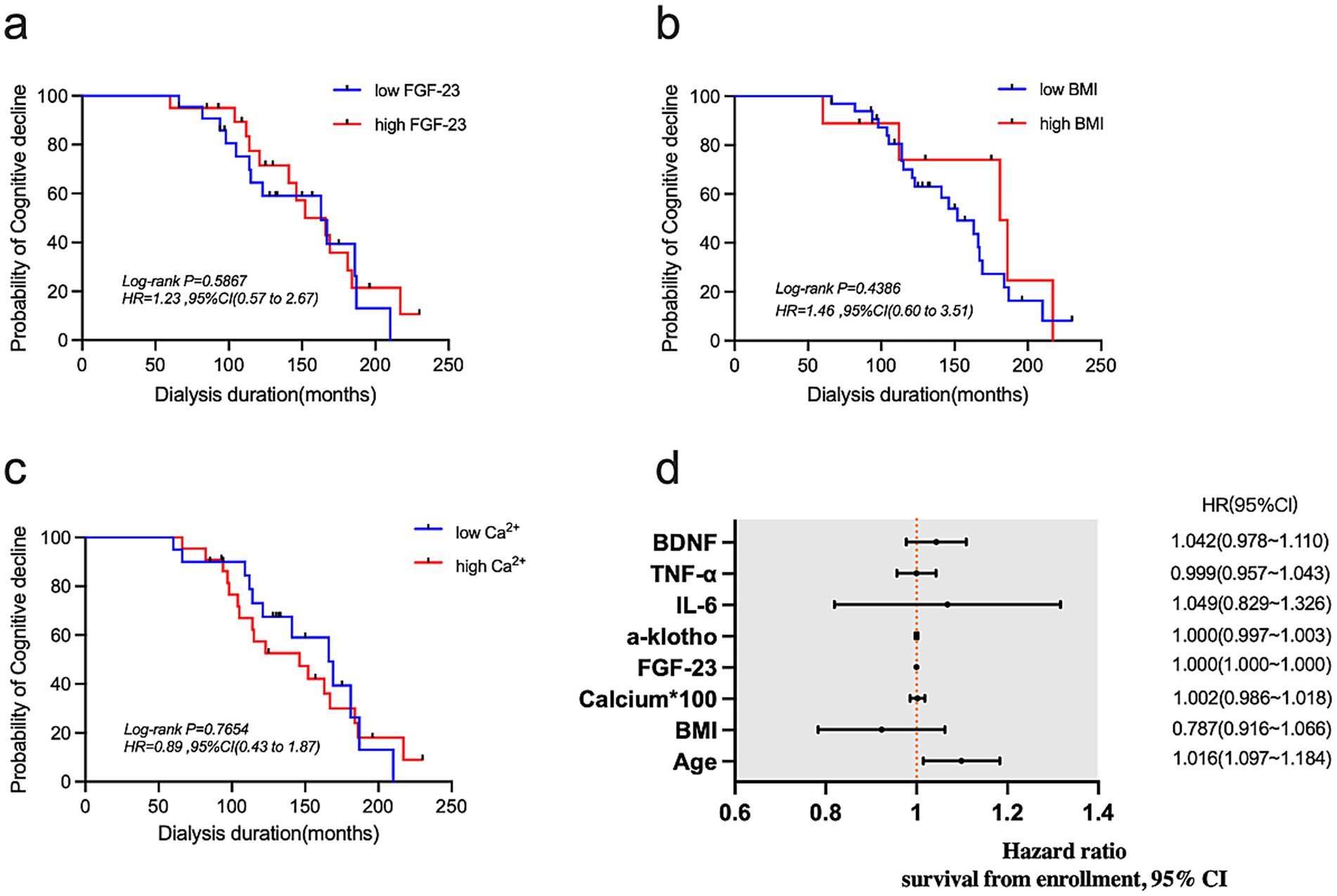
Figure 3. (a–c) Kaplan–Meier curves for FGF-23, BMI, and calcium. (d) Forest plot showing results of multivariate Cox proportional hazard analysis.
Discussion
In our initial study in 2018, we concluded that the Montreal Cognitive Assessment (MoCA) is broader and more sensitive than the Mini-Mental State Examination (MMSE), as well as more reasonable for people with severe cognitive impairment. Therefore, for this re-evaluation of cognitive function in maintenance hemodialysis (MHD) patients, we chose to use MoCA, considering its wider availability and affordability compared to other common cognitive impairment screening tests (6). The results of our study showed a significantly higher prevalence of cognitive impairment, at 69.0%, compared to 61.9% 4 years ago.
