Abstract
Introduction:
Discoid lupus erythematosus (DLE) is a chronic autoimmune skin disorder. Research fragmentation in DLE limits cohesive clinical and scientific progress. This bibliometric analysis aimed to clarify publication trends, collaboration networks, and emergent research themes in DLE from 2010 to 2024.
Methods:
A comprehensive search of the Web of Science Core Collection (WoSCC) used the terms “discoid lupus erythematosus” OR “lupus erythematosus discoid.” English-language articles and reviews (n = 861) were identified and analyzed via the Bibliometrix package in R to examine annual output, authorship, core journals, and keywords evolution.
Results:
Annual publications increased notably after 2018, although average citation rates declined. A small group of prolific authors, led by WERTH VP, contributed disproportionately. The United States dominated publication volume and international collaboration, followed by Italy, the United Kingdom, Germany, and China. Keywords analysis showed a shift from initial emphasis on disease classification and diagnosis toward advanced therapies, immunological mechanisms.
Conclusion:
Despite growing interest in DLE, it remains underrepresented compared with systemic lupus erythematosus. Broader collaborations, refined diagnostic criteria, and robust clinical trials are essential to enhance therapeutic strategies and patient outcomes in DLE.
1 Introduction
Discoid lupus erythematosus (DLE) is a chronic autoimmune skin disorder that predominantly affects sun-exposed areas, such as the face, ears, and scalp (1, 2). It is clinically characterized by erythematous, scaly plaques, which, if left untreated, may progress to scarring, atrophy, and dyspigmentation (3). Although DLE can occur as an isolated condition, it is also recognized as a cutaneous manifestation of systemic lupus erythematosus (SLE), a complex autoimmune disease that can affect multiple organ systems (4, 5).
The epidemiology of DLE is influenced by a combination of genetic, environmental, and immunological factors. While DLE is observed in all racial and ethnic groups, studies suggest a higher prevalence in African American populations compared to Caucasians. Additionally, the disease disproportionately affects women, with a female-to-male ratio of approximately 3:1 (6, 7). The pathogenesis of DLE is multifactorial, involving genetic predisposition, ultraviolet radiation exposure, and other environmental triggers (8, 9). Recent studies have also highlighted the role of epigenetic modifications in the development of DLE, adding a layer of complexity to its pathophysiology (10). This intricate etiology underscores the need for a comprehensive and individualized approach to the management and treatment of DLE.
Hydroxychloroquine has long been established as the cornerstone of DLE therapy, demonstrating efficacy in reducing disease activity and preventing disease progression (11, 12). In recent years, advances in understanding the underlying pathophysiological mechanisms of DLE have paved the way for exploring novel therapeutic strategies (13). Emerging biologic agents, such as belimumab, which have shown effectiveness in treating SLE, are currently being investigated for their potential application in DLE management (14, 15). However, the majority of clinical trials in lupus research focus on SLE, leaving DLE underrepresented in the literature. This research gap highlights the need for greater focus on DLE in future studies to improve outcomes for individuals affected by this condition.
Bibliometric analysis is a valuable tool for systematically evaluating trends, collaborations, and emerging research hotspots within a specific domain (16). By quantitatively analyzing large volumes of scholarly literature, bibliometric methods offer insights into the developmental trajectory of a field, identify influential publications and authors, and uncover underexplored areas of research (17). Moreover, bibliometric analysis can accelerate therapeutic innovation by revealing key studies and emerging research trends. By identifying influential clinical trials and mechanistic research, it can uncover overlooked therapeutic targets or drug classes, guiding resource allocation toward promising interventions (18). Additionally, bibliometric insights promote interdisciplinary collaboration and help researchers focus on high-impact studies addressing clinical gaps (19). This approach is particularly useful for DLE research, given its multidisciplinary nature and the fragmented distribution of studies across dermatology, immunology, and rheumatology journals. Tools such as CiteSpace, VOSviewer, and Bibliometrix allow researchers to construct co-authorship networks, visualize keywords co-occurrence, and analyze citation patterns (20, 21). These methods can help map the research landscape of DLE and provide a foundation for future investigations.
Despite the growing interest in DLE, the literature remains fragmented, with limited integration across different research domains. Moreover, there is a lack of comprehensive bibliometric analyses that synthesize current knowledge and identify emerging trends in DLE research. Such analyses are crucial for guiding future studies, optimizing resource allocation, and promoting international collaboration (22).
This study aims to address this gap by conducting a comprehensive bibliometric analysis of DLE-related literature, focusing on publication trends, influential authors, co-authorship and collaboration networks, keywords co-occurrence, major research themes, and emerging trends in the field. The goal is to provide a systematic and intuitive overview of the DLE research landscape, serving as a valuable resource for clinicians, researchers, and policymakers. The findings will not only enhance our understanding of the field but also inform the development of targeted strategies to improve patient outcomes.
2 Methods
2.1 Data sources and search strategies
In this study, a bibliometric analysis of the literature on DLE was conducted using data retrieved from the Web of Science Core Collection (WoSCC) database (23). It is a multidisciplinary citation database that provides comprehensive citation metadata. By contrast, PubMed focuses on biomedical literature and includes preprints, while Google Scholar aggregates a broader array of sources, including gray literature and non-English publications, but lacks consistent metadata (24). WoSCC was chosen for its detailed citation network capabilities, and suitability for evaluating trends in high-impact, peer-reviewed research, although this may exclude some emerging preprints or region-specific journals (25).
The search was performed on November 26, 2024, covering the period from January 1, 2010, to November 26, 2024. The search strategy employed was as follows: TS = (“discoid lupus erythematosus”) OR TS = (“lupus erythematosus discoid”). The document types included articles and reviews, with non-relevant literature, conference proceedings, and non-research outputs excluded. Only documents published in English were considered. The search results were exported in txt format, containing complete metadata for further analysis (Figure 1).
Figure 1

Flowchart for the bibliometric analysis of discoid lupus erythematosus.
2.2 Bibliometric analysis
The retrieved dataset was processed and analyzed using the Bibliometrix package (version 4.2.3) in R (26). This software was utilized to conduct various bibliometric analyses, enabling a comprehensive exploration of trends and patterns within the DLE research literature.
The initial bibliometric analysis was conducted using the “biblioAnalysis()” and “summary()” functions from the Bibliometrix package. These functions provided key metrics, including the total number of publications, citation counts, and the distribution of publications over time. The “biblioAnalysis()” function enabled the identification of leading authors, journals, and countries contributing to the field, while the “summary()” function summarized key indicators. To examine research collaborations, the “metaTagExtraction()” and “Biblionetwork()” functions were employed to identify collaboration patterns among countries, and authors. The results were visually represented using the “NetworkPlot()” function, which generated network maps displaying relationships based on co-authorship and co-citation patterns. The analysis also incorporated a keywords co-occurrence network, offering insights into the thematic evolution of DLE research. This was achieved through the “Biblioshiny()” function, which provides an interactive platform for visualizing bibliometric data. Additionally, thematic maps and co-occurrence networks were generated, facilitating a detailed exploration of emerging research hotspots within the field of DLE.
3 Results
3.1 Annual distribution
A total of 861 publications on DLE were retrieved from the WoSCC database, comprising both articles and reviews. From 2010 to 2017, the annual publication volume fluctuated, with fewer than 60 publications per year. However, from 2018 to 2024, the number of publications increased, with an average of more than 60 articles per year. Despite the rise in publication volume, the total number of citations showed a downward trend from 2010 to 2024 (Figure 2), gradually dropping from over 2,000.
Figure 2
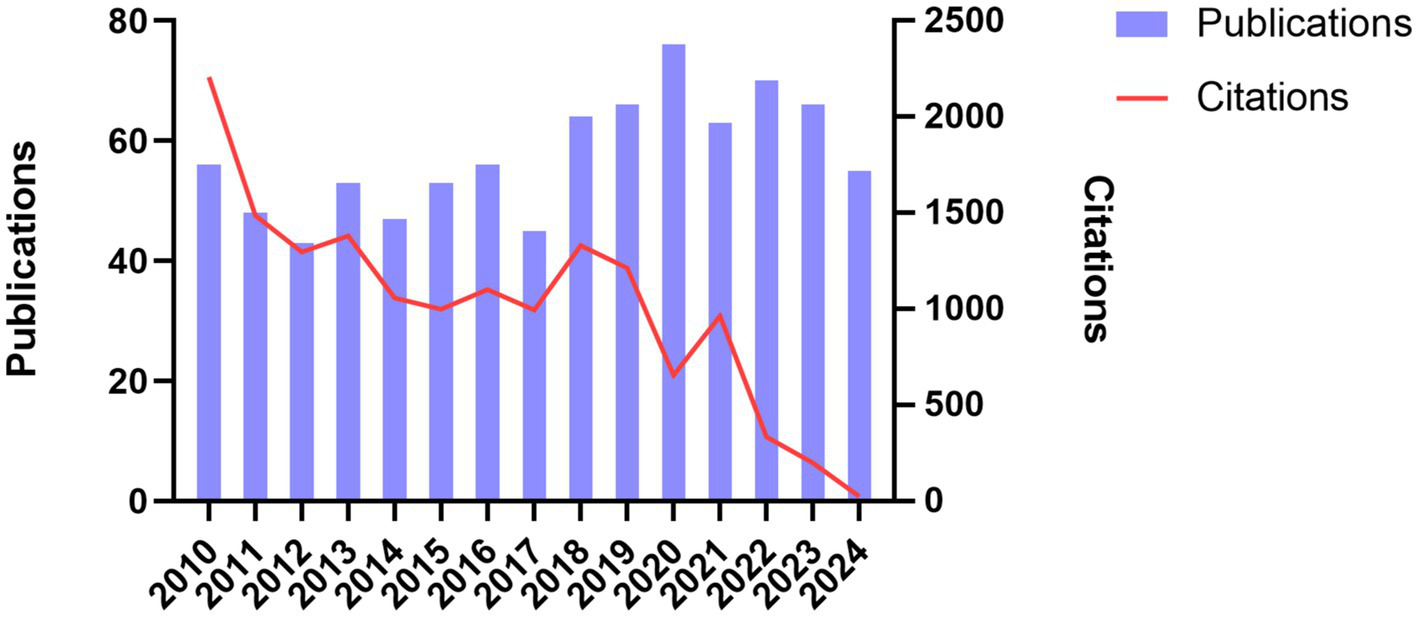
Annual distribution of publications and total citations on discoid lupus erythematosus.
3.2 Three-factor analysis
Figure 3 offers a comprehensive visualization of the interdisciplinary collaborations among authors, institutions, and journals within this research domain. Remarkably, the author WERTH VP has formed significant collaborations with leading institutions, such as the University of Pennsylvania, Northwestern University, and the Feinberg School of Medicine, among others. These collaborative efforts are prominently reflected in several key journals, including Lupus, Journal of Investigative Dermatology, and Veterinary Dermatology. Furthermore, the analysis reveals a marked concentration of research output in these select journals, underscoring their pivotal role in advancing the field. This pattern of collaboration and publication reflects broader trends within the discipline, highlighting the critical interconnections between institutions and journals.
Figure 3

Three factor analysis. The rectangle diagram illustrates the main elements. The larger the rectangle, the stronger the relationship between the shown element and the others. AU, authors; AU_UN, institutions; SO, journals.
3.3 Distribution of journals
A total of 861 publications on DLE were published across 329 different journals. The top 10 journals by publication volume are listed in Figure 4. The journal with the highest publication volume was Lupus from England, with 78 publications, followed by the American Journal of Dermatopathology and British Journal of Dermatology, each contributing 23 publications.
Figure 4
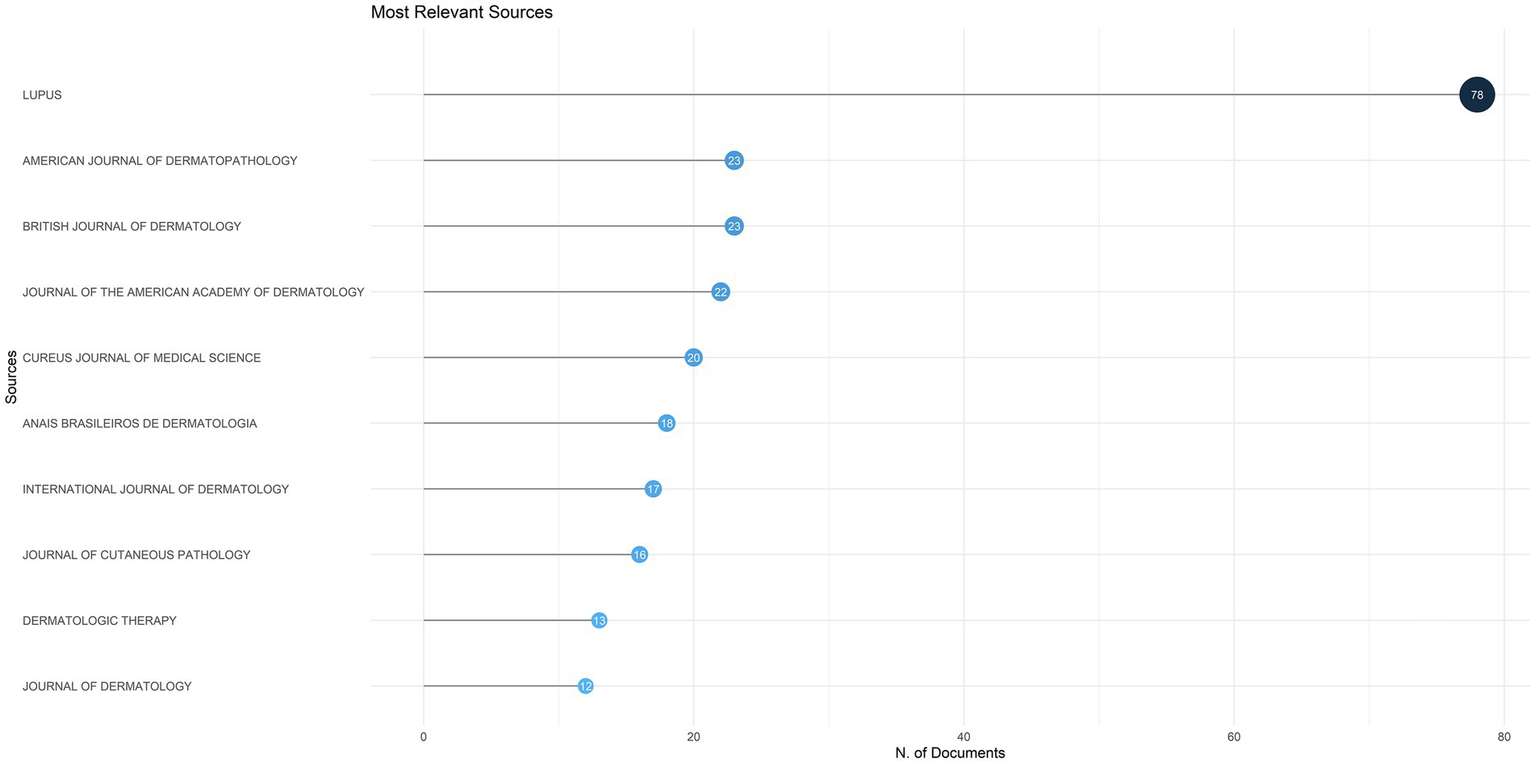
The top 10 journals by publication volume on discoid lupus erythematosus.
3.4 Distribution of authors and collaboration network
The collected literature concerning DLE comprised contributions from a total of 4,149 authors, with the leading 13 authors ranked by their publication output displayed in Table 1. Significantly, the author with the most substantial publication output was WERTH VP from the United States, followed by ZHAO M and CHONG BF from China.
Table 1
| Authors | Articles |
|---|---|
| Werth VP | 24 |
| Zhao M | 11 |
| Chong BF | 10 |
| Lu QJ | 9 |
| Tosti A | 9 |
| Wu HJ | 9 |
| Miteva M | 8 |
| Chasset F | 7 |
| Errichetti E | 7 |
| Kahlenberg JM | 7 |
| Merola JF | 7 |
| Olivry T | 7 |
| Wenzel J | 7 |
The top 13 authors by publication volume on discoid lupus erythematosus.
The collaborative network among authors was categorized into 13 distinct clusters (Figure 5), where each color signifies a unique group of authors working together. The largest nodes, representing the most prolific contributors in this domain, include WERTH VP, CHONG BF, MEROLA JF, among others, reflecting their extensive publication records in this area of research.
Figure 5
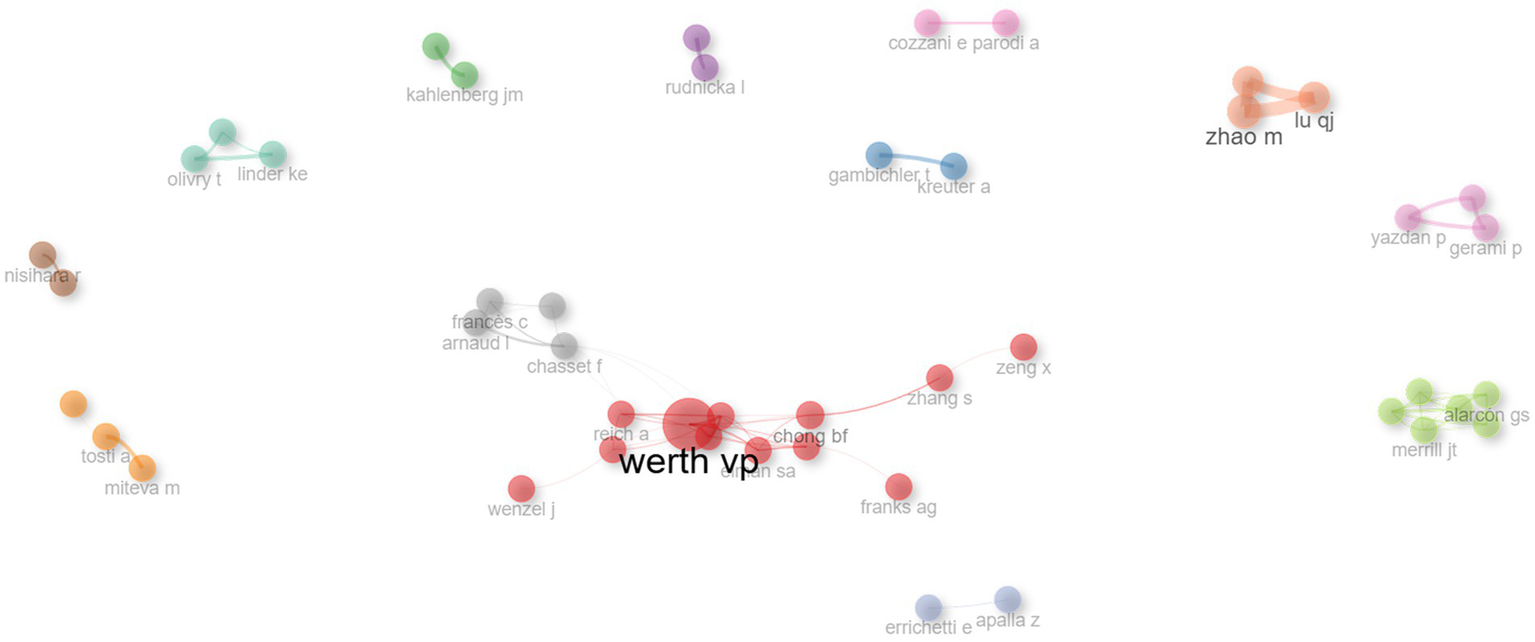
Author collaboration network. Nodes represent authors, with node size indicating publication count. Links reflect co-authorships, and link thickness corresponds to collaboration strength. Clusters highlight groups of closely collaborating researchers within the field.