Based on the analysis of baseline information, we found that the age and dialysis age of patients 4 years later compared to 4 years ago were significantly different (p < 0.05). Advanced age is an independent risk factor for the onset of cognitive impairment (10–14), with cognitive change being a normal aging process. Some cognitive abilities, such as vocabulary, may improve with age, while reasoning, memory capacity, and processing speed decline over time (15). The occurrence of cognitive impairment is mainly observed in elderly patients due to common age-related diseases, such as atherosclerosis and hypertension, which can lead to cerebral blood deficiency and brain cell death, thereby affecting cognitive function. In addition, the hemodialysis treatment for patients with end-stage renal disease has been shown to effectively prolong survival. However, it is associated with potential adverse brain reactions such as transient ischemic attack (TIA) due to fluctuations in blood volume, blood pressure, and electrolyte levels during the process (16). We believe that the likelihood of iatrogenic adverse brain events increases with the age and duration of dialysis, resulting in a higher incidence of cognitive impairment. Therefore, elderly hemodialysis patients have a higher prevalence of cognitive impairment compared to the general elderly population.
Studies have shown that in non-diabetic dialysis patients, fat mass increases with age and muscle mass decreases (17). The increase of fat mass was indirectly related to the atrophy of frontal lobe, temporal lobe, and hippocampus (18). The hippocampus, which has key functions in memory, spatial level, and cognition (19), is recognized as a potential early biomarker for early memory decline and clinical dementia due to its reduced volume. In this study, we observed that the average BMI of 42 patients increased 4 years later compared to 4 years ago, with statistical differences (p < 0.05). Patients with high BMI also have a higher risk of cognitive impairment during dialysis, 2.28 times higher than those with low BMI. Thus, we propose that the increase in BMI among MHD patients is the result of increased fat mass, which can be further explored through human component analysis.
It is worth noting that the average blood calcium levels of 42 patients decreased 4 years later compared to 4 years ago, with statistical differences (p < 0.05). We also found in the survival curve that patients with low blood calcium levels were 1.31 times more at risk of cognitive impairment than those with high levels. This decrease in blood calcium levels among MHD patients could be attributed to various factors, including vitamin D deficiency, hypothyroidism, and the usage of calcium-containing phosphorus binders, vitamin D receptor agonists, and calcium-sensitive receptor agonists. The absorption of calcium is greatly affected by the prevalence of vitamin D deficiency in MHD patients. Vitamin D has various benefits for brain health, such as protecting neurons and having anti-inflammatory and anti-oxidative effects (20). Study has shown that vitamin D can maintain and enhance hippocampal synaptic function, preventing the decline of cognitive function, and vitamin D deficiency can potentially accelerate age-related cognitive decline (21). However, in this study, we did not measure the levels of vitamin D in MHD patients, and further research is needed to investigate the role of vitamin D in hemodialysis. Additionally, patients with hypothyroidism are more susceptible to intracranial vascular calcification. It is widely known that cognitive decline in aspects such as memory, emotion, execution, and spatial cognition is closely associated with intracranial and extracranial arterial stenosis caused by vascular calcification (22).
FGF-23, a serum biomarker, plays a crucial role in maintaining phosphate homeostasis and is synthesized and secreted by bone cells. In this study, we examined the serum biomarkers of patients with and without cognitive decline 4 years ago. The average FGF-23 levels were found to be higher in patients with cognitive decline, indicating a significant difference (p < 0.05). Our findings suggest a potential relationship between cognitive impairment in MHD patients and FGF-23 levels. The ROC curve analysis further revealed that FGF-23 has diagnostic value for cognitive impairment. But in the survival curve, we found that patients with high FGF-23 levels have a lower risk of cognitive decline later in life. It is not consistent with our previous study, and we may consider it because of the small sample size of the study and the large fluctuation range of FGF-23. The level of FGF-23 progressively increases in CKD patients as renal function deteriorates, aiming to maintain serum phosphate levels. In end-stage renal disease, FGF-23 levels may be up to 1,000 times higher than normal (23), which is consistent with previous research results (24). Li et al. (25) demonstrated that high phosphorus is a risk factor for dementia. Due to incomplete removal of excess phosphorus through hemodialysis treatment, MHD patients experience increased phosphorus deposition in the body, leading to a higher risk of cardiovascular and cerebrovascular diseases as well as varying degrees of neuropsychiatric or cognitive impairment (26). FGF-23 has also been found to play a significant role in neuronal morphology and synaptic density (27). Experimental studies have shown that FGF-23 can induce hippocampal atrophy and directly damage the cognitive function of CKD patients through its toxic effect on hippocampal neurons and synapses (28). The physiological role of FGF-23 is primarily mediated through the classic klotho-dependent pathway. Reduced expression of klotho in CKD patients increases the risk of cognitive decline as high klotho expression is considered neuroprotective (29, 30). Additionally, FGF-23 inhibits the activity of 1-α hydroxylase, leading to decreased vitamin D levels (31), which can further impact cognitive function.