3.5 Cooperation of countries and regions
Publications on DLE originated from 72 countries/regions. The bar chart in Figure 6A clearly illustrates that the top five countries with the highest levels of international collaboration are the United States, Italy, the United Kingdom, Germany, and China. Among them, the United States leads with 33 internationally co-authored publications, accounting for 14.5% of its total output. This trend highlights the growing importance of international cooperation in DLE research. Figure 6B depicts the cooperation patterns between different countries/regions, with dark blue representing higher publication volumes and stronger red indicating closer collaboration. Particularly, the United States not only has the most publications but also engages in the most extensive cooperation with other countries.
Figure 6
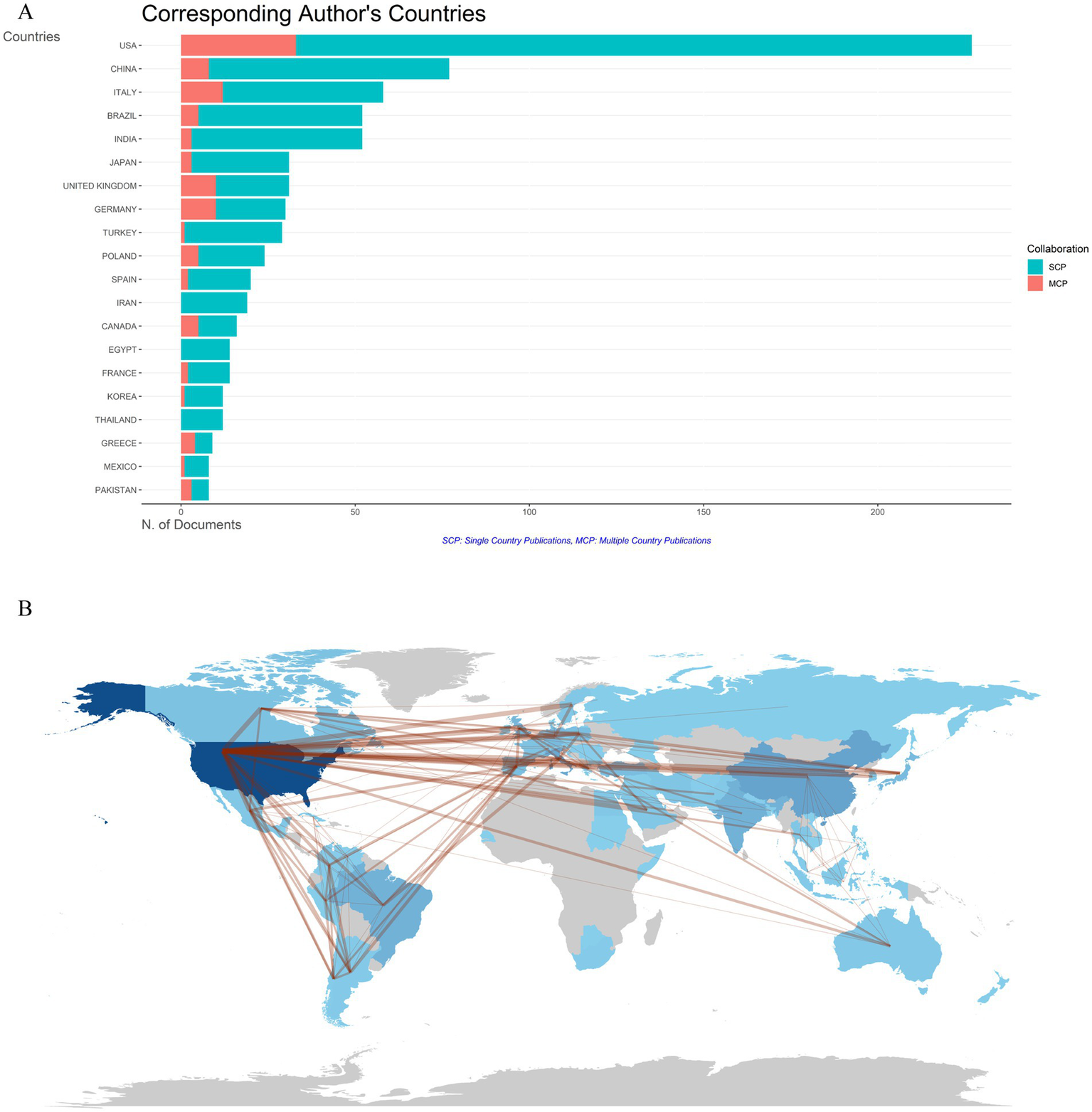
Countries/regions co-authorship analysis. (A) Histogram of cooperation in the top 20 productive countries/regions. The red section represents multiple country/region collaborative publications (MCP), and the green section defines single country/region publications (SCP). (B) Geographic maps in publication and collaboration of countries/regions.
3.6 Most cited articles
Table 2 presents the 20 most frequently cited articles in DLE research. The article with the highest citation count, titled “Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer,” was authored by Warnakulasuriya et al. and published in Oral Diseases in 2021 (27).
Table 2
| Article | Total citations |
|---|---|
| Warnakulasuriya et al. (27), Oral Dis | 496 |
| Thakor et al. (63), Nano Lett | 375 |
| Merrill et al. (64), Arthritis Rheum-US | 289 |
| Holland (65), Clin Rev Allerg Immu | 261 |
| Errichetti et al. (66), Dermatology Ther | 205 |
| Heckmann et al. (67), J Mol Biol | 187 |
| Holland (68), Hematol Oncol Clin N | 178 |
| Okon et al. (35), Best Pract Res Cl Rh | 177 |
| Sarkar et al. (69), Ann Rheum Dis | 161 |
| Frangou et al. (70), Ann Rheum Dis | 160 |
| Fallah et al. (71), Ann Oncol | 142 |
| Stefanato (72), Histopathology | 134 |
| Carrozzo et al. (73), Periodontol 2000 | 127 |
| Min et al. (74), Exp Dermatol | 125 |
| Osio-Salido et al. (75), Lupus | 117 |
| Prencipe et al. (76), J Allergy Clin Immun | 117 |
| Braunstein et al. (77), Brit J Dermatol | 114 |
| Wenzel et al. (36), Nat Rev Rheumatol | 114 |
| Gill et al. (78), J Am Acad Dermatol | 113 |
| Hemminki et al. (79), J Autoimmun | 110 |
Top 20 most highly cited articles of discoid lupus erythematosus.
3.7 Lotka’s and Bradford’s law
Lotka’s law describes the distribution of author productivity across various scientific disciplines, typically indicating that a small proportion of authors is responsible for the majority of scholarly output, while a larger group contributes relatively few publications (28). An analysis of author productivity in line with Lotka’s law (Figure 7A) shows that only a small subset of authors has published 10 or more articles (n = 3, 0.07%), whereas the vast majority (n = 3,624, 87.35%) have authored just a single publication. Notably, one author stands out with a remarkable contribution of 24 articles.
Figure 7
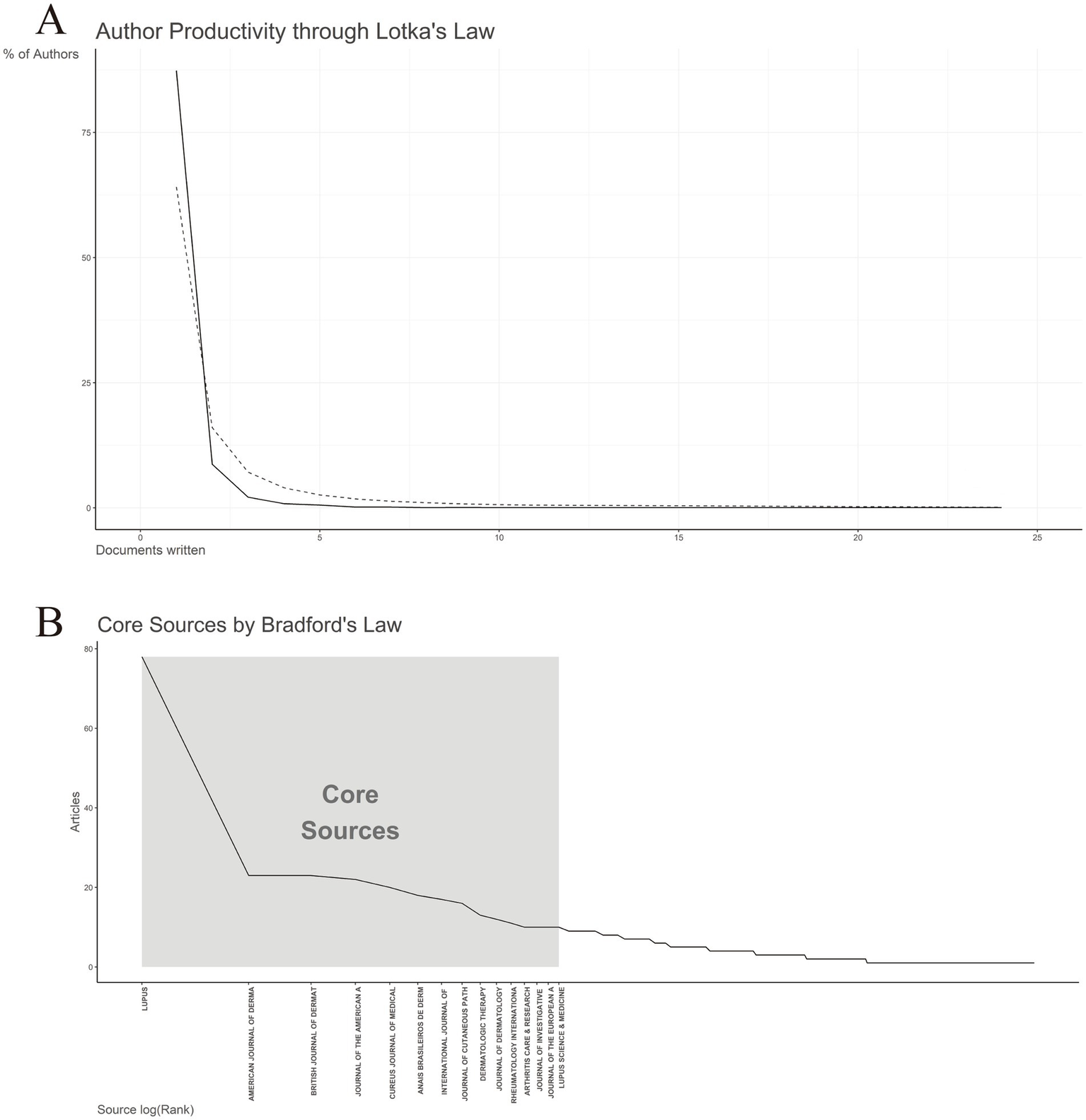
Lotka’s and Bradford’s law. (A) Author’s productivity analysis by Lotka’s Law. (B) Journals clustering through Bradford’s law.
Bradford’s law posits that journal citations are unevenly distributed across journals in any given subject area, with a small number of core journals accounting for a disproportionately large share of publications (29). As illustrated in Figure 7B, a few key journals, such as Lupus and American Journal of Dermatopathology, contributed approximately 60% of the total articles analyzed. These journals constitute the “core sources,” characterized by their high productivity and central role in disseminating research findings in the field. Beyond this core group, a set of secondary journals contributed a moderate number of publications, followed by a long tail of tertiary journals, each accounting for only a small fraction of the total output. This distribution underscores the concentration of knowledge in the field in a limited number of high-impact sources, aligning with Bradford’s theoretical framework. Identifying these journal zones (core, secondary, and tertiary) enhances researchers’ ability to target key publications and optimize literature searches in the field.
3.8 Co-cited references
Each of the co-cited references listed in Table 3 has been cited at least 40 times, with the most cited being HOCHBERG MC, 1997, ARTHRITIS RHEUM which has been co-cited over 100 times. A co-citation network analysis of the literature, as shown in Figure 8, identified two clusters, and the most cited article in the blue cluster, Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus, was published in Arthritis Rheumatology in 1997 (30), is central to the understanding of SLE and has significantly influenced the classification criteria for lupus-related disorders. This article’s extensive citation indicates its foundational role in advancing diagnostic criteria for lupus. The most cited article in the red cluster, Follicular red dots: a novel dermoscopic pattern observed in scalp discoid lupus erythematosus, was published in Archives of Dermatology in 2009 (31). Its high citation count underscores its significant impact on DLE diagnosis, providing valuable insights that have since been widely adopted in clinical practice. These articles underscore the critical contributions of diagnostic criteria to the ongoing research and clinical management of DLE.
Table 3
| Cited reference | Citations |
|---|---|
| Hochberg (30), Arthritis Rheum | 114 |
| Tan et al. (80), Arthritis Rheum | 81 |
| Petri et al. (81), Arthritis Rheum-US | 68 |
| Gilliam et al. (82), J Am Acad Dermatol | 61 |
| Albrecht et al. (83), J Invest Dermatol | 56 |
| Grönhagen et al. (84), Brit J Dermatol | 54 |
| Walling et al. (85), Am J Clin Dermatol | 53 |
| Durosaro et al. (86), Arch Dermatol | 52 |
| Okon et al. (35), Best Pract Res Cl Rh | 43 |
| Kuhn et al. (87), J Autoimmun | 40 |
Top 10 co-cited references in the field of discoid lupus erythematosus.
Figure 8
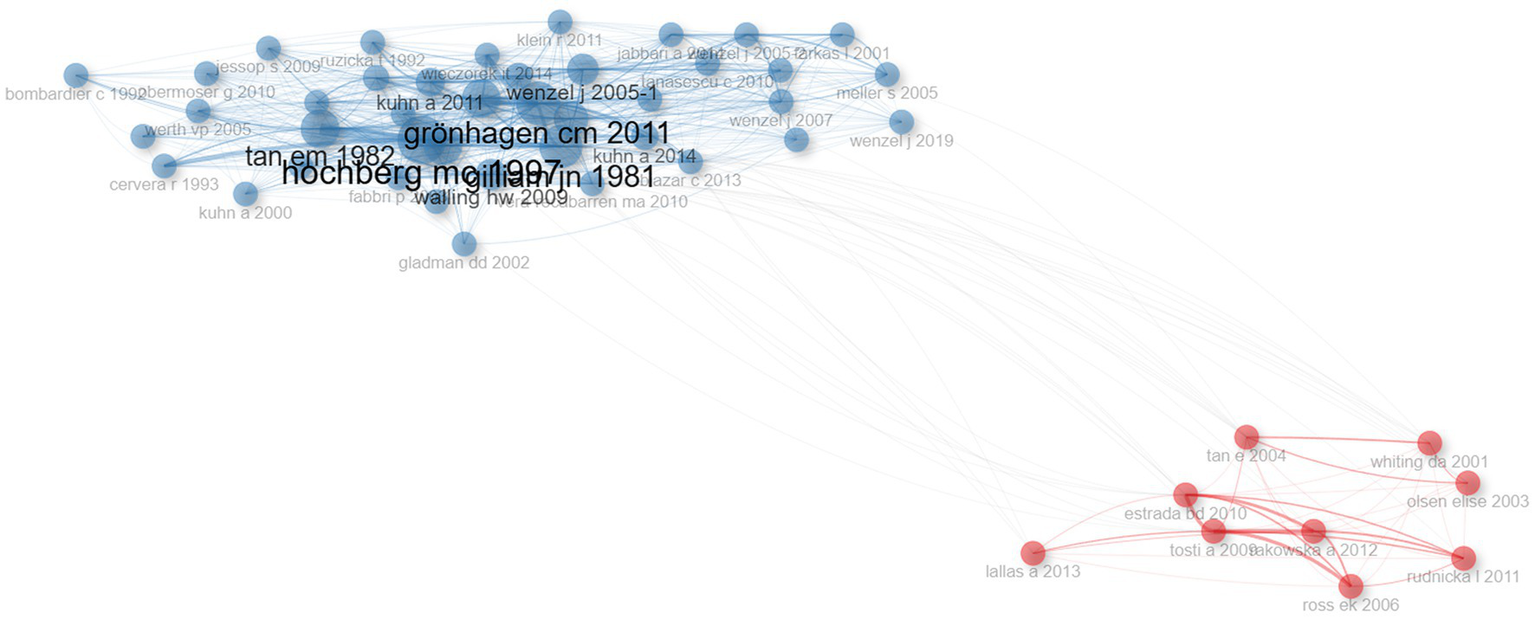
Co-citation network. Each node represents an article, and the connections between them reflect how often they are cited together. The size of the nodes corresponds to the number of citations, and the thickness of the lines indicates the strength of the co-citation relationship. The network helps to identify key studies and emerging research trends in the field.
3.9 Cluster analysis
By analyzing the coupling relationships between literature or authors, such as mutual citation, collaboration, or shared focus, we can categorize them into distinct groups (32). As shown in Figure 9A, two primary clusters were identified, each characterized by distinct research foci and attributes. The first cluster is labeled “classification - conf 68.3% disease - conf 65.9% revised criteria - conf 100%” The second cluster is labeled “diagnosis - conf 77.5% classification - conf 31.7% double-blind - conf 100%,” conf (confidence) represents the degree to which a particular keyword is associated with a given cluster. The first cluster predominantly includes key studies such as CHONG BF, 2012, BRIT J DERMATOL, WIECZOREK IT, 2014, JAMA DERMATOL (33, 34), which are fundamental in refining lupus classification. The second cluster primarily features important works by OKON LG, 2013, BEST PRACT RES CL RH, WENZEL J, 2019, NAT REV RHEUMATOL (35, 36), contributing to advancements in diagnostic techniques and clinical trials (Figure 9B). These clusters emphasize the continued focus on refining DLE diagnosis and classification.
Figure 9
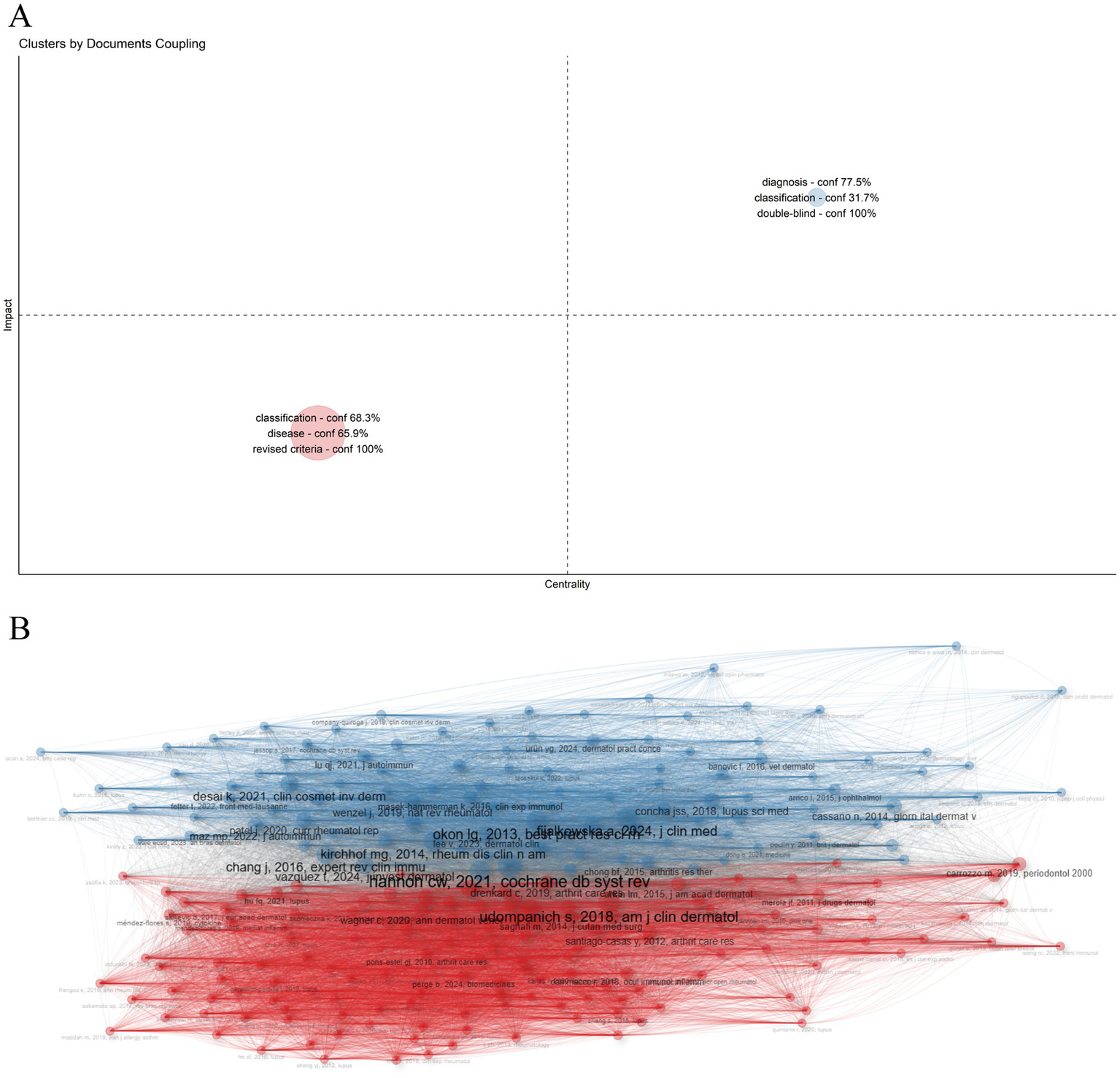
Cluster analysis. The network is divided into clusters, each representing a group of articles with similar co-citation patterns. Different colors distinguish the clusters, which highlight key research topics or trends in the field. (A) Cluster map. (B) Cluster by coupling network.