Neuroinflammation is increasingly recognized as a contributing factor in neurodegenerative diseases, with numerous studies reporting a relationship between proinflammatory cytokines, such as tumor necrosis factor (TNF-α) and interleukin-6 (IL-6), and dementia and cognitive impairment (32–35). IL-6 plays a dual role by indirectly stimulating central nervous system inflammation through the blood–brain barrier and influencing cognition through its effect on cardiovascular disease (36, 37). Higher levels of IL-6 are thought to be associated with poorer cognitive function, while TNF-α. It indirectly affects cognitive function through macrophage mediated release of IL-6 (38). Brain-derived neurotrophic factor (BDNF) is widely distributed in the central nervous system, particularly in the hippocampus and cerebral cortex (39). BDNF impacts neuron survival, function, synaptic plasticity, long-term memory, and indirectly influences cognitive function by mediating neuroinflammation (40). While IL-6 and TNF-α levels are thought to negatively correlate with cognitive function, BDNF levels are positively correlated. Nonetheless, in our study, diagnostic accuracy of α-klotho, IL-6, TNF-α, and BDNF is low, and there is no statistical difference between the cognitive decline and cognitive undiminished groups. We attribute this to the small sample size of the study.
Conclusion
Age, dialysis age, increased BMI, decreased blood calcium, and the abnormal level of FGF-23 may be independent risk factors for cognitive impairment in hemodialysis patients. BMI and decreased blood calcium can be used as predictive factors of hemodialysis-related cognitive impairment. Therefore, a longer follow-up study on the prognosis of MHD patients participating in this experiment is needed to further explore the effects of age, dialysis age, BMI, blood calcium, and the level of FGF-23 on cognitive impairment in MHD patients. Detection, diagnosis, and treatment should be prioritized in the clinic to prevent and delay the occurrence and development of cognitive impairment in MHD patients, thereby improving their quality of life.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Changzhou First People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. RJ: Formal analysis, Supervision, Writing – review & editing. BZ: Formal analysis, Funding acquisition, Supervision, Writing – review & editing. JY: Data curation, Supervision, Writing – review & editing. LM: Conceptualization, Formal analysis, Funding acquisition, Methodology, Supervision, Writing – review & editing. JS: Conceptualization, Formal analysis, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from Major Science and Technology Projects of Changzhou Municipal Committee of Health and Family Planning (to LM, ZD201601), the Program of Major Science and Technology Project of Changzhou Health Commission (no. ZD202101), Changzhou Sci&Tech (to LM, CJ20210090; to BZ, CJ20230054), Top Talent of Changzhou medical innovation talent training (no. 201627), and the Program of Medical Young Talent of Jiangsu (no. QNRC2016276).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haileamlak, A. Chronic kidney disease is on the rise. Ethiop J Health Sci. (2018) 28:681–2. doi: 10.4314/ejhs.v28i6.1
2. Lv, JC, and Zhang, LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. (2019) 1165:3–15. doi: 10.1007/978-981-13-8871-2_1
3. Tanaka, S, and Okusa, MD. Crosstalk between the nervous system and the kidney. Kidney Int. (2020) 97:466–76. doi: 10.1016/j.kint.2019.10.032
4. Zhang, CY, He, FF, Su, H, Zhang, C, and Meng, XF. Association between chronic kidney disease and Alzheimer's disease: an update. Metab Brain Dis. (2020) 35:883–94. doi: 10.1007/s11011-020-00561-y
5. Murray, AM, Tupper, DE, Knopman, DS, Gilbertson, DT, Pederson, SL, Li, S, et al. Cognitive impairment in hemodialysis patients is common. Neurology. (2006) 67:216–23. doi: 10.1212/01.wnl.0000225182.15532.40
6. Drew, DA, Tighiouart, H, Rollins, J, Duncan, S, Babroudi, S, Scott, T, et al. Evaluation of screening tests for cognitive impairment in patients receiving maintenance hemodialysis. J Am Soc Nephrol. (2020) 31:855–64. doi: 10.1681/ASN.2019100988
7. Angermann, S, Baumann, M, Steubl, D, Lorenz, G, Hauser, C, Suttmann, Y, et al. Cognitive impairment in hemodialysis patients: implementation of cut-off values for the Montreal cognitive assessment (MoCA)-test for feasible screening. PLoS One. (2017) 12:e0184589. doi: 10.1371/journal.pone.0184589
8. Drew, DA, Weiner, DE, Tighiouart, H, Scott, T, Lou, K, Kantor, A, et al. Cognitive function and all-cause mortality in maintenance hemodialysis patients. Am J Kidney Dis. (2015) 65:303–11. doi: 10.1053/j.ajkd.2014.07.009
9. Zhu, B, Jin, LN, Shen, JQ, Liu, JF, Jiang, RY, Yang, L, et al. Differential expression of serum biomarkers in hemodialysis patients with mild cognitive decline: a prospective single-center cohort study. Sci Rep. (2018) 8:12250. doi: 10.1038/s41598-018-29760-5
10. Jaroudi, W, Garami, J, Garrido, S, Hornberger, M, Keri, S, and Moustafa, AA. Factors underlying cognitive decline in old age and Alzheimer's disease: the role of the hippocampus. Rev Neurosci. (2017) 28:705–14. doi: 10.1515/revneuro-2016-0086
11. Giau, VV, Bagyinszky, E, and An, SSA. Potential fluid biomarkers for the diagnosis of mild cognitive impairment. Int J Mol Sci. (2019) 20:4149. doi: 10.3390/ijms20174149
12. Lisko, I, Kulmala, J, Annetorp, M, Ngandu, T, Mangialasche, F, and Kivipelto, M. How can dementia and disability be prevented in older adults: where are we today and where are we going? J Intern Med. (2021) 289:807–30. doi: 10.1111/joim.13227
13. Pais, R, Ruano, L, Moreira, C, Carvalho, OP, and Barros, H. Prevalence and incidence of cognitive impairment in an elder Portuguese population (65-85 years old). BMC Geriatr. (2020) 20:470. doi: 10.1186/s12877-020-01863-7
14. Kim, H, Lee, S, Ku, BD, Ham, SG, and Park, WS. Associated factors for cognitive impairment in the rural highly elderly. Brain Behav. (2019) 9:e01203. doi: 10.1002/brb3.1203
15. Harada, CN, Natelson Love, MC, and Triebel, KL. Normal cognitive aging. Clin Geriatr Med. (2013) 29:737–52. doi: 10.1016/j.cger.2013.07.002
16. Prohovnik, I, Post, J, Uribarri, J, Lee, H, Sandu, O, and Langhoff, E. Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab. (2007) 27:1861–9. doi: 10.1038/sj.jcbfm.9600478
17. Ohkawa, S, Odamaki, M, Ikegaya, N, Hibi, I, Miyaji, K, and Kumagai, H. Association of age with muscle mass, fat mass and fat distribution in non-diabetic haemodialysis patients. Nephrol Dial Transplant. (2005) 20:945–51. doi: 10.1093/ndt/gfh643
18. Kim, W, Jang, H, Kim, YT, Cho, J, Sohn, J, Seo, G, et al. The effect of body fatness on regional brain imaging markers and cognitive function in healthy elderly mediated by impaired glucose metabolism. J Psychiatr Res. (2021) 140:488–95. doi: 10.1016/j.jpsychires.2021.06.011
19. Lisman, J, Buzsáki, G, Eichenbaum, H, Nadel, L, Ranganath, C, and Redish, AD. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. (2017) 20:1434–47. doi: 10.1038/nn.4661
20. Sultan, S, et al. Low vitamin D and its association with cognitive impairment and dementia. J Aging Res. (2020) 2020:6097820. doi: 10.1155/2020/6097820
21. Latimer, CS, Brewer, LD, Searcy, JL, Chen, KC, Popović, J, Kraner, SD, et al. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci USA. (2014) 111:E4359–66. doi: 10.1073/pnas.1404477111
22. Chu, Z, Cheng, L, and Tong, Q. Carotid artery calcification score and its association with cognitive impairment. Clin Interv Aging. (2019) 14:167–77. doi: 10.2147/CIA.S192586
23. Faul, C, Amaral, AP, Oskouei, B, Hu, MC, Sloan, A, Isakova, T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. (2011) 121:4393–408. doi: 10.1172/JCI46122
24. Miao, LY, et al. Effects of three blood purification methods on serum fibroblast growth factor-23 clearance in patients with hyperphosphatemia undergoing maintenance hemodialysis. Exp Ther Med. (2014) 7:947–52. doi: 10.3892/etm.2014.1543