3.10 Identification and analysis of keywords
Keywords in the retrieved articles were analyzed to assess the key topics within the field of DLE. The word cloud in Figure 10A and the tree diagram in Figure 10B illustrate the most frequently occurring keywords. Markedly the top three keywords—diagnosis disease and classification—highlight the primary focus areas of research in this domain. The changing trends of hot keywords related to DLE over the past 15 years are depicted in Figure 10C. Conspicuously t-cells was the most persistent keyword appearing consistently from 2011 to 2020. In recent years emerging keywords such as trichoscopy interferon and clobetasol propionate have gained prominence highlighting treatment and diagnosis as the forefront of DLE research.
Figure 10
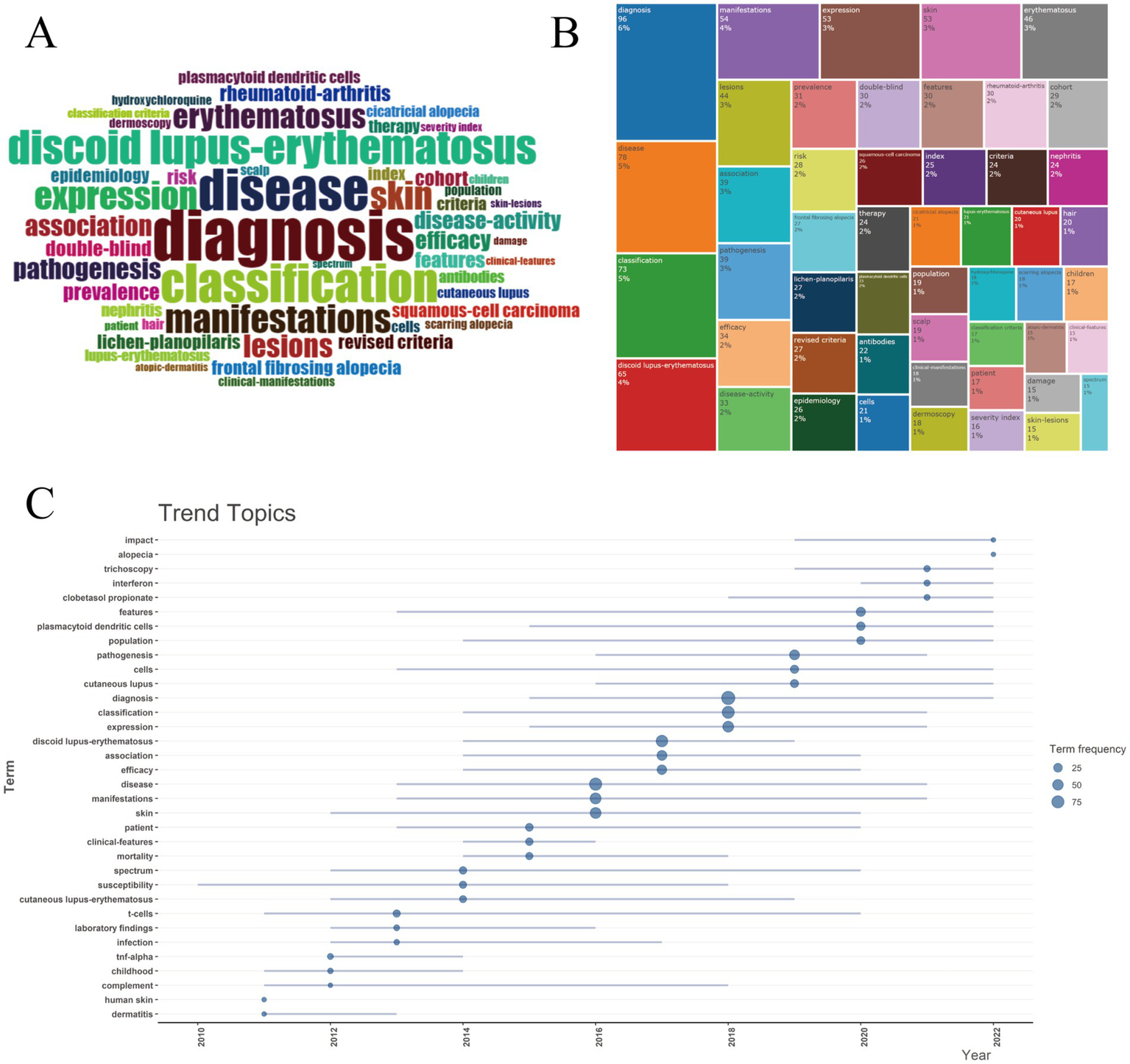
Keywords and trend topics. (A) Word cloud. (B) Tree map. (C) Trend topics. Circle size corresponds to keyword frequency counts (scale: 25 = 25 occurrences, 50 = 50 occurrences, 75 = 75 occurrences), and line length quantifies temporal span.
A comprehensive overview of DLE research themes, their structural relationships, and temporal evolution is provided in Figure 11. The co-occurrence network (Figure 11A) reveals 49 keywords grouped into five distinct clusters, highlighting key topics such as diagnosis, expression, classification, and manifestations. The thematic map (Figure 11B) categorizes research topics into four quadrants based on their relevance and stage of development. Motor themes (upper-right quadrant), such as therapy, efficacy, and double-blind studies, exhibit higher centrality and density values. Basic themes (lower-right quadrant), including disease, classification, and manifestations, are fundamental to understanding DLE. Emerging or decline themes (lower-left quadrant), such as squamous-cell carcinoma and lichen planus, are still under development. Niche themes (upper-left quadrant), including frontal fibrosing alopecia and cicatricial alopecia, are well-developed but remain at the periphery of the research domain. Factor analysis (Figure 11C) revealed four main clusters of research: diagnostic methods (discoid lupus erythematosus, diagnosis), clinical features and classification (classification, manifestations), basic research on molecular mechanisms (expression, therapy, pathogenesis), and specific scalp manifestations (frontal fibrosing alopecia, hair). These clusters emphasize that advancements in diagnosis and clinical classification remain central to understanding DLE. The thematic evolution (Figure 11D) reveals distinct temporal phases in DLE research: early studies (2010–2015) primarily focused on clinical descriptions and diagnosis, followed by a growing emphasis on classification and efficacy (2016–2020). In recent years (2021–2024), there has been rapid growth in niche topics, advanced therapies, and rare manifestations. This trend reflects the expanding scope of DLE research, encompassing both fundamental studies and clinical applications.
Figure 11

Thematic structure and evolution. (A) Co-occurrence network. (B) Thematic map. The x-axis represents the center and measures the relevance between topics, while the y-axis indicates the density. A higher density signifies a more mature area of research. (C) Factor analysis. Each factor represents a key theme in the research, helping to identify major trends and simplify complex data. (D) Thematic evolution.
4 Discussion
Discoid lupus erythematosus (DLE) presents both a medical challenge due to its physical manifestations and a significant psychosocial burden. The conspicuous facial lesions, in particular, can cause considerable psychological distress and reduce self-esteem, especially among young women who may be more concerned about their appearance (37). DLE recognized as a cutaneous manifestation of systemic lupus erythematosus (SLE) (38). It remains relatively underrepresented in the broader lupus literature compared to systemic lupus erythematosus (SLE), leading to notable gaps in our understanding of its pathogenesis, management, and outcomes. This bibliometric study aimed to address these gaps by comprehensively analyzing DLE-related publications from 2010 to 2024, focusing on scientific output trends, collaboration patterns, core journals, key authors, and emerging research fronts. The findings yield valuable insights into the developmental trajectory of DLE scholarship and provide a roadmap for future investigations.
4.1 Overall growth trends in DLE research
A total of 861 publications were retrieved from the Web of Science Core Collection (WoSCC) database, covering the period from January 2010 to November 2024. Our analysis reveals a fluctuating yet generally increasing annual output of DLE-related articles, with a more pronounced rise observed after 2018. The observed acceleration in DLE-related publications has coincided with the increasing clinical adoption of biologic therapies, such as anifrolumab, which were initially developed for SLE but also have shown efficacy in refractory cutaneous diseases, including DLE. Emerging case reports and clinical studies highlighting the novel application of these biologics may have contributed to renewed research interest in DLE therapies (39, 40). This upward trend indicates a growing scholarly interest in DLE, consistent with the broader global emphasis on autoimmune and autoinflammatory conditions (41, 42). However, the total number of citations demonstrated a downward trajectory from 2010 to 2024. This paradox—rising publication counts accompanied by decreasing average citations—may reflect several factors. Firstly, the proliferation of specialized journals and open-access platforms has increased the overall volume of output but resulted in a dispersion of citations across a broader range of venues (43). Additionally, DLE research often intersects with SLE and other autoimmune diseases, which often results in citation practices favoring high-impact journals focused on broader autoimmune research rather than those specifically dedicated to DLE. Moreover, articles published toward the end of the study period (e.g., 2022–2024) have had less time to accumulate citations, resulting in a temporal lag in citation metrics. Overall, while the growing quantity of DLE-related research reflects increased awareness, the declining citation density presents a potential challenge in consolidating findings, bridging research domains, and directing attention toward highly impactful works.
4.2 Core journals and application of Bradford’s law
Bradford’s law posits that literature within a specialized domain is primarily concentrated in a limited number of core journals, with productivity declining sharply in secondary and tertiary sources (44). In our study, Lupus (England) emerged as the highest-volume journal, publishing 78 articles, followed by the American Journal of Dermatopathology with 23 articles. This distribution exemplifies Bradford’s law, whereby key journals in a given area account for a disproportionately large share of publications—approximately 60% of the total—as depicted in Figure 7B. The concentration of DLE literature in these leading journals highlights the domain specialization inherent in the field, as journals like Lupus and American Journal of Dermatopathology serve as essential conduits for disseminating findings related to lupus pathogenesis, immunology, and dermatologic manifestations. Additionally, authors preferentially submit high-quality work to these journals due to their long-standing reputations, established editorial boards, and recognized expertise in lupus research. However, while this specialization fosters in-depth coverage, it may also limit cross-disciplinary integration, making it more challenging for fields such as immunology, rheumatology, and dermatology to converge around DLE-focused studies. Therefore, expanding DLE research dissemination across multidisciplinary fields remains critical, especially as emerging therapeutic strategies increasingly intersect with rheumatology, immunology, and genetics (45, 46).
4.3 Leading authors and collaboration networks
Consistent with Lotka’s law, our study revealed that a small number of authors produced the majority of publications (47), while the vast majority authored only a single article (87.35%). This pattern is particularly evident in a condition such as DLE, which remains relatively niche compared to more common dermatological or autoimmune disorders (e.g., rheumatoid arthritis). A few highly engaged experts such as WERTH VP and CHONG BF lead the field through highly cited publications.
Bibliometric mapping uncovered 13 distinct author clusters (Figure 5), reflecting cohesive research collaborations. The structure of these author networks indicates that certain clusters are centered around shared institutional affiliations (e.g., University of Pennsylvania, Northwestern University), while others are driven by thematic overlaps, such as immunological aspects of lupus. Identifying these clusters allows new and aspiring investigators to pinpoint established teams, thereby fostering meaningful partnerships and multi-center collaborations. Strong collaborative patterns are often correlated with increased scientific impact (48). Indeed, multi-institutional and international collaborations not only amplify sample sizes and geographic diversity but also integrate interdisciplinary expertise—from immunologists and rheumatologists to dermatologists and geneticists. Given the complexity of lupus pathogenesis, robust collaborations are essential for advancing personalized therapies, refining diagnostic criteria, and improving patient quality of life.
4.4 Countries and international cooperation
As illustrated in Figures 6A,B, the United States leads in both publication volume and collaborative linkages, followed by Italy, the United Kingdom, Germany, and China. This geopolitical distribution aligns with broader trends in lupus and immunology research, where high-income countries with well-established research infrastructures dominate the field. Especially, China’s emergence among the top five contributors highlights its growing focus on autoimmune diseases, driven by substantial governmental funding, rapid economic development, and a significant increase in academic output (49). Autoimmune diseases demonstrate varying prevalence and distinct clinical presentations across regions, shaped by genetic and environmental factors (50, 51). Strengthening international partnerships can accelerate the identification of novel biomarkers, epigenetic mechanisms, and advanced therapeutics to address diverse disease phenotypes.
4.5 Keyword and cluster analysis
Keyword analysis revealed shifting research emphases in DLE over time. Early studies (2010–2015) focused on basic clinical descriptions such as disease and diagnosis alongside broad immunopathological processes. Between 2016 and 2020 the focus expanded to include classification efficacy and therapy. Recent years have witnessed a rise in niche topics including trichoscopy interferon and clobetasol propionate reflecting advancements in dermatologic diagnostic methods for scalp lesions and novel immunotherapeutic strategies targeting the interferon pathway (52, 53). These trends highlight significant strides in specialized areas like advanced diagnostic techniques (e.g., dermoscopy trichoscopy) and immunopathological research including cytokine and chemokine profiling (54, 55). While clobetasol propionate a potent topical corticosteroid is a novel treatment for DLE (56). Its efficacy stems from its strong anti-inflammatory and immunosuppressive properties which help reduce interface dermatitis and prevent irreversible scarring clinical studies have demonstrated that in patients with DLE topical 0.05% clobetasol propionate ointment offers significantly greater therapeutic benefits than 0.1% tacrolimus ointment (57). To improve its safety profile novel delivery systems are under investigation. For instance clobetasol propionate–loaded nanosponge hydrogels enable controlled drug release and markedly reduce adverse effects such as skin atrophy (58). However these innovations remain in preclinical or early-phase development with no registered clinical trials specifically targeting DLE to date.
Cluster analysis identified distinct thematic groupings in DLE research. The first cluster centered on classification and revised diagnostic criteria, highlighting ongoing efforts to refine disease definitions and outcomes, particularly given the overlap between DLE and SLE. The second cluster focused on diagnostic methods and double-blind studies, aiming to differentiate DLE from conditions such as psoriasis, lichen planus, and other forms of cutaneous lupus. These studies emphasize the growing demand for evidence-based interventions. The others showcased scalp-specific presentations of DLE, including frontal fibrosing alopecia, cicatricial alopecia, and associated dermoscopy patterns. Typical dermoscopic findings in DLE include white structureless areas, arborizing vessels, white scales, and follicular keratin plugs—features that help distinguish it from other alopecias (59, 60). Dermoscopy can also reveal characteristic features in non-scalp regions, including the face, trunk, and limbs, where keratotic follicular plugs, peripilar white halos, and surface scaling are frequently observed. Recognizing these patterns is essential for early diagnosis and timely intervention in DLE (61).
4.6 Most cited articles and co-citation analysis
Interestingly, the most cited article, authored by Warnakulasuriya in 2021 (27), focuses on oral potentially malignant disorders. While this topic may initially seem tangential to DLE, it highlights the intersection between mucocutaneous lesions and the broader field of oral diseases. Consistent co-citations indicate that these works provide foundational conceptual or methodological contributions, such as defining DLE, advancing imaging techniques for diagnosis, or refining classification criteria. The strong co-citation of the 1997 American College of Rheumatology criteria (30) reflects their enduring relevance. These criteria align with ongoing efforts to refine lupus classification, aiming to distinguish systemic and discoid manifestations and guide clinical trial enrollment effectively.
4.7 Clinical and research significance
DLE presents diagnostic and therapeutic challenges due to its variable clinical spectrum, overlap with SLE, and distinct subtypes (62). This bibliometric study highlights diagnosis and classification as crucial themes, emphasizing the need for refined diagnostic algorithms to improve early detection, reduce misdiagnosis, and optimize interventions. Thematic evolution reveals a growing focus on therapy and efficacy, while hydroxychloroquine remain standard treatments, future strategies are likely to incorporate novel immunomodulators in combination regimens.
4.8 Strengths and limitations
This study offers several strengths. The analysis spans from 2010 to 2024, capturing the recent surge in DLE research and providing a comprehensive overview of current priorities and collaboration patterns. It incorporates diverse metrics such as co-authorship, co-citation, keywords co-occurrence, thematic mapping, and factor analyses. Additionally, identifying leading authors, core journals, and key topics establishes a structured knowledge base that clinicians and researchers can use to guide new projects, prioritize funding, and advance translational research.
However, some limitations should be noted. Restricting data retrieval to WoSCC may exclude relevant articles indexed in PubMed, Scopus, or other databases, potentially underrepresenting the global literature. The inclusion of only English-language articles introduces a bias against research from non-English-speaking regions. Finally, bibliometric measures may undervalue recent high-quality studies due to citation lag and do not account for the sentiment of citations (positive, neutral, or negative).
4.9 Future directions
Refining diagnostic criteria for DLE remains a critical priority. Advanced molecular and genetic studies, such as single-cell RNA sequencing, can provide insights into disease heterogeneity and identify personalized therapeutic targets. Large-scale studies are needed to evaluate the efficacy of biologics and small-molecule inhibitors while exploring combination therapies with conventional agents like hydroxychloroquine. Expanding collaborations with underrepresented regions could improve the global applicability of findings and uncover unique genetic or environmental factors influencing disease expression. Furthermore, leveraging AI and machine learning with clinical datasets and imaging repositories has the potential to transform diagnostics, refine classification systems, and accelerate drug discovery.
5 Conclusion
This bibliometric study highlights the increasing scholarly focus on discoid lupus erythematosus (DLE) from 2010 to 2024, despite relatively low average citation rates. Key journals, such as Lupus, dominate publication output, and the United States lead in research collaborations. The initial emphasis on diagnostic criteria and disease classification has progressively shifted toward advanced therapies and immunological mechanisms. Expanding collaborations and conducting deeper molecular investigations are essential for refining DLE management and improving patient outcomes.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DZ: Conceptualization, Formal analysis, Writing – original draft. CL: Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. QY: Data curation, Validation, Writing – review & editing. YL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ugonabo N Levin M . Treatment of hyperpigmented discoid lupus erythematosus with Jessner's peel: a case report. J Drugs Dermatol. (2022) 21:92–3. doi: 10.36849/JDD.5521
2.
Molomo EM Bouckaert M Khammissa RA Motswaledi HM Lemmer J Feller L . Discoid lupus erythematosus-related squamous cell carcinoma of the lip in an HIV-seropositive black male. J Cancer Res Ther. (2015) 11:1036. doi: 10.4103/0973-1482.146107
3.