25. Li, T, Xie, Y, Bowe, B, Xian, H, and al-Aly, Z. Serum phosphorus levels and risk of incident dementia. PLoS One. (2017) 12:e0171377. doi: 10.1371/journal.pone.0171377
26. Scialla, JJ, and Wolf, M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol. (2014) 10:268–78. doi: 10.1038/nrneph.2014.49
27. Hensel, N, Schön, A, Konen, T, Lübben, V, Förthmann, B, Baron, O, et al. Fibroblast growth factor 23 signaling in hippocampal cells: impact on neuronal morphology and synaptic density. J Neurochem. (2016) 137:756–69. doi: 10.1111/jnc.13585
28. Laszczyk, AM, Nettles, D, Pollock, TA, Fox, S, Garcia, ML, Wang, J, et al. FGF-23 deficiency impairs hippocampal-dependent cognitive function. eNeuro. (2019) 6:ENEURO.0469–18.2019. doi: 10.1523/ENEURO.0469-18.2019
29. Koh, N, Fujimori, T, Nishiguchi, S, Tamori, A, Shiomi, S, Nakatani, T, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. (2001) 280:1015–20. doi: 10.1006/bbrc.2000.4226
30. Shardell, M, Semba, RD, Rosano, C, Kalyani, RR, Bandinelli, S, Chia, CW, et al. Plasma klotho and cognitive decline in older adults: findings from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. (2016) 71:677–82. doi: 10.1093/gerona/glv140
31. Razzaque, MS. Interactions between FGF23 and vitamin D. Endocr Connect. (2022) 11:e220239. doi: 10.1530/EC-22-0239
32. Custodero, C, Ciavarella, A, Panza, F, Gnocchi, D, Lenato, GM, Lee, J, et al. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: a systematic review and meta-analysis. Geroscience. (2022) 44:1373–92. doi: 10.1007/s11357-022-00556-w
33. Zhao, J, Bi, W, Xiao, S, Lan, X, Cheng, X, Zhang, J, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. (2019) 9:5790. doi: 10.1038/s41598-019-42286-8
34. Metti, AL, Aizenstein, H, Yaffe, K, Boudreau, RM, Newman, A, Launer, L, et al. Trajectories of peripheral interleukin-6, structure of the hippocampus, and cognitive impairment over 14 years in older adults. Neurobiol Aging. (2015) 36:3038–44. doi: 10.1016/j.neurobiolaging.2015.07.025
35. Ferrucci, L, and Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
36. Wright, CB, Sacco, RL, Rundek, TR, Delman, JB, Rabbani, LRE, and Elkind, MSV. Interleukin-6 is associated with cognitive function: the northern Manhattan study. J Stroke Cerebrovasc Dis. (2006) 15:34–8. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009
37. Gianaros, PJ, Marsland, AL, Kuan, DCH, Schirda, BL, Jennings, JR, Sheu, LK, et al. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biol Psychiatry. (2014) 75:738–45. doi: 10.1016/j.biopsych.2013.10.012
38. Ma, K, Zhang, H, and Baloch, Z. Pathogenetic and therapeutic applications of tumor necrosis factor-alpha (TNF-alpha) in major depressive disorder: a systematic review. Int J Mol Sci. (2016) 17:733. doi: 10.3390/ijms17050733
39. Weinstein, G, Beiser, AS, Choi, SH, Preis, SR, Chen, TC, Vorgas, D, et al. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham heart study. JAMA Neurol. (2014) 71:55–61. doi: 10.1001/jamaneurol.2013.4781
Keywords: hemodialysis, cognitive decline, serum biomarkers, risk factors, quality of life
Citation: Li Y, Jiang R, Zhu B, Yu J, Miao L and Shen J (2025) The role of serum biomarkers in the decline of cognitive function in hemodialysis patients: a single-center retrospective study. Front. Med. 12:1556584. doi: 10.3389/fmed.2025.1556584
Edited by:
Evangelos C. Fradelos, University of Thessaly, GreeceReviewed by:
Natalija Filipovic, University of Split, CroatiaJordana Fradico, Oswaldo Cruz Foundation, Brazil
Copyright © 2025 Li, Jiang, Zhu, Yu, Miao and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianqin Shen, aWN1c2pxQDE2My5jb20=; Liying Miao, bHltaWFvQHNpbmEuY29t
†These authors have contributed equally to this work
 Yulu Li
Yulu Li Riyue Jiang
Riyue Jiang Bin Zhu
Bin Zhu Jie Yu
Jie Yu Liying Miao
Liying Miao Jianqin Shen
Jianqin Shen