Shah A Albrecht J Bonilla-Martinez Z Okawa J Rose M Rosenbach M et al . Lenalidomide for the treatment of resistant discoid lupus erythematosus. Arch Dermatol. (2009) 145:303–6. doi: 10.1001/archdermatol.2009.30
4.
Chen J Gao Y Chai Y Gao H . A 30-year-old woman with a history of autoimmune hyperthyroidism presenting with fever and Oral ulcers, diagnosed with discoid lupus erythematosus. Am J Case Rep. (2023) 24:e938988. doi: 10.12659/ajcr.938988
5.
Zhang B Zhou T Wu H Zhao M Lu Q . Difference of IFI44L methylation and serum IFN-a1 level among patients with discoid and systemic lupus erythematosus and healthy individuals. J Transl Autoimmun. (2021) 4:100092. doi: 10.1016/j.jtauto.2021.100092
6.
Patil MK Salazar CE Nayudu K Rohan TZ Nambudiri VE . Comorbidities associated with discoid lupus erythematosus: a case-control study in the all of us research program. Exp Dermatol. (2024) 33:e15162. doi: 10.1111/exd.15162
7.
Faden DF Xie L Stone C Lopes Almeida Gomes L Le T Ezeh N et al . Area deprivation and disease severity in adult patients with discoid lupus erythematosus. JAMA Dermatol. (2024) 160:984–8. doi: 10.1001/jamadermatol.2024.2355
8.
Motswaledi MH Khammissa RA Wood NH Meyerov R Lemmer J Feller L . Discoid lupus erythematosus as it relates to cutaneous squamous cell carcinoma and to photosensitivity. SADJ. (2011) 66:340–3. PMID:
9.
Sáenz-Corral CI Vega-Memíje ME Martínez-Luna E Cuevas-González JC Rodríguez-Carreón AA de la Rosa JJ et al . Apoptosis in chronic cutaneous lupus erythematosus, discoid lupus, and lupus profundus. Int J Clin Exp Pathol. (2015) 8:7260–5. PMID:
10.
Wu H Chang C Lu Q . The epigenetics of lupus erythematosus. Adv Exp Med Biol. (2020) 1253:185–207. doi: 10.1007/978-981-15-3449-2_7
11.
Wahie S Meggitt SJ . Long-term response to hydroxychloroquine in patients with discoid lupus erythematosus. Br J Dermatol. (2013) 169:653–9. doi: 10.1111/bjd.12378
12.
Jessop S Whitelaw DA Delamere FM . Delamere: drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. (2009) 7:Cd002954. doi: 10.1002/14651858.CD002954.pub2
13.
Domingo S Solé C Moliné T Ferrer B Ordi-Ros J Cortés-Hernández J . Efficacy of thalidomide in discoid lupus erythematosus: insights into the molecular mechanisms. Dermatology. (2020) 236:467–76. doi: 10.1159/000508672
14.
Singh JA Shah NP Mudano AS . Belimumab for systemic lupus erythematosus. Cochrane Database Syst Rev. (2021) 2:Cd010668. doi: 10.1002/14651858.CD010668.pub2
15.
Depascale R Gatto M Zen M Saccon F Larosa M Zanatta E et al . Belimumab: a step forward in the treatment of systemic lupus erythematosus. Expert Opin Biol Ther. (2021) 21:563–73. doi: 10.1080/14712598.2021.1895744
16.
Wei T Xiao F He X Peng P He W He M et al . A bibliometric analysis and visualization of research trends on periacetabular osteotomy. J Hip Preserv Surg. (2023) 10:181–91. doi: 10.1093/jhps/hnad038
17.
Ganesh C Victor Samuel A Purushothaman D Magesh KT Vivek N . Bibliometric analysis of dental caries detection. Cureus. (2023) 15:e40741. doi: 10.7759/cureus.40741
18.
Kim SJ Lee DW . Publication trends in osteoporosis treatment: a 20-year bibliometric analysis. J Bone Metab. (2024) 31:90–100. doi: 10.11005/jbm.2024.31.2.90
19.
Berlinberg A Bilal J Riaz IB Kurtzman DJB . The 100 top-cited publications in psoriatic arthritis: a bibliometric analysis. Int J Dermatol. (2019) 58:1023–34. doi: 10.1111/ijd.14261
20.
Liu GY Yan MD Mai YY Fu FJ Pan L Zhu JM et al . Frontiers and hotspots in anxiety disorders: a bibliometric analysis from 2004 to 2024. Heliyon. (2024) 10:e35701. doi: 10.1016/j.heliyon.2024.e35701
21.
Du Q Zhao R Wan Q Li S Li H Wang D et al . Protocol for conducting bibliometric analysis in biomedicine and related research using cite space and VOSviewer software. STAR Protoc. (2024) 5:103269. doi: 10.1016/j.xpro.2024.103269
22.
Vaitsi GA Bourganou MV Lianou DT Kiouvrekis Y Michael CC Gougoulis DA et al . Scientometric analysis: an emerging tool in veterinary and animal scientific research. Animals. (2024) 14:3132. doi: 10.3390/ani14213132
23.
Zhou Q Pei J Poon J Lau AY Zhang L Wang Y et al . Worldwide research trends on aristolochic acids (1957-2017): suggestions for researchers. PLoS One. (2019) 14:e0216135. doi: 10.1371/journal.pone.0216135
24.
Nourbakhsh E Nugent R Wang H Cevik C Nugent K . Medical literature searches: a comparison of PubMed and Google scholar. Health Inf Libr J. (2012) 29:214–22. doi: 10.1111/j.1471-1842.2012.00992.x
25.
Anker MS Hadzibegovic S Lena A Haverkamp W . The difference in referencing in web of science, Scopus, and Google scholar. ESC Heart Fail. (2019) 6:1291–312. doi: 10.1002/ehf2.12583
26.
Aria M Cuccurullo C . Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2017) 11:959–75. doi: 10.1016/j.joi.2017.08.007
27.
Warnakulasuriya S Kujan O Aguirre-Urizar JM Bagan JV González-Moles M Kerr AR et al . Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO collaborating Centre for oral cancer. Oral Dis. (2021) 27:1862–80. doi: 10.1111/odi.13704
28.
Kushairi N Ahmi A . Flipped classroom in the second decade of the Millenia: a Bibliometrics analysis with Lotka's law. Educ Inf Technol. (2021) 26:4401–31. doi: 10.1007/s10639-021-10457-8
29.
Venable GT Shepherd BA Loftis CM McClatchy SG Roberts ML Fillinger ME et al . Bradford's law: identification of the core journals for neurosurgery and its subspecialties. J Neurosurg. (2016) 124:569–79. doi: 10.3171/2015.3.Jns15149
30.
Hochberg MC . Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1997) 40:1725. doi: 10.1002/art.1780400928
31.
Tosti A Torres F Misciali C Vincenzi C Starace M Miteva M et al . Follicular red dots: a novel dermoscopic pattern observed in scalp discoid lupus erythematosus. Arch Dermatol. (2009) 145:1406–9. doi: 10.1001/archdermatol.2009.277
32.
Lv Z Hou J Wang Y Wang X Wang Y Wang K . Knowledge-map analysis of bladder cancer immunotherapy. Hum Vaccin Immunother. (2023) 19:2267301. doi: 10.1080/21645515.2023.2267301
33.
Chong BF Song J Olsen NJ . Determining risk factors for developing systemic lupus erythematosus in patients with discoid lupus erythematosus. Br J Dermatol. (2012) 166:29–35. doi: 10.1111/j.1365-2133.2011.10610.x
34.
Wieczorek IT Propert KJ Okawa J Werth VP . Systemic symptoms in the progression of cutaneous to systemic lupus erythematosus. JAMA Dermatol. (2014) 150:291–6. doi: 10.1001/jamadermatol.2013.9026
35.
Okon LG Werth VP . Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. (2013) 27:391–404. doi: 10.1016/j.berh.2013.07.008
36.
Wenzel J . Cutaneous lupus erythematosus: new insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol. (2019) 15:519–32. doi: 10.1038/s41584-019-0272-0
37.
Martins PR Skare T Ferrari TA Silva AP Alessio BF . Comparative analysis of the quality of life of patients with discoid lupus erythematosus and systemic lupus erythematosus with skin injuries. An Bras Dermatol. (2012) 87:326–8. doi: 10.1590/s0365-05962012000200028
38.
Drucker AM Su J Mussani F Siddha SK Gladman DD Urowitz MB . Prognostic implications of active discoid lupus erythematosus and malar rash at the time of diagnosis of systemic lupus erythematosus: results from a prospective cohort study. Lupus. (2016) 25:376–81. doi: 10.1177/0961203315610645
39.
Ambrogio F Foti C Noviello S Cazzato G Meduri AR Marasco C et al . Can Dermoscopy be a useful follow-up tool in patients with discoid lupus treated with Anifrolumab?Diagnostics. (2025) 15:522. doi: 10.3390/diagnostics15050522
40.
Karagenova R Vodusek Z Krimins R Krieger A Timlin H . Treatment with Voclosporin and Anifrolumab in a patient with lupus nephritis and refractory discoid lupus erythematosus: a case report and literature review. Cureus. (2024) 16:e55321. doi: 10.7759/cureus.55321
41.
Szekanecz Z McInnes IB Schett G Szamosi S Benkő S Szűcs G . Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat Rev Rheumatol. (2021) 17:585–95. doi: 10.1038/s41584-021-00652-9
42.
Havnaer A Han G . Autoinflammatory disorders: a review and update on pathogenesis and treatment. Am J Clin Dermatol. (2019) 20:539–64. doi: 10.1007/s40257-019-00440-y
43.
Clayson PE Baldwin SA Larson MJ . The open access advantage for studies of human electrophysiology: impact on citations and Altmetrics. Int J Psychophysiol. (2021) 164:103–11. doi: 10.1016/j.ijpsycho.2021.03.006
44.
Desai N Veras L Gosain A . Using Bradford's law of scattering to identify the core journals of pediatric surgery. J Surg Res. (2018) 229:90–5. doi: 10.1016/j.jss.2018.03.062
45.
Di Cerbo A Giusti S Mariotti F Spaterna A Rossi G Magi GE . Increased expression of toll-like receptor 4 in skin of dogs with discoid lupus erythematosus (DLE). Animals. (2021) 11:1044. doi: 10.3390/ani11041044
46.
Bays A Gardner GC . Newer therapies in rheumatology. Med Clin North Am. (2024) 108:829–42. doi: 10.1016/j.mcna.2024.02.004
47.
Schubert A Schubert G . Law-making "pioneers" of scientometrics. Orv Hetil. (2016) 157:74–8. doi: 10.1556/650.2015.30279
48.
Mitrović I Mišić M Protić J . Exploring high scientific productivity in international co-authorship of a small developing country based on collaboration patterns. J Big Data. (2023) 10:64. doi: 10.1186/s40537-023-00744-1
49.
Marten R McIntyre D Travassos C Shishkin S Longde W Reddy S et al . An assessment of progress towards universal health coverage in Brazil, Russia, India, China, and South Africa (BRICS). Lancet. (2014) 384:2164–71. doi: 10.1016/s0140-6736(14)60075-1
50.
Conrad N Misra S Verbakel JY Verbeke G Molenberghs G Taylor PN et al . Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. (2023) 401:1878–90. doi: 10.1016/s0140-6736(23)00457-9
51.
Solomon O Lanata CM Adams C Nititham J Taylor KE Chung SA et al . Local ancestry at the major histocompatibility complex region is not a major contributor to disease heterogeneity in a multiethnic lupus cohort. Arthritis Rheumatol. (2024) 76:614–9. doi: 10.1002/art.42766
52.
Milan E Vezzoni R Starace MVR . Trichoscopy of discoid lupus erythematosus in Caucasian scalp: a review. Skin Appendage Disord. (2024) 10:363–9. doi: 10.1159/000539189
53.
Azuma N Natsuaki M Hashimoto N Abe T Ueda S Ohno Y et al . Efficacy of anifrolumab in long-term intractable alopecia due to discoid lupus erythematosus. Mod Rheumatol Case Rep. (2024) 8:267–71. doi: 10.1093/mrcr/rxae018
54.
Gowda SK Errichetti E Thakur V Panda M Dash S Agarwal A et al . Trichoscopic features of scalp discoid lupus erythematosus versus lichen planopilaris: a systematic review. Clin Cosmet Investig Dermatol. (2024) 17:805–27. doi: 10.2147/ccid.S460742
55.
Amudzi AA Piedra-Mora C Ma DJ Wong NB David CN Robinson NA et al . Using gene expression analysis to understand complex autoimmune skin disease patients: a series of four canine cutaneous lupus erythematosus cases. Front Vet Sci. (2022) 9:778934. doi: 10.3389/fvets.2022.778934
56.
Fukasawa T Yoshizaki-Ogawa A Enomoto A Miyagawa K Sato S Yoshizaki A . Efficient topical treatments of cutaneous lupus erythematosus: a systematic review and network -meta-analysis. Clin Exp Dermatol. (2024) 50:21–8. doi: 10.1093/ced/llae236
57.
Pothinamthong P Janjumratsang P . A comparative study in efficacy and safety of 0.1% tacrolimus and 0.05% clobetasol propionate ointment in discoid lupus erythematosus by modified cutaneous lupus erythematosus disease area and severity index. J Med Assoc Thail. (2012) 95:933–40. PMID:
58.
Kumar S Prasad M Rao R . Topical delivery of clobetasol propionate loaded nanosponge hydrogel for effective treatment of psoriasis: formulation, physicochemical characterization, antipsoriatic potential and biochemical estimation. Mater Sci Eng C Mater Biol Appl. (2021) 119:111605. doi: 10.1016/j.msec.2020.111605
59.
Duque-Estrada B Tamler C Sodré CT Barcaui CB Pereira FB . Dermoscopy patterns of cicatricial alopecia resulting from discoid lupus erythematosus and lichen planopilaris. An Bras Dermatol. (2010) 85:179–83. doi: 10.1590/s0365-05962010000200008
60.
Lallas A Apalla Z Lefaki I Sotiriou E Lazaridou E Ioannides D et al . Dermoscopy of discoid lupus erythematosus. Br J Dermatol. (2013) 168:284–8. doi: 10.1111/bjd.12044
61.
Żychowska M Żychowska M . Dermoscopy of discoid lupus erythematosus - a systematic review of the literature. Int J Dermatol. (2021) 60:818–28. doi: 10.1111/ijd.15365
62.
Prieto-Torres L Morales-Moya AL Garcia-Garcia M Aldea-Manrique B Ara M Requena L . Discoid lupus erythematosus presenting with disfiguring acneiform plaques: a diagnostic challenge. Am J Dermatopathol. (2021) 43:e95–7. doi: 10.1097/dad.0000000000001945
63.
Thakor AS Jokerst J Zavaleta C Massoud TF Gambhir SS . Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett. (2011) 11:4029–36. doi: 10.1021/nl202559p
64.
Merrill JT Burgos-Vargas R Westhovens R Chalmers A D’Cruz D Wallace DJ et al . The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. (2010) 62:3077–87. doi: 10.1002/art.27601
65.
Holland SM . Chronic granulomatous disease. Clin Rev Allergy Immunol. (2010) 38:3–10. doi: 10.1007/s12016-009-8136-z
66.
Errichetti E Stinco G . Dermoscopy in General Dermatology: A Practical Overview, Dermatol Ther (Heidelb). (2016) 6:471–507. doi: 10.1007/s13555-016-0141-6
67.
Heckmann BL Boada-Romero E Cunha LD Magne J Green DR . LC3-Associated Phagocytosis and Inflammation. J Mol Biol. (2017) 429:3561–3576. doi: 10.1016/j.jmb.2017.08.012
68.
Holland SM . Chronic granulomatous disease. Hematol Oncol Clin North Am. (2013) 27:89–99. doi: 10.1016/j.hoc.2012.11.002
69.
Sarkar MK Hile GA Tsoi LC Xing X Liu J Liang Y et al . Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann Rheum Dis. (2018) 77:1653–1664. doi: 10.1136/annrheumdis-2018-213197
70.
Frangou E Chrysanthopoulou A Mitsios A Kambas K Arelaki S Angelidou I et al . REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann Rheum Dis. (2019) 78:238–248. doi: 10.1136/annrheumdis-2018-213181
71.
Fallah M Liu X Ji J Försti A Sundquist K Hemminki K . Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. (2014) 25:2025–2030. doi: 10.1093/annonc/mdu365
72.
Stefanato CM . Histopathology of alopecia: a clinicopathological approach to diagnosis. Histopathology. (2010) 56:24–38. doi: 10.1111/j.1365-2559.2009.03439.x
73.
Carrozzo M Porter S Mercadante V Fedele S . Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontol. (2019) 2000:105–125. doi: 10.1111/prd.12260
74.
Min D Lee W Bae IH Lee TR Croce P Yoo SS . Bioprinting of biomimetic skin containing melanocytes. Exp Dermatol. (2018) 27:453–459. doi: 10.1111/exd.13376
75.
Osio-Salido E Manapat-Reyes H . Epidemiology of systemic lupus erythematosus in Asia. Lupus. (2010) 19:1365–73. doi: 10.1177/0961203310374305
76.
Prencipe G Caiello I Pascarella A Grom AA Bracaglia C Chatel L et al . Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol. (2018) 141:1439–1449. doi: 10.1016/j.jaci.2017.07.021
77.
Braunstein I Klein R Okawa J Werth VP . The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol. (2012) 166:971–5. doi: 10.1111/j.1365-2133.2012.10825.x
78.
Gill L Zarbo A Isedeh P Jacobsen G Lim HW Hamzavi I . Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. J Am Acad Dermatol. (2016) 74:295–302. doi: 10.1016/j.jaad.2015.08.063
79.
Hemminki K Li X Sundquist J Sundquist K . The epidemiology of Graves’ disease: evidence of a genetic and an environmental contribution. J Autoimmun. (2010) 34:J307–13. doi: 10.1016/j.jaut.2009.11.019
80.
Tan EM Cohen AS Fries JF Masi AT McShane DJ Rothfield NF et al . The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. (1982) 25:1271–7. doi: 10.1002/art.1780251101
81.
Petri M Orbai AM Alarcón GS Gordon C Merrill JT Fortin PR et al . Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis & Rheumatism, (2012) 64:2677–2686.
82.
Gilliam JN Sontheimer RD . Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol. (1981) 4:471–5. doi: 10.1016/s0190-9622(81)80261-7
83.
Albrecht J Taylor L Berlin JA Dulay S Ang G Fakharzadeh S et al . The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. (2005) 125:889–94. doi: 10.1111/j.0022-202X.2005.23889.x
84.
Grönhagen CM Fored CM Granath F Nyberg F . Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. (2011) 164:1335–41. doi: 10.1111/j.1365-2133.2011.10272.x
85.
Walling HW Sontheimer RD . Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. (2009) 10:365–81. doi: 10.2165/11310780-000000000-00000
86.
Durosaro O Davis MD Reed KB Rohlinger AL . Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. (2009) 145:249–53. doi: 10.1001/archdermatol.2009.21
87.
Kuhn A Landmann A . The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. (2014) 48:14–19. doi: 10.1016/j.jaut.2014.01.021
Summary
Keywords
discoid lupus erythematosus, bibliometric analysis, collaboration networks, diagnostic criteria, immunology
Citation
Zhang D, Liang C, Yin Q and Li Y (2025) Trends and collaborations in discoid lupus erythematosus research: a bibliometric analysis from 2010 to 2024. Front. Med. 12:1556976. doi: 10.3389/fmed.2025.1556976
Received
07 January 2025
Accepted
30 May 2025
Published
20 June 2025
Volume
12 - 2025
Edited by
Robert Gniadecki, University of Alberta, Canada
Reviewed by
Albert E. Zhou, UCONN Health, United States
João Ferreira, Universidade de Lisboa, Portugal
Gustavo Almeida-Silva, Santa Maria Hospital, Portugal
Updates
Copyright
© 2025 Zhang, Liang, Yin and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaoran Liang, 15513632930@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